Salmon Protein Hydrolysate Potentiates the Growth Inhibitory Effect of Bicalutamide on Human Prostate Cancer Cell Lines LNCaP and PC3 by Modulating Iron Homeostasis
Abstract
:1. Introduction
2. Results
2.1. Effects of SPH Monotreatment on the Proliferation of LNCaP and PC3 Cells
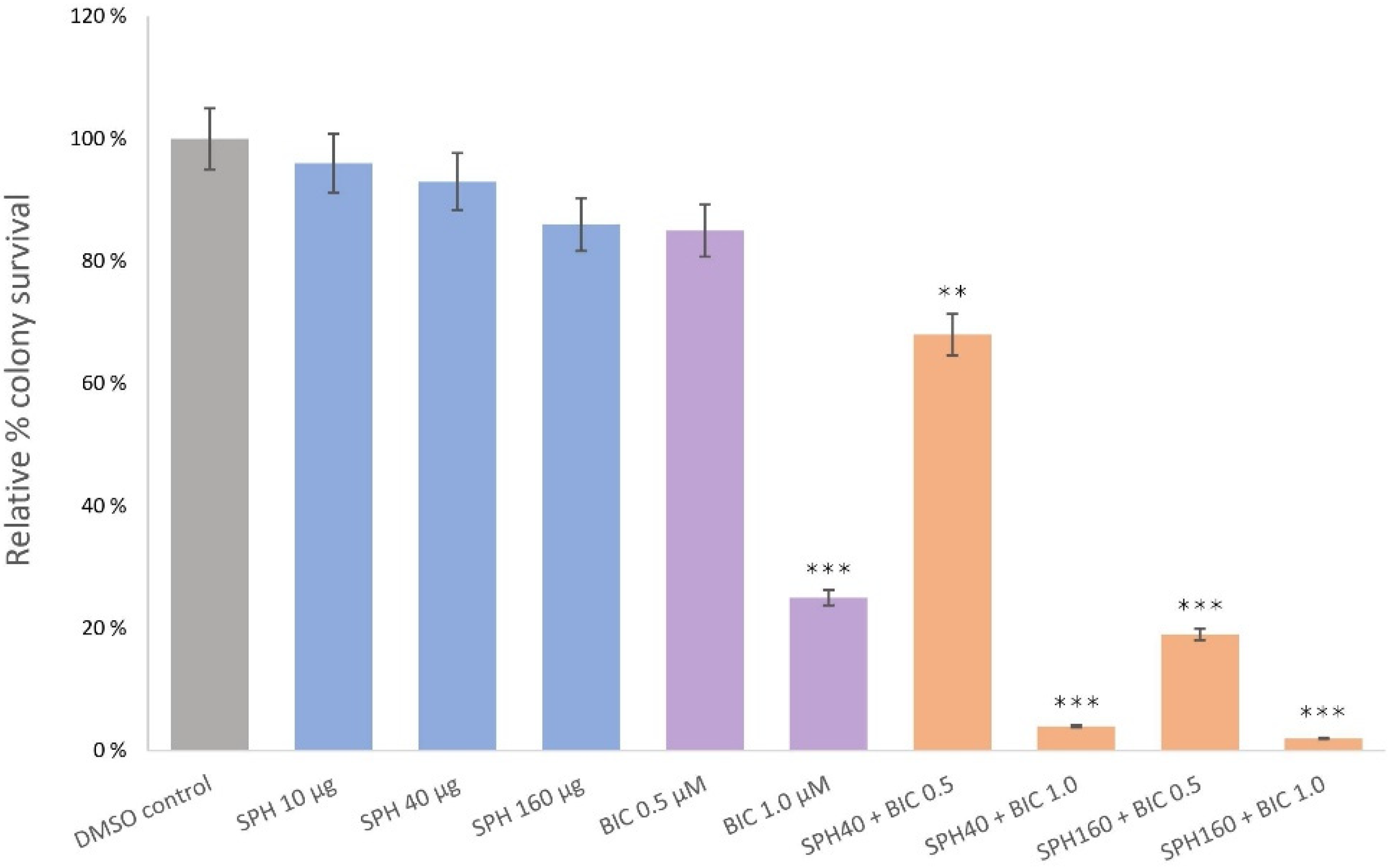
2.2. SPH and Bicalutamide Co-Treatment Significantly Reduces Relative Colony Survival of AR-Positive LNCaP Prostate Cancer Cells
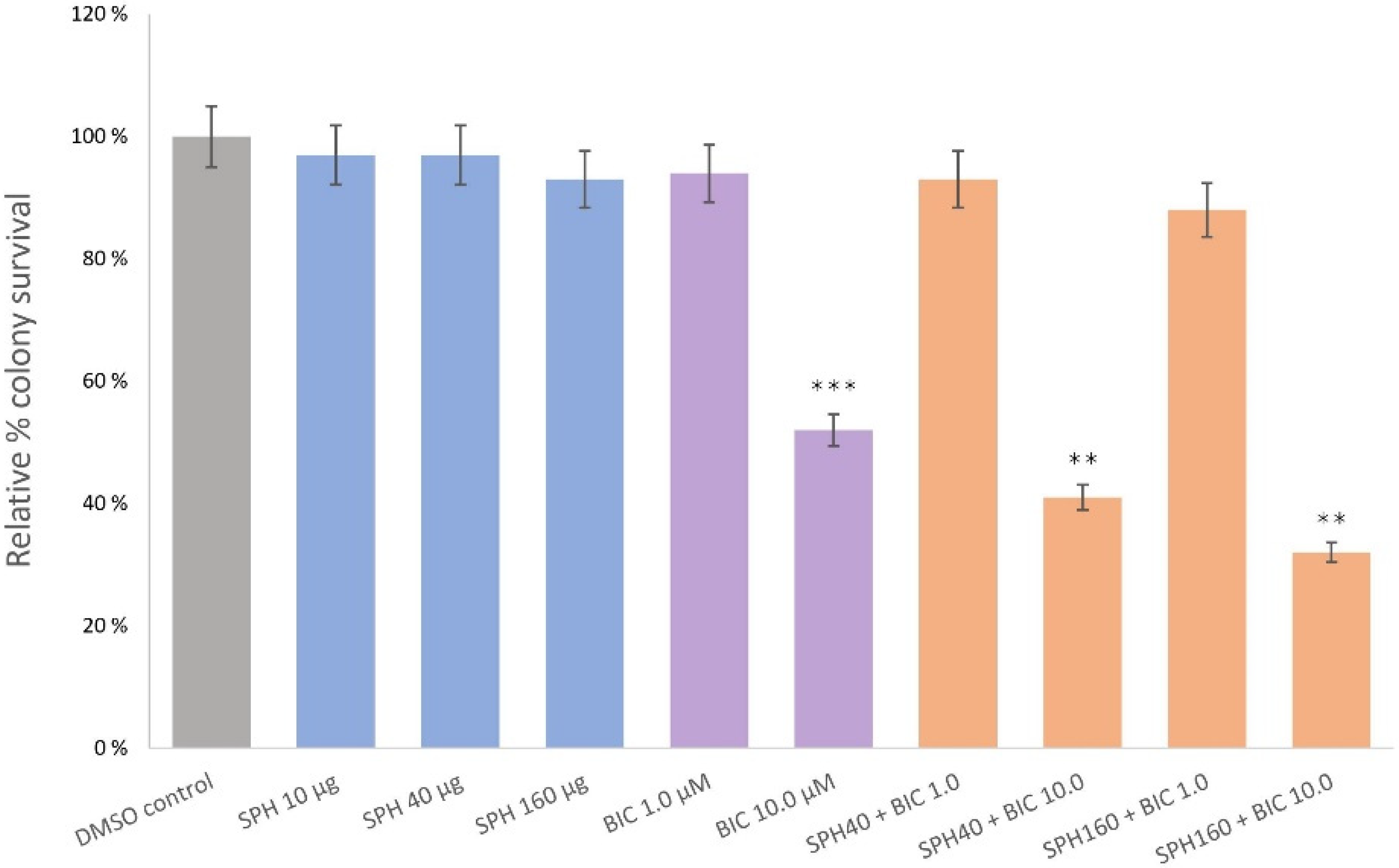
2.3. SPH and Bicalutamide Co-Treatment Significantly Reduces Relative Colony Survival of AR-Negative PC3 Prostate Cancer Cells
2.4. Gene Expression Profiles of AR-Positive LNCaP and AR-Negative Prostate Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Cell Culture Preparation
4.3. Test Solutions
4.4. Colony Formation Assay
4.5. Gene Expression Assay
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Sandhu, S.; Moore, C.M.; Chiong, E.; Beltran, H.; Bristow, R.G.; Williams, S.G. Prostate cancer. Lancet 2021, 398, 1075–1090. [Google Scholar] [CrossRef]
- Baade, P.D.; Youlden, D.R.; Krnjacki, L.J. International epidemiology of prostate cancer: Geographical distribution and secular trends. Mol. Nutr. Food Res. 2009, 53, 171–184. [Google Scholar] [CrossRef]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin. 1972, 22, 232–240. [Google Scholar] [CrossRef]
- Schröder, F.; Crawford, E.D.; Axcrona, K.; Payne, H.; Keane, T.E. Androgen deprivation therapy: Past, present and future. BJU Int. 2012, 109 (Suppl. 6), 1–12. [Google Scholar] [CrossRef]
- Ehsani, M.; David, F.O.; Baniahmad, A. Androgen Receptor-Dependent Mechanisms Mediating Drug Resistance in Prostate Cancer. Cancers 2021, 13, 1534. [Google Scholar] [CrossRef]
- Quigley, D.A.; Dang, H.X.; Zhao, S.G.; Lloyd, P.; Aggarwal, R.; Alumkal, J.J.; Foye, A.; Kothari, V.; Perry, M.D.; Bailey, A.M.; et al. Genomic Hallmarks and Structural Variation in Metastatic Prostate Cancer. Cell 2018, 174, 758–769.e9. [Google Scholar] [CrossRef] [Green Version]
