Abstract
Bisindolyl alkaloids represent a large family of natural and synthetic products that display various biological activities. Among the bisindole compounds, 6,7,12,13-tetrahydro-5H-cyclohepta[2,1-b:3,4-b’]diindoles have received little attention. Only two methods have been developed for the construction of the 6,7,12,13-tetrahydro-5H-cyclohepta[2,1-b:3,4-b’]diindole scaffold thus far, including the classical Fischer indole synthesis conducted by reacting indole-fused cycloheptanone and hydrazines, and the condensation reaction to build the seven-membered ring. Here, we report for the first time a new route to synthesize 6,7,12,13-tetrahydro-5H-cyclohepta[2,1-b:3,4-b’]diindoles through intramolecular oxidative coupling of 1,3-di(1H-indol-3-yl)propanes in the presence of PIFA, DDQ and TMSCl with moderate to excellent yields.
1. Introduction
The bisindole core constitutes a valuable class of biologically active molecules that are prevalent in nature [1,2,3,4,5,6]. These natural and synthetic compounds display various biological activities such as antitumor, antimicrobial and antidiabetic. For example, Iheyamine A (Figure 1A), an alkaloid isolated from a colonial ascidian Polycitorella sp., shows interesting moderate cytotoxicity [7], and Rebeccamycin (Figure 1B), an antitumor antibiotic isolated from cultures of Saccharotrix aerocolonigenes, is well-known for its inhibitory potency toward topoisomerase I [8,9,10]. In addition, natural product derivative Midostaurin (Figure 1C) has been approved by the US FDA for the treatment of FLT3-mutated acute myelogenous leukemia (AML) [11], and synthetic indolocarbazole derivative D (Figure 1D) shows excellent antibacterial activity and good antifungal activity [12]. Furthermore, Caulersin (Figure 1E) from the alga Caulerpa serrulate [13,14,15] and Racemosin C (Figure 1F) isolated from the green alga Caulerpa racemose [16] exhibit significant PTP1B inhibitory activity. It is worth noting that both compounds E and F contain a medium ring besides the bisindole structure. The bisindole core also has applications in material science. For example, the synthetic cyclopenta[2,1-b:3,4-b’]diindole derivatives (Figure 1G) show excellent luminous efficiency as organic electroluminescent compounds [17].
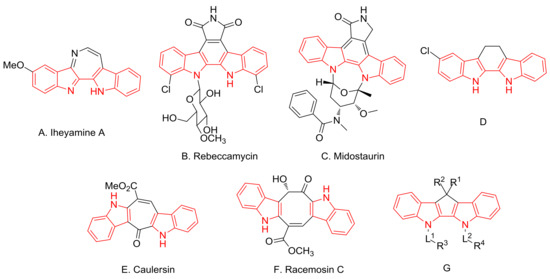
Figure 1.
Some bioactive bisindolyl alkaloids and synthetic compounds.
Among the bisindole compounds, the rarely explored 6,7,12,13-tetrahydro-5H-cyclohepta[2,1-b:3,4-b’]diindoles attracted our interest. To the best of our knowledge, only two methods have been reported to construct 6,7,12,13-tetrahydro-5H-cyclohepta[2,1-b:3,4-b’]diindoles. One was the establishment of the second indole by classic Fischer indole synthesis, which usually suffered from an excess of acids, hazardous hydrazines, and a limited number of indole-fused cycloheptanones (Scheme 1a) [18,19,20]. The other approach was the construction of seven-membered ring by condensation reaction, and this process strictly required specific functional groups on the substrates (Scheme 1b) [21].
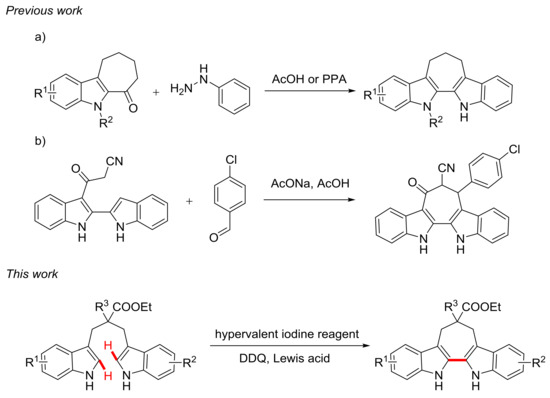
Scheme 1.
Methods for the synthesis of 6,7,12,13-tetrahydro-5H-cyclohepta[2,1-b:3,4-b’]diindoles.
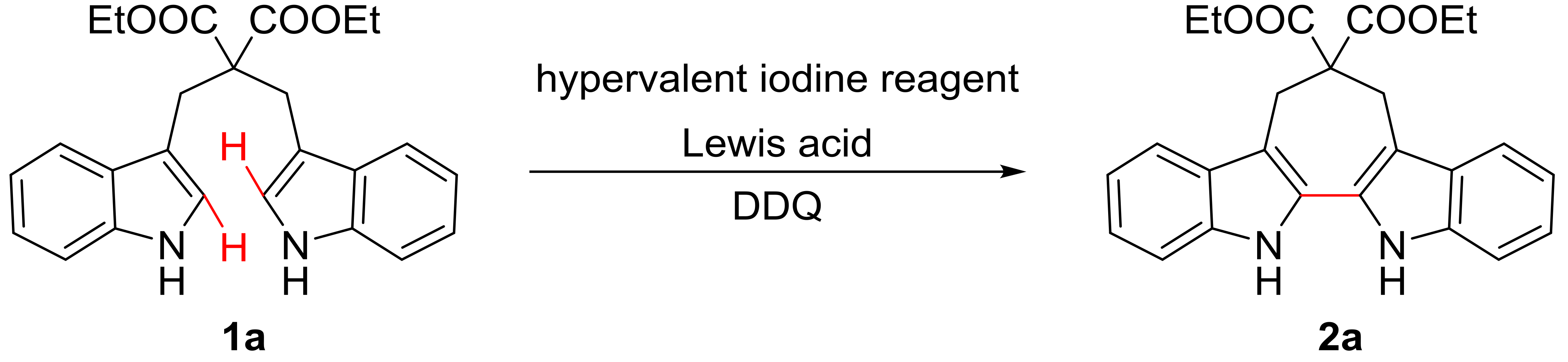
Our group has been focused on the synthesis of natural product analogues for bioactive screening for many years [22,23,24,25,26]. Thus we hoped to develop a convenient and practical method for the synthesis of 6,7,12,13-tetrahydro-5H-cyclohepta[2,1-b:3,4-b’]diindoles. Inspired by previous work, the hypervalent iodine reagents, such as phenyliodine(III)bis(trifluoroacetate) (PIFA) and phenyliodine(III)diacetate (PIDA), have been widely applied to organic synthesis due to its mild oxidation capacity, low toxicity and environmentally benign properties [27,28]. Intermolecular oxidative coupling reactions of aromatic rings mediated by hypervalent iodine reagents have been studied by many researchers and many excellent examples have been reported, including heteroaromatic couplings [29,30,31,32,33,34], cross-couplings of heteroaromatic and aromatic rings [35,36,37] and aromatic couplings [38,39,40,41]. In the meantime, some progress have been achieved in intramolecular oxidative coupling reactions mediated by hypervalent iodine reagents, including the establishment of spiro structures [42,43,44] and fused rings [45,46,47,48]. Constructing a seven-membered ring through intramolecular oxidation is still highly desirable. Herein, we report for the first time a new route to synthesize 6,7,12,13-tetrahydro-5H-cyclohepta[2,1-b:3,4-b’]diindoles by intramolecular oxidative coupling of 1,3-di(1H-indol-3-yl)propanes in the presence of phenyliodine(III)bis(trifluoroacetate) (PIFA), 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ), and trimethylsilyl chloride (TMSCl). This convenient and practical protocol shows good functional-group tolerance with moderate to excellent yields.
