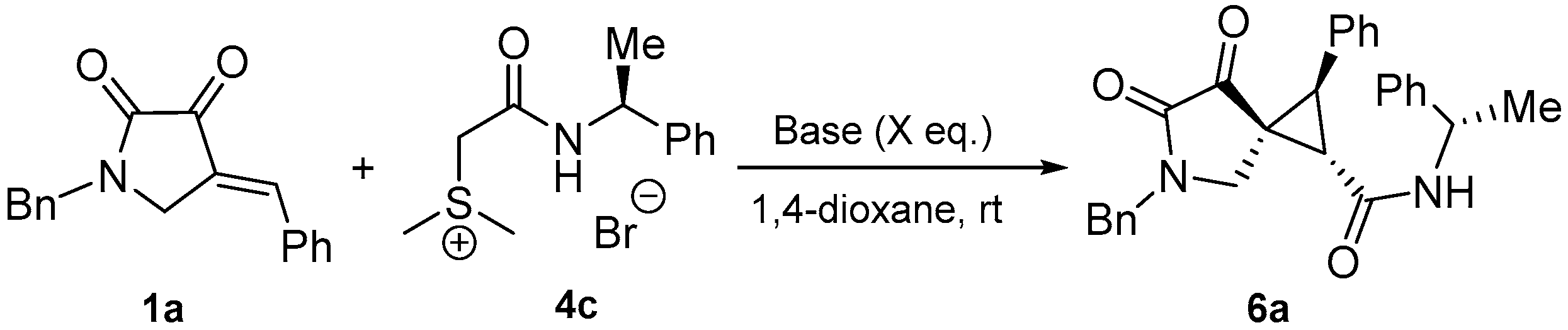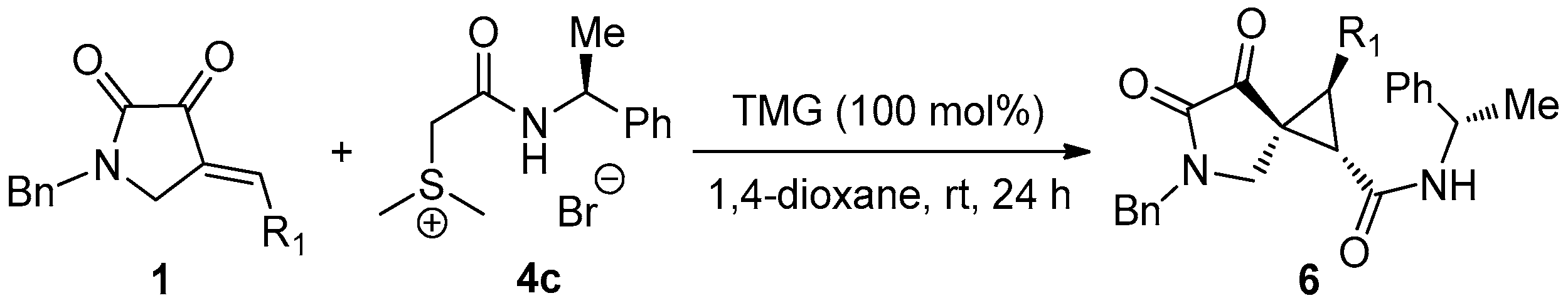Diastereoselective Synthesis of Spirocyclopropanes under Mild Conditions via Formal [2 + 1] Cycloadditions Using 2,3-Dioxo-4-benzylidene-pyrrolidines
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Information
3.2. Synthesis
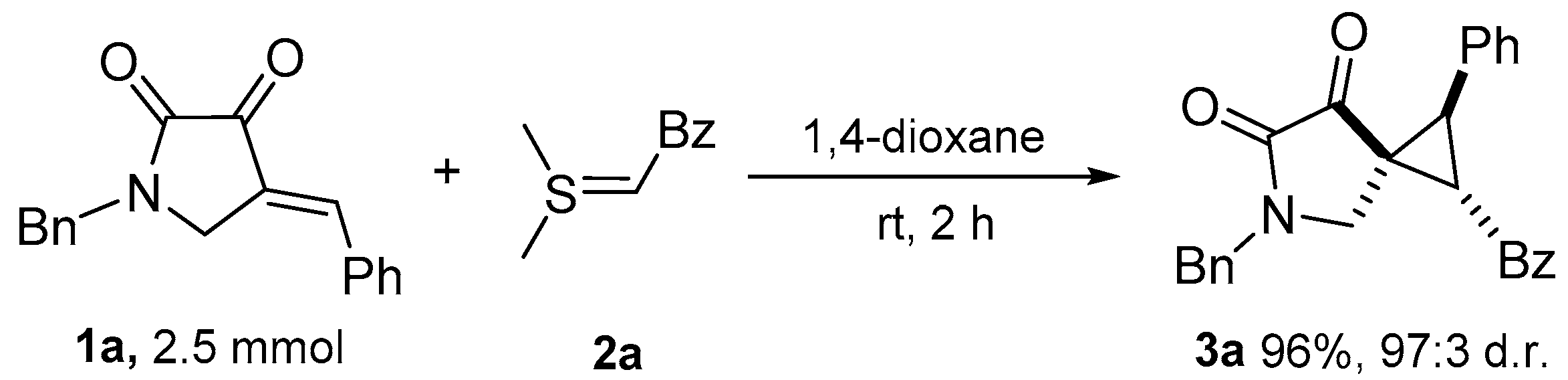
3.2.1. General Procedure for the Synthesis of Multi-Substituted Spirocyclopropane 3
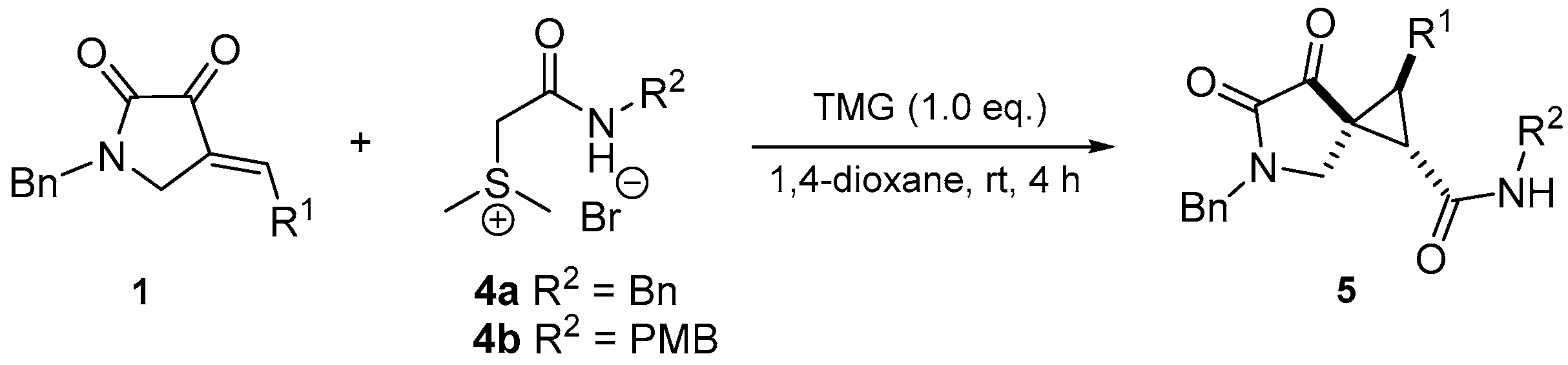
3.2.2. General Procedure for the Synthesis of Multi-Substituted Spirocyclopropane 5
3.2.3. General Procedure for the Synthesis of Multi-substituted Chiral Spirocyclopropane 6
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Note
- Rajesh, S.M.; Perumal, S.; Menéndez, J.C.; Yogeeswari, P.; Sriram, D. Antimycobacterial activity of spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio- and stereoselective 1,3-dipolar cycloaddition. Med. Chem. Commun. 2011, 2, 626–630. [Google Scholar] [CrossRef]
- Saha, S.; Acharya, C.; Pal, U.; Chowdhury, S.R.; Sarkar, K.; Maiti, N.C.; Jaisankar, P.; Majumder, H.K. A Novel spirooxindole derivative inhibits the growth of leishmania donovani parasites both in vitro and in vivo by targeting type IB topoisomerase. Antimicrob. Agents Chemother. 2016, 60, 6281–6293. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-Y.; Chang, C.-W.; Tseng, Y.-J.; Lee, J.; Sung, P.-J.; Su, J.-H.; Hwang, T.-L.; Dai, C.-F.; Wang, H.-C.; Sheu, J.-H. Bioactive steroids from the formosan soft coral umbellulifera petasites. Mar. Drugs 2016, 14, 180. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xing, X.-Y.; Sha, F.; Wu, Z.-Y.; Wu, X.-Y. Enantioselective synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole] derivatives via an organocatalytic asymmetric Michael/cyclization cascade reaction. Org. Biomol. Chem. 2016, 14, 8346–8355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-X.; Wang, H.-Y.; Jin, Q.-W.; Zheng, C.-W.; Zhao, G.; Shang, Y.-J. Thiourea-Quaternary Ammonium salt catalyzed asymmetric 1, 3-dipolar cycloaddition of imino esters to construct spiro[pyrrolidin-3,3′-oxindoles]. Org. Lett. 2016, 18, 4774–4777. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Yang, B.; Zhu, C.; Bäckvall, J.E. Highly efficient cascade reaction for selective formation of spirocyclobutenes from dienallenes via palladium-catalyzed oxidative double carbocyclization-carbonylation-alkynylation. J. Am. Chem. Soc. 2016, 138, 13846–13849. [Google Scholar] [CrossRef] [PubMed]
