Dihydroquinolines, Dihydronaphthyridines and Quinolones by Domino Reactions of Morita-Baylis-Hillman Acetates
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Methods
3.2. Syntheses of the MBH Acetates
3.2.1. 2-Cyano-1-(2-fluoropyridin-3-yl)allyl Acetate (6)
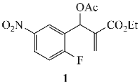
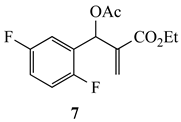
3.2.2. Ethyl-2-(acetoxy(2,5-difluorophenyl)methyl)acrylate (7)
3.3. Representative Procedure for the Synthesis of Dihydroheteroaromatics Using MBH Acetates and 1° Alkyl or Aromatic Amines
3.3.1. Ethyl-1-methyl-6-nitro-1,2-dihydroquinoline-3-carboxylate (9a) from 1 and Methylamine (8a)
3.3.2. Ethyl-1-hexyl-6-nitro-1,2-dihydroquinoline-3-carboxylate (9b) from 1 and n-hexylamine (8b)
3.3.3. Ethyl-1-isobutyl-6-nitro-1,2-dihydroquinoline-3-carboxylate (9c) from 1 and Isobutylamine (8c)
3.3.4. Ethyl-1-benzyl-6-nitro-1,2-dihydroquinoline-3-carboxylate (9d) from 1 and Benzylamine (8d)
3.3.5. Ethyl-6-nitro-1-phenethyl-1,2-dihydroquinoline-3-carboxylate (9e) from 1 and Phenethylamine (8e)
3.3.6. Ethyl-6-nitro-1-phenyl-1,2-dihydroquinoline-3-carboxylate (9f) from 1 and Aniline (8f)
3.3.7. 1-Methyl-6-nitro-1,2-dihydroquinoline-3-carbonitrile (10a) from 2 and 8a
3.3.8. 1-Hexyl-6-nitro-1,2-dihydroquinoline-3-carbonitrile (10b) from 2 and 8b
3.3.9. 1-Isobutyl-6-nitro-1,2-dihydroquinoline-3-carbonitrile (10c) from 2 and 8c
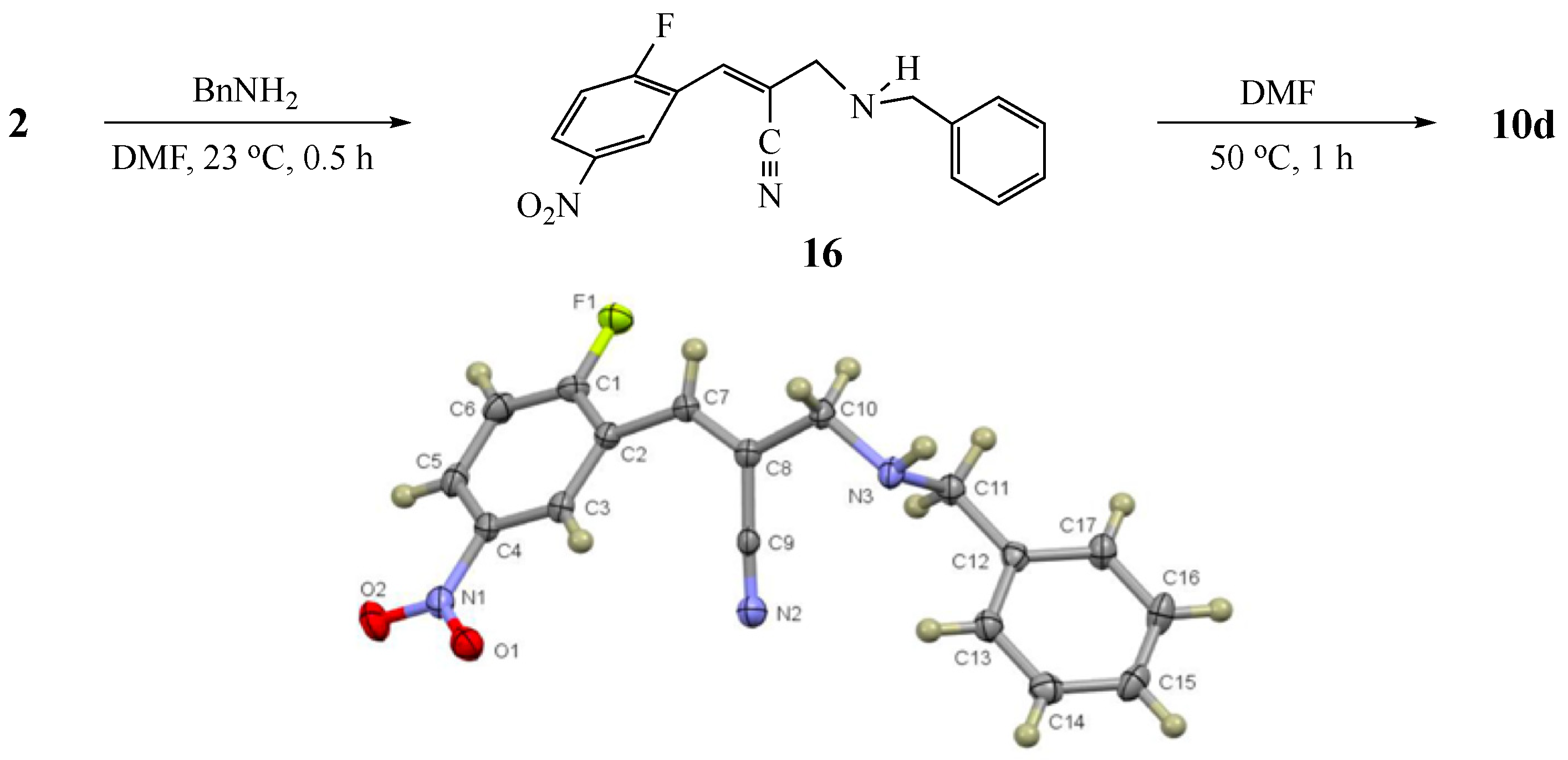
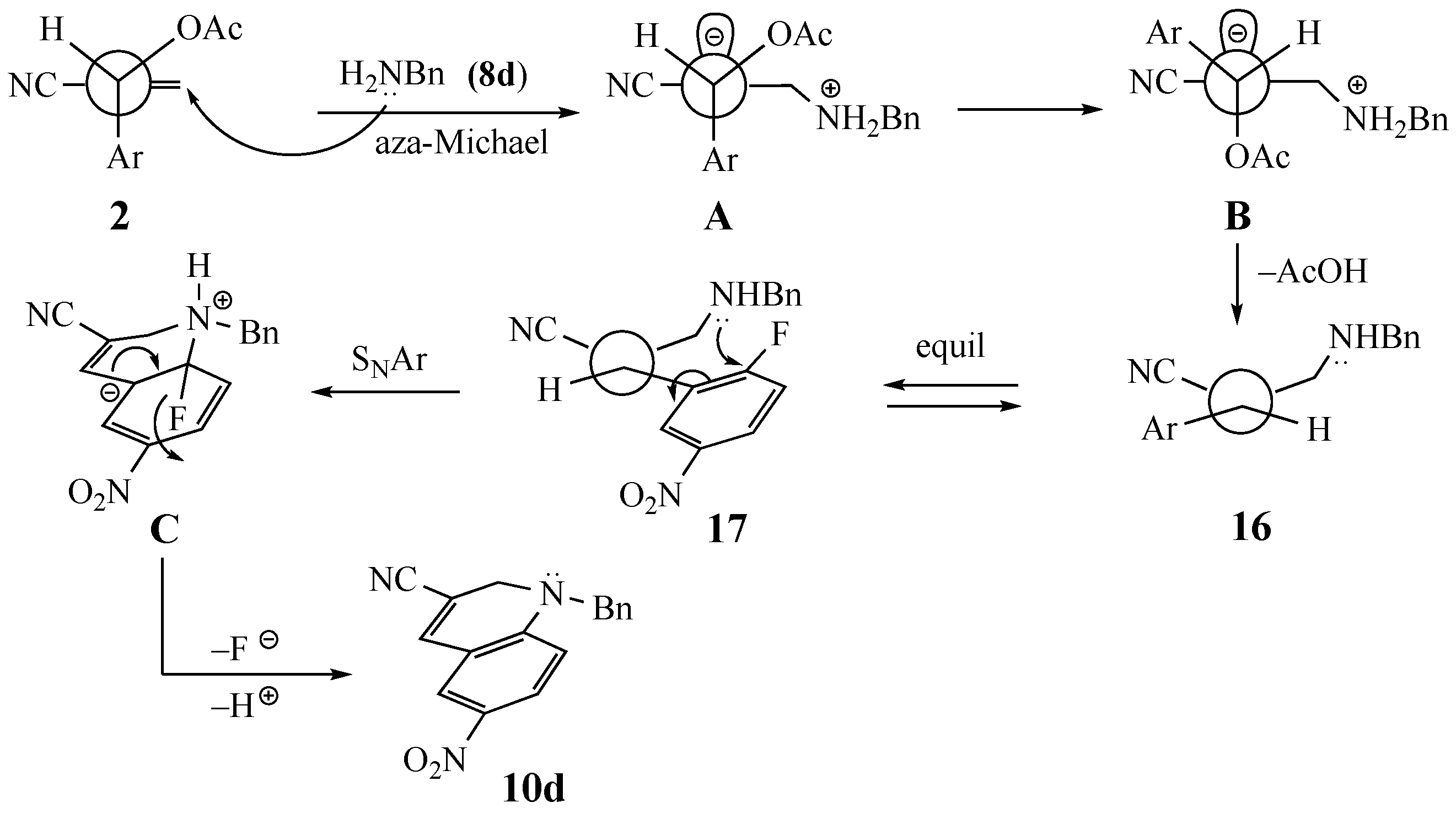
3.3.10. 1-Benzyl-6-nitro-1,2-dihydroquinoline-3-carbonitrile (10d) from 2 and 8d
3.3.11. 6-Nitro-1-phenethyl-1,2-dihydroquinoline-3-carbonitrile (10e) from 2 and 8e
3.3.12. 6-Nitro-1-phenyl-1,2-dihydroquinoline-3-carbonitrile (10f) from 2 and 8f
3.3.13. Ethyl-6-cyano-1-methyl-1,2-dihydroquinoline-3-carboxylate (11a) from 3 and 8a
3.3.14. Ethyl-6-cyano-1-hexyl-1,2-dihydroquinoline-3-carboxylate (11b) from 3 and 8b
3.3.15. Ethyl-6-cyano-1-isobutyl-1,2-dihydroquinoline-3-carboxylate (11c) from 3 and 8c
3.3.16. Ethyl-1-benzyl-6-cyano-1,2-dihydroquinoline-3-carboxylate (11d) from 3 and 8d
