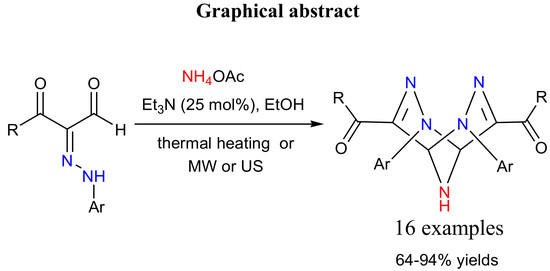
Facile Assembling of Novel 2,3,6,7,9-pentaazabicyclo- [3.3.1]nona-3,7-diene Derivatives under Microwave and Ultrasound Platforms
Abstract
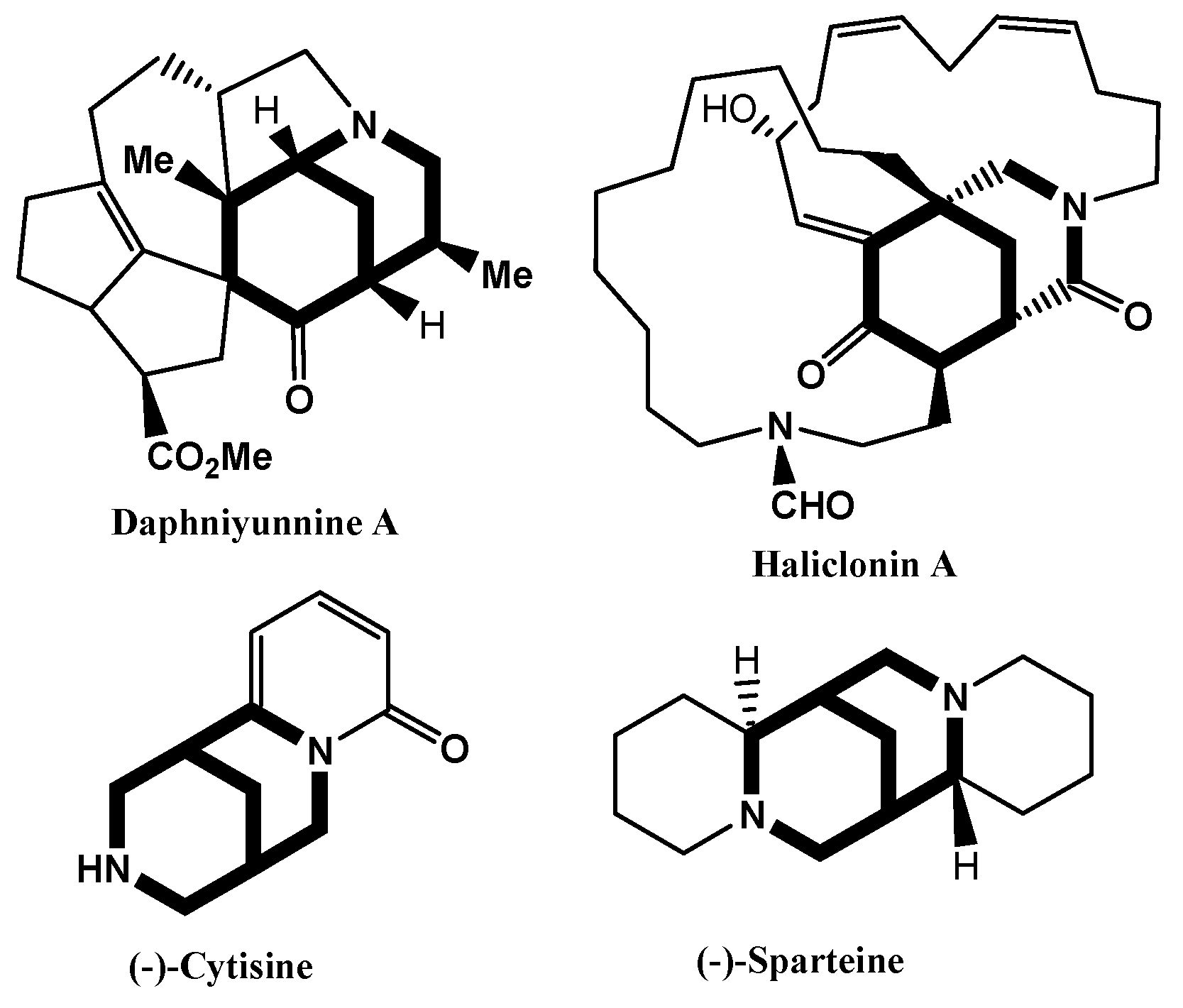
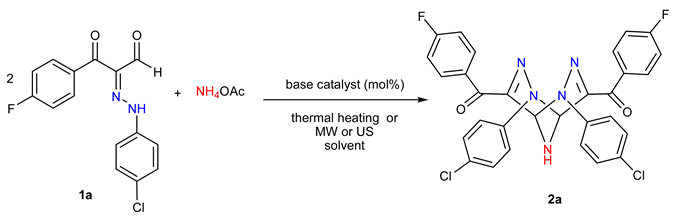
1. Introduction
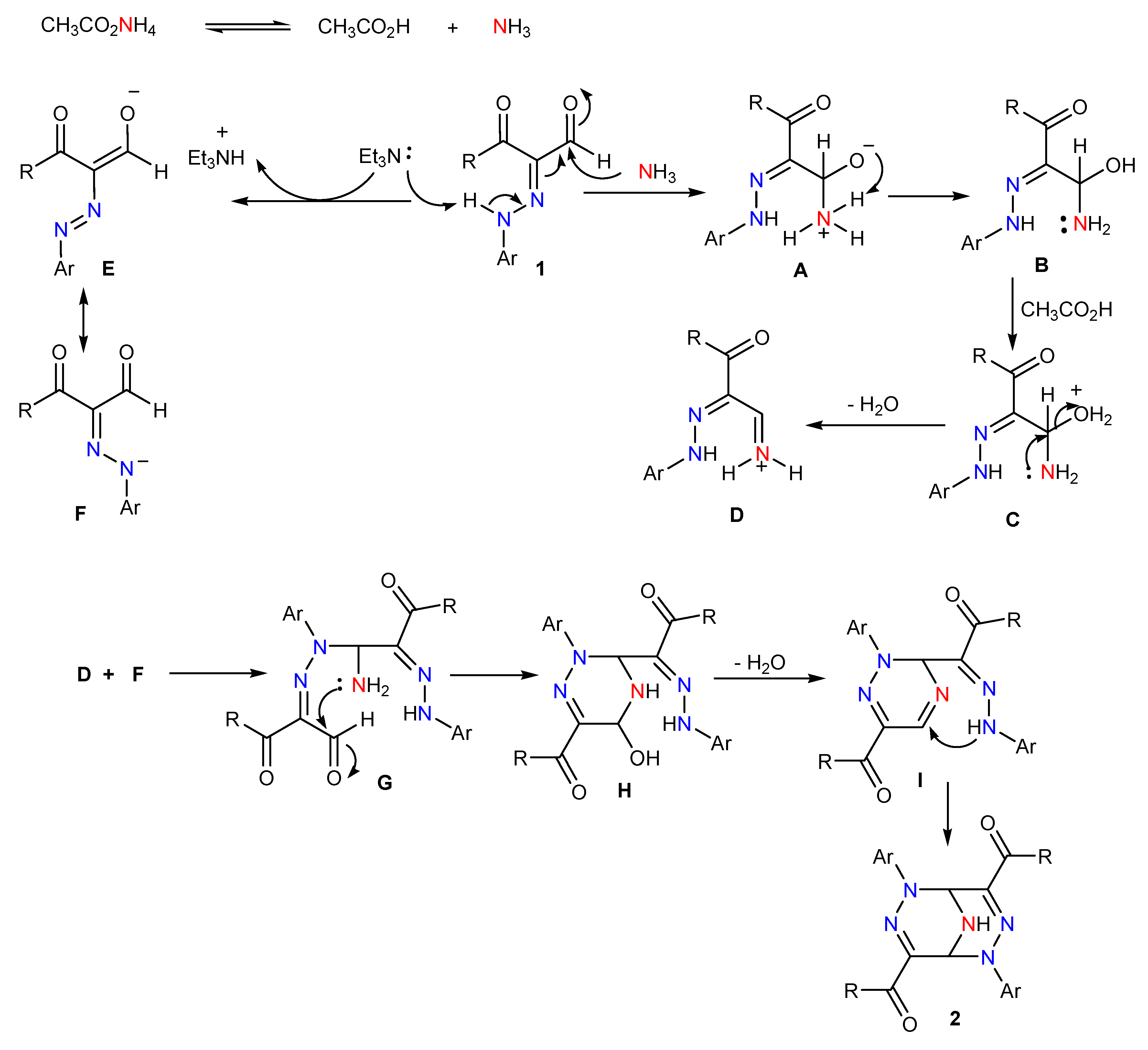
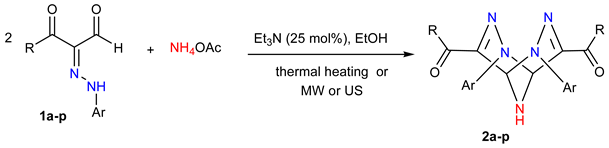
2. Results
3. Materials and Methods
3.1. General Information
3.2. Synthesis of 2,3,6,7,9-Pentaazabicyclo[3.3.1]nona-3,7-diene Derivatives 2a–p
3.2.1. General Method A
3.2.2. General Method B
3.2.3. General Method C
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamaker, L.K.; Cook, J.M. The Synthesis of Macroline Related Sarpagine Alkaloids. Alkaloids: Chemical and Biological Perspectives; Elsevier Science: Amsterdam, The Netherlands, 1995; Volume 9. [Google Scholar]
- Keawpradub, N.; Kirby, G.C.; Steele, J.C.P.; Houghton, P.J. Antiplasmodial Activity of Extracts and Alkaloids of Three Alstonia Species from Thailand. Planta Med. 1999, 65, 690–694. [Google Scholar] [CrossRef] [PubMed]
- Novartis, A.-G.; Novartis Pharma GmbH, Switz. (1-Aza-bicyclo[3.3.1]non-4-yl)-[5-(1H-indol-5-yl)-heteroaryl]amines as Cholinergic Ligands of the n-AChR for the Treatment of Psychotic and Neurodegenerative Disorders. Patent WO2007068475, 21 June 2007. [Google Scholar]
- Novartis, A.-G.; Novartis Pharma GmbH, Switz. [(1H-Indol-5-yl)-heteroaryloxy]-(1-azabicyclo-[3.3.1]nonanes as Cholinergic Ligands of the n-AChR for the Treatment of Psychotic and Neurodegenrative Disorders. Patent WO2007068476, 21 June 2007. [Google Scholar]
- Bonjoch, J.; Diaba, F.; Bradshaw, B. Synthesis of 2-Azabicyclo[3.3.1]nonanes. Synthesis 2011, 993–1018. [Google Scholar] [CrossRef]
- Trost, B.M.; Tang, W.; Toste, F.D. Divergent Enantioselective Synthesis of (−)-Galanthamine and (−)-Morphine. J. Am. Chem. Soc. 2005, 127, 14785–14803. [Google Scholar] [CrossRef] [PubMed]
- Proto, S.; Amat, M.; Pérez, M.; Ballette, R.; Romagnoli, F.; Mancinelli, A.; Bosch, J. Model Studies on the Synthesis of Madangamine Alkaloids. Assembly of the Macrocyclic Rings. Org. Lett. 2012, 14, 3916–3919. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.H.; Kang, G.W.; Jeon, J.-E.; Lim, C.; Lee, H.-S.; Sim, C.J.; Oh, K.-B.; Shin, J. Haliclonin A, a New Macrocyclic Diamide from the Sponge Haliclona sp. Org. Lett. 2009, 11, 1713–1716. [Google Scholar] [CrossRef] [PubMed]
