Iron Stress Reprograms Enterocyte Metabolism
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. XTT Assay
2.3. Total Protein Extraction and Western Blot
2.4. RT-qPCR
2.5. BrdU Cell Proliferation Assay
2.6. Untargeted Metabolomics
2.7. Statistics
3. Results
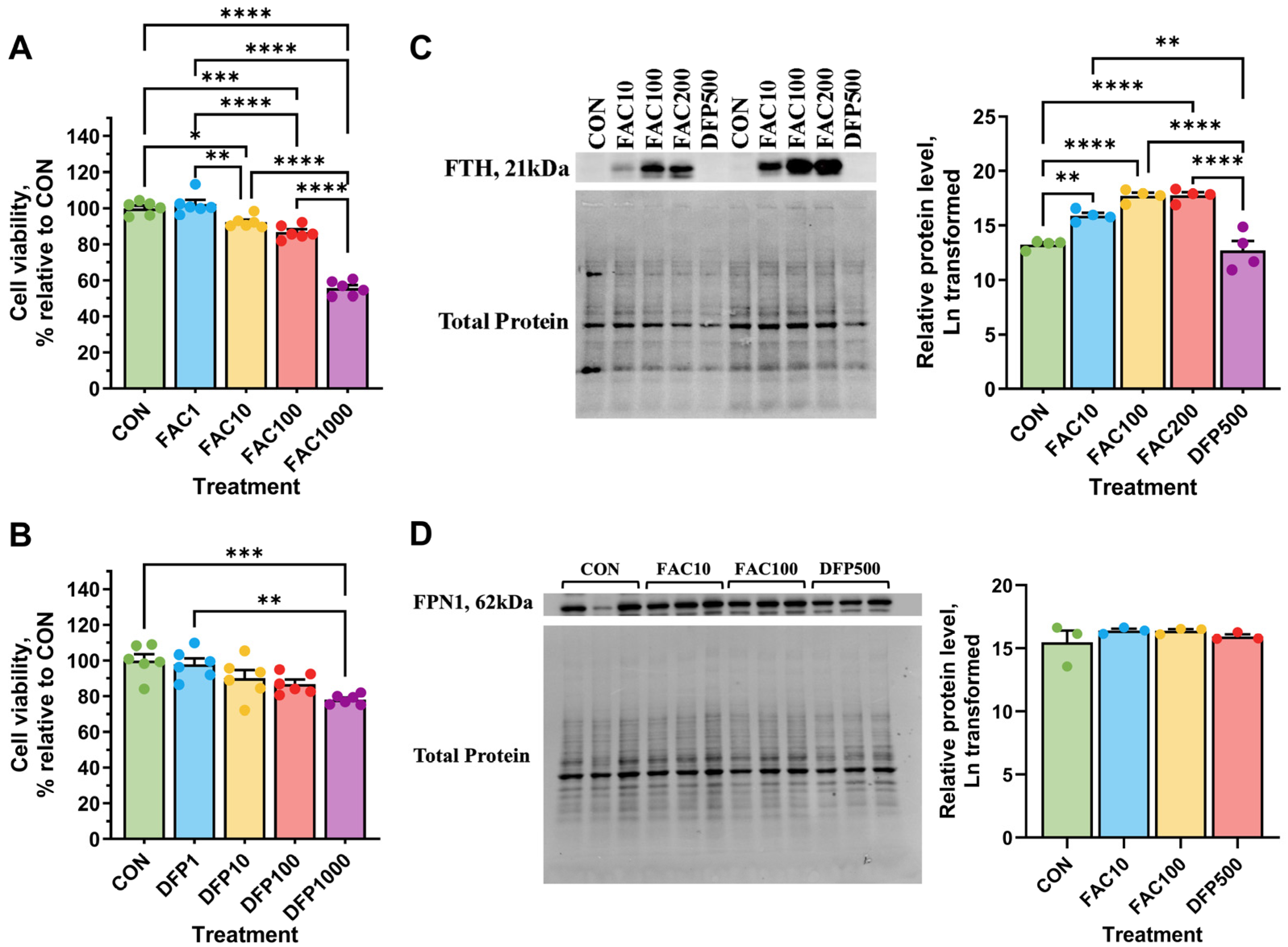
3.1. Effects of Iron Excess and Deficiency on Viability and Cellular Iron Status
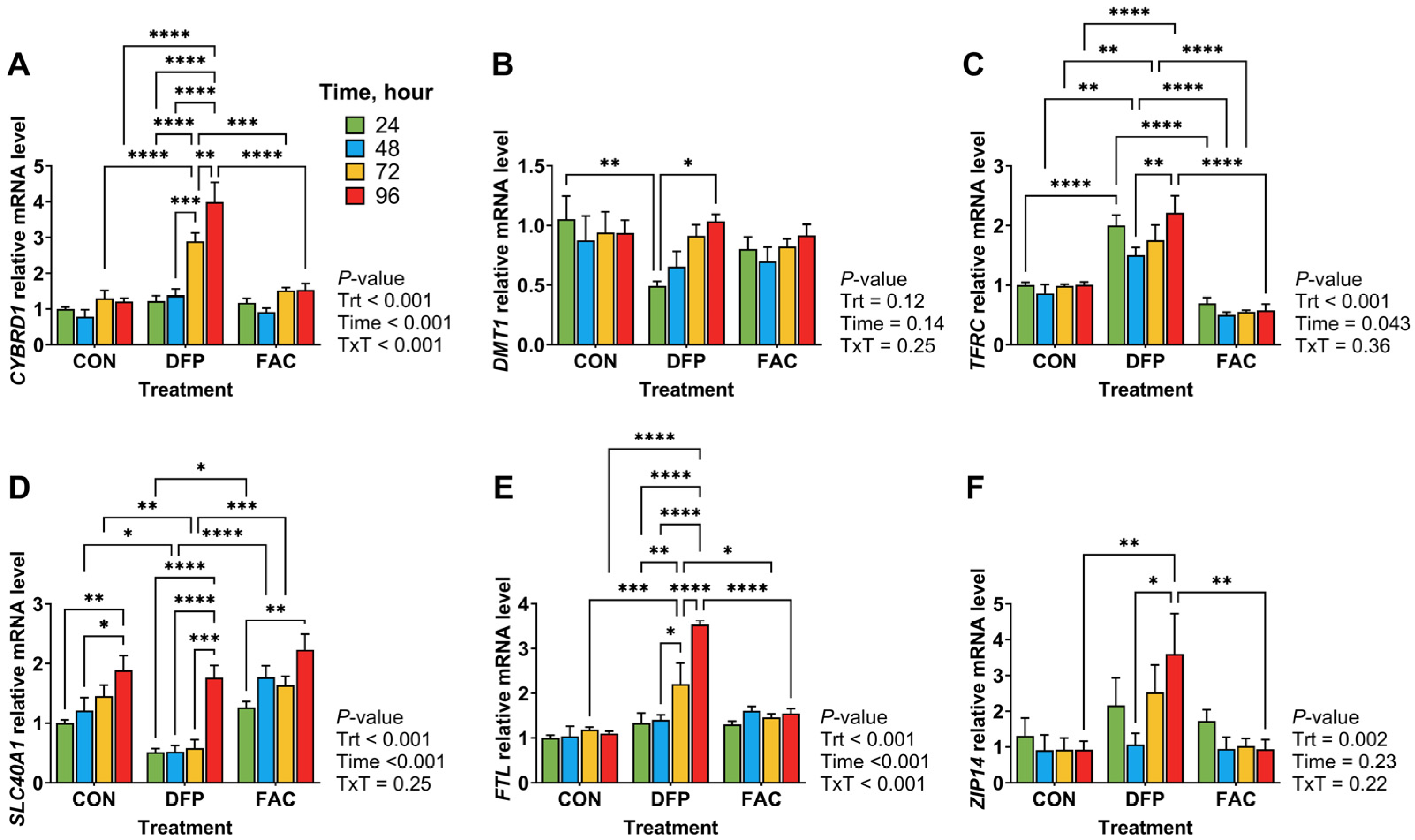
3.2. Effects of Iron Excess and Deficiency on Iron Transporter Gene Expression
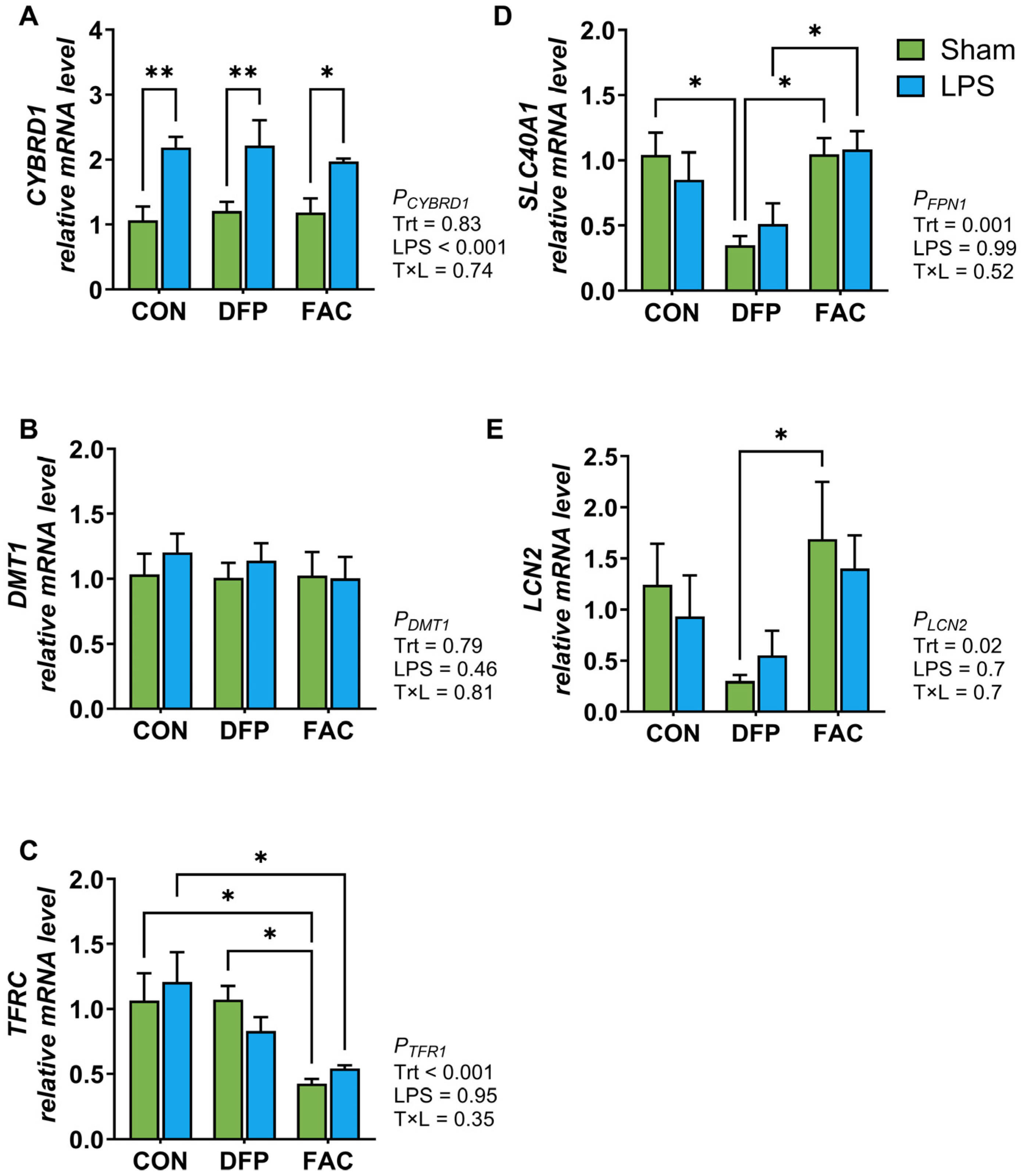
3.3. Effects of Iron Imbalance and LPS Treatment on Cellular Proliferation and the Expression of Genes Encoding for Iron Regulatory Proteins and Inflammatory Mediators
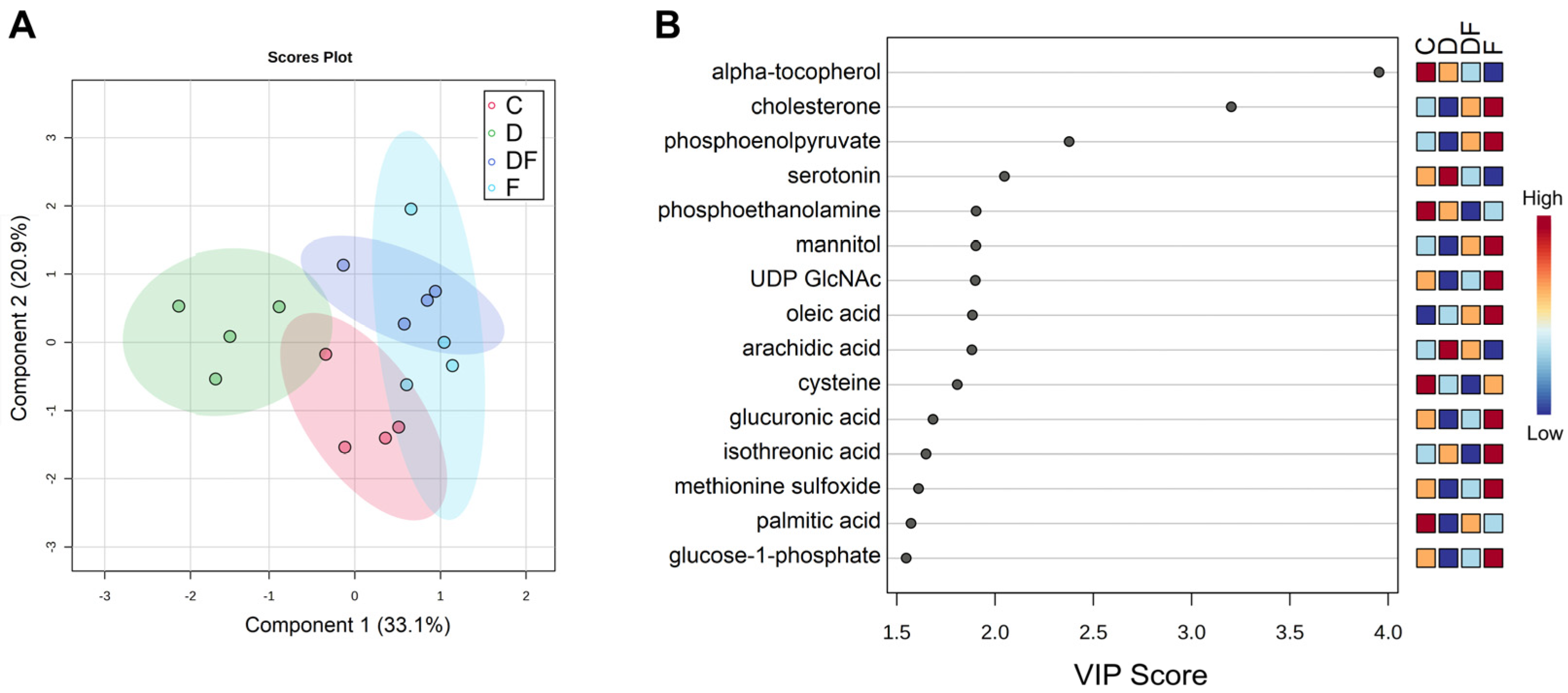
3.4. Metabolic Responses to Iron Excess, Deficiency, and Restoration After Deprivation
4. Discussion
4.1. Effects of Iron Excess and Deficiency on Cell Viability and Iron Metabolism
4.2. Iron Imbalance Has Limited Effects on the Inflammatory Response
4.3. Effects of Iron Imbalance on Intermediary Metabolism
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BrdU | Bromodeoxyuridine |
| CYBRD1 | Duodenal Cytochrome B |
| DFP | Deferiprone |
| DMT1 | Divalent Metal Transporter 1 |
| FAC | Ferric Ammonium Citrate |
| FPN1 | Ferroportin |
| FTH | Ferritin Heavy Chain |
| FTL | Ferritin Light Chain |
| HIF | Hypoxia-Inducible Factor |
| ID | Iron Deficiency |
| IE | Iron Excess |
| IL8 | C-X-C motif chemokine ligand 8, CXCL8 |
| IRP | Iron Regulatory Protein |
| LCN2 | Lipocalin-2 |
| LPS | Lipopolysaccharide |
| PCA | Principle Components Analysis |
| PLSDA | Partial Least Squares Discriminant Analysis |
| ROS | Reactive Oxidative Species |
| SLC40A1 | Solute carrier family 40 member 1 (Ferroportin) |
| TFRC | Transferrin receptor 1 |
| TLR4 | Toll like Receptor 4 |
| TNF | Tumor Necrosis Factor alpha |
| UTR | Untranslated Region |
| VIP | Variable Importance in Projection |
| ZIP14 | Solute carrier family 39 member 14 |
References
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar]
- Dessie, G.; Li, J.; Nghiem, S.; Doan, T. Prevalence and Determinants of Stunting-Anemia and Wasting-Anemia Comorbidities and Micronutrient Deficiencies in Children Under 5 in the Least-Developed Countries: A Systematic Review and Meta-analysis. Nutr. Rev. 2025, 83, e178–e194. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Choudhry, V.P. Iron deficiency and infection. Indian J. Pediatr. 2010, 77, 789–793. [Google Scholar] [CrossRef]
- Oppenheimer, S.J. Iron and its relation to immunity and infectious disease. J. Nutr. 2001, 131, 616S–633S, discussion 633S–635S. [Google Scholar] [CrossRef] [PubMed]
