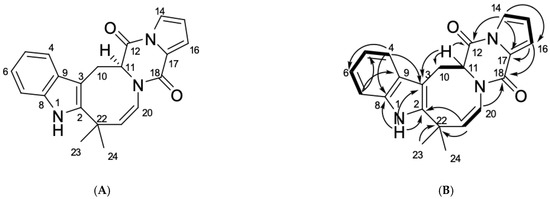
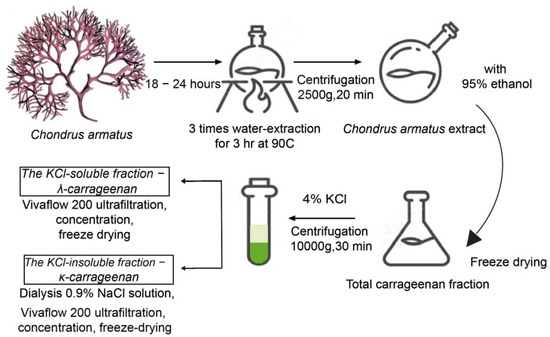
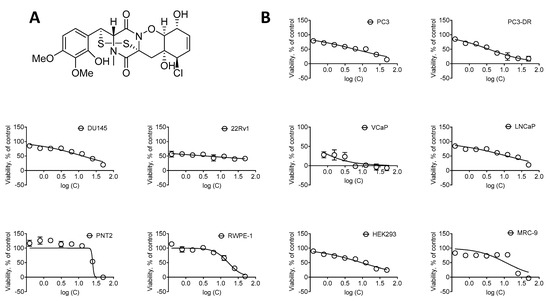
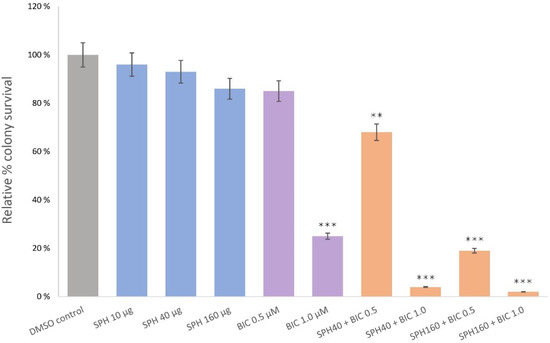
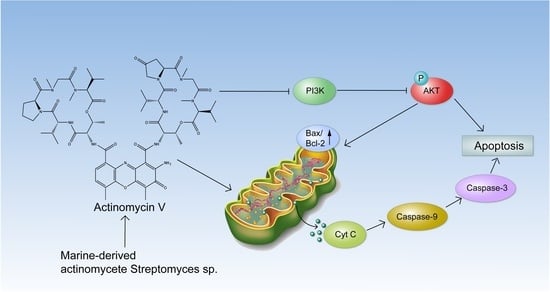
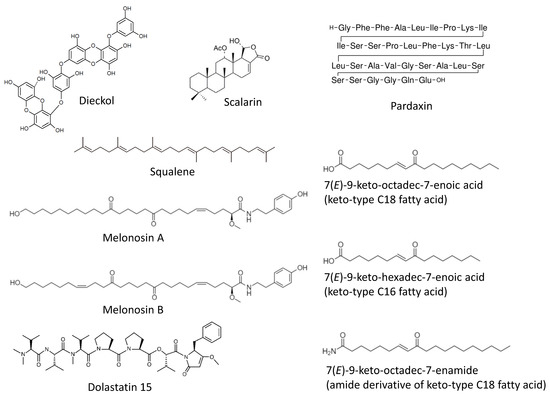
