Fucoidan Does Not Exert Anti-Tumorigenic Effects on Uveal Melanoma Cell Lines
Abstract
:1. Introduction
2. Results
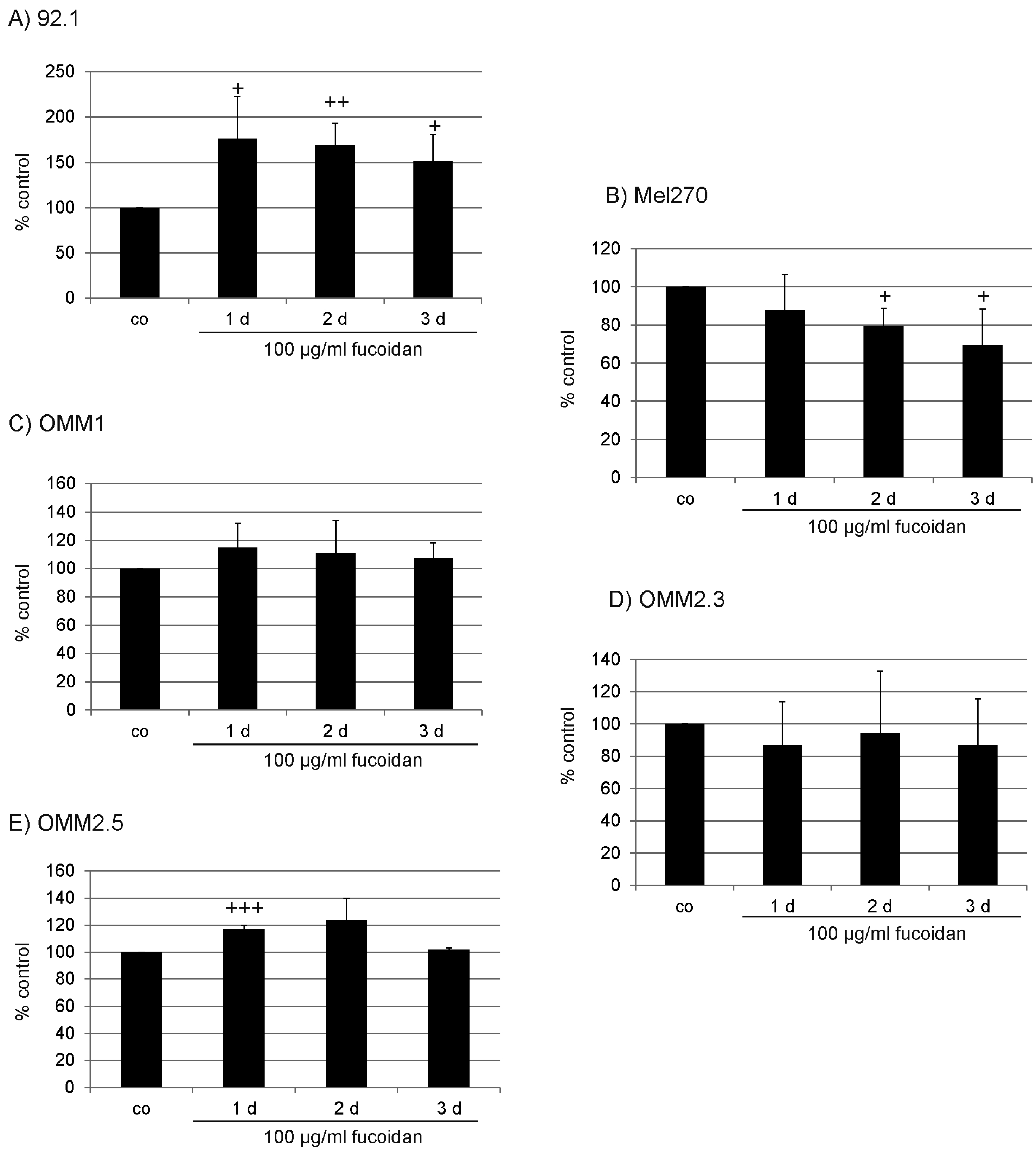
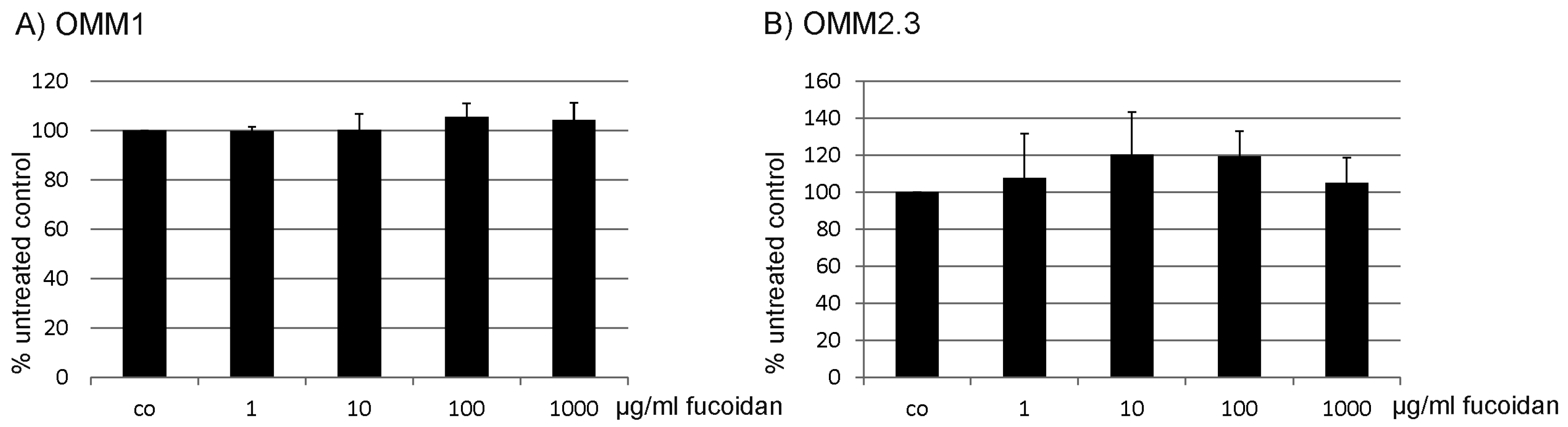
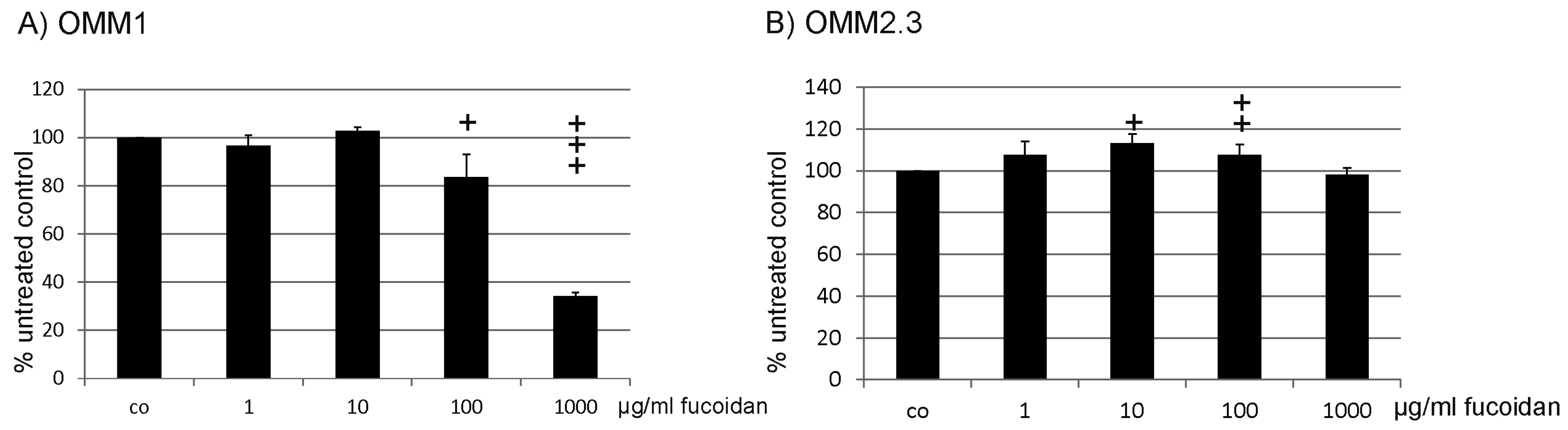
2.1. Proliferation
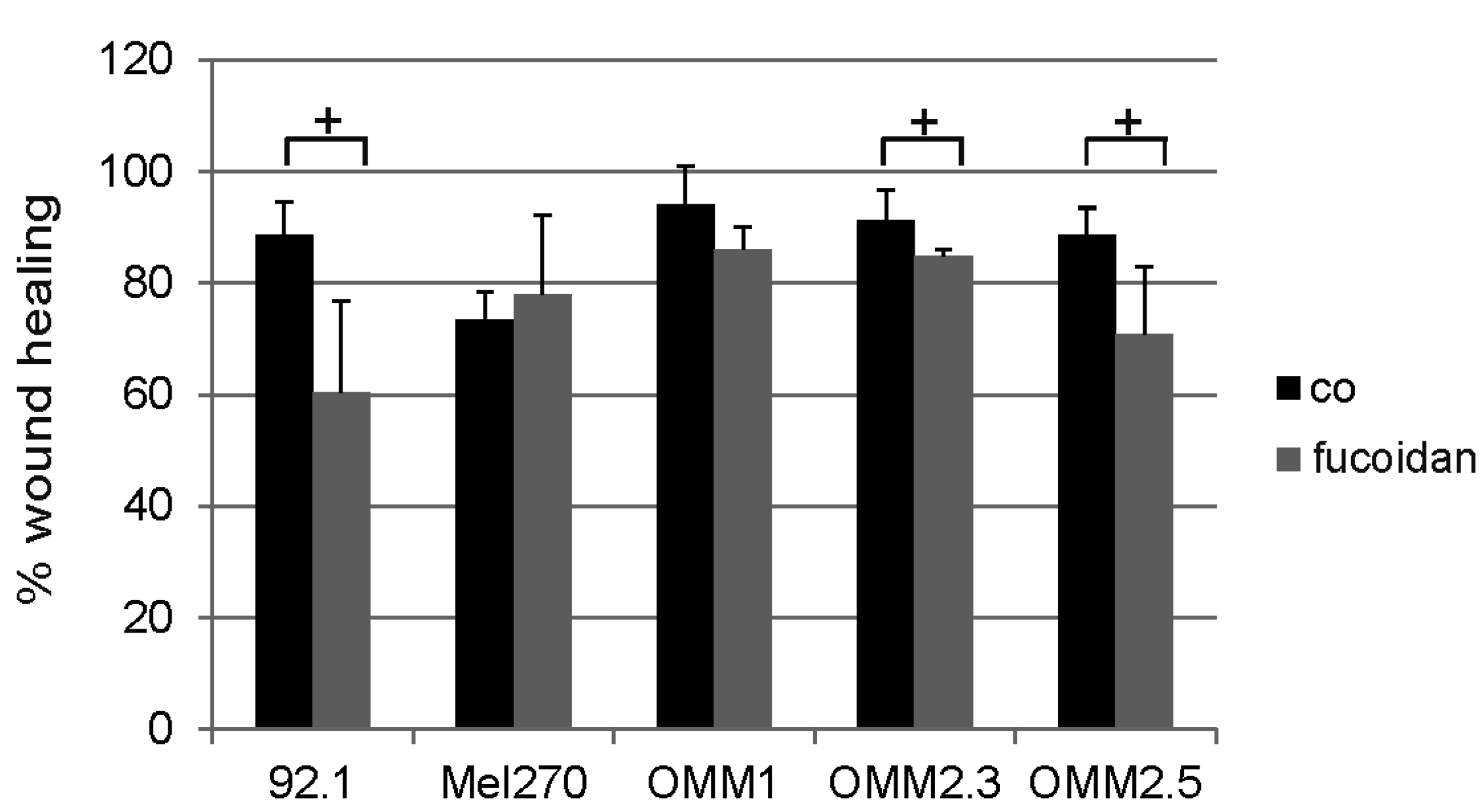
2.2. Wound Healing/Migration
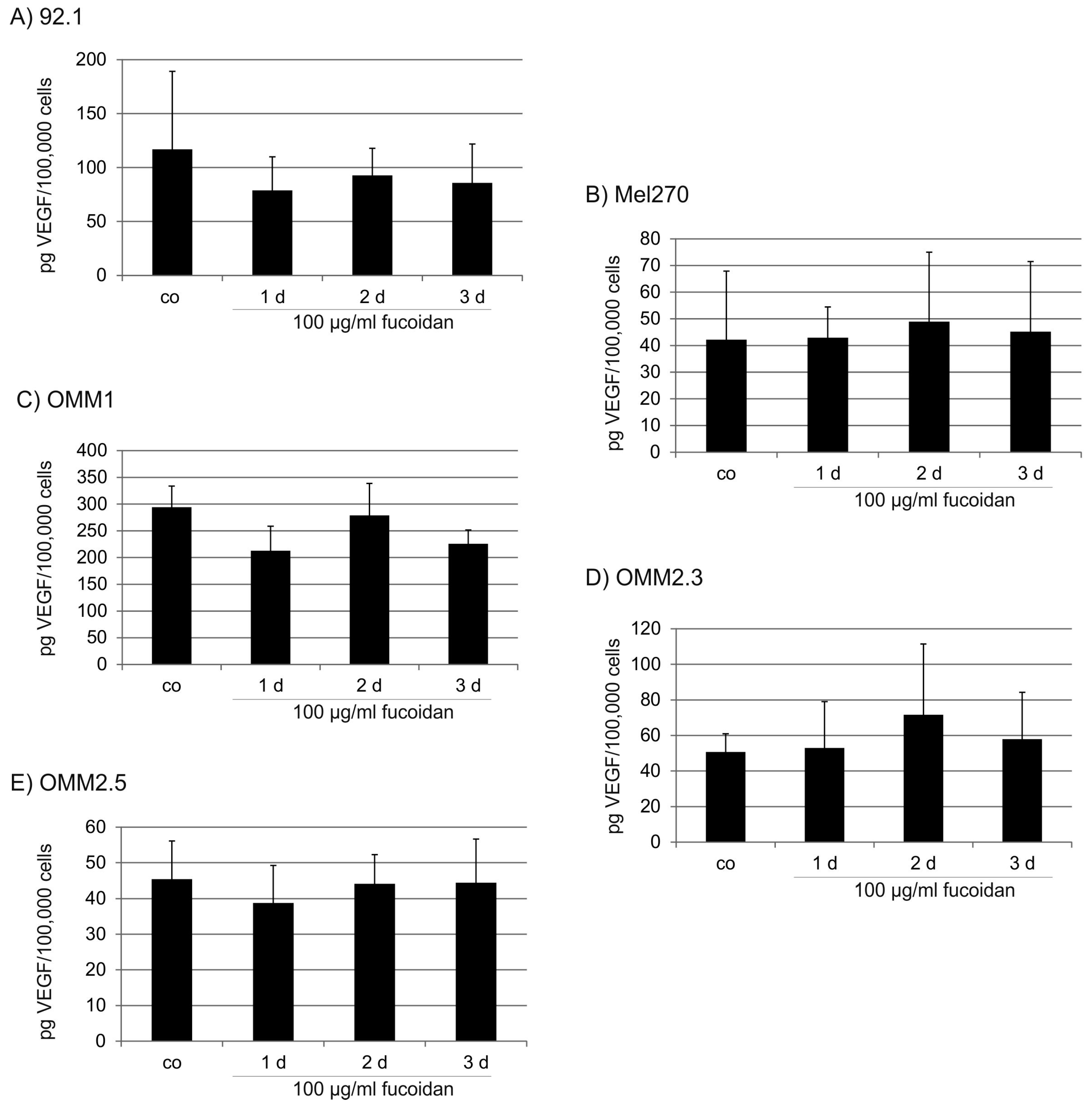
2.3. VEGF Secretion
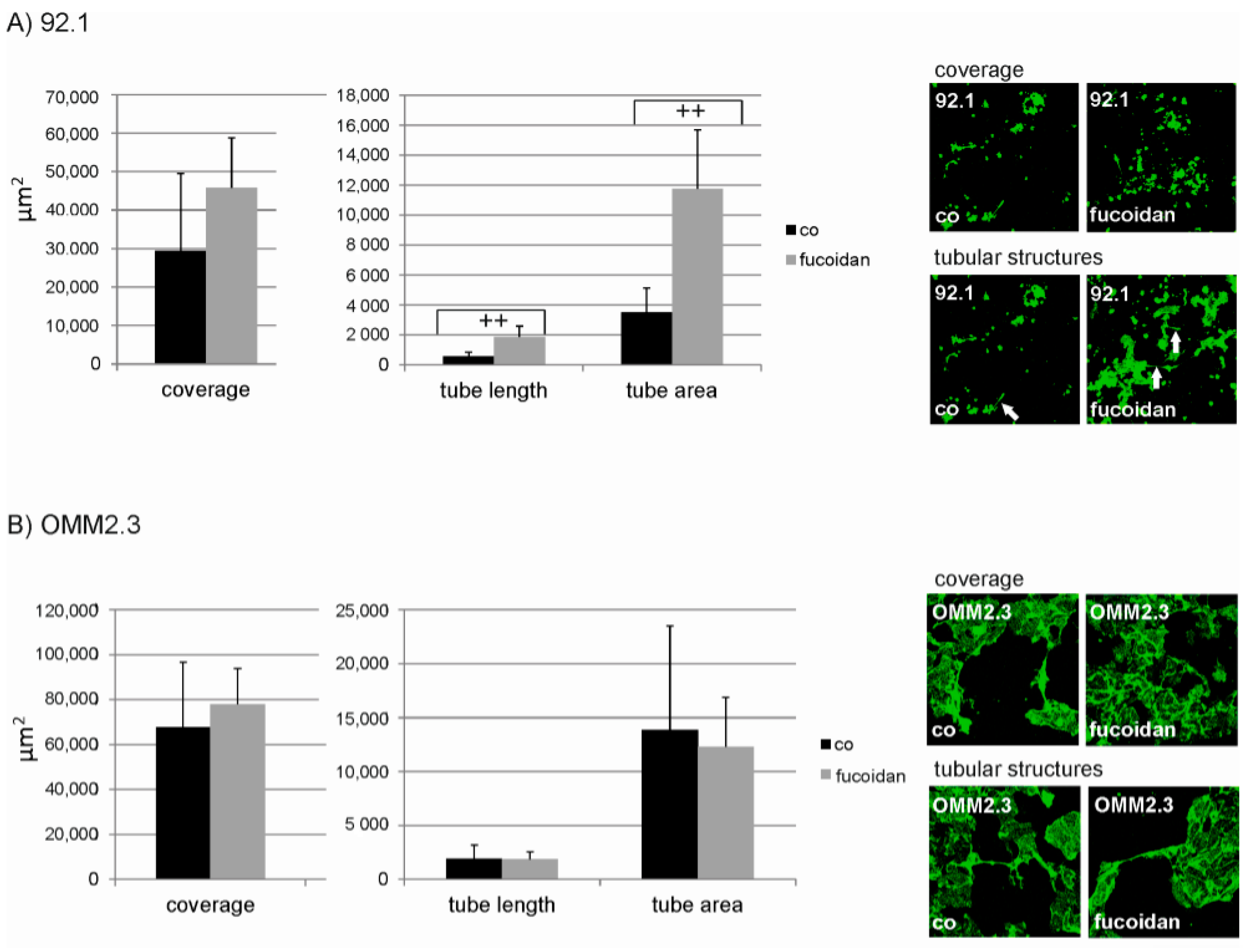
2.4. Angiogenesis
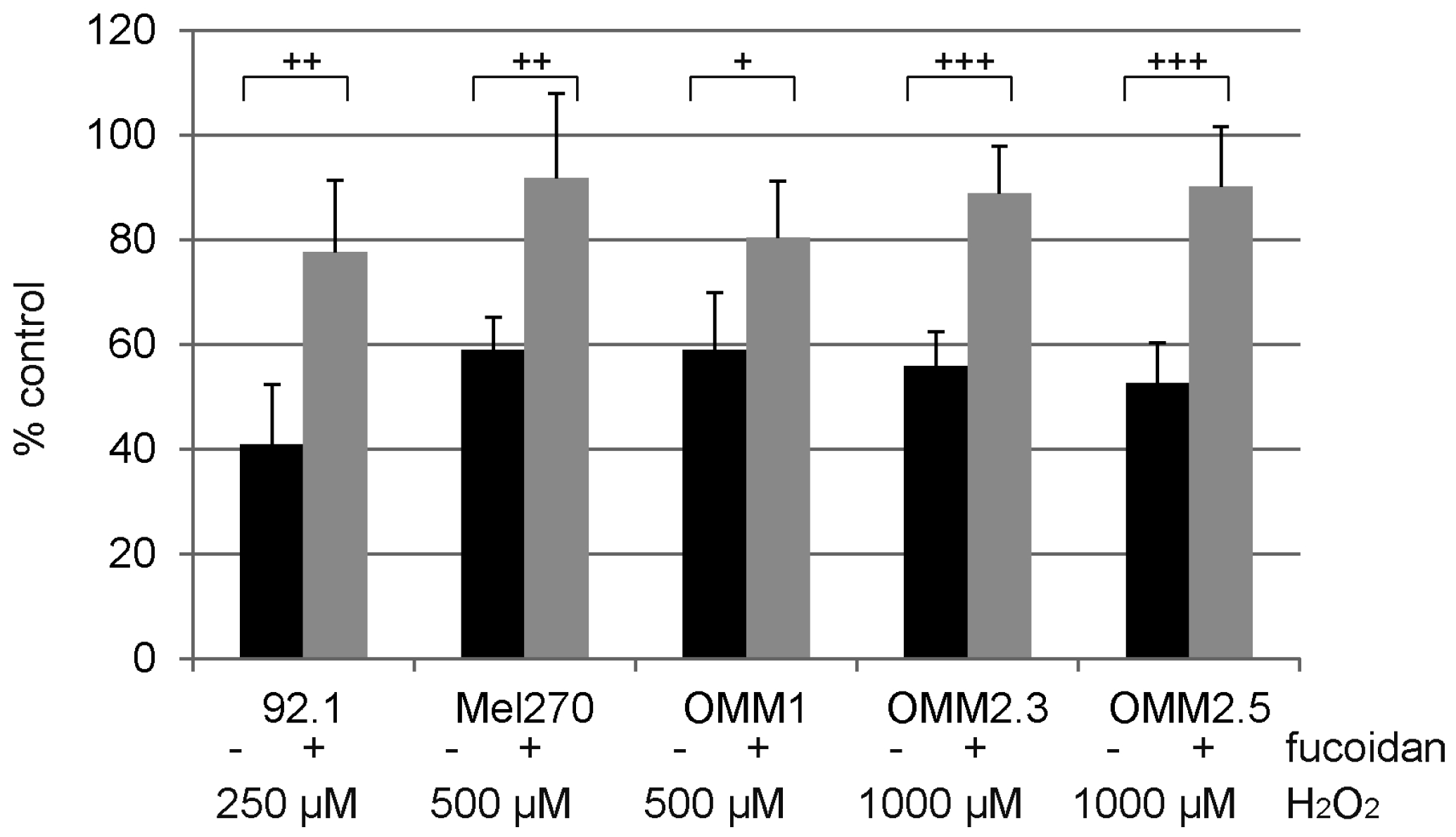
2.5. Protection
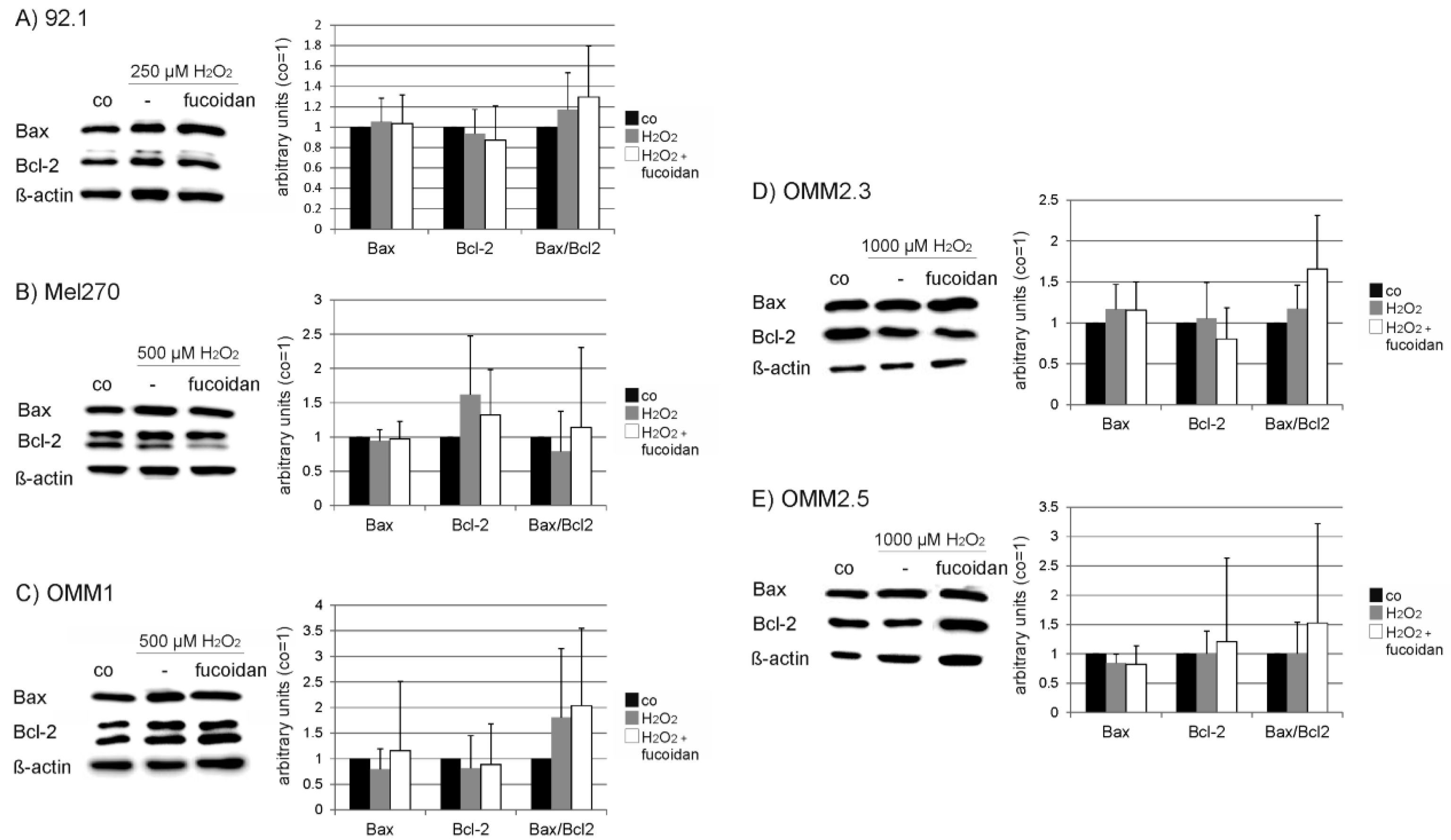
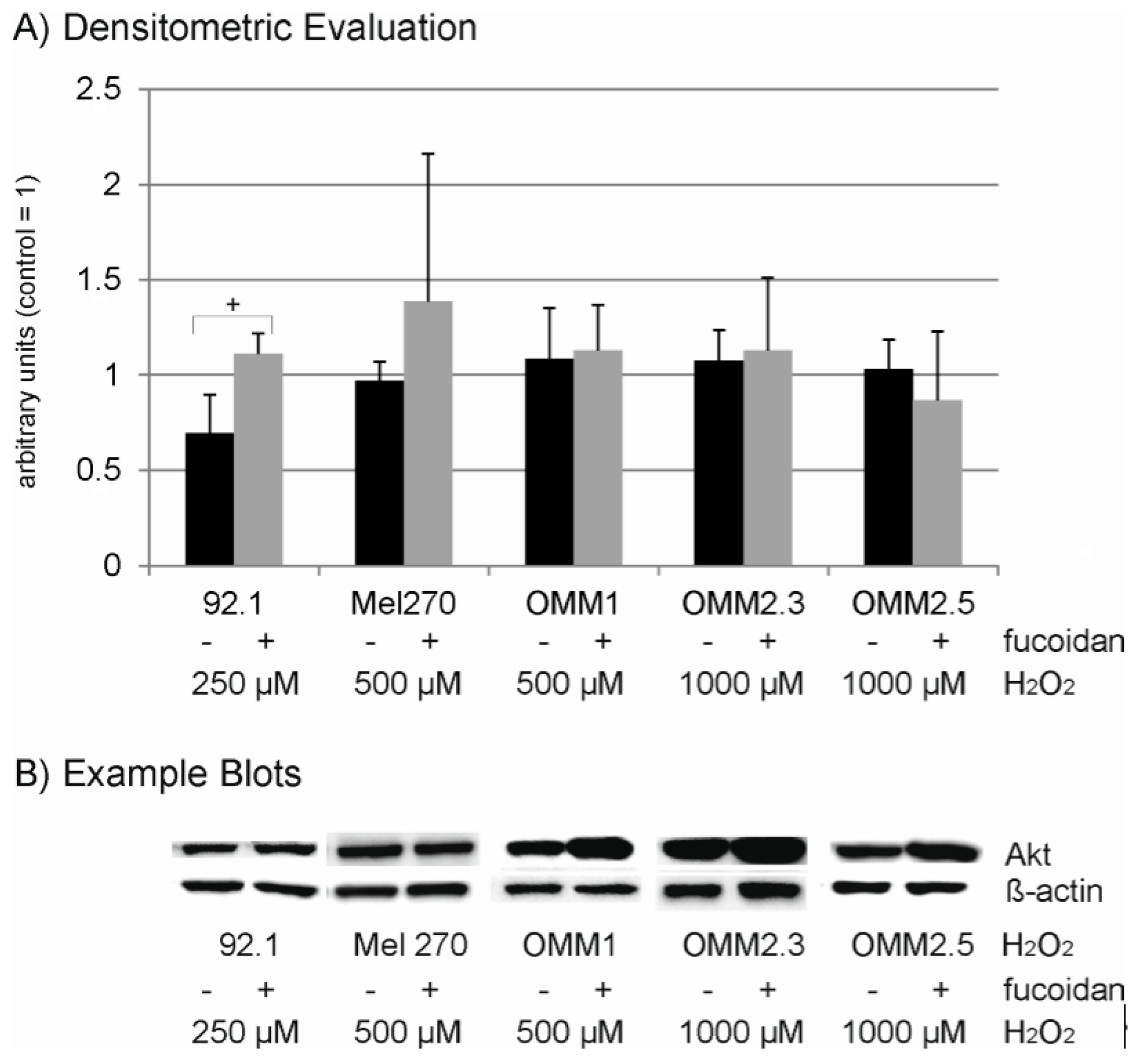
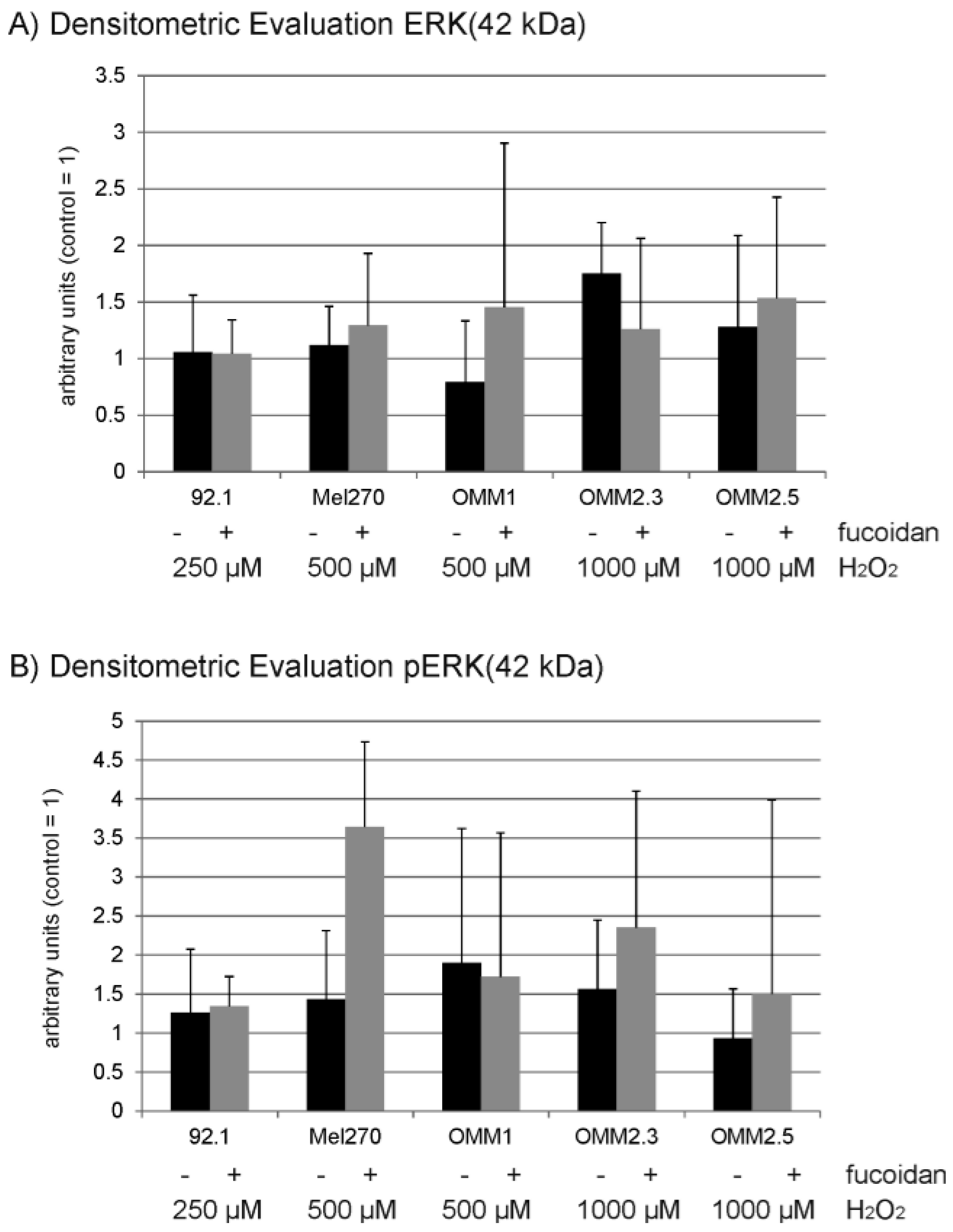
2.6. Protein Expression
3. Discussion
4. Conclusions
5. Material and Methods
5.1. Cell Culture of Melanoma Cells
5.2. Fucoidan
5.3. Proliferation
5.4. WST-Assay
5.5. Scratch Assay
5.6. VEGF-ELISA
5.7. Angiogenesis Assay
5.8. Image Analysis
5.9. Cytotoxicity
5.10. Whole Cell Lysate
5.11. Western Blot
5.12. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shields, J.A.; Shields, C.L. Management of posterior uveal melanoma: Past, present, and future: The 2014 Charles L. Schepens lecture. Ophthalmology 2015, 122, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Shields, C.L.; Kels, J.G.; Shields, J.A. Melanoma of the eye: Revealing hidden secrets, one at a time. Clin. Dermatol. 2015, 33, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Seregard, S.; Pelayes, D.E.; Singh, A.D. Radiation therapy: Uveal tumors. Dev. Ophthalmol. 2013, 52, 36–57. [Google Scholar] [PubMed]
- Spagnolo, F.; Caltabiano, G.; Queirolo, P. Uveal melanoma. Cancer Treat. Rev. 2012, 38, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Sun, J.; Su, X.; Yu, Q.; Yu, Q.; Zhang, P. A review about the development of fucoidan in antitumor activity: Progress and challenges. Carbohydr. Polym. 