- Orihara, K.; Kawagishi, F.; Yokoshima, S.; Fukuyama, T. Synthetic Studies of Haliclonin A: Construction of the 3-Azabicyclo[3.3.1]nonane Skeleton with a Bridge that Forms the 17-Membered Ring. Synlett 2018, 29, 769–772. [Google Scholar]
- Keawpradub, N.; Eno-Amooquaye, E.; Burke, P.J.; Houghton, P.J. Cytotoxic activity of indole alkaloids from Alstonia macrophylla. Planta Med. 1999, 65, 311–315. [Google Scholar] [CrossRef]
- Boeckler, F.; Gmeiner, P. The structural evolution of dopamine D3 receptor ligands: Structure–activity relationships and selected neuropharmacological aspects. Pharmacol. Ther. 2006, 112, 281–333. [Google Scholar] [CrossRef]
- Wu, Z.-W.; Song, S.-Y.; Li, L.; Lu, H.-L.; Lieberman, B.; Huang, Y.-S.; Mach, R.H. Synthesis and evaluation of tetrahydroindazole derivatives as sigma-2 receptor ligands. Bioorg. Med. Chem. 2015, 23, 1463–1471. [Google Scholar] [CrossRef]
- Arora, S.; Sinha, N.; Nair, P.; Chakka, S.K.; Sai, K.; Hajare, A.; Reddy, A.; Patil, P.; Sayyed, M.; Kamboj, R.K.; et al. Novel Compounds as DIPEPTIDYL Peptidase IV (DPP IV) inhibitors. US Patent 2010/0291020, 18 November 2010. [Google Scholar]
- Kolhatkar, R.; Cook, C.D.; Ghorai, S.K.; Deschamps, J.; Beardsley, P.M.; Reith, M.E.; Dutta, A.K. Further structurally constrained analogues of cis-(6-benzhydrylpiperidin-3-yl)benzylamine with elucidation of bioactive conformation: discovery of 1,4-diazabicyclo[3.3.1]nonane derivatives and evaluation of their biological properties for the monoamine transporters. J. Med. Chem. 2004, 47, 5101–5113. [Google Scholar] [PubMed]
- Mishra, M.; Kolhatkar, R.; Zhen, J.; Parrington, I.; Reith, M.E.A.; Dutta, A.K. Further structural optimization of cis-(6-benzhydryl-piperidin-3-yl)-benzylamine and 1,4-diazabicyclo-[3.3.1]nonane derivatives by introducing an exocyclic hydroxyl group: Interaction with dopamine, serotonin, and norepinephrine transporters. Bioorg. Med. Chem. 2008, 16, 2769–2778. [Google Scholar] [CrossRef]
- Annika, B.; Magnus, B.; Torbjorn, H.; Kurt-Jürgen, H.; Bertil, S.; Gert, S. Bispidine Compounds Useful in the Treatment of Cardiac Arrhythmias. US Patent 6,887,881 B1, 3 May 2005. [Google Scholar]
