New Zosteropenillines and Pallidopenillines from the Seagrass-Derived Fungus Penicillium yezoense KMM 4679
Abstract
1. Introduction
2. Results
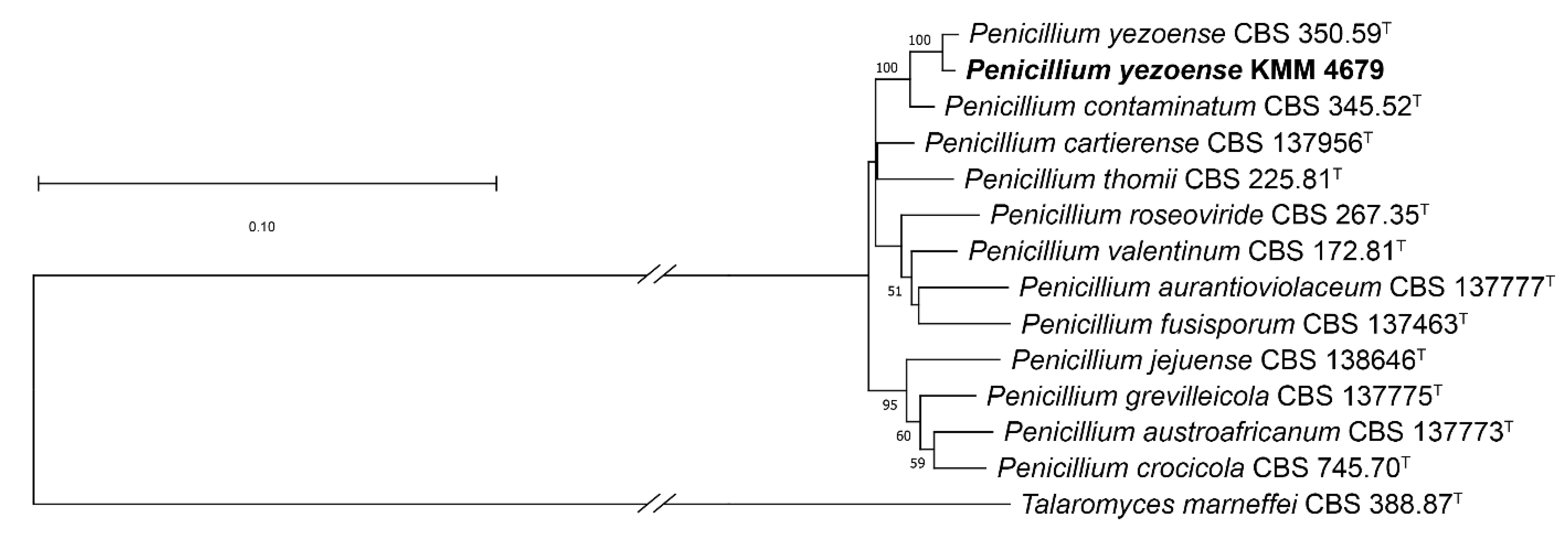
2.1. Molecular Identification of the Fungal Strain/Identification of Penicillium yezoense KMM 4679
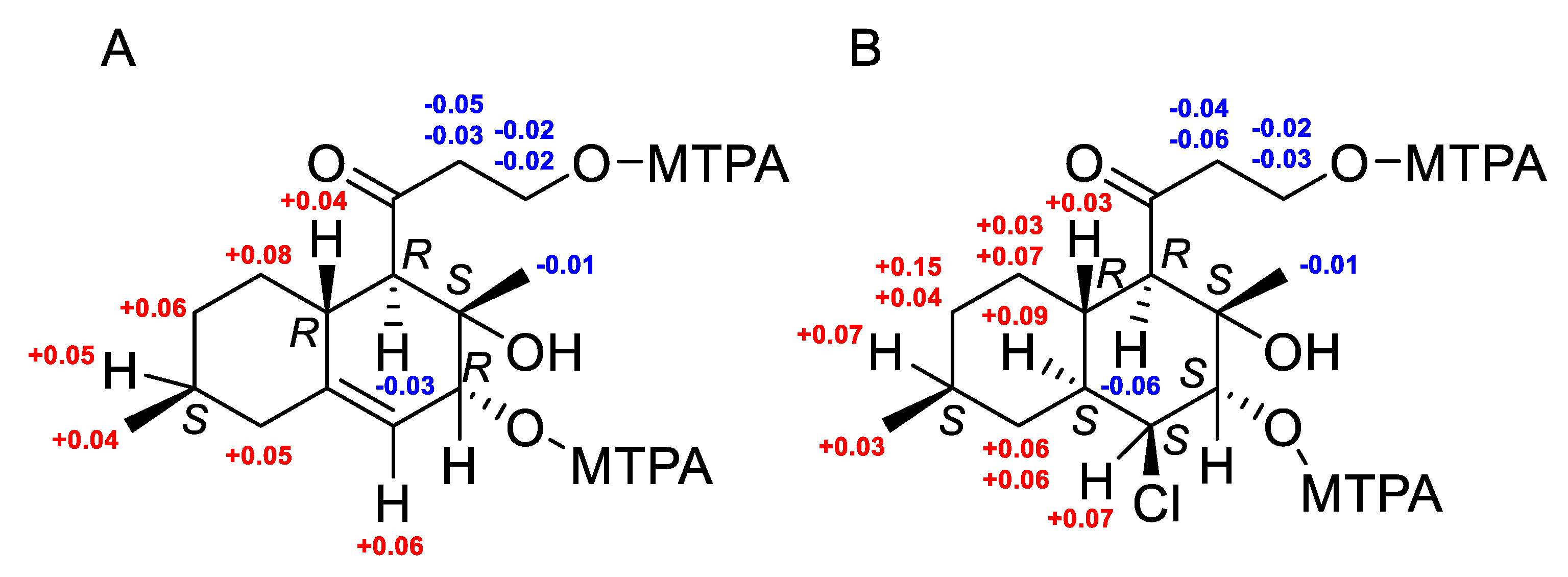
2.2. Structural Elucidation
2.3. Biological Activity of Isolated Compounds
2.3.1. Antimicrobial Activity
2.3.2. Cytotoxic Activity of Isolated Compounds
2.3.3. Cytoprotective Activity
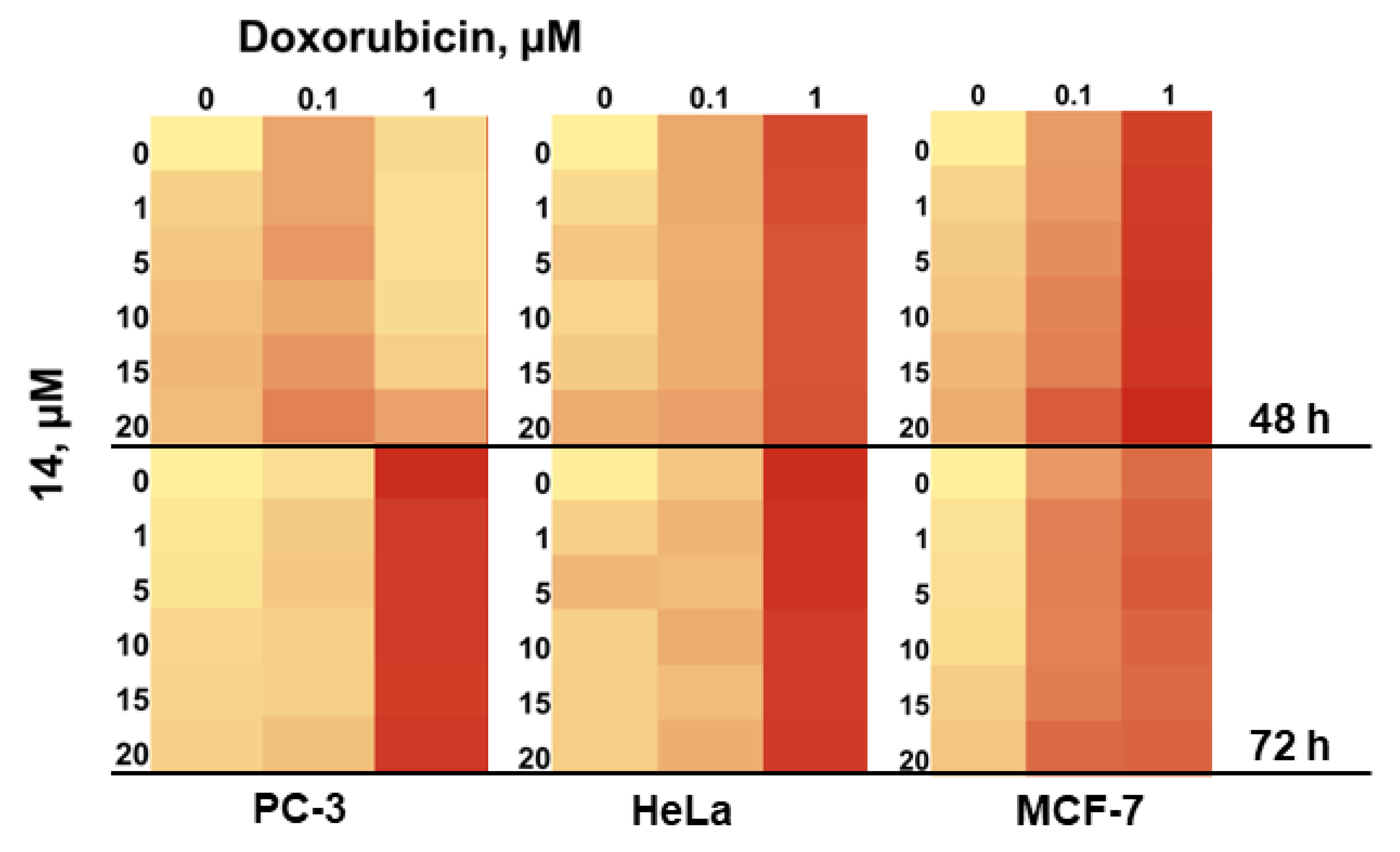
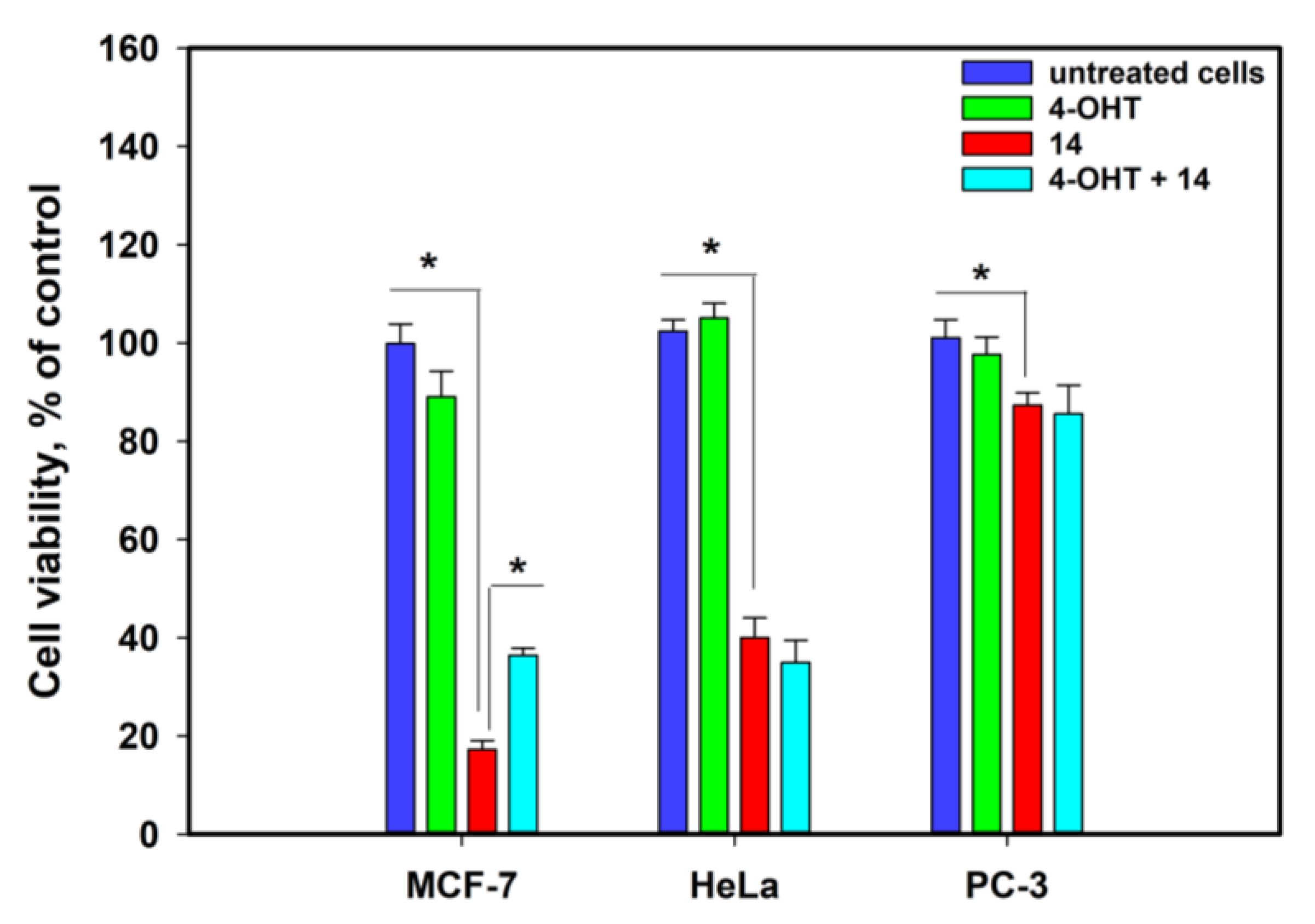
2.3.4. Anticancer Activity of 1-Acetylpallidopenilline A (14)
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungal Strain
4.3. DNA Extraction and Amplification
4.4. Phylogenetic Analysis
4.5. Cultivation of P. yezoense KMM 4679
4.6. Extraction and Isolation
4.7. Spectral Data
4.8. Preparation of (S)-MTPA and (R)-MTPA Esters of Zosteropenillines Q (7), R (9) and S (10)
4.9. Antimicrobial Activity
4.10. Biofilm Formation
4.11. Cell Culture
4.12. Cytotoxic Activity (MTT Assay)
4.13. Colony Formation Assay
4.14. Migration Assay
4.15. Drug Combination Study
4.16. Hypoxia
4.17. Lipid Peroxidation Level
4.18. Statistical Data Evaluation
4.19. Quantum Chemical Modeling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carroll, A.R.; Copp, B.R.; Grkovic, T.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2024, 41, 162–207. [Google Scholar] [CrossRef] [PubMed]
- Houbraken, J.; Visagie, C.M.; Meijer, M.; Frisvad, J.C.; Busby, P.E.; Pitt, J.I.; Seifert, K.A.; Louis-Seize, G.; Demirel, R.; Yilmaz, N.; et al. A taxonomic and phylogenetic revision of Penicillium section Aspergilloides. Stud. Mycol. 2014, 78, 373–451. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Fong, J.J.; Oh, S.Y.; Houbraken, J.; Sohn, J.H.; Hong, S.B.; Lim, Y.W. Penicillium jejuense sp. nov., isolated from the marine environments of Jeju Island, Korea. Mycologia 2015, 107, 209–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, D.; Vaughan, D.; McHardy, W.J. A critical examination of SEM ultrastructural features in two Penicillium thomii isolates from soil. Mycol. Res. 1996, 100, 223–228. [Google Scholar] [CrossRef]
- Grum-Grzhimaylo, O.A.; Bilanenko, E.N. The micromycete complexes of bogs at the Kandalaksha bay of the White sea. Mikol. Fitopatol. 2012, 46, 297–305. [Google Scholar]
- Kirichuk, N.; Mikhail, P. Secondary marine fungi associated with brown algae Sargassum spp. from Peter the Great Bay (the Sea of Japan). Mycol. Phytopathol. 2015, 49, 146–150. [Google Scholar]
