Abstract
A series of symmetrical dibenzylidene derivatives of cyclobutanone were synthesized with the goal of studying the physicochemical properties of cross-conjugated dienones (ketocyanine dyes). The structures of the products were established and studied by X-ray diffraction and by NMR and electronic spectroscopy. All the products had E,E-geometry. The oxidation and reduction potentials of the dienones were determined by cyclic voltammetry. The potentials were shown to depend on the nature, position, and number of substituents in the benzene rings. A linear correlation was found between the difference of the electrochemical oxidation and reduction potentials and the energy of the long-wavelength absorption maximum. This correlation can be employed to analyze the properties of other compounds of this type. Quantum chemistry was used to explain the observed regularities in the electrochemistry, absorption, and fluorescence of the dyes. The results are in good agreement with the experimental redox potentials and spectroscopy data.
1. Introduction
The enormous synthetic potential of the carbon–carbon bond conjugated to a carbonyl group has been long and successfully used in organic chemistry. A typical example is the Michael reaction and its numerous varieties [1]. The introduction of two double bonds in conjugation with a keto group enables some additional reactions [2]. These compounds, called cross-conjugated dienones, ketocyanine dyes, or diarylidene ketone derivatives, attract researchers’ attention for their versatile synthetic chemistry and extensive applicability, first of all, in biology and medicine [3,4,5]. One more potential application of cross-conjugated dienones is the design of photoactive materials [6,7,8,9].
The known methods for the synthesis of these compounds have been developed primarily for dienones derived from cyclopentanone or cyclohexanone and various aromatic aldehydes. Their homologues with larger or smaller rings have been rarely addressed, which may be attributable to the poor availability of the corresponding cycloalkanones as well as side reactions decreasing the yield of the target compound. The first attempts to synthesize a diarylidene derivative of cyclobutanone through aldol condensation, catalyzed by 60% KOH in ethanol, were described in [10]. However, it was shown later that the dienone obtained in that paper was a dimer of 2,4-dibenzylidenecyclobutanone. Upon the action of the hydroxide, a fast dimerization of the resulting dienone takes place. It was found that the susceptibility to dimerization increases with the temperature and the basicity of the reaction mixture [11,12]. Another possible reason for the low reaction yield can be side processes arising from the high reactivity of cyclobutanone, which is susceptible to self-condensation in basic media [13,14]. Therefore, only single examples of 2,4-dibenzylidenecyclobutanones have been characterized in the literature [11,12,13,14,15]. Meanwhile, recently, cross-conjugated dienones derived from cyclobutanone have attracted attention as sensitizers to the generation of singlet oxygen for the photodynamic therapy of cancer [16,17].
Apart from participation in additional reactions, double bonds are also responsible for two important properties of dienones. First, a prominent feature of dienones is the existence of E- and Z-isomers, which can be interconverted under the action of various stimuli such as light, acids, or transition metals [18,19,20,21,22]. Most often, the E,E-isomer is most stable in the series of cyclopentanone and cyclohexanone derivatives. The proportion of E,Z- and Z,Z-isomers increases with the increasing ring size [3,20]. For cyclobutanone-derived dienones, information of this type is scarce.
One more feature of this class of compounds is the ability to undergo [2 + 2]-photocycloaddition (PCA) reaction [20,23,24]. In the case of free dienones, this reaction can proceed both in the crystal and in solution. The possibility and the stereoselectivity of PCA can be controlled by the supramolecular preorganization of double bonds, which may ensure the most appropriate geometry for the preceding dimer pair [25,26,27]. In the case of crystalline pyridine derivatives, such reactions can be accomplished using supramolecular templating (initiation) by metal complexes [28], resorcinol (pyridine-containing monoenones and acyclic dienones [29]), or silver ions (only pyridine-containing acyclic dienones were studied) [30,31] The possibility of PCA for 2,4-dibenzylidenecyclobutanones has not been discussed in the literature.
With the purpose of developing photoswitchable supramolecular systems, we started a comprehensive study of cross-conjugated dienones containing crown-ether moieties as ionophore substituents [32,33]. This research is meant to design hybrid molecules combining two functional moieties: an ionophore able to bind metal and ammonium cations and guest molecules, and a photoswitchable moiety needed for controlled binding, using light as an energy source.
Gaining a more in-depth understanding of the involved photochemical transformations required a detailed investigation of the properties and physicochemical characteristics of the model compounds. In addition, it was necessary to elucidate the possibility of a PCA reaction in the crystal without supramolecular preorganization of the reacting double bonds.
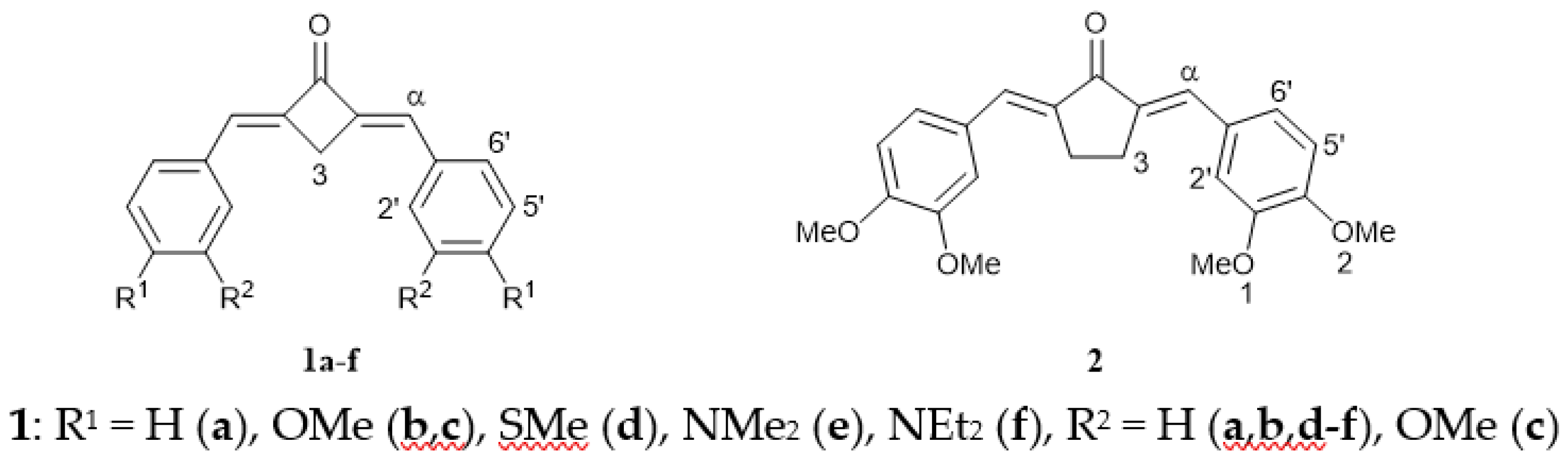
Here, we synthesized and studied dienones 1a–f, differing in the nature and number of alkoxy, alkylthio, and dialkylamino substituents (Figure 1). The structures in question are chromophore models of crown ethers and aza/thia-crown ethers. Cyclobutanone was chosen as the central moiety as an analog of cyclopentanone derivatives, such as 2, and cyclohexanone derivatives, addressed in our previous studies [34,35].

Figure 1.
Structure of compounds 1a–f and 2.
The structures of compounds were determined by X-ray diffraction analysis and by NMR and electronic spectroscopy. X-ray diffraction analysis was used to find out whether the arrangement of double bonds of neighboring molecules is favorable for the PCA to occur in the crystal without a supramolecular effect. Cyclic voltammetry was employed to determine the oxidation and reduction potentials in order to elucidate the dependences of the energy characteristics of the molecules on the position, nature, and number of substituents in the benzene rings. Quantum chemistry was used to find the preferred conformation of 1c, to relate the electrochemical and photochemical data, and to elucidate the mechanism of luminescence emission and quenching in 1a–f. In addition, we attempted to identify a correlation between the photophysical and electrochemical characteristics of dienones 1a–f.
2. Results and Discussion
2.1. Synthesis of 1a–f
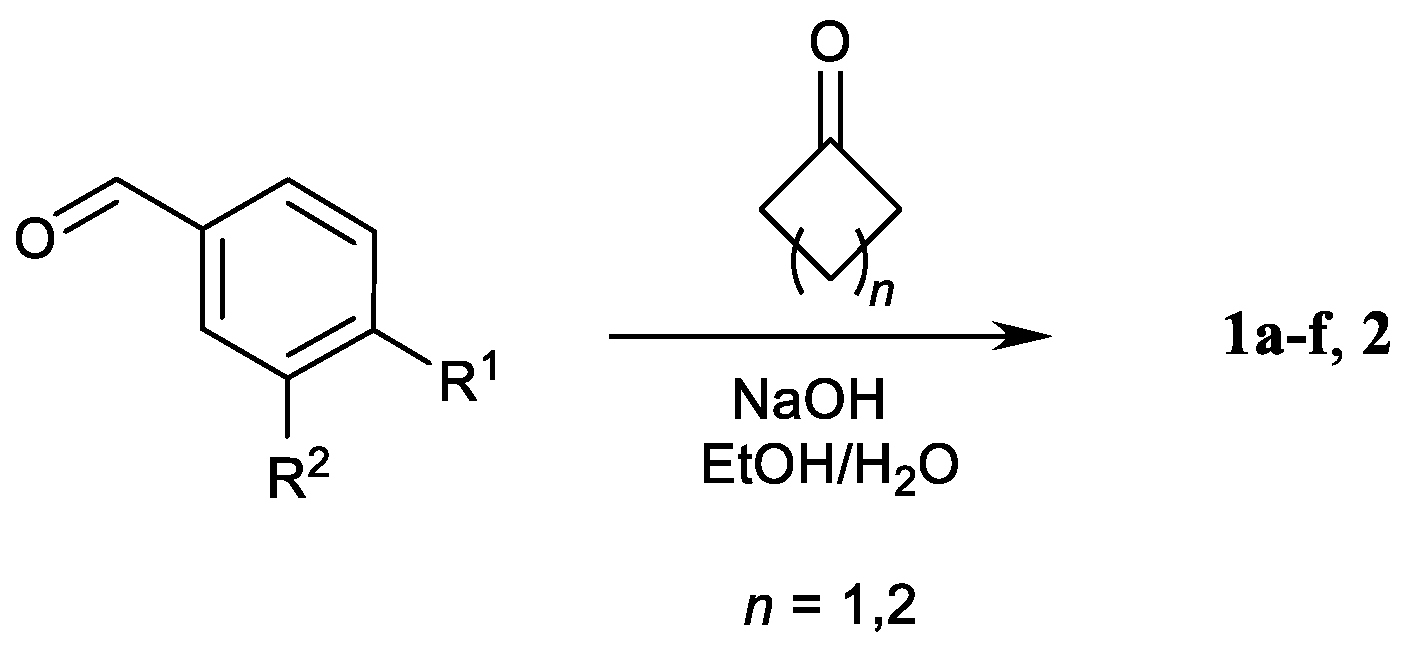
Compounds 1a–f and 2 were synthesized by alkaline aldol-crotonic condensation of cyclobutanone and cyclopentanone, respectively, with two equivalents of substituted benzaldehyde (Claisen–Schmidt reaction, Scheme 1), carried out similarly to the reported procedures [10,11,12,13,14,15,20,34].

Scheme 1.
Synthesis of dienones 1a–f,2.
Compounds 1a–f were isolated as bright-colored crystalline solids. X-ray diffraction data were obtained for all the compounds, except for 1a (for details, see below), and indicated that all the dienones were formed as E,E-isomers.
A similar conclusion can be drawn from the data of NMR spectroscopy. Indeed, the chemical shifts of the olefinic protons of dienones 1a–f in the 7.01–7.19 ppm range attest to the E,E-isomers [20].
2.2. X-ray Diffraction Analysis
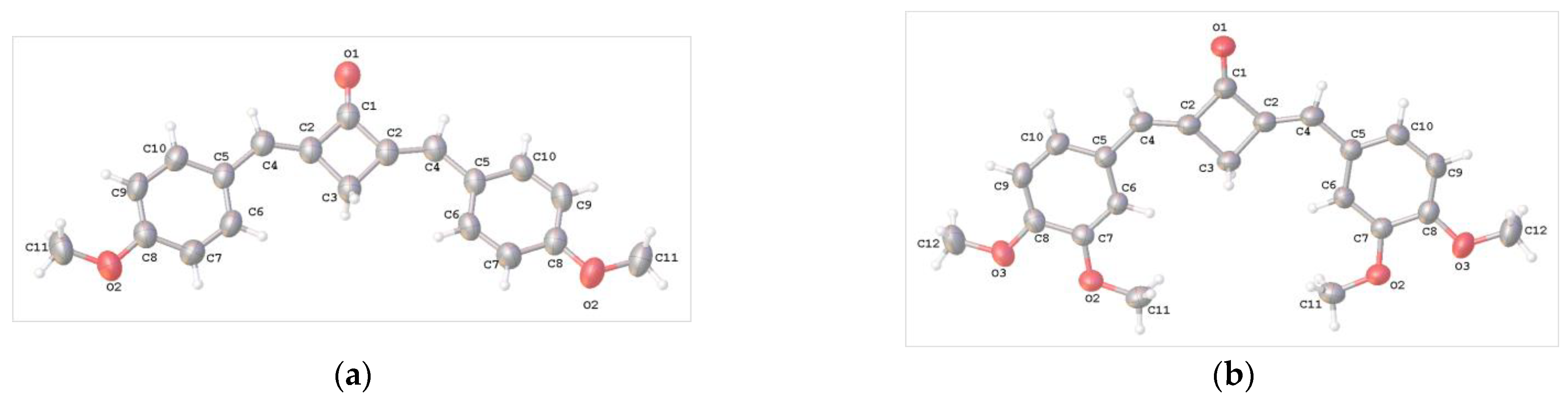
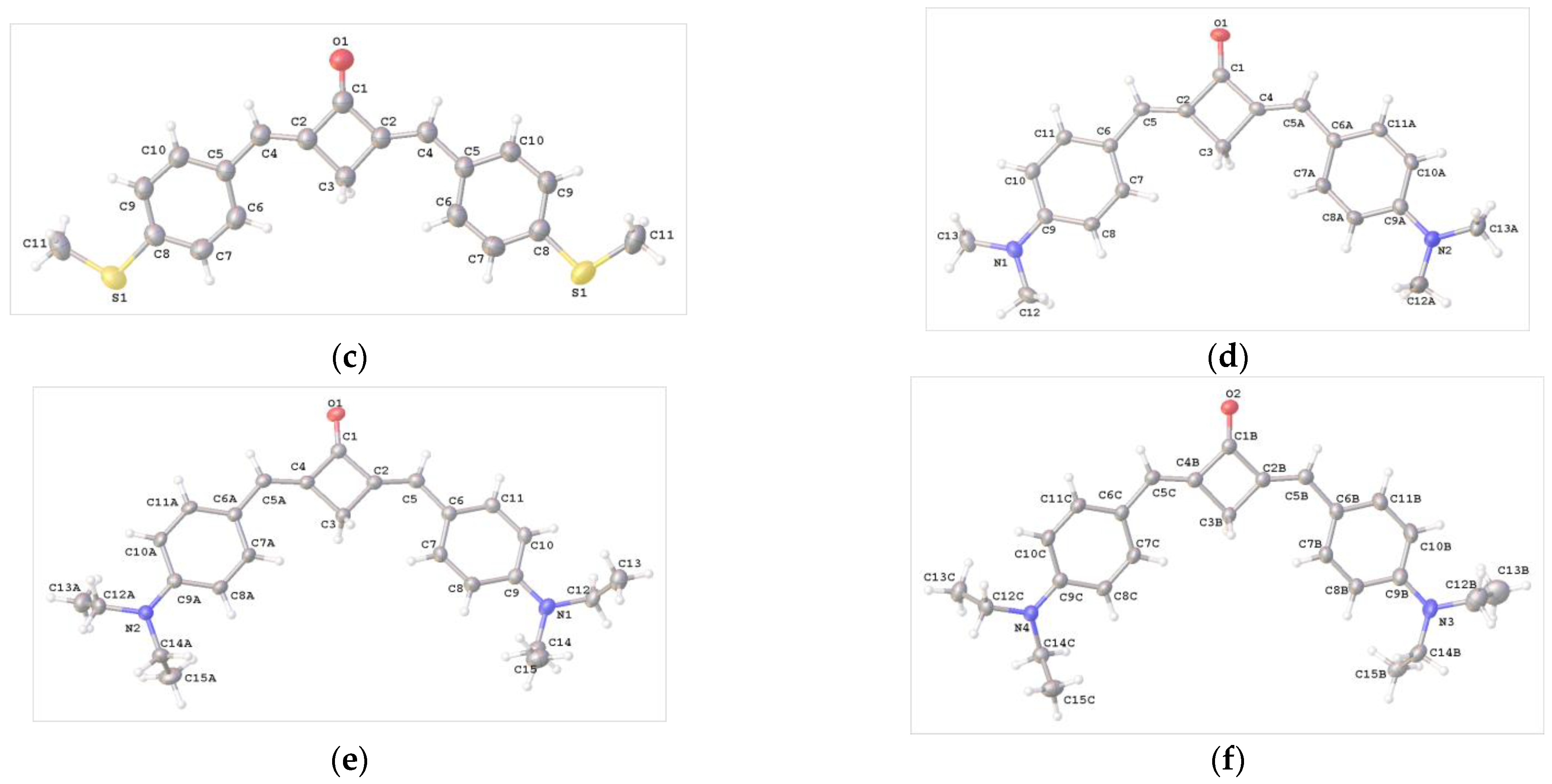
The crystals of compounds 1b–f and 2 (syn,syn- and anti,anti-conformers) suitable for X-ray diffraction study were grown from MeCN solutions. Each of these crystals was subjected to X-ray diffraction analysis. The solved structures of 1b-f supported the results of the NMR spectroscopic study, indicating that only E,E-isomers formed for the dibenzylidenecyclobutanone molecules. The structures of compounds 1b–f are depicted in Figure 2.
