Comparative Evaluation of the Antibacterial and Antitumor Activities of 9-Phenylfascaplysin and Its Analogs
Abstract
1. Introduction
2. Results and Discussion
2.1. Preparation of 9-Phenylfascaplysin, Its Isomers, and Some Analogs
2.2. Antibacterial and Antiproliferative Activities of 9-Phenylfascaplysin and Its Isomers and Analogs In Vitro
2.3. Study of the Therapeutic Potential of 9-Phenylfascaplysin In Vivo
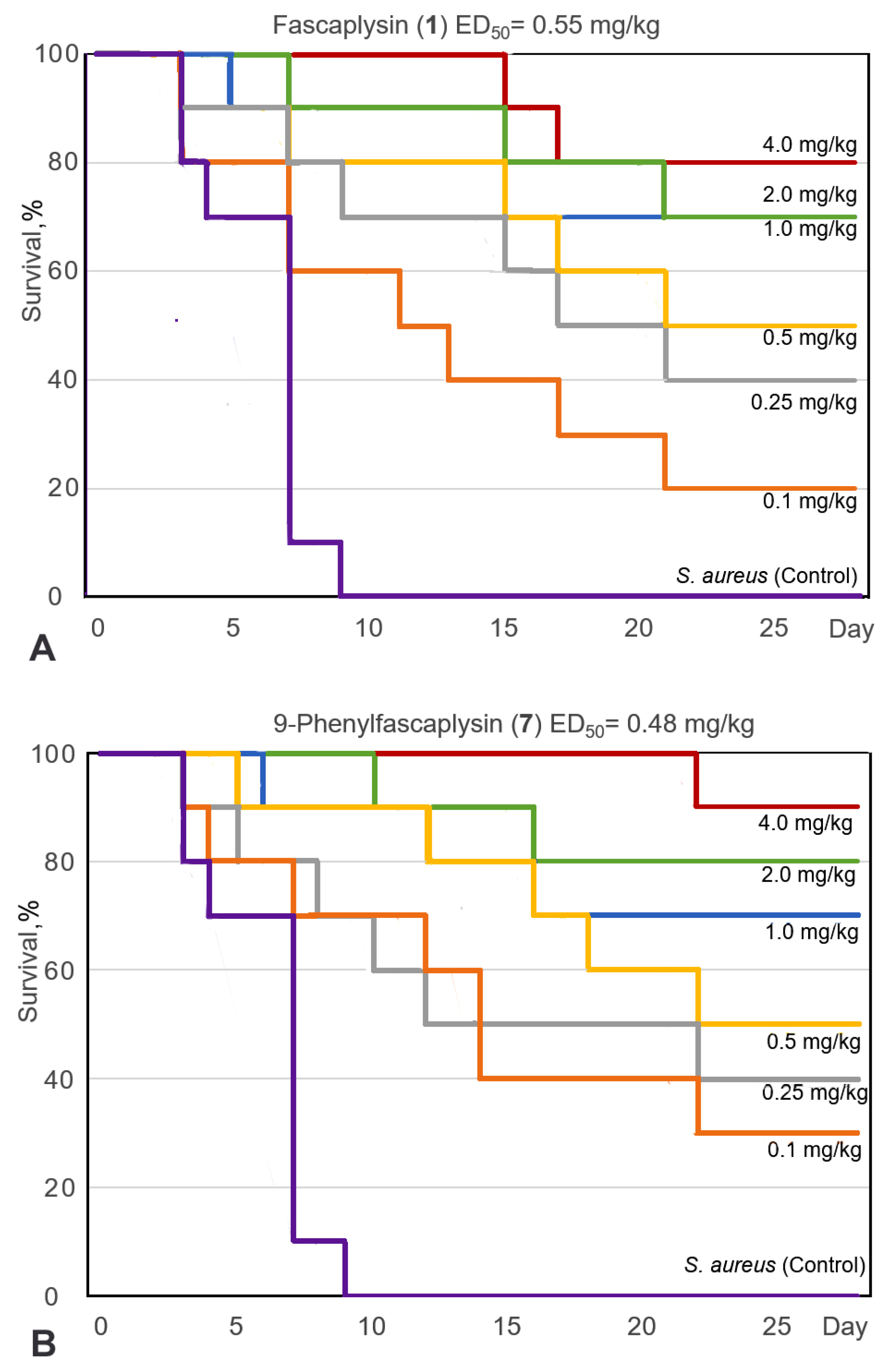
2.3.1. Study of the Antibacterial Efficacy of 9-Phenylfascaplysin In Vivo
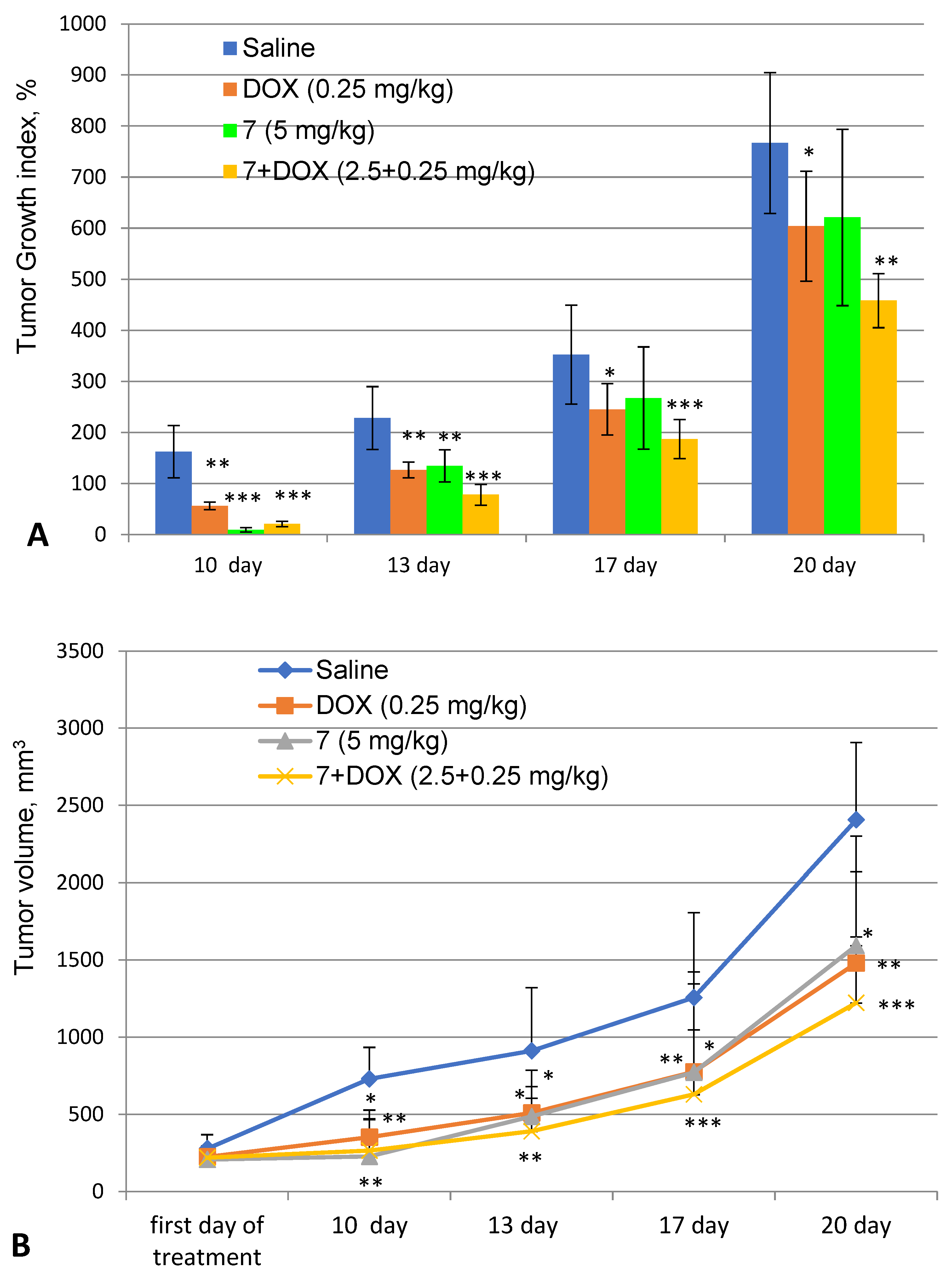
2.3.2. Evaluation of the Antitumor Activity of 9-Phenylfascaplysin (7) In Vivo
2.4. Acute Toxicity of 9-Phenylfascaplysin (7)
3. Materials and Methods
3.1. Chemistry
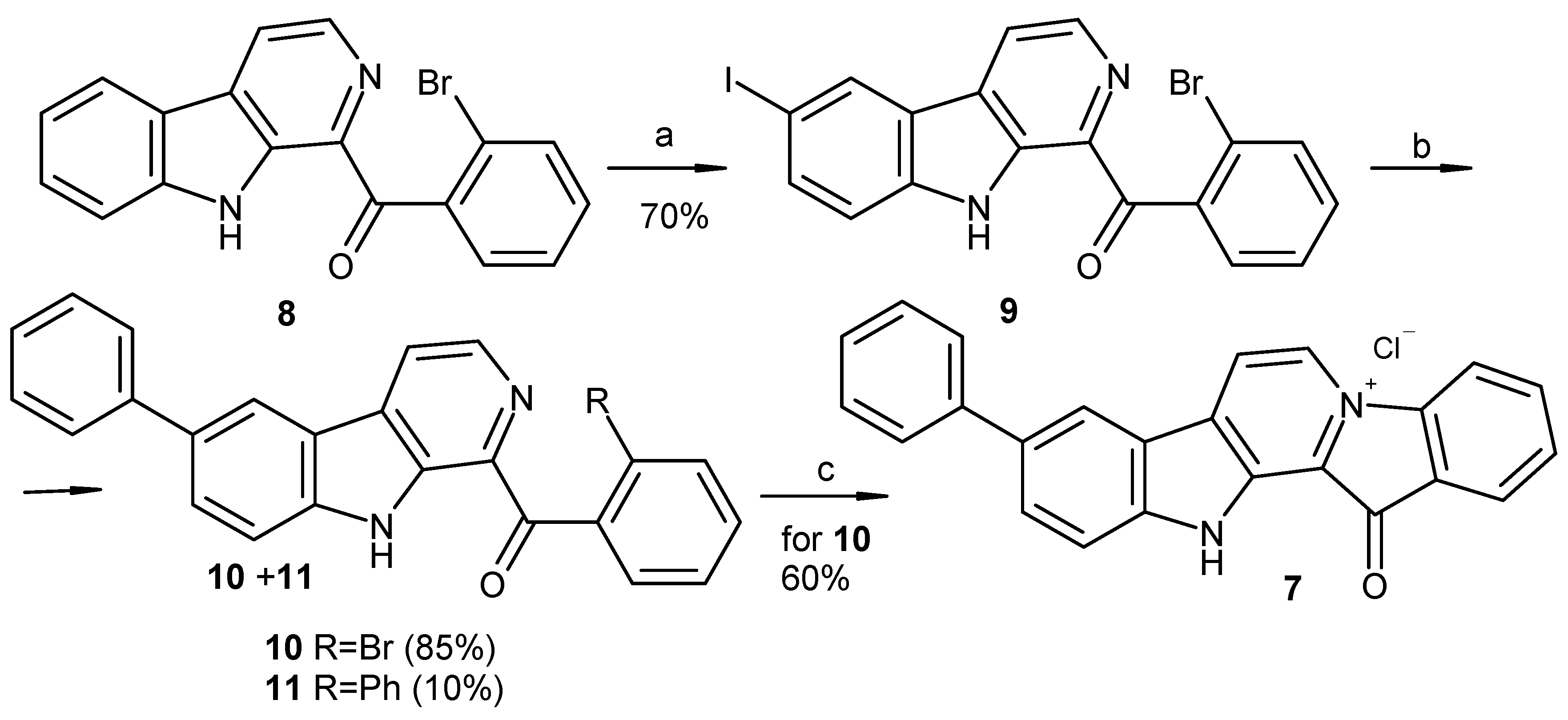
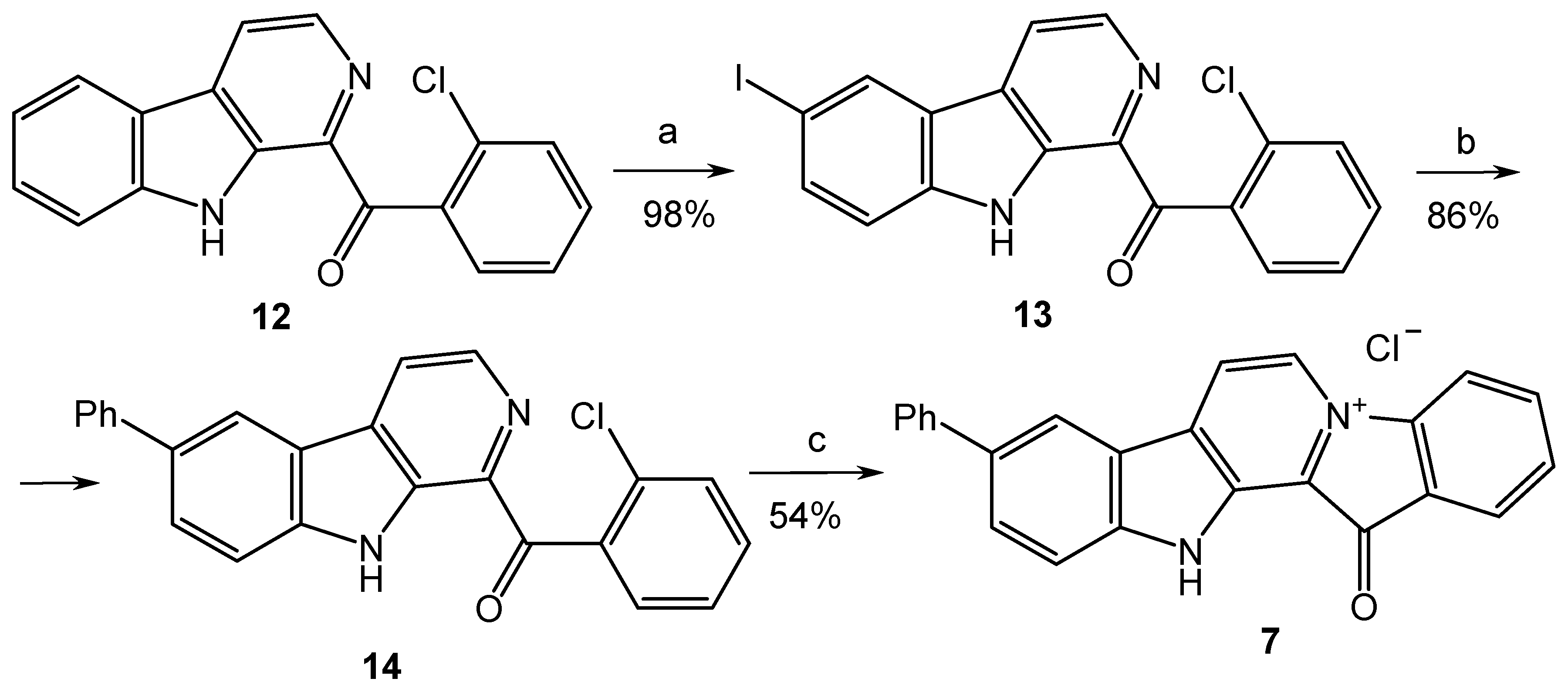
3.1.1. Preparation of Compounds 9, 13
3.1.2. Preparation of 1-(2′-Bromobenzoyl)-6-phenyl-β-carboline (10)
3.1.3. Preparation of 9-Phenylfascaplysin (7) from Compound 10
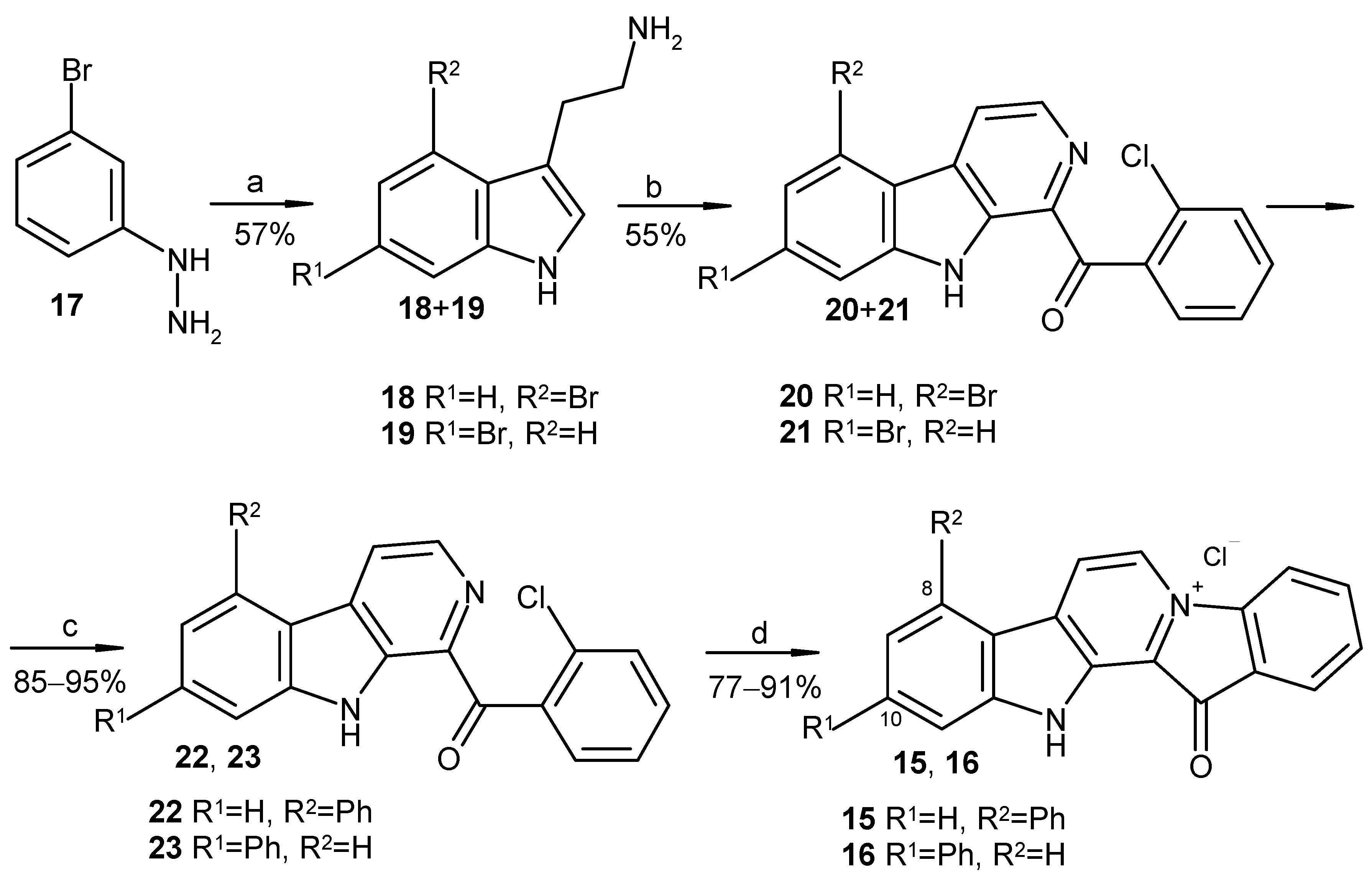
3.1.4. Preparation of Mixture of Tryptamines 18 and 19
3.1.5. Preparation of Substituted 1-Benzoyl-β-carbolines 20, 21
3.1.6. Preparation of Substituted 1-Benzoyl-β-carbolines 14, 22, 23, 24a–24h
3.1.7. Preparation of Substituted Fascaplysins 7, 15, 16, and 25a–25h
3.2. Biological Assay
3.2.1. MIC Values Determination
3.2.2. Antiproliferative Activity against Tumor Cells
3.2.3. Molecular Docking
3.2.4. In Vivo Efficiency Study of Antibacterial Activity
3.2.5. In Vivo Study of Antitumor Activity
3.2.6. Acute Toxicity
3.3. Statistic Calculation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mc Carlie, S.; Boucher, C.E.; Bragg, R.R. Molecular basis of bacterial disinfectant resistance. Drug Resist. Updates 2020, 48, 100672. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary ammonium compounds: An antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef]
- Brochado, A.R.; Telzerow, A.; Bobonis, J.; Banzhaf, M.; Mateus, A.; Selkrig, J.; Huth, E.; Bassler, S.; Zamarreno Beas, J.; Zietek, M.; et al. Species-specific activity of antibacterial drug combinations. Nature 2018, 559, 259–263. [Google Scholar] [CrossRef]
- Davies, J.; Davies, D. Origins and evolution of antibiotic resistance. Microbiol. Mol. Biol. Rev. 2010, 74, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Fares, M.; Abd El Hadi, S.R.S.; Eladwy, R.A.; Shoun, A.A.; Abdel-Aziz, M.M.; Eldehna, W.M.; Abdel-Aziz, H.A.; Keller, P.A. An improved synthesis of pyrido [2,3-d]pyrimidin-4(1H)-ones and their antimicrobial activity. Org. Biomol. Chem. 2018, 16, 3389–3395. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Yang, Y. Recent advances in antibacterial agents. Bioorg. Med. Chem. Lett. 2021, 35, 127799. [Google Scholar] [CrossRef]