- Kirichuk, N.; Mikhail, P. Filamentous fungi associated with the seagrass Zostera marina Linnaeus, 1753 of Rifovaya Bay (Peter the Great Bay, the Sea of Japan). Russ. J. Mar. Biol. 2015, 41, 351–355. [Google Scholar] [CrossRef]
- Cabello, M.; Irrazabal, G.; Bucsinszky, A.M.; Saparrat, M.; Schalamuk, S. Effect of an arbuscular mycorrhizal fungus, Glomus mosseae, and a rock-phosphate-solubilizing fungus, Penicillium thomii, on Mentha piperita growth in a soilless medium. J. Basic. Microbiol. 2005, 45, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Morales, A.; Alvear, M.; Valenzuela, E.; Castillo, C.; Borie, F. Screening, evaluation and selection of phosphate-solubilising fungi as potential biofertiliser. J. Soil Sci. Plant Nutr. 2011, 11, 89–103. [Google Scholar] [CrossRef]
- Jiang, T.; Tian, L.; Guo, A.; Fu, H.; Pei, Y.; Lin, W. Chemical constituents from marine fungus Penicillium thomii. Yao Xue Xue Bao Acta Pharm. Sin. 2002, 37, 271–274. [Google Scholar]
- Li, Q.; Xu, W.; Fan, R.; Zhang, J.; Li, Y.; Wang, X.; Han, S.; Liu, W.; Pan, M.; Cheng, Z. Penithoketone and penithochromones A–L, polyketides from the deep-sea-derived fungus Penicillium thomii YPGA3. J. Nat. Prod. 2020, 83, 2679–2685. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Liu, Y.; Liu, W.; Yang, F.; Zhang, J.; Liu, R.; Zhao, F.; Xu, W.; Cheng, Z. Chromone derivatives with α-glucosidase inhibitory activity from the marine fungus Penicillium thomii Maire. Molecules 2021, 26, 5273. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Liu, Y.; Zhang, X.; Liu, W.; Qiao, Y.; Xu, W.; Li, Q.; Cheng, Z. Three new chromone derivatives from the deep-sea-derived fungus Penicillium thomii. Rec. Nat. Prod. 2023, 17, 174–178. [Google Scholar]
- Cheng, Z.; Liu, W.; Fan, R.; Han, S.; Li, Y.; Cui, X.; Zhang, J.; Wu, Y.; Lv, X.; Zhang, Y. Terpenoids from the deep-sea-derived fungus Penicillium thomii YPGA3 and their bioactivities. Mar. Drugs 2020, 18, 164. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, W.; Han, S.; Zhang, J.; Xu, W.; Li, Q.; Cheng, Z. Penitholabene, a rare 19-nor labdane-type diterpenoid from the deep-sea-derived fungus Penicillium thomii YPGA3. Fitoterapia 2020, 146, 104691. [Google Scholar] [CrossRef] [PubMed]
- Zhuravleva, O.I.; Sobolevskaya, M.P.; Leshchenko, E.V.; Kirichuk, N.N.; Denisenko, V.A.; Dmitrenok, P.S.; Dyshlovoy, S.A.; Zakharenko, A.M.; Kim, N.Y.; Afiyatullov, S.S. Meroterpenoids from the alga-derived fungi Penicillium thomii maire and Penicillium lividum westling. J. Nat. Prod. 2014, 77, 1390–1395. [Google Scholar] [CrossRef] [PubMed]