2. Results and Discussion
2.1. Preparation of 1,3-di(1H-indol-3-yl)propanes
First, we managed to prepare 1,3-di(1H-indol-3-yl)propanes 1 by two steps with high yielded substitution reaction between the indole trimethyl quaternary ammonium salts and esters 5 [49]. The synthetic route is shown in Scheme 2. The experimental characterization data are given in the Supplementary Materials.
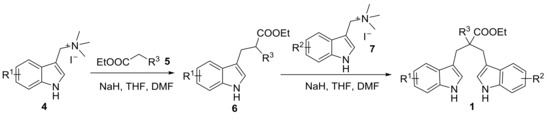
Scheme 2.
Synthetic route to 1,3-di(1H-indol-3-yl)propanes 1.
2.2. Intramolecular Oxidative Coupling of 1,3-di(1H-indol-3-yl)propanes
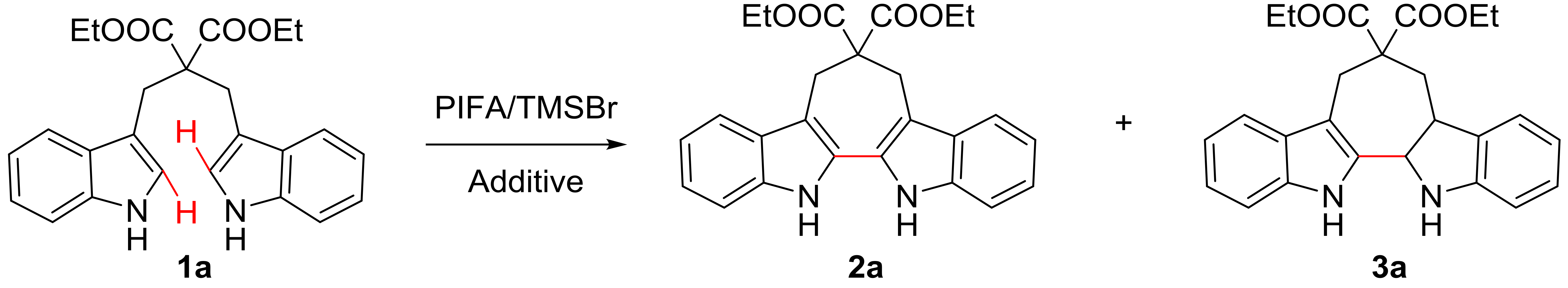
Our studies initiated with the reaction of diethyl 2,2-bis((1H-indol-3-yl)methyl)malonate (1a) in the presence of PIFA and TMSBr in dichloromethane (DCM), the reaction mixture was stirred at 0 °C for 4 h, and then warmed to room temperature to stir for 12 h (Table 1, entry 1). To our delight, the desired product 2a was isolated with 55% yield, and a trace of nonaromatic intermediate 3a was detected. Interestingly, reducing the usage of PIFA did not affect the total yield of 2a and 3a, but the yield of 3a was slightly increased (Table 1, entries 2–3). When 0.3 equiv. of PIFA was used and the reaction was performed under argon atmosphere, the total yield was decreased, but the yield of 3a was improved (Table 1, entry 4 vs. 3). To suppress the formation of intermediate 3a and decrease the loading of PIFA, the additional oxidant DDQ was employed (Table 1, entries 5–7). When 0.3 equiv. of PIFA and 0.5 equiv. of DDQ were added, the reaction yield of 2a was improved to 60% (Table 1, entry 7). By replacing DDQ with BQ (1,4-benzoquinone) or CAN (ceric ammonium nitrate), the yield of desired product was decreased (Table 1, entries 8–9). Moreover, increasing the DDQ loading resulted in a reduced yield (Table 1, entries 5–6). Notably, when only DDQ was added as oxidant, the reaction also generated 2a with 48% yield (Table 1, entry 10). In general, we found that 0.3 equiv. of PIFA and 0.5 equiv. of DDQ was the most favorable combination.

Table 1.
Optimization of PIFA mediated oxidative cyclization/ aromatization.
Next, a set of Lewis acids were examined at 0 °C. The yield was slightly increased when TMSBr was replaced by TMSCl (Table 2, entry 2 vs 1), while TMSOTf and BF3 OEt2 reduced the yield down to 23% and 49% (Table 2, entries 3–4). Moreover, lower temperatures turned out to be better for the reaction with a yield of 88% at −40 °C and 70% at −78 °C, respectively (Table 2, entries 5–6). Subsequently, an investigation of different hypervalent iodine reagents was performed at −40 °C. It revealed that the use of PIDA decreased the reaction yield slightly (85%, Table 2, entry 7), and the yield was reduced when IBX (2-iodoxybenzoic acid) and DMP (Dess-Martin periodinane) were used (Table 2, entries 8, 9). Finally, the optimized condition for the synthesis of 6,7,12,13-tetrahydro-5H-cyclohepta[2,1-b:3,4-b’]diindoles from 1,3- di(1H-indol-3-yl)propanes was obtained: ethyl 2-((1H-indol-3-yl)methyl)-3-(1H-indol-3-yl)propanoate 1a (1 equiv.), PIFA (0.3 equiv.), DDQ (0.5 equiv.), and TMSCl (1 equiv.), the reaction mixture was stirred at −40 °C under air atmosphere for 4 h, and then warmed to room temperature and stirred for 12 h.

Table 2.
Optimization of reaction conditions.
With the optimized reaction conditions in hand, we further examined the substrate scope of the 1,3-di(1H-indol-3-yl)propane derivatives as shown in Scheme 3. The 1,3-di(1H-indol-3-yl)propanes substituted by both electron-donating groups (-Me and -OMe) and electron-withdrawing groups (-F, -Cl, -Br and -CF3) on the indole ring were investigated. Both the electron-donating (-Me) and electron-withdrawing groups (-F, -Cl, and -Br) at the C5/C6 positions of the indole substrates reacted smoothly to provide the target products with good to excellent yields. The substitution position had significant influence on the reaction yield: C6-substituted indoles generated the corresponding products (2c, 2f, 2i, and 2l) in higher yields than the C5 substituted substrates (2b, 2e, 2h, and 2k). It is worth noting that, as an interesting exception, a -OMe group on the C5, C6 and C7 positions gave similar yields under the optimized conditions (2n, 2o and 2p). Moreover, for substituents -Me, -F, -Cl, -Br and -OMe, reactions conducted on symmetrical substrates (2d, 2g, 2j, 2m and 2q) showed moderate to excellent yields similar to asymmetrical ones (2c, 2f, 2i, 2l and 2o). As for strong electron-withdrawing groups, the indole bearing a C5 CF3 group showed a moderate yield (2r, 55%). These results may be determined by the stability of cationic radical intermediate involved in the reaction (Scheme 4). To further explore the scope of the substrates, the starting materials bearing -SO2CH3 or -CN in place of -COOEt for R3 were also employed and were found to afford good yields, respectively (2s, 65% and 2t, 63%).
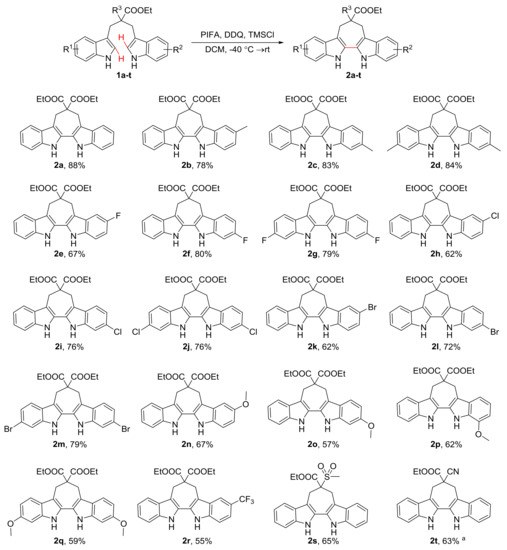
Scheme 3.
The substrate scope for the synthesis of 6,7,12,13-tetrahydro-5H-cyclohepta[2,1-b:3,4-b’]diindoles. a Conversion yield.
