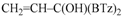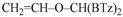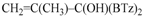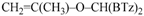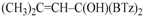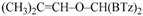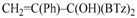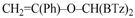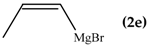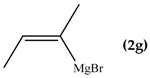Abstract
The reaction between bis(2-benzothiazolyl)ketone and vinyl Grignard reagents bearing different substituents on the vinyl moiety gave the product derived from attack on the carbonylic carbon- and/or oxygen-atom. The regioselectivity of the attack depends on the kind of substituents bound to the vinylic carbon atoms and on their relative position. The reaction between vinylmagnesium bromide and 2-methyl-1-propenylmagnesium bromide was carried out under different experimental conditions and in the presence of radical scavengers. The results indicate a plausible mechanistic pathway involving radical intermediates in the case of O-alkylation, but a polar ones in the case of classic C-alkylation. This agrees with our previous reports indicating a key role played by the delocalization ability of the substituents bound to the carbonyl group in driving the regioselectivity of the vinylmagnesium bromide attack towards O-alkylation. Further support of this was obtained by diffractometric analysis of four distinct bis(heteroaryl)ketones.
1. Introduction
Traditionally, Grignard reagents [1] are considered as potential anions able to easily react to hetero double bonds. The most important reaction is the nucleophilic 1,2-addition to the carbonylic carbon atom that gives access to a plethora of alcohol derivatives (Scheme 1).
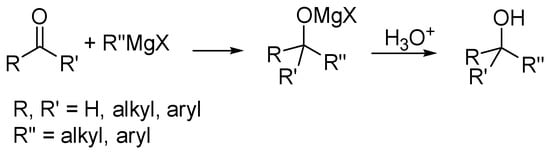
Scheme 1.
Classic pathway of the addition of Grignard reagents to carbonyl compounds.
Depending on the structure of Grignard reagents, substrates or the nature of solvents, alternative reactions can take place [2]. For example, benzophenone reacts with cyclohexyl or isobutyl Grignard reagents forming alcohols [3] or bimolecular reduction to pinacol by using neopentylmagnesium halides; both the classic 1,2-addition and the conjugate addition on the phenyl ring can occur with tert-butyl Grignard reagents. O-alkylation by Grignard reagents [2] to carbonylic oxygen atom is rarely observed, although it occurred to a minor extent in the case of substrates such as orthoquinones [4], orthoquinolacetates [5,6,7], 9,10-phenanthraquinone [8], and benzil [9]. In our previous studies, we found unexpected reactivity of vinylmagnesium bromide (Scheme 2) and iso-propenylmagnesium bromide with di(1,3-benzothiazol-2-yl)ketone (1) which gave an exclusive attack to the oxygen carbonyl atom [10]. By reacting 1 and 2-methyl-1-propenylmagnesium bromide, mixtures of C- and O-alkylation species were obtained in almost equimolar amount. Conversely, alkyl, allyl, or alkynyl Grignard reagents gave exclusively the expected carbinol [11]. O-alkylation and concomitant C-alkylation [12] occurred with azaheteroaryl ketones such as di(thiazol-2-yl)ketone (5) or di(1,3-benzoxazol-2-yl)ketone (8). Conversely, di-[N-methyl-1,3-benzimidazol-2-yl]ketone (11) gave the carbinol as unique compound (see Scheme 2).
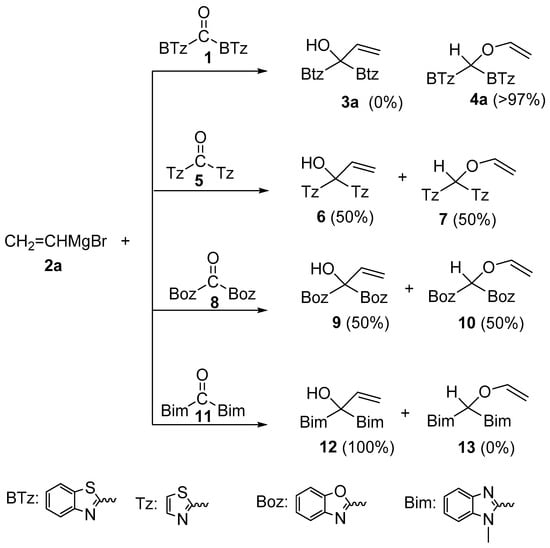
Scheme 2.
C- and O-alkylation reactions between vinylmagnesium bromide and several azaheteroaryl ketones.
More recently, we discovered that phenyl lithium and phenyl Grignard reagents also produced a mixture of C- and O-alkylation species; the relative ratio is dependent on the substituent on the phenyl ring [13].
Based on the above considerations, to verify the influence of the geometry and the nature of substituents bound to vinyl moiety to C- versus O-alkylation selectivity, we planned to run reactions between 1 and a series of 1-alkenylmagnesium reagents. Further, we designed an investigation of the crystal structure of the aza-heteroaryl ketones reported in Scheme 2, in order to try to rationalize their different behaviour when they react with vinylmagnesium bromide.
2. Results and Discussion
For the sake of simplicity, this part was divided in sub-headings, examining separately the factors that might direct the regioselectivity of the vinyl Grignard reagent attack towards the carbonylic oxygen- or carbon-atom.