- Rossiter, S.E.; Fletcher, M.H.; Wuest, W.M. Natural products as platforms to overcome antibiotic resistance. Chem. Rev. 2017, 117, 12415–12474. [Google Scholar] [CrossRef]
- Roll, D.M.; Ireland, C.M.; Lu, H.S.M.; Clardy, J. Fascaplysin, an unusual antimicrobial pigment from the marine sponge Fascaplysinopsis sp. J. Org. Chem. 1988, 53, 3276–3278. [Google Scholar] [CrossRef]
- Bharate, S.B.; Manda, S.; Mupparapu, N.; Battini, N.; Vishwakarma, R.A. Chemistry and biology of fascaplysin, a potent marine-derived CDK-4 inhibitor. Mini-Rev. Med. Chem. 2012, 12, 650–664. [Google Scholar] [CrossRef]
- Popov, A.M.; Stonik, V.A. Physiological activity of fascaplisine--an unusual pigment from tropical sea sponges. Antibiot. Chemother. 1991, 36, 12–14. [Google Scholar]
- Wang, C.; Wang, S.; Li, H.; Hou, Y.; Cao, H.; Hua, H.; Li, D. Marine-derived lead fascaplysin: Pharmacological activity, total synthesis, and structural modification. Mar. Drugs 2023, 21, 226. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiu, H.; Yang, N.; Xie, H.; Liang, W.; Lin, J.; Zhu, H.; Zhou, Y.; Wang, N.; Tan, X.; et al. Fascaplysin derivatives binding to DNA via unique cationic five-ring coplanar backbone showed potent antimicrobial/antibiofilm activity against MRSA in vitro and in vivo. Eur. J. Med. Chem. 2022, 230, 114099. [Google Scholar] [CrossRef] [PubMed]
- Fretz, H.; Ucci-Stoll, K.; Hug, P.; Schoepfer, J.; Lang, M. Investigations on the reactivity of fascaplysin. Part I. Aromatic electrophilic substitutions occur at position 9. Helv. Chim. Acta 2001, 83, 3065–3068. [Google Scholar] [CrossRef]
- Pan, H.; Qiu, H.; Zhang, K.; Zhang, P.; Liang, W.; Yang, M.; Mou, C.; Lin, M.; He, M.; Xiao, X.; et al. Fascaplysin derivatives are potent multi-target agents against Alzheimer’s disease: In vitro and in vivo evidence. ACS Chem. Neurosci. 2019, 10, 4741–4756. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Zhao, X.; Jiang, Y.; Liang, W.; Wang, W.; Jiang, X.; Jiang, M.; Wang, X.; Cui, W.; Li, Y.; et al. Design and synthesis of fascaplysin derivatives as inhibitors of FtsZ with potent antibacterial activity and mechanistic study. Eur. J. Med. Chem. 2023, 254, 115348. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; She, M.T.; Guo, X.C.; Zheng, B.X.; Huang, X.H.; Zhang, Y.H.; Ser, H.L.; Wong, W.L.; Sun, N.; Lu, Y.J. Design and synthesis of quinolinium-based derivatives targeting FtsZ for antibacterial evaluation and mechanistic study. Eur. J. Med. Chem. 2022, 236, 114360. [Google Scholar] [CrossRef] [PubMed]