- Misra, A.; Kumar, K.S.A.; Jain, M.; Bajaj, K.; Shandilya, S.; Srivastava, S.; Shukla, P.; Barthwal, M.K.; Dikshit, M.; Dikshit, D.K. Synthesis and evaluation of dual antiplatelet activity of bispidine derivatives of N-substituted pyroglutamic acids. Eur. J. Med. Chem. 2016, 110, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gündisch, D.; Eibl, C. Nicotinic acetylcholine receptor ligands, a patent review. Expert Opin. Ther. Patents 2011, 21, 1867–1896. [Google Scholar] [CrossRef] [PubMed]
- Eibl, C.; Munoz, L.; Tomassoli, I.; Stokes, C.; Papke, R.L.; Gündisch, D. The 3,7-diazabicyclo-[3.3.1]nonane scaffold for subtype selective nicotinic acetylcholine receptor ligands. Part 2: Carboxamide derivatives with different spacer motifs. Bioorg. Med. Chem. 2013, 21, 7309–7329. [Google Scholar] [CrossRef]
- Tomassoli, I.; Gündisch, D. The twin drug approach for novel nicotinic acetylcholine receptor Ligands. Bioorg. Med. Chem. 2015, 23, 4375–4389. [Google Scholar] [CrossRef]
- Bermudez, J.; Gaster, L.; Gregory, J.; Jerman, J.; Joiner, G.F.; King, F.D.; Rahman, S.K. Synthesis and 5-HT 3 receptor antagonist potency of novel (endo) 3,9-diazabicyclo-[3.3.1]nonan-7-amino Derivatives. Bioorg. Med. Chem. Lett. 1994, 4, 2373–2376. [Google Scholar] [CrossRef]
- Pinna, G.A.; Cignarella, G.; Loriga, G.; Murineddu, G.; Mussinu, J.-M.; Ruiu, S.; Faddad, P.; Frattad, W. N-3(9)-Arylpropenyl-N-9(3)-propionyl-3,9-diazabicyclo-[3.3.1]nonanes as μ-Opioid Receptor Agonists. Effects on μ-Affinity of Arylalkenyl Chain Modifications. Bioorg. Med. Chem. 2002, 10, 1929–1937. [Google Scholar] [CrossRef]
- Loriga, G.; Lazzari, P.; Manca, I.; Ruiu, S.; Falzoi, M.; Murineddu, G.; Bottazzi, M.E.H.; Pinna, G.; Pinna, G.A. Novel diazabicycloalkane delta opioid agonists. Bioorg. Med. Chem. 2015, 23, 5527–5538. [Google Scholar] [CrossRef]
- Tanaka, K.; Siwu, E.R.O.; Hirosaki, S.; Iwata, T.; Matsumoto, R.; Kitagawa, Y.; Pradipta, A.R.; Okamura, M.; Fukase, K. Efficient synthesis of 2,6,9-triazabicyclo[3.3.1]nonanes through amine-mediated formal [4+4] reaction of unsaturated imines. Tetrahedron Lett. 2012, 53, 5899–5902. [Google Scholar] [CrossRef]
