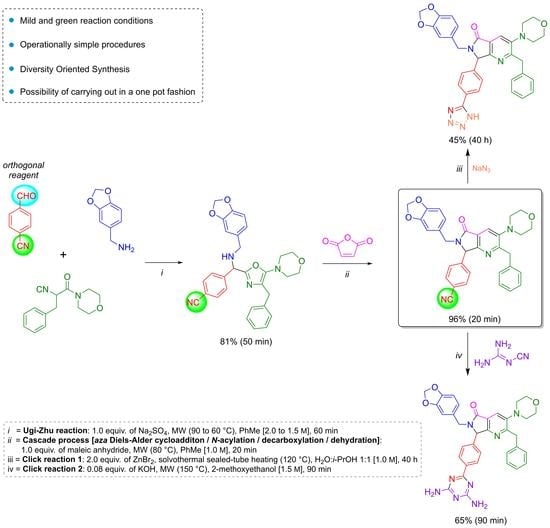
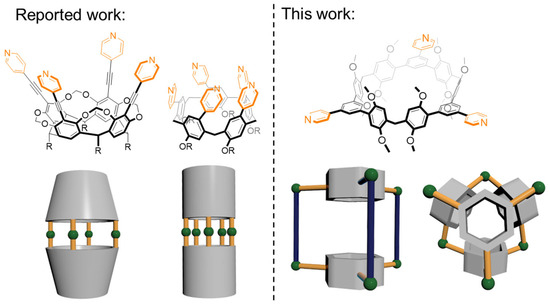
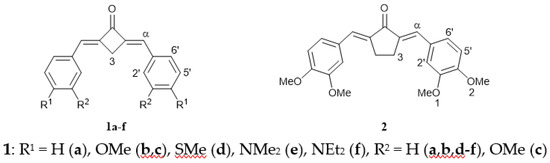
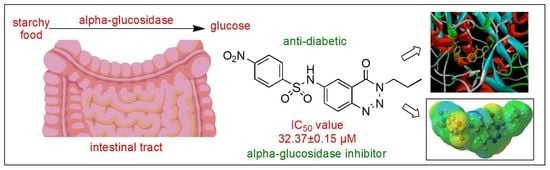
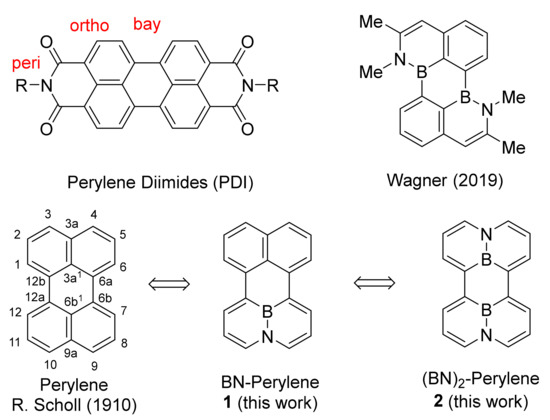
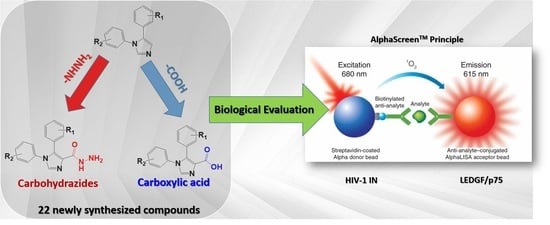
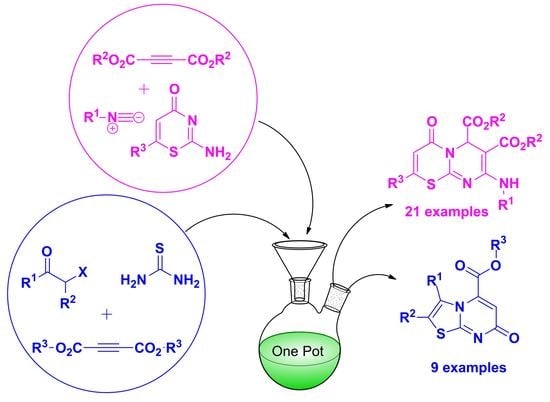
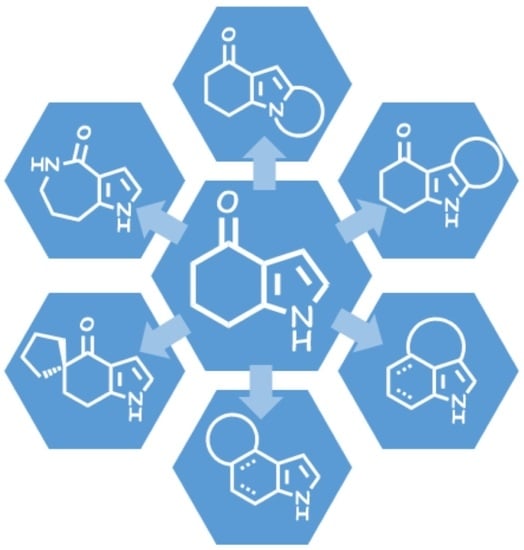
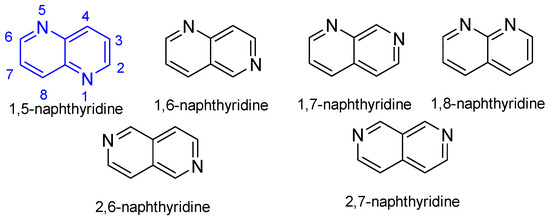
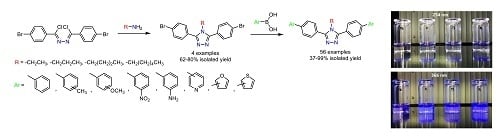