- Schobert, R.; Knauer, S.; Seibt, S.; Biersack, B. Anticancer active illudins: Recent developments of a potent alkylating compound class. Curr. Med. Chem. 2011, 18, 790–807. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.C.; Cong, Q.; Kelner, M.J. Structure−activity relationship studies of illudins: Analogues possessing a spiro-cyclobutane ring. J. Org. Chem. 2003, 68, 9648–9653. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T.C.; Kelner, M.J.; Wang, W.; Diaz, M.A.; Estes, L.A.; Taetle, R. Acylfulvenes, a new class of potent antitumor agents. Experientia 1996, 52, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Tanasova, M.; Sturla, S.J. Chemistry and biology of acylfulvenes: Sesquiterpene-derived antitumor agents. Chem. Rev. 2012, 112, 3578–3610. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Ferreirinha, P.; Sousa, H.; Ribeiro, J.; Bastos, M.M.; Neto, T.; Oliveira, P.A.; Medeiros, R.; Vilanova, M.; da Costa, R.M.G. Ptaquiloside from bracken (Pteridium spp.) inhibits tumour-infiltrating CD8+ T cells in HPV-16 transgenic mice. Food Chem. Toxicol. 2016, 97, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Boger, D.L.; Hughes, T.V.; Hedrick, M.P. Synthesis, chemical properties, and biological evaluation of CC-1065 and duocarmycin analogues incorporating the 5-methoxycarbonyl-1,2,9,9a-tetrahydrocyclopropa. J. Org. Chem. 2001, 66, 2207–2216. [Google Scholar] [CrossRef] [PubMed]
- Boger, D.L.; Boyce, C.W.; Garbaccio, R.M.; Goldberg, J.A. CC-1065 and the duocarmycins: Synthetic studies. Chem. Rev. 1997, 97, 787–828. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Nguyen, M.H. Fixed-dose combination of sofosbuvir and ledipasvir for the treatment of chronic hepatitis C genotype 1. Expert. Opin. Pharmacother. 2015, 16, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M.; Stepanova, M.; Marcellin, P.; Afdhal, N.; Kowdley, K.V.; Zeuzem, S.; Hunt, S.L. Treatment with ledipasvir and sofosbuvir improves patient-reported outcomes: Results from the ION-1, -2, and -3 clinical trials. Hepatology 2015, 61, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The protein data bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed]
- René, O.; Stepek, I.A.; Gobbi, A.; Fauber, B.P.; Gaines, S. Palladium-catalyzed ring expansion of spirocyclopropanes to form caprolactams and azepanes. J. Org. Chem. 2015, 80, 10218–10225. [Google Scholar]
- Gopalakrishnan, B.; Mohan, S.; Parella, R.; Babu, S.A. Diastereoselective Pd(II)-catalyzed sp3 C-H arylation followed by ring opening of cyclopropanecarboxamides: Construction of anti β-Acyloxy carboxamide derivatives. J. Org. Chem. 2016, 81, 8988–9005. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Zhang, J.; Wang, D.Z. Gold-catalyzed rearrangement of alkynyl donor-acceptor cyclopropanes to construct highly functionalized alkylidenecyclopentenes. Org. Lett. 2015, 17, 2098–2101. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Lin, L.-L.; Xia, Y.; Zhou, P.-F.; Liu, X.-H.; Feng, X.-M. Catalytic asymmetric [3 + 3] annulation of cyclopropanes with mercaptoacetaldehyde. Org. Biomol. Chem. 2016, 14, 5914–5917. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liang, Y.-J.; Zhou, G.-Y.; Wang, K.-W.; Dong, D.-W. Ring-enlargement of dimethylaminopropenoyl cyclopropanes: An efficient route to substituted 2,3-dihydrofurans. J. Org. Chem. 2008, 73, 8089–8092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-G.; Zhang, W.; Li, J.-L.; Liu, Q.-F.; Liu, T.-X.; Zhang, G.-S. Synthesis of multisubstituted pyrroles from doubly activated cyclopropanes using an iron-mediated oxidation domino reaction. J. Org. Chem. 2014, 79, 11226–11233. [Google Scholar] [CrossRef] [PubMed]
- Rösner, C.; Hennecke, U. Homohalocyclization: Electrophilic bromine-induced cyclizations of cyclopropanes. Org. Lett. 2015, 17, 3226–3229. [Google Scholar] [CrossRef] [PubMed]
- Tsunoi, S.; Maruoka, Y.; Suzuki, I.; Shibata, I. Catalytic [3 + 2] cycloaddition through ring cleavage of simple cyclopropanes with isocyanates. Org. Lett. 2015, 17, 4010–4013. [Google Scholar] [CrossRef] [PubMed]
- Kaicharla, T.; Roy, T.; Thangaraj, M.; Gonnade, R.G.; Biju, A.T. Lewis acid catalyzed selective reactions of donor-acceptor cyclopropanes with 2-naphthols. Angew. Chem. Int. Ed. 2016, 55, 10061–10064. [Google Scholar] [CrossRef] [PubMed]
- Nambu, H.; Ono, N.; Yakura, T. Acid-catalyzed ring-opening cyclization of spirocyclopropanes for the construction of a 2-arylbenzofuran skeleton: Total synthesis of cuspidan B. Synthesis 2016, 48, 1892–1901. [Google Scholar] [CrossRef]
- Nambu, H.; Fukumoto, M.; Hirota, W.; Yakura, T. Ring-opening cyclization of cyclohexane-1,3-dione-2-spirocyclopropanes with amines: Rapid access to 2-substituted 4-hydroxyindole. Org. Lett. 2014, 16, 4012–4015. [Google Scholar] [CrossRef] [PubMed]
- Chanthamath, S.; Iwasa, S. Enantioselective cyclopropanation of a wide variety of olefins catalyzed by Ru(II)-Pheox complexes. Acc. Chem. Res. 2016, 49, 2080–2090. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F. Present and future of cyclopropanations in fragrance chemistry. Chem. Biodivers. 2014, 11, 1734–1751. [Google Scholar] [CrossRef] [PubMed]
- Lebel, H.; Marcoux, J.F.; Molinaro, C.; Charette, A.B. Stereoselective cyclopropanation reactions. Chem. Rev. 2003, 103, 977–1050. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, W.A. Synthesis of cyclopropane containing natural products. Tetrahedron 2001, 57, 8589–8627. [Google Scholar] [CrossRef]
- Shchepin, V.V.; Stepanyan, Y.G.; Silaichev, P.S.; Ezhikova, M.A.; Kodess, M.I. Reactions of bromine-containing organozinc compounds derived from α,α-dibromo ketones with 2-arylmethylideneindan-1,3-diones and 5-arylmethylidene-2,2-dimethyl-1,3-dioxane-4,6-diones. Russ. J. Org. Chem. 2010, 46, 499–502. [Google Scholar] [CrossRef]
