Abstract
Efficient synthesis of phenanthridin-6(5H)-one derivatives 12a–n in a four-component reaction of aldehyde hydrazone, aromatic aldehydes and malononitrile in Q-Tubes is reported. The results showed that the methodology has the advantage of being a one-pot synthesis of tricyclic systems in good yields. Potential routes leading to formation of compounds 12 are discussed. The structures of the synthesized compounds could be unequivocally established via X-ray crystal structure determination and spectroscopic methods.
1. Introduction
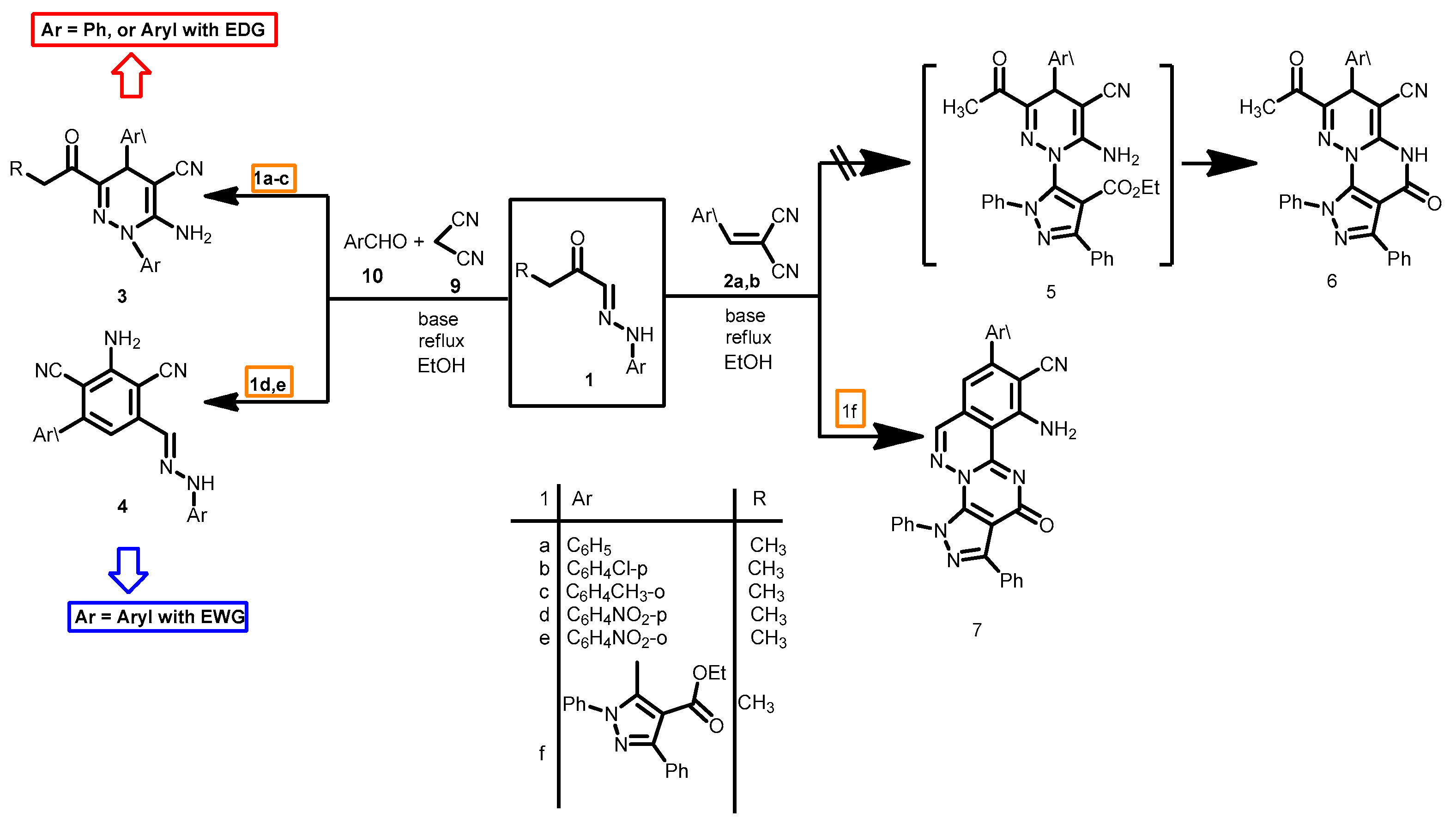
The considerable biological and medicinal activities of pyridazines has stimulated considerable research on efficient syntheses of these derivatives in past years [1,2,3,4]. Elnagdi et al. reported synthesis of 2-amino-1,4-dihydropyridazine, an isoelectronic derivative of 1,4-dihydropyrimidines of established biological activities [5,6,7,8], via 3 + 3 atom combination of arylhydrazones 1a and α,β-unsaturated nitriles 2 [9] or by reacting a mixture of 1, 4 and 5 in one pot (Scheme 1). Subsequent studies [10,11] on this novel route revealed however that it is of a limited scope as the reaction products proved to be dependent on the nature of the reacting aryl hydrazones. Multicomponent reaction of 1a–c with α,β-unsaturated nitriles 2 in presence of a base has been reported to yield 3, while the reaction of 1f with 2 in basic medium afforded the new substituted pyrazolo[4′,3′-5,6]pyrimido[2,1-a]phthalazine-9-carbonitriles ring system 7 [12] (Scheme 1).
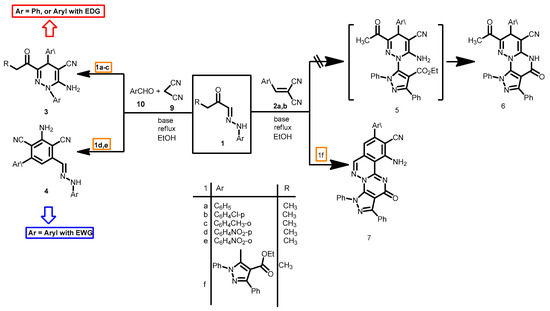
Scheme 1.
The reactivity of aryl hydrazones 1 towards α,β-functionally substituted cinnamonitriles.
Microwave energy has been reported to be effective in the synthesis of small molecules in many of our previous works [13,14,15,16]. However, we noted that microwaves technology is expensive to scale up [17], in contrast to using “Q-Tube” pressure reactors, which proved to accelerate reactions of negative activation volume in a more optimal and safer manner, compared to microwaves [18].
