Abstract
Background/Objectives: The association between plasma metabolites derived from dietary substrates and inflammatory processes remains underexplored, despite its potential relevance in the prevention of non-communicable diseases. This systematic review aimed to examine the relationship between blood metabolites and the modulation of inflammatory biomarkers. Methods: A total of 25 randomized controlled trials, published between 2019 and 2024, were included from an initial pool of 111 records. These studies investigated the effects of dietary patterns, specific food groups, or nutritional supplements on the human metabolome and their potential links to inflammation. Results: Metabolomic analyses were predominantly performed using mass spectrometry (MS)-based platforms (17 out of 25), with liquid chromatography–mass spectrometry as the most frequently employed method. Both targeted (n = 14) and untargeted (n = 11) approaches were represented, and samples were drawn from plasma, urine, and feces. Across the interventions, 64 metabolites were modulated, including fatty acyls, glycerolipids, benzenoids, and organic acids, reflecting potential changes in pathways related to oxidative stress, lipid and carbohydrate metabolism, and inflammatory signaling. Several studies also assessed classical inflammatory biomarkers such as C-reactive protein (CRP), tumor necrosis factor alpha (TNFα), interleukin-6 (IL-6), and monocyte chemoattractant protein-1 (MCP-1). Interventions involving healthy traditional dietary patterns, improvements in dietary fat quality, or the use of specific probiotic strains were often associated with favorable immunometabolic outcomes. In contrast, some interventions, such as Mohana Choorna, elicited upregulation of immune-related gene expression in adipose tissue without improvements in glucose or lipid metabolism. Conclusions: While metabolomic responses varied across studies, the evidence highlights the value of dietary interventions in modulating systemic metabolism and inflammation. These findings support the integration of metabolomics into clinical nutrition to define more personalized and effective dietary strategies for inflammation-related chronic disease prevention.
1. Introduction
Metabolomics is the study of the metabolome, the complete set of small molecules produced by cells [1,2]. Metabolomic data enable the correlation of metabolite profiles with phenotypes, known as metabotypes [3,4]. These profiles can fluctuate in response to genetic variation [5], environmental exposures [6], and lifestyle factors [7,8], all of which interact to influence health outcomes. Among these, diet is widely recognized as one of the most influential lifestyle factors [9]. Therefore, the characterization of small molecules derived from different dietary exposures enables researchers to evaluate how diet-induced alterations in metabolic profiles contribute to interindividual variation in metabotypes [10,11].
The identification of biomarkers through high-throughput metabolomic techniques has become a valuable strategy for unraveling the complex, bidirectional relationships between diet, metabolism, and inflammation [12,13]. This information supports the development of targeted nutritional strategies aimed at modulating inflammation and improving health outcomes.
The two primary analytical platforms in nutritional metabolomics are mass spectrometry (MS) and nuclear magnetic resonance (NMR) spectroscopy. On the one hand, MS-based techniques provide high sensitivity and broad coverage but typically require a prior separation method, such as liquid chromatography (LC) or gas chromatography (GC), to manage sample complexity [14,15,16,17]. GC-MS is effective for volatile and thermally stable compounds, including organic acids and amino acids, but often requires prior chemical derivatization, which limits its utility for less volatile metabolites [18]. LC-MS is more versatile, enabling the analysis of both polar and non-polar compounds without derivatization, and is now the most widely used platform in metabolomics [19]. However, LC-MS can be affected by ion suppression, where co-eluting compounds interfere with analyte ionization and reduce signal intensity [20]. On the other hand, NMR spectroscopy provides detailed structural information based on the magnetic properties of atomic nuclei. It is accurate, reproducible, and non-destructive, requiring minimal sample preparation and allowing for the analysis of living systems [21,22]. Moreover, NMR can identify unknown compounds using spectral information, even without reference standards [15]. Its main limitations are lower sensitivity compared to MS, necessitating higher metabolite concentrations, and signal overlap in complex mixtures [23]. Given these characteristics, NMR is frequently used as a complementary technique, while MS-based approaches remain dominant in clinical metabolomics.
The main approaches commonly used in metabolomics are untargeted and targeted [24]. Untargeted metabolomics seeks to detect a broad array of metabolites without prior selection, enabling the discovery of unanticipated compounds and generating comprehensive metabolic “fingerprints” of nutritional states [25]. However, it also presents important challenges: (a) it produces large, complex datasets that require advanced data processing; (b) it is prone to false discoveries, (c) many detected features remain unidentified (the so-called “dark metabolome”) [26,27], and additionally, (d) lower-abundance metabolites may be masked by more abundant compounds [20]. In contrast, targeted metabolomics focuses on a predefined set of compounds, offering higher reproducibility and simpler data analysis, making it ideal for hypothesis-driven studies [28]. Thus, while untargeted approaches are more suited for biomarker discovery and pathway exploration, targeted approaches provide the possibility to quantify a set of well-characterized metabolites, often those with known biological importance. These two strategies are therefore complementary and have been combined to yield broader insights [18,29].
Advances in these metabolomic platforms have made them essential tools for assessing individual health status [30]. As thousands of metabolites in human plasma reflect complex interactions between genetic, environmental, and lifestyle factors [31], metabolomic profiling is increasingly used to identify novel biomarkers for monitoring the impact of medical treatments and lifestyle changes. This is especially relevant in the context of chronic non-communicable diseases, such as diabetes, cardiovascular diseases, and cancer, which are now the leading causes of death worldwide [32].
A common underlying factor in the development of these diseases is chronic low-grade inflammation [33], characterized by persistently elevated levels of pro-inflammatory mediators and circulating immune cells [34,35,36]. Although this inflammatory state may not immediately damage tissues, it contributes significantly to disease progression [37]. Metabolomic analyses have identified specific molecules that could be involved in inflammation. These include metabolites that are significantly associated with both inflammatory and anti-inflammatory processes. This highlights their potential for use in prevention and therapeutic strategies [38].
Among the key factors influencing metabolism and the production of inflammation-modulating metabolites, diet plays a pivotal role [39]. Nutritional intake shapes both host and microbiota-derived metabolites, and diet-derived metabolites generated by the gut microbiota substantially affect host metabolism [40]. Diets high in saturated fatty acids (SFAs), for example, are associated with decreased microbial diversity and increased proliferation of pathogenic species [41]. This microbial imbalance damages the gut barrier, facilitating the translocation of bacterial components into the bloodstream, triggering systemic inflammation and promoting insulin resistance. In contrast, several diet-derived metabolites exert anti-inflammatory effects [42]. For instance, short-chain fatty acids (SCFAs), such as butyrate, produced by microbial fermentation of dietary fiber, have been shown to reduce inflammation, strengthen the gut barrier, and positively modulate the immune response [43,44,45].
Although numerous dietary intervention studies have explored these mechanisms, randomized controlled trials (RCTs) are considered the gold standard for establishing causal relationships. Unlike observational or cross-sectional designs, RCTs minimize bias, reduce confounding, and provide a temporal sequence between dietary exposures, metabolite responses, and inflammatory outcomes, making them essential to validate mechanistic pathways [46,47]. Yet, the role of host and gut-derived metabolites as mediators of inflammatory processes and the efficacy of targeted dietary interventions to modulate these pathways have not been sufficiently studied through RCTs. Moreover, despite clear evidence that diet and microbiota-derived metabolites modulate inflammation, the lack of long-term RCTs with mechanistic and translational approaches has led to fragmented knowledge. Addressing these causal relationships, while considering host–diet–microbiota interactions, is crucial to understanding the long-term metabolic effects of dietary patterns on gut health and inflammation.
While previous reviews have explored the effects of diet on inflammation or the human metabolome separately, few have comprehensively integrated evidence from randomized controlled trials examining both metabolomic responses and inflammatory biomarkers across diverse dietary interventions. This review addresses this gap by systematically synthesizing recent findings on how specific foods, dietary patterns, and supplements modulate metabolites linked to inflammation. By highlighting novel metabolite-biomarker associations and methodological innovations, this work advances understanding of the mechanistic links between diet and immunometabolic regulation and provides a foundation for more personalized nutritional strategies aimed at preventing inflammation-related chronic diseases.
In this context, a structured analysis of findings from randomized trials is needed to fill these knowledge gaps and provide a deeper understanding of how dietary intake influences metabolomic profiles and inflammation.
Accordingly, this systematic review aims to examine the impact of different dietary substrates on metabolomic signatures and how effects on metabolism modulate inflammatory responses.
2. Materials and Methods
The systematic review was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist guidelines (Table S1) [48]. This review protocol was not registered on any platform.
2.1. Search Strategy
An electronic search was performed using PubMed, Cochrane Library, and Epistemonikos databases (last searched in September 2024) to identify all articles related to the role of different dietary substrates on the profiles of metabolites present in humans and how they influence inflammatory tone. The following combination of Medical Subject Heading (MeSH) terms and text words were used: (Inflammation) AND (Nutrition OR Food OR Nutrient* OR Diet) AND (Metabolomic OR Metabolomics OR Metabolome OR Lipidomic OR Lipidomics OR Lipidome OR “Metabolic Profile” OR “Metabolic Profiles” OR “Metabolic Profiling” OR “Metabolite Profile” OR “Metabolite Profiles” OR “Metabolite Profiling”).
2.2. Eligibility Criteria
This study only included original publications written in English. Reviews and meta-analyses, comments, guidelines, editorials or letters, conference summaries, and non-randomized or non-controlled studies were excluded. The inclusion and exclusion criteria are outlined in Table 1.

Table 1.
Inclusion and exclusion criteria.
2.3. Data Collection Process
Data was extracted in a standardized format into a Microsoft Excel® spreadsheet. The following information was collected from each included study: Title, Authors, Year of Publication, Journal, DOI, Search Query, Date of Inclusion in the Database, Filters, RCT Research, Source, Metabolomic Technique, Samples, availability of the paper Free/Not Free, and Additional Comments.
A quantitative meta-analysis was not conducted due to several limiting factors. First, the majority of the included studies did not provide access to raw metabolomic data, preventing reanalysis or data harmonization. Second, substantial heterogeneity existed in the metabolites quantified and in the analytical platforms employed, including liquid chromatography–tandem mass spectrometry (LC–MS/MS), nuclear magnetic resonance (NMR), and other targeted or untargeted approaches. Additionally, differences in sample types, reporting formats, and normalization strategies further restricted data comparability across trials.
2.4. Screening and Selection
Four reviewers independently assessed and selected the studies based on the inclusion, exclusion, and quality assessment criteria outlined in the Risk of Bias Tool (Rob2). Discrepancies were resolved by discussion to reach a consensus.
2.5. Risk of Bias Assessment
The quality of the included studies was assessed using the Cochrane risk of bias tool for randomized trials (Rob2) (https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials, accessed on 17 November 2024). The Rob2 tool allows for the assessment of bias that may arise at different stages of an RCT across five distinct domains. The five assessed domains represent: (1) bias in the randomization process; (2) bias due to deviation from intended interventions; (3) bias due to missing outcome data; (4) bias in the measurement of the outcome; and (5) bias in the selection and reporting of results. Each domain was rated as having a “low risk”, “some concerns”, or “high risk” of bias.
3. Results
3.1. Study Selection
The flow diagram of the study selection process is shown in Figure 1. In the original search, 111 records were published from 2019 to 2024. Before screening, 14 records were removed: 2 duplicates, 7 trial registrations, and 5 records with inaccessible full text. A total of 97 records remained and were screened by title and abstract. Of these, 34 were excluded for not meeting eligibility criteria. The remaining 63 articles were assessed in full-text form. Among those, 38 were excluded for the following reasons: inadequate outcome (n = 9), inadequate study design (n = 2), no conventional metabolomics instrument used (i.e., methods other than MS or NMR) (n = 12), not metabolomics-focused (studies centered on biomarkers rather than direct metabolite profiling) (n = 12), questionable study integrity (n = 1), and absence of a comparison group between intervention and control (n = 2). Ultimately, 25 studies met all inclusion criteria and were included in the systematic review. Of these, 8 were crossover RCTs and 17 were parallel-group RCTs.
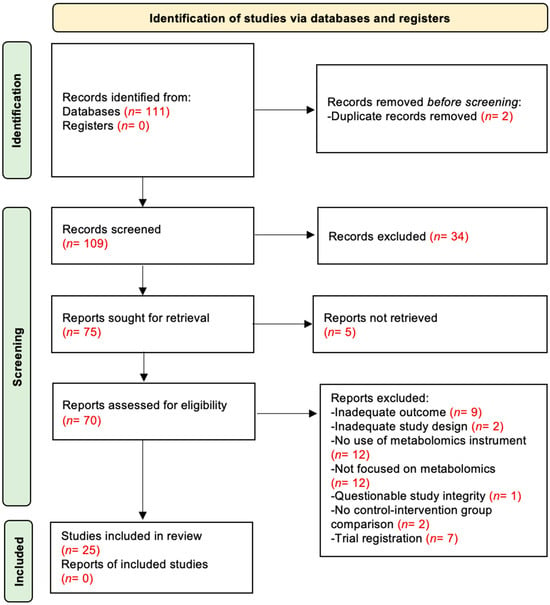
Figure 1.
PRISMA flow chart illustrating the selection process of the studies.
3.2. Study Characteristics
The main characteristics of the included studies are summarized in Table S2, while comprehensive details are provided in Table S3. Ten studies were published in 2019 [49,50,51,52,53,54,55,56,57,58], three in 2020 [59,60,61], six in 2021 [62,63,64,65,66,67], one in 2023 [68], and five in 2024 [69,70,71,72,73]. The geographical origins of the studies were diverse. Most were conducted in Europe, particularly in Greece [62], Italy [51,62], Serbia [62], Spain [52,69,70], the United Kingdom [49,60], Netherlands [53,61,63], Denmark [55,58], Portugal [72], Finland [55], Sweden [54,55], Iceland [55], and Norway [56]. Three were conducted in the United States [64,65,70], two in Australia [67,71], and the rest in Asia: Iran [50], Korea [59,73,74], and China [57,66]. Of these, three were multi-centric: two international [55,62] and one intercontinental [70]. The sample sizes ranged from 10 [67] to 217 [57], with a total of 1654 individuals across all studies. The analysis included healthy individuals as well as those diagnosed with conditions such as non-alcoholic fatty liver disease, overweight or obesity, elevated blood pressure, impaired glucose tolerance, vitamin D deficiency, hypercholesterolemia, depressive symptomatology, rheumatoid arthritis, asthma, cirrhosis, and hypertension.
One study included only men [67], while four were conducted exclusively in women [52,53,54,59]. The remaining studies included participants of both sexes, except for one [68] that did not report on sex. The mean age of the participants ranged between 41 [73] and 81 [51] years. The duration of the intervention varied from 7 days [53] to 2 years [70].
Eight of the interventions focused on food or dietary patterns, such as the Mediterranean diet or dietary fat control. Fourteen involved supplementations, such as vitamin D and Korean red ginseng, and three involved probiotics (Table 2).

Table 2.
Main characteristics of the randomized controlled trials analyzed, organized according to the type of dietary intervention.
3.3. Analytical and Biological Heterogeneity
The selected studies show substantial methodological variability in crucial aspects such as: (1) sample type and preparation approaches, (2) choice of analytical platform, instrumentation, and optimization details, (3) calibration practices, and (4) reporting (or lack of) detection limits, all of which can affect comparability between the studies.
