Methylglyoxal, a Knot to Be Untied in Brain Glucose Hypometabolism
Abstract
1. Introduction
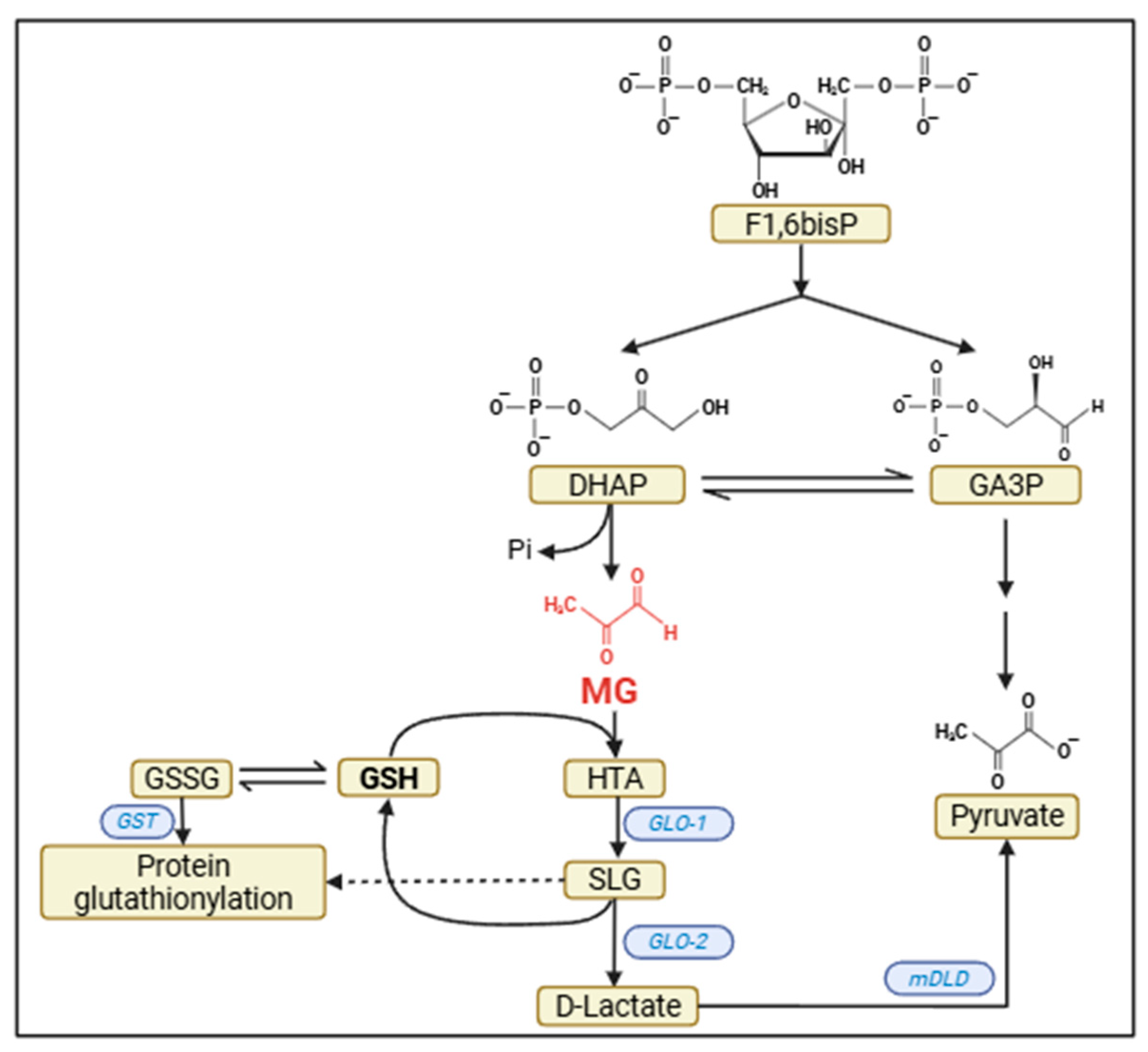
2. Sources of Brain MG
3. Brain Detoxification
4. Extracellular MG Signaling via RAGE
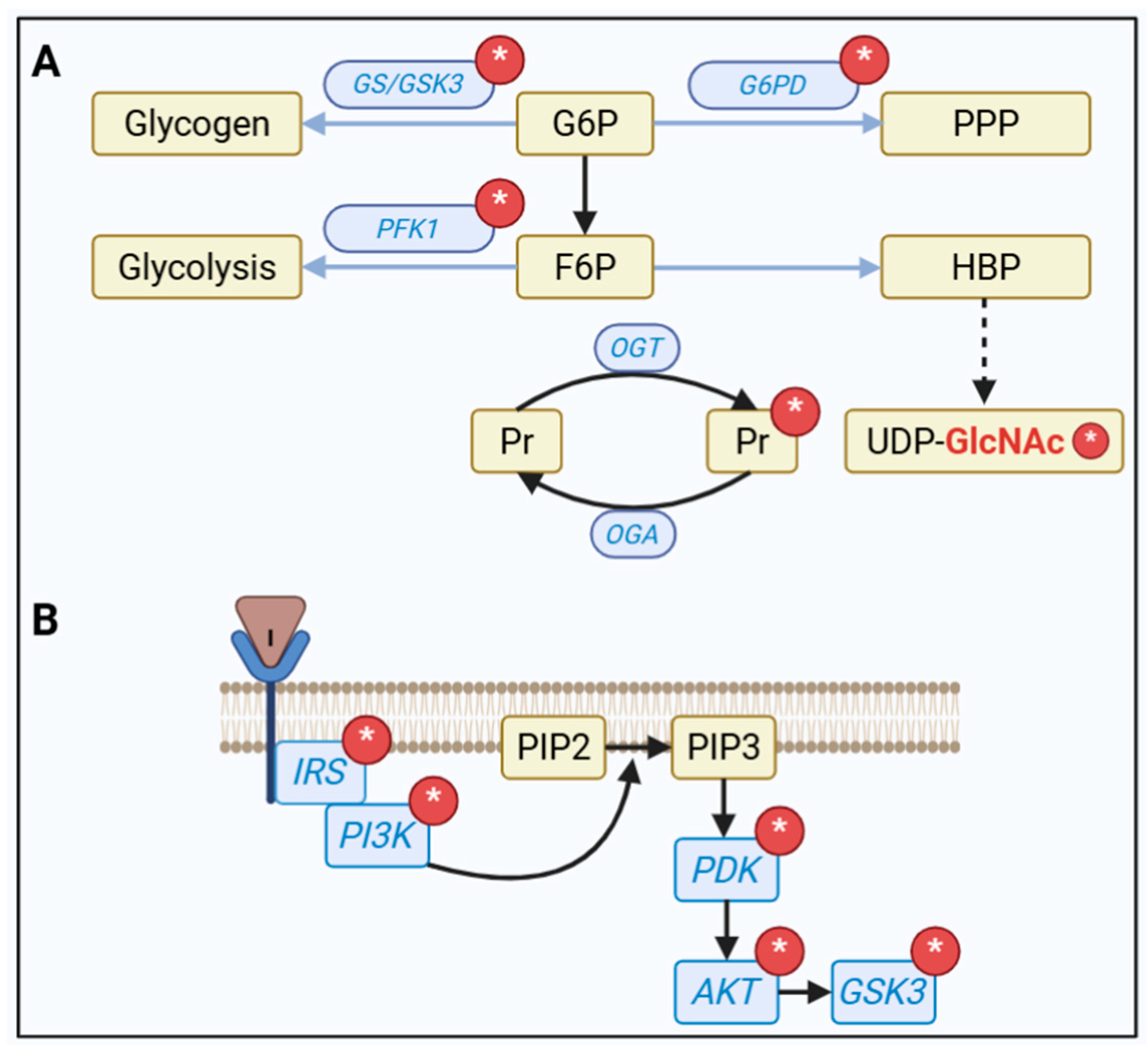
5. Direct Intracellular Consequences of Increased MG in the UPR
6. But What Is the Knot in the MG Metabolism?
7. Pharmacological and Nonpharmacological Strategies for Controlling MG
8. Final Comments and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| AGEs | advanced glycation end products |
| ATF6 | activating transcription factor 6 |
| CDK5 | cyclin-dependent kinase 5 |
| CYP2E1 | cytochrome P450 oxidase 2E1 |
| DE | diabetic encephalopathy |
| DHAP | dihydroxyacetone phosphate |
| DM | diabetes mellitus |
| G6PD | glucose-6-phosphate dehydrogenase |
| GA3P | glyceraldehyde 3-phosphate |
| GAPD | glyceraldehyde 3-phosphate dehydrogenase |
| GCL | glutamate-cysteine ligase |
| GLO1 | glyoxalase 1 |
| GLO2 | glyoxalase 2 |
| GSH | glutathione |
| GSK-3β | glycogen synthase kinase 3β |
| HBP | hexosamine biosynthesis pathway |
| IRE1α | inositol-requiring transmembrane kinase/endoribonuclease 1α |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| MG | methyliglyoxal |
| NFκB | nuclear factor kappa B |
| NOX | NADPH oxidase |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| PERK | PKR-like ER kinase |
| PPP | pentose phosphate pathway |
| RAGE | advanced glycation end products receptor |
| ROS | reactive oxygen species |
| S100B | S100 calcium binding protein B |
| UPR | unfolded protein response |
References
- Li, W.Y.; Lee, C.Y.; Lee, K.M.; Zhang, G.; Lyu, A.; Yue, K.K.M. Advanced Glycation End-Product Precursor Methylglyoxal May Lead to Development of Alzheimer’s Disease. Diabetes Metab. Syndr. Obes. 2022, 15, 3153–3166. [Google Scholar] [CrossRef] [PubMed]
- Allaman, I.; Bélanger, M.; Magistretti, P.J. Methylglyoxal, the dark side of glycolysis. Front. Neurosci. 2015, 9, 23. [Google Scholar] [CrossRef]
- Hansen, F.; Galland, F.; Lirio, F.; De Souza, D.F.; Da Ré, C.; Pacheco, R.F.; Vizuete, A.F.; Quincozes-Santos, A.; Leite, M.C.; Gonçalves, C.-A. Methylglyoxal Induces Changes in the Glyoxalase System and Impairs Glutamate Uptake Activity in Primary Astrocytes. Oxidative Med. Cell Longev. 2017, 2017, 9574201. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.P.; Vizuete, A.F.K.; Zin, L.E.F.; de Marques, C.O.; Pacheco, R.F.; Leal, M.B.; Gonçalves, C.-A. The Methylglyoxal/RAGE/NOX-2 Pathway is Persistently Activated in the Hippocampus of Rats with STZ-Induced Sporadic Alzheimer’s Disease. Neurotox. Res. 2022, 40, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, L.; Fenech, M.; Dhillon, V.S.; Young, C.; Hoffmann, P.; Deo, P. Role of methylglyoxal protein modifications in DNA damage and chromosomal instability: Emerging molecular mechanisms. Mutat. Res. Rev. Mutat. Res. 2025, 796, 108558. [Google Scholar] [CrossRef]