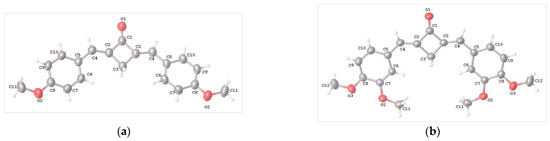
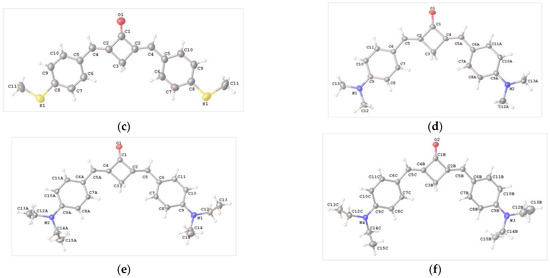
Figure 2.
Molecular structure and atom numbering in crystals 1b (a), 1c (b), 1d (c), 1e (d) and two independent units 1f (e,f).
In the crystals of 1b–d, the molecule occupies a special position on a twofold axis, whereas in 1e, the molecule is in a general position. In the crystal of 1f, there are two crystallographically independent molecules [1f (A) and 1f (B)]. In all of the compounds, the molecular skeleton is nearly planar.
Selected bond lengths and bond angles for 1b–f are listed in Table S1, Supplementary Materials.
The geometric parameters of both the independent molecules of 1f are actually identical. Figure 3 shows a superposition of these molecules. Only small differences in the torsion angles associated with the benzyl-ring orientation can be observed.

Figure 3.
Superposition of independent molecules (A and B) in crystal structure 1f.
It is seen from the data of Table S1 (Supplementary Materials) that the most important corresponding geometric parameters are well reproduced for molecules 1b–f, containing the central four-membered ring. In particular, a significant deformation of the exocyclic angles at the C2 atoms of the four-membered ring is observed, with the C1-C2-C4 angle being ~10° smaller than the C3-C2-C4 angle. Some deformation of the cyclobutane ring angles is also noted: the C2-C3-C2 angle is smaller than the other angles of the ring. One would assume that the above-mentioned non-equivalence in the exocyclic bond angles might be minimized via the rotation of the benzene rings around the C4-C5 bonds. However, this is not observed. The reason may be a significant conjugation over the whole Ph-C=C-C(O)-C=C-Ph moiety, although the bond length distribution does not display an elongation of the carbonyl bond and C=C double bonds. These bond lengths are close to the normal values for localized bonds. The 1-2-4-5 moieties are nearly planar, the corresponding torsion angles are close to 180° or 0°.
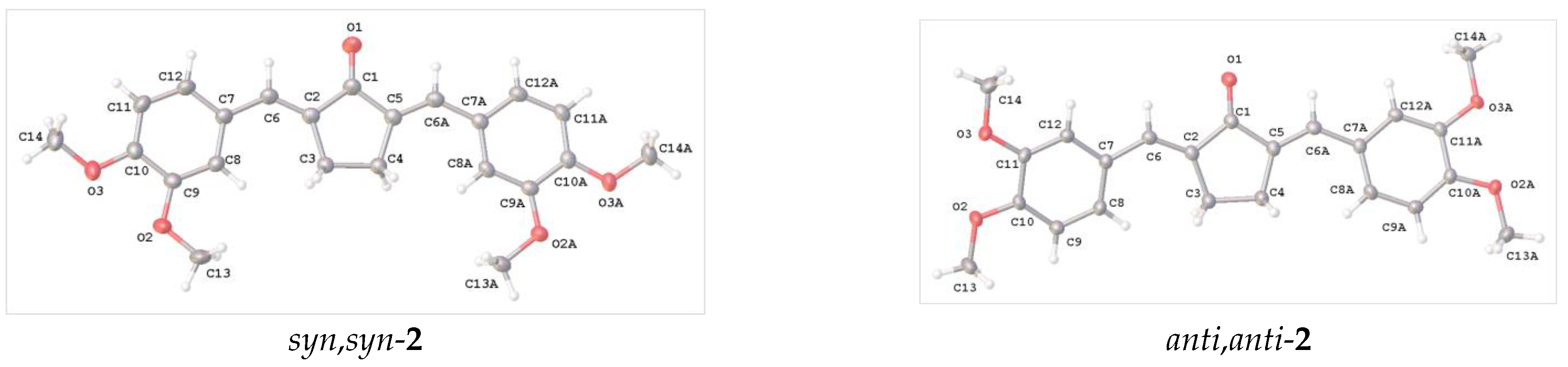
It is pertinent to compare the data of Table S1 (Supplementary Materials) with the corresponding data for compounds 2 based on a five-membered central ring (2,5-dibenzylidenecyclopentanones) (syn,syn- and anti,anti-conformers). Figure 4 shows the molecular structures of two such compounds (syn,syn-2 and anti,anti-2), while Table S2 (Supplementary Materials) lists selected geometric parameters of the molecules.

Figure 4.
Structure and atom numbering of molecules syn,syn-2 and anti,anti-2.
The geometric parameters from Table S2 (Supplementary Materials) demonstrate close similarity for both (syn,syn- and anti,anti-) conformers of 2. These data well agree with the corresponding values for other derivatives of dibenzylidenecyclopentanones [34,36]. Moreover, the most important features are reproduced for both five- and four-membered ring systems. In particular, this is the planarity of the molecule, bond-length alternation within the Ph-C=C-C(O)-C=C-Ph moiety, and also the same type of exocyclic angle deformation.
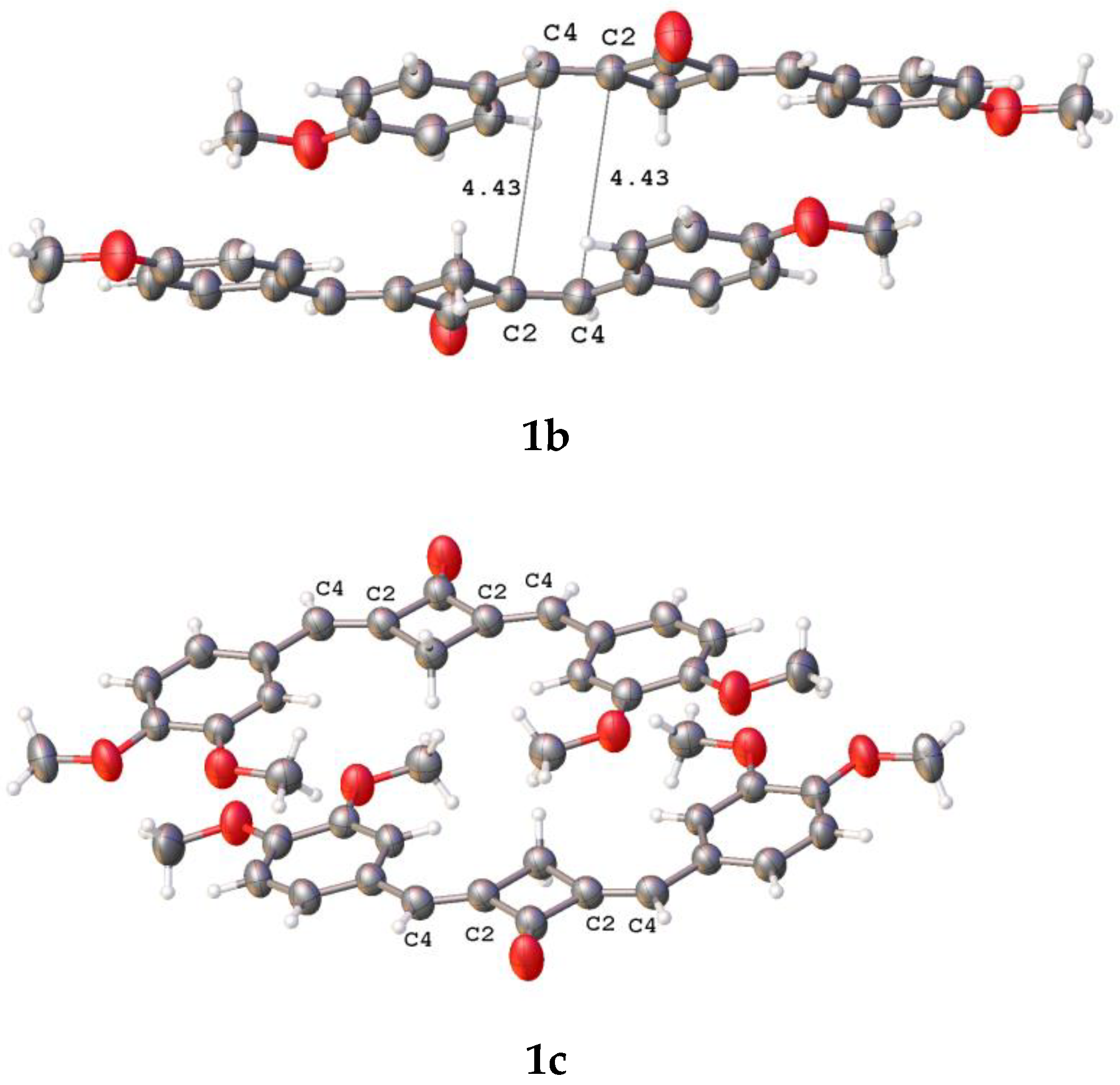
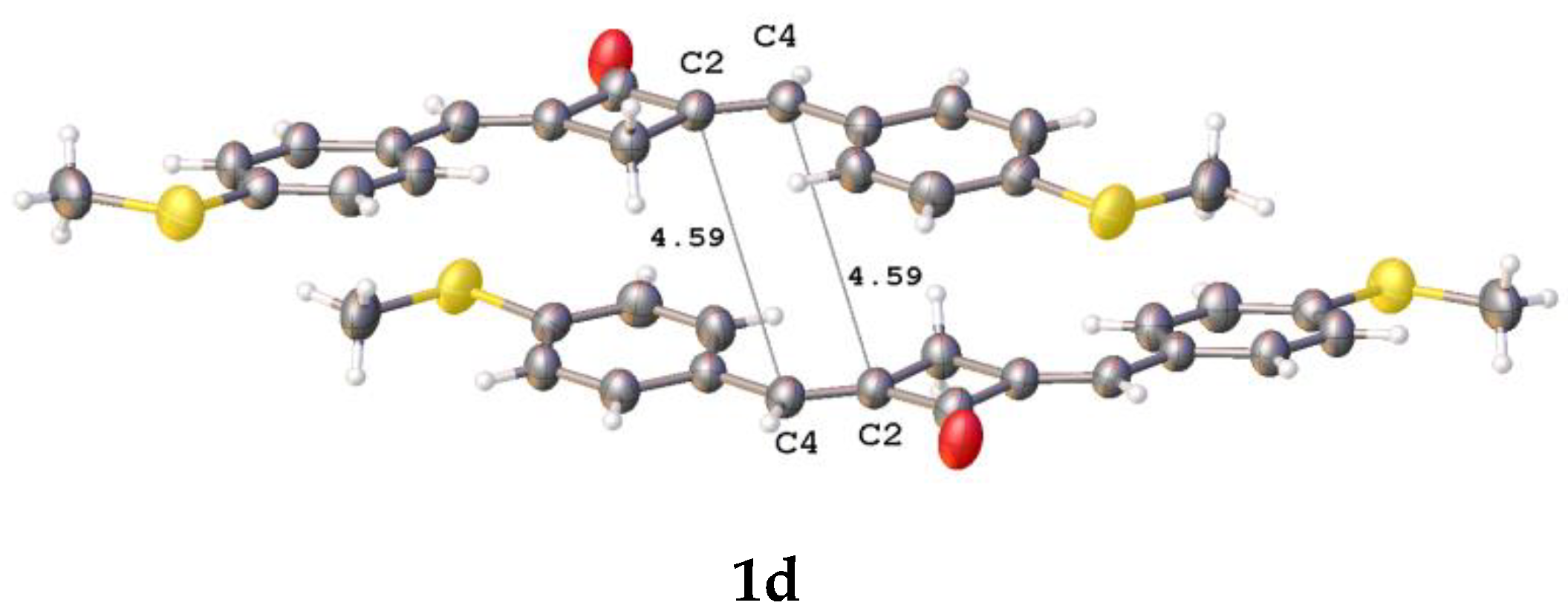
Earlier, it was established that the vast majority of conjugated planar molecules form six canonic types of crystal packings, two of which (stacking and parallel-dimeric packing) are favorable for the solid-state [2 + 2] photochemical reaction [27]. In spite of the planarity of these molecular systems (1b–f and 2), they do not form the canonic packing motifs in the crystalline state. Relatively close contacts between the ethylene bonds of the adjacent molecules were observed only in the structures of 1b–d (Figure 5).