2016, 154, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.S.; Lee, J.H.; Lee, S.H. Antitumor Effects of Fucoidan on Human Colon Cancer Cells via Activation of Akt Signaling. Biomol. Ther. 2015, 23, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Hayakawa, M.; Koga, H.; Torimura, T. Effects of fucoidan on proliferation, AMP-activated protein kinase, and downstream metabolism- and cell cycle-associated molecules in poorly differentiated human hepatoma HLF cells. Int. J. Oncol. 2015, 46, 2216–2222. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Kim, G.Y.; Moon, S.K.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. Fucoidan inhibits the proliferation of human urinary bladder cancer T24 cells by blocking cell cycle progression and inducing apoptosis. Molecules 2014, 19, 5981–5998. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Ge, Y.; Zhang, J.; Wang, Q.; Hou, L.; Liu, Y.; Sun, L.; Li, Q. Anticancer properties and mechanisms of fucoidan on mouse breast cancer in vitro and in vivo. PLoS ONE 2012, 7, e43483. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int. J. Biol. Macromol. 2011, 49, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Boo, H.J.; Hong, J.Y.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Kim, E.J.; Hyun, J.W.; Koh, Y.S.; Yoo, E.S.; Kwon, J.M.; et al. The anticancer effect of fucoidan in PC-3 prostate cancer cells. Mar. Drugs 2013, 11, 2982–2999. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, H.; Zhang, L.; Qin, Y.; Cong, Q.; Wang, P.; Ding, K. A fucoidan from Nemacystus decipiens disrupts angiogenesis through targeting bone morphogenetic protein 4. Carbohydr. Polym. 2016, 144, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, J.; Chang, A.K.; Liu, B.; Yang, L.; Li, Q.; Wang, P.; Zou, X. Fucoidan extract derived from Undaria pinnatifida inhibits angiogenesis by human umbilical vein endothelial cells. Phytomedicine 2012, 19, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Atashrazm, F.; Lowenthal, R.M.; Woods, G.M.; Holloway, A.F.; Dickinson, J.L. Fucoidan and cancer: A multifunctional molecule with anti-tumor potential. Mar. Drugs 2015, 13, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Missotten, G.S.; Notting, I.C.; Schlingemann, R.O.; Zijlmans, H.J.; Lau, C.; Eilers, P.H.; Keunen, J.E.; Jager, M.J. Vascular endothelial growth factor a in eyes with uveal melanoma. Arch. Ophthalmol. 2006, 124, 1428–1434. [Google Scholar] [CrossRef] [PubMed]
- Boyd, S.R.; Tan, D.; Bunce, C.; Gittos, A.; Neale, M.H.; Hungerford, J.L.; Charnock-Jones, S.; Cree, I.A. Vascular endothelial growth factor is elevated in ocular fluids of eyes harbouring uveal melanoma: Identification of a potential therapeutic window. Br. J. Ophthalmol. 2002, 86, 448–452. [Google Scholar] [CrossRef] [PubMed]
- El Filali, M.; Missotten, G.S.; Maat, W.; Ly, L.V.; Luyten, G.P.; van der Velden, P.A.; Jager, M.J. Regulation of VEGF-A in uveal melanoma. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2329–2337. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Kuang, X.; Pan, Y.; Tan, M.; Lu, B.; Lu, J.; Cheng, Q.; Li, J. Clinicopathological characteristics of vascular endothelial growth factor expression in uveal melanoma: A meta-analysis. Mol. Clin. Oncol. 2014, 2, 363–368. [Google Scholar] [PubMed]
- Huang, T.H.; Chiu, Y.H.; Chan, Y.L.; Chiu, Y.H.; Wang, H.; Huang, K.C.; Li, T.L.; Hsu, K.H.; Wu, C.J. Prophylactic administration of fucoidan represses cancer metastasis by inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in Lewis tumor-bearing mice. Mar. Drugs 2015, 13, 1882–1900. [Google Scholar] [CrossRef] [PubMed]