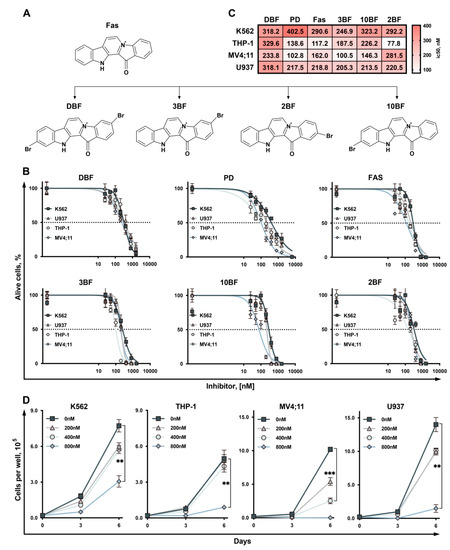
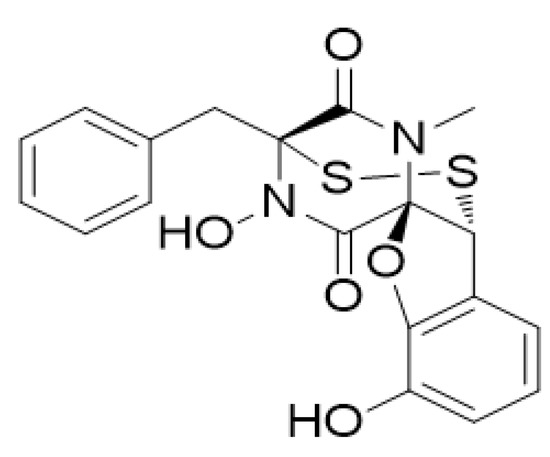
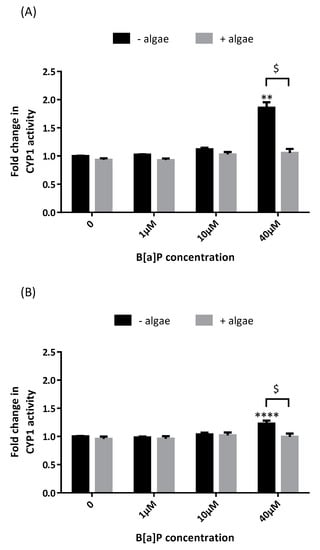
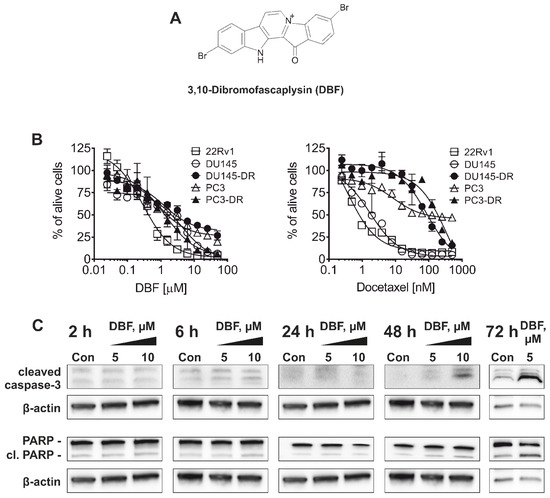
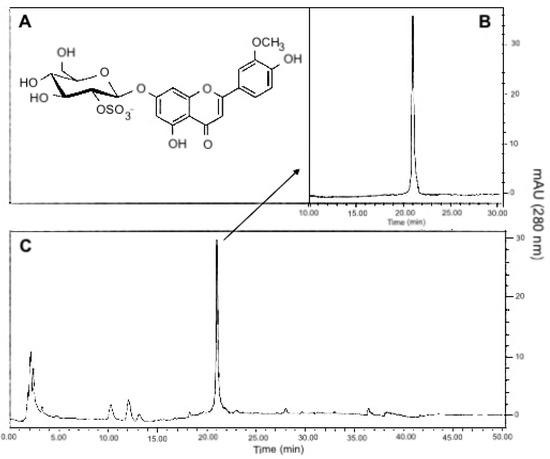
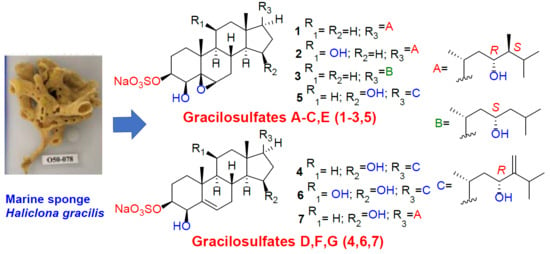