- Tsutsui, A.; Tanaka, K. 2,6,9-Triazabicyclo[3.3.1]nonanes as overlooked amino-modification products by acrolein. Org. Biomol. Chem. 2013, 11, 7208–7211. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, A.; Pradipta, A.R.; Saigitbatalova, E.; Kurbangalieva, A.; Tanaka, K. Exclusive formation of imino[4+4]cycloaddition products with biologically relevant amines: plausible candidates for acrolein biomarkers and biofunctional modulators. Med. Chem. Commun. 2015, 6, 431–436. [Google Scholar] [CrossRef]
- Revesz, L.; Blum, E.; Wicki, R. Synthesis of Novel Piperazine Based Building Blocks: 3,7,9-Triazabicyclo[3.3.1]nonane, 3,6,8-Triazabicyclo[3.2.2]nonane, 3-Oxa-7,9-diazabicyclo-[3.3.1]nonane and 3-Oxa-6,8-diazabicyclo[3.2.2]nonane. Tetrahedron Lett. 2005, 46, 5577–5580. [Google Scholar] [CrossRef]
- Pradipta, A.R.; Tanaka, K. Synthesis of 3,7,9- and 2,6,9-triazabicyclo[3.3.1]nonane derivatives. Heterocycles 2013, 87, 2001–2014. [Google Scholar]
- Herpel, M.; Rehse, K. Nitrosation Products of Hexamethylenetetramine. Arch. Pharm. Pharm. Med. Chem. 1999, 332, 255–257. [Google Scholar] [CrossRef]
- Bryukhanov, A.Y.; Golod, E.L. Synthesis and Transformations of 1,3,5,7-Tetraazabicyclo-[3. 3.1]nonanes, Russ. J. Gen. Chem. 2002, 72, 1299–1305. [Google Scholar] [CrossRef]
- Vagenknecht, J.; Zeman, S. Some characteristics of 3,7-dinitro-, 3,7-dinitroso- and dinitrate compounds derived from 1,3,5,7-tetraazabicyclo[3. 3.1]nonane, J. Hazard. Mat. 2005, A119, 1–11. [Google Scholar] [CrossRef]
- Cordes, A.W.; Oakley, R.T.; Boeri, R.T. Structure of a Bicyclic Sulfur-Nitrogen-Carbon Heterocyclic Molecule. Acta Cryst. 1985, C41, 1833–1834. [Google Scholar] [CrossRef]
- Behbehani, H.; Ibrahim, H.M. An efficient ultrasonic-mediated one-pot synthesis of 2,3,6,7,9-pentaazabicyclo[3.3.1]nonanes via a N,N-dimethylformamide dimethylacetal catalyzed Mannich-like reaction. RSC Adv. 2016, 6, 52700–52709. [Google Scholar] [CrossRef]
- De la Hoz, A.; Loupy, A. Microwaves in Organic Synthesis, 3rd ed.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Pollastri, M.P.; Devine, W.G. Microwave Synthesis in Green Techniques for Organic Synthesis and Medicinal Chemistry; Zhang, W., Cue, B., Eds.; Wiley: Chichester, UK, 2012; Charpter 12; pp. 325–342. [Google Scholar]