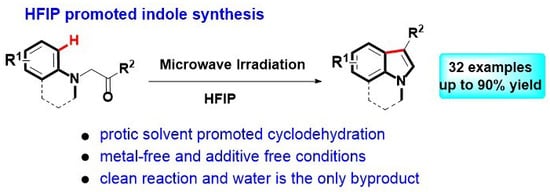
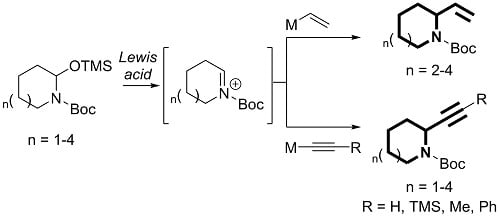
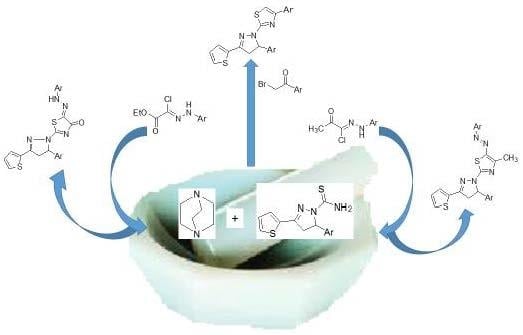
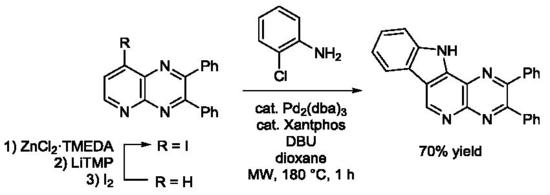
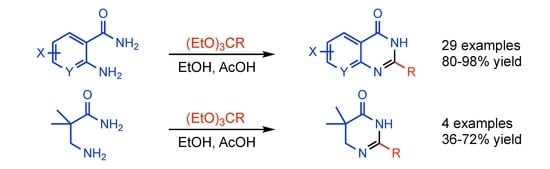
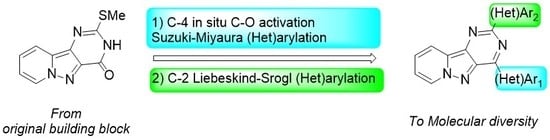
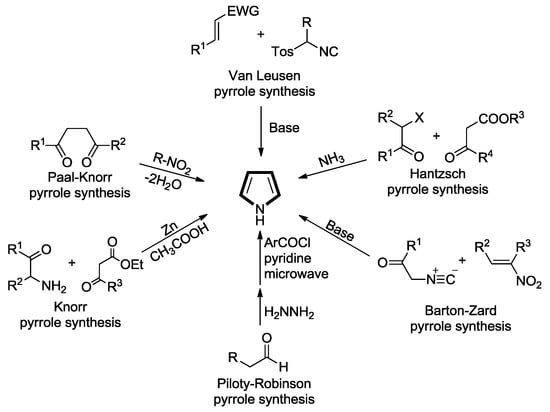
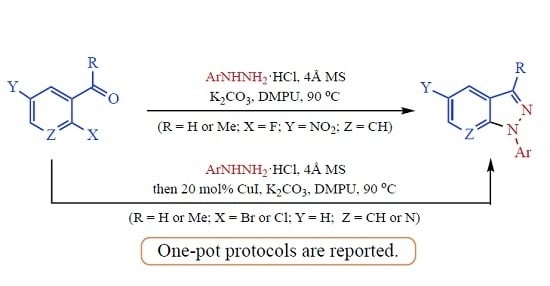
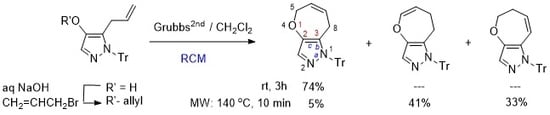
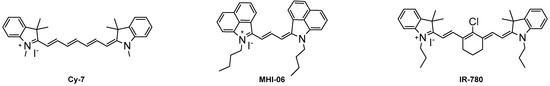