- WHO Guidelines Approved by the Guidelines Review Committee. In Guideline: Daily Iron Supplementation in Infants and Children; World Health Organization: Geneva, Switzerland, 2016.
- Dewey, K.G.; Domellöf, M.; Cohen, R.J.; Landa Rivera, L.; Hernell, O.; Lönnerdal, B. Iron supplementation affects growth and morbidity of breast-fed infants: Results of a randomized trial in Sweden and Honduras. J. Nutr. 2002, 132, 3249–3255. [Google Scholar] [CrossRef] [PubMed]
- Puntarulo, S. Iron, oxidative stress and human health. Mol. Aspects Med. 2005, 26, 299–312. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Chassard, C.; Rohner, F.; N’Goran E, K.; Nindjin, C.; Dostal, A.; Utzinger, J.; Ghattas, H.; Lacroix, C.; Hurrell, R.F. The effects of iron fortification on the gut microbiota in African children: A randomized controlled trial in Cote d’Ivoire. Am. J. Clin. Nutr. 2010, 92, 1406–1415. [Google Scholar] [CrossRef]
- Peterson, L.W.; Artis, D. Intestinal epithelial cells: Regulators of barrier function and immune homeostasis. Nat. Rev. Immunol. 2014, 14, 141–153. [Google Scholar] [CrossRef]
- Vitale, S.; Picascia, S.; Gianfrani, C. The cross-talk between enterocytes and intraepithelial lymphocytes. Mol. Cell. Pediatr. 2016, 3, 20. [Google Scholar] [CrossRef]
- Vereecke, L.; Beyaert, R.; van Loo, G. Enterocyte death and intestinal barrier maintenance in homeostasis and disease. Trends Mol. Med. 2011, 17, 584–593. [Google Scholar] [CrossRef]
- Ashy, A.A.; Salleh, M.; Ardawi, M. Glucose, glutamine, and ketone-body metabolism in human enterocytes. Metabolism 1988, 37, 602–609. [Google Scholar] [CrossRef]
- Yang, H.; Söderholm, J.D.; Larsson, J.; Permert, J.; Lindgren, J.; Wirén, M. Bidirectional supply of glutamine maintains enterocyte ATP content in the in vitro using chamber model. Int. J. Colorectal Dis. 2000, 15, 291–296. [Google Scholar] [CrossRef]
- Sheikh, A.; Tumala, B.; Vickers, T.J.; Martin, J.C.; Rosa, B.A.; Sabui, S.; Basu, S.; Simoes, R.D.; Mitreva, M.; Storer, C.; et al. Enterotoxigenic Escherichia coli heat-labile toxin drives enteropathic changes in small intestinal epithelia. Nat. Commun. 2022, 13, 6886. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, J.M.; Chen, W.; Gookin, J.; Wu, G.Y.; Fu, Q.; Blikslager, A.T.; Rippe, R.A.; Argenzio, R.A.; Cance, W.G.; Weaver, E.M.; et al. Arginine stimulates intestinal cell migration through a focal adhesion kinase dependent mechanism. Gut 2004, 53, 514–522. [Google Scholar] [CrossRef]
- Sakiyama, T.; Musch, M.W.; Ropeleski, M.J.; Tsubouchi, H.; Chang, E.B. Glutamine increases autophagy under Basal and stressed conditions in intestinal epithelial cells. Gastroenterology 2009, 136, 924–932. [Google Scholar] [CrossRef]
