Hypoxia Reduces the Efficiency of Elisidepsin by Inhibiting Hydroxylation and Altering the Structure of Lipid Rafts
Abstract
:1. Introduction
2. Results
2.1. The Elisidepsin Sensitivity of Cell Lines is Reduced under Hypoxic Conditions
| Cell lines | IC50 (µM) | ||
|---|---|---|---|
| Normoxia | 4-day hypoxia | 14-day hypoxia | |
| A431 | 7.8 ± 0.8 | 15.4 ± 1.8 * | n. d. |
| CHO | 15 ± 0.8 | 66 ± 9.3 * | n. d. |
| HaCaT | 7.5 ± 0.9 | 27.6 ± 4.2 * | n. d. |
| HeLa | 9.1 ± 1.2 | 16.5 ± 1.7 * | n. d. |
| MCF-7 | 1.4 ± 0.3 | 1.6 ± 0.5 | 1.7 ± 0.6 |
| MDA-MB-453 | 3.6 ± 0.5 | 3.9 ± 0.7 | 6.1 ± 0.5 * |
| SKBR-3 | 2.4 ± 0.4 | 2.3 ± 0.5 | 5.2 ± 0.2 * |
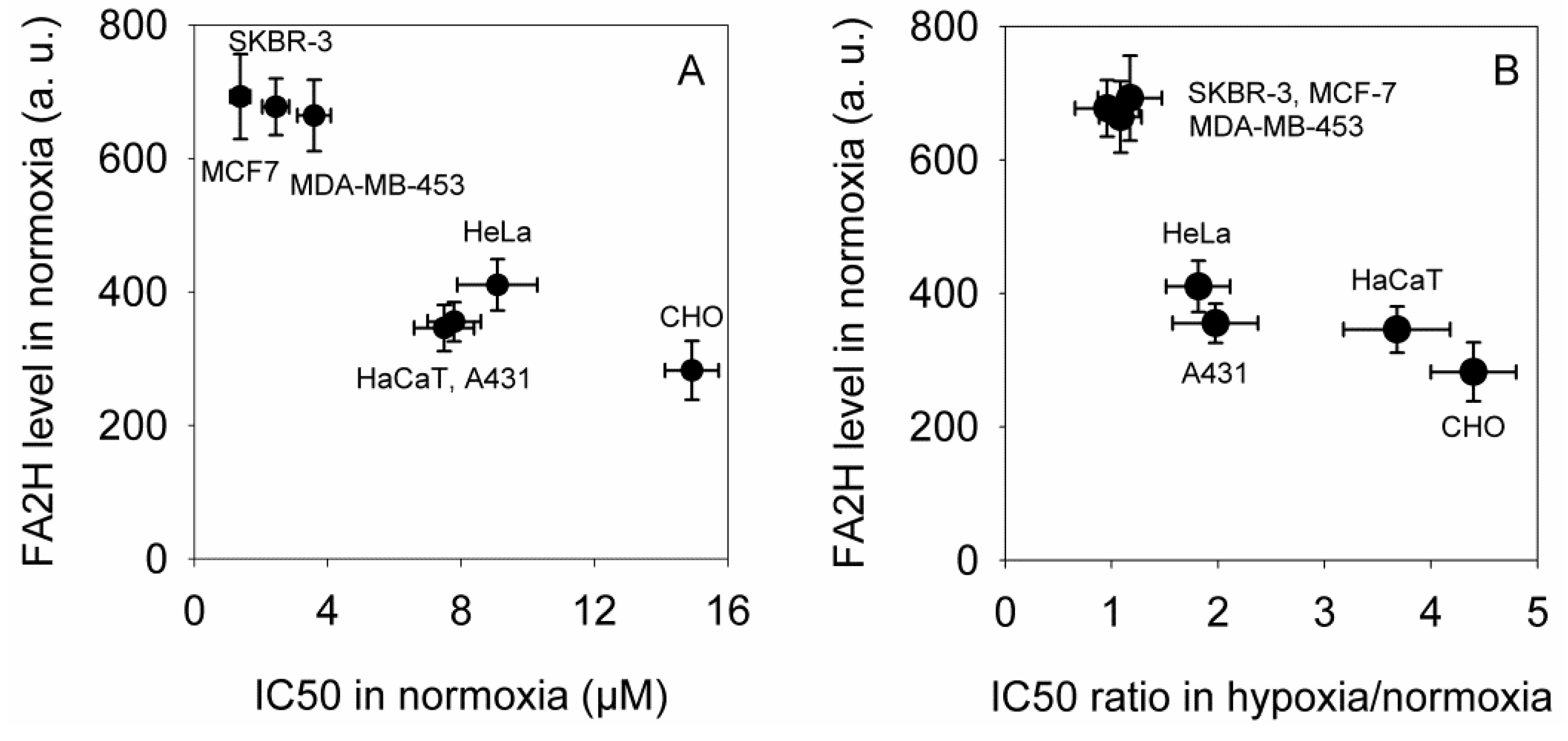
2.2. The Expression of FA2H Correlates with Elisidepsin Sensitivity in Normoxia and Determines the Hypoxia-Induced Increase in the IC50 Values
2.3. 2-Hydroxy Palmitic Acid Reverses the Effect of Hypoxia on Elisidepsin Sensitivity
| Cell lines | IC50 (µM) | |||
|---|---|---|---|---|
| Normoxia | Hypoxia | MeOH in hypoxia | 2-OH-PA in hypoxia | |
| A431 | 9.0 ± 1.7 | 17.9 ± 2.8 | 15.6 ± 3.1 | 7.75 ± 2.0 |
| CHO | 15.6 ± 2.1 | 34.6 ± 4.7 | 38.9 ± 5.3 | 12.3 ± 2.4 |
| HaCaT | 7.6 ± 1.9 | 18.7 ± 2.5 | 16.4 ± 2.8 | 9.5 ± 1.7 |
| HeLa | 9.4 ± 1.0 | 21.0 ± 3.3 | 19.0 ± 2.4 | 10.1 ± 1.8 |
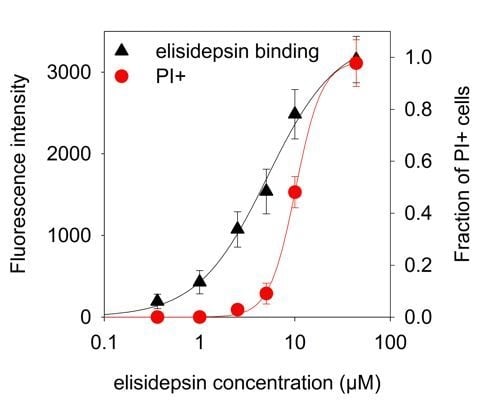
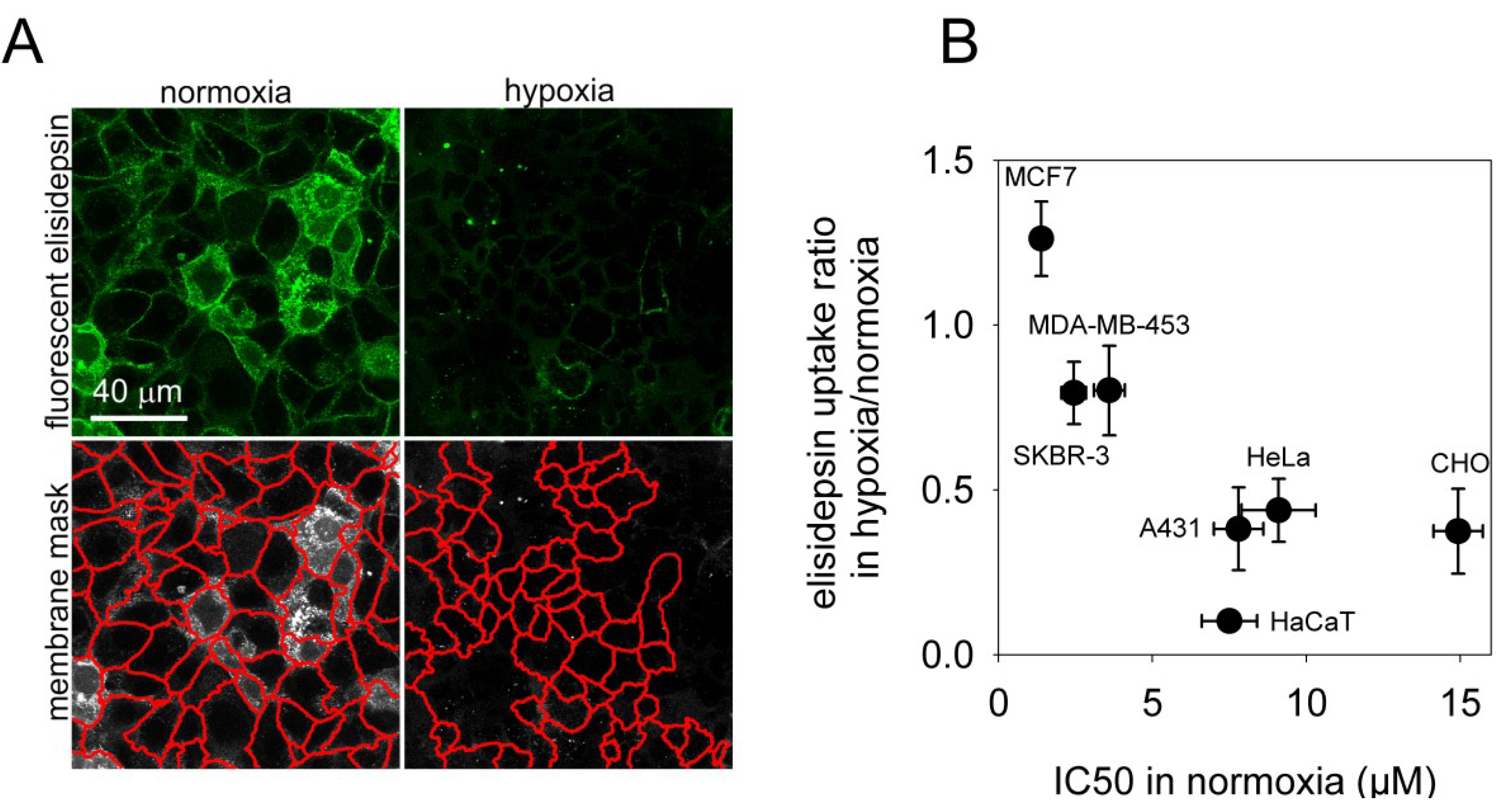
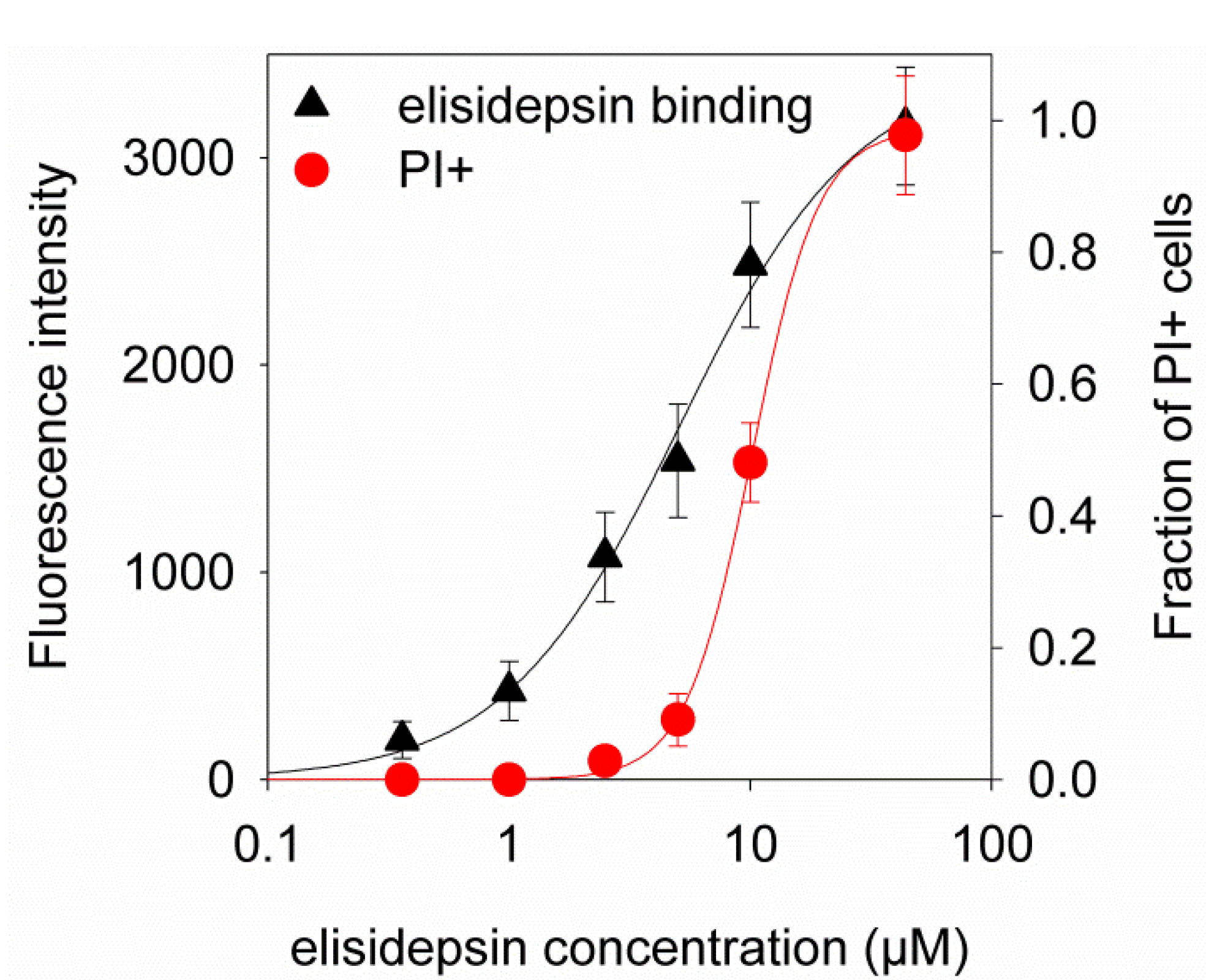
2.4. Hypoxia Reduces the Binding of Fluorescent Elisidepsin
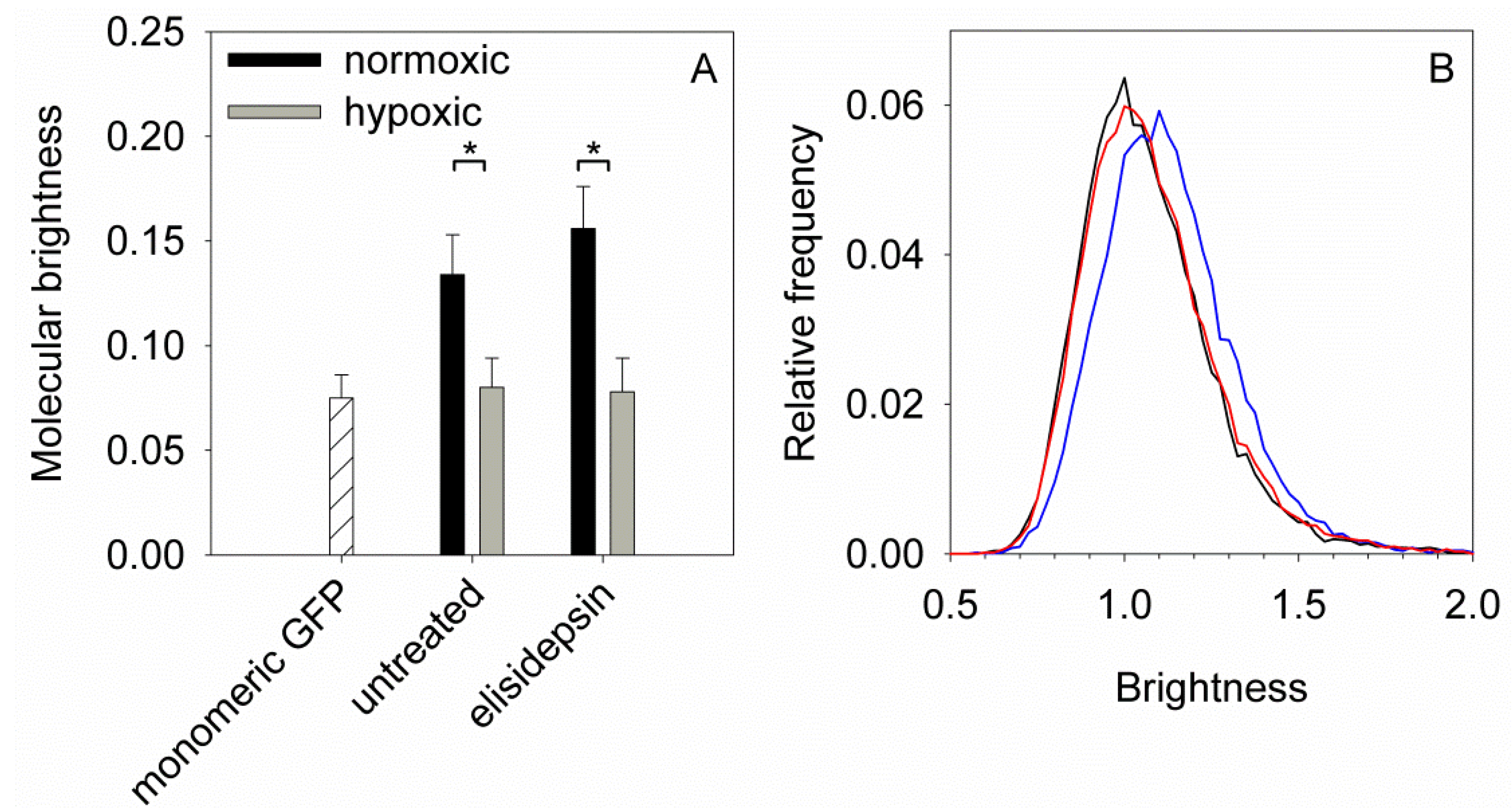
2.5. Elisidepsin Induces Clustering of GPI-Anchored GFP
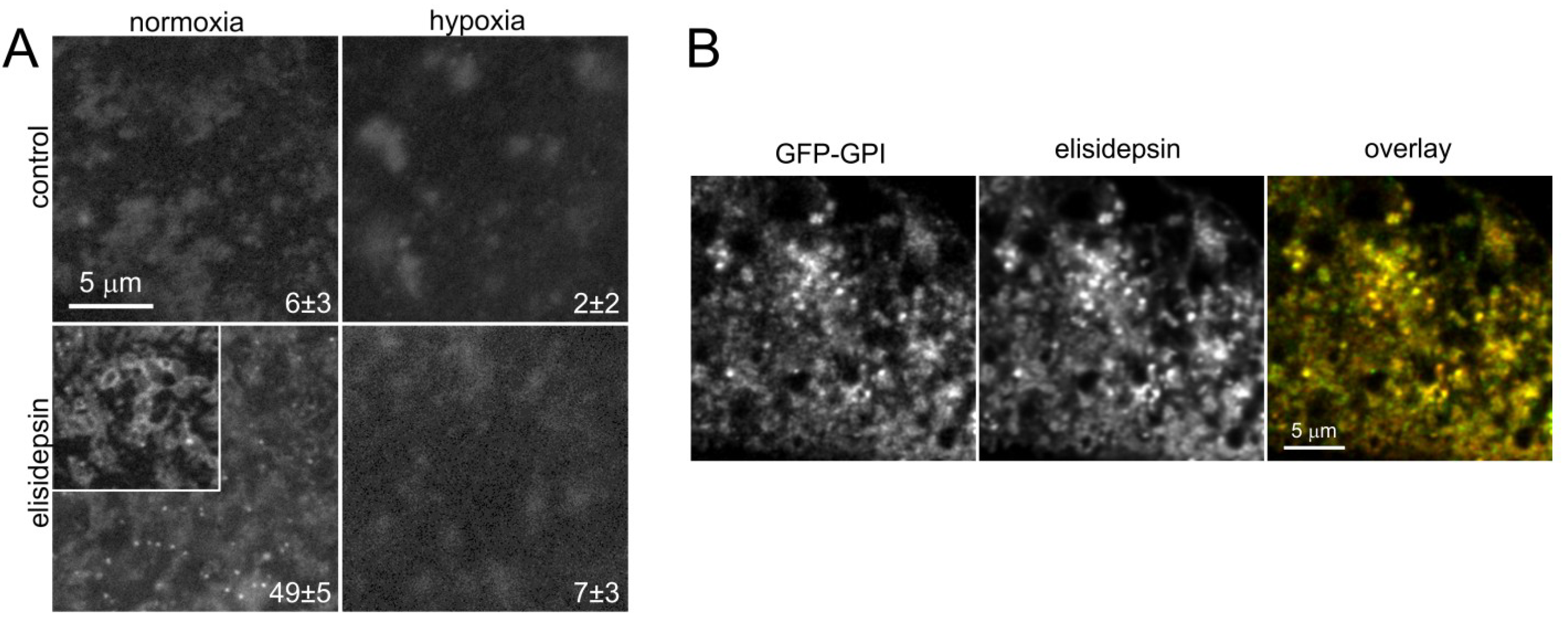
2.6. Hypoxia Decreases the Clustering of Lipid Rafts and Induces Changes in the Fluidity and Compactness of the Membrane
3. Discussion