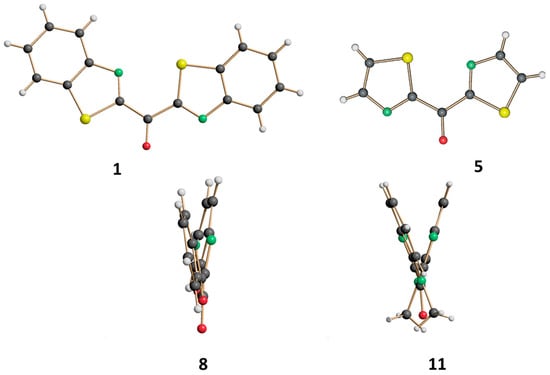
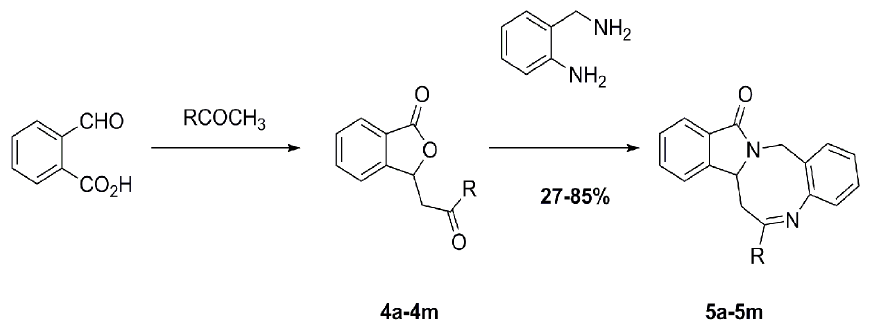
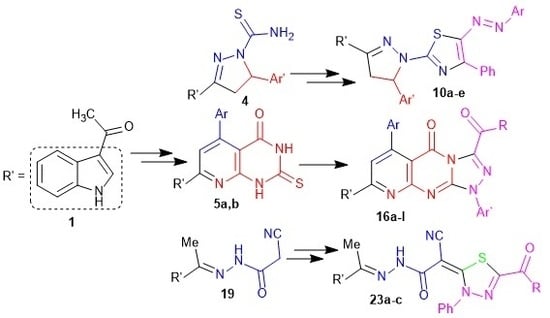
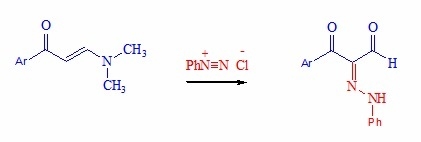
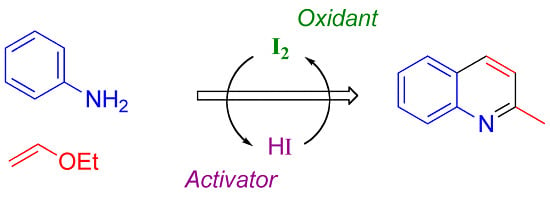
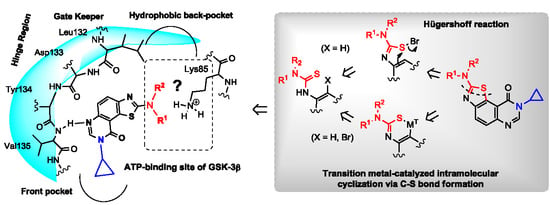
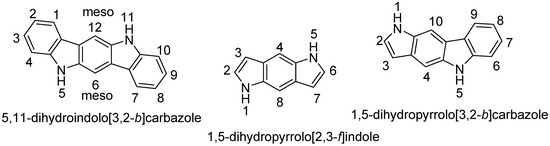
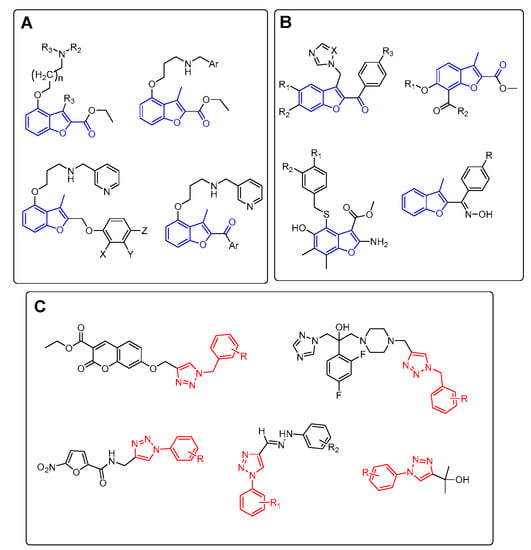
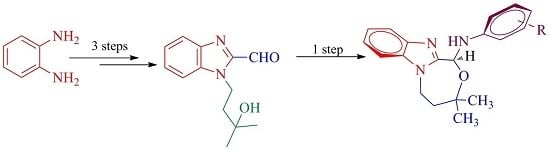
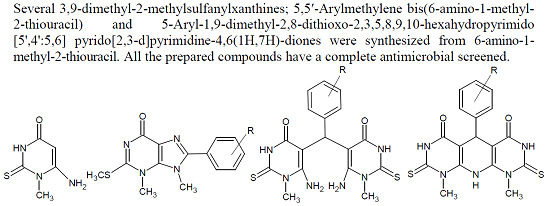
