3. Materials and Methods
3.1. General
Infrared (IR) spectra were obtained using a Perkin Elmer Fourier Transformation-Infrared (FT-IR) spectrometer 1720X (Perkin Elmer, Walttham, MA, USA). High Resolution Mass Spectra (HRMS) were recorded using a JEOL JMS-700 (2) mass spectrometer (JEOL, Tokyo, Japan). NMR spectra were recorded at 27 °C using Agilent 300-, 400-MR-DD2, and 600-DD2 spectrometers (Agilent Technologies, Santa Clara, CA, USA) in CDCl3 using tetramethylsilane (TMS) as the internal standard. Liquid column chromatography was conducted using silica gel FL-60D (Fuji Silysia, Tokyo, Japan). Analytical TLC was performed using precoated plates WAKO silica gel 70 F254 (Wako Pure Chemical Industries, Tokyo, Japan) and the compounds were detected by dipping the plates in an ethanol solution of phosphomolybdic acid, followed by heating. Preparative TLC was performed using precoated glass plates silica gel 60 F254 (Merck & Co., Inc., Darmstadt, Germany). MW-assisted reactions were carried out using a Biotage Initiator® (Basel, Switzerland). Anhydrous CH2CH2 was purchased from Wako Pure Chemical Industries (Osaka, Japan).
3.2. O-Allylation of 4-Hydroxy-1H-pyrazoles (Scheme 1)
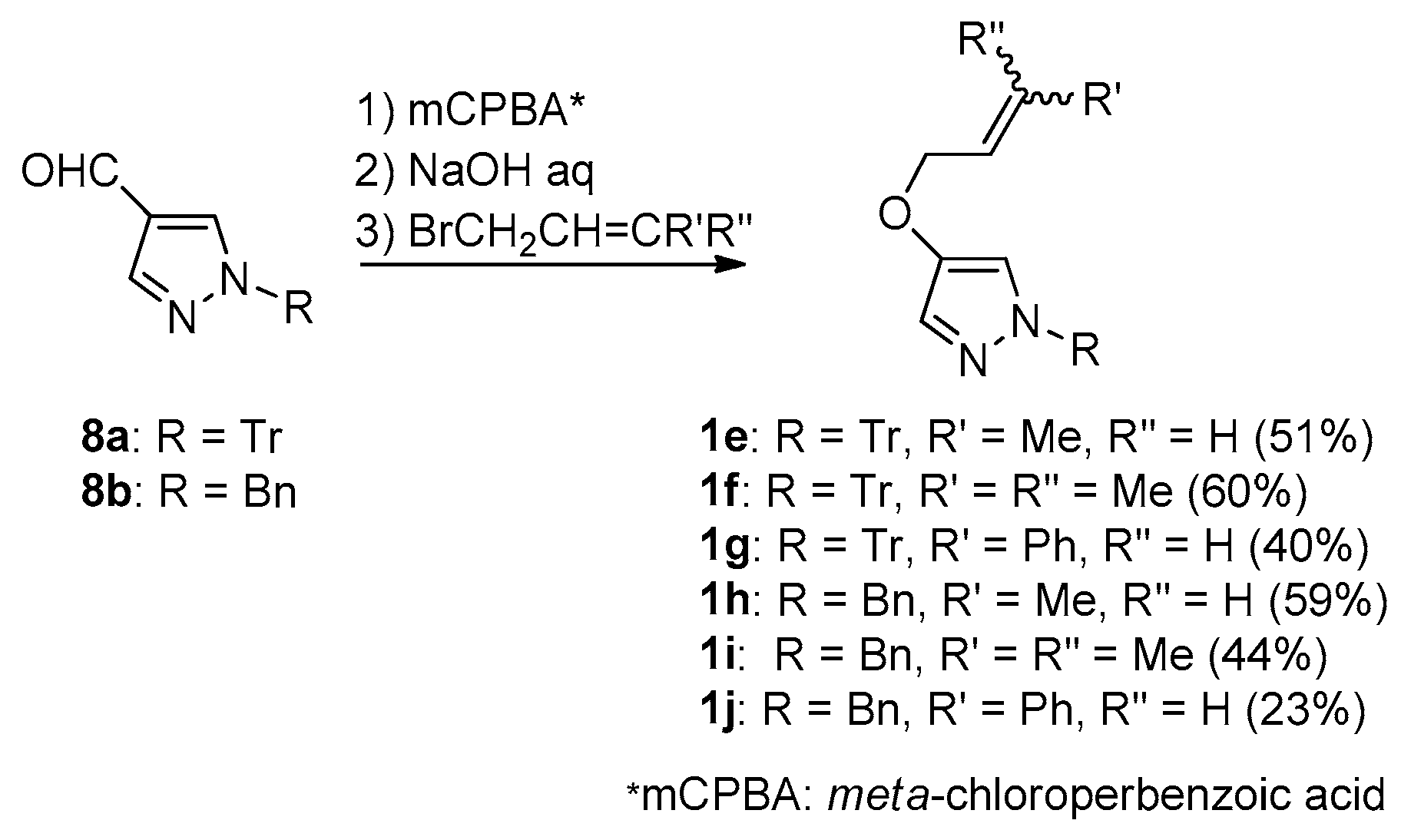
General procedure: To a solution of 4-formyl-1H-1-tritylprazole (8a) (94.6 mg, 0.28 mmol) in CH2Cl2 (6 mL) was added 70% mCPBA (131.8 mg, 0.53 mmol) at 0 °C, with stirring. After 5 h, saturated NaHCO3 aq (10 mL) was added to quench the reaction mixture. The mixture was extracted with CH2Cl2 3 times. Combined organic layer was dried over MgSO4, filtered, and condensed under reduced pressure to give a crude formate. To an acetone solution of the crude formate (6 mL), 20% NaOH aq (4 mL) was added, then the mixture was heated under reflux for 1 h, then crotyl bromide (48 µL, 0.42 mmol) was added to the cooled mixture. After stirring for 3 h, saturated NH4Cl aq was added to the reaction mixture to quench, the mixture was condensed under reduced pressure, extracted with CH2Cl2 for 3 times. The combined CH2Cl2 layer was dried over MgSO4, filtered, and condensed under reduced pressure to give a crude residue, which was purified with flash column chromatography (EtOAc:Hexane = 1:10) to give 4-(2-butenyl)oxy-1H-1-tritylpyrazole (1e) (54.5 mg, 51%).
4-(2-butenyl)oxy-1H-1-tritylpyrazole (1e): white powder; melting point (m.p.) 84–87 °C; IR (KBr) vmax 1571 (C=C), 1490 (C=C), 1445 (C=C) cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.63 (0.5 H, dd, J = 6.5, 1.0 Hz, (Z)-CH3CH=CH-), 1.71 (2.5 H, dd, J = 6.4, 1.0 Hz, (E)-CH3CH=CH-), 4.26 (1.7 H, br d, J = 6.4 Hz, (E)-CH=CHCH2O-), 4.41 (0.3 H, dd, J = 6.4 Hz, (Z)-CH=CHCH2O-), 5.31–5.63 (1H, m, -CH=CH-), 5.63–5.78 (1H, m, -CH=CH-), 7.01 (0.83H, d, J = 0.8 Hz, pyrazole-H), 7.03 (0.17H, d, J = 0.8 Hz, pyrazole-H), 7.10–7.20 (6H, m, Tr-H), 7.22–7.38 (9 H, m, Tr-H), 7.40 (0.83H, d, J = 0.6 Hz, pyrazole-H), 7.41 (0.17H, d, J = 0.7 Hz, pyrazole-H); 13C-NMR (100 MHz, CDCl3): δ (13.2), 17.7, 53.4, (67.0), 72.2, 78.5, (81.9), (118.2), 118.3, (125.4), 126.0, 127.2, 128.1, (128.8), 130.0, 130.7, 130.9, 143.1, 144.1, 146.8; High Resolution Electron Impact Mass Spectrum (HREIMS) m/z calcd. for C26H24N2O [M+] 380.1189, found 380.1185.