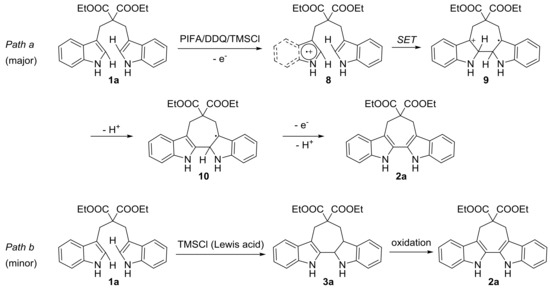
Scheme 4.
Plausible reaction mechanism.
Inspired by the mature mechanism research of oxidative coupling reaction mediated by hypervalent iodine reagents [29,30], a possible mechanism for the synthesis of 6,7,12,13-tetrahydro-5H-cyclohepta[2,1-b:3,4-b’]diindoles from 1,3-di(1H-indol-3-yl)propanes in the presence of PIFA, DDQ and TMSCl was proposed in Scheme 4. Initially, the radical cation was formed from 1a with PIFA/DDQ/TMSCl. Intermediate 9 was then produced by intramolecular single electron transfer (SET), and deprotonated intermediate 10 was formed subsequently. Finally, product 2a was generated via a further one-electron oxidation and deprotonation process (Scheme 4, Path a). Additionally, a small amount of intermediate 3a was formed during the reaction (confirmed by LC-MS) [30,50], which may have resulted from the protonation and Friedel-Crafts type reaction of substrate 1a in the presence of Lewis acid. This intermediate was subsequently oxidized to give product 2a (Scheme 4, path b).
3. Materials and Methods
3.1. General Information
All the chemical reagents were commercial products and were used without purification in all cases. TLC was performed on silica gel plates (0.15–0.2 mm thickness, Yantai Huiyou Company, Yantai, China) and detected with UV light at 254 nM, Column chromatography was carried out on silica gel (200–300 mesh). Proton and carbon magnetic resonance spectra (1H-NMR and 13C-NMR) were recorded on Varian Mercury-300, Varian Mercury-400, Varian Mercury-500 and/or Varian Mercury-600 spectrometers (Palo Alto, CA, United States). NMR experiments were conducted in CDCl3, CD3OD and DMSO-d6. Tetramethylsilane (TMS) was used as the internal standard. Chemical shifts (δ) are reported in parts per million (ppm). Data are reported as follows: chemical shift, multiplicity (br s = broad singlet, d = doublet, dd = doublet of doublet, dt = doublet of triplet, m = multiple, s = singlet and t = triplet), coupling constants (Hz). Low-resolution mass spectra (ESI) were obtained using Agilent HPLC-MS (Palo Alto, CA, United States) (1200-6110). High resolution mass spectra (HRMS) were obtained using Agilent 1290-6545 UHPLC-QTOF. Melting points (mp) were measured by Büchi 510 melting point apparatus without further correction.
3.2. General Procedure for the Synthesis of 6,7,12,13-Tetrahydro-5H-Cyclohepta[2,1-b:3,4-b’]diindoles (2a–t)
PIFA (0.1 mmol, 43 mg), DDQ (0.15 mmol, 34 mg) and TMSCl (0.3 mmol, 38 μL) were added to the stirred solution of 1,3-di(1H-indol-3-yl)propane 1 (0.3 mmol) in dichloromethane (2 mL) at −40 °C in sequence. The mixture was stirred under air at the same temperature for 4 h and then warmed to room temperature (25 °C) and stirred for an additional 12 h. After completion of the reaction, the mixture was diluted with aqueous NaHCO3 solution and extracted with dichloromethane. After drying with anhydrous Na2SO4, dichloromethane was removed under reduced pressure. The residue was purified by column chromatography on silica gel using petroleum ether/ethyl acetate (10:1 to 5:1, v/v) as the eluent to provide the product 2.
Diethyl 5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-dicarboxylate (2a). Yellow solid, 110 mg (yield 88%). mp 187–189 °C. 1H-NMR (400 MHz, DMSO-d6) δ 10.93 (s, 2H, NH), 7.54 (d, J = 7.9 Hz, 2H, Ar-H), 7.41 (d, J = 8.0 Hz, 2H, Ar-H), 7.12 (ddd, J = 8.1, 6.9, 1.2 Hz, 2H, Ar-H), 7.04 (ddd, J = 7.9, 6.9, 1.1 Hz, 2H, Ar-H), 3.94 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.54 (s, 4H, Cq-CH2Ar), 0.88 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (125 MHz, DMSO-d6) δ 170.40 (C=O), 135.67 (C), 128.75 (C), 127.22 (C), 121.75 (CH), 119.11 (CH), 117.76 (CH), 110.95 (CH), 108.62 (C), 60.79 (OCH2), 54.36 (C), 30.72 (CH2), 13.54 (CH3). HRMS (ESI): m/z calcd for C25H25N2O4 [M + H]+: 417.1809, found: 417.1820.
Diethyl 3-methyl-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-dicarboxylate (2b). Yellow solid, 101 mg (yield 78%). mp 203–205 °C. 1H-NMR (400 MHz, Methanol-d4) δ 7.52 (d, J = 7.8 Hz, 1H, Ar-H), 7.33 (d, J = 8.0 Hz, 1H, Ar-H), 7.31 (s, 1H, Ar-H), 7.23 (d, J = 8.2 Hz, 1H, Ar-H), 7.12 (t, J = 7.5 Hz, 1H, Ar-H), 7.05 (t, J = 7.4 Hz, 1H, Ar-H), 6.97 (d, J = 8.0 Hz, 1H, Ar-H), 4.00 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.61 (s, 2H, Cq-CH2Ar), 3.59 (s, 2H, Cq-CH2Ar), 2.44 (s, 3H, Ar-CH3), 0.98 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (150 MHz, Methanol-d4) δ 172.83 (C=O), 137.67 (C), 136.06 (C), 130.76 (C), 130.54 (C), 129.57 (C), 128.85 (C), 128.78 (C), 124.69 (CH), 123.00 (CH), 120.39 (CH), 118.75 (CH), 118.49 (CH), 111.67 (CH), 111.44 (CH), 110.04 (C), 109.86 (C), 62.45 (OCH2), 56.56 (C), 32.31 (CH2), 32.25 (CH2), 21.70 (ArCH3), 14.10 (CH3). HRMS (ESI): m/z calcd for C26H27N2O4 [M + H]+: 431.1965, found: 431.1976.
Diethyl 2-methyl-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-dicarboxylate (2c). Yellow solid, 107 mg (yield 83%). mp 174–176 °C. 1H-NMR (400 MHz, Methanol-d4) δ 7.51 (d, J = 7.7 Hz, 1H, Ar-H), 7.39 (d, J = 8.1 Hz, 1H, Ar-H), 7.33 (d, J = 7.9 Hz, 1H, Ar-H), 7.15 – 7.09 (m, 2H, Ar-H), 7.05 (t, J = 7.5 Hz, 1H, Ar-H), 6.90 (dd, J = 8.0, 1.1 Hz, 1H, Ar-H), 3.99 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.60 (s, 2H, Cq-CH2Ar), 3.58 (s, 2H, Cq-CH2Ar), 2.44 (s, 3H, Ar-CH3), 0.97 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (125 MHz, Methanol-d4) δ 172.83 (C=O), 138.14 (C), 137.65 (C), 132.95 (C), 130.57 (C), 128.92 (C), 128.53 (C), 128.06 (C), 122.91 (CH), 122.16 (CH), 120.38 (CH), 118.70 (CH), 118.56 (CH), 111.65 (2CH), 110.27 (C), 109.81 (C), 62.44 (OCH2), 56.54 (C), 32.38 (CH2), 32.26 (CH2), 21.88 (ArCH3), 14.09 (CH3). HRMS (ESI): m/z calcd for C26H27N2O4 [M + H]+: 431.1965, found: 431.1971.