- Dan, W.; Gao, J.; Qi, X.; Wang, J.; Dai, J. Antibacterial quaternary ammonium agents: Chemical diversity and biological mechanism. Eur. J. Med. Chem. 2022, 243, 114765. [Google Scholar] [CrossRef]
- Hurley, K.A.; Santos, T.M.; Nepomuceno, G.M.; Huynh, V.; Shaw, J.T.; Weibel, D.B. Targeting the bacterial division protein FtsZ. J. Med. Chem. 2016, 59, 6975–6998. [Google Scholar] [CrossRef]
- Du, R.L.; Sun, N.; Fung, Y.H.; Zheng, Y.Y.; Chen, Y.W.; Chan, P.H.; Wong, W.L.; Wong, K.Y. Discovery of FtsZ inhibitors by virtual screening as antibacterial agents and study of the inhibition mechanism. RSC Med. Chem. 2022, 13, 79–89. [Google Scholar] [CrossRef]
- Kaul, M.; Parhi, A.K.; Zhang, Y.; LaVoie, E.J.; Tuske, S.; Arnold, E.; Kerrigan, J.E.; Pilch, D.S. A bactericidal guanidinomethyl biaryl that alters the dynamics of bacterial FtsZ polymerization. J. Med. Chem. 2012, 55, 10160–10176. [Google Scholar] [CrossRef]
- Jiang, Y.; Qiu, H.; Liang, W.; Lin, J.; Lin, J.; Liu, W.; Wang, X.; Cui, W.; Chen, X.; Wang, H.; et al. Derivatization of marine-derived fascaplysin via highly regioselective Suzuki-Miyaura coupling contributing to the enhanced antibacterial activity. ChemistrySelect 2022, 7, e202201441. [Google Scholar] [CrossRef]
- Gribble, G.W.; Pelcman, B. Total syntheses of the marine sponge pigments fascaplysin and homofascaplysin B and C. J. Org. Chem. 1992, 57, 3636–3642. [Google Scholar] [CrossRef]
- Rocca, P.; Marsais, F.; Godard, A.; Quéguiner, G. A short synthesis of the antimicrobial marine sponge pigment fascaplysin. Tetrahedron Lett. 1993, 34, 7917–7918. [Google Scholar] [CrossRef]
- Molina, P.; Fresneda, P.M.; García-Zafra, S.; Almendros, P. Iminophosphorane-mediated syntheses of the fascaplysin alkaloid of marine origin and nitramarine. Tetrahedron Lett. 1994, 35, 8851–8854. [Google Scholar] [CrossRef]
- Radchenko, O.S.; Novikov, V.L.; Elyakov, G.B. A simple and practical approach to the synthesis of the marine sponge pigment fascaplysin and related compounds. Tetrahedron Lett. 1997, 38, 5339–5342. [Google Scholar] [CrossRef]