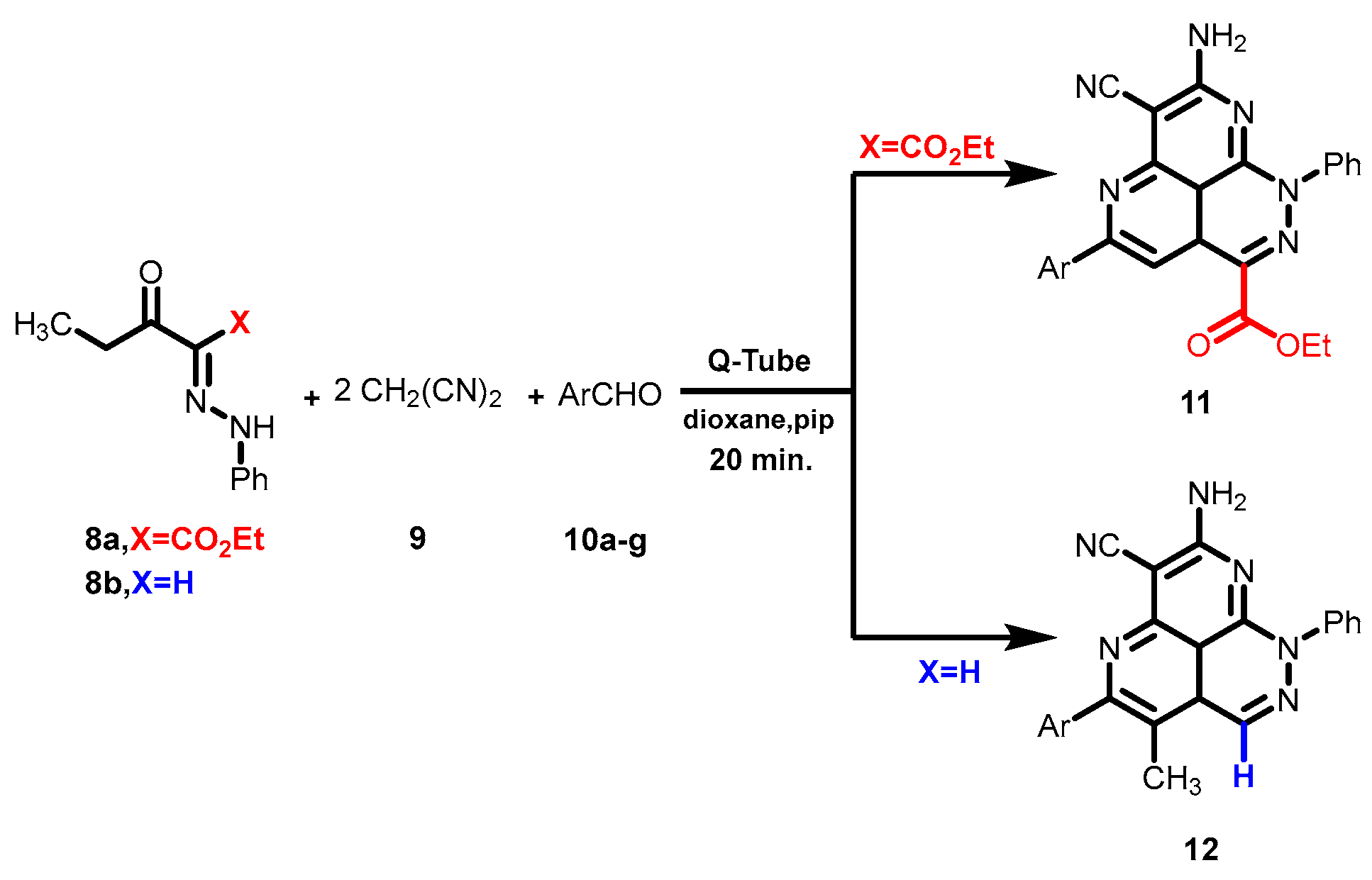
Our research group has previously reported extensively on the use of Q-Tubes to synthesize such compounds but the reaction conditions in these published works had many limitations that altered the nature of the synthesized products [19,20] (Scheme 2). The human urge to find cures for challenging diseases and improve human life leads organic chemists to be in a continuous quest to develop novel polyfunctional heterocycles, as well as developing new economical and greener technologies. Considering the promising biological activity of new compounds 12 where the ring system combines pyridazine and napthyridine rings, both with vast biological activities [21,22,23,24], we sought to expand this work to prove that the method proposed for the synthesis of these novel compounds is a general one by making more