4. Experimental Section
4.1. Cell Culture and Transfection
4.2. Antibodies and Chemicals
4.3. Determination of Elisidepsin Sensitivity
4.4. Flow Cytometric Measurement of Fatty Acid 2-Hydroxylase Expression
4.5. Confocal Microscopy
4.6. Determination of the Binding of Fluorescent Elisidepsin to the Membrane
4.7. Number and Brightness (N&B) Analysis of Cells Transfected by GFP-GPI

4.8. Measurement of Fluorescence Anisotropy and Generalized Polarization

4.9. Determination of 2-Hydroxylated Fatty Acids Using Mass Spectrometry
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Albreht, T.; McKee, M.; Alexe, D.M.; Coleman, M.P.; Martin-Moreno, J.M. Making progress against cancer in europe in 2008. Eur. J. Cancer 2008, 44, 1451–1456. [Google Scholar] [CrossRef]
- Suarez, Y.; Gonzalez, L.; Cuadrado, A.; Berciano, M.; Lafarga, M.; Munoz, A. Kahalalide f, a new marine-derived compound, induces oncosis in human prostate and breast cancer cells. Mol. Cancer Ther. 2003, 2, 863–872. [Google Scholar]
- Faircloth, G.; Cuevas, C. Kahalalide f and es285: Potent anticancer agents from marine molluscs. Prog. Mol. Subcell Biol. 2006, 43, 363–379. [Google Scholar]
- Provencio, M.; Sanchez, A.; Gasent, J.; Gomez, P.; Rosell, R. Cancer treatments: Can we find treasures at the bottom of the sea? Clin. Lung Cancer 2009, 10, 295–300. [Google Scholar] [CrossRef]
- Molina-Guijarro, J.M.; Macias, A.; Garcia, C.; Munoz, E.; Garcia-Fernandez, L.F.; David, M.; Nunez, L.; Martinez-Leal, J.F.; Moneo, V.; Cuevas, C.; et al. Irvalec inserts into the plasma membrane causing rapid loss of integrity and necrotic cell death in tumor cells. PLoS One 2011, 6, e19042. [Google Scholar] [CrossRef]