- Russo, A.; Meninno, S.; Tedesco, C.; Lattanzi, A. Synthesis of Activated Cyclopropanes by an MIRC Strategy: An enantioselective organocatalytic approach to spirocyclopropanes. Eur. J. Org. Chem. 2011, 2011, 5096–5103. [Google Scholar] [CrossRef]
- Wang, G.-W.; Gao, J. Selective formation of spiro dihydrofurans and cyclopropanes through unexpected reaction of aldehydes with 1,3-dicarbonyl compounds. Org. Lett. 2009, 11, 2385–2388. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, J.-I.; Yamamoto, M.; Kawabata, N. Electrochemical reduction of dibromodiketones facile [3 + 2] cycloaddition with olefins. Tetrahedron Lett. 1985, 26, 6217–6220. [Google Scholar] [CrossRef]
- Qian, P.; Du, B.-N.; Song, R.-C.; Wu, X.-D.; Mei, H.-B.; Han, J.-L.; Pan, Y. N-Iodosuccinimide-initiated spirocyclopropanation of styrenes with 1,3-dicarbonyl compound for the synthesis of spirocyclopropanes. J. Org. Chem. 2016, 81, 6546–6553. [Google Scholar] [CrossRef] [PubMed]
- Lautens, M.; Klute, W.; Tam, W. Transition metal-mediated cycloaddition reactions. Chem. Rev. 1996, 96, 49–92. [Google Scholar] [CrossRef] [PubMed]
- Young, I.S.; Qiu, Y.-P.; Smith, M.J.; Hay, M.B.; Doubleday, W.W. Preparation of a tricyclopropylamino acid derivative via Simmons–Smith cyclopropanation with downstream intramolecular aminoacetoxylation for impurity control. Org. Process Res. Dev. 2016, 20, 2108–2115. [Google Scholar] [CrossRef]
- Lévesque, E.; Goudreauand, S.R.; Charette, A.B. Improved zinc-catalyzed Simmons-Smith reaction: Access to various 1,2,3-trisubstituted cyclopropanes. Org. Lett. 2014, 16, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Hayashi, R.; Hsung, R.P.; DeKorver, K.A.; Lohse, A.G.; Song, Z.; Tang, Y. Synthesis of amido-spiro[2.2]pentanes via Simmons-Smith cyclopropanation of allenamides. Org. Biomol. Chem. 2009, 7, 3331–3337. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.G.; Ling, K.B.; Roberts, P.M.; Russell, A.J.; Thomson, J.E. Diastereoselective Simmons-Smith cyclopropanations of allylic amines and carbamates. Chem. Commun. 2007, 39, 4029–4031. [Google Scholar] [CrossRef] [PubMed]
- Pellissier, H. Recent developments in asymmetric cyclopropanation. Tetrahedron 2008, 64, 7041–7095. [Google Scholar] [CrossRef]
- Su, Y.; Li, Q.-F.; Zhao, Y.-M.; Gu, P.-M. Preparation of optically active cis-cyclopropane carboxylates: Cyclopropanation of α-silyl stryenes with aryldiazoacetates and desilylation of the resulting silyl cyclopropanes. Org. Lett. 2016, 18, 4356–4359. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, M.J.; Shankar, B.K.R.; Shechter, H. Rhodium(II) acetate-catalyzed reactions of 2-diazo-1,3-indandione and 2-diazo-1-indanone with various substrates. J. Org. Chem. 1988, 53, 2699–2705. [Google Scholar] [CrossRef]