- Sobolevskaya, M.P.; Leshchenko, E.V.; Hoai, T.P.T.; Denisenko, V.A.; Dyshlovoy, S.A.; Kirichuk, N.N.; Khudyakova, Y.V.; Kim, N.Y.; Berdyshev, D.V.; Pislyagin, E.A.; et al. Pallidopenillines: Polyketides from the alga-derived fungus Penicillium thomii Maire KMM 4675. J. Nat. Prod. 2016, 79, 3031–3038. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Leshchenko, E.V.; Berdyshev, D.V.; Sobolevskaya, M.P.; Antonov, A.S.; Denisenko, V.A.; Popov, R.S.; Pivkin, M.V.; Udovenko, A.A.; Pislyagin, E.A.; et al. Zosteropenillines: Polyketides from the marine-derived fungus Penicillium thomii. Mar. Drugs 2017, 15, 46. [Google Scholar] [CrossRef]
- Sobolevskaya, M.P.; Zhuravleva, O.I.; Leshchenko, E.V.; Afiyatullov, S.S.; Khudyakova, Y.V.; Kim, N.Y.; Kirichuk, N.N.; Dyshlovoy, S.A. Spiroketals from marine isolates of the fungi Penicillium thomii KMM 4645 and P. lividum KMM 4663. Chem. Nat. Compd. 2014, 50, 1122–1124. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Leshchenko, E.V.; Sobolevskaya, M.P.; Denisenko, V.A.; Kirichuk, N.N.; Khudyakova, Y.V.; Hoai, T.P.T.; Dmitrenok, P.S.; Menchinskaya, E.S.; Pislyagin, E.A.; et al. New eudesmane sesquiterpenes from the marine-derived fungus Penicillium thomii. Phytochem. Lett. 2015, 14, 209–214. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Leshchenko, E.V.; Sobolevskaya, M.P.; Antonov, A.S.; Denisenko, V.A.; Popov, R.S.; Khudyakova, Y.V.; Kirichuk, N.N.; Kuz’mich, A.S.; Pislyagin, E.A.; et al. New thomimarine E from marine isolate of the fungus Penicillium thomii. Chem. Nat. Compd. 2017, 53, 290–294. [Google Scholar] [CrossRef]
- Afiyatullov, S.S.; Leshchenko, E.V.; Antonov, A.S.; Zhuravleva, O.I. Secondary metabolites of fungus Penicillium thomii associated with eelgrass Zostera marina. Chem. Nat. Compd. 2018, 54, 1029–1030. [Google Scholar] [CrossRef]
- Li, G.; Kusari, S.; Spiteller, M. Natural products containing ‘decalin’motif in microorganisms. Nat. Prod. Rep. 2014, 31, 1175–1201. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Sato, M.; Osada, H. Recent advances in the chemo-biological characterization of decalin natural products and unraveling of the workings of Diels–Alderases. Fungal Biol. Biotechnol. 2022, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Tobert, J.A. Lovastatin and beyond: The history of the HMG-CoA reductase inhibitors. Nat. Rev. Drug Discov. 