2.1. Influence of the Stereochemistry of Methyl Substituents on Vinyl Moiety
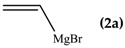
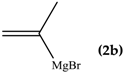
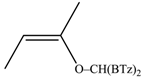
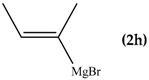
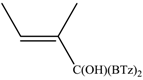
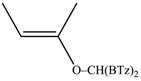
The reaction between di(1,3-benzothiazol-2-yl)ketone (1) and vinyl magnesium reagents bearing different substituents on the vinyl moiety (Scheme 3) was carried out at −70 °C by adding the Grignard reagent to a solution of ketone in anhydrous tetrahydrofuran. Grignard reagents 2a–c were commercially available whereas 2d–h were synthesized from the suitable vinylbromide and magnesium turnings and titrated prior the use. After 15 min from the addition of the Grignard reagent, the reaction was quenched with water and, after fast work-up (see experimental) the reaction mixture was analysed by 1H-NMR spectroscopy and relative molar C/O-alkylation ratio was inferred from the spectrum. The procedure has been developed since it is known that the O-alkylation species can easily undergo air-oxidation of benzylic CH group forming a hemiketal which decomposes to 1 and vinyl alcohol that immediately collapse to the aldehyde tautomeric form. A similar behaviour was also observed for bis(2-benzothiazolyl)methane that spontaneously undergoes oxidation to 1 [14]. Every reaction between 1 and the selected vinyl Grignard was repeated three times and the ratio reported in Table 1 is the average of the three measurements. Compounds 4a–g resulted to be stable enough to be purified by column chromatography on silica gel and stored for at least two months at −18 °C. However, it decomposes as above indicated within 2 days when dissolved in deuterochloroform solvent.
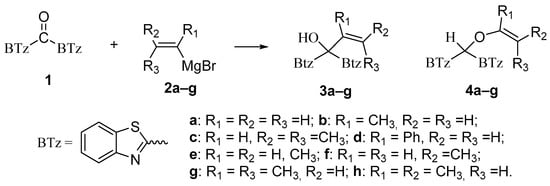
Scheme 3.
Reactions between 1 and different vinylmagnesium bromides.

Table 1.
Reaction of 1 with vinyl Grignard reagents 2a–h. a.
Results obtained are gathered in Table 1. For the sake of comparison, previous data [10] obtained by reacting 1 with vinylmagnesium bromides 2a–c are also reported.
2.1.1. Methyl Substituents in β-Position
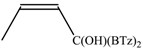
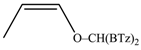
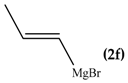
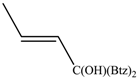
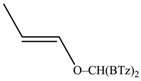
The regioselectivity of the reaction between 1 and vinylmagnesium reagents bearing one methyl group in β-position with respect to the C–Mg bond resulted strongly influenced by the stereogeometry and by the electronic feature of the substituent which both affect the alkene stability. In the case of the Grignard Z-isomer (compound 2e, Table 1, entry 5) the carbinol 3e and the vinyl ether 4e were formed in 70/30 relative molar ratio whereas almost complete O-alkylation was observed with the E-isomer (compound 2f, Table 1, entry 6).
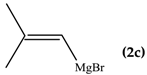
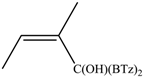
When the β-dimethyl substituted Grignard reagent 2c was used (Table 1, entry 3) a 57/43 molar ratio of the C/O-alkylated products was obtained thus suggesting the prevalence of the e steric hindrance effect (compare entries 3, 5, and 6 in Table 1). This was further confirmed by the experiment with 2g which gave 90:10 C-alkylation:O-alkylation ratio indicating that the effect due to the β-substituent prevails over the α-substituent (compare entry 7 with entry 2 in Table 1). To further confirm the influence of the stability of the alkene (mainly due to the substituent electronic effect) and of his geometry in driving the regioselectivity of the attack to the carbonyl group of 1 we attempted to prepare compound 2h from which we expected to recover almost exclusively the O-alkylation product 4h. Unfortunately, presence of the latter was not detected but the two carbinols 3g and 3h in 95:5 relative molar ratio were recovered ascribed to the relevant isomerization occurred during the preparation of the organometallic reagent [15].
2.1.2. Effect of the Substituent in α-Position
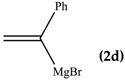
Concerning the influence of the substituent in α-position with respect to Mg atom of vinyl Grignard reagents which rules the regioselectivity of the attack towards C- or O-alkylation, we compared the results reported in entries 1, 2, and 4 of Table 1. Vinylmagnesium bromide (2a) and 1-propenylmagnesium bromide (2b) reacted with 1 giving exclusively O-alkylation, whereas 1-phenylmagnesium bromide gave the carbinol 3d and the vinyl ether 4d in 7/3 molar ratio. These findings might be explained by invoking competition between the C- and the O-attack reactions. From the literature survey about effect of α-substituents to form and stabilize vinyl radicals from the corresponding bromides we found [16] that the configuration of vinyl radicals depends on the nature of the α-substituent. Vinyl radicals with an α-hydrogen, -alkyl, -alkoxy, or-halogen substituent have a bent structure [17,18,19,20] while the cyano, carboxyl, phenyl, and alkenyl group are π-delocalized and thought able to impose linearity on the radical center to effect maximum resonance stabilization, as supported by ESR data [21,22]. Thus, the α-(phenyl)vinyl radical results more stabilized than the α-methyl and vinyl analogues and this might explain the difference found in the behavior with 1. In other words, the occurrence of both the O- and the C-alkylation with 2d, and only the O-alkylation with 2a and 2b might be explained by the fact that the vinyl radical from 2a and 2b would be less stabilized and then more reactive than that derived from 2d allowing competition between O- and C-alkylation.
This is in line with the common opinion that anomalous behaviour of Grignard reagents can be explained by invoking a single electron transfer (SET) mechanism [23,24]. It has been reported [2] that the C–Mg bond dissociation energy is well related to the oxidation potential values of different Grignard reagents (the C–Mg dissociation energy of 23 Kcal/mol corresponds an oxidation potential difference of 1.00 Volt [2]) and the vinyl- and aryl-Grignard reagents possess the strongest among the C–Mg dissociation energies [2]. Few cases of O-alkylation on alpha-dicarbonyl substrates have been reported, but the reaction occurs with vinyl- and phenyl-Grignard reagents only with 9,10-phenanthraquinone [8]. The authors hypothesized that the nature of C-Mg bond is an important requisite to determine the fashion-mode of addition.
2.1.3. Effect of the Substituent in α-Position
It globally emerges from the reported data that the regioselectivity of the vinyl Grignard addition to 1 cannot be exclusively attributed to both steric and electronic features of the vinyl moiety substituents but also to mechanistic path effects. In the context, distinct intermediate species can be involved in O- and C-alkylation deriving by single electron transfer (SET) or polar mechanism. Some of them are drawn in Scheme 4.