- Kalapos, M.P. The tandem of free radicals and methylglyoxal. Chem. Biol. Interact. 2008, 171, 251–271. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Dicarbonyls and glyoxalase in disease mechanisms and clinical therapeutics. Glycoconj. J. 2016, 33, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Vašková, J.; Kováčová, G.; Pudelský, J.; Palenčár, D.; Mičková, H. Methylglyoxal Formation-Metabolic Routes and Consequences. Antioxidants 2025, 14, 212. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef]
- Barros, L.F.; Schirmeier, S.; Weber, B. The Astrocyte: Metabolic Hub of the Brain. Cold Spring Harb. Perspect. Biol. 2024, 16, a041355. [Google Scholar] [CrossRef]
- Dringen, R.; Arend, C. Glutathione Metabolism of the Brain-The Role of Astrocytes. J. Neurochem. 2025, 169, e70073. [Google Scholar] [CrossRef]
- Bélanger, M.; Yang, J.; Petit, J.-M.; Laroche, T.; Magistretti, P.J.; Allaman, I. Role of the glyoxalase system in astrocyte-mediated neuroprotection. J. Neurosci. 2011, 31, 18338–18352. [Google Scholar] [CrossRef] [PubMed]
- de Bari, L.; Atlante, A.; Armeni, T.; Kalapos, M.P. Synthesis and metabolism of methylglyoxal, S-D-lactoylglutathione and D-lactate in cancer and Alzheimer’s disease. Exploring the crossroad of eternal youth and premature aging. Ageing Res. Rev. 2019, 53, 100915. [Google Scholar] [CrossRef] [PubMed]
- Wątroba, M.; Grabowska, A.D.; Szukiewicz, D. Effects of Diabetes Mellitus-Related Dysglycemia on the Functions of Blood-Brain Barrier and the Risk of Dementia. Int. J. Mol. Sci. 2023, 24, 10069. [Google Scholar] [CrossRef]
- Ahmed, N.; Ahmed, U.; Thornalley, P.J.; Hager, K.; Fleischer, G.; Münch, G. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer’s disease and link to cognitive impairment. J. Neurochem. 2005, 92, 255–263. [Google Scholar] [CrossRef]
- Yan, S.D.; Chen, X.; Fu, J.; Chen, M.; Zhu, H.; Roher, A.; Slattery, T.; Zhao, L.; Nagashima, M.; Morser, J.; et al. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature 1996, 382, 685–691. [Google Scholar] [CrossRef]
- Leerach, N.; Harashima, A.; Munesue, S.; Kimura, K.; Oshima, Y.; Goto, H.; Yamamoto, H.; Higashida, H.; Yamamoto, Y. Glycation reaction and the role of the receptor for advanced glycation end-products in immunity and social behavior. Glycoconj. J. 2021, 38, 303–310. [Google Scholar] [CrossRef]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common neurodegenerative pathways in obesity, diabetes, and Alzheimer’s disease. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef]
- Tang, H.; Ma, T.; Wang, Y.; Zhang, C.; Chu, Y.; Guo, Y.; Xi, J.; Jiao, D.; Li, B.; Xie, C.; et al. Paeoniflorin modulates AGEs/RAGE/P38MAPK/ERK/mTOR autophagy pathway to improve cognitive dysfunction in MRL/lpr mice. Int. J. Biol. Macromol. 2025, 307, 141765. [Google Scholar] [CrossRef] [PubMed]
- Berends, E.; van Oostenbrugge, R.J.; Foulquier, S.; Schalkwijk, C.G. Methylglyoxal, a highly reactive dicarbonyl compound, as a threat for blood brain barrier integrity. Fluids Barriers CNS 2023, 20, 75. [Google Scholar] [CrossRef]
- Vizuete, A.F.K.; Gonçalves, C.-A. Is Methylglyoxal a Potential Biomarker for the Warburg Effect Induced by the Lipopolysaccharide Neuroinflammation Model? Neurochem. Res. 2024, 49, 1823–1837. [Google Scholar] [CrossRef]