- Ling, Y.H.; Aracil, M.; Jimeno, J.; Perez-Soler, R.; Zou, Y. Molecular pharmacodynamics of pm02734 (elisidepsin) as single agent and in combination with erlotinib; synergistic activity in human non-small cell lung cancer cell lines and xenograft models. Eur. J. Cancer 2009, 45, 1855–1864. [Google Scholar] [CrossRef]
- Teixido, C.; Arguelaguet, E.; Pons, B.; Aracil, M.; Jimeno, J.; Somoza, R.; Mares, R.; Ramon, Y.C.S.; Hernandez-Losa, J. Erbb3 expression predicts sensitivity to elisidepsin treatment: In vitro synergism with cisplatin, paclitaxel and gemcitabine in lung, breast and colon cancer cell lines. Int. J. Oncol. 2012, 41, 317–324. [Google Scholar]
- Martin-Algarra, S.; Espinosa, E.; Rubio, J.; Lopez Lopez, J.J.; Manzano, J.L.; Carrion, L.A.; Plazaola, A.; Tanovic, A.; Paz-Ares, L. Phase ii study of weekly kahalalide f in patients with advanced malignant melanoma. Eur. J. Cancer 2009, 45, 732–735. [Google Scholar] [CrossRef]
- Pardo, B.; Paz-Ares, L.; Tabernero, J.; Ciruelos, E.; Garcia, M.; Salazar, R.; Lopez, A.; Blanco, M.; Nieto, A.; Jimeno, J.; et al. Phase i clinical and pharmacokinetic study of kahalalide f administered weekly as a 1-hour infusion to patients with advanced solid tumors. Clin. Cancer Res. 2008, 14, 1116–1123. [Google Scholar] [CrossRef]
- Rademaker-Lakhai, J.M.; Horenblas, S.; Meinhardt, W.; Stokvis, E.; de Reijke, T.M.; Jimeno, J.M.; Lopez-Lazaro, L.; Lopez Martin, J.A.; Beijnen, J.H.; Schellens, J.H. Phase i clinical and pharmacokinetic study of kahalalide f in patients with advanced androgen refractory prostate cancer. Clin. Cancer Res. 2005, 11, 1854–1862. [Google Scholar] [CrossRef]
- Salazar, R.; Jones, R.J.; Oaknin, A.; Crawford, D.; Cuadra, C.; Hopkins, C.; Gil, M.; Coronado, C.; Soto-Matos, A.; Cullell-Young, M.; et al. A phase i and pharmacokinetic study of elisidepsin (pm02734) in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2012, 70, 673–681. [Google Scholar] [CrossRef]