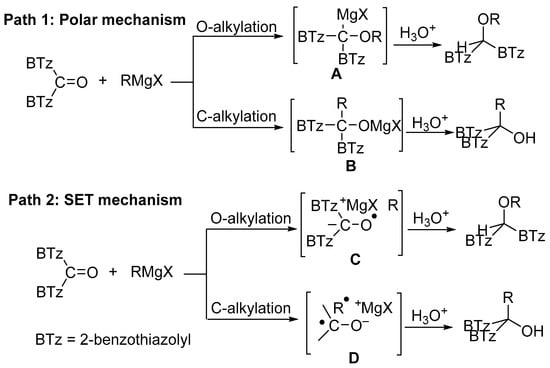
Scheme 4.
Possible reaction pathways of the currently considered Grignard addition.
To shed light on the mechanistic aspects of the reaction we planned to run some experiments and results obtained are reported in Table 2. In particular, compound 1 was reacted with vinylmagnesium bromide (2a), and 2-methyl-1-propenylmagnesium bromide (2c) under different experimental conditions. We selected to run experiments by using these latter since they are representative of limit cases and exclude isomerization, thus avoiding further complications. Indeed, 2a gave only O-alkylation, while both C- and O-alkylation occurred with 2c.

Table 2.
Reactions of 1 with 2a and 2c under different experimental conditions. a
From data reported in Table 2 emerges that the conversion of 1 decreased in the presence of 2,2,6,6-tetramethylpiperidinoxyl (TEMPO) as radical scavenger (comparison between entries 1 and 2); the recovery of the O-alkylation product even in the presence of the scavenger might be due to the coupling rate of the vinyl radical with 1, likely faster than the diffusion rate of the scavenger within the solvent cage. When the radical trapper was present in half equivalent amount with respect to the reactant amount, the reaction between 1 and 2c (Table 2, entries 3–5) gives 3c:4c = 2:1 in minor conversion, whereas only the carbinol 3c was detected when TEMPO was added in equimolar amount. These results can be considered an indication that the O-alkylation involves the intervention of C-like radical species (Scheme 4). The recovery of the totally deuterated species 4a from the crude reaction mixture from 1 and 2a upon quenching with D2O [12] supported the above speculation (Scheme 5).
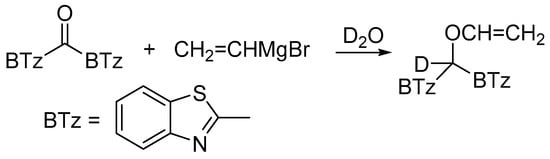
Scheme 5.
Formation of 4a deuterated on the benzylic position upon quenching with D2O of the reaction between 1 and 2a.
Finally, we made a further experiment aiming to support the hypothesis that the reaction between 1 and vinyl Grignard reagents gives C-alkylation through a polar mechanism while O-alkylation proceeds involving radical species. Ashby reported fundamental studies on the mechanism of reaction of Grignard reagents with ketones. In particular, he reported experiments in which propenylmagnesium reagents having different cis/trans ratios were reacted with benzophenone excess [25,26]. The cis-propenylmagnesium bromide was selected as vinyl Grignard probe. If a free radical would be formed during the reaction course, the cis-propenyl radical would isomerize to the thermodynamically stable trans-propenyl radical, resulting in a product with trans stereochemistry. The result [25] did not indicate isomerization of the propenyl group as evidenced by the fact that the starting Grignard reagent cis/trans ratio was exactly reflected in the reaction products. Thus, the retention of configuration of the probe was considered an indication that the reaction is not proceeding via an SET mechanism.
In line with these considerations, we prepared a THF solution of propenylmagnesium bromide by reacting cis-propenylbromide with magnesium turnings dividing it in two parts: one to be reacted with benzophenone while the other with bis(2-benzothiazolyl)ketone (1). The 1H-NMR analysis of the reaction mixture with benzophenone showed two carbinol signals derived from the addition of cis- and trans-propenylmagnesium bromide to benzophenone. In agreement with the result reported in Table 1, entry 5, the cis:trans ratio was 1:2, indicating that the isomerization occurs in Grignard formation but not during the addition to the ketone.
In the case of reaction with 1, the 1H-NMR spectrum exhibited signals ascribed to carbinols 3e and 3f in relative 1:2 molar ratio and of O-alkylated products 4e and 4f. These findings indicate that carbinols derive from a polar mechanism, as in the case of the reaction with benzophenone. The detection of vinyl ethers suggests the occurrence of a radical mechanism when bis(2-benzothiazolyl)ketone was used as substrate.
2.2. Influence of the Solvent and of the Metal Bound to Vinyl Group
It has been reported [9] that the reaction between benzil and phenylmagnesium bromide in THF gives O-alkylation (25% yield), whereas this reaction occurred in negligible amount in Et2O. Therefore, addition of 2a to a solution of 1 in diethyl ether afforded the C- and O-alkylation in equimolar extent, although the reaction is slow due to the low solubility of 1 in Et2O confirming a strong influence of the solvent polarity to the regioselectivity.
To verify the role of the alkylating agent on the regioselectivity we run the reaction between 1 and vinyl lithium, prepared in situ from tetravinylstannane and n-butyllithium. After purification by silica gel chromatography the carbinol 3a in 25% yield was recovered and no O-alkylated product was detected. This finding can be considered of synthetic interest since the vinyllithium offers an alternative synthetic path to obtain the C-alkylation product 3a (Scheme 6).
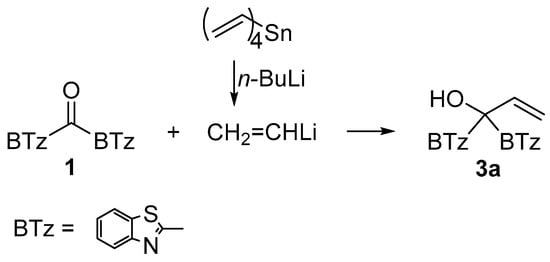
Scheme 6.
Synthesis of carbinol 3a.