- Waldmann, H.; Eberhardt, L.; Wittstein, K.; Kumar, K. Silver catalyzed cascade synthesis of alkaloid ring systems: Concise total synthesis of fascaplysin, homofascaplysin C and analogues. Chem. Commun. 2010, 46, 4622–4624. [Google Scholar] [CrossRef]
- Zhidkov, M.E.; Baranova, O.V.; Kravchenko, N.S.; Dubovitskii, S.V. A new method for the synthesis of the marine alkaloid fascaplysin. Tetrahedron Lett. 2010, 51, 6498–6499. [Google Scholar] [CrossRef]
- Tryapkin, O.A.; Kantemirov, A.V.; Dyshlovoy, S.A.; Prassolov, V.S.; Spirin, P.V.; von Amsberg, G.; Sidorova, M.A.; Zhidkov, M.E. A new mild method for synthesis of marine alkaloid fascaplysin and its therapeutically promising derivatives. Mar. Drugs 2023, 21, 424. [Google Scholar] [CrossRef]
- Zhidkov, M.E.; Kaminskii, V.A. A new method for the synthesis of the marine alkaloid fascaplysin based on the microwave-assisted Minisci reaction. Tetrahedron Lett. 2013, 54, 3530–3532. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Liu, M.-C.; Cai, Q.; Jia, F.-C.; Wu, A.-X. A cascade coupling strategy for one-pot total synthesis of β-carboline and isoquinoline-containing natural products and derivatives. Chem. A Eur. J. 2013, 19, 10132–10137. [Google Scholar] [CrossRef]
- Zhidkov, M.E.; Kantemirov, A.V.; Koisevnikov, A.V.; Andin, A.N.; Kuzmich, A.S. Syntheses of the marine alkaloids 6-oxofascaplysin, fascaplysin and their derivatives. Tetrahedron Lett. 2018, 59, 708–711. [Google Scholar] [CrossRef]
- Zhidkov, M.E.; Smirnova, P.A.; Tryapkin, O.A.; Kantemirov, A.V.; Khudyakova, Y.V.; Malyarenko, O.S.; Ermakova, S.P.; Grigorchuk, V.P.; Kaune, M.; Von Amsberg, G.; et al. Total syntheses and preliminary biological evaluation of brominated fascaplysin and reticulatine alkaloids and their analogues. Mar. Drugs 2019, 17, 496. [Google Scholar] [CrossRef] [PubMed]
- Zhidkov, M.E.; Kaune, M.; Kantemirov, A.V.; Smirnova, P.A.; Spirin, P.V.; Sidorova, M.A.; Stadnik, S.A.; Shyrokova, E.Y.; Kaluzhny, D.N.; Tryapkin, O.A.; et al. Study of structure–activity relationships of the marine alkaloid fascaplysin and its derivatives as potent anticancer agents. Mar. Drugs 2022, 20, 185. [Google Scholar] [CrossRef] [PubMed]
- Volodina, Y.L.; Dezhenkova, L.G.; Tikhomirov, A.S.; Tatarskiy, V.V.; Kaluzhny, D.N.; Moisenovich, A.M.; Moisenovich, M.M.; Isagulieva, A.K.; Shtil, A.A.; Tsvetkov, V.B.; et al. New anthra[2,3-b]furancarboxamides: A role of positioning of the carboxamide moiety in antitumor properties. Eur. J. Med. Chem. 2019, 165, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Tikhomirov, A.S.; Tsvetkov, V.B.; Volodina, Y.L.; Litvinova, V.A.; Andreeva, D.V.; Dezhenkova, L.G.; Kaluzhny, D.N.; Treshalin, I.D.; Shtil, A.A.; Shchekotikhin, A.E. Heterocyclic ring expansion yields anthraquinone derivatives potent against multidrug resistant tumor cells. Bioorg. Chem. 2022, 127, 105925. [Google Scholar] [CrossRef]
