Synthesis and Antibacterial Evaluation of Novel 1,3,4-Oxadiazole Derivatives Containing Sulfonate/Carboxylate Moiety
Abstract
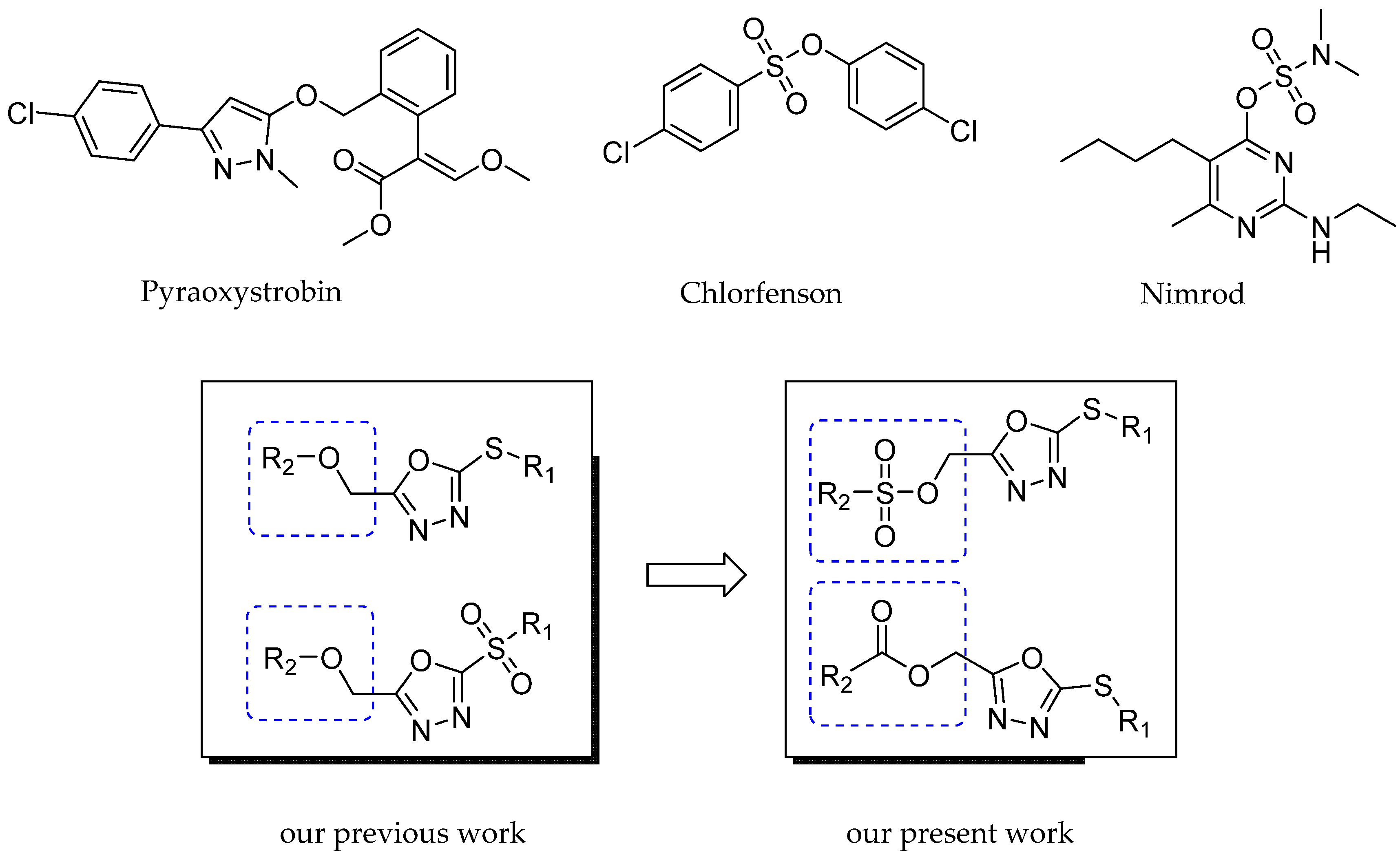
1. Introduction
2. Results and Discussion
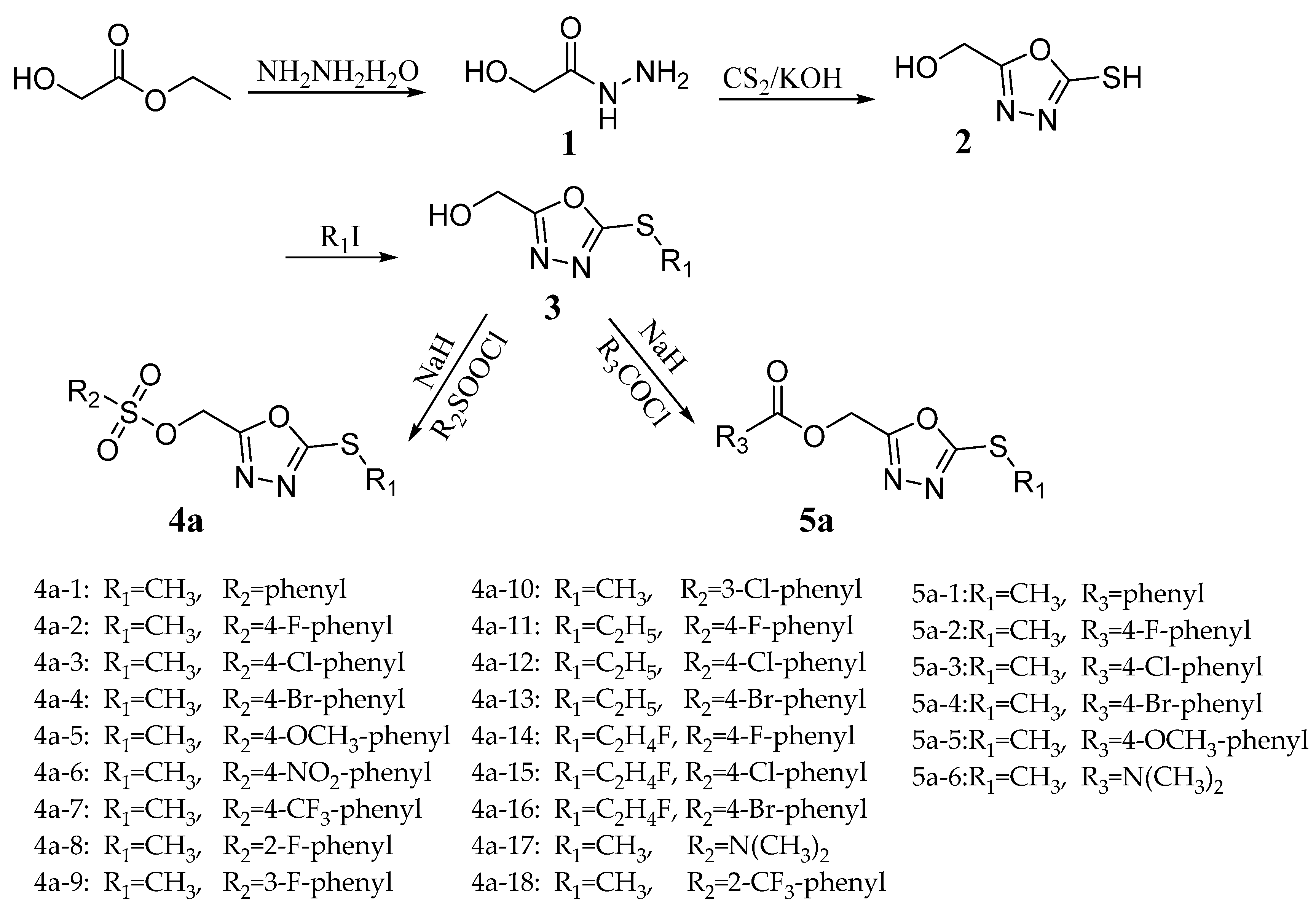
2.1. Chemistry
2.2. In Vitro Antibacterial Activity
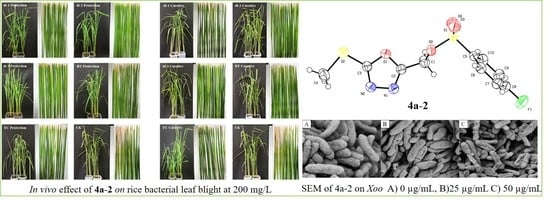
2.3. In Vivo Antibacterial Activity
2.4. Scanning Electron Microscopy Studies
2.5. Structure-Activity Relationship (SAR) Analyses
3. Experimental
3.1. Chemicals and Instruments
3.2. General Synthetic Procedure for the Target Compounds
3.2.1. Preparation of Intermediate 1
3.2.2. Preparation of Intermediate 2
3.2.3. Preparation of Intermediate 3
3.2.4. Preparation of Target Compound 4a/5a
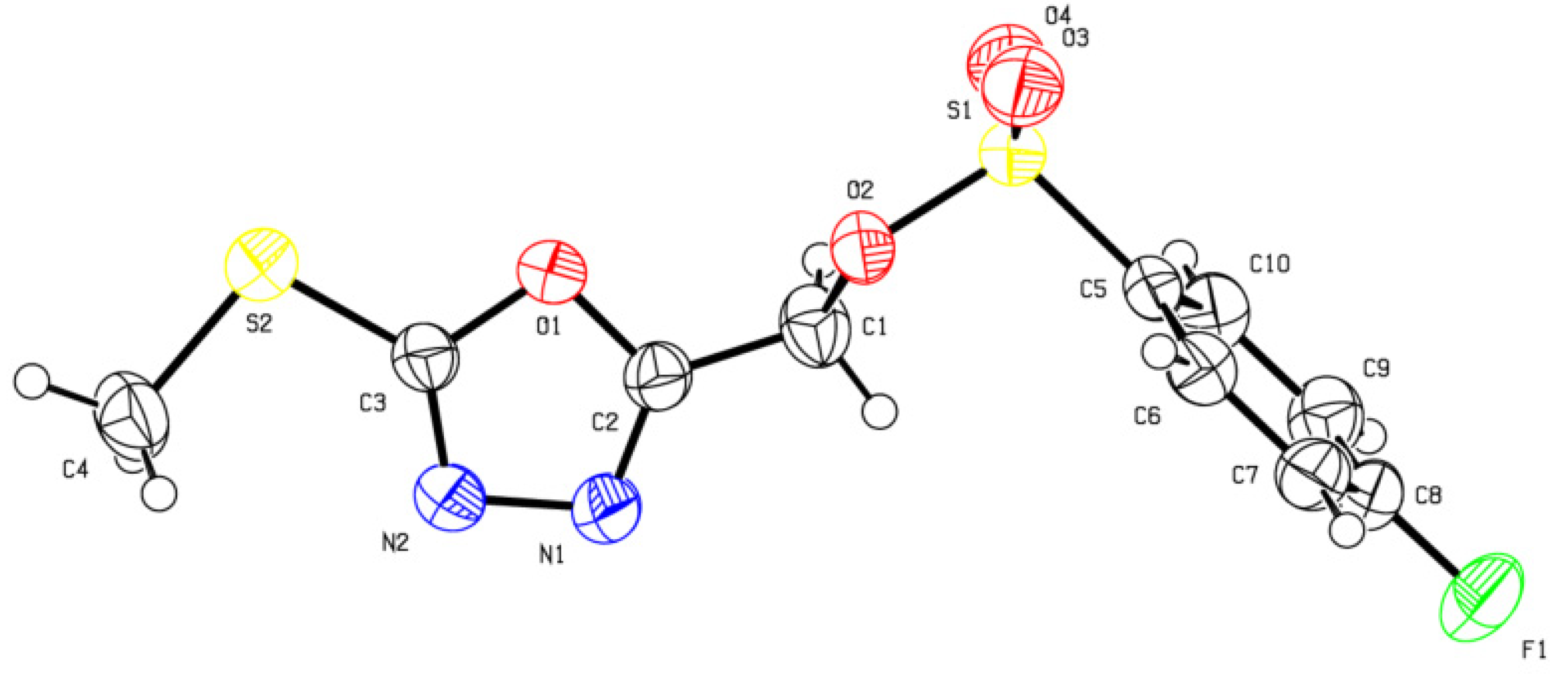
3.3. X-ray Diffraction Analysis
3.4. Antibacterial Bioassay by Scanning Electron Microscopy
3.5. Antibacterial Bioassay In Vitro
3.6. Antibacterial Activity Bioassay In Vivo
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ji, Z.Y.; Wang, C.L.; Zhao, K.J. Rice routes of countering Xanthomonas oryzae. Int. J. Mol. Sci. 2018, 19, 14. [Google Scholar] [CrossRef]
- Sun, Y. Identification and characterization of genes responsive to apoptosis: Application of DNA chip technology and mRNA differential display. Histol. Histopathol. 2000, 15, 1271–1284. [Google Scholar] [PubMed]
- Quibod, I.L.; Atieza-Grande, G.; Oreiro, E.G.; Palmos, D.; Nguyen, M.H.; Coronejo, S.T.; Aung, E.E.; Nugroho, C.; Roman-Reyna, V.; Burgos, M.R. The green revolution shaped the population structure of the rice pathogen Xanthomonas oryzae pv. oryzae. ISME J. 2019. [Google Scholar] [CrossRef]