- Subedi, L.; Lee, J.H.; Gaire, B.P.; Kim, S.Y. Sulforaphane Inhibits MGO-AGE-Mediated Neuroinflammation by Suppressing NF-κB, MAPK, and AGE-RAGE Signaling Pathways in Microglial Cells. Antioxidants 2020, 9, 792. [Google Scholar] [CrossRef]
- Vizuete, A.F.K.; Fróes, F.; Seady, M.; Hansen, F.; Ligabue-Braun, R.; Gonçalves, C.-A.; Souza, D.O. A Mechanism of Action of Metformin in the Brain: Prevention of Methylglyoxal-Induced Glutamatergic Impairment in Acute Hippocampal Slices. Mol. Neurobiol. 2024, 61, 3223–3239. [Google Scholar] [CrossRef]
- Lissner, L.J.; Wartchow, K.M.; Rodrigues, L.; Bobermin, L.D.; Borba, E.; Dias, V.G.; Hansen, F.; Quincozes-Santos, A.; Gonçalves, C.-A. Acute Methylglyoxal-Induced Damage in Blood-Brain Barrier and Hippocampal Tissue. Neurotox. Res. 2022, 40, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-Y.; Lin, Y.-P.; Lin, Y.-S.; Chang, S.-S. Advanced glycation end products enhance amyloid precursor protein expression by inducing reactive oxygen species. Free Radic. Biol. Med. 2010, 49, 474–480. [Google Scholar] [CrossRef]
- More, S.S.; Vince, R. Potential of a γ-glutamyl-transpeptidase-stable glutathione analogue against amyloid-β toxicity. ACS Chem. Neurosci. 2012, 3, 204–210. [Google Scholar] [CrossRef]
- de Almeida, G.R.L.; Szczepanik, J.C.; Selhorst, I.; Cunha, M.P.; Dafre, A.L. The expanding impact of methylglyoxal on behavior-related disorders. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 120, 110635. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Akhter, F.; Xue, R.; Sosunov, A.; Wu, L.; Chen, D.; Arancio, O.; Yan, S.F.; Yan, S.S. Synaptic mitochondria glycation contributes to mitochondrial stress and cognitive dysfunction. Brain 2025, 148, 262–275. [Google Scholar] [CrossRef]
- Hansen, F.; Battú, C.E.; Dutra, M.F.; Galland, F.; Lirio, F.; Broetto, N.; Nardin, P.; Gonçalves, C.-A. Methylglyoxal and carboxyethyllysine reduce glutamate uptake and S100B secretion in the hippocampus independently of RAGE activation. Amino Acids 2016, 48, 375–385. [Google Scholar] [CrossRef]
- Prantner, D.; Nallar, S.; Richard, K.; Spiegel, D.; Collins, K.D.; Vogel, S.N. Classically activated mouse macrophages produce methylglyoxal that induces a TLR4- and RAGE-independent proinflammatory response. J. Leukoc. Biol. 2021, 109, 605–619. [Google Scholar] [CrossRef] [PubMed]
- Distler, M.G.; Plant, L.D.; Sokoloff, G.; Hawk, A.J.; Aneas, I.; Wuenschell, G.E.; Termini, J.; Meredith, S.C.; Nobrega, M.A.; Palmer, A.A. Glyoxalase 1 increases anxiety by reducing GABAA receptor agonist methylglyoxal. J. Clin. Investig. 2012, 122, 2306–2315. [Google Scholar] [CrossRef] [PubMed]
- Vizuete, A.F.K.; Hansen, F.; Da Ré, C.; Leal, M.B.; Galland, F.; Leite, M.C.; Gonçalves, C.-A. GABAA Modulation of S100B Secretion in Acute Hippocampal Slices and Astrocyte Cultures. Neurochem. Res. 2019, 44, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Sims, S.G.; Cisney, R.N.; Lipscomb, M.M.; Meares, G.P. The role of endoplasmic reticulum stress in astrocytes. Glia 2022, 70, 5–19. [Google Scholar] [CrossRef]
