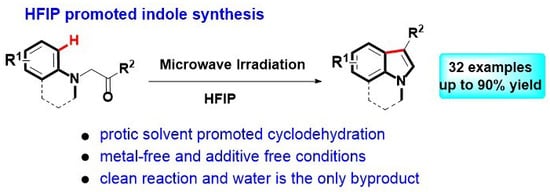
HFIP-Promoted Bischler Indole Synthesis under Microwave Irradiation
Abstract
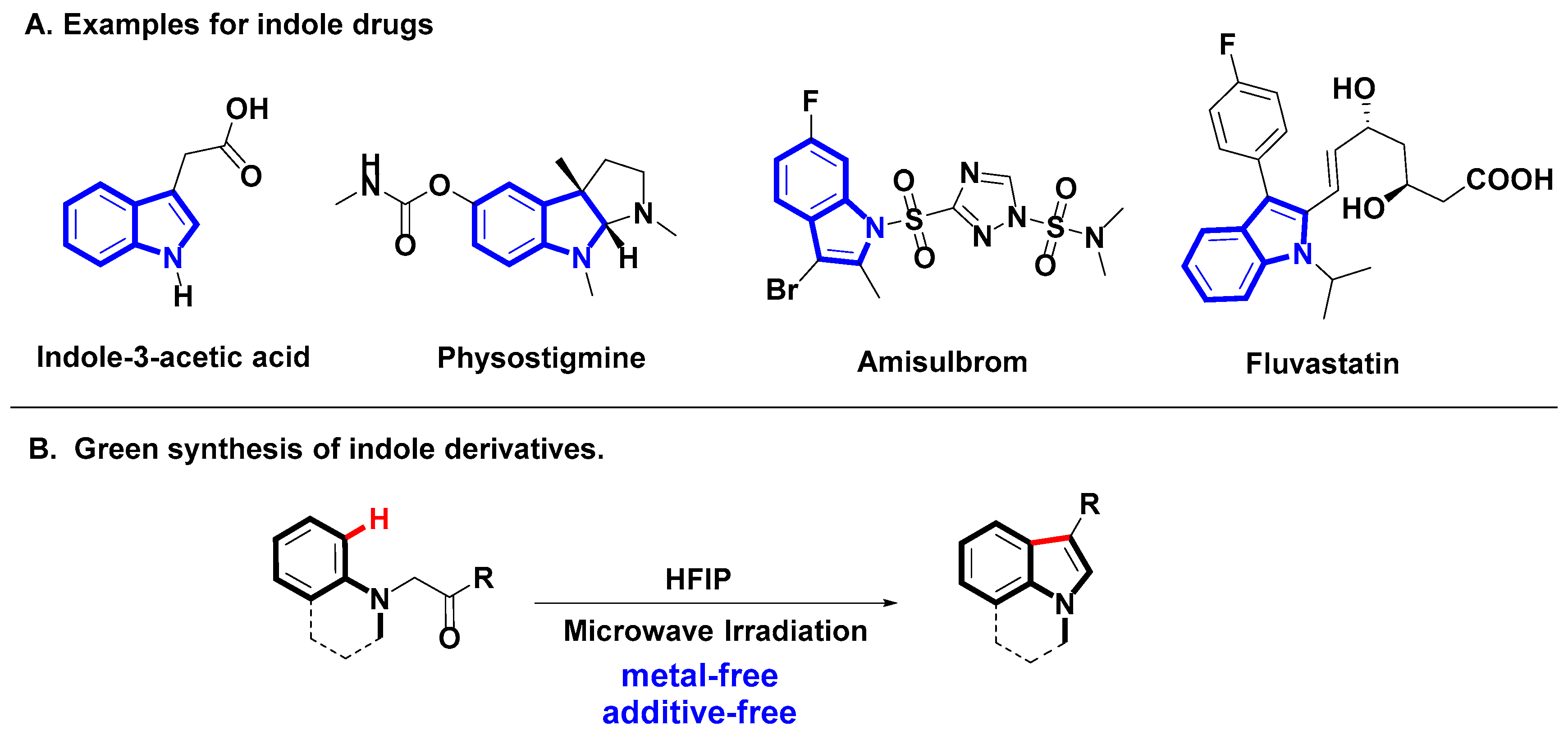
1. Introduction
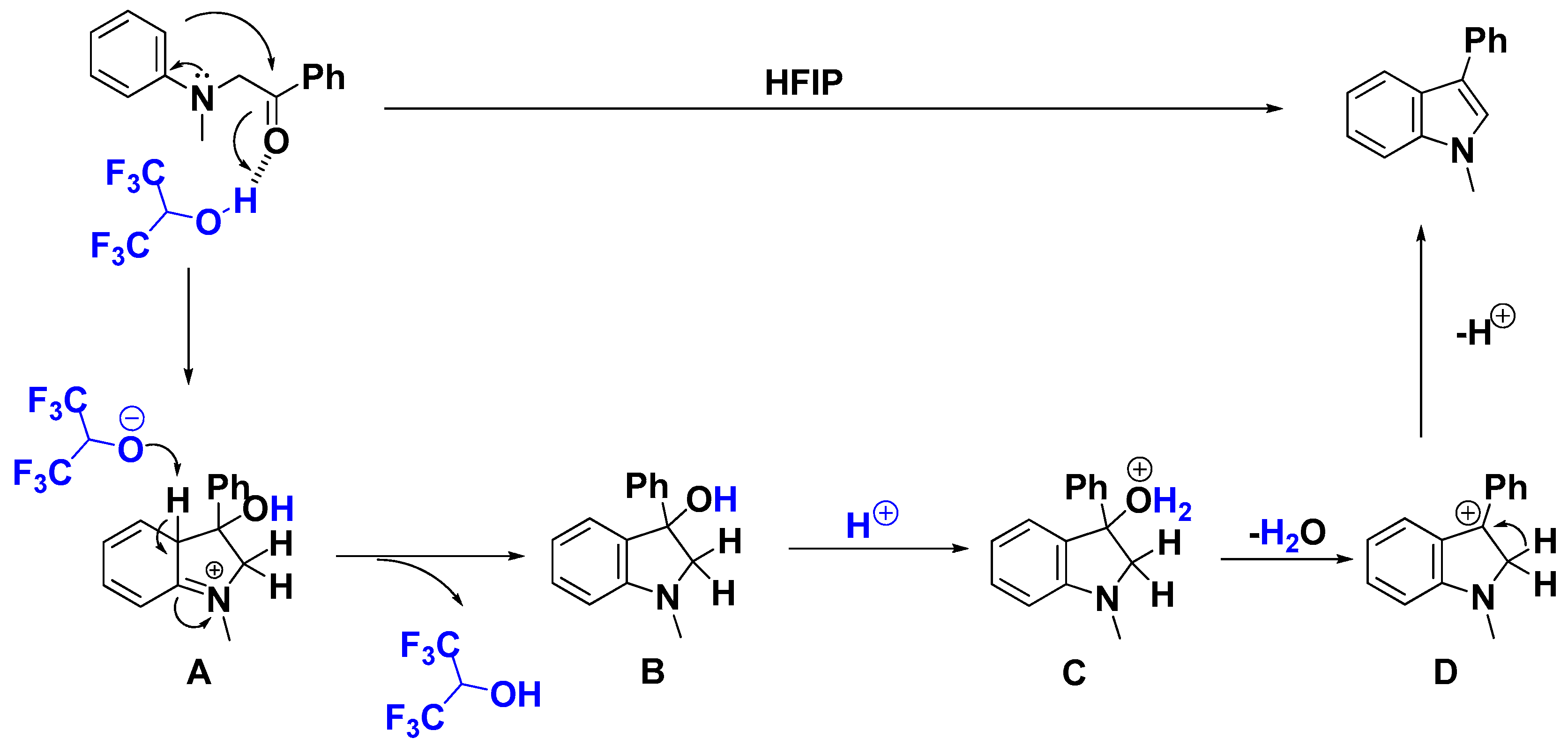
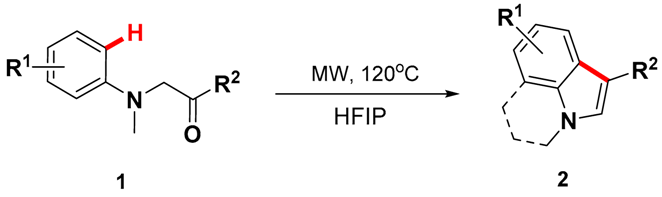
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. General Procedure for the Synthesis of Indoles and Pyrrolo[3,2,1-ij]quinolones
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Marine indole alkaloids: Potential new drug leads for the control of depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Imperatore, C.; Aiello, A.; D’Aniello, F.; Senese, M.; Menna, M. Alkaloids from marine invertebrates as important leads for anticancer drugs discovery and development. Molecules 2014, 19, 20391–20423. [Google Scholar] [CrossRef] [PubMed]
- Bariwal, J.; Voskressensky, L.G.; van der Eycken, E.V. Recent advances in spirocyclization of indole derivatives. Chem. Soc. Rev. 2018, 47, 3831–3848. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Müller, J. Auxin conjugates: Their role for plant development and in the evolution of land plants. J. Exp. Bot. 2011, 62, 1757–1773. [Google Scholar] [CrossRef]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical trials and late-stage drug development for Alzheimer’s disease: An appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef] [PubMed]
- Dreinert, A.; Wolf, A.; Mentzel, T.; Meunier, B.; Fehr, M. The cytochrome bc1 complex inhibitor ametoctradin has an unusual binding mode. BBA-Bioenerg. 2018, 1859, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Fuenfschilling, P.C.; Hoehn, P.; Mutz, J.-P. An improved manufacturing process for fluvastatin. Org. Process Res. Dev. 2007, 11, 13–18. [Google Scholar] [CrossRef]
- Inman, M.; Moody, C.J. Indole synthesis-something old, something new. Chem. Sci. 2013, 4, 29–41. [Google Scholar] [CrossRef]