Diethyl 2,10-dimethyl-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-di carboxylate (2d), 112 mg (yield 84%). Yellow solid. mp 212–214 °C. 1H-NMR (400 MHz, DMSO-d6) δ 10.72 (s, 2H, NH), 7.39 (d, J = 8.0 Hz, 2H, Ar-H), 7.19 (s, 2H, Ar-H), 6.87 (d, J = 8.1 Hz, 2H, Ar-H), 3.94 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.50 (s, 4H, Cq-CH2Ar), 2.42 (s, 6H, Ar-CH3), 0.89 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (125 MHz, DMSO-d6) δ 170.43 (C=O), 136.04 (C), 130.76 (C), 126.83 (C), 126.76 (C), 120.81 (CH), 117.40 (CH), 110.71 (CH), 108.11 (C), 60.75 (OCH2), 54.31 (C), 30.79 (CH2), 21.44 (ArCH3), 13.57 (CH3). HRMS (ESI): m/z calcd for C27H29N2O4 [M + H]+: 445.2122, found: 445.2131.
Diethyl 3-fluoro-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-dicarboxylate (2e). Yellow solid, 87 mg (yield 67%). mp 202–204 °C. 1H-NMR (400 MHz, Methanol-d4) δ 7.53 (d, J = 7.8 Hz, 1H, Ar-H), 7.35 (d, J = 8.0 Hz, 1H, Ar-H), 7.30 (dd, J = 8.8, 4.4 Hz, 1H, Ar-H), 7.18 (dd, J = 9.8, 2.5 Hz, 1H, Ar-H), 7.16–7.12 (m, 1H, Ar-H), 7.07 (ddd, J = 8.0, 7.0, 1.1 Hz, 1H, Ar-H), 6.89 (td, J = 9.1, 2.5 Hz, 1H, Ar-H), 4.00 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.62 (s, 2H, Cq-CH2Ar), 3.56 (s, 2H, Cq-CH2Ar), 0.98 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (125 MHz, Methanol-d4) δ 172.68 (C=O), 159.38 (d, J = 233.3 Hz, CF), 137.80 (C), 134.23 (C), 130.96 (d, J = 9.6 Hz, C), 130.77 (C), 130.46 (C), 128.28 (C), 123.35 (CH), 120.51 (CH), 118.94 (CH), 112.51 (d, J = 9.8 Hz, CH), 111.80 (CH), 110.90 (d, J = 26.5 Hz, CH), 110.90 (C), 110.35 (d, J = 5.0 Hz, C), 103.49 (d, J = 23.9 Hz, CH), 62.50 (OCH2), 56.56 (C), 32.26 (CH2), 32.20 (CH2), 14.09 (CH3). HRMS (ESI): m/z calcd for C25H24FN2O4 [M + H]+: 435.1715, found: 435.1715.
Diethyl 2-fluoro-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-dicarboxylate (2f). Yellow solid, 104 mg (yield 80%). mp 219–221 °C. 1H-NMR (400 MHz, Methanol-d4) δ 7.52 (d, J = 7.9 Hz, 1H, Ar-H), 7.48 (dd, J = 8.7, 5.2 Hz, 1H, Ar-H), 7.34 (d, J = 8.0 Hz, 1H, Ar-H), 7.13 (ddd, J = 8.1, 7.0, 1.2 Hz, 1H, Ar-H), 7.09–7.03 (m, 2H, Ar-H), 6.85 (ddd, J = 9.7, 8.7, 2.3 Hz, 1H, Ar-H), 4.00 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.61 (s, 2H, Cq-CH2Ar), 3.59 (s, 2H, Cq-CH2Ar), 0.98 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (125 MHz, Methanol-d4) δ 172.70 (C=O), 161.54 (d, J = 236.2 Hz, CF), 137.74 (C), 137.68 (d, J = 14.0 Hz, C), 130.49 (C), 129.27 (d, J = 3.5 Hz, C), 128.48 (C), 127.30 (C), 123.13 (CH), 120.48 (CH), 119.72 (d, J = 10.3 Hz, CH), 118.82 (CH), 111.76 (CH), 110.34 (C), 110.14 (C), 108.75 (d, J = 24.9 Hz, CH), 97.88 (d, J = 26.2 Hz, CH), 62.49 (OCH2), 56.51 (C), 32.26 (CH2), 32.17 (CH2), 14.09 (CH3). HRMS (ESI): m/z calcd for C25H24FN2O4 [M + H]+: 435.1715, found: 435.1723.
Diethyl 2,10-difluoro-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-dicarboxylate (2g). Yellow solid, 107 mg (yield 79%). mp 254–256 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.05 (s, 2H, NH), 7.53 (dd, J = 8.7, 5.4 Hz, 2H, Ar-H), 7.25 (dd, J = 9.9, 2.4 Hz, 2H, Ar-H), 6.89 (ddd, J = 9.9, 8.7, 2.4 Hz, 2H, Ar-H), 3.94 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.52 (s, 4H, Cq-CH2Ar), 0.88 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (125 MHz, DMSO-d6) δ 170.30 (C=O), 159.28 (d, J = 235.2 Hz, CF), 135.60 (d, J = 12.8 Hz, C), 127.58 (d, J = 3.5 Hz, C), 125.63 (C), 118.89 (d, J = 10.4 Hz, CH), 108.61 (C), 107.56 (d, J = 24.5 Hz, CH), 97.27 (d, J = 25.9 Hz, CH), 60.86 (OCH2), 54.24 (C), 30.64 (CH2), 13.56 (CH3). HRMS (ESI): m/z calcd for C25H23F2N2O4 [M + H]+: 453.1620, found: 453.1629.
Diethyl 3-chloro-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-dicarboxylate (2h). Yellow solid, 84 mg (yield 62%). mp 229–231 °C. 1H-NMR (400 MHz, Methanol-d4) δ 7.54 (d, J = 7.8 Hz, 1H, Ar-H), 7.49 (d, J = 2.0 Hz, 1H, Ar-H), 7.35 (d, J = 8.0 Hz, 1H, Ar-H), 7.31 (d, J = 8.5 Hz, 1H, Ar-H), 7.15 (t, J = 7.5 Hz, 1H, Ar-H), 7.10–7.04 (m, 2H, Ar-H), 4.00 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.62 (s, 2H, Cq-CH2Ar), 3.56 (s, 2H, Cq-CH2Ar), 0.98 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (150 MHz, Methanol-d4) δ 172.61 (C=O), 137.82 (C), 136.05 (C), 131.64 (C), 130.47 (C), 130.42 (C), 128.07 (C), 126.19 (C), 123.43 (CH), 123.00 (CH), 120.54 (CH), 118.98 (CH), 118.18 (CH), 112.93 (CH), 111.83 (CH), 111.10 (C), 109.83 (C), 62.52 (OCH2), 56.48 (C), 32.21 (CH2), 32.05 (CH2), 14.10 (CH3). HRMS (ESI): m/z calcd for C25H24ClN2O4 [M + H]+: 451.1419, found: 451.1426.
Diethyl 2-chloro-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-dicarboxylate (2i). Yellow solid, 103 mg (yield 76%). mp 231–233 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.08 (s, 1H, NH), 10.99 (s, 1H, NH), 7.55 (d, J = 8.4 Hz, 1H, Ar-H), 7.54 (d, J = 7.7 Hz, 1H, Ar-H), 7.50 (d, J = 1.9 Hz, 1H, Ar-H), 7.41 (d, J = 8.0 Hz, 1H, Ar-H), 7.14 (ddd, J = 8.0, 6.9, 1.2 Hz, 1H, Ar-H), 7.08–7.01 (m, 2H, Ar-H), 3.94 (q, J = 7.0 Hz, 4H, -CO2CH2CH3), 3.54 (s, 2H, Cq-CH2Ar), 3.53 (s, 2H, Cq-CH2Ar), 0.88 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (125 MHz, DMSO-d6) δ 170.32 (C=O), 136.06 (C), 135.77 (C), 128.69 (C), 128.32 (C), 127.60 (C), 126.70 (C), 126.27 (C), 122.02 (CH), 119.49 (CH), 119.22 (CH), 119.14 (CH), 117.91 (CH), 111.06 (CH), 110.66 (CH), 109.20 (C), 108.77 (C), 60.86 (OCH2), 54.28 (C), 30.73 (CH2), 30.56 (CH2), 13.57 (CH3). HRMS (ESI): m/z calcd for C25H24ClN2O4 [M + H]+: 451.1419, found: 451.1422.