- Su, Y.; Bai, M.; Qiao, J.-B.; Li, X.-J.; Li, R.; Tu, Y.-Q.; Gu, P.-M. Diastereo- and enantioselective cyclopropanation of alkyenyl fluorides with benzyl diazoarylacetates. Tetrahedron Lett. 2015, 56, 1805–1807. [Google Scholar] [CrossRef]
- Arai, S.; Nakayama, K.; Hatano, K.; Shioiri, T. Stereoselective synthesis of cyclopropane rings under phase-transfer-catalyzed conditions. J. Org. Chem. 1998, 63, 9572–9575. [Google Scholar] [CrossRef]
- Miyagawa, T.; Tatenuma, T.; Tadokoro, M.; Satoh, T. A short and stereoselective synthesis of highly substituted cyclopropanes from α,β-unsaturated carbonyl compounds with dichloromethyl p-tolyl sulfoxide. Tetrahedron 2008, 64, 5279–5284. [Google Scholar] [CrossRef]
- Elinson, M.N.; Feducovich, S.K.; Vereshchagin, A.N.; Gorbunov, S.V.; Belyakov, P.A.; Nikishin, G.I.; Wang, Q.-F.; Song, X.-K.; Chen, J.; Yan, C.-G. Pyridinium ylide-assisted one-pot two-step tandem synthesis of polysubstituted cyclopropanes. J. Comb. Chem. 2009, 11, 1007–1010. [Google Scholar]
- Li, A.-H.; Dai, L.-X.; Aggarwal, V.K. Asymmetric ylide reactions: Epoxidation, cyclopropanation, aziridination, olefination, and rearrangement. Chem. Rev. 1997, 97, 2341–2372. [Google Scholar] [CrossRef] [PubMed]
- McGarrigle, E.M.; Myers, E.L.; Illa, O.; Shaw, M.A.; Riches, S.L.; Aggarwal, V.K. Chalcogenides as organocatalysts. Chem. Rev. 2007, 107, 5841–5883. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wu, B.; Chen, M.-W.; Jiang, G.-F.; Zhou, Y.-G. The concise synthesis of spiro-cyclopropane compounds via the dearomatization of indole derivatives. Org. Lett. 2014, 16, 2578–2581. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.-B.; Fang, X.-X.; Li, X.-Y.; Wu, J.; Yao, H.-Q.; Lin, A.-J. 1,6-Conjugated addition-mediated [2 + 1] annulation: Approach to spiro[2.5]octa-4,7-dien-6-one. J. Org. Chem. 2015, 80, 11123–11130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hu, X.-Q.; Wang, Z.-Y.; Xu, P.-F. Reactions of sulfonium salts with 2,3-dioxopyrrolidine derivatives: A concise synthesis of spirocyclopropane. Synthesis 2015, 47, 2529–2537. [Google Scholar]
- Nambu, H.; Ono, N.; Hirota, W.; Fukumoto, M.; Yakura, T. An efficient method for the synthesis of 2′,3′-nonsubstituted cycloalkane-1,3-dione-2-spirocyclopropanes using (2-bromoethyl)diphenylsulfonium trifluoromethanesulfonate. Chem. Pharm. Bull. (Tokyo) 2016, 64, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Nambu, H.; Fukumoto, M.; Hirota, W.; Ono, N.; Yakura, T. An efficient synthesis of cycloalkane-1,3-dione-2-spirocyclopropanes from 1,3-cycloalkanediones using (1-aryl-2-bromoethyl)-dimethylsulfonium bromides: Application to a one-pot synthesis of tetrahydroindol-4(5H)-one. Tetrahedron Lett. 2015, 56, 4312–4315. [Google Scholar] [CrossRef]