3.3.17. Ethyl-6-cyano-1-phenethyl-1,2-dihydroquinoline-3-carboxylate (11e) from 3 and 8e
3.3.18. Ethyl-6-cyano-1-phenyl-1,2-dihydroquinoline-3-carboxylate (11f) from 3 and 8f
3.3.19. 1-Methyl-1,2-dihydroquinoline-3,6-dicarbonitrile (12a) from 4 and 8a
3.3.20. 1-Hexyl-1,2-dihydroquinoline-3,6-dicarbonitrile (12b) from 4 and 8b
3.3.21. 1-Isobutyl-1,2-dihydroquinoline-3,6-dicarbonitrile (12c) from 4 and 8c
3.3.22. 1-Benzyl-1,2-dihydroquinoline-3,6-dicarbonitrile (12d) from 4 and 8d
3.3.23. 1-Phenethyl-1,2-dihydroquinoline-3,6-dicarbonitrile (12e) from 4 and 8e
3.3.24. Ethyl-1-methyl-1,2-dihydro-1,8-naphthyridine-3-carboxylate (13a) from 5 and 8a
3.3.25. Ethyl-1-hexyl-1,2-dihydro-1,8-naphthyridine-3-carboxylate (13b) from 5 and 8b
3.3.26. Ethyl-1-isobutyl-1,2-dihydro-1,8-naphthyridine-3-carboxylate (13c) from 5 and 8c
3.3.27. Ethyl-1-benzyl-1,2-dihydro-1,8-naphthyridine-3-carboxylate (13d) from 5 and 8d
3.3.28. Ethyl-1-phenethyl-1,2-dihydro-1,8-naphthyridine-3-carboxylate (13e) from 5 and 8e
3.3.29. Ethyl-1-phenyl-1,2-dihydro-1,8-naphthyridine-3-carboxylate (13f) from 5 and 8f
3.3.30. 1-Hexyl-1,2-dihydro-1,8-naphthyridine-3-carbonitrile (14b) from 6 and 8b
3.3.31. 1-Benzyl-1,2-dihydro-1,8-naphthyridine-3-carbonitrile (14d) from 6 and 8d
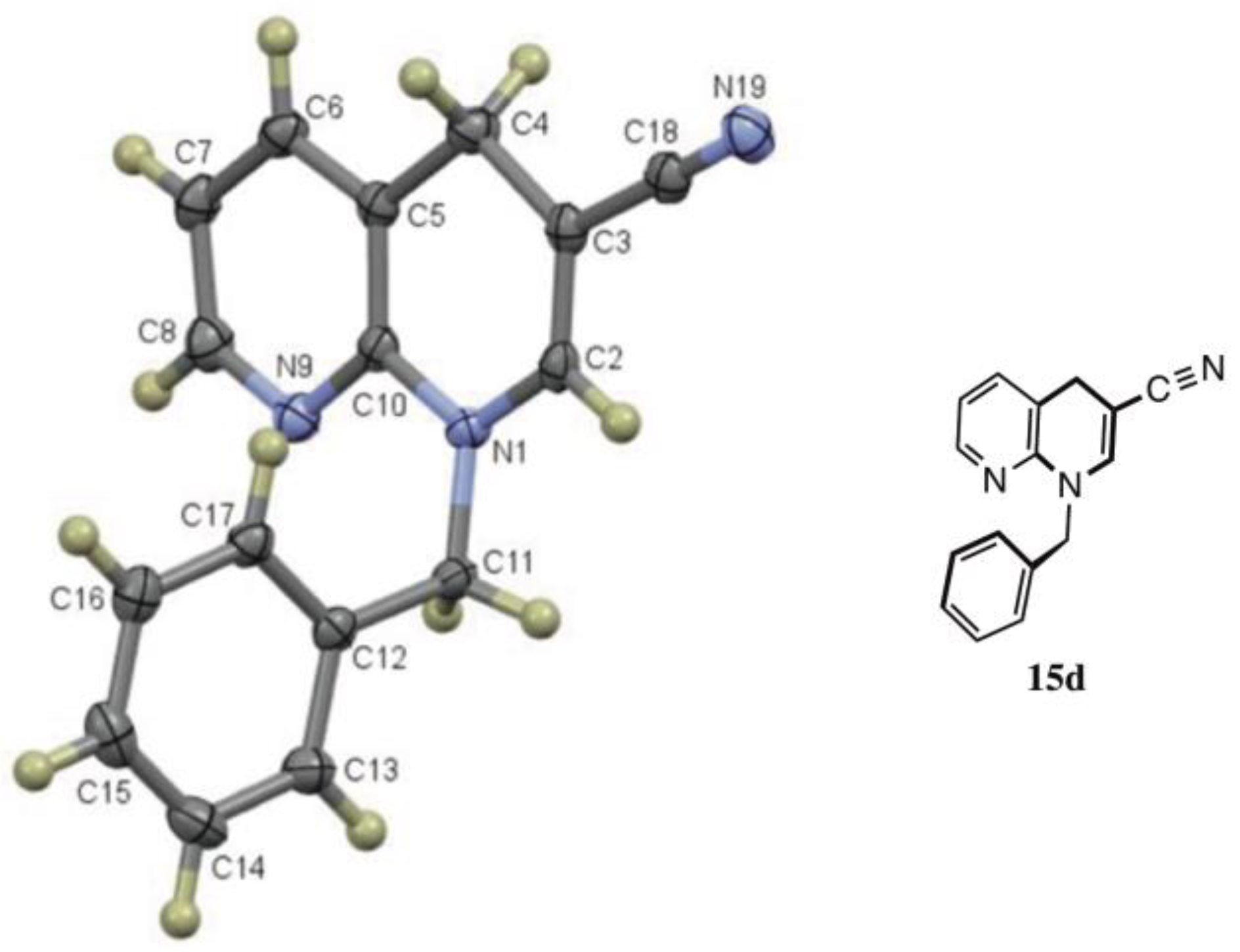
3.3.32. 1-Benzyl-1,4-dihydro-1,8-naphthyridine-3-carbonitrile (15d) from 6 and 8d
3.3.33. 1-Phenethyl-1,2-dihydro-1,8-naphthyridine-3-carbonitrile (14e) from 6 and 8e
3.3.34. 1-Phenyl-1,4-dihydro-1,8-naphthyridine-3-carbonitrile (15f) from 6 and 8f
3.4. Control Experiment Attempted Substitution of Benzylamine (8d) on 2-fluoropyridine and other SNAr Activated Rings
3.5. Control Experiment Capture of Intermediate (Z)-2-((benzylamino)methyl)-3-(2-fluoro-5-nitrophenyl)acrylonitrile (16)
3.6. Representative Procedure for Preparation of Fluorodihydroquinolines Using MBH Acetates and Primary Amines
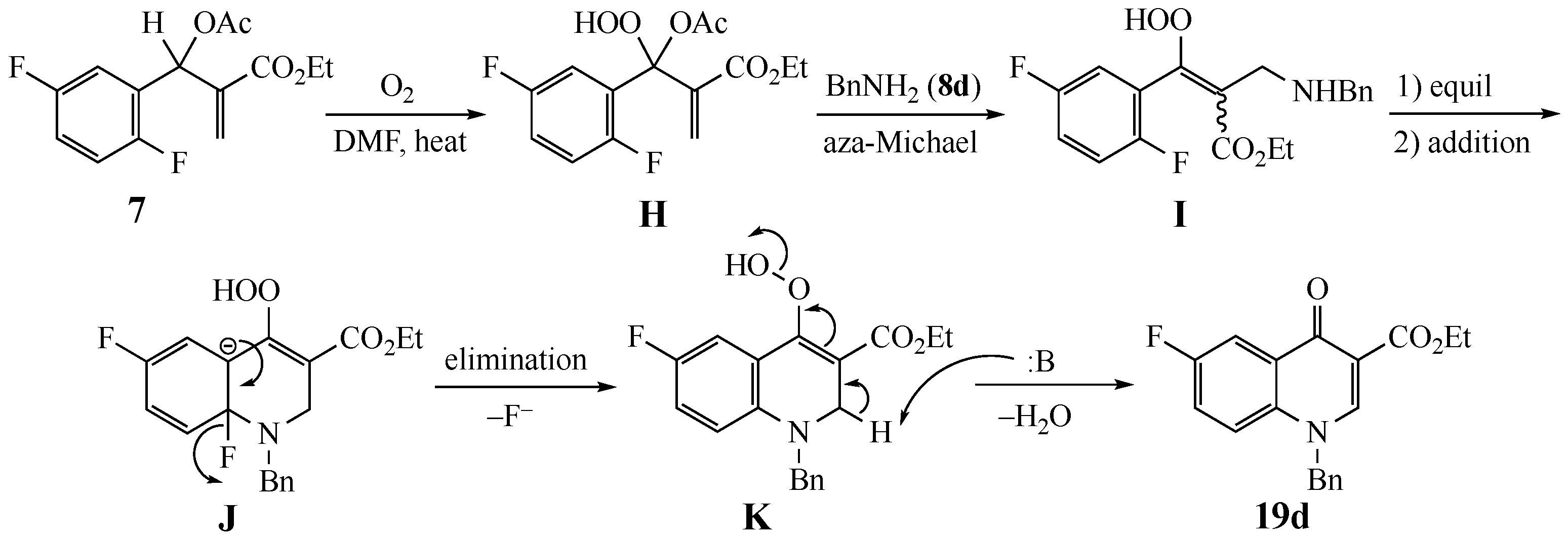