examples. Moreover this work has led to the proposal of a plausible reaction mechanism that would clarify and lead the way for any further work on such new ring systems.
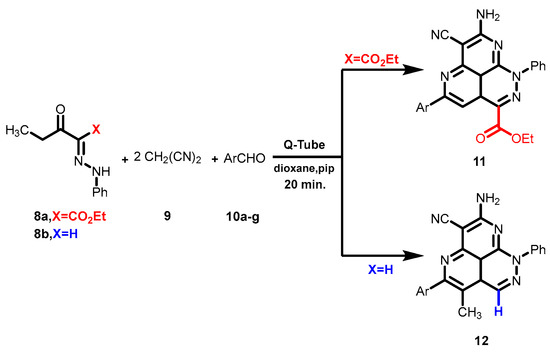
Scheme 2.
Novel synthesis of the tricyclic system 11 by reacting ethyl-3-oxo-2-(2-phenylhydrazono) pentanoate (8a) with malononitrile (9) and aromatic aldehyde derivatives 10 in a Q-Tube.
2. Results
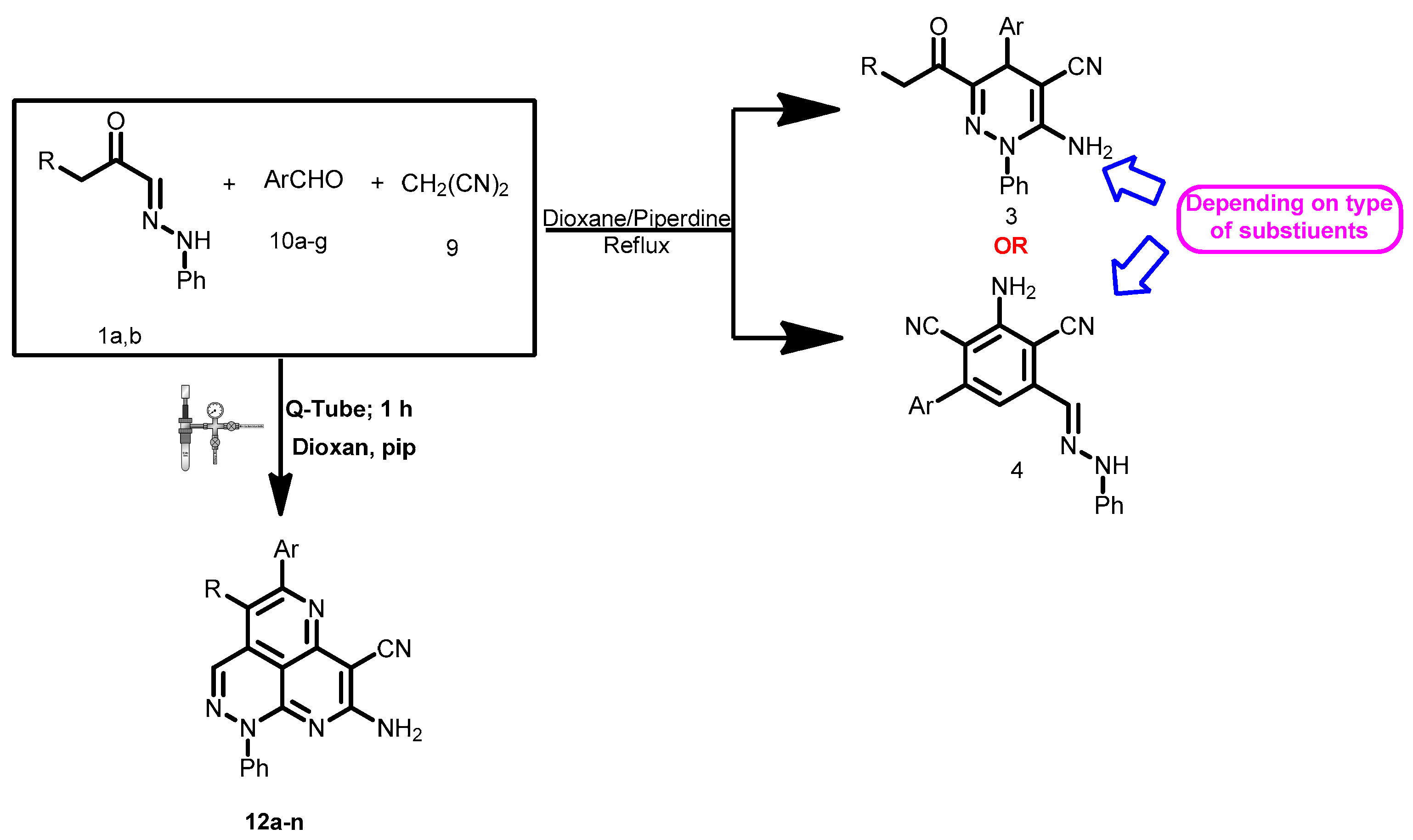
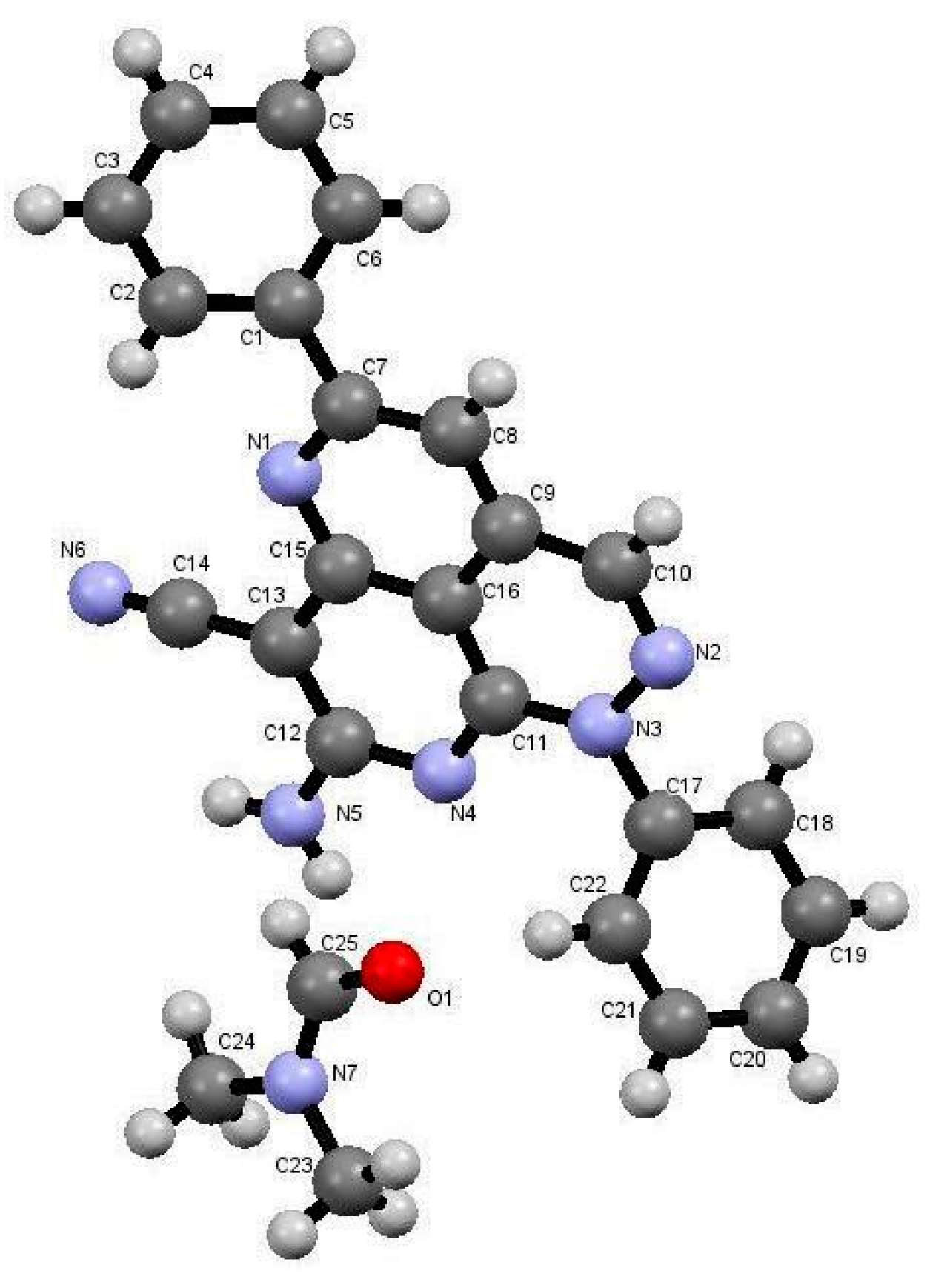
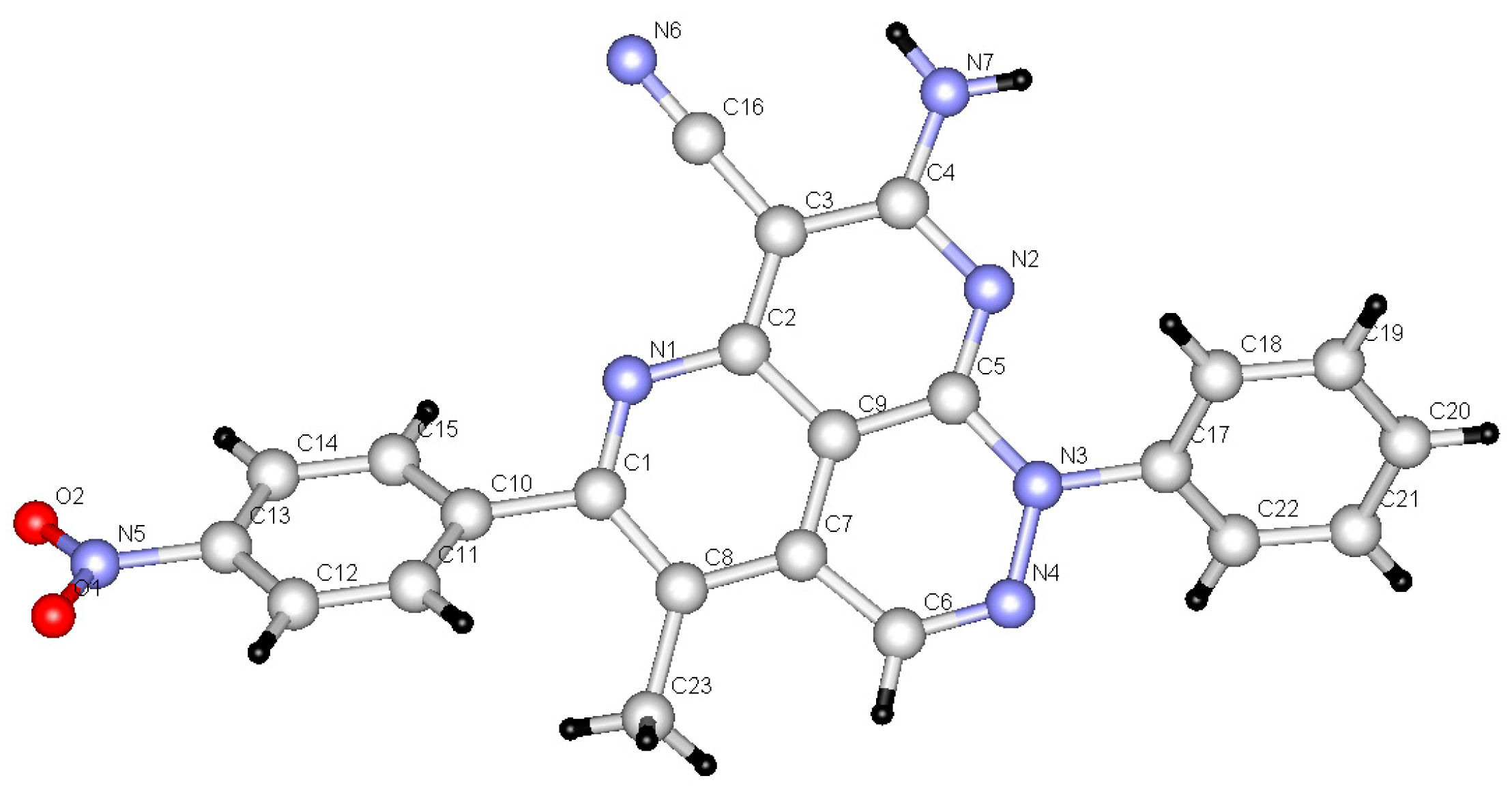
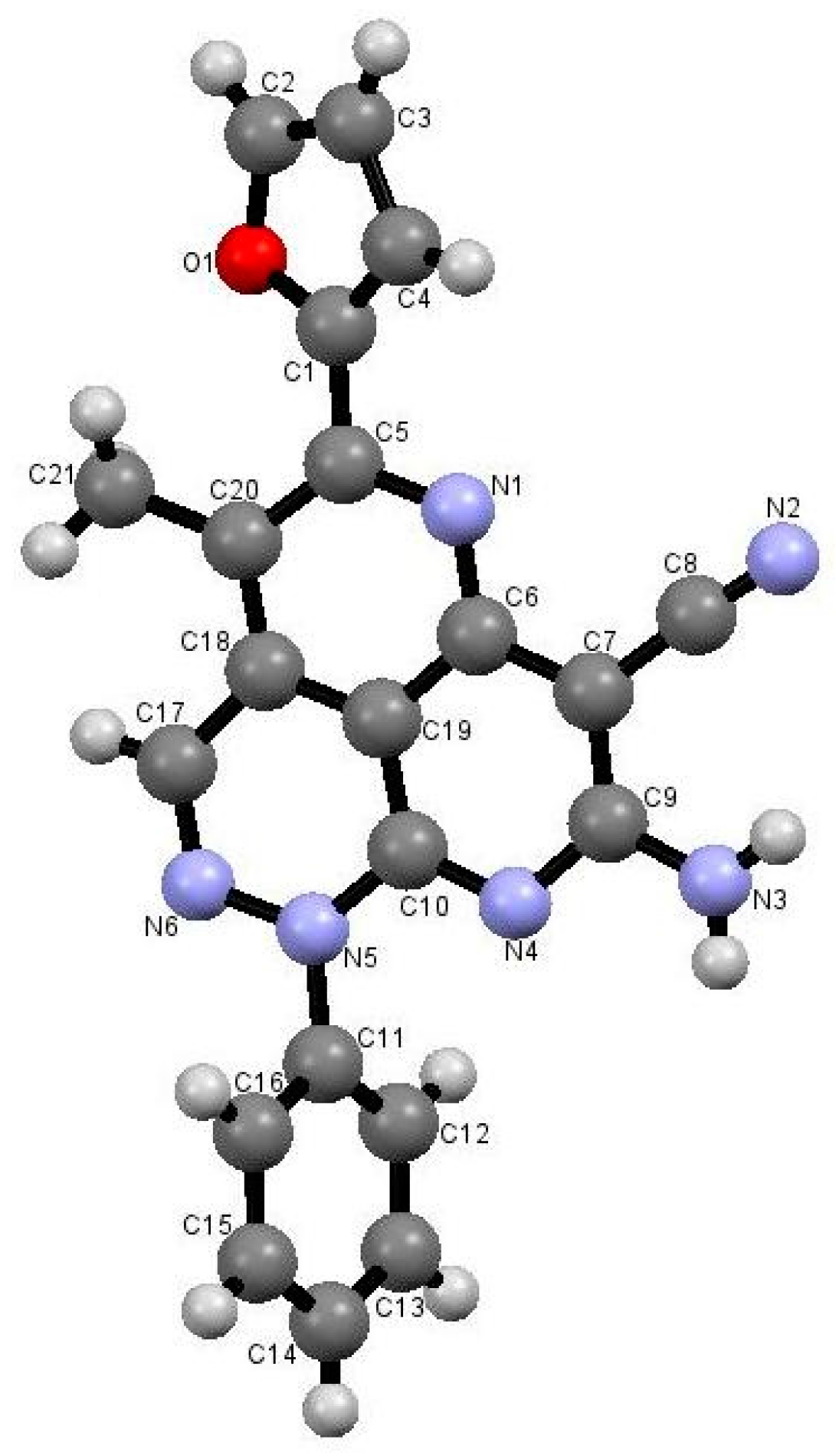
The reactions in Q-Tubes (cf. Figure 1) at 150 °C and 20 psi of 2-oxo-2-arylhydrazonals 1a,b with aromatic aldehydes 10a–g and malononitrile (9) in dioxane in the presence of piperidine afforded compounds 12a–n. The tricyclic systems 12 are formed in 72–85% yield (Scheme 3, Table 