Facile Access to Fe(III)-Complexing Cyclic Hydroxamic Acids in a Three-Component Format
Abstract
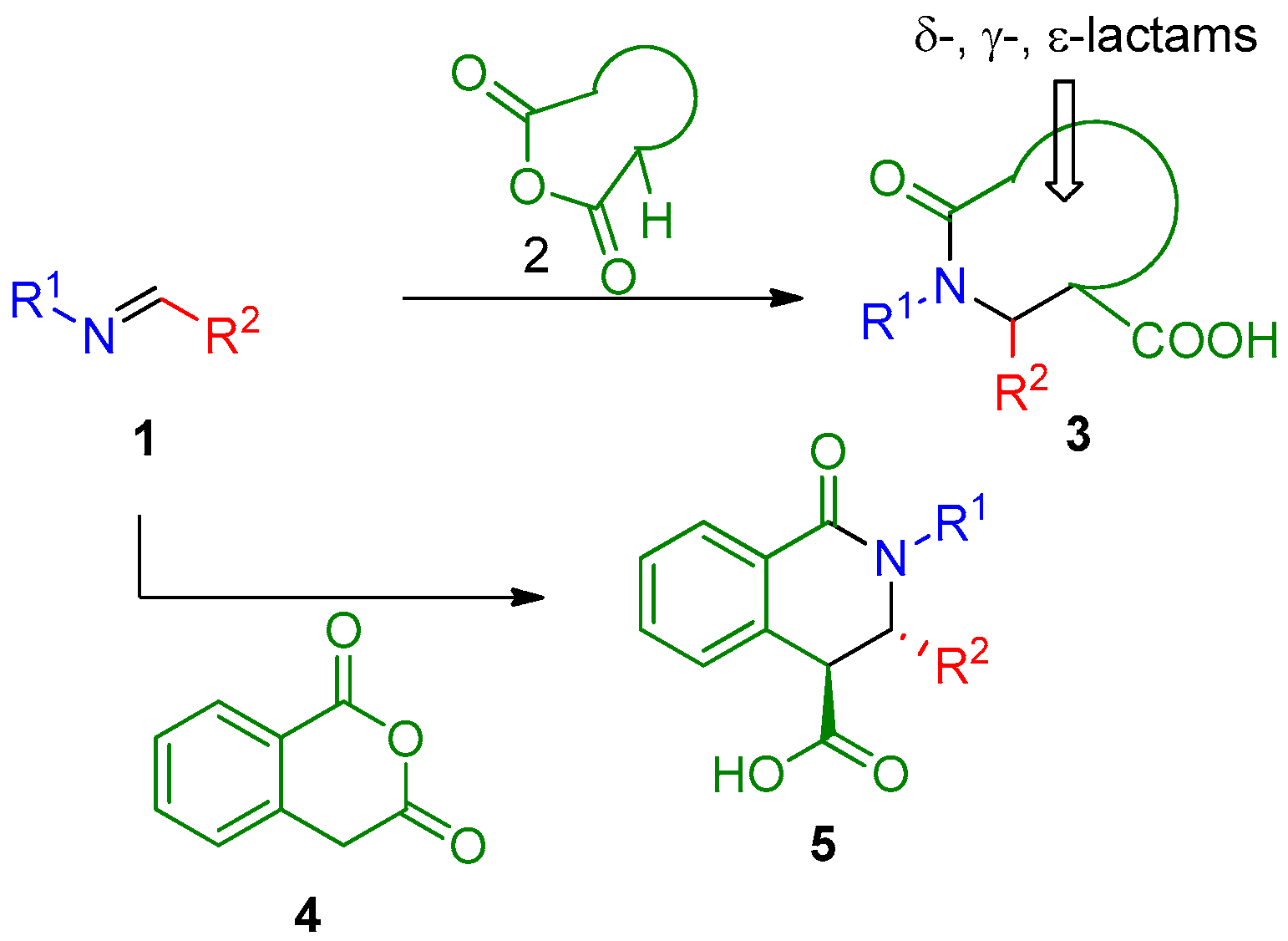
1. Introduction
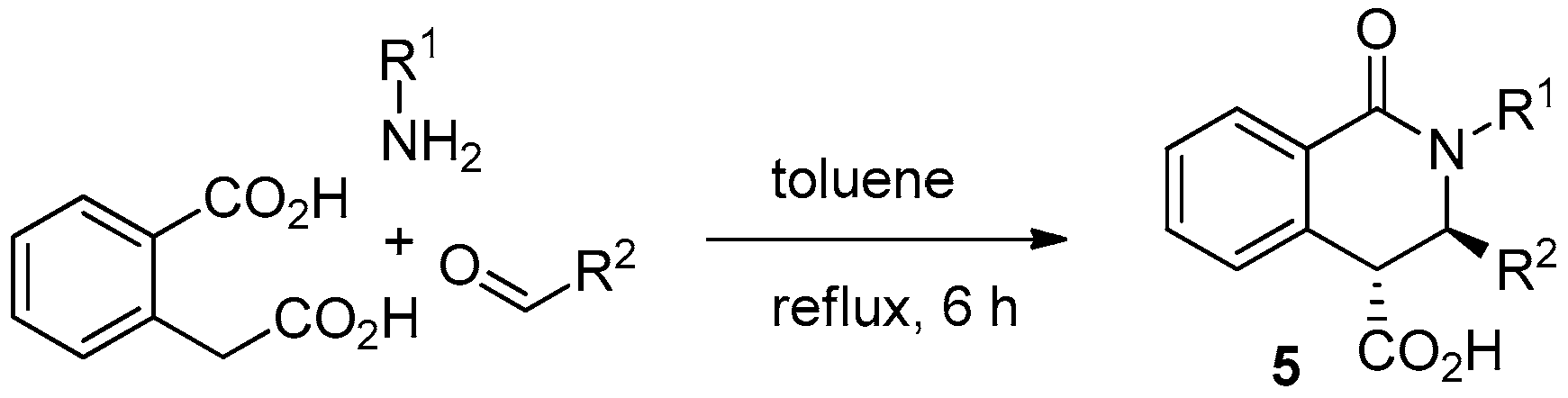
2. Results and Discussion
3. Materials and Methods
3.1. General Considerations
3.2. Synthesis
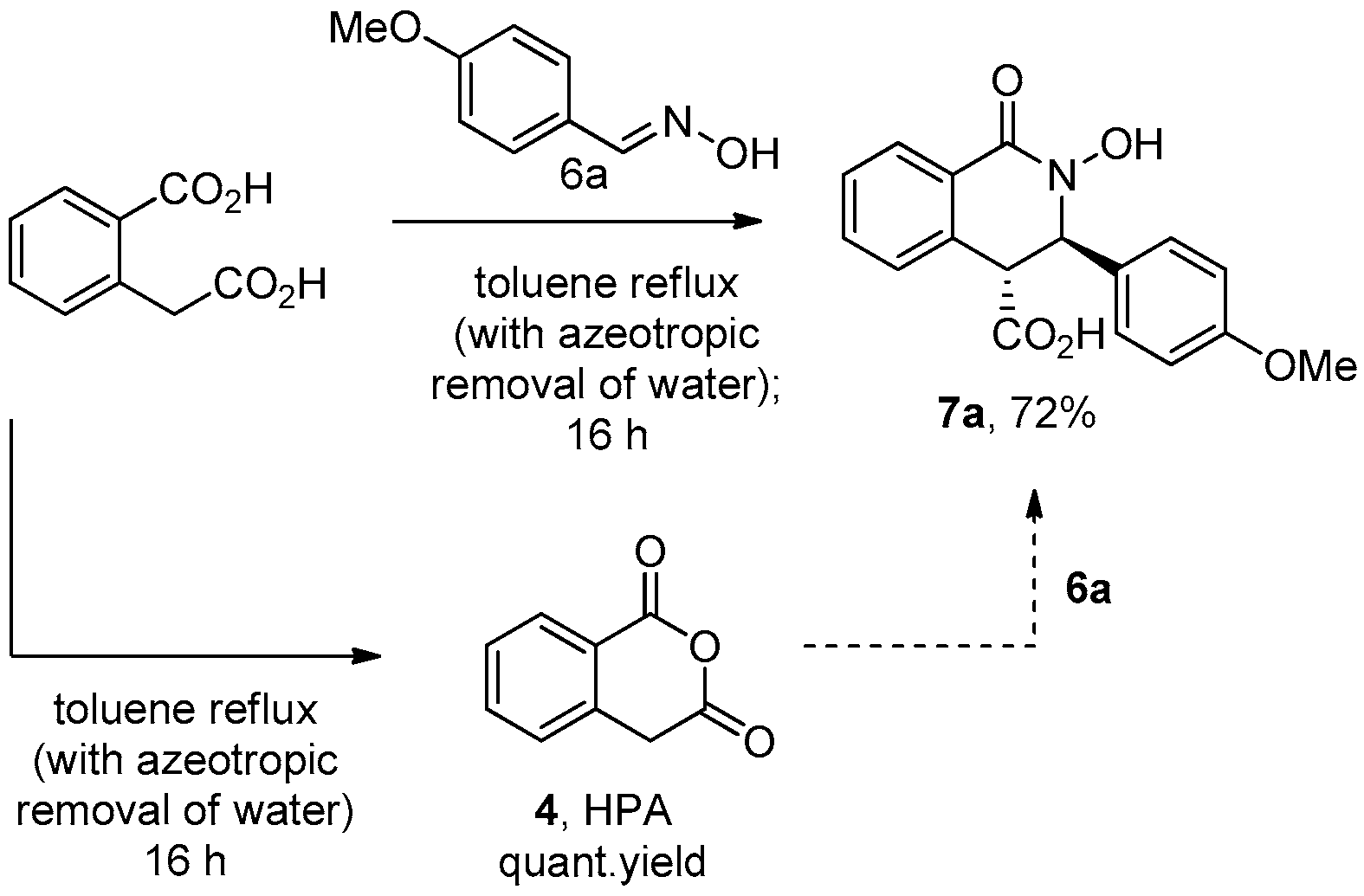
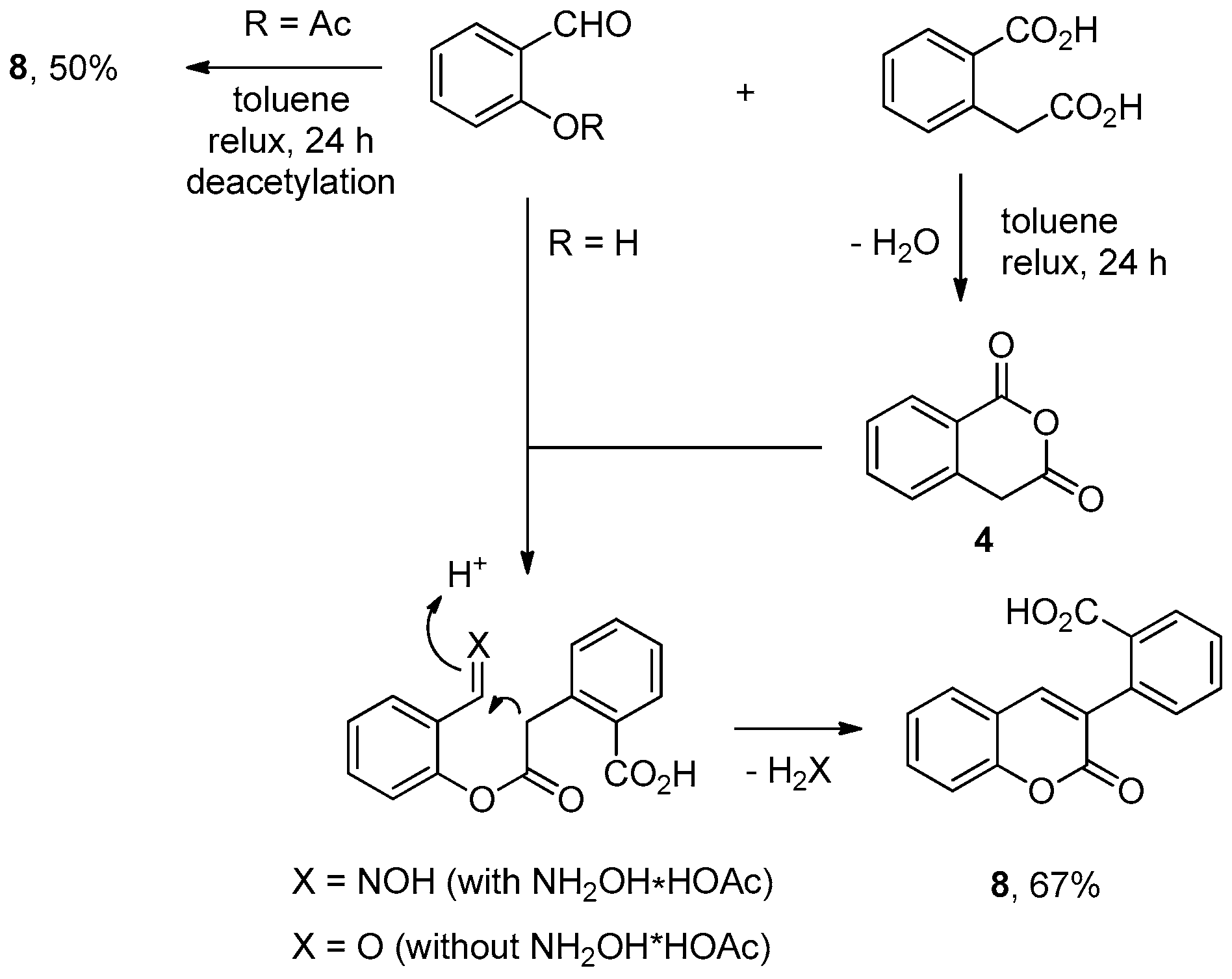
3.2.1. Homophthalic Anhydride 4 (Scheme 3)
3.2.2. Hydroxylamine Acetate
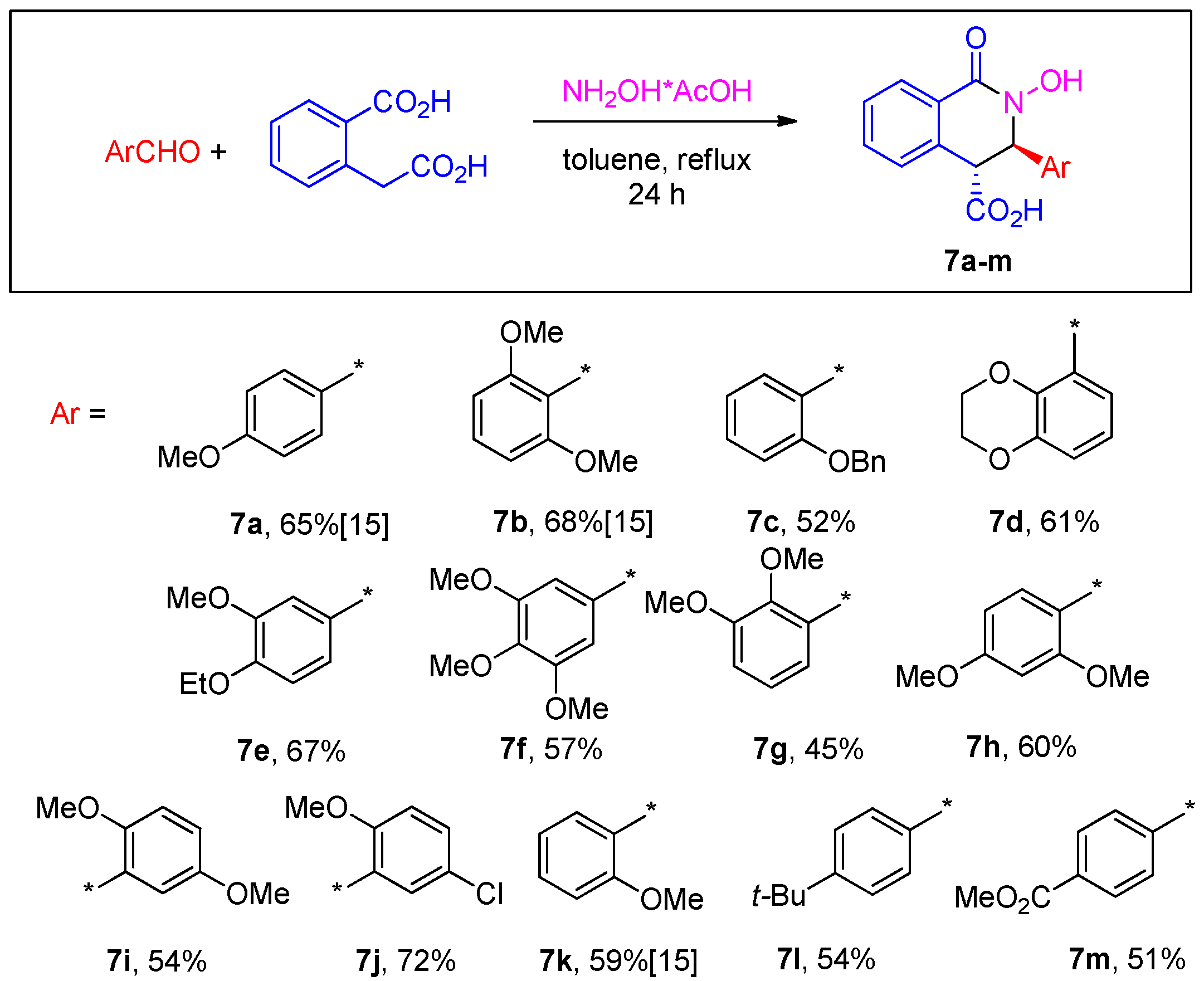
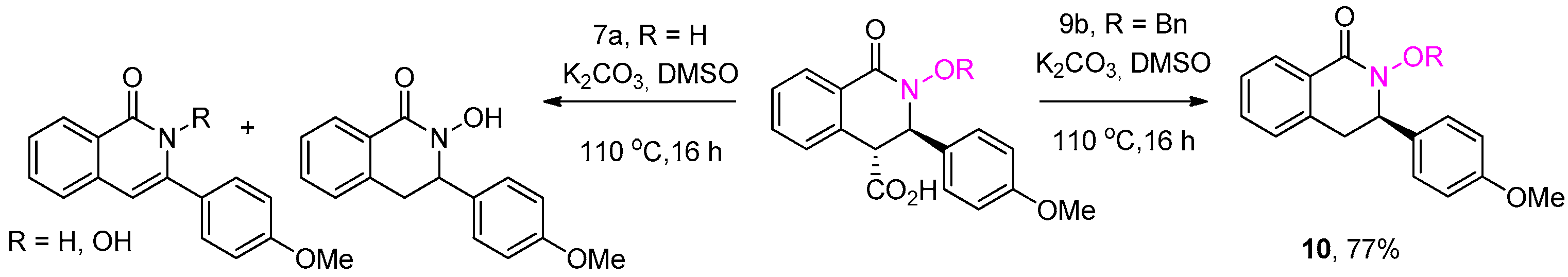
3.2.3. General Procedure for the Preparation of N-Hydroxy THIQ Acids 7a–k
3.2.4. General Procedure for the Preparation of N-Hydroxy THIQ Acids 7l,m
3.2.5. 2-(2-Oxo-2H-chromen-3-yl)benzoic Acid 8
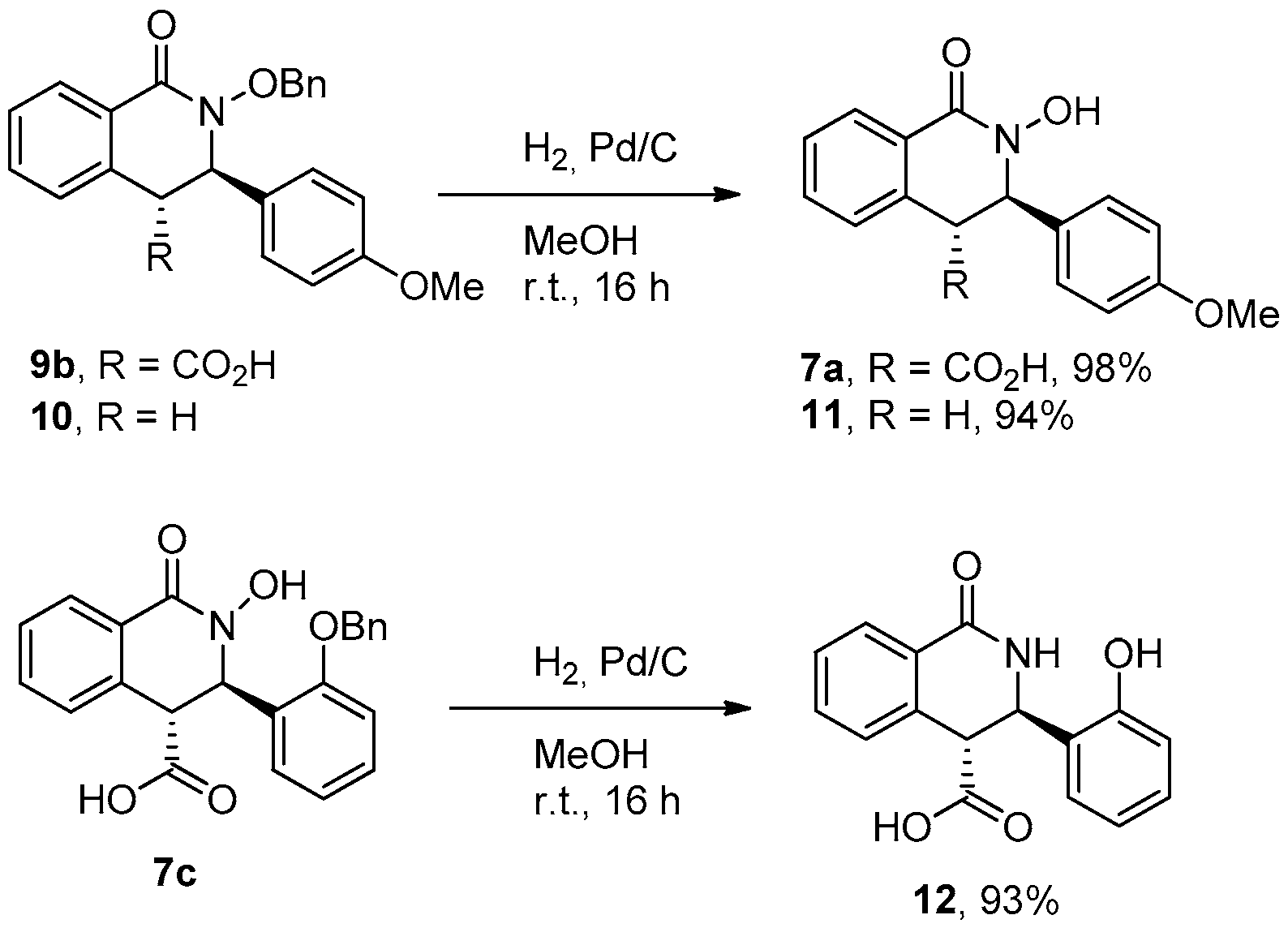
3.2.6. General Procedure for the Preparation of N-Benzyloxy THIQ Acids 9a–g
3.2.7. 2-(Benzyloxy)-3-(4-methoxyphenyl)-3,4-dihydroisoquinolin-1(2H)-one (10)
3.2.8. General Procedure for O-debenzylation
Synthesis of Compounds 7a, 11, and 12
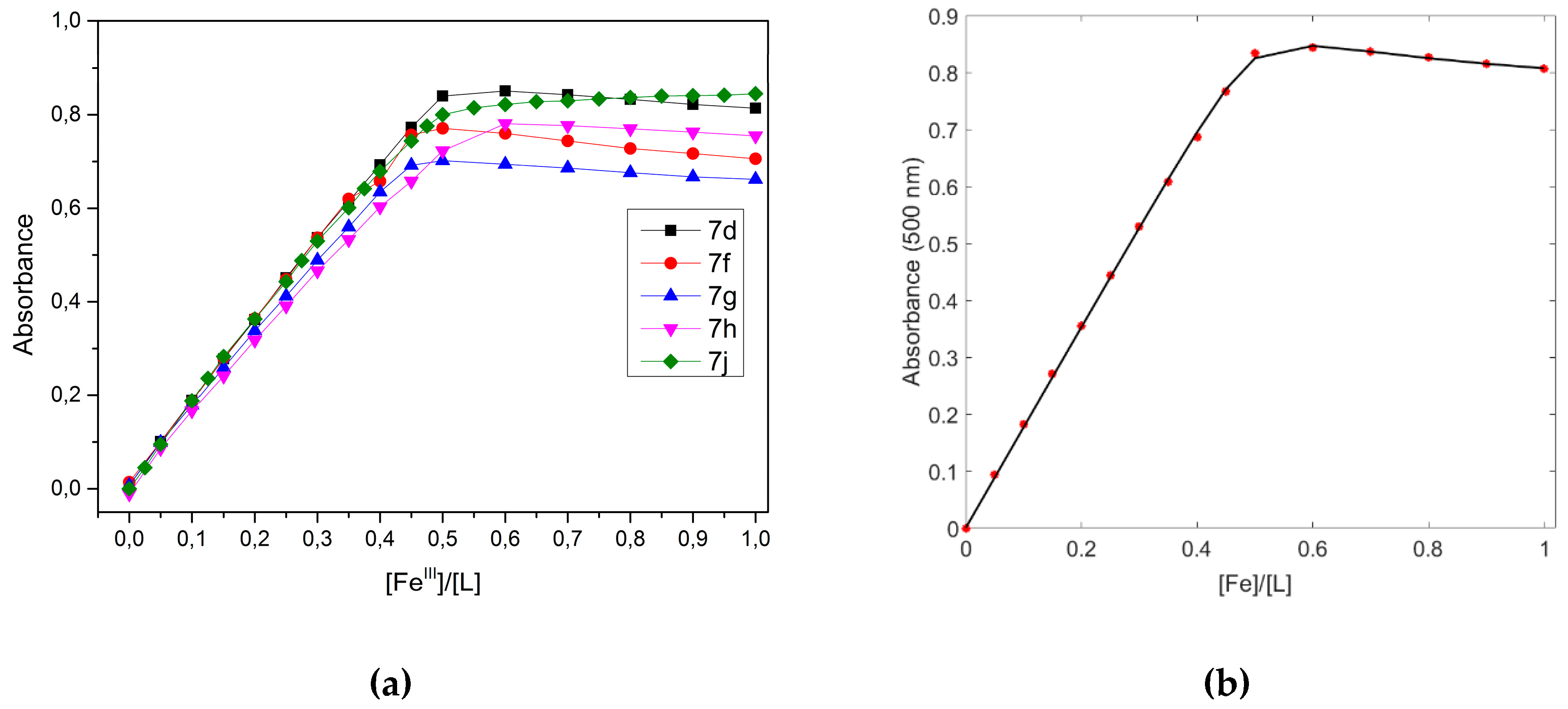