- Harris, I.S.; Treloar, A.E.; Inoue, S.; Sasaki, M.; Gorrini, C.; Lee, K.C.; Yung, K.Y.; Brenner, D.; Knobbe-Thomsen, C.B.; Cox, M.A.; et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell 2015, 27, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Piskounova, E.; Agathocleous, M.; Murphy, M.M.; Hu, Z.; Huddlestun, S.E.; Zhao, Z.; Leitch, A.M.; Johnson, T.M.; DeBerardinis, R.J.; Morrison, S.J. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature 2015, 527, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Leach, J.K.; Van Tuyle, G.; Lin, P.S.; Schmidt-Ullrich, R.; Mikkelsen, R.B. Ionizing radiation-induced, mitochondria-dependent generation of reactive oxygen/nitrogen. Cancer Res. 2001, 61, 3894–3901. [Google Scholar] [PubMed]
- Hyun, J.H.; Kim, S.C.; Kang, J.I.; Kim, M.K.; Boo, H.J.; Kwon, J.M.; Koh, Y.S.; Hyun, J.W.; Park, D.B.; Yoo, E.S.; et al. Apoptosis inducing activity of fucoidan in HCT-15 colon carcinoma cells. Biol. Pharm. Bull. 2009, 32, 1760–1764. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Hwang, H.J.; Kim, G.Y.; Cha, H.J.; Kim, W.J.; Kim, N.D.; Yoo, Y.H.; Choi, Y.H. Induction of apoptosis by fucoidan in human leukemia U937 cells through activation of p38 MAPK and modulation of Bcl-2 family. Mar. Drugs 2013, 11, 2347–2364. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, G.; Babchia, N.; Calipel, A.; Mouriaux, F.; Faussat, A.M.; Mrzyk, S.; Mascarelli, F. Activation of the FGF2/FGFR1 autocrine loop for cell proliferation and survival in uveal melanoma cells. Investig. Ophthalmol. Vis. Sci. 2009, 50, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Babchia, N.; Calipel, A.; Mouriaux, F.; Faussat, A.M.; Mascarelli, F. The PI3K/Akt and mTOR/P70S6K signaling pathways in human uveal melanoma cells: Interaction with B-Raf/ERK. Investig. Ophthalmol. Vis. Sci. 2010, 51, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.K.; Cohen, S.M.; Mukerji, R.; Chaguturu, V.; Zhang, X.; Timmermann, B.N.; Cohen, M.S.; Person, E.A. Natural withanolide withaferin A induces apoptosis in uveal melanoma cells by suppression of Akt and c-MET activation. Tumour Biol. 2012, 33, 1179–1189. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.L.; Musi, E.; Ambrosini, G.; Nair, J.S.; Deraje Vasudeva, S.; de Stanchina, E.; Schwartz, G.K. Impact of combined mTOR and MEK inhibition in uveal melanoma is driven by tumor genotype. PLoS ONE 2012, 7, e40439. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, R.; Zhang, Y.; Xu, X.; Song, X.; Zhou, Y.; Zhang, H.; Ge, S.; Fan, X. The role of Bax and Bcl-2 in gemcitabine-mediated cytotoxicity in uveal melanoma cells. Tumour Biol. 2014, 35, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Sulkowska, M.; Famulski, W.; Bakunowicz-Lazarczyk, A.; Chyczewski, L.; Sulkowski, S. Bcl-2 expression in primary uveal melanoma. Tumori 2001, 87, 54–57. [Google Scholar] [PubMed]
- Dithmer, M.; Kirsch, A.M.; Gräfenstein, L.; Wang, F.; Schmidt, H.; Coupland, S.E.; Fuchs, S.; Roider, J.; Klettner, A. Uveale Melanomzellen unter oxidativen Stress—Einfluss von VEGF und VEGF-Inhibitoren. Klin. Monatsbl. Augenhkd. 2017. [Google Scholar] [CrossRef]
- Dithmer, M.; Fuchs, S.; Shi, Y.; Schmidt, H.; Richert, E.; Roider, J.; Klettner, A. Fucoidan reduces secretion and expression of vascular endothelial growth factor in the retinal pigment epithelium and reduces angiogenesis in vitro. PLoS ONE 2014, 9, e89150. [Google Scholar] [CrossRef] [PubMed]
- Riou, D.; Colliec-Jouault, S.; Pinczon du Sel, D.; Bosch, S.; Siavoshian, S.; Le Bert, V.; Tomasoni, C.; Sinquin, C.; Durand, P.; Roussakis, C. Antitumor and antiproliferative effects of a fucan extracted from ascophyllum nodosum against a non-small-cell bronchopulmonary carcinoma line. Anticancer Res. 1996, 16, 1213–1218. [Google Scholar] [PubMed]