3.6.1. Ethyl-6-fluoro-1-methyl-1,2-dihydroquinoline-3-carboxylate (18a) from 7 and 8a
3.6.2. Ethyl-6-fluoro-1-hexyl-1,2-dihydroquinoline-3-carboxylate (18b) from 7 and 8b
3.6.3. Ethyl-6-fluoro-1-isobutyl-1,2-dihydroquinoline-3-carboxylate (18c) from 7 and 8c
3.6.4. Ethyl-1-benzyl-6-fluoro-1,2-dihydroquinoline-3-carboxylate (18d) from 7 and 8d
3.6.5. Ethyl-6-fluoro-1-phenethyl-1,2-dihydroquinoline-3-carboxylate (18e) from 7 and 8e
3.7. Representative Procedure for Synthesis of Fluoroquinolones
3.7.1. Ethyl-6-fluoro-1-methyl-4-oxo-1,4-dihydroquinoline-3-carboxylate (19a) from 7 and 8a
3.7.2. Ethyl-6-fluoro-1-isobutyl-4-oxo-1,4-dihydroquinoline-3-carboxylate (19c) from 7 and 8c
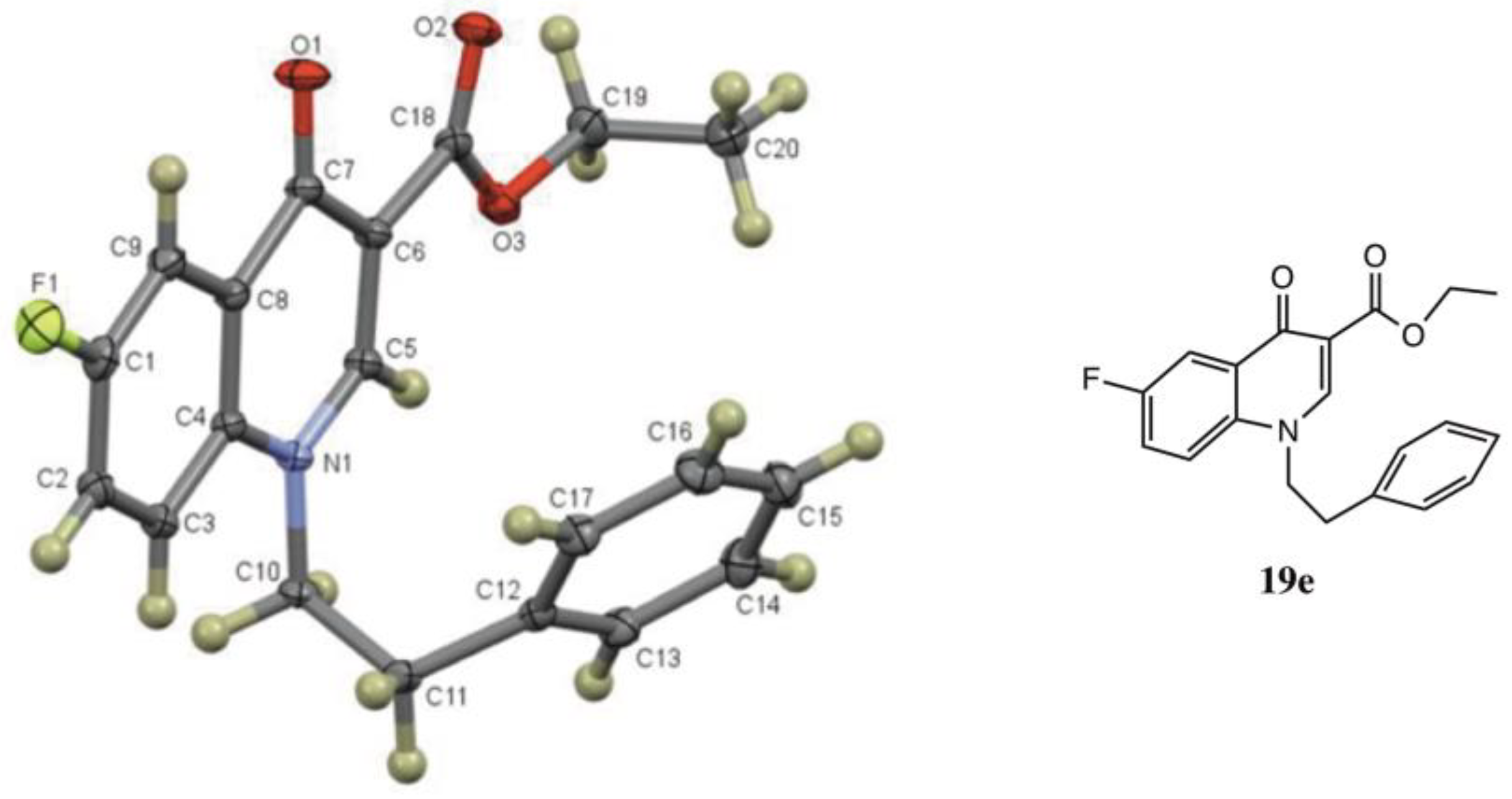
3.7.3. Ethyl-1-benzyl-6-fluoro-4-oxo-1,4-dihydroquinoline-3-carboxylate (19d) from 7 and 8d
3.7.4. Ethyl-6-fluoro-4-oxo-1-phenethyl-1,4-dihydroquinoline-3-carboxylate (19e) from 7 and 8e
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Annor-Gyamfi, J.K.; Ametsetor, E.; Meraz, K.; Bunce, R.A. Naphthalenes and quinolines by domino reactions of Morita-Baylis-Hillman acetates. Molecules 2020, 25, 5168. [Google Scholar] [CrossRef] [PubMed]
- Bunce, R.A.; Nago, T. 1-Alkyl-2,3-dihydro-4(1H)-quinolinones by a tandem Michael-SNAr annulation reaction. J. Heterocyclic Chem. 2009, 46, 623–628. [Google Scholar] [CrossRef]
- Bunce, R.A.; Squires, S.T.; Nammalwar, B. 1-Alkyl- and (±)-1,2-dialkyl-2,3-dihydro-1,8-naphthyridine-4(1H)-ones by a tandem Michael-SNAr annulation reaction. J. Org. Chem. 2013, 78, 2144–2148. [Google Scholar] [CrossRef]
- Narendar Reddy, T.; Jayathirtha Rao, V. Importance of Baylis-Hillman adducts in modern drug discovery. Tetrahedron Lett. 2018, 59, 2859–2875. [Google Scholar] [CrossRef]
- Lima-Junior, C.G.; Vasconcellos, M.A.A. Morita-Baylis-Hillman adducts: Biological activities and potentialities to the discovery of new cheaper drugs. Bioog. Med. Chem. 2012, 20, 3954–3971. [Google Scholar] [CrossRef] [PubMed]