4-(3-Methyl-2-butenyl)oxy-1H-1-tritylpyrazole (1f): Colorless crystals (CH2Cl2); m.p. 60–70 °C; IR (KBr) vmax 1576 (C=C), 1492 (C=C), 1445 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3): δ 1.63 (3H, d, J = 0.6 Hz, =CMeMe), 1.74 (3H, d, J = 0.9 Hz, =CMeMe), 4.33 (2H, dt, J = 7.0 Hz, OCH2CH=), 5.40 (1H, tqq, J = 7.0, 0.9, 0.6 Hz, -OCH2CH=C(CH3)2), 7.00 (1H, d, J = 0.9 Hz, pyrazole-H), 7.14–7.17 (6H, m, Tr-H), 7.26–7.31 (9 H, m, Tr-H), 7.40 (1H, d, J = 0.9 Hz, pyrazole-H); 13C-NMR (125 MHz, CDCl3): δ 18.1, 25.7, 68.1, 78.5, 118.2, 119.7, 127.6, 127.9, 128.2, 130.1, 138.4, 143.2, 144.2; HREIMS m/z calcd. for C28H26N2O [M+] 394.2045, found 394.2047.
(E/Z)-4-(3-Phenyl-2-propenyl)oxy-1H-1-tritylpyrazoles (1g): Colorless needles (CH2Cl2); m.p. 115–120 °C; IR (KBr) vmax 1565 (C=C), 1445 (C=C) cm−1; 1H-NMR (400 MHz, CDCl3): δ 4.50 (1.8H, dd, J = 6.1, 1.2 Hz, -OCH2CH=), 4.86 (0.2H, dd, J = 6.5, 1.2 Hz, -OCH2CH=), 6.31 (1H, dt, J = 16.0, 6.1 Hz, -CH2CH=CH-), 6.62 (1H, d, J = 16.0 Hz, -CH=CHPh), 7.05 (1H, d, J = 0.5 Hz, pyrazole-H), 7.10–7.19 (8H, m, Tr-H, Ph-H), 7.22–7.40 (12H, m, Tr-H, Ph-H), 7.44 (1H, d, J = 0.5 Hz, pyrazole-H); 13C-NMR (100 MHz, CDCl3): δ 29.7, 72.3, 78.7, 118.7, 124.3, 126.6, 127.6, 127.9, 128.3, 128.6, 130.1, 136.3, 143.1, 144.0; HREIMS m/z calcd. for C31H26N2O [M+] 442.2045, found 442.2046.
(E/Z)-1-Benzyl-4-(2-butenyl)oxy-1H-pyrazoles (1h): Oil; IR (film) vmax 1575 (C=C), 1496 (C=C) cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.66 (0.5 H, dd, J = 5.8, 0.5 Hz, (Z)-CH3CH=CH-), 1.70 (2.5H, dd, J = 6.5, 0.6 Hz, (E)-CH3CH=CH-), 4.28 (1.7 H, dd, J = 6.2, 1.0 Hz, (E)-OCH2CH=CH-), 4.42 (0.3 H, dd, J = 6.2, 0.6 Hz, (Z)-OCH2CH=CH-), 5.18 (2 H, s, ArCH2Ph), 5.60–5.72 (1H, m, -CH=CH-), 5.72–5.84 (1H, m, -CH=CH-), 7.01 (0.83 H, s, pyrazole-H), 7.03 (0.17 H, s, pyrazole-H), 7.17 (2H, dd, J = 6.9, 1.1 Hz, Ph-H), 7.20–7.40 (6 H, m, Ph-H, pyrazole-H); 13C-NMR (100 MHz, CDCl3): δ (13.3), 17.8, (49.7), 56.6, (67.2), 72.4, (114.96), 150.02, (125.5), 126.0, (126.9), 127.5, 127.6, (128.0), (128.3), 128.5, 128.7, (128.88), (128,.92), (129.0), 131.0, 136.7, (143.5), 145.6; HREIMS m/z calcd. for C14H16N2O [M+] 228.1263, found 228.1263.
1-Benzyl-4-(3-methyl-2-butenyl)oxy-1H-pyrazoles (1i): Oil; IR (film) vmax 1574 (C=C), 1496 (C=C), 1455 (C=C) cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.67 (3H, s, =CMeMe), 1.74 (3H, s, =CMeMe), 4.34 (2H, d, J = 6.9 Hz, -OCH2CH=), 5.18 (2H, s, ArCH2Ph), 5.42 (1H, m, -CH2CH=CMe2), 7.01 (1H, s, pyrazole-H), 7.18 (2H, d, J = 7.3 Hz, Ph-H), 7.24–7.34 (4 H, m, Ph-H, pyrazole-H); 13C-NMR (100 MHz, CDCl3): δ 18.1, 25.7, 56.6, 68.2, 114.9, 119.6, 127.50, 127.54, 128.0, 128.7, 136.7, 138.6, 145.8; HREIMS m/z calcd. for C15H18N2O [M+] 242.1419, found 242.1420.
(E/Z)-1-Benzyl-4-(3-phenyl(2-propenyl))oxy-1H-pyrazoles (1j): white powder; m.p. 68–71 °C; IR (KBr) vmax 1565 (C=C), 1490 (C=C), 1445 (C=C) cm−1; 1H-NMR (400 MHz, CDCl3): δ 4.50 (1.9H, dd, J = 7.1, 1.4 Hz, -OCH2CH=), 4.58 (0.1H, dd, J = 5.8, 1.4 Hz, -OCH2CH=), 5.17 (1.9H, s, ArCH2Ph), 5.21 (0.1H, s, ArCH2Ph), 6.31 (1H, dt, J = 16.0, 5.9 Hz, -CH2CH=CH-), 6.63 (1H, d, J = 16.0 Hz, -CH=CHPh), 7.04 (1H, s, pyrazole-H), 7.13–7.36 (11H, m, Ph-H, pyrazole-H); 13C-NMR (100 MHz, CDCl3): δ 56.7, 72.4, 115.3, 124.3, 126.6, 127.5, 127.7, 127.95, 128.0, 128.6, 128.8, 133.4, 136.3, 136.6, 145.6; HREIMS m/z calcd. for C19H18N2O [M+] 290.1419, found 290.1418.
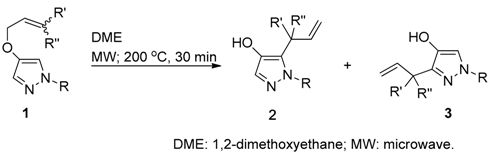
3.3. Claisen Rearrangement of 1-Protected 4-Allyloxy-1H-pyrazoles with DME (Table 1)
General procedure (Table 1, entry 2): A sealed vial containing a solution of
1b (146.1 mg, 0.68 mmol) in DME (2 mL) was heated at 200 °C for 30 min under MW irradiation. After the cooling, the reaction mixture was quenched with NH
4Cl aq. and extracted with EtOAc. The organic layer was washed with brine, dried over MgSO
4, and filtered. The solvent was removed under reduced pressure; the crude residue was subsequently purified by column chromatography (hexane/EtOAc = 3:1
v/
v), affording
2b (143.2 mg, 98% yield) as an oil.
3-Allyl-4-allyloxy-1H-1-toluenesulfonylpyrazole (3c); Oil; IR (liquid film) vmax 1593 (C=C), 1491 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3): δ 2.41 (3H, s, PhCH3), 3.68 (2H, dt, J = 5.9, 1.5 Hz, ArCH2CH=CH2), 4.41 (2H, dt, J = 5.3, 1.5 Hz, -OCH2CH=CH2), 5.01 (1H, dq, J = 17.0, 1.4 Hz, -CH=CHH), 5.06 (1H, dq, J = 10.0, 1.5 Hz, -CH=CHH), 5.25 (1H, dq, J = 10.6, 1.4 Hz, -CH=CHH), 5.32 (1H, dq, J = 17.3, 1.5 Hz, -CH=CHH), 5.89–5.97 (2H, m, ArCH2CH=CH2), 5.91 (1H, m, -CH2CH=CH2), 7.29 (2H, br d, J = 8.5 Hz, Ph-H), 7.52 (1H, s, pyrazole-H), 7.83 (2H, br d, J = 8.5 Hz, Ph-H); 13C-NMR (125 MHz, CDCl3): δ 21.7, 27.8, 73.1, 116.6, 118.3, 128.0, 129.8, 130.7, 132.8, 133.8, 134.4, 134.8, 143.7, 145.4; HREIMS m/z calcd. for C16H18N2O3S [M+] 318.1038, Found 318.1035.