2003, 2, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-F.; Sun, Z.-C.; Xiao, L.; Zhou, Y.-M.; Du, F.-Y. Herbicidal polyketides and diketopiperazine derivatives from Penicillium viridicatum. J. Agric. Food Chem. 2019, 67, 14102–14109. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, J.; Huang, M.; Liu, L.; Wang, J.; Lin, Y. Six new polyketide decalin compounds from mangrove endophytic fungus Penicillium aurantiogriseum 328#. Mar. Drugs 2015, 13, 6306–6318. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Z.; Kurtán, T.; Mándi, A.; Tang, H.; Chou, Y.; Soong, K.; Su, L.; Sun, P.; Zhuang, C.-L.; Zhang, W. Immunomodulatory Polyketides from a Phoma-like fungus isolated from a soft coral. J. Nat. Prod. 2017, 80, 2930–2940. [Google Scholar] [CrossRef]
- Kusumi, T.; Ooi, T.; Ohkubo, Y.; Yabuuchi, T. The modified Mosher’s method and the sulfoximine method. Bull. Chem. Soc. Jpn. 2006, 79, 965–980. [Google Scholar] [CrossRef]
- Guo, H.; Feng, T.; Li, Z.-H.; Liu, J.-K. Five new polyketides from the basidiomycete Craterellus odoratus. Nat. Prod. Bioprospect. 2012, 2, 170–173. [Google Scholar] [CrossRef]
- Stocking, E.M.; Williams, R.M. Chemistry and biology of biosynthetic Diels-Alder reactions. Angew. Chem. Int. Ed. 2003, 42, 3078–3115. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, M.; Donato, M.; D’Amato, A.L.; Rosanova, M.; Russo, A.O.M.; Scafetta, R.; De Angelis, C.; Trivedi, M.V.; André, F.; Arpino, G.; et al. New steps on an old path: Novel estrogen receptor inhibitors in breast cancer. Crit. Rev. Oncol./Hematol. 2022, 180, 103861. [Google Scholar] [CrossRef] [PubMed]
- Shagufta; Ahmad, I.; Mathew, S.; Rahman, S. Recent progress in selective estrogen receptor downregulators (SERDs) for the treatment of breast cancer. RSC Med. Chem. 2020, 11, 438–454. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Liu, W. Selective Estrogen Receptor Degraders (SERDs): A promising strategy for estrogen receptor positive endocrine-resistant breast cancer. J. Med. Chem. 2020, 63, 15094–15114. [Google Scholar] [CrossRef] [PubMed]
- Powell, E.; Huang, S.-X.; Xu, Y.; Rajski, S.R.; Wang, Y.; Peters, N.; Guo, S.; Eric Xu, H.; Hoffmann, F.M.; Shen, B.; et al. Identification and characterization of a novel estrogenic ligand actinopolymorphol A. Biochem. Pharmacol. 2010, 80, 1221–1229. [Google Scholar] [CrossRef][Green Version]