- Tan, B.; Yin, Y.; Kong, X.; Li, P.; Li, X.; Gao, H.; Huang, R.; Wu, G. L-Arginine stimulates proliferation and prevents endotoxin-induced death of intestinal cells. Amino Acids 2010, 38, 1227–1235. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Z.; Ji, Y.; Sun, K.; Dai, Z.; Wu, G. L-Glutamine Enhances Tight Junction Integrity by Activating CaMK Kinase 2-AMP-Activated Protein Kinase Signaling in Intestinal Porcine Epithelial Cells. J. Nutr. 2016, 146, 501–508. [Google Scholar] [CrossRef]
- Perng, V.; Navazesh, S.E.; Park, J.; Arballo, J.R.; Ji, P. Iron Deficiency and Overload Modulate the Inflammatory Responses and Metabolism of Alveolar Macrophages. Nutrients 2022, 14, 3100. [Google Scholar] [CrossRef]
- Fang, S.; Zhuo, Z.; Yu, X.; Wang, H.; Feng, J. Oral administration of liquid iron preparation containing excess iron induces intestine and liver injury, impairs intestinal barrier function and alters the gut microbiota in rats. J. Trace Elem. Med. Biol. 2018, 47, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hansen, S.L.; Borst, L.B.; Spears, J.W.; Moeser, A.J. Dietary Iron Deficiency and Oversupplementation Increase Intestinal Permeability, Ion Transport, and Inflammation in Pigs. J. Nutr. 2016, 146, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; He, Y.; Lin, L.; Chen, P.; Chen, M.; Zhang, S. The emerging role of ferroptosis in intestinal disease. Cell Death Dis. 2021, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Cerda, S.R.; Bissonnette, M.; Scaglione-Sewell, B.; Lyons, M.R.; Khare, S.; Mustafi, R.; Brasitus, T.A. PKC-delta inhibits anchorage-dependent and -independent growth, enhances differentiation, and increases apoptosis in CaCo-2 cells. Gastroenterology 2001, 120, 1700–1712. [Google Scholar] [CrossRef]
- Vergauwen, H. The IPEC-J2 Cell Line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Springer: Cham, Switzerland, 2015; pp. 125–134. [Google Scholar]
- Nossol, C.; Barta-Böszörményi, A.; Kahlert, S.; Zuschratter, W.; Faber-Zuschratter, H.; Reinhardt, N.; Ponsuksili, S.; Wimmers, K.; Diesing, A.K.; Rothkötter, H.J. Comparing Two Intestinal Porcine Epithelial Cell Lines (IPECs): Morphological Differentiation, Function and Metabolism. PLoS ONE 2015, 10, e0132323. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Nonnecke, E.B.; Doan, N.; Lönnerdal, B.; Tan, B. Excess Iron Enhances Purine Catabolism Through Activation of Xanthine Oxidase and Impairs Myelination in the Hippocampus of Nursing Piglets. J. Nutr. 2019, 149, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Fiehn, O. Metabolomics by Gas Chromatography-Mass Spectrometry: Combined Targeted and Untargeted Profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30.34.31–30.34.32. [Google Scholar] [CrossRef]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M.; Kind, T.; Lee, D.Y.; Lu, Y.; Moon, S.; Nikolau, B. Quality control for plant metabolomics: Reporting MSI-compliant studies. Plant J. 2008, 53, 691–704. [Google Scholar] [CrossRef]