- Kappe, C.O.; Stadler, A. Microwaves in Organic and Medicinal Chemistry, 2nd ed.; Wiley: Weinheim, Germany, 2012. [Google Scholar]
- Zhang, W.; Cue, B.W. Green Techniques for Organic Synthesis and Medicinal Chemistry, 2nd ed.; Wiley & Sons, Ltd.: Hoboken, NK, USA, 2018; Chapter 17. [Google Scholar]
- Baig, R.B.N.; Varma, R.S. Alternative energy input: mechanochemical, microwave and ultrasound-assisted organic synthesis. Chem. Soc. Rev. 2012, 41, 1559–1584. [Google Scholar] [CrossRef]
- Pedersen, S.L.; Tofteng, A.P.; Malik, L.; Jensen, K.J. Microwave heating in solid-phase peptide synthesis. Chem. Soc. Rev. 2012, 41, 1826–1844. [Google Scholar] [CrossRef] [PubMed]
- Takkellapati, S.R. Microwave-assisted Chemical Transformations. Curr. Org. Chem. 2013, 17, 2305–2322. [Google Scholar] [CrossRef]
- Kappe, C.O. How to measure reaction temperature in microwave-heated transformations. Chem. Soc. Rev. 2013, 42, 4977–4990. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P. Applied Sonochemistry: Uses of Power Ultrasound in Chemistry and Processing; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Ruano, J.L.G.; Parra, A.; Marzo, L.; Yuste, F.; Mastranzo, V.M. One-pot synthesis of sulfonamides from methyl sulfinates using ultrasound. Tetrahedron 2011, 67, 2905–2910. [Google Scholar] [CrossRef]
- Puri, S.; Kaur, B.; Parmar, A.; Kumar, H. Applications of Ultrasound in Organic Synthesis—A Green Approach. Curr. Org. Chem. 2013, 17, 1790–1828. [Google Scholar] [CrossRef]
- Banerjee, B. Recent developments on ultrasound-assisted one-pot multicomponent synthesis of biologically relevant heterocycles. Ultrason. Sonochem. 2017, 35, 15–35. [Google Scholar] [CrossRef]
- Chatel, G. How sonochemistry contributes to green chemistry. Ultrason. Sonochem. 2018, 40, 117–122. [Google Scholar] [CrossRef]
- Al-Matar, H.M.; Dawood, K.M.; Tohamy, W.M. Tandem one-pot synthesis of 2-arylcinnolin-6-one derivatives from arylhydrazonopropanals and acetoacetanilides using sustainable ultrasound and microwave platforms. RSC Adv. 2018, 8, 34459–34467. [Google Scholar] [CrossRef]
- Behbehani, H.; Dawood, K.M.; Ibrahim, H.M.; Mostafa, N.S. Regio- and stereoselective route to bis-[3-methyl-1,1′,4′-triaryl-5-oxo-spiro-pyrazoline-4,5′-pyrazoline] derivatives via 1,3-dipolar cycloaddition under sonication. Arab. J. Chem. 2018, 11, 1053–1060. [Google Scholar] [CrossRef]
