Anticancer and Cancer Preventive Properties of Marine Polysaccharides: Some Results and Prospects
Abstract
:1. Introduction
2. Polysaccharides from Brown Algae
2.1. Fucoidans
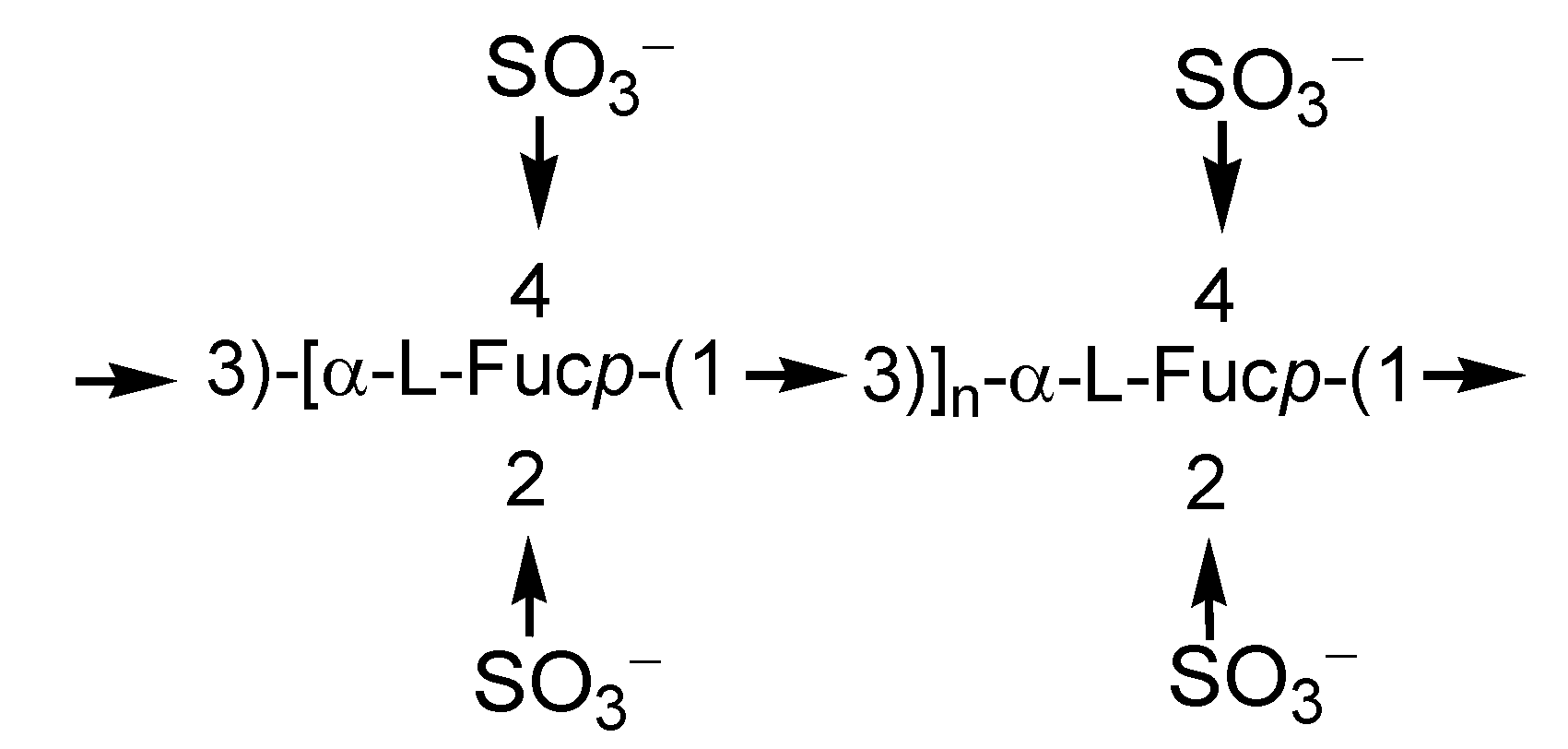
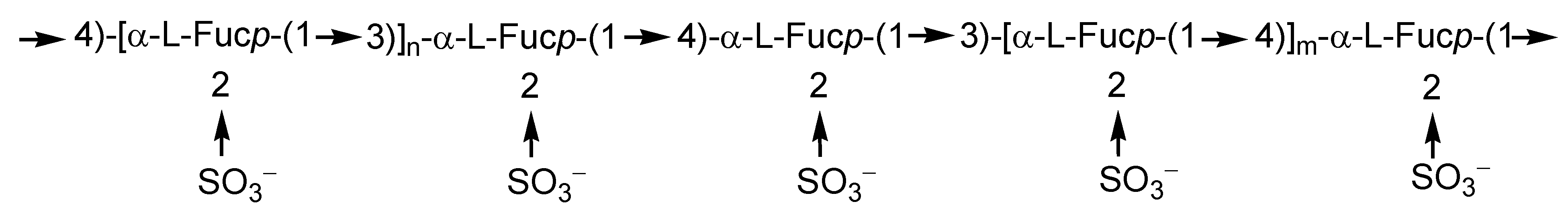
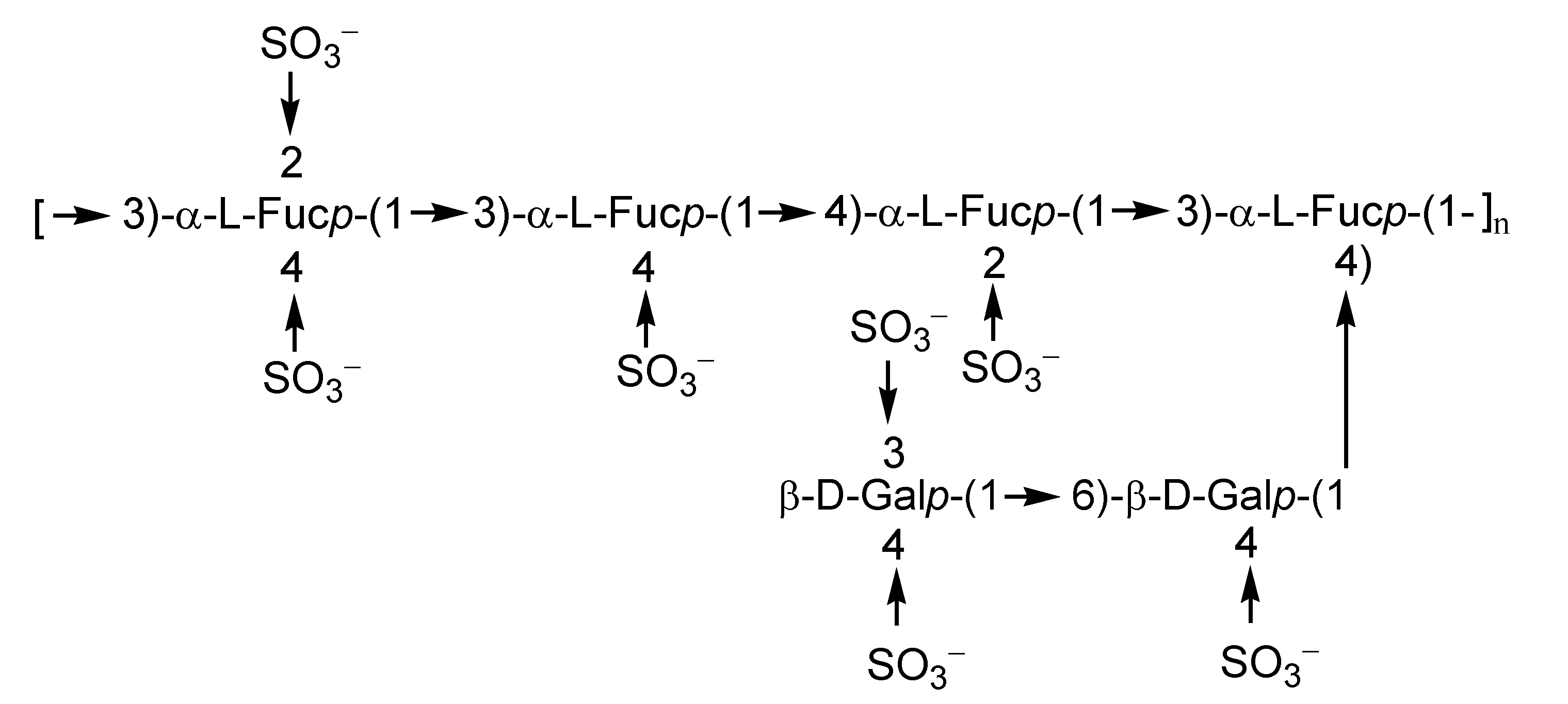
2.2. Laminarans
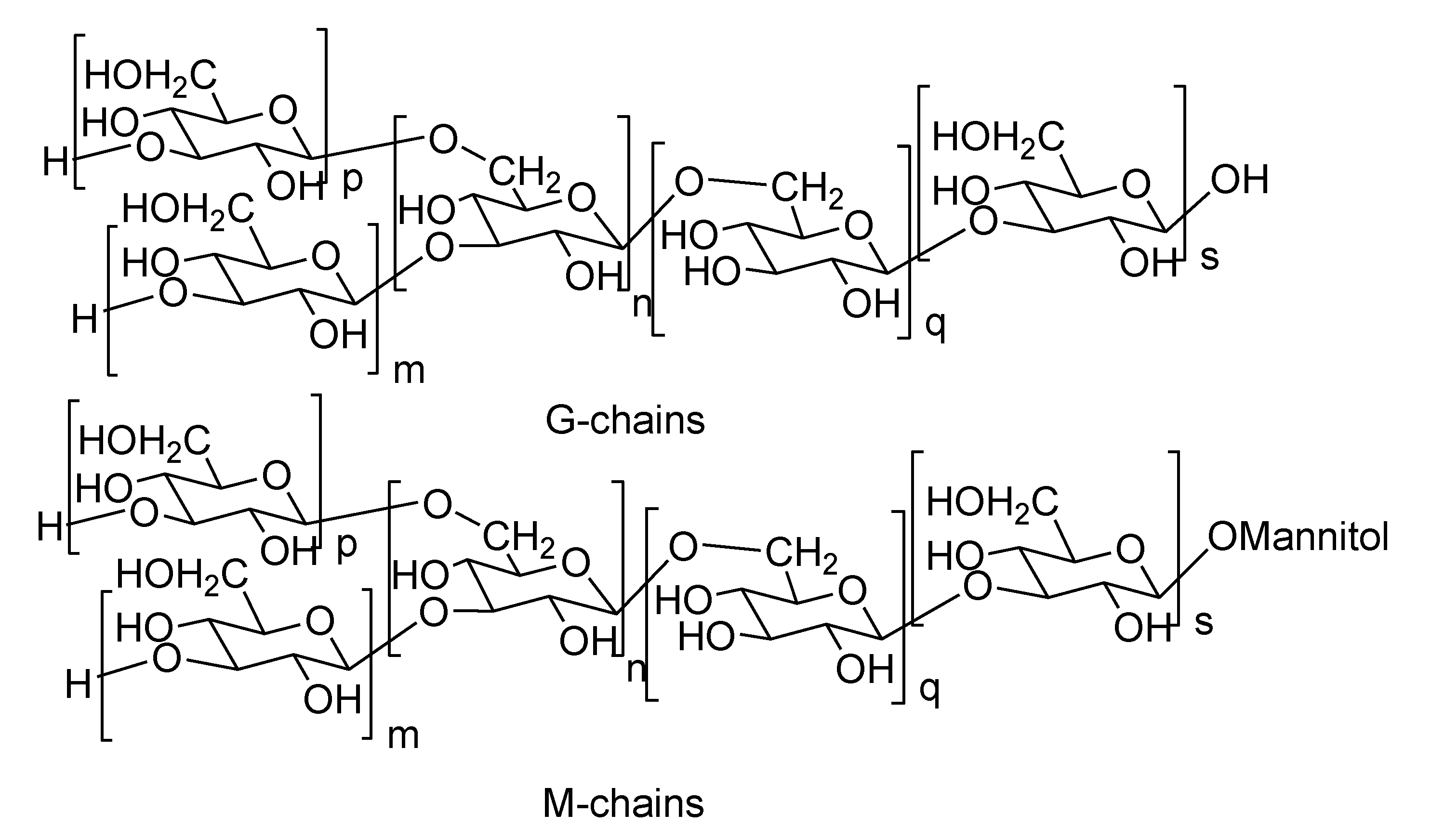
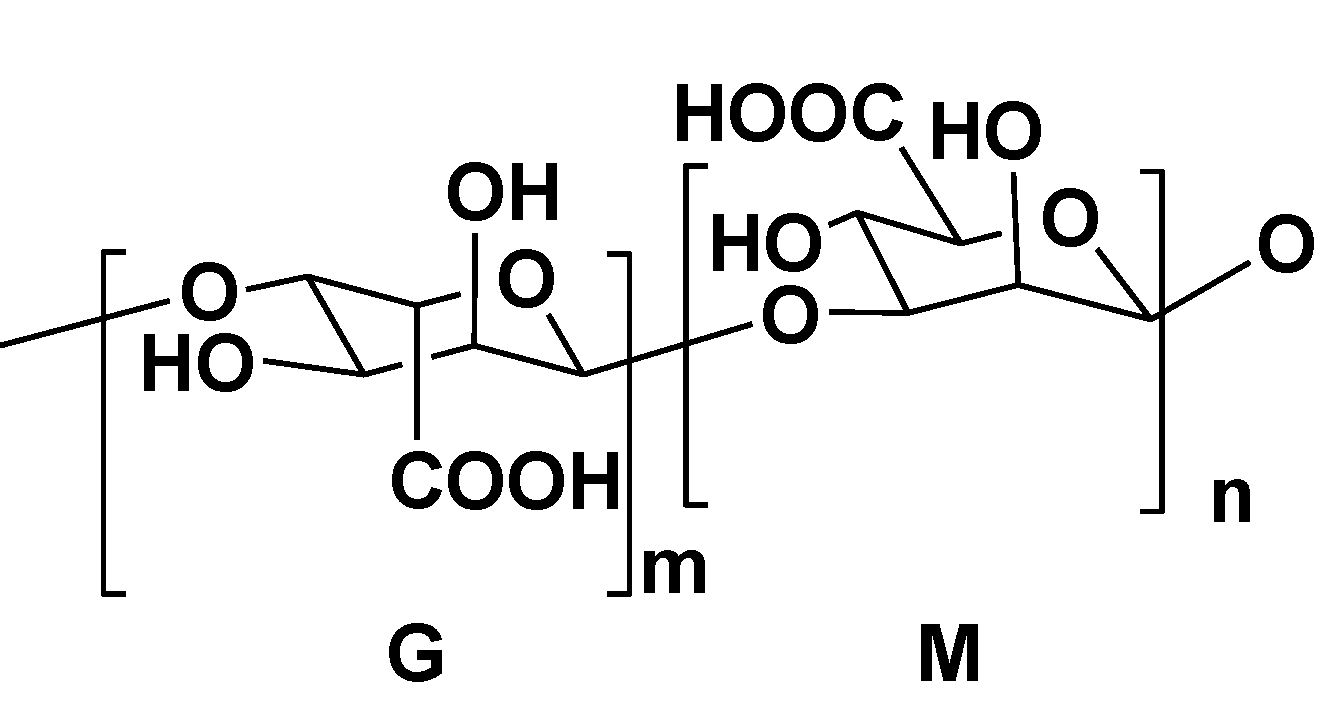
2.3. Alginic Acids
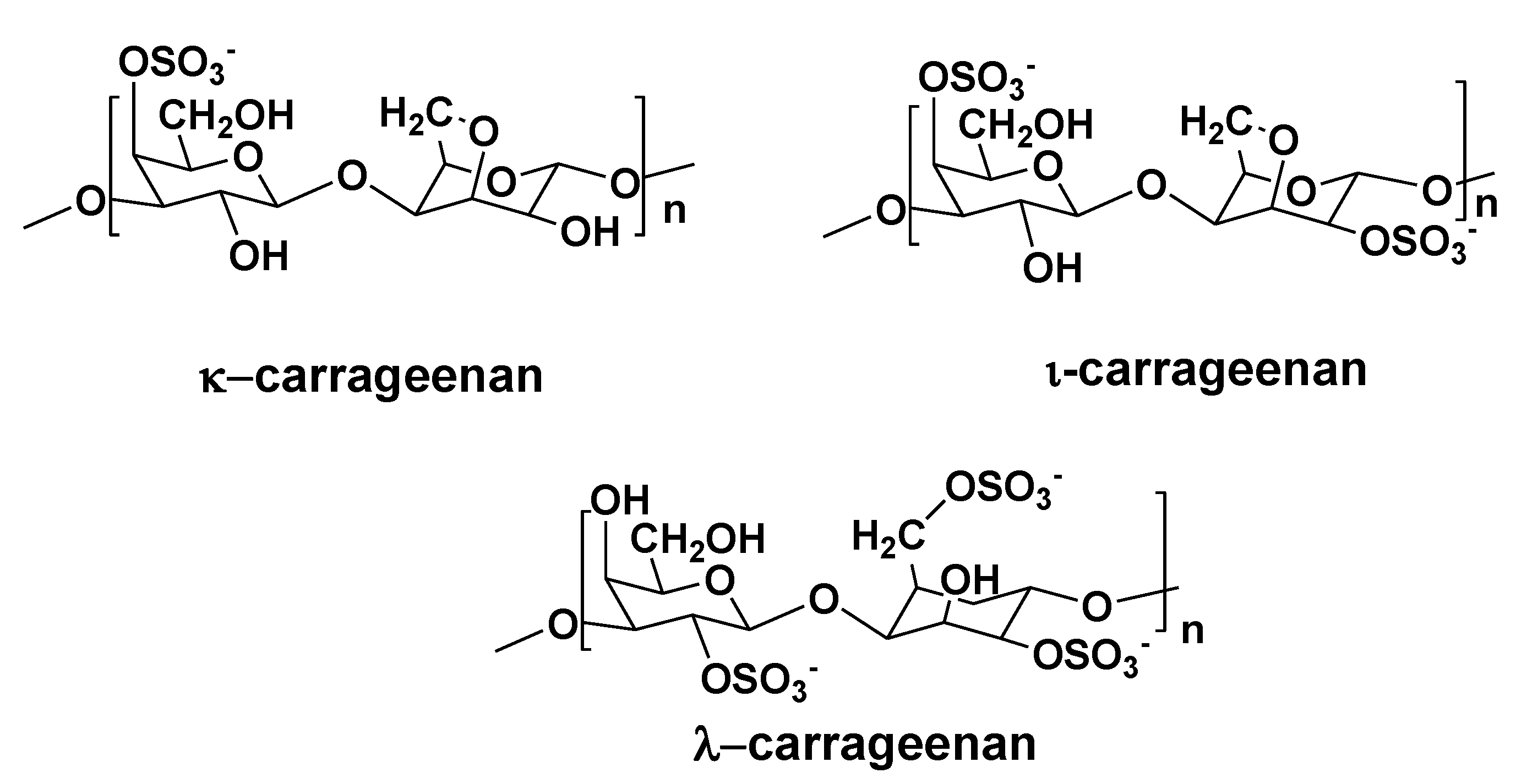
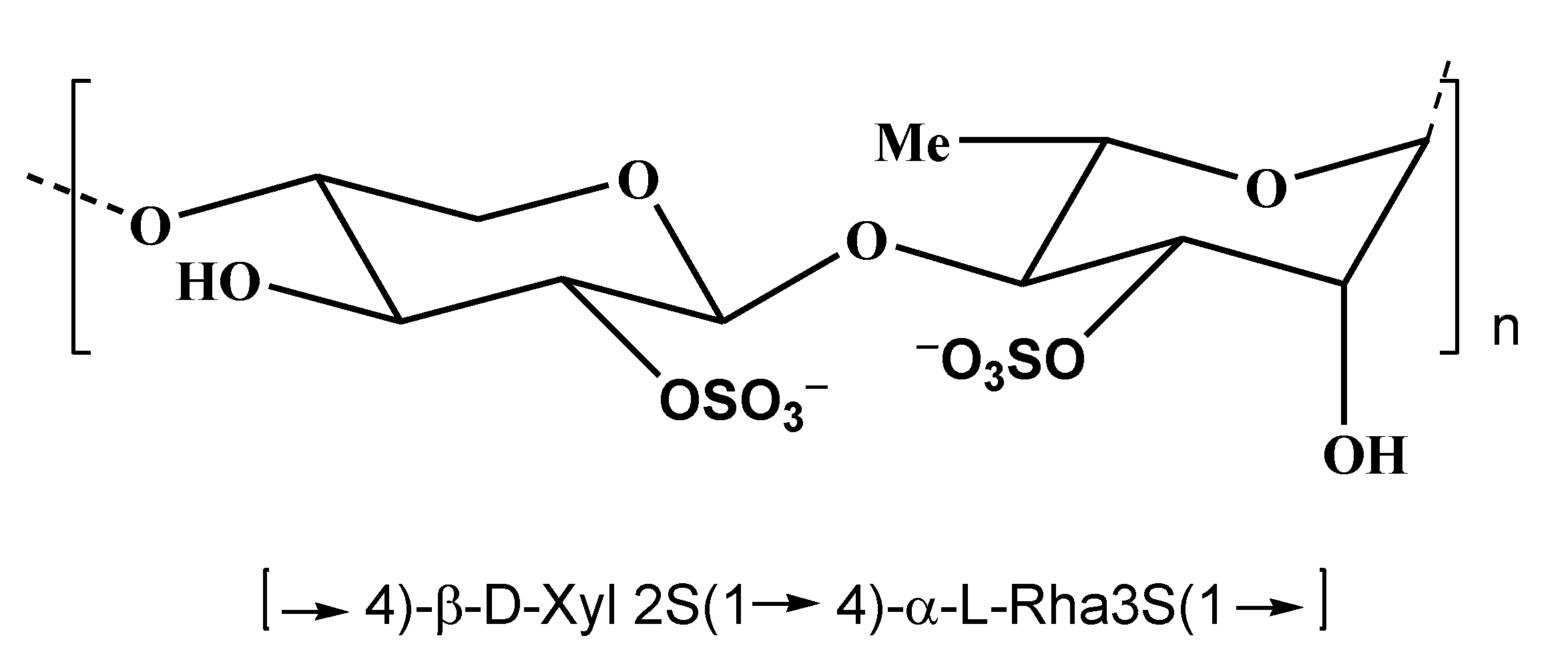
3. Polysaccharides from Red Algae
4. Polysaccharides from Green Algae
5. Polysaccharides from Microalgae
6. Polysaccharides from Marine Bacteria and Fungi
7. Polysaccharides from Marine Animals
8. Conclusion
Acknowledgments
Conflicts of Interest
References
- Laurienzo, P. Marine polysaccharides in pharmaceutical application: An overview. Mar. Drugs 2010, 8, 2435–2465. [Google Scholar] [CrossRef]
- Senni, K.; Pereira, J.; Gueniche, F.; Delbarre-Ladrat, C.; Sinquin, C.; Ratiskol, J.; Godeau, G.; Fischer, A.M.; Helley, D.; Colliec-Jouault, S. Marine polysaccharides: A source of bioactive molecules for cell therapy and tissue engineering. Mar. Drugs 2011, 9, 1664–1681. [Google Scholar] [CrossRef]
- Stonik, V.A.; Fedorov, S.N. Cancer preventive marine natural product. In Cellular and Genetic Practices for Translational Medicine; Kwak, H., Ed.; Research Signpost: Karalla, India, 2011; pp. 1–36. [Google Scholar]
- Beress, A.; Wassermann, O.; Bruhn, T.; Beress, L.; Kraiselburd, E.N.; Gonzalez, L.V.; de Motta, G.E.; Chavez, P.I. A new procedure for the isolation of anti-HIV compounds (polysaccharides and polyphenols) from the marine alga Fucus vesiculosus. J. Nat. Prod. 1993, 56, 478–488. [Google Scholar] [CrossRef]
- Ponce, N.M.A.; Pujol, C.A.; Damonte, E.B.; Flores, M.L.; Stortz, C.A. Fucoidans from the brown seaweed Adenocystis utricularis: Extraction methods, antiviral activity and structural studies. Carbohydr. Res. 2003, 338, 153–165. [Google Scholar] [CrossRef]
- Ray, B. Polysaccharides from Enteromorpha compressa: Isolation, purification and structural features. Carbohydr. Polym. 2006, 66, 408–416. [Google Scholar] [CrossRef]
- Ye, H.; Wang, K.; Zhou, C.; Liu, J.; Zeng, X. Purification, antitumor and antioxidant activities in vitro of polysaccharides from the brown seaweed Sargassum pallidum. Food Chem. 2008, 111, 428–432. [Google Scholar] [CrossRef]
- Esteves, A.I.S.; Nicolai, M.; Humanes, M.; Gonsalves, J. Sulfated polysaccharides in marine sponges: Extraction methods and anti-HIV activity. Mar. Drugs 2011, 9, 139–153. [Google Scholar]
- Smit, A.J. Medicinal and pharmaceutical uses of seaweed natural products: A review. J. Appl. Phycol. 2004, 16, 245–262. [Google Scholar] [CrossRef]
- Andrade, L.R.; Leal, R.N.; Noseda, M.; Duarte, M.E.; Pereira, M.S.; Mourao, P.A.; Farina, M.; Amado Filho, G.M. Brown algae overproduce cell wall polysaccharides as a protection mechanism against the heavy metal toxicity. Mar. Pollut. Bull. 2010, 60, 1482–1488. [Google Scholar]
- Berteau, O.; Mulloy, B. Sulfated fucans, fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology 2003, 13, 29R–40R. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–223. [Google Scholar] [CrossRef]
- Li, B.; Lu, F.; Wei, X.; Zhao, R. Fucoidan: Structure and bioactivity. Molecules 2008, 13, 1671–1695. [Google Scholar] [CrossRef]
- Bilan, M.I.; Grachev, A.A.; Ustuzhanina, N.E.; Shashkov, A.S.; Nifantiev, N.E.; Usov, A.I. Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydr. Res. 2002, 337, 719–730. [Google Scholar] [CrossRef]
- Chizhov, A.O.; Dell, A.; Morris, H.R.; Haslam, S.M.; McDowell, R.A.; Shashkov, A.S.; Nifant’ev, N.E.; Khatuntseva, E.A.; Usov, A.I. A study of fucoidan from the brown seaweed Chorda filum. Carbohydr. Res. 1999, 320, 108–119. [Google Scholar] [CrossRef]
- Li, B.; Wei, X.J.; Sun, J.L.; Xu, S.Y. Structural investigation of a fucoidan containing a fucose-free core from the brown seaweed Hizikia fusiforme. Carbohydr. Res. 2006, 341, 1135–1146. [Google Scholar] [CrossRef]
- Usov, A.I.; Smirnova, G.P.; Bilan, M.I.; Shashkov, A.S. Polysaccharides of algae. 53. Brown alga Laminaria cicchorioides. Bioorg. Khim. 1998, 24, 382–389. [Google Scholar]
- Shevchenko, N.M.; Anastiuk, S.D.; Gerasimenko, N.I.; Dmitrenok, P.S.; Isakov, V.V.; Zvyagintseva, T.N. Polysaccharide and lipid composition of the brown seaweed Laminaria gurjanovae. Rus. J. Bioorg. Chem. 2007, 33, 88–98. [Google Scholar] [CrossRef]
- Thinh, P.D.; Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Ly, B.M.; Zvyagintseva, T.N. Structural characteristics and anticancer activity of fucoidan from the brown alga Sargassum mcclurei. Mar. Drugs 2013, 11, 1456–1476. [Google Scholar] [CrossRef]
- Synytsya, A.; Kim, W.J.; Kim, S.M.; Pohl, R.; Synytsya, A.; Kvasnicka, F.; Copikova, J.; Park, Y.I. Structure and antitumour activity of fucoidan isolated from sporophyll of Korean brown seaweed Undaria pinnatifida. Carbohydr. Polym. 2010, 81, 41–48. [Google Scholar]