- Iversen, P. Antiandrogen monotherapy: Indications and results. Urology 2002, 60 (Suppl. 1), 64–71. [Google Scholar] [CrossRef]
- Gourdin, T. Recent progress in treating advanced prostate cancer. Curr. Opin. Oncol. 2020, 32, 210–215. [Google Scholar] [CrossRef]
- Lelièvre, P.; Sancey, L.; Coll, J.-L.; Deniaud, A.; Busser, B. Iron Dysregulation in Human Cancer: Altered Metabolism, Biomarkers for Diagnosis, Prognosis, Monitoring and Rationale for Therapy. Cancers 2020, 12, 3524. [Google Scholar] [CrossRef]
- Guo, Q.; Li, L.; Hou, S.; Yuan, Z.; Li, C.; Zhang, W.; Zheng, L.; Li, X. The Role of Iron in Cancer Progression. Front. Oncol. 2021, 11, 778492. [Google Scholar] [CrossRef]
- Zhou, L.; Zhao, B.; Zhang, L.; Wang, S.; Dong, D.; Lv, H.; Shang, P. Alterations in Cellular Iron Metabolism Provide More Therapeutic Opportunities for Cancer. Int. J. Mol. Sci. 2018, 19, 1545. [Google Scholar] [CrossRef] [Green Version]
- Adachi, M.; Kai, K.; Yamaji, K.; Ide, T.; Noshiro, H.; Kawaguchi, A.; Aishima, S. Transferrin receptor 1 overexpression is associated with tumour de-differentiation and acts as a potential prognostic indicator of hepatocellular carcinoma. Histopathology 2019, 75, 63–73. [Google Scholar] [CrossRef]
- Shen, Y.; Li, X.; Dong, D.; Zhang, B.; Xue, Y.; Shang, P. Transferrin receptor 1 in cancer: A new sight for cancer therapy. Am. J. Cancer Res. 2018, 8, 916–931. [Google Scholar]
- Bordini, J.; Morisi, F.; Elia, A.R.; Santambrogio, P.; Pagani, A.; Cucchiara, V.; Ghia, P.; Bellone, M.; Briganti, A.; Camaschella, C.; et al. Iron Induces Cell Death and Strengthens the Efficacy of Antiandrogen Therapy in Prostate Cancer Models. Clin. Cancer Res. 2020, 26, 6387–6398. [Google Scholar] [CrossRef]
