Abstract
The alkylation of a series of nitroindazole derivatives with 1,2-dibromoethane afforded the corresponding N-(2-bromoethyl)- and N-vinyl-nitro-1H-indazoles. The Cu(I)-catalysed azide- alkyne 1,3-dipolar cycloaddition was selected to substitute the nitroindazole core with 1,4-disubstituted triazole units after converting one of the N-(2-bromoethyl)nitroindazoles into the corresponding azide. The reactivity in 1,3-dipolar cycloaddition reactions with nitrile imines generated in situ from ethyl hydrazono-α-bromoglyoxylates was studied with nitroindazoles bearing a vinyl unit. The corresponding nitroindazole-pyrazoline derivatives were obtained in good to excellent yields.
1. Introduction
1,3-Dipolar cycloadditions have been successfully exploited as a facile route for the construction of five-membered heterocyclic rings [1,2]. These reactions are considered relevant alternatives to heterocyclic classic routes and allows one to install biologically significant functionalities like triazole and pyrazoline units in different scaffolds in a single step [3,4,5,6,7,8,9]. An attractive approach giving rise to substituted triazole derivatives is based on the 1,3-dipolar cycloaddition of azides to terminal alkynes catalysed by copper(I), the so-called Copper(I)-catalysed azide-alkyne (CuAAC) reaction [10,11,12]. In this click chemistry approach the presence of copper(I) is a crucial requirement to afford selectively only one of the regioisomers - the 1,4-disubstituted 1,2,3-triazole [11,13,14].
On the other hand, different cycloaddition procedures have been established to afford pyrazoline/pyrazole derivatives [4,15,16]. Among them, it is the one based on the reaction of N-aryl-C-ethoxycarbonylnitrile imines, generated in situ by base-induced dehydrobromination of ethyl hydrazono-α-bromoglyoxylates, with templates bearing vinyl units [17,18,19,20].
Pyrazoline and pyrazole derivatives have demonstrated a broad spectrum of interesting biological properties, and some of them were shown to have analgesic, anti-hyperglycemic, hypotensive, antipyretic, antioxidant, antiparasitic, antimicrobial, antitumoral and anti-inflammatory activities, among others [5,6,7,21,22,23].
Indazoles are another important group of N-heterocycles with significant biological activities as nitric oxide synthase (NOS) inhibitors, kinase inhibitors, anti-inflammatory, anticancer, antimicrobial, antifungal, antimalarial, and antileishmanial agents, among others. Some anticancer and anti-inflammatory drugs based in indazole scaffolds are commercially available [24,25,26,27,28,29,30]. Besides the biological properties presented by indazole derivatives, this family of N-heterocycles also showed potential to be used in other fields as corrosion inhibitors, components for OLEDs and battery applications, and as copolymerizing molecules for new materials [31,32,33,34].
Following our interest on developing synthetic approaches to functionalize the indazole core [35,36], we decided to follow the 1,3-dipolar cycloaddition methodology to substitute nitroindazoles with triazole or pyrazoline moieties, aiming in such way to obtain new compounds with improved biological features for different applications. In the strategy envisaged it was considered that the alkylation of indazole with 1,2-dibromoethane using liquid-liquid phase transfer catalysis, could afford not only the N-1- and N-2-bromoethylindazoles, but also the corresponding N-1- and N-2-vinylindazoles [37]. The annular tautomerisation of two nitrogen atoms (N-1, N-2) present in the indazole ring, has been explored in synthetic and theoretical studies concerning the substitution of N-H-indazole [38]. More recently, it was reported by our group that the reactivity of N-1 and N-2 alkylated indazole isomers is strongly dependent on the reaction conditions, namely, solvent proticity and pH, as well as, electronic and steric effects [39,40].
Herein it is described the synthesis of a series of N-bromoethyl-nitroindazoles and N-vinyl-nitroindazoles reacting the corresponding nitroindazoles with 1,2-dibromoethane. A detailed analysis of the reaction conditions allowed to develop a simple, fast and inexpensive protocol to obtain both the vinyl and the bromoethyl derivatives in just one step in reasonable amounts. One of the bromoethyl derivatives was used to afford the corresponding azide, and its reactivity in CuAAC reactions with different alkynes was studied. Additionally, the reactivity of one of the vinyl derivatives as dipolarophile was studied in the presence of N-aryl-C-ethoxycarbonylnitrile-imines.
2. Results
2.1. Reaction of Nitroindazoles 1a–1d with 1,2-Dibromoethane
The reaction of the nitroindazoles 1a–1d with 1,2-dibromoethane performed at room temperature, in acetone and in the presence of Cs2CO3 (1.1 equiv.) afforded the corresponding N-bromoethylnitroindazoles 2 and 4 and the N-vinyl-nitroindazoles 3 and 5 (Scheme 1) in overall yields ranging from 68% to 82%. The starting nitroindazoles 1 were obtained in excellent yields by diazotization of the adequate 2-methyl-nitroanilines according to the procedure described by Noelting (Scheme 1) [41].
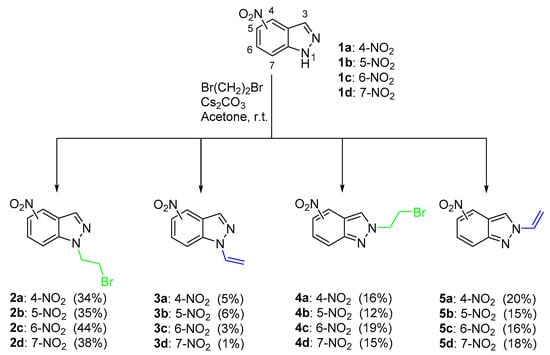
Scheme 1.
Synthetic access to N-bromoethyl-nitro-indazoles and N-vinyl-nitro-indazoles.
The conditions indicated in Scheme 1 (cesium carbonate/acetone, room temperature) were selected considering that in the optimisation studies performed with derivative 1b (see Table 1), the N-bromoethylnitroindazoles 2b and 4b and the elimination products 3b and 5b were obtained in just 1 h of reaction in reasonable amounts. From the results summarised in Table 1, it is patent that the distribution of the products 2b–5b is strongly dependent on the experimental conditions used.

Table 1.
Conditions studied for the optimization of the N-alkylation reaction of compound 1b and yields of compounds 2b–4b.
The first experiments were performed with 1b and using KOH (1 equiv.) in acetone at room temperature; such conditions gave rise, after 48 h, to the synthesis of compound 2b and to 3b, respectively, in 39% and 9% yields (entry 1). Two other products 4b and 5b were obtained; those are due to the reaction of the N-2 of the indazole moiety, the vinyl derivative 5b being obtained in higher yield than 4b (25% versus 12%).
In a previous study performed with 4-nitroindazole 1a at low temperature (0 °C), only the substituted compounds 2a and 4a were obtained in a total yield of 58% [39]. Therefore, the elimination process with higher activation energy than the substitution route [42] is favoured by the higher temperature used in the reactions reported in the present work. It was also observed (entries 2 and 3) that the formation of the elimination products was strongly favoured when the reactions were performed in the presence of three equiv. of KOH either at room temperature (4 h of reaction) or at reflux (2 h of reaction). In fact, under these conditions, only traces of 4b were detected, and the yield of 3b increased to 25% and 21%, respectively. The change of the solvent to tetrahydrofuran (THF) or methanol had no positive effect on the yield/distribution of the products obtained. When methanol was used (entry 5) it was not observed the full consumption of the starting material and a significant amount was recovered (ca. 35%).
In the reactions performed in acetone and 1 equiv. of Cs2CO3 (entry 6), compound 2b was isolated in 35% in just 1 h of reaction time and its elimination product 3b in 6% yield; under those conditions, the vinyl derivative 5b was isolated in slightly better yield (15%) than compound 4b (12%). Interestingly, the preferential formation of the N-bromoethylnitroindazole 4b towards its elimination product 5b (20% vs. 8%) can be obtained in the reaction performed with K2CO3 at room temperature, although a longer reaction time was required (68 h) until the full consumption of the starting nitroindazole 1b has been detected (entry 7). With this base, an improvement in the yield of 2b to 47% was observed, and 3b was isolated in 10% yield.
The conditions of entry 6 were extended to the other nitroindazoles since these conditions showed the best relationship between the reaction time and total yield. In general, the product distribution and the moderate selectivity towards N-1 was maintained (Scheme 1). The N-bromoethylnitroindazoles 2a–2d (34–44%) were always isolated as the major products and in much higher yields than the corresponding elimination derivatives 3a–3d (1-6%), while the yield values of 4a–4d (12-19%) were obtained in the same range of the vinyl derivatives 5a–5d (15-20%). The elimination reaction leading to the N-vinyl substituted derivatives is strongly favoured in the N-2 alkylated derivatives, probably due to higher instability of the corresponding N-bromoethyl-nitroindazole [43,44].
The structures of all derivatives were unambiguously confirmed by using 1D (1H and 13C spectra) and 2D [(1H,1H) COSY, (1H,13C) HSQC and (1H,13C) HMBC] NMR techniques, and by mass spectrometry (see experimental section and supporting information SI, Figures S1–S67).
The 1H-NMR spectra of derivatives 2a–2d and 4a–4d are consistent with N-alkylated indazole derivatives with a bromoethyl moiety showing, in the aliphatic region, two characteristic triplets at ca. δ 5.0 ppm and δ 4.0 ppm due to the resonances of the methylene protons. The 13C-NMR spectra of isomers 2a–2d and 4a–4d showed two signals at ca. δ 50 and δ 32 ppm due to the resonances of the two methylene carbons from the bromoethyl moiety. The assignments of these signals were confirmed by DEPT 135 studies.
The 1H-NMR spectra of N-substituted vinylic products 3a–3d and 5a–5d present in the aliphatic region two doublets of doublets due to the resonances of the methylene protons from the vinylic units at ca. δ 5.8 ppm and δ 5.1 ppm for derivatives 3a–3d, while for compounds 5a–5d these signals are slightly deshielded to ca. δ 6.2 ppm and δ 5.4 ppm. The resonance of the methinic proton from the vinylic group generates a doublet of doublets signal ranging from δ 7.9 to δ 7.3 ppm. These three NMR signals present characteristic constant couplings due to the geminal (Jgem ≈ 1 Hz), cis (Jcis ≈ 9 Hz) and trans (Jtrans ≈ 15 Hz) correlations between the three protons from the vinyl unit. The resonances of all the remaining protons from the nitroindazole moiety generate signals in the aromatic region, as expected, being the proton from the position 3 of the pyrazolic ring (δ 9.2-8.2 ppm) the most deshielded one.
Additionally, the 13C-NMR spectra of the compounds 3a–3d and 5a–5d present the signals due to the resonance of the methinic carbons from the alkene groups at ca. δ 130 ppm. The signals corresponding to the resonances of the secondary carbons from the vinylic double bonds were easily identified by DEPT 135 NMR spectra ranging from δ 107.4 to δ 100.5 ppm.
The structures of all compounds isolated from the alkylation/elimination reactions of nitroindazoles 1a–1d with 1,2-dibromoethane were confirmed by ESI(+) mass spectrometry, showing the peaks corresponding to the expected [M]+• or [M + H]+ molecular ions.
2.2. CuAAC of Azidoethyl-Nitroindazoles with Terminal Alkynes
The CuAAC of organic azides and terminal alkynes is considered to be a versatile and promising approach to prepare new bioactive compounds, pharmaceutical lead compounds, bio-probes, soft materials among others [45,46,47,48,49,50].
