The Anticancer Effect of (1S,2S,3E,7E,11E)-3,7,11, 15-Cembratetraen-17,2-olide(LS-1) through the Activation of TGF-β Signaling in SNU-C5/5-FU, Fluorouracil-Resistant Human Colon Cancer Cells
Abstract
:1. Introduction
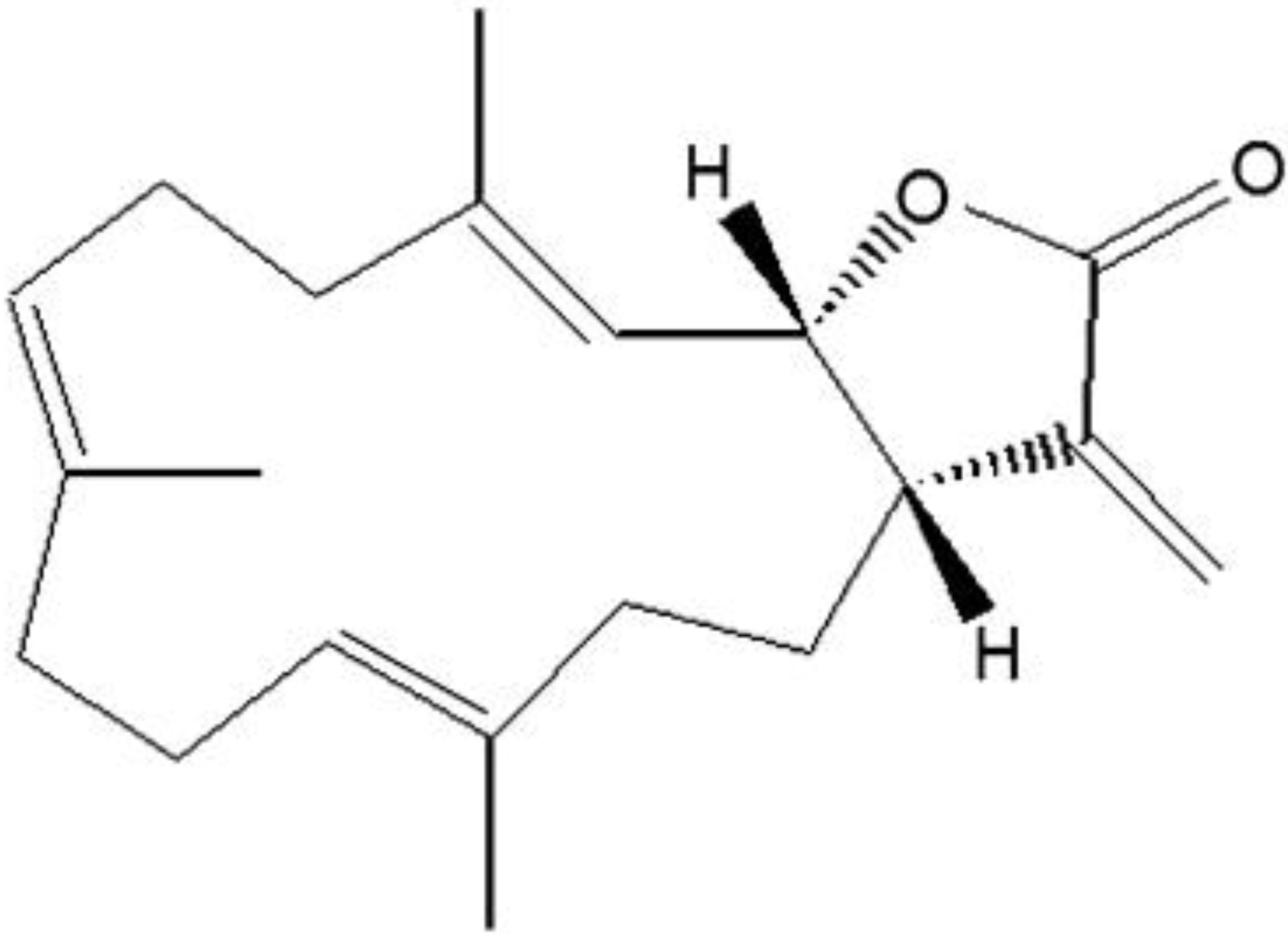
2. Results and Discussion
2.1. Results
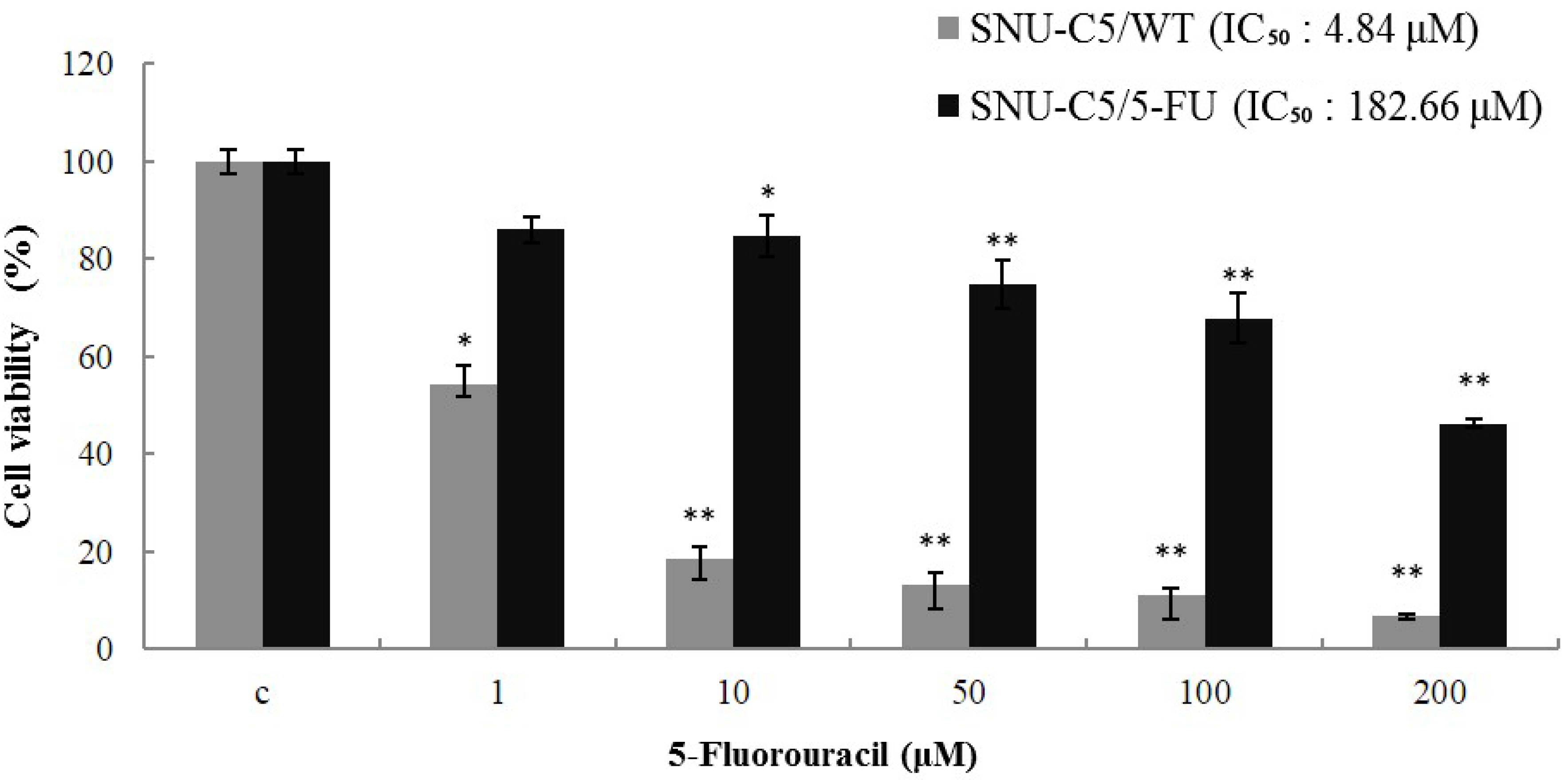
2.1.1. Effect of LS-1 on the Growth of SNU-C5/5-FU

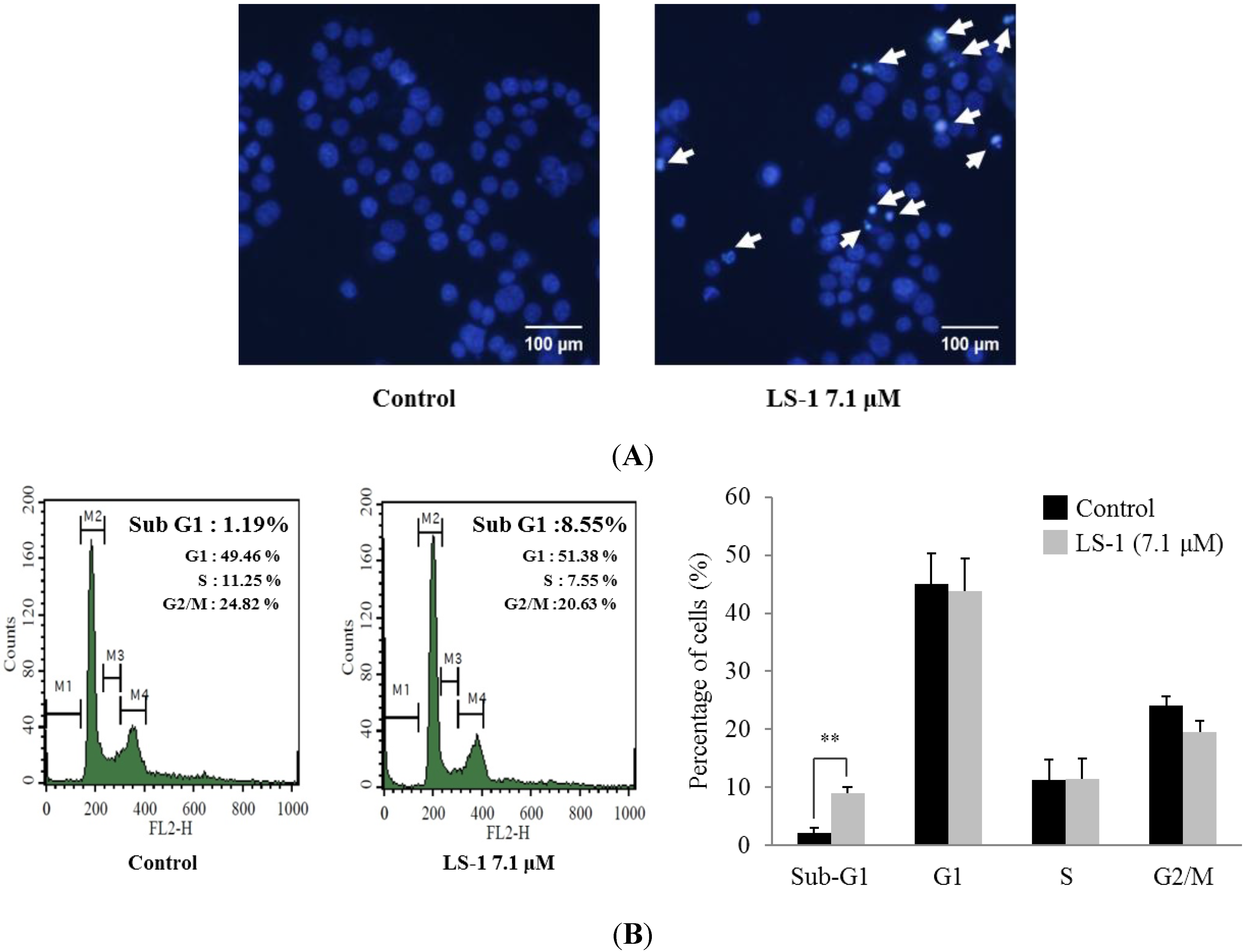
2.1.2. Effect of LS-1 on the Apoptosis Induction of SNU-C5/5-FU Cells

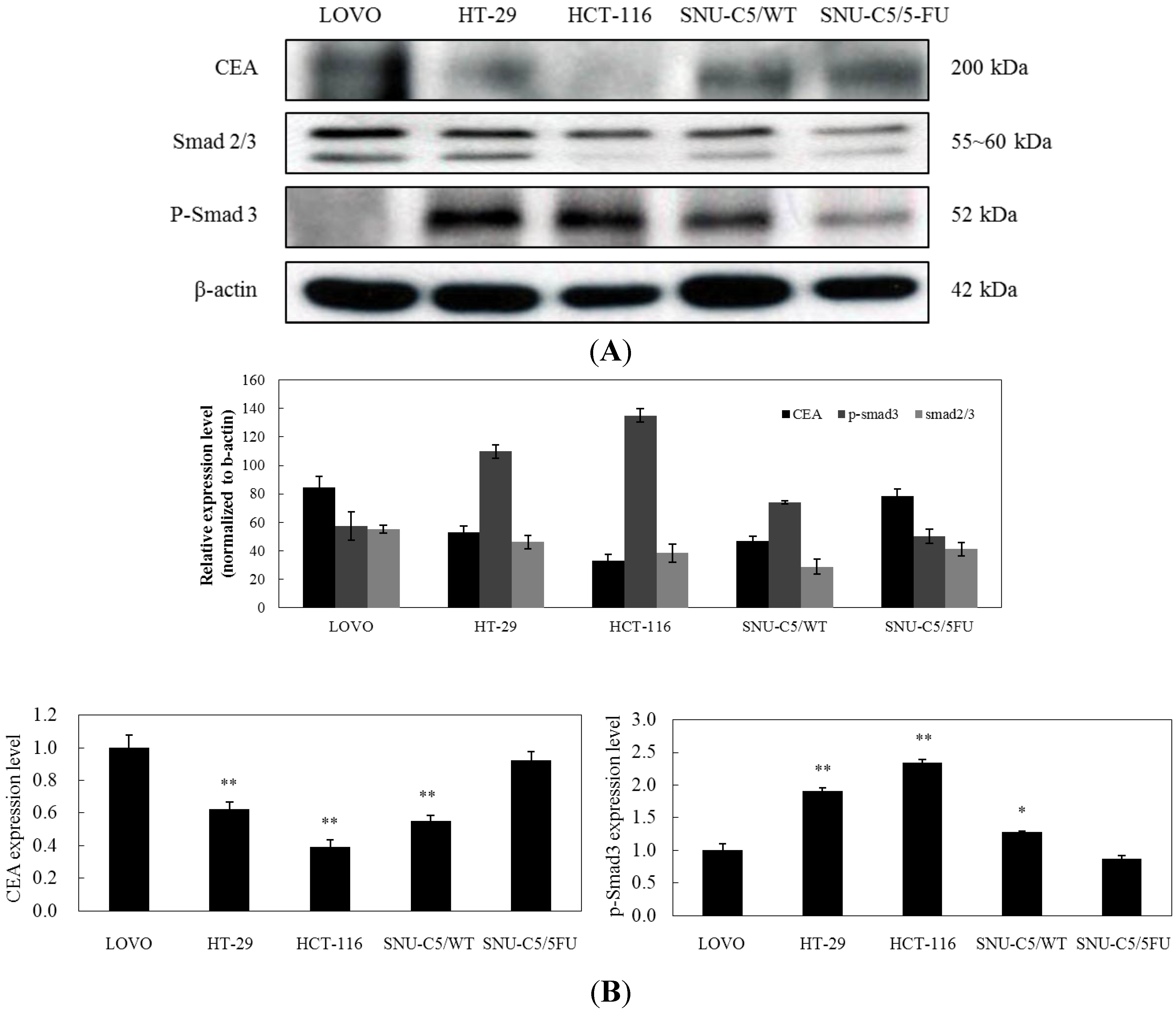
2.1.3. Effect of LS-1 on the TGF-β Signaling in SNU-C5/5-FU
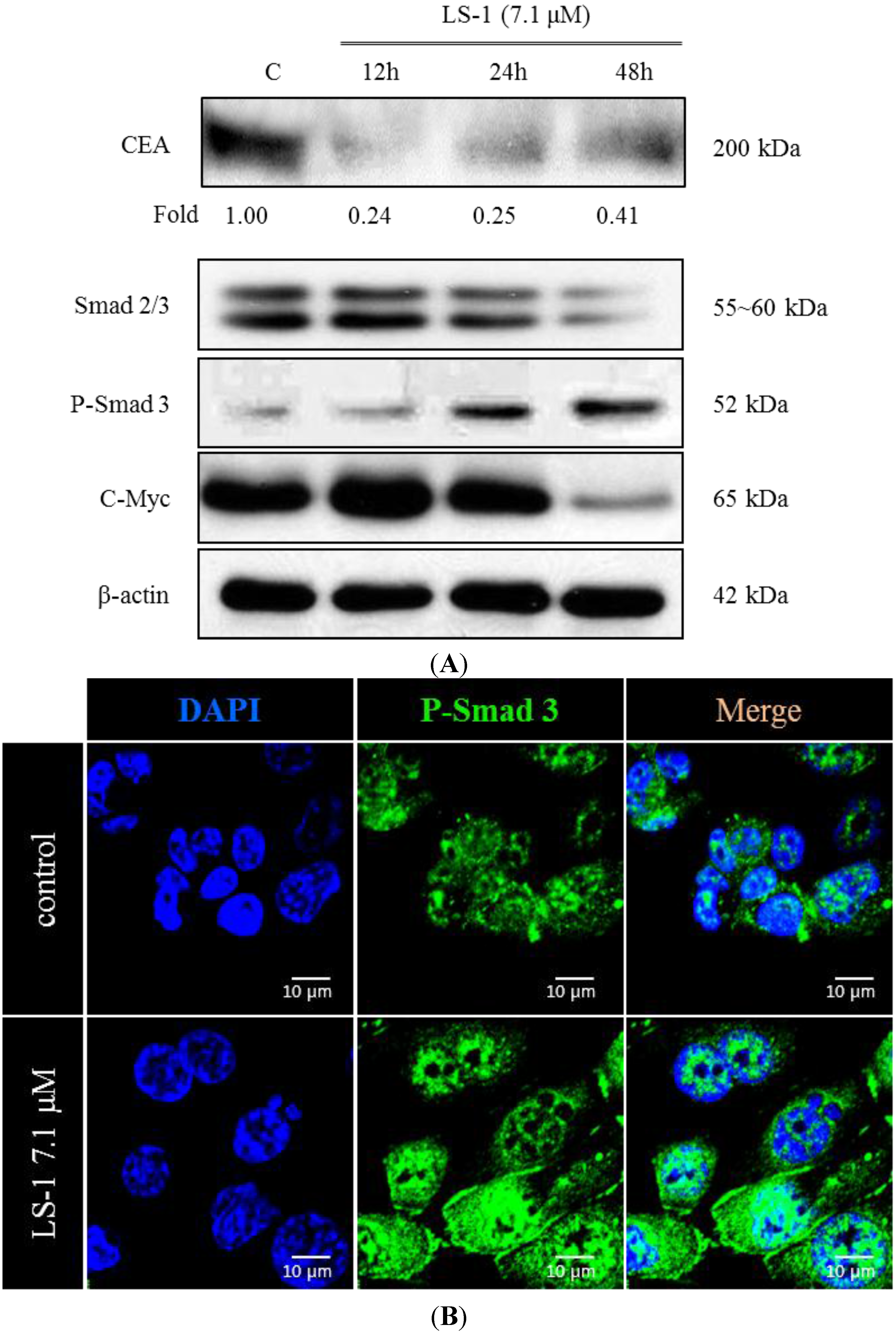
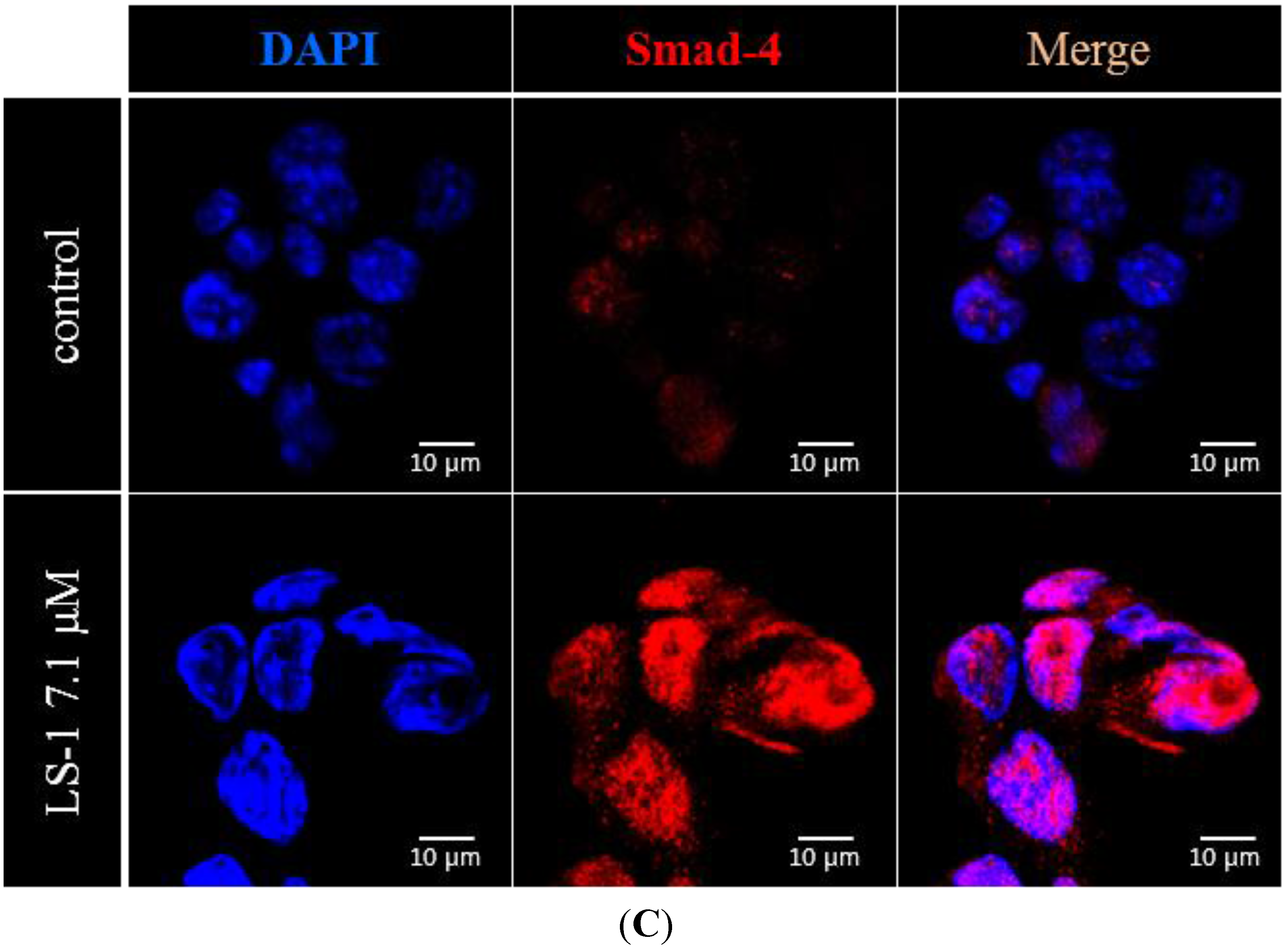
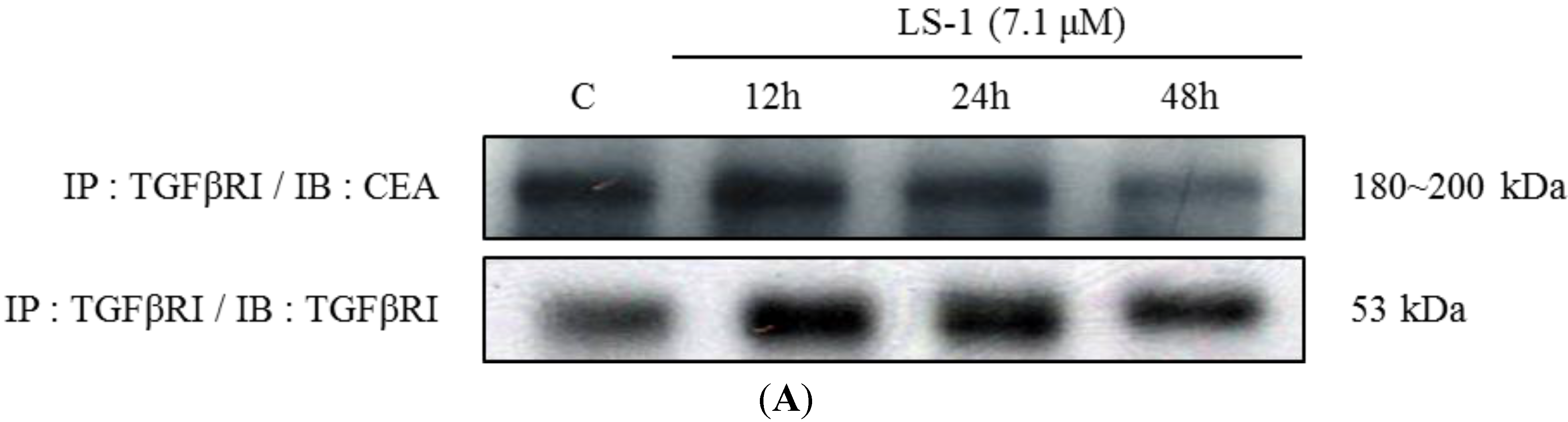
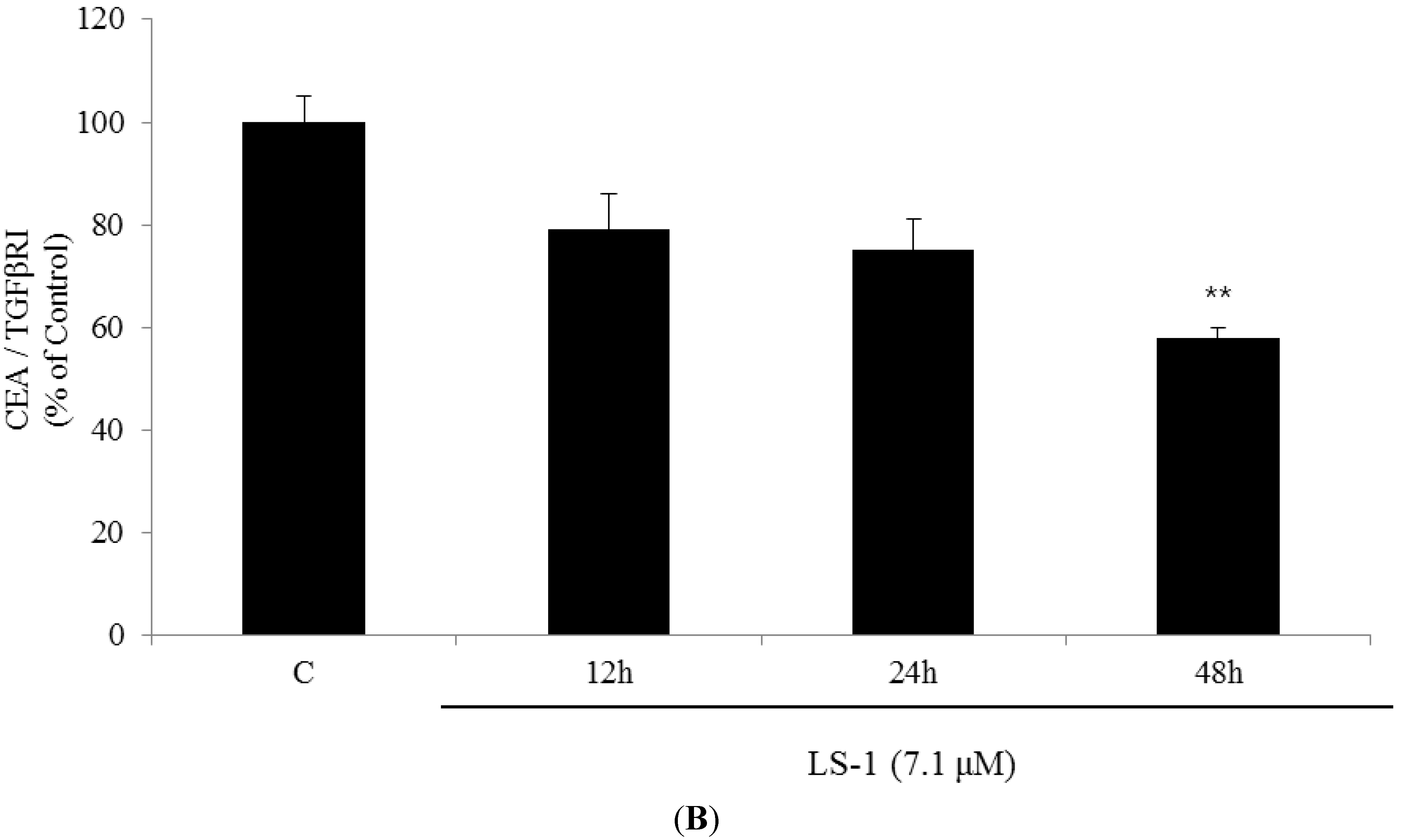
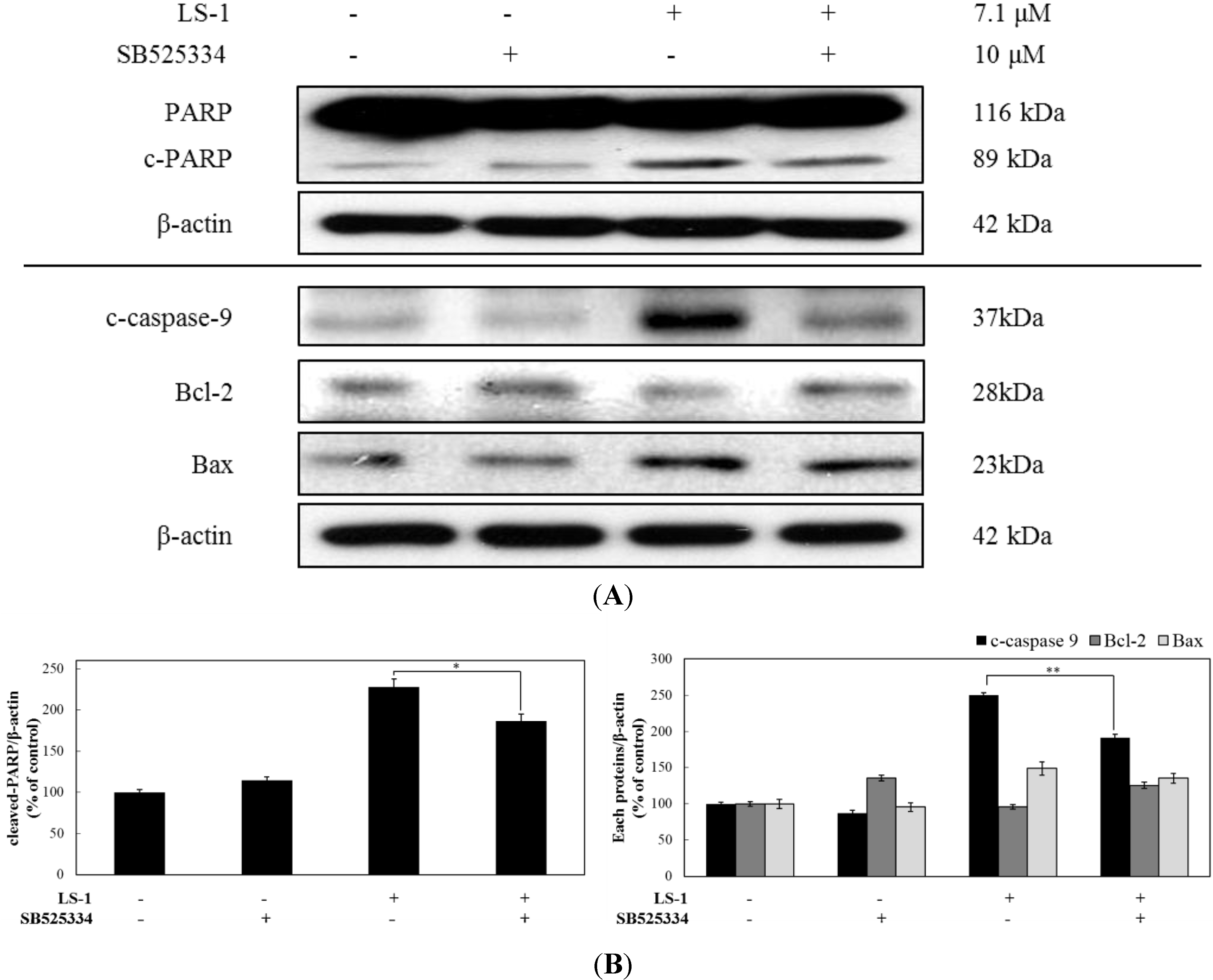
2.1.4. LS-1 Induced Apoptosis of SNU-C5/5-FU via Activation of TGF-β Signaling
2.2. Discussion
3. Experimental Section
3.1. Materials
3.2. Cell Culture
3.3. Cell Viability Assay
3.4. Morphological Analysis of Apoptosis by Hoechst 33342 Staining
3.5. Flow Cytometric Analysis of Apoptosis
3.6. Western Blot Analysis
3.7. Co-Immunoprecipitation Assay
3.8. Confocal Microscopy
3.9. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jemal, A.; Siegel, R.; Ward, E.; Hao, Y.; Xu, J.; Murray, T.; Thun, M.J. Cancer Statistics, 2008. CA Cancer J. Clin. 2008, 58, 71–96. [Google Scholar] [CrossRef] [PubMed]
- Masi, G.; Allegrini, G.; Cupini, S.; Marcucci, L.; Cerri, E.; Brunetti, I.; Fontana, E.; Ricci, S.; Andreuccetti, M.; Falcone, A. First-Line Treatment of Metastatic Colorectal Cancer with Irinotecan, Oxaliplatin and 5-Fluorouracil/Leucovorin (FOLFOXIRI): Results of a Phase II Study with a Simplified Biweekly Schedule. Ann. Oncol. 