Regarding sample type, the most used was plasma (eight studies), followed by serum (five studies) and urine (four studies). A few studies included stool, muscle biopsies, or erythrocyte membrane fractions. Sample preparation strategies varied greatly between studies, as they are highly dependent on the analytical platform of choice (NMR or MS). A trend was evident regarding the choice of analytical platform, with most of the studies using MS (17/25) compared to NMR (6/25). Moreover, most studies (15/25) used LC as the prior separation technique. Among those, the most reported LC method was UPLC (13/15) with a C18 reverse phase column (10/15), and the most utilized detection system for MS was triple quadrupole (QQQ) (10/15) with electrospray ionization (ESI) (12/15). One study employed both GC-MS and LC-MS, while another study utilized GC-MS alone. Two studies used a combination of NMR and LC-MS to maximize metabolome coverage. The remaining study used GC but coupled with flame ionization instead of MS for metabolite detection (Table S2).
Targeted approaches (14/25) were slightly more common than untargeted approaches (10/25) in the analyzed studies. One study reported both targeted and untargeted approaches. However, no clear bias towards one type was observed. Differences instead reflected a choice based on whether there is a prior hypothesis being tested involving a given set of metabolites or not.
The dominant approach for sample preparation in MS-based studies was protein precipitation with an organic solvent, generally acetonitrile or methanol (15/17). Solid-phase extraction (SPE) with commercial cartridges was used in four studies. The three studies using GC-MS typically employed a two-step derivatization, but reagents and conditions were not uniform across these interventions. For NMR analysis, all studies reporting sample preparation used a similar and simpler approach consisting of the addition of an inorganic buffered solution prepared in deuterated water (D2O) for proton exchange (Table S2).
Regarding calibration practices, fourteen studies used relative quantification and seven employed absolute quantification using multi-point calibration curves or authentic standards. Moreover, 10 studies explicitly reported using an internal standard (spiked-in during sample preparation) for calibration. Most studies (11/25) used statistical methods for quality control (QC) of the data. However, analytical QC practices, such as the use of pooled QC samples, run throughout batches to monitor instrument stability and drift, which are essential to ensure proper data quality, are reported much less frequently (4/25). Four studies did not report any calibration practices. Almost all studies (23/25) did not report limits of detection for the detected metabolites (Table S2).
Among the 25 studies included, plasma was the most analyzed sample type, being used in 14 studies (56%). Serum samples were analyzed in 8 studies (32%), and urine samples in 5 studies (20%). Red blood cell (RBC) membranes and feces were each analyzed in 2 studies (8%). White blood cells and muscle biopsy specimens were the least commonly analyzed, each appearing in only 1 study (4%) (Table S2). These findings highlight a predominant focus on plasma and serum in the current literature, while other biological matrices remain underrepresented.
3.4. Metabolic Biomarkers Associated with Diet
Table 3 shows the dietary interventions carried out in each study and the associated metabolites that showed either increases or decreases in responses, and Figure 2 represents this graphically. In every intervention, the type of sample analyzed is explained, and the metabolites are grouped into families. The RefMet classification [75] provided by Metabolomics Workbench (https://www.metabolomicsworkbench.org/, accessed on 1 February 2025), an international repository for metabolomics data and metadata, was used to place each metabolite according to the highest hierarchical level of classification of a metabolite (superclass). From this point forward, consensus on the classifications used will be based on those extracted from this source instead of those presented in each article. Metabolites that could not be classified in this source were assigned to a superclass using the Human Metabolome Database (HMDB) [76].

Table 3.
Effects of dietary factors on metabolites and inflammatory biomarkers according to the type of dietary intervention.
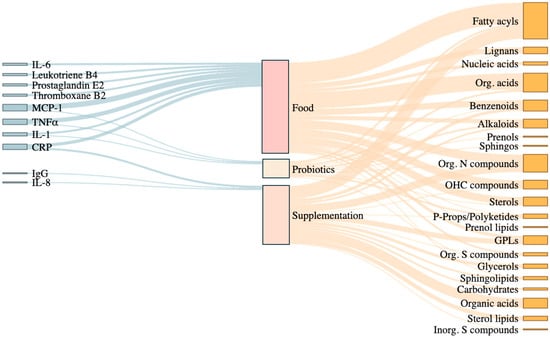
Figure 2.
Sankey diagram illustrates the relationships between inflammatory biomarkers, dietary intervention types, and affected metabolite classes in the reviewed RCTs. The diagram shows the connections between key inflammatory biomarkers (e.g., IL-6, CRP, TNFα), the type of dietary interventions applied (Food, Supplementation, Probiotics), and the metabolite classes modulated in response. Food-based interventions are broadly associated with fatty acyls, organic acids, and nitrogen compounds, while supplementation and probiotics modulate particular classes of metabolites such as glycerophospholipids, benzenoids, and sphingolipids. The width of each flow reflects the frequency of associations reported across the reviewed studies, highlighting which metabolite classes are most consistently linked to dietary patterns and inflammatory outcomes. Org. Acids: organic acids; Sphingos: sphingolipids; Org. N compounds: organic nitrogen compounds; OHC compounds: organoheterocyclic compounds; P-props/Polyketides: phenylpropanoids and polyketides; GPLs: glycerophospholipids; Org. S compounds: organic sulfur compounds; Inorg. S compounds: inorganic sulfur compounds. Figure created using R (version 4.4.1) within RStudio (version 2024.09.0+375).
A total of 64 metabolites of 17 metabolite superclasses were found to be modulated by different dietary interventions: alkaloids, benzenoids, carbohydrates, fatty acyls, glycerolipids, glycerophospholipids, lignans, nucleic acids, organic acids, organic nitrogen compounds, organic oxygen compounds, organic heterocyclic compounds, organosulfur compound, phenylpropanoids and polyketides, prenol lipids, sphingolipids, sulfur inorganic compounds, and sterol lipids.
Diets targeting oxidative stress have been shown to increase amino acids such as leucine, methionine, and glutamine, as well as allantoin, while decreasing fatty acyls such as oleic acid and nitrogen compounds such as trimethylamine N-oxide. Interventions targeting microbiota metabolism showed an increase in long-chain fatty acids, such as docosahexaenoic acid, and also essential amino acids, while reducing organic nitrogen compounds, such as 8-oxo-2’-deoxyguanosine. Similarly, diets targeting lipid metabolism were associated with increased polyunsaturated fatty acids (PUFAs). On the other hand, those diets targeting carbohydrate metabolism increased oxidized low-density lipoprotein (ox-LDL) and glutamate, while reducing oleic acid, pseudouridine, and nitrogen-containing compounds such as choline. Interventions aimed to improve inflammatory biomarkers also increased ox-LDL, and essential amino acids, while reducing lysophosphatidylcholines (a type of glycerophospholipid) and oxidative DNA markers [78] like Oxo-2’-deoxyguanosine and N-acetylglycoproteins [69].
Other general dietary interventions targeting specific cultural or national patterns were associated with changes in key metabolic pathways. For instance, the typical Western diet observed in the U.S. population was associated with an increased presence of benzenoids, such as hippuric acid, fatty acyls, organoheterocyclic compounds, organic acids, and steroid lipids, while reducing the presence of lignans, such as enterodiol-glucuronide [73]. In contrast, the Healthy Nordic Diet, characterized by a high intake of whole grains, canola oil, berries, and fish, showed increased levels of pipecolic acid, betaine, and nitrogen compounds [55]. The balanced Korean diet was associated with increased benzenoids, fatty acyls, lignans, sphingolipids, and sterol lipids. With a similar metabolomic profile, the 2010 Dietary Guidelines for Americans promoted an increase in organoheterocyclic compounds, like creatinine [73].
Dietary interventions that modify nutrient intake or the consumption of specific food groups were associated with distinct changes in metabolite profiles across various biological matrices. For example, walnut-enriched diets have been shown to increase oxylipins like 5,6-DiHETE and 5-HETE, while reducing fatty acyls such as 9-HOTrE and 12,13Epoxy9(E)octadecenoic acid. The addition of cruciferous vegetables to the diet increases S-methyl cysteine sulfoxide in urine, while reducing it in plasma and serum carotenoids (prenol lipids) [70]. Whole grain-rich diets, on the other hand, lead to an increase in urine metabolites such as benzenoids and fatty acyls, as well as organic acids like 2-aminophenol sulfate [58]. Replacing SFAs with PUFAs in the diet increases short chain fatty acids such as acetate, water-soluble vitamin such as thiamine, and organic acids such as serine and proline in plasma, while reducing lipoproteins, cholesterol, and other fatty acids such as palmitoylcarnitine [56]. Moreover, fat-controlled diets changed the levels of different alkaloids, such as indole and indoleacetic acid, and fatty acids, such as palmitic acid, stearic acid, and arachidonic acid, in fecal samples [57].
Supplementation interventions revealed several notable metabolomic shifts. In particular, many of the studies reported increases in specific fatty acids and lipids. For example, high-dose vitamin D3 supplementation increased phosphatidylcholine and sphingomyelin. Moreover, the effect of vitamin D3 supplementation doses on glycerolipids varied depending on the dose. While the low dose tended to decrease 40:4 diglycerol, the high dose increased it and decreased 36:3 [53]. Eicosapentaenoic acid supplementation increased and decreased different types of PUFAs [65]. Cholecalciferol and blue mussel supplementation led to a reduction in some types of carbohydrates, such as glucose. Additionally, with cholecalciferol supplementation, certain organic acids (aminoacids) were consistently modulated. For example, the levels of glutamine and histidine increased [72], whereas Bifidobacterium lactis Probio-M8 combined with Symbicort Turbuhaler supplementation increased the levels of succinic acid, L-tryptophan, and curcumin–phospholipid complex, resulting in decreased levels of various metabolites belonging to the superclass of organic acids [66].
Although less commonly reported, some interventions affected specific metabolite subclasses such as organic oxygen compounds, benzenoids, phenylpropanoids and polyketides. For instance, supplementation with curcumin–phospholipid complex showed a decrease in kynurenine [50], and the probiotics Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI showed a decrease in indole-3-acetic acid [74]. In addition to probiotic interventions, supplementation with Korean red ginseng [57], curcumin–phospholipid complex [50], and shark liver oil [67] decreased sterol lipids. Supplementation with liquid essential amino acids combined with whey protein and vitamin D [51] increased an organic oxygen compound (N-acetyl-L-leucine), while supplementation with xanthophylls, anthocyanins combined with xanthophylls [52], nicotinamide riboside [61], and Bifidobacterium lactis Probio-M8 powder combined with Symbicort Turbuhaler, a nasal inhaler used for asthma prevention, decreased organic nitrogen compounds [66]. Conversely, benzenoids like phenol sulfate increased in diet supplemented with anthocyanins and xanthophylls [52], strawberry powder [64], and Bifidobacterium lactis Probio-M8 powder combined with Symbicort Turbuhaler [66], and decreased with curcumin–phospholipid complex consumption [50]. Phenylpropanoids and polyketides were only present when urolithin A increased with strawberry supplementation [64]. Prenol lipids only decreased with cruciferous vegetables servings added to diet [71].
3.5. Dietary Interventions and Their Effects on Inflammation
Table 3 also shows the inflammatory biomarkers measured in each study. The most frequently assessed biomarkers across the studies were C-reactive protein (CRP), tumor necrosis factor-alpha (TNFα), monocyte chemoattractant protein-1 (MCP-1), and various interleukins (IL-6, IL-1β, and IL-8).
Personalized diets designed to optimize carbohydrate and oxidative stress biomarkers were linked to higher TNFα and MCP-1 levels than a Mediterranean diet-based control, while diets focused on lipid metabolism were associated with a reduction in CRP but an increase in TNFα [69]. Additionally, some studies explored genetic biomarkers related to inflammation and lipid metabolism [56,63]. In one case, during the Mohana Choorna supplementation, where SFAs were replaced with PUFAs, the expression of genes involved in inflammation was upregulated [63].
The balanced Korean diet, the diets recommended by the 2010 Dietary Guidelines for Americans, and even the typical U.S. diet, all demonstrated consistent decreases in MCP-1 levels [73]. Additionally, the healthy Nordic diet was found to reduce IL-1 through negative associations with pipecolic acid, betaine, and trigonelline [55]. Depending on the specific fat content, fat-controlled diets also showed reductions in CRP, thromboxane B2, prostaglandin E2, and leukotriene B4, while whole grain-rich diets were associated with decreased fasting serum IL-6, IL-1β, and TNFα [57]. Eicosapentaenoic acid and shark liver oil supplementation both showed a decrease in CRP levels [65]. On the other hand, some interventions showed no clear effects on classical inflammatory markers. For example, personalized diets targeting inflammation-related and microbiota metabolism biomarkers [69], addition of cruciferous vegetables [71], inulin [49], strawberries [64], nicotinamide riboside [61], and supplements rich in anthocyanins or xanthophylls [52], did not significantly change key markers such as IL-6, CRP, or Apo levels. However, probiotic interventions using strains like Bifidobacterium bifidum BGN4, Bifidobacterium longum BORI [68], and Lactobacillus casei Shirota [60] were associated with decreased MCP-1 levels.
3.6. The Impact on the Gut Microbiota
Data on gut microbiota were only obtained in some of the dietary interventions. Lower-fat diets have been associated with increased relative abundances of the genera Blautia and Faecalibacterium. In contrast, higher-fat diets tend to reduce Faecalibacterium, while increasing the abundance of Alistipes and Bacteroides [57].
Whole grain diets do not significantly alter overall bacterial diversity, but they do promote the growth of specific strains such as Faecalibacterium prausnitzii and Prevotella copri, while reducing Bacteroides thetaiotaomicron [58]. Inulin and inulin-propionate ester supplementation appear to decrease microbial diversity while selectively enriching certain beneficial taxa, including Bifidobacterium faecale, Anaerostipes hadrus, and Fusicatenibacter saccharivorans, while reducing others, such as Blautia obeum and Prevotella copri [49].
Probiotic interventions demonstrated taxon-specific shifts accompanied by changes in metabolite profiles. Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI increased indole-3-propionic acid, a tryptophan-derived microbial metabolite associated with reduced IL-1β and TNFα levels [74]. B. lactis Probio-M8 promoted the growth of Bifidobacterium animalis and Roseburia hominis alongside increased sphingomyelin and tryptophan derivatives [66].
Mastiha supplementation showed an increase in the Bray–Curtis microbiota dissimilarity index, indicating broader shifts in microbial community structure, alongside a reduction in Flavonifractor abundance [62].
4. Discussion
4.1. Metabolites Derived from Dietary Intervention in RCTs and Their Association with Inflammation
Integrating targeted and untargeted metabolomics into dietary intervention research provides a rigorous framework for identifying molecular mediators underlying the health effects of specific dietary patterns and supplements [79]. Rather than relying solely on clinical endpoints, combining both approaches allows to identify bioactive compounds, host-microbiota co-metabolites, and signaling molecules that reflect real-time biological responses to nutritional exposure [80].
Across the reviewed studies, diverse interventions ranging from whole dietary patterns to targeted supplementation, consistently modulated the levels of various metabolites and showed potential links to inflammatory biomarkers.