For the incorporation of triazole units in the nitroindazole core (Scheme 2) it was selected the N-bromoethylnitroindazole 2b that was efficiently converted into the required 5-nitroindazole azide 6 (88%) by reaction with an excess of sodium azide in DMF. The structure of this synthon was confirmed by mass spectrometry and 1H-NMR and 13C data (see experimental data and Figures S68–S71 in SI).
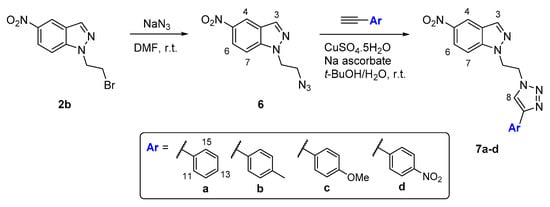
Scheme 2.
Synthetic access to new N-substituted triazolonitroindazoles by the copper(I)-catalysed azide-alkyne cycloaddition approach.
The copper(I)-catalysed 1,3-dipolar cycloaddition reactions involving the azide 6 were performed in the presence of the terminal alkynes, 1-ethynylbenzene, 1-ethynyl-4-methylbenzene, 1-ethynyl-4-metoxybenzene and 1-ethynyl-4-nitrobenzene (Scheme 2). All these reactions were accomplished at room temperature in a mixture of tert-butyl alcohol-water (1:1) using sodium ascorbate as the reducing agent and CuSO4 as the copper source. After reaction times ranging from 12 to 16 h, the desired adducts 7a–7d were isolated in excellent yields (71–87%) (Table 2). The yield of the CuAAC reaction and the consequent formation of the 1,4-disubstituted triazole ring is favoured by the presence of electron donor groups instead of electron-withdrawing groups in the para position of the phenyl ring from the alkyne reagent.

Table 2.
Copper(I)-catalysed azide-alkyne cycloaddition reactions of compound 6 with a series of ethynylbenzene derivatives.
The excellent performance of 6 in this CuAAC reactions prompted us to extend our study to 1,3-diethynylbenzene (Scheme 3). With this terminal alkyne, the reaction leads to the formation of 1,4-disubstituted triazole 8 and the bis-nitroindazolyl-triazole 9 in 23% and 58% yields, respectively. These two adducts were easily separated by column chromatography using hexane-ethyl acetate as solvent (Scheme 3).
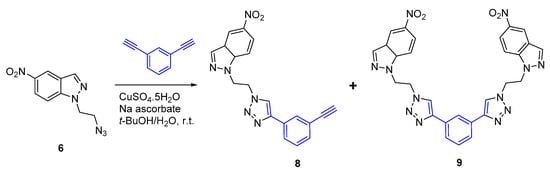
Scheme 3.
CuAAC reaction of derivative 6 with 1,3-diethynylbenzene.
The structure of all the newly synthesised compounds were unambiguously confirmed by using 1D (1H and 13C spectra) and 2D [(1H,1H) COSY, (1H,13C) HSQC and (1H,13C) HMBC] NMR techniques, and by mass spectrometry (see experimental section and supporting information SI, Figures S72–S97).
In this series of compounds, the success of the reaction was easily confirmed from the 1H-NMR spectra analysis. It shows the resonance of the protons from the ethylenic bridge as two multiplets at δ 5.10–5.01 ppm and δ 5.00–4.92 ppm and a remarkable singlet in the aromatic region due to the resonance of the proton from the triazole ring. In the 1H-NMR spectrum of compound 8, an additional peak at δ 4.25 ppm due to the resonance of the proton from the alkyne unit was observed. The resonance of the two carbons from the triple bond generates two characteristic peaks at δ 83.1 and δ 81.2 ppm in the 13C-NMR spectrum.
The structure of each the indazole-triazole derivatives 7b and 9 were unambiguously established by single-crystal X-ray diffraction studies (vide infra).
2.3. 1,3-Dipolar Cycloaddition Reactions of N-Vinyl-Nitroindazoles with Nitrile Imines
Considering the easy accessibility to N-vinylnitroindazoles we envisaged an extra functionalization of the indazole nucleus with pyrazoline units by using the vinyl moiety to trap nitrile imines generated from the ethyl hydrazono-α-bromoglyoxylates (Scheme 4).
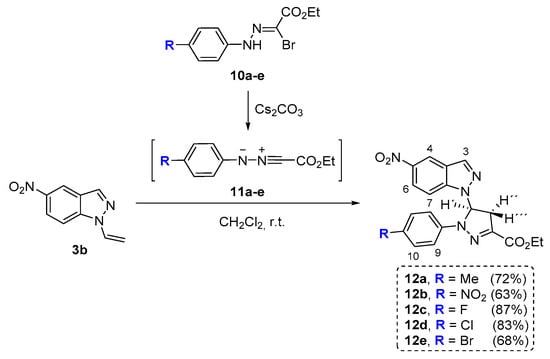
Scheme 4.
Reaction of N-vinyl-nitroindazole 3b with nitrile imines 11a-e.
The ethyl hydrazono-α-bromoglyoxylates 10a–10e selected to generate in situ the corresponding N-aryl-C-ethoxycarbonylnitrile imines 11a–11e were obtained using the approach developed by Hamilton and co-workers. The preparation of the nitrile imine precursors was carried out by the reaction of ethyl acetoacetate with the adequate diazonium salts followed by bromination of the resulting azoacetoacetic esters [51].
The cycloaddition reactions involving the indazole 3b and the N-aryl-C-ethoxycarbonylnitrile imines 11a–11e were performed in dichloromethane at room temperature, in the presence of Cs2CO3 (2 equiv.). After 24 h of reaction, it was observed by TLC the total, or almost total, consumption of the starting indazole and this being accompanied by the formation of the main product. After the workup and purification of the reaction mixture by column chromatography, we were able to conclude by a detailed spectroscopic analysis that the major products were the pyrazoline cycloadducts 12a–12e which were isolated in yields ranging from 63% to 83%. It is worth to refer that in neither case was isolated the corresponding pyrazole derivative from the dehydrogenation of the pyrazoline ring from derivatives 12a–12e.
When the reaction was performed with the vinyl-nitroindazole 3c and the nitrile imine 11d, obtained in situ from the corresponding ethyl hydrazono-α-bromoglyoxylates 10d, the expected pyrazoline derivative 13 (Figure 1) was obtained in 81% yield. This yield is similar to the one obtained with the indazole 3b, showing that the position of the electron-withdrawing nitro group in the indazole moiety does not have a significant influence in the reaction yield.
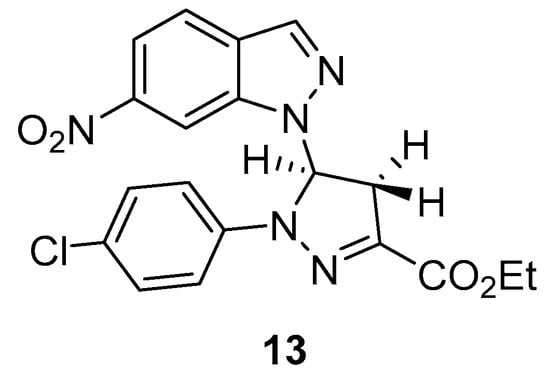
Figure 1.
Structure of the pyrazoline-indazole cycloadduct 13 obtained from 3c.
The structural assignments of the new cycloadducts 12a–12e and 13 relied on 1D (1H and13C spectra) and 2D [(1H,1H) COSY, NOESY, (1H,13C) HSQC and (1H,13C) HMBC] NMR spectra and on their mass spectra (see experimental section and Figures S98–S119 in the SI).
The resonances due to the protons from the indazole core were not significantly affected by the presence of the introduced pyrazoline unit. Important diagnostic peaks confirming the presence of the pyrazoline unit are the two double doublets at ca. δ 7.58–7.76 ppm and δ 3.82–3.85 ppm and a multiplet at ca. δ 3.33–3.38 ppm, corresponding respectively to the pyrazoline protons H’, H′′ and H′′′. In the aliphatic region, there is also the expected quartet (ca. δ 4.3 ppm) and triplet (ca. δ 1.3 ppm) due to the resonance of the protons from the ethyl ester group. The 13C-NMR spectra show a distinctive signal near δ 161 ppm corresponding to the resonance of the carbonyl carbon from the ethyl ester group. The structure of the cycloadduct 13 was also unequivocally established by single-crystal X-ray diffraction studies (vide infra).
2.4. X-Ray Diffraction
Single-crystal X-ray diffraction analysis was used to study the structural features of three of the reported compounds in this paper, namely compounds 7b, 9 and 13. The N-substituted triazolo-nitroindazole 7b crystallises in the centrosymmetric monoclinic space group P21/n with the asymmetric unit being composed of a whole molecular unit as depicted in Figure 2. The small number of hydrogen bonding donors and acceptors in this compound leads to a close packing in the solid-state achieved, mainly, by weak hydrogen bonding interactions of the C‒H···N and C‒H···O types, having typical geometrical parameters: dC···N distances found in the 3.195(3)-3.325(3) Å range with <(CHN) interaction angles ranging from 114 to139°; the dC···O distances were found instead in the 3.277(3)–3.411(4) Å range with the corresponding <(CHO) interaction angles in the range 123–142°. Despite the presence of several aromatic moieties, only weak π···π contacts are present, most of them between aromatic rings composing the molecular unit itself [intercentroid distances of dπ···π = 3.9112(14)–3.9511(14) Å].
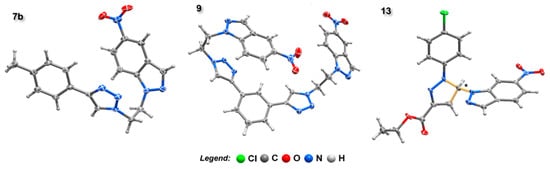
Figure 2.
Schematic representation of molecular units presents in the crystal structures of compounds 7b, 9 and 13. Non-hydrogen atoms are represented as thermal ellipsoids drawn at the 50% probability level and hydrogen atoms as small spheres with arbitrary radii. The chiral carbon present in 13 is denoted by an asterisk.
From the reaction of derivative 6 with 1,3-diethynylbenzene, we were able to crystallise the bis-nitroindazolyl-triazole 9. Despite its intrinsic molecular symmetry, 9 crystallises in the triclinic P-1 space group, with the asymmetric unit being composed of a complete molecular unit (Figure 2). As for 7b, the close packing in the solid-state is achieved mainly by weak C‒H···N and C‒H···O hydrogen bonding interactions [dC···N distances found in the 3.237(3)–3.530(3) Å range with <(CHN) interaction angles ranging from 123 to158°; the dC···O distances were found instead in the 3.291(4)–3.594(3) Å range with the corresponding <(CHO) interaction angles in the range 132-168°]. Interestingly, despite the presence of a high number of aromatic moieties, only a few highly offset π···π contacts are observed, all between aromatic rings within the molecular unit [intercentroid distances of dπ···π = 3.5320(14)–3.7007(15) Å].