- Li, P.; Hu, D.Y.; Xie, D.D.; Chen, J.X.; Jin, L.H.; Song, B.A. Design, synthesis, and evaluation of new sulfone derivatives containing a 1,3,4-oxadiazole moiety as active antibacterial agents. J. Agric. Food Chem. 2018, 66, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Li, C.; Zhang, Y.Y.; Qiao, C.H.; Ye, Y.H. Synthesis and biological evaluation of benzofuroxan derivatives as fungicides against phytopathogenic fungi. J. Agric. Food Chem. 2013, 61, 8632–8640. [Google Scholar] [CrossRef] [PubMed]
- Kharde, R.R.; Lavale, S.A.; Ghorpade, B.B. Molecular diversity among the isolates of Xanthomonas axonopodis pv. citri causing bacterial canker in citrus. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2375–2384. [Google Scholar] [CrossRef]
- Abhang, P.B.; Totawar, M.V.; Katkar, M.M.; Atram, P.C.; Mane, S.S. Efficacy of fungicides botanicals bioagents against Xanthomonas axonopodis pv. citri. Int. J. Chem. Stud. 2018, 6, 1108–1111. [Google Scholar]
- Chen, L.J.; Guo, T.; Xia, R.J. Tang, X.; Chen, Y.; Zhang, C.; Xue, W.; Novel phosphorylated penta-1,4-dien-3-one derivatives: Design, synthesis, and biological activity. Molecules 2019, 24, 12. [Google Scholar]
- Wang, S.B.; Gan, X.H.; Wang, Y.J.; Li, S.Y.; Yi, C.F.; Chen, J.X.; He, F.C.; Yang, Y.Y.; Hu, D.Y.; Song, B.A. Novel 1,3,4-oxadiazole derivatives containing a cinnamic acid moiety as potential bactericide for rice bacterial diseases. Int. J. Mol. Sci. 2019, 20, 1020. [Google Scholar] [CrossRef]
- Su, S.H.; Zhou, X.; Liao, G.P.; Qi, P.Y.; Jin, L.H. Synthesis and antibacterial evaluation of new sulfone derivatives containing 2-aroxymethyl-1,3,4-oxadiazole/thiadiazole moiety. Molecules 2017, 22, 64. [Google Scholar] [CrossRef]