Diethyl 2,10-dichloro-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-di carboxylate (2j). Yellow solid, 111 mg (yield 76%). mp 273–275 °C. 1H-NMR (300 MHz, DMSO-d6) δ 11.16 (s, 2H, NH), 7.56 (d, J = 8.5 Hz, 2H, Ar-H), 7.50 (d, J = 1.9 Hz, 2H, Ar-H), 7.05 (dd, J = 8.5, 1.9 Hz, 2H, Ar-H), 3.94 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.52 (s, 4H, Cq-CH2Ar), 0.88 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (150 MHz, DMSO-d6) δ 170.21 (C=O), 136.13 (C), 127.77 (C), 127.52 (C), 126.52 (C), 119.59 (CH), 119.28 (CH), 110.73 (CH), 109.33 (C), 60.91 (OCH2), 54.18 (C), 30.56 (CH2), 13.58 (CH3). HRMS (ESI): m/z calcd for C25H21Cl2N2O4 [M − H]−: 483.0884, found: 483.0880.
Diethyl 3-bromo-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-dicarboxylate (2k). Yellow solid, 92 mg (yield 62%). mp 253–255 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.14 (s, 1H, NH), 10.96 (s, 1H, NH), 7.73 (s, 1H, Ar-H), 7.55 (d, J = 7.9 Hz, 1H, Ar-H), 7.42 (d, J = 7.5 Hz, 1H, Ar-H), 7.39 (d, J = 8.6 Hz, 1H, Ar-H), 7.22 (d, J = 8.5 Hz, 1H, Ar-H), 7.14 (t, J = 7.7 Hz, 1H, Ar-H), 7.05 (t, J = 7.5 Hz, 1H, Ar-H), 3.94 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.55 (s, 2H, Cq-CH2Ar), 3.51 (s, 2H, Cq-CH2Ar), 0.89 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (125 MHz, DMSO-d6) δ 170.30 (C=O), 135.80 (C), 134.38 (C), 130.60 (C), 128.79 (C), 128.65 (C), 126.59 (C), 124.02 (CH), 122.10 (CH), 120.11 (CH), 119.23 (CH), 117.95 (CH), 112.96 (CH), 111.74 (C), 111.08 (CH), 109.50 (C), 108.30 (C), 60.85 (OCH2), 54.33 (C), 30.69 (CH2), 30.54 (CH2), 13.54 (CH3). HRMS (ESI): m/z calcd for C25H24BrN2O4 [M + H]+: 495.0914, found: 495.0918.
Diethyl 2-bromo-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-dicarboxylate (2l). Yellow solid, 107 mg (yield 72%). mp 230–232 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.08 (s, 1H, NH), 11.00 (s, 1H, NH), 7.63 (d, J = 1.8 Hz, 1H, Ar-H), 7.54 (d, J = 7.9 Hz, 1H, Ar-H), 7.50 (d, J = 8.5 Hz, 1H, Ar-H), 7.41 (d, J = 8.1 Hz, 1H, Ar-H), 7.19–7.11 (m, 2H, Ar-H), 7.05 (t, J = 7.3 Hz, 1H, Ar-H), 3.94 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.54 (s, 2H, Cq-CH2Ar), 3.52 (s, 2H, Cq-CH2Ar), 0.88 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (125 MHz, DMSO-d6) δ 170.31 (C=O), 136.52 (C), 135.78 (C), 128.70 (C), 128.23 (C), 127.83 (C), 126.64 (C), 122.05 (2CH), 119.53 (CH), 119.23 (CH), 117.92 (CH), 114.31 (C), 113.55 (CH), 111.07 (CH), 109.31 (C), 108.80 (C), 60.87 (OCH2), 54.27 (C), 30.73 (CH2), 30.52 (CH2), 13.57 (CH3). HRMS (ESI): m/z calcd for C25H24BrN2O4 [M + H]+: 495.0914, found: 495.0915.
Diethyl 2,10-dibromo-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-di carboxylate (2m). Yellow solid, 136 mg (yield 79%). mp 269–271 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.15 (s, 2H, NH), 7.64 (d, J = 1.8 Hz, 2H, Ar-H), 7.52 (d, J = 8.4 Hz, 2H, Ar-H), 7.17 (dd, J = 8.4, 1.8 Hz, 2H, Ar-H), 3.94 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.52 (s, 4H, Cq-CH2Ar), 0.88 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (150 MHz, DMSO-d6) δ 170.19 (C=O), 136.59 (C), 127.76 (C), 127.62 (C), 122.16 (CH), 119.66 (CH), 114.59 (C), 113.62 (CH), 109.47 (C), 60.90 (OCH2), 54.19 (C), 30.52 (CH2), 13.57 (CH3). HRMS (ESI): m/z calcd for C25H21Br2N2O4 [M − H]−: 570.9874, found: 570.9867.
Diethyl 3-methoxy-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-dicarboxylate (2n). Yellow solid, 90 mg (yield 67%). mp 192–194 °C. 1H-NMR (400 MHz, DMSO-d6) δ 10.89 (s, 1H, NH), 10.76 (s, 1H, NH), 7.52 (d, J = 7.8 Hz, 1H, Ar-H), 7.40 (d, J = 8.0 Hz, 1H, Ar-H), 7.30 (d, J = 8.7 Hz, 1H, Ar-H), 7.12 (t, J = 7.1 Hz, 1H, Ar-H), 7.06–6.99 (m, 2H, Ar-H), 6.75 (dd, J = 8.7, 2.4 Hz, 1H, Ar-H), 3.95 (q, J = 7.0 Hz, 4H, -CO2CH2CH3), 3.80 (s, 3H, -OCH3), 3.53 (s, 2H, Cq-CH2Ar), 3.51 (s, 2H, Cq-CH2Ar), 0.90 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (125 MHz, DMSO-d6) δ 170.48 (C=O), 153.61 (C), 135.67 (C), 130.76 (C), 129.15 (C), 128.77 (C), 127.86 (C), 127.36 (C), 121.71 (CH), 119.09 (CH), 117.74 (CH), 112.03 (CH), 111.74 (CH), 110.93 (CH), 108.52 (C), 108.45 (C), 99.43 (CH), 60.81 (OCH2), 55.39 (C), 54.47 (OCH3), 30.84 (CH2), 30.75 (CH2), 13.58 (CH3). HRMS (ESI): m/z calcd for C26H27N2O5 [M + H]+: 447.1914, found: 447.1920.
Diethyl 2-methoxy-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-dicarboxylate (2o). Yellow solid, 76 mg (yield 57%). mp 118–120 °C. 1H-NMR (400 MHz, Methanol-d4) δ 7.50 (d, J = 7.8 Hz, 1H, Ar-H), 7.39 (d, J = 8.7 Hz, 1H, Ar-H), 7.32 (d, J = 7.9 Hz, 1H, Ar-H), 7.10 (t, J = 7.5 Hz, 1H, Ar-H), 7.04 (t, J = 7.4 Hz, 1H, Ar-H), 6.87 (d, J = 2.2 Hz, 1H, Ar-H), 6.73 (dd, J = 8.7, 2.2 Hz, 1H, Ar-H), 3.99 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.84 (s, 3H, -OCH3), 3.58 (s, 2H, Cq-CH2Ar), 3.57 (s, 2H, Cq-CH2Ar), 0.97 (t, J = 7.2 Hz, 6H, -CO2CH2CH3). 13C-NMR (150 MHz, Methanol-d4) δ 172.82 (C=O), 158.23 (C), 138.47 (C), 137.61 (C), 130.60 (C), 129.01 (C), 127.52 (C), 125.07 (C), 122.76 (CH), 120.35 (CH), 119.48 (CH), 118.61 (CH), 111.59 (CH), 110.44 (CH, C), 109.22 (C), 95.14 (CH), 62.44 (OCH2), 56.51 (C), 55.99 (OCH3), 32.38 (CH2), 32.20 (CH2), 14.10 (CH3). HRMS (ESI): m/z calcd for C26H27N2O5 [M + H]+: 447.1914, found: 447.1924.