- Fotie, J.; Kaiser, M.; Delfin, D.A.; Manley, J.; Reid, C.S.; Paris, J.-M.; Wenzler, T.; Maes, L.; Mahasenan, K.V.; Li, C.; et al. Antitrypanosomal activity of 1,2-dihydroquinolin-6-ols and their ester derivatives. J. Med. Chem. 2010, 53, 966–982. [Google Scholar] [CrossRef]
- Bhanja, C.; Jena, S.; Nayak, S.; Mohapatra, S. Organocatalytic tandem Michael addition reactions: A powerful access to the enantioselective synthesis of functionalized chromenes, thiochromenes and 1,2-dihydroquinolines. Beilstein J. Org. Chem. 2012, 8, 1668–1694. [Google Scholar] [CrossRef]
- Pucheta, A.; Mendieta, A.; Madrigal, D.A.; Hernández-Benitez, R.I.; Romero, L.; Garduño-Siciliano, L.; Rugerio-Escalona, C.; Cruz-Lopez, M.C.; Jimenez, F.; Ramirez-Villalva, A.; et al. Synthesis and biological activity of fibrate-based acyl- and alkyl- phenoxyacetic methyl esters and 1,2-dihydroquinones. Med. Chem. Res. 2020, 29, 459–478. [Google Scholar] [CrossRef]
- Matsuda, M.; Mori, T.; Kawashima, K.; Nagatsuka, M.; Kobayashi, S.; Yamamoto, M.; Kato, M.; Takai, M.; Oda, T. Preparation of Novel 1,2-dihydroquinoline Derivatives Having Glucocorticoid Receptor Binding Activity. World Patent WO 2007032556 A1, 22 March 2007. [Google Scholar]
- Buschmann, N.; Fairhurst, R.A.; Furet, P.; Knoepfel, T.; LeBlanc, C.; Mah, R.; Mallet, F.; Martz, J.; Liao, L.; Xiong, J.; et al. Preparation of N-(5-cyano-4-((2-methoxyethyl)amino)pyridin-2-yl)-7-formyl-6-((4-methyl-2-oxopiperazin-1-yl)methyl)-3,4-dihydro-1,8-naphthyridine-1(2H)-carboxamide Particles Useful in Pharmaceutical Compositions for the Treatment of Cancer. World Patent WO 2016151500 A1, 29 September 2016. [Google Scholar]
- Baruah, A.; Khanna, I.; Pillarisetti, S. Preparation and Pharmaceutical Compositions of Dihydro-Naphthyridine and Tetrahydropyrazolopyridine derivatives as Cholesteryl Ester-Transfer Protein Inhibitors. U.S. Patent 20060135551 A1, 22 June 2006. [Google Scholar]
- Zhanel, G.G.; Walkty, A.; Vercaigne, L.; Karlowsky, J.A.; Embil, J.; Gin, A.S.; Hoban, D.J. The new fluoroquinolones: A critical review. Can. J. Infect. Dis. 1999, 10, 207–238. [Google Scholar] [CrossRef]