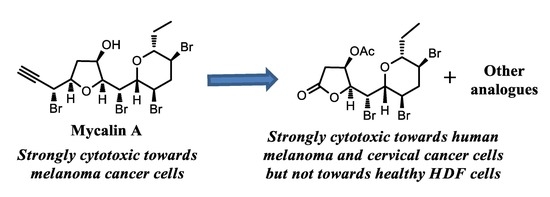
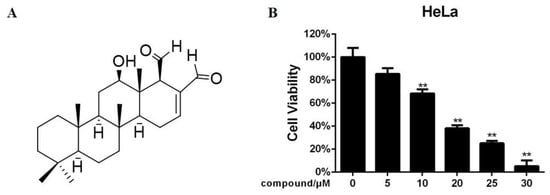
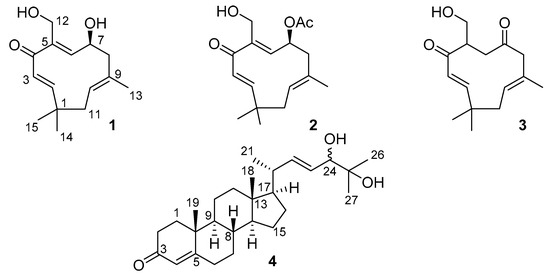
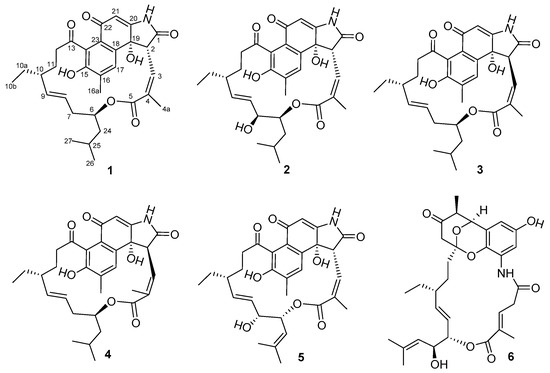
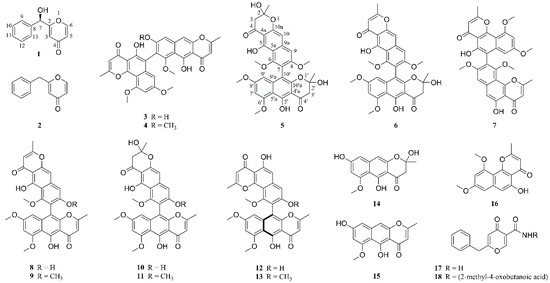
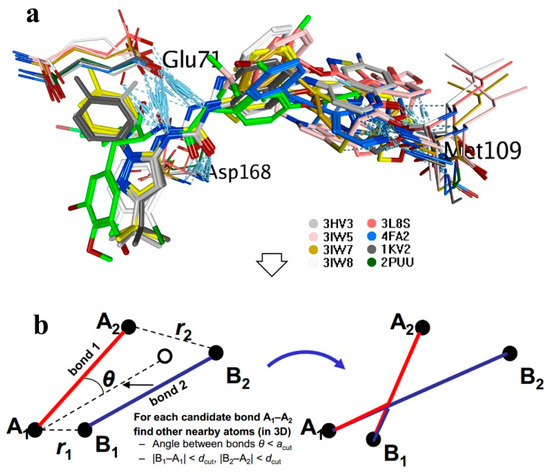
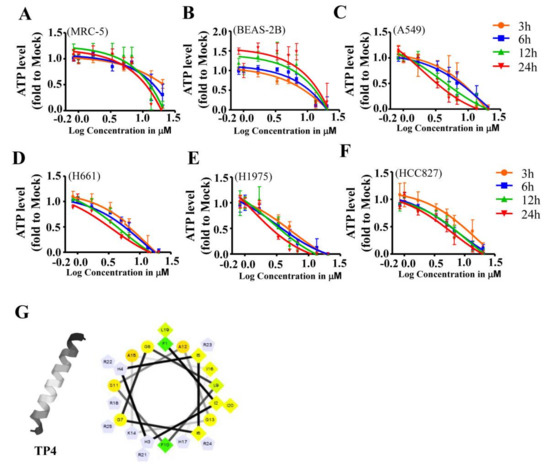