- Wang, J.; Zhang, Q.; Zhang, Z.; Zhang, H.; Niu, X. Structural studies on a novel fucogalactan sulfate extracted from the brown seaweed Laminaria japonica. Int. J. Biol. Macromol. 2010, 47, 126–131. [Google Scholar] [CrossRef]
- Sakai, T.; Kimura, H.; Kojima, K.; Shimanaka, K.; Ikai, K.; Kato, I. Marine bacterial sulfated fucoglucuronomannan (SFGM) lyase digests brown algal SFGM into trisaccharides. Mar. Biotechnol. 2003, 5, 70–78. [Google Scholar] [CrossRef]
- Pomin, V.H. Fucanomics and galactanomics: Marine distribution, medicinal impact, conceptions, and challenges. Mar. Drugs 2012, 10, 793–811. [Google Scholar] [CrossRef]
- Pomin, V.H.; Mourao, P.A. Structure, biology, evolution, and medical importance of sulfated fucans and galactans. Glycobiology 2008, 18, 1016–1027. [Google Scholar] [CrossRef]
- Chung, H.J.; Jeun, J.; Houng, S.J.; Jun, H.J.; Kweon, D.K.; Lee, S.J. Toxicological evaluation of fucoidan from Undaria pinnatifida in vitro and in vivo. Phytother. Res. 2010, 24, 1078–1083. [Google Scholar]
- Kim, K.J.; Lee, O.H.; Lee, B.Y. Genotoxicity studies on fucoidan from Sporophyll of Undaria pinnatifida. Food Chem. Toxicol. 2010, 48, 1101–1104. [Google Scholar]
- Kim, K.J.; Lee, O.H.; Lee, H.H.; Lee, B.Y. A 4-week repeated oral dose toxicity study of fucoidan from the sporophyll of Undaria pinnatifida in Sprague-Dawley rats. Toxicology 2010, 267, 154–158. [Google Scholar] [CrossRef]
- Mizuno, T.; Kinoshita, T.; Zhuang, C.; Ito, H.; Mayuzumi, Y. Antitumor-active heteroglycans from niohshimeji mushroom, Tricholoma giganteum. Biosci. Biotechnol. Biochem. 1995, 59, 568–571. [Google Scholar]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The fucoidans from brown algae of Far-Eastern seas: Anti-tumor activity and structure-function relationship. Food Chem. 2013, 141, 1211–1217. [Google Scholar]
- Wijesekara, I.; Pangestuti, R.; Kim, S. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011, 84, 14–21. [Google Scholar] [CrossRef]
- Alekseyenko, T.V.; Zhanayeva, S.Y.; Venediktova, S.Y.; Zvyagintseva, T.N.; Kuznetsova, T.A.; Besednova, N.N.; Korolenko, T.A. Antitumor and antimetastatic activity of fucoidan, a sulfated polysaccharide from the Okhotsk sea Fucus evanescens brown alga. Bull. Exp. Biol. Med. 2007, 143, 730–732. [Google Scholar]
- Itoh, H.; Noda, H.; Amano, H.; Zhuaug, C.; Mizuno, T.; Ito, H. Antitumor activity and immunological properties of marine algal polysaccharides, especially fucoidan, prepared from Sargassum thunbergii of Phaeophyceae. Anticancer Res. 1993, 13, 2045–2052. [Google Scholar]
- Kusaykin, M.; Bakunina, I.; Sova, V.; Ermakova, S.; Kuznetsova, T.; Besednova, N.; Zaporozhets, T.; Zvyagintseva, T. Structure, biological activity, and enzymatic transformation of fucoidans from the brown seaweeds. Biotechnol. J. 2008, 3, 904–915. [Google Scholar] [CrossRef]
- Shibata, H.; Iimuro, M.; Uchiya, N.; Kawamori, T.; Nagaoka, M.; Ueyama, S.; Hashimoto, S.; Yokokura, T.; Sugimura, T.; Wakabayashi, K. Preventive effects of Cladosiphon fucoidan against Helicobacter pylori infection in Mongolian gerbils. Helicobacter 2003, 8, 59–65. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, G.Y.; Nam, T.J.; Deuk Kim, N.; Hyun Choi, Y. Antiproliferative activity of fucoidan was associated with the induction of apoptosis and autophagy in AGS human gastric cancer cells. J. Food Sci. 2011, 76, T77–T83. [Google Scholar] [CrossRef]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef]
- Foley, S.A.; Szegezdi, E.; Mulloy, B.; Samali, A.; Tuohy, M.G. An unfractionated fucoidan from Ascophyllum nodosum: Extraction, characterization, and apoptotic effects in vitro. J. Nat. Prod. 2011, 74, 1851–1861. [Google Scholar]
- Athukorala, Y.; Ahn, G.N.; Jee, Y.H.; Kim, G.Y.; Kim, S.H.; Ha, J.H.; Kang, J.S.; Lee, K.W.; Jeon, Y.J. Antiproliferative activity of sulfated polysaccharide isolated from an enzymatic digest of Ecklonia cava on the U-937 cell line. J. Appl. Phycol. 2009, 21, 307–314. [Google Scholar] [CrossRef]
- Costa, L.S.; Fidelis, G.P.; Telles, C.B.; Dantas-Santos, N.; Camara, R.B.; Cordeiro, S.L.; Costa, M.S.; Almeida-Lima, J.; Melo-Silveira, R.F.; Oliveira, R.M.; Albuquerque, I.R.L; Andrade, G.P.V.; Rocha, H.A.O. Antioxidant and antiproliferative activities of heterofucans from the seaweed Sargassum filipendula. Mar. Drugs 2011, 9, 952–966. [Google Scholar] [CrossRef]
- Costa, L.S.; Telles, C.B.; Oliveira, R.M.; Nobre, L.T.; Dantas-Santos, N.; Camara, R.B.; Costa, M.S.; Almeida-Lima, J.; Melo-Silveira, R.F.; Albuquerque, I.R.; et al. Heterofucan from Sargassum filipendula induces apoptosis in HeLa cells. Mar. Drugs 2011, 9, 603–614. [Google Scholar] [CrossRef]
- Zhang, Z.; Teruya, K.; Eto, H.; Shirahata, S. Fucoidan extract induces apoptosis in MCF-7 cells via a mechanism involving the ROS-dependent JNK activation and mitochondria-mediated pathways. PLoS One 2011, 6, e27441. [Google Scholar]