- Fiehn, O.; Wohlgemuth, G.; Scholz, M. Setup and Annotation of Metabolomic Experiments by Integrating Biological and Mass Spectrometric Metadata. In Data Integration in the Life Sciences, Proceedings of the Second International Workshop, DILS 2005, San Diego, CA, USA, 20–22 July 2005; Springer: Berlin/Heidelberg, Germany, 2005; pp. 224–239. [Google Scholar]
- Snoeck, V.; Goddeeris, B.; Cox, E. The role of enterocytes in the intestinal barrier function and antigen uptake. Microbes Infect. 2005, 7, 997–1004. [Google Scholar] [CrossRef]
- Mobarra, N.; Shanaki, M.; Ehteram, H.; Nasiri, H.; Sahmani, M.; Saeidi, M.; Goudarzi, M.; Pourkarim, H.; Azad, M. A Review on Iron Chelators in Treatment of Iron Overload Syndromes. Int. J. Hematol. Oncol. Stem Cell Res. 2016, 10, 239–247. [Google Scholar]
- Mastrogiannaki, M.; Matak, P.; Keith, B.; Simon, M.C.; Vaulont, S.; Peyssonnaux, C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J. Clin. Investig. 2009, 119, 1159–1166. [Google Scholar] [CrossRef]
- Shah, Y.M.; Matsubara, T.; Ito, S.; Yim, S.H.; Gonzalez, F.J. Intestinal hypoxia-inducible transcription factors are essential for iron absorption following iron deficiency. Cell Metab. 2009, 9, 152–164. [Google Scholar] [CrossRef]
- Taylor, M.; Qu, A.; Anderson, E.R.; Matsubara, T.; Martin, A.; Gonzalez, F.J.; Shah, Y.M. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology 2011, 140, 2044–2055. [Google Scholar] [CrossRef]
- Shawki, A.; Anthony, S.R.; Nose, Y.; Engevik, M.A.; Niespodzany, E.J.; Barrientos, T.; Öhrvik, H.; Worrell, R.T.; Thiele, D.J.; Mackenzie, B. Intestinal DMT1 is critical for iron absorption in the mouse but is not required for the absorption of copper or manganese. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G635–G647. [Google Scholar] [CrossRef]
- Erlitzki, R.; Long, J.C.; Theil, E.C. Multiple, conserved iron-responsive elements in the 3′-untranslated region of transferrin receptor mRNA enhance binding of iron regulatory protein 2. J. Biol. Chem. 2002, 277, 42579–42587. [Google Scholar] [CrossRef]
- Park, J.; Wickramasinghe, S.; Mills, D.A.; Lönnerdal, B.L.; Ji, P. Iron Fortification and Inulin Supplementation in Early Infancy: Evaluating the Impact on Iron Metabolism and Trace Mineral Status in a Piglet Model. Curr. Dev. Nutr. 2024, 8, 102147. [Google Scholar] [CrossRef] [PubMed]