- Rabbani, N.; Xue, M.; Thornalley, P.J. Dicarbonyl stress, protein glycation and the unfolded protein response. Glycoconj. J. 2021, 38, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Hemagirri, M.; Chen, Y.; Gopinath, S.C.B.; Sahreen, S.; Adnan, M.; Sasidharan, S. Crosstalk between protein misfolding and endoplasmic reticulum stress during ageing and their role in age-related disorders. Biochimie 2024, 221, 159–181. [Google Scholar] [CrossRef]
- Palsamy, P.; Bidasee, K.R.; Ayaki, M.; Augusteyn, R.C.; Chan, J.Y.; Shinohara, T. Methylglyoxal induces endoplasmic reticulum stress and DNA demethylation in the Keap1 promoter of human lens epithelial cells and age-related cataracts. Free Radic. Biol. Med. 2014, 72, 134–148. [Google Scholar] [CrossRef]
- Xue, M.; Irshad, Z.; Rabbani, N.; Thornalley, P.J. Increased cellular protein modification by methylglyoxal activates endoplasmic reticulum-based sensors of the unfolded protein response. Redox Biol. 2024, 69, 103025. [Google Scholar] [CrossRef]
- Bollong, M.J.; Lee, G.; Coukos, J.S.; Yun, H.; Zambaldo, C.; Chang, J.W.; Chin, E.N.; Ahmad, I.; Chatterjee, A.K.; Lairson, L.L.; et al. A metabolite-derived protein modification integrates glycolysis with KEAP1–NRF2 signalling. Nature 2018, 562, 600–604. [Google Scholar] [CrossRef]
- Liu, T.; Lv, Y.-F.; Zhao, J.-L.; You, Q.-D.; Jiang, Z.-Y. Regulation of Nrf2 by phosphorylation: Consequences for biological function and therapeutic implications. Free Radic. Biol. Med. 2021, 168, 129–141. [Google Scholar] [CrossRef]
- Schultz, B.; Taday, J.; Menezes, L.; Cigerce, A.; Leite, M.C.; Gonçalves, C.-A. Calpain-Mediated Alterations in Astrocytes Before and During Amyloid Chaos in Alzheimer’s Disease. J. Alzheimers Dis. 2021, 84, 1415–1430. [Google Scholar] [CrossRef]
- Jimenez-Blasco, D.; Santofimia-Castaño, P.; Gonzalez, A.; Almeida, A.; Bolaños, J.P. Astrocyte NMDA receptors’ activity sustains neuronal survival through a Cdk5-Nrf2 pathway. Cell Death Differ. 2015, 22, 1877–1889. [Google Scholar] [CrossRef]
- Soltani, S.; Dolatshahi, M.; Soltani, S.; Khazaei, K.; Rahmani, M.; Raji, C.A. Relationships Between Brain Glucose Metabolism Patterns and Impaired Glycemic Status: A Systematic Review of FDG-PET Studies With a Focus on Alzheimer’s Disease. Hum. Brain Mapp. 2025, 46, e70180. [Google Scholar] [CrossRef]
- Gonçalves, C.-A.; Rodrigues, L.; Bobermin, L.D.; Zanotto, C.; Vizuete, A.; Quincozes-Santos, A.; Souza, D.O.; Leite, M.C. Glycolysis-Derived Compounds From Astrocytes That Modulate Synaptic Communication. Front. Neurosci. 2018, 12, 1035. [Google Scholar] [CrossRef]
- Escartin, C.; Guillemaud, O.; Carrillo-de Sauvage, M.-A. Questions and (some) answers on reactive astrocytes. Glia 2019, 67, 2221–2247. [Google Scholar] [CrossRef] [PubMed]
- Sharallah, O.A.; Poddar, N.K.; Alwadan, O.A. Delineation of the role of G6PD in Alzheimer’s disease and potential enhancement through microfluidic and nanoparticle approaches. Ageing Res. Rev. 2024, 99, 102394. [Google Scholar] [CrossRef]