- Ale, M.T.; Maruyama, H.; Tamauchi, H.; Mikkelsen, J.D.; Meyer, A.S. Fucose-containing sulfated polysaccharides from brown seaweeds inhibit proliferation of melanoma cells and induce apoptosis by activation of caspase-3 in vitro. Mar. Drugs 2011, 9, 2605–2621. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Zhang, Q.; Kong, Y.; Xie, B.; Gao, M.; Tao, Y.; Xu, H.; Zhan, F.; Dai, B.; Shi, J.; et al. Antitumor activity of fucoidan against diffuse large B cell lymphoma in vitro and in vivo. Acta Biochim. Biophys. Sin. 2015, 47, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.M.; Kim, W.J.; Moon, S.K. AKT signaling is involved in fucoidan-induced inhibition of growth and migration of human bladder cancer cells. Food Chem. Toxicol. 2014, 64, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kim, J.S.; Kim, E. Fucoidan from Seaweed Fucus vesiculosus Inhibits Migration and Invasion of Human Lung Cancer Cell via PI3K-Akt-mTOR Pathways. PLoS ONE 2012, 7, e50624. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, S.; Tanigawa, N.; Nakagawa, H.; Soeda, S.; Shimeno, H. Oversulfation of fucoidan enhances its anti-angiogenic and antitumor activities. Biochem. Pharmacol. 2003, 65, 173–179. [Google Scholar] [CrossRef]
- Klettner, A.; Westhues, D.; Lassen, J.; Bartsch, S.; Roider, J. Regulation of constitutive vascular endothelial growth factor secretion in retinal pigment epithelium/choroid organ cultures: P38, nuclear factor κB, and the vascular endothelial growth factor receptor-2/phosphatidylinositol 3 kinase pathway. Mol. Vis. 2013, 19, 281–291. [Google Scholar] [PubMed]
- Han, Y.S.; Lee, J.H.; Jung, J.S.; Noh, H.; Baek, M.J.; Ryu, J.M.; Yoon, Y.M.; Han, H.J.; Lee, S.H. Fucoidan protects mesenchymal stem cells against oxidative stress and enhances vascular regeneration in a murine hindlimb ischemia model. Int. J. Cardiol. 