- Sondergaard, T.E.; Klitgaard, L.G.; Purup, S.; Kobayashi, H.; Giese, H.; Sørensen, J.L. Estrogenic effects of fusarielins in human breast cancer cell lines. Toxicol. Lett. 2012, 214, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef]
- Leshchenko, E.V.; Antonov, A.S.; Borkunov, G.V.; Hauschild, J.; Zhuravleva, O.I.; Khudyakova, Y.V.; Menshov, A.S.; Popov, R.S.; Kim, N.Y.; Graefen, M.; et al. New bioactive beta-resorcylic acid derivatives from the alga-derived fungus Penicillium antarcticum KMM 4685. Mar. Drugs 2023, 21, 178. [Google Scholar] [CrossRef]
- Kifer, D.; Mužinić, V.; Klarić, M.Š. Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1, 8-cineole against Staphylococcus aureus planktonic and biofilm growth. J. Antibiot. 2016, 69, 689–696. [Google Scholar] [CrossRef]
- Zhu, P.; Zhao, N.; Sheng, D.; Hou, J.; Hao, C.; Yang, X.; Zhu, B.; Zhang, S.; Han, Z.; Wei, L. Inhibition of growth and metastasis of colon cancer by delivering 5-fluorouracil-loaded pluronic P85 copolymer micelles. Sci. Rep. 2016, 6, 20896. [Google Scholar] [CrossRef] [PubMed]
- Dyshlovoy, S.A.; Tabakmakher, K.M.; Hauschild, J.; Shchekaleva, R.K.; Otte, K.; Guzii, A.G.; Makarieva, T.N.; Kudryashova, E.K.; Fedorov, S.N.; Shubina, L.K. Guanidine alkaloids from the marine sponge Monanchora pulchra show cytotoxic properties and prevent EGF-induced neoplastic transformation in vitro. Mar. Drugs 2016, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.T.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; Li, X.; et al. Gaussian 16, Revision A.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
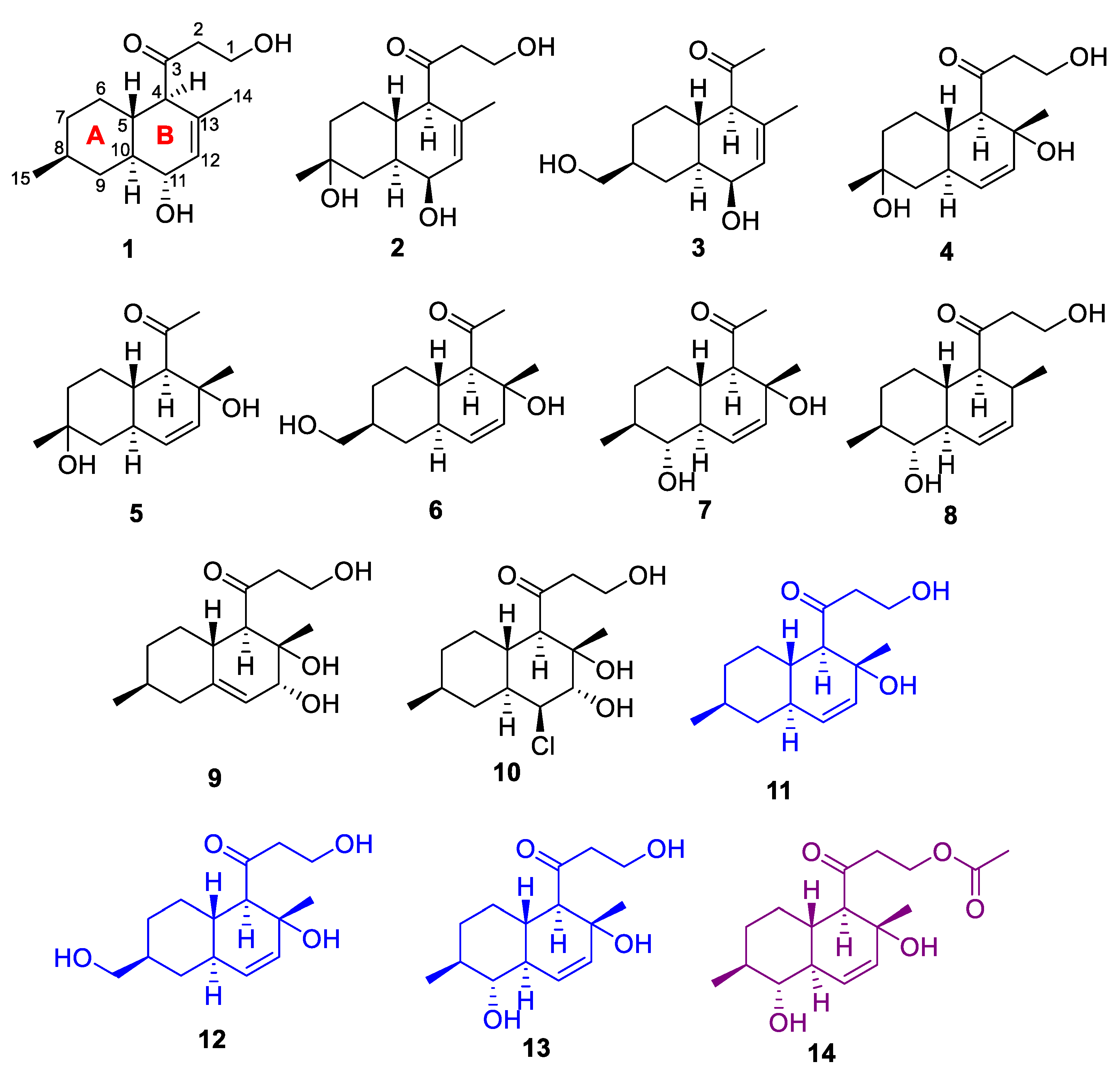
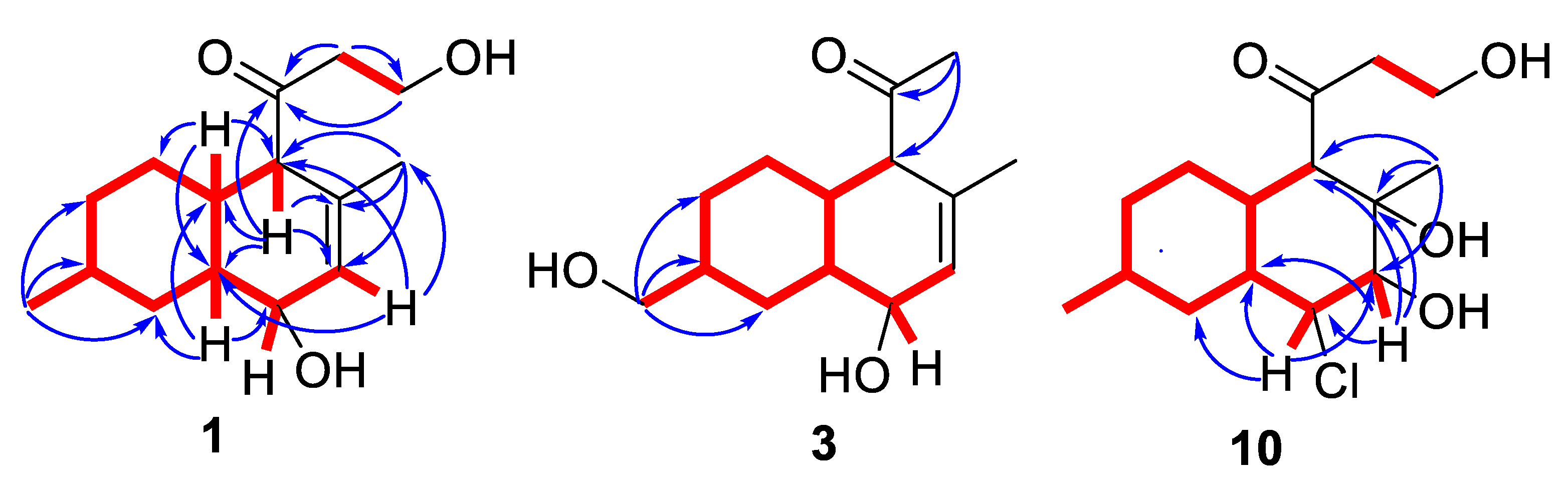



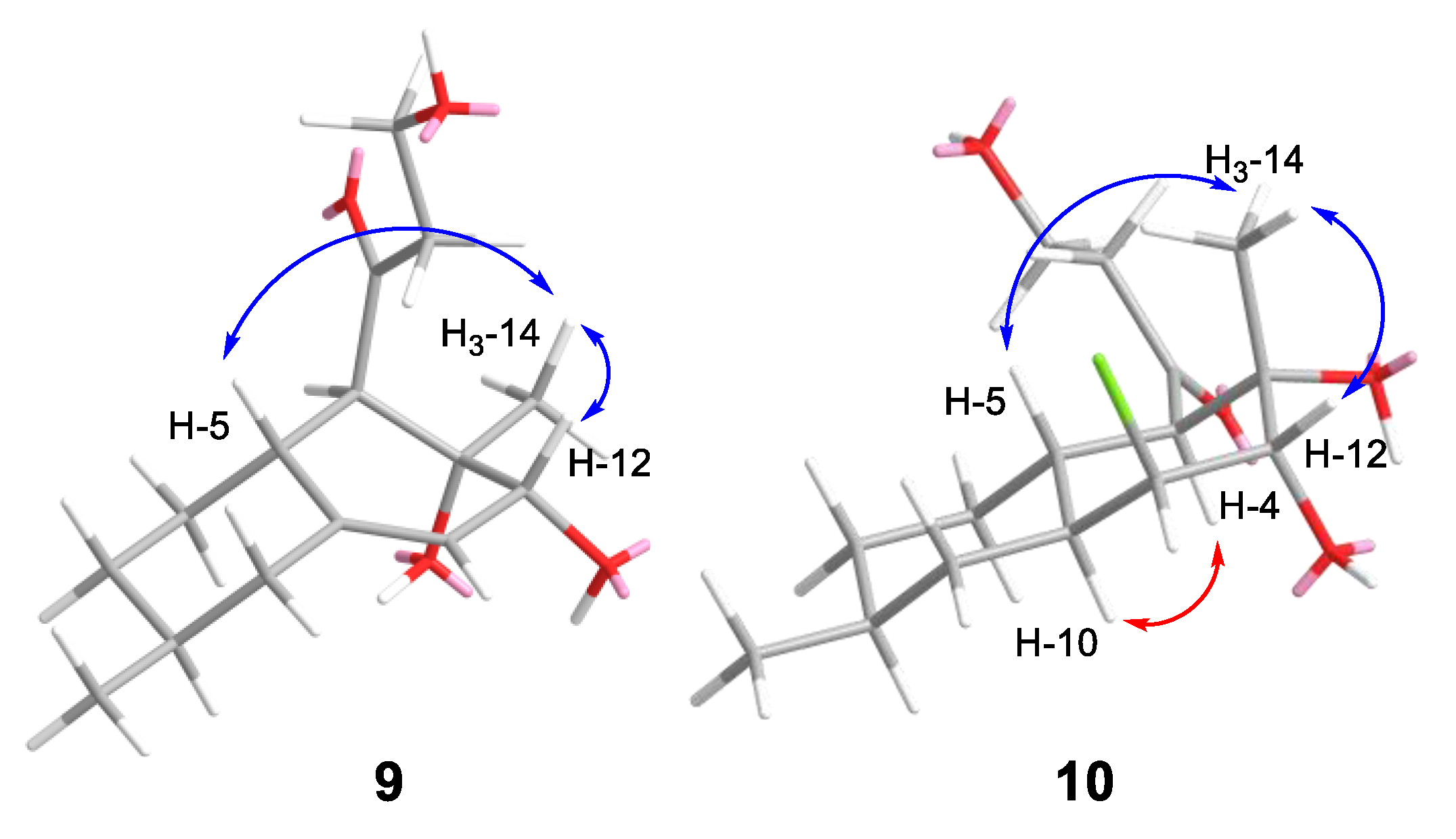





| No. | 1 * | 2 * | 3 * | 4 ** | 5 * |
|---|---|---|---|---|---|
| 1 | 57.8, CH2 | 58.0, CH2 | – | 58.1, CH2 | – |
| 2 | 43.1, CH2 | 43.7, CH2 | 28.0, CH3 | 49.2, CH2 | 34.7, CH3 |
| 3 | 214.6, C | 214.5, C | 211.2, C | 214.1, C | 211.1, C |
| 4 | 62.2, CH | 62.4, CH | 63.4, CH | 63.2, CH | 63.5, CH |
| 5 | 38.9, CH | 33.6, CH | 34.1, CH | 41.0, CH | 40.8, CH |
| 6 | 31.4, CH2 | 27.0, CH2 | 31.3, CH2 | 24.9, CH2 | 24.9, CH2 |
| 7 | 34.3, CH2 | 38.0, CH2 | 28.8, CH2 | 38.7, CH2 | 38.7, CH2 |
| 8 | 31.7, CH | 70.0, C | 40.2, C | 69.8, C | 69.8, C |
| 9 | 38.5, CH2 | 40.4, CH2 | 31.3, CH2 | 44.6, CH2 | 44.7, CH2 |
| 10 | 45.3, CH | 33.6, CH | 41.4, CH | 37.0, CH | 37.0, CH |
| 11 | 72.6, CH | 66.8, CH | 68.6, CH | 131.6, CH | 131.5, CH |
| 12 | 129.8, CH | 127.5, CH | 127.1, CH | 134.2, CH | 134.4, CH |
| 13 | 132.0, C | 135.4, C | 135.7, C | 73.0, C | 72.7, C |