- Li, P.; Tian, P.Y.; Chen, Y.Z.; Song, X.P.; Xue, W.; Jin, L.H.; Hu, D.Y.; Yang, S.; Song, B.A. Novel bisthioether derivatives containing a 1,3,4-oxadiazole moiety: Design, synthesis, antibacterial and nematocidal activities. Pest Manag. Sci. 2018, 74, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.M.; Han, F.F.; He, M.; Hu, D.Y.; He, J.; Yang, S.; Song, B.A. Inhibition of tobacco bacterial wilt with sulfone derivatives containing an 1,3,4-oxadiazole moiety. J. Agric. Food Chem. 2012, 60, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.W.; Zhu, H.H.; Wang, P.Y.; Zeng, D.; Wu, Y.Y.; Liu, L.W.; Wu, Z.B.; Li, Z.; Yang, S. Synthesis of thiazolium-labeled 1,3,4-oxadiazole thioethers as prospective antimicrobials: In vitro and in vivo bioactivity and mechanism of action. J. Agric. Food Chem. 2019, 67, 12696–12708. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.Y.; Shao, W.B.; Zhu, J.Z.; Long, Z.Q.; Liu, L.W.; Wang, P.Y.; Li, Z.; Yang, S. Novel 1,3,4-oxadiazole-2-carbohydrazides as prospective agricultural antifungal agents potentially targeting succinate dehydrogenase. J. Agric. Food Chem. 2019, 67, 13892–13903. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Luo, N.; Ding, M.H.; Bao, X.P. Synthesis, in vitro antibacterial and antifungal evaluation of novel 1,3,4-oxadiazole thioether derivatives bearing the 6-fluoroquinazolinylpiperidinyl moiety. Chin. Chem. Lett. 2019. [Google Scholar] [CrossRef]