2004, 15, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Grivicich, I.; Mans, D.R.; Peters, G.J.; Schwartsmann, G. Irinotecan and Oxaliplatin: An Overview of the Novel Chemotherapeutic Options for the Treatment of Advanced Colorectal Cancer. Braz. J. Med. Biol. Res. 2001, 34, 1087–1103. [Google Scholar] [CrossRef] [PubMed]
- Jette, L.; Bissoon-Haqqani, S.; le Francois, B.; Maroun, J.A.; Birnboim, H.C. Resistance of Colorectal Cancer Cells to 5-FUdR and 5-FU Caused by Mycoplasma Infection. Anticancer Res. 2008, 28, 2175–2180. [Google Scholar] [PubMed]
- Lee, H.C.; Ling, Q.D.; Yu, W.C.; Hung, C.M.; Kao, T.C.; Huang, Y.W.; Higuchi, A. Drug-Resistant Colon Cancer Cells Produce High Carcinoembryonic Antigen and might Not be Cancer-Initiating Cells. Drug Des. Dev. Ther. 2013, 7, 491–502. [Google Scholar]
- Correale, P.; Aquino, A.; Giuliani, A.; Pellegrini, M.; Micheli, L.; Cusi, M.G.; Nencini, C.; Petrioli, R.; Prete, S.P.; de Vecchis, L.; et al. Treatment of Colon and Breast Carcinoma Cells with 5-Fluorouracil Enhances Expression of Carcinoembryonic Antigen and Susceptibility to HLA-A(*)02.01 Restricted, CEA-Peptide-Specific Cytotoxic T Cells in Vitro. Int. J. Cancer 2003, 104, 437–445. [Google Scholar] [CrossRef]
- Aquino, A.; Prete, S.P.; Guadagni, F.; Greiner, J.W.; Giuliani, A.; Orlando, L.; Masci, G.; de Santis, S.; Bonmassar, E.; Graziani, G. Effect of 5-Fluorouracil on Carcinoembryonic Antigen Expression and Shedding at Clonal Level in Colon Cancer Cells. Anticancer Res. 2000, 20, 3475–3484. [Google Scholar] [PubMed]