- Khalil, K.D.; Al-Matar, H.M. Studies on 2-Arylhydrazononitriles: Synthesis of 3-Aryl-2-arylhydrazopropanenitriles and Their Utility as Precursors to 2-Substituted Indoles, 2-Substituted-1,2,3-Triazoles, and 1-Substituted Pyrazolo[4,3-d]pyrimidines. Molecules 2012, 17, 12225–12233. [Google Scholar] [CrossRef]
- Al-Matar, H.M.; Riyadh, S.M.; Elnagdi, M.H. Studies with enamines: Reactivity of N,N-dimethyl-N-[(E)-2-(4-nitrophenyl)-1-ethenyl]amine towards nitrilimine and aromatic diazonium salts. J. Heterocycl. Chem. 2007, 44, 603–607. [Google Scholar] [CrossRef]
- Dawood, K.M.; El-Deftar, M.M. Microwave-Assisted Synthesis of 2-Substituted 4-Biarylyl-1,3-thiazoles by Carbon–Carbon Cross-Coupling in Water. Synthesis 2010, 1030–1038. [Google Scholar] [CrossRef]
- Darweesh, A.F.; Shaaban, M.R.; Farag, A.M.; Metz, P.; Dawood, K.M. Facile Access to Biaryls and 2-Acetyl-5-Arylbenzofurans via Suzuki Coupling in Water under Thermal and Microwave Conditions. Synthesis 2010, 3163–3173. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.A.; El-Zahabi, H.S.A.; Dawood, K.M. Microwave-assisted synthesis and in-vitro anti-tumor activity of 1,3,4-triaryl-5-N-arylpyrazole-carboxamides. Eur J. Med. Chem. 2010, 45, 2427–2432. [Google Scholar] [CrossRef]
- Dawood, K.M.; Farag, A.M.; El-Deftar, M.M.; Gardiner, M.; Abdelaziz, H.A. Microwave-assisted synthesis of 5-arylbenzofuran-2-carboxylates via Suzuki coupling using a 2-quinolinealdoxime-Pd(II)-complex. Arkivoc 2013, 2013, 210–226. [Google Scholar]
- Dawood, K.M.; Elamin, M.B.; Farag, A.M. Microwave-assisted synthesis of 2-acetyl-5-arylthiophenes and 4-(5-arylthiophen-2-yl)thiazoles via Suzuki coupling in water. Arkivoc 2015, 7, 50–62. [Google Scholar]
- Behbehani, H.; Ibrahim, H.M.; Dawood, K.M. Ultrasound-Assisted Regio- and Stereoselective Synthesis of Bis-[1′,4′-diaryl-1-oxo-spiro-benzosuberane-2,5′-pyrazoline] Derivatives via 1,3-Dipolar Cycloaddition. RSC Adv. 2015, 5, 25642–25649. [Google Scholar] [CrossRef]
- Basyouni, W.M.; El-Bayouki, K.A.M.; Tohamy, W.M.; Abbas, S.Y. Silica Sulfuric Acid: An Efficient, Reusable, Heterogeneous Catalyst for the One-Pot, Five-Component Synthesis of Highly Functionalized Piperidine Derivatives. Synth. Commun. 2015, 45, 1073–1081. [Google Scholar] [CrossRef]