- Hyun, J.H.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Boo, H.J.; Kwon, J.M.; Koh, Y.S.; Hyun, J.W.; Park, D.B.; Yoo, E.S.; et al. Apoptosis inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol. Pharm. Bull. 2009, 32, 1760–1764. [Google Scholar] [CrossRef]
- Kim, E.J.; Park, S.Y.; Lee, J.Y.; Park, J.H. Fucoidan present in brown algae induces apoptosis of human colon cancer cells. BMC Gastroenterol. 2010, 10, 96. [Google Scholar] [CrossRef]
- Yamasaki-Miyamoto, Y.; Yamasaki, M.; Tachibana, H.; Yamada, K. Fucoidan induces apoptosis through activation of caspase-8 on human breast cancer MCF-7 cells. J. Agric. Food Chem. 2009, 57, 8677–8682. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. Sulfated polysaccharides from brown seaweeds Saccharina japonica and Undaria pinnatifida: isolation, structural characteristics, and antitumor activity. Carbohydr. Res. 2011, 346, 2769–2776. [Google Scholar] [CrossRef]
- Jin, J.O.; Song, M.G.; Kim, Y.N.; Park, J.I.; Kwak, J.Y. The mechanism of fucoidan-induced apoptosis in leukemic cells: Involvement of ERK1/2, JNK, glutathione, and nitric oxide. Mol. Carcinog. 2010, 49, 771–782. [Google Scholar]
- Aisa, Y.; Miyakawa, Y.; Nakazato, T.; Shibata, H.; Saito, K.; Ikeda, Y.; Kizaki, M. Fucoidan induces apoptosis of human HS-sultan cells accompanied by activation of caspase-3 and down-regulation of ERK pathways. Am. J. Hematol. 2005, 78, 7–14. [Google Scholar] [CrossRef]
- Carmeliet, P. Angiogenesis in health and disease. Nat. Med. 2003, 9, 653–660. [Google Scholar] [CrossRef]
- Narazaki, M.; Segarra, M.; Tosato, G. Sulfated polysaccharides identified as inducers of neuropilin-1 internalization and functional inhibition of VEGF165 and semaphorin3A. Blood 2008, 111, 4126–4136. [Google Scholar] [CrossRef]
- Cho, M.L.; Lee, B.Y.; You, S.G. Relationship between oversulfation and conformation of low and high molecular weight fucoidans and evaluation of their in vitro anticancer activity. Molecules 2011, 16, 291–297. [Google Scholar]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Teruya, T.; Konishi, T.; Uechi, S.; Tamaki, H.; Tako, M. Anti-proliferative activity of oversulfated fucoidan from commercially cultured Cladosiphon okamuranus TOKIDA in U937 cells. Int. J. Biol. Macromol. 2007, 41, 221–226. [Google Scholar] [CrossRef]
- Yang, C.; Chung, D.; Shin, I.S.; Lee, H.; Kim, J.; Lee, Y.; You, S. Effects of molecular weight and hydrolysis conditions on anticancer activity of fucoidans from sporophyll of Undaria pinnatifida. Int. J. Biol. Macromol. 2008, 43, 433–437. [Google Scholar] [CrossRef]
- Ye, J.; Li, Y.; Teruya, K.; Katakura, Y.; Ichikawa, A.; Eto, H.; Hosoi, M.; Hosoi, M.; Nishimoto, S.; Shirahata, S. Enzyme-digested fucoidan extracts derived from seaweed Mozuku of Cladosiphon novae-caledoniae kylin inhibit invasion and angiogenesis of tumor cells. Cytotechnology 2005, 47, 117–126. [Google Scholar] [CrossRef]
- Liu, J.M.; Bignon, J.; Haroun-Bouhedja, F.; Bittoun, P.; Vassy, J.; Fermandjian, S.; Wdzieczak-Bakala, J.; Boisson-Vidal, C. Inhibitory effect of fucoidan on the adhesion of adenocarcinoma cells to fibronectin. Anticancer Res. 2005, 25, 2129–2133. [Google Scholar]
- Philchenkov, A.; Zavelevich, M.; Imbs, T.; Zaporozhets, T.; Zvyagintseva, T. Sensitization of human malignant lymphoid cells to etoposide by fucoidan, a brown seaweed polysaccharide. Exp. Oncol. 2007, 29, 181–185. [Google Scholar]
- Jiang, Z.; Okimura, T.; Yokose, T.; Yamasaki, Y.; Yamaguchi, K.; Oda, T. Effects of sulfated fucan, ascophyllan, from the brown alga Ascophyllum nodosum on various cell lines: A comparative study on ascophyllan and fucoidan. J. Biosci. Bioeng. 2010, 110, 113–117. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Tarbeeva, D.V.; Ermakova, S.P.; Zvyagintseva, T.N. The structural characteristics and biological activity of fucoidans from the brown algae Alaria sp. and Saccharina japonica of different reproductive status. Chem. Biodivers. 2012, 9, 817–828. [Google Scholar] [CrossRef]
- Vishchuk, O.S.; Ermakova, S.P.; Zvyagintseva, T.N. The effect of sulfated (1→3)-alpha-l-fucan from the brown alga Saccharina cichorioides Miyabe on resveratrol-induced apoptosis in colon carcinoma cells. Mar. Drugs 2013, 11, 194–212. [Google Scholar] [CrossRef]
- Jeong, B.E.; Ko, E.J.; Joo, H.G. Cytoprotective effects of fucoidan, an algae-derived polysaccharide on 5-fluorouracil-treated dendritic cells. Food Chem. Toxicol. 2012, 50, 1480–1484. [Google Scholar] [CrossRef]
- Yang, M.; Ma, C.; Sun, J.; Shao, Q.; Gao, W.; Zhang, Y.; Li, Z.; Xie, Q.; Dong, Z.; Qu, X. Fucoidan stimulation induces a functional maturation of human monocyte-derived dendritic cells. Int. Immunopharmacol. 2008, 8, 1754–1760. [Google Scholar] [CrossRef]