- Vicente, R. Recent advances in indole syntheses: New routes for a classic target. Org. Biomol. Chem. 2011, 9, 6469–6480. [Google Scholar] [CrossRef]
- Humphrey, G.R.; Kuethe, J.T. Practical methodologies for the synthesis of indoles. Chem. Rev. 2006, 106, 2875–2911. [Google Scholar] [CrossRef]
- Buu-Hoï, N.P.; Saint-Ruf, G.; Deschamps, D.; Hieu, H.T. Carcinogenic nitrogen compounds. Part LXXII. The Möhlau-Bischler reaction as a preparative route to 2-arylindoles. J. Chem. Soc. C Org. 1971, 2606–2609. [Google Scholar] [CrossRef]
- Bigot, P.; Saint-Ruf, G.; Buu-Hoï, N.P. Carcinogenic nitrogen compounds. Part LXXXII. Polycyclic indoles by means of the Möhlau-Bischler synthesis. J. Chem. Soc. Perkin Trans. 1 1972, 2573–2576. [Google Scholar] [CrossRef]
- Robinson, B. The fischer indole synthesis. Chem. Rev. 1963, 63, 373–401. [Google Scholar] [CrossRef]
- Heravi, M.M.; Rohani, S.; Zadsirjan, V.; Zahedi, N. Fischer indole synthesis applied to the total synthesis of natural products. RSC Adv. 2017, 7, 52852–52887. [Google Scholar] [CrossRef]
- Bartoli, G.; Palmieri, G.; Bosco, M.; Dalpozzo, R. The reaction of vinyl grignard reagents with 2-substituted nitroarenes: A new approach to the synthesis of 7-substituted indoles. Tetrahedron Lett. 1989, 30, 2129–2132. [Google Scholar] [CrossRef]
- Bartoli, G.; Dalpozzo, R.; Nardi, M. Applications of Bartoli indole synthesis. Chem. Soc. Rev. 2014, 43, 4728–4750. [Google Scholar] [CrossRef] [PubMed]
- Bashford, K.E.; Cooper, A.L.; Kane, P.D.; Moody, C.J.; Muthusamy, S.; Swann, E. N-H Insertion reactions of rhodium carbenoids. Part 3.1. The development of a modified Bischler indole synthesis and a new protecting-group strategy for indoles. J. Chem. Soc. Perkin Trans. 1 2002, 1672–1687. [Google Scholar] [CrossRef]
- Tsuchikama, K.; Hashimoto, Y.-K.; Endo, K.; Shibata, T. Iridium-catalyzed selective synthesis of 4-substituted benzofurans and indoles via directed cyclodehydration. Adv. Synth. Catal. 2009, 351, 2850–2854. [Google Scholar] [CrossRef]