1). The structure of the reaction products could be established to be pyridazino[5,4,3-de][1,6]naphthyridine derivatives 12a–n via spectroscopic methods (available in the supplementary materials) as well as X-ray crystal structure determination of products 12a, 12m and 12n (Figure 1, Figure 2 and Figure 3).
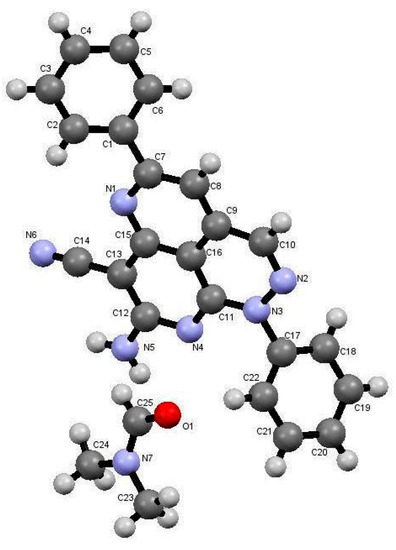
Figure 1.
X-ray crystallographic structure of compound 12a [25].
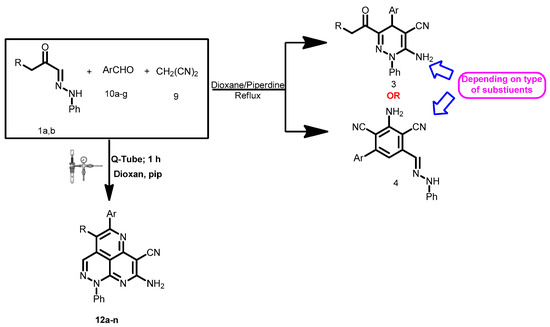
Scheme 3.
A new reaction path for the reaction of aldehyde hydrazones with malononitrile and aldehydes.

Table 1.
Synthesis of pyridazino[5,4,3-de][1,6]naphthyridine derivatives 12a–n.
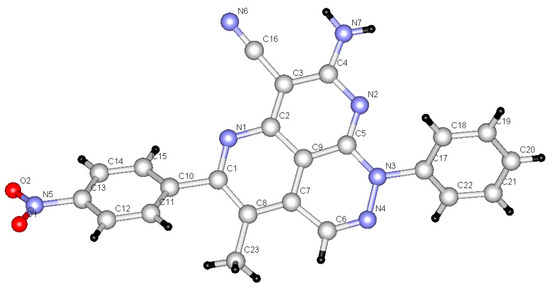
Figure 2.
X-ray crystallographic structure of compound 12m [26].
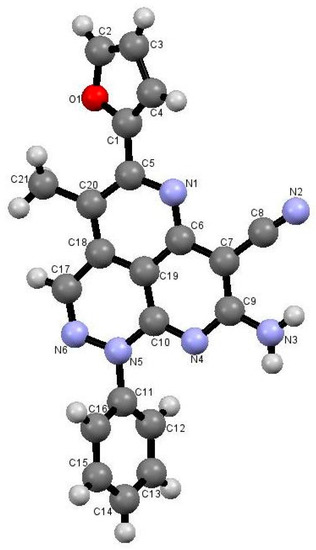
Figure 3.
X-ray crystallographic structure of compound 12n [27].