1-Benzyl-4-hydroxy-5-(1-methyl-2-propenyl)-1H-pyrazoles (2h): White powder; m.p. 95–100 °C; IR (KBr) vmax 1497 (OH), 1591 (C=C), 1455 (C=C) cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.25 (3H, d, J = 7.2 Hz, CH2CH-), 3.51 (1H, m, ArCHCH3CH=), 4.99 (1H, br d, J = 17. 4 Hz, -CH=CHH), 5.06 (1H, br d, J = 10.4 Hz, -CH=CHH), 5.21 (1H, d, J = 17.2 Hz, ArCHAHBPh), 5.25 (1H, d, J = 17.2 Hz, ArCHAHBPh), 5.96 (1H, ddd, J = 17.2, 10.2, 5.5 Hz, -CHCH=CH2), 7.03 (2H, d, J = 6.8 Hz, Ph-H), 7.18 (1H, s, pyrazole-H), 6.00 (1H, ddt, J = 17.2, 10.5, 5.3 Hz, -OCH2CH=CH2), 7.25–7.31 (3H, m, Ph-H); 13C-NMR (100 MHz, CDCl3): δ 17.6, 33.6, 53.9, 114.5, 126.5, 127.6, 128.5, 128.6, 129.9, 137.4, 138.6, 139.2; HREIMS m/z calcd. for C14H16N2O [M+] 228.1263, found 228.1262.
1-Benzyl-4-hydroxy-5-(1-phenyl-2-propenyl)-1H-pyrazoles (2j): Powder; m.p. 88–93 °C; IR (KBr) vmax 3458 (OH), 1591 (C=C), 1496 (C=C), 1455 (C=C) cm−1; 1H-NMR (400 MHz, CDCl3): δ 4.68 (1H, d, J = 6.6 Hz, ArCHPhCH=), 4.89 (1H, dt, J = 17.2, 1.4 Hz, -CH=CHH), 5.06 (1H, d, J = 16.2 Hz, ArCHAHBPh), 5.15 (1H, d, J = 16.2 Hz, ArCHAHBPh), 5.18 (1H, d, J = 10.3 Hz, -CH=CHH), 5.29 (1H, ddd, J = 17.0, 10.2, 6.8 Hz, -CHCH=CH2), 6.98 (2H, br d, J = 6.7 Hz, Ph-H), 7.10 (2H, br d, J = 6.9 Hz, Ph-H), 7.11–7.28 (8H, m, Ph-H, pyrazole-H); 13C-NMR (100 MHz, CDCl3): δ 45.2, 54.1, 17.4, 126.6, 127.1, 127.6, 127.9, 128.5, 128.7, 128.8, 136.8, 137.0, 139.4, 139.7; HREIMS m/z calcd. for C19H18N2O [M+] 290.1419, found 290.1415.
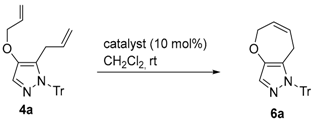
3.4. O-Allylation of 1-Protected 5- or 3-Allyl-4-allyloxy-1H-pyrazoles (Scheme 2)
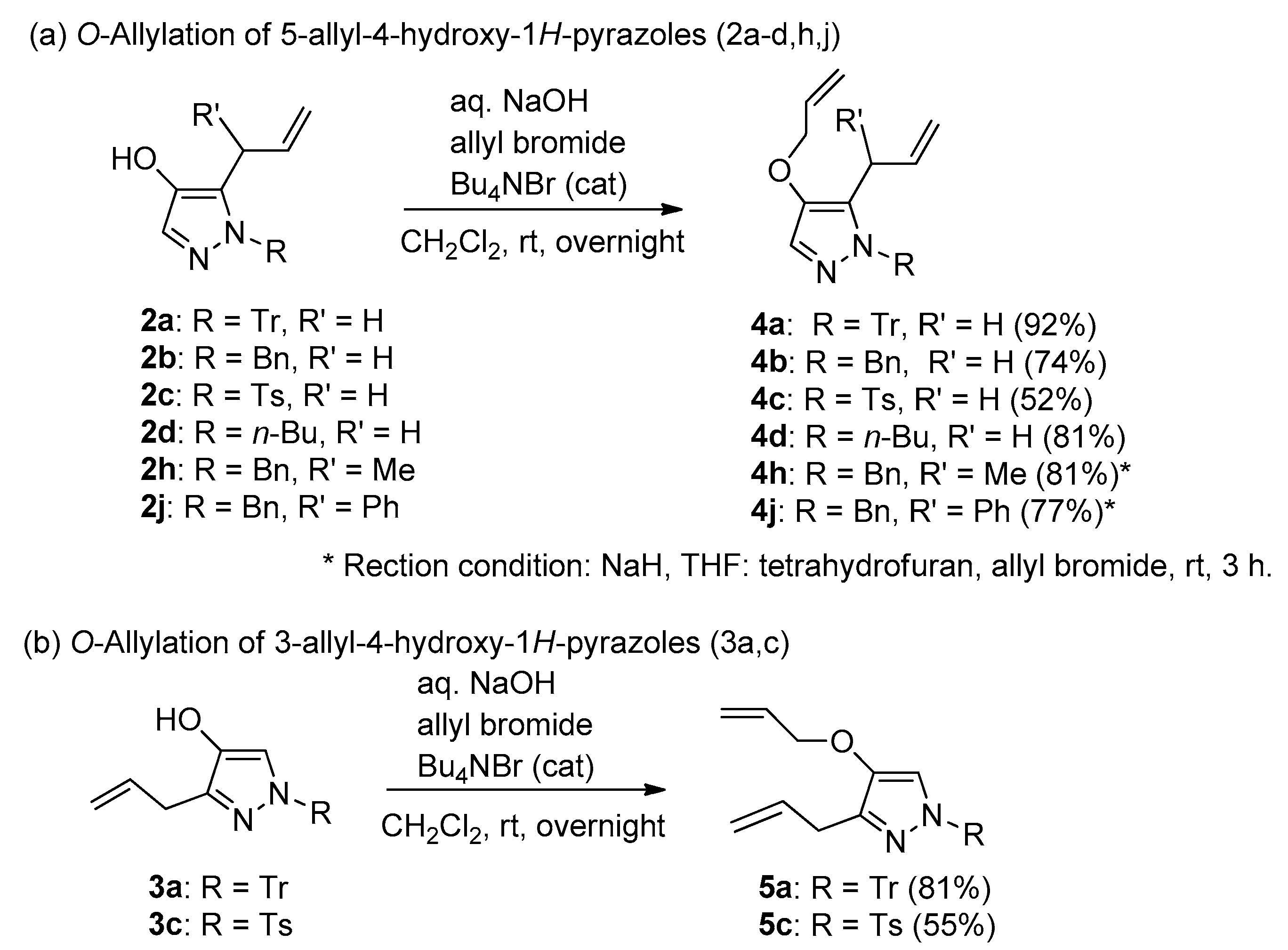
General procedure: To a solution of 5-allyl-4-hydroxy-1-trityl-1H-pyrazole (2a) (0.21 g, 0.56 mmol) in CH2Cl2 (4 mL), 20% NaOH aq. (3 mL) and allyl bromide (120 µL, 1.4 mmol) were added. After stirring at room temperature (r.t.) overnight, the reaction mixture was quenched with sat. NH4Cl aq., and then extracted with CH2Cl2. The organic layer was dried over anhydrous MgSO4, filtered, and evaporated. The crude residue was purified using flash chromatography (eluent:hexane/EtOAc gradient), affording 4a (0.21 g, 92% yield).
5-Allyl-4-allyloxy-1-trityl-1H-pyrazole (4a): Colorless crystals (CH2Cl2); m.p. 110–114 °C; IR (film) vmax 1578 (C=C), 1492 (C=C), 1445 (C=C) cm−1; 1H-NMR (500 MHz, CDCl3): δ 2.85 (2H, br dt, J = 6.6, 1.1 Hz, ArCH2CH=CH2), 4.43 (2H, dt, J = 5.3, 1.6 Hz, OCH2CH=CH2), 4.62 (1H, ddd, J = 17.2, 3.2, 1.4 Hz, -CH=CHH), 4.65 (1H, ddd, J = 10.1, 3.2, 1.3 Hz, -CH=CHH), 5.00 (1H, ddt, J = 17.2, 10.1, 6.6 Hz, ArCH=CH2), 5.23 (1H, ddt, J = 10.5, 3.0, 1.4 Hz, -CH2CH=CHH), 5.36 (1H, dq, J = 17.2, 1.6 Hz, -CH2CH=CHH), 6.00 (1H, ddt, J = 17.2, 10.5, 5.3 Hz, -OCH2CH=CH2), 7.10–7.14 (6H, m, Tr-H), 7.23–7.31 (9 H, m, Tr-H), 7.33 (1H, s, pyrazole-H); 13C-NMR (125 MHz, CDCl3): δ 31.2, 72.4, 78.5, 115.6, 117.2, 125.3, 125.4, 127.5, 129.1, 130.1, 132.5, 133.7, 143.0, 144.2; HREIMS m/z calcd. for C28H26N2O [M+] 406.2045, found 406.2049.