- Samara, R.N.; Laguinge, L.M.; Jessup, J.M. Carcinoembryonic Antigen Inhibits Anoikis in Colorectal Carcinoma Cells by Interfering with TRAIL-R2 (DR5) Signaling. Cancer Res. 2007, 67, 4774–4782. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Leal, P.; Stanners, C.P. The Human Carcinoembryonic Antigen (CEA) GPI Anchor Mediates Anoikis Inhibition by Inactivation of the Intrinsic Death Pathway. Oncogene 2008, 27, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cao, H.; Jiao, Z.; Pakala, S.B.; Sirigiri, D.N.; Li, W.; Kumar, R.; Mishra, L. Carcinoembryonic Antigen Interacts with TGF-{Beta} Receptor and Inhibits TGF-{Beta} Signaling in Colorectal Cancers. Cancer Res. 2010, 70, 8159–8168. [Google Scholar] [CrossRef] [PubMed]
- Nicklas, D.; Saiz, L. Characterization of Negative Feedback Network Motifs in the TGF-Beta Signaling Pathway. PLoS One 2013, 8, e83531. [Google Scholar] [CrossRef] [PubMed]
- Sheen, Y.Y.; Kim, M.J.; Park, S.A.; Park, S.Y.; Nam, J.S. Targeting the Transforming Growth Factor-Beta Signaling in Cancer Therapy. Biomol. Ther. (Seoul) 2013, 21, 323–331. [Google Scholar] [CrossRef]
- Thuault, S.; Valcourt, U.; Petersen, M.; Manfioletti, G.; Heldin, C.H.; Moustakas, A. Transforming Growth Factor-Beta Employs HMGA2 to Elicit Epithelial-Mesenchymal Transition. J. Cell Biol. 2006, 174, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Portella, G.; Cumming, S.A.; Liddell, J.; Cui, W.; Ireland, H.; Akhurst, R.J.; Balmain, A. Transforming Growth Factor Beta is Essential for Spindle Cell Conversion of Mouse Skin Carcinoma in Vivo: Implications for Tumor Invasion. Cell Growth Differ. 1998, 9, 393–404. [Google Scholar] [PubMed]