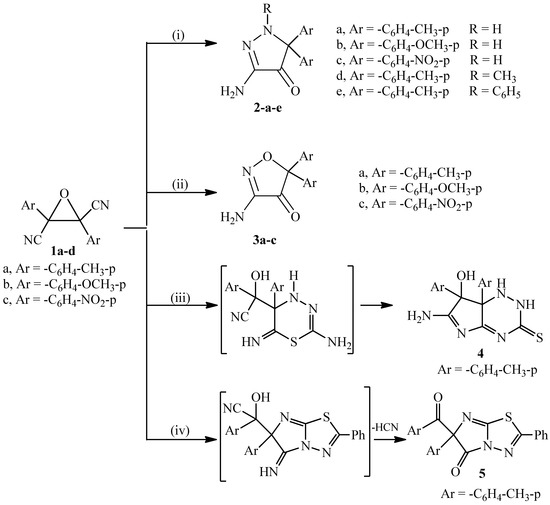
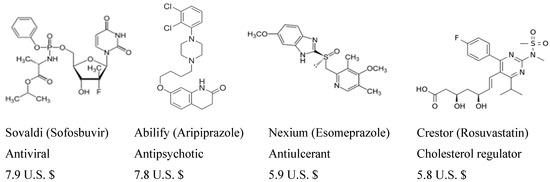
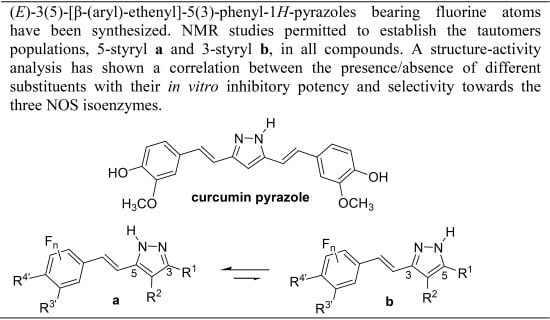
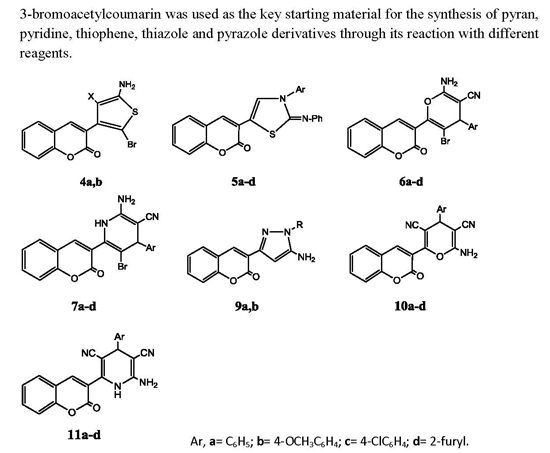
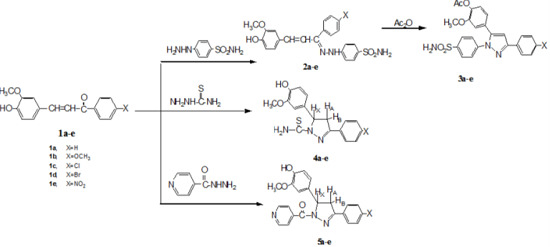
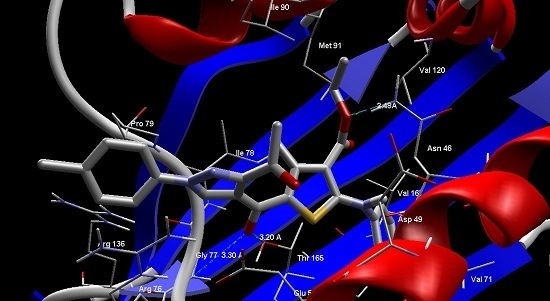
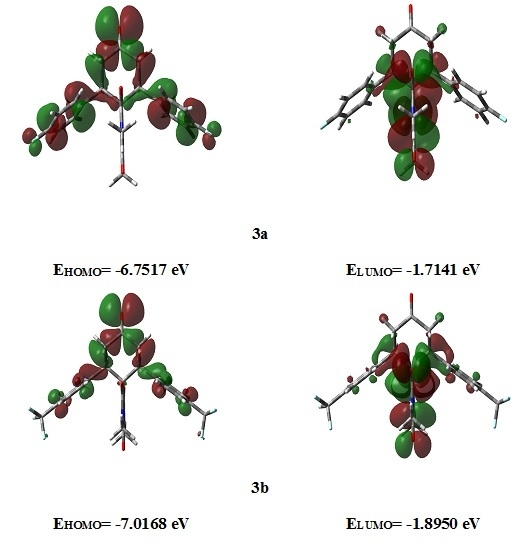
Heterocyclic Compounds
A topical collection in Molecules (ISSN 1420-3049). This collection belongs to the section "Organic Chemistry".