3. Discussion
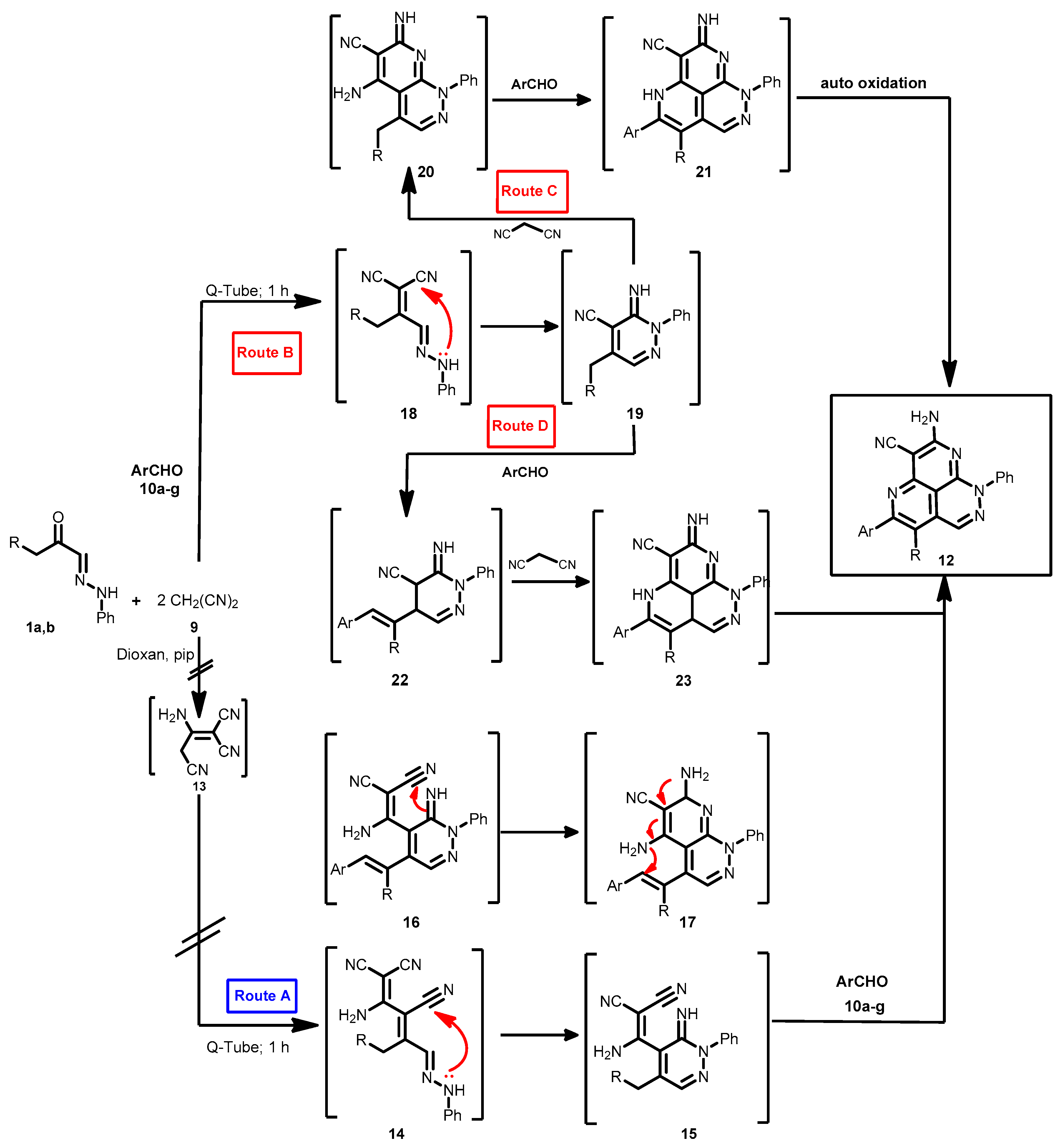
Two mechanistic pathways seem possible (Scheme 4): initial dimerization of malononitrile to yield dimer 13, that then condenses with the acyl carbonyl yielding 14 that cyclizes to form 15 (route A) [19]. This route could readily be eliminated as in our hands malononitrile could not be dimerized under the reported conditions moreover when it is considered that this dimerization it probably impossible in the absence of ethoxide or sodium hydroxide which are thought necessary for the dimerization of malononitrile [27].
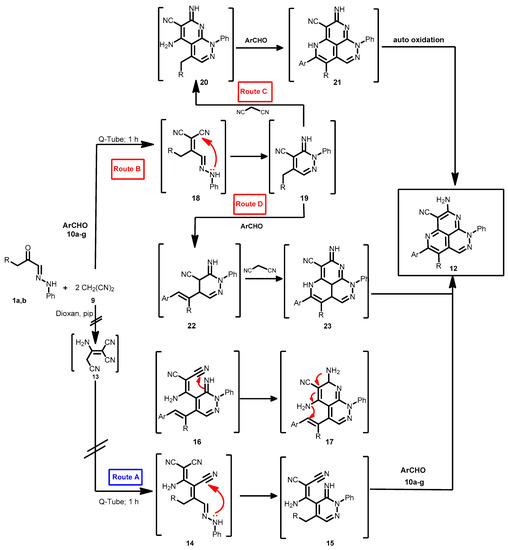
Scheme 4.
The proposed mechanism for the formation of pyridazino[5,4,3-de][1,6]naphthyridine derivatives 12a–n.
Moustafa et al. also reported that the dimer 13 alone reacts with their arylhydrazones 1a,b yielding pyridazino[5,4,3-de][1,6]naphthyridine derivatives, not condensed with pyridazine derivatives. Thus, it is almost certain that the initial step leading to formation of 12 is the condensation of malononitrile (9) with acyl carbonyl 18. The product 19 can then either cyclize into 20 and then 21 (route C) or condense with an aromatic aldehyde to give 22 and then 23 (route D). Neither route C or D can completely be ruled out, although we believe that the aromatic aldehyde condenses initially with 19 then subsequent reactions lead to 22 that then reacts with malononitrile (9) to form 23 which cyclizes to the final product 12 (Scheme 4).
4. Materials and Methods
4.1. General Information
Q-tube assisted reactions were performed in a Q-tube safe pressure reactor from Q Labtech (East Lyme, CT 06333, CT, USA, equipped with a cap/sleeve, pressure adapter (120 psi), needle adapter/needle, borosilicate glass tube, Teflon septum, and catch bottle. All reactions were monitored by using TLC with 1:1 ethyl acetate-petroleum ether as eluent and were carried out until starting materials were completely consumed. Melting points are reported uncorrected and were determined with a Sanyo (Gallenkamp, Osaka, Japan) instrument. Infrared spectra were recorded using KBr pellets and a FT–IR 6300 instrument (Jasco, Tokyo, Japan) and absorption bands are reported in cm−1. 1H- and 13C-NMR spectra were determined by using a DPX instrument (Bruker, Billerica, MA, USA) at 400 MHz or 600 MHz for 1H-NMR and 100 MHz for 13C-NMR and either CDCl3 or DMSO-d6 solutions with TMS as internal standards. Chemical shifts are reported in ppm. Mass spectra and accurate mass measurements were made using a GCMS DFS spectrometer (Thermo, Bremen, Germany) with the EI (70 EV) mode. X-ray crystallographic structure determinations were performed by using Rapid II (Rigaku, Tokyo, Japan) and X8 Prospector (Bruker, Karlsruhe, Germany) single crystal X-ray diffractometers. All X-ray crystal structure data can be obtained free of charge from the Cambridge Crystallographic Data Centre [26,27,28] via www.ccdc.cam.ac.uk. The data and material are available in the Supplementary material and manuscript. Supplementary material is attached as PDF format and submitted along with the manuscript.
4.2. General Procedures for Q-Tube-Assisted Synthesis of 12a–n
2-Oxo-2-arylhydrazonals 1a,b (0.01 mol), aromatic aldehydes 13a–g (0.01 mol) and malononitrile (9) was 9 before (0.02 mol) in the presence of piperidine (1 mL) and dioxin (20 mL) as solvent were sequentially added in a 35 mL Q-tube pressure tube, furnished by Q Labtech. A Teflon septum was placed on top of the tube, and an appropriate cap was used. The mixture was heated in an oil bath at 150 °C. After about 60 min, the reaction mixture was monitored by TLC. The mixture was cooled and poured into ice-water. The solid was collected by filtration and purified by column chromatography and crystallized from ethanol.
8-Amino-1,5-diphenyl-1H-pyridazino[5,4,3-de][1,6]naphthyridine-7-carbonitrile (12a). Dark yellow crystals, Yield 85%; m.p. 314–315 °C; Anal. Calcd. for C22H14N6 (362.13): C, 72.92; H, 3.89; N, 23.19. Found: C, 72.83; H, 3.79; N, 23.25. EI-HRMS: m/z = 362.1274 (MH+); C22H14N6 requires: m/z = 362.1279 (MH+); 1H-NMR (400 MHz, DMSO-d6): δ = 7.06 (br, 2H, NH2, D2O exchangeable), 7.44–7.66 (m, H, Ph-H, CH), 8.19–8.22 (m, 2H, Ph-H), 8.38 (s, 1H, CH); 13C-NMR (100 MHz, DMSO-d6): δ = 162.0, 161.2, 154.5, 150.9, 141.7, 138.7, 137.8, 133.2, 130.3, 128.8 (2C), 128.7 (2C), 128.1, 127.1 (2C), 126.3 (2C), 116.8, 108.8, 104.6, 73.3. MS: m/z (%) 362.2 (M+, 100), 334 (10), 181 (10), 77 (5).