- Funauchi, Y.; Tanikawa, C.; Yi Lo, P.H.; Mori, J.; Daigo, Y.; Takano, A.; Miyagi, Y.; Okawa, A.; Nakamura, Y.; Matsuda, K. Regulation of iron homeostasis by the p53-ISCU pathway. Sci. Rep. 2015, 5, 16497. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.Q.; De Marchi, T.; Timmermans, A.M.; Beekhof, R.; Trapman-Jansen, A.M.; Foekens, R.; Look, M.P.; van Deurzen, C.H.; Span, P.N.; Sweep, F.C.; et al. Ferritin heavy chain in triple negative breast cancer: A favorable prognostic marker that relates to a cluster of differentiation 8 positive (CD8+) effector T-cell response. Mol. Cell. Proteom. 2014, 13, 1814–1827. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.J.; Kwok, Z.H.; Chew, X.H.; Zhang, B.; Liu, C.; Soong, T.W.; Yang, H.; Tay, Y. A FTH1 gene:pseudogene:microRNA network regulates tumorigenesis in prostate cancer. Nucleic Acids Res. 2018, 46, 1998–2011. [Google Scholar] [CrossRef]
- Tai, S.; Sun, Y.; Squires, J.M.; Zhang, H.; Oh, W.K.; Liang, C.-Z.; Huang, J. PC3 is a cell line characteristic of prostatic small cell carcinoma. Prostate 2011, 71, 1668–1679. [Google Scholar] [CrossRef] [Green Version]
- Falanga, A.; Lombardi, L.; Galdiero, E.; Genio, V.D.; Galdiero, S. The world of cell penetrating: The future of medical applications. Future Med. Chem. 2020, 12, 1431–1446. [Google Scholar] [CrossRef]
- Framroze, B.; Havaldar, F.; Misal, S. An in-vitro study on the regulation of oxidative protective genes in human gingival and intestinal epithelial cells after treatment with salmon protein hydrolysate peptides. Funct. Foods Health Dis. 2018, 8, 398–411. [Google Scholar]
- Framroze, B. A Placebo-Controlled Study of the Impact of Dietary Salmon Protein Hydrolysate Supplementation in Increasing Ferritin and Hemoglobin Levels in Iron-Deficient Anemic Subjects. J. Nutr. Food Sci. 2015, 5, 2. [Google Scholar]