- Wu, Z.X.; Zhang, J.; Chen, J.X.; Pan, J.K.; Zhao, L.; Liu, D.Y.; Zhang, A.W.; Chen, J.; Hu, D.Y.; Song, B.A. Design, synthesis, antiviral bioactivity and three–dimensional quantitative structure–activity relationship study of novel ferulic acid ester derivatives containing quinazoline moiety. Pest Manag. Sci. 2017, 73, 2079–2089. [Google Scholar] [CrossRef]
- Chen, J.X.; Gan, X.H.; Yi, C.F.; Wang, S.B.; Yang, Y.Y.; He, F.C.; Hu, D.Y.; Song, B.A. Synthesis, nematicidal activity, and 3D-QSAR of novel 1,3,4-oxadiazole/thiadiazole thioether derivatives. Chin. J. Chem. 2018, 36, 939–944. [Google Scholar] [CrossRef]
- Yang, Z.B.; Li, P.; He, Y.J.; Luo, J.; Zhou, J.; Wu, Y.H.; Chen, L.T. Novel pyrethrin derivatives containing an 1,3,4-oxadiazole thioether moiety: Design, synthesis, and insecticidal activity. J. Heterocycl. Chem. 2019, 1–8. [Google Scholar] [CrossRef]
- Yang, Z.; Li, P.; He, Y.J.; Luo, J.; Zhou, J.; Wu, Y.H.; Chen, L.T. Design, synthesis, and biological evaluation of novel pyrethrin derivatives containing 1,3,4-oxadiazole and thioether moieties as active insecticidal agents. Chem. Pap. 2019. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, Y. Design, synthesis, acaricidal activities, and structure-activity relationship studies of novel oxazolines containing sulfonate moieties. J. Agric. Food Chem. 2019, 67, 13544–13549. [Google Scholar] [CrossRef]
- Guo, T.; Xia, R.J.; Chen, M.; Su, S.J.; He, J.; He, M.; Wang, H.; Xue, W. Biological activity evaluation and action mechanism of 1,4-Pentadien-3-one derivatives containing thiophene sulfonate. Phosphorus Sulfur Silicon Relat. Elem. 2020, 195, 123–130. [Google Scholar] [CrossRef]