- Tan, C.M.; Therien, A.G.; Lu, J.; Lee, S.H.; Caron, A.; Gill, C.J.; Lebeau-Jacob, C.; Benton-Perdomo, L.; Monteiro, J.M.; Pereira, P.M.; et al. Restoring methicillin-resistant Staphylococcus aureus susceptibility to β-lactam antibiotics. Sci. Transl. Med. 2012, 4, 126ra35. [Google Scholar] [CrossRef] [PubMed]
- Lovering, F.; Bikker, J.; Humblet, C. Escape from flatland: Increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Muller, L.; Furet, P.; Schoepfer, J.; Stephan, C.; Zumstein-Mecker, S.; Fretz, H.; Chaudhuri, B. Inhibition of cyclin-dependent kinase 4 (Cdk4) by fascaplysin, a marine natural product. Biochem. Biophys. Res. Commun. 2000, 275, 877–884. [Google Scholar] [CrossRef]
- Oh, T.-I.; Lee, Y.-M.; Nam, T.-J.; Ko, Y.-S.; Mah, S.; Kim, J.; Kim, Y.; Reddy, R.H.; Kim, Y.J.; Hong, S.; et al. Fascaplysin exerts anti-cancer effects through the downregulation of survivin and HIF-1 and Inhibition of VEGFR2 and TRKA. Int. J. Mol. Sci. 2017, 18, 2074. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Lu, X.L.; Lin, J.; Chen, H.M.; Yan, X.J.; Wang, F.; Xu, W.F. Direct effects of fascaplysin on human umbilical vein endothelial cells attributing the anti-angiogenesis activity. Biomed. Pharmacother. 2010, 64, 527–533. [Google Scholar] [CrossRef]
- Meng, N.; Mu, X.; Lv, X.; Wang, L.; Li, N.; Gong, Y. Autophagy represses fascaplysin-induced apoptosis and angiogenesis inhibition via ROS and p8 in vascular endothelia cells. Biomed. Pharmacother. 2019, 114, 108866. [Google Scholar] [CrossRef] [PubMed]
- Hörmann, A.; Chaudhuri, B.; Fretz, H. DNA binding properties of the marine sponge pigment fascaplysin. Bioorgan. Med. Chem. 2001, 9, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Guru, S.K.; Pathania, A.S.; Manda, S.; Kumar, A.; Bharate, S.B.; Vishwakarma, R.A.; Malik, F.; Bhushan, S. Fascaplysin induces caspase mediated crosstalk between apoptosis and autophagy through the inhibition of PI3K/AKT/mTOR signaling cascade in human leukemia HL-60 cells. J. Cell. Biochem. 2015, 116, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, B.; Nakeff, A.; Tenney, K.; Crews, P.; Gunatilaka, L.; Valeriote, F. A new paradigm for the development of anticancer agents from natural products. J. Exp. Ther. Oncol. 2006, 5, 195–204. [Google Scholar] [PubMed]