5-Allyl-4-allyloxy-1H-1-benzylpyrazole (4b): Oil; IR (film) vmax 1580 (C=C), 1492 (C=C), 1408 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3): δ 3.26 (2H, br dt, J = 5.8, 1.6 Hz, ArCH2CH=CH2), 4.43 (2H, dt, J = 5.6, 1.5 Hz, -OCH2CH=CH2), 4.97 (1H, ddd, J = 17.0, 3.2, 1.7 Hz, -CH=CHH), 5.04 (1H, ddd, J = 10.3, 3.3, 1.5 Hz, -CH=CHH), 5.21 (2H, s, PhCH2O-), 5.24 (1H, ddd, J = 10.2, 3.0, 1.5 Hz, -CH=CHH), 5.35 (1H, ddd, J = 17.0, 3.3, 1.5 Hz, -CH=CHH), 5.77 (1H, ddt, J = 17.0, 10.5, 5.8 Hz, ArCH2CH=CH2), 6.01 (1H, ddt, J = 17.3, 10.5, 5.3 Hz, -OCH2CH=CH2), 7.15 (2H, br d, J = 7.4 Hz, Ph-H), 7.25 (1H, br t, J = 7.4 Hz, Ph-H), 7.29 (2H, br t, J = 7.4 Hz, Ph-H), 7.31 (1H, s, pyrazole-H); 13C-NMR (150 MHz, CDCl3): δ 27.1, 53.8, 73.3, 116.3, 117.6, 126.7, 126.9, 127.0, 127.5, 128.6, 133.69, 133.71, 137.1,142.3; HREIMS m/z calcd. for C16H18N2O [M+] 254.1419, found 254.1416.
5-Allyl-4-allyloxy-1H-1-toluenesulfonylpyrazole (4c): Oil; IR (liquid film) vmax 1593 (C=C), 1491 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3): δ 2.41 (3H, s, PhCH3), 3.68 (2H, dt, J = 5.9, 1.5 Hz, ArCH2CH=CH2), 4.41 (2H, dt, J = 5.3, 1.5 Hz, -OCH2CH=CH2), 5.01 (1H, dq, J = 17.0, 1.4 Hz, -CH=CHH), 5.06 (1H, dq, J = 10.0, 1.5 Hz, -CH=CHH), 5.25 (1H, dq, J = 10.6, 1.4 Hz, -CH=CHH), 5.32 (1H, dq, J = 17.3, 1.5 Hz, -CH=CHH), 5.89–5.97 (2H, m, ArCH2CH=CH2), 5.91 (1H, m, -CH2CH=CH2), 7.29 (2H, br d, J = 8.5 Hz, Ph-H), 7.52 (1H, s, pyrazole-H), 7.83 (2H, br d, J = 8.5 Hz, Ph-H); 13C-NMR (125 MHz, CDCl3): δ 21.7, 27.8, 73.1, 116.6, 118.3, 128.0, 129.8, 130.7, 132.8, 133.8, 134.4, 134.8, 143.7, 145.4; HREIMS m/z calcd. for C16H18N2O3S [M+] 318.1038, found 318.1035.
5-Allyl-4-allyloxy-1-butyl-1H-pyrazole (4d): Oil; IR (film) vmax 1588 (C=C), 1413 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3): δ 0.92 (3H, t, J = 7.3 Hz, -CH2CH3), 1.32 (2H, m, -CH2CH2CH3), 1.32 (2H, m, -CH2CH2CH3), 1.76 (2H, m, -CH2CH2CH2-), 3.37 (2H, dt, J = 5.6, 1.5 Hz, ArCH2CH=CH2), 3.93 (2H, br t, J = 7.3 Hz, ArCH2CH2-), 4.41 (2H, dt, J = 5.9, 1.8 Hz, OCH2CH=CH2), 5.01 (1H, dq, J = 17.0, 1.8 Hz, -CH=CHH), 5.09 (1H, dq, J = 10.0, 1.5 Hz, -CH=CHH), 5.24 (1H, dq, J = 10.6, 1.5 Hz, -CH=CHH), 5.35 (1H, dq, J = 17.4, 1.8 Hz, -CH=CHH), 5.86 (1H, ddt, J = 17.0, 10.6, 5.6 Hz, ArCH2CH=CH2), 6.01 (1H, ddt, J = 17.4, 10.0, 5.9 Hz, -OCH2CH=CH2), 7.23 (1H, s, pyrazole-H); 13C-NMR (150 MHz, CDCl3): δ 13.7, 19.9, 27.1, 32.2, 49.5, 73.3, 116.2, 117.6, 126.3, 133.9, 134.1; HREIMS m/z calcd. for C13H20N2O [M]+ 220.1575, found 220.1575.
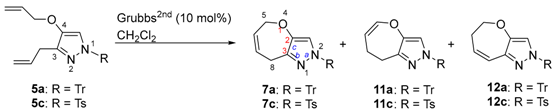
3-Allyl-4-allyloxy-1H-1-tritylpyrazole (5a): Colorless needles (CH2Cl2/hexane); m.p. 62–65 °C; IR (KBr) vmax 1568 (C=C), 1488 (C=C), 1442 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 3.38 (2H, dt, J = 6.2, 1.7 Hz, ArCH2CH=CH2), 4.24 (2H, dt, J = 5.6, 1.5 Hz, -OCH2CH=CH2), 4.99 (1H, dq, J = 10.0, 1.7 Hz, ArCH2CH=CHAHB), 5.03 (1H, dq, J = 17.0, 1.7 Hz, ArCH2CH=CHAHB), 5.18 (1H, dq, J = 10.5, 1.4 Hz, -OCH2CH=CHAHB), 5.26 (1H, dq, J = 17.3, 1.7 Hz, -OCH2CH=CHAHB), 5.93 (1H, ddt, J = 17.3, 10.4, 5.6 Hz, -OCH2CH=CH2), 5.99 (1H, ddt, J = 17.0, 10.0, 6.2 Hz, ArCH2CH=CH2), 6.88 (1H, s, pyr-H), 7.15–7.19 (6H, m, Tr-H), 7.26–7.30 (9H, m Tr-H); 13C-NMR (125 MHz, CDCl3): δ 30.0, 72.9, 78.1, 115.1, 117.4, 118.5, 127.4, 127.6, 130.1, 133.5, 135.8, 140.1, 141.6, 143.5; HREIMS m/z calcd. for C28H26N2O [M+] 406.2045, found 406.2043.
3-Allyl-4-allyloxy-1-toluenesulfonyl-1H-pyrazole (5c): Colorless needles (CH2Cl2/hexane); m.p. 82–84 °C; IR (KBr) vmax 1540 (C=C), 1500 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 2.41 (3H, s, ArCH3), 3.33 (2H, dt, J = 6.5, 1.7 Hz, ArCH2CH=CH2), 4.38 (2H, dt, J = 5.3, 1.5 Hz, -OCH2CH=CH2), 5.00 (1H, dq, J = 8.8, 1.5 Hz, -CH=CHH), 5.02–5.03 (1H, m, -CH=CHH), 5.29 (1H, dq, J = 10.5, 1.5 Hz, -CH=CHH), 5.37 (1H, dq, J = 17.3, 1.5 Hz, -CH=CHH), 5.93–5.98 (2H, m, 2 × -CH2CH=CH2), 7.28 (2H, d, J = 7.5 Hz, Ts-H), 7.51 (1H, s, pyr-H), 7.81 (2H, d, J = 7.5 Hz, Ts-H); 13C-NMR (150 MHz, CDCl3) δ 22.7, 30.1, 72.3, 113.5, 116.7, 118.3, 127.7, 129.8, 132.2, 133.3, 134.3, 145.2, 145.5, 148.9; HREIMS m/z calcd. for C16H18N2O3S [M]+ 318.1038, found 318.1041.
General procedure under aprotic condition: To a solution of 2h (57.7 mg, 0.27 mmol) in dry THF (2 mL), 60%NaH (16.0 mg, 0.40 mmol) was added at rt. 20 Min later, allyl bromide (34 µL, 0.40 mmol) was added to the reaction flask. After stirring at rt for 3 h, the reaction mixture was quenched with sat. NH4Cl aq., then extracted with CH2Cl2. The organic layer was dried over anhydrous MgSO4, filtered, and evaporated. The crude residue was purified using column chromatography (eluent:hexane/EtOAc = 3:1), affording 4h as a colorless oil (49.9 mg, 81% yield).