The pyrazoline-indazole cycloadduct 13 crystallises in the noncentrosymmetric monoclinic space group P21. The asymmetric unit is composed of a whole molecular unit containing one asymmetric carbon (C14, denoted with an asterisk in Figure 2). The crystal quality and the absence of heavy atomic elements did not allow a precise calculation of the Flack parameter. However, we believe that the crystal should consist of a racemic mixture since there is no reason for the performed reaction to exhibit any enantiomeric excess. As for the previous compounds, the close packing of 13 is achieved by weak C‒H···N and C‒H···O hydrogen-bonding interactions [dC···N distances found in the 3.160(6)–3.329(6) Å range with <(CHN) interaction angles ranging from 115 to164°; dC···O distance of 3.288(6) Å with the corresponding <(CHO) interaction angle of 128°]. No π···π contacts are observed.
3. Materials and Methods
3.1. General Remarks
Melting points were measured using a B-540 melting point apparatus (Buchi, Flawil, Switzerland). Electrospray ionization mass spectra (ESI) were acquired with a Micromass Q-Tof 2 (Micromass, Manchester, UK), operating in the positive ion mode, equipped with a Z-spray source, an electrospray probe and a syringe pump. Source and desolvation temperatures were 80 °C and 150 °C, respectively. Capillary voltage was 3000 V. The spectra were acquired at a nominal resolution of 9000 and at cone voltages of 30 V. Nebulisation and collision gases were N2 and Ar, respectively. Compound solutions in methanol were introduced at a 10 μL min−1 flow rate. 1H and 13C solution NMR spectra were recorded on an Avance 300 spectrometer at 300.13 and 75.47 MHz, respectively (Bruker, Wissembourg, France). DMSO-d6 was used as solvent and tetramethylsilane (TMS) as the internal reference; the chemical shifts are expressed in δ (ppm) and the coupling constants (J) in Hertz (Hz). Unequivocal 1H assignments were made using 2D COSY (1H/1H), while 13C assignments were made on the basis of 2D HSQC (1H/13C) and HMBC (delay for long-range J C/H couplings were optimized for 7 Hz) experiments. Elemental analyses were performed on a CHNS-932 apparatus (LECO, Madrid, Spain). Column chromatography was carried out using silica gel (Merck, 35–70 mesh). Analytical TLC was carried out on 0.2 mm thick sheets precoated with silica gel 60 (Merck, city, Darmstadt, Germany). All chemicals were used as supplied. Solvents were purified or dried according to the literature procedures [52].
3.2. N-alkylation of Nitroindazole Derivatives 1a-d with 1,2-Dibromoethane. General Procedure
To a solution of the appropriate nitroindazole 1a–1d (100 mg, 0.62 mmol) in acetone (10.0 mL) it was added a small excess of cesium carbonate (1.1 equiv., 0.67 mmol, 218 mg). Then, the 1,2-dibromoethane alkylating agent (1.1 equiv., 0.67 mmol, 58 μL) was added dropwise and the resulting reactional mixture was maintained under stirring at room temperature until the TLC control showed the total consumption of the starting material (1 h). Then, the solvent was evaporated and the crude product was purified by column chromatography (silica gel) using hexane:toluene (1:1) as the eluent.
1-(2-Bromoethyl)-4-nitro-1H-indazole, 2a. Yield: 34% (56.3 mg), orange solid, Rf: 0.17, m.p.: 92–94 °C. 1H-NMR (DMSO-d6): δ 8.60 (1H, s, H-3), 8.35 (1H, d, J = 8.0 Hz, 1H-5), 8.19 (1H, d, J = 8.0 Hz, H-7), 7.68 (1H, t, J = 8.0 Hz, H-6), 5.00 (2H, t, J = 5.8 Hz, N-CH2), 4.00 (2H, t, J = 5.8 Hz, Br-CH2). 13C-NMR (DMSO-d6): δ 141.6 (C7a), 139.7 (C4), 132.4 (C3), 125.9 (C6), 118.6 (C5), 118.2 (C7), 115.8 (C3a), 50.0 (N-CH2), 32.0 (Br-CH2) ppm. MS-ESI(+): m/z 270.1 [M]+•. Elemental analysis calcd (%) for C9H8BrN3O2.1/20 PhCH3 C 40.98, H 3.09, N 15.27; found C 40.93, H 3.15, N 15.64.
1-(2-Bromoethyl)-5-nitro-1H-indazole, 2b. Yield: 35% (57.9 mg), yellow solid, Rf: 0.18, m.p.: 109–111 °C. 1H-NMR (DMSO-d6): δ 8.84 (1H, d, J = 2.2 Hz, H-4), 8.48 (1H, s, H-3), 8.25 (H-6, dd, J = 9.3 and 2.2 Hz, 1H), 7.97 (1H, d, J = 9.3 Hz, H-7), 4.95 (2H, t, J = 5.8 Hz, N-CH2), 3.99 (2H, t, J = 5.8 Hz, Br-CH2) ppm. 13C-NMR (DMSO-d6): δ 141.9 (C7a), 141.8 (C5), 136.9 (C3), 122.6 (C3a), 121.1 (C6), 119.1 (C4), 110.9 (C7), 49.9 (N-CH2), 32.1 (Br-CH2) ppm. MS-ESI(+): m/z (81Br) 272.0 [M + H]+. Elemental analysis calcd (%) for C9H8BrN3O2.1/20 PhCH3 C 40.98, H 3.09, N 15.27; found C 40.61, H 3.17, N 15.64.
1-(2-Bromoethyl)-6-nitro-1H-indazole, 2c. Yield: 44% (72.8 mg), orange solid, Rf: 0.20, m.p.: 117–119 °C. 1H-NMR (CDCl3): δ 8.46 (1H, t, J = 1.0 Hz, H-7), 8.19 (1H, d, J = 1.0 Hz, H-3), 8.05 (1H, dd, J = 8.9 and 1.1 Hz, H-5), 7.86 (1H, dd, J = 8.9 and 1.1 Hz, H-4), 4.85 (N-CH2, t, J = 6.3 Hz, 2H), 3.87 (Br-CH2, t, J = 6.3 Hz, 2H) ppm. 13C-NMR (CDCl3); δ 146.8 (C7a), 139.0 (C6), 134.6 (C3), 127.0 (C3a), 122.1 (C4), 115.8 (C5), 105.9 (C7), 50.6 (N-CH2), 29.6 (Br-CH2) ppm. MS-ESI(+): m/z (81Br) 272.0 [M + H]+. Elemental analysis calcd (%) for C9H8BrN3O2.1/40 PhCH3 C 40.48, H 3.04, N 15.42; found C 40.70, H 3.05, N 15.85.
1-(2-Bromoethyl)-7-nitro-1H-indazole, 2d.Yield: 38% (62.9), yellow solid, Rf: 0.37, m.p.: 77–79 °C. 1H-NMR (CDCl3): δ 8.25 (1H, s, H-3), 8.19 (1H, dd, J = 7.9 and 1.1 Hz, H-6), 8.06 (1H, dd, J = 7.9 and 1.1 Hz, H-4), 7.28 (1H, t, J = 7.9 Hz, H-5), 5.07 (2H, t, J = 6.6 Hz, N-CH2), 3.74 (2H, t, J = 6.6 Hz, Br-CH2) ppm. 13C-NMR (CDCl3): δ 135.6 (C3), 135.4 (C7a), 131.0 (C7), 129.0 (C3a), 128.4 (C4), 125.4 (C6), 120.3 (C5), 54.0 (N-CH2), 30.2 (Br-CH2) ppm.
MS-ESI(+): m/z 270.0 [M]+•. Elemental analysis calcd (%) for C9H8BrN3O2 C 40.02, H 2.99, N 15.56; found C 40.24, H 3.06, N 15.48.
4-Nitro-1-vinyl-1H-indazole, 3a. Yield: 5% (5.8 mg), orange solid, Rf: 0.40, m.p.: 135–137 °C. 1H-NMR (DMSO-d6): δ 8.71 (1H, s, H-3), 8.46 (1H, d, J = 8.1 Hz, H-5), 8.23 (1H, d, J = 8.1 Hz, H-7), 7.87 (1H, dd, J = 15.2, 8.8 Hz, N-CH=), 7.74 (1H, t, J = 8.1 Hz, H-6), 5.80 (1H, d, J = 15.2 Hz, H′′), 5.08 (1H, d, J = 8.8 Hz, H′).13C-NMR (DMSO-d6): δ 139.93 (C7a),139.86 (C4), 134.4 (C3), 129.9 (N-CH=), 127.2 (C6), 119.4 (C5), 117.9 (C7), 116.7 (C3a), 100.5 (CH2) ppm. MS-ESI(+): m/z 190.1 [M + H]+. Elemental analysis calcd (%) for C9H7N3O2.1/10 H2O C 56.60, H 3.80, N 22.00; found C 56.30, H 3.79, N 22.29.
5-Nitro-1-vinyl-1H-indazole, 3b. Yield: 6% (7.0 mg), yellow solid, Rf: 0.42, m.p.: 160–162 °C. 1H-NMR (DMSO-d6): δ 8.85 (1H, d, J = 2.2 Hz, H-4), 8.59 (1H, s, H-3), 8.31 (1H, dd, J = 9.2 and 2.2 Hz, H-6), 8.10 (1H, d, J = 9.2 Hz, H-7), 7.81 (1H, dd, J = 15.2 Hz and 8.8 Hz, N-CH=), 5.77 (1H, d, J = 15.2 Hz, H′′), 5.05 (1H, d, J = 8.8 Hz, H′) ppm. 13C-NMR (DMSO-d6): δ 142.5 (C7a), 140.0 (C4), 138.7 (C3), 130.0 (N-CH=), 123.6 (C3a), 122.1 (C6), 119.3 (C4), 110.8 (C7), 100.6 (CH2) ppm. MS-ESI(+): m/z 190.1 [M + H]+. Elemental analysis calcd (%) for C9H7N3O2.1/11 H2O C 56.65, H 3.79, N 22.02; found C 57.06, H 4.18, N 21.63.
6-Nitro-1-vinyl-1H-indazole, 3c. Yield: 3% (3.5 mg), yellow solid, Rf: 0.33, m.p.: 127–129 °C. 1H-NMR (CDCl3): δ 8.53 (1H, d, J = 1.6 Hz, H-7), 8.25 (1H, s, H-3), 8.09 (1H, dd, J = 8.8 and 1.6 Hz, H-5), 7.88 (1H, d, J = 8.8 Hz, H-4), 7.40 (1H, dd, J = 15.4 and 8.9 Hz, N-CH=), 5.87 (1H, dd, J = 15.4 and 1.2 Hz, H′′), 5.10 (1H, dd, J = 8.9 and 1.2 Hz, H’) ppm. 13C-NMR (CDCl3): δ 147.1 (C7a), 137.3 (C6), 135.7 (C3), 129.3 (N-CH=), 127.9 (C3a), 122.1 (C4), 116.6 (C5), 106.1 (C7), 101.4 (CH2) ppm. MS-ESI(+): m/z 190.1 [M + H]+. Elemental analysis calcd (%) C9H7N3O2.1/5 H2O C 56.07, H 3.87, N 21.80; found C 55.70, H 3.49, N 22.14.