- Yan, X.; Chen, H.; Lu, X.; Wang, F.; Xu, W.; Jin, H.; Zhu, P. Fascaplysin exert anti-tumor effects through apoptotic and anti-angiogenesis pathways in sarcoma mice model. Eur. J. Pharm Sci. 2011, 43, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Guru, S.K.; Manda, S.; Kumar, A.; Mintoo, M.J.; Prasad, V.D.; Sharma, P.R.; Mondhe, D.M.; Bharate, S.B.; Bhushan, S. A marine sponge alkaloid derivative 4-chloro fascaplysin inhibits tumor growth and VEGF mediated angiogenesis by disrupting PI3K/Akt/mTOR signaling cascade. Chem. Biol. Interact. 2017, 275, 43–60. [Google Scholar] [CrossRef]
- Berezovskaya, I.V. Classification of Substances with Respect to Acute Toxicity for Parenteral Administration. Pharm. Chem. J. 2003, 37, 139–141. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standarts Institute M100 Performance Standards for Antimicrobial Susceptibility Testing. 2020. Available online: https://clsi.org/standards/products/microbiology/documents/m100/ (accessed on 23 March 2021).
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Koes, D.R.; Baumgartner, M.P.; Camacho, C.J. Lessons Learned in Empirical Scoring with smina from the CSAR 2011 Benchmarking Exercise. J. Chem. Inf. Model. 2013, 53, 1893–1904. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminformatics 2011, 3, 1–14. [Google Scholar] [CrossRef]
- Adasme, M.F.; Linnemann, K.L.; Bolz, S.N.; Kaiser, F.; Salentin, S.; Haupt, V.J.; Schroeder, M. PLIP 2021: Expanding the scope of the protein–ligand interaction profiler to DNA and RNA. Nucleic Acids Res. 2021, 49, W530–W534. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera: A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Purposes. Strasbourg: 1986, 18.III.1986, Council of Europe, ETS No.123. Available online: https://rm.coe.int/168007a67b (accessed on 28 August 2018).
- Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes EN. Official Journal of the European Union, L 276/33-276/79 (20.10.2010). Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010:276:0033:0079:EN:PDF (accessed on 27 April 2020).
- National State Standard GOST 33044-2014 the Russian Federation Standard “The Principles of Good Laboratory Practice” (Approved and Put into Effect by the Order of the Federal Agency for Technical Regulation and Metrology of October 20, 2014), No 1700. Available online: https://docs.cntd.ru/document/1200115791 (accessed on 1 August 2015). (In Russian).