- Guo, T.; Xia, R.J.; Chen, M.; He, J.; Su, S.J.; Liu, L.W.; Li, X.Y.; Xue, W. Biological activity evaluation and action mechanism of chalcone derivatives containing thiophene sulfonate. RSC Adv. 2019, 9, 24942–24950. [Google Scholar] [CrossRef]
- Flampouri, E.; Theodosi-Palimeri, D.; Kintzios, S. Strobilurin fungicide kresoxim-methyl effects on a cancerous neural cell line: Oxidant/antioxidant responses and in vitro migration. Toxicol. Mech. Methods. 2018, 28, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wu, H.M.; Hu, T.T.; Chen, X.; Ding, X.C. Adsorption and leaching of novel fungicide pyraoxystrobin on soils by 14C tracing method. Environ. Monit. Assess. 2018, 190, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ramalingam, S.; Periandy, S.; Sugunakala, S.; Prabhu, T.; Bououdina, M. Insilico molecular modeling, docking and spectroscopic [FT-IR/FT-Raman/UV/NMR] analysis of Chlorfenson using computational calculations. Spectrochim. Acta Part A 2013, 115, 118–135. [Google Scholar] [CrossRef] [PubMed]
- Melcarne, C.; Ramond, E.; Dudzic, J.; Bretscher, A.J.; Kurucz, E.; Ando, I.; Lemaitre, B. Two Nimrod receptors, NimC1 and Eater, synergistically contribute to bacterial phagocytosis in Drosophila melanogaster. FEBS J. 2019, 286, 2670–2691. [Google Scholar] [CrossRef]
- Wang, R.; Zhi, X.; Li, J.; Xu, H. Synthesis of novel oxime sulfonate derivatives of 2′(2′,6′)-(Di)chloropicropodophyllotoxins as insecticidal Agents. J. Agric. Food Chem. 2015, 63, 6668–6674. [Google Scholar] [CrossRef]