7-Nitro-1-vinyl-1H-indazole, 3d. Yield: 1% (1.2 mg), orange solid, Rf: 0.46, m.p.: 97–99 °C. 1H-NMR (CDCl3): δ 8.30 (1H, s, H-3),8.13 (1H, dd, J = 7.8 and 1.0 Hz, H-6), 8.05 (1H, dd, J = 7.8 and 1.0 Hz, H-4), 7.46 (1H, dd, J = 15.0 and 8.6 Hz, N-CH=), 7.30 (1H, t, J = 7.8 Hz, H-5), 5.80 (1H, dd, J = 15.0 and 0.6 Hz, H′′), 5.04 (1H, dd, J = 8.6 and 0.6 Hz, H’) ppm. 13C-NMR (CDCl3): δ 136.6 (C3), 132.1 (N-CH=), 129.4 (C7a), 129.1 (C7), 128.0 (C3a), 1278.0 (C4), 125.4 (C6), 120.9 (C5), 103.3 (CH2) ppm. MS-ESI(+): m/z 190.1 [M + H]+. Elemental analysis calcd (%) C9H7N3O2.1/11 H2O C 56.65, H 3.79, N 22.02; found C 56.96, H 4.11, N 22.23.
2-(2-Bromoethyl)-4-nitro-2H-indazole, 4a. Yield: 16% (26.5 mg), orange solid, Rf: 0.12, m.p.: 93–95 °C. 1H-NMR (DMSO-d6): δ 9.00 (1H, s, H-3), 8.22 (2H, d, J = 8.0 Hz, H-5 and H-7), 7.53 (1H, t, J = 8.0 Hz, H-6), 5.00 (2H, t, J = 5.9 Hz, N-CH2), 4.10 (2H, t, J = 5.9 Hz, Br-CH2) ppm. 13C-NMR (DMSO-d6): δ 149.3 (C7a),140.1 (C4), 126.3 (C5), 125.8 (C3), 124.9 (C6), 120.7 (C7), 113.7 (C3a), 54.5 (N-CH2), 31.8 (Br-CH2) ppm. MS-ESI(+): m/z 270.1 [M]+•. Elemental analysis calcd (%) for C9H8BrN3O2.1/15 PhCH3 C 41.16, H 3.11, N 15.21; found C 41.04, H 3.09, N 15.65.
2-(2-Bromoethyl)-5-nitro-2H-indazole, 4b. Yield: 12% (19.9 mg), yellow solid, Rf: 0.07, m.p.: 136–138 °C. 1H-NMR (DMSO-d6): δ 8.94 (1H, dd, J = 2.2 and 0.9 Hz, H-4), 8.89 (1H, d, J = 0.9, H-3), 8.04 (1H, dd, J = 9.5 and 2.2 Hz, H-6), 7.81 (1H, dt, J = 9.5 and 0.9 Hz, H-7), 4.96 (2H, t, J = 5.8 Hz, N-CH2), 4.07 (2H, t, J = 5.8 Hz, Br-CH2) ppm. 13C-NMR (DMSO-d6): δ 149.3 (C7a), 142.1 (C5), 130.4 (C3), 120.7 (C6), 119.9 (C4), 119.6 (C3a), 118.2 (C7), 54.6 (N-CH2), 31.9 (Br-CH2) ppm. MS-ESI(+): m/z (81Br) 272.4 [M + H]+. Elemental analysis calcd (%) for C9H8BrN3O2.1/20 PhCH3 C 40.98, H 3.09, N 15.27; found C 40.94, H 3.19, N 15.67.
2-(2-Bromoethyl)-6-nitro-2H-indazole, 4c. Yield: 19% (31.5 mg), orange solid, Rf: 0.08, m.p.: 161-163 °C.1H-NMR (CDCl3): δ 8.73 (1H, d, J = 1.0 Hz, H-3), 8.66-8.65 (1H, m, H-7), 8.02 (1H, dd, J = 9.2 and 1.0 Hz, H-5), 7.83 (1H, dd, J = 9.2 and 2.0 Hz, H-4), 4.98 (2H, t, J = 5.8 Hz, N-CH2), 4.08 (2H, t, J = 5.8 Hz, Br-CH2) ppm. 13C-NMR (CDCl3): δ 146.2 (C7a), 146.1 (C6), 126.7 (C3), 123.8 (C3a), 122.9 (C4), 114.9 (C5), 114.8 (C7), 54.8 (N-CH2), 31.9 (Br-CH2) ppm. MS-ESI(+): m/z 270.1 [M]+•. Elemental analysis calcd (%) for C9H8BrN3O2.1/20 PhCH3 C 40.98, H 3.09, N 15.27; found C 40.61, H 3.25, N 15.43.
2-(2-Bromoethyl)-7-nitro-2H-indazole, 4d. Yield: 15% (24.8 mg), orange solid, Rf: 0.05, m.p.: 149–151 °C. 1H-NMR (DMSO-d6): δ 8.89 (1H, s, H-3), 8.47-8.18 (2H, m, H-6 and H-4), 7.29 (1H, t, J = 7.8 Hz, H-5), 4.99 (2H, t, J = 5.8 Hz, N-CH2), 4.07 (2H, t, J = 5.8 Hz, Br-CH2) ppm. 13C-NMR (DMSO-d6): δ 139.9 (C7a), 136.6 (C7), 130.4 (C3), 128.2 (C4), 125.2 (C6), 124.9 (C3a), 120.0 (C5), 54.6 (N-CH2), 32.0 (Br-CH2). MS-ESI(+): m/z 270.0 [M]+•. Elemental analysis calcd (%) for C9H8BrN3O2.1/20 PhCH3 C 40.98, H 3.09, N 15.27; found C 40.99, H 2.89, N 15.60.
4-Nitro-2-vinyl-2H-indazole, 5a. Yield: 20% (23.2 mg), yellow solid, Rf: 0.25, m.p.: 133–135 °C. 1H-NMR (DMSO-d6): δ 9.16 (1H, d, J = 1.0 Hz, H-3), 8.34-8.05 (2H, m, H-5 and H-7), 7.73 (1H, dd, J = 15.5 and 8.7 Hz, N-CH=), 7.56 (1H, dd, J = 8.6 Hz and 7.5 Hz, H-6), 6.22 (1H, dd, J = 15.5 Hz and 1.1 Hz, H′′), 5.38 (1H, dd, J = 8.7 and 1.1 Hz, H′) ppm. 13C-NMR (DMSO-d6): δ 149.6 (C7a), 140.3 (C4), 133.7 (C3), 126.4 (N-CH=), 126.0 (C6), 124.3 (C5), 121.5 (C7), 114.3 (C3a), 107.2 (CH2) ppm. MS-ESI(+): m/z 190.1 [M + H]+. Elemental analysis calcd (%) for C9H7N3O2.1/10 H2O C 56.60, H 3.80, N 22.00; found C 56.44, H 3.51, N 22.27.
5-Nitro-2-vinyl-2H-indazole, 5b. Yield: 15% (17.4 mg), yellow solid, Rf: 0.13, m.p.: 128–130 °C. 1H-NMR (DMSO-d6): δ 9.03 (1H, s, H-3), 8.91 (1H, d, J = 1.7 Hz, H-4), 8.05 (1H, dd, J = 9.5 and 1.7 Hz, H-6), 7.83 (1H, d, J = 9.5 Hz, H-7), 7.70 (1H, dd, J = 15.4 and 8.7 Hz, N-CH=), 6.16 (1H, dd, J = 15.4 and 1.1 Hz, H′′), 5.37 (1H, dd, J = 8.7 and 1.1 Hz, H′) ppm. 13C-NMR (DMSO-d6): δ 149.4 (C7a), 142.5 (C4), 133.7 (C3), 128.6 (N-CH=), 120.9 (C6), 120.9 (C4), 120.1 (C3a), 118.4 (C7), 107.2 (CH2) ppm. MS-ESI(+): m/z 190.1 [M + H]+. Elemental analysis calcd (%) for C9H7N3O2.1/11 H2O C 56.65, H 3.79, N 22.02; found C 56.23, H 3.78, N 22.41.
6-Nitro-2-vinyl-2H-indazole, 5c. Yield: 16% (18.6 mg), yellow solid, Rf: 0.17, m.p.: 109–111 °C. 1H-NMR (CDCl3): δ 8.72 (1H, d, J = 1.9 Hz, H-7), 8.22 (1H, d, J = 0.8 Hz, H-3), 7.91 (1H, dd, J = 9.2 and 1.9 Hz, H-5), 7.77 (1H, dd, J = 9.2 and 0.8 Hz, H-4), 7.36 (1H, dd, J = 15.6 and 8.7 Hz, N-CH=), 6.11 (1H, dd, J = 15.6 and 1.7 Hz, H′′), 5.33 (1H, dd, J = 8.7 and 1.7 Hz, H′) ppm. 13C-NMR (CDCl3): δ 147.5 (C7a), 147.3 (C6), 133.5 (N-CH=), 124.4 (C3a), 122.0 (C3), 121.8 (C4), 116.56(C5), 115.9 (C7), 107.3 (CH2) ppm. MS-ESI(+): m/z 190.1 [M + H]+. Elemental analysis calcd (%) C9H7N3O2.1/6 H2O C 56.25, H 3.85, N 21.87; found C 56.15, H 4.08, N 22.01.
7-Nitro-2-vinyl-2H-indazole, 5d. Yield: 15% (17.4 mg), yellow solid, Rf: 0.07, m.p.: 149–151 °C. 1H-NMR (CDCl3): δ 8.39 (1H, s, H-3), 8.37 (1H, dd, J = 7.9 and 1.0 Hz, H-6), 8.06 (1H, dd, J = 7.9 and 1.0 Hz, H-4), 7.46 (1H, dd, J = 15.7 and 8.8 Hz, N-CH=), 7.22 (1H, t, J = 7.9 Hz, H-5), 6.12 (1H, dd, J = 15.7 and 1.8 Hz, H′′), 5.35 (1H, dd, J = 8.8 and 1.8 Hz, H’) ppm. 13C-NMR (CDCl3): δ 141.3 (C7a), 137.9 (C7), 133.7 (C3), 128.9 (HC-N N-CH=), 126.2 (C4), 125.6 (C3a), 122.8 (C6), 121.0 (C5), 107.4 (CH2) ppm. MS-ESI(+): m/z 190.1 [M + H]+. Elemental analysis calcd (%).C9H7N3O2.2/5 H2O C 55.05, H 4.00, N 21.40; found C 54.97, H 3.76, N 21.33.
3.3. Procedure for the Preparation of 1-(2-Azidoethyl)-5-nitro-1H-indazole Intermediate 6
A mixture of 1-(2-bromoethyl)-5-nitro-1H-indazole (2b, 0.1 g, 0.37 mmol) and sodium azide NaN3 (10 equiv., 3.7 mmol, 130 μL) in 5 mL of DMF was maintained under stirring at room temperature for 24 h. After this period, the TLC control confirmed the disappearance of the starting material and the formation of a main product. Then, the reaction mixture was washed with water and the desired product was extracted with diethyl ether. The organic layer was separated, dried under Na2SO4 and the solvent evaporated under reduced pressure. The residue was crystallized in hexane affording compound 6 pure in 88% yield. Yield: 88% (78.2 mg), yellow solid, Rf: 0.57, m.p.: 100–102 °C. 1H-NMR (DMSO-d6): δ 8.85 (1H, dd, J = 2.2 and 0.8 Hz, H-4), 8.48 (1H, d, J = 0.8 Hz, H-3), 8.27 (1H, dd, J = 9.3 and 2.2 Hz, H-6), 7.96 (1H, dt, J = 9.3 and 0.8 Hz, H-7), 4.71 (2H, t, J = 5.4 Hz, N-CH2), 3.83 (2H, t, J = 5.4 Hz, N3-CH2) ppm. 13C-NMR (DMSO-d6): δ 141.8 (C7a), 141.7 (C5), 136.8 (C3), 122.8 (C3a), 121.1 (C6), 119.1 (C4), 110.7 (C7), 50.2 (N-CH2), 48.00 (CH2-N3) ppm. MS-ESI(+): m/z 233.1 [M + H]+. Elemental analysis calcd (%) for C9H8N6O2.1/3 H2O C 45.38, H 3.67, N 35.28; found C 45.37, H 3.48, N 35.08.
