Metabolic Features of Saliva Before and After Breast Cancer Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Collection, Storage, Pre-Treatment and Analysis of Saliva
2.3. Statistical Analysis
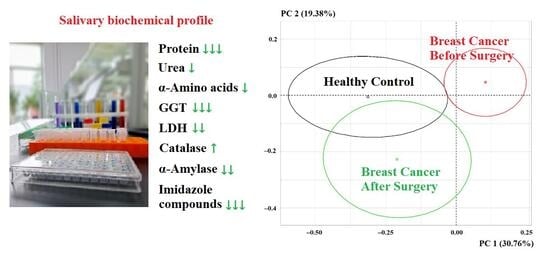
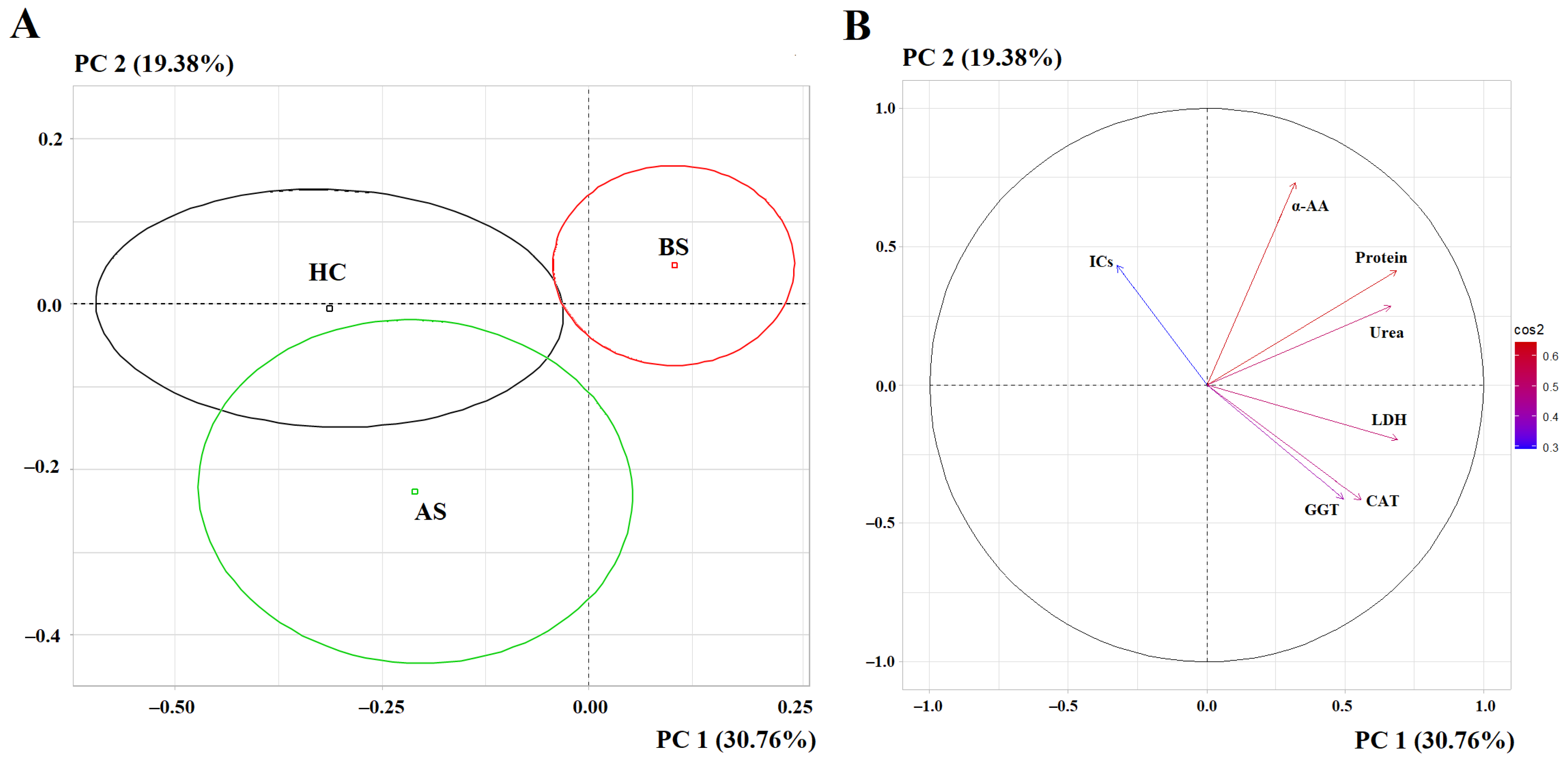
3. Results
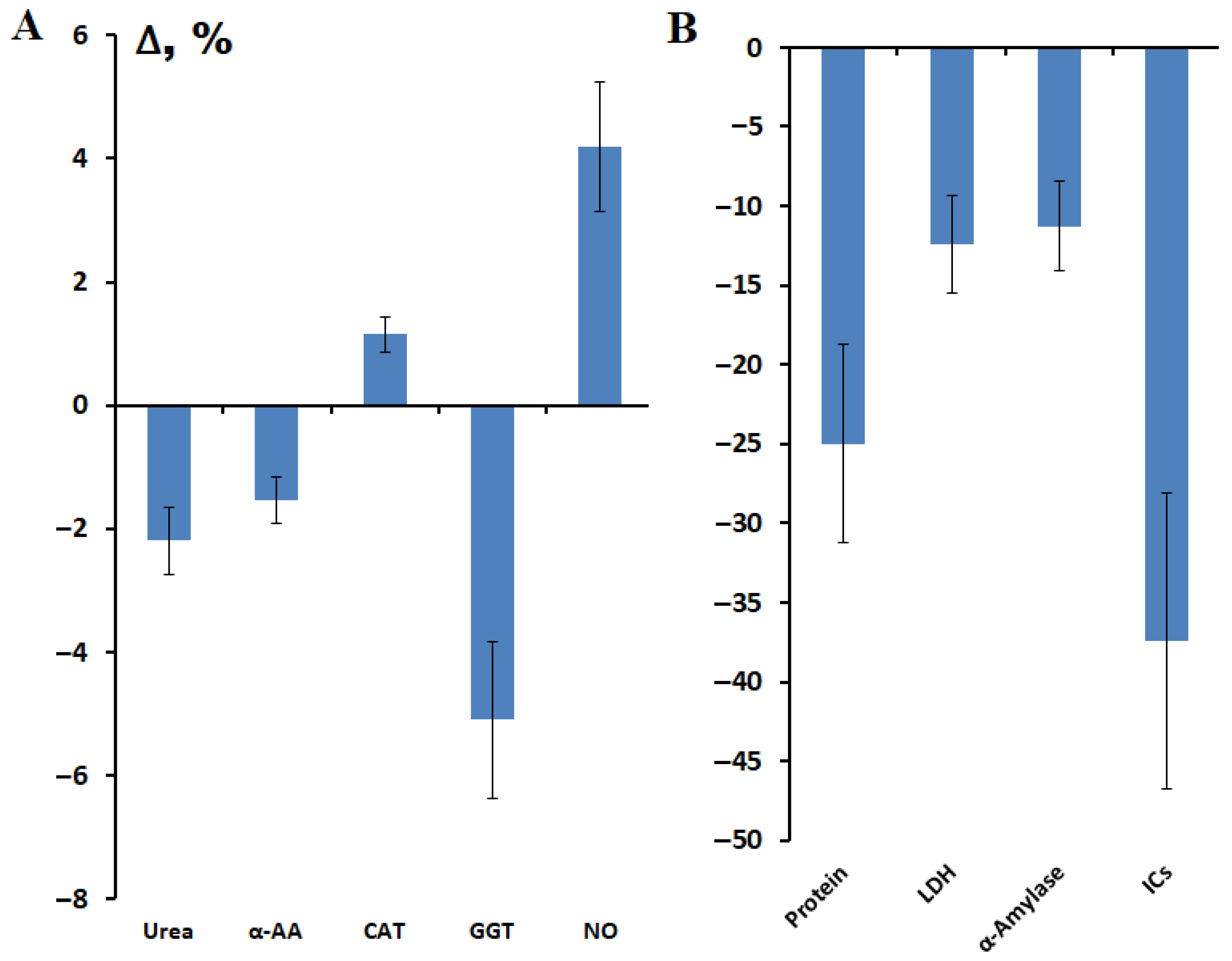
3.1. Changes in the Biochemical Composition of Saliva After Breast Cancer Surgery
| Indicators | Breast Cancer, n = 660 | Healthy Controls, n = 127 | p-Value |
|---|---|---|---|
| Protein, g/L | 0.75 [0.51; 1.00] | 0.66 [0.48; 0.97] | 0.0432 |
| Urea, mmol/L | 9.31 [6.27; 12.23] | 7.47 [5.30; 10.58] | 0.0006 |
| α-AAs, mmol/L | 3.97 [3.74; 4.34] | 3.85 [3.70; 4.07] | <0.0001 |
| Catalase, ncat/mL | 3.43 [2.51; 5.15] | 2.99 [2.47; 4.03] | 0.0485 |
| LDH, U/L | 1062.0 [628.0; 1623.0] | 1277.0 [833.7; 1563.0] | <0.0001 |
| GGT, U/L | 22.7 [19.7; 25.9] | 19.3 [17.0; 22.7] | <0.0001 |
| α-Amylase, U/L | 256.2 [149.1; 535.1] | 199.6 [103.4; 421.8] | 0.5378 |
| ICs, mmol/L | 0.246 [0.131; 0.405] | 0.329 [0.211; 0.531] | <0.0001 |
| NO, μmol/L | 75.25 [67.00; 84.83] | 73.61 [69.72; 79.35] | 0.8070 |
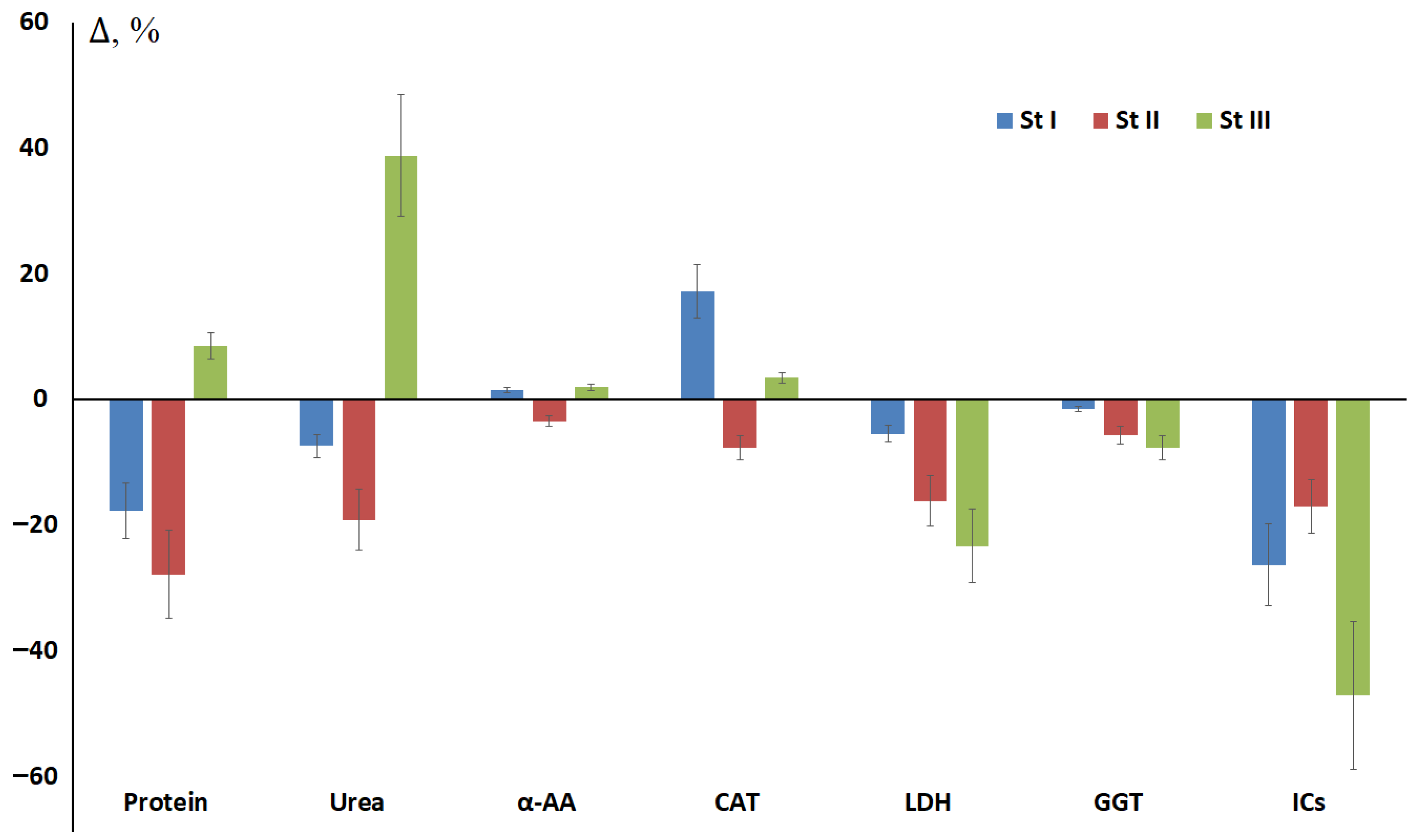
| Indicators | Surgery | St I, n = 228/40 | St II, n = 272/72 | St III, n = 97/27 | St IV, n = 63/0 |
|---|---|---|---|---|---|
| Protein, g/L | BS | 0.78 [0.59; 1.06] | 0.77 [0.53; 1.05] | 0.66 [0.39; 0.81] | 0.72 [0.47; 0.93] |
| p = 0.0087 * | p = 0.0336 | - | - | ||
| AS | 0.64 [0.48; 0.81] | 0.55 [0.38; 0.77] | 0.72 [0.50; 0.93] | - | |
| - | p = 0.0330 | - | - | ||
| Urea, mmol/L | BS | 10.19 [7.41; 12.73] | 9.57 [6.61; 12.22] | 7.59 [4.50; 10.50] | 7.32 [4.08; 11.68] |
| p = 0.0000 | p = 0.0001 | - | - | ||
| AS | 9.43 [6.99; 12.28] | 7.74 [5.38; 11.67] | 10.53 [6.51; 12.72] | - | |
| p = 0.0050 | - | p = 0.0080 | - | ||
| α-AAs, mmol/L | BS | 4.02 [3.76; 4.40] | 4.01 [3.78; 4.42] | 3.88 [3.67; 4.16] | 3.87 [3.69; 4.32] |
| p = 0.0001 | p = 0.0000 | - | - | ||