5-(1-methyl-2-propenyl)-4-allyloxy-1-benzyk-1H-pyrazole (4h): Oil; IR (film) vmax 1575 (C=C), 1496 (C=C), 1456 (C=C) cm−1; 1H-NMR (400 MHz, CDCl3): δ 1.28 (3H, d, J = 7.3 Hz, -CHCH3), 3.49 (1H, br quint, J = 7.3 Hz, ArCH (CH3)CH=), 4.41 (2H, br d, J = 4.9 Hz, -OCH2CH=CH2), 4.86 (1H, dt, J = 17.2, 1.4 Hz, -CHCH=CHH), 4.94 (1H, dt, J = 10.2, 1.4 Hz, -CHCH=CHH), 5.20 (1H, br d, J = 16.2 Hz, ArCHAHBPh), 5.22 (1H, dq, J = 10.4, 1.4 Hz, -CH2CH=CHH), 5.23 (1H, br d, J = 16.2 Hz, ArCHAHBPh), 5.36 (1H, dq, J = 17.2, 1.4 Hz, -CH2CH=CHH), 5.80–6.06 (2H, m (overlapped), 2 × -CH=CH2), 7.02 (2H, br d, J = 6.7 Hz, Ph-H), 7.23–7.31 (4 H, m, Ph-H, pyrazole-H); 13C-NMR (100 MHz, CDCl3): δ 18.1, 34.3, 54.1, 72.8, 113.9, 117.2, 126.5, 126.9, 127.5, 128.6, 131.0, 133.7, 137.5, 139.6, 142.2; HREIMS m/z calcd. for C17H20N2O [M+] 268.1576, found 268.1579.
5-(1-phenyl-2-propenyl)-4-allyloxy-1-benzyk-1H-pyrazole (4j): Oil; IR (film) vmax 1576 (C=C), 1496 (C=C) cm−1; 1H-NMR (400 MHz, CDCl3): δ 4.33 (1H, br dd, J = 11.5, 4.5 Hz, -OCHAHBCH= CH2), 4.36 (1H, br dd, J = 11.5, 4.5 Hz, -OCHAHBCH= CH2), 4.68 (1H, d, J = 7.4 Hz, ArCHPhCH=), 4.86 (1H, br d, J = 17.0 Hz, -CH=CHH), 5.06–5.14 (3H, overlapped, 2 × benzyl methylene, -CH=CH), , 5.20 (1H, br dq, J = 10.4, 1.4 Hz, -CH2CH=CHH), 5.29 (1H, br dq, J = 17.2, 1.5 Hz, -CH2CH=CHH), 5.91 (1H, ddt, J = 15.8, 10.0, 4.7 Hz, -OCH2CH=CH2), 6.30 (1H, ddd, J = 17.0, 9.4, 7.9 Hz, -CH2CH=CH2), 5.80–6.06 (2H, m (overlapped), 2 × -CH=CH2), 6.96 (2H, br d, J = 7.1 Hz, Ph-H), 7.09 (2H, br d, J = 7.2 Hz, Ph-H), 7.15–7.27 (6H, m, Ph-H), 7.33 (1 H, s, pyrazole-H); 13C-NMR (100 MHz, CDCl3): δ 45.7, 54.3, 73.0, 116.3, 117.3, 126.7, 127.3, 127.6, 127.9, 128.5, 128.6, 129.6, 133.7, 136.9, 137.1, 140.4, 142.7; HREIMS m/z calcd. for C22H22N2O [M+] 330.1732, found 330.1732.
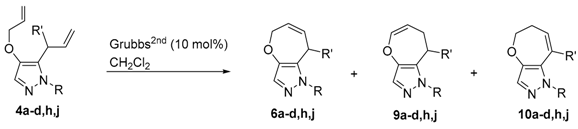
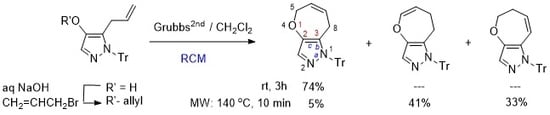
3.5. RCM of 1-Protected 4-Allyloxy- 5- or 3-Allyl-1H-pyrazoles (Table 1, Table 2 and Table 3)
General procedure for the reactions at room temperature (
Table 2, entry 2): To a solution of
4a (18.5 mg, 0.046 mmol) in CH
2Cl
2 (4 mL) was added Grubbs
2nd (3.9 mg, 0.0046 mmol) under argon atmosphere. After stirring for 120 min, the solvent was removed under reduced pressure. The crude residue was purified by preparative TLC (eluent: hexane/AcOEt = 5:1
v/
v), affording
6a (12. 7 mg, 74% yield).
General procedure for the MW-assisted reactions (
Table 3, entry 5): To a solution of
4a (18.5 mg, 0.046 mmol) in CH
2Cl
2 (4 mL) in a MW reactor vial was added Grubbs
2nd (4.2 mg, 0.0049 mmol, 10 mol %) under argon atmosphere. The reaction vial was irradiated at 140 °C for 120 min. After cooling the reaction mixture, the solvent was removed under reduced pressure. The crude residue was purified by preparative TLC (eluent: hexane/AcOEt = 5:1
v/
v), affording
6a (0.9 mg, 5% yield),
9a (7.5 mg, 41% yield), and
10a (5.7 mg, 33% yield).
5,8-Dihydro-1H-1-trityloxepino[3,2-c]pyrazole (6a): Colorless crystals (CH2Cl2/hexane); m.p. 92–95 °C; IR (KBr) vmax 1584 (C=C), 1492 (C=C), 1445 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 2.63 (2H, m, ArCH2CH=), 4.45 (2H, m, -OCH2CH2=), 5.40 (1H, dtt, J = 11.8, 5.0, 1.4 Hz, -CH=CHCH2Ar), 5.68 (1H, dtt, J = 11.8, 5.0, 1.8 Hz, -OCH2CH=CH-), 7.10–7.13 (6H, m, Tr-H), 7.26–7.32 (10H, m, Tr-H, py-H); 13C-NMR (150 MHz, CDCl3) δ 27.3, 68.2, 78.5, 126.2, 127.4, 127.5, 127.6, 127.9, 128.3, 129.96, 130.03, 130.7, 142.8, 144.9; HREIMS m/z calcd. for C26H22N2O [M]+ 378.1732, found 378.1730.
7,8-Dihydro-1H-1-trityloxepino[3,2-c]pyrazole (9a): Colorless crystals (CH2Cl2/hexane); m.p. 154–158 °C; IR (KBr) vmax 1658 (C=C), 1579 (C=C), 1492 (C=C), 1447 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 1.74 (2H, m, =CHCH2CH2-), 2.25 (2H, m, -CH2CH2Ar), 4.60 (1H, dt, J = 7.6, 5.6 Hz, -CH=CHCH2-), 6.19 (1H, dt, J = 7.6, 1.5 Hz, -OCH=CHCH2-), 7.12–7.15 (6H, m, Tr-H), 7.25–7.32 (10H, m, Tr-H, pyr-H); 13C-NMR (150 MHz, CDCl3) δ 23.5, 27.9, 78.5, 105.7, 127.3, 127.6, 127.9, 129.9, 131.3, 141.7, 142.2, 143.1; HREIMS m/z calcd. for C26H23N2O [M + H]+ 379.1810, found 379.1813.
5,6-Dihydro-1H-oxepino[3,2-c]pyrazole (10a): Colorless crystals (CH2Cl2/hexane); m.p. 144–148 °C; IR (KBr) vmax 1564 (C=C), 1489 (C=C), 1446 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 2.54 (2H, m, =CHCH2CH2-), 4.08 (2H, m, -OCH2CH2-), 5.30 (1H, dt, J = 11.5, 5.2 Hz, -CH=CHCH2-), 5.70 (1H, br d, J = 11.5 Hz, ArCH=CHCH2-), 7.12–7.15 (6H, m, Tr-H), 7.25–7.32 (10H, m, Tr-H, py-H); 13C-NMR (150 MHz, CDCl3) δ 33.8, 68.8, 78.9, 119.5, 126.3, 127.28, 127.33, 127.5, 128.9, 130.0, 143.1, 145.9; HREIMS m/z calcd. for C26H22N2O [M]+ 378.1732, found 378.1736.