- Liao, W.-W.; Li, K.; Tang, Y. Controllable diastereoselective cyclopropanation. Enantioselective synthesis of vinylcyclopropanes via chiral telluronium ylides. J. Am. Chem. Soc. 2003, 125, 13030–13031. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.-C.; Liao, W.-W.; Tang, Y.; Sun, X.-L.; Dai, L.-X. The michael addition-elimination of ylides to α,β-unsaturated imines. Highly stereoselective synthesis of vinylcyclopropanecarbaldehydes and vinylcyclopropylaziridines. J. Am. Chem. Soc. 2005, 127, 12222–12223. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.-G.; Zhang, H.; Chen, J.; Zhou, X.-H.; Shao, M.; McMills, M.C. Stereoselective synthesis of highly substituted trans-2,3-dihydrofuran and trans-1,2-cyclopropane derivatives containing sulfonyl groups. Tetrahedron 2008, 64, 163–167. [Google Scholar] [CrossRef]
- Wu, X.-Y.; Cao, W.-G.; Zhang, H.; Chen, J.; Jiang, H.-Y.; Deng, H.-M.; Shao, S.; Zhang, J.-P.; Chen, H.-Y. Highly stereoselective synthesis of β,γ-disubstituted and α,β,γ-trisubstituted butyrolactones. Tetrahedron 2008, 64, 10331–10338. [Google Scholar] [CrossRef]
- Zhao, Y.-H.; Zheng, C.-W.; Zhao, G.; Cao, W.-G. Highly enantioselective tandem cyclopropanation/Wittig reaction of α,β-unsaturated aldehydes with arsonium ylides catalyzed by recyclable dendritic catalyst. Tetrahedron Asymmetry 2008, 19, 701–708. [Google Scholar] [CrossRef]
- Cao, W.-G.; Zhang, H.; Chen, J.; Deng, H.-M.; Shao, M.; Lei, L.; Qian, J.-X.; Zhu, Y. A facile preparation of trans-1,2-cyclopropanes containing p-trifluoromethylphenyl group and its application to the construction of pyrazole and cyclopropane ring fused pyridazinone derivatives. Tetrahedron 2008, 64, 6670–6674. [Google Scholar] [CrossRef]
- Papageorgiou, C.D.; Cubillo de Dios, M.A.; Ley, S.V.; Gaunt, M.J. Enantioselective organocatalytic cyclopropanation via ammonium ylides. Angew. Chem. Int. Ed. 2004, 43, 4641–4644. [Google Scholar] [CrossRef] [PubMed]
- Vanecko, J.A.; Wan, H.; West, F.G. Recent advances in the Stevens rearrangement of ammonium ylides. Application to the synthesis of alkaloid natural products. Tetrahedron 2006, 62, 1043–1062. [Google Scholar] [CrossRef]
- Jończyk, A.; Konarska, A. Generation and reactions of ammonium ylides in basic two-phase systems: Convenient synthesis of cyclopropanes, oxiranes and alkenes substituted with electron-withdrawing groups. Synlett 1999, 7, 1085–1087. [Google Scholar] [CrossRef]
- Kowalkowska, A.; Sucholbiak, D.; Jończyk, A. Generation and reaction of ammonium ylides in basic two-phase systems. Eur. J. Org. Chem. 2005, 925–933. [Google Scholar]
- Li, J.-L.; Yang, K.-C.; Li, Y.; Li, Q.; Zhu, H.-P.; Han, B.; Peng, C.; Zhi, Y.-G.; Gou, X.-J. Asymmetric synthesis of bicyclic dihydropyrans via organocatalytic inverse-electron-demand oxo-Diels-Alder reactions of enolizable aliphatic aldehydes. Chem. Commun. 2016, 52, 10617–10620. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-L.; Li, Q.; Yang, K.-C.; Li, Y.; Zhou, L.; Han, B.; Peng, C.; Gou, X.-J. A practical green chemistry approach to synthesize fused bicyclic 4H-pyranes via an amine catalysed 1,4-addition and cyclization cascade. RSC Adv. 2016, 6, 38875–38879. [Google Scholar] [CrossRef]