2.3. X-ray Diffraction Analysis of Azaheteroaryl Ketones Showing Different C-/O-Alkylation Regioselectivity towards Vinylmagnesium Bromide
It has been reported [10,12] that the reaction between 2a and a series of azaheteroaryl ketones exhibited O-vinylation strongly dependent on the groups bound to the carbonyl moiety. It occurred completely only with the ketone 1, bearing two benzothiazolyl substituents while the reaction with bis(2-thiazolyl)ketone (5) produced both O- and C-alkylation products in equimolar amount (Scheme 2) evidencing the importance of the benzocondensation. The presence of a benzene ring fused with the heterocycle might favor the delocalization of a negative charge in the O-alkylated C-like intermediate (Scheme 4). Equimolar amount of two distinct O- and C products were obtained on bis(2-benzoxazolyl)ketone (8) substrate. Conversely, in the case of bis(1-methylbenzimidazolyl)ketone (11) the carbinol species derived from the classic Grignard addition was exclusively obtained. These findings suggested us to investigate the structure of ketones 1, 5, 8, and 11 by diffractometric analysis. Suitable crystals for the purpose were obtained from acetone (1 and 11), methanol (8), or by the vapor diffusion technique (methanol/n-hexane) in the case of 6.
X-ray study showed distinct stereogeometries characterized by different dihedral angles (Table 3) between the planes defined by the two carbonyl substituents, as shown in Figure 1.

Table 3.
Crystal data and structure-refinement details for 1, 5, 8, and 11.2H2O.
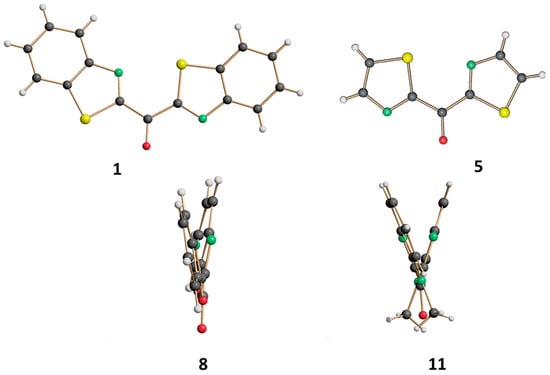
Figure 1.
Molecular structures of compounds 1, 5 (front-view), 8, 11 (side-view) determined by X-ray studies.
In compounds 1 and 5 in which the ketone group is bound to two benzothiazolyl and thiazolyl rings, respectively, the dihedral angles are 12.9(1)° and 5.0(1)° [8.3(1)°, for the second molecule in 5]. At a difference with the previously observed values, in 8 the dihedral angles formed by the benzoxazolyl rings bound to the carbonyl group are slightly wider being 22.76(7)° whereas the largest angle is found between the methylbenzoimidazolyl rings in 11 [39.76(7)°]. This latter result is also in agreement with the analogous dihedral angle between the methylimidazole rings in the bis(1-methyl-2-imidazolyl)ketone that is even larger being 49.4(1)° [27].
Although we are aware that conformations in the solid state can differ from the ones in solution, by roughly comparing the structure geometry of the benzocondensed azaheteroaryl ketones 1, 8, and 11, it emerges that the attack at the oxygen atom may be sterically impeded by the presence of two N-methyl groups in the case of 11.
A further possible explanation about the difference between relative C-and O-alkylation ratio passing from 1 to 8 until 11 might reside in the distinct ability to delocalize a negative charge on the benzylic carbon atom. In the case of 11 the presence of nitrogen atoms makes the imidazole ring more aromatic thus less prone to delocalize the charge within the ring; when the nitrogen is replaced by the oxygen such as in 8 this feature might be less important, whereas the intervention of the sulfur d-orbitals in the case of 1 might likely favor the delocalization charge.
3. Materials and Methods
3.1. General Methods
The 1H spectra were recorded with a Varian Gemini 300 (Varian, Palo Alto, CA, USA) spectrometer operating at 300 MHz for 1H-NMR and 75.56 MHz for 13C-NMR. Signal multiplicities were established by Distortionless Enhanced by Polarization Transfer (DEPT) experiments. Chemical shifts were measured in δ (ppm) with reference to the solvent (δ = 7.26 ppm and 77.00 ppm for CDCl3, for 1H-, and 13C-NMR, respectively). J values are given in Hz. MS analyses were performed with a gaschromatograph Agilent 6890 equipped with a (5%-phenyl)methyl-polysiloxane column (30 m length, 0.250 mm i.d., 0.25 µm thickness) interfaced to a quadrupole mass detector Agilent 5973N. Mass and HR-MS spectra were recorded on a VG 7070E instrument (VG Instruments, Inc., Stamford, CT, USA) at an ionization voltage of 70 eV in the EI mode. Electron spray ionization mass spectra (ESI-MS) were recorded with a WATERS 2Q 4000 instrument (Waters Corporation, Milford, MA, USA). IR spectra were recorded on a Perkin–Elmer model 1600 FT-IR spectrophotometer (Perkin Elmer, Waltham, MA, USA). Compounds 3a–h showed characteristic IR signals at 3650–3300 cm−1 (OH) and 4a–g (oils) at 1050–1300 cm−1 (O–C). Melting points were measured with a Büchi 535 apparatus and were not corrected. Chromatographic purifications (FC) were carried out on glass columns packed with silica gel (Merck grade 9385, 230–400 mesh particle size, 60 Å pore size) at medium pressure. Thin layer chromatography (TLC) was performed on silica gel 60 F254 coated aluminum foils (Fluka Chemie GmbH, Buchs, Switzerland). The solvents and reagents used are commercially available (Sigma-Aldrich (Milan, Italy) or Fluka Chemie GmbH, Buchs, Switzerland). THF and diethyl ether were distilled from sodium/benzophenone ketyl. Air- and moisture-sensitive solutions and reagents were handled in dried apparatus under an atmosphere of dry argon using standard Schlenk-type techniques. Compounds 1, 5, 8, and 11 are synthesized as previously reported [14] and their characterization data agreed with those reported. The Grignard reagents, if not commercially available, were prepared from the vinyl bromide precursor and their solution titrated immediately prior to use according to standard procedure [28].