| 14 | 21.1, CH3 | 21.7, CH3 | 21.6, CH3 | 26.0, CH3 | 25.7, CH3 |
| 15 | 22.5, CH3 | 32.0, CH3 | 67.0, CH2 | 31.6, CH3 | 31.6, CH2 |
| Position | 1 * | 2 * | 3 * | 4 ** | 5 * |
|---|---|---|---|---|---|
| 1 | 3.85, brs (2H) | 3.86, d (5.5) 3.85, d (5.5) | – | 3.92, ddd (11.0, 7.3, 3.7) 3.86, ddd (10.5, 6.7, 3.8) | – |
| 2 | 2.70, ddd (18.1, 6.5, 4.6) 2.64, ddd (18.1, 6.2, 4.5) | 2.79, ddd (18.3, 10.8, 5.4) 2.68, ddd (18.3, 10.7, 5.3) | 2.14, s | 3.07, ddd (17.9, 6.7, 3.7) 2.62, ddd (18.1, 7.1, 3.8) | 2.29, s |
| 4 | 2.83, brd (10.0) | 2.86, brd (9.9) | 2.74, brd (9.9) | 2.95, d (11.6) | 2.90, d (11.6) |
| 5 | 1.52, qd (11.5, 4.0) | 1.76, m | 1.76, m | 1.50, m | 1.47, m |
| 6 | 1.65, dq (13.1, 3.4) 1.14, qd (13.0, 3.7) | 1.55, m 1.51, m | 1.73–1.82, m 1.13, m | 1.58, m 1.27, m | 1.62, tq (16.7, 3.0) 1.26, m |
| 7 | 1.69, dq (13.1, 3.1) 0.89, qd (13.1, 3.4) | 1.61, m 1.38, td (13.4, 5.0) | 1.79, m 0.96, m | 1.64, dq (14.0, 3.1) 1.44, qd (13.6, 4.1) | 1.64, m 1.45, m |
| 8 | 1.40, m | – | 1.60, m | – | – |
| 9 | 2.21, dq (12.8, 3.4) 0.70, dd (12.7, 12.0) | 1.65, m 1.59, m | 1.73–1.82, m 1.27, m | 1.70, dt (13.5, 3.0) 1.19 (13.3) | 1.70, dt (13.5, 2.9) 1.20, t (13.3) |
| 10 | 1.16, m | 1.68, tt (12.0, 3.4) | 1.32, tt (11.5, 3.3) | 2.31, m | 2.29, m |
| 11 | 3.88, brd (9.0) | 3.86, dd (5.5, 3.3) | 3.92, dd (5.6, 3.1) | 5.41, dd (9.9, 1.7) | 5.40, dd (9.9, 1.5) |
| 12 | 5.59, q (1.6) | 5.89, dt (5.8, 1.6) | 5.88, brd (5.8) | 5.46, dd (9.9, 2.9) | 5.48, dd (9.9, 2.9) |
| 14 | 1.60, s | 1.63, brs | 1.63, brs | 1.20, s | 1.19, s |
| 15 | 0.92, d (6.5) | 1.28, s | 3.51, dd (5.7, 1.2) 3.50, dd (5.7, 1.3) | 1.24, s | 1.24, s |
| No. | 6 * | 7 * | 8 * | 9 * | 10 * |
|---|---|---|---|---|---|
| 1 | – | – | 57.8, CH2 | 58.1, CH2 | 58.3, CH2 |
| 2 | 34.8, CH3 | 34.7, CH3 | 45.2, CH2 | 49.4, CH2 | 49.1, CH2 |
| 3 | 211.1, C | 210.9, C | 213.1, C | 214.5, C | 214.5, C |
| 4 | 63.7, CH | 63.5, CH | 55.6, CH | 57.9, CH | 60.4, CH |
| 5 | 41.4, CH | 39.3, CH | 33.9, CH | 39.4, CH | 34.7, CH |
| 6 | 28.8, CH2 | 28.7, CH2 | 28.8, CH2 | 32.8, CH2 | 30.8, CH2 |
| 7 | 29.0, CH2 | 32.8, CH2 | 33.1, CH2 | 33.9, CH2 | 33.9, CH2 |
| 8 | 40.6, CH | 40.6, CH | 40.6, CH | 33.6, CH | 32.4, CH |
| 9 | 35.3, CH2 | 78.1, CH | 78.6, CH | 43.0, CH2 | 38.1, CH2 |
| 10 | 41.5, CH | 48.5, CH | 48.4, CH | 145.3, C | 39.4, CH |
| 11 | 131.6, CH | 127.0, CH | 126.1, CH | 118.2, CH | 63.3, CH |
| 12 | 134.2, CH | 134.8, CH | 131.7, CH | 72.3, CH | 78.5, CH |