- Sun, L.; Wu, G.; Willson, J.K.; Zborowska, E.; Yang, J.; Rajkarunanayake, I.; Wang, J.; Gentry, L.E.; Wang, X.F.; Brattain, M.G. Expression of Transforming Growth Factor Beta Type II Receptor Leads to Reduced Malignancy in Human Breast Cancer MCF-7 Cells. J. Biol. Chem. 1994, 269, 26449–26455. [Google Scholar] [PubMed]
- Kim, S.J.; Im, Y.H.; Markowitz, S.D.; Bang, Y.J. Molecular Mechanisms of Inactivation of TGF-Beta Receptors during Carcinogenesis. Cytokine Growth Factor Rev. 2000, 11, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Chau, V.M.; Phan, V.K.; Hoang, T.H.; Nguyen, H.N.; Nguyen, X.C.; Tran, H.Q.; Nguyen, X.N.; Hyun, J.H.; Kang, H.K.; et al. Chemical Components from the Vietnamese Soft Coral Lobophytum sp. Arch. Pharm. Res. 2010, 33, 503–508. [Google Scholar] [CrossRef]
- Hong, J.Y.; Boo, H.J.; Kang, J.I.; Kim, M.K.; Yoo, E.S.; Hyun, J.W.; Koh, Y.S.; Kim, G.Y.; Maeng, Y.H.; Hyun, C.L.; et al. (1S,2S,3E,7E,11E)-3,7,11,15-Cembratetraen-17,2-Olide, a Cembrenolide Diterpene from Soft Coral Lobophytum sp., Inhibits Growth and Induces Apoptosis in Human Colon Cancer Cells through Reactive Oxygen Species Generation. Biol. Pharm. Bull. 2012, 35, 1054–1063. [Google Scholar] [CrossRef]
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; Kroemer, G. Mitochondrial Control of Cellular Life, Stress, and Death. Circ. Res. 2012, 111, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Seal, S.; Chatterjee, P.; Bhattacharya, S.; Pal, D.; Dasgupta, S.; Kundu, R.; Mukherjee, S.; Bhattacharya, S.; Bhuyan, M.; Bhattacharyya, P.R.; et al. Vapor of Volatile Oils from Litsea Cubeba Seed Induces Apoptosis and Causes Cell Cycle Arrest in Lung Cancer Cells. PLoS One 2012, 7, e47014. [Google Scholar] [CrossRef]