- Jin, J.O.; Park, H.Y.; Xu, Q.; Park, J.I.; Zvyagintseva, T.; Stonik, V.A.; Kwak, J.Y. Ligand of scavenger receptor class A indirectly induces maturation of human blood dendritic cells via production of tumor necrosis factor-alpha. Blood 2009, 113, 5839–5847. [Google Scholar] [CrossRef]
- Hu, Y.; Cheng, S.C.; Chan, K.T.; Ke, Y.; Xue, B.; Sin, F.W.; Zeng, C.; Xie, Y. Fucoidin enhances dendritic cell-mediated T-cell cytotoxicity against NY-ESO-1 expressing human cancer cells. Biochem. Biophys. Res. Commun. 2010, 392, 329–334. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.S.; Kim, E. Fucoidan from seaweed Fucus vesiculosus inhibits migration and invasion of human lung cancer cell via PI3K-Akt-mTOR pathways. PLoS One 2012, 7, e50624. [Google Scholar]
- Xue, M.; Ge, Y.; Zhang, J.; Wang, Q.; Hou, L.; Liu, Y.; Sun, L.; Li, Q. Anticancer properties and mechanisms of fucoidan on mouse breast cancer in vitro and in vivo. PLoS One 2012, 7, e43483. [Google Scholar]
- Lee, N.Y.; Ermakova, S.P.; Choi, H.K.; Kusaykin, M.I.; Shevchenko, N.M.; Zvyagintseva, T.N.; Choi, H.S. Fucoidan from Laminaria cichorioides inhibits AP-1 transactivation and cell transformation in the mouse epidermal JB6 cells. Mol. Carcinog. 2008, 47, 629–637. [Google Scholar] [CrossRef]
- Lee, N.Y.; Ermakova, S.P.; Zvyagintseva, T.N.; Kang, K.W.; Dong, Z.; Choi, H.S. Inhibitory effects of fucoidan on activation of epidermal growth factor receptor and cell transformation in JB6 Cl41 cells. Food Chem. Toxicol. 2008, 46, 1793–1800. [Google Scholar] [CrossRef]
- Moon, H.J.; Lee, S.H.; Ku, M.J.; Yu, B.C.; Jeon, M.J.; Jeong, S.H.; Stonik, V.A.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 promoter expression and down regulation of type I procollagen synthesis in human skin fibroblasts. Eur. J. Dermatol. 2009, 19, 129–134. [Google Scholar]
- Moon, H.J.; Lee, S.R.; Shim, S.N.; Jeong, S.H.; Stonik, V.A.; Rasskazov, V.A.; Zvyagintseva, T.; Lee, Y.H. Fucoidan inhibits UVB-induced MMP-1 expression in human skin fibroblasts. Biol. Pharm. Bull. 2008, 31, 284–289. [Google Scholar] [CrossRef]
- Moon, H.J.; Park, K.S.; Ku, M.J.; Lee, M.S.; Jeong, S.H.; Imbs, T.I.; Zvyagintseva, T.N.; Ermakova, S.P.; Lee, Y.H. Effect of Costaria costata fucoidan on expression of matrix metalloproteinase-1 promoter, mRNA, and protein. J. Nat. Prod. 2009, 72, 1731–1734. [Google Scholar] [CrossRef]
- Jintang, S.; Alei, F.; Yun, Z.; Weixu, H.; Meixiang, Y.; Fengcai, W.; Xun, Q. Fucoidan increases TNF-alpha-induced MMP-9 secretion in monocytic cell line U937. Inflamm. Res. 2010, 59, 271–276. [Google Scholar] [CrossRef]
- Cumashi, A.; Ushakova, N.A.; Preobrazhenskaya, M.E.; D’Incecco, A.; Piccoli, A.; Totani, L.; Tinari, N.; Morozevich, G.E.; Berman, A.E.; Bilan, M.I.; et al. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology 2007, 17, 541–552. [Google Scholar]
- Hemmingson, J.A.; Falshaw, R.; Furneaux, R.H.; Thompson, K. Structure and antiviral activity of the galactofucans from Undaria pinnatifida. J. Appl. Phycol. 2006, 18, 185–193. [Google Scholar] [CrossRef]
- Makarenkova, I.D.; Deriabin, P.G.; L’vov, D.K.; Zvyagintseva, T.N.; Besednova, N.N. Antiviral activity of sulfated polysaccharide from the brown alga Laminaria japonica against avian influenza A (H5N1) virus infection in the cultured cells. Vopr. Virusol. 2010, 55, 41–45. [Google Scholar]
- Wang, J.; Zhang, Q.; Zhang, Z.; Song, H.; Li, P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010, 46, 6–12. [Google Scholar]
- Zhu, W.; Ooi, V.E.; Chan, P.K.; Ang, P.O., Jr. Isolation and characterization of a sulfated polysaccharide from the brown alga Sargassum patens and determination of its anti-herpes activity. Biochem. Cell. Biol. 2003, 81, 25–33. [Google Scholar]
- Teas, J.; Vena, S.; Cone, D.L.; Irhimeh, M. The consumption of seaweed as a protective factor in the etiology of breast cancer: proof of principle. J. Appl. Phycol. 2013, 25, 771–779. [Google Scholar] [CrossRef]
- Ikeguchi, M.; Yamamoto, M.; Arai, Y.; Maeta, Y.; Ashida, K.; Katano, K.; Miki, Y.; Kimura, T. Fucoidan reduces the toxicities of chemotherapy for patients with unresectable advanced or recurrent colorectal cancer. Oncol. Lett. 2011, 2, 319–322. [Google Scholar]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef]
- Imbs, T.I.; Shevchenko, N.M.; Semenova, T.L.; Sukhoverkhov, S.V.; Zvyagintseva, T.N. Compositional heterogeneity of sulfated polysaccharides synthesized by the brown alga Costaria costata. Chem. Nat. Comp. 2011, 47, 96–97. [Google Scholar] [CrossRef]
- Skriptsova, A.V.; Shevchenko, N.M.; Tarbeeva, D.V.; Zvyagintseva, T.N. Comparative study of polysaccharides from reproductive and sterile tissues of five brown seaweeds. Mar. Biotechnol. 2012, 14, 304–311. [Google Scholar] [CrossRef]
- Anastyuk, S.D.; Shevchenko, N.M.; Nazarenko, E.L.; Dmitrenok, P.S.; Zvyagintseva, T.N. Structural analysis of a fucoidan from the brown alga Fucus evanescens by MALDI-TOF and tandem ESI mass spectrometry. Carbohydr. Res. 2009, 344, 779–787. [Google Scholar] [CrossRef]