| 13 | 72.6, C | 72.1, C | 31.5, CH | 73.4, CH | 73.4, C |
| 14 | 25.7, CH3 | 25.5, CH3 | 17.4, CH3 | 19.9, CH3 | 24.3, CH3 |
| 15 | 68.2, CH2 | 18.4, CH3 | 18.5, CH3 | 22.3, CH3 | 22.4, CH3 |
| Position | 6 * | 7 * | 8 * | 9 * | 10 * |
|---|---|---|---|---|---|
| 1 | 3.88, ddd (11.2, 6.8, 3.8) 3.84, ddd (11.2, 6.8, 3.8) | 3.91, ddd (11.2, 7.0, 3.6) 3.86, ddd (11.2, 7.0, 3.6) | 3.90, ddd (11.0, 7.5, 3.6) 3.86, ddd (10.5, 6.5, 3.6) | ||
| 2 | 2.28, s | 2.29, s | 2.78, ddd (18.1, 6.8, 3.8) 2.55, ddd (18.1, 6.8, 3.8) | 3.08, ddd (18.0, 6.8, 3.8) 2.61, ddd (18.0, 6.8, 3.8) | 3.05, ddd (17.8, 7.5, 3.6) 2.54, ddd (17.8, 6.4, 3.6) |
| 4 | 2.86, d (11.7) | 2.88, d (11.8) | 2.82, dd (11.2, 6.0) | 2.90, d (10.2) | 2.87, d (11.3) |
| 5 | 1.49, qd (11.7, 3.0) | 1.59, m | 1.59, qd (11.2, 3.0) | 2.38, td (10.2, 5.0) | 1.77, qd (11.3, 3.5) |
| 6 | 1.83, m 0.94, qd (12.1, 3.4) | 1.71, m 0.94, m | 1.85, dq (12.6, 3.1) 0.84, qd (12.6, 3.5) | 1.67, m 1.02, m | 1.66, m 0.90, m |
| 7 | 1.80, m 1.03, qd (12.5, 4.0) | 1.73, m 1.13, m | 1.75, dq (13.8, 3.6) 1.17, qd (13.3, 3.9) | 1.65, m 1.03, m | 1.61, m 0.90, m |
| 8 | 1.61, m | 1.39, m | 1.39, m | 1.43, m | 1.43, m |
| 9 | 1.87, m 0.82, q (12.5) | 2.83, t (9.8) | 2.86, t (9.9) | 2.22, m 1.65, m | 1.50, dq (13.1, 3.1) 1.22, m |
| 10 | 1.86, m | 1.80, tt (10.0, 2.2) | 1.71, tq (10.6, 2.3) | – | 1.91, tt (11.3, 3.4) |
| 11 | 5.45, s | 6.02, dd (10.0, 1.4) | 5.99, brd (10.0) | 5.50, dt (5.8, 2.1) | 4.15, t (3.1) |
| 12 | 5.45, s | 5.54, dd (10.0, 2.7) | 5.67, ddd (10.0, 4.4, 2.7) | 3.61, dd (4.8, 0.9) | 3.76, dd (2.8) |
| 13 | – | – | 2.57, dd (6.0, 4.0) | – | – |
| 14 | 1.18, s | 1.18, s | 0.83, d (7.2) | 1.06, s | 1.42, s |
| 15 | 3.48, dd (10.5, 6.9) 3.45, dd (10.5, 6.6) | 1.03, d (6.4) | 1.04, d (6.4) | 0.90, d (6.5) | 0.91, d (6.5) |
| Compound | Inhibition of Microbial Growth, % of Control | ||
|---|---|---|---|
| S. aureus | E. coli | C. albicans | |
| 1 | 30.08 ± 2.42 | 0 | 8.32 ± 0.62 |
| 2 | 0 | 0 | 6.12 ± 1.89 |
| 4 | 0 | 0 | 10.98 ± 2.31 |
| 5 | 0 | 0 | 35.31 ± 1.16 |
| 6 | 28.99 ± 1.66 | 0 | 17.34 ± 2.55 |
| 7 | 29.95 ± 4.50 | 0 | 19.21 ± 3.12 |
| 8 | 19.86 ± 1.82 | 0 | 0 |
| 9 | 11.66 ± 1.71 | 0 | 12.03 ± 1.14 |
| 10 | 15.73 ± 0.36 | 0 | 0 |
| 11 | 16.95 ± 4.12 | 0 | 18.10 ± 2.58 |
| 12 | 20.31 ± 1.20 | 0 | 6.61 ± 1.12 |
| 13 | 15.49 ± 1.35 | 0 | 0 |
| 14 | 31.65 ± 7.80 | 0 | 0 |
| Gentamicin/nitrofungin | 98.61 ± 1.15 | 97.56 ± 2.10 | 98.13 ± 0.69 |
| Compound | Cell Viability, % of Control | |||
|---|---|---|---|---|