- Han, S.U.; Kwak, T.H.; Her, K.H.; Cho, Y.H.; Choi, C.; Lee, H.J.; Hong, S.; Park, Y.S.; Kim, Y.S.; Kim, T.A.; et al. CEACAM5 and CEACAM6 are Major Target Genes for Smad3-Mediated TGF-Beta Signaling. Oncogene 2008, 27, 675–683. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Tobon, A.; Varani, J.; Brattain, M.G. Induction of Carcinoembryonic Antigen Secretion and Modulation of Protein Secretion/Expression and Fibronectin/Laminin Expression in Human Colon Carcinoma Cells by Transforming Growth Factor-Beta. Cancer Res. 1988, 48, 4059–4064. [Google Scholar] [PubMed]
- Fabregat, I.; Fernando, J.; Mainez, J.; Sancho, P. TGF-Beta Signaling in Cancer Treatment. Curr. Pharm. Des. 2014, 20, 2934–2947. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.S.; Duh, C.Y.; Chiang, M.Y.; Lin, C.N. Sinugibberol, a new cytotoxic cembranoid diterpene from the soft coral Sinularia gibberosa. J. Nat. Prod. 1995, 58, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- Duh, C.Y.; Wang, S.K.; Weng, Y.L.; Chiang, M.Y.; Dai, C.F. Cytotoxic terpenoids from the Formosan soft coral Nephthea brassica. J. Nat. Prod. 1999, 62, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Duh, C.Y.; Wang, S.K.; Huang, B.T.; Dai, C.F. Cytotoxic Cembrenolide Diterpenes from the Formosan Soft Coral Lobophytum Crassum. J. Nat. Prod. 2000, 63, 884–885. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Yin, J.; Jiang, W.; Ma, M.; Lei, X.; Xiang, Z.; Dong, J.; Huang, K.; Yan, P. Cytotoxic and Antibacterial Cembranoids from a South China Sea Soft Coral, Lobophytum sp. Mar. Drugs 2013, 11, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Al-Lihaibi, S.S.; Alarif, W.M.; Abdel-Lateff, A.; Ayyad, S.E.; Abdel-Naim, A.B.; El-Senduny, F.F.; Badria, F.A. Three new cembranoid-type diterpenes from Red Sea soft coral Sarcophyton glaucum: Isolation