- Tramontini, M.; Angiolini, L. Mannich-Bases, Chemistry and Uses; CRC: Boca Raton, FL, USA, 1994. [Google Scholar]
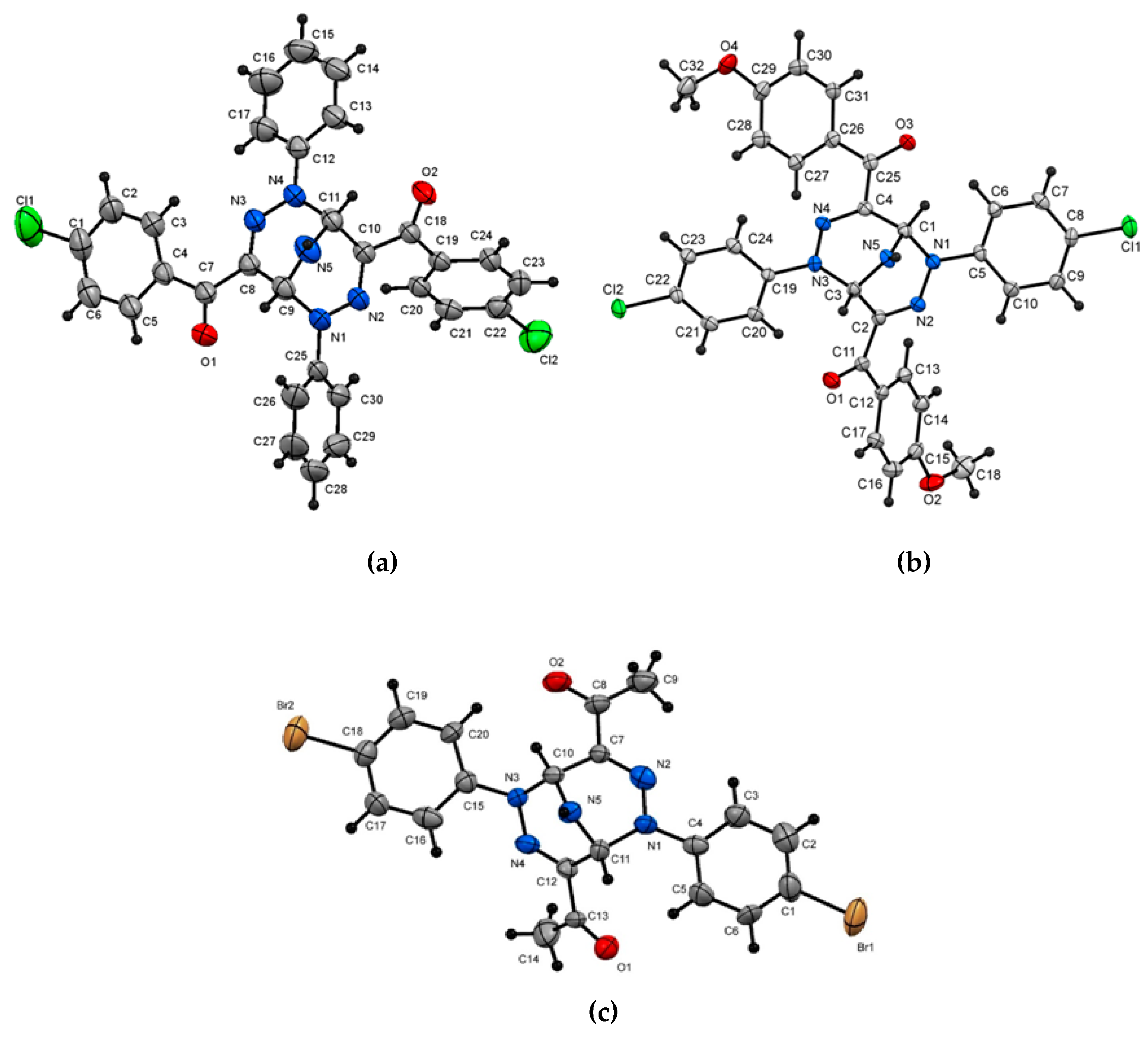
- The Crystallographic Data for Compounds 2f (ref. CCDC 1885322), 2k (ref. CCDC 1885339) and 2p (ref. CCDC 1888859) Can Be Obtained on Request from the Director; Cambridge Crystallographic Data Center: Cambridge, UK.
- Bishop, R. Supramolecular Host–Guest Chemistry of Heterocyclic V-Shaped Molecules. Top. Heterocycl. Chem. 2009, 18, 37–74. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- Al-Shiekh, M.A.; Medrassi, H.Y.; Elnagdi, M.H.; Hafez, E.A. Substituted hydrazonals as building blocks in heterocyclic synthesis: A new route to arylhydrazonocinnolines. J. Chem. Res. 2007, 432–436. [Google Scholar] [CrossRef]
Sample Availability: Samples of the synthesized compounds are available from the corresponding authors. |
| Run | Base-Catalyst (mol%) | Solvent | Conv. Heating | Sonication | MW Irradiation | |||
|---|---|---|---|---|---|---|---|---|
| Yield b % | Time (h) | Yield b % | Time (min) | Yield b % | Time (min) | |||
| 1 | No catalyst | EtOH | trace | 15 | trace | 120 | trace | 30 |
| 2 | Et3N (15) | EtOH | 70 | 4 | 82 | 60 | 87 | 5 |
| 3 | Et3N (25) | EtOH | 78 c | 3 | 89 d | 50 | 94 | 3 |
| 4 | Et3N (30) | EtOH | 71 | 3 | 85 | 60 | 90 | 4 |
| 5 | Et3N (25) e | EtOH | 66 e | 3 | 73 e | 50 | 80e | 3 |
| 6 | Et3N (25) f | EtOH | 60 f | 3 | 68 f | 50 | 73f | 3 |
| 7 | Et3N (25) | MeOH | 71 | 4 | 82 | 50 | 89 | 4 |
| 8 | Et3N (25) | isopropanol | 70 | 4 | 80 | 60 | 88 | 4 |
| 9 | Et3N (25) | n-hexane | 34 | 6 | 52 | 80 | 63 | 10 |
| 10 | Et3N (25) | acetic acid | 32 | 4 | 40 | 70 | 50 | 6 |
| 11 | Et3N (25) | DMF | 30 | 6 | 45 | 100 | 55 | 10 |
| 12 | Et3N (25) | toluene | 35 | 5 | 50 | 90 | 65 | 10 |
| 13 | pyridine (25) | EtOH | 20 | 4 | 28 | 60 | 35 | 5 |
| 14 | DABCO (25) | EtOH | 17 | 5 | 20 | 80 | 28 | 7 |
| 15 | DBU (25) | EtOH | 15 | 5 | 18 | 70 | 25 | 8 |
| 16 | NaHCO3 (25) | EtOH | 10 | 6 | 10 | 80 | 15 | 10 |
| 17 | K2CO3 (25) | EtOH | 10 | 5 | 12 | 90 | 15 | 10 |
| 18 | NaOH (25) | EtOH | 12 | 5 | 14 | 80 | 18 | 9 |