- Perng, V.; Li, C.; Klocke, C.R.; Navazesh, S.E.; Pinneles, D.K.; Lein, P.J.; Ji, P. Iron Deficiency and Iron Excess Differently Affect Dendritic Architecture of Pyramidal Neurons in the Hippocampus of Piglets. J. Nutr. 2021, 151, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Yang, W.; Fang, C.; He, H.; Liu, J.; Fang, R. New insights into the mechanisms of iron absorption: Iron dextran uptake in the intestines of weaned pigs through glucose transporter 5 (GLUT5) and divalent metal transporter 1 (DMT1) transporters. Anim. Nutr. 2024, 19, 25–40. [Google Scholar] [CrossRef]
- Gunshin, H.; Allerson, C.R.; Polycarpou-Schwarz, M.; Rofts, A.; Rogers, J.T.; Kishi, F.; Hentze, M.W.; Rouault, T.A.; Andrews, N.C.; Hediger, M.A. Iron-dependent regulation of the divalent metal ion transporter. FEBS Lett. 2001, 509, 309–316. [Google Scholar] [CrossRef]
- Martini, L.A.; Tchack, L.; Wood, R.J. Iron treatment downregulates DMT1 and IREG1 mRNA expression in Caco-2 cells. J. Nutr. 2002, 132, 693–696. [Google Scholar] [CrossRef]
- Yeh, K.Y.; Yeh, M.; Watkins, J.A.; Rodriguez-Paris, J.; Glass, J. Dietary iron induces rapid changes in rat intestinal divalent metal transporter expression. Am. J. Physiol. Gastrointest. Liver Physiol. 2000, 279, G1070–G1079. [Google Scholar] [CrossRef] [PubMed]
- Canonne-Hergaux, F.; Gruenheid, S.; Ponka, P.; Gros, P. Cellular and subcellular localization of the Nramp2 iron transporter in the intestinal brush border and regulation by dietary iron. Blood 1999, 93, 4406–4417. [Google Scholar] [CrossRef]
- Lee, P.L.; Gelbart, T.; West, C.; Halloran, C.; Beutler, E. The human Nramp2 gene: Characterization of the gene structure, alternative splicing, promoter region and polymorphisms. Blood Cells Mol. Dis. 1998, 24, 199–215. [Google Scholar] [CrossRef]
- Bae, T.; Hallis, S.P.; Kwak, M.K. Hypoxia, oxidative stress, and the interplay of HIFs and NRF2 signaling in cancer. Exp. Mol. Med. 2024, 56, 501–514. [Google Scholar] [CrossRef]
- Drakesmith, H.; Nemeth, E.; Ganz, T. Ironing out Ferroportin. Cell Metab. 2015, 22, 777–787. [Google Scholar] [CrossRef]
- Kasai, S.; Mimura, J.; Ozaki, T.; Itoh, K. Emerging Regulatory Role of Nrf2 in Iron, Heme, and Hemoglobin Metabolism in Physiology and Disease. Front. Vet. Sci. 2018, 5, 242. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdian, V.; Tapadar, S.; MacDonald, I.A.; Xu, Y.; Ho, P.Y.; Bridges, A.; Rajpurohit, P.; Sanghani, B.A.; Fan, Y.; Thangaraju, M.; et al. Deferiprone: Pan-selective Histone Lysine Demethylase Inhibition Activity and Structure Activity Relationship Study. Sci. Rep. 2019, 9, 4802. [Google Scholar] [CrossRef] [PubMed]