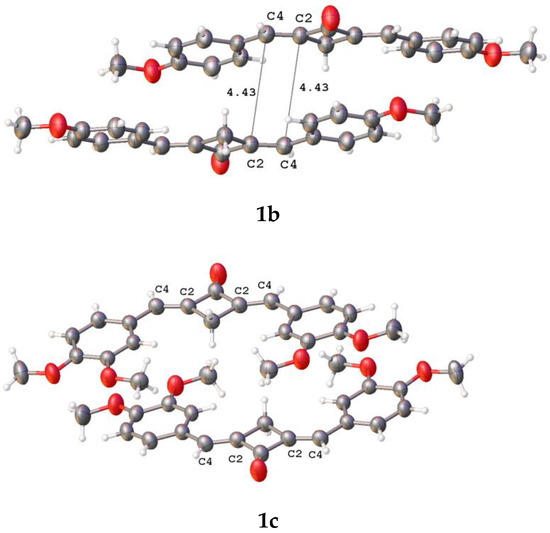
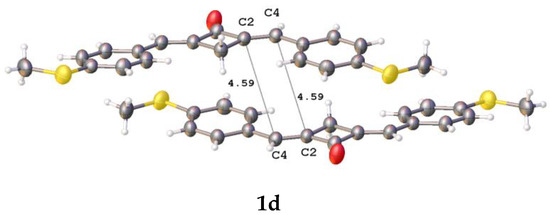
Figure 5.
Fragments of crystal packings involving two adjacent molecules with close intermolecular ethylene-ethylene contacts in crystals of 1b–d; the distances are given in Å.
The distance between the ethylenic bonds in 1c is longer than 5 Å. In all the cases, the molecules in a pair are shifted with respect to one another in parallel planes. The distances between the ethylenes are much longer than 4.2 Å, which makes the PCA reaction in these molecules impossible [27]. Apparently, the PCA reaction of compounds 1b–f and 2 in the solid state requires the use of a supramolecular template.
The results of the quantum chemical calculations are in line with the X-ray diffraction data. The 5-4-3-2 torsion angles decrease from 25° for the unsubstituted dienone 1a to 16–17° for 1e,f. This trend is correlated with the variation of the electron-donating properties of the substituents in the benzene rings of the dienones.
2.3. NMR Spectroscopy
NMR spectroscopy can serve to elucidate the fine structure of organic molecules and molecular assemblies in solutions [37]. The NMR data are rarely compared with the structures of compounds known from a crystallographic analysis because it is impossible to obtain high-quality crystals for the whole series. In the case of dienones, the determination of their conformational behavior in solutions is especially important for the prediction and determination of the structures of supramolecular systems based on bis-crown-containing dienones [32]. Therefore, we studied the structural characteristics of (E,E)-dienones 1c and 2 using various NMR techniques.
In the crystalline state, (E,E)-tetramethoxydienone 1c and (E,E)-tetramethoxydienone 2 occur as nearly planar symmetrical syn,syn-, syn,syn-, and anti,anti-conformers, respectively, which is probably attributable to the requirement of the close packing of molecules. Upon dissolution, a fast, conformational equilibrium may be established between the symmetrical syn,syn- and anti,anti- and unsymmetrical syn,anti-conformers (Figure 4), by analogy with the previously studied bis-crown-containing stilbenes and distyrylbenzenes [38,39].
The NOESY spectrum of compound 1c, which is given in Figure S9, Supplementary Materials (the atom numbering differing from IUPAC rules is presented in Figure 1), and the spectrum of 2 in CD2Cl2 show averaged signals from the different conformers. The spectrum of dienone 1c has an intense cross-peak, corresponding to the through-space intramolecular interaction of the H(2′) protons of the benzene ring with the H(3) methylene protons of the cyclobutanone moiety, and a less intense cross-peak between these protons and the H(6′) aromatic protons. It is noteworthy that the spectrum contains no cross-peaks between the H(2′) and H(6′) protons of the benzene ring and the H(α) protons of the ethylene bonds (Figure S9, Supplementary Materials). This overall spectral pattern can be interpreted by assuming that the syn,(syn/anti)-conformers predominate in the equilibrium.
The NOESY spectrum of dienone 2 also exhibits intense intramolecular cross-peaks, indicating the coupling of the H(2′) and H(6′) protons of the benzene ring and the H(3) methylene protons of the cyclopentanone moiety. Apart from the indicated cross-peaks, the H(6′) aromatic protons of dienone 2 give a small NOESY cross-peak with the H(α) ethylene protons [35].
Although the NOESY spectra do not enable an accurate estimate of the contribution of each conformer of 1c and 2 to the equilibria due to the strong coupling of the methylene protons with both type H(2′) and type H(6′) protons in all the conformers, the revealed spectral features provide the conclusion that syn,(syn/anti)-conformers predominate in the solution in both cases.
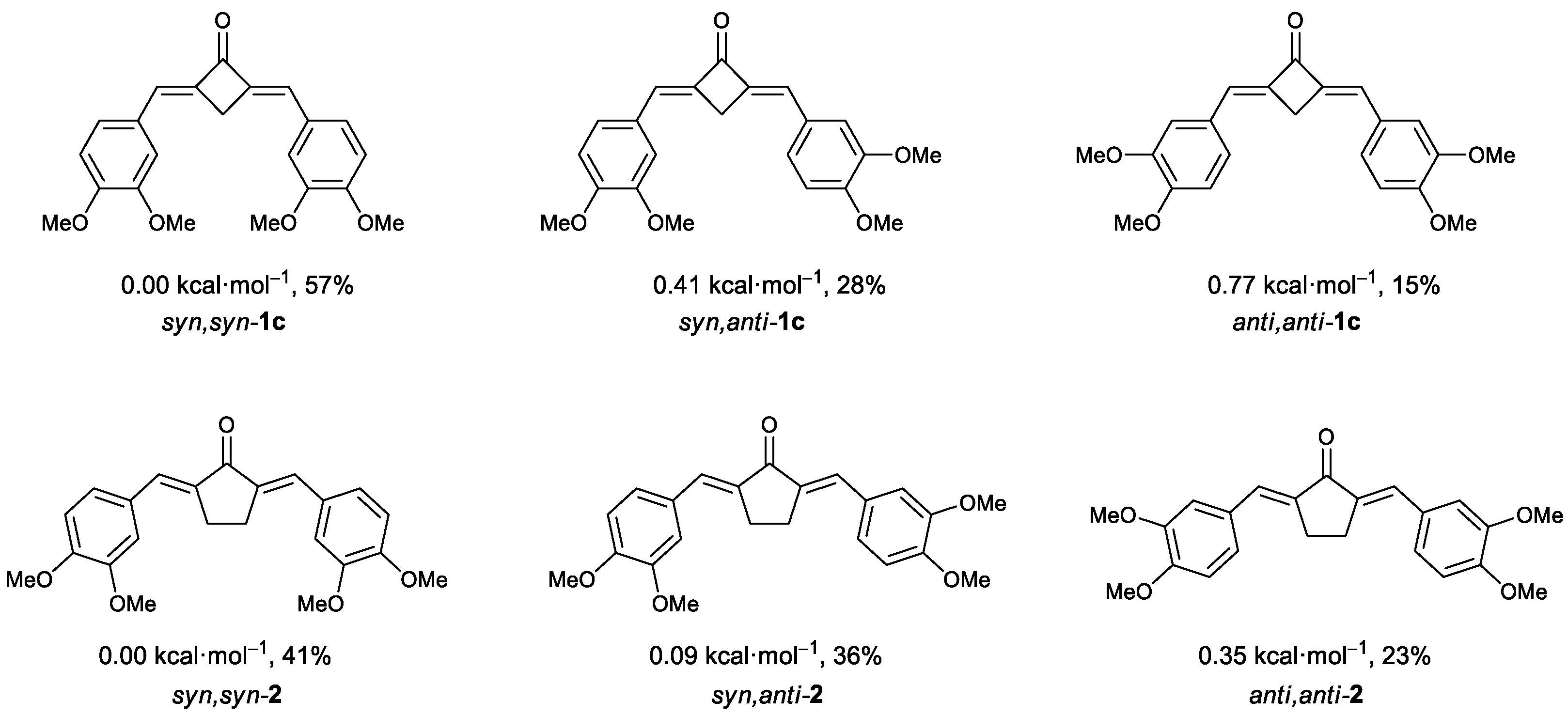
Using the data on the energies of stable structures for these compounds found by the FireFly program package, we calculated the theoretical ratio between the three possible conformers for dienones 1c and 2 (Figure 6).
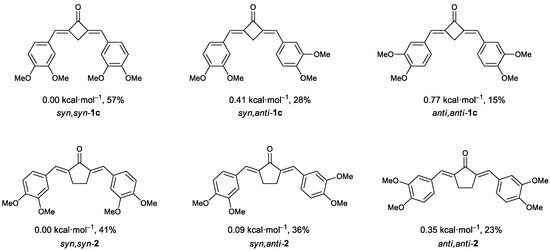
Figure 6.
Possible conformers of (E,E)-dienones 1c and 2 and their stability relative to the most favorable syn,syn-conformers. The mole fractions of conformers at room temperature in CD2Cl2 are given in %; the values were determined by the Boltzmann equations using the relative energies of the conformers.
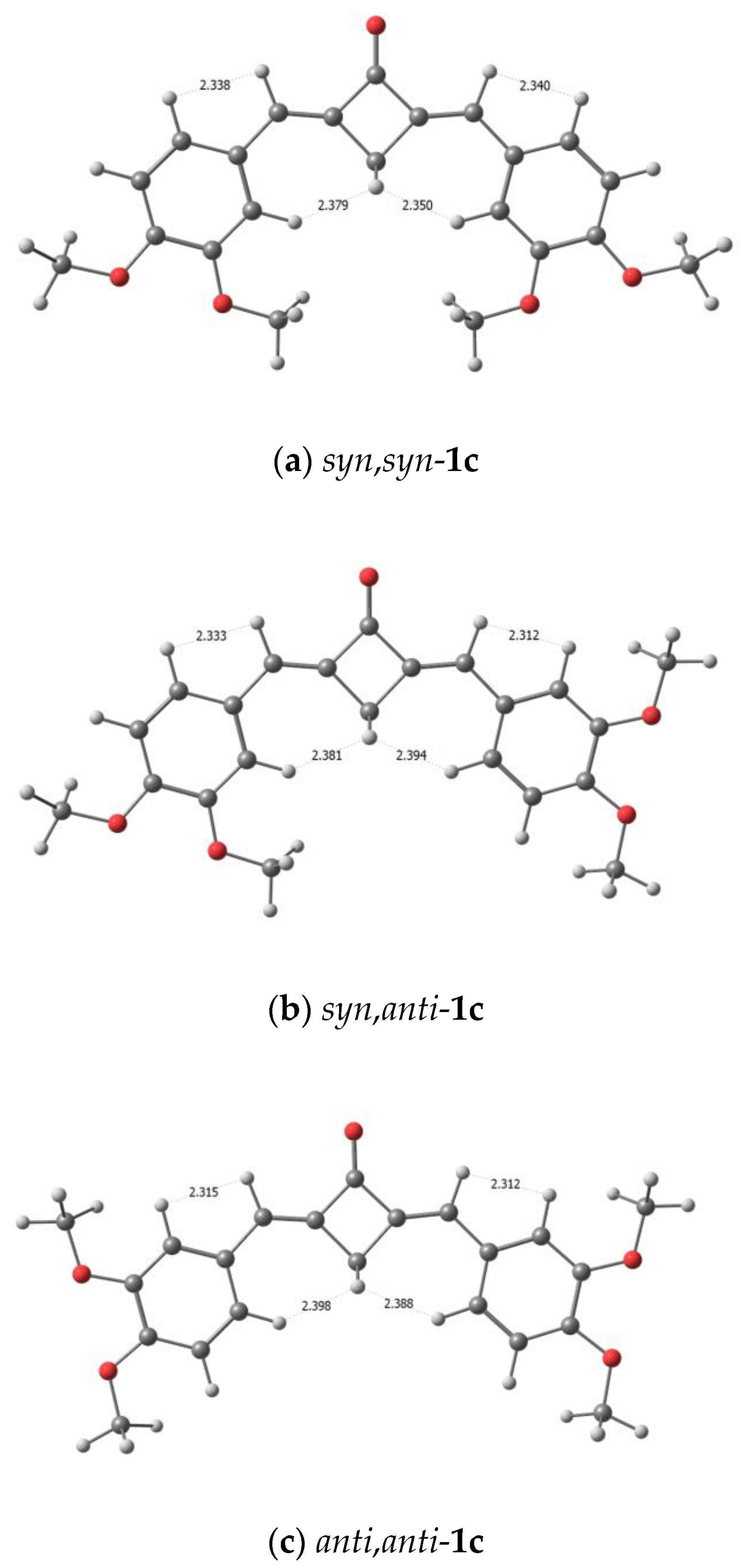
The theoretical 1H NMR spectra of conformers of (E,E)-dienone 1c and 2 simulated using their mole fractions are in good agreement with the experimental data (Table 1); in particular, the calculations predict higher field positions for the H(5′) meta-protons than for the H(6′) and H(2′) ortho-protons. The calculated distances between the H(2’), H(6’) protons and the H(α) methine protons, or between the H(2’), H(6’) protons and the H(3) methylene protons, are sufficient for the manifestation of NOE interactions, see Figure 7. The conformer models predict the possible appearance of NOESY cross-peaks between these groups of protons.

Table 1.
Proton chemical shifts of compounds 1c and 2 averaged taking account of the mole fractions of conformers in comparison with experimental data (in CD2Cl2).
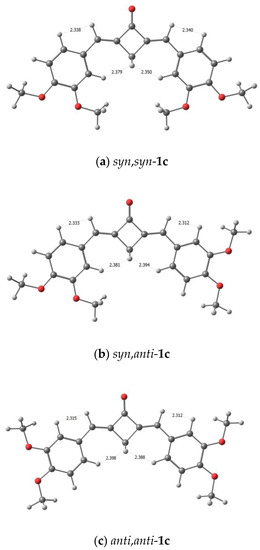
Figure 7.
Calculated distances between the protons in the syn,syn-1c (a), syn,anti-1c (b), and anti,anti-1c (c).
The predominance of syn,(syn/anti)-conformers of dienone 1c can be explained by competition between the steric factor and the stabilization via dipole–dipole interactions with polar molecules of the medium. According to calculations, these conformers have the highest theoretical dipole moments. Despite the sterically unfavorable conformation, additional stabilization is brought about by strong interactions with the molecules of the medium. Ongoing to the anti,anti-conformation, the energy benefit caused by the decrease in the steric strain is counterbalanced by the trend towards a decrease in the dipole moment of the molecule, which leads to destabilization in polar dichloromethane. In the case of anti,anti-conformer, the dipole moment decreases by a few units. As a result, destabilization starts to predominate.
2.4. Electrochemistry
A cyclic voltammetry (CV) study of compounds 1a–d,f was carried out on a cleaned surface of a glassy carbon (GC) electrode in MeCN in order to reveal the effect of substituents in the aromatic rings of cross-conjugated dibenzylidene cyclobutanones on the frontier orbital energies in comparison with the same characteristics of the cyclopentanone- and cyclohexanone-based dienones studied previously [34,35]. The CV curves were recorded starting from 0 V and moving towards the cathodic and anodic potentials. Table 2 gives the first peak potentials determined in the MeCN for comparison of the electrochemical characteristics with the data of other physicochemical investigations in the same solvent. The same Table 2 presents the differences (shifts) of the reduction and oxidation potentials of the substrates relative to those of the unsubstituted compound 1a.

Table 2.
Electrochemical potentials of compounds 1a–d,f in MeCN versus Ag/AgCl/KCl(sat.) measured by CV in the presence of 0.1 M Bu4NClO4 on a GC electrode at a potential sweep rate of 100 mV s−1.