Diethyl 1-methoxy-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-dicarboxylate (2p). Yellow solid, 83 mg (yield 62%). mp 224–226 °C. 1H-NMR (400 MHz, DMSO-d6) δ 11.09 (s, 1H, NH), 10.97 (s, 1H, NH), 7.53 (d, J = 7.8 Hz, 1H, Ar-H), 7.39 (d, J = 8.1 Hz, 1H, Ar-H), 7.16–7.09 (m, 2H, Ar-H), 7.03 (t, J = 7.4 Hz, 1H, Ar-H), 6.97 (t, J = 7.8 Hz, 1H, Ar-H), 6.71 (d, J = 7.7 Hz, 1H, Ar-H), 3.97 (s, 3H, -OCH3), 3.95 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.54 (s, 2H, Cq-CH2Ar), 3.53 (s, 2H, Cq-CH2Ar), 0.89 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (125 MHz, DMSO-d6) δ 170.38 (C=O), 145.63 (C), 135.46 (C), 130.05 (C), 128.67 (C), 127.11 (C), 126.81 (C), 125.66 (C), 121.69 (CH), 119.73 (CH), 119.04 (CH), 117.73 (CH), 110.82 (CH), 110.68 (CH), 109.09 (C), 108.31 (C), 102.40 (CH), 60.79 (OCH2), 55.14 (C), 53.99 (OCH3), 31.00 (CH2), 30.82 (CH2), 13.55 (CH3). HRMS (ESI): m/z calcd for C26H27N2O5 [M + H]+: 447.1914, found: 447.1927.
Diethyl 2,10-dimethoxy-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-di carboxylate (2q). Yellow solid, 84 mg (yield 59%). mp 199–201 °C. 1H-NMR (400 MHz, Methanol-d4) δ 7.37 (d, J = 8.6 Hz, 2H, Ar-H), 6.86 (d, J = 2.2 Hz, 2H, Ar-H), 6.72 (dd, J = 8.6, 2.3 Hz, 2H, Ar-H), 3.99 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.83 (s, 6H, -OCH3), 3.54 (s, 4H, Cq-CH2Ar), 0.98 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (150 MHz, Methanol-d4) δ 172.85 (C=O), 158.01 (C), 138.37 (C), 127.86 (C), 125.16 (C), 119.28 (CH), 110.26 (CH), 109.37 (C), 95.19 (CH), 62.42 (OCH2), 56.52 (C), 56.01 (OCH3), 32.31 (CH2), 14.12 (CH3). HRMS (ESI): m/z calcd for C27H29N2O6 [M + H]+: 477.2020, found: 477.2025.
Diethyl 3-(trifluoromethyl)-5,7,12,13-tetrahydro-6H-cyclohepta[2,1-b:3,4-b’]diindole-6,6-di carboxylate (2r). Yellow solid, 80 mg (yield 55%). mp 263–265 °C. 1H-NMR (300 MHz, DMSO-d6) δ 11.41 (s, 1H, NH), 11.00 (s, 1H, NH), 7.94 (s, 1H, Ar-H), 7.61 (d, J = 8.4 Hz, 1H, Ar-H), 7.57 (d, J = 8.3 Hz, 1H, Ar-H), 7.47 –7.37 (m, 2H, Ar-H), 7.16 (t, J = 7.0 Hz, 1H, Ar-H), 7.06 (t, J = 7.5 Hz, 1H, Ar-H), 3.94 (q, J = 7.1 Hz, 4H, -CO2CH2CH3), 3.59 (s, 2H, Cq-CH2Ar), 3.57 (s, 2H, Cq-CH2Ar), 0.88 (t, J = 7.1 Hz, 6H, -CO2CH2CH3). 13C-NMR (125 MHz, DMSO-d6) δ 170.29 (C=O), 137.22 (C), 135.86 (C), 129.50 (C), 128.64 (C), 128.14 (C), 126.38 (C), 125.58 (q, J = 271.4 Hz, CF3), 122.26 (CH), 120.09 (q, J = 31.1 Hz, CCF3), 119.30 (CH), 118.04 (CH), 117.93 (d, J = 3.1 Hz, CH), 115.40 (d, J = 3.9 Hz, CH), 111.68 (CH), 111.16 (CH), 109.87 (C), 109.49 (C), 60.87 (OCH2), 54.34 (C), 30.75 (CH2), 30.43 (CH2), 13.51 (CH3). HRMS (ESI): m/z calcd for C26H24F3N2O4 [M + H]+: 485.1683, found: 485.1682.
Ethyl 6-(methylsulfonyl)-6,7,12,13-tetrahydro-5H-cyclohepta[2,1-b:3,4-b’]diindole-6-carboxylate (2s). Yellow solid, 82 mg (yield 65%). mp 227–229 °C. 1H-NMR (400 MHz, Methanol-d4) δ 7.61 (d, J = 7.8 Hz, 2H, Ar-H), 7.36 (d, J = 8.1 Hz, 2H, Ar-H), 7.16 (t, J = 7.5 Hz, 2H, Ar-H), 7.09 (t, J = 7.4 Hz, 2H, Ar-H), 4.17 (d, J = 14.7 Hz, 2H, Cq-CHHAr), 3.76 (q, J = 7.1 Hz, 2H, -CO2CH2CH3), 3.48 (d, J = 14.7 Hz, 2H, Cq-CHHAr), 3.33 (s, 3H, -SO2CH3), 0.53 (t, J = 7.1 Hz, 3H, -CO2CH2CH3). 13C-NMR (125 MHz, DMSO-d6) δ 165.95 (C=O), 135.73 (C), 128.73 (C), 126.88 (C), 122.03 (CH), 119.27 (CH), 118.04 (CH), 111.03 (CH), 107.36 (C), 71.30 (C), 61.26 (OCH2), 37.23 (SO2CH3), 27.31 (CH2), 12.84 (CH3). HRMS (ESI): m/z calcd for C23H23N2O4S [M + H]+: 423.1373, found: 423.1373.
Ethyl 6-cyano-6,7,12,13-tetrahydro-5H-cyclohepta[2,1-b:3,4-b’]diindole-6-carboxylate (2t). Yellow solid, 45 mg (recycled raw material 39 mg, conversion yield 63%). mp 252–254 °C. 1H-NMR (300 MHz, DMSO-d6) δ 11.19 (s, 2H, NH), 7.60 (d, J = 7.9 Hz, 2H, Ar-H), 7.47 (d, J = 8.0 Hz, 2H, Ar-H), 7.17 (ddd, J = 8.1, 7.0, 1.2 Hz, 2H, Ar-H), 7.07 (ddd, J = 8.0, 7.0, 1.1 Hz, 2H, Ar-H), 4.32 (q, J = 7.1 Hz, 2H, -CO2CH2CH3), 3.87 (d, J = 15.9 Hz, 2H, Cq-CHHAr), 3.54 (d, J = 16.0 Hz, 2H, Cq-CHHAr), 1.28 (t, J = 7.1 Hz, 3H, -CO2CH2CH3). 13C-NMR (150 MHz, DMSO-d6) δ 169.11 (C=O), 135.63 (C), 128.47 (C), 127.52 (C), 122.25 (CH), 119.50 (CH), 118.49 (CN), 117.98 (CH), 111.18 (CH), 106.70 (C), 62.79 (OCH2), 42.75 (C), 32.97 (CH2), 13.81 (CH3). HRMS (ESI): m/z calcd for C23H20N3O2 [M + H]+: 370.1550, found: 370.1553.