| НЕК-298 | РС-3 | HeLa | MCF-7 | |
| 1 | 95.16 ± 4.01 | 89.25 ± 0.44 | 66.49 ± 6.19 | 86.42 ± 1.76 |
| 2 | 90.98 ± 2.82 | 90.98 ± 2.82 | 44.12 ± 5.13 | 57.46 ± 1.82 |
| 4 | 94.71 ± 1.53 | 94.71 ± 1.53 | 81.80 ± 6.76 | 81.92 ± 3.92 |
| 5 | 85.64 ± 1.85 | 91.20 ± 3.37 | 37.12 ± 3.85 | 77.40 ± 2.73 |
| 6 | 83.26 ± 3.78 | 83.15 ± 1.72 | 71.44 ± 3.99 | 82.05 ± 1.31 |
| 7 | 80.00 ± 0.99 | 88.70 ± 1.10 | 80.80 ± 3.25 | 76.23 ± 4.53 |
| 8 | 86.10 ± 2.24 | 83.60 ± 1.81 | 72.66 ± 2.49 | 81.21 ± 5.56 |
| 9 | 96.45 ± 7.64 | 96.44 ± 7.63 | 79.14 ± 6.36 | 79.64 ± 1.67 |
| 10 | 98.10 ± 1.15 | 85.98 ± 2.13 | 68.69 ± 6.21 | 84.85 ± 4.90 |
| 11 | 93.97 ± 2.36 | 93.97 ± 2.36 | 34.35 ± 1.90 | 81.00 ± 2.38 |
| 12 | 87.01 ± 2.71 | 87.01 ± 2.71 | 66.70 ± 6.64 | 82.77 ± 1.36 |
| 13 | 89.76 ± 3.31 | 89.76 ± 3.31 | 71.85 ± 3.02 | 95.05 ± 1.51 |
| 14 | 96.92 ± 4.83 | 45.91 ± 0.67 | 53.44 ± 2.14 | 23.71 ± 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leshchenko, E.V.; Chingizova, E.A.; Antonov, A.S.; Shlyk, N.P.; Borkunov, G.V.; Berdyshev, D.V.; Chausova, V.E.; Kirichuk, N.N.; Khudyakova, Y.V.; Chingizov, A.R.; et al. New Zosteropenillines and Pallidopenillines from the Seagrass-Derived Fungus Penicillium yezoense KMM 4679. Mar. Drugs 2024, 22, 317. https://doi.org/10.3390/md22070317
Leshchenko EV, Chingizova EA, Antonov AS, Shlyk NP, Borkunov GV, Berdyshev DV, Chausova VE, Kirichuk NN, Khudyakova YV, Chingizov AR, et al. New Zosteropenillines and Pallidopenillines from the Seagrass-Derived Fungus Penicillium yezoense KMM 4679. Marine Drugs. 2024; 22(7):317. https://doi.org/10.3390/md22070317
Chicago/Turabian StyleLeshchenko, Elena V., Ekaterina A. Chingizova, Alexandr S. Antonov, Nadezhda P. Shlyk, Gleb V. Borkunov, Dmitrii V. Berdyshev, Viktoria E. Chausova, Natalya N. Kirichuk, Yuliya V. Khudyakova, Artur R. Chingizov, and et al. 2024. "New Zosteropenillines and Pallidopenillines from the Seagrass-Derived Fungus Penicillium yezoense KMM 4679" Marine Drugs 22, no. 7: 317. https://doi.org/10.3390/md22070317
APA StyleLeshchenko, E. V., Chingizova, E. A., Antonov, A. S., Shlyk, N. P., Borkunov, G. V., Berdyshev, D. V., Chausova, V. E., Kirichuk, N. N., Khudyakova, Y. V., Chingizov, A. R., Kalinovsky, A. I., Popov, R. S., Kim, N. Y., Chadova, K. A., Yurchenko, E. A., Isaeva, M. P., & Yurchenko, A. N. (2024). New Zosteropenillines and Pallidopenillines from the Seagrass-Derived Fungus Penicillium yezoense KMM 4679. Marine Drugs, 22(7), 317. https://doi.org/10.3390/md22070317