2015, 198, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, H.; Wang, Q.; Liang, H.; Jiang, X. Fucoidan protects ARPE-19 cells from oxidative stress via normalization of reactive oxygen species generation through the Ca2+-dependent ERK signaling pathway. Mol. Med. Rep. 2015, 11, 3746–3752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Teruya, K.; Yoshida, T.; Eto, H.; Shirahata, S. Fucoidan extract enhances the anti-cancer activity of chemotherapeutic agents in MDA-MB-231 and MCF-7 breast cancer cells. Mar. Drugs 2013, 11, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Lu, J.; Roscilli, G.; Aurisicchio, L.; Cappelletti, M.; Pavoni, E.; White, W.L.; Bedogni, B. The natural compound fucoidan from New Zealand Undaria pinnatifida synergizes with the ERBB inhibitor lapatinib enhancing melanoma growth inhibition. Oncotarget 2017, 8, 17887–17896. [Google Scholar] [CrossRef] [PubMed]
- Oh, B.; Kim, J.; Lu, W.; Rosenthal, D. Anticancer Effect of Fucoidan in Combination with Tyrosine Kinase Inhibitor Lapatinib. Evid.-Based Complement. Altern. Med. 2014, 2014, 865375. [Google Scholar] [CrossRef] [PubMed]
- De Waard-Siebinga, I.; Blom, D.J.; Griffioen, M.; Schrier, PI.; Hoogendoorn, E.; Beverstock, G.; Danen, E.H.; Jager, M.J. Establishment and characterization of an uveal-melanoma cell line. Int. J. Cancer 1995, 62, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Verbik, D.J.; Murray, T.G.; Tran, J.M.; Ksander, B.R. Melanomas that develop within the eye inhibit lymphocyte proliferation. Int. J. Cancer 1997, 73, 470–478. [Google Scholar] [CrossRef]
- Luyten, G.P.; Naus, N.C.; Mooy, C.M.; Hagemeijer, A.; Kan-Mitchell, J.; Van Drunen, E.; Vuzevski, V.; De Jong, P.T.; Luider, T.M. Establishment and characterization of primary and metastatic uveal melanoma cell lines. Int. J. Cancer 1996, 66, 380–387. [Google Scholar] [CrossRef]
- Fuchs, S.; Motta, A.; Migliaresi, C.; Kirkpatrick, C.J. Outgrowth endothelial cells isolated and expanded from human peripheral blood progenitor cells as a potential source of autologous cells for endothelialization of silk fibroin biomaterials. Biomaterials 2006, 27, 5399–5408. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Hofmann, A.; Kirkpartrick, C. Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng. 2007, 13, 2577–2588. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, S.; Jiang, X.; Schmidt, H.; Dohle, E.; Ghanaati, S.; Orth, C.; Hofmann, A.; Motta, A.; Migliaresi, C.; Kirkpatrick, C.J. Dynamic processes involved in the pre-vascularization of silk fibroin constructs for bone regeneration using outgrowth endothelial cells. Biomaterials 2009, 30, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Faby, H.; Hillenkamp, J.; Roider, J.; Klettner, A. Hyperthermia-induced upregulation of vascular endothelial growth factor in retinal pigment epithelial cells is regulated by mitogen-activated protein kinases. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dithmer, M.; Kirsch, A.-M.; Richert, E.; Fuchs, S.; Wang, F.; Schmidt, H.; Coupland, S.E.; Roider, J.; Klettner, A. Fucoidan Does Not Exert Anti-Tumorigenic Effects on Uveal Melanoma Cell Lines. Mar. Drugs 2017, 15, 193. https://doi.org/10.3390/md15070193
Dithmer M, Kirsch A-M, Richert E, Fuchs S, Wang F, Schmidt H, Coupland SE, Roider J, Klettner A. Fucoidan Does Not Exert Anti-Tumorigenic Effects on Uveal Melanoma Cell Lines. Marine Drugs. 2017; 15(7):193. https://doi.org/10.3390/md15070193
Chicago/Turabian StyleDithmer, Michaela, Anna-Maria Kirsch, Elisabeth Richert, Sabine Fuchs, Fanlu Wang, Harald Schmidt, Sarah E. Coupland, Johann Roider, and Alexa Klettner. 2017. "Fucoidan Does Not Exert Anti-Tumorigenic Effects on Uveal Melanoma Cell Lines" Marine Drugs 15, no. 7: 193. https://doi.org/10.3390/md15070193
APA StyleDithmer, M., Kirsch, A.-M., Richert, E., Fuchs, S., Wang, F., Schmidt, H., Coupland, S. E., Roider, J., & Klettner, A. (2017). Fucoidan Does Not Exert Anti-Tumorigenic Effects on Uveal Melanoma Cell Lines. Marine Drugs, 15(7), 193. https://doi.org/10.3390/md15070193