4. Conclusions
In summary, we have developed a novel oxidative cyclization/ aromatization of 1,3-di(1H-indol-3-yl)propanes to synthesize 6,7,12,13-tetrahydro-5H-cyclohepta[2,1-b:3,4-b’] diindoles. By employing PIFA/DDQ as coordinative oxidants in the presence of TMSCl as Lewis acid, the efficient intramolecular cyclization/aromatization generated the corresponding products with moderate to excellent yields. This protocol tolerated a broad range of functional-groups and the reaction could be carried out in air. Notably, no expensive transition metal catalysts/reagents were used for this transformation and this provided a new approach for these valuable compounds. Most importantly, the existing ester/CN groups in the target compounds could be transformed into other functional groups. Further biological evaluation of these compounds is in progress in our lab and the results will be reported soon.
Supplementary Materials
The NMR spectra of the obtained compounds and some additional experimental details are available online.
Author Contributions
Conceptualization, C.Y.; methodology, L.P. and X.Z.; chemical experiments, L.P.; inspiration and discussions, L.P., X.Z. and C.Y.; writing—original draft preparation, L.P.; writing—review and editing, X.Z. and C.Y.
Funding
This study was supported by Shanghai Sailing Program (17YF1423400) and China Postdoctoral Science Foundation Grant (2017M621572).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Praveen, P.; Parameswaran, P.; Majik, M. Bis(indolyl)methane Alkaloids: Isolation, Bioactivity, and Syntheses. Synthesis 2015, 47, 1827–1837. [Google Scholar] [CrossRef]
- Damodiran, M.; Muralidharan, D.; Perumal, P.T. Regioselective synthesis and biological evaluation of bis(indolyl)methane derivatized 1,4-disubstituted 1,2,3-bistriazoles as anti-infective agents. Bioorg. Med. Chem. Lett. 2009, 19, 3611–3614. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Mei, W.; Guo, Z.; Liu, S.; Zhao, Y.; Yang, D.; Zeng, Y.; Jiang, B.; Dai, H. Two New Types of Bisindole Alkaloid from Trigonostemon lutescens. Org. Lett. 2013, 15, 1492–1495. [Google Scholar] [CrossRef] [PubMed]
- Ling, F.; Xiao, L.; Fang, L.; Feng, C.; Xie, Z.; Lv, Y.; Zhong, W. B(C6F5)3-catalyzed Markovnikov addition of indoles to aryl alkynes: An approach toward bis(indolyl)alkanes. Org. Biomol. Chem. 2018, 16, 9274–9278. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Jiang, F.; Sheng, F.T.; Jiao, Y.; Mei, G.J.; Shi, F. Design and Catalytic Asymmetric Construction of Axially Chiral 3,3’-Bisindole Skeletons. Angew. Chem. Int. Ed. 2018, 57, 1–7. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, X.; Wu, Y.; Zhang, W.; Chen, X.; You, X.; Hu, L. Synthesis and structure-activity relationship of novel bisindole amidines active against MDR Gram-positive and Gram-negative bacteria. Eur. J. Med. Chem. 2018, 150, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Ohtani, I.I.; Tanaka, J.; Higa, T. Iheyamines, new cytotoxic bisindole pigments from a colonial ascidian, Polycitorella sp. Tetrahedron Lett. 1999, 40, 303–306. [Google Scholar] [CrossRef]
- Nettleton, D.E.; Doyle, T.W.; Krishnan, B. Isolation and structure of rebeccamycin—A new antitumor antibiotic from nocardia aerocoligenes. Tetrahedron Lett. 1985, 26, 4011–4014. [Google Scholar] [CrossRef]
- Bush, J.A.; Long, B.H.; Catino, J.J.; Bradner, W.T. Production and biological activity of rebeccamycin, a novel antitumor agent. J. Antibiot. 1987, 40, 668–678. [Google Scholar] [CrossRef]
- Yamashita, Y.; Fujii, N.; Murakata, C.; Ashizawa, T.; Okabe, M.; Nakano, H. Induction of Mammalian DNA Topoisomerase I Mediated DNA Cleavage by Antitumor Indolocarbazole Derivatives. Biochemistry 1992, 31, 12069–12075. [Google Scholar] [CrossRef]
- Wu, M.; Li, C.; Zhu, X. FLT3 inhibitors in acute myeloid leukemia. J. Hematol. Oncol. 2018, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Balamurali, R.; Prasad, K.J.R. Synthesis, characterization and pharmacological activities of 5,6,11,12-tetrahydroindolo[2,3-a]carbazole derivatives. II Farmaco 2001, 56, 229–232. [Google Scholar] [CrossRef]
- Su, J.; Zhu, Y.; Zeng, L.; Xu, X. A New Bisindole from Alga Caulerpa serrulata. J. Nat. Prod. 1997, 60, 1043–1044. [Google Scholar] [CrossRef]
- Wahlström, N.; Stensland, B.; Bergman, J. Synthesis of the marine alkaloid caulersin. Tetrahedron 2004, 60, 2147–2153. [Google Scholar] [CrossRef]
- Miki, Y.; Aoki, Y.; Miyatake, H.; Minematsu, T.; Hibino, H. Synthesis of caulersin and its isomers by reaction of indole-2,3-dicarboxylic anhydrides with methyl indoleacetates. Tetrahedron Lett. 2006, 47, 5215–5218. [Google Scholar] [CrossRef]
- Yang, H.; Liu, D.Q.; Liang, T.J.; Li, J.; Liu, A.H.; Yang, P.; Lin, K.; Yu, X.Q.; Guo, Y.W.; Mao, S.C.; et al. Racemosin C, a novel minor bisindole alkaloid with protein tyrosine phosphatase-1B inhibitory activity from the green alga Caulerpa racemosa. J. Asian Nat. Prod. Res. 2014, 16, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Lee, D.H.; Park, S.H.; Ham, J.S.; Nam, H.G.; Jang, S.H.; Baek, Y.G.; Cho, G.O. Novel Organic Electroluminescent Compound and Organic Electroluminescent Element Including the Same; KR 2016126150; 2 November 2016. Available online: https://worldwide.espacenet.com/publicationDetails/originalDocument?CC=KR&NR=20160126150A&KC=A&FT=D&ND=3&date=20161102&DB=EPODOC&locale=en_EP# (accessed on 8 March 2019).