- Salazar, R.; Cuadra, C.; Gil-Martin, M.; Vandermeeren, A.; Alfaro, V.; Coronado, C. Complete and sustained objective response per recist to irvalec (pm02734) in undifferentiated large cell esophageal adenocarcinoma: A case report and a review of the literature. Case Rep. Oncol. 2012, 5, 354–358. [Google Scholar] [CrossRef]
- Janmaat, M.L.; Rodriguez, J.A.; Jimeno, J.; Kruyt, F.A.; Giaccone, G. Kahalalide f induces necrosis-like cell death that involves depletion of erbb3 and inhibition of akt signaling. Mol. Pharmacol. 2005, 68, 502–510. [Google Scholar]
- Váradi, T.; Roszik, J.; Lisboa, D.; Vereb, G.; Molina-Guijarro, J.M.; Galmarini, C.M.; Szöllősi, J.; Nagy, P. Erbb protein modifications are secondary to severe cell membrane alterations induced by elisidepsin treatment. Eur. J. Pharmacol. 2011, 667, 91–99. [Google Scholar] [CrossRef]
- Herrero, A.B.; Astudillo, A.M.; Balboa, M.A.; Cuevas, C.; Balsinde, J.; Moreno, S. Levels of scs7/fa2h-mediated fatty acid 2-hydroxylation determine the sensitivity of cells to antitumor pm02734. Cancer Res. 2008, 68, 9779–9787. [Google Scholar] [CrossRef]
- Ling, Y.H.; Aracil, M.; Zou, Y.; Yuan, Z.; Lu, B.; Jimeno, J.; Cuervo, A.M.; Perez-Soler, R. Pm02734 (elisidepsin) induces caspase-independent cell death associated with features of autophagy, inhibition of the akt/mtor signaling pathway, and activation of death-associated protein kinase. Clin. Cancer Res. 2011, 17, 5353–5366. [Google Scholar] [CrossRef]
- Garcia-Rocha, M.; Bonay, P.; Avila, J. The antitumoral compound kahalalide f acts on cell lysosomes. Cancer Lett. 1996, 99, 43–50. [Google Scholar] [CrossRef]