| AS | 4.08 [3.80; 4.45] | 3.87 [3.71; 4.16] | 3.95 [3.74; 4.28] | - | |
| p = 0.0032 | - | - | - | ||
| Catalase, ncat/mL | BS | 3.35 [2.40; 5.05] | 3.61 [2.61; 5.05] | 3.34 [2.34; 5.51] | 3.09 [2.44; 4.24] |
| - | p = 0.0150 | - | - | ||
| AS | 3.93 [2.83; 5.13] | 3.33 [2.57; 4.40] | 3.46 [2.20; 4.90] | - | |
| LDH, U/L | BS | 1049.5 [571.4; 1605.5] | 1106.0 [674.8; 1713.0] | 1023.0 [661.2; 1503.0] | 930.4 [542.0; 1452.0] |
| - | - | - | p = 0.0178 | ||
| AS | 992.2 [753.6; 1657.0] | 927.9 [513.6; 1393.0] | 784.3 [627.4; 1474.0] | - | |
| - | p = 0.0039 | - | - | ||
| GGT, U/L | BS | 23.4 [20.9; 26.7] | 22.7 [19.6; 26.1] | 22.0 [18.3; 24.7] | 22.4 [18.5; 24.5] |
| p = 0.0000 | p = 0.0000 | p = 0.0048 | p = 0.0059 | ||
| AS | 23.0 [19.0; 25.1] | 21.4 [17.8; 24.1] | 20.3 [18.8; 23.3] | - | |
| p = 0.0012 | p = 0.0183 | - | - | ||
| ICs, mmol/L | BS | 0.234 [0.136; 0.388] | 0.234 [0.123; 0.413] | 0.289 [0.145; 0.436] | 0.252 [0.159; 0.357] |
| p = 0.0000 | p = 0.0003 | - | p = 0.0121 | ||
| AS | 0.172 [0.087; 0.361] | 0.195 [0.118; 0.392] | 0.153 [0.080; 0.285] | - | |
| p = 0.0028 | p = 0.0007 | p = 0.0002 | - |
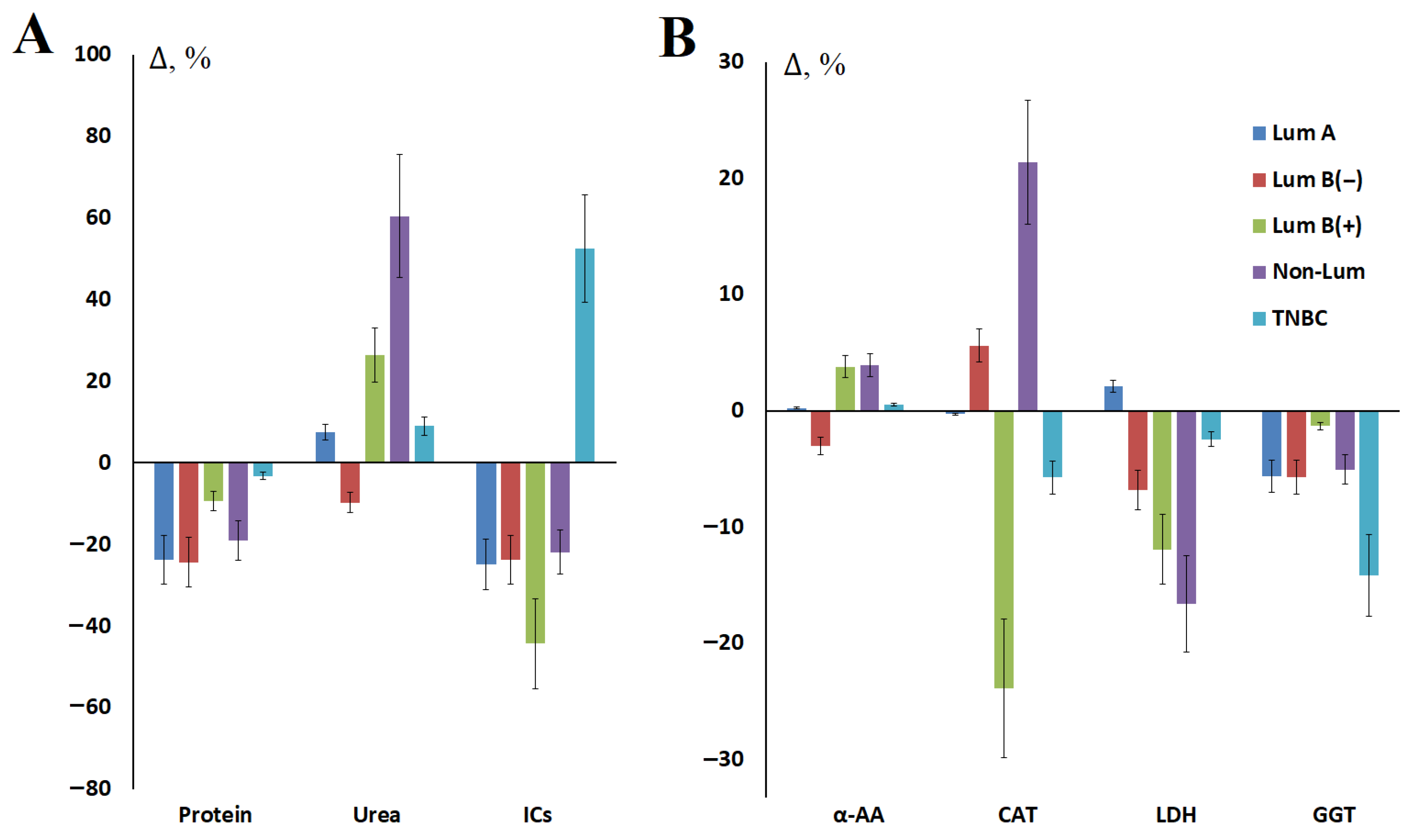
| Indicators | Sur | Lum A, n = 234/27 | Lum B(−), n = 200/74 | Lum B(+), n = 60/10 | Non-Lum, n = 42/6 | TNBC, n = 122/22 |
|---|---|---|---|---|---|---|
| Protein, g/L | BS | 0.84 [0.59; 1.11] | 0.78 [0.59; 1.02] | 0.75 [0.50; 0.88] | 0.63 [0.45; 0.78] | 0.63 [0.42; 0.86] |
| p = 0.0007 | p = 0.0193 | - | - | - | ||
| AS | 0.64 [0.47; 0.93] | 0.59 [0.38; 0.85] | 0.68 [0.43; 0.88] | 0.51 [0.39; 0.68] | 0.61 [0.46; 0.78] | |
| Urea, mmol/L | BS | 10.46 [7.69; 12.62] | 9.52 [6.72; 12.52] | 8.26 [5.27; 12.07] | 7.20 [4.72; 9.86] | 6.87 [4.08; 10.48] |
| p = 0.0000 | p = 0.0002 | - | - | - | ||
| AS | 11.24 [7.19; 12.67] | 8.59 [5.98; 11.19] | 10.44 [5.32; 13.99] | 11.55 [9.77; 13.59] | 7.49 [5.53; 12.35] | |
| p = 0.0008 | - | - | p = 0.0453 | - | ||