- Bilan, M.I.; Usov, A.I. Structural analysis of fucoidans. Nat. Prod. Commun. 2008, 3, 1639–1648. [Google Scholar]
- Usov, A.I.; Chizhov, A.O. New data on the structure of laminaran from Chorda filum (L.) Lam. and reserve glycans from other brown algae. Russ. Chem. Bull. 1993, 42, 1597–1601. [Google Scholar] [CrossRef]
- Rioux, L.E.; Turgeon, S.L.; Beaulieu, M. Structural characterization of laminaran and galactofucan extracted from the brown seaweed Saccharina longicruris. Phytochemistry 2010, 71, 1586–1595. [Google Scholar] [CrossRef]
- Menshova, R.V.; Ermakova, S.P.; Anastyuk, S.D.; Isakov, V.V.; Dubrovskaya, Y.V.; Kusaikin, M.I.; Um, B.H.; Zvyagintseva, T.N. Structure, enzymatic transformation and anticancer activity of branched high molecular weight laminaran from brown alga Eisenia bicyclis. Carbohydr. Polym. 2014, 99, 101–109. [Google Scholar] [CrossRef]
- Akramiene, D.; Aleksandraviciene, C.; Grazeliene, G.; Zalinkevicius, R.; Suziedelis, K.; Didziapetriene, J.; Simonsen, U.; Stankevicius, E.; Kevelaitis, E. Potentiating effect of beta-glucans on photodynamic therapy of implanted cancer cells in mice. Tohoku J. Exp. Med. 2010, 220, 299–306. [Google Scholar] [CrossRef]
- Kuda, T.; Yano, T.; Matsuda, N.; Nishizawa, M. Inhibitory effects of laminaran and low molecular alginate against the putrefactive compounds produced by intestinal microflora in vitro and in rats. Food Chem. 2005, 91, 745–749. [Google Scholar] [CrossRef]
- Mantovani, M.S.; Bellini, M.F.; Angeli, J.P.; Oliveira, R.J.; Silva, A.F.; Ribeiro, L.R. beta-Glucans in promoting health: prevention against mutation and cancer. Mutat. Res. 2008, 658, 154–161. [Google Scholar] [CrossRef]
- Miao, H.Q.; Elkin, M.; Aingorn, E.; Ishai-Michaeli, R.; Stein, C.A.; Vlodavsky, I. Inhibition of heparanase activity and tumor metastasis by laminarin sulfate and synthetic phosphorothioate oligodeoxynucleotides. Int. J. Cancer 1999, 83, 424–431. [Google Scholar] [CrossRef]
- Liu, Z.; Lv, D.; Liu, S.; Gong, J.; Wang, D.; Xiong, M.; Chen, X.; Xiang, R.; Tan, X. Alginic acid-coated chitosan nanoparticles loaded with legumain DNA vaccine: effect against breast cancer in mice. PLoS One 2013, 8, e60190. [Google Scholar]
- Usov, A.I. Polysaccharides of the red algae. Adv. Carbohydr. Chem. Biochem. 2011, 65, 115–217. [Google Scholar]
- Buck, C.B.; Thompson, C.D.; Roberts, J.N.; Muller, M.; Lowy, D.R.; Schiller, J.T. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006, 2, e69. [Google Scholar] [CrossRef]
- Sun, T.; Tao, H.; Xie, J.; Zhang, S.; Xu, X. Degradation and antioxidant activity of κ-Carrageenans. J. Appl. Polym. Sci. 2010, 117, 194–199. [Google Scholar]
- Hu, X.; Jiang, X.; Aubree, E.; Boulenguer, P.; Critchley, A.T. Preparation and in vivo antitumor activity of kappa-carrageenan oligosaccharides. Pharm. Biol. 2006, 44, 646–650. [Google Scholar] [CrossRef]
- Zhou, G.; Xin, H.; Sheng, W.; Li, Z.; Xu, Z. In vivo growth-inhibition of S180 tumor by mixture of 5-Fu and low molecular lambda-carrageenan from Chondrus ocellatus. Pharmacol. Res. 2005, 51, 153–157. [Google Scholar] [CrossRef]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Dai, J. Immunomodulation and antitumor activity of kappa-carrageenan oligosaccharides. Cancer Lett. 2006, 243, 228–234. [Google Scholar] [CrossRef]
- Lins, K.O.; Bezerra, D.P.; Alves, A.P.; Alencar, N.M.; Lima, M.W.; Torres, V.M.; Farias, W.R.; Pessoa, C.; de Moraes, M.O.; Costa-Lotufo, L.V. Antitumor properties of a sulfated polysaccharide from the red seaweed Champia feldmannii (Diaz-Pifferer). J. Appl. Toxicol. 2009, 29, 20–26. [Google Scholar] [CrossRef]
- Tobacman, J.K. Review of harmful gastrointestinal effects of carrageenan in animal experiments. Environ. Health Perspect. 2001, 109, 983–994. [Google Scholar] [CrossRef]
- Benard, C.; Cultrone, A.; Michel, C.; Rosales, C.; Segain, J.P.; Lahaye, M.; Galmiche, J.P.; Cherbut, C.; Blottiere, H.M. Degraded carrageenan causing colitis in rats induces TNF secretion and ICAM-1 upregulation in monocytes through NF-kappaB activation. PLoS One 2010, 5, e8666. [Google Scholar]
- Cohen, S.M.; Ito, N. A critical review of the toxicological effects of carrageenan and processed eucheuma seaweed on the gastrointestinal tract. Crit. Rev. Toxicol. 2002, 32, 413–444. [Google Scholar] [CrossRef]
- Weiner, M.L.; Nuber, D.; Blakemore, W.R.; Harriman, J.F.; Cohen, S.M. A 90-day dietary study on kappa carrageenan with emphasis on the gastrointestinal tract. Food Chem. Toxicol. 2007, 45, 98–106. [Google Scholar] [CrossRef]
- Lahaye, M.; Robic, A. Structure and functional properties of ulvan, a polysaccharide from green seaweeds. Biomacromolecules 2007, 8, 1765–1774. [Google Scholar] [CrossRef]
- Bilan, M.I.; Vinogradova, E.V.; Shashkov, A.S.; Usov, A.I. Structure of a highly pyruvylated galactan sulfate from the Pacific green alga Codium yezoense (Bryopsidales, Chlorophyta). Carbohydr. Res. 2007, 342, 586–596. [Google Scholar] [CrossRef]