4.2. The Impact of a Whole-Food Diet on Metabolite Expression
Dietary patterns centered on whole, minimally processed foods, abundant in plants and healthy fats, sufficient lean and plant-based proteins, and limited alcohol, red meat, refined and ultra-processed items, induced consistent changes in metabolites associated with lipid metabolism [81], immune signaling, and microbial fermentation [55,58,69,73,82]. In Nordic and wholegrain diets, there are increases in trigonelline, pyrocatechol sulfate, and DHPPA-glucuronide, a phenylpropanoid derived from microbial processing of plant polyphenols and fibers. This bacterial activity promotes anti-inflammatory enterocytic communication [83]. Meanwhile, trigonelline, is recognized for its antioxidant and anti-inflammatory properties, as well as its role in lipid metabolism [84]. On the other hand, elevated levels of hippuric acid, vanillic acid 4-o-sulfate, and isobutyryl carnitine in a balanced Korean diet and the 2010 Dietary Guidelines for Americans have shown an increased β-oxidation and benzenoid processing, which may influence immune responses, either by contributing the resolution of inflammation or, conversely, by exacerbating it, depending on the context [85,86,87]. The increase in organoheterocyclic compounds, such as creatinine, associated with the 2010 Dietary Guidelines for Americans and unlike the healthy Korean diet, could be explained by its higher proportion of creatine-rich foods, such as lean meats, fish, and eggs, whose metabolism and cooking favor the generation of these metabolites [88].
Metabolic shifts promoted by healthy dietary types were associated with reductions in inflammatory cytokines (Figure 2), particularly IL-6, IL-1β, MCP-1, and TNFα [55,69,73], highlighting the interaction between microbial/host-derived metabolites and the canonical inflammatory cascade mediated by whole grain-derived metabolites [89]. Interestingly, in healthy individuals following the healthy Korean, 2010 Dietary Guidelines for Americans, and personalized diets designed to improve markers of carbohydrate metabolism and biomarkers of oxidative stress, changes in MCP-1 levels aligned with alterations in lipid-related metabolites, specifically carnitine derivatives, sphingolipids, and sterol lipids such as n,n-dimethyl-safingol and 11β-hydroxyandrosterone-3-glucuronide, respectively. This pattern may imply that lipid-derived signaling molecules or bile-acid pathways may influence chemokine regulation [90], resulting in reduced inflammation under controlled dietary conditions [73].
Similarly, diets enriched in walnuts decreased the levels of oxylipins (e.g., 5,6-DiHETrE and 5-HETE), which are mediators involved in the resolution of inflammation [91], while also reducing pro-oxidative fatty acid derivatives [70]. While other inflammation markers were not directly evaluated, these results could suggest that the beneficial effects of walnuts on vascular and brain health are mediated, at least in part, by dietary modulation of oxylipins and by inhibition of the soluble epoxide hydrolase enzyme [92].
Dietary sulfur-containing compounds are widely distributed and highly enriched in cruciferous vegetables [93]. The consumption of cruciferous vegetables was associated with increased levels of sulphur-containing metabolites (e.g., s-methylcysteine sulfoxide and sulforaphane), which possess anti-inflammatory properties and may contribute to reduced risk of chronic diseases, such as colorectal cancer [94]. Alongside the presence of sulphur-containing metabolites, no association with IL-6 or CRP was observed. The results of this type of intervention emphasize that even including specific food components in the diet may reshape those metabolic pathways relevant to inflammatory processes. In consonance with these results, it has also been observed that plant- and whole foods-based dietary patterns consistently reduce inflammatory biomarkers, such as CRP, IL-6, and TNFα, while simultaneously decreasing oxidative stress biomarkers [95,96], which points to them as valid anti-inflammatory and antioxidant approaches for their use in clinical settings. Accordingly, recent metabolomic studies have identified specific molecules, such as trigonelline, DHPPA-glucuronide family metabolites, acylcarnitines/carnitines, sphingolipids, and steroid glucuronides, as potential mediators of anti-inflammatory effects. These molecules act through β-oxidation, benzenoid metabolism, sphingolipid, and bile acid signaling pathways. For example, trigonelline is inversely associated with dietary inflammatory potential, while sphingomyelins and bile acids are altered in inflammatory diets [97].
Interestingly, a significant reduction in serum carotenoids, such as lutein, lycopene, and α- and β-carotene, was documented when cruciferous vegetables were added to the diet [71], which may reflect differences in antioxidant composition between the interventions rather than a loss of nutritional benefits from sulfur-containing compounds from cruciferous vegetables [98].
4.3. The Impact of Fatty Acids on Metabolite Expression
The second group of food interventions, which focused specifically on the quality and quantity of dietary fats, revealed distinct metabolomic signatures. Specifically, the substitution of SFAs with PUFAs increased metabolites such as acetate, bile acids, citrate, cystathionine, and enzymes like PCSK9 [56], suggesting the activation of cholesterol efflux mechanisms and the LXRα-LDLR axis [99]. Concomitantly, there was a reduction in atherogenic lipoproteins, specifically, very low-density lipoprotein and low-density lipoprotein, cholesterol esters, carnitine derivatives, and lipoproteins, reflecting improvements in lipid metabolism and probably a reduction in lipid-induced inflammation [100]. However, it should be noted that specific inflammation markers were not measured.
Similarly, diets with varying fat content (20–40% of energy) revealed that high-fat diets were associated with lower levels of microbial-derived metabolites, such as butyric and valeric acid [57]. These patterns suggest that both the quality and content of fat can influence host–microbiota interactions and downstream inflammatory signaling by altering lipid, amino acid, and SCFAs metabolism [101,102].
All of these findings are consistent with the previous literature emphasizing the metabolic and inflammatory consequences of modulating dietary fat [103]. For example, recent studies show that diets lower in saturated fat and richer in unsaturated fats are associated with improved lipid profiles and reduced inflammatory biomarkers, such CRP and IL-6, supporting the notion that fat quality plays a key role in metabolic and inflammatory regulation [104,105]. Similarly, it has been shown that replacing SFAs with PUFAs or monounsaturated fatty acids (MUFAs) lowers cardiovascular risk by improving lipid metabolism and reducing inflammation-related gene expression [106]. Low-fat diets also modulate gut microbiota-derived metabolites, such as butyric acid and indole derivatives, suggesting an attenuation of systemic inflammation [21]. The consistency of these findings across various dietary contexts highlights the sensitivity of the metabolome to manipulation of dietary fat consumption and its potential for understanding the effects of dietary interventions on inflammation.
4.4. The Impact of Supplementation on Metabolite Expression
Further nuances were added to the context by supplementation studies. Vitamin D supplementation induces metabolomic alterations that strongly suggest immunoinflammatory modulation. Low-dose vitamin D3 supplementation was found to reduce circulating sphingomyelins and ether-linked phosphatidylcholines/phosphatidylethanolamines, both of which are precursors of ceramides [107], which play a crucial role both in cellular signaling and inflammation [108]. Therefore, the observed increase in sphingomyelin turnover [53], may contribute to reduced postprandial inflammation [109]. In contrast, high-dose supplementation reversed these benefits [53], highlighting the importance of appropriate dosing [110,111]. Moreover, vitamin D3 alone decreased blood glucose and acetate while increasing glutamine and histidine [72], all metabolites known to support immune cell function and inversely correlated with inflammatory biomarkers [112,113]. When combined with essential amino acids and whey protein, this intervention also resulted in increased oleic, palmitic, and stearic acids and glutathione reductase, and decreased omega-6 PUFAs [51]. Altogether, these changes suggest an improved redox balance and reduced lipid-mediated inflammation [114].
While two of the three interventions with vitamin D3 did not directly measure inflammation, the metabolite profiles indicated the presence of compounds with known anti-inflammatory properties, such as glutamine and histidine. Also, evidence of sphingolipid remodeling, shifts in fatty acid composition, and increased antioxidant enzyme activity suggests modulation of eicosanoid-mediated responses [115]. These changes point to enhanced macrophage regulation, reduced endothelial activation, and potential reductions in CRP and TNFα following vitamin D3 supplementation [116].
Several supplementation interventions revealed metabolite profiles indicative of anti-inflammatory or pro-resolution of inflammation activity. Eicosapentaenoic acid supplementation, for example, was associated with increased levels of n-3 PUFAs (20:5, 22:5, and 22:6), as well as decreases in certain n-6 PUFAs. There was also an increase in specialized mediators of inflammation resolution, such as LXB4 [65]. This supports the role of eicosapentaenoic acid in pathways involved in the resolution of inflammation [117]. On the other side, shark liver oil supplementation altered glycerophospholipid subclasses and reduced CRP, suggesting potential benefits through remodeling of the lipidome [22].
Curcumin–phospholipid complex consumption, although not directly assessed for inflammatory markers, reduced levels of metabolites such as indoxyl sulfate and kynurenine, both commonly elevated in inflammatory and microbiota-related metabolic dysregulation [118]. This suggests an indirect modulation of inflammation through the gut-liver axis and mitochondrial metabolism. In contrast, consumption of inulin-propionate ester, compared to inulin or cellulose, elevated circulating propionate and significantly reduced IL-8 levels, aligning with propionate’s known immunoregulatory properties [119]. These findings highlight the value of metabolomics in detecting systemic metabolic shifts that conventional inflammatory markers may overlook.
By contrast, other interventions were associated with either neutral or potentially adverse inflammatory profiles. This was the case of Mohana Choorna consumption, which, despite prior preclinical evidence of metabolic benefits [63], induced the upregulation of numerous inflammation and immune-related genes in human adipose tissue which might point to early disruptions in tissue health and progression towards metabolic dysfunction [36], without improving glucose or lipid metabolism, as previously reported [63]. This highlights the need to validate positive evidence obtained with animal models in properly designed human studies.
Supplementation with nicotinamide riboside reduced plasma acetylcarnitine, but no significant changes were observed across a broad range of inflammatory cytokines [61], suggesting a limited systemic impact on inflammation under the conditions studied [120].
4.5. The Impact of Probiotics Supplementation on Metabolite Expression
The reviewed probiotic interventions reported distinct metabolomic signatures, suggesting diverse potential immunomodulatory roles, through microbiota-derived metabolites with recognized anti-inflammatory properties [66,68]. For example, Bifidobacterium lactis Probio-M8 supplementation increased the levels of metabolites involved in anti-inflammatory pathways and mucosal immune homeostasis, such as sphingomyelin, syringic acid, and tryptophan [66] while decreasing the levels of molecules linked to metabolic stress and inflammation, such as tetracosanoic acid and 3-methylglutarylcarnitine [121].
Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI led to a significant increase in some tryptophan-derived metabolites, such as indole-3-propionic acid, produced by gut bacteria. This metabolite has been shown to reduce IL-1β and TNFα levels [68], and to help maintain the intestinal barrier integrity [122]. Additionally, the observed increase in secondary bile acids may reflect enhanced microbial activity and modulation of the gut-liver axis [123]. In contrast, Lactobacillus casei Shirota did not induce detectable changes in the metabolome, but reduced MCP-1 levels [60], suggesting immune modulation via mechanisms not directly related to metabolome remodeling [109]. These findings underscore how shifts in tryptophan metabolism, indole derivatives, and lipid mediators induced by probiotics may underlie anti-inflammatory effects [124,125].
Taken together, the reviewed evidence suggests that dietary modulation of the gut microbiota may represent a key mechanism underlying metabolomic and inflammatory responses. Diets promoting the growth of Faecalibacterium, Roseburia, and Bifidobacterium, major producers of butyrate and other SCFAs, are typically associated with enhanced intestinal barrier function and reduced expression of pro-inflammatory cytokines [126]. Conversely, the increased abundance of Alistipes or Flavonifractor has been linked to the generation of oxidative metabolites and elevated LPS-mediated signaling, consistent with low-grade inflammation [127].
These interactions may also extend beyond SCFAs, as several trials reported shifts in tryptophan metabolism and sphingolipid pathways that coincide with microbial changes [128]. For instance, Bifidobacterium-based interventions increased circulating indole-3-propionic acid and sphingomyelin, both recognized for their anti-inflammatory and epithelial-protective roles [74,129]. This convergence of microbiota and metabolomic changes supports the concept of an integrated diet–microbiota–metabolite–inflammation axis, though the small number of RCTs combining multi-omic analyses precludes causal inference. Further mechanistic and longitudinal studies are warranted to clarify these relationships.
4.6. Nutrient-Derived Metabolites in the Regulation of Inflammation
When nutrients and food-derived compounds are ingested, they undergo metabolic processing that can lead to the accumulation of metabolites linked with chronic inflammation. Examples include ceramides [130], diacylglycerols (DAGs) [131], and quinone derivatives of polycyclic aromatic hydrocarbons (PAH-quinones) [132,133]. During normal cellular metabolism, dietary biomolecules are directed toward the Krebs cycle, the central hub of energy production. Within this cycle, substrates are broken down to generate electron carriers, NADH and FADH2, which subsequently fuel ATP synthesis through the electron transport chain [134]. In inflammatory contexts, however, diet-derived molecules such as saturated fatty acids disrupt these processes. For instance, saturated fats can be metabolized in macrophages into ceramides and palmitate, which activate immune receptors, including Toll-like receptors (TLR2/4) and NLRP3/6 inflammasomes. This promotes polarization toward pro-inflammatory M1 macrophages. Under these conditions, the Krebs cycle becomes disrupted at key nodes, leading to the accumulation of intermediates such as citrate, succinate, and itaconate [135]. These shifts drive metabolic reprogramming toward aerobic glycolysis and enhance the production of reactive oxygen species (ROS) [136], further triggering pro-inflammatory pathways that contribute to chronic disease [137].
The resulting inflammation impacts multiple organ systems, including the liver [138], muscle [139], adipose tissue [140], pancreas [141], heart [142], and the nervous system [39], thereby contributing to disease pathogenesis. By contrast, consumption of diets rich in fiber and phytochemicals (e.g., vitamins and flavonoids) supports polarization toward an M2 macrophage phenotype, associated with immune resolution and tissue repair [143]. For example, vitamin C, metabolized to ascorbate, can promote M2-like macrophage differentiation at appropriate levels, while catechins exert similar effects. Additionally, fermentation of dietary fiber by the gut microbiota yields short-chain fatty acids (SCFAs), which further favor M2 macrophage polarization [144] (Figure 3).
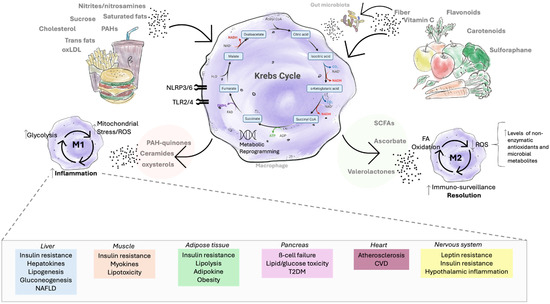
Figure 3.