3.4. General Procedure for 1,3-Dipolar Cycloaddition of Azides with Terminal Alkynes
To a stirred solution of azide 6 (0.1 g, 0.43 mmol) and the appropriate terminal alkynes (0.64 mmol) in 5 mL of a mixture H2O/t-BuOH (1:1), it was added copper sulphate (0.02 mmol) and sodium ascorbate (0.04 mmol). The reaction mixture was stirred at room temperature until the TLC control showed the total consumption of the starting material (12–16 h). After this period, the reaction mixture was washed with water and the organic phase was extracted with dichloromethane. The combined organic layers were dried with anhydrous sodium sulfate and the solvent was removed under reduced pressure. The desired products 7a–7d were obtained pure after crystallization in ethanol. The compounds 8 and 9 were purified by column chromatography (silica gel) using hexane:ethyl acetate (1:1) as the eluent.
5-Nitro-1-(2-(4-phenyl-1H-1,2,3-triazol-1-yl)ethyl)-1H-indazole, 7a. Yield: 74% (106.5 mg), white solid, Rf: 0.30, m.p.: 182–184 °C. 1H-NMR (DMSO-d6): δ 8.78 (1H, d, J = 2.2 Hz, H-4), 8.41 (1H, d, J = 0.9 Hz, H-3), 8.39 (1H, s, H-8), 8.13 (1H, dd, J = 9.3 and 2.2 Hz, H-6), 7.79–7.59 (3H, m, H-7, H-11 and H-15), 7.50-–7.36 (2H, m, H-12 and H-14), 7.34–7.25 (1H, m, H-13), 5.08–5.04 (2H, m, N-CH2), 4.96-4.92 (2H, m, CH2-triazole) ppm. 13C-NMR (DMSO-d6): δ 146.3 (C9), 141.7 (C7a), 141,5 (C5), 137.0 (C3), 130.5 (C10), 128.9 (C12 and C12′), 127.9 (C13), 125.0 (C11 and C11′), 122.6 (C3a), 121.8 (C8), 120.9 (C6), 119.1 (C4), 110.2 (C7), 49.2 (N-CH2), 48.5 (CH2-triazole) ppm. MS-ESI(+): m/z 335.2 [M + H]+. Elemental analysis calcd (%) for C17H14N6O2.9/8 H2O C 57.58, H 4.62, N 23.70; found C 57.16, H 4.53, N 24.21.
5-Nitro-1-(2-(4-p-tolyl-1H-1,2,3-triazol-1-yl)ethyl)-1H-indazole, 7b. Yield: 82% (123.0 mg), white solid, Rf: 0.34, m.p.: 188–190 °C. MS-ESI(+): m/z 349.2 [M + H]+. 1H-NMR (CDCl3): δ 8.66 (1H, dd, J = 2.1 and 0.8 Hz, H-4), 8.28 (1H, d, J = 0.8 Hz, H-3), 8.13 (1H, dd, J = 9.2 and 2.1 Hz, H-6), 7.44 (2H, dd, J = 6.6 Hz, H-11 and H-15), 7.21–7.09 (4H, m, H-7, H-8, H-12 and H-14), 4.98 (4H, s, N-CH2-CH2-triazole), 2.33 (3H, s, CH3) ppm. 13C-NMR (CDCl3): δ 142.7 (C7a), 142.0 (C5), 138.3 (C9), 137.2 (C3), 129.5 (C12 and C12′), 127.0 (C13), 126.7 (C10), 125.5 (C11 and C11′), 122.8 (C3a), 122.1 (C6), 120.2 (C8), 118.8 (C4), 108.8 (C7), 49.5 (N-CH2), 48.9 (CH2-triazole), 21.3 (CH3) ppm.
1-(2-(4-(4-Methoxyphenyl)-1H-1,2,3-triazol-1-yl)ethyl)-5-nitro-1H-indazole, 7c. Yield: 87% (136.5 mg), yellow solid, Rf: 0.22, m.p.: 210–212 °C. 1H-NMR (DMSO-d6): δ 8.78 (1H, dd, J = 2.2 and 0.8 Hz, H-4), 8.41 (1H, d, J = 0.8 Hz, H-3), 8.28 (1H, s, H-8), 8.13 (1H, dd, J = 9.3 and 2.2 Hz, H-6), 7.69 (1H, dt, J = 9.3 and 0.8 Hz, H-7), 7.64–7.56 (2H, m, H-11 and H-15), 7.00–6.93 (2H, m, H-12 and H-14), 5.07–5.01 (2H, m, N-CH2), 4.98–4.90 (2H, m, CH2-trizole), 3.76 (CH3, s, 3H) ppm. 13C-NMR (DMSO-d6): δ 159.0 (C13), 146.2 (C9), 141.7 (C7a), 141,5 (C5), 137.0 (C3), 126.4 (C12 and C12′), 123.1 (C3a), 122.7 (C10), 120.9 (C8), 120.8 (C6), 119.1 (C4), 114.3 (C11 and C11′), 110.2 (C7), 55.2 (CH3), 49.2 (N-CH2), 48.5 (CH2-triazole) ppm. MS-ESI(+): m/z 365.2 [M + H]+. Elemental analysis calcd (%) for C18H16N6O3.3/2 H2O C 57.44, H 4.64, N 22.33; found C 57.75, H 4.52, N 21.97.
5-Nitro-1-(2-(4-(4-nitrophenyl)-1H-1,2,3-triazol-1-yl)ethyl)-1H-indazole, 7d. Yield: 71% (116.0 mg), orange solid, Rf: 0.15, m.p.: 281–283 °C. 1H-NMR (DMSO-d6): δ 8.79 (1H, dd, J = 2.2 and 0.8 Hz, H-4), 8.68 (1H, s, H-8), 8.40 (1H, d, J = 0.8 Hz, H-3), 8.35–8.25 (2H, m, H-12 and H-14), 8.14 (2H, dd, J = 9.3 and 2.2 Hz, H-6), 8.02-–7.93 (2H, m, H-11 and H-15), 7.73 (1H, dt, J = 9.3 and 0.8 Hz, H-7), 5.10-5.06 (2H, m, N-CH2), 5.00-4.96 (CH2-triazole) ppm. 13C-NMR (DMSO-d6): δ 146.6 (C13), 144.3 (C9), 141.7 (C7a), 141,5 (C5), 137.0 (C10), 136.9 (C3), 125.8 (C12 and C12′), 124.4 (C11 and C11′), 123.9 (C8), 122.7 (C3a), 120.9 (C6), 119.1 (C4), 110.2 (C7), 49.4 (N-CH2), 48.5 (CH2-triazole) ppm. MS-ESI(+): m/z 380.1 [M + H]+. Elemental analysis calcd (%) for C17H13N7O4.1/2 H2O C 52.58, H 3.63, N 25.25; found C 52.68, H 3.24, N 24.91.
1-(2-(4-(3-Ethynylphenyl)-1H-1,2,3-triazol-1-yl)ethyl)-5-nitro-1H-indazole, 8. Yield: 23% (35.5 mg), white solid, Rf: 0.32, m.p.: 176–178 °C. 1H-NMR (DMSO-d6): δ 8.79 (1H, d, J = 2.2 Hz, H-4), 8.48 (1H, s, H-8), 8.41 (1H, s, H-3), 8.13 (1H, dd, J = 9.3 and 2.2 Hz, H-6), 7.78–7.69 (3H, m, H-7, H-13 and H-15), 7.476–7.38 (2H, m, H-11 and H-14), 5.08–5.04 (2H, m, N-CH2), 4.96–4.92 (2H, m, CH2-triazole), 4.25 (1H, s, -C≡CH) ppm. 13C-NMR (DMSO-d6): δ 145.3 (C9), 141.7 (C7a), 141,5 (C5), 137.0 (C3), 131.00 (C11), 130.99 (C10), 129.4 (C14), 128.0 (C13), 125.5 (C15), 122.7 (C12), 122.5 (C3a), 122.3 (C8), 120.9 (C6), 119.1 (C4), 110.2 (C7), 83.1 (Ph-C≡), 81.2 (≡CH), 49.3 (N-CH2), 48.5 (CH2-triazole) ppm. MS-ESI(+): m/z 359.2 [M + H]+. Elemental analysis calcd (%) for C19H14N6O2.3/4 H2O C 61.37, H 4.20, N 22.60; found C 61.39, H 3.80, N 22.25.
1,3-bis(1-(2-(5-Nitro-1H-indazol-1-yl)ethyl)-1H-1,2,3-triazol-4-yl)benzene, 9. Yield: 58% (73.9 mg), yellow solid, Rf: 0.08, m.p.: 173–175 °C. 1H-NMR (DMSO-d6): δ 8.79 (2H, d, J = 2.1 Hz, H-4), 8.49 (2H, s, H-8), 8.41 (2H, s, H-3), 8.14 (2H, dd, J = 9.3 and 2.1 Hz, H-6), 8.10 (1H, t, J = 1.6 Hz, H-11), 7.73 (2H, d, J = 9.3 Hz, H-7), 7.61 (2H, dd, J = 7.7 and 1.6 Hz, 2H H-12 and H-14), 7.44 (1H, t, J = 7.7 Hz, H-13), 5.10–5.06 (4H, m, N-CH2), 4.97-4.94 (2H, m, CH2-triazole) ppm. 13C-NMR (DMSO-d6): δ 146.0 (C9), 141.7 (C7a), 141.5 (C5), 137.0 (C3), 131.1 (C10), 129.5 (C13), 124.5 (C12 and C14), 122.6 (C3a), 122.0 (C8), 121.5 (C11), 120.9 (C6), 119.1 (C4), 110.2 (C7), 49.2 (N-CH2), 48.5 (CH2-triazole) ppm. MS-ESI(+): m/z 591.3 [M + H]+. Elemental analysis calcd (%) for C28H22N12O4.2/3 H2O.1/3 C4H10O C 56.17, H 4.28, N 26.80; found C 56.17, H 3.96, N 26.46.
3.5. General Procedure for 1,3-Dipolar Cycloaddition Reactions of N-Vinyl-Nitroindazoles with Nitrile Imines to Give Access to Compounds 12a–12e
A solution of 5-nitro-1-vinyl-1H-indazole 3b or 6-nitro-1-vinyl-1H-indazole, 3 (0.02 g, 0.10 mmol) and the appropriate hydrazonyl bromide 10a–10e (1.5 equiv., 0.15 mmol) in dichloromethane (5 mL) was treated with cesium carbonate (0.20 mmol) and then stirred for 24 h at room temperature. After this period, the solvent was removed, and the crude product was purified by column chromatography on silica gel (EtOAc:hexane 2:8) to afford the corresponding products 12a–12e.