- Sun, R.F.; Wang, Z.W.; Li, Y.Q.; Xiong, L.X.; Liu, Y.X.; Wang, Q.M. Design, synthesis and insecticidal evaluation of new benzoylureas containing amide and sulfonate groups based on the sulfonylurea receptor protein binding site for diflubenzuron and glibenclamide. J. Agric. Food Chem. 2013, 61, 517–522. [Google Scholar] [CrossRef]
- Kumari, A.; Singh, N.; Ramakrishnan, B. Parameters affecting azoxystrobin and imidacloprid degradation in biobed substrates in the North Indian tropical environment. J. Environ. Sci. Health Part B. 2019, 54, 843–857. [Google Scholar] [CrossRef]
- Tao, Q.Q.; Liu, L.W.; Wang, P.Y.; Long, Q.S.; Zhao, Y.L.; Jin, L.H.; Xu, W.M.; Chen, Y.; Li, Z.; Yang, S. Synthesis and in vitro and in vivo biological activity evaluation and quantitative proteome profiling of oxadiazoles bearing flexible heterocyclic patterns. J. Agric. Food Chem. 2019, 67, 7626–7639. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 4a/5a are available from the authors. |



| Compd. | Xanthomonas Oryzae Pv. Oryzae | Xanthomonas Axonopodis Pv. Citri | ||
|---|---|---|---|---|
| 100 µg/mL | 50 µg/mL | 200 µg/mL | 100 µg/mL | |
| 4a-1 | 98.3 ± 1.2 | 90.4 ± 1.4 | 94.2 ± 0.2 | 79.4 ± 1.3 |
| 4a-2 | 99.5 ± 1.8 | 94.1 ± 2.1 | 97.1 ± 0.3 | 82.1 ± 2.0 |
| 4a-3 | 94.0 ± 1.1 | 85.4 ± 0.9 | 93.8 ± 0.4 | 69.3 ± 2.7 |
| 4a-4 | 91.2 ± 2.8 | 78.7 ± 2.2 | 80.4 ± 0.2 | 45.8 ± 4.6 |
| 4a-5 | 91.8 ± 2.6 | 41.5 ± 2.3 | 62.4 ± 3.6 | 48.1 ± 3.0 |
| 4a-6 | 20.0 ± 1.9 | 5.0 ± 2.2 | 20.2 ± 2.7 | 15.9 ± 2.7 |
| 4a-7 | 10.1 ± 1.2 | 72.7 ± 1.1 | 22.9 ± 4.1 | 19.1 ± 4.7 |
| 4a-8 | 76.8 ± 1.5 | 39.8 ± 2.1 | 92.3 ± 4.6 | 25.5 ± 2.7 |
| 4a-9 | 60.7 ± 1.5 | 20.2 ± 2.1 | 18.8 ± 1.5 | 10.5 ± 2.1 |
| 4a-10 | 48.9 ± 2.6 | 18.5 ± 1.1 | 17.3 ± 2.3 | 15.2 ± 1.8 |
| 4a-11 | 97.5 ± 1.2 | 82.2 ± 1.4 | 47.3 ± 1.8 | 33.2 ± 1.2 |