- Hama, H. Fatty acid 2-hydroxylation in mammalian sphingolipid biology. Biochim. Biophys. Acta 2010, 1801, 405–414. [Google Scholar] [CrossRef]
- Hoshi, M.; Kishimoto, Y. Synthesis of cerebronic acid from lignoceric acid by rat brain preparation. Some properties and distribution of the -hydroxylation system. J. Biol. Chem. 1973, 248, 4123–4130. [Google Scholar]
- Davda, S.; Bezabeh, T. Advances in methods for assessing tumor hypoxia in vivo: Implications for treatment planning. Cancer Metastasis Rev. 2006, 25, 469–480. [Google Scholar] [CrossRef]
- Goldstein, R.F.; Stryer, L. Cooperative polymerization reactions. Analytical approximations, numerical examples, and experimental strategy. Biophys. J. 1986, 50, 583–599. [Google Scholar] [CrossRef]
- Guo, L.; Zhou, D.; Pryse, K.M.; Okunade, A.L.; Su, X. Fatty acid 2-hydroxylase mediates diffusional mobility of raft-associated lipids, glut4 level, and lipogenesis in 3t3-l1 adipocytes. J. Biol. Chem. 2010, 285, 25438–25447. [Google Scholar]
- Guo, L.; Zhang, X.; Zhou, D.; Okunade, A.L.; Su, X. Stereospecificity of fatty acid 2-hydroxylase and differential functions of 2-hydroxy fatty acid enantiomers. J. Lipid Res. 2012, 53, 1327–1335. [Google Scholar] [CrossRef]
- Behn, C.; Araneda, O.F.; Llanos, A.J.; Celedon, G.; Gonzalez, G. Hypoxia-related lipid peroxidation: Evidences, implications and approaches. Respir. Physiol. Neurobiol. 2007, 158, 143–150. [Google Scholar] [CrossRef]
- Bagchi, M.; Prasad, M.R.; Engelman, R.M.; Das, D.K. Effects of free radicals on the fluidity of myocardial membranes. Free Radic. Res. Commun. 1989, 7, 375–380. [Google Scholar] [CrossRef]