- Yanagiya, R.; Kato, H.; Ninomiya, A.; Ueno, M.; Kanamori, A.; Miyatake, Y.; Oka, M.; Ishii, K.; Matsuura, T.; Nakagawa, S.; et al. Revised model for cell cycle regulation by iron: Differential roles between transferrin and ferritin. Redox Biol. 2025, 85, 103727. [Google Scholar] [CrossRef] [PubMed]
- Yasumoto, E.; Nakano, K.; Nakayachi, T.; Morshed, S.R.; Hashimoto, K.; Kikuchi, H.; Nishikawa, H.; Kawase, M.; Sakagami, H. Cytotoxic activity of deferiprone, maltol and related hydroxyketones against human tumor cell lines. Anticancer Res. 2004, 24, 755–762. [Google Scholar]
- Arce, C.; Ramírez-Boo, M.; Lucena, C.; Garrido, J.J. Innate immune activation of swine intestinal epithelial cell lines (IPEC-J2 and IPI-2I) in response to LPS from Salmonella typhimurium. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, 161–174. [Google Scholar] [CrossRef]
- Huang, Q.; You, R.; Tan, M.; Cai, D.; Zou, H.; Zhang, S.; Huang, H. HIF-1α is an important regulator of IL-8 expression in human bone marrow stromal cells under hypoxic microenvironment. Protoplasma 2024, 261, 543–551. [Google Scholar] [CrossRef]
- Xu, L.; Xie, K.; Mukaida, N.; Matsushima, K.; Fidler, I.J. Hypoxia-induced elevation in interleukin-8 expression by human ovarian carcinoma cells. Cancer Res. 1999, 59, 5822–5829. [Google Scholar]
- Deschemin, J.C.; Vaulont, S. Role of hepcidin in the setting of hypoferremia during acute inflammation. PLoS ONE 2013, 8, e61050. [Google Scholar] [CrossRef] [PubMed]
- Krijt, J.; Vokurka, M.; Sefc, L.; Duricová, D.; Necas, E. Effect of lipopolysaccharide and bleeding on the expression of intestinal proteins involved in iron and haem transport. Folia Biol. 2006, 52, 1–5. [Google Scholar] [CrossRef]
- Schwartz, A.J.; Das, N.K.; Ramakrishnan, S.K.; Jain, C.; Jurkovic, M.T.; Wu, J.; Nemeth, E.; Lakhal-Littleton, S.; Colacino, J.A.; Shah, Y.M. Hepatic hepcidin/intestinal HIF-2α axis maintains iron absorption during iron deficiency and overload. J. Clin. Investig. 2019, 129, 336–348. [Google Scholar] [CrossRef]
- Paneque, A.; Fortus, H.; Zheng, J.; Werlen, G.; Jacinto, E. The Hexosamine Biosynthesis Pathway: Regulation and Function. Genes 2023, 14, 933. [Google Scholar] [CrossRef]
- Oexle, H.; Gnaiger, E.; Weiss, G. Iron-dependent changes in cellular energy metabolism: Influence on citric acid cycle and oxidative phosphorylation. Biochim. Biophys. Acta 1999, 1413, 99–107. [Google Scholar] [CrossRef]
- Munn, N.J.; Arnio, E.; Liu, D.; Zoeller, R.A.; Liscum, L. Deficiency in ethanolamine plasmalogen leads to altered cholesterol transport. J. Lipid Res. 2003, 44, 182–192. [Google Scholar] [CrossRef]