- Bak, L.K.; Walls, A.B.; Schousboe, A.; Waagepetersen, H.S. Astrocytic glycogen metabolism in the healthy and diseased brain. J. Biol. Chem. 2018, 293, 7108–7116. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Zhao, G.; Zhao, Y. Dysregulation of energy metabolism in Alzheimer’s disease. J. Neurol. 2024, 272, 2. [Google Scholar] [CrossRef]
- Dos Santos, J.P.A.; Vizuete, A.; Hansen, F.; Biasibetti, R.; Gonçalves, C.-A. Early and Persistent O-GlcNAc Protein Modification in the Streptozotocin Model of Alzheimer’s Disease. J. Alzheimers Dis. 2018, 61, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Manyevitch, R.; Protas, M.; Scarpiello, S.; Deliso, M.; Bass, B.; Nanajian, A.; Chang, M.; Thompson, S.M.; Khoury, N.; Gonnella, R.; et al. Evaluation of Metabolic and Synaptic Dysfunction Hypotheses of Alzheimer’s Disease (AD): A Meta-Analysis of CSF Markers. Curr. Alzheimer Res. 2018, 15, 164–181. [Google Scholar] [CrossRef]
- Ahmed, H.; Wang, Y.; Griffiths, W.J.; Levey, A.; Pikuleva, I.; Liang, S.H.; Haider, A. Brain cholesterol and Alzheimer’s disease: Challenges and opportunities in probe and drug development. Brain 2024, 147, 1622–1635. [Google Scholar] [CrossRef]
- Haddad, M.; Perrotte, M.; Ben Khedher, M.R.; Madec, E.; Lepage, A.; Fülöp, T.; Ramassamy, C. Levels of Receptor for Advanced Glycation End Products and Glyoxalase-1 in the Total Circulating Extracellular Vesicles from Mild Cognitive Impairment and Different Stages of Alzheimer’s Disease Patients. J. Alzheimers Dis. 2021, 84, 227–237. [Google Scholar] [CrossRef]
- Peters, A.S.; Wortmann, M.; Fleming, T.H.; Nawroth, P.P.; Bruckner, T.; Böckler, D.; Hakimi, M. Effect of metformin treatment in patients with type 2 diabetes with respect to glyoxalase 1 activity in atherosclerotic lesions. Vasa 2019, 48, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.L.; de Oliveira, M.G.; Medeiros, M.L.; Mónica, F.Z.; Antunes, E. Metformin abrogates the voiding dysfunction induced by prolonged methylglyoxal intake. Eur. J. Pharmacol. 2021, 910, 174502. [Google Scholar] [CrossRef]
- Kender, Z.; Fleming, T.; Kopf, S.; Torzsa, P.; Grolmusz, V.; Herzig, S.; Schleicher, E.; Rácz, K.; Reismann, P.; Nawroth, P.P. Effect of metformin on methylglyoxal metabolism in patients with type 2 diabetes. Exp. Clin. Endocrinol. Diabetes 2014, 122, 316–319. [Google Scholar] [CrossRef]
- Nikray, N.; Abharian, N.; Jafari Ashtiani, S.; Kobarfard, F.; Faizi, M. Comparative Evaluation of Aminoguanidine, Semicarbazide and Thiosemicarbazide Treatment for Methylglyoxal-Induced Neurological Toxicity in Experimental Models. Iran. J. Pharm. Res. 2024, 23, e153322. [Google Scholar] [CrossRef] [PubMed]
- Lo, T.W.; Selwood, T.; Thornalley, P.J. The reaction of methylglyoxal with aminoguanidine under physiological conditions and prevention of methylglyoxal binding to plasma proteins. Biochem. Pharmacol. 1994, 48, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.; Urban, C.; Berbaum, K.; Loske, C.; Alpar, A.; Gärtner, U.; De Arriba, S.G.; Arendt, T.; Münch, G. The carbonyl scavengers aminoguanidine and tenilsetam protect against the neurotoxic effects of methylglyoxal. Neurotox. Res. 2005, 7, 95–101. [Google Scholar] [CrossRef]