- Park, J.S.; Jung, T.S.; Noh, Y.H.; Kim, W.S.; Park, W.I.; Kim, Y.S.; Chung, I.K.; Sohn, U.D.; Bae, S.K.; Bae, M.K.; et al. The effect of lidocaine. Hcl on the fluidity of native and model membrane lipid bilayers. Korean J. Physiol. Pharmacol. 2012, 16, 413–422. [Google Scholar] [CrossRef]
- Edvardson, S.; Hama, H.; Shaag, A.; Gomori, J.M.; Berger, I.; Soffer, D.; Korman, S.H.; Taustein, I.; Saada, A.; Elpeleg, O. Mutations in the fatty acid 2-hydroxylase gene are associated with leukodystrophy with spastic paraparesis and dystonia. Am. J. Hum. Genet. 2008, 83, 643–648. [Google Scholar] [CrossRef]
- Zoller, I.; Meixner, M.; Hartmann, D.; Bussow, H.; Meyer, R.; Gieselmann, V.; Eckhardt, M. Absence of 2-hydroxylated sphingolipids is compatible with normal neural development but causes late-onset axon and myelin sheath degeneration. J. Neurosci. 2008, 28, 9741–9754. [Google Scholar] [CrossRef]
- Lopez, P.H.; Schnaar, R.L. Gangliosides in cell recognition and membrane protein regulation. Curr. Opin. Struct. Biol. 2009, 19, 549–557. [Google Scholar] [CrossRef]
- Yin, J.; Hashimoto, A.; Izawa, M.; Miyazaki, K.; Chen, G.Y.; Takematsu, H.; Kozutsumi, Y.; Suzuki, A.; Furuhata, K.; Cheng, F.L.; et al. Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer Res. 2006, 66, 2937–2945. [Google Scholar] [CrossRef]
- Yin, J.; Miyazaki, K.; Shaner, R.L.; Merrill, A.H., Jr.; Kannagi, R. Altered sphingolipid metabolism induced by tumor hypoxia—New vistas in glycolipid tumor markers. FEBS Lett. 2010, 584, 1872–1878. [Google Scholar] [CrossRef]
- Young, R.J.; Moller, A. Immunohistochemical detection of tumour hypoxia. Methods Mol. Biol. 2010, 611, 151–159. [Google Scholar]
- Pályi-Krekk, Z.; Barok, M.; Isola, J.; Tammi, M.; Szöllősi, J.; Nagy, P. Hyaluronan-induced masking of erbb2 and cd44-enhanced trastuzumab internalisation in trastuzumab resistant breast cancer. Eur. J. Cancer 2007, 43, 2423–2433. [Google Scholar] [CrossRef]