The effect of food metabolites on inflammation. Unhealthy lifestyle exposures such as high-palatable foods rich in trans fats, cholesterol, oxidized LDL (low-density lipoprotein), nitrites, nitrosamines, and polycyclic aromatic hydrocarbons (PAHs) activate innate immune receptors (e.g., TLR2/4, NLRP3/6) and drive metabolic reprogramming toward aerobic glycolysis, accumulation of Krebs cycle intermediates (e.g., citrate, succinate), mitochondrial stress, and augmented reactive oxygen species (ROS) production. For instance, metabolites derived from saturated fats (e.g., palmitic acid → ceramides) promote M1 macrophage polarization and chronic inflammation. This, in turn, contributes to systemic complications such as insulin resistance, steatosis, and non-alcoholic fatty liver disease (NAFLD) in the liver; myokine alterations and lipotoxicity in muscle; adipokine imbalance, obesity, and excessive lipolysis in adipose tissue; β-cell failure and type 2 diabetes in the pancreas; atherosclerosis and cardiovascular disease; and leptin resistance, insulin resistance, and hypothalamic inflammation in the nervous system. By contrast, healthy dietary compounds such as vitamin C and flavonoids promote fatty acid oxidation and enhanced antioxidant capacity (via non-enzymatic antioxidants: vitamin C → ascorbate; or via antioxidant microbial metabolites: catechins → valerolactones), thereby favoring M2 macrophage polarization, which favors immune resolution and tissue repair. Furthermore, fermentation of dietary fiber by gut microbiota releases short-chain fatty acids (SCFAs) (e.g., fiber → butyrate), while microbial catabolism of polyphenols produces phenolic metabolites (e.g., bound polyphenols → ferulic acid). Both classes of compounds reinforce M2 macrophage polarization and anti-inflammatory pathways.
4.7. The Effects of Analytical and Biological Heterogeneity on the Comparability of Metabolomic Studies
The reviewed interventions showed important analytical variability, making comparability across studies more difficult. However, it is possible to observe a clear preference to use LC–MS-based approaches in the reviewed RCTs. This is in line with their higher sensitivity and versatility, as it can be used for both polar and non-polar compounds of a wide molecular mass range, making them highly popular in general for metabolomic studies [19]. Even though most interventions converged towards the use of LC–MS as their analytical platform, there was important heterogeneity regarding calibration practices, use of QC procedures, and reporting practices. This calls for a standardized checklist with minimum reporting requirements to be included in RCTs such as sample preparation details, full instrument details, acquisition parameters, calibration practices, and QC strategies [4,145]. This is crucial not only to improve reproducibility but to allow cross-study comparability [146]. Also, the limit of detection should be included, as the lack of this hinders the ability to determine whether a metabolite is absent or below the limit of detection, which makes it very difficult to assess the reliability of the reported findings [145]. Although reporting critical instrument parameters was slightly better than calibration practices, QC strategies, and detection limits in the reviewed studies, it was still inconsistent among them. Moreover, all studies using MS, even those using high-resolution methods, failed to transparently report practices on internal mass calibration, lock-mass correction, or mass accuracy performance. These are essential to demonstrate instrument stability and reliable mass assignments used for metabolite identification [20]. Researchers should also be encouraged to always share calibration curves and raw/processed data to improve transparency, reproducibility, and facilitate comparability of the results [147].
Metabolomic signatures are strongly matrix-dependent, which can limit the reliability, accuracy, and interpretation of results [148,149]. In the reviewed articles, plasma and serum samples were commonly used, as compared to urine, where high concentrations of salts, such as sodium chloride, and other compounds make metabolite detection more challenging. Urine’s matrix effect is problematic, often time-consuming for equipment cleaning, and prone to adduct formation [150]. Plasma and serum primarily reflect systemic lipid and amino acid metabolism [151,152,153], whereas urine captures short-term fluctuations in microbial- and diet-derived metabolites, particularly phenolic derivatives [154,155]. Fecal metabolomics, although less frequently applied, provides complementary insights into gut-derived metabolites that influence, for example, host inflammatory responses [156,157,158,159].
Importantly, researchers should consider not only matrix effects but also the biological distribution of the metabolite of interest, as different compounds are more likely to be detected in specific matrices. The choice of biological matrix plays a critical role in shaping metabolite–inflammation associations, as plasma, urine, and feces capture distinct metabolic processes and time scales of exposure [160,161,162]. Plasma often reflects short-term metabolic responses and circulating bioactive compounds [163,164], while urine primarily captures excreted metabolites and may provide a cumulative measure of dietary intake or microbial catabolism [165,166,167]. In contrast, fecal samples better represent gut microbial activity and local metabolite production, although their relationship to systemic inflammation is less direct [168,169]. This heterogeneity complicates comparisons across studies, as the same metabolite may show different associations depending on the matrix analyzed. Moreover, the interpretation of statistical versus biological significance remains an important challenge. While some studies report significant associations at the nanomolar level, such changes may not always translate into physiologically relevant anti-inflammatory effects [170,171]. Distinguishing meaningful biological signals from minor fluctuations is therefore essential to advance metabolomics toward clinical and translational applications.
These matrix-specific differences underscore the importance of standardized biospecimen selection and multi-matrix experimental designs to capture comprehensive metabolic pathway interactions. Consequently, biomarker validation in nutritional metabolomics requires harmonization across matrices to enhance comparability and reproducibility of findings.
4.8. Conflicting Findings and Possible Moderators
Although many interventions suggest anti-inflammatory metabolic effects, results across RCTs remain inconsistent. Some studies reported increases in pro-inflammatory or neutral metabolites (e.g., ox-LDL, specific fatty acids, kinases) without corresponding changes in CRP or IL-6 [52,69]. Such discrepancies may reflect differences in nutrient dose [172,173], intervention duration [174,175], baseline metabolic or microbiota profiles, and the biological matrix analyzed (e.g., plasma vs. feces) [176]. Moreover, individual variability in absorption and metabolism may also contribute [177,178].
Recent dose–response metabolomics studies show that metabolic effects generate a stagnation beyond certain points, suggesting nonlinear relationships between intake and biochemical response [164]. However, few RCTs explore this systematically, and some lack statistical power to detect changes in inflammatory biomarkers. Additionally, metabolomic shifts do not always translate into corresponding cytokine responses, suggesting, in some contexts, a potential uncoupling between metabolic and inflammatory outcomes [107].
Vitamin D-related interventions illustrate this heterogeneity. In one trial differing only by dose, phosphatidylcholines and sphingomyelins changed in opposite directions [53]; others showed increases in glucose, creatinine, and fatty acyls [72], or positive correlations between palmitic/stearic acids and CRP [51]. These divergent responses probably are the reflection of differences in formulation, dose, duration, or co-interventions [179].
Only three of the included trials explicitly tested different doses or dietary levels of the same intervention: (i) fat-controlled diets with varying proportions of energy from fat (20%, 30%, 40%) [57], (ii) vitamin D3 supplementation at low vs. high doses [53], and (iii) EPA supplementation at 1 g/day, 2 g/day, and 4 g/day [65]. These studies provide valuable insights but also underscore the complexity of dose–response relationships in metabolomics. For instance, while the low-fat diet (20% energy) increased protective metabolites such as butyric acid, the high-fat diet (40% energy) led to increases in arachidonic acid and indole derivatives [57], which are more closely linked to pro-inflammatory pathways [115].
The duration of dietary interventions is another crucial factor shaping metabolomic and inflammatory outcomes; in the analyzed clinical trials, intervention durations ranged from seven days to 24 months. Short-term RCTs (e.g., ≤4 weeks) may capture acute metabolic responses or transient shifts in intermediary metabolites [180], whereas long-term interventions (≥6 months) are more likely to reflect adaptive remodeling of lipid and amino acid metabolism, as well as stable anti-inflammatory effects [181]. This temporal variability can partly explain why some trials reported early metabolite changes without concurrent alterations in inflammatory biomarkers, while others observed delayed but sustained improvements over time [182]. Such heterogeneity, further influenced by participants’ baseline metabolic status [183], methodological variability in metabolomic platforms [184], and differences in the metabolites measured through targeted techniques, highlights the complexity of diet–metabolite–inflammation interactions and underscores the need for harmonized study designs and standardized metabolomic approaches to allow more reliable cross-trial comparisons.
Overall, these findings indicate that heterogeneity stems from a combination of dose, duration, baseline metabolic status, and methodological variability in metabolomic profiling. Future RCTs should incorporate multiple dosage arms, standardized metabolite quantification, and longer follow-up to clarify whether responses scale with dose or plateau beyond a threshold. Meta-regression of dose, duration, and participant characteristics could further define functional metabolic thresholds linked to clinically relevant anti-inflammatory effects.
4.9. Clinical Implications
The findings of this review highlight the clinical potential of integrating metabolomic profiling into dietary interventions aimed at modulating inflammation. The consistent identification of metabolite patterns associated with anti-inflammatory effects, including trigonelline, carnitines, sphingolipids, and microbial-derived compounds, suggests that specific dietary strategies can induce measurable biological changes well before conventional clinical markers would detect them. This positions metabolomics as a valuable early detection tool for evaluating dietary efficacy and tailoring personalized nutrition approaches in clinical practice.
While metabolomics offers considerable promise for early disease detection and personalized nutrition, its implementation in clinical practice remains challenging. High analytical costs, limited accessibility of specialized instrumentation, and the absence of standardized protocols currently restrict its routine clinical use [185,186]. Moreover, variability in sample processing, data normalization, and metabolite annotation complicates cross-laboratory comparability and clinical validation [187,188]. To move toward clinical translation, efforts should focus on cost reduction, the development of standardized workflows, and integration with existing clinical platforms [83,189]. Establishing shared databases and reference ranges for key metabolites will also be essential to enable consistent interpretation and facilitate the incorporation of metabolomic profiling into personalized dietary counseling and preventive medicine.
4.10. Strengths and Limitations
The clinical RCTs included in this systematic review exhibited several methodological strengths, for instance: (a) some studies had relatively large participant numbers [56,57,70], (b) implementation of robust intervention designs [56,57,70,71], including double-blind protocols, which improved internal validity and reduced bias, (c) long intervention periods [57], (d) rigorous adherence monitoring that enabled the detection of long-term metabolic adaptations [70,71], and (e) confirmatory analyses to support metabolomic findings, contributing to the reliability of the reported results [54,64]. Ultimately, the novelty of their objectives and the originality of the metabolic signatures reported added significant value to the field [59,63,68,71,73].
Several methodological limitations were consistently observed across the included studies, which may affect the strength of the evidence synthesized in this review: (a) several trials were affected by pandemic-related constraints, such as limited in-person contact and reliance on electronic participation, which might have impacted adherence and reporting [69,71]; (b) small sample sizes, limited ethnic representation, wide age ranges, and lack of personalized or representative diets reduced generalizability [50,54,60,62,63,65,72,73]; (c) incomplete dietary controls posed risks of unreported food intake or medication use, introducing confounding factors [56,59,65,72]; (d) short intervention durations, absence of follow-up, single-blind designs, and inconsistent handling of biological samples, all potentially affecting data quality [50,59,61,63,69,70,73]; and (e) the absence of complementary analyses like biopsies or a comprehensive lipidome profiling, use of diverse metabolomic techniques, limits the understanding of the underlying mechanisms associated with the metabolomic observed metabolomics changes.
Building on the factors discussed in Section 4.6, several methodological aspects further constrain the interpretation and comparability of results across RCTs. This systematic review explored a broad range of dietary exposures and metabolites measured by several techniques, and while the heterogeneity in terms of study population and endpoints of each RCT makes it difficult to generalize findings between studies, some consistent patterns can still be identified. Another important limitation relates to the lack of standardization in metabolite identification and nomenclature across RCTs. In our synthesis, we observed that individual studies often relied on different reference databases, and in many cases, the database used was not reported at all. This inconsistency complicates efforts to unify metabolite names and categories, particularly in untargeted metabolomics, where metabolite annotation is inherently challenging. Although we used RefMet (a reference nomenclature for metabolomics) [75] as the primary framework for classification, not all metabolites could be retrieved from this source, and we therefore complemented our classification using HMDB [76]. The absence of a single comprehensive and universally adopted resource makes it difficult to achieve full consistency when comparing across multiple trials. Nevertheless, we recommend that future studies systematically adopt reliable and standardized resources, such as RefMet, HMDB, or PubChem [180], when reporting metabolite identities, as this would substantially improve clarity, reproducibility, and comparability between clinical studies.
5. Conclusions
The reviewed evidence supports dietary recommendations centered on whole-food-based patterns and improved fat quality, such as Mediterranean, Nordic, and low saturated fat diets, for their capacity to favorably modulate metabolic and inflammatory pathways. Metabolomic findings strengthen the evidence linking these dietary approaches with whole-food-based and improved-fat-quality in shaping metabolites-health associations, even though not each intervention showed a consistent reduction in chronic inflammation. Importantly, the results highlight the value of incorporating multiomic analyses to unravel the complex interactions between diet, gut microbiota, metabolism, and immune responses.
For a clinical standpoint, the findings reinforce the potential of incorporating personalized dietary advice to promote immune balance trough nutrient-microbiota-host interactions. Presenting such connections, in clinically relevant terms, helps bridge the gap between molecular evidence and nutritional guidance, facilitating their interpretation and application in clinical practice.
Future research should prioritize integrative designs that combine metabolomics with conventional clinical endpoints, such as inflammatory and metabolic markers, in long-term randomized trials. Observational studies are also encouraged to include metabolomic assessments to capture real-world dietary exposures and interindividual variability to complement experimental evidence. In parallel, the adoption of standardized protocols, shared data repositories, and harmonized analytic pipelines will be essential to ensure reproducibility and comparability across studies, thereby advancing precision nutrition strategies tailored to individual metabolic profiles.
Together, these approaches will help translate metabolomic insights into effective, personalized dietary strategies for the prevention of chronic inflammation and related diseases.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/metabo15110705/s1, Table S1: PRISMA 2020 Checklist; Table S2: Main characteristics of the included studies—Complete table; Table S3: Analytical approaches of the included studies.