- Pham, T.D.M.; Ziora, Z.M.; Blaskovich, M.A.T. Quinolone antibiotics. Med. Chem. Commun. 2019, 10, 1719–1739. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Pennefather, P.M.; Kaye, S.B.; Hart, C.A. Fluoroquinolones: Place in Ocular therapy. Drugs 2001, 61, 747–761. [Google Scholar] [CrossRef]
- Falagas, M.E.; Matthaiou, D.K.; Bliziotis, I.A. Systematic review: Fluoroquinolones for the treatment of intra-abdominal surgical infections. Aliment. Pharmacol. Ther. 2007, 25, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.; Smyth, A. Fluoroquinolones in the treatment of bronchopulmonary disease in cystic fibrosis. Ther. Adv. Respir. Dis. 2012, 6, 363–373. [Google Scholar] [CrossRef]
- Raz-Pasteur, A.; Shasha, D.; Paul, M. Fluoroquinolones or macrolides alone versus combined with β-lactams for adults with community-acquired pneumonia: Systematic review and meta-analysis. Int. J. Antimicrob. Agt. 2015, 46, 242–248. [Google Scholar] [CrossRef] [PubMed]
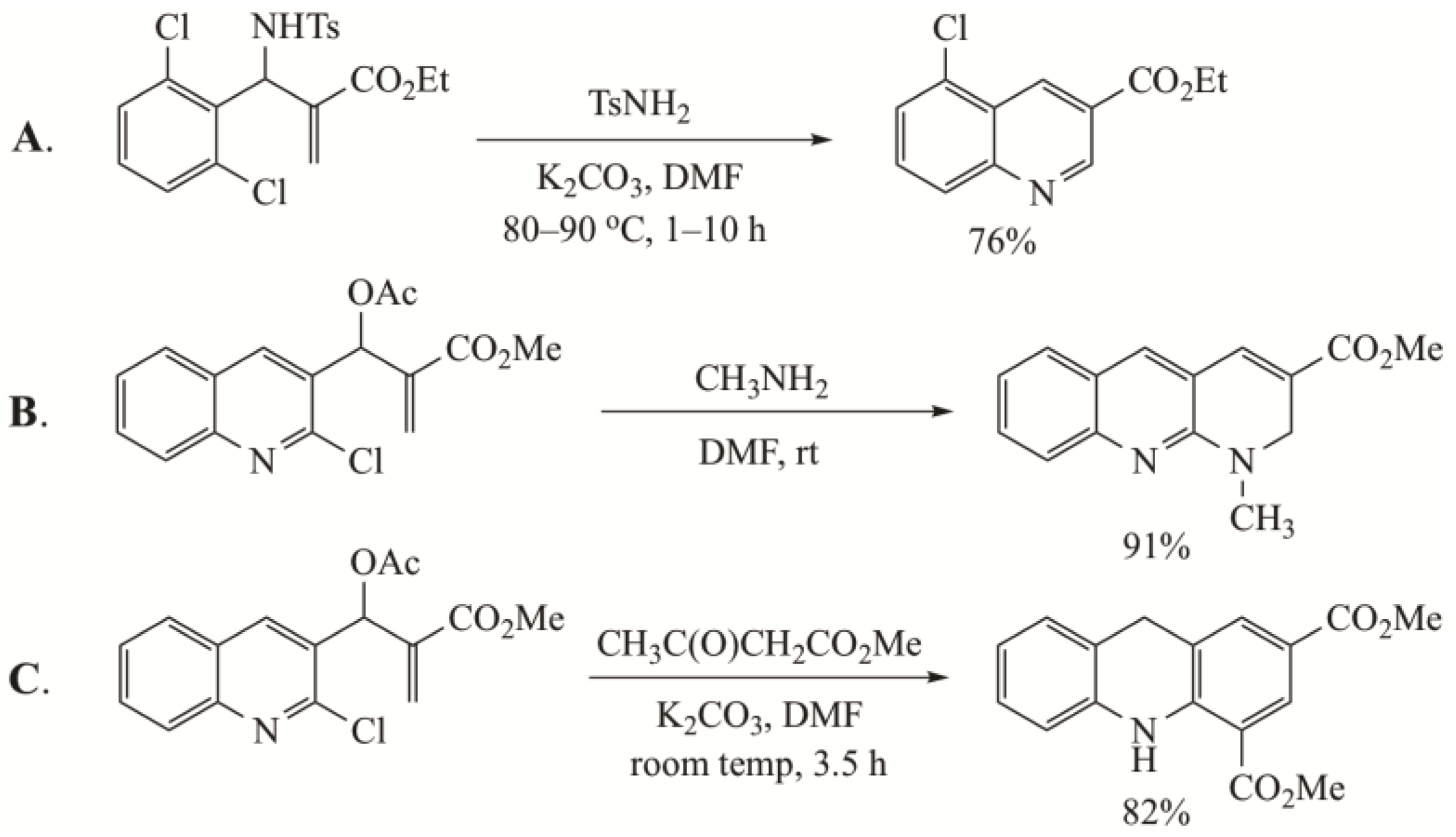
- Kim, J.N.; Lee, H.J.; Lee, K.Y.; Kim, H.S. Synthesis of 3-quinolinecarboxylic acid esters from the Baylis-Hillman adducts of 2-halobenzaldehyde N-tosylimines. Tetrahedron Lett. 2001, 42, 3737–3740. [Google Scholar] [CrossRef]
- Singh, B.; Chandra, A.; Singh, R.H. Base-free amination of BH acetates of 2-chloroquinolinyl-3-carboxaldehydes: A facile route to the synthesis of N-substituted-1,2-dihydrobenzo[b][1.8]naphthyridines. Tetrahedron 2011, 67, 2441–2446. [Google Scholar] [CrossRef]
- Gupta, T.; Bharadwaj, K.C.; Singh, R.M. Cascade SN2’-SNAr, Elimination and 1,5-hydride shift reactions by acetylacetone/acetoacetic esters: Synthesis of 9,10-dihydroacridines. Eur. J. Org. Chem. 2016, 4981–4984. [Google Scholar] [CrossRef]
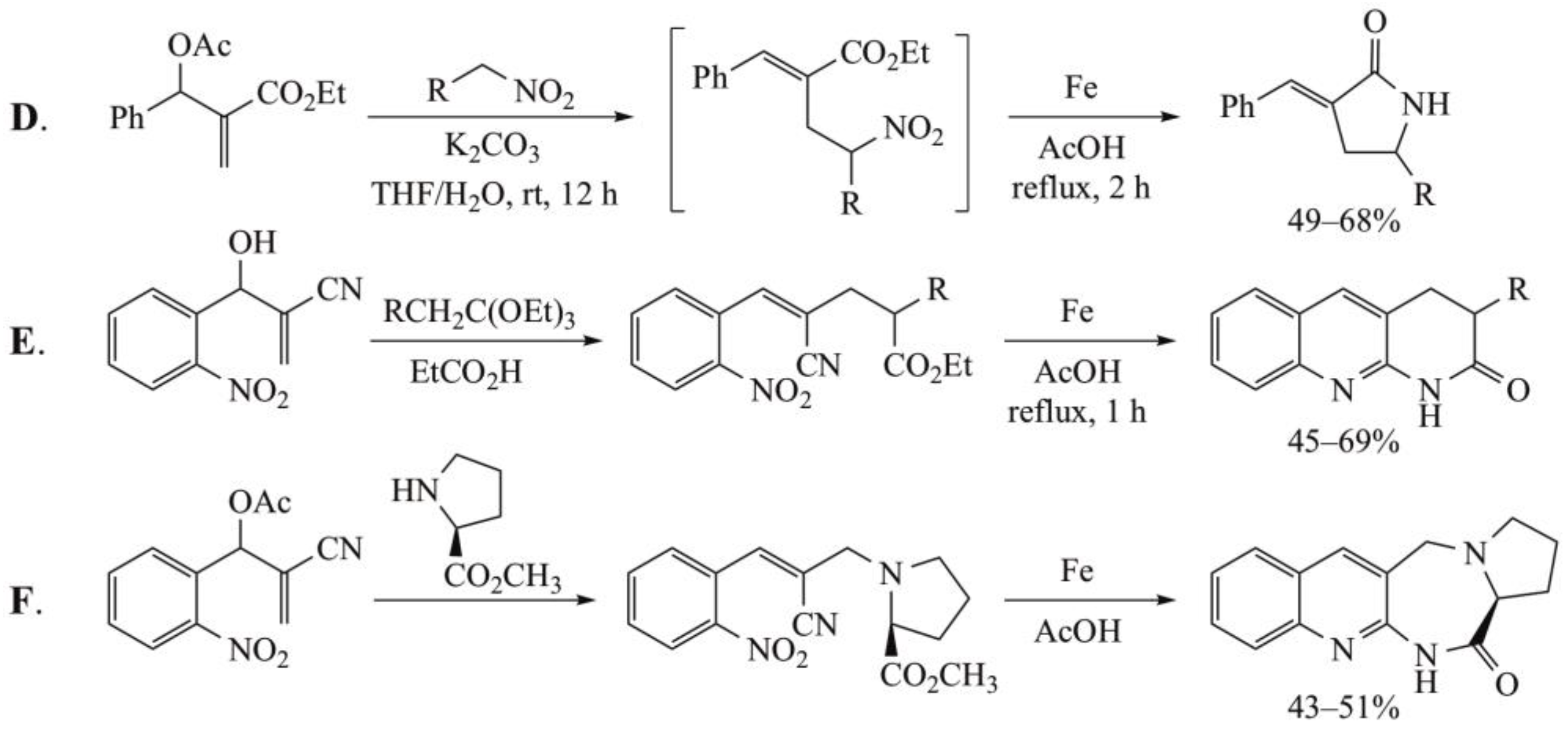
- Basavaiah, D.; Rao, J.S. Applications of Baylis-Hillman acetates: One pot, facile and convenient synthesis of substituted γ-lactams. Tetrahedron Lett. 2004, 45, 1621–1625. [Google Scholar] [CrossRef]