| α-AAs, mmol/L | BS | 4.05 [3.79; 4.39] | 4.00 [3.74; 4.41] | 3.96 [3.72; 4.24] | 3.81 [3.62; 4.09] | 3.88 [3.69; 4.19] |
| p = 0.0000 | p = 0.0003 | - | - | - | ||
| AS | 4.06 [3.68; 4.32] | 3.88 [3.71; 4.22] | 4.11 [3.94; 4.61] | 3.96 [3.74; 4.22] | 3.90 [3.78; 4.33] | |
| - | - | p = 0.0096 | - | - | ||
| Catalase, ncat/mL | BS | 3.47 [2.57; 5.30] | 3.39 [2.37; 5.07] | 3.98 [2.43; 5.95] | 3.09 [2.45; 4.79] | 3.49 [2.59; 4.90] |
| p = 0.0242 | - | p = 0.0416 | - | p = 0.0375 | ||
| AS | 3.46 [2.23; 4.51] | 3.58 [2.67; 4.80] | 3.03 [2.43; 3.52] | 3.75 [2.00; 6.73] | 3.29 [2.74; 5.06] | |
| LDH, U/L | BS | 1203.0 [686.2; 1727.0] | 1020.0 [598.1; 1652.0] | 1050.0 [674.8; 1353.0] | 1009.5 [710.1; 1513.0] | 931.7 [522.8; 1431.5] |
| - | - | - | - | p = 0.0025 | ||
| AS | 1228.0 [747.4; 1629.0] | 950.8 [578.5; 1380.0] | 924.9 [539.3; 1219.0] | 842.1 [757.0; 968.4] | 909.0 [709.6; 1717.0] | |
| GGT, U/L | BS | 23.1 [20.0; 26.8] | 22.8 [19.6; 25.9] | 22.9 [20.0; 26.2] | 21.7 [18.4; 23.8] | 22.6 [19.3; 24.9] |
| p = 0.0000 | p = 0.0000 | p = 0.0001 | p = 0.0254 | p = 0.0000 | ||
| AS | 21.8 [18.3; 25.9] | 21.5 [18.8; 23.6] | 22.6 [21.2; 26.6] | 20.6 [18.8; 22.5] | 19.4 [17.2; 24.3] | |
| p = 0.0441 | p = 0.0024 | p = 0.0133 | - | - | ||
| ICs, mmol/L | BS | 0.237 [0.131; 0.396] | 0.219 [0.104; 0.376] | 0.307 [0.207; 0.418] | 0.279 [0.160; 0.362] | 0.272 [0.153; 0.459] |
| p = 0.0001 | p = 0.0000 | - | p = 0.0476 | p = 0.0344 | ||
| AS | 0.178 [0.083; 0.393] | 0.167 [0.101; 0.319] | 0.171 [0.071; 0.219] | 0.218 [0.052; 0.320] | 0.415 [0.171; 0.601] | |
| - | - | p = 0.0150 | - | - |
3.2. The Influence of Breast Cancer Stage on Changes in Salivary Biochemical Parameters Before and After Surgery
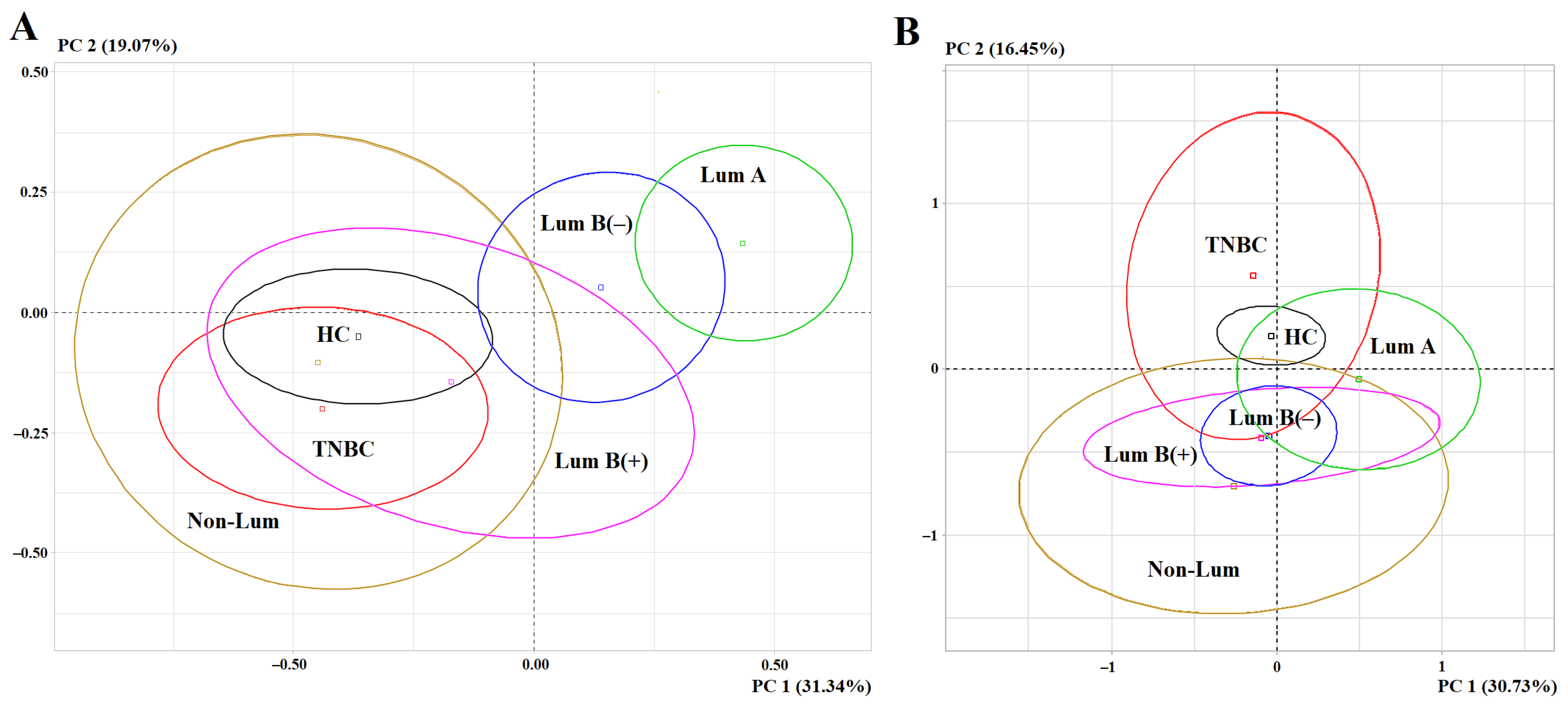
3.3. The Influence of Molecular Biological Subtype of Breast Cancer on Changes in Biochemical Parameters of Saliva Before and After Surgery