Author Contributions
Conceptualization, M.L.M., C.N., G.N.G.-F., and B.C.; methodology, M.L.M., C.N., G.N.G.-F. and B.C.; literature search and study selection, M.L.M., C.N., G.N.G.-F. and B.C.; data curation, G.N.G.-F. and B.C.; original draft preparation, G.N.G.-F. and B.C.; writing, review, and editing, M.L.M., C.N., G.N.G.-F., B.C. and G.S.-S.; visualization, G.N.G.-F. and B.C.; supervision, M.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a Doctoral scholarship awarded to B.C. by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Ministry of Science and Technology, Argentina (RESOL-2021-154-APN-DIR#CONICET) This work was also supported by intramural funding from Universidad Adventista del Plata.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CRP | C-reactive protein |
| GC | Gas chromatography |
| IL | Interleukin |
| LC | Liquid chromatography |
| MS | Mass spectrometry |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MUFAs | Monounsaturated fatty acids |
| NAFLD | Non-alcoholic fatty liver disease |
| NMS | Nuclear magnetic resonance |
| ox-LDL | Oxidized low-density lipoprotein |
| PUFAs | Polyunsaturated fatty acids |
| RCTs | Randomized controlled trials |
| SCFAs | Short-chain fatty acids |
| SFAs | Saturated fatty acids |
| TNFα | Tumor necrosis factor-alpha |
References
- Ebrahim, S. Metabolomics, Nutrition and Why Epidemiology Matters. Int. J. Epidemiol. 2016, 45, 1307–1310. [Google Scholar] [CrossRef]
- Lee, J.; Banerjee, D. Metabolomics and the Microbiome as Biomarkers in Sepsis. Crit. Care Clin. 2020, 36, 105–113. [Google Scholar] [CrossRef]
- Kortesniemi, M.; Noerman, S.; Kårlund, A.; Raita, J.; Meuronen, T.; Koistinen, V.; Landberg, R.; Hanhineva, K. Nutritional Metabolomics: Recent Developments and Future Needs. Curr. Opin. Chem. Biol. 2023, 77, 102400. [Google Scholar] [CrossRef]
- González-Domínguez, Á.; Domínguez-Riscart, J.; Savolainen, O.; Lechuga-Sancho, A.; Landberg, R.; González-Domínguez, R. Identifying Metabotypes of Insulin Resistance Severity in Children with Metabolic Syndrome. Cardiovasc. Diabetol 2024, 23, 315. [Google Scholar] [CrossRef]
- Troisi, J.; Cavallo, P.; Colucci, A.; Pierri, L.; Scala, G.; Symes, S.; Jones, C.; Richards, S. Metabolomics in Genetic Testing. Adv. Clin. Chem. 2020, 94, 85–153. [Google Scholar] [CrossRef]
- Deng, P.; Li, X.; Petriello, M.C.; Wang, C.; Morris, A.J.; Hennig, B. Application of Metabolomics to Characterize Environmental Pollutant Toxicity and Disease Risks. Rev. Environ. Health 2019, 34, 251. [Google Scholar] [CrossRef]
- Fernandes Silva, L.; Vangipurapu, J.; Oravilahti, A.; Lusis, A.J.; Laakso, M. Metabolomics, Genetics, and Environmental Factors: Intersecting Paths in Abdominal Aortic Aneurysm. Int. J. Mol. Sci. 2025, 26, 1498. [Google Scholar] [CrossRef] [PubMed]
- Suárez, M.; Caimari, A.; del Bas, J.M.; Arola, L. Metabolomics: An Emerging Tool to Evaluate the Impact of Nutritional and Physiological Challenges. TrAC Trends Anal. Chem. 2017, 96, 79–88. [Google Scholar] [CrossRef]
- Richards, J.L.; Yap, Y.A.; McLeod, K.H.; MacKay, C.R.; Marinõ, E. Dietary Metabolites and the Gut Microbiota: An Alternative Approach to Control Inflammatory and Autoimmune Diseases. Clin. Transl. Immunol. 2016, 5, e82. [Google Scholar] [CrossRef] [PubMed]
- Abdul Rahim, M.B.H.; Chilloux, J.; Martinez-Gili, L.; Neves, A.L.; Myridakis, A.; Gooderham, N.; Dumas, M.-E. Diet-Induced Metabolic Changes of the Human Gut Microbiome: Importance of Short-Chain Fatty Acids, Methylamines and Indoles. Acta Diabetol. 2019, 56, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Cortés-Martín, A.; Colmenarejo, G.; Selma, M.V.; Espín, J.C. Genetic Polymorphisms, Mediterranean Diet and Microbiota-Associated Urolithin Metabotypes Can Predict Obesity in Childhood-Adolescence. Sci. Rep. 2020, 10, 7850. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small Molecule Metabolites: Discovery of Biomarkers and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef]
- Tandon, P.; Abrams, N.D.; Carrick, D.M.; Chander, P.; Dwyer, J.; Fuldner, R.; Gannot, G.; Laughlin, M.; McKie, G.; PrabhuDas, M.; et al. Metabolic Regulation of Inflammation and Its Resolution: Current Status, Clinical Needs, Challenges, and Opportunities. J. Immunol. 2021, 207, 2625. [Google Scholar] [CrossRef]
- Nagana Gowda, G.A.; Zhu, W.; Raftery, D. NMR-Based Metabolomics: Where Are We Now and Where Are We Going? Prog. Nucl. Magn. Reson. Spectrosc. 2025, 150–151, 101564. [Google Scholar] [CrossRef]
- Nagana Gowda, G.A.; Raftery, D. NMR Based Metabolomics. In Cancer Metabolomics. Advances in Experimental Medicine and Biology; Hu, S., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Volume 150–151, p. 19. ISBN 978-3-030-51651-2. [Google Scholar]
- Zeki, Ö.C.; Eylem, C.C.; Reçber, T.; Kır, S.; Nemutlu, E. Integration of GC–MS and LC–MS for Untargeted Metabolomics Profiling. J. Pharm. Biomed. Anal. 2020, 190, 113509. [Google Scholar] [CrossRef]
- Wang, X.; Ouyang, Y.; Liu, J.; Zhu, M.; Zhao, G.; Bao, W.; Hu, F.B. Fruit and Vegetable Consumption and Mortality from All Causes, Cardiovascular Disease, and Cancer: Systematic Review and Dose-Response Meta-Analysis of Prospective Cohort Studies. BMJ 2014, 349, g4490. [Google Scholar] [CrossRef] [PubMed]
- Ulaszewska, M.M.; Weinert, C.H.; Trimigno, A.; Portmann, R.; Andres Lacueva, C.; Badertscher, R.; Brennan, L.; Brunius, C.; Bub, A.; Capozzi, F.; et al. Nutrimetabolomics: An Integrative Action for Metabolomic Analyses in Human Nutritional Studies. Mol. Nutr. Food Res. 2019, 63, e1800384. [Google Scholar] [CrossRef]
- Ding, J.; Feng, Y.-Q. Mass Spectrometry-Based Metabolomics for Clinical Study: Recent Progresses and Applications. TrAC Trends Anal. Chem. 2023, 158, 116896. [Google Scholar] [CrossRef]
- Alseekh, S.; Aharoni, A.; Brotman, Y.; Contrepois, K.; D’Auria, J.; Ewald, J.; Ewald, J.C.; Fraser, P.D.; Giavalisco, P.; Hall, R.D.; et al. Mass Spectrometry-Based Metabolomics: A Guide for Annotation, Quantification and Best Reporting Practices. Nat. Methods 2021, 18, 747–756. [Google Scholar] [CrossRef]
- Emwas, A.-H.M. The Strengths and Weaknesses of NMR Spectroscopy and Mass Spectrometry with Particular Focus on Metabolomics Research. In Metabonomics: Methods and protocols; Springer: New York, NY, USA, 2015; pp. 161–193. [Google Scholar]
- Parri, A.; Fitó, M.; Torres, C.F.; Muñoz-Aguayo, D.; Schröder, H.; Cano, J.F.; Vázquez, L.; Reglero, G.; Covas, M.-I. Alkylglycerols Reduce Serum Complement and Plasma Vascular Endothelial Growth Factor in Obese Individuals. Inflammopharmacology 2016, 24, 127–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Markley, J.L.; Brüschweiler, R.; Edison, A.S.; Eghbalnia, H.R.; Powers, R.; Raftery, D.; Wishart, D.S. The Future of NMR-Based Metabolomics. Curr. Opin. Biotechnol. 2017, 43, 34–40. [Google Scholar] [CrossRef]
- Guggisberg, D.; Joosten, H.; Fuchsmann, P.; Vergères, G. Chapter 16. Nutrimetabolomics: Concepts and Applications. In Foodomics: Omic Strategies and Applications in Food Science; The Royal Society of Chemistry: London, UK, 2021; pp. 374–415. [Google Scholar]
- Ivey, K.L.; Rimm, E.B.; Kraft, P.; Clish, C.B.; Cassidy, A.; Hodgson, J.; Croft, K.; Wolpin, B.; Liang, L. Identifying the Metabolomic Fingerprint of High and Low Flavonoid Consumers. J. Nutr. Sci. 2017, 6, e34. [Google Scholar] [CrossRef]
- Sindelar, M.; Patti, G.J. Chemical Discovery in the Era of Metabolomics. J. Am. Chem. Soc. 2020, 142, 9097–9105. [Google Scholar] [CrossRef]
- Giera, M.; Aisporna, A.; Uritboonthai, W.; Siuzdak, G. The Hidden Impact of In-Source Fragmentation in Metabolic and Chemical Mass Spectrometry Data Interpretation. Nat. Metab. 2024, 6, 1647–1648. [Google Scholar] [CrossRef] [PubMed]
- González-Peña, D.; Brennan, L. Recent Advances in the Application of Metabolomics for Nutrition and Health. Annu. Rev. Food Sci. Technol. 2019, 10, 479–519. [Google Scholar] [CrossRef] [PubMed]
- Cajka, T.; Fiehn, O. Toward Merging Untargeted and Targeted Methods in Mass Spectrometry-Based Metabolomics and Lipidomics. Anal. Chem. 2016, 88, 524–545. [Google Scholar] [CrossRef]
- Pathmasiri, W.; Rushing, B.R.; McRitchie, S.; Choudhari, M.; Du, X.; Smirnov, A.; Pelleigrini, M.; Thompson, M.J.; Sakaguchi, C.A.; Nieman, D.C.; et al. Untargeted Metabolomics Reveal Signatures of a Healthy Lifestyle. Sci. Rep. 2024, 14, 13630. [Google Scholar] [CrossRef]
- Chen, J.; Yi, Q.; Wang, Y.; Wang, J.; Yu, H.; Zhang, J.; Hu, M.; Xu, J.; Wu, Z.; Hou, L.; et al. Long-Term Glycemic Variability and Risk of Adverse Health Outcomes in Patients with Diabetes: A Systematic Review and Meta-Analysis of Cohort Studies. Diabetes Res. Clin. Pract. 2022, 192, 110085. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Noncommunicable Diseases. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 6 July 2025).
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef] [PubMed]
- Herrero-Cervera, A.; Soehnlein, O.; Kenne, E. Neutrophils in Chronic Inflammatory Diseases. Cell. Mol. Immunol. 2022, 19, 177–191. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L.; Chen, L.; et al. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef] [PubMed]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The Interplay between Cytokines, Inflammation, and Antioxidants: Mechanistic Insights and Therapeutic Potentials of Various Antioxidants and Anti-Cytokine Compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef]
- Us-Medina, U.; Millán Linares, M.d.C.; Arana Argáez, V.; Segura-Campos, M.R. In Vitro Antioxidant and Anti-Inflammatory Activity of Chaya Extracts (Cnidoscolus Aconitifolius (Mill.) I.M. Johnst). Nutr. Hosp. 2019, 37, 46–55. [Google Scholar] [CrossRef]
- Kurowska, A.; Ziemichód, W.; Herbet, M.; Piątkowska-Chmiel, I. The Role of Diet as a Modulator of the Inflammatory Process in the Neurological Diseases. Nutrients 2023, 15, 1436. [Google Scholar] [CrossRef]
- Ohue-Kitano, R.; Banno, Y.; Masujima, Y.; Kimura, I. Gut Microbial Metabolites Reveal Diet-Dependent Metabolic Changes Induced by Nicotine Administration. Sci. Rep. 2024, 14, 1056. [Google Scholar] [CrossRef]
- Hamamah, S.; Iatcu, O.C.; Covasa, M. Dietary Influences on Gut Microbiota and Their Role in Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). Nutrients 2024, 17, 143. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Flores, G.N.; Carlino, B.; Gili, R.V.; Leeson, S.; Mayta, M. Phytochemicals for Preventing and Treating Chronic Diseases. In Medicinal Applications of Phytopharmaceuticals; Khoobchandani, M., Ghosh, S., Eds.; Springer Nature: Cham, Switzerland, 2024. [Google Scholar]
- Siddiqui, M.T.; Cresci, G.A.M. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 14, 6025. [Google Scholar] [CrossRef]
- Wastyk, H.C.; Fragiadakis, G.K.; Perelman, D.; Dahan, D.; Merrill, B.D.; Yu, F.B.; Topf, M.; Gonzalez, C.G.; Van Treuren, W.; Han, S.; et al. Gut Microbiota-Targeted Diets Modulate Human Immune Status. Cell 2021, 184, 4137. [Google Scholar] [CrossRef]
- Nogal, A.; Valdes, A.M.; Menni, C. The Role of Short-Chain Fatty Acids in the Interplay between Gut Microbiota and Diet in Cardio-Metabolic Health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Tsirogianni, A.M.; Bouzalmate-Hajjaj, A.; Van ’T Hooft, J.; Saeed Khan, K.; Bueno-Cavanillas, A.; Cano-Ibáñez, N. The Usefulness of Randomized Trials of Lifestyle Interventions for Overweight, Obesity, or Metabolic Syndrome: A Systematic Review. Clin. Nutr. ESPEN 2024, 63, 936–943. [Google Scholar] [CrossRef]
- Lu, S.; Sun, X.; Zhang, W.; Li, X.; Zhou, Z.; Xiao, R.; Lv, Q.; Tang, H.; Wang, B.; Qu, J.; et al. Effects of the Mediterranean Diet on Metabolic Indices and Quality of Life in Cancer Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Funct. Foods 2024, 114, 106074. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; the PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264–269. [Google Scholar] [CrossRef]
- Chambers, E.S.; Byrne, C.S.; Morrison, D.J.; Murphy, K.G.; Preston, T.; Tedford, C.; Garcia-Perez, I.; Fountana, S.; Serrano-Contreras, J.I.; Holmes, E.; et al. Dietary Supplementation with Inulin-Propionate Ester or Inulin Improves Insulin Sensitivity in Adults with Overweight and Obesity with Distinct Effects on the Gut Microbiota, Plasma Metabolome and Systemic Inflammatory Responses: A Randomised Cross-over Trial. Gut 2019, 68, 1430–1438. [Google Scholar] [CrossRef]