1-Benzyl-5,8-dihydro-1H-oxepino[3,2-c]pyrazole (6b): Oil; IR (film) vmax 1654 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 3.38 (2H, m, ArCH2CH=), 4.45 (2H, dq, J = 5.5, 1.2 Hz, -OCH2CH=CHCH2-), 5.20 (2H, s, NCH2Ph), 5.80 (1H, m, -CH=CHCH2-), 6.28 (1H, m, -CH=CHCH2-), 7.06 (2H, br d, J = 7.3 Hz, Ph-H), 7.25 (1H, s, pyr-H), 7.26 (1H, br t, J = 7.3 Hz, Ph-H), 7.33 (1H, br t, J = 7.3 Hz, Ph-H); 13C-NMR (150 MHz, CDCl3) δ 25.9, 29.7, 53.9, 68.2, 126.6, 126.8, 127.7128.5, 128.8, 129.0, 136.9, 144.6; HREIMS m/z calcd. for C14H14N2O [M]+ 226.1106, found 226.1105.
1-Benzyl-7,8-dihydro-1H-oxepino[3,2-c]pyrazole (9b): Oil; IR (liquid film) vmax 1652 (C=C), 1583 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 2.33 (2H, m, -=CHCH2CH2-), 2.75 (2H, m, -CH2CH2Ar), 4.85 (1H, dt, J = 7.4, 6.4 Hz, -CH=CHCH2-), 5.24 (2H, s, -CH2Ph), 6.30 (1H, dt, J = 7.4, 1.1 Hz, -OCH=CHCH2-), 7.25 (2H, br d, J = 7.7 Hz, Ph-H), 7.26 (1H, br t, J = 7.4 Hz, Ph-H), 7.27 (1H, s, pyr-H), 7.31 (2H, br t, J = 7.7 Hz, Ph-H); 13C-NMR (150 MHz, CDCl3) δ 23.2, 24.9, 54.1, 106.8, 126.4, 127.6, 128.1 128.4, 128.8, 137.2, 140.7, 143.3; HREIMS m/z calcd. for C14H15N2O [M + H]+ 227.1184, found 227.1182.
1-Benzyl-5,6-dihydro-1H-oxepino[3,2-c]pyrazole (10b): Oil; IR (liquid film) vmax 1564 (C=C), 1496 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 2.67 (2H, m, -=CHCH2CH2-), 4.13 (2H, m, -CH2CH2O-), 5.30 (2H, br s, NCH2Ph), 5.80 (1H, dt, J = 11.1, 5.3 Hz, -CH=CHCH2-), 6.28 (1H, br d, J = 11.1 Hz, ArCH=CH-), 7.11 (2H, br d, J = 7.0 Hz, Ph-H), 7.22 (1H, d, J = 0.5 Hz, pyr-H), 7.26 (1H, br t, J = 7.0 Hz, Ph-H), 7.31 (2H, br t, J = 7.0 Hz, Ph-H); 13C-NMR (150 MHz, CDCl3) δ 34.2, 53.9, 68.5, 116.4, 126.5, 126.9, 127.6, 127.7, 128.7, 129.0, 137.2, 144.8; HREIMS m/z calcd. for C14H15N2O [M + H]+ 227.1184, found 227.1184.
5,8-Dihydro-1-toluenesulfonyl-1H-oxepino[3,2-c]pyrazole (6c): Oil; IR (liquid film) vmax 1595 (C=C) cm−1; 1H-NMR (400 MHz, CDCl3) δ 2.42 (3H, s, ArCH3), 3.93 (2H, m, -OCH2CH=), 4.42 (2H, d, J = 4.3 Hz, =CHCH2Ar), 5.94–5.95 (2H, m, -OCH2CH=CHCH2Ar, -OCH2CH=CHCH2Ar), 7.32 (2H, d, J = 8.0 Hz, Ph-H), 7.44 (1H, s, pyr-H), 7.81 (2H, d, J = 8.4 Hz, Ph-H); 13C-NMR (100 MHz, CDCl3) δ 21.7, 26.5, 67.7, 118.5, 127.6, 127.7, 128.4, 130.0, 134.7, 136.1, 137.5, 145.5; HREIMS m/z calcd. for C14H14N2O [M]+ 226.1106, found 226.1105.
7,8-Dihydro-1H-oxepino[3,2-c]-1-tolenesulfonylpyrazole (9c): Oil; IR (film) vmax 1579 (C=C), 1480 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 2.39–2.43 (2H, m, =CHCH2CH2-), 2.42 (3H, s, CH3-Ar), 3.26–3.29 (2H, m, ArCH2CH2-), 5.06 ( 1H, q, J = 6.7 Hz, -CH=CHCH2-), 6.29 (1H, br d, J = 7.0 Hz, -OCH=CH-), 7.31 (2H, br d, J = 8.0 Hz, Ph-H), 7.48 (1H, s, pyr-H), 7.69 (2H, br d, J = 8.0 Hz, Ph-H); 13C-NMR (150 MHz, CDCl3) δ 21.7, 33.8, 68.8, 116.9, 127.7, 128.7, 129.8, 130.1, 131.7, 134.7, 136.7, 145.3, 146.5; HREIMS m/z calcd. for C14H15N2O3S [M]+ 290.0725, found 290.0725.
5,6-Dihydro-1-toluenesulfonyl-1H-oxepino[3,2-c]pyrazole (10c): Oil; IR (film) vmax 1579 (C=C), 1480 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 2.41 (3H, s, CH3-Ar), 2.66–2.70 (2H, m, =CHCH2CH2-), 4.09–4.13 (2H, m, -OCH2CH2-), 6.05 (1H, dt, J = 9.8, 5.3 Hz, -CH=CHCH2-), 7.24 (1H, br d, J = 10.0 Hz, ArCH=CH-), 7.30 (2H, br d, J = 8.2 Hz, Ph-H), 7.44 (1H, d, J = 0.6 Hz, pyr-H), 7.80 (2H, br d, J = 8.2 Hz, Ph-H); 13C-NMR (150 MHz, CDCl3) δ 21.7, 33.8, 68.8, 116.9, 127.7, 128.7, 129.8, 130.1, 131.7, 134.7, 136.7, 145.3, 146.5; HREIMS m/z calcd. for C14H15N2O3S [M]+ 290.0725, found 290.0724.
1-n-Butyl-5,8-dihydro-1H-oxepino[3,2-c]pyrazole (6d): Oil; IR (film) vmax 1658 (C=C) cm−1; 1H-NMR (400 MHz, CDCl3) δ 0.91 (3H, t, J = 7.4 Hz, CH3CH2-), 1.30 (2H, sext, J = 7.4 Hz, CH3CH2CH2-), 1.273 (2H, quint, J = 7.4 Hz, -CH2CH2CH2N-), 3.48–3.50 (2H, m, =CHCH2Ar), 3.90 (2H, t, J = 7.4 Hz, -NCH2CH2-), 4.40–4.44 (2H, m, -OCH2CH), 5.82–5.91 (2H, m, 2 × -CH=CH-), 7.14 (1H, s, pyrazole-H); 13C-NMR (100 MHz, CDCl3) δ 13.7, 19.8, 25.7, 32.2, 49.4, 68.2, 125.9, 126.3, 128.3, 128.7, 143.9; HREIMS m/z calcd. for C11H16N2O [M]+ 192.1263, found 192.1264. * Compound 6d is so unstable to isomerize at room temperature in a couple of days.
Inseparable mixture of 1-n-butyl-7,8-dihydro-1H-oxepino[3,2-c] pyrazole (9d) and 1-n-butyl-5,6-dihydro-1H-oxepino[3,2-c]pyrazole (10d): Oil; IR (film) vmax 1654 (C=C), 1565 (C=C), 1492 (C=C) cm−1; HREIMS m/z calcd. for C26H23N2O [M]+ 192.1263, found 192.1262; 9d: 1H-NMR (600 MHz, CDCl3) δ 1.26 (3H, t, J = 7.3 Hz, CH3CH2-), 1.70–1.78 (2H, m, -CH2CH2N-), 2.42–2.46 (2H, m, =CHCH2CH2-), 2.87–2.90 (2H, m, ArCH2CH2-), 3.98 (2H, t, J = 7.3 Hz, -NCH2CH2-), 4.89 (2H, br q. J = 7.3 Hz, -OCH=CHCH2-), 6.31 (1H, d, J = 7.3 Hz, -OCH=CH-), 7.19 (1H, s, pyrazole-H); 13C-NMR (150 MHz, CDCl3) δ 14.2, 19.1, 23.4, 24.9, 43.3, 60.4, 106.6, 126.5, 127.6, 143.4, 144.4; 10d: 1H-NMR (600 MHz, CDCl3) δ 0.93 (3H, t, J = 7.6 Hz, CH3CH2-), 1.11–1.38 (2H, m, CH3CH2CH2-), 1.70–1.78 (2H, m, -CH2CH2N-), 2.70 (2H, dddd, J = 9.1, 5.3, 1.4 Hz, =CHCH2CH2-), 4.05 (2H, t, J = 7.3 Hz, -NCH2CH2-), 4.13 ( 2H, br dd, J = 4.6, 4.4 Hz, -OCH2CH2-), 5.87 (1H, dt, J = 11.2, 5.5 Hz, -CH2CH=CH-), 6.35 (1H, dd, J =11.2, 0.9 Hz, ArCH=CH-), 7.14 (1H, s, pyrazole-H); 13C-NMR (150 MHz, CDCl3) δ 13.7, 19.9, 32,5, 34.2, 49.7, 68.5, 116.3, 126.8, 128.5, 143.4, 144.4.