- Gonzalez, R.C.; Woods, R.E.; Eddins, S.L. Segmentation Using the Watershed Algorithm. In Digital Image Processing Using Matlab; Gonzalez, R.C., Woods, R.E., Eddins, S.L., Eds.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2004; pp. 417–425. [Google Scholar]
- Digman, M.A.; Dalal, R.; Horwitz, A.F.; Gratton, E. Mapping the number of molecules and brightness in the laser scanning microscope. Biophys. J. 2008, 94, 2320–2332. [Google Scholar] [CrossRef]
- Kuhry, J.G.; Fonteneau, P.; Duportail, G.; Maechling, C.; Laustriat, G. Tma-dph: A suitable fluorescence polarization probe for specific plasma membrane fluidity studies in intact living cells. Cell Biophys. 1983, 5, 129–140. [Google Scholar]
- Lakowicz, J.R. Fluorescence Anisotropy. In Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; pp. 353–382. [Google Scholar]
- Harris, F.M.; Best, K.B.; Bell, J.D. Use of laurdan fluorescence intensity and polarization to distinguish between changes in membrane fluidity and phospholipid order. Biochim. Biophys. Acta 2002, 1565, 123–128. [Google Scholar] [CrossRef]
- Sanchez, S.A.; Tricerri, M.A.; Gunther, G.; Gratton, G. Laurdan Generalized Polarization: From Cuvette to Microscope. In Modern Research and Educational Topics in Microscopy. Applications in Biology and Medicine; Méndez-Vilas, A., Díaz, J., Eds.; Formatex: Badajoz, Spain, 2007; Volume 1, pp. 1007–1014. [Google Scholar]
- Parasassi, T.; de Stasio, G.; Ravagnan, G.; Rusch, R.M.; Gratton, E. Quantitation of lipid phases in phospholipid vesicles by the generalized polarization of laurdan fluorescence. Biophys. J. 1991, 60, 179–189. [Google Scholar] [CrossRef]