- Chashmniam, S.; Mirhafez, S.R.; Dehabeh, M.; Hariri, M.; Azimi Nezhad, M.; Nobakht, M.; Gh, B.F. A Pilot Study of the Effect of Phospholipid Curcumin on Serum Metabolomic Profile in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Trial. Eur. J. Clin. Nutr. 2019, 73, 1224–1235. [Google Scholar] [CrossRef]
- Corsetto, P.A.; Montorfano, G.; Klersy, C.; Massimino, L.; Infantino, V.; Iannello, G.; Anna Faliva, M.; Lukaski, H.; Perna, S.; Alalwan, T.A.; et al. Fatty Acid Profile and Antioxidant Status Fingerprint in Sarcopenic Elderly Patients: Role of Diet and Exercise. Nutrients 2019, 11, 2569. [Google Scholar] [CrossRef]
- Estévez-Santiago, R.; Silván, J.M.; Can-Cauich, C.A.; Veses, A.M.; Alvarez-Acero, I.; Martinez-Bartolome, M.A.; San-Román, R.; Cámara, M.; Olmedilla-Alonso, B.; de Pascual-Teresa, S. Lack of a Synergistic Effect on Cardiometabolic and Redox Markers in a Dietary Supplementation with Anthocyanins and Xanthophylls in Postmenopausal Women. Nutrients 2019, 11, 1533. [Google Scholar] [CrossRef]
- Fernández-Arroyo, S.; Hernández-Aguilera, A.; de Vries, M.A.; Burggraaf, B.; van der Zwan, E.; Pouw, N.; Joven, J.; Castro Cabezas, M. Effect of Vitamin D3 on the Postprandial Lipid Profile in Obese Patients: A Non-Targeted Lipidomics Study. Nutrients 2019, 11, 1194. [Google Scholar] [CrossRef]
- Lindqvist, H.M.; Gjertsson, I.; Andersson, S.; Calder, P.C.; Bärebring, L. Influence of Blue Mussel (Mytilus Edulis) Intake on Fatty Acid Composition in Erythrocytes and Plasma Phospholipids and Serum Metabolites in Women with Rheumatoid Arthritis. Prostaglandins Leukot. Essent. Fat. Acids 2019, 150, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Tuomainen, M.; Kärkkäinen, O.; Leppänen, J.; Auriola, S.; Lehtonen, M.; Savolainen, M.J.; Hermansen, K.; Risérus, U.; Åkesson, B.; Thorsdottir, I.; et al. Quantitative Assessment of Betainized Compounds and Associations with Dietary and Metabolic Biomarkers in the Randomized Study of the Healthy Nordic Diet (SYSDIET). Am. J. Clin. Nutr. 2019, 110, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Ulven, S.M.; Christensen, J.J.; Nygård, O.; Svardal, A.; Leder, L.; Ottestad, I.; Lysne, V.; Laupsa-Borge, J.; Ueland, P.M.; Midttun, Ø.; et al. Using Metabolic Profiling and Gene Expression Analyses to Explore Molecular Effects of Replacing Saturated Fat with Polyunsaturated Fat—A Randomized Controlled Dietary Intervention Study. Am. J. Clin. Nutr. 2019, 109, 1239–1250. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, F.; Yuan, J.; Li, J.; Jiang, D.; Zhang, J.; Li, H.; Wang, R.; Tang, J.; Huang, T.; et al. Effects of Dietary Fat on Gut Microbiota and Faecal Metabolites, and Their Relationship with Cardiometabolic Risk Factors: A 6-Month Randomised Controlled-Feeding Trial. Gut 2019, 68, 1417–1429. [Google Scholar] [CrossRef] [PubMed]
- Roager, H.M.; Vogt, J.K.; Kristensen, M.; Hansen, L.B.S.; Ibrügger, S.; Mærkedahl, R.B.; Bahl, M.I.; Lind, M.V.; Nielsen, R.L.; Frøkiær, H.; et al. Whole Grain-Rich Diet Reduces Body Weight and Systemic Low-Grade Inflammation without Inducing Major Changes of the Gut Microbiome: A Randomised Cross-over Trial. Gut 2019, 68, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.-J.; Jang, S.-N.; Liu, K.-H.; Jung, D.-H. Effect of Korean Red Ginseng on Cholesterol Metabolites in Postmenopausal Women with Hypercholesterolemia: A Pilot Randomized Controlled Trial. Nutrients 2020, 12, 3423. [Google Scholar] [CrossRef]
- Macnaughtan, J.; Figorilli, F.; García-López, E.; Lu, H.; Jones, H.; Sawhney, R.; Suzuki, K.; Fairclough, S.; Marsden, J.; Moratalla, A.; et al. A Double-Blind, Randomized Placebo-Controlled Trial of Probiotic Lactobacillus Casei Shirota in Stable Cirrhotic Patients. Nutrients 2020, 12, 1651. [Google Scholar] [CrossRef]
- Remie, C.M.; Roumans, K.H.; Moonen, M.P.; Connell, N.J.; Havekes, B.; Mevenkamp, J.; Lindeboom, L.; de Wit, V.H.; van de Weijer, T.; Aarts, S.A.; et al. Nicotinamide Riboside Supplementation Alters Body Composition and Skeletal Muscle Acetylcarnitine Concentrations in Healthy Obese Humans. Am. J. Clin. Nutr. 2020, 112, 413–426. [Google Scholar] [CrossRef]
- Amerikanou, C.; Kanoni, S.; Kaliora, A.C.; Barone, A.; Bjelan, M.; D’Auria, G.; Gioxari, A.; Gosalbes, M.J.; Mouchti, S.; Stathopoulou, M.G.; et al. Effect of Mastiha Supplementation on NAFLD: The MAST4HEALTH Randomised, Controlled Trial. Mol. Nutr. Food Res. 2021, 65, 2001178. [Google Scholar] [CrossRef]
- Esser, D.; Matualatupauw, J.; de Vos, R.C.H.; Wehrens, R.; van der Stappen, J.; van der Meer, I.; Afman, L.A. Ayurvedic Herbal Preparation Supplementation Does Not Improve Metabolic Health in Impaired Glucose Tolerance Subjects; Observations from a Randomised Placebo Controlled Trial. Nutrients 2021, 13, 260. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiao, D.; Zhang, X.; Sandhu, A.K.; Chandra, P.; Kay, C.; Edirisinghe, I.; Burton-Freeman, B. Strawberry Consumption, Cardiometabolic Risk Factors, and Vascular Function: A Randomized Controlled Trial in Adults with Moderate Hypercholesterolemia. J. Nutr. 2021, 151, 1517–1526. [Google Scholar] [CrossRef]
- Lamon-Fava, S.; So, J.; Mischoulon, D.; Ziegler, T.R.; Dunlop, B.W.; Kinkead, B.; Schettler, P.J.; Nierenberg, A.A.; Felger, J.C.; Maddipati, K.R.; et al. Dose- and Time-Dependent Increase in Circulating Anti-Inflammatory and pro-Resolving Lipid Mediators Following Eicosapentaenoic Acid Supplementation in Patients with Major Depressive Disorder and Chronic Inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2021, 266, 102219. [Google Scholar] [CrossRef]
- Liu, A.; Ma, T.; Xu, N.; Jin, H.; Zhao, F.; Kwok, L.-Y.; Zhang, H.; Zhang, S.; Sun, Z. Adjunctive Probiotics Alleviates Asthmatic Symptoms via Modulating the Gut Microbiome and Serum Metabolome. Microbiol. Spectr. 2021, 9, e0085921. [Google Scholar] [CrossRef]
- Paul, S.; Smith, A.A.T.; Culham, K.; Gunawan, K.A.; Weir, J.M.; Cinel, M.A.; Jayawardana, K.S.; Mellett, N.A.; Lee, M.K.S.; Murphy, A.J.; et al. Shark Liver Oil Supplementation Enriches Endogenous Plasmalogens and Reduces Markers of Dyslipidemia and Inflammation. J. Lipid Res. 2021, 62, 100092. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-S.; Jung, S.; Hwang, G.-S.; Shin, D.-M. Gut Microbiota Indole-3-Propionic Acid Mediates Neuroprotective Effect of Probiotic Consumption in Healthy Elderly: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial and in Vitro Study. Clin. Nutr. 2023, 42, 1025–1033. [Google Scholar] [CrossRef]
- Calderón-Pérez, L.; Escoté, X.; Companys, J.; Alcaide-Hidalgo, J.M.; Bosch, M.; Rabassa, M.; Crescenti, A.; Valls, R.M.; Pedret, A.; Solà, R.; et al. A Single-Blinded, Randomized, Parallel Intervention to Evaluate Genetics and Omics-Based Personalized Nutrition in General Population via an e-Commerce Tool: The PREVENTOMICS e-Commerce Study. Am. J. Clin. Nutr. 2024, 120, 129–144. [Google Scholar] [CrossRef]
- Cofán, M.; Checa, A.; Serra-Mir, M.; Roth, I.; Valls-Pedret, C.; Lopez-Illamola, A.; Doménech, M.; Rajaram, S.; Lázaro, I.; Sabaté, J.; et al. A Walnut-Enriched Diet for 2 Years Changes the Serum Oxylipin Profile in Healthy Older Persons. J. Nutr. 2024, 154, 395–402. [Google Scholar] [CrossRef]
- Connolly, E.L.; Liu, A.H.; Radavelli-Bagatini, S.; Shafaei, A.; Boyce, M.C.; Wood, L.G.; McCahon, L.; Koch, H.; Sim, M.; Hill, C.R.; et al. Cruciferous Vegetables Lower Blood Pressure in Adults with Mildly Elevated Blood Pressure in a Randomized, Controlled, Crossover Trial: The VEgetableS for vaScular hEaLth (VESSEL) Study. BMC Med. 2024, 22, 353. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Carvalho, R.; Fonseca, A.M.; Castelo Branco, M.; Alves, M.; Jarak, I. Standard Doses of Cholecalciferol Reduce Glucose and Increase Glutamine in Obesity-Related Hypertension: Results of a Randomized Trial. Int. J. Mol. Sci. 2024, 25, 3416. [Google Scholar] [CrossRef]
- Singh, D.; Ham, D.; Kim, S.-A.; Kothari, D.; Park, Y.J.; Joung, H.; Lee, C.H. Urine Metabolomics Unravel the Effects of Short-Term Dietary Interventions on Oxidative Stress and Inflammation: A Randomized Controlled Crossover Trial. Sci. Rep. 2024, 14, 15277. [Google Scholar] [CrossRef]
- Kim, C.H. Complex Regulatory Effects of Gut Microbial Short-Chain Fatty Acids on Immune Tolerance and Autoimmunity. Cell. Mol. Immunol. 2023, 20, 341–350. [Google Scholar] [CrossRef]
- Fahy, E.; Subramaniam, S. RefMet: A Reference Nomenclature for Metabolomics. Nat. Methods 2020, 17, 1173–1174. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Guo, A.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- Santos, P. The Role of Cardiovascular Risk Assessment in Preventive Medicine: A Perspective from Portugal Primary Health-Care Cardiovascular Risk Assessment. J. Environ. Public Health 2020, 2020, 1639634. [Google Scholar] [CrossRef]
- Chiorcea-Paquim, A.-M. 8-Oxoguanine and 8-Oxodeoxyguanosine Biomarkers of Oxidative DNA Damage: A Review on HPLC–ECD Determination. Molecules 2022, 27, 1620. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, Y. Precision Dietary Intervention: Gut Microbiome and Meta-Metabolome as Functional Readouts. Phenomics 2025, 5, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Menni, C.; Berry, S.E.; Valdes, A.M.; Spector, T.D.; Segata, N. Cardiometabolic Health, Diet and the Gut Microbiome: A Meta-Omics Perspective. Nat. Med. 2023, 29, 551–561. [Google Scholar] [CrossRef]
- Gonzalez-Granda, A.; Damms-Machado, A.; Basrai, M.; Bischoff, S.C. Changes in Plasma Acylcarnitine and Lysophosphatidylcholine Levels Following a High-Fructose Diet: A Targeted Metabolomics Study in Healthy Women. Nutrients 2018, 10, 1254. [Google Scholar] [CrossRef]
- Koemel, N.A.; Senior, A.M.; Benmarhnia, T.; Holmes, A.; Okada, M.; Oulhote, Y.; Parker, H.M.; Shah, S.; Simpson, S.J.; Raubenheimer, D.; et al. Diet Quality, Microbial Lignan Metabolites, and Cardiometabolic Health among US Adults. Nutrients 2023, 15, 1412. [Google Scholar] [CrossRef]
- Jonsson, K.; Andersson, R.; Bach Knudsen, K.E.; Hallmans, G.; Hanhineva, K.; Katina, K.; Kolehmainen, M.; Kyrø, C.; Langton, M.; Nordlund, E.; et al. Rye and Health—Where Do We Stand and Where Do We Go? Trends Food Sci. Technol. 2018, 79, 78–87. [Google Scholar] [CrossRef]
- Kambalapally, C.; Suthar, P.K.; Patale, P.; Dhiman, S.; Gupta, V.; Thongire, V.; Sarmah, D.; Datta, A.; Kalia, K.; Bhattacharya, P. Trigonelline and Its Uses in Stroke. In Treatments, Nutraceuticals, Supplements, and Herbal Medicine in Neurological Disorders; Martin, C., Patel, V., Preedy, V., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 979–992. ISBN 9780323900522. [Google Scholar]
- Pruss, K.M.; Chen, H.; Liu, Y.; Van Treuren, W.; Higginbottom, S.K.; Jarman, J.B.; Fischer, C.R.; Mak, J.; Wong, B.; Cowan, T.M.; et al. Host-Microbe Co-Metabolism via MCAD Generates Circulating Metabolites Including Hippuric Acid. Nat. Commun. 2023, 14, 512. [Google Scholar] [CrossRef]
- Magiera, A.; Kołodziejczyk-Czepas, J.; Olszewska, M.A. Antioxidant and Anti-Inflammatory Effects of Vanillic Acid in Human Plasma, Human Neutrophils, and Non-Cellular Models In Vitro. Molecules 2025, 30, 467. [Google Scholar] [CrossRef]
- Ghasemzadeh Rahbardar, M.; Ferns, G.A.; Ghayour Mobarhan, M. Vanillic Acid as a Promising Intervention for Metabolic Syndrome: Preclinical Studies. Iran. J. Basic Med. Sci. 2025, 28, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Khodorova, N.; Calvez, J.; Pilard, S.; Benoit, S.; Gaudichon, C.; Rutledge, D.N. Urine Metabolite Profiles after the Consumption of a Low- and a High-Digestible Protein Meal, and Comparison of Urine Normalization Techniques. Metabolites 2024, 14, 177. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Gul, P.; Rashid, M.T.; Li, Q.; Liu, K. Composition of Whole Grain Dietary Fiber and Phenolics and Their Impact on Markers of Inflammation. Nutrients 2024, 16, 1047. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Distrutti, E. Bile Acid-Activated Receptors, Intestinal Microbiota, and the Treatment of Metabolic Disorders. Trends Mol. Med. 2015, 21, 702–714. [Google Scholar] [CrossRef]
- Wagner, K.M.; McReynolds, C.B.; Schmidt, W.K.; Hammock, B.D. Soluble Epoxide Hydrolase as a Therapeutic Target for Pain, Inflammatory and Neurodegenerative Diseases. Pharmacol. Ther. 2017, 180, 62–76. [Google Scholar] [CrossRef]
- Caligiuri, S.P.B.; Parikh, M.; Stamenkovic, A.; Pierce, G.N.; Aukema, H.M. Dietary Modulation of Oxylipins in Cardiovascular Disease and Aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H903–H918. [Google Scholar] [CrossRef]