3.2. Synthesis of 1,1-Di(1,3-benzothiazol-2-yl)-2-propen-1-ol (3a)
In a three necked round bottom flask equipped with two drip funnels and with a neck settled with a sintered glass filter shut with a Teflon valve, under argon atmosphere and magnetic stirring, a solution of tetravinyltin (97% purity grade, 0.36 mL, 1.92 mmol) in anhydrous n-pentane (120 mL) was introduced. A solution of n-butyllithium 2.5 M in n-hexane (1.8 mL) was added dropwise and after 30 min the white solid obtained was separated by filtration from the liquid through the filter and washed with 2.0 mL of n-pentane. The solid remained within the flask was dissolved in anhydrous THF (5.0 mL) and the apparatus was cooled at −70 °C through immersion in a liquid nitrogen/acetone bath. After about 5 min, a solution of compound 1 (0.065, 0.22 mmol) in anhydrous THF (10 mL) was added dropwise. Immediately the pale yellow solution became red. The reaction course was monitored through TLC and after 2 h 10 mL of water was added; the crude was extracted with diethyl ether and the organic layer dried over anhydrous Na2SO4. Compound 3a was recovered in 20% yield after purification by column chromatography on silica gel (eluent: light petroleum/diethyl ether 75/25).
1,1-Di(1,3-benzothiazol-2-yl)-2-propen-1-ol (3a): 1H-NMR (CDCl3, 300 MHz, 25 °C): δ (ppm): 8.08–8.02 (m, 2H), 7.90–7.85 (m, 2H), 7.52–7.44 (m, 2H), 7.42–7.35 (m, 2H), 6.77 (dd, 1H, J = 17.0 Hz, J = 10.4 Hz), 5.77 (d, 1H, J = 17.0 Hz), 5.63 (bs, 1H, OH), 5.42 (d, 1H, J = 10.4 Hz); 13C-NMR (CDCl3: 75.56 MHz, 25 °C): δ (ppm): 173.7, 152.4, 139.2, 135.9, 126.1, 125.4, 123.3, 121.9, 116.1, 78.9; MS (m/z): 324 (M+, 76), 307 (25), 296 (25), 268 (27), 190 (38), 162 (100), 136 (41). HREIMS: m/z 324.0395 (calculated for C17H12N2OS2, 324.0391).
3.3. Reactions between 1 and Vinylmagnesium Bromide Derivatives (2a–h)—General Procedure
Vinylmagnesiumbromide derivative (0.41 mmol) in THF (3.0 mL) was added dropwise to a stirred solution of carbonyl compound (0.34 mmol) in THF (3.0 mL), cooled at −70 °C. After about 20 min. the reaction mixture was quenched with saturated aqueous (NH4)2SO4, stirred while being warmed to room temperature, and extracted with diethyl ether. The organic layers were washed with “brine”, dried over anhydrous Na2SO4, and concentrated “in vacuo” without warming. The time employed for this workup was about 15 min. The residue was analyzed by 1H-NMR and then the products were isolated by FC and fully characterized. The vinyl ethers, stored at −18 °C, are stable for several months. Reactions of 1 with 2a–c and related products have been reported in the literature [10]. Characterization data for new compounds are reported below and the relative 3:4 ratio is reported in Table 1.
1,1-Di(1,3-benzothiazol-2-yl)-2-phenyl-2-propen-1-ol (3d): 1H-NMR (CDCl3, 300 MHz, 25 °C): δ (ppm): 8.03 (d, J = 8.0 Hz, 2H), 7.83 (d, J = 7.4 Hz, 2H), 7.50–7.30 (m, 6H), 7.22–7.14 (m, 3H), 5.96 (bs, 1H, OH, exchange with D2O), 5.56 (s, 1H), 5.47 (s, 1H); 13C-NMR (CDCl3: 75.56 MHz, 25 °C): δ (ppm): 173.8, 152.2, 150.6, 138.7, 136.1, 128.8, 127.8, 127.6, 126.1, 125.4, 123.4, 121.7, 120.1, 81.3; ESI-MS (m/z): 401 [M + H]+, 423 [M + Na]+; HREIMS: m/z 400.0711 (calculated for C23H16N2OS2, 400.0704).
Z-1,1-Bis(2-benzothiazolyl)-2-buten-1-ol (3e): 1H-NMR (CDCl3, 300 MHz, 25 °C): δ (ppm): 8.08–7.98 (m, 2H), 7.88–7.82 (m, 2H), 7.50–7.40 (m, 2H), 7.40–7.30 (m, 2H), 6.46 (dq, 1H, J = 11.2 Hz, J = 1.8 Hz), 6.05–5.90 (m, 1H), 5.73 (bs, 1H, OH), 1.76 (dd, 3H, J = 7.3 Hz, J = 1.8 Hz); 13C-NMR (CDCl3: 75.56 MHz, 25 °C): δ (ppm): 175.6, 152.4, 136.0, 132.8, 131.7, 126.1, 125.3, 123.3, 121.8, 78.3, 14.7; MS (m/z): 338 (M+), 321, 297, 281, 176, 136; HREIMS: m/z 338.0543 (calculated for C18H14N2OS2, 338.05475).
E-1,1-Bis(2-benzothiazolyl)-2-buten-1-ol (3f): 1H-NMR (CDCl3, 300 MHz, 25 °C): δ (ppm): 8.10–7.95 (m, 2H), 7.90–7.80 (m, 2H), 7.55–7.42 (m, 2H), 7.42–7.35 (m, 2H), 6.40 (dq, J = 15.3 Hz, J = 1.5 Hz, 1H), 6.22–6.05 (m, 1H), 5.63 (br.s., 1H, OH, exchange with D2O), 1.78 (dd, J = 6.6 Hz, J = 1.5 Hz, 3H); 13C-NMR (CDCl3: 75.56 MHz, 25 °C): δ (ppm): 174.5, 152.4, 135.9, 132.5, 128.2, 126.1, 125.3, 123.2, 121.8, 78.6, 17.6; MS (m/z): 338 (M+), 321, 297, 281, 176, 136; HREIMS: m/z 338.0546 (calculated for C18H14N2OS2, 338.05475).