3.3. Spectrophotometric Investigation of Compounds 7d, 7f, 7g, 7h, 7j, and Their Complexation with Iron(III) Nitrate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farkas, E.; Enyedy, É.A.; Micera, G.; Garribba, E. Coordination modes of hydroxamic acids in copper(II), nickel(II) and zinc(II) mixed-ligand complexes in aqueous solution. Polyhedron 2000, 19, 1727–1736. [Google Scholar] [CrossRef]
- Schalk, I.J.; Hannauer, M.; Braud, A. New roles for bacterial siderophores in metal transport and tolerance. Environ. Microbiol. 2011, 13, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, K.; Hu, H.-Y.; Fetz, V.; Prochnow, H.; Rais, B.; Müller, P.P.; Brönstrup, M. Multivalent Siderophore–DOTAM Conjugates as Theranostics for Imaging and Treatment of Bacterial Infections. Angew. Chem. Int. Ed. 2017, 56, 8272–8276. [Google Scholar] [CrossRef] [PubMed]
- Italia, K.; Colah, R.; Ghosh, K. Experimental animal model to study iron overload and iron chelation and review of other such models. Blood Cells Mol. Dis. 2015, 55, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jacobs, H.K.; Gopalan, A.S. A new approach to cyclic hydroxamic acids: Intramolecular cyclization of N-benzyloxy carbamates with carbon nucleophiles. Tetrahedron 2011, 67, 2206–2214. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; King, S.B. Synthesis of Cyclic Hydroxamic Acids through−NOH Insertion of Ketones. Organic Lett. 2009, 11, 4580–4583. [Google Scholar] [CrossRef] [PubMed]
- Jewula, P.; Berthet, J.-C.; Chambron, J.-C.; Rousselin, Y.; Thuéry, P.; Meyer, M. Synthesis and Structural Study of Tetravalent (Zr4+, Hf4+, Ce4+, Th4+, U4+) Metal Complexes with Cyclic Hydroxamic Acids. European J. Inorganic Chem. 2015, 2015, 1529–1541. [Google Scholar] [CrossRef]
- González-López, M.; Shaw, J.T. Cyclic Anhydrides in Formal Cycloadditions and Multicomponent Reactions. Chem. Rev. 2009, 109, 164–189. [Google Scholar] [CrossRef] [PubMed]
- Dar’in, D.; Bakulina, O.; Chizhova, M.; Krasavin, M. New Heterocyclic Product Space for the Castagnoli–Cushman Three-Component Reaction. Organic Lett. 2015, 17, 3930–3933. [Google Scholar] [CrossRef] [PubMed]
- Castagnoli, N. Condensation of succinic anhydride with N-benzylidene-N-methylamine. Stereoselective synthesis of trans- and cis-1-methyl-4-carboxy-5-phenyl-2-pyrrolidinone. J. Organic Chem. 1969, 34, 3187–3189. [Google Scholar] [CrossRef]
- Cushman, M.; Castagnoli, N. Novel approach to the synthesis of nitrogen analogs of the tetrahydrocannabinols. J. Organic Chem. 1973, 38, 440–448. [Google Scholar] [CrossRef]
- Mohammadi, M.H.; Mohammadi, A.A. One-Pot, Three-Component Synthesis of Cis-Isoquinolonic Acids Using ZnCl2, AlCl3-SiO2 as Catalyst. Synth. Commun. 2011, 41, 523–527. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.; Tian, H.; Qian, C.; Sun, J. One-Pot Synthesis of cis-Isoquinolonic Acid Derivatives via Three-Component Reaction of Homophthalic Anhydride with Aldehydes and Amines using Ytterbium(III) Triflate as Catalyst. Adv. Synth. Cataly. 2005, 347, 689–694. [Google Scholar] [CrossRef]
- Pommier, Y. DNA Topoisomerase I Inhibitors: Chemistry, Biology and Interfacial Inhibition. Chem. Rev. 2009, 109, 2894–2902. [Google Scholar] [CrossRef] [PubMed]