- Basavaiah, D.; Reddy, K.R. Simple one-pot synthesis of tri and tetracyclic frameworks containing [1,8]-naphthyridin-2-one moiety from the Baylis-Hillman adducts. Tetrahedron 2010, 66, 1215–1219. [Google Scholar] [CrossRef]
- Singh, V.; Hutait, S.; Batra, S. Reductive cyclization-mediated synthesis of fused polycyclic quinolines from Baylis-Hillman adducts of acrylonitrile: Scope and limitations. Eur. J. Org. Chem. 2009, 3454–3466. [Google Scholar] [CrossRef]
- Procopiou, P.A.; Baugh, S.P.D.; Flack, S.S.; Inglis, G.G.A. An extremely powerful acylation reaction of alcohols with acid anhydrides catalyzed by trimethylsilyl trifluoromethanesulfonate. J. Org. Chem. 1998, 63, 2342–2347. [Google Scholar] [CrossRef]
- Genest, A.; Portinha, D.; Fleury, E.; Ganachaud, F. The aza-Michael reaction as an alternative strategy to generate advanced silicon-based (macro)molecules and materials. Prog. Polym. Sci. 2017, 72, 61–110. [Google Scholar] [CrossRef]
- Buchholz, R.; Hoffmann, H.M.R. α-Methylidene and α-Alkylidene-β-lactams from nonproteinogenic amino acids. Helv. Chim. Acta 1991, 74, 1213–1220. [Google Scholar] [CrossRef]
- Stohrer, W.-D. On the stereochemistry of the SN2’ reaction. Angew. Chem. Int. Ed. 1983, 22, 613–614. [Google Scholar] [CrossRef]
- Bergman, E.D.; Ginsburg, D.; Pappo, R. The Michael Reaction. Org. React. 1959, 10, 179–555. [Google Scholar] [CrossRef]
- Werner, R.M.; Williams, L.M.; Davis, J.T. The C-glycosyl analog of an N-linked glycoamino acid. Tetrahedron Lett. 1998, 39, 9135–9138. [Google Scholar] [CrossRef]
- Shao, H.; Ekthawatchai, S.; Chen, C.-S.; Wu, S.-H.; Zou, W. 1,2-Migration of 2’-oxoalkyl group and concomitant synthesis of 2-C-branched O-, S-glycosides and glycosyl azides via 1,2-cyclopropanated sugars. J. Org. Chem. 2005, 70, 4726–4734. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.P.; Bleasdale, C.; Delaney, K.; Lindstrom, A.B.; Rappaport, S.M.; Waidyanatha, S.; Watson, W.P.; Golding, B.T. Evidence for the formation of Michael adducts from reactions of [E,E]-muconaldehyde with glutathione and other thiols. Bioorg. Chem. 2005, 33, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Hintermann, L.; Dittmer, C. Asymmetric ion-pairing catalysis of the reversible cyclization of 2’- hydroxychalcone to flavanone: Asymmetric catalysis of an equilibrating reaction. Eur. J. Org. Chem. 2012, 5573–5584. [Google Scholar] [CrossRef]
- Bach, R.D.; Wolber, G.J. Stereochemistry of the concerted SN2’ reaction of 3-chloropropene: A theoretical study. J. Am. Chem. Soc. 1985, 107, 1352–1357. [Google Scholar] [CrossRef]
- Achord, J.M.; Hussey, C.L. Determination of dissolved oxygen in nonaqueous electrochemical solvents. Anal. Chem. 1980, 52, 601–602. [Google Scholar] [CrossRef]
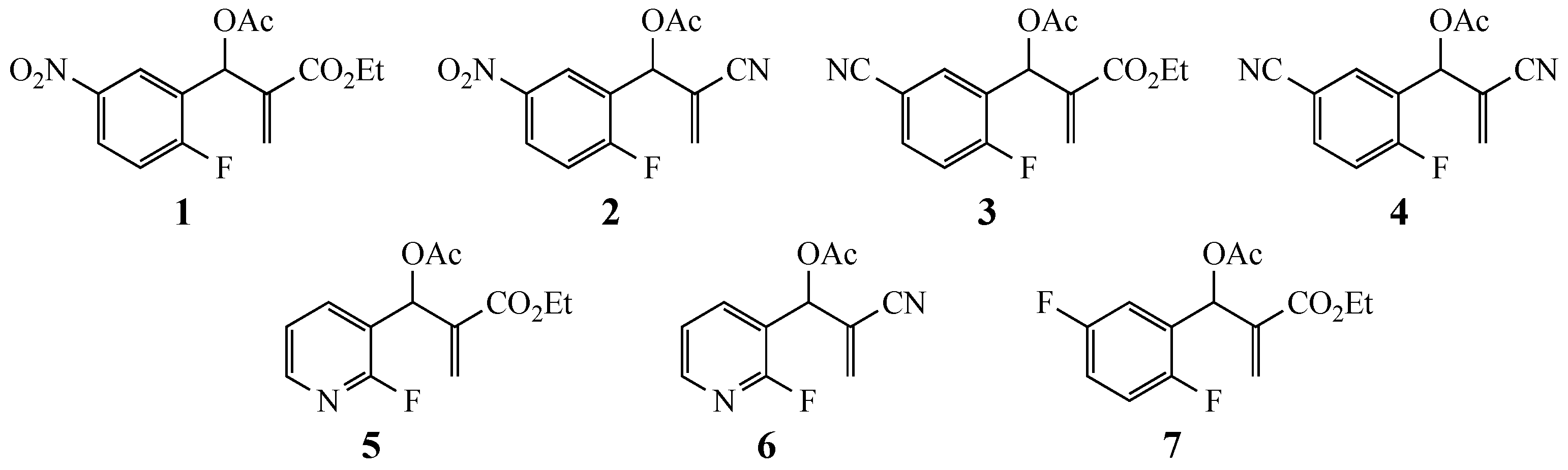
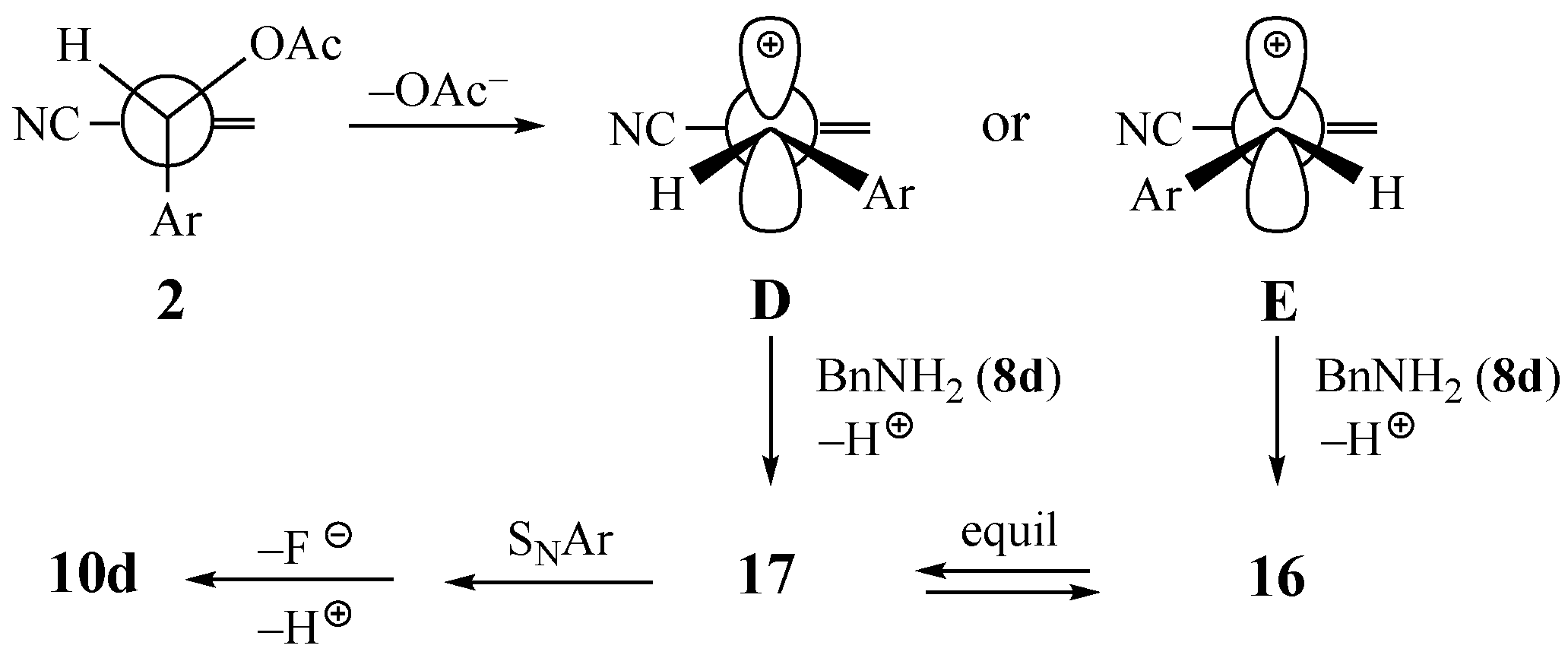

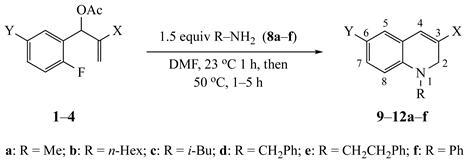
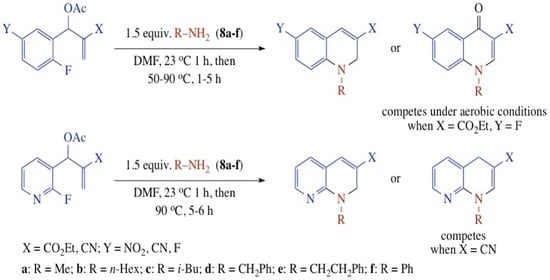
| Substrate | Amine | R a | X | Y | Product (%) |
|---|---|---|---|---|---|
 | 8a | CH3 | CO2Et | NO2 | 9a (93) |
| 8b | n-Hex | CO2Et | NO2 | 9b (87) | |
| 8c | i-Bu | CO2Et | NO2 | 9c (93) | |
| 8d | CH2Ph | CO2Et | NO2 | 9d (92) | |
| 8e | CH2CH2Ph | CO2Et | NO2 | 9e (83) | |
| 8f | Ph | CO2Et | NO2 | 9f (89) | |
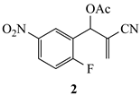 | 8a | CH3 | CN | NO2 | 10a (88) |
| 8b | n-Hex | CN | NO2 | 10b (78) | |
| 8c | i-Bu | CN | NO2 | 10c (79) | |
| 8d | CH2Ph | CN | NO2 | 10d (80) | |
| 8e | CH2CH2Ph | CN | NO2 | 10e (82) | |
| 8f | Ph | CN | NO2 | 10f (81) | |
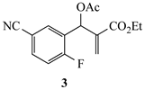 | 8a | CH3 | CO2Et | CN | 11a (91) |
| 8b | n-Hex | CO2Et | CN | 11b (82) | |
| 8c | i-Bu | CO2Et | CN | 11c (85) | |
| 8d | CH2Ph | CO2Et | CN | 11d (79) | |
| 8e | CH2CH2Ph | CO2Et | CN | 11e (85) | |
| 8f | Ph | CO2Et | CN | 11f (83) | |
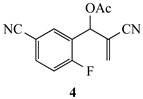 | 8a | CH3 | CN | CN | 12a (82) |
| 8b | n-Hex | CN | CN | 12b (77) | |
| 8c | i-Bu | CN | CN | 12c (81) | |
| 8d | CH2Ph | CN | CN | 12d (86) | |