Z-1,1-Bis(2-benzothiazolyl)-2-methyl-2-buten-1-ol (3g): 1H-NMR (CDCl3, 300 MHz, 25 °C): δ (ppm): 8.05 (d, 2H, J = 7.4 Hz), 7.89 (d, 2H, J = 8.22 Hz), 7.53–7.35 (m, 4H), 5.81–5.77 (m, 1H), 5.62 (br.s., 1H, OH), 1.85 (s, 3H), 1.47–1.43 (m, 3H); 13C-NMR (CDCl3: 75.56 MHz, 25 °C): δ (ppm): 175.1, 152.3, 136.6, 136.6, 136.0, 128.6, 126.1, 125.4, 123.4, 121.8, 80.4, 23.1, 15.0; MS (m/z): 352 (M+), 335, 320, 297, 190, 162; HREIMS: m/z 352.0707 (calculated for C19H16N2OS2, 352.0704).
E-1,1-Bis(2-benzothiazolyl)-2-methyl-2-buten-1-ol (3h): m.p.: 123–124 °C. 1H-NMR (CDCl3, 300 MHz, 25 °C): δ (ppm): 8.05 (d, 2H, J = 8.1 Hz), 7.90 (d, 2H, J = 8.4 Hz), 7.50 (td, 2H, J = 8.1 Hz, J = 1.3 Hz), 7.40 (td, 2H, J = 8.4 Hz, J = 1.2 Hz), 5.70 (bs, 1H, OH), 5.70–5.55 (m, 1H), 1.80 (s, 3H), 1.70 (d, 3H, J = 6.8 Hz); 13C-NMR (CDCl3: 75.56 MHz, 25 °C): δ (ppm): 174.1, 152.3, 137.8, 136.2, 126.1, 125.4, 125.3, 123.3, 121.8, 82.3, 13.9, 12.8; MS (m/z): 352 (M+), 335, 320, 297, 190, 162; HREIMS: m/z 352.0708 (calculated for C19H16N2OS2, 352.0704).
2-[1,3-Benzothiazol-2-yl((1-phenylvinyl)oxy)methyl]-1,3-benzothiazole (4d): 1H-NMR (CDCl3, 300 MHz, 25 °C): δ (ppm): 8.09 (d, 2H, J = 8.2 Hz), 7.88 (d, 2H, J = 8.0 Hz), 7.80–7.35 (m, 9H), 7.03 (s,1H), 4.91 (d, 1H, J = 3.9 Hz), 4.62 (d, 1H, J = 4.0 Hz); 13C-NMR (CDCl3: 75.56 MHz, 25 °C): δ (ppm): 168.1, 157.6, 153.0, 135.2, 129.1, 128.4, 126.2, 125.6 (two signals overlapped), 123.9, 121.8, 87.6, 77.8; MS (m/z): 400 (M+), 399 (M+–1), 281; HREIMS: m/z 400.0709 (calculated for C23H16N2OS2, 400.0704).
Z-Bis-(2-benzothiazolyl)methyl 1-propenyl ether (4e): 1H-NMR (CDCl3, 300 MHz, 25 °C): δ (ppm): 8.10–8.00 (m, 2H), 7.92–7.80 (m, 2H), 7.52–7.30 (m, 4H), 6.53 (s, 1H), 6.29 (dq, 1H, J = 6.1 Hz, J = 1.8 Hz), 4.72 (dq, 1H, J = 12.8 Hz, J = 6.9 Hz), 1.80 (dd, 3H, J = 6.9 Hz, J = 1.8 Hz); 13C-NMR (CDCl3: 75.56 MHz, 25 °C): δ (ppm): 168.3, 152.9, 142.8, 135.3, 126.2, 125.6, 123.8, 121.8, 106.3, 80.6, 9.6; MS (m/z): 400 (M+), 338 (M+), 281, 162, 134; HREIMS: m/z 338.0543 (calculated for C18H14N2OS2, 338.05475).
E-Bis-(2-benzothiazolyl)methyl 1-propenyl ether (4f): 1H-NMR (CDCl3, 300 MHz, 25 °C): δ (ppm): 8.12–8.02 (m, 2H), 7.92–7.82 (m, 2H), 7.52–7.30 (m, 4H), 6.58 (s, 1H), 6.42 (dq, 1H, J = 12.4 Hz, J = 1.7 Hz), 5.22 (dq, 1H, J = 12.4 Hz, J = 6.8 Hz), 1.54 (dd, 3H, J = 6.8 Hz, J = 1.6 Hz); 13C-NMR (CDCl3: 75.56 MHz, 25 °C): δ (ppm): 168.3, 153.2, 143.7, 135.2, 126.2, 125.6, 123.8, 121.8, 105.2, 79.2, 12.4; MS (m/z): 338 (M+), 281, 162, 134; HREIMS: m/z 338.0541 (calculated for C18H14N2OS2, 338.05475).
Z-Bis(2-benzothiazolyl)methyl 2-butenyl ether (4g): 1H-NMR (CDCl3, 300 MHz, 25 °C): δ (ppm): 8.10–8.00 (m, 2H), 7.90–7.80 (m, 2H), 7.50–7.35 (m, 4H), 6.80 (s, 1H), 4.75–4.65 (m, 1H), 1.98–1.94 (m, 3H), 1.78–1.75 (m, 3H); REIMS: m/z 352.0700 (calculated for C19H16N2OS2, 352.0704).