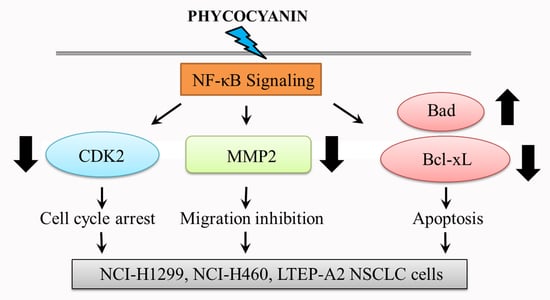
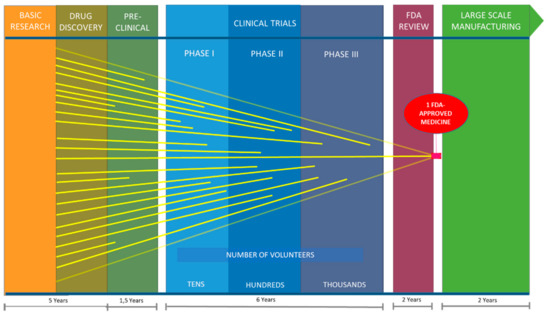
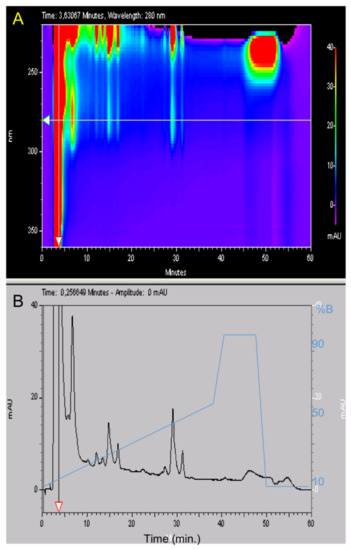
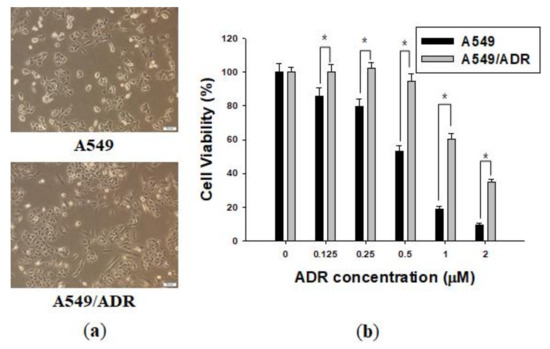
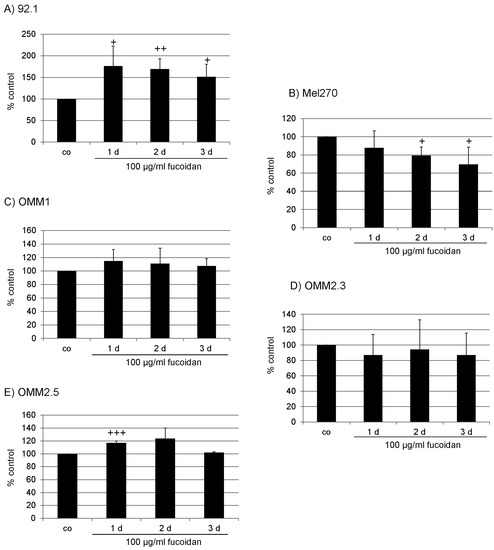
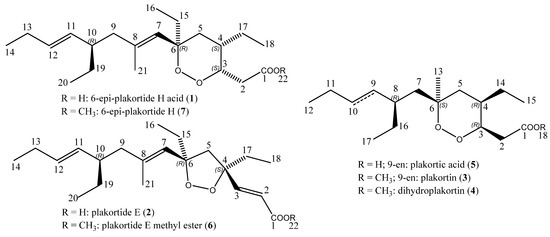