- Bobermin, L.D.; Sesterheim, P.; da Costa, D.S.; Rezena, E.; Schmitz, I.; da Silva, A.; de Moraes, A.D.M.; Souza, D.O.; Wyse, A.T.; Leipnitz, G.; et al. Simvastatin Differentially Modulates Glial Functions in Cultured Cortical and Hypothalamic Astrocytes Derived from Interferon α/β Receptor Knockout mice. Neurochem. Res. 2024, 49, 732–743. [Google Scholar] [CrossRef]
- Zanotto, C.; Hansen, F.; Galland, F.; Batassini, C.; Federhen, B.C.; da Silva, V.F.; Leite, M.C.; Nardin, P.; Gonçalves, C.-A. Glutamatergic Alterations in STZ-Induced Diabetic Rats Are Reversed by Exendin-4. Mol. Neurobiol. 2019, 56, 3538–3551. [Google Scholar] [CrossRef]
- Rodrigues, T.; Borges, P.; Mar, L.; Marques, D.; Albano, M.; Eickhoff, H.; Carrêlo, C.; Almeida, B.; Pires, S.; Abrantes, M.; et al. GLP-1 improves adipose tissue glyoxalase activity and capillarization improving insulin sensitivity in type 2 diabetes. Pharmacol. Res. 2020, 161, 105198. [Google Scholar] [CrossRef]
- Tseng, Y.-T.; Tsai, Y.-H.; Fülöp, F.; Chang, F.-R.; Lo, Y.-C. 2-Iodo-4’-Methoxychalcone Attenuates Methylglyoxal-Induced Neurotoxicity by Activation of GLP-1 Receptor and Enhancement of Neurotrophic Signal, Antioxidant Defense and Glyoxalase Pathway. Molecules 2019, 24, 2249. [Google Scholar] [CrossRef] [PubMed]
- Sovrani, V.; Bobermin, L.D.; Sesterheim, P.; Rezena, E.; Cioccari, M.S.; Netto, C.A.; Gonçalves, C.-A.; Leipnitz, G.; Quincozes-Santos, A. Glioprotective effects of resveratrol in hypothalamic astrocyte cultures obtained from interferon receptor knockout (IFNα/βR−/−) mice. In Vitr. Cell Dev. Biol. Anim. 2023, 59, 366–380. [Google Scholar] [CrossRef]
- Quincozes-Santos, A.; Bobermin, L.D.; Tramontina, A.C.; Wartchow, K.M.; Da Silva, V.-F.; Gayger-Dias, V.; Thomaz, N.K.; de Moraes, A.D.M.; Schauren, D.; Nardin, P.; et al. Glioprotective Effects of Resveratrol Against Glutamate-Induced Cellular Dysfunction: The Role of Heme Oxygenase 1 Pathway. Neurotox. Res. 2025, 43, 7. [Google Scholar] [CrossRef]
- Santini, S.J.; Cordone, V.; Mijit, M.; Bignotti, V.; Aimola, P.; Dolo, V.; Falone, S.; Amicarelli, F. SIRT1-Dependent Upregulation of Antiglycative Defense in HUVECs Is Essential for Resveratrol Protection against High Glucose Stress. Antioxidants 2019, 8, 346. [Google Scholar] [CrossRef]
- Alfarano, M.; Pastore, D.; Fogliano, V.; Schalkwijk, C.G.; Oliviero, T. The Effect of Sulforaphane on Glyoxalase I Expression and Activity in Peripheral Blood Mononuclear Cells. Nutrients 2018, 10, 1773. [Google Scholar] [CrossRef]