3.4. Reactions of Propenylmagnesium Bromide with 1 or Benzophenone
In a flame-dried apparatus and under argon atmosphere, a solution of cis-propenylbromide (0.425 mL, 0.005 mol) in anhydrous THF (30 mL) was added dropwise to magnesium turnings (0.005 mol) in THF (5.0 mL). After a gentle warming the Grignard formation was observed and the reaction was allowed until the magnesium was consumed. The resulting solution was withdrawn with a graduate syringe and a half amount was inserted in a dropping funnel and the other half in another one. Each funnel was connected with a flame-dried round-bottomed flask in which 0.00025 mol of ketone were dissolved in 5.0 mL of anhydrous THF and kept at −70 °C. After about 15 min from the addition of the Grignard solution, the reaction was quenched with aqueous (NH4)2SO4 and extracted with diethyl ether. The organic layer was dried over anhydrous Na2SO4, and the solvent was removed after filtration. The reaction mixture was analyzed through 1H-NMR spectroscopy. The reaction products were recognized by comparison with literature data in case of reaction with benzophenone [25] and with data obtained in experiments reported in Table 1, entries 5 and 6.
3.5. X-ray Diffraction Data
Crystallographic Data Collection and Structure Determination
The X-ray intensity data of 1, 5, 8, and 11 were measured with a Bruker Apex II CCD diffractometer. The cell dimensions and the orientation matrix were initially determined by a least squares refinement of reflections measured in three sets of 20 exposures, which were collected in three different ω regions, and eventually refined against all data. A full sphere of reciprocal space was scanned in 0.3°ω steps. The SMART [29] software was used to collect frames of data, index reflections, and determine the lattice parameters. The collected frames were then processed for integration by the SAINT program, [29] and an empirical absorption correction was applied with SADABS [30]. The structures were solved by direct methods (SIR2004) [31] and subsequent Fourier syntheses and refined by full-matrix least-squares techniques on F2 (SHELXTL) [32] with anisotropic thermal parameters for all non-hydrogen atoms. All hydrogen atoms were added in calculated positions in the final stage of refinement and refined with U(H) = 1.2 Ueq(C) and allowed to ride on their carrier atoms. In the asymmetric unit of 5 there are two independent molecules. In 11.2H2O two water molecules are present in the asymmetric unit. Crystal data and details of the data collections for 1, 5, 8, and 11 are reported in Table 3.
CCDC 1587827 (for 1), 1587828 (for 5), 1587829 (for 8), and 1587830 (for 11) contain the Supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/, Tel.: +44 (0)1223 336408, Fax: +44 (0)1223 336033).
4. Conclusions
The regioselectivity of the attack of vinyl Grignard reagents to bis(benzothiazolyl)ketone (1) strongly depends on the alkene geometry and on its substitution grade: the exclusive attack to the carbonylic oxygen atom occurring with vinyl Grignard reagents 2a and 2b indicates that the absence of substituents on the vinyl moiety or the presence of an alkyl substituent on the same carbon atom bound to the magnesium might play a key role in driving the regioselectivity of the attack towards the O-alkylation. In the presence of a methyl group bound to the carbon atom in β-position with respect to the Mg atom an almost complete O-alkylation was observed; the alkene was E stereoisomer. However, the Z-isomer undergoes both C-and O-alkylation. From the current data it emerges that the regioselectivity of the addition of vinyl Grignard reagents to 1 cannot be explained only by the steric effect or the geometry of the vinyl moiety but that it can be due to the concomitance of different effects on the mechanistic pathway. The latter was also investigated by reaction between 1 and vinylmagnesium bromide (and 2-methyl-1-propenylmagnesium bromide) under different experimental conditions and in the presence of radical scavengers: the results observed suggest a mechanistic pathway in the O-alkylation involving an intermediate bearing radical oxygen atom and negative charge in benzylic position. By comparing the results from the reaction between 2-propenylmagnesium bromide and benzophenone or bis(2-benzothiazolyl)ketone with the literature data we infer that a polar mechanism is likely involved in the formation of carbinols.
The X-ray diffraction studies on four bis(heteroaryl)ketones, i.e., bis(benzothiazolyl)ketone (1), bis(2-thiazolyl)ketone (5), bis(2-benzoxazolyl)ketone (8), and bis(1-methylbenzimidazolyl)ketone (11) and comparison of the structural data supported the speculation that the ability in delocalizing the negative charge in the benzylic position of intermediate may play a key role in driving the attack of the vinyl Grignard reactant towards the O-alkylation.
Supplementary Materials
Supplementary materials are available online.
Acknowledgments
Work supported by Alma Mater Studiorum—Università di Bologna (RFO funds). Authors thank Luca Zuppiroli for the mass spectra.