- Farias, E.H.; Pomin, V.H.; Valente, A.P.; Nader, H.B.; Rocha, H.A.; Mourao, P.A. A preponderantly 4-sulfated, 3-linked galactan from the green alga Codium isthmocladum. Glycobiology 2008, 18, 250–259. [Google Scholar]
- Fernandez, P.V.; Estevez, J.M.; Cerezo, A.S.; Ciancia, M. Sulfated β-d-mannan from green seaweed Codium vermilara. Cabohydr. Polym. 2012, 87, 916–919. [Google Scholar]
- Costa, L.S.; Fidelis, G.P.; Cordeiro, S.L.; Oliveira, R.M.; Sabry, D.A.; Camara, R.B.; Nobre, L.T.; Costa, M.S.; Almeida-Lima, J.; Farias, E.H.; et al. Biological activities of sulfated polysaccharides from tropical seaweeds. Biomed. Pharmacother. 2010, 64, 21–28. [Google Scholar] [CrossRef]
- Ji, H.; Shao, H.; Zhang, C.; Hong, P.; Xiong, H. Separation of the polysaccharides in Caulerpa racemosa and their chemical composition and antitumor activity. J. Appl. Polym. Sci. 2008, 110, 1435–1440. [Google Scholar] [CrossRef]
- Kim, J.K.; Cho, M.L.; Karnjanapratum, S.; Shin, I.S.; You, S.G. In vitro and in vivo immunomodulatory activity of sulfated polysaccharides from Enteromorpha prolifera. Int. J. Biol. Macromol. 2011, 49, 1051–1058. [Google Scholar] [CrossRef]
- Leiro, J.M.; Castro, R.; Arranz, J.A.; Lamas, J. Immunomodulating activities of acidic sulphated polysaccharides obtained from the seaweed Ulva rigida C. Agardh. Int. Immunopharmacol. 2007, 7, 879–888. [Google Scholar] [CrossRef]
- Tabarsa, M.; Han, J.H.; Kim, C.Y.; You, S.G. Molecular characteristics and immunomodulatory activities of water-soluble sulfated polysaccharides from Ulva pertusa. J. Med. Food 2012, 15, 135–144. [Google Scholar] [CrossRef]
- Devaki, T.; Sathivel, A.; BalajiRaghavendran, H.R. Stabilization of mitochondrial and microsomal function by polysaccharide of Ulva lactuca on d-galactosamine induced hepatitis in rats. Chem. Biol. Interact. 2009, 177, 83–88. [Google Scholar] [CrossRef]
- Sathivel, A.; Raghavendran, H.R.; Srinivasan, P.; Devaki, T. Anti-peroxidative and anti-hyperlipidemic nature of Ulva lactuca crude polysaccharide on d-galactosamine induced hepatitis in rats. Food Chem. Toxicol. 2008, 46, 3262–3267. [Google Scholar]
- Shimura, H.; Itoh, K.; Sugiyama, A.; Ichijo, S.; Ichijo, M.; Furuya, F.; Nakamura, Y.; Kitahara, K.; Kobayashi, K.; Yukawa, Y.; et al. Absorption of radionuclides from the Fukushima nuclear accident by a novel algal strain. PLoS One 2012, 7, e44200. [Google Scholar]
- de Jesus Raposo, M.F.; de Morais, R.M.; de Morais, A.M. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef]
- Talyshinsky, M.M.; Souprun, Y.Y.; Huleihel, M.M. Anti-viral activity of red microalgal polysaccharides against retroviruses. Cancer Cell Int. 2002, 2, 8–14. [Google Scholar] [CrossRef]
- Hasui, M.; Matsuda, M.; Okutani, K.; Shigeta, S. In vitro antiviral activities of sulfated polysaccharides from a marine microalga (Cochlodinium polykrikoides) against human immunodeficiency virus and other enveloped viruses. Int. J. Biol. Macromol. 1995, 17, 293–297. [Google Scholar] [CrossRef]
- Fabregas, J.; Carcia, D.; Fernandez-Alonso, M.; Rocha, A.I.; Gomez-Puertas, P.; Escribano, J.M.; Otero, A.; Coll, J.M. In vitro inhibition of the replication of haemorrhagic septicaemia virus (VHSV) and African swine fever virus (ASFV) by extracts from marine microalgae. Antiviral Res. 1999, 44, 67–73. [Google Scholar] [CrossRef]
- Huleihel, M.; Ishanu, V.; Tal, J.; Arad, S.M. Antiviral effects of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J. Appl. Phycol. 2001, 13, 127–134. [Google Scholar] [CrossRef]
- Pugh, N.; Ross, S.A.; ElSohly, H.N.; ElSohly, M.A.; Pasco, D.S. Isolation of three high molecular weight polysaccharide preparations with potent immunostimulatory activity from Spirulina platensis, aphanizomenon flos-aquae and Chlorella pyrenoidosa. Planta Med. 2001, 67, 737–742. [Google Scholar] [CrossRef]
- Sun, L.; Wang, C.; Shi, Q.; Ma, C. Preparation of different molecular weight polysaccharides from Porphyridium cruentum and their antioxidant activities. Int. J. Biol. Macromol. 2009, 45, 42–47. [Google Scholar] [CrossRef]
- Park, E.J.; Pezzuto, J.M. Antioxidant marine products in cancer chemoprevention. Antioxid. Redox Signal. 2013, 19, 115–138. [Google Scholar] [CrossRef]
- Gardeva, E.; Toshkova, R.; Minkova, K.; Gigova, L. Cancer protective action of polysaccharide derived from microalga Porphyridium cruentum—biological background. Biotechnol. Biotechnol. Equip. 2009, 23, 783–787. [Google Scholar]
- Choi, W.Y.; Kang, D.H.; Lee, H.Y. Enhancement of immune activation activities of Spirulina maxima grown in deep-sea water. Int. J. Mol. Sci. 2013, 14, 12205–12221. [Google Scholar] [CrossRef]
- Ismail, M.F.; Ali, D.A.; Fernando, A.; Abdraboh, M.E.; Gaur, R.L.; Ibrahim, W.M.; Raj, M.H.; Ouhtit, A. Chemoprevention of rat liver toxicity and carcinogenesis by Spirulina. Int. J. Biol. Sci. 2009, 5, 377–387. [Google Scholar]