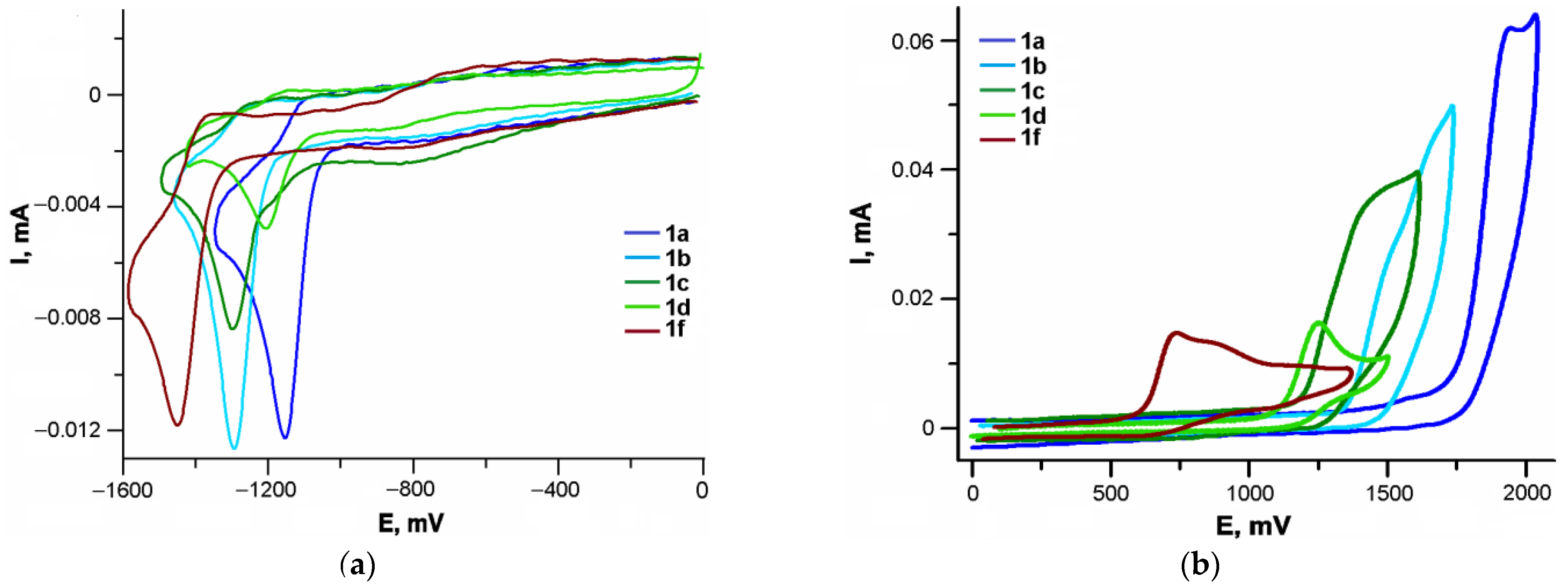
The cathodic and anodic processes of compounds 1a–d,f are irreversible (Figure 8a). The compounds 1b–d,f containing substituents in the aromatic rings are reduced with more difficulty than the unsubstituted 1a. The first cathodic peak potentials of dienones 1b–d,f are shifted by 140–310 mV to more negative values relative to that of 1a (Table 2, Figure 8a). The most pronounced electron-donating effect on the cathodic potential is observed for the diethylamino group (310 mV), while the least pronounced effect was found for the methylthio group (50 mV).

Figure 8.
Voltammetric curves for the reduction (a) and oxidation (b) of dienones: 1a–d,f.
The cathodic peak potentials of dibenzylidene cyclobutanones shift to the anodic region relative to the potentials of similar cyclopentanone derivatives and, to a larger extent, relative to cyclohexanone derivatives (Table 3 [34,35]). This may be due to the more planar molecular geometry of the dibenzylidene cyclobutanone derivatives, resulting in a higher degree of conjugation in comparison with similar cyclopentanone and cyclohexanone derivatives.

Table 3.
Electrochemical potentials of dibenzylidene cyclobutanones 1a–d,f and previously studied cyclopentanone and cyclohexanone derivatives with the same substituents measured in MeCN under the same conditions (Ag/AgCl/KCl(sat.) in the presence of 0.1 M Bu4NClO4 on a GC electrode at a potential sweep rate of 100 mV s−1).
The electron-donating ability of the para-substituents in the benzylidene moieties of dienones has a more pronounced effect on the anodic potentials than on the cathodic potentials. The shifts of the oxidation peak potentials of 1b–d,f to lower anodic values relative to that of the unsubstituted dienone 1a are 430–1190 mV.
The frontier orbital energies corresponding to the ionization potentials (HOMO) and electron affinities (LUMO) calculated by quantum chemical techniques are summarized in Table 2. The theoretical values are correlated with the oxidation and reduction potentials and qualitatively reproduce the pattern of variation of these values in the series of dienones found experimentally (the shift relative to 1a). The calculation confirms the conclusion about the effect of the electron-donating properties of the substituents on the shifts of the cathodic and anodic peaks and the band gap ΔE. The ionization mechanism is similar to that found for dienones based on cyclopentanone and cyclohexanone [34,35]. An electron is removed from the HOMO-1 quasi-degenerate with HOMO and is accepted by the LUMO.
2.5. Photophysics
Electronic absorption and fluorescence spectra were measured to determine the effect of substituents on the spectral properties of dienones 1a–f (Table 4, Figure 9, Figure S20, Supplementary Materials).

Table 4.
Parameters of the electronic absorption and fluorescence spectra of dienones 1a–f, MeCN.
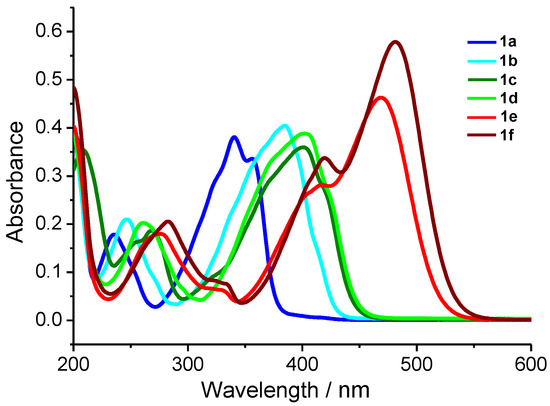
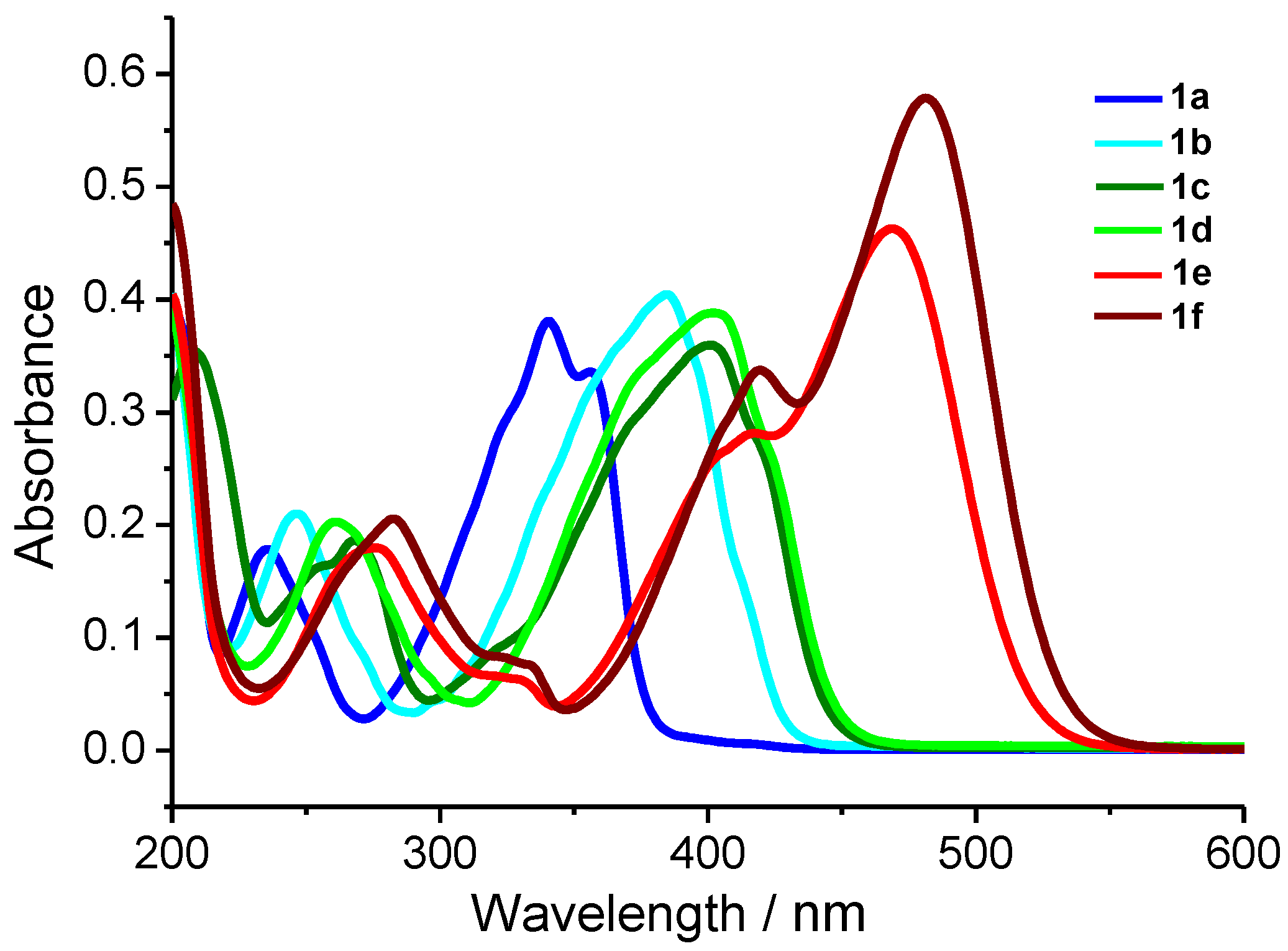
Figure 9.
Absorption spectra of dienones 1a–f, in MeCN, C = 1 × 10−5 M.
All dienones exhibit a long-wavelength absorption band (LWAB) (λmax from 341 nm for 1a to 481 nm for 1f) and several more bands in the shorter wavelength range. LWABs can be assigned to the HOMO–LUMO transitions [40,41], while the shorter wavelength bands can be attributed to local electron transitions in the aromatic rings. Attention is attracted by the qualitative dependence of the LWAB maxima on the electron-donating ability of the substituents in the para-position of the benzylidene moieties. The LWABs of para-dialkylamino-substituted 1e,f are red-shifted relative to that of the unsubstituted 1a, to the greatest extent, which is in good agreement with the high electron-donating ability of the substituents and is correlated with the lowest oxidation potentials among the considered series (Table 4). In the case of meta-methoxy-substituted compound 1c, the LWAB maximum shifts to 401 nm, and the oxidation potential increases relative to those of 1e,f. The further red shift of LWAB of dienone 1d is also accompanied by increasing Eox and is caused by the higher electron-donating ability of the SMe substituent.
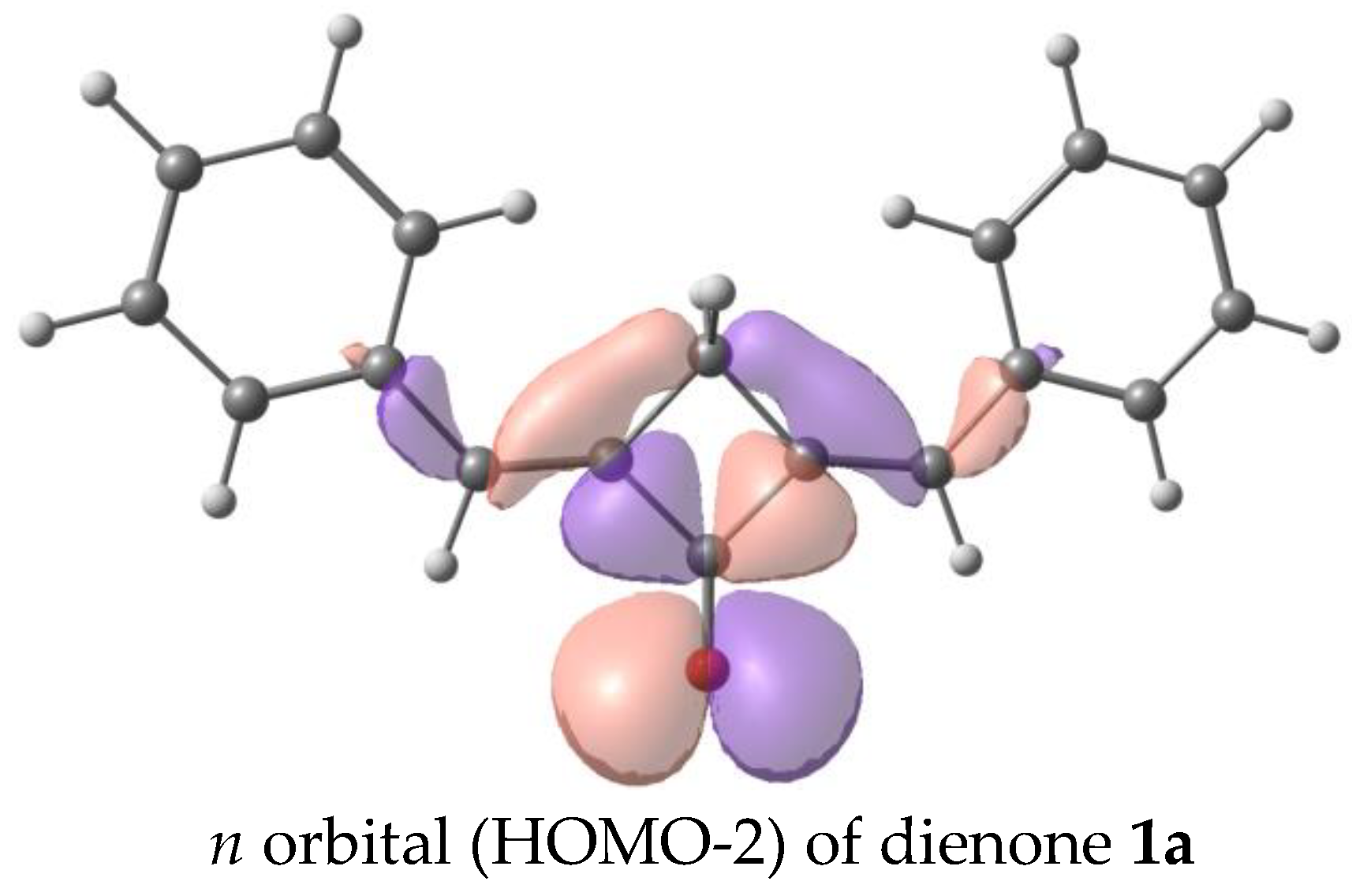
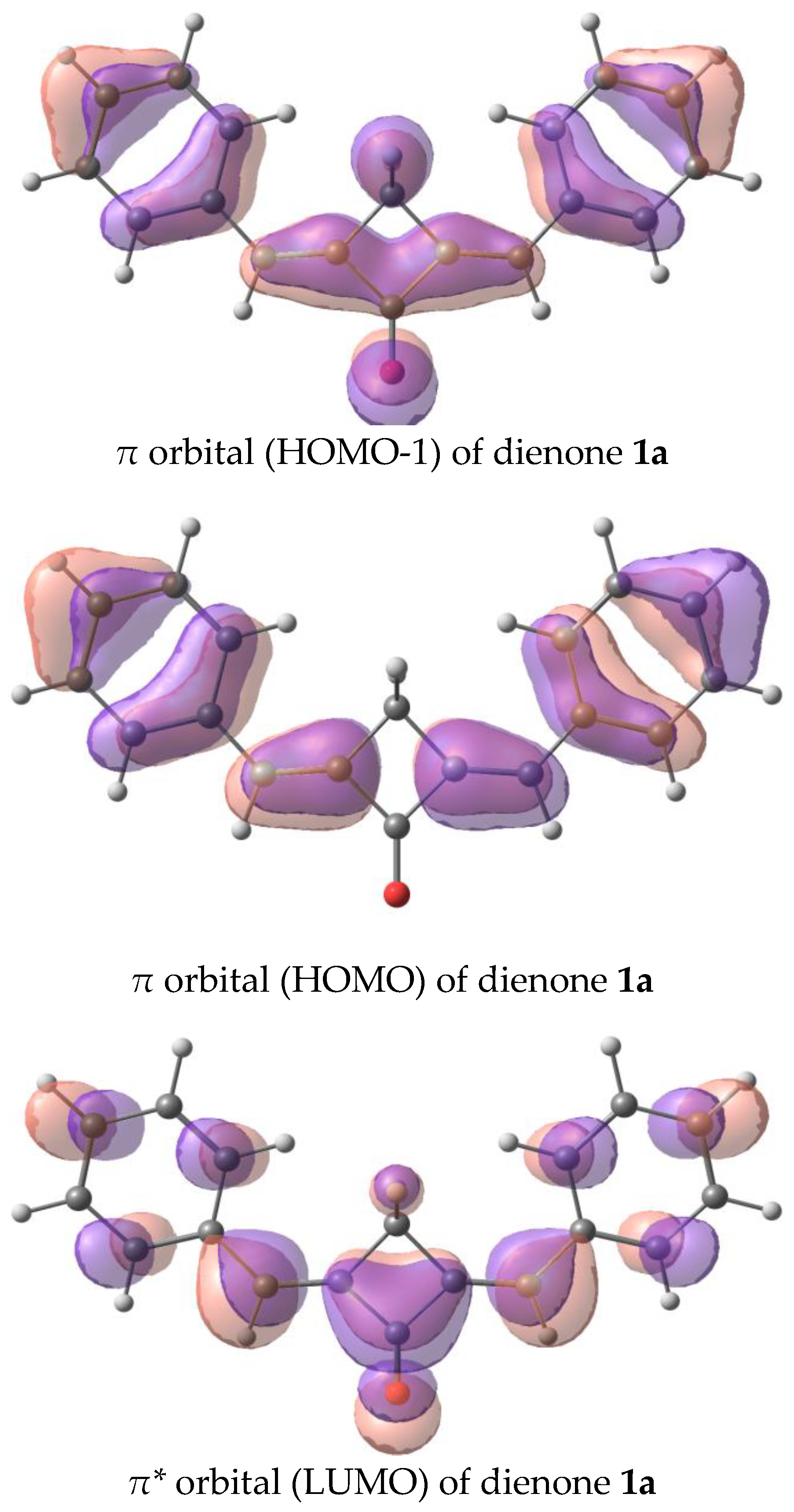
The calculated absorption and fluorescence spectra are in qualitative agreement with the experiment and reproduce the trend in the series. The first electron transition for all the dyes, except for 1a, is the π-π* transition. In 1a, the first electron transition is the dark n-π* transition. This accounts for the absence of fluorescence in dye 1a. The corresponding orbitals are depicted in Figure 10. Generally, the red shifts of the LWABs of dienones 1a–f, relative to those of dienones based on cyclopentanone and cyclohexanone, are caused by the more planar geometry of the π-electron system of cyclobutanones.