- Floyd, D.M.; Stein, P.; Wang, Z.; Liu, J.; Castro, S.; Clark, J.A.; Connelly, M.; Zhu, F.; Holbrook, G.; Matheny, A.; et al. Hit-to-Lead Studies for the Antimalarial Tetrahydroisoquinolone Carboxanilides. J. Med. Chem. 2016, 59, 7950–7962. [Google Scholar] [CrossRef] [PubMed]
- Humphries, P.S.; Benbow, J.W.; Bonin, P.D.; Boyer, D.; Doran, S.D.; Frisbie, R.K.; Piotrowski, D.W.; Balan, G.; Bechle, B.M.; Conn, E.L.; et al. Synthesis and SAR of 1,2,[3,4-tetrahydroisoquinolin-1-ones as novel G-protein-coupled receptor 40 (GPR40) antagonists. Bioorg. Med. Chem. Lett. 2009, 19, 2400–2403. [Google Scholar] [CrossRef] [PubMed]
- Bakulina, O.; Bannykh, A.; Dar’in, D.; Krasavin, M. Cyclic Hydroxamic Acid Analogues of Bacterial Siderophores as Iron-Complexing Agents prepared through the Castagnoli–Cushman Reaction of Unprotected Oximes. Chem. A Eur. J. 2017, 23, 17667–17673. [Google Scholar] [CrossRef] [PubMed]
- Chupakhin, E.; Dar’in, D.; Krasavin, M. The Castagnoli-Cushman reaction in a three-component format. Tetrahedron Lett. 2018, 59, 2595–2599. [Google Scholar] [CrossRef]