- Matsuda, M.; Yamori, T.; Naitoh, M.; Okutani, K. Structural revision of sulfated polysaccharide B-1 isolated from a marine Pseudomonas species and its cytotoxic activity against human cancer cell lines. Mar. Biotechnol. 2003, 5, 13–19. [Google Scholar] [CrossRef]
- Sun, C.; Wang, J.W.; Fang, L.; Gao, X.D.; Tan, R.X. Free radical scavenging and antioxidant activities of EPS2, an exopolysaccharide produced by a marine filamentous fungus Keissleriella sp. YS 4108. Life Sci. 2004, 75, 1063–1073. [Google Scholar] [CrossRef]
- Heath-Heckman, E.A.C.; McFall-Ngai, M.J. The occurrence of chitin in the hemocytes of invertebrates. Zoology 2011, 114, 191–198. [Google Scholar] [CrossRef]
- Kozlowski, E.O.; Gomes, A.M.; Silva, C.S.; Pereira, M.S.; de Vilela Silva, A.C.; Pavao, M.S.G. Structure and biological activities of glycosaminoglycan analogs from marine invertebrates: New therapeutic agents? In Glycans in Diseases and Therapeutics; Pavao, M.S.G., Ed.; Springer-Verlag: Heidelberg, Berlin, Germany, 2011; pp. 159–184. [Google Scholar]
- Yin, H.; Du, Y.; Zhang, J. Low molecular weight and oligomeric chitosans and their bioactivities. Curr. Top. Med. Chem. 2009, 9, 1546–1559. [Google Scholar] [CrossRef]
- Pomin, V.H.; de Souza Mourao, P.A. Structure versus anticoagulant and antithrombotic actions of marine sulfated polysaccharides. Braz. J. Pharmacogn. 2012, 22, 921–928. [Google Scholar]
- Luo, L.; Wu, M.; Xu, L.; Lian, W.; Xiang, J.; Lu, F.; Gao, N.; Xiao, C.; Wang, S.; Zhao, J. Comparison of physicochemical characteristics and anticoagulant activities of polysaccharides from three sea cucumbers. Mar. Drugs 2013, 11, 399–417. [Google Scholar] [CrossRef]
- Chen, S.; Li, G.; Wu, N.; Guo, X.; Liao, N.; Ye, X.; Liu, D.; Xue, C.; Chai, W. Sulfation pattern of the fucose branch is important for the anticoagulant and antithrombotic activities of fucosylated chondroitin sulfates. Biochim. Biophys. Acta 2013, 1830, 3054–3066. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, J.; Jin, W.; Zhang, Q. The antioxidant activities and neuroprotective effects of polysaccharides from the starfish Asterias rollestoni. Carbohydr. Polym. 2013, 95, 9–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, S.; Liang, H.; Wang, Y.; Wang, W.; Ji, A. Enchancing effect of a sea cucumber Stichopus japonicus sulfated polysaccharide on neurosphere formation in vitro. J. Biosci. Bioeng. 2010, 110, 479–486. [Google Scholar] [CrossRef]
- Sheng, X.; Zhang, N.; Song, S.; Li, M.; Liang, H.; Zhang, Y.; Wang, Y.; Ji, A. Morphological transformation and proliferation of rat astrocytes as induced by sulfated polysaccharides from the sea cucumber Stichopus japonicus. Neurosci. Lett. 2011, 503, 37–42. [Google Scholar] [CrossRef]
- Lian, W.; Wu, M.; Huang, N.; Gao, N.; Li, Z.; Zhang, Z.; Zheng, Y.; Peng, W.; Zhao, J. Anti-HIV-1 activity and structure-activity-relationship study of a fucosylated glycosaminoglycan from an echinoderm by targeting the conserved CD4 induced epitope. Biochim. Biophys. Acta 2013, 1830, 4681–4691. [Google Scholar] [CrossRef]
- Liu, C.H.; Lin, Q.X.; Gao, Y.; Ye, L.; Xing, Y.Y.; Xi, T. Characterization and antitumor activity of a polysaccharide from Strongylocentrotus nudus eggs. Carbohydr. Polym. 2007, 67, 313–318. [Google Scholar] [CrossRef]
- Liu, C.; Xi, T.; Lin, Q.; Xing, Y.; Ye, L.; Luo, X.; Wang, F. Immunomodulatory activity of polysaccharides isolated from Strongylocentrotus nudus eggs. Int. Immunopharmacol. 2008, 8, 1835–1841. [Google Scholar] [CrossRef]
- Wang, M.; Wang, H.; Tang, Y.; Kang, D.; Gao, Y.; Ke, M.; Dou, J.; Xi, T.; Zhou, C. Effective inhibition of a Strongylocentrotus nudus eggs polysaccharide against hepatocellular carcinoma is mediated via immunoregulation in vivo. Immunol. Lett. 2011, 141, 74–82. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Chen, J.; Tang, Y.; Dou, J.; Yu, J.; Xi, T.; Zhou, C. A polysaccharide from Strongylocentrotus nudus eggs protect against myelosuppression and immunosuppression in cyclophosphamide-treated mice. Int. Immunopharmacol. 2011, 11, 1946–1953. [Google Scholar] [CrossRef]
- Suna, L.; Zhua, B.; Lia, D.; Wanga, L.; Donga, X.; Muratab, Y.; Xingc, R.; Dongd, Y. Purification and bioactivity of a sulfated polysaccharide conjugate from viscera of abalone Haliotis discus hannai Ino. Food Agric. Immunol. 2010, 21, 15–26. [Google Scholar]
- Azmi, A.S.; Ahmad, A.; Banerjee, S.; Rangnekar, V.M.; Mohammad, R.M.; Sarkar, F.H. Chemoprevention of pancreatic cancer: Characterization of Par-4 and its modulation by 3,3′-diindolylmethane (DIM). Pharm. Res. 2008, 25, 2117–2124. [Google Scholar] [CrossRef]
- Surh, Y.-J. Molecular mechanisms of chemopreventive effects of selected dietary and medicinal phenolic compounds. Mutat. Res. 1999, 428, 305–327. [Google Scholar] [CrossRef]