- Behrens, B. Zur Auswertung der Digitalisblätter im Forschversuch. Arch. Exper. Path. Pharm. 1929, 140, 237. [Google Scholar] [CrossRef]
- National State Standard GOST 33216-2014 the Russian Federation Standard “Guidelines for Accommodation and Care of Animals. Species-Specific Provisions for Laboratory Rodents and Rabbits” (Approved and Put into Effect by the Order of the Federal Agency for Technical Regulation and Metrology of December 22, 2014), No 73P. Available online: https://docs.cntd.ru/document/1200127506 (accessed on 1 August 2015). (In Russian).
- Elsherbiny, N.M.; Younis, N.N.; Shaheen, M.A.; Elseweidy, M.M. The synergistic effect between vanillin and doxorubicin in Ehrlich ascites carcinoma solid tumour and MCF-7 human breast cancer cell line. Pathol. Res. Pract. 2016, 212, 767–777. [Google Scholar] [CrossRef]
- Akhila, J.S.; Deepa, S.; Alwar, M.C. Acute toxicity studies and determination of median lethal dose. Curr. Sci. 2007, 93, 917–920. Available online: http://www.jstor.org/stable/24099255 (accessed on 12 December 2023).





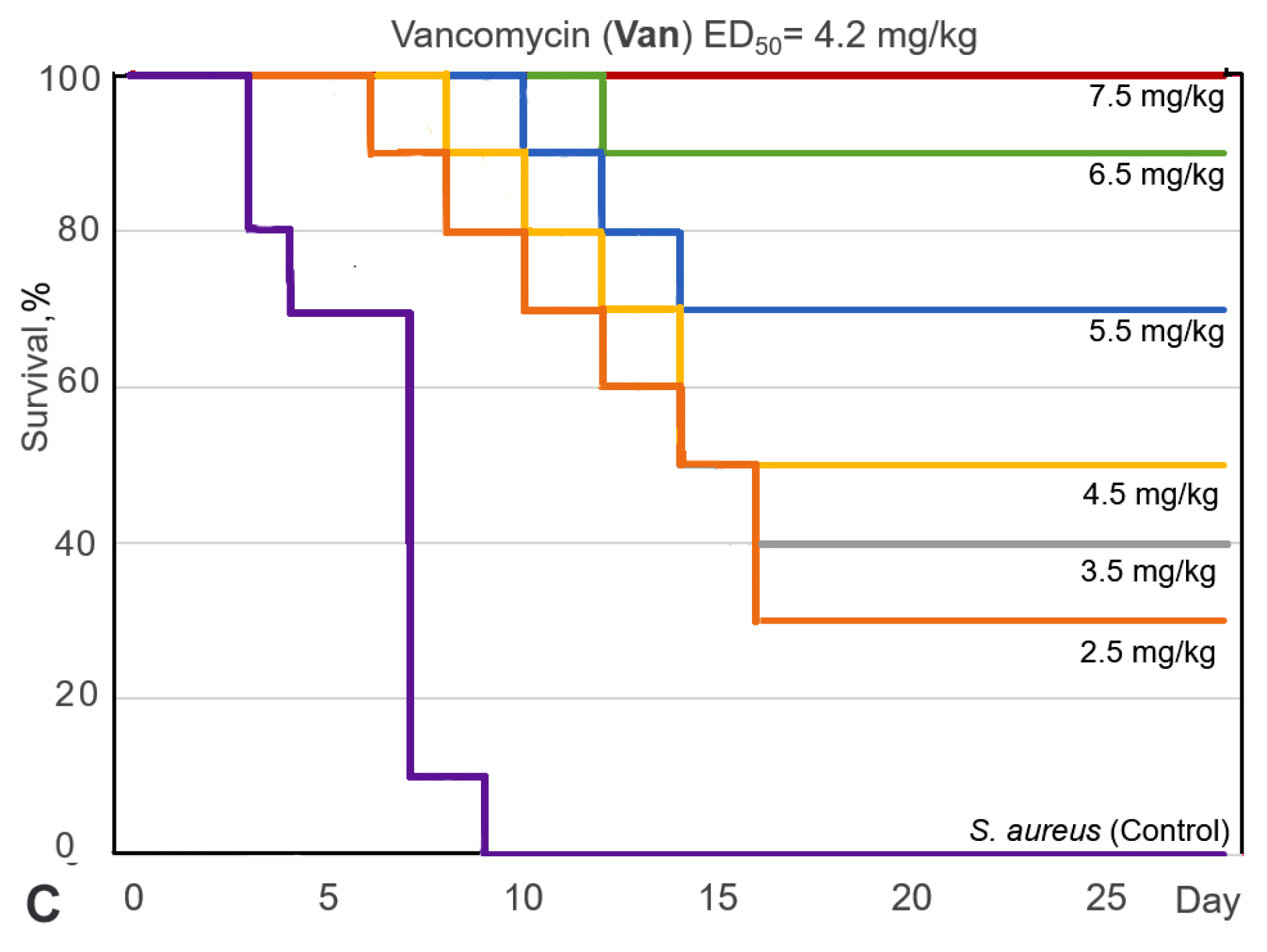
| Compound | MIC, µg/mL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus ATCC 29213 | B. cereus ATCC 10702 | E. faecalis ATCC 29212 | E. faecium 132 | E. faecium 130 (VRE) | E. faecalis 583 (VRE) | S. aureus 88 (MRSA) | S. aureus PE3R (MRSA) | S. epidermidis 2001 MR | S. aureus 21555 | M. smegmatis ATCC 607 | E. coli ATCC 25922 | |
| Van | 0.5 | 1.0 | 2.0 | 0.5 | >32 | 32.0 | 0.5 | 1.0 | 1.0 | 2.0 | - | - |
| Rif | 0.018 | 0.25 | - | - | - | - | - | - | - | - | 0.03 | 8.0 |
| 1 | 1.0 | 0.125 | 8.0 | 1.0 | 1.0 | ≥8.0 | 1.0 | 0.5 | 0.0075 | 0.03 | 0.03 | 8.0 |
| 7 | 0.03 | 0.03 | 0.25 | 4.0 | 2.0 | 0.25 | 0.015 | 0.03 | 0.00375 | 0.03 | 0.25 | 8.0 |
| 15 | 0.125 | 2.0 | 16.0 | 8.0 | 8.0 | 16.0 | 0.5 | 0.5 | 0.0075 | 0.25 | 2.0 | >16.0 |
| 16 | 0.125 | 0.125 | 0.25 | 8.0 | 16.0 | 0.13 | 0.125 | 0.06 | 0.03 | 0.03 | 1.0 | >16.0 |
| 25a | 0.06 | 0.03 | 0.25 | 8.0 | 8.0 | 0.125 | 0.03 | 0.03 | 0.03 | 0.03 | 0.5 | >16.0 |
| 25b | 0.06 | 0.06 | 0.125 | 0.5 | 0.5 | 0.125 | 0.03 | 0.06 | 0.015 | 0.03 | 0.5 | >16.0 |
| 25c | 0.25 | 0.25 | 2.0 | 16 | 16 | 2.0 | 0.25 | 0.25 | 0.25 | 0.25 | 4.0 | >16.0 |
| 25d | 0.06 | 0.5 | 0.5 | 16 | 8.0 | 1.0 | 0.125 | 0.06 | 0.015 | 0.06 | 8.0 | >16.0 |
| 25e | 0.06 | 0.25 | 0.125 | 0.5 | 0.5 | 0.25 | 0.06 | 0.06 | 0.015 | 0.06 | 0.5 | 8.0 |
| 25f | 0.25 | 1.0 | 0.25 | 0.25 | 0.25 | 0.25 | 0.5 | 0.25 | 0.03 | 0.06 | 16.0 | >16.0 |
| 25g | 0.5 | 0.125 | >16.0 | 16.0 | 16.0 | >16.0 | 1.0 | 0.5 | 0.06 | 0.25 | 8.0 | >16.0 |
| 25h | 4.0 | 0.25 | >16.0 | >16.0 | >16.0 | >16.0 | 8.0 | 2.0 | 0.25 | 0.5 | 4.0 | >16.0 |