and antiproliferative activity against HepG2 cells. Eur. J. Med. Chem. 2014, 81, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Elkhateeb, A.; El-Beih, A.A.; Gamal-Eldeen, A.M.; Alhammady, M.A.; Ohta, S.; Pare, P.W.; Hegazy, M.E. New terpenes from the Egyptian soft coral Sarcophyton shrenbergi. Mar. Drugs 2014, 12, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.W.; Reed, J.C. Bcl-2 Family Proteins and Cancer. Oncogene 2008, 27, 6398–6406. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Guerrero, A.D.; Huang, L.; Shabier, Z.; Pan, M.; Tan, T.H.; Wang, J. Caspase-9-induced mitochondrial disruption through cleavage of anti-apoptotic BCL-2 family members. J. Biol. Chem. 2007, 282, 33888–33895. [Google Scholar] [CrossRef] [PubMed]
- Bhola, N.E.; Balko, J.M.; Dugger, T.C.; Kuba, M.G.; Sanchez, V.; Sanders, M.; Stanford, J.; Cook, R.S.; Arteaga, C.L. TGF-Beta Inhibition Enhances Chemotherapy Action Against Triple-Negative Breast Cancer. J. Clin. Investig. 2013, 123, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Hoosein, N.M.; McKnight, M.K.; Levine, A.E.; Mulder, K.M.; Childress, K.E.; Brattain, D.E.; Brattain, M.G. Differential Sensitivity of Subclasses of Human Colon Carcinoma Cell Lines to the Growth Inhibitory Effects of Transforming Growth Factor-Beta 1. Exp. Cell Res. 1989, 181, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Soeth, E.; Wirth, T.; List, H.J.; Kumbhani, S.; Petersen, A.; Neumaier, M.; Czubayko, F.; Juhl, H. Controlled Ribozyme Targeting Demonstrates an Antiapoptotic Effect of Carcinoembryonic Antigen in HT29 Colon Cancer Cells. Clin. Cancer Res. 2001, 7, 2022–2030. [Google Scholar] [PubMed]