1-Benzyl-5,8-dihydro-8-methyl-1H-oxepino[3,2-c]pyrazole (6h): Oil; IR (film) vmax 1583 (C=C), 1496 (C=C), 1456 (C=C) cm−1; 1H-NMR (400 MHz, CDCl3) δ 1.21 (3H, d, J = 7.0 Hz, 8-Me), 3.41 (1H, br quint, J = 6.8 Hz, ArCH (CH3)CH=), 4.36 (1H, dd, J = 15.3, 1.9 Hz, -OCHHCH=), 4.50 (1H, dd, J = 15.3, 5.7 Hz, -OCHHCH=), 5.19 (1H, d, J = 6.3 Hz, -NCHHPh), 5.24 (1H, d, J = 6.3 Hz, -NCHHPh), 5.67 (1H, br dt, J = 12.0, 5.0 Hz, -CH=CHCH2-), 5.77 (1H, dd, J = 12.0, 6.4 Hz, -CH=CHCH-), 7.07 (2H, br d, J = 7.4 Hz, Ph-H), 7.23-7.33 (4H, m, Ph-H, pyr-H); 13C-NMR (100 MHz, CDCl3) δ 21.9, 31.6, 53.5, 68.3, 126.5, 126.7, 127.6, 128.7, 129.4, 131.6, 133.4, 137.1, 142.7; HREIMS m/z calcd. for C15H16N2O [M]+ 240.1263, found 240.1264.
1-Benzyl-7,8-dihydro-8-methyl-1H-oxepino[3,2-c]pyrazole (9h): Oil; IR (liquid film) vmax 1654 (C=C), 1578 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 1.15 (3H, d, J = 7.3 Hz, 8-CH3), 2.03 (1H, ddd, J = 15.0, 8.8, 3.5 Hz, =CHCHHCH-), 2.54 (1H, ddd, J = 15.3, 6.4, 2.9 Hz, -=CHCHHCH-), 3.14–3.1 (1H, m, -CH2CH(CH3)Ar), 4.71 (1H, dddd, J = 7.4, 6.4 Hz, -CH=CHCH2-), 5.22 (1H, d, J = 16.2 Hz, ArCHHPh), 5.29 (1H, d, J =15.9 Hz, ArCHHPh), 6.29 (1H, dd, J = 7.3, 2.6 Hz, -OCH=CH-), 7.05 (2H, br d, J = 7.0 Hz, Ph-H), 7.24–7.327 (4H, s, Ph-H, pyr-H); 13C-NMR (150 MHz, CDCl3) δ 20.9, 30.4, 30.7, 53.9, 103.2, 126.4, 127.6, 128.5, 128.7, 132.7, 137.6, 139.9, 142.2; HREIMS m/z calcd. for C15H16N2O [M]+ 240.1263, found 240.1267.
1-Benzyl-5,6-dihydro-8-methyl-1H-oxepino[3,2-c]pyrazole (10h): Oil; IR (liquid film) vmax 1551 (C=C), 1496 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 2.05 (3H, q, J = 1.2 Hz, 8-CH3), 2.40–2.44 (2H, m, -CH2CH2CH=), 4.13 (2H, br dd, J = 6.6, 4.8 Hz, -CH2CH2O-), 5.45 (2H, br s, NCH2Ph), 5.77 (1H, tq, J = 6.4, 1.2 Hz, -C(CH3)=CHCH2-), 6.95 (2H, br d, J = 7.1 Hz, Ph-H), 7.21–7.31 (3H, m, Ph-H), 7.30 (1H, s, pyr-H); 13C-NMR (150 MHz, CDCl3) δ 22.2, 30.3, 56.2, 73.8, 125.8, 126.4, 126.5, 127.3, 128.6, 129.7, 129.8, 138.2, 143.5; HREIMS m/z calcd. for C15H16N2O [M]+ 240.1263, found 240.1267.
1-Benzyl-5,8-dihydro-8-phenyl-1H-oxepino[3,2-c]pyrazole (6j): Oil; IR (film) vmax 1585 (C=C), 1495 (C=C), 1455 (C=C) cm−1; 1H-NMR (400 MHz, CDCl3) δ(400 MHz, CDCl3) δ 4.41 (1H, ddt, J = 15.0, 4.1, 1.5 Hz, -OCHHCH=), 4.51 (1H, d, J = 4.9 Hz, ArCHPhCH=), 4.52 (1H, br dd, J = 15.0, 5.6 Hz, -OCHHCH=), 4.80 (1H, d, J = 16.0 Hz, NCHHPh), 5.13 (1H, d, J = 16.0 Hz, NCHHPh), 5.70 (1H, dddd, J = 12.0, 5.1, 3.5, 0.8 Hz, -OCH2CH=CH-), 5.79 (1H, br dd, J = 12.0, 6.0 Hz, -CH=CHCHPh), 6.92 (2H, br d, J = 8.0 Hz, Ph-H), 7.12–7.39 (8H, m, Ph-H), 7.32 (1H, s, pyr-H); 13C-NMR (100 MHz, CDCl3) δ 43.6, 53.8, 68.2, 126.3, 126.5, 127.1, 127.5, 127.6, 128.7, 128.7, 129.4, 129.9, 131.0, 136.7, 141.2, 144.5; HREIMS m/z calcd. for C20H18N2O [M]+ 302.1419, found 302.1412.
1-Benzyl-7,8-dihydro-8-phenyl-1H-oxepino[3,2-c]pyrazole (9j): Oil; IR (liquid film) vmax 1653 (C=C), 1577 (C=C), 1559 (C=C) cm−1; 1H-NMR (400 MHz, CDCl3) δ 2.33 (1H, ddd, J = 14.2, 9.2, 4.1 Hz, -CHCHHCH=), 2.78–2.84 (1H, m, -CHCHHCH=), 4.25 (1H, br t, J = 3.9 Hz, ArCH(Ph)CH2-), 4.67 (1H, ddd, J = 8.8, 6.9, 4.9 Hz, -OCH=CHCH2-), 4.75 (1H, d, J = 16.0 Hz, NCHHPh), 5.15 (1H, d, J = 16.0 Hz, NCHHPh), 6.43 (1H, dd, J = 6.7, 2.2 Hz, -OCH=CH-), 6.93 (2H, br d, J = 5.7 Hz, Ph-H), 7.07 (2H, br d, J = 6.5 Hz, Ph-H), 7.20–7.39 (6H, m, Ph-H), 7.39 (1H, s, pyr-H); HREIMS m/z calcd. for C20H18N2O [M]+ 302.1419, found 302.1415. 13C-NMR spectrum of 9j could not be measured due to poor amount of the material.
5,8-Dihydro-2H-2-trityloxepino[3,2-c]pyrazole (7a); White powder (CH2Cl2); m.p. 42–48 °C; IR (film) vmax 1670 (C=C), 1494 (C=C), 1446 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 3.60 (2H, m, =CHCH2Ar), 4.47 (2H, m, 4.7 Hz, -OCH2CH=), 5.80 (1H, m, -CH=CHCH2Ar), 6.00 (1H, m, -OCH2CH=CH-), 6.98 (1H, s, py-H), 7.14–7.18 (6H, m, Tr-H), 7.27–7.34 (9H, m, Tr-H); 13C-NMR (150 MHz, CDCl3) δ 27.8, 68.6, 78.6, 121.5, 127.1, 127.56, 127.62, 129.5, 130.1, 140.1, 142.6, 143.3; HREIMS m/z calcd. for C26H22N2O [M]+ 378.1732, found 378.1730.