Submission Status: Closed | Viewed by 921554Editors
Interests: heterocycles; antibacterial agents; anticancer agents; enzyme inhibitors; medicinal chemistry; drug discovery; drug development; synthetic methodology
Special Issues, Collections and Topics in MDPI journals
Interests: heterocyclic synthesis; nitrogen-containing heterocycles; methodologies using organic or organometallic reagents; organometallic catalysis; cyclo-isomerization reactions; cyclo-functionalization reactions; benzannulation; aminobenzannulation; pi-acid metal catalysis; silver chemistry; gold chemistry; cobalt chemistry; Pauson-Khand reaction; bioactive compounds; alkaloids; kinases inhibition
Special Issues, Collections and Topics in MDPI journals
Interests: organic synthesis; heterocycles; supramolecular chemistry; porphyrin analogues; natural products; helicenes
Special Issues, Collections and Topics in MDPI journals
Interests: heterocyclic chemistry; periodic table
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
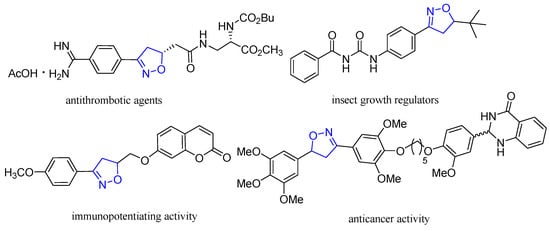
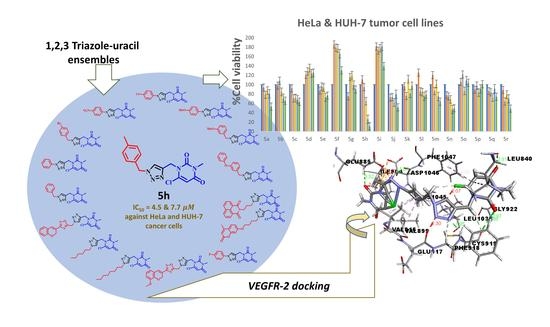
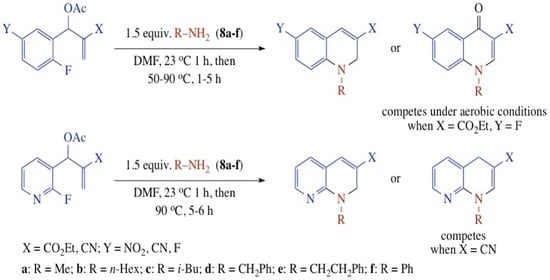
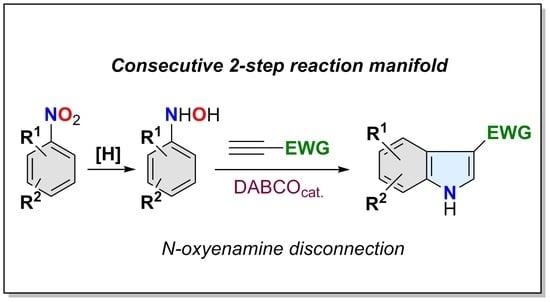