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merabishvili, V.M.; Semiglazov, V.F.; Busko, E.A. Age-related features of the prevalence, early diagnosis and survival of breast cancer patients (clinical and population-based study). Vopr. Onkol. Probl. Oncol. 2024, 70, 450–459. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast cancer statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef]
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef] [PubMed]
- Haukaas, T.H.; Euceda, L.R.; Giskeødegård, G.F.; Lamichhane, S.; Krohn, M.; Jernström, S.; Aure, M.R.; Lingjærde, O.C.; Schlichting, E.; Garred, Ø.; et al. Metabolic clusters of breast cancer in relation to gene- and protein expression subtypes. Cancer Metab. 2016, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; Davis, J.B.; Espina, V. Protein biomarkers for subtyping breast cancer and implications for future research: A 2024 update. Expert Rev. Proteom. 2024, 21, 401–416. [Google Scholar] [CrossRef]
- Li, J.; Wu, J.; Han, J. Analysis of Tumor Microenvironment Heterogeneity among Breast Cancer Subtypes to Identify Subtype-Specific Signatures. Genes 2022, 14, 44. [Google Scholar] [CrossRef] [PubMed]
- Rios-Hoyo, A.; Shan, N.L.; Karn, P.L.; Pusztai, L. Clinical Implications of Breast Cancer Intrinsic Subtypes. Adv. Exp. Med. Biol. 2025, 1464, 435–448. [Google Scholar]
- Colomer, R.; González-Farré, B.; Ballesteros, A.I.; Peg, V.; Bermejo, B.; Pérez-Mies, B.; de la Cruz, S.; Rojo, F.; Pernas, S.; Palacios, J. Biomarkers in breast cancer 2024: An updated consensus statement by the Spanish Society of Medical Oncology and the Spanish Society of Pathology. Clin. Transl. Oncol. 2024, 26, 2935–2951. [Google Scholar] [CrossRef]
- Sørlie, T.; Perou, C.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA. 2001, 98, 10869–10874. [Google Scholar] [CrossRef]