- Szklenar, M.; Kalkowski, J.; Stangl, V.; Lorenz, M.; Rühl, R. Eicosanoids and docosanoids in plasma and aorta of healthy and atherosclerotic rabbits. J. Vasc. Res. 2013, 50, 372–382. [Google Scholar] [CrossRef]
- Rühl, R. Method to determine 4-oxo-retinoic acids, retinoic acids and retinol in serum and cell extracts by liquid chromatography/diode-array detection atmospheric pressure chemical ionisation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 2497–2504. [Google Scholar] [CrossRef]
Supplementary Files
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Király, A.; Váradi, T.; Hajdu, T.; Rühl, R.; Galmarini, C.M.; Szöllősi, J.; Nagy, P. Hypoxia Reduces the Efficiency of Elisidepsin by Inhibiting Hydroxylation and Altering the Structure of Lipid Rafts. Mar. Drugs 2013, 11, 4858-4875. https://doi.org/10.3390/md11124858
Király A, Váradi T, Hajdu T, Rühl R, Galmarini CM, Szöllősi J, Nagy P. Hypoxia Reduces the Efficiency of Elisidepsin by Inhibiting Hydroxylation and Altering the Structure of Lipid Rafts. Marine Drugs. 2013; 11(12):4858-4875. https://doi.org/10.3390/md11124858
Chicago/Turabian StyleKirály, Anna, Tímea Váradi, Tímea Hajdu, Ralph Rühl, Carlos M. Galmarini, János Szöllősi, and Peter Nagy. 2013. "Hypoxia Reduces the Efficiency of Elisidepsin by Inhibiting Hydroxylation and Altering the Structure of Lipid Rafts" Marine Drugs 11, no. 12: 4858-4875. https://doi.org/10.3390/md11124858
APA StyleKirály, A., Váradi, T., Hajdu, T., Rühl, R., Galmarini, C. M., Szöllősi, J., & Nagy, P. (2013). Hypoxia Reduces the Efficiency of Elisidepsin by Inhibiting Hydroxylation and Altering the Structure of Lipid Rafts. Marine Drugs, 11(12), 4858-4875. https://doi.org/10.3390/md11124858