- Kuckländer, U.; Töberich, H. Eine neue Synthese von 7,8,9,10-Tetrahydrocyclohept[b]indol-6(5H)-onen. Chem. Ber. 1981, 114, 2238–2244. [Google Scholar] [CrossRef]
- Thummel, R.P.; Hegde, V. Polyaza-Cavity Shaped Molecules. 14. Annelated 2-(2’-Pyridyl)indoles, 2,2’-Biindoles, and Related Systems. J. Org. Chem. 1989, 54, 1720–1725. [Google Scholar] [CrossRef]
- Kavitha, C.; Prasad, K.J.R. Synthesis, Characterization and Biological Activity of Some Indolo[2′,3′:7,6] cyclohept[b]indoles and 1,2,3-Selenadiazolo[4′,5′:6,7]cyclohept[b]indoles. Asian J. Chem. 2004, 16, 40–48. [Google Scholar]
- Wahlström, N.; Slätt, J.; Stensland, B.; Ertan, A.; Bergman, J.; Janosik, T. Synthetic Applications of Cyanoacetylated Bisindoles: Synthesis of Novel Cycloheptadiindoles, Indolocarbazoles, and Related Aza Analogues. J. Org. Chem. 2007, 72, 5886–5889. [Google Scholar] [CrossRef]
- Yang, X.; Sun, G.; Yang, C.; Wang, B. Novel Rhein Analogues as Potential Anticancer Agents. ChemMedChem 2011, 6, 2294–2301. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Xiong, B.; Shen, Y.; Yang, C. Design, synthesis, and preliminary biological evaluation of novel ketone derivatives of shikimic acid. RSC Adv. 2013, 3, 20599. [Google Scholar] [CrossRef]
- Zhang, X.; He, Q.; Xiang, H.; Song, S.; Miao, Z.; Yang, C. Rapid access to α-carbolines via a one-pot tandem reaction of α,β-unsaturated ketones with 2-nitrophenylacetonitrile and the anti-proliferative activities of the products. Org. Biomol. Chem. 2014, 12, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Chen, H.; Xu, X.; Zhu, F.; Guo, L.; Jiang, M.; Yang, C.; Deng, L. Synthesis of Rigid Analogues of Flavone by Intramolecular Heck Reaction. Eur. J. Org. Chem. 2015, 2015, 3040–3043. [Google Scholar] [CrossRef]
- Xu, X.; Qi, X.; Yan, Y.; Qi, J.; Qian, N.; Guo, L.; Li, C.; Wang, F.; Huang, P.; Zhou, H.; et al. Synthesis and biological evaluation of rhein amides as inhibitors of osteoclast differentiation and bone resorption. Eur. J. Med. Chem. 2016, 123, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Zhdankin, V.V. Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev. 2016, 116, 3328–3435. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Dohi, T.; Kita, Y. Metal-free Oxidative Cross-Coupling Reaction of Aromatic Compounds Containing Heteroatoms. Synlett 2017, 28, 1680–1694. [Google Scholar] [CrossRef]
- Tohma, H.; Iwata, M.; Maegawa, T.; Kiyono, Y.; Maruyama, A.; Kita, Y. A novel and direct synthesis of alkylated 2,2′-bithiophene derivatives using a combination of hypervalent iodine(III) reagent and BF3·Et2O. Org. Biomol. Chem. 2003, 1, 1647–1649. [Google Scholar] [CrossRef]
- Dohi, T.; Morimoto, K.; Maruyama, A.; Kita, Y. Direct Synthesis of Bipyrroles Using Phenyliodine Bis(trifluoroacetate) with Bromotrimethylsilane. Org. Lett. 2006, 8, 2007–2010. [Google Scholar] [CrossRef]
- Morimoto, K.; Yamaoka, N.; Ogawa, C.; Nakae, T.; Fujioka, H.; Dohi, T.; Kita, Y. Metal-Free Regioselective Oxidative Biaryl Coupling Leading to Head-to-Tail Bithiophenes: Reactivity Switching, a Concept Based on the Iodonium(III) Intermediate. Org. Lett. 2010, 12, 3804–3807. [Google Scholar] [CrossRef]
- Rana, A.; Panda, P.K. β-Octamethoxyporphycenes. Org. Lett. 2014, 16, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Rana, A.; Lee, S.; Kim, D.; Panda, P.K. β-Octakis(methylthio)porphycenes: Synthesis, characterisation and third order nonlinear optical studies. Chem. Commun. 2015, 51, 7705–7708. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.S.; Tsai, M.T.; Tsai, M.H.; Ong, C.W. The regioselective homocoupling of meta-hydroxypyridines with hypervalent iodine(III). Chem. Asian. J. 2015, 10, 849–852. [Google Scholar] [CrossRef]
- Jean, A.; Cantat, J.; Bérard, D.; Bouchu, D.; Canesi, S. Novel Method of Aromatic Coupling between N-Aryl Methanesulfonamide and Thiophene Derivatives. Org. Lett. 2007, 9, 2553–2556. [Google Scholar] [CrossRef] [PubMed]
- Dohi, T.; Ito, M.; Yamaoka, N.; Morimoto, K.; Fujioka, H.; Kita, Y. Unusual ipso substitution of diaryliodonium bromides initiated by a single-electron-transfer oxidizing process. Angew. Chem. Int. Ed. Engl. 2010, 49, 3334–3337. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, K.; Ohnishi, Y.; Koseki, D.; Nakamura, A.; Dohi, T.; Kita, Y. Stabilized pyrrolyl iodonium salts and metal-free oxidative cross-coupling. Org. Biomol. Chem. 2016, 14, 8947–8951. [Google Scholar] [CrossRef]
- Kantak, A.A.; Potavathri, S.; Barham, R.A.; Romano, K.M.; DeBoef, B. Metal-free intermolecular oxidative C-N bond formation via tandem C-H and N-H bond functionalization. J. Am. Chem. Soc. 2011, 133, 19960–19965. [Google Scholar] [CrossRef]
- Parumala, S.K.R.; Peddinti, R.K. Reversal of Polarity in Masked o-Benzoquinones: Rapid Access to Unsymmetrical Oxygenated Biaryls. Org. Lett. 2013, 15, 3546–3549. [Google Scholar] [CrossRef]
- Morimoto, K.; Sakamoto, K.; Ohshika, T.; Dohi, T.; Kita, Y. Organo-Iodine(III)-Catalyzed Oxidative Phenol-Arene and Phenol-Phenol Cross-Coupling Reaction. Angew. Chem. Int. Ed. 2016, 55, 3652–3656. [Google Scholar] [CrossRef]
- Sharma, S.; Parumala, S.K.R.; Peddinti, R.K. Lewis Acid-Mediated Site-Selective Synthesis of Oxygenated Biaryls from Methoxyphenols and Electron-Rich Arenes. J. Org. Chem. 2017, 82, 9367–9383. [Google Scholar] [CrossRef]
- Arisawa, M.; Ramesh, N.G.; Nakajima, M.; Tohma, H.; Kita, Y. Hypervalent Iodine(III)-Induced Intramolecular Cyclization of α-(Aryl)alkyl-β-dicarbonyl Compounds: A Convenient Synthesis of Benzannulated and Spirobenzannulated Compounds. J. Org. Chem. 2001, 66, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, H.; Anilkumar, G.; Tohma, H.; Kita, Y. A Novel and Useful Oxidative Intramolecular Coupling Reaction of Phenol Ether Derivatives on Treatment with a Combination of Hypervalent Iodine(III) Reagent and Heteropoly Acid. Chem. Eur. J. 2002, 8, 5377–5383. [Google Scholar] [CrossRef]
- Hamamoto, H.; Shiozaki, Y.; Hata, K.; Tohma, H.; Kita, Y. A Novel and Concise Synthesis of Spirodienone Alkaloids Using Hypervalent Iodine(III) Reagents. Chem. Pharm. Bull. 2004, 52, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Moreno, I.; Tellitu, I.; Domínguez, E.; SanMartín, R.l. A Simple Route to New Phenanthro- and Phenanthroid-Fused Thiazoles by a PIFA-Mediated (Hetero)biaryl Coupling Reaction. Eur. J. Org. Chem. 2002, 2126–2135. [Google Scholar] [CrossRef]
- Barrett, T.N.; Braddock, D.C.; Monta, A.; Webb, M.R.; White, A.J. Total synthesis of the marine metabolite (±)-polysiphenol via highly regioselective intramolecular oxidative coupling. J. Nat. Prod. 2011, 74, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, S.; Andrez, J.C.; Canesi, S. A Stereoselective Oxidative Polycyclization Process Mediated by a Hypervalent Iodine Reagent. Org. Lett. 2011, 13, 3406–3409. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhang, X.; Zhang, Y.; Ruan, L.; Zhang, J.; Zhang-Negrerie, D.; Du, Y. Iodocyclization of N-Arylpropynamides Mediated by Hypervalent Iodine Reagent: Divergent Synthesis of Iodinated Quinolin-2-ones and Spiro[4,5]trienones. Org. Lett. 2017, 19, 150–153. [Google Scholar] [CrossRef]
- Suvorov, N.N.; Velezheva, V.S.; Vampilova, V.V. Indole derivatives. LXXXVII. Improved methods for the synthesis of skatyl- and substituted skatylmalonic esters. Chem. Heterocycl. Compd. 1973, 9, 1367–1369. [Google Scholar] [CrossRef]
- Pelcman, B.; Gribble, G.W. Total Synthesis of The Marine Sponge Pigment Fascaplysin. Tetrahedron Lett. 1990, 31, 2381–2384. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1a–t and 2a–t are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).