- Bastien, R.R.; Rodríguez-Lescure, Á.; Ebbert, M.T.; Prat, A.; Munárriz, B.; Rowe, L.; Miller, P.; Ruiz-Borrego, M.; Anderson, D.; Lyons, B.; et al. PAM50 breast cancer subtyping by RT-qPCR and concordance with standard clinical molecular markers. BMC Med. Genom. 2012, 5, 44. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Siddiqui, S.; Smith, K.; Singh, P.; Kumar, B.; Chouaib, S.; Chandrasekaran, S. Metabolic stratification of human breast tumors reveal subtypes of clinical and therapeutic relevance. iScience 2023, 26, 108059. [Google Scholar] [CrossRef]
- Borgan, E.; Sitter, B.; Lingjærde, O.C.; Johnsen, H.; Lundgren, S.; Bathen, T.F.; Sørlie, T.; Børresen-Dale, A.L.; Gribbestad, I.S. Merging transcriptomics and metabolomics-advances in breast cancer profiling. BMC Cancer 2010, 10, 628. [Google Scholar] [CrossRef]
- Bai, M.; Sun, C. Determination of Breast Metabolic Phenotypes and Their Associations With Immunotherapy and Drug-Targeted Therapy: Analysis of Single-Cell and Bulk Sequences. Front. Cell Dev. Biol. 2022, 10, 829029. [Google Scholar] [CrossRef] [PubMed]
- Dyachenko, E.I.; Bel’skaya, L.V. Salivary Metabolites in Breast Cancer and Fibroadenomas: Focus on Menopausal Status and BMI. Metabolites 2024, 14, 531. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Dyachenko, E.I. Salivary Biomarkers in Breast Cancer: From Salivaomics to Salivaoncoomics. Front. Biosci. 2024, 29, 253. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V. HER2-low Breast Cancer: Salivary Metabolic Features and Therapeutic Perspectives. Front. Biosci. 2025, 17, 36652. [Google Scholar] [CrossRef]
- Takamori, S.; Ishikawa, S.; Sato, K.; Watanabe, H.; Suzuki, J.; Oizumi, H.; Shiono, S.; Uchida, T.; Okuyama, N.; Edamatsu, K.; et al. Salivary metabolites as potential predictive biomarkers for lung surgery complications: A retrospective cross-sectional study. Surg. Today 2024, 54, 866–873. [Google Scholar] [CrossRef]
- Komori, E.; Kato-Kogoe, N.; Imai, Y.; Sakaguchi, S.; Taniguchi, K.; Omori, M.; Ohmichi, M.; Nakamura, S.; Nakano, T.; Lee, S.W.; et al. Changes in salivary microbiota due to gastric cancer resection and its relation to gastric fluid microbiota. Sci. Rep. 2023, 13, 15863. [Google Scholar] [CrossRef]