| Group | MST, Days | ILS, % | Survival, % |
|---|---|---|---|
| Control (-) | 16.1 ± 5.0 | - | 0 |
| 7 (1.25 mg/kg) | 24.9 ± 19.6 | 54.6 | 20 |
| 7 (2.5 mg/kg) | 31.9 ± 20.8 | 98.0 | 30 |
| 7 (5.0 mg/kg) | 58.4 ± 1.1 * | 262.5 | 80 |
| DOX (0.25 mg/kg) | 62.0 ± 0.0 ** | 284.8 | 100 |
| 7 + DOX (1.25 + 0.25 mg/kg) | 54.0 ± 10.7 * | 235.2 | 50 |
| Group | Dose, mg/kg | Tumor Weight, g | TGI, % |
|---|---|---|---|
| Control (-) | - | 2.54 ± 0.71 | - |
| DOX | 0.25 | 1.76 ± 0.94 * | 31 |
| 7 | 5 | 1.75 ± 0.89 * | 31 |
| 7 + DOX | 2.5 + 0.25 | 1.67 ± 0.75 * | 34 |
| Group | Body Weight of Animals, g | |||
|---|---|---|---|---|
| Initial | 6 Day | 13 Day | 20 Day | |
| Saline | 32.74 ± 3.09 | 32.32 ± 3.65 | 30.71 ± 5.70 | 27.56 ± 6.95 |
| 7 (5.00 mg/kg) | 30.04 ± 2.69 | 30.11 ± 3.90 | 29.06 ± 4.85 | 28.49 ± 7.14 |
| DOX (0.25) | 31.48 ± 2.90 | 31.36 ± 3.25 | 29.95 ± 5.31 | 28.05 ± 4.91 |
| 7 + DOX (2.50 + 0.25) | 30.12 ± 3.31 | 30.10 ± 2.53 | 29.84 ± 2.53 | 27.94 ± 5.79 |
| Parameter | 9-Phenylfascaplysin (7) |
|---|---|
| LD50 (mg/kg) | 25 |
| MTD (LD10) (mg/kg) | 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhidkov, M.E.; Sidorova, M.A.; Smirnova, P.A.; Tryapkin, O.A.; Kachanov, A.V.; Kantemirov, A.V.; Dezhenkova, L.G.; Grammatikova, N.E.; Isakova, E.B.; Shchekotikhin, A.E.; et al. Comparative Evaluation of the Antibacterial and Antitumor Activities of 9-Phenylfascaplysin and Its Analogs. Mar. Drugs 2024, 22, 53. https://doi.org/10.3390/md22020053
Zhidkov ME, Sidorova MA, Smirnova PA, Tryapkin OA, Kachanov AV, Kantemirov AV, Dezhenkova LG, Grammatikova NE, Isakova EB, Shchekotikhin AE, et al. Comparative Evaluation of the Antibacterial and Antitumor Activities of 9-Phenylfascaplysin and Its Analogs. Marine Drugs. 2024; 22(2):53. https://doi.org/10.3390/md22020053
Chicago/Turabian StyleZhidkov, Maxim E., Maria A. Sidorova, Polina A. Smirnova, Oleg A. Tryapkin, Andrey V. Kachanov, Alexey V. Kantemirov, Lyubov G. Dezhenkova, Natalia E. Grammatikova, Elena B. Isakova, Andrey E. Shchekotikhin, and et al. 2024. "Comparative Evaluation of the Antibacterial and Antitumor Activities of 9-Phenylfascaplysin and Its Analogs" Marine Drugs 22, no. 2: 53. https://doi.org/10.3390/md22020053
APA StyleZhidkov, M. E., Sidorova, M. A., Smirnova, P. A., Tryapkin, O. A., Kachanov, A. V., Kantemirov, A. V., Dezhenkova, L. G., Grammatikova, N. E., Isakova, E. B., Shchekotikhin, A. E., Pak, M. A., Styshova, O. N., Klimovich, A. A., & Popov, A. M. (2024). Comparative Evaluation of the Antibacterial and Antitumor Activities of 9-Phenylfascaplysin and Its Analogs. Marine Drugs, 22(2), 53. https://doi.org/10.3390/md22020053