- Tham, Y.K.; Huynh, K.; Mellett, N.A.; Henstridge, D.C.; Kiriazis, H.; Ooi, J.Y.Y.; Matsumoto, A.; Patterson, N.L.; Sadoshima, J.; Meikle, P.J.; et al. Distinct lipidomic profiles in models of physiological and pathological cardiac remodeling, and potential therapeutic strategies. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Cedillo, L.; Ahsan, F.M.; Li, S.; Stuhr, N.L.; Zhou, Y.; Zhang, Y.; Adedoja, A.; Murphy, L.M.; Yerevanian, A.; Emans, S.; et al. Ether lipid biosynthesis promotes lifespan extension and enables diverse pro-longevity paradigms in Caenorhabditis elegans. eLife 2023, 12, e82210. [Google Scholar] [CrossRef]
- Perez, M.A.; Magtanong, L.; Dixon, S.J.; Watts, J.L. Dietary Lipids Induce Ferroptosis in Caenorhabditiselegans and Human Cancer Cells. Dev. Cell 2020, 54, 447–454.e4. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Henry, W.S.; Ricq, E.L.; Graham, E.T.; Phadnis, V.V.; Maretich, P.; Paradkar, S.; Boehnke, N.; Deik, A.A.; Reinhardt, F.; et al. Plasticity of ether lipids promotes ferroptosis susceptibility and evasion. Nature 2020, 585, 603–608. [Google Scholar] [CrossRef]
- Chen, S.; Shen, C.; Zeng, X.; Sun, L.; Luo, F.; Wan, R.; Zhang, Y.; Chen, X.; Hou, Y.; Wang, W.; et al. Energy metabolism and the intestinal barrier: Implications for understanding and managing intestinal diseases. Front. Microbiol. 2025, 16, 1515364. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin E in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar] [CrossRef]
- Tucker, J.M.; Townsend, D.M. Alpha-tocopherol: Roles in prevention and therapy of human disease. Biomed. Pharmacother. 2005, 59, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zhang, Y.; Lou, H.; Ou, Z.; Liu, J.; Duan, W.; Wang, H.; Ge, Y.; Min, J.; Wang, F.; et al. GPX4 and vitamin E cooperatively protect hematopoietic stem and progenitor cells from lipid peroxidation and ferroptosis. Cell Death Dis. 2021, 12, 706. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Zeng, Y.; Chen, B.; Yan, J.; Ma, F.; Zhuang, G.; Hao, H.; Cao, G.; Xiao, X.; Li, S. Vitamin E and GPX4 cooperatively protect treg cells from ferroptosis and alleviate intestinal inflammatory damage in necrotizing enterocolitis. Redox Biol. 2024, 75, 103303. [Google Scholar] [CrossRef] [PubMed]
- Fisher, A.L.; Srole, D.N.; Palaskas, N.J.; Meriwether, D.; Reddy, S.T.; Ganz, T.; Nemeth, E. Iron loading induces cholesterol synthesis and sensitizes endothelial cells to TNFα-mediated apoptosis. J. Biol. Chem. 2021, 297, 101156. [Google Scholar] [CrossRef]


| Metabolite | C 1 | D | DF | F | q-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | ||