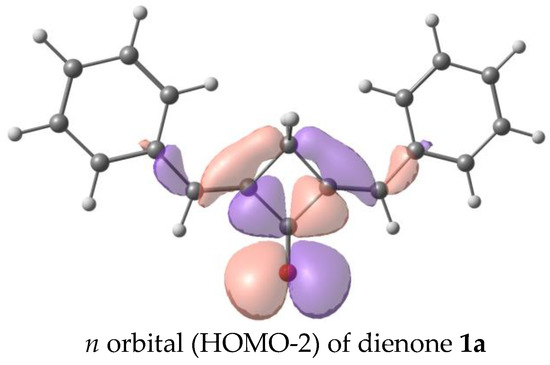
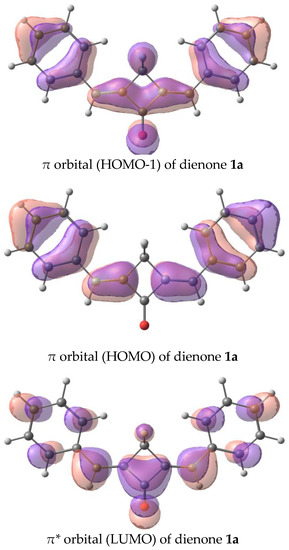
Figure 10.
Orbitals involved in the first electron transition of dienone 1a, oxidation, and reduction.
The dependence of fluorescence properties of compounds 1a–f on their oxidation potentials is generally similar to the λmax(LWAB)–Eox dependence. The brightest and longest-wavelength fluorescence is observed for the amino derivatives 1e,f (Table 4).
The trends of variation of the fluorescence properties of cyclobutanones 1a–f differ from those described for a series of related compounds [35]. Unlike cyclopentanone- and cyclohexanone-based dienones, most compounds with electron-donating substituents 1b–f, except for 1a,b, exhibit fluorescence.
According to the calculations, the E,E-isomer is dominant for each of the compounds in the S0 state. In the S1 state, a relatively fast E-Z isomerization can proceed, resulting in several isomers being present in comparable amounts (Table 5). Dienone 1d contains significant amounts of all three possible isomers. However, unlike the other series, the dominant isomers of compounds 1b–f are characterized by short radiation lifetimes. Therefore, fluorescence is the preferable deactivation pathway for the excited states as compared to the nonradiative deactivation pathways such as internal conversion or intersystem crossing (Table 4). This is in line with the experimental data.

Table 5.
Relative energies of (E,E), (E,Z), and (Z,Z) isomers in kcal·mol−1 and mole fractions of the isomers of dienones 1a–f in the S1 state.
We have analyzed the factors affecting the fluorescence quantum yield in the cyclobutanone series. Our calculations showed that the dyes in study have two low-lying excited states of the π-π* and n-π* types. The n-π* transition is forbidden, and the corresponding excited state is dark, while the π-π* transition corresponds to an intense absorption and emission band. In the unsubstituted dienone 1a, the dark n-π* state lies below the bright π-π* state, which results in the lack of emission. Previously, we observed a similar picture in a cyclohexanone analog of dienone 1a and attributed its lack of fluorescence to the same reason [35,42].
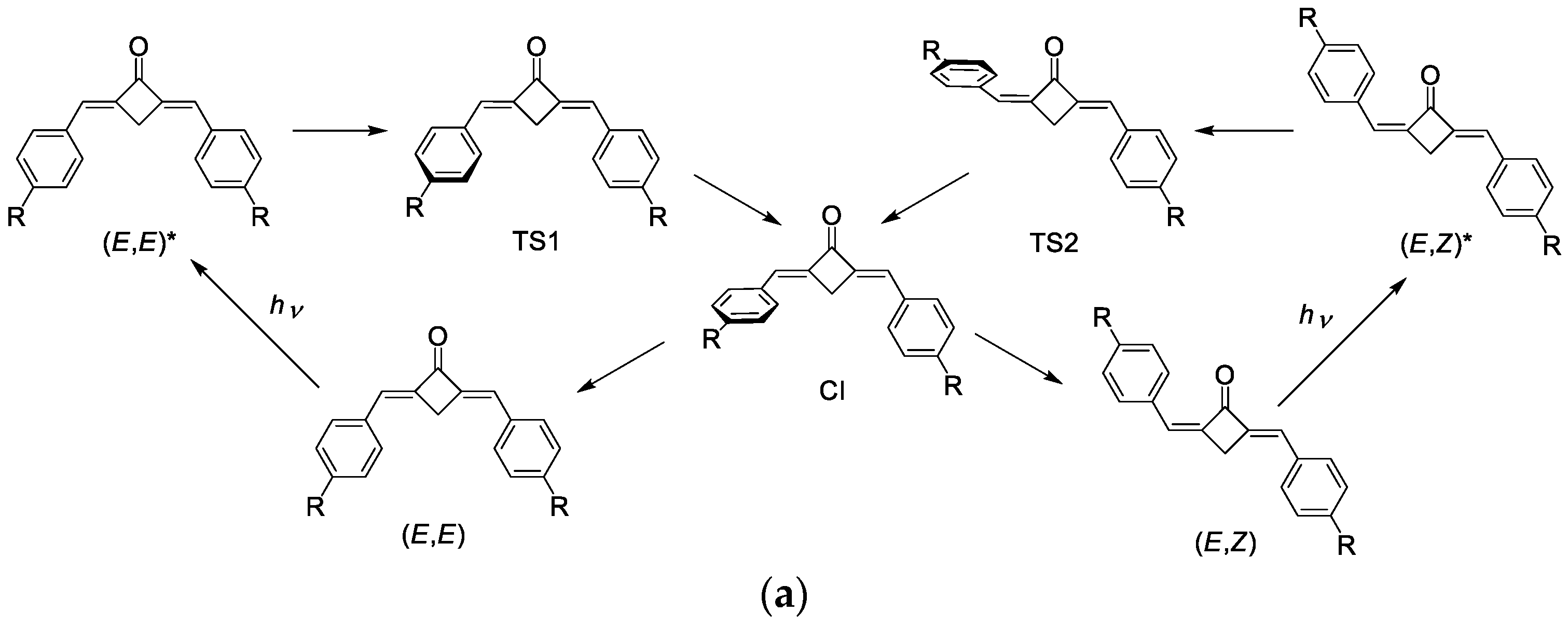
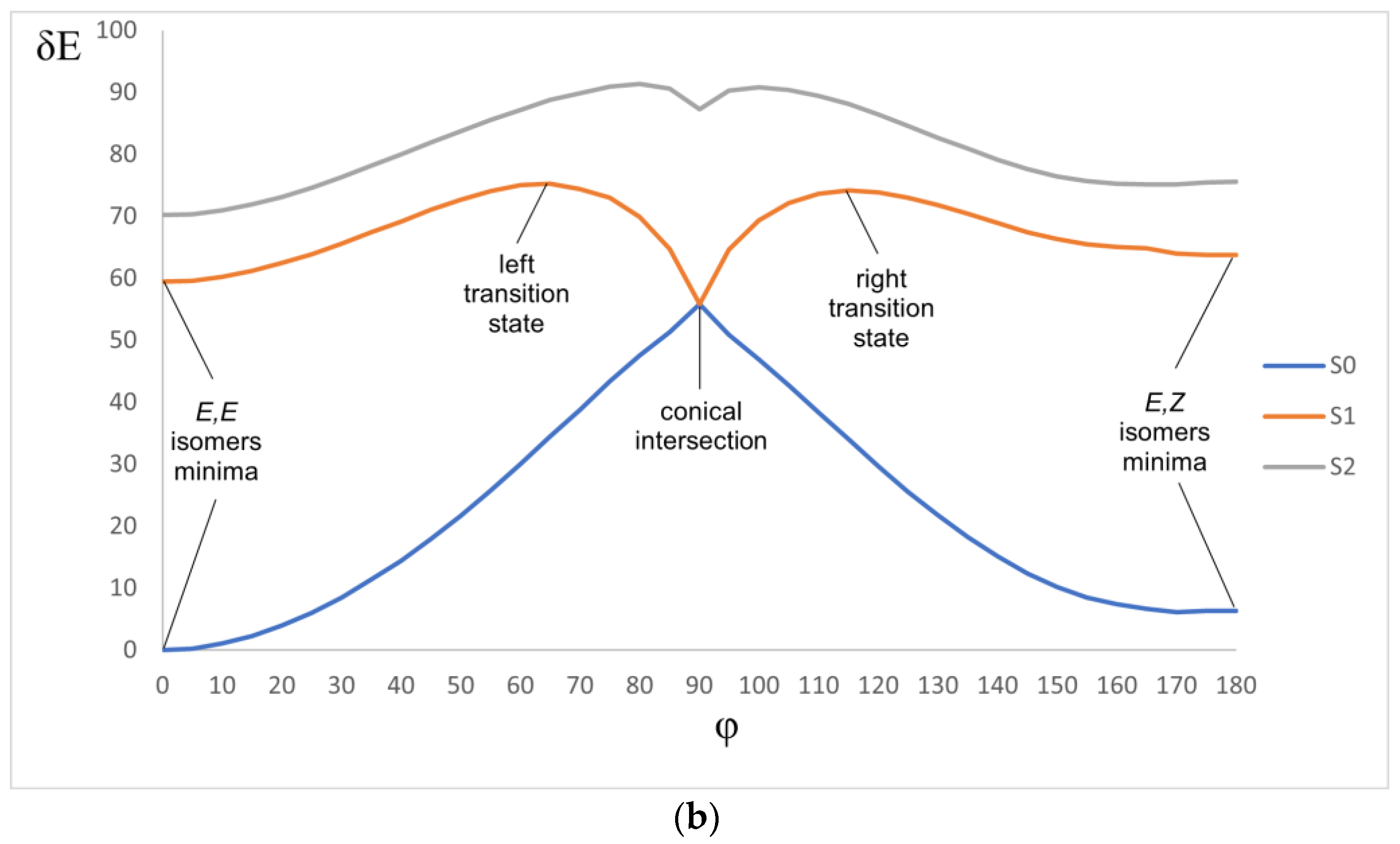
The main mechanism of deactivation in the studied dienones is the structural relaxation resulting in the distortion of the conjugation. Our calculation showed that the twisting of the formally double bond during E-E-E-Z isomerization is the most probable way (Figure 11a). The (E,E)-dienone is excited, and its relaxation on the S1 potential energy surface leads to the twisting of the formally double bond. After overcoming a relatively low barrier, the molecule reaches the S1-S0 conical intersection, which corresponds to the ~90-degree twist. This conical intersection is of the funnel type, and further relaxation may proceed on the S0 potential energy surface towards either of the (E,Z) or (E,E) forms. We have built the potential energy profiles for the ground and two lowest excited states (Figure 11b shows the general scheme common for all the compounds in the series). Note that TDDFT poorly describes the behavior of the potential energy surfaces near conical intersections, therefore, these profiles can only be used for qualitative conclustions.


Figure 11.
(a) Scheme of the photoisomerization mechanism. (b) Typical potential energy profile (kcal/mol) of the ground S0 and lowest excited π-π* and n-π* states. φ is the dihedral angle at the C=C bond.
All the profiles show a conical intersection (CI) between the S0 and S1 states and two barriers on the S1 state, from the E,E form to CI and from the E,Z form to CI. The energy of the CI point is lower than the energy of either the E,E or E,Z minima on the S1 surface. This means that the relaxation should proceed nonradiatively, hindered only by a barrier. The depth of the CI relative to the deepest minimum (E,E form) is the driving force of the relaxation. This depth decreases from 1a to 1f (Table 6). From the CI point, the molecule nonradiatively goes to the ground state. The transition states (TSs) separate the S1 minima from the CI point. A TDDFT calculation gives the TS position at φ ~ 60–70°. The barriers hinder the rotation, and their height increases from 1a to 1f (Table 6). This means that increasing the donor capacity of the substituent makes nonradiative relaxation less probable, both thermodynamically and kinetically, and facilitates fluorescence.