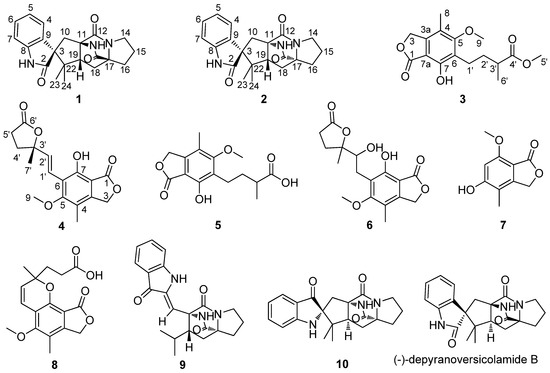
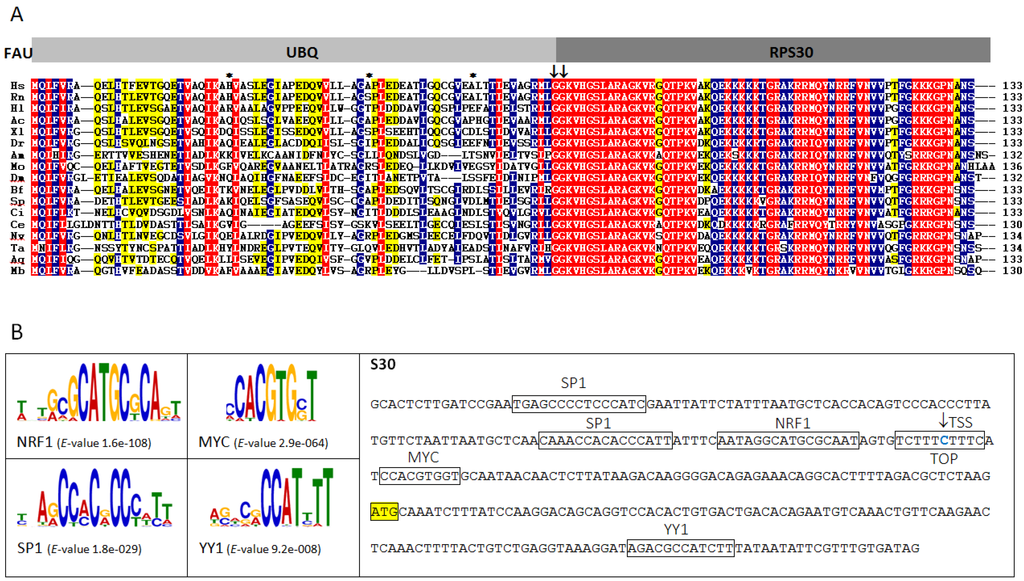
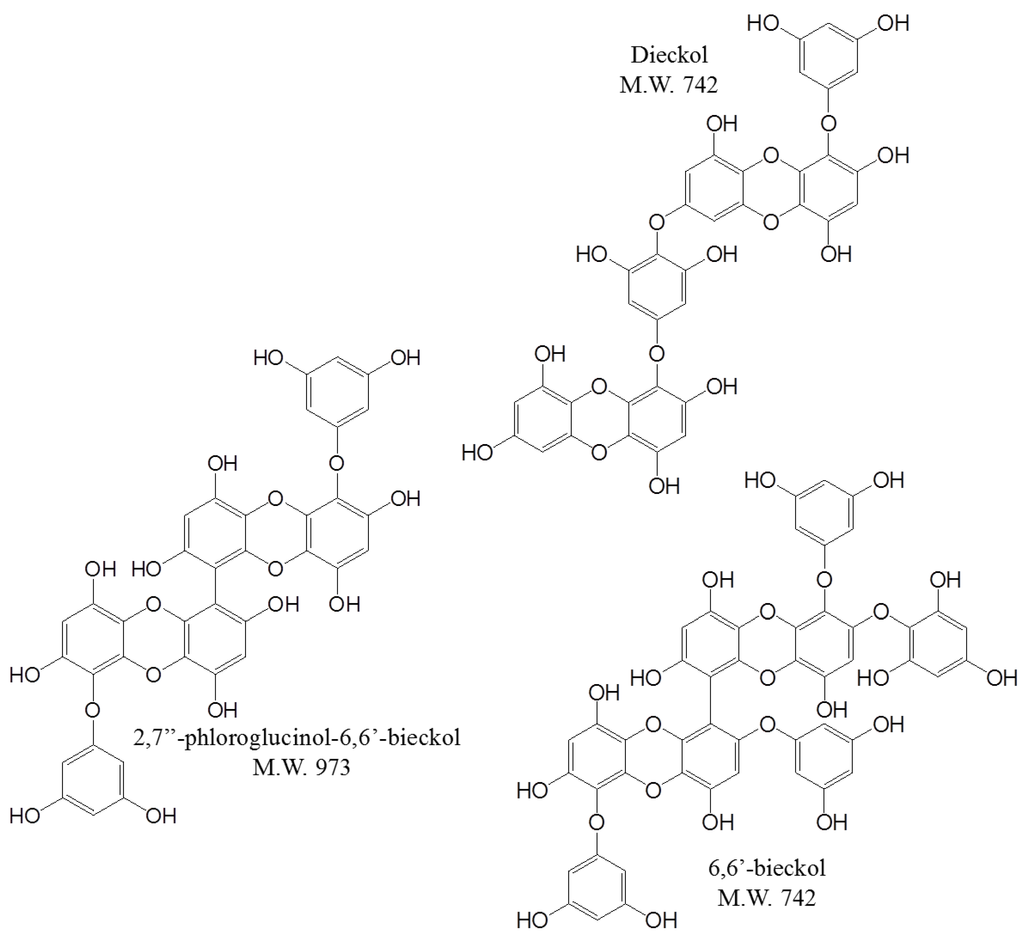
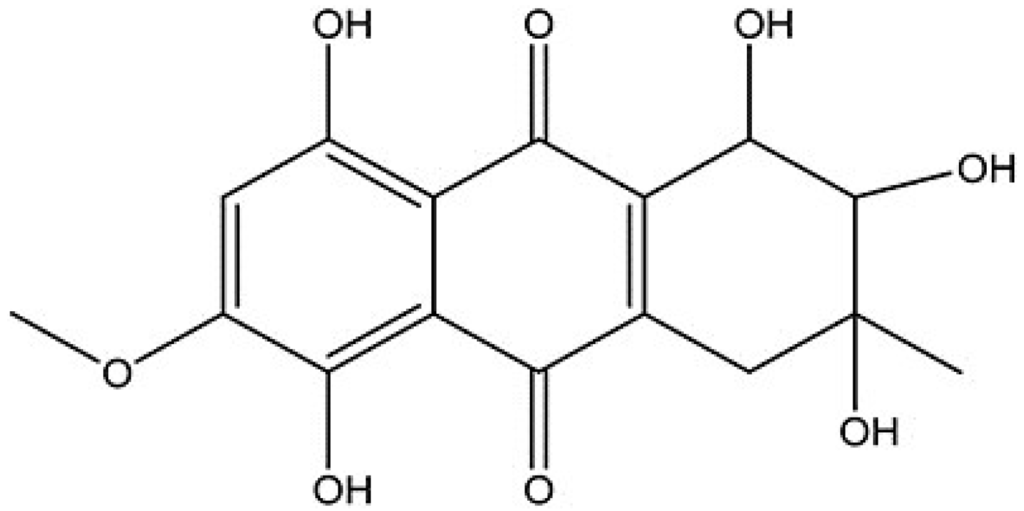
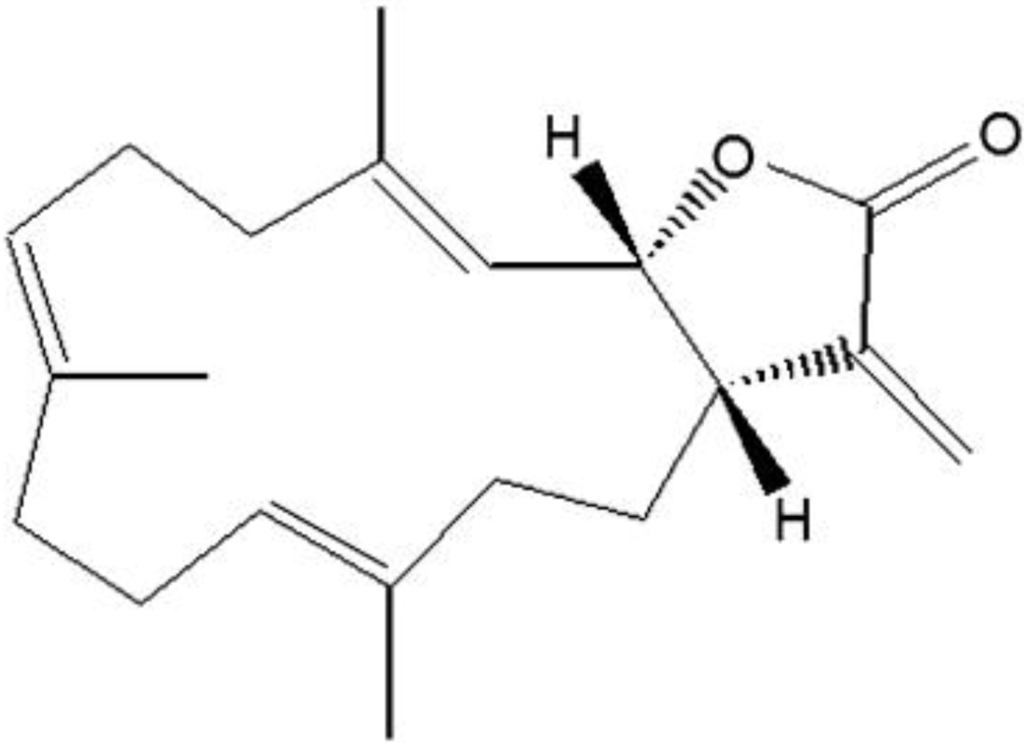
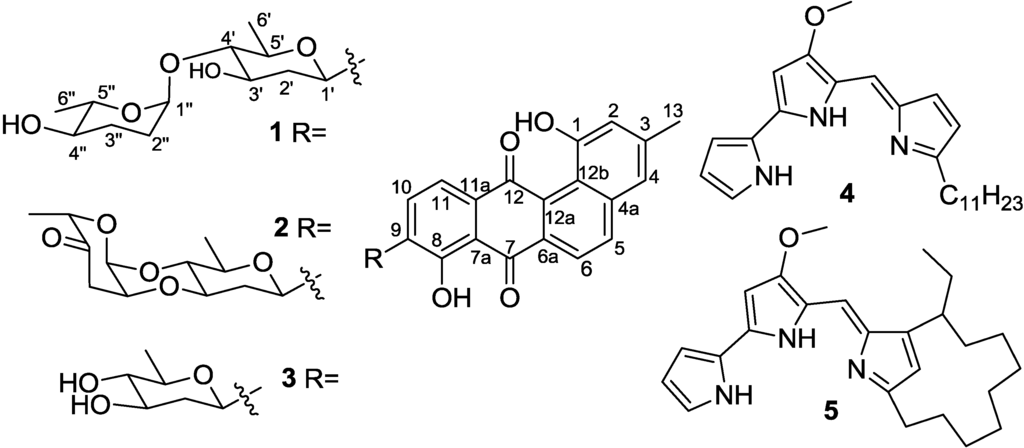