Author Contributions
C.B. conceived and designed the experiments, analyzed the data and wrote the paper; G.M. and L.C. performed the experiments and analyzed the data; M.M. made crystallographic analyses and analyzed the relevant data; S.B. and M.M. contributed in writing the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Silverman, G.S.; Rakita, P.E. Handbook of Grignard Reagents Dekker; CRC Press: New York, NY, USA, 1996. [Google Scholar]
- Holm, T.; Crossland, I. Mechanistic Features of the Reactions of Organomagnesium Compounds. In Grignard Reagents-New Developments; Richey, H.G., Jr., Ed.; John Wiley and Sons: Chichester, UK, 2000. [Google Scholar]
- Laird, T. Aromatic Ketones in Comprehensive Organic Chemistry; Barton, D., Ollis, W.D., Stoddart, J.F., Eds.; Pergamon Press: Oxford, UK, 1979; Chapter 5.4; Volume 1, pp. 1174–1181. [Google Scholar]
- Blomberg, C.; Grootveld, H.H.; Gerner, T.H.; Bickelhaupt, F. Radical formation during reactions of Grignard reagents with quinones. J. Organomet. Chem. 1970, 24, 549–553. [Google Scholar] [CrossRef]
- Wessely, F.; Kotlan, J. Über die Einwirkung von metallorganischen Verbindungen auf Chinole II. Monatshefte Chem. 1953, 84, 124–133. [Google Scholar] [CrossRef]
- Miller, B. Reactions of cyclohexadienones. 37. Attack of Grignard and lithium reagents at carbonyl oxygens of o-quinol acetates. J. Org. Chem. 1977, 42, 1402–1408. [Google Scholar] [CrossRef]
- Miller, B. Reactions of cyclohexadienones. 38. Substituent effects on reactions of benzylmagnesium chlorides with o-quinol acetates. J. Org. Chem. 1977, 42, 1408–1415. [Google Scholar] [CrossRef]
- Wege, D. Abnormal addition of vinylmagnesium bromide to 9,10-phenanthraquinone. Aust. J. Chem. 1971, 24, 1531–1535. [Google Scholar] [CrossRef]
- Holm, T. The reaction of benzil with Grignard reagents. Acta Chem. Scand. Ser. B 1987, 41, 278–284. [Google Scholar] [CrossRef]
- Boga, C.; Forlani, L.; Todesco, P.E. Unexpected regioselectivity in the attack of vinyl Grignard reagents to bis(2-benzothiazolyl) ketone. Tetrahedron Lett. 1997, 38, 4845–4848. [Google Scholar] [CrossRef]
- Boga, C.; Forlani, L.; Todesco, P.E. A simple synthesis of new carbinols from bis(2-benzothiazolyl)ketone. Gazz. Chim. Ital. 1997, 127, 197–199. [Google Scholar]
- Boga, C.; Stengel, R.; Abdayem, R.; Del Vecchio, E.; Forlani, L.; Todesco, P.E. Regioselectivity in the Addition of Vinylmagnesium Bromide to Heteroarylic Ketones: C-versus O-Alkylation. J. Org. Chem. 2004, 69, 8903–8909. [Google Scholar] [CrossRef] [PubMed]
- Boga, C.; Micheletti, G. Regioselectivity in the Addition of Grignard Reagents to Bis(2-benzothiazolyl)Ketone: C- vs. O-Alkylation Using Aryl Grignard Reagents. Eur. J. Org. Chem. 2010, 2010, 5659–5665. [Google Scholar]
- Forlani, L.; Boga, C.; Del Vecchio, E.; Padovani, M. Spontaneous Oxidation of bis(heteroaryl)methanes and bis(heteroaryl)carbinols to Ketones. Arkivoc 2003, 2003, 75–91. [Google Scholar]
- Galli, C.; Guarnieri, A.; Koch, H.; Mencarelli, P.; Rappoport, Z. Effect of Substituents on the Structure of the Vinyl Radical: Calculations and Experiments. J. Org. Chem. 1997, 62, 4072–4077. [Google Scholar] [CrossRef]
- Goumans, T.P.M.; van Alem, K.; Lodder, G. Photochemical Generation and Structure of Vinyl Radicals. Eur. J. Org. Chem. 2008, 435–443. [Google Scholar] [CrossRef]
- Singer, L.A. Selective Organic Transformations; Thyagarajan, B.S., Ed.; Wiley-Interscience: New York, NY, USA, 1972; Volume 2, pp. 239–268. [Google Scholar]
- Beckwith, A.L.J.; Ingold, K.U. Rearrangements in Ground and Excited States; De Mayo, P., Ed.; Academic Press: New York, NY, USA, 1980; Volume 1, pp. 280–310. [Google Scholar]
- Simumara, O. The Stereochemistry of Cyclohexyl and Vinylic Radicals. Top. Stereochem. 1969, 4, 1–37. [Google Scholar]
- Galli, C.; Rappoport, Z. Unequivocal SRN1 Route of Vinyl Halides with a Multitude of Competing Pathways: Reactivity and Structure of the Vinyl Radical Intermediate. Acc. Chem. Res. 2003, 36, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Bonazzola, L.; Feinstein, S.; Marx, R. Electronic structure of α-substituted vinyl radicals. Mol. Phys. 1971, 22, 689–695. [Google Scholar] [CrossRef]
- Neilson, G.W.; Symons, M.C.R. Radicals formed from acetylenes by high energy radiation and hydrogen atom bombardment: An electron spin resonance study. J. Chem. Soc. Perkin Trans. 2 1973, 1405–1410. [Google Scholar] [CrossRef]
- Ashby, E.C.; Laemmle, J.; Neumann, H.M. The Mechanisms of Grignard Reagent Addition to Ketones. Acc. Chem. Res. 1974, 7, 272–280. [Google Scholar] [CrossRef]
- McKinley, J.; Aponick, A.; Raber, J.C.; Fritz, C.; Montgomery, D.; Wigal, C.T. Reactions of Alkyl lithium and Grignard Reagents with Benzoquinone: Evidence for an Electron-Transfer Mechanism. J. Org. Chem. 1997, 62, 4874–4876. [Google Scholar] [CrossRef]
- Ashby, E.C.; Bowers, J.R., Jr. Organometallic Reaction Mechanisms. 17. Nature of Alkyl Transfer in Reactions of Grignard Reagents with Ketones. Evidence for Radical Intermediates in the Formation of 1,2-Addition Product Involving Tertiary and Primary Grignard Reagents. J. Am. Chem. Soc. 1981, 103, 2242–2250. [Google Scholar] [CrossRef]
- Ashby, E.C. A detailed description of the mechanism of reaction of Grignard reagents with ketones. Pure Appl. Chem. 1980, 52, 545–569. [Google Scholar] [CrossRef]
- Bulak, E.; Sarper, O.; Dogan, A.; Lissner, F.; Schleid, T.; Kaim, W. Dichlorogold(III) complexes of bis(1-methyl-2-imidazolyl)ketone and related ligands: Geometrical and electronic structures. Polyhedron 2006, 25, 2577–2582. [Google Scholar] [CrossRef]
- Bergbreiter, D.E.; Pendergrass, E. Analysis of Organomagnesium and Organolithium Reagents Using N-Phenyl-1-naphthylamin. J. Org. Chem. 1981, 49, 219–220. [Google Scholar] [CrossRef]
- SMART & SAINT Software Reference Manuals; Version 5.051 (Windows NT Version); Bruker Analytical X-ray Instruments Inc.: Madison, WI, USA, 1998.
- Sheldrick, G.M. SADABS, Program for Empirical Absorption Correction; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Cryst. 2005, 38, 381–388. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELX-2014; University of Göttingen and Bruker AXS: Karlsruhe, Germany, 2014. [Google Scholar]
Sample Availability: Not available. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).