- Tokunaga, M.; Ota, M.; Haga, M.-A.; Wakatsuki, Y. A practical one-pot synthesis of 2,3-disubstituted indoles from unactivated anilines. Tetrahedron Lett. 2001, 42, 3865–3868. [Google Scholar] [CrossRef]
- Kumar, M.P.; Liu, R.-S. Zn(OTf)2-catalyzed cyclization of proparyl alcohols with anilines, phenols, and amides for synthesis of indoles, benzofurans, and oxazoles through different annulation mechanisms. J. Org. Chem. 2006, 71, 4951–4955. [Google Scholar] [CrossRef]
- Bunescu, A.; Piemontesi, C.; Wang, Q.; Zhu, J. Heteroannulation of arynes with N-aryl-α-aminoketones for the synthesis of unsymmetrical N-aryl-2,3-disubstituted indoles: An aryne twist of Bischler-Möhlau indole synthesis. Chem. Commun. 2013, 49, 10284–10286. [Google Scholar] [CrossRef] [PubMed]
- MacDonough, M.T.; Shi, Z.; Pinney, K.G. Mechanistic considerations in the synthesis of 2-aryl-indole analogues under Bischler-Mohlau conditions. Tetrahedron Lett. 2015, 56, 3624–3629. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, V.; Perumal, S.; Avendaño, C.; Menéndez, J.C. Microwave-assisted, solvent-free Bischler indole synthesis. Synlett 2006, 91–95. [Google Scholar] [CrossRef]
- Liao, Y.-Y.; Gao, Y.-C.; Zheng, W.; Tang, R.-Y. Oxidative radical cyclization of N-methyl-N-arylpropiolamide to isatins via cleavage of the carbon-carbon triple bond. Adv. Synth. Catal. 2018, 360, 3391–3400. [Google Scholar] [CrossRef]
- Tang, R.-Y.; Guo, X.-K.; Xiang, J.-N.; Li, J.-H. Palladium-catalyzed synthesis of 3-acylated indoles involving oxidative cross-coupling of indoles with α-amino carbonyl compounds. J. Org. Chem. 2013, 78, 11163–11171. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Zhang, J.-R.; Liao, Y.-Y.; Xu, L.; Hu, M.; Tang, R.-Y. CuCl/air-mediated oxidative coupling reaction of imidazoheterocycles with N-aryl glycine esters. RSC Adv. 2017, 7, 30152–30159. [Google Scholar] [CrossRef]