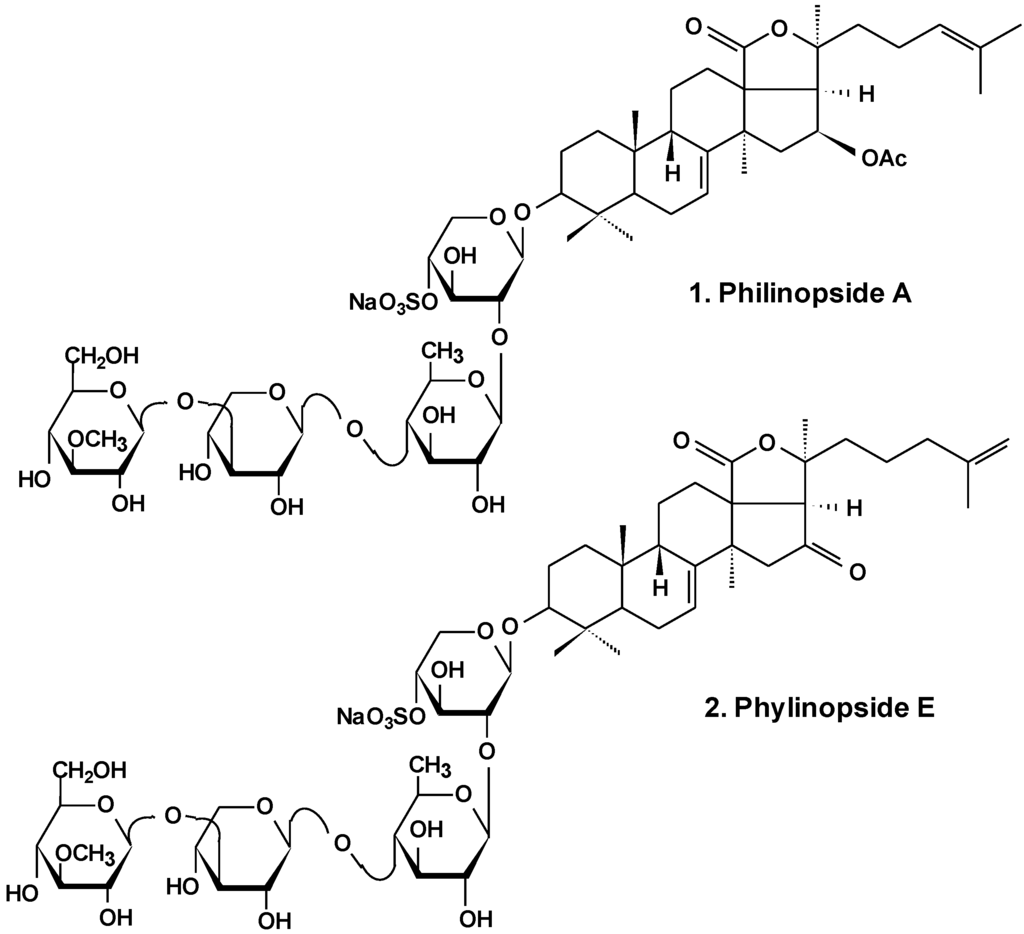
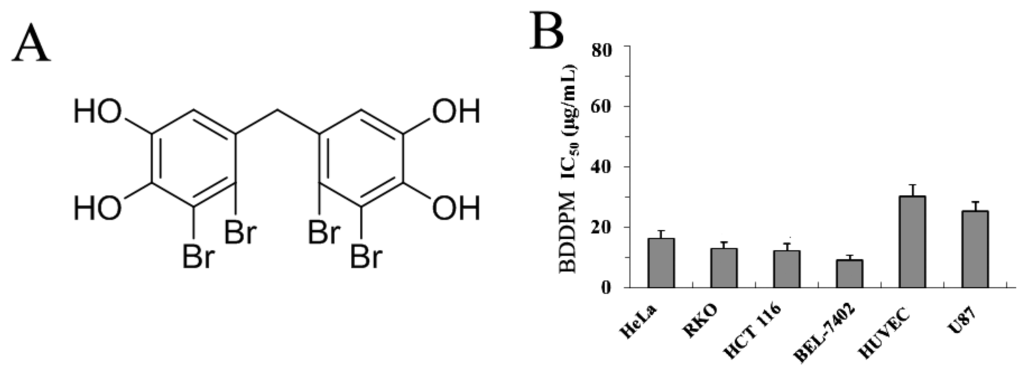
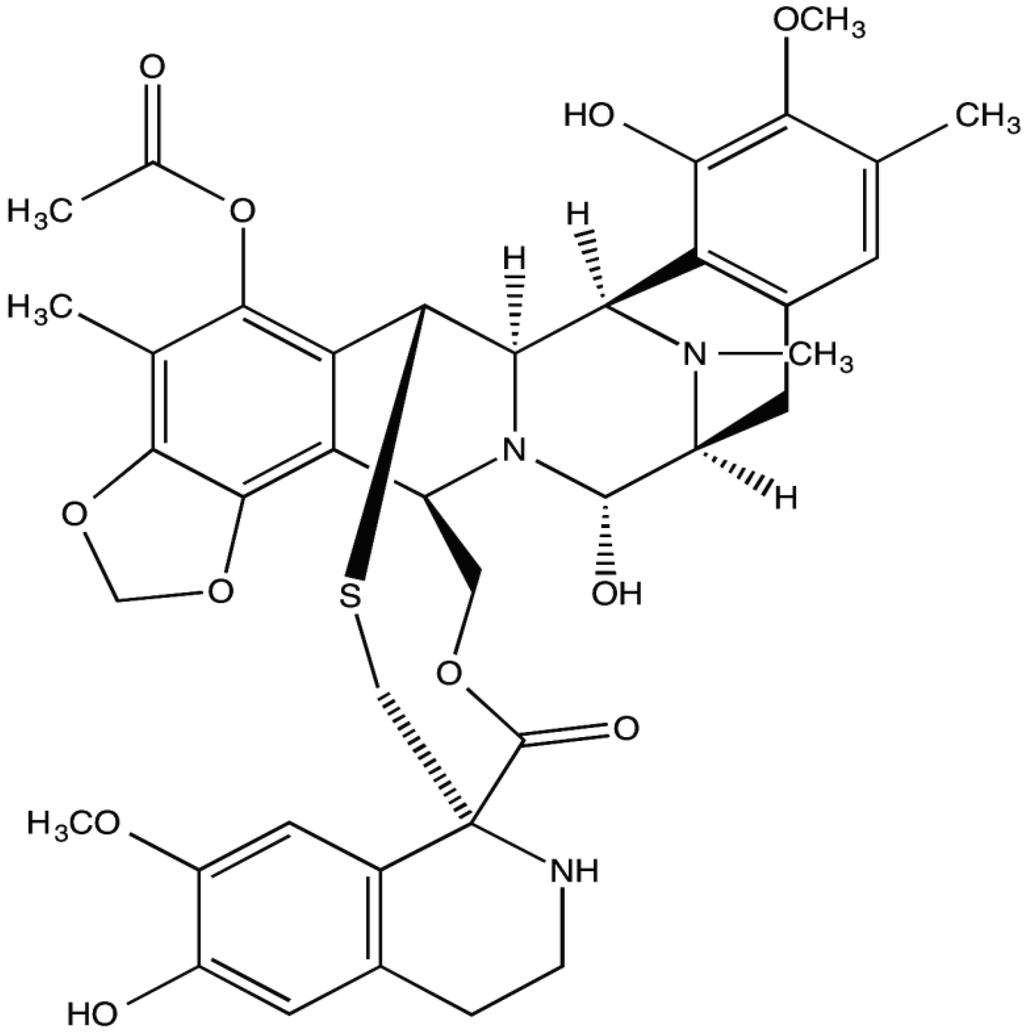
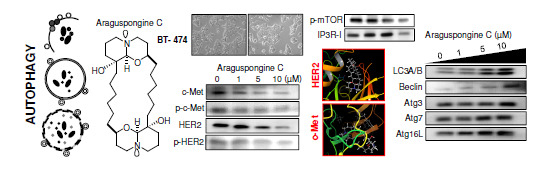
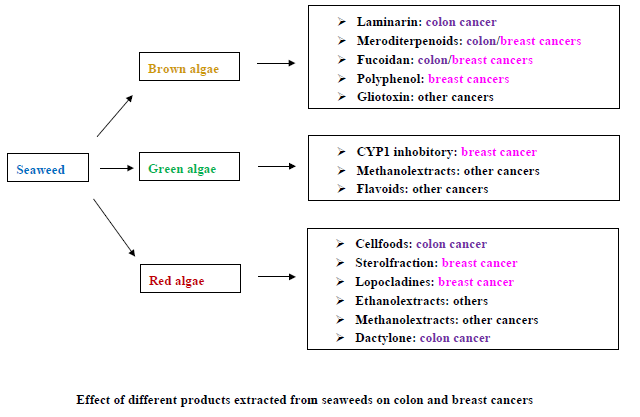
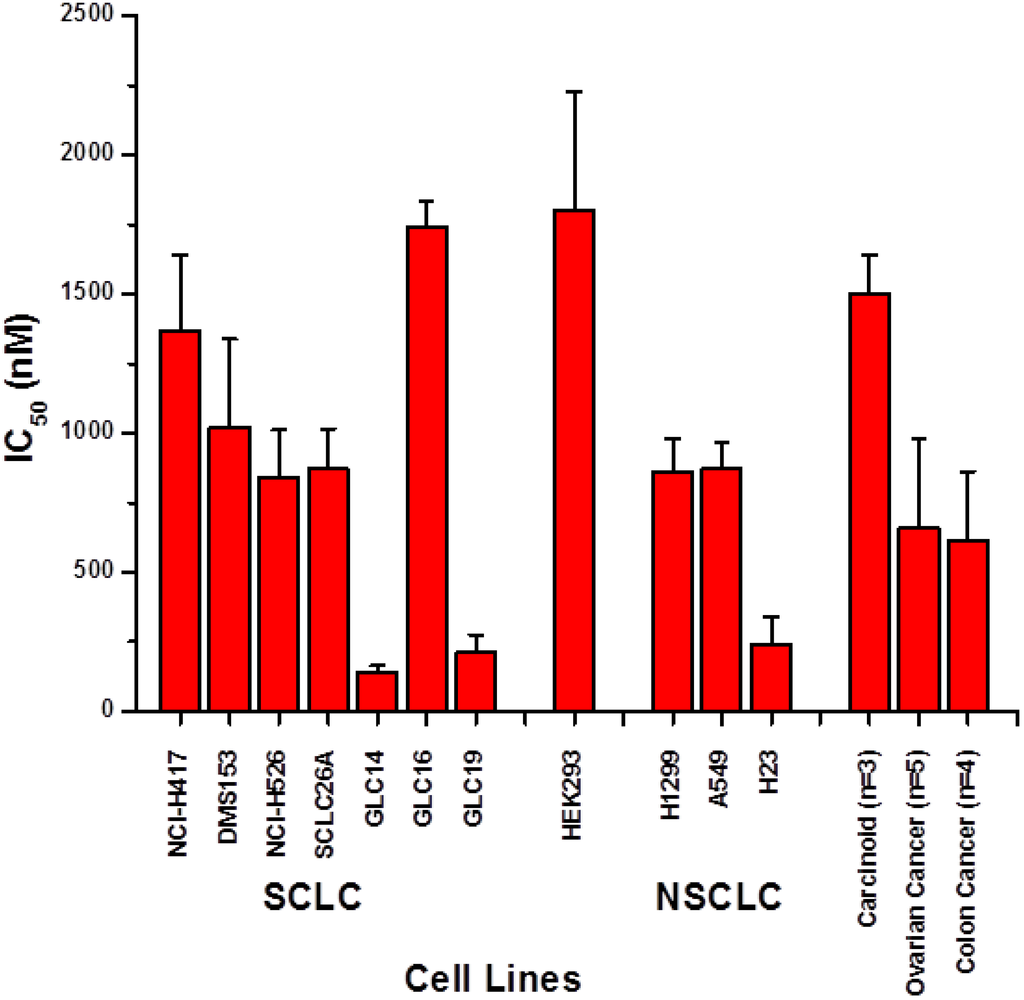