8-Amino-5-(4-chlorophenyl)-1-phenyl-1H-pyridazino[5,4,3-de][1,6]naphthyridine-7-carbonitrile (12b). Green crystals, yield 75%; m.p. 340–341 °C; Anal. Calcd. for C22H13ClN6 (396.06): C, 66.59; H, 3.30; N, 21.18. Found: C, 66.70; H, 3.35; N, 21.20. EI-HRMS: m/z = 396.0885 (MH+); C22H13N635Cl requires: m/z 396.0890 (MH+); 1H-NMR (400 MHz, DMSO-d6): δ = 7.10 (br, 2H, NH2, D2O exchangeable), 7.45–7.66 (m, 8H, Ph-H, CH), 8.22–8.24 (m, 2H, Ph-CH), 8.38 (s, 1H, CH); 13C-NMR (100 MHz, DMSO-d6): δ = 162.1, 160.0, 154.6, 151.0, 141.7, 138.7, 136.7, 133.4, 130.3, 129.1 (2C), 128.9 (2C), 128.2, 126.6 (2C), 124.6 (2C), 118.0, 109.1, 104.6, 56.0. MS: m/z (%) 396.1 (M+, 100), 368 (10), 198 (10), 166 (5), 77 (5).
8-Amino-5-(3-chlorophenyl)-1-phenyl-1H-pyridazino[5,4,3-de][1,6]naphthyridine-7-carbonitrile (12c). Green crystals, yield 80%; m.p. 314–316 °C; Anal. Calcd. for C22H13ClN6 (396.06): C, 66.59; H, 3.30; N, 21.18. Found: C, 66.57; H, 3.33; N, 21.12. EI-HRMS: m/z = 396.0885 (MH+); C22H13N635Cl requires: m/z = 396.0890 (MH+); 1H-NMR (400 MHz, DMSO-d6): δ = 7.11 (br, 2H, NH2, D2O exchangeable), 7.27 (s, 1H, CH), 7.45–7.67 (m, 9H, Ph-H), 8.45 (s, 1H, CH); 13C-NMR (100 MHz, DMSO-d6): δ = 162.2, 162.0, 154.6, 151.1, 141.7, 138.8, 138.5, 132.4, 131.4, 131.0, 130.6, 129.9 (2C), 128.8, 128.2, 127.4, 126.4 (2C), 116.8, 108.9, 108.8, 73.1. MS: m/z (%) 396.1 (M+, 100), 361 (15), 334 (5), 198 (10), 166 (5), 77 (5).
8-Amino-1-phenyl-5-p-tolyl-1H-pyridazino[5,4,3-de][1,6]naphthyridine-7-carbonitrile (12d). Faint green crystals, yield 77%; m.p. 340–341 °C; Anal. Calcd. for C23H16N6 (376.14): C, 73.39; H, 4.28; N, 22.33. Found: C, 73.34; H, 4.30; N, 22.28. EI-HRMS: m/z = 376.1430 (MH+); C23H16N6 requires: m/z = 376.1436 (MH+); 1H-NMR (400 MHz, DMSO-d6): δ = 2.39, 2.51 (s, 3H, CH3), 7.05 (br, 2H, NH2, D2O exchangeable), 7.34–7.65 (m, 8H, Ph-H, CH), 8.09–8.11 (m, 2H, Ph-H), 8.36 (s, 1H, CH); 13C-NMR (100 MHz, DMSO-d6): δ = 162.2, 161.2, 154.4, 150.9, 141.7, 140.2, 138.8, 135.1, 133.2, 129.4 (2C), 128.8 (2C), 128.1, 127.1 (2C), 126.3 (2C), 116.9, 108.7, 104.2, 73.3, 20.9. MS: m/z (%) 376.2 (M+, 100), 348 (10), 188 (10), 77 (5).
8-Amino-1-phenyl-5-m-tolyl-1H-pyridazino[5,4,3-de][1,6]naphthyridine-7-carbonitrile (12e). Green crystals, yield 73%; m.p. 280–281 °C; Anal. Calcd. for C23H16N6 (376.14): C, 73.39; H, 4.28; N, 22.33. Found: C, 73.38; H, 4.27; N, 22.31. EI-HRMS: m/z = 376.1431 (MH+); C23H16N6 requires: m/z = 376.1436 (MH+); 1H-NMR (400 MHz, DMSO-d6): δ = 2.43,2.51 (s, 3H, CH3), 7.07 (br, 2H, NH2, D2O exchangeable), 7.18 (s, 1H, CH), 7.34–7.66 (m, 9H, Ph-H), 8.40 (s, 1H, CH); 13C-NMR (100 MHz, DMSO-d6): δ = 165.2, 162.0, 154.3, 151.1, 141.7, 139.6, 138.7, 135.8, 133.7, 130.8, 129.3, 128.9, 128.2 (2C), 128.1, 126.3 (2C), 125.9, 116.9, 108.3, 108.2, 73.3, 20.3. MS: m/z (%) 376 (M+, 50), 375 (100), 348 (10), 255 (10), 187 (10), 77 (5).
8-Amino-5-(4-nitrophenyl)-1-phenyl-1H-pyridazino[5,4,3-de][1,6]naphthyridine-7-carbonitrile (12f). Dark brown crystals, yield 82%; m.p. 397–398 °C; Anal. Calcd. for C22H13N7O2 (407.11): C, 64.86; H, 3.22; N, 24.07. Found: C, 64.75; H, 3.10; N, 24.12. EI-HRMS: m/z = 407.1125 (MH+); C22H13O2N7 requires: m/z = 407.1131 (MH+); 1H-NMR (400 MHz, DMSO-d6): δ = 7.18 (br, 2H, NH2, D2O exchangeable), 7.48–7.68 (m, 5H, Ph-H, CH), 7.93, 8.244 (d, 1H, CH-pyridazine), 8.40–8.48 (m, 4H, Ph-H, CH), 9.20, 9.41 (s, 1H, CH). MS: m/z (%) 407.2 (M+, 100), 361 (20), 334 (10), 180 (10), 77 (5).
8-Amino-5-(furan-2-yl)-1-phenyl-1H-pyridazino[5,4,3-de][1,6]naphthyridine-7-carbonitrile (12g). Dark green crystals, yield 86%; m.p. 345–346 °C; Anal. Calcd. for C20H12N6O (352.11): C, 68.18; H, 3.43; N, 23.85. Found: C, 68.21; H, 3.52; N, 23.77. EI-HRMS: m/z = 352.1066 (MH+); C20H12O1N6 requires: m/z = 352.1072 (MH+); 1H-NMR (400 MHz, DMSO-d6): δ = 6.74–6.75 (m, 1H, furyl-H), 7.05 (br, 2H, NH2, D2O exchangeable), 7.24–7.97 (m, 8H, Ph-H, furyl-H, CH), 8.42 (s, 1H, CH); 13C-NMR (100 MHz, DMSO-d6): δ = 162.0, 154.6, 153.2, 152.7, 150.8, 145.6, 141.7, 138.6, 133.2, 128.8 (2C), 128.2, 126.3 (2C), 116.8, 112.7, 11.8, 108.6, 102.7, 73.0. MS: m/z (%) 352.1 (M+, 100), 324 (5), 176 (10), 77 (5).
8-Amino-4-methyl-1,5-diphenyl-1H-pyridazino[5,4,3-de][1,6]naphthyridine-7-carbonitrile (12h). Yellow crystals, yield 83%; m.p. 364–365 °C; Anal. Calcd. for C23H16N6 (376.14): C, 73.39; H, 4.28; N, 22.33. Found: C, 73.35; H, 4.15; N, 22.41. EI-HRMS: m/z = 376.1431 (MH+); C23H16N6 requires: m/z = 376.1436 (MH+); 1H-NMR (400 MHz, DMSO-d6): δ = 2.34, 2.50 (s, 3H, CH3), 6.95 (br, 2H, NH2, D2O exchangeable), 7.45–7.66 (m, 10H, Ph-H, CH), 8.56 (s, 1H, CH); 13C-NMR (100 MHz, DMSO-d6): δ = 164.5, 161.6, 152.3, 150.6, 141.8, 140.0, 136.9, 131.0, 129.0 (2C), 128.8 (2C), 128.5, 128.2, 128.0 (2C), 126.3 (2C), 117.1, 114.1, 108.9, 72.6, 13.9. MS: m/z (%) 376.2 (M+, 100), 368 (10), 348 (5), 255 (5), 188 (10), 97 (10), 57 (5).