Table 6.
E,E-to-CI (1) and E,Z -to-CI (2) activation energies and CI depths for 1a–f in the S1 state.
The calculated activation energies were used to assess the isomerization rate constants (Table 7). A comparison of the characteristic isomerization times with the radiative lifetime shows that E-E-E-Z isomerization pathway can explain partial fluorescence quenching in 1b and the lack of fluorescence in 1a. The left barrier on the S1 potential energy surface can be overcome via thermal vibrations, and rotation around the double bond can be activated. Increasing the donor capacity of the substituents from 1a to 1f hinders isomerization, with almost unchanged τr, which leads to the noticeable fluorescence of 1c–f, in excellent agreement with the experiment.

Table 7.
Radiative lifetime of the (E,E) isomer, E-Z isomerization rate constant and time.
In addition, we considered an alternative deactivation channel proposed for styryl dyes in [43], namely, the twisting of the phenyl ring around the formally single bond. Our calculations showed that, in dienones, such twisting gives neither stable structures on the S1 surface nor S1-S0 conical intersections.
The absorption and emission of compound 1f and its aza-crown ether analog are described in detail elsewhere [42].
2.6. Correlations
Previously, we studied the relationships between a number of calculated and experimental characteristics for a broad range of compounds, including dienones. In particular, we demonstrated the existence of a linear relationship between the electrochemical and spectrophotometric characteristics of dienone molecules [34,35,44,45].
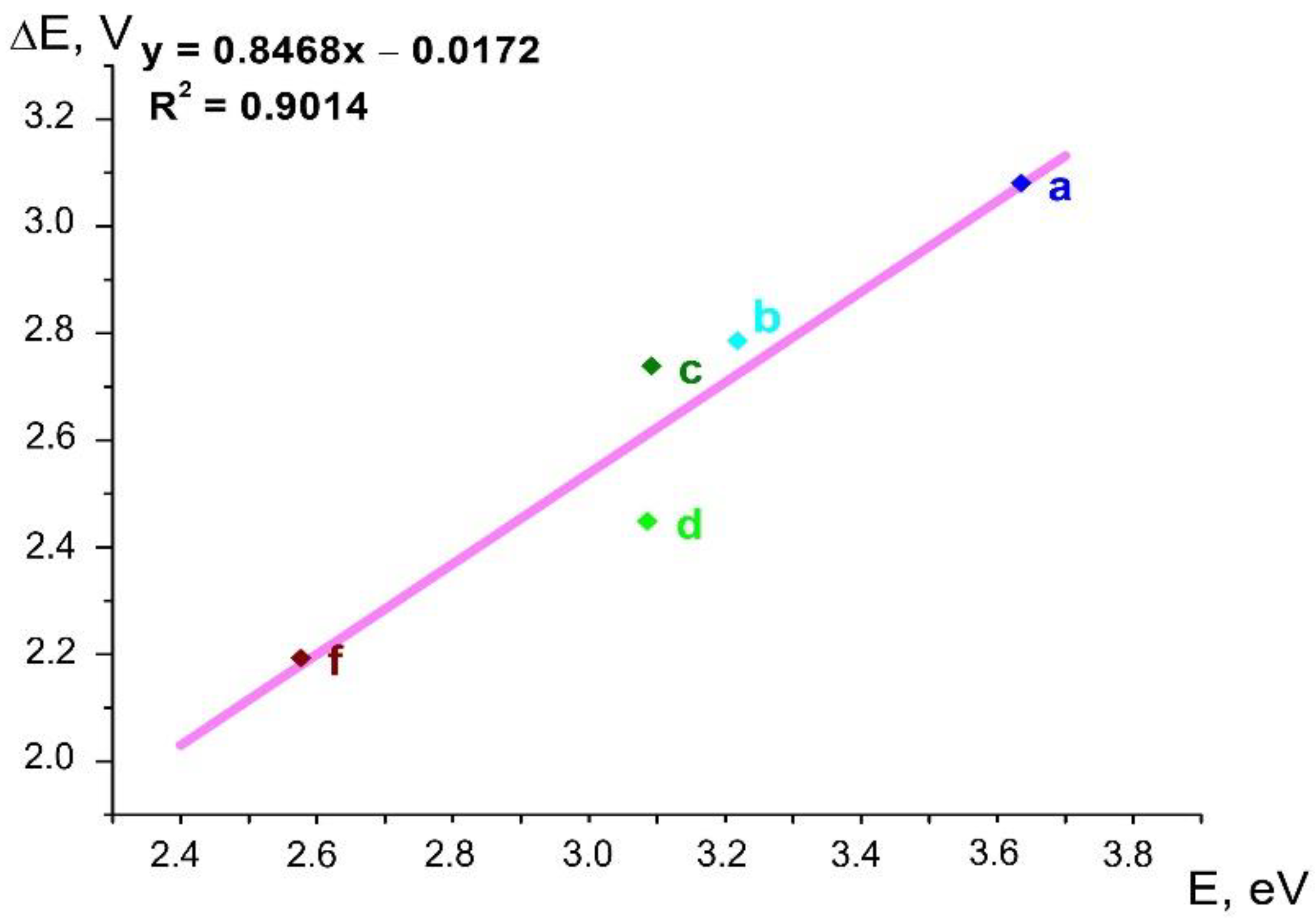
In order to establish the relationship between the data of absorption spectra and electrochemical measurements, and to elucidate the possible dependences of these results on the electronic properties of the substituents in the benzene rings of dienones 1a–f, we carried out a correlation analysis of the relationship between the long-wavelength absorption maximum and oxidation/reduction potential difference (Figure 12).

Figure 12.
Linear correlation between the energy E of the long-wavelength absorption maximum and the difference ΔE of the oxidation/reduction potentials for dienones 1a–d,f. The coefficient of determination R2 and regression equation are given.
The results presented here indicate that the energy characteristics of frontier orbitals in a series of related compounds, such as cross-conjugated dienones, can be adequately described by both electrochemical and spectrophotometric results, despite the irreversibility of the electrochemical reduction step. The results obtained in this work can be used to analyze new dienones and to design the desired characteristics of new molecules.
3. Materials and Methods
3.1. Materials
MeCN (extra high purity, water content < 0.3%, Cryochrom) was used to prepare the solutions. The Bu4NClO4 (≥99%, for electrochemical analysis) was purchased from Sigma-Aldrich and used as the supporting electrolyte. The cyclobutanone, cyclopentanone, benzaldehyde, 4-methoxybenzaldehyde, 3,4-dimethoxybenzaldehyde, 4-(methylthio)benzaldehyde, 4-dimethylaminobenzaldehyde, and 4-diethylaminobenzaldehyde (Sigma–Aldrich) were used as received. The EtOH (chemically pure grade) was used without additional purification, (2E,5E)-2,5-bis(3,4-dimethoxybenzylidene)cyclopentanone (2) was prepared as described previously [34]. The DC-Alufolien Aluminiumoxid 60 F254 neutral was purchased from Merck.
Synthesis of 2,6-Dibenzylidenecyclobutanone Derivatives 1a-f
(2E,4E)-2,4-Dibenzylidenecyclobutanone (1a). A mixture of cyclobutanone (70 mg, 1 mmol) and benzaldehyde (212 mg, 2 mmol) in 75% EtOH (875 μL) was added dropwise with stirring at 5 °C to a 0.03 M solution of NaOH in 75% EtOH (3.5 mL). The reaction mixture was stirred for 35 min, and a dilute solution of acetic acid in EtOH was added to pH 6. The precipitate thus formed was collected on a filter, washed with an EtOH–H2O mixture (1:1 v/v), and dried in air. This gave 30.1 mg of dienone 1a as a light-yellow crystalline powder. Yield 12%, m.p. 185–187 °C (cf. Ref. [11]: 191–192 °C). 1H NMR (δ, ppm, J/Hz): 3.89 (m, 2 H, C(3)H2), 7.19 (m, 2 H, 2 C(α)H), 7.39–7.46 (m, 6 H, 2 H(3′), 2 H(4′), 2 H(5′)), 7.59 (dd, 4 H, 2 H(2′), 2 H(6′), J = 1.3, J = 6.7). 13C NMR (δ, ppm): 36.57 (C(3)), 127.35 (2 C(α)H), 129.53 (2 C(3′), 2 C(5′)), 130.43 (2 C(2′), 2 C(6′)), 130.58 (2 C(4′)), 135.37 (2 C(1′)), 146.95 (C(2), C(4)), 190.93 (C(1)). UV–vis (MeCN) λmax 341 nm (ε = 38,000 M−1 cm−1), 356 nm (ε = 33,600 M−1 cm−1). Fluorescence: none. IR (CH2Cl2, ν): 1714 cm−1 (C=O). HRMS (ESI+) m/z calcd for C18H15O [M + H]+: 247.1117. Found: 247.1125. Elemental analysis calcd (%) for C18H14O 0.25H2O: C, 86.20, H, 5.83. Found: C, 86.06, H, 5.88.
(2E,4E)-2,4-Bis(4-methoxybenzylidene)cyclobutanone (1b). A 3 M solution of NaOH (250 μL) in an EtOH–H2O mixture (2:1 v/v) was added with cooling and stirring to a mixture of cyclobutanone (0.35 mg, 0.5 mmol) and 4-methoxybenzaldehyde (0.143 mg, 1 mmol) in EtOH (100 μL). The reaction mixture was kept at 5 °C for 5 h, and the precipitate thus formed was collected on a filter, washed on the filter with an EtOH–H2O mixture (2:1 v/v), and recrystallized from EtOH containing some CH2Cl2. This gave 89 mg of dienone 1b as a yellow crystalline powder. Yield 58%, m.p. 190–193 °C (from EtOH–CH2Cl2) (cf. Ref. [11]: 193–194 °C). 1H NMR (δ, ppm, J/Hz): 3.78 (br.s, 2 H, C(3)H2), 3.83 (s, 6 H, 2 Me), 6.95 (d, 4 H, 2 H(3′), 2 H(5′), J = 8.5), 7.11 (m, 2 H, 2 C(α)H), 7.53 (d, 4 H, 2 H(2′), 2 H(6′), J = 8.5). 13C NMR (δ, ppm): 36.00 (C(3)), 55.97 (2 C, 2 Me), 115.02 (2 C(3′), 2 C(5′)), 126.63 (2 C(α)H), 128.16 (2 C (4′)), 132.09 (2 C (2′), 2 C (6′)), 144.69 (2 C(1′)), 161.79 (C(2), C(4)), 190.73 (C(1)). UV–vis (MeCN) λmax 385 nm (ε = 40,400 M−1 cm−1). Fluorescence: none. IR (CH2Cl2, ν): 1714 cm−1 (C=O). HRMS (ESI+) m/z calcd for C20H19O3 [M + H]+: 307.1328. Found: 307.1337. Elemental analysis calcd (%) for C20H18O3: C, 78.41, H, 5.92. Found: C, 78.22, H, 5.94.
(2E,4E)-2,4-Bis(3,4-dimethoxybenzylidene)cyclobutanone (1c). A mixture of cyclobutanone (35 mg, 0.5 mmol) and 3,4-dimethoxybenzaldehyde (167 mg, 1 mmol) in EtOH (650 μL) was added dropwise with stirring to a 0.15 M solution of NaOH in 75% EtOH (2.75 mL). The reaction mixture was stirred at room temperature for 4 h, and the precipitate was collected on a filter, washed on the filter with water, and dried in the air. This gave 80.5 mg of dienone 1c as a yellow-orange crystalline powder. Yield 46%, m.p. 187–190 °C (cf. Ref. [12]: 191–193 °C). 1H NMR (δ, ppm, J/Hz): 3.81 (m, 2 H, C(3)H2), 3.86 and 3.87 (2 s, 12 H, 2 3′-MeO, 2 4′-MeO), 6.92 (d, 2 H, 2 H(5′), J = 8.2), 7.06 (d, 2 H, 2 H(2′), J = 1.8), 7.10 (m, 2 H, 2 C(α)H), 7.19 (dd, 2 H, 2 H(6′), J = 8.2, J = 1.8). 13C NMR (δ, ppm): 35.81 (C(3)), 56.39 (2 C, 2 3′-MeO), 56.43 (2 C, 2 4′-MeO), 112.10 (2 C(5′)), 113.17 (2 C(2′)), 124.26 (2 C (6′)), 127.06 (2 C(α)H), 128.36 (2 C(1′)), 144,76 (C(2), C(4)), 149.95 (2 C(3′)), 151.76 (2 C(4′)), 190.52 (C(1)). UV–vis (MeCN) λmax 401 nm (ε = 36,000 M−1 cm−1), fluorescence (MeCN, λex 390 nm) λflmax 505 nm. IR (CH2Cl2, ν): 1705 cm−1 (C=O). HRMS (ESI+) m/z calcd for C22H23O5 [M + H]+: 367.1540. Found: 367.1544. Elemental analysis calcd (%) for C22H22O5: C, 72.12, H, 6.05. Found: C, 71.79, H, 6.33.
(2E,4E)-2,4-Bis[4-(methylthio)benzylidene]cyclobutanone (1d). A solution of cyclobutanone (35 mg, 0.5 mmol) in EtOH (200 μL) was added dropwise with stirring at 5 °C to a mixture of 4-methylthiobenzaldehyde (157 mg, 1 mmol) and a 0.25 M solution of NaOH in an EtOH–H2O mixture (2:1 v/v) (350 μL). The reaction mixture was kept at 5 °C for 20 h, Et2O (2 mL) was added, and the resulting thick precipitate was ground to form a powder, which was collected on a filter and recrystallized from EtOH containing some CH2Cl2. This gave 78 mg of dienone 1d as a yellow crystalline powder. Yield 46%, m.p. 215–217 °C (from EtOH–CH2Cl2). 1H NMR (δ, ppm, J/Hz): 2.51 (s, 6 H, 2 Me,), 3.82 (br.s, 2 H, C(3)H2), 7.13 (br.s, 2 H, 2 C(α)H), 7.27 (d, 4 H, 2 H(3′), 2 H(5′), J = 8.3), 7.50 (d, 4 H, 2 H(2′), 2 H(6′), J = 8.3). 13C NMR (δ, ppm): 15.46 (2 C, 2 Me), 36.41 (C(3)), 126.44 (2 C(3′), 2 C(5′)), 126.72 (2 C(α)H), 130.70 (2 C (2′) 2 C (6′)), 131.82 (2 C (4′)), 142.64 (2 C(1′)), 146,04 (C(2), C(4)), 190.59 (C(1)). UV–vis (MeCN) λmax 402 nm (ε = 39,000 M−1 cm−1), fluorescence (MeCN, λex 396 nm) λflmax 522 nm. IR (CH2Cl2, ν): 1708 cm−1 (C=O). HRMS (ESI+) m/z calcd for C20H19OS2 [M + H]+: 339.0871. Found: 339.0869. Elemental analysis calcd (%) for C20H18OS2: C, 70.97, H, 5.36, S, 18.95. Found: C, 70.74, H, 5.50, S, 18.89.