Ethyl 5-(5-nitro-1H-indazol-1-yl)-1-(p-tolyl)-4,5-dihydro-1H-pyrazole-3-carboxylate, 12a. Yield: 72% (29.9 mg), yellow solid, Rf: 0.38, m.p.: 167–169 °C. 1H-NMR (DMSO-d6): δ 8.78 (1H, d, J = 2.0 Hz, H-4), 8.39 (1H, s, H-3), 8.34 (1H, dd, J = 9.3 and 2.0 Hz, H-6), 8.09 (1H, d, J = 9.3 Hz, H-7), 7.59 (1H, dd, J = 11.3 Hz and 3.1 Hz, H’), 7.06–7.98 (2H, m, H-9 and H-9′), 6.97–6.91 (2H, m, H-10 and H-10′), 4.31 (2H, q, J = 7.1 Hz, -OCH2CH3), 3.82 (1H, dd, J = 18.9 and 11.2 Hz, H′′′), 3.39–3.31 (1H, m, H′′), 2.09 (3H, s, Ph-CH3), 1.32 (3H, t, J = 7.1 Hz, -OCH2CH3) ppm. 13C-NMR (DMSO-d6): δ 161.6 (C=O), 142.1 (C7a), 140.8 (C12), 139.5 (C5), 138.6 (C3), 138.4 (C8), 131.0 (C11), 129.6 (C9 and C9′), 122.8 (C3a), 122.0 (C6), 119.4 (C4), 114.9 (C10 and C10′), 110.3 (C7), 72.1 (CH’), 60.8 (O-CH2CH3), 39.1 (CH2), 20.1 (Ph-CH3),14.2 (O-CH2CH3) ppm. MS-ESI(+): m/z 416.2 [M + Na]+. Elemental analysis calcd (%) for C20H19N5O4.1/2 H2O C 59.69, H 5.01, N 17.40; found C 59.30, H 4.71, N 17.83.
Ethyl 5-(5-nitro-1H-indazol-1-yl)-1-(4-nitrophenyl)-4,5-dihydro-1H-pyrazole-3-carboxylate, 12b. Yield: 63% (28.3 mg), orange solid, Rf: 0.22, m.p.: 183–185 °C. 1H-NMR (DMSO-d6): δ 8.82 (1H, d, J = 2.2 Hz, H-4), 8.44 (1H, s, H-3), 8.39 (1H, dd, J = 9.3 and 2.2 Hz, H-6), 8.17 (1H, d, J = 9.3 Hz, H-7), 8.08 (2H, d, J = 9.3 Hz, H-10 and H-10′), 7.76 (1H, dd, J = 10.9 Hz and 2.9 Hz, H’), 7.25 (H-9 and H-9′, d, J = 9.3 Hz, 2H), 4.35 (2H, q, J = 7.1 Hz, -OCH2CH3), 3.94 (1H, dd, J = 19.2 and 10.9 Hz, H′′′), 3.45–3071 (1H, m, H′′), 1.34 (3H, t, J = 7.1 Hz, -OCH2CH3) ppm. 13C-NMR (DMSO-d6): δ 161.0 (C=O), 146.2 (C11), 144.4 (C8), 142.4 (C7a), 141.0 (C12), 140.9 (C5), 138.9 (C3), 125.7 (C9 and C9′), 123.0 (C3a), 122.4 (C6), 119.5 (C4), 114.0 (C10 and C10′), 110.4 (C7), 71.4 (CH’), 61.4 (O-CH2CH3), 40.0 (CH2), 14.2 (O-CH2CH3) ppm. MS-ESI(+): m/z 447.1 [M + Na]+. Elemental analysis calcd (%) for C19H16N6O6 C 53.78, H 3.80, N 19.80; found C 53.84, H 4.17, N 20.07.
Ethyl 1-(4-fluorophenyl)-5-(5-nitro-1H-indazol-1-yl)-4,5-dihydro-1H-pyrazole-3-carboxylate, 12c. Yield: 87% (36.5 mg), white solid, Rf: 0.27, m.p.: 177–179 °C. 1H-NMR (DMSO-d6): δ 8.78 (1H, d, J = 2.2 Hz, H-4), 8.40 (1H, s, H-3), 8.34 (1H, dd, J = 9.3 and 2.2 Hz, H-6), 8.10 (1H, d, J = 9.3 Hz, H-7,), 7.60 (1H, H’, dd, J = 11.2 Hz and 3.0 Hz), 7.15–7.11 (2H, m, H-9 and H-9′), 7.07–6.97 (2H, m, H-10 and H-10′), 4.31 (2H, q, J = 7.1 Hz, -OCH2CH3), 3.83 (1H, dd, J = 18.9 and 11.2 Hz, H′′′), 3.41-3.40 (1H, m, H′′), 1.32 (3H, t, J = 7.1 Hz, -OCH2CH3) ppm. 13C-NMR (DMSO-d6): δ 161.5 (C=O), 142.1 (C7a), 140.9 (C12), 140.5 (C5), 138.5 (C3), 137.6 (C8), 125.7 (C11), 122.8 (C3a), 122.1 (C6), 119.4 (C4), 116.7, 116.6 (C9 and C9′), 116.0, 115.7 (C10 and C10′), 110.3 (C7), 72.4 (CH’), 60.9 (O-CH2CH3), 39.3 (CH2), 14.2 (O-CH2CH3) ppm. MS-ESI(+): m/z 420.2 [M + Na]+. Elemental analysis calcd (%) for C19H16FN5O4.1/2 H2O C 56.16, H 4.24, N 17.23; found C 56.48, H 4.24, N 17.06.
Ethyl 1-(4-chlorophenyl)- 5-(5-nitro-1H-indazol-1-yl)- 4,5-dihydro-1H-pyrazole-3-carboxylate, 12d. Yield: 83% (36.3 mg), white solid, Rf: 0.30, m.p.: 179–181 °C. 1H-NMR (DMSO-d6): δ 8.80 (1H, d, J = 2.2 Hz, H-4), 8.41 (1H, s, H-3), 8.35 (1H, dd, J = 9.3 and 2.2 Hz, H-6), 8.11 (1H, d, J = 9.3 Hz, H-7), 7.62 (1H, dd, J = 11.0 Hz and 3.0 Hz, H’), 7.23–7.18 (2H, m, H-9 and H-9′), 7.15–7.10 (2H, m, H-10 and H-10′), 4.32 (2H, q, J = 7.1 Hz, -OCH2CH3), 3.85 (1H, dd, J = 19.1 Hz and 11.0 Hz, H′′′), 3.40 (1H, dd, J = 19.1 and 3.0 Hz, H′′), 1.33 (3H, t, J = 7.1 Hz, -OCH2CH3) ppm. 13C-NMR (DMSO-d6): δ 161.3 (C=O), 142.2 (C7a), 141.1 (C12), 140.8 (C5), 139.9 (C8), 138.6 (C3), 129.1 (C9 and C9′), 125.7 C11), 122.9 (C3a), 122.2 (C6), 119.4 (C4), 116.3 (C10 and C10′), 110.3 (C7), 72.0 (CH’), 61.0 (O-CH2CH3), 39.4 (CH2), 14.2 (O-CH2CH3) ppm. MS-ESI(+): m/z 436.1 [M + Na]+. Elemental analysis calcd (%) for C19H16ClN5O4.1/6 H2O C 54.75, H 3.95, N 16.80; found C 54.28, H 4.27, N 17.30.
Ethyl 1-(4-bromophenyl)-5-(5-nitro-1H-indazol-1-yl)-4,5-dihydro-1H-pyrazole-3-carboxylate, 12e. Yield: 68% (32.9 mg), white solid, Rf: 0.32, m.p.: 152–154 °C. 1H-NMR (DMSO-d6): δ 8.80 (1H, d, J = 2.2 Hz, H-4), 8.41 (1H, s, H-3), 8.35 (1H, dd, J = 9.3 and 2.2 Hz, H-6), 8.10 (1H, d, J = 9.3 Hz, H-7), 7.62 (1H, dd, J = 11.1 Hz and 3.0 Hz, H’), 7.37–7.27 (2H, m, H-9 and H-9′), 7.13-7.01 (2H, m, H-10 and H-10′), 4.32 (2H, q, J = 7.1 Hz, -OCH2CH3), 3.85 (1H, dd, J = 19.0 and 11.1 Hz, H′′′), 3.40–3.39 (1H, m, H′′), 1.33 (3H, t, J = 7.1 Hz, -OCH2CH3) ppm. 13C-NMR (DMSO-d6): δ 161.3 (C=O), 142.2 (C7a), 141.1 (C12), 140.8 (C5), 140.3 (C8), 138.6 (C3), 131.9 (C9 and C9′), 122.9 (C3a), 122.2 (C6), 119.4 (C4), 116.7 (C10 and C10′), 113.6 (C11), 110.3 (C7), 71.9 (CH’), 61.1 (O-CH2CH3), 39.4 (CH2), 14.2 (O-CH2CH3) ppm. MS-ESI(+): m/z 482.1 [M + Na]+. Elemental analysis calcd (%) for C19H16BrN5O4 C 49.80, H 3.52, N 15.28; found C 50.08, H 3.64, N 15.31.
Ethyl 1-(4-chlorophenyl)-5-(6-nitro-1H-indazol-1-yl)-4,5-dihydro-1H-pyrazole-3-carboxylate, 13. Yield: 81% (35.4 mg), white solid, Rf: 0.35, m.p.: 150–152 °C. 1H-NMR (DMSO-d6); δ 9.05 (1H, s, H-7), 8.33 (1H, s, H-3), 8.00 (1H, s, H-5 and H-4), 7.77 (1H, dd, J = 11.0, 2.7 Hz, H’), 7.20 (1H, d, J = 8.9 Hz, H-9 and H-9′), 7.15 (1H, d, J = 8.9 Hz, H-10 and H-10′), 4.32 (2H, q, J = 7.1 Hz, -OCH2CH3), 3.83 (1H, dd, J = 19.0 and 10.9 Hz, H′′′), 3.32–3.31 (1H, m, H′′), 1.32 (3H, t, J = 7.1 Hz, -OCH2CH3) ppm. 13C-NMR (DMSO-d6): δ 161.4 (C=O), 146.7 (C7a), 141.0 (C12), 140.0 (C8), 138.0 (C6), 136.4 (C3), 129.1 (C9 and C9′), 126.7 (C11), 125.7 (C3a), 122.8 (C4), 116.4 (C10 and C10′), 115.9 (C5), 106.6 (C7), 71.8 (CH’), 61.0 (O-CH2), 39.6 (CH2), 14.2 (CH3) ppm. MS-ESI(+): m/z 436.1 [M + Na]+. Elemental analysis calcd (%) for C19H16ClN5O4 C 55.12, H 3.90, N 16.92; found C 54.88, H 4.03, N 16.60.
3.6. Single-Crystal X-Ray Diffraction Studies
Single crystals of compounds 7b, 9 and 13 were manually harvested from the crystallization vials and immersed in highly viscous FOMBLIN Y perfluoropolyether vacuum oil (LVAC 140/13, Sigma-Aldrich) to avoid degradation caused by the evaporation of the solvent [53]. Crystals were mounted on either Hampton Research CryoLoops or MiTeGen MicroLoops, typically with the help of a Stemi 2000 stereomicroscope equipped with Carl Zeiss lenses (San Francisco, CA, USA).
X-ray diffraction data for 9 and 13 were collected at 150(2)K on a D8 QUEST system (Bruker, Flawil, Switzerland) equipped with a Mo Kα sealed tube (λ = 0.71073 Å), a multilayer TRIUMPH X-ray mirror (Concord, CA, USA), a PHOTON 100 CMOS detector, and an Oxford Instruments Cryostrem 700+ Series low temperature device (Grenoble, Switzerland). Crystal data for 7b was instead collected at 150(2)K on a Bruker X8 Kappa APEX II CCD area-detector diffractometer (Mo Kα graphite-monochromated radiation, λ = 0.71073 Å, controlled by the APEX3 software package (Bruker, Wissembourg, France) [54] and equipped with an Oxford Cryosystems Series 700 cryostream monitored remotely using the software interface Cryopad [55]. In both cases, diffraction images were processed using the software package SAINT+ [56], and data were corrected for absorption by the multiscan semi-empirical method implemented in SADABS 2016/2 [57].