8-Amino-5-(4-chlorophenyl)-4-methyl-1-phenyl-1H-pyridazino[5,4,3-de][1,6]naphthyridine-7-carbonitrile (12j). Dark yellow crystals, yield 78%; m.p. 345–346 °C; Anal. Calcd. for C23H15ClN6 (410.1): C, 67.24; H, 3.68; N, 20.45. Found: C, 67.27; H, 3.56; N, 20.45. EI-HRMS: m/z = 410.1041 (MH+); C23H15N635Cl requires: m/z = 410.1047 (MH+); 1H-NMR (400 MHz, DMSO-d6): δ = 2.35, 2.50 (s, 3H, CH3), 6.90 (br, 2H, NH2, D2O exchangeable), 7.45–7.66 (m, 9H, Ph-H, CH), 8.58 (s, 1H, CH); 13C-NMR (100 MHz, DMSO-d6): δ = 163.8, 162.1, 156.4, 151.1, 142.3, 139.4, 137.3, 134.0, 132.5, 131.7, 131.3 (2C), 129.3 (2C), 128.6 (2C), 126.7 (2C), 117.2, 114.8, 109.7, 73.5, 14.3. MS: m/z (%) 410.1 (M+, 100), 374 (10), 346 (5), 255 (5), 205 (5), 187 (10), 173 (5), 97 (5), 77 (5).
8-Amino-4-methyl-1-phenyl-5-p-tolyl-1H-pyridazino[5,4,3-de][1,6]naphthyridine-7-carbonitrile (12k). Dark orange crystals, yield 80%; m.p. 370–371 °C; Anal. Calcd. for C24H18N6 (390.16): C, 73.83; H, 4.65; N, 21.52. Found: C, 73.88; H, 4.59; N, 21.60. EI-HRMS: m/z = 390.1587 (MH+); C24H18N6 requires: m/z = 390.1593 (MH+); 1H-NMR (400 MHz, DMSO-d6): δ = 2.36 (s, 3H, CH3), 2.41 (s, 3H, CH3), 6.93 (br, 2H, NH2, D2O exchangeable), 7.34-7.67 (m, 9H, Ph-H, CH), 8.56 (s, 1H, CH); 13C-NMR (100 MHz, DMSO-d6): δ = 163.9, 161.6, 152.4, 150.7, 141.8, 140.2, 137.2, 129.0 (2C), 128.8 (2C), 128.6 (2C), 127.1, 126.3 (2C), 129.1, 124.5, 119.1, 114.8, 109.7, 72.6, 20.8, 14.0. MS: m/z (%) 390.2 (M+, 100), 375 (5), 269 (5), 187 (5), 77 (5).
8-Amino-4-methyl-1-phenyl-5-m-tolyl-1H-pyridazino[5,4,3-de][1,6]naphthyridine-7-carbonitrile (12l). Yellow crystals, yield 72%; m.p. 284–285 °C; Anal. Calcd. for C24H18N6 (390.16): C, 73.83; H, 4.65; N, 21.52. Found: C, 73.81; H, 4.68; N, 21.55. EI-HRMS: m/z = 390.1587 (MH+); C24H18N6 requires: m/z = 390.1592 (MH+); IR: 3489, 3336 (NH2), 2200 (CN); 1H-NMR (400 MHz, DMSO-d6): δ = 2.08 (s, 3H, CH3), 2.11 (s, 3H, CH3), 6.96 (br, 2H, NH2, D2O exchangeable), 7.19–7.66 (m, 9H, Ph-H, CH), 8.53 (s, 1H, CH); 13C-NMR (100 MHz, DMSO-d6): δ = 165.5, 161.5, 152.4, 150.7, 141.8, 139.8, 136.9, 134.8, 130.6, 130.0, 128.9 (2C), 128.2 (2C), 126.3 (2C), 125.6 (2C), 117.1, 114.7, 109.0, 72.6, 19.0, 13.0. MS: m/z (%) 390.2 (M+, 50), 375 (100), 346 (5), 255 (5), 195 (5), 187 (15), 173 (10), 129 (5), 77 (5).
8-Amino-4-methyl-5-(4-nitrophenyl)-1-phenyl-1H-pyridazino[5,4,3-de][1,6]naphthyridine-7-carbonitrile (12m). Dark yellow crystals, yield 82%; m.p. 368–369 °C; Anal. Calcd. for C23H15N7O2 (421.13): C, 65.55; H, 3.59; N, 23.27. Found: C, 65.59; H, 3.63; N, 23.31. EI-HRMS: m/z = 421.1282 (MH+); C23H15O2N7 requires: m/z = 421.1287 (MH+); 1H-NMR (400 MHz, DMSO-d6): δ = 2.37, 2.53 (s, 3H, CH3), 6.82 (br, 2H, NH2, D2O exchangeable), 7.47–8.38 (m, 9H, Ph-H, CH), 8.58, 8.58 (s, 1H, CH); 13C-NMR (100 MHz, DMSO-d6): δ = 14.56, 49.5, 105, 124.12 (2C) , 127.10 (2C), 129.08, 129.70 (2C), 131.31 (4C), 135.61, 137.67, 142.69, 162.56, 163.25; MS: m/z (%) 421.2 (M+, 100), 390 (15), 374 (25), 348 (10), 255 (5), 187 (10), 77 (5).
8-Amino-5-(furan-2-yl)-4-methyl-1-phenyl-1H-pyridazino[5,4,3-de][1,6]naphthyridine-7-carbonitrile (12n). Dark green crystals, yield 86%; m.p. 368–369 °C; Anal. Calcd. for C21H14N6O (366.12): C, 68.84; H, 3.85; N, 22.94. Found: C, 68.89; H, 3.78; N, 22.88. EI-HRMS: m/z = 366.1223 (MH+); C21H14O1N6 requires: m/z = 366.1229 (MH+); 1H-NMR (400 MHz, DMSO-d6): δ = 2.50 (s, 3H, CH3), 6.72–6.73 (m, 1H, furyl-H), 6.91 (br, 2H, NH2, D2O exchangeable), 7.19–7.97 (m, 7H, Ph-H, furyl-H), 8.52 (s, 1H, CH); 13C-NMR (100 MHz, DMSO-d6): δ = 161.6, 153.0, 152.4, 152.2, 150.4, 145.1, 141.8, 136.8, 131.8, 128.9 (2C), 128.2, 126.3 (2C), 117.0, 114.3, 113.2, 112.0, 108.8, 72.3, 13.2. MS: m/z (%) 366.1 (M+, 100), 337 (5), 311 (5), 183 (5), 77 (10).
5. Conclusions
Synthesis of 2-amino-1,4-dihydropyridazines by reacting arylhydrazonals, active methylene nitriles and aromatic aldehydes has been found of very limited scope. We show here that under pressure the sequence of this multicomponent reaction changes as the initial step with the least activation volume predominates, and in this way a novel route to pyridazino[5,4,3-de][1,6]naphthyridines could be developed. Our observations open the route for discovering new multicomponent reactions under pressure, thus it is recommended to expand this technique.
Supplementary Materials
Supplementary Materials are available online.
Acknowledgments
This project was funded by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. (2-363-36-RG). The authors, therefore, would like to express their deep gratitude to the DSR technical and financial support.