- Bogdanov, M.G.; Mitrev, Y.; Tiritiris, I. New Highly Diastereoselective Perkin/Michael Addition Domino Reaction between Homophthalic Anhydride and Aromatic Aldehydes: A Facile Approach to Blue-Fluorescent Dibenzo[c,h]chromenones. Eur. J. Organic Chem. 2010, 2011, 377–384. [Google Scholar] [CrossRef]
- Senadi, G.C.; Mutra, M.R.; Lu, T.-Y.; Wang, J.-J. Oximes as reusable templates for the synthesis of ureas and carbamates by an in situ generation of carbamoyl oximes. Green Chem. 2017, 19, 4272–4277. [Google Scholar] [CrossRef]
- Svinyarov, I.; Bogdanov, M.G. One-pot synthesis and radical scavenging activity of novel polyhydroxylated 3-arylcoumarins. European J. Med. Chem. 2014, 78, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Tutov, A.; Bakulina, O.; Dar’in, D.; Krasavin, M. Concise synthesis of 2-N-hydroxy-3,4-dihydroisoquinol-2-one: A bacterial siderophore and human 5-lipooxygenase inhibitor. Tetrahedron Lett. 2018, 59, 1511–1512. [Google Scholar] [CrossRef]
- Gräfe, U.; Ritzau, M.; Ihn, W.; Möllmann, U.; Fleck, W.F.; Groth, J.; Reissbrodt, R. A new microbial isoquinoline iron chelator from Streptomyces spec. 2002-104. J. Basic Microbiol. 1994, 34, 351–355. [Google Scholar] [CrossRef]