| Run | Products | R | Ar | Conv. Heating a | Sonication a | MW a Irradiation | |||
|---|---|---|---|---|---|---|---|---|---|
| Yield b% | Time (h) | Yield b % | Time (min) | Yield b % | Time (min) | ||||
| 1 | 2a | 4-FC6H4 | 4-ClC6H4 | 78 | 3 | 89 | 50 | 94 | 3 |
| 2 | 2b | 4-FC6H4 | 4-BrC6H4 | 77 | 4 | 87 | 60 | 93 | 4 |
| 3 | 2c | C6H5 | 4-ClC6H4 | 70 | 3 | 81 | 50 | 89 | 4 |
| 4 | 2d | C6H5 | 4-BrC6H4 | 72 | 5 | 82 | 70 | 90 | 6 |
| 5 | 2e | C6H5 | 2-NO2C6H4 | 68 | 4 | 80 | 70 | 86 | 5 |
| 6 | 2f | 4-ClC6H4 | C6H5 | 71 | 3 | 83 | 40 | 90 | 3 |
| 7 | 2g | 4-ClC6H4 | 4-BrC6H4 | 73 | 5 | 84 | 70 | 91 | 5 |
| 8 | 2h | 4-BrC6H4 | 4-ClC6H4 | 86 | 4 | 87 | 70 | 92 | 5 |
| 9 | 2i | 4-BrC6H4 | 4-BrC6H4 | 84 | 5 | 86 | 80 | 92 | 6 |
| 10 | 2j | 4-OMeC6H4 | C6H5 | 66 | 5 | 77 | 70 | 82 | 5 |
| 11 | 2k | 4-OMeC6H4 | 4-ClC6H4 | 65 | 6 | 77 | 90 | 81 | 6 |
| 12 | 2l | 4-OMeC6H4 | 4-BrC6H4 | 67 | 8 | 78 | 100 | 83 | 7 |
| 13 | 2m | 4-NO2C6H4 | 4-ClC6H4 | 67 | 7 | 79 | 110 | 84 | 7 |
| 14 | 2n | 4-NO2C6H4 | 4-BrC6H4 | 71 | 8 | 80 | 100 | 86 | 8 |
| 15 | 2o | CH3 | 4-ClC6H4 | 62 | 7 | 73 | 90 | 81 | 8 |
| 16 | 2p | CH3 | 4-BrC6H4 | 64 | 6 | 75 | 110 | 81 | 9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Matar, H.M.; Dawood, K.M.; Tohamy, W.M.; Shalaby, M.A. Facile Assembling of Novel 2,3,6,7,9-pentaazabicyclo- [3.3.1]nona-3,7-diene Derivatives under Microwave and Ultrasound Platforms. Molecules 2019, 24, 1110. https://doi.org/10.3390/molecules24061110
Al-Matar HM, Dawood KM, Tohamy WM, Shalaby MA. Facile Assembling of Novel 2,3,6,7,9-pentaazabicyclo- [3.3.1]nona-3,7-diene Derivatives under Microwave and Ultrasound Platforms. Molecules. 2019; 24(6):1110. https://doi.org/10.3390/molecules24061110
Chicago/Turabian StyleAl-Matar, Hamad M., Kamal M. Dawood, Wael M. Tohamy, and Mona A. Shalaby. 2019. "Facile Assembling of Novel 2,3,6,7,9-pentaazabicyclo- [3.3.1]nona-3,7-diene Derivatives under Microwave and Ultrasound Platforms" Molecules 24, no. 6: 1110. https://doi.org/10.3390/molecules24061110
APA StyleAl-Matar, H. M., Dawood, K. M., Tohamy, W. M., & Shalaby, M. A. (2019). Facile Assembling of Novel 2,3,6,7,9-pentaazabicyclo- [3.3.1]nona-3,7-diene Derivatives under Microwave and Ultrasound Platforms. Molecules, 24(6), 1110. https://doi.org/10.3390/molecules24061110