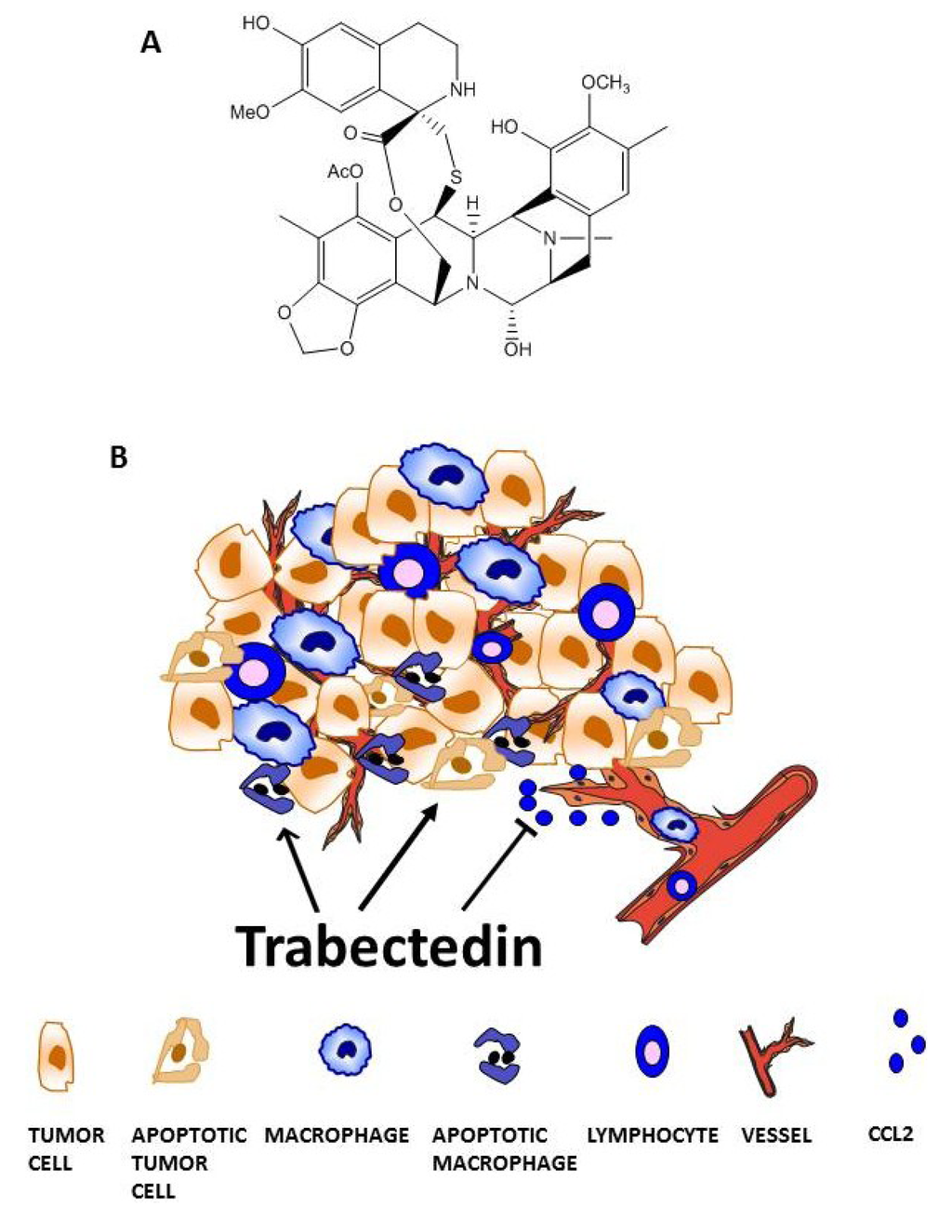
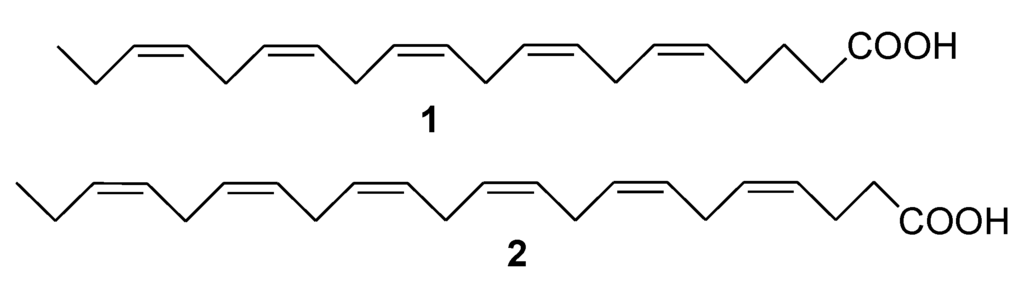
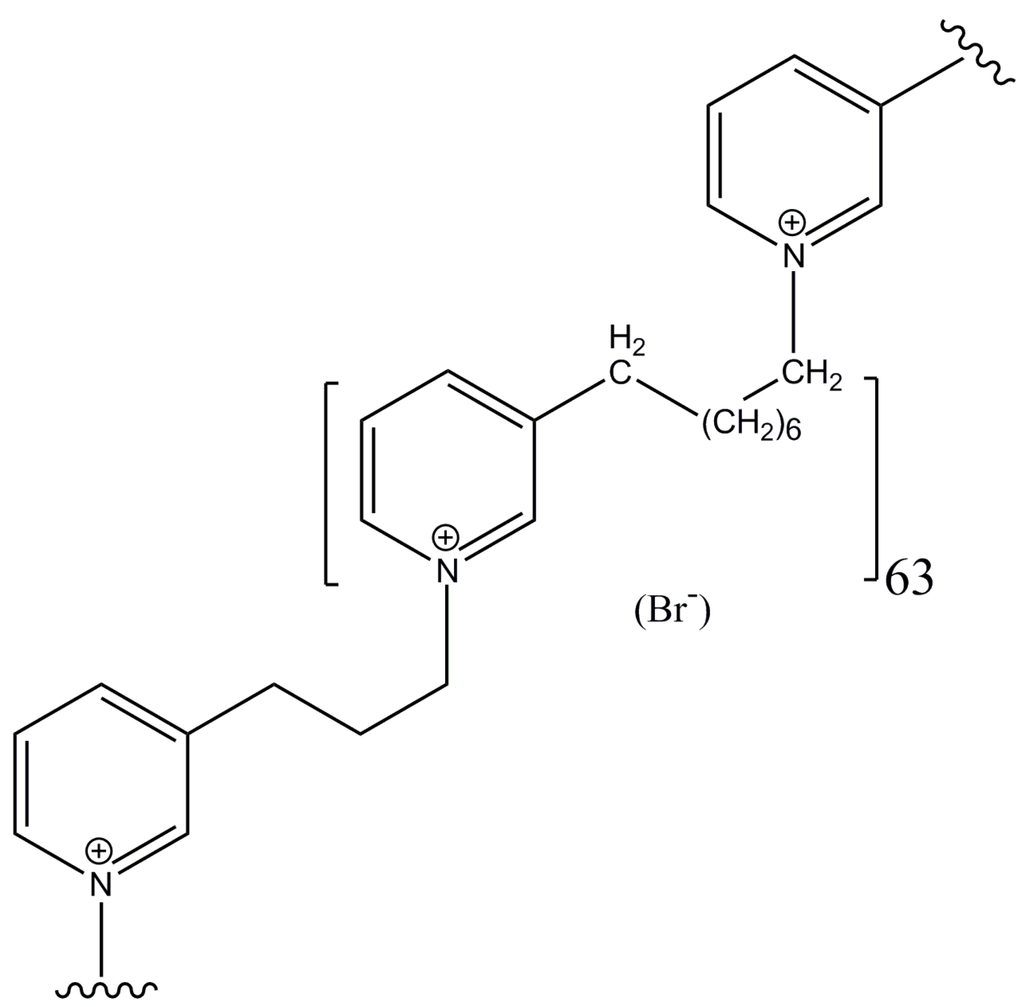
Marine Compounds and Cancer
A topical collection in Marine Drugs (ISSN 1660-3397). This collection belongs to the section "Marine Pharmacology".