(2E,4E)-2,4-Bis[4-(dimethylamino)benzylidene]cyclobutanone (1e). A 1.33 M solution of NaOH in an EtOH–H2O mixture (2:1 v/v) (250 μL) was added with stirring to a mixture of cyclobutanone (35 mg, 0.5 mmol) and 4-dimethylaminobenzaldehyde (149 mg, 1 mmol) in EtOH (250 μL). The reaction mixture was allowed to stand at room temperature for 96 h, and the precipitate was collected on a filter, washed with an EtOH–H2O mixture (1:10 v/v) and Et2O, and dried in the air. This gave 120 mg of dienone 1e as a red-orange crystalline powder. Yield 72%, m.p. 268–271 °C. (cf. Ref. [12]: 274–275 °C). 1H NMR (δ, ppm, J/Hz): 3.01 (s, 12 H, 4 Me), 3.71 (br. s, 2 H, C(3)H2), 6.71 (d, 4 H, 2 H(3′), 2 H(5′), J = 8.7), 7.04 (m, 2 H, 2 C(α)H), 7.46 (d, 4 H, 2 H(2′), 2 H(6′), J = 8.7). 13C NMR (δ, ppm): 35.76 (C(3)), 40.47 (4 C, 4 Me), 112.45 (2 C(3′), 2 C(5′)), 123.31 (2 C (4′)), 126.83 (2 C(α)H), 131.97 (2 C (2′) 2 C (6′)), 142.28 (2 C(1′)), 151,97 (C(2), C(4)), 190.50 (C(1)). UV–vis (MeCN) λmax 418 nm (ε = 28,000 M−1 cm−1), 469 nm (ε = 46,000 M−1 cm−1), fluorescence (MeCN, λex 460 nm) λflmax 576 nm. IR (CH2Cl2, ν): 1695 cm−1 (C=O). HRMS (ESI+) m/z calcd for C22H25N2O [M + H]+: 333.1961 Found: 333.1966. Elemental analysis calcd (%) for C22H24N2O: C, 79.48, H, 7.28, N, 8.43. Found: C, 79.29, H, 7.16, N, 8.20.
(2E,4E)-2,4-Bis[4-(diethylamino)benzylidene]cyclobutanone (1f). A 1.33 M solution of NaOH in an EtOH–H2O mixture (2:1 v/v) (250 μL) was added with stirring to a mixture of cyclobutanone (35 mg, 0.5 mmol) and 4-diethylaminobenzaldehyde (177 mg, 1 mmol) in EtOH (250 μL). The reaction mixture was allowed to stand at room temperature for 96 h, and the precipitate was collected on a filter, washed with an EtOH–H2O mixture (1:10 v/v), and dried in air. This gave 145 mg of dienone 1f as a red-orange crystalline powder. Yield 75%, m.p. 185–187 °C. 1H NMR (δ, ppm, J/Hz): 1.18 (t, 12 H, 4 Me, J = 7.1), 3.40 (q, 8 H, 4 CH2N, J = 7.1), 3.70 (br.s., 2 H, C(3)H2), 6.67 (d, 4 H, 2 H(3′), 2 H(5′), J = 8.9), 7.01 (m, 2 H, 2 C(α)H), 7.44 (d, 4 H, 2 H(2′), 2 H(6′), J = 8.9). 13C NMR (δ, ppm): 12.94 (4 C, 4 Me), 35.70 (C(3)), 45.04 (4 C, 4 CH2N), 111.99 (2 C(3′), 2 C(5′)), 122.57 (2 C (4′)), 126.75 (2 C(α)H), 132.28 (2 C (2′) 2 C (6′)), 141.79 (2 C(1′)), 149,53 (C(2), C(4)), 190.44 (C(1)). UV–vis (MeCN) λmax 419 nm (ε = 33,800 M−1 cm−1), 481 nm (ε = 58,000 M−1 cm−1), fluorescence (MeCN, λex 472 nm) λflmax 575 nm. IR (CH2Cl2, ν): 1691 cm−1 (C=O). HRMS (ESI+) m/z calcd. for C26H33N2O [M + H]+: 389.2587. Found: 389.2590. Elemental analysis calcd (%) for C26H32N2O: C, 80.37, H, 8.30, N, 7.21. Found: C, 80.20, H, 8.21, N, 7.07.
3.2. Methods
The reactions were monitored by thin layer chromatography using DC–Alufolien Aluminiumoxid 60 F254 neutral plates, Merck. The melting points (uncorrected) were determined on a Mel-Temp II instrument. The 1H and 13C NMR spectra were measured on a Bruker DRX-600 spectrometer (operating at 500.13, 600.22, and 125.76 MHz, respectively) in CD2Cl2 at 25–30 °C, using the solvent as an internal standard (δH 5.30 and δC 54.00 ppm, respectively). The proton and carbon signals were assigned using homonuclear 1H-1H COSY and heteronuclear 1H-13C COSY (HSQC and HMBC) 2D spectra. The chemical shifts were determined with an accuracy of 0.01 ppm, and the spin–spin coupling constants were measured with an accuracy of 0.1 Hz.
IR spectra were recorded using the Fourier transform spectrometer Nicolet iS5 (Thermo Fisher Scientific, using an internal reflectance attachment with diamond optical element – attenuated total reflection (ATR, iD7) with 45° angle of incidence. Resolution 4 cm−1, the number of scansis 20.
The electronic absorption spectra were recorded on a Cary 4000 spectrophotometer in the MeCN. The fluorescence spectra were obtained on a Cary Eclipse spectrofluorometer at room temperature. All the manipulations with solutions of dyes 1a–f were performed in a darkroom under red light (daylight induces the E-Z photoisomerization).
High-resolution mass spectra (HR MS) were run on a Bruker micrOTOF II instrument using electrospray ionization (ESI) [37]. The measurements were done in a positive ion mode (interface capillary voltage of −4500 V), mass range from m/z 50 to 3000 Da, and external or internal calibration was done with Electrospray Calibrant Solution (Fluka). A syringe injection was used for the solutions in MeCN (flow rate of 3 mL min−1). Nitrogen was applied as a dry gas, and the interface temperature was set at 180 °C.
The elemental analysis was carried out at the Microanalytical Laboratory of the A. N. Nesmeyanov Institute of Organoelement Compounds (Russian Academy of Sciences, Moscow, Russian Federation). The samples for the elemental analysis were dried at 80 °C in vacuo.
3.3. Cyclic Voltammetry
The electrochemical measurements were carried out using an IPC_Pro M potentiostat in a three-electrode system. A glassy carbon disk (d = 2 mm) served as the working electrode, a 0.1 M Bu4NClO4 solution in MeCN was used as the supporting electrolyte, and an Ag/AgCl/KCl(aq., sat.) reference electrode and a platinum plate auxiliary electrode were used. The working electrode surface was polished by alumina powder with a particle size of less than 0.5 μm (Sigma-Aldrich). In the CV measurements, the potential sweep rate was 100 mV s−1. The potentials are presented with iR-compensation. The number of transferred electrons was determined by comparing the peak current in the substrate and the current of single-electron oxidation of ferrocene taken in the same concentration. The concentration of compounds 1a–d,f was 1 × 10−4 M.
3.4. X-ray Diffraction Experiments
A suitable single crystal of each of compounds 1b–f and 2 was mounted on a CCD SMART APEX-II diffractometer under a stream of cooled nitrogen, and the crystallographic parameters and X-ray reflection intensities were measured (MoKα-radiation (λ = 0.71073 Å), graphite monochromator, ω-scan mode). The reduction of the experimental data was performed using the SAINT program [46].
The structures were solved by direct methods and refined by least squares on F2 in the anisotropic approximation for non-hydrogen atoms. The hydrogen atom positions were calculated geometrically and refined, at the final stage, using the riding model.
The crystallographic characteristics and structure refinement details are summarized in Tables S3–S5, Supplementary Materials.
The calculations were performed using OLEX-2 and SHELXTL-Plus software [47,48]. The X-ray diffraction studies were done at the Center for Collective Use of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences. The structural data were deposited with the Cambridge Crystallographic Data Centre with numbers: CCDC 2181814 (1b), CCDC 2181815 (1c), CCDC 2181816 (1d), CCDC 1892909 (1e), CCDC 2181818 (1f), CCDC 2181820 (syn,syn-2) и CCDC 2181819 (anti,anti-2).
3.5. Density Functional Theory (DFT) Calculations
The structures and energies of the molecules were calculated using the density-functional theory (DFT) with the PBE0 functional and 6-31 + G(d,p) basis set by the FireFly program [49], partially based on GAMESS code [50]. The solvent (MeCN) effects were taken into account using the dielectric polarizable continuum model (D-PCM) [51]. The vertical absorption and emission spectra and E-E-E-Z isomerization energy profiles were calculated by the time-dependent DFT (TDDFT) with the same functional, basis set, and solvent model. The vertical absorption spectra were calculated by the TDDFT after DFT optimization of the ground state geometry, while the vertical emission spectra were calculated in a similar way after geometry optimization of the π-π* excited state using the TDDFT and D-PCM. The radiative lifetimes were calculated using the formula:
where f0i and νi are oscillator strength and the frequency of the electronic transition of the ith isomer respectively; kr is the radiation constant. The isomerization periods were calculated using the formula:
where xi is the vibrational mode frequency of the ith isomer, and EAi is the activation barrier of this isomer.
kr = (⅔)f0iν2i0, τr = 1/kr
ktc = cνi·exp(−EAi/RT), ttc = 1/ktc
We considered the (E,E), (E,Z), and (Z,Z) isomers of dyes 1a–f and 2. We have found that the (E,E) isomers have the lowest energy and account for >99.9% of the isomer mixture. The spectral and ionization properties were calculated only for the (E,E) isomer.
In dyes 1c and 2, free rotation around the C4-C5 bond is possible. The mole fractions of the three possible rotamers of dienones 1c and 2 were calculated using the partition function:
where xi is the mole fraction of the ith conformer and Ei is the ground state energy of this conformer. The calculated UV–Vis absorption and emission properties of the rotamers are almost the same (within 2 nm), therefore, we give only the values for the syn,syn-conformer.
We simulated the structural relaxation only for the E,E isomers. It was found that, in the equilibrium mixture of the ground state, the fraction of this isomer is above 90%. It is the main source of photoinduced transformation products.
To construct the profiles of the E-E-E-Z isomerization, we used a simple (unrelaxed) scan of the potential energy surface along the dihedral angle, corresponding to the rotation around the formally double C=C bond with 5-degree increments. The energy values correspond to the non-optimized structures obtained by twisting of the initial isomer.
The left rotation barriers were estimated by the energy difference of the saddle points of the left transition state and stable structures of the E,E isomers (left minimum). The right ones were estimated as the difference between the top of the right peak of the S1 curve and the local minimum observed on the way to the E,Z geometry.
We understand that phototransformation, which proceeds via a conical intersection, requires multireference quantum chemistry for an adequate description of the potential energy profiles [52]. Nevertheless, our semi-quantitative description gives insights into the mechanism of phototransformations in organic dyes [53].
The vertical ionization potentials (IP) and electron affinities (EA) were calculated by restricted-open-shell DFT (RO-DFT) for the corresponding monocation and monoanion of each dye. The functional, basis set, and solvation model were the same.
1H NMR spectra were calculated using Priroda program package [54,55] with the PBE functional and triple-zeta quality basis set. The optimized geometries were taken from the B3LYP/6-31 + G(d,p)/DPCM calculation. Previously [33], we have shown that, for dienones, the solvent effects are important to properly reproduce the structures and conformation energies.
4. Conclusions
The effect of the structure on the photophysical and electrochemical properties of a series of symmetrical dibenzylidene derivatives of cyclobutanone containing electron-donating substituents in the benzene rings of dienones has been studied. It was shown that the products of the condensation of cyclobutanone with benzaldehyde derivatives tend to exist as E,E-isomers. The conformational analysis of (E,E)-dienones using X-ray diffraction and NMR data revealed the structural features of these compounds in the crystal and in solution. Quantum chemical calculations confirmed more favorable syn,(syn/anti)-conformations of the conjugated moieties for dibenzylidenecyclobutanones with four methoxy groups. It was found that the PCA reaction of 1a–f in the solid state requires the use of a supramolecular template. Using electronic spectroscopy, the spectral properties of the dienone derivatives were compared. Quantum chemical calculations explained the observed regularities of the luminescence of 1a–f as a function of the donor capacity of the substituent and elucidated the mechanism of luminescence emission and quenching. The dependence of the redox potentials on the position, nature, and number of substituents in the benzene ring, and their correlation with photophysical and quantum chemical characteristics, are of considerable interest for the subsequent investigation of photoactive dienone derivatives. The studied structure and properties of cyclobutanone-based dienones can also be used in the design of photoactive supramolecular systems.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27217602/s1, Tables S1–S5: The geometric parameters of molecules 1b–d and 2, X-ray diffraction experiments, crystallographic characteristics and structure refinement details of compound 1b–f and 2; Figures S1–S19: The 1H NMR, 13C NMR, NOESY NMR, and HRMS spectra of compounds 1a–f, NOESY NMR spectrum of compound 1c; Figure S20: Fluorescence spectra of compounds 1c–f; Figures S21–S25: Quantum chemical calculations, orbitals involved in the first electron transition of compounds 1b–f; Figures S26–S32: Quantum chemical calculations, potential energy profiles of the ground S0 and lowest excited states of compounds 1a–f; Figures S33–S35: Correlations between the calculated frontier orbital energies, ionization potential, and electron affinities, and experimental oxidation and reduction potentials can be found in Supplementary Materials.
Author Contributions
Conceptualization, S.P.G.; formal analysis, A.Y.F., R.O.S. and M.V.F.; funding acquisition, S.P.G.; investigation, M.V.F., L.G.K., A.A.M., N.A.K. and V.N.N.; methodology, M.V.F. and V.N.N.; project administration, S.P.G.; writing—original draft: M.V.F., A.Y.F. and L.G.K.; writing—reviewing and editing: M.V.F. and S.P.G.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Russian Science Foundation (grant number 22-13-00064, except synthesis). The synthesis of dienones 1a–f and 2 was done under the financial support of the Ministry of Science and Higher Education of the Russian Federation (State Assignment FSRC “Crystallography and Photonics” of RAS).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
CCDC No. 2181814 (1b), CCDC No. 2181815 (1c), CCDC No. 2181816 (1d), CCDC No. 1892909 (1e), CCDC No. 2181818 (1f), CCDC No. 2181820 (syn,syn-2) и CCDC No. 2181819 (anti,anti-2) contain the supplementary crystallographic data for this article. These data can be obtained free of charge at the Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif (accessed on 1 October 2022).
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Not applicable.