- Ji, X.-M.; Zhou, S.-J.; Deng, C.-L.; Chen, F.; Tang, R.-Y. NH4PF6-promoted cyclodehydration of α-amino carbonyl compounds: Efficient synthesis of pyrrolo [3,2,1-ij] quinoline and indole derivatives. RSC Adv. 2014, 4, 53837–53841. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Patil, S.A.; Patil, R.; Miller, D.D. Microwave-assisted synthesis of medicinally relevant indoles. Curr. Med. Chem. 2011, 18, 615–637. [Google Scholar] [CrossRef]
- Driowya, M.; Saber, A.; Marzag, H.; Demange, L.; Benhida, R.; Bougrin, K. Microwave-assisted synthesis of bioactive six-membered heterocycles and their fused analogues. Molecules 2016, 21, 492. [Google Scholar] [CrossRef]
- Brancale, A.; Silvestri, R. Indole, a core nucleus for potent inhibitors of tubulin polymerization. Med. Res. Rev. 2007, 27, 209–238. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.L.; Zhang, W.M.; Lv, P.C.; Zhu, H.L. Indole-based, Antiproliferative Agents Targeting Tublin Polymerizaton. Curr. Top. Med. Chem. 2017, 17, 120–137. [Google Scholar] [CrossRef] [PubMed]
- Sakami, S.; Kawai, K.; Maeda, M.; Aoki, T.; Fujii, H.; Ohno, H.; Ito, T.; Saitoh, A.; Nakao, K.; Izumimoto, N.; et al. Design and synthesis of a metabolically stable and potent antitussive agent, a novel δ opioid receptor antagonist, TRK-851. Bioorg. Med. Chem. 2008, 16, 7956–7967. [Google Scholar] [CrossRef] [PubMed]
- Matesic, L.; Locke, J.M.; Vine, K.L.; Ranson, M.; Bremner, J.B.; Skropeta, D. Synthesis and anti-leukaemic activity of pyrrolo[3,2,1-ij]indole-1,2-diones, pyrrolo[3,2,1-ij]quinoline-1,2-diones and other polycyclic isatin derivatives. Tetrahedron 2012, 68, 6810–6819. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, W.; Feng, C.; Liu, Z.; Bai, E.; Wang, X.; Lei, M.; Cheng, H.; Feng, H.; Shi, J.; et al. Design, synthesis, SAR discussion, in vitro and in vivo evaluation of novel selective EGFR modulator to inhibit L858R/T790M double mutants. Eur. J. Med. Chem. 2017, 135, 12–23. [Google Scholar] [CrossRef]