- Schalk, I.J. A Trojan-Horse Strategy Including a Bacterial Suicide Action for the Efficient Use of a Specific Gram-Positive Antibiotic on Gram-Negative Bacteria. J. Med. Chem. 2018, 61, 3842–3844. [Google Scholar] [CrossRef] [PubMed]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
- Özcan, S.; Balci, M. The chemistry of homophthalic acid: A new synthetic strategy for construction of substituted isocoumarin and indole skeletons. Tetrahedron 2008, 64, 5531–5540. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Barber, P.S.; Gurau, G.; Griggs, C.S.; Rogers, R.D. Pulping of Crustacean Waste Using Ionic Liquids: To Extract or Not To Extract. ACS Sustainable Chem. Eng. 2016, 4, 6072–6081. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 7a–m, 9a–g are available from the authors. |



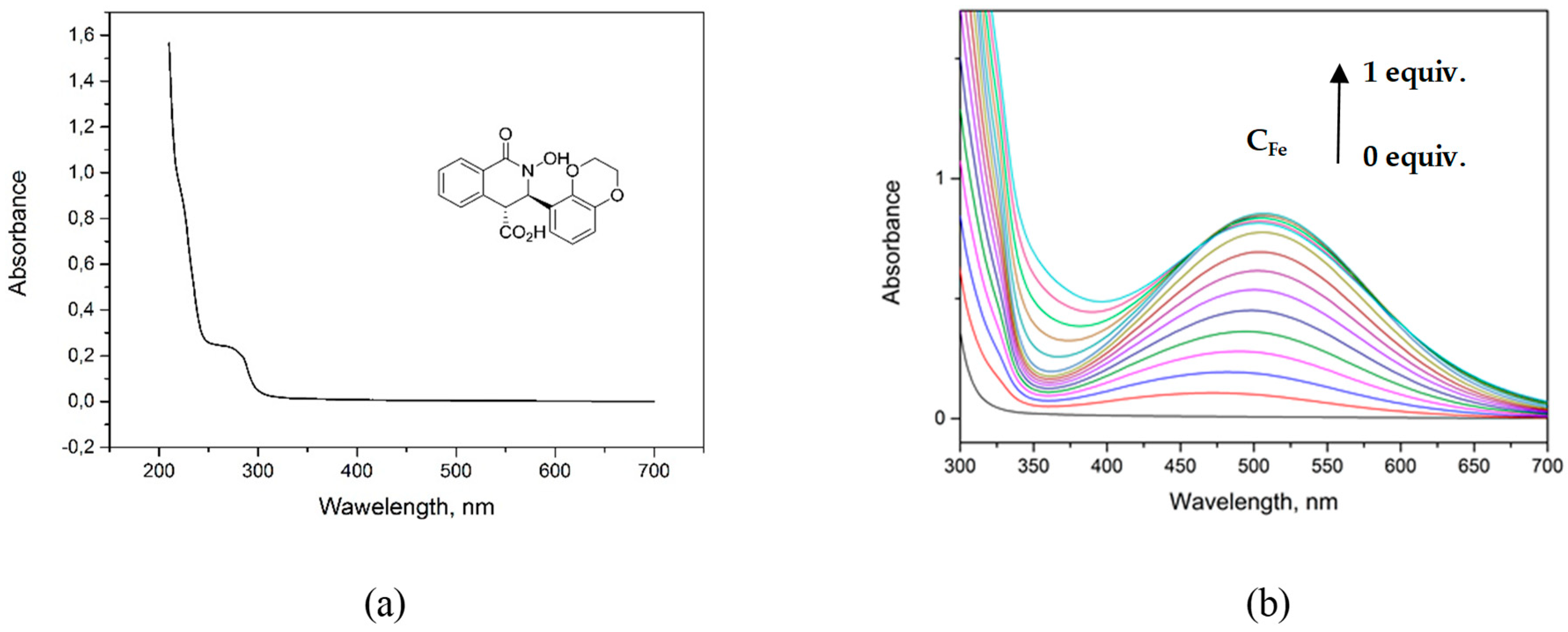
| Ligand | M:L | K1,2 | log K1,2 | λ [nm] a |
|---|---|---|---|---|
| 7d | 1:1 | 1.67 × 106 | 6.22 | 500 |
| 1:2 | 2.32 × 105 | 5.36 | ||
| 7f | 1:1 | 5.09 × 106 | 6.71 | 500 |
| 1:2 | 6.04 × 105 | 5.78 | ||
| 7g | 1:1 | 2.16 × 107 | 7.33 | 500 |
| 1:2 | 5.38 × 104 | 4.73 | ||