- Tesfay, L.; Clausen, K.A.; Kim, J.W.; Hegde, P.; Wang, X.; Miller, L.D.; Deng, Z.; Blanchette, N.; Arvedson, T.; Miranti, C.K.; et al. Hepcidin regulation in prostate and its disruption in prostate cancer. Cancer Res. 2015, 75, 2254–2263. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Shafarin, J.; Abu Jabal, R.; Aljabi, N.; Hamad, M.; Sualeh Muhammad, J.; Unnikannan, H.; Hamad, M. Ferritin heavy chain (FTH1) exerts significant antigrowth effects in breast cancer cells by inhibiting the expression of c-MYC. FEBS Open bio 2021, 11, 3101–3114. [Google Scholar] [CrossRef]
- Hentze, M.W.; Muckenthaler, M.U.; Galy, B.; Camaschella, C. Two to Tango: Regulation of Mammalian Iron Metabolism. Cell 2010, 142, 24–38. [Google Scholar] [CrossRef] [Green Version]
- Udenigwe, C.C.; Abioye, R.O.; Okagu, I.U.; Obeme-Nmom, J.I. Bioaccessibility of bioactive peptides: Recent advances and perspectives. Curr. Opin. Food Sci. 2021, 39, 182–189. [Google Scholar] [CrossRef]
- Pavlicevic, M.; Maestri, E.; Marmiroli, M. Marine Bioactive Peptides—An Overview of Generation, Structure and Application with a Focus on Food Sources. Mar. Drugs 2020, 18, 424. [Google Scholar] [CrossRef]
- Feng, S.; Tang, Q.; Sun, M.; Chun, J.Y.; Evans, C.P.; Gao, A.C. Interleukin-6 increases prostate cancer cells resistance to bicalutamide via TIF2. Mol. Cancer Ther. 2009, 8, 665–671. [Google Scholar] [CrossRef] [Green Version]
- Colquhoun, A.J.; Venier, N.A.; Vandersluis, A.D.; Besla, R.; Sugar, L.M.; Kiss, A.; Fleshner, N.E.; Pollak, M.; Klotz, L.H.; Venkateswaran, V. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis. 2012, 15, 346–352. [Google Scholar] [CrossRef] [Green Version]
| Cell Line | Treatment | Gene | Fold Change | Average | SD | ||
|---|---|---|---|---|---|---|---|
| LNCaP | 1 µM BIC | FTH1 | 1.0 | 1.1 | 1.0 | 1.0 | 0.1 |
| 1 µM BIC | TFRC | 1.0 | 1.0 | 1.2 | 1.1 | 0.1 | |
| LNCaP | 40 µg/mL + 1 µM BIC | FTH1 | 2.3 | 2.2 | 2.3 | 2.3 | 0.1 |
| 40 µg/mL + 1 µM BIC | TFRC | 0.5 | 0.6 | 0.5 | 0.5 | 0.1 | |