- Park, S.; Kim, H.W.; Joo Lee, C.; Kim, Y.; Sung, J. Profiles of Volatile Sulfur Compounds in Various Vegetables Consumed in Korea Using HS-SPME-GC/MS Technique. Front. Nutr. 2024, 11, 1409008. [Google Scholar] [CrossRef]
- Miekus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Swiergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef]
- Steckhan, N.; Hohmann, C.-D.; Kessler, C.; Dobos, G.; Michalsen, A.; Cramer, H. Effects of Different Dietary Approaches on Inflammatory Markers in Patients with Metabolic Syndrome: A Systematic Review and Meta-Analysis. Nutrition 2016, 32, 338–348. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Koelman, L.; Rodrigues, C.E. Dietary Patterns and Biomarkers of Oxidative Stress and Inflammation: A Systematic Review of Observational and Intervention Studies. Redox Biol. 2021, 42, 101869. [Google Scholar] [CrossRef]
- Tabung, F.K.; Liang, L.; Huang, T.; Balasubramanian, R.; Zhao, Y.; Chandler, P.D.; Manson, J.E.; Cespedes Feliciano, E.M.; Hayden, K.M.; Van Horn, L.; et al. Identifying Metabolomic Profiles of Inflammatory Diets in Postmenopausal Women. Clin. Nutr. 2020, 39, 1478–1490. [Google Scholar] [CrossRef]
- Camfield, D.A.; Nolidin, K.; Savage, K.; Timmer, J.; Croft, K.; Tangestani Fard, M.; Simpson, T.; Downey, L.; Scholey, A.; Pipingas, A.; et al. Higher Plasma Levels of F2 -Isoprostanes Are Associated with Slower Psychomotor Speed in Healthy Older Adults. Free Radic. Res. 2019, 53, 377–386. [Google Scholar] [CrossRef]
- Christensen, J.J.; Ulven, S.M.; Retterstøl, K.; Narverud, I.; Bogsrud, M.P.; Henriksen, T.; Bollerslev, J.; Halvorsen, B.; Aukrust, P.; Holven, K.B. Comprehensive Lipid and Metabolite Profiling of Children with and without Familial Hypercholesterolemia: A Cross-Sectional Study. Atherosclerosis 2017, 266, 48–57. [Google Scholar] [CrossRef]
- Strand, E.; Pedersen, E.R.; Svingen, G.F.T.; Olsen, T.; Bjørndal, B.; Karlsson, T.; Dierkes, J.; Njølstad, P.R.; Mellgren, G.; Tell, G.S.; et al. Serum Acylcarnitines and Risk of Cardiovascular Death and Acute Myocardial Infarction in Patients With Stable Angina Pectoris. J. Am. Heart Assoc. 2017, 6, e003620. [Google Scholar] [CrossRef]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative Metabolomics in Vegans and Omnivores Reveal Constraints on Diet-Dependent Gut Microbiota Metabolite Production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef]
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and Their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed]
- Mora-Ortiz, M.; Yubero-Serrano, E.; Priego-Capote, F.; Gutierrez-Mariscal, F.; Alcala-Diaz, J.; Torres-Peña, J.; Arenas de-Larriva, A.; Delgado-Lista, J.; Perez-Martinez, P.; Roche, H.; et al. Dietary Lipid Quantity and Quality Modulate the Postprandial Metabolomic Profile in Patients with Metabolic Syndrome. Nutrients 2024, 16, 4267. [Google Scholar] [CrossRef]
- Yu, Z.; Nan, F.; Wang, L.Y.; Jiang, H.; Chen, W.; Jiang, Y. Effects of High-Protein Diet on Glycemic Control, Insulin Resistance and Blood Pressure in Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Nutr. 2020, 39, 1724–1734. [Google Scholar] [CrossRef] [PubMed]
- Eichelmann, F.; Prada, M.; Sellem, L.; Jackson, K.G.; Salas Salvadó, J.; Razquin Burillo, C.; Estruch, R.; Friedén, M.; Rosqvist, F.; Risérus, U.; et al. Lipidome Changes Due to Improved Dietary Fat Quality Inform Cardiometabolic Risk Reduction and Precision Nutrition. Nat. Med. 2024, 30, 2867–2877. [Google Scholar] [CrossRef]
- Imamura, F.; Micha, R.; Wu, J.H.Y.; de Oliveira Otto, M.C.; Otite, F.O.; Abioye, A.I.; Mozaffarian, D. Effects of Saturated Fat, Polyunsaturated Fat, Monounsaturated Fat, and Carbohydrate on Glucose-Insulin Homeostasis: A Systematic Review and Meta-Analysis of Randomised Controlled Feeding Trials. PLoS Med. 2016, 13, e1002087. [Google Scholar] [CrossRef] [PubMed]
- Mousa, H.; Elrayess, M.A.; Diboun, I.; Jackson, S.K.; Zughaier, S.M. Metabolomics Profiling of Vitamin D Status in Relation to Dyslipidemia. Metabolites 2022, 12, 771. [Google Scholar] [CrossRef]
- Delcheva, G.; Stefanova, K.; Stankova, T. Ceramides—Emerging Biomarkers of Lipotoxicity in Obesity, Diabetes, Cardiovascular Diseases, and Inflammation. Diseases 2024, 12, 195. [Google Scholar] [CrossRef]
- Norris, G.; Blesso, C. Dietary and Endogenous Sphingolipid Metabolism in Chronic Inflammation. Nutrients 2017, 9, 1180. [Google Scholar] [CrossRef]
- Taylor, P.N.; Davies, J.S. A Review of the Growing Risk of Vitamin D Toxicity from Inappropriate Practice. Br. J. Clin. Pharmacol. 2018, 84, 1121–1127. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Valenti, M.; Del Forno, F.; Caneva, E.; Pietrobelli, A. Vitamin D: Daily vs. Monthly Use in Children and Elderly—What Is Going On? Nutrients 2017, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.; Monteiro, A.; Rodrigues, R.; Ferreira, C.; Coutinho, J.; Filipe, R.; Ramos, S.; Branco, M.C.; Fonseca, M. In Obese Hypertensives Cholecalciferol Inhibits Circulating TH17 Cells but Not Macrophage Infiltration on Adipose Tissue. Clin. Immunol. 2023, 247, 109244. [Google Scholar] [CrossRef]
- Vanherwegen, A.-S.; Gysemans, C.; Mathieu, C. Vitamin D Endocrinology on the Cross-Road between Immunity and Metabolism. Mol. Cell. Endocrinol. 2017, 453, 52–67. [Google Scholar] [CrossRef]
- Abreu, P.; Pinheiro, C.H.J.; Vitzel, K.F.; Vasconcelos, D.A.A.; Torres, R.P.; Fortes, M.S.; Marzuca-Nassr, G.N.; Mancini-Filho, J.; Hirabara, S.M.; Curi, R. Contractile Function Recovery in Severely Injured Gastrocnemius Muscle of Rats Treated with Either Oleic or Linoleic Acid. Exp. Physiol. 2016, 101, 1392–1405. [Google Scholar] [CrossRef]
- Das, U.N. Essential Fatty Acids and Their Metabolites in the Pathobiology of Inflammation and Its Resolution. Biomolecules 2021, 11, 1873. [Google Scholar] [CrossRef] [PubMed]
- Akbari, M.; Ostadmohammadi, V.; Tabrizi, R.; Mobini, M.; Lankarani, K.B.; Moosazadeh, M.; Heydari, S.T.; Chamani, M.; Kolahdooz, F.; Asemi, Z. The Effects of Alpha-Lipoic Acid Supplementation on Inflammatory Markers among Patients with Metabolic Syndrome and Related Disorders: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. 2018, 15, 39. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine Omega-3 Fatty Acids and Inflammatory Processes: Effects, Mechanisms and Clinical Relevance. Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Hewlings, S.; Kalman, D. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, 92. [Google Scholar] [CrossRef]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of Targeted Delivery of Propionate to the Human Colon on Appetite Regulation, Body Weight Maintenance and Adiposity in Overweight Adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef]
- Dollerup, O.L.; Chubanava, S.; Agerholm, M.; Søndergård, S.D.; Altıntaş, A.; Møller, A.B.; Høyer, K.F.; Ringgaard, S.; Stødkilde-Jørgensen, H.; Lavery, G.G.; et al. Nicotinamide Riboside Does Not Alter Mitochondrial Respiration, Content or Morphology in Skeletal Muscle from Obese and Insulin-resistant Men. J. Physiol. 2020, 598, 731–754. [Google Scholar] [CrossRef]
- McCoin, C.S.; Knotts, T.A.; Ono-Moore, K.D.; Oort, P.J.; Adams, S.H. Long-Chain Acylcarnitines Activate Cell Stress and Myokine Release in C2 C12 Myotubes: Calcium-Dependent and -Independent Effects. Am. J. Physiol.-Endocrinol. Metab. 2015, 308, E990–E1000. [Google Scholar] [CrossRef]
- Agus, A.; Planchais, J.; Sokol, H. Gut Microbiota Regulation of Tryptophan Metabolism in Health and Disease. Cell Host Microbe 2018, 23, 716–724. [Google Scholar] [CrossRef]
- Guzior, D.V.; Quinn, R.A. Review: Microbial Transformations of Human Bile Acids. Microbiome 2021, 9, 140. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Nieuwdorp, M.; de Vos, W.M.; Rampanelli, E. Microbial Tryptophan Metabolism Tunes Host Immunity, Metabolism, and Extraintestinal Disorders. Metabolites 2022, 12, 834. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The Intestinal Microbiota Fuelling Metabolic Inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef]
- Fu, J.; Li, G.; Li, X.; Song, S.; Cheng, L.; Rui, B.; Jiang, L. Gut Commensal Alistipes as a Potential Pathogenic Factor in Colorectal Cancer. Discov. Onc. 2024, 15, 473. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Ying, S. Tryptophan Metabolism and Gut Microbiota: A Novel Regulatory Axis Integrating the Microbiome, Immunity, and Cancer. Metabolites 2023, 13, 1166. [Google Scholar] [CrossRef]
- Peron, G.; Meroño, T.; Gargari, G.; Hidalgo-Liberona, N.; Miñarro, A.; Lozano, E.V.; Castellano-Escuder, P.; González-Domínguez, R.; Del Bo’, C.; Bernardi, S.; et al. A Polyphenol-Rich Diet Increases the Gut Microbiota Metabolite Indole 3-Propionic Acid in Older Adults with Preserved Kidney Function. Mol. Nutr. Food Res. 2022, 66, 2100349. [Google Scholar] [CrossRef] [PubMed]
- Turpin-Nolan, S.M.; Brüning, J.C. The Role of Ceramides in Metabolic Disorders: When Size and Localization Matters. Nat. Rev. Endocrinol. 2020, 16, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Dzobo, K.E.; Cupido, A.J.; Mol, B.M.; Stiekema, L.C.A.; Versloot, M.; Winkelmeijer, M.; Peter, J.; Pennekamp, A.-M.; Havik, S.R.; Vaz, F.M.; et al. Diacylglycerols and Lysophosphatidic Acid, Enriched on Lipoprotein(a), Contribute to Monocyte Inflammation. Arter. Thromb. Vasc. Biol. 2024, 44, 720–740. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, G.R.; Guizellini, G.M.; Da Silva, S.A.; De Almeida, A.P.; Pinaffi-Langley, A.C.C.; Rogero, M.M.; De Camargo, A.C.; Torres, E.A.F.S. Polycyclic Aromatic Hydrocarbons in Foods: Biological Effects, Legislation, Occurrence, Analytical Methods, and Strategies to Reduce Their Formation. Int. J. Mol. Sci. 2021, 22, 6010. [Google Scholar] [CrossRef]
- Mirza Alizadeh, A.; Mohammadi, M.; Hashempour-baltork, F.; Hosseini, H.; Shahidi, F. Process-Induced Toxicants in Food: An Overview on Structures, Formation Pathways, Sensory Properties, Safety and Health Implications. Food Prod. Process. Nutr. 2025, 7, 7. [Google Scholar] [CrossRef]
- Alabduladhem, T.O.; Bordoni, B. Physiology, Krebs Cycle. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Ryan, D.G.; O’Neill, L.A.J. Krebs Cycle Reborn in Macrophage Immunometabolism. Annu. Rev. Immunol. 2020, 38, 289–313. [Google Scholar] [CrossRef]
- Williams, N.C.; O’Neill, L.A.J. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front. Immunol. 2018, 9, 141. [Google Scholar] [CrossRef]
- Rogero, M.; Calder, P. Obesity, Inflammation, Toll-Like Receptor 4 and Fatty Acids. Nutrients 2018, 10, 432. [Google Scholar] [CrossRef]
- Petrescu, M.; Vlaicu, S.I.; Ciumărnean, L.; Milaciu, M.V.; Mărginean, C.; Florea, M.; Vesa, Ș.C.; Popa, M. Chronic Inflammation-A Link between Nonalcoholic Fatty Liver Disease (NAFLD) and Dysfunctional Adipose Tissue. Medicina 2022, 58, 641. [Google Scholar] [CrossRef]
- Wang, W.; Kou, J.; Long, J.; Wang, T.; Zhang, M.; Wei, M.; Xie, Q. GC/MS and LC/MS Serum Metabolomic Analysis of Chinese LN Patients. Sci. Rep. 2024, 14, 1523. [Google Scholar] [CrossRef]
- Alexaki, V.I. Adipose Tissue-Derived Mediators of Systemic Inflammation and Metabolic Control. Curr. Opin. Endocr. Metab. Res. 2024, 37, 100560. [Google Scholar] [CrossRef]
- Chegini, M.; Tabar, M.S.; Behrouz, V.; Bahrizadeh, M.; Sadeghi, A.; Hekmatdoost, A.; Yari, Z. Diet-Induced Inflammation Is Associated with Fatty Pancreas in Patients with Common Bile Duct Stones. Sci. Rep. 2025, 15, 15620. [Google Scholar] [CrossRef] [PubMed]
- Maki, K.C.; Dicklin, M.R.; Kirkpatrick, C.F. Saturated Fats and Cardiovascular Health: Current Evidence and Controversies. J. Clin. Lipidol. 2021, 15, 765–772. [Google Scholar] [CrossRef]
- Saqib, U.; Sarkar, S.; Suk, K.; Mohammad, O.; Baig, M.S.; Savai, R. Phytochemicals as Modulators of M1-M2 Macrophages in Inflammation. Oncotarget 2018, 9, 17937–17950. [Google Scholar] [CrossRef]
- Lee, R.; Yoon, B.-I.; Hunter, C.A.; Kwon, H.M.; Sung, H.W.; Park, J. Short Chain Fatty Acids Facilitate Protective Immunity by Macrophages and T Cells during Acute Fowl Adenovirus-4 Infection. Sci. Rep. 2023, 13, 17999. [Google Scholar] [CrossRef]