| 7h | 1:1 | 8.22 × 105 | 5.91 | 500, 550, 600 |
| 1:2 | 3.39 × 105 | 5.53 | ||
| 7j | 1:1 | 2.31 × 105 | 5.36 | 470 |
| 1:2 | 4.06 × 105 | 5.61 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chupakhin, E.; Bakulina, O.; Dar’in, D.; Krasavin, M. Facile Access to Fe(III)-Complexing Cyclic Hydroxamic Acids in a Three-Component Format. Molecules 2019, 24, 864. https://doi.org/10.3390/molecules24050864
Chupakhin E, Bakulina O, Dar’in D, Krasavin M. Facile Access to Fe(III)-Complexing Cyclic Hydroxamic Acids in a Three-Component Format. Molecules. 2019; 24(5):864. https://doi.org/10.3390/molecules24050864
Chicago/Turabian StyleChupakhin, Evgeny, Olga Bakulina, Dmitry Dar’in, and Mikhail Krasavin. 2019. "Facile Access to Fe(III)-Complexing Cyclic Hydroxamic Acids in a Three-Component Format" Molecules 24, no. 5: 864. https://doi.org/10.3390/molecules24050864
APA StyleChupakhin, E., Bakulina, O., Dar’in, D., & Krasavin, M. (2019). Facile Access to Fe(III)-Complexing Cyclic Hydroxamic Acids in a Three-Component Format. Molecules, 24(5), 864. https://doi.org/10.3390/molecules24050864