References
- Adams, R.V. Organic Reactions; Adams, R., Ed.; Wiley & Sons, Inc.: London, UK; New York, NY, USA, 1959; Volume 10, 576p. [Google Scholar]
- Vatsadze, S.Z.; Golikov, A.G.; Kriven’ko, A.P.; Zyk, N.V. Chemistry of cross-conjugated dienones. Russ. Chem. Rev. 2008, 77, 707–728. [Google Scholar] [CrossRef]
- Cui, J.; Crich, D.; Wink, D.; Lam, M.; Rheingold, A.L.; Case, D.A.; Fu, W.T.; Zhou, Y.; Rao, M.; Olson, A.J.; et al. Design and synthesis of highly constrained factor Xa inhibitors: Amidine-Substituted bis(benzoyl)-1,3-diazepan-2-ones and bis(benzylidene)-bis(gem-dimethyl)cycloketones. Bioorg. Med. Chem. 2003, 11, 3379–3392. [Google Scholar] [CrossRef]
- Jin, R.; Chen, Q.; Yao, S.; Bai, E.; Fu, W.; Wang, L.; Wang, J.; Du, X.; Wei, T.; Xu, H.; et al. Synthesis and anti-tumor activity of EF24 analogues as IKKβ inhibitors. Eur. J. Med. Chem. 2018, 144, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, U.; Sgorbissa, A.; Foti, C.; Drioli, S.; Angelica, R.; Tomasella, A.; Picco, R.; Semrau, M.S.; Storici, P.; Benedetti, F.; et al. Synthesis, Characterization, and Optimization for in Vivo Delivery of a Nonselective Isopeptidase Inhibitor as New Antineoplastic Agent. J. Med. Chem. 2015, 58, 1691–1704. [Google Scholar] [CrossRef] [PubMed]
- Gangadhara; Kishore, K. Novel photocrosslinkable liquid-crystalline polymers: Poly[bis(benzylidene)] esters. Macromolecules 1993, 26, 2995–3003. [Google Scholar] [CrossRef]
- Kannan, P.; Gangadhara; Kishore, K. Novel photocrosslinkable flame retardant polyvanillylidene arylphosphate esters. Polymer 1997, 38, 4349–4355. [Google Scholar] [CrossRef]
- Yakimansky, A.V.; Tenkovtsev, A.V.; Dudkina, M.M.; Voigt-Martin, I.G.; Kolb, U.; Lukoshkin, V.A.; Böhme, F. Studies of structures and properties of polymeric systems containing bis-(hydroxy-arylidene)alkanones as NLO-active chromophores. J. Non-Cryst. Solids 2002, 303, 237–245. [Google Scholar] [CrossRef]
- Doroshenko, A.O.; Sychevskaya, L.B.; Grygorovych, A.V.; Pivovarenko, V.G. Fluorescence Probing of Cell Membranes with Azacrown Substituted Ketocyanine Dyes. J. Fluoresc. 2002, 12, 455–464. [Google Scholar] [CrossRef]
- Demianov, M.J.; Dojarenko, M. Darstellung von Cyclobutanon durch pyrochemische Zersetzung der 1-Oxy-cyclobutan-1-carbonsäure. Ber. Dtsch. Chem. Ges. 1922, 55, 2737. [Google Scholar]
- Thieme, P. Notiz zur Darstellung von 2.4-Dibenzyliden-cyclobutanonen. Chem. Ber. B 1968, 101, 378–380. [Google Scholar] [CrossRef]
- Nielsen, A.T.; Weiss, R.C.; Moore, D.W. Base-Catalyzed Intermolecular Condensation of α,β-Unsaturated Ketones. Dimerization of 2,4-Diarylidenecyclobutanones to 2-Spiro(2 oxocyclobutyl)bicyclo[3.2.0]heptan-6-one Derivatives. J. Org. Chem. 1972, 37, 1086–1092. [Google Scholar] [CrossRef]
- Clark, G.R.; Lin, J.; Nikaido, M. Aldole reactions of a cyclobutanone enolate. Tetrahedron Lett. 1984, 25, 2645–2648. [Google Scholar] [CrossRef]
- Vidal, J.; Huet, F. Synthesis of α-Methylenecyclobutanones. The First Preparation of Norsarkomycin Methyl Ester. J. Org. Chem. 1988, 53, 611–616. [Google Scholar] [CrossRef]
- Zou, Q.; Zhao, Y.; Makarov, N.S.; Campo, J.; Yuan, H.; Fang, D.-C.; Perry, J.W.; Feipeng, W. Effect of alicyclic ring size on the photophysical and photochemical properties of bis(arylidene)cycloalkanone compounds. Phys. Chem. Chem. Phys. 2012, 14, 11743–11752. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Zhao, H.; Zhao, Y.; Fang, Y.; Chen, D.; Ren, J.; Wang, X.; Wang, Y.; Gu, Y.; Feipeng, W. Effective Two-Photon Excited Photodynamic Therapy of Xenograft Tumors Sensitized by Water-Soluble Bis(arylidene)cycloalkanone Photosensitizers. J. Med. Chem. 2015, 58, 7949–7958. [Google Scholar] [CrossRef]
- Lin, Y.; Jiang, X.-F.; Duan, X.; Zeng, F.; Wu, B.; Wu, S. Therapeutic Nanosystem Consisting of Singlet-Oxygen-Responsive Prodrug and Photosensitizer Excited by Two-Photon Light. ACS Med. Chem. Lett. 2018, 9, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Sanford, E.M.; Paulisse, K.W.; Reeves, J.T. A computational study of 2,5-dibenzylidenecyclopentanone and 2,6-dibenzylidenecyclohexanone, model compounds for poly(arylidenecycloalkanones). J. Appl. Polym. Sci. 1999, 74, 2255–2257. [Google Scholar] [CrossRef]
- Grandeury, A.; Petit, S.; Coste, S.; Coquerel, G.; Perrio, C.; Gouhier, G. New synthesis of (Z,E)-2,7-bis(4-cyanobenzylidene)cycloheptan-1-one under stereospecific constraints induced by host-guest interactions. Chem. Commun. 2005, 31, 4007–4009. [Google Scholar] [CrossRef]
- Vatsadze, S.Z.; Manaenkova, M.A.; Sviridenkova, N.V.; Zyk, N.V.; Krut’ko, D.P.; Churakov, A.V.; Antipin, M.Y.; Howard, J.A.K.; Lang, H. Synthesis and spectroscopic and structural studies of cross-conjugated dienones derived from cyclic ketones and aromatic aldehydes. Russ. Chem. Bull. 2006, 55, 1184–1194. [Google Scholar] [CrossRef]
- Aizenshtat, Z.; Hausman, M.; Pickholtz, Y.; Tal, D.; Blum, J. Chlorocarbonylbis(triphenylphosphine)iridium-catalyzed isomerization, isoaromatization, and disproportionation of some cycloalkanones having exocyclic double bonds. J. Org. Chem. 1977, 42, 2386–2394. [Google Scholar] [CrossRef]
- George, H.; Roth, H.J. Photoisomerisierung und Cyclo-1,2-Addition α,β-ungesättigter Cyclanone. Tetrahedron Lett. 1971, 12, 4057–4060. [Google Scholar] [CrossRef]
- Kaupp, G.; Zimmermann, I. First Detection of a π-Coupled 1,5-Diradical via Cycloaddition. Angew. Chem. 1981, 20, 1018–1019. [Google Scholar] [CrossRef]
- Ovchinnikova, I.G.; Nikulov, D.K.; Bartashevich, E.V.; Matochkina, E.G.; Kodess, M.I.; Slepukhin, P.A.; Druzhinin, A.V.; Fedorova, O.V.; Rusinov, G.L.; Charushin, V.N. Pre-organization of diarylideneacetonyl crownophanes in single crystals to photochemical transformations. Russ. Chem. Bull. 2011, 60, 824–840. [Google Scholar] [CrossRef]
- Alfimov, M.V.; Gromov, S.P.; Stanislavskii, O.B.; Ushakov, E.N.; Fedorova, O.A. Crown-containing styryl dyes. 8. Cation-dependent concerted [2 + 2]-autophotocycloaddition of photochromic 15-crown-5 ether betaines. Russ. Chem. Bull. 1993, 42, 1385–1389. [Google Scholar] [CrossRef]
- Gromov, S.P.; Vedernikov, A.I.; Lobova, N.A.; Kuz’mina, L.G.; Dmitrieva, S.N.; Strelenko, Y.A.; Howard, J.A.K. Synthesis, Structure, and Properties of Supramolecular Photoswitches Based on Ammonioalkyl Derivatives of Crown-Ether Styryl Dyes. J. Org. Chem. 2014, 79, 11416–11430. [Google Scholar] [CrossRef]
- Kuz’mina, L.G.; Vedernikov, A.I.; Gromov, S.P.; Alfimov, M.V. Crystallographic Approach to the [2 + 2] Photocycloaddition Topochemical Reactions of Unsaturated Compounds with Single Crystal Retention. Crystallogr. Rep. 2019, 64, 691–712. [Google Scholar] [CrossRef]
- Biradha, K.; Santra, R. Crystal engineering of topochemical solid state reactions. Chem. Soc. Rev. 2013, 42, 950–967. [Google Scholar] [CrossRef]
- Elacqua, E.; Kaushik, P.; Groeneman, R.H.; Sumrak, J.C.; Bučar, D.-K.; MacGillivray, L.R. A Supramolecular Protecting Group Strategy Introduced to the Organic Solid State: Enhanced Reactivity through Molecular Pedal Motion. Angew. Chem. Int. Ed. 2012, 51, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Santra, R.; Garai, M.; Mondal, D.; Biradha, K. Anion Influence in Directing and Altering the Stereochemistry of the Double [2+2] Reaction of Bis-Pyridyl Dienes in their Silver Complexes: A Green Synthetic Route. Chem. Eur. J. 2013, 19, 489–493. [Google Scholar] [CrossRef]
- Santra, R.; Biradha, K. Solid state double [2 + 2] photochemical reactions in the co-crystal forms of 1,5-bis(4-pyridyl)-1,4-pentadiene-3-one: Establishing mechanism using single crystal X-ray, UV and H-1 NMR. CrystEngComm 2011, 13, 3246–3257. [Google Scholar] [CrossRef]
- Vatsadze, S.Z.; Gromov, S.P. Novel Linear Bis-Crown Receptors with Cross-Conjugated and Conjugated Central Cores. Macroheterocycles 2017, 10, 432–445. [Google Scholar] [CrossRef][Green Version]
- Fomina, M.V.; Kurchavov, N.A.; Freidzon, A.Y.; Nuriev, V.N.; Vedernikov, A.I.; Strelenko, Y.A.; Gromov, S.P. Self-assembly involving hydrogen bonds. Spectral properties and structure of supramolecular complexes of bis-aza-18-crown-6-containing dienones with alkanediammonium salts. J. Photochem. Photobiol. A 2020, 402, 112801. [Google Scholar] [CrossRef]
- Vatsadze, S.Z.; Gavrilova, G.V.; Zyuz’kevich, F.S.; Nuriev, V.N.; Krut’ko, D.P.; Moiseeva, A.A.; Shumyantsev, A.V.; Vedernikov, A.I.; Churakov, A.V.; Kuz’mina, L.G.; et al. Synthesis, structure, electrochemistry, and photophysics of 2,5-dibenzylidenecyclopentanones containing in benzene rings substituents different in polarity. Russ. Chem. Bull. 2016, 65, 1761–1772. [Google Scholar] [CrossRef]
- Fomina, M.V.; Vatsadze, S.Z.; Freidzon, A.Y.; Kuz’mina, L.G.; Moiseeva, A.A.; Starostin, R.O.; Nuriev, V.N.; Gromov, S.P. Structure–Property Relationships of dibenzylidenecyclohexanones. ACS Omega 2022, 7, 10087–10099. [Google Scholar] [CrossRef]
- Butcher, R.J.; Jasinski, J.P.; Narayana, B.; Sarojini, B.K.; Bindya, S.; Yathirajan, H.S. 2,5-Bis(3,4-dimethoxybenzylidene) cyclopentanone. Acta Cryst. 2007, E63, o3270–o3271. [Google Scholar] [CrossRef]
- Tsedilin, A.M.; Fakhrutdinov, A.N.; Eremin, D.B.; Zalesskiy, S.S.; Chizhov, A.O.; Kolotyrkina, N.G.; Ananikov, V.P. How sensitive and accurate are routine NMR and MS measurements? Mendeleev Commun. 2015, 25, 454–456. [Google Scholar] [CrossRef]
- Vedernikov, A.I.; Basok, S.S.; Gromov, S.P.; Kuz’mina, L.G.; Avakyan, V.G.; Lobova, N.A.; Kulygina, E.Y.; Titkov, T.V.; Strelenko, Y.A.; Ivanov, E.I.; et al. Synthesis and structure of bis-crown-containing stilbenes. Russ. J. Org. Chem. 2005, 41, 843–854. [Google Scholar] [CrossRef]
- Nuriev, V.N.; Fedorov, O.V.; Moiseeva, A.A.; Freidzon, A.Y.; Kurchavov, N.A.; Vedernikov, A.I.; Medved’ko, A.V.; Pod’yacheva, E.S.; Vatsadze, S.Z.; Gromov, S.P. Synthesis, structure, spectral properties, and electrochemistry of bis(crown ether) containing 1,3-distyrylbenzenes. Russ. J. Org. Chem. 2017, 53, 1726–1737. [Google Scholar] [CrossRef]
- Tsukerman, S.V.; Kutulya, L.A.; Lavrushin, V.F. Spectra and halo-chromism of dibenzylidenecycloalkanones and their thiophene and furan analogs. Zurn. Obshch. Khim. (Russ. J. Obshch. Chem.) 1964, 34, 3597. (In Russian) [Google Scholar]
- Issa, R.M.; Etaiw, S.H.; Issa, I.M.; El-Shafie, A.K. Electronic Absorption Spectra of some Diarylidene-Cyclopentanones and -Cyclohexanones. Acta Chim. Acad. Sceint. Hung. 1976, 89, 381–391. [Google Scholar]
- Gutrov, V.N.; Zakharova, G.V.; Fomina, M.V.; Nuriev, V.N.; Gromov, S.P.; Chibisov, A.K. Molecular photonics of dienones based on cycloalkanones and their derivatives. J. Photochem. Photobiol. A 2022, 425, 113678. [Google Scholar] [CrossRef]
- Freidzon, A.Y.; Bagatur’yants, A.A.; Gromov, S.P.; Alfimov, M.V. Recoordination of a metal ion in the cavity of a crown compound: A theoretical study 3. Absorption spectra and excited states of azacrown-containing styryl dyes and their complexes. Russ. Chem. Bull. 2008, 57, 2045–2055. [Google Scholar] [CrossRef]
- Al-Anber, M.; Vatsadze, S.Z.; Holze, R.; Lang, H.; Thiel, W.R. π-Conjugated N-heterocyclic compounds: Correlation of computational and electrochemical data. Dalton Trans. 2005, 22, 3632–3637. [Google Scholar] [CrossRef] [PubMed]
- Vatsadze, S.Z.; Al-Anber, M.; Thiel, W.R.; Lang, H.; Holze, R. Electrochemical studies and semiempirical calculations on pi-conjugated dienones and heterocyclic nitrogen containing donor ligand molecules. J. Solid. State. Electrochem. 2005, 9, 764–777. [Google Scholar] [CrossRef]
- Bruker. APEX2, SADABS and SAINT; Bruker AXS Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Pushman, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- SHELXTL-Plus, Version 5.10; Bruker AXS, Inc.: Madison, WI, USA, 1997.
- Granovsky, A.A. Firefly Version 8.2.0. Available online: http://classic.chem.msu.su/gran/firefly/index.html (accessed on 28 October 2022).
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.J.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef]
- Freidzon, A.Y.; Safonov, A.A.; Bagaturyants, A.A.; Alfimov, M.V. Solvatofluorochromism and Twisted Intramolecular Charge-Transfer State of the Nile Red Dye. Int. J. Quantum Chem. 2012, 112, 3059–3067. [Google Scholar] [CrossRef]
- Quentin, C.; Gerasimaitė, R.; Freidzon, A.; Atabekyan, L.S.; Lukinavičius, G.; Belov, V.N.; Mitronova, G.Y. Direct Visualization of Amlodipine Intervention into Living Cells by Means of Fluorescence Microscopy. Molecules 2021, 26, 2997. [Google Scholar] [CrossRef]
- Laikov, D.N. Fast evaluation of density functional exchange-correlation terms using the expansion of the electron density in auxiliary basis sets. Chem. Phys. Lett. 1997, 281, 151–156. [Google Scholar] [CrossRef]
- Laikov, D.N.; Ustynyuk, Y.A. PRIRODA-04: A quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ. Chem. Bull. 2005, 54, 820–826. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).