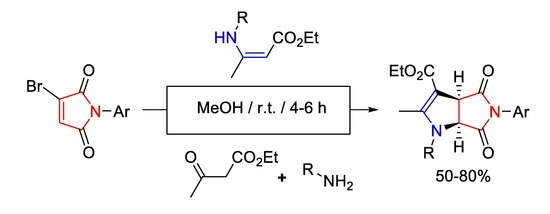
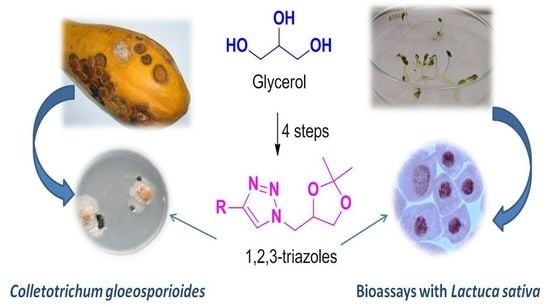
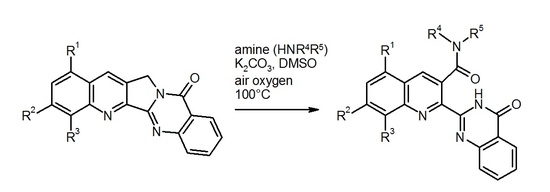
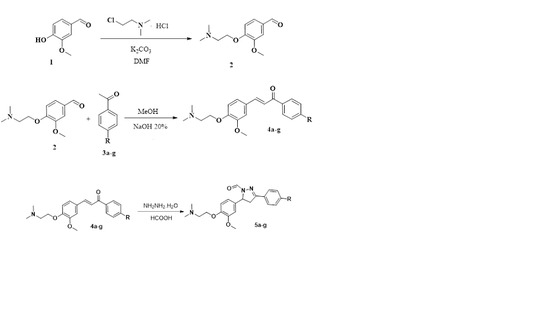
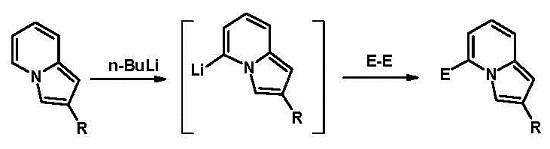
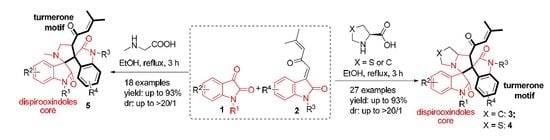
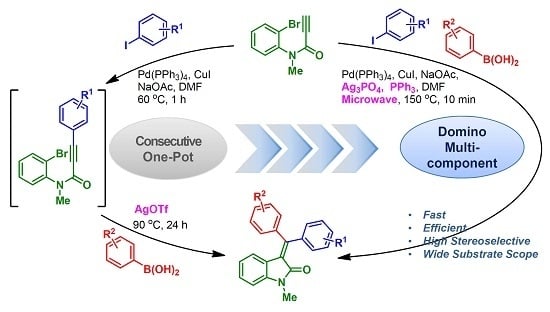
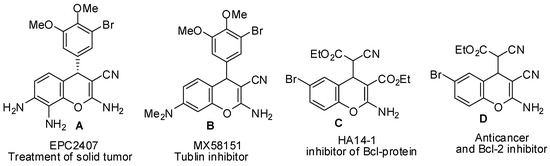
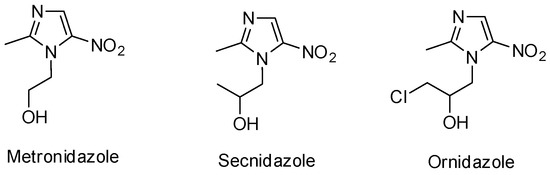
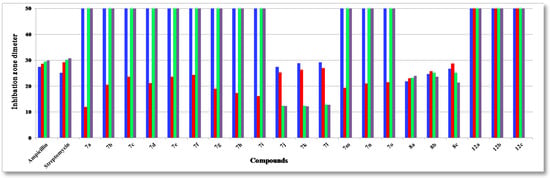
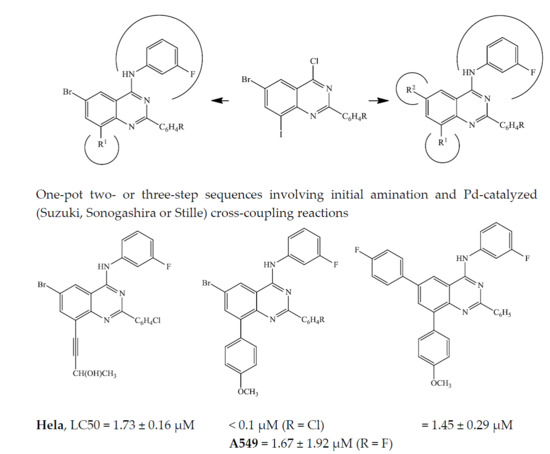
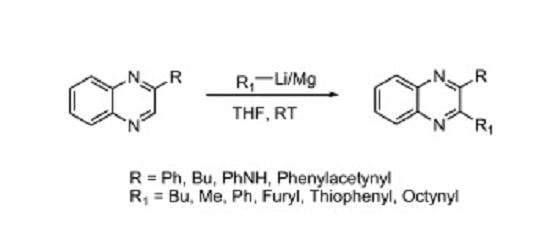
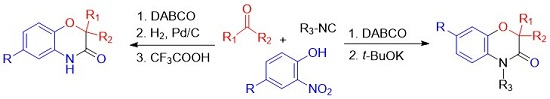
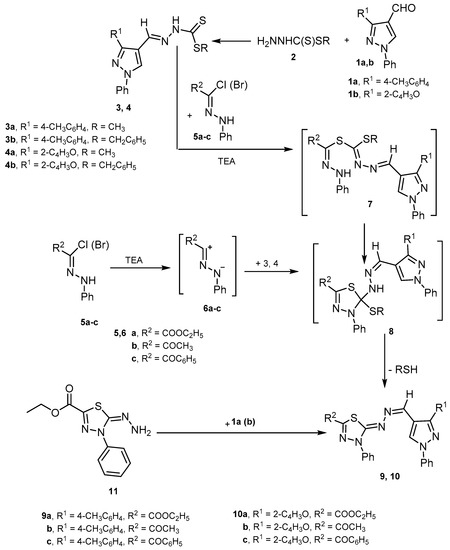
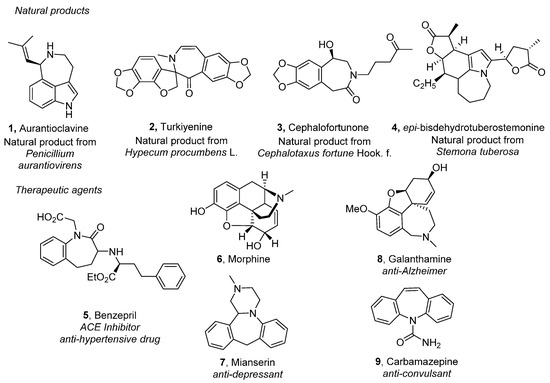
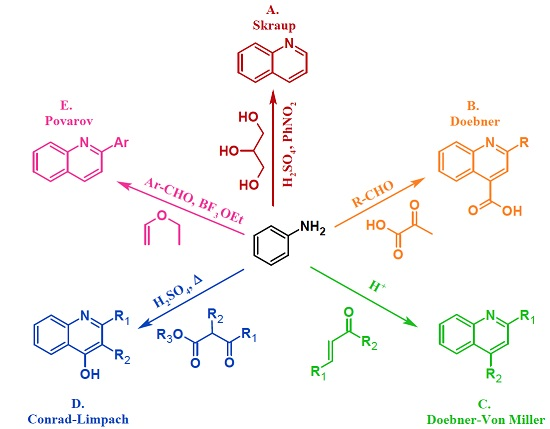
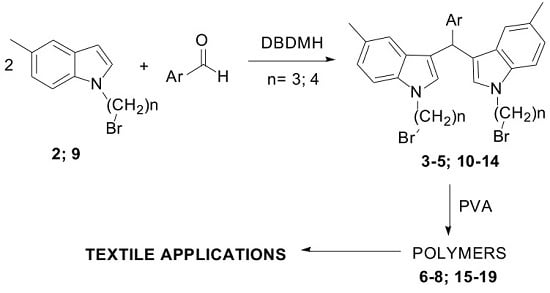
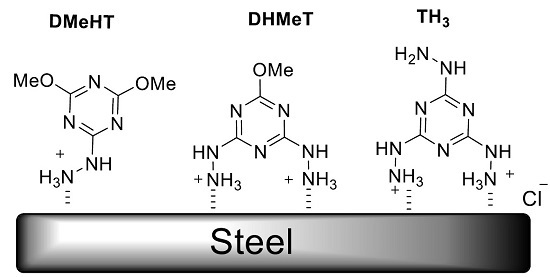
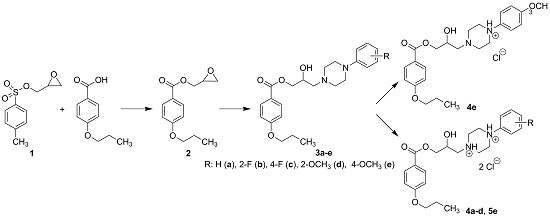
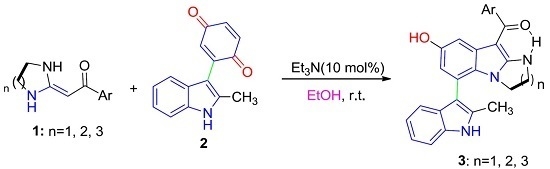
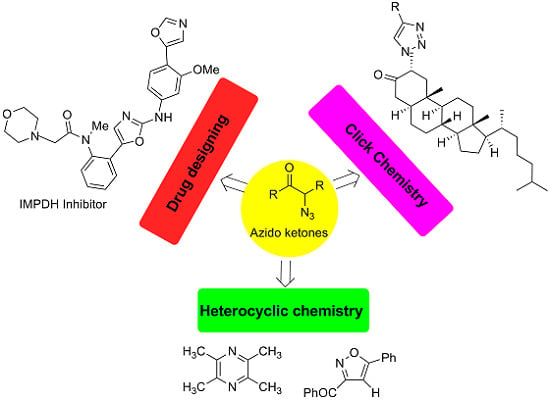
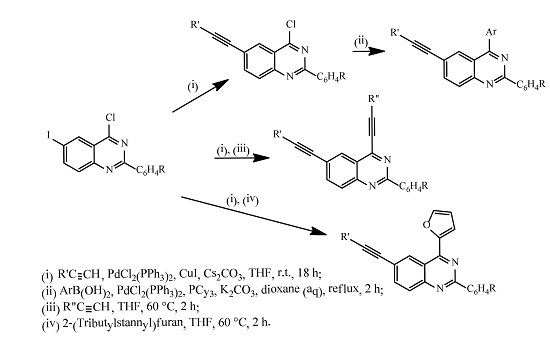
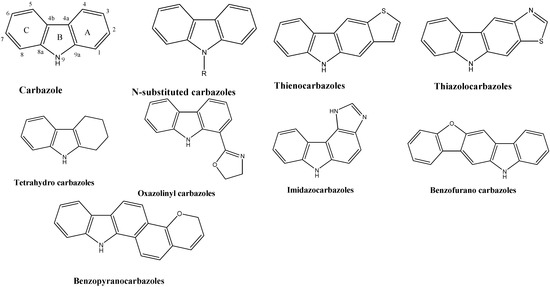
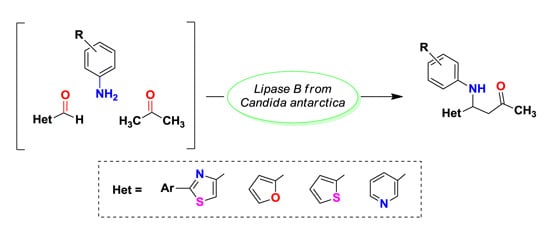
Heterocyclic compounds are an important class of molecules in organic chemistry, due to their presence in natural products and their use in pharmaceuticals and new materials. Heterocycles