- Bobermin, L.D.; Weber, F.B.; Dos Santos, T.M.; Belló-Klein, A.; Wyse, A.T.S.; Gonçalves, C.-A.; Quincozes-Santos, A. Sulforaphane Induces Glioprotection After LPS Challenge. Cell Mol. Neurobiol. 2022, 42, 829–846. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.F.O.; Costa, M.C.; Figueiredo, I.D.; Inácio, M.D.; Rodrigues, M.R.; Assis, R.P.; Baviera, A.M.; Brunetti, I.L. Curcumin, Alone or in Combination with Aminoguanidine, Increases Antioxidant Defenses and Glycation Product Detoxification in Streptozotocin-Diabetic Rats: A Therapeutic Strategy to Mitigate Glycoxidative Stress. Oxidative Med. Cell Longev. 2020, 2020, 1036360. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, J.; Chen, L.; Li, J.; Zhang, H.; Guo, X. Glycine Suppresses AGE/RAGE Signaling Pathway and Subsequent Oxidative Stress by Restoring Glo1 Function in the Aorta of Diabetic Rats and in HUVECs. Oxidative Med. Cell Longev. 2019, 2019, 4628962. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, D.; Chen, L.; Li, J.; Yuan, G.; Yang, G.; Zhang, H.; Guo, X.; Zhang, J. Glycine increases glyoxalase-1 function by promoting nuclear factor erythroid 2-related factor 2 translocation into the nucleus of kidney cells of streptozotocin-induced diabetic rats. J. Diabetes Investig. 2019, 10, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Yakupu, A.; Guan, H.; Dong, J.; Liu, Y.; Song, F.; Tang, J.; Tian, M.; Niu, Y.; Lu, S. Pyridoxamine ameliorates methylglyoxal-induced macrophage dysfunction to facilitate tissue repair in diabetic wounds. Int. Wound J. 2022, 19, 52–63. [Google Scholar] [CrossRef]
- Chen, Y.-J.; Kong, L.; Tang, Z.-Z.; Zhang, Y.-M.; Liu, Y.; Wang, T.-Y.; Liu, Y.-W. Hesperetin ameliorates diabetic nephropathy in rats by activating Nrf2/ARE/glyoxalase 1 pathway. Biomed. Pharmacother. 2019, 111, 1166–1175. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.-M.; Zhang, M.-Y.; Chen, Y.-J.; Liu, Y.-W. Hesperetin ameliorates diabetes-associated anxiety and depression-like behaviors in rats via activating Nrf2/ARE pathway. Metab. Brain Dis. 2021, 36, 1969–1983. [Google Scholar] [CrossRef]
- Frandsen, J.; Narayanasamy, P. Flavonoid Enhances the Glyoxalase Pathway in Cerebellar Neurons to Retain Cellular Functions. Sci. Rep. 2017, 7, 5126. [Google Scholar] [CrossRef]
- Zhu, X.; Cheng, Y.-Q.; Lu, Q.; Du, L.; Yin, X.-X.; Liu, Y.-W. Enhancement of glyoxalase 1, a polyfunctional defense enzyme, by quercetin in the brain in streptozotocin-induced diabetic rats. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Dieter, B.P.; Vella, C.A. A proposed mechanism for exercise attenuated methylglyoxal accumulation: Activation of the ARE-Nrf pathway and increased glutathione biosynthesis. Med. Hypotheses 2013, 81, 813–815. [Google Scholar] [CrossRef]
- Falone, S.; D’Alessandro, A.; Mirabilio, A.; Petruccelli, G.; Cacchio, M.; Di Ilio, C.; Di Loreto, S.; Amicarelli, F. Long term running biphasically improves methylglyoxal-related metabolism, redox homeostasis and neurotrophic support within adult mouse brain cortex. PLoS ONE 2012, 7, e31401. [Google Scholar] [CrossRef]
- Falone, S.; D’Alessandro, A.; Mirabilio, A.; Cacchio, M.; Di Ilio, C.; Di Loreto, S.; Amicarelli, F. Late-onset running biphasically improves redox balance, energy- and methylglyoxal-related status, as well as SIRT1 expression in mouse hippocampus. PLoS ONE 2012, 7, e48334. [Google Scholar] [CrossRef]