Structures were solved using the algorithm implemented in SHELXT-2014/5 [58], which allowed the immediate location of almost all of the heaviest atoms composing the asymmetric unit of the three compounds. The remaining missing and misplaced non-hydrogen atoms were located from difference Fourier maps calculated from successive full-matrix least-squares refinement cycles on F2 using the latest SHELXL from the 2018/3 release [59], All structural refinements were performed using the graphical interface ShelXle [60].
Hydrogen atoms bound to carbon and oxygen were placed at their idealized positions using appropriate HFIX instructions in SHELXL: 43 (aromatic carbon atoms), 13 (tertiary carbon atoms), 23 (–CH2– carbon atoms) and 137 (for terminal methyl groups). These hydrogen atoms were included in subsequent refinement cycles with isotropic thermal displacements parameters (Uiso) fixed at 1.2 (for the three first families of groups) or 1.5×Ueq (for the methyl groups) of the parent non-hydrogen atoms. Structural drawings have been created using the software package Crystal Impact Diamond [61].
The last difference Fourier map synthesis showed: for 7b, the highest peak (0.199 eÅ−3) and the deepest hole (-0.191 eÅ−3) located at 0.91 and 0.83 Å from C13 and C11, respectively; for 9, the highest peak (0.225 eÅ−3) and the deepest hole (-0.256 eÅ−3) located at 0.87 and 0.41 Å from C24 and H19, respectively; and for 13, the highest peak (0.310 eÅ−3) and the deepest hole (-0.631 eÅ−3) located at 1.00 and 1.01 Å from Cl1.
Crystal data for 7b: C18H16N6O2, M = 348.37, monoclinic, space group P21/n, Z = 4, a = 9.4190(7) Å, b = 5.4701(4) Å, c = 32.403(3) Å, β = 95.259(5)°, V = 1662.5(2) Å3, μ(Mo-Kα) = 0.096 mm−1, Dc = 1.392 g cm−3, colourless plate with crystal size of 0.16 × 0.06 × 0.04 mm3. Of a total of 26,545 reflections collected, 3038 were independent (Rint = 0.0761). Final R1 = 0.0504 [I > 2σ(I)] and wR2 = 0.1242 (all data). Data completeness to theta = 25.24°, 99.8%. CCDC 1957399.
Crystal data for 9: C28H22N12O4, M = 590.57, triclinic, space group P-1, Z = 2, a = 5.2677(7) Å, b = 13.2391(17) Å, c = 19.680(3) Å, α = 73.232(4)º, β = 87.663(4)°, γ = 86.022(4)°, V = 1310.6(3) Å3, μ(Mo-Kα) = 0.107 mm−1, Dc = 1.496 g cm−3, colourless needle with crystal size of 0.27 × 0.09 × 0.06 mm3. Of a total of 21,961 reflections collected, 4765 were independent (Rint = 0.0507). Final R1 = 0.0619 [I > 2σ(I)] and wR2 = 0.1275 (all data). Data completeness to theta = 25.24°, 99.7%. CCDC 1957400.
Crystal data for 13: C19H16ClN5O4, M = 413.82, monoclinic, space group P21, Z = 2, a = 12.6613(17) Å, b = 4.9988(6) Å, c = 14.716(2) Å, β = 102.355(4)°, V = 909.8(2) Å3, μ(Mo-Kα) = 0.249 mm−1, Dc = 1.511 g cm−3, colourless needle with crystal size of 0.21 × 0.07 × 0.03 mm3. Of a total of 12,618 reflections collected, 3298 were independent (Rint = 0.0520). Final R1 = 0.0550 [I > 2σ(I) and wR2 = 0.1331 (all data). Data completeness to theta = 25.24°, 99.3%. CCDC 1957398.
4. Conclusions
In summary, the N-alkylation of nitroindazole derivatives with dibromoethane afforded N-(2-bromoethyl)- and N-vinyl-nitro-1H-indazoles. The distribution of the N-substituted derivatives depends on the experimental conditions although the N-1 bromoethyl derivatives are always isolated as the major components. The elimination reaction at N-2 seems to be more favourable than the elimination reaction at the N-1 position. Both types of derivatives showed to be excellent templates for further functionalization via 1,3-dipolar cycloaddition approaches.
The N-bromoethylnitroindazole derivative 2c after being efficiently converted into the corresponding azide 6 afforded, in the presence of terminal ethynylbenzene derivatives and under CuAAC conditions, the expected triazolo derivatives in yields ranging from 71 to 87%. This reaction seems to be favoured by the presence of electron-donating groups in the ethynylbenzene derivative.
The reaction of the N-vinyl-nitroindazole 3b with N-aryl-C-ethoxycarbonylnitrile imines generated in situ from ethyl hydrazono-α-bromoglyoxylates, afforded the corresponding nitroindazole-pyrazoline derivatives 12 with yields ranging from 63 to 87%. The presence of the nitro group in a different position of the indazole core did not seem to affect its reactivity as dipolarophile since derivative 13 was also obtained in excellent yield from the nitroindazole 3c. The expected regioselectivity in these cycloaddition reactions was further supported by single-crystal X-ray diffraction analysis with some single crystals of the compounds obtained.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/25/1/126/s1, Copies Figures S1 to S124: Copies of 1H, 13C, 2D NMR and MS spectra of compounds 2a–2d, 3a–2d, 4a–4d, 5a–5d, 6, 7a–5d, 8, 9, 12a–12e and 13.
Author Contributions
Investigation – M.E. (nitroindazole acetonitriles functionalization and characterization), N.M.M.M. (nitroindazole acetonitriles functionalization and characterization), O.A. (Synthesis of the 1,3-dipoles precursors), R.F.M. (X-Ray data analysis); Supervision – F.A.A.P., E.M.R. and M.G.P.M.S.N.; Validation – J.A.S.C., M.A.F.F.; Writing – original draft, N.M.M.M., L.B. and R.F.M.; Writing-review and editing, N.M.M.M., J.A.S.C., M.A.F.F., E.M.R. and M.G.P.M.S.N. All authors have read and agreed to the published version of the manuscript.
Funding
Thanks are due to the University of Aveiro and FCT/MCT for the financial support for the QOPNA research Unit (FCT UID/QUI/00062/2019) through national founds and, where applicable, co-financed by the FEDER, within the PT2020 Partnership Agreement, and to the Portuguese NMR Network. The authors also thank CICECO-Aveiro Institute of Materials FCT Ref. UID/CTM/50011/2019, Sultan Moulay Slimane University and the Transnational cooperation programs, FCT-CNRST (Morocco), for financial assistance (2019-2020). NMM Moura thanks his research contract (CDL-CTTRI-88-89-97-ARH/2018) which is funded by national funds (OE), through FCT – Fundação para a Ciência e a Tecnologia, I.P., in the scope of the framework contract foreseen in numbers 4, 5 and 6 of the article 23, of the Law Decree 57/2016, of August 29, changed by Law 57/2017, of July 19. RF Mendes gratefully acknowledges FCT for a Junior Research Position (CEECIND/00553/2017). APC was sponsored by MDPI.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martina, K.; Tagliapietra, S.; Veselov, V.V.; Cravotto, G. Green Protocols in Heterocycle Syntheses via 1,3-Dipolar Cycloadditions. Front. Chem. 2019, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Arrastia, I.; Arrieta, A.; Cossío, F.P. Application of 1,3-Dipolar Reactions between Azomethine Ylides and Alkenes to the Synthesis of Catalysts and Biologically Active Compounds. Eur. J. Org. Chem. 2018, 2018, 5889–5904. [Google Scholar] [CrossRef] [PubMed]
- Bozorov, K.; Zhao, J.Y.; Aisa, H.A. 1,2,3-Triazole-containing hybrids as leads in medicinal chemistry: A recent overview. Bioorg. Med. Chem. 2019, 27, 3511–3531. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.W. Advances in Heterocyclic Chemistry; Academic Press: Cambridge, MA, USA, 2018; Volume 126. [Google Scholar]
- Faisal, M.; Saeed, A.; Hussain, S.; Dar, P.; Larik, F.A. Recent developments in synthetic chemistry and biological activities of pyrazole derivatives. J. Chem. Sci. 2019, 131, 70. [Google Scholar] [CrossRef]
- Varghese, B.; Al-Busafi, S.N.; Suliman, F.O.; Al-Kindy, S.M.Z. Unveiling a versatile heterocycle: pyrazoline a review. RSC Adv. 2017, 7, 46999–47016. [Google Scholar] [CrossRef]
- Vinutha, V.S.; Badiadka, N.; Balladka, K.S.; Kullaiah, B. A Comprehensive Review on Recent Developments in the Field of Biological Applications of Potent Pyrazolines Derived from Chalcone Precursors. Lett. Drug Des. Discovery 2018, 15, 516–574. [Google Scholar]
- Pramod, S.; Jitendra, S.; Geeta, J.P.; Mohan, S.M.R. 2-Pyrazolines as Biologically Active and Fluorescent Agents, An Overview. Anti-Cancer Agents Med. Chem. 2018, 18, 1366–1385. [Google Scholar]
- Prasher, P.; Sharma, M. Tailored therapeutics based on 1,2,3-1H-triazoles: A mini review. MedChemComm 2019, 10, 1302–1328. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Kacprzak, K.; Skiera, I.; Piasecka, M.; Paryzek, Z. Alkaloids and Isoprenoids Modification by Copper(I)-Catalyzed Huisgen 1,3-Dipolar Cycloaddition (Click Chemistry): Toward New Functions and Molecular Architectures. Chem. Rev. 2016, 116, 5689–5743. [Google Scholar] [CrossRef]
- Dervaux, B.; Du Prez, F.E. Heterogeneous azide-alkyne click chemistry: towards metal-free end products. Chem. Sci. 2012, 3, 959–966. [Google Scholar] [CrossRef]
- Zheng, Z.J.; Wang, D.; Xu, Z.; Xu, L.W. Synthesis of bi- and bis-1,2,3-triazoles by copper-catalyzed Huisgen cycloaddition: A family of valuable products by click chemistry. Beilstein J. Org. Chem. 2015, 11, 2557–2576. [Google Scholar] [CrossRef] [PubMed]
- Struthers, H.; Mindt, T.L.; Schibli, R. Metal chelating systems synthesized using the copper(I) catalyzed azide-alkyne cycloaddition. Dalton Trans. 2010, 39, 675–696. [Google Scholar] [CrossRef] [PubMed]
- Fustero, S.; Simón-Fuentes, A.; Sanz-Cervera, J.F. Recent Advances in the Synthesis of Pyrazoles. A Review. Org. Prep. Proced. Int. 2009, 41, 253–290. [Google Scholar] [CrossRef]
