- Review
Lipidomic Signatures in Pediatric Metabolic Disorders
- Monica Narvaez-Rivas and
- Kenneth D. R. Setchell
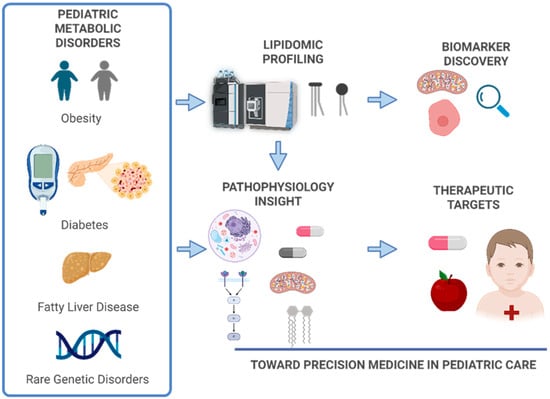
Lipids are essential biomolecules involved in membrane structure, energy storage, and intracellular signaling. Dysregulation of lipid metabolism (dyslipidemia) plays a central role in a wide spectrum of pediatric metabolic disorders, including both inherited and acquired conditions. Recent and rapid advances in mass spectrometry-based lipidomics have enabled high-resolution profiling of more than one-thousand lipid species, facilitating the discovery of disease-specific lipid signatures that were previously undetectable with conventional biochemical assays. In parallel, the rising prevalence of pediatric obesity, diabetes, asthma, metabolic dysfunction-associated steatotic liver disease (MASLD; formerly referred to as non-alcoholic fatty liver disease or NAFLD) and cancers has accelerated research aimed at uncovering molecular pathways underlying these conditions. Lipidomic approaches have also improved the identification and characterization of rare metabolic disorders. As analytical technologies continue to advance, lipidomics is poised to become a cornerstone of precision medicine in pediatrics, offering new opportunities for early diagnosis, risk stratification, and therapeutic targeting.
28 December 2025