- Phipps, R.J.; Grimster, N.P.; Gaunt, M.J. Cu(II)-catalyzed direct and site-selective arylation of indoles under mild conditions. J. Am. Chem. Soc. 2008, 130, 8172–8174. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, K.; Tanaka, S.; Konno, M.; Onozawa, S.; Chiba, M.; Tanaka, Y.; Sasaki, Y.; Okubo, R.; Hattori, T. Me2AlCl-mediated carboxylation, ethoxycarbonylation, and carbamoylation of indoles. Tetrahedron 2016, 72, 734–745. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2–22, 24–34 are available from the authors. |

| Entry | Solvent | Time/(min) | T/°C | Yield (%) b |
|---|---|---|---|---|
| 1 | HFIP | 30 | 120 | 76 |
| 2 | CF3CH2OH | 30 | 120 | 45 |
| 3 | i-PrOH | 30 | 120 | 21 |
| 4 | EtOH | 30 | 120 | 23 |
| 5 | HFIP | 30 | 80 | 51 |
| 6 | HFIP | 30 | 100 | 72 |
| 7 | HFIP | 20 | 120 | 58 |
| 8 | HFIP | 40 | 120 | 88 |
| 9 c | HFIP | 960 | 120 | 86 |
 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, G.; Zhang, Z.-X.; Zhang, C.-B.; Xu, H.-H.; Tang, R.-Y. HFIP-Promoted Bischler Indole Synthesis under Microwave Irradiation. Molecules 2018, 23, 3317. https://doi.org/10.3390/molecules23123317
Yao G, Zhang Z-X, Zhang C-B, Xu H-H, Tang R-Y. HFIP-Promoted Bischler Indole Synthesis under Microwave Irradiation. Molecules. 2018; 23(12):3317. https://doi.org/10.3390/molecules23123317
Chicago/Turabian StyleYao, Guangkai, Zhi-Xiang Zhang, Cheng-Bei Zhang, Han-Hong Xu, and Ri-Yuan Tang. 2018. "HFIP-Promoted Bischler Indole Synthesis under Microwave Irradiation" Molecules 23, no. 12: 3317. https://doi.org/10.3390/molecules23123317
APA StyleYao, G., Zhang, Z.-X., Zhang, C.-B., Xu, H.-H., & Tang, R.-Y. (2018). HFIP-Promoted Bischler Indole Synthesis under Microwave Irradiation. Molecules, 23(12), 3317. https://doi.org/10.3390/molecules23123317