Viewed by 784459Editors
Interests: medical oncology; drug resistance; marine anti-cancer compounds; drug development; tumor biology; proteomics
Special Issues, Collections and Topics in MDPI journals
2. Martini-Klinik, Prostate Cancer Center, University Hospital Hamburg-Eppendorf, Hamburg, Germany
Interests: marine natural compounds; secondary metabolites; anticancer activity; mechanism of action; autophagy; drug combinational studies
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
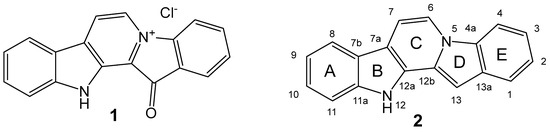
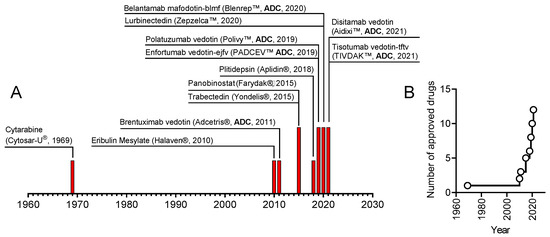
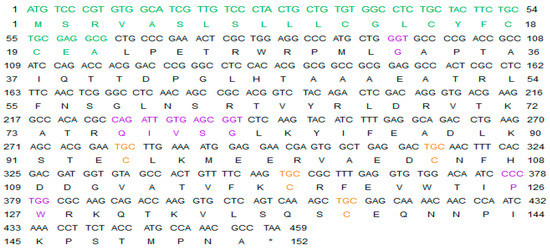
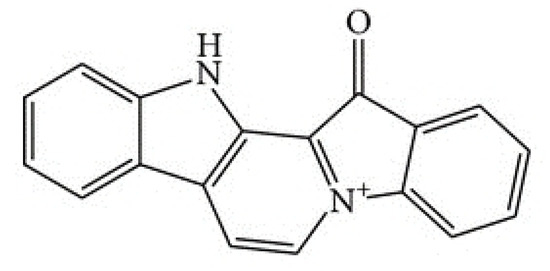
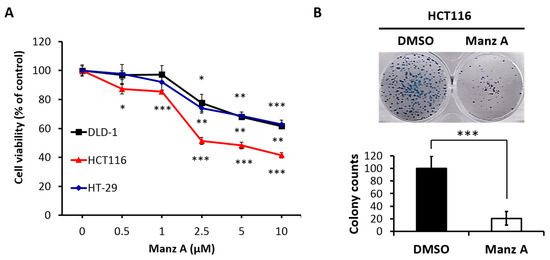
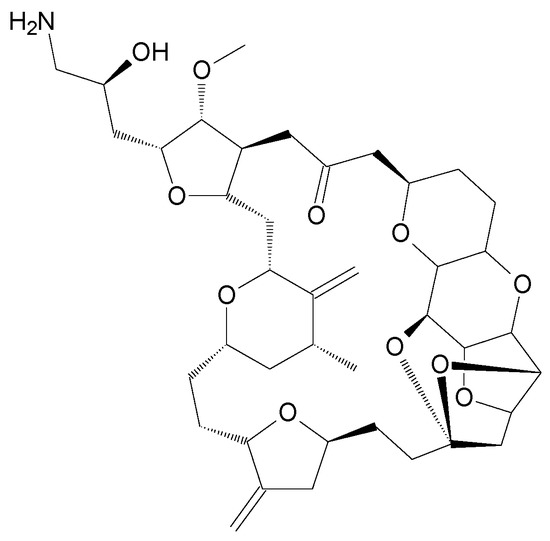
In 2019, the scientific and medical community celebrated the 50th anniversary of the introduction of the very first marine drug, Cytarabine (aka Ara-C, Cytosar-U®), into the clinics. In 1969, it was approved by the Food and Drug Administration (FDA) for the treatment of leukemia. Nowadays, the list of approved marine-derived anticancer drugs consists of seven medications. Many more are in all phases of clinical testing, and a plethora of substances have already been examined for in vitro and in vivo activity.
Increasingly precise research tools allow the dissection of the molecular mode of action of these cytotoxic substances, thereby uncovering the specific drug targets in cancer cells. This development will blur the edges between targeted and untargeted therapy, and will hopefully lead to a more directed use of cancer medicine based on a molecular rationale of activity in the future.
This Topical Collection will cover the whole scope from agents with cancer-preventive activity, to novel and previously characterized compounds with anti-cancer activity, both in vitro and in vivo, and the latest status of clinical development from drug trials. Notably, compounds possessing pro-carcinogenic activity or mediating cancer cell survival are also within the scope of this Topical Collection. In addition, special focus will be placed on current shortfalls and possible strategies to overcome obstacles in the area of marine anti-cancer drug development.
Dr. Sergey A. DyshlovoyDr. Friedemann Honecker
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as short communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Marine Drugs is an international peer-reviewed open access monthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- cancer
- marine natural compounds and their derivatives
- marine toxins
- drug discovery
- cancer-preventive activity
- molecular effects
- molecular targets
- drug resistance
- drug combination
- toxicology
- xenograft models