- Dong, Z.; Chen, Q.; Zhao, D.; Zhang, S.; Yu, K.; Wang, G.; Wang, D. Utilizing Saliva Metabolomics for Diagnosing Gastric Cancer and Exploring the Changes in Saliva Metabolites After Surgery. Onco Targets Ther. 2024, 17, 933–948. [Google Scholar] [CrossRef]
- Bel’skaya, L.V.; Sarf, E.A.; Solomatin, D.V.; Kosenok, V.K. Metabolic Features of Saliva in Breast Cancer Patients. Metabolites 2022, 12, 166. [Google Scholar] [CrossRef]
- Gardner, A.; Parkes, H.G.; So, P.W.; Carpenter, G.H. Determining bacterial and host contributions to the human salivary metabolome. J. Oral. Microbiol. 2019, 11, 1617014. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Wei, Z.; Tian, T.; Bose, D.; Shih, N.N.C.; Feldman, M.D.; Khoury, T.; De Michele, A.; Robertson, E.S. Prognostic correlations with the microbiome of breast cancer subtypes. Cell Death Dis. 2021, 12, 831. [Google Scholar] [CrossRef] [PubMed]
- Bel’skaya, L.V.; Sarf, E.A.; Solomatin, D.V. Free Salivary Amino Acid Profile in Breast Cancer: Clinicopathological and Molecular Biological Features. Curr. Issues Mol. Biol. 2024, 46, 5614–5631. [Google Scholar] [CrossRef] [PubMed]
- Medina, V.; Croci, M.; Crescenti, E.; Mohamad, N.; Sanchez-Jiménez, F.; Massari, N.; Nuñez, M.; Cricco, G.; Martin, G.; Bergoc, R.; et al. The role of histamine in human mammary carcinogenesis: H3 and H4 receptors as potential therapeutic targets for breast cancer treatment. Cancer Biol. Ther. 2008, 7, 28–35. [Google Scholar] [CrossRef]
- Massari, N.A.; Nicoud, M.B.; Medina, V.A. Histamine receptors and cancer pharmacology: An update. Br. J. Pharmacol. 2020, 177, 516–538. [Google Scholar] [CrossRef]
- Vázquez-Meza, H.; Vilchis-Landeros, M.M.; Vázquez-Carrada, M.; Uribe-Ramírez, D.; Matuz-Mares, D. Cellular Compartmentalization, Glutathione Transport and Its Relevance in Some Pathologies. Antioxidants 2023, 12, 834. [Google Scholar] [CrossRef]