- Kondoh, Y.; Kawase, M.; Ohmori, S. D-lactate concentrations in blood, urine and sweat before and after exercise. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 65, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Ildarabadi, A.; Mir Mohammad Ali, S.N.; Rahmani, F.; Mosavari, N.; Pourbakhtyaran, E.; Rezaei, N. Inflammation and oxidative stress in epileptic children: From molecular mechanisms to clinical application of ketogenic diet. Rev. Neurosci. 2024, 35, 473–488. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, A.K. Molecular mechanism of caloric restriction mimetics-mediated neuroprotection of age-related neurodegenerative diseases: An emerging therapeutic approach. Biogerontology 2023, 24, 679–708. [Google Scholar] [CrossRef] [PubMed]
- Ezkurdia, A.; Ramírez, M.J.; Solas, M. Metabolic Syndrome as a Risk Factor for Alzheimer’s Disease: A Focus on Insulin Resistance. Int. J. Mol. Sci. 2023, 24, 4354. [Google Scholar] [CrossRef]
- Meng, F.; Wang, J.; Wang, L.; Zou, W. Glucose metabolism impairment in major depressive disorder. Brain Res. Bull. 2025, 221, 111191. [Google Scholar] [CrossRef]
- Beisswenger, P.J. Methylglyoxal in diabetes: Link to treatment, glycaemic control and biomarkers of complications. Biochem. Soc. Trans. 2014, 42, 450–456. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra Bhat, L.; Vedantham, S.; Krishnan, U.M.; Rayappan, J.B.B. Methylglyoxal—An emerging biomarker for diabetes mellitus diagnosis and its detection methods. Biosens. Bioelectron. 2019, 133, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Krautwald, M.; Münch, G. Advanced glycation end products as biomarkers and gerontotoxins—A basis to explore methylglyoxal-lowering agents for Alzheimer’s disease? Exp. Gerontol. 2010, 45, 744–751. [Google Scholar] [CrossRef] [PubMed]
- McMurray, K.M.J.; Ramaker, M.J.; Barkley-Levenson, A.M.; Sidhu, P.S.; Elkin, P.K.; Reddy, M.K.; Guthrie, M.L.; Cook, J.M.; Rawal, V.H.; A Arnold, L.; et al. Identification of a novel, fast-acting GABAergic antidepressant. Mol. Psychiatry 2018, 23, 384–391. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gayger-Dias, V.; Da Silva, V.-F.; Sobottka, T.M.; Leite, M.C.; Vizuete, A.F.K.; Gonçalves, C.-A. Methylglyoxal, a Knot to Be Untied in Brain Glucose Hypometabolism. Metabolites 2025, 15, 690. https://doi.org/10.3390/metabo15110690
Gayger-Dias V, Da Silva V-F, Sobottka TM, Leite MC, Vizuete AFK, Gonçalves C-A. Methylglyoxal, a Knot to Be Untied in Brain Glucose Hypometabolism. Metabolites. 2025; 15(11):690. https://doi.org/10.3390/metabo15110690
Chicago/Turabian StyleGayger-Dias, Vitor, Vanessa-Fernanda Da Silva, Thomas Michel Sobottka, Marina Concli Leite, Adriana Fernanda K. Vizuete, and Carlos-Alberto Gonçalves. 2025. "Methylglyoxal, a Knot to Be Untied in Brain Glucose Hypometabolism" Metabolites 15, no. 11: 690. https://doi.org/10.3390/metabo15110690
APA StyleGayger-Dias, V., Da Silva, V.-F., Sobottka, T. M., Leite, M. C., Vizuete, A. F. K., & Gonçalves, C.-A. (2025). Methylglyoxal, a Knot to Be Untied in Brain Glucose Hypometabolism. Metabolites, 15(11), 690. https://doi.org/10.3390/metabo15110690