| 8e | CH2CH2Ph | CN | CN | 12e (88) |
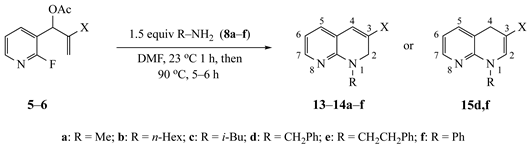
| Substrate | Amine | R a | X | Product (%) |
|---|---|---|---|---|
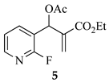 | 8a | CH3 | CO2Et | 13a (88) |
| 8b | n-Hex | CO2Et | 13b (80) | |
| 8c | i-Bu | CO2Et | 13c (80) | |
| 8d | CH2Ph | CO2Et | 13d (81) | |
| 8e | CH2CH2Ph | CO2Et | 13e (83) | |
| 8f | Ph | CO2Et | 13f (77) | |
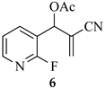 | 8b | n-Hex | CN | 14b (82) |
| 8d | CH2Ph | CN | 14d (6) + 15d (82) | |
| 8e | CH2CH2Ph | CN | 14e (80) | |
| 8f | Ph | CN | 15f (72) b |
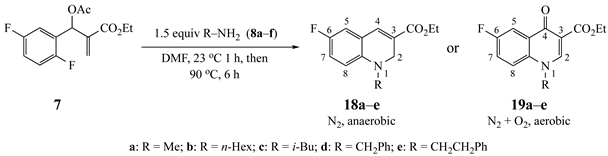
| Substrate | Amine | R a | Product (%) |
|---|---|---|---|
 | 8a | CH3 | 18a (91) |
| 8b | n-Hex | 18b (80) | |
| 8c | i-Bu | 18c (88) | |
| 8d | CH2Ph | 18d (84) | |
| 8e | CH2CH2Ph | 18e (89) | |
| 8a | CH3 | 19a (85) | |
| 8b | n-Hex | b | |
| 8c | i-Bu | 19c (86) | |
| 8d | CH2Ph | 19d (83) | |
| 8e | CH2CH2Ph | 19e (84) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annor-Gyamfi, J.K.; Ametsetor, E.; Meraz, K.; Bunce, R.A. Dihydroquinolines, Dihydronaphthyridines and Quinolones by Domino Reactions of Morita-Baylis-Hillman Acetates. Molecules 2021, 26, 890. https://doi.org/10.3390/molecules26040890
Annor-Gyamfi JK, Ametsetor E, Meraz K, Bunce RA. Dihydroquinolines, Dihydronaphthyridines and Quinolones by Domino Reactions of Morita-Baylis-Hillman Acetates. Molecules. 2021; 26(4):890. https://doi.org/10.3390/molecules26040890
Chicago/Turabian StyleAnnor-Gyamfi, Joel K., Ebenezer Ametsetor, Kevin Meraz, and Richard A. Bunce. 2021. "Dihydroquinolines, Dihydronaphthyridines and Quinolones by Domino Reactions of Morita-Baylis-Hillman Acetates" Molecules 26, no. 4: 890. https://doi.org/10.3390/molecules26040890
APA StyleAnnor-Gyamfi, J. K., Ametsetor, E., Meraz, K., & Bunce, R. A. (2021). Dihydroquinolines, Dihydronaphthyridines and Quinolones by Domino Reactions of Morita-Baylis-Hillman Acetates. Molecules, 26(4), 890. https://doi.org/10.3390/molecules26040890