- Kaufmann, D.; West, P.J.; Smith, M.D.; Yagen, B.; Bialer, M.; Devor, M.; White, H.S.; Brennan, K.C. sec-Butylpropylacetamide (SPD), a new amide derivative of valproic acid for the treatment of neuropathic and inflammatory pain. Pharmacol. Res. 2016, 117, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, S.R.; Livingstone, V.; Low, E.; Pressler, R.; Rennie, J.M.; Boylan, G.B. Phenobarbital reduces EEG amplitude and propagation of neonatal seizures but does not alter performance of automated seizure detection. Clin. Neurophysiol. 2016, 127, 3343–3350. [Google Scholar] [CrossRef] [PubMed]
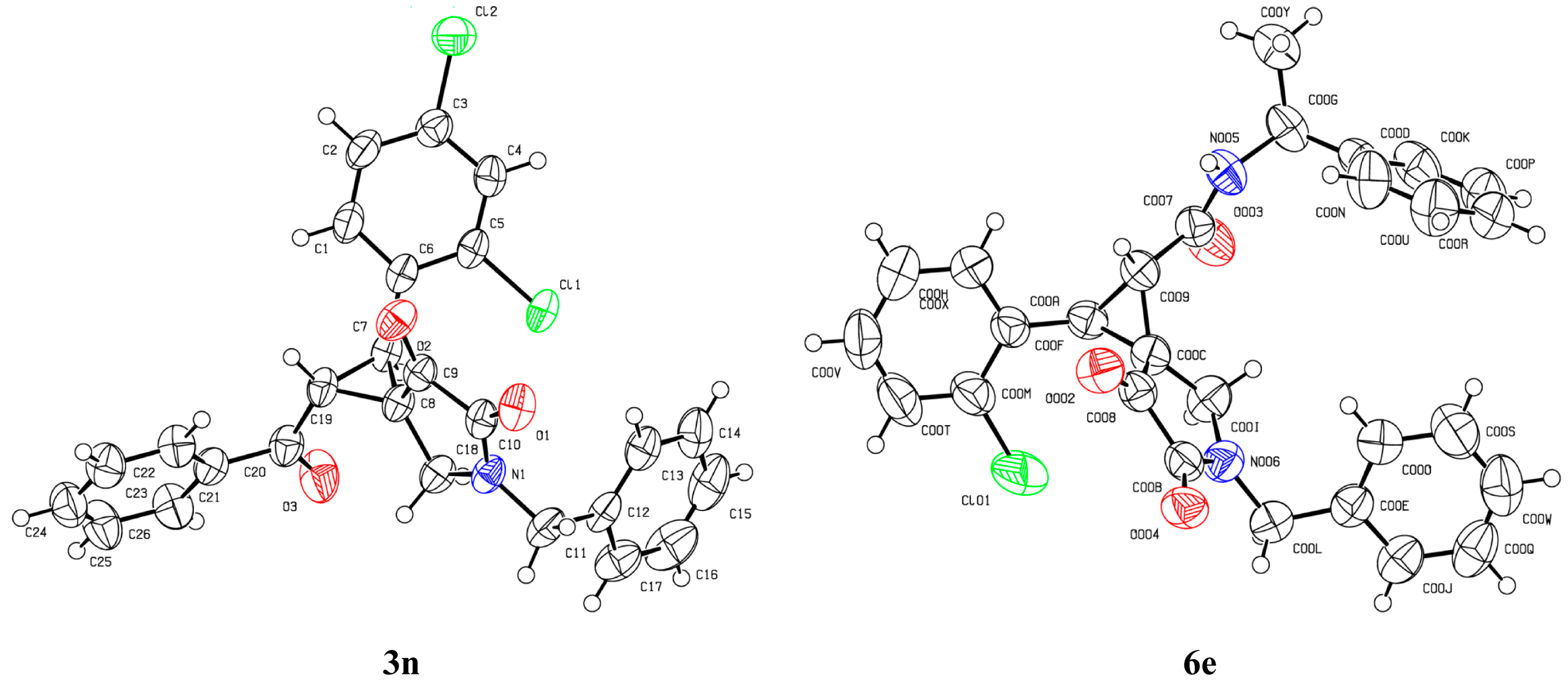
- CCDC 1478324 (3n) and CCDC 1524538 (6e) Contain the Supplementary Crystallographic Data for This Paper. These Data can Be Obtained Free of Charge from the Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- Krell, E. Handbook of Laboratory Distillation; Elsevier Publishing Company: Amsterdam, The Netherlands, 1963. [Google Scholar]
- Rosengart, M.J. The Technique of Distillation and Rectification in the Laboratory; VEB Verlag Technik: Berlin, Germany, 1954. [Google Scholar]
- Stage, H. Columns for laboratory distillation. Angew. Chem. 1947, B19, 175. [Google Scholar] [CrossRef]
- Southwick, P.L.; Barnas, E.F. 4-Benzylidine-2,3-dioxopyrrolidines and 4-benzyl-2,3-dioxopyrrolidines. synthesis and experiments on reduction and alkylation. J. Org. Chem. 1962, 27, 98–106. [Google Scholar] [CrossRef]
- Sample Availability: All samples 3, 5 and 6 are available from the authors.

| Entry | Solvent | d.r. b | Yield (%) c |
|---|---|---|---|
| 1 | DCM | 93:7 | 86 |
| 2 | CHCl3 | 96:4 | 94 |
| 3 | DCE | 94:6 | 90 |
| 4 | THF | 97:3 | 79 |
| 5 | 1,4-dioxane | 98:2 | 92 |
| 6 | MeOH | 53:47 | 76 |
| 7 | EtOH | 68:32 | 74 |
| 8 | MeCN | 84:16 | 92 |
| 9 | Toluene | 87:13 | 88 |
| Entry | R1 | R2 | R3 | Product | d.r. b | Yield (%) c |
|---|---|---|---|---|---|---|
| 1 | Ph | Bn | Ph | 3a | 98:2 | 92 |
| 2 | 3-MeC6H4 | Bn | Ph | 3b | 92:8 | 83 |
| 3 | 4-MeC6H4 | Bn | Ph | 3c | 98:2 | 93 |
| 4 | 2-MeOC6H4 | Bn | Ph | 3d | 92:8 | 83 |
| 5 | 3-MeOC6H4 | Bn | Ph | 3e | 97:3 | 91 |
| 6 | 4-MeOC6H4 | Bn | Ph | 3f | 96:4 | 94 |
| 7 | 4-FC6H4 | Bn | Ph | 3g | 96:4 | 88 |
| 8 | 2-ClC6H4 | Bn | Ph | 3h | 97:3 | 98 |
| 9 | 3-ClC6H4 | Bn | Ph | 3i | 97:3 | 94 |
| 10 | 4-ClC6H4 | Bn | Ph | 3j | 96:4 | 59 |
| 11 | 3-BrC6H4 | Bn | Ph | 3k | 94:6 | 84 |
| 12 | 4-BrC6H4 | Bn | Ph | 3l | 96:4 | 57 |
| 13 | 3,4-(MeO)2C6H3 | Bn | Ph | 3m | 98:2 | 81 |
| 14 d | 2,4-Cl2C6H3 | Bn | Ph | 3n | >99:1 | 99 |