- Jang, C.W.; Chen, C.H.; Chen, C.C.; Chen, J.Y.; Su, Y.H.; Chen, R.H. TGF-Beta Induces Apoptosis through Smad-Mediated Expression of DAP-Kinase. Nat. Cell Biol. 2002, 4, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Rhyu, D.Y.; Park, J.; Sharma, B.R.; Ha, H. Role of reactive oxygen species in transforming growth factor-beta1-induced extracellular matrix accumulation in renal tubular epithelial cells. PLoS One 2012, 44, 625–628. [Google Scholar]
- Yoon, Y.S.; Lee, J.H.; Hwang, S.C.; Choi, K.S.; Yoon, G. TGF beta1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene 2005, 24, 1895–1903. [Google Scholar] [CrossRef] [PubMed]
- Datto, M.B.; Yu, Y.; Wang, X.F. Functional analysis of the transforming growth factor beta responsive elements in the WAF1/Cip1/p21 promoter. J. Biol. Chem. 1995, 270, 28623–28628. [Google Scholar] [CrossRef] [PubMed]
- Masgras, I.; Carrera, S.; de Verdier, P.J.; Brennan, P.; Majid, A.; Makhtar, W.; Tulchinsky, E.; Jones, G.D.; Roninson, I.B.; Macip, S. Reactive oxygen species and mitochondrial sensitivity to oxidative stress determine induction of cancer cell death by p21. J. Biol. Chem. 2012, 287, 9845–9854. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Cao, Z.; Xu, X.; van Meir, E.G.; Lambeth, J.D. Homologs of gp91phox: Cloning and tissue expression of Nox3, Nox4, and Nox5. Gene 2001, 269, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a Soluble Tetrazolium/Formazan Assay for Cell Growth and Drug Sensitivity in Culture using Human and Other Tumor Cell Lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Fried, J.; Perez, A.G.; Clarkson, B.D. Flow Cytofluorometric Analysis of Cell Cycle Distributions using Propidium Iodide. Properties of the Method and Mathematical Analysis of the Data. J. Cell Biol. 1976, 71, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, E.