- Akaydın, S.Y.; Salihoğlu, E.M.; Güngör, D.G.; Karanlık, H.; Demokan, S. Correlation Between Gamma-Glutamyl Transferase Activity and Glutathione Levels in Molecular Subgroups of Breast Cancer. Eur. J. Breast Health 2019, 16, 72–76. [Google Scholar] [CrossRef]
- King, J.B.; West, M.B.; Cook, P.F.; Hanigan, M.H. A novel, species-specific class of uncompetitive inhibitors of gamma-glutamyl transpeptidase. J. Biol. Chem. 2009, 284, 9059–9065. [Google Scholar] [CrossRef]
- Gupta, R.K.; Patel, A.K.; Kumari, R.; Chugh, S.; Shrivastav, C.; Mehra, S.; Sharma, A.N. Interactions between oxidative stress, lipid profile and antioxidants in breast cancer: A case control study. Asian Pac. J. Cancer Prev. 2012, 13, 6295–6298. [Google Scholar] [CrossRef]
- Jablonska, E.; Gromadzinska, J.; Peplonska, B.; Fendler, W.; Reszka, E.; Krol, M.B.; Wieczorek, E.; Bukowska, A.; Gresner, P.; Galicki, M.; et al. Lipid peroxidation and glutathione peroxidase activity relationship in breast cancer depends on functional polymorphism of GPX1. BMC Cancer 2015, 15, 657. [Google Scholar] [CrossRef]
- Gholizadeh, N.; Ramandi, M.A.; Motiee-Langroudi, M.; Jafari, M.; Sharouny, H.; Sheykhbahaei, N. Serum and salivary levels of lactate dehydrogenase in oral squamous cell carcinoma, oral lichen planus and oral lichenoid reaction. BMC Oral. Health. 2020, 20, 314. [Google Scholar] [CrossRef]
- López-Pintor, R.M.; González-Serrano, J.; Vallina, C.; Serkedzhieva, K.I.; Virto, L.; Nuevo, P.; Caponio, V.C.A.; Iniesta, M.; Santamarta, T.R.; Fernández, P.L.; et al. Factors influencing salivary lactate dehydrogenase levels in oral squamous cell carcinoma and oral potentially malignant disorders. Front. Oral. Health 2025, 5, 1525936. [Google Scholar] [CrossRef]
- Sarode, S.C.; Sarode, G.S.; Kalele, K. Oral lichenoid reaction: A review. Int. J. Oral. Maxillofac. Pathol. 2012, 3, 17–26. [Google Scholar]