| 3-Aminopiperidine-2,6-dione | 3124.8 a | 299.4 | 1430.8 b | 161.6 | 2903.8 a | 299.1 | 3502.8 a | 524.6 | 0.013 |
| aconitic acid | 524.8 b | 72.5 | 3322.3 a | 964.7 | 496.0 b | 103.8 | 797.3 b | 141.6 | 0.006 |
| cholesterone | 921.0 b | 177.3 | 533.8 c | 104.8 | 1706.3 a | 110.3 | 1874.0 a | 274.4 | 0.012 |
| citric acid 2 | 154,670.3 b | 13,419.7 | 1,408,826.8 a | 357,909.6 | 101,176.8 b | 20,492.3 | 164,589.5 b | 11,606.3 | <0.001 |
| cytidine-5-monophosphate | 42,332.5 a | 6691.8 | 6888.8 b | 2799.4 | 27,693.0 a | 8910.2 | 53,602.0 a | 11,961.3 | 0.017 |
| glucose-1-phosphate | 10,421.5 a | 1378.1 | 3750.5 b | 980.5 | 10,443.5 a | 1644.5 | 12,552.3 a | 1133.7 | 0.017 |
| glucuronic acid | 1057.3 a | 166.9 | 619.5 b | 74.5 | 915.8 ab | 107.6 | 1320.3 a | 186 | 0.097 |
| hexadecylglycerol | 1249.3 b | 136.5 | 7011.5 a | 2372.2 | 1230.3 b | 28.3 | 1072.3 b | 130.2 | 0.012 |
| ile-ile | 2824.0 a | 609.7 | 783.5 b | 261.2 | 1716.0 a | 155.9 | 2098.8 a | 450.3 | 0.097 |
| inosine | 1023.8 a | 161.6 | 298.3 b | 51.2 | 559.3 b | 147 | 1082.0 a | 117.4 | 0.017 |
| inositol-4-monophosphate2 | 27,104.5 a | 2906.9 | 7251.5 b | 1097.1 | 22,921.5 a | 2277.6 | 31,200.5 a | 2461 | <0.001 |
| isopropylbenzene | 15,517.3 a | 1345.4 | 10,806.0 b | 920.1 | 11,053.0 b | 1357.7 | 15,495.3 a | 628.4 | 0.097 |
| lanosterol | 614.3 a | 89.7 | 244.8 b | 55.2 | 463.8 a | 73.2 | 853.0 a | 166.8 | 0.070 |
| octadecylglycerol | 929.5 b | 111.6 | 7322.8 a | 2267.1 | 1554.8 b | 581.5 | 922.5 b | 110.4 | 0.019 |
| shikimic acid | 7532.8 a | 851.5 | 2443.5 b | 593.5 | 4236.8 ab | 653.7 | 5393.3 a | 1569.2 | 0.097 |
| sorbitol | 37,957.0 a | 4338.3 | 18,056.8 b | 4348.2 | 18,732.0 b | 3177.9 | 40,249.3 a | 5834.5 | 0.088 |
| tocopherol alpha- | 3966.0 a | 134 | 3717.8 a | 356.8 | 2778.0 b | 78.3 | 2687.3 b | 228.2 | 0.022 |
| UDP GlcNAc | 2566.0 a | 691.5 | 1268.3 b | 149.2 | 2322.5 a | 367.4 | 3286.3 a | 348.6 | 0.097 |
| UDP-glucuronic acid 2 | 5516.3 a | 731.1 | 1331.5 b | 6.3 | 6296.3 a | 825.6 | 6201.0 a | 761.4 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navazesh, S.E.; Ji, P. Iron Stress Reprograms Enterocyte Metabolism. Metabolites 2025, 15, 691. https://doi.org/10.3390/metabo15110691
Navazesh SE, Ji P. Iron Stress Reprograms Enterocyte Metabolism. Metabolites. 2025; 15(11):691. https://doi.org/10.3390/metabo15110691
Chicago/Turabian StyleNavazesh, Shya E., and Peng Ji. 2025. "Iron Stress Reprograms Enterocyte Metabolism" Metabolites 15, no. 11: 691. https://doi.org/10.3390/metabo15110691
APA StyleNavazesh, S. E., & Ji, P. (2025). Iron Stress Reprograms Enterocyte Metabolism. Metabolites, 15(11), 691. https://doi.org/10.3390/metabo15110691