-J.; Kang, J.-I.; Kwak, J.-W.; Jeon, C.-H.; Tung, N.-H.; Kim, Y.-H.; Choi, C.-H.; Hyun, J.-W.; Koh, Y.-S.; Yoo, E.-S.; et al. The Anticancer Effect of (1S,2S,3E,7E,11E)-3,7,11, 15-Cembratetraen-17,2-olide(LS-1) through the Activation of TGF-β Signaling in SNU-C5/5-FU, Fluorouracil-Resistant Human Colon Cancer Cells. Mar. Drugs 2015, 13, 1340-1359. https://doi.org/10.3390/md13031340
Kim E-J, Kang J-I, Kwak J-W, Jeon C-H, Tung N-H, Kim Y-H, Choi C-H, Hyun J-W, Koh Y-S, Yoo E-S, et al. The Anticancer Effect of (1S,2S,3E,7E,11E)-3,7,11, 15-Cembratetraen-17,2-olide(LS-1) through the Activation of TGF-β Signaling in SNU-C5/5-FU, Fluorouracil-Resistant Human Colon Cancer Cells. Marine Drugs. 2015; 13(3):1340-1359. https://doi.org/10.3390/md13031340
Chicago/Turabian StyleKim, Eun-Ji, Jung-Il Kang, Jeon-Won Kwak, Chan-Hee Jeon, Nguyen-Huu Tung, Young-Ho Kim, Cheol-Hee Choi, Jin-Won Hyun, Young-Sang Koh, Eun-Sook Yoo, and et al. 2015. "The Anticancer Effect of (1S,2S,3E,7E,11E)-3,7,11, 15-Cembratetraen-17,2-olide(LS-1) through the Activation of TGF-β Signaling in SNU-C5/5-FU, Fluorouracil-Resistant Human Colon Cancer Cells" Marine Drugs 13, no. 3: 1340-1359. https://doi.org/10.3390/md13031340
APA StyleKim, E.-J., Kang, J.-I., Kwak, J.-W., Jeon, C.-H., Tung, N.-H., Kim, Y.-H., Choi, C.-H., Hyun, J.-W., Koh, Y.-S., Yoo, E.-S., & Kang, H.-K. (2015). The Anticancer Effect of (1S,2S,3E,7E,11E)-3,7,11, 15-Cembratetraen-17,2-olide(LS-1) through the Activation of TGF-β Signaling in SNU-C5/5-FU, Fluorouracil-Resistant Human Colon Cancer Cells. Marine Drugs, 13(3), 1340-1359. https://doi.org/10.3390/md13031340