- Nagler, R.M.; Lischinsky, S.; Diamond, E.; Klein, I.; Reznick, A.Z. New insights into salivary lactate dehydrogenase of human subjects. J. Lab. Clin. Med. 2001, 137, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Sarf, E.A.; Dyachenko, E.I.; Bel’skaya, L.V. The Role of Salivary Vascular Endothelial Growth Factor A, Cytokines, and Amino Acids in Immunomodulation and Angiogenesis in Breast Cancer. Biomedicines 2024, 12, 1329. [Google Scholar] [CrossRef]
- Feng, K.; Ren, F.; Wang, X. Relationships among breast, gut, and oral microbiota across diverse pathological types of breast cancer, a Chinese cohort study. Front. Mol. Biosci. 2023, 10, 1325552. [Google Scholar] [CrossRef]
- Nater, U.M.; Rohleder, N. Salivary alpha-amylase as a non-invasive biomarker for the sympathetic nervous system: Current state of research. Psychoneuroendocrinology 2009, 34, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Fedrowitz, M.; Hass, R.; Bertram, C.; Löscher, W. Salivary α-amylase exhibits antiproliferative effects in primary cell cultures of rat mammary epithelial cells and human breast cancer cells. J. Exp. Clin. Cancer Res. 2011, 30, 102. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solomatin, D.V.; Sarf, E.A.; Bel’skaya, L.V. Metabolic Features of Saliva Before and After Breast Cancer Surgery. Metabolites 2025, 15, 693. https://doi.org/10.3390/metabo15110693
Solomatin DV, Sarf EA, Bel’skaya LV. Metabolic Features of Saliva Before and After Breast Cancer Surgery. Metabolites. 2025; 15(11):693. https://doi.org/10.3390/metabo15110693
Chicago/Turabian StyleSolomatin, Denis V., Elena A. Sarf, and Lyudmila V. Bel’skaya. 2025. "Metabolic Features of Saliva Before and After Breast Cancer Surgery" Metabolites 15, no. 11: 693. https://doi.org/10.3390/metabo15110693
APA StyleSolomatin, D. V., Sarf, E. A., & Bel’skaya, L. V. (2025). Metabolic Features of Saliva Before and After Breast Cancer Surgery. Metabolites, 15(11), 693. https://doi.org/10.3390/metabo15110693