7,8-Dihydro-2H-2-trityloxepino[3,2-c]pyrazole (11a): Colorless crystals (CH2Cl2/hexane); m.p. 120–123 °C; IR (KBr) vmax 1650 (C=C), 1506 (C=C), 1492 (C=C), 1445 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 2.39 (2H, m, -=CHCH2CH2-), 2.97 (2H, m, ArCH2CH2-), 4.80 (1H, dt, J = 7.8, 5.9 Hz, -CH=CHCH2-), 6.21 (1H, br dt, J = 7.8, 1.5 Hz, -OCH=CHCH2-), 7.01 (1H, s, py-H), 7.04–7.08 (6H, m, Tr-H), 7.28–7.31 (9H, m, Tr-H); 13C-NMR (150 MHz, CDCl3) δ 24.8, 27.8, 78.2, 106.1, 121.3, 127.61, 127.64, 130.2, 139.8, 141.4, 142.0, 143.2; HREIMS m/z calcd. for C26H22N2O [M]+ 379.1732, found 378.1730.
5,6-Dihydro-2H-2-trityloxepino[3,2-c]pyrazole (12a): Colorless crystals (CH2Cl2/hexane); m.p. 147–151 °C; IR (KBr) vmax 1565 (C=C), 1492 (C=C), 1445 (C=C) cm−1; 1H-NMR (600 MHz, CDCl3) δ 2.67 (2H, m, =CHCH2CH2-), 4.14 (2H, br t, J = 4.7 Hz, -OCH2CH2-), 5.88 (1H, dt, J = 11.2, 5.0 Hz, -CH=CHCH2-), 6.54 (1H, br d, J = 11.2 Hz, ArCH=CHCH2-), 6.95 (1H, d, J = 0.6 Hz, py-H), 7.15–7.19 (6H, m, Tr-H), 7.27–7.30 (9H, m, Tr-H); 13C-NMR (150 MHz, CDCl3) δ 34.1, 69.6, 78.5, 120.3, 123.1, 127.61, 127.65, 128.4, 130.2, 140.1, 142.8, 143.2; HREIMS m/z calcd. for C26H22N2O [M]+ 378.1732, found 378.1728.
5,8-Dihydro-2H-2-toluenesulfonyloxepino[3,2-c]pyrazole (7c): Oil; IR (film) vmax 1670 (C=C), 1494 (C=C), 1446 (C=C) cm−1; 1H-NMR (CDCl3) δ 2.42 (3H, s, Ar-CH3), 3.56 (2H, dq, J = 5.3, 1.8 Hz, =CHCH2Ar), 4.48 (2H, dq, J = 5.3, 1.2 Hz, =CHCH2O-), 5.80 (1H, dtt, J = 10.3, 5.3, 1.8 Hz, -CH=CHCH2Ar), 5.97 (1H, dtt, J = 10.3, 5.0, 1.2 Hz, -OCH2CH=CH-), 7.30 (2H, br d, J = 8.6 Hz, Ar-H), 7.66 (1H, s, py-H), 7.84 (2H, br d, J = 8.2 Hz, Ar-H); 13C-NMR (CDCl3) δ 21.7, 27.2, 68.1, 118.4, 127.3, 127.9, 128.6, 129.9, 134.3, 145.5, 145.8, 148.2; HREIMS m/z calcd. for C14H14N2O3S [M]+ 290.0725, found 290.0723.
Dihydro-2H-2-toluenesulfonyloxepino[3,2-c]pyrazole (11c): White power; m.p. 98–102 °C; IR (KBr) vmax 1657 (C=C), 1589 (C=C) cm−1; 1H-NMR (CDCl3) δ2.31–2.34 (2H, m, -CH2CH2CH2-), 2.42 (3H, s, Ar-CH3), 2.93–2.96 (2H, m, -CH2CH2Ar), 4.84 (1H, dt, J = 7.6, 5.9 Hz, -CH=CHCH2-), 6.20 (1H, dt, J = 7.6, 1.5 Hz, -CH=CHAr), 7.33 (2H, br d, J = 8.5 Hz, Ar-H), 7.72 (1H, s, py-H), 7.85 (2H, br d, J = 8.5 Hz, Ar-H); 13C-NMR (100 Hz, CDCl3) δ 21.7, 23.6, 27.8, 106.7, 118.2, 128.0, 130.0, 134.1, 141.6, 143.3, 145.6, 149.4; HREIMS m/z calcd. for C14H14N2O3S [M]+ 290.0725, found 290.0722.
5,6-Dihydro-2H-2-toluenesulfonyloxepino[3,2-c]pyrazole (12c): White power; m.p. 114–117 °C; IR (KBr) vmax 1587 (C=C), 1481 (C=C) cm−1; 1H-NMR (CDCl3) δ 2.41 (3H, s, Ar-CH3), 2.64–2.68 (2H, m, -CH2CH2CH2-), 4.11 (2H, br t, J = 14.7 Hz, -CH2CH2O-), 6.10 (1H, dt, J = 11.4, 5.0 Hz, -CH=CHCH2-), 6.51 (1H, br d, J = 11.4, Hz, ArCH=CH-), 7.31 (2H, br d, J = 8.2 Hz, Ar-H), 7.63 (1H, d, J = 0.8 Hz, py-H), 7.85 (2H, br d, J = 8.2 Hz, Ar-H); 13C-NMR (CDCl3) δ 21.7, 33.9, 69.8, 117.1, 121.7, 128.0, 129.9, 128.6, 129.9, 133.7, 145.2, 147.6; HREIMS m/z calcd. for C14H14N2O3S [M]+ 290.0725, found 290.0725.
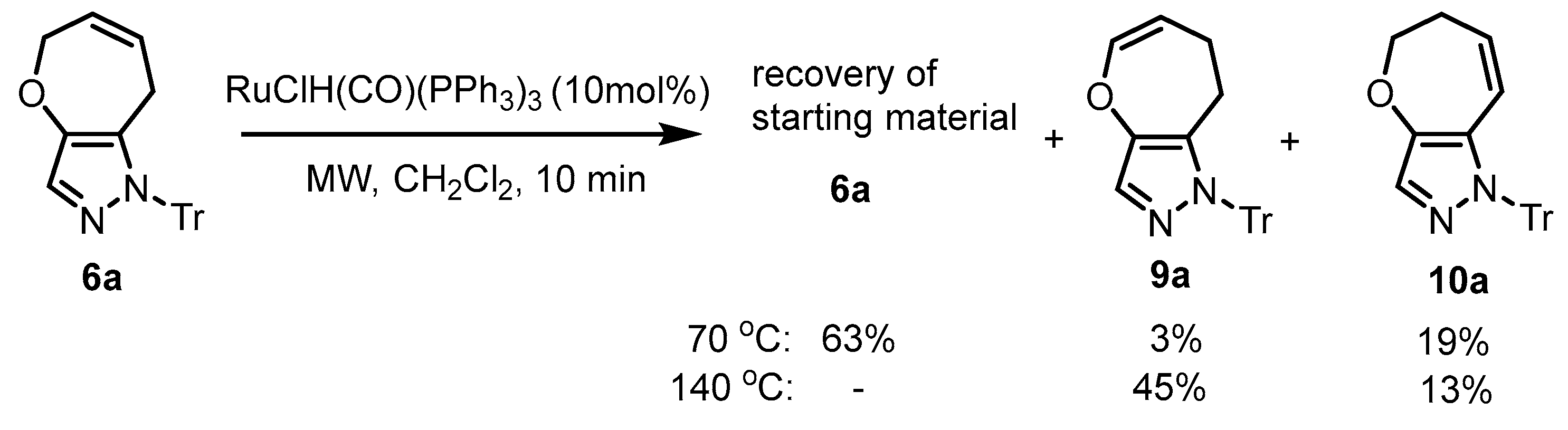
3.6. Double-Bond Migration of 5,8-Dihydro-1H-1-trityloxepino[3,2-c]pyrazole (6a) Catalyzed by Ruthenium Hydride Species
To a CH2Cl2 solution (4 mL) of 6a (9.6 mg, 0.025 mmol) in a MW vial (2–5 mL), RuClH(CO)(PPh3)3 (2.6 mg, 0.0027 mmol) was added. The vial was sealed and heated under MW irradiation at 140 °C for 10 min. The cooled reaction mixture was evaporated under reduced pressure. The residue was purified by preparative TLC (eluent: hexane/EtOAc = 10:1 v/v), affording 9a (4.3 mg, 45%) and 10a (1.2 mg, 13%).