- Karrouchi, K.R.S.; Ramli, Y.; Taoufik, J.; Mabkhot, Y.N.; Al-aizari, F.A.; Ansar, M. Synthesis and Pharmacological Activities of Pyrazole Derivatives: A Review. Molecules 2018, 23, 134. [Google Scholar] [CrossRef] [PubMed]
- Sibi, M.P.; Stanley, L.M.; Jasperse, C.P. An Entry to a Chiral Dihydropyrazole Scaffold: Enantioselective [3 + 2] Cycloaddition of Nitrile Imines. J. Am. Chem. Soc. 2005, 127, 8276–8277. [Google Scholar] [CrossRef]
- Mojahidi, S.; Sekkak, H.; Rakib, E.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Cavaleiro, J.A.S.; Zouihri, H. Alkylation and 1,3-Dipolar Cycloaddition of 6-Styryl-4,5-dihydro-2H-pyridazin-3-one: Synthesis of Novel N-Substituted Pyridazinones and Triazolo [4,3-b]pyridazinones. J. Chem. 2013, 2013, 7. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Tomé, A.C.; Rakib, E.M.; Hannioui, A.; Mojahidi, S.; Hackbarth, S.; Röder, B.; Almeida Paz, F.A.; et al. Novel pyrazoline and pyrazole porphyrin derivatives: synthesis and photophysical properties. Tetrahedron 2012, 68, 8181–8193. [Google Scholar] [CrossRef]
- Moura, N.M.M.; Giuntini, F.; Faustino, M.A.F.; Neves, M.G.P.S.; Tomé, A.C.; Silva, A.M.S.; Rakib, E.M.; Hannioui, A.; Abouricha, S.; Röder, B.; et al. 1,3-Dipolar cycloaddition of nitrile imines to meso-tetraarylporphyrins. ARKIVOC 2010, 5, 24–33. [Google Scholar]
- Shaaban, M.R.; Mayhoub, A.S.; Farag, A.M. Recent advances in the therapeutic applications of pyrazolines. Expert Opin. Ther. Patents 2012, 22, 253–291. [Google Scholar] [CrossRef]
- Havrylyuk, D.; Roman, O.; Lesyk, R. Synthetic approaches, structure activity relationship and biological applications for pharmacologically attractive pyrazole/pyrazoline–thiazolidine-based hybrids. Eur. J. Med. Chem. 2016, 113, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Dwivedi, J.; Sharma, S.; Jain, S.; Singh, A. The Synthetic and Biological Attributes of Pyrazole Derivatives: A Review. Mini-Rev. Med. Chem. 2018, 18, 918–947. [Google Scholar] [CrossRef] [PubMed]
- Larina, L.; Lopyrev, V. Nitroazoles: Synthesis, Structure and Applications; Springer: London, UK, 2009. [Google Scholar]
- Shrivastava, A.; Upmanyu, A.K.C.N.; Singh, A. Recent Progress in Chemistry and Biology of Indazole and its Derivatives: A Brief Review. Austin J. Anal. Pharm. Chem. 2016, 3, 1076. [Google Scholar]
- Claramunt, R.M.; Santa María, D.; Alkorta, I.; Elguero, J. The Structure of N-phenyl-pyrazoles and Indazoles: Mononitro, Dinitro, and Trinitro Derivatives. J. Heterocycl. Chem. 2018, 55, 44–64. [Google Scholar] [CrossRef]
- Denya, I.; Malan, S.F.; Joubert, J. Indazole derivatives and their therapeutic applications: A patent review (2013-2017). Expert Opin. Ther. Patents 2018, 28, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, D.D.; Chapolikar, A.D.; Devkate, C.G.; Warad, K.D.; Tayade, A.P.; Pawar, R.P.; Domb, A.J. Synthesis of indazole motifs and their medicinal importance: An overview. Eur. J. Med.Chem. 2015, 90, 707–731. [Google Scholar] [CrossRef]
- Zhang, S.-G.; Liang, C.-G.; Zhang, W.-H. Recent Advances in Indazole-Containing Derivatives: Synthesis and Biological Perspectives. Molecules 2018, 23, 2783. [Google Scholar] [CrossRef]
- Dong, J.; Zhang, Q.; Wang, Z.; Huang, G.; Li, S. Recent Advances in the Development of Indazole-based Anticancer Agents. ChemMedChem 2018, 13, 1490–1507. [Google Scholar] [CrossRef]
- Kang, Y.-S.; Yoon, T.; Mun, J.; Park, M.S.; Song, I.-Y.; Benayad, A.; Oh, S.M. Effective passivation of a high-voltage positive electrode by 5-hydroxy-1H-indazole additives. J. Mater. Chem. A 2014, 2, 14628–14633. [Google Scholar] [CrossRef]
- Ahmed, Z.; Iftikhar, K. Efficient Layers of Emitting Ternary Lanthanide Complexes for Fabricating Red, Green, and Yellow OLEDs. Inorg. Chem. 2015, 54, 11209–11225. [Google Scholar] [CrossRef]
- Fan, K.W.; Roberts, J.J.; Martens, P.J.; Stenzel, M.H.; Granville, A.M. Copolymerization of an indazole ligand into the self-polymerization of dopamine for enhanced binding with metal ions. J. Mater. Chem. B 2015, 3, 7457–7465. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Xu, S.; Li, W. Experimental and theoretical studies on the corrosion inhibition of copper by two indazole derivatives in 3.0% NaCl solution. J. Colloid Interface Sci. 2016, 472, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Kouakou, A.; Chicha, H.; Rakib, E.M.; Gamouh, A.; Hannioui, A.; Chigr, M.; Viale, M. SnCl2/RSH: a versatile catalytic system for the synthesis of 4-alkylsulfanyl-indazole derivatives. J. Sulfur Chem. 2015, 36, 86–95. [Google Scholar] [CrossRef]
- Bassou, O.; Chicha, H.; Allam, A.; Monticone, M.; Gangemi, R.; Maric, I.; Viale, M.; Rakib, E.M. Synthesis and Anti-proliferative Activity of Novel Polysubstitued Indazole Derivatives. J. Heterocycl. Chem. 2019, 56, 343–348. [Google Scholar] [CrossRef]
- López, P.; Seipelt, C.G.; Merkling, P.; Sturz, L.; Álvarez, J.; Dölle, A.; Zeidler, M.D.; Cerdán, S.; Ballesteros, P. N-2-(Azol-1(2)-yl)ethyliminodiacetic acids: a novel series of Gd(III) chelators as T2 relaxation agents for magnetic resonance imaging. Bioorg. Med. Chem. 1999, 7, 517–527. [Google Scholar] [CrossRef]
- Micheletti, G.; Kouakou, A.; Boga, C.; Franchi, P.; Calvaresi, M.; Guadagnini, L.; Lucarini, M.; Rakib, E.M.; Spinelli, D.; Tonelli, D.; et al. Comparative spectroscopic and electrochemical study of N-1 or N-2-alkylated 4-nitro and 7-nitroindazoles. Arabian J. Chem. 2017, 10, 823–836. [Google Scholar] [CrossRef]
- Abbassi, N.; Rakib, E.M.; Hannioui, A. Alkylation and Reduction of N-Alkyl-4-nitroindazoles with Anhydrous SnCl2 in Ethanol: Synthesis of Novel 7-Ethoxy-N-alkylindazole Derivatives. Heterocycles 2011, 83, 891–900. [Google Scholar] [CrossRef]
- Abbassi, N.; Rakib, E.M.; Chicha, H.; Bouissane, L.; Hannioui, A.; Aiello, C.; Gangemi, R.; Castagnola, P.; Rosano, C.; Viale, M. Synthesis and Antitumor Activity of Some Substituted Indazole Derivatives. Arch. Pharm. 2014, 347, 423–431. [Google Scholar] [CrossRef]
- Noelting, E. Ueber Bildung von Indazolen aus nitrirten orthomethylirten Aminen. Ber. Dtsch. Chem. Ges. 1904, 37, 2556–2597. [Google Scholar] [CrossRef]
- Cooper, K.A.; Hughes, E.D.; Ingold, C.K.; MacNulty, B.J. 418. Mechanism of elimination reactions. Part VIII. Temperature effects on rates and product-proportions in uni- and bi-molecular substitution and elimination reactions of alkyl halides and sulphonium salts in hydroxylic solvents. J. Chem. Soc. 1948, 2049–2054. [Google Scholar] [CrossRef]
- Catalan, J.; del Valle, J.C.; Claramunt, R.M.; Boyer, G.; Laynez, J.; Gomez, J.; Jimenez, P.; Tomas, F.; Elguero, J. Acidity and Basicity of Indazole and its N-Methyl Derivatives in the Ground and in the Excited State. J. Phys. Chem. 1994, 98, 10606–10612. [Google Scholar] [CrossRef]
- Schmidt, A.; Beutler, A.; Snovydovych, B. Recent Advances in the Chemistry of Indazoles. Eur. J. Org. Chem. 2008, 2008, 4073–4095. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Hao, X.; Jing, L.L.; Wu, G.C.; Kang, D.W.; Liu, X.Y.; Zhan, P. Recent applications of click chemistry in drug discovery. Expert. Opin. Drug Discov. 2019, 14, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Arslan, M.; Acik, G.; Tasdelen, M.A. The emerging applications of click chemistry reactions in the modification of industrial polymers. Polym. Chem. 2019, 10, 3806–3821. [Google Scholar] [CrossRef]
- Yanez-Sedeno, P.; Gonzalez-Cortes, A.; Campuzano, S.; Pingarron, J.M. Copper(I)-Catalyzed Click Chemistry as a Tool for the Functionalization of Nanomaterials and the Preparation of Electrochemical (Bio)Sensors. Sensors 2019, 19, 29. [Google Scholar] [CrossRef]
- Hong, T.T.; Liu, W.F.; Li, M.; Chen, C.P. Click chemistry at the microscale. Analyst 2019, 144, 1492–1512. [Google Scholar] [CrossRef]
- Li, P.Z.; Wang, X.J.; Zhao, Y.L. Click chemistry as a versatile reaction for construction and modification of metal-organic frameworks. Coord. Chem. Rev. 2019, 380, 484–518. [Google Scholar] [CrossRef]
- Takayama, Y.; Kusamori, K.; Nishikawa, M. Click Chemistry as a Tool for Cell Engineering and Drug Delivery. Molecules 2019, 24, 20. [Google Scholar] [CrossRef]
- Sharp, D.B.; Hamilton, C.S. Derivatives of 1,2,4-Triazole and of Pyrazole. J. Am. Chem. Soc. 1946, 68, 588–591. [Google Scholar] [CrossRef]
- Armarego, W.L.F.; Chai, C. Purification of Laboratory Chemicals, 7th ed.; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]
- Kottke, T.; Stalke, D.J. Crystal handling at low temperatures. Appl. Crystallogr. 1993, 26, 615–619. [Google Scholar] [CrossRef]
- APEX3. Data Collection Software Version 2016.9-0; Bruker AXS: Delft, The Netherlands, 2005–2016. [Google Scholar]
- Cryopad. Remote monitoring and control, Version 1.451; Oxford Cryosystems: Oxford, UK, 2006. [Google Scholar]
- SAINT+. Data Integration Engine v. 8.37a©; Bruker AXS: Madison, WI, USA, 1997–2015. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT - Integrated space-group and crystal-structure determination. Acta Cryst. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hübschle, C.B.; Sheldrick, G.M.; Dittric, B. ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef]
- Brandenburg, K. DIAMOND, Version 3.2f; Crystal Impact GbR: Bonn, Germany, 1997–2010. [Google Scholar]
Sample Availability: Samples of the compounds 1–5, 6, 7a–7d, 8, 9, 10a–10e, 12a–12e and 13 are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).