- Pan, M.-H.; Ho, C.-T. Chemopreventive effects of natural dietary compounds on cancer development. Chem. Soc. Rev. 2008, 37, 2558–2574. [Google Scholar]
- Cerella, C.; Sobolewski, C.; Dicato, M.; Diederich, M. Targeting COX-2 expression by natural compounds: A promising alternative strategy to synthetic COX-2 inhibitors for cancer chemoprevention and therapy. Biochem. Pharmacol. 2010, 80, 1801–1815. [Google Scholar]
- Schumacher, M.; Kelkel, M.; Dicato, M.; Diederich, M. Gold from the sea: Marine compounds as inhibitors of the hallmarks of cancer. Biotechnol. Adv. 2011, 29, 531–547. [Google Scholar] [CrossRef]
- Kiprushina, Yu.O.; Lukyanov, P.A.; Odintsova, N.A. Effect of mytilan on the UV-radiation resistance of marine invertebrate larvae and human lymphocytes. Russ. J. Mar. Biol. 2010, 36, 305–310. [Google Scholar] [CrossRef]
- Kale, V.; Freysdottir, J.; Paulsen, B.S.; Fridjonsson, O.H.; Hreggvidsson, G.O.; Omarsdottir, S. Sulfated polysaccharide from the sea cucumber Cucumaria frondosa affect maturation of human dendritic cells and their activation of allogeneic CD4(+) T cells in vitro. Bioact. Carbohydr. Diet. Fibre 2013, 2, 108–117. [Google Scholar] [CrossRef]
- Borsig, L.; Wang, L.; Cavalcante, M.C.M.; Cardilo-Reis, L.; Ferreira, P.L.; Mourao, P.A.S.; Esko, J.D.; Pavao, M.S.G. Selectin bloking activity of a fucosylated chondroitin sulfate glycosaminoglycan from sea cucumber. Effect on tumor metastasis and neutrophil recruitment. J. Biol. Chem. 2007, 282, 14984–14991. [Google Scholar] [CrossRef]
- Kawashima, H.; Atarashi, K.; Hirose, M.; Hirose, J.; Yamada, S.; Sugahara, K.; Miyasaka, M. Oversulfated chondroitin/dermatan sulfates containing glcabeta1/iodaalpha1-3galnac(4,6-o-disulfate) interact with L- and P-selectin and chemokines. J. Biol. Chem. 2002, 277, 12921–12930. [Google Scholar]
- Wang, L.; Brown, J.R.; Vaki, A.; Esko, J.D. Heparin’s anti-inflammatory effects require glucosamine 6-o-sulfation and are mediated by blockade of L- and P-selectins. J. Clin. Invest. 2002, 110, 127–136. [Google Scholar]
- Wang, Y.; Su, W.; Zhang, C.; Xue, C.; Chang, Y.; Wu, X.; Tang, Q.; Wang, J. Protective effect of sea cucumber (Acaudina molpadioides) fucoidan against ethanol-induced gastric damage. Food Chem. 2012, 133, 1414–1419. [Google Scholar] [CrossRef]
- Xu, C.X.; Jin, H.; Chung, Y.S.; Shin, J.Y.; Lee, K.H.; Beck, G.R., Jr.; Palmos, G.N.; Choi, B.D.; Cho, M.H. Chondroitin sulfate extracted from ascidian tunic inhibits phorbol ester-induced expression of inflammatory factors VCAM-1 and COX-2 by blocking NF-κB activation in mouse skin. J. Agric. Food Chem. 2008, 56, 9667–9675. [Google Scholar] [CrossRef]
- Xu, C.X.; Jin, H.; Chung, Y.S.; Shin, J.Y.; Woo, M.A.; Lee, K.H.; Palmos, G.N.; Choi, B.D.; Cho, M.H. Chondroitin sulfate extracted from the Styela clava tunic suppresses TNF-α-induced expression of inflammatory factors, VCAM-1 and iNOS by blocking Akt/NF-κB signal in JB6 cells. Cancer Lett. 2008, 264, 93–100. [Google Scholar] [CrossRef]
- Brito, A.S.; Arimateia, D.S.; Souza, L.R.; Lima, M.A.; Santos, V.O.; Medeiros, V.P.; Ferreira, P.A.; Silva, R.A.; Ferreira, C.V.; Justo, G.Z.; et al. Ant-inflammatory properties of a geparin-like glycosaminoglycan with reduced anti-coagulant activity isolated from a marine shrimp. Bioorg. Med. Chem. 2008, 16, 9588–9595. [Google Scholar] [CrossRef]
- Belmiro, C.L.; Castelo-Branco, M.T.; Melim, L.M.; Schanaider, A.; Elia, C.; Madi, K.; Pavao, M.S.; de Souza, H.S. Unfractionated heparin and new heparin analogues from ascidians (chordate-tunicate) ameliorate colitis in rats. J. Biol. Chem. 2009, 284, 11267–11278. [Google Scholar]
- Dreyfuss, J.L.; Regatieri, C.V.; Lima, M.A.; Paredes-Gamero, E.J.; Brito, A.S.; Chavante, S.F.; Belfort, R.; Farah, M.E.; Nader, H.B. A heparin mimetic isolated from a marine shrimp suppresses neovascularization. J. Thromb. Haemost. 2010, 8, 1828–1837. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fedorov, S.N.; Ermakova, S.P.; Zvyagintseva, T.N.; Stonik, V.A. Anticancer and Cancer Preventive Properties of Marine Polysaccharides: Some Results and Prospects. Mar. Drugs 2013, 11, 4876-4901. https://doi.org/10.3390/md11124876
Fedorov SN, Ermakova SP, Zvyagintseva TN, Stonik VA. Anticancer and Cancer Preventive Properties of Marine Polysaccharides: Some Results and Prospects. Marine Drugs. 2013; 11(12):4876-4901. https://doi.org/10.3390/md11124876
Chicago/Turabian StyleFedorov, Sergey N., Svetlana P. Ermakova, Tatyana N. Zvyagintseva, and Valentin A. Stonik. 2013. "Anticancer and Cancer Preventive Properties of Marine Polysaccharides: Some Results and Prospects" Marine Drugs 11, no. 12: 4876-4901. https://doi.org/10.3390/md11124876
APA StyleFedorov, S. N., Ermakova, S. P., Zvyagintseva, T. N., & Stonik, V. A. (2013). Anticancer and Cancer Preventive Properties of Marine Polysaccharides: Some Results and Prospects. Marine Drugs, 11(12), 4876-4901. https://doi.org/10.3390/md11124876