| 4a-12 | 96.4 ± 2.2 | 78.4 ± 2.1 | 45.5 ± 2.1 | 32.4 ± 1.8 |
| 4a-13 | 94.9 ± 1.7 | 81.2 ± 2.4 | 41.9 ± 0.8 | 30.0 ± 2.8 |
| 4a-14 | 99.1 ± 1.5 | 94.0 ± 1.9 | 41.2 ± 4.8 | 33.0 ± 3.8 |
| 4a-15 | 97.0 ± 0.9 | 72.5 ± 1.0 | 38.7 ± 3.6 | 32.1 ± 1.8 |
| 4a-16 | 94.0 ± 1.2 | 48.0 ± 1.5 | 36.9 ± 2.8 | 20.0 ± 1.9 |
| 4a-17 | 30.2 ± 1.8 | 18.1 ± 0.9 | 25.6 ± 1.5 | 11.4 ± 1.2 |
| 4a-18 | 10.4 ± 2.3 | 6.2 ± 1.5 | 48.3 ± 3.7 | 24.2 ± 5.3 |
| 5a-1 | 35.1 ± 1.2 | 18.2 ± 1.5 | 47.9 ± 6.0 | 23.8 ± 4.0 |
| 5a-2 | 96.4 ± 2.5 | 11.5 ± 1.5 | 55.1 ± 1.5 | 35.5 ± 3.9 |
| 5a-3 | 15.2 ± 5.3 | 7.3 ± 4.9 | 68.5 ± 1.4 | 38.5 ± 1.2 |
| 5a-4 | 12.5 ± 2.1 | 7.0 ± 3.2 | 32.4 ± 1.7 | 12.0 ± 2.2 |
| 5a-5 | 11.0 ± 1.1 | 2.3 ± 2.1 | 45.1 ± 1.8 | 29.8 ± 2.7 |
| 5a-6 | 7.2 ± 2.8 | 3.5 ± 1.7 | 32.2 ± 2.5 | 18.8 ± 1.5 |
| Bismerthiazol b | 60.2 ± 3.1 | 28.3 ± 2.8 | 72.5 ± 2.8 | 58.2 ± 2.1 |
| Thiodiazole Copper b | 57.3 ± 1.9 | 30.2 ± 2.1 | 79.1 ± 1.8 | 53.1 ± 1.1 |
| Compd. | Xanthomonas Oryzae Pv. Oryzae | Xanthomonas Axonopodis Pv. Citri |
|---|---|---|
| EC50 (µM) c | EC50 (µM) c | |
| 4a-1 | 63.4 ± 3.8 | 114.0 ± 6.6 |
| 4a-2 | 50.1 ± 4.2 | 95.8 ± 4.6 |
| 4a-3 | 87.2 ± 4.7 | 132.5 ± 7.5 |
| 4a-4 | 99.4 ± 4.7 | 155.2 ± 5.8 |
| 4a-11 | 98.0 ± 6.6 | / |
| 4a-12 | 95.3 ± 3.9 | / |
| 4a-13 | 86.4 ± 4.8 | / |
| 4a-14 | 69.0 ± 4.4 | / |
| 4a-15 | 83.4 ± 6.0 | / |
| 4a-16 | 112.5 ± 6.0 | / |
| Bismerthiazol b | 253.5 ± 7.6 | 274.3 ± 8.6 |
| Thiodiazole copper b | 467.4 ± 15.5 | 406.3 ± 13.0 |
| Treatment | 14 Days after Spraying | ||
|---|---|---|---|
| Morbidity (%) | Disease Index (%) | Control Efficiency (%) a | |