| LNCaP | 160 µg/mL + 1 µM BIC | FTH1 | 2.8 | 3.0 | 2.7 | 2.8 | 0.2 |
| 160 µg/mL + 1 µM BIC | TFRC | 0.3 | 0.4 | 0.5 | 0.4 | 0.1 | |
| PC3 | 10 µM BIC | FTH1 | 0.9 | 1.0 | 1.2 | 1.0 | 0.2 |
| 10 µM BIC | TFRC | 1.0 | 1.1 | 1.2 | 1.1 | 0.1 | |
| PC3 | 40 µg/mL + 10 µM BIC 40 µg/mL + 10 µM BIC | FTH1 TFRC | 2.2 | 2.4 | 2.5 | 2.4 | 0.2 |
| 0.5 | 0.5 | 0.4 | 0.5 | 0.1 | |||
| PC3 | 160 µg/mL + 10 µM BIC 160 µg/mL + 10 µM BIC | FTH1 TFRC | 2.8 | 2.6 | 2.4 | 2.6 | 0.2 |
| 0.3 | 0.3 | 0.4 | 0.3 | 0.1 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjerknes, C.; Framroze, B.; Currie, C.; Pettersen, C.H.H.; Axcrona, K.; Hermansen, E. Salmon Protein Hydrolysate Potentiates the Growth Inhibitory Effect of Bicalutamide on Human Prostate Cancer Cell Lines LNCaP and PC3 by Modulating Iron Homeostasis. Mar. Drugs 2022, 20, 228. https://doi.org/10.3390/md20040228
Bjerknes C, Framroze B, Currie C, Pettersen CHH, Axcrona K, Hermansen E. Salmon Protein Hydrolysate Potentiates the Growth Inhibitory Effect of Bicalutamide on Human Prostate Cancer Cell Lines LNCaP and PC3 by Modulating Iron Homeostasis. Marine Drugs. 2022; 20(4):228. https://doi.org/10.3390/md20040228
Chicago/Turabian StyleBjerknes, Christian, Bomi Framroze, Crawford Currie, Caroline Hild Hakvåg Pettersen, Karol Axcrona, and Erland Hermansen. 2022. "Salmon Protein Hydrolysate Potentiates the Growth Inhibitory Effect of Bicalutamide on Human Prostate Cancer Cell Lines LNCaP and PC3 by Modulating Iron Homeostasis" Marine Drugs 20, no. 4: 228. https://doi.org/10.3390/md20040228
APA StyleBjerknes, C., Framroze, B., Currie, C., Pettersen, C. H. H., Axcrona, K., & Hermansen, E. (2022). Salmon Protein Hydrolysate Potentiates the Growth Inhibitory Effect of Bicalutamide on Human Prostate Cancer Cell Lines LNCaP and PC3 by Modulating Iron Homeostasis. Marine Drugs, 20(4), 228. https://doi.org/10.3390/md20040228