are the key to biological activity in many small molecule drugs, due to their ability to hydrogen bond, alter polarity, and modulate lipophilicity at specific sites in the pathogen or host, with the overall effect of inhibiting the biological processes that lead to the programmed progressions of diseases. Similar advantages can be cited in the area of materials chemistry, as heterocycles can impart unique properties to new materials, due to their polarity, solubility, and their electronic and optical properties. In this Molecules Topical Collection, entitled "Heterocyclic Compounds", we invite manuscript submissions that focus on the synthesis of natural and unnatural heterocyclic compounds and their potential uses in medicinal and materials chemistry. It is expected that most submissions will focus on nitrogen, oxygen, and sulfur heterocycles, but structures incorporating other heteroatoms will also be considered. Original research articles or reviews that discuss synthetic methodologies for preparing heterocyclic compounds, total synthesis, and structure studies of heterocycle-containing substances, as well as potential new applications to medicinal and materials chemistry, are particularly welcome.
Prof. Dr. Richard A. Bunce
Prof. Dr. Philippe Belmont
Prof. Dr. Wim Dehaen
Prof. Dr. Eugene Babaev
Collection Editors
Manuscript Submission Information
Manuscripts for the topical collection can be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. All papers will be peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on this website. The topical collection considers regular research articles, short communications and review articles. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page.
Please visit the Instructions for Authors page before submitting a manuscript. The article processing charge (APC) for publication in this open access journal is 2700 CHF (Swiss francs).
Keywords
- new synthetic approaches to heterocycles
- synthesis of heterocyclic natural products
- biologically active heterocycles
- medicinal studies of heterocycles
- structure-activity relationships of drugs containing heterocycles
- new heterocycle-based materials
- structure-property relationships of materials containing heterocycles
- heterocycles with unique properties
- applications of heterocycle-based materials