- Cheng, W.L.; Markus, C.; Lim, C.Y.; Tan, R.Z.; Sethi, S.K.; Loh, T.P.; the IFCC Working Group on Method Evaluation Protocols. Calibration Practices in Clinical Mass Spectrometry: Review and Recommendations. Ann. Lab. Med. 2023, 43, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Mosley, J.D.; Dunn, W.B.; Kuligowski, J.; Lewis, M.R.; Monge, M.E.; Ulmer Holland, C.; Vuckovic, D.; Zanetti, K.A.; Schock, T.B.; the Metabolomics Quality Assurance, Quality Control Consortium (mQACC). Metabolomics 2023 Workshop Report: Moving toward Consensus on Best QA/QC Practices in LC–MS-Based Untargeted Metabolomics. Metabolomics 2024, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Nian, M.; Chen, X.; Fang, M. Addressing Pitfalls of Metabolomics for Toxicology: A Call for Standardization, Reproducibility and Data Sharing. Chem. Res. Toxicol. 2025, 38, 1150–1156. [Google Scholar] [CrossRef]
- Zhu, M.; Lamont, L.; Maas, P.; Harms, A.C.; Beekman, M.; Slagboom, P.E.; Dubbelman, A.-C.; Hankemeier, T. Matrix Effect Evaluation Using Multi-Component Post-Column Infusion in Untargeted Hydrophilic Interaction Liquid Chromatography-Mass Spectrometry Plasma Metabolomics. J. Chromatogr. A 2025, 1740, 465580. [Google Scholar] [CrossRef]
- Ćeranić, A.; Bueschl, C.; Doppler, M.; Parich, A.; Xu, K.; Lemmens, M.; Buerstmayr, H.; Schuhmacher, R. Enhanced Metabolome Coverage and Evaluation of Matrix Effects by the Use of Experimental-Condition-Matched 13C-Labeled Biological Samples in Isotope-Assisted LC-HRMS Metabolomics. Metabolites 2020, 10, 434. [Google Scholar] [CrossRef]
- Huppertz, B.; Möller-Friedrich, S.; Baum, K. Matrix Effects of Urine Marker Substances in LC-MS/MS Analysis of Drug of Abuse. Ther. Drug Monit. 2024, 46, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Páez-Franco, J.C.; Torres-Ruiz, J.; Sosa-Hernández, V.A.; Cervantes-Díaz, R.; Romero-Ramírez, S.; Pérez-Fragoso, A.; Meza-Sánchez, D.E.; Germán-Acacio, J.M.; Maravillas-Montero, J.L.; Mejía-Domínguez, N.R.; et al. Metabolomics Analysis Reveals a Modified Amino Acid Metabolism That Correlates with Altered Oxygen Homeostasis in COVID-19 Patients. Sci. Rep. 2021, 11, 6350. [Google Scholar] [CrossRef] [PubMed]
- Hagn, G.; Meier-Menches, S.M.; Plessl-Walder, G.; Mitra, G.; Mohr, T.; Preindl, K.; Schlatter, A.; Schmidl, D.; Gerner, C.; Garhöfer, G.; et al. Plasma Instead of Serum Avoids Critical Confounding of Clinical Metabolomics Studies by Platelets. J. Proteome Res. 2024, 23, 3064–3075. [Google Scholar] [CrossRef]
- Kaluarachchi, M.; Boulangé, C.L.; Karaman, I.; Lindon, J.C.; Ebbels, T.M.D.; Elliott, P.; Tracy, R.P.; Olson, N.C. A Comparison of Human Serum and Plasma Metabolites Using Untargeted 1H NMR Spectroscopy and UPLC-MS. Metabolomics 2018, 14, 32. [Google Scholar] [CrossRef]
- Mosele, J.I.; Motilva, M.-J. Phenol Biological Metabolites as Food Intake Biomarkers, a Pending Signature for a Complete Understanding of the Beneficial Effects of the Mediterranean Diet. Nutrients 2021, 13, 3051. [Google Scholar] [CrossRef] [PubMed]
- Jackson, M.K.; Wang, B.; Rasmussen, H.; Natarajan, S.K.; Bilek, L.D.; Ehlers, D.K.; Graeff-Armas, L.; D’Angelo, C.; Cochran, T.; Harp, K.; et al. Urinary Metabolites as Biomarkers of Dietary Intake: A Systematic Review. Front. Nutr. 2025, 12, 1596543. [Google Scholar] [CrossRef]
- Taubenheim, J.; Kadibalban, A.S.; Zimmermann, J.; Taubenheim, C.; Tran, F.; Schreiber, S.; Rosenstiel, P.; Aden, K.; Kaleta, C. Metabolic Modeling Reveals a Multi-Level Deregulation of Host-Microbiome Metabolic Networks in IBD. Nat. Commun. 2025, 16, 5120. [Google Scholar] [CrossRef]
- Vich Vila, A.; Hu, S.; Andreu-Sánchez, S.; Collij, V.; Jansen, B.H.; Augustijn, H.E.; Bolte, L.A.; Ruigrok, R.A.A.A.; Abu-Ali, G.; Giallourakis, C.; et al. Faecal Metabolome and Its Determinants in Inflammatory Bowel Disease. Gut 2023, 72, 1472–1485. [Google Scholar] [CrossRef]
- Zheng, F.; Yang, Y.; Lu, G.; Tan, J.S.; Mageswary, U.; Zhan, Y.; Ayad, M.E.; Lee, Y.-Y.; Xie, D. Metabolomics Insights into Gut Microbiota and Functional Constipation. Metabolites 2025, 15, 269. [Google Scholar] [CrossRef]
- Zierer, J.; Jackson, M.A.; Kastenmüller, G.; Mangino, M.; Long, T.; Telenti, A.; Mohney, R.P.; Small, K.S.; Bell, J.T.; Steves, C.J.; et al. The Fecal Metabolome as a Functional Readout of the Gut Microbiome. Nat. Genet. 2018, 50, 790–795. [Google Scholar] [CrossRef]
- Spick, M.; Lewis, H.-M.; Frampas, C.F.; Longman, K.; Costa, C.; Stewart, A.; Dunn-Walters, D.; Greener, D.; Evetts, G.; Wilde, M.J.; et al. An Integrated Analysis and Comparison of Serum, Saliva and Sebum for COVID-19 Metabolomics. Sci. Rep. 2022, 12, 11867. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yu, Y.; Shen, W.; Tan, Z.; Tang, S.; Yao, H.; He, J.; Wan, F. Metabolomics Analysis Across Multiple Biofluids Reveals the Metabolic Responses of Lactating Holstein Dairy Cows to Fermented Soybean Meal Replacement. Front. Vet. Sci. 2022, 9, 812373. [Google Scholar] [CrossRef] [PubMed]
- Evans, E.D.; Duvallet, C.; Chu, N.D.; Oberst, M.K.; Murphy, M.A.; Rockafellow, I.; Sontag, D.; Alm, E.J. Predicting Human Health from Biofluid-Based Metabolomics Using Machine Learning. Sci. Rep. 2020, 10, 17635. [Google Scholar] [CrossRef]
- Donatti, A.; Canto, A.M.; Godoi, A.B.; Da Rosa, D.C.; Lopes-Cendes, I. Circulating Metabolites as Potential Biomarkers for Neurological Disorders—Metabolites in Neurological Disorders. Metabolites 2020, 10, 389. [Google Scholar] [CrossRef]
- Wang, T.; Holscher, H.D.; Maslov, S.; Hu, F.B.; Weiss, S.T.; Liu, Y.-Y. Predicting Metabolite Response to Dietary Intervention Using Deep Learning. Nat. Commun. 2025, 16, 815. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.C.Y.; Groves, R.A.; Rydzak, T.; Lewis, I.A. Metabolomics Survey of Uropathogenic Bacteria in Human Urine. Front. Microbiol. 2024, 15, 1507561. [Google Scholar] [CrossRef]
- Li, T.; Ihanus, A.; Ohukainen, P.; Järvelin, M.-R.; Kähönen, M.; Kettunen, J.; Raitakari, O.T.; Lehtimäki, T.; Mäkinen, V.-P.; Tynkkynen, T.; et al. Clinical and Biochemical Associations of Urinary Metabolites: Quantitative Epidemiological Approach on Renal-Cardiometabolic Biomarkers. Int. J. Epidemiol. 2024, 53, dyad162. [Google Scholar] [CrossRef]
- Griffin, L.E.; Kohrt, S.E.; Rathore, A.; Kay, C.D.; Grabowska, M.M.; Neilson, A.P. Microbial Metabolites of Flavanols in Urine Are Associated with Enhanced Anti-Proliferative Activity in Bladder Cancer Cells In Vitro. Nutr. Cancer 2022, 74, 194–210. [Google Scholar] [CrossRef]
- Deng, K.; Xu, J.; Shen, L.; Zhao, H.; Gou, W.; Xu, F.; Fu, Y.; Jiang, Z.; Shuai, M.; Li, B.; et al. Comparison of Fecal and Blood Metabolome Reveals Inconsistent Associations of the Gut Microbiota with Cardiometabolic Diseases. Nat. Commun. 2023, 14, 571. [Google Scholar] [CrossRef]
- Sangermani, M.; Desiati, I.; Jørgensen, S.M.; Li, J.V.; Andreassen, T.; Bathen, T.F.; Giskeødegård, G.F. Stability in Fecal Metabolites amid a Diverse Gut Microbiome Composition: A One-Month Longitudinal Study of Variability in Healthy Individuals. Gut Microbes 2024, 16, 2427878. [Google Scholar] [CrossRef]
- Montero, O.; Hedeland, M.; Balgoma, D. Trials and Tribulations of Statistical Significance in Biochemistry and Omics. Trends Biochem. Sci. 2023, 48, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Rosner, B.A.; Colditz, G.A. Statistically Significant Association Does Not Imply Improvement in Prediction of Clinical Outcomes. Cancer Prev. Res. 2025. Available online: https://aacrjournals.org/cancerpreventionresearch/article/doi/10.1158/1940-6207.CAPR-25-0056/766659/Statistically-Significant-Association-Does-not (accessed on 6 July 2025).
- Sostare, E.; Bowen, T.J.; Lawson, T.N.; Freier, A.; Li, X.; Lloyd, G.R.; Najdekr, L.; Jankevics, A.; Smith, T.; Varshavi, D.; et al. Metabolomics Simultaneously Derives Benchmark Dose Estimates and Discovers Metabolic Biotransformations in a Rat Bioassay. Chem. Res. Toxicol. 2024, 37, 923–934. [Google Scholar] [CrossRef]
- Yao, C.-H.; Wang, L.; Stancliffe, E.; Sindelar, M.; Cho, K.; Yin, W.; Wang, Y.; Patti, G.J. Dose-Response Metabolomics To Understand Biochemical Mechanisms and Off-Target Drug Effects with the TOXcms Software. Anal. Chem. 2020, 92, 1856–1864. [Google Scholar] [CrossRef] [PubMed]
- Correia, B.S.B.; Sandby, K.; Krarup, T.; Magkos, F.; Geiker, N.R.W.; Bertram, H.C. Changes in Plasma, Urine, and Fecal Metabolome after 16 Weeks of Consuming Dairy With Different Food Matrixes—A Randomized Controlled Trial. Mol. Nutr. Food Res. 2024, 68, 2300363. [Google Scholar] [CrossRef]
- Mohr, A.E.; Sweazea, K.L.; Bowes, D.A.; Jasbi, P.; Whisner, C.M.; Sears, D.D.; Krajmalnik-Brown, R.; Jin, Y.; Gu, H.; Klein-Seetharaman, J.; et al. Gut Microbiome Remodeling and Metabolomic Profile Improves in Response to Protein Pacing with Intermittent Fasting versus Continuous Caloric Restriction. Nat. Commun. 2024, 15, 4155. [Google Scholar] [CrossRef] [PubMed]
- Muijsenberg, A.; Canfora, E.E.; Blaak, E.E. Metabolic Phenotypes, Genotypes, and Gut Microbiome Signatures in Obesity: Implications for Precision Nutrition Strategies in Type 2 Diabetes Prevention. Nutr. Rev. 2025, 100, nuaf088. [Google Scholar] [CrossRef]
- Morand, C. How to Better Consider and Understand Interindividual Variability in Response to Polyphenols in Clinical Trials. Front. Nutr. 2024, 11, 1522516. [Google Scholar] [CrossRef]
- Kviatcovsky, D.; Zheng, D.; Elinav, E. Gut Microbiome and Its Potential Link to Personalized Nutrition. Curr. Opin. Physiol. 2021, 22, 100439. [Google Scholar] [CrossRef]
- Chen, L.; Dong, Y.; Bhagatwala, J.; Raed, A.; Huang, Y.; Zhu, H. Vitamin D3 Supplementation Increases Long-Chain Ceramide Levels in Overweight/Obese African Americans: A Post-Hoc Analysis of a Randomized Controlled Trial. Nutrients 2020, 12, 981. [Google Scholar] [CrossRef]
- Smith, E.; Ottosson, F.; Ericson, U.; Hellstrand, S.; Rizzo, M.; Sukruang, K.; Pizza, V.; Orho-Melander, M.; Nilsson, P.M.; Kennbäck, C.; et al. Impact of a Short-Term Mediterranean Diet Intervention on Plasma Metabolites: A Pilot Study. Metabolomics 2024, 20, 82. [Google Scholar] [CrossRef]
- Zheng, H.; Lorenzen, J.; Astrup, A.; Larsen, L.; Yde, C.; Clausen, M.; Bertram, H. Metabolic Effects of a 24-Week Energy-Restricted Intervention Combined with Low or High Dairy Intake in Overweight Women: An NMR-Based Metabolomics Investigation. Nutrients 2016, 8, 108. [Google Scholar] [CrossRef]
- Hengist, A.; Davies, R.G.; Walhin, J.-P.; Buniam, J.; Merrell, L.H.; Rogers, L.; Bradshaw, L.; Moreno-Cabañas, A.; Rogers, P.J.; Brunstrom, J.M.; et al. Ketogenic Diet but Not Free-Sugar Restriction Alters Glucose Tolerance, Lipid Metabolism, Peripheral Tissue Phenotype, and Gut Microbiome: RCT. Cell Rep. Med. 2024, 5, 101667. [Google Scholar] [CrossRef] [PubMed]
- Gram-Kampmann, E.M.; Olesen, T.B.; Hansen, C.D.; Hugger, M.B.; Jensen, J.M.; Handberg, A.; Beck-Nielsen, H.; Krag, A.; Olsen, M.H.; Højlund, K. A Six-Month Low-Carbohydrate Diet High in Fat Does Not Adversely Affect Endothelial Function or Markers of Low-Grade Inflammation in Patients with Type 2 Diabetes: An Open-Label Randomized Controlled Trial. Cardiovasc. Diabetol. 2023, 22, 212. [Google Scholar] [CrossRef]
- Rhee, E.P.; Waikar, S.S.; Rebholz, C.M.; Zheng, Z.; Perichon, R.; Clish, C.B.; Evans, A.M.; Avila, J.; Denburg, M.R.; Anderson, A.H.; et al. Variability of Two Metabolomic Platforms in CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 40–48. [Google Scholar] [CrossRef]
- Adekoya, A.; Okezue, M.A.; Menon, K. Medical Laboratories in Healthcare Delivery: A Systematic Review of Their Roles and Impact. Laboratories 2025, 2, 8. [Google Scholar] [CrossRef]
- Di Minno, A.; Gelzo, M.; Caterino, M.; Costanzo, M.; Ruoppolo, M.; Castaldo, G. Challenges in Metabolomics-Based Tests, Biomarkers Revealed by Metabolomic Analysis, and the Promise of the Application of Metabolomics in Precision Medicine. Int. J. Mol. Sci. 2022, 23, 5213. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.; Noureldein, M.; Bezdeková, D.; Schram, A.; Howard, R.; Powers, R. A Reproducibility Crisis for Clinical Metabolomics Studies. TrAC Trends Anal. Chem. 2024, 180, 117918. [Google Scholar] [CrossRef]
- Li, S.; Looby, N.; Chandran, V.; Kulasingam, V. Challenges in the Metabolomics-Based Biomarker Validation Pipeline. Metabolites 2024, 14, 200. [Google Scholar] [CrossRef]
- Castelli, F.A.; Rosati, G.; Moguet, C.; Fuentes, C.; Marrugo-Ramírez, J.; Lefebvre, T.; Volland, H.; Merkoçi, A.; Simon, S.; Fenaille, F.; et al. Metabolomics for Personalized Medicine: The Input of Analytical Chemistry from Biomarker Discovery to Point-of-Care Tests. Anal. Bioanal. Chem. 2022, 414, 759–789. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).