| 4a-1 | 100 | 34.6 | 48.1 ± 2.5 |
| 4a-2 | 100 | 16.7 | 68.6 ± 3.5 |
| 4a-3 | 100 | 22.2 | 62.3 ± 4.3 |
| Bismerthiazol | 100 | 33.3 | 49.6 ± 3.1 |
| Thiodiazole copper | 100 | 39.9 | 42.2 ± 3.0 |
| CK (negative control) | 100 | 87.6 | / |
| Treatment | 14 Days after Spraying | ||
|---|---|---|---|
| Morbidity (%) | Disease Index (%) | Control Efficiency (%) a | |
| 4a-1 | 100 | 37.8 | 44.6 ± 2.9 |
| 4a-2 | 100 | 22.2 | 62.3 ± 3.8 |
| 4a-3 | 100 | 27.8 | 56.0 ± 3.5 |
| Bismerthiazol | 100 | 39.2 | 42.9 ± 2.4 |
| Thiodiazole copper | 100 | 45.2 | 36.1 ± 2.5 |
| CK (negative control) | 100 | 87.6 | / |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhou, X.; Lu, H.; Mu, X.; Jin, L. Synthesis and Antibacterial Evaluation of Novel 1,3,4-Oxadiazole Derivatives Containing Sulfonate/Carboxylate Moiety. Molecules 2020, 25, 1488. https://doi.org/10.3390/molecules25071488
Wang L, Zhou X, Lu H, Mu X, Jin L. Synthesis and Antibacterial Evaluation of Novel 1,3,4-Oxadiazole Derivatives Containing Sulfonate/Carboxylate Moiety. Molecules. 2020; 25(7):1488. https://doi.org/10.3390/molecules25071488
Chicago/Turabian StyleWang, Lei, Xia Zhou, Hui Lu, Xianfu Mu, and Linhong Jin. 2020. "Synthesis and Antibacterial Evaluation of Novel 1,3,4-Oxadiazole Derivatives Containing Sulfonate/Carboxylate Moiety" Molecules 25, no. 7: 1488. https://doi.org/10.3390/molecules25071488
APA StyleWang, L., Zhou, X., Lu, H., Mu, X., & Jin, L. (2020). Synthesis and Antibacterial Evaluation of Novel 1,3,4-Oxadiazole Derivatives Containing Sulfonate/Carboxylate Moiety. Molecules, 25(7), 1488. https://doi.org/10.3390/molecules25071488