Author Contributions
The main part of the work was carried out by Majdah A. AL-Johani, Sameera M. Mousally and Noha Hilmy Elnagdi, under the direct supervision of Khadijah M. Al-Zaydi and Norah F. Alqahtani. Conceptually the work was designed by Mohamed H. Elnagdi, Khadijah M. Al-Zaydi and Norah F. Alqahtani. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Asif, M. Some Recent Approaches of Biologically Active Substituted Pyridazine and Phthalazine Drugs. Curr. Med. Chem. 2012, 19, 2984–2991. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Hegde, G.L.; Khanum, S.A.; Shashikanth, S. Synthesis and Pharmacological Activity of 4-Aryl-Thieno-(2,3-d)-Pyridazines. Indian J. Pharm. Sci. 2005, 67, 210–215. [Google Scholar]
- Tucaliuc, R.A.; Cotea, V.V.; Niculaua, M.; Tuchilus, C.; Mantu, D.; Mangalagiu, I.I. New pyridazine–fluorine derivatives: Synthesis, chemistry and biological activity. Eur. J. Med. Chem. 2013, 67, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Zhu, Y.; Lian, M.; Liu, M.; Yuan, J.; Yin, G.; Wu, A. Unexpected C–C Bond Cleavage: A Route to 3, 6-Diarylpyridazines and 6-Arylpyridazin-3-ones from 1,3-Dicarbonyl Compounds and Methyl Ketones. J. Org. Chem. 2012, 77, 9865–9870. [Google Scholar] [CrossRef] [PubMed]
- Kessler, S.N.; Wegner, H.A. One-Pot Synthesis of Phthalazines and Pyridazino-aromatics: A Novel Strategy for Substituted Naphthalenes. Org. Lett. 2012, 14, 3268–3271. [Google Scholar] [CrossRef] [PubMed]
- Poschenrieder, H.; Stachel, H.-D. Synthesis of pyrrolo[3,4-c]pyridazines. J. Heterocycl. Chem. 1995, 32, 1457–1460. [Google Scholar] [CrossRef]
- Behbehani, H.; Ibrahim, H.M. Microwave-Assisted Synthesis in Water: First One-Pot Synthesis of A Novel Class of Polysubstituted benzo[4,5]imidazo[1,2-b]pyridazines via Intramolecular SNAr. RSC Adv. 2015, 5, 89226–89237. [Google Scholar] [CrossRef]
- Elnagdi, M.H.; Moustafa, M.S.; Sadek, K.U. Green Synthesis of Biologically Relevant Azoles and Azines Derivatives, 1st ed.; Lap Lambert Acadimic Publishing: Saarbrücken, Germany, 2014; p. 64. ISBN 9783659551086. [Google Scholar]
- Ghozlan, S.A.S.; Abdelhamid, I.A.; Hassaneen, H.M.; Elnagdi, M.H. Studies with Enamines and Azaenamines: A novel Efficient Route to 6-amino-1,4-dihydropyridazines and their Condensed Derivatives. J. Heterocycl. Chem. 2007, 44, 105–108. [Google Scholar] [CrossRef]
- Al-Mousawi, S.M.; Moustafa, M.S.; Abdelhamid, I.A.; Elnagdi, M.H. Reassignment of the Structures of Condensation Products of α-keto α′-formylarylhydrazones with Ethyl Cyanoacetate: A Novel Route to ethyl 5-arylazo-2-hydroxynicotinates. Tetrahedron Lett. 2011, 52, 202–204. [Google Scholar] [CrossRef]
- Ackermann, L.; Gunnoe, T.B.; Habgood, L.G. Catalytic Hydroarylation of Carbon-Carbon Multiple Bonds; Wiley-VCH Verlag Gmbh & Co. KGaA: Weinheim, Germany, 2017; pp. 51–58. ISBN 9783527340132. [Google Scholar]
- Ghozlan, S.A.S.; Abdelmoniem, A.M.; Butenschon, H.; Abdelhamid, I.A. Discrepancies in The Reactivity Pattern of Azaenamines towards Cinnamonitriles: Synthesis of Novel aza-steroid Analogues. Tetrahedron 2015, 71, 1413–1418. [Google Scholar] [CrossRef]
- Al-Zaydi, K.M. Microwave Assisted Synthesis, Part 1: Rapid Solventless Synthesis of 3-Substituted Coumarins and Benzocoumarins by Microwave Irradiation of the Corresponding Enaminones. Molecules 2003, 8, 541–555. [Google Scholar] [CrossRef]
- Al-Zaydi, K.M.; Borik, R.M.; Elnagdi, M.H. Arylhydrazononitriles as Precursors to 2-Substituted 1,2,3-triazoles and 4-amino-5-cyano-pyrazole Derivatives Utilizing Microwave and Ultrasound Irradiation. Green Chem. Lett. Rev. 2012, 5, 241–250. [Google Scholar] [CrossRef]
- Al-Zaydi, K.M.; Nhari, L.M.; Borik, R.M.; Elnagdi, M.H. Green Technologies in Organic Synthesis: Self-Condensation of Enamines, Enaminones and EnaminoestersUnder Microwave Irradiation in Ionic Liquid. Green Chem. Lett. Rev. 2010, 3, 93–99. [Google Scholar] [CrossRef]
- Al-Zaydi, K.M.; Borik, R.M.; Elnagdi, M.H. 2-Arylhydrazonopropanals as Building Blocks in Heterocyclic Chemistry: Microwave Assisted Condensation of 2-Aryl-hydrazonopropanals with Amines and Active Methylene Reagents. Molecules 2003, 8, 910–923. [Google Scholar] [CrossRef]
- Kappe, C.O.; Stadler, A. Microwaves in Organic and Medicinal Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 3–5. ISBN 9783527606559. [Google Scholar] [CrossRef]
- Kappe, C.O.; Dallinger, D.; Murphree, S.S. Practical Microwave Synthesis for Organic Chemists—Strategies; John Wiley & Sons: Hoboken, NJ, USA, 2009; pp. 2–4. ISBN 9783527623907. [Google Scholar] [CrossRef]
- Moustafa, M.S.; Al-Mousawi, S.M.; Abdelhamid, I.A.; Elnagdi, M.H. Use of A Novel Multicomponent Reaction Under High Pressure for The Efficient Construction of A New Pyridazino[5,4,3-de][1,6]naphthyridine Tricyclic System. RSC Adv. 2016, 93, 90840–90845. [Google Scholar] [CrossRef]
- Sadek, K.U.; Selim, M.A.; .Alnajjar, A.; Atallah, M.; Elnagdi, M.H. Multicomponent Reactions under Increased Pressure: On the Reaction of Arylhydrazonals, Aromatic Aldehydes and Malononitrile in Q-Tube. Chem. Eur. J. 2016, 7, 468–472. [Google Scholar] [CrossRef]
- Almansour, A.I.; Kumar, R.S.; Arumugam, R.; Basiri, A.; Kia, Y.; Ali, M.A. An Expedient Synthesis, Acetylcholinesterase Inhibitory Activity, and Molecular Modeling Study of Highly Functionalized Hexahydro-1,6-naphthyridines. BioMed Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Raboisson, P.; Desjarlais, R.L.; Reed, R.; Lattanze, J.; Chaikin, M.; Manthey, C.L.; Tomczuk, B.E.; Marugan, J.J. Identification of novel short chain 4-substituted indoles as potent alphavbeta3 antagonist using structure-based drug design. Eur. J. Med. Chem. 2007, 42, 334. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, D.; Wu, N.; Zhang, A.; Jia, Z.; Li, X. The synthesis and biological evaluation of a novel series of C7 non-basic substituted fluoroquinolones as antibacterial agents. Bioorg. Med. Chem. Lett. 2009, 19, 4130. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Wang, Y.; Kazemi, R.; Xu, S.; Xu, Z.; Sanchez, T.W.; Yang, L.; Debnath, B.; Odde, S.; Xie, H. Repositioning HIV-1 Integrase Inhibitors for Cancer Therapeutics: 1,6-Naphthyridine-7-carboxamide as a Promising Scaffold with Drug-like Properties. J. Med. Chem. 2012, 55, 9492–9509. [Google Scholar] [CrossRef] [PubMed]
- CCDC 1434604 Contains the X-ray Structure Crystallographic Data for This Paper. Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk).
- CCDC 1434605 Contains the X-ray Structure Crystallographic Data for This Paper. Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk).
- CCDC 1493165 Contains the X-ray Structure Crystallographic Data for This Paper. Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk).
- Mittelbach, M. An improved and facile synthesis of 2-Amino-1,1,3-trycyanopropene. Monatsh. Chem. 1985, 116, 689–691. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 12a–n are available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).