| 15 | 1-Naphthyl | Bn | Ph | 3o | 94:6 | 94 |
| 16 | 2-Thienyl | Bn | Ph | 3p | 98:2 | 86 |
| 17 | Ph | PMB | Ph | 3q | 98:2 | 90 |
| 18 e | Ph | Bn | OEt | 3r | 92:8 | 99 |
| 19 e | Ph | Bn | Ot-Bu | 3s | 90:10 | 99 |
| Entry | R1 | R2 | Product | d.r. b | Yield (%) c |
|---|---|---|---|---|---|
| 1 | Ph | Bn | 5a | 96:4 | 98 |
| 2 | Ph | PMB | 5b | >99:1 | 99 |
| 3 | 3-MeC6H4 | Bn | 5c | 97:3 | 99 |
| 4 | 3-MeC6H4 | PMB | 5d | 94:6 | 92 |
| 5 | 4-ClC6H4 | Bn | 5e | 91:9 | 99 |
| 6 | 2-ClC6H4 | PMB | 5f | 94:6 | 98 |
| 7 d | 2-Naphthyl | Bn | 5g | > 99:1 | 85 |
| 8 d | 2-Naphthyl | PMB | 5h | > 99:1 | 90 |
| 9 d | 2-Thienyl | Bn | 5i | > 99:1 | 83 |
| 10 d | 2-Thienyl | PMB | 5j | > 99:1 | 81 |
| Entry | Base | X (eq.) | Time | d.r. b | Yield (%) c |
|---|---|---|---|---|---|
| 1 | TMG | 1.0 | 24 h | 72:28 | 99 |
| 2 | DBU | 1.0 | 24 h | 62:38 | 99 |
| 3 | KOH | 1.5 | 15 min | 65:35 | 99 |
| 4 | tBuOK | 1.5 | 10 min | 68:32 | 95 |
| 5 | K2CO3 | 2.0 | 72 h | 68:32 | 92 |
| Entry | R1 | Product | d.r. b | Yield(%) c |
|---|---|---|---|---|
| 1 | Ph | 6a | 72:28 | 99 |
| 2 | 4-MeC6H4 | 6b | 70:30 | 97 |
| 3 | 4-MeOC6H4 | 6c | 64:36 | 91 |
| 4 | 4-FC6H4 | 6d | 81:19 | 92 |
| 5 d | 2-ClC6H4 | 6e | 70:30 | 90 |
| 6 | 4-BrC6H4 | 6f | 62:38 | 81 |
| 7 e | 1-Naphthyl | 6g | 72:28 | 97 |
| 8 e | 2-Naphthyl | 6h | 77:23 | 99 |
| 9 e | 2-Thienyl | 6i | 60:40 | 90 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, Q.-Z.; Huang, L.; Liang, H.; Yang, K.-C.; Leng, H.-J.; Liu, Y.; Shen, X.-D.; Gou, X.-J.; Li, J.-L. Diastereoselective Synthesis of Spirocyclopropanes under Mild Conditions via Formal [2 + 1] Cycloadditions Using 2,3-Dioxo-4-benzylidene-pyrrolidines. Molecules 2017, 22, 328. https://doi.org/10.3390/molecules22020328
Li Y, Li Q-Z, Huang L, Liang H, Yang K-C, Leng H-J, Liu Y, Shen X-D, Gou X-J, Li J-L. Diastereoselective Synthesis of Spirocyclopropanes under Mild Conditions via Formal [2 + 1] Cycloadditions Using 2,3-Dioxo-4-benzylidene-pyrrolidines. Molecules. 2017; 22(2):328. https://doi.org/10.3390/molecules22020328
Chicago/Turabian StyleLi, Yi, Qing-Zhu Li, Li Huang, Hong Liang, Kai-Chuan Yang, Hai-Jun Leng, Yue Liu, Xu-Dong Shen, Xiao-Jun Gou, and Jun-Long Li. 2017. "Diastereoselective Synthesis of Spirocyclopropanes under Mild Conditions via Formal [2 + 1] Cycloadditions Using 2,3-Dioxo-4-benzylidene-pyrrolidines" Molecules 22, no. 2: 328. https://doi.org/10.3390/molecules22020328
APA StyleLi, Y., Li, Q.-Z., Huang, L., Liang, H., Yang, K.-C., Leng, H.-J., Liu, Y., Shen, X.-D., Gou, X.-J., & Li, J.-L. (2017). Diastereoselective Synthesis of Spirocyclopropanes under Mild Conditions via Formal [2 + 1] Cycloadditions Using 2,3-Dioxo-4-benzylidene-pyrrolidines. Molecules, 22(2), 328. https://doi.org/10.3390/molecules22020328