Metabolomics Analysis Uncovers Distinct Profiles of Liver Post-Transplant Patients by Immunosuppression Regimen
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Processing and Analytical Methods
3. Results
3.1. Study Population and Clinical Characteristics
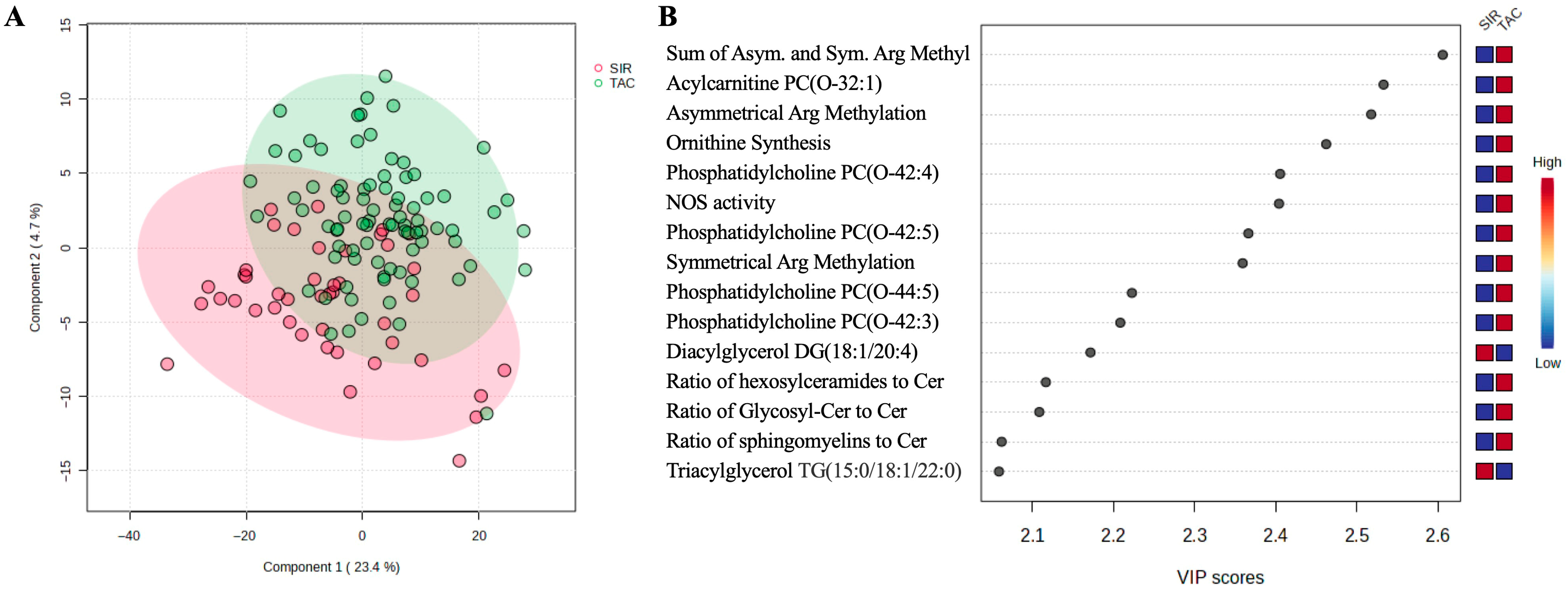
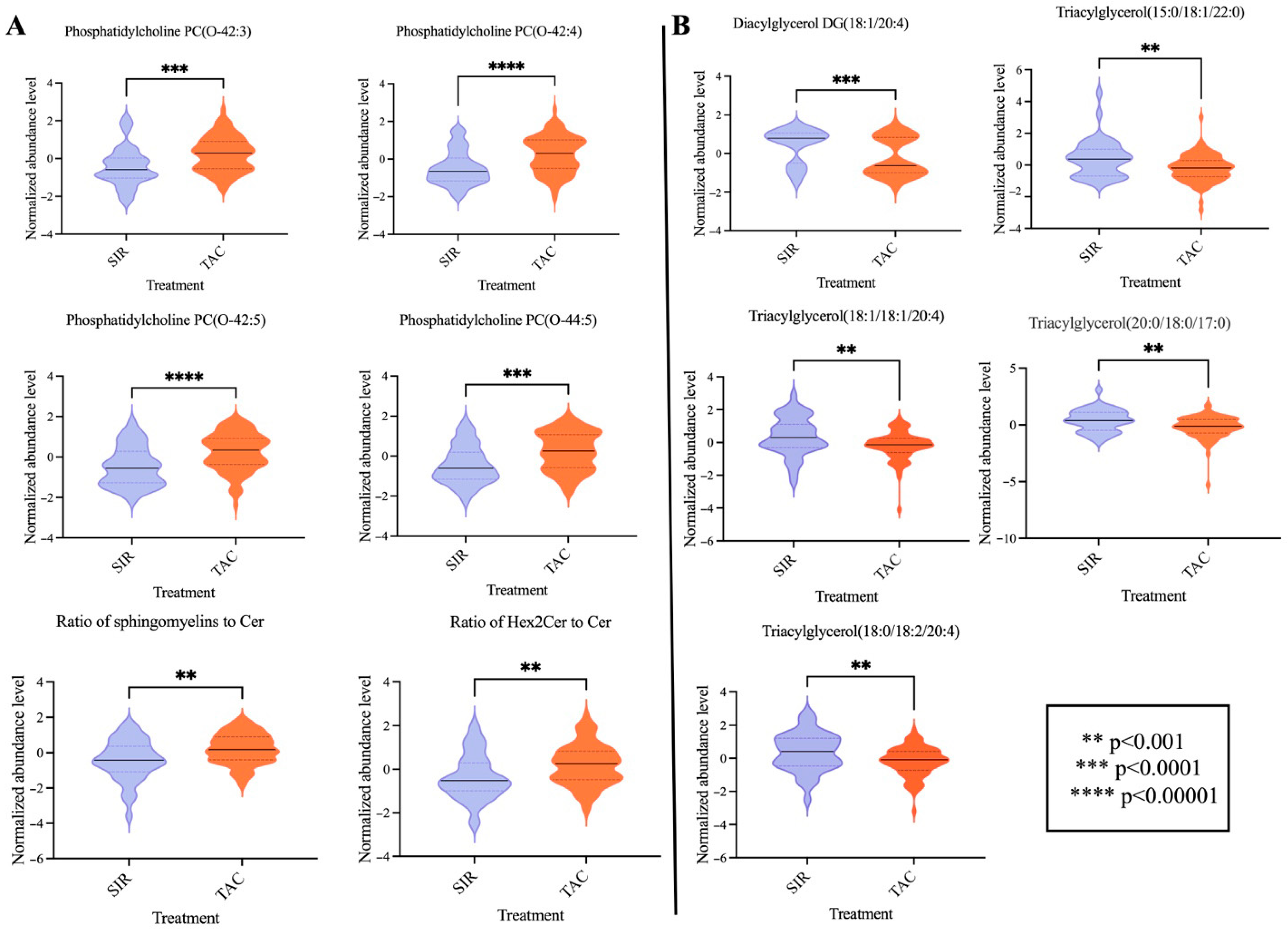
3.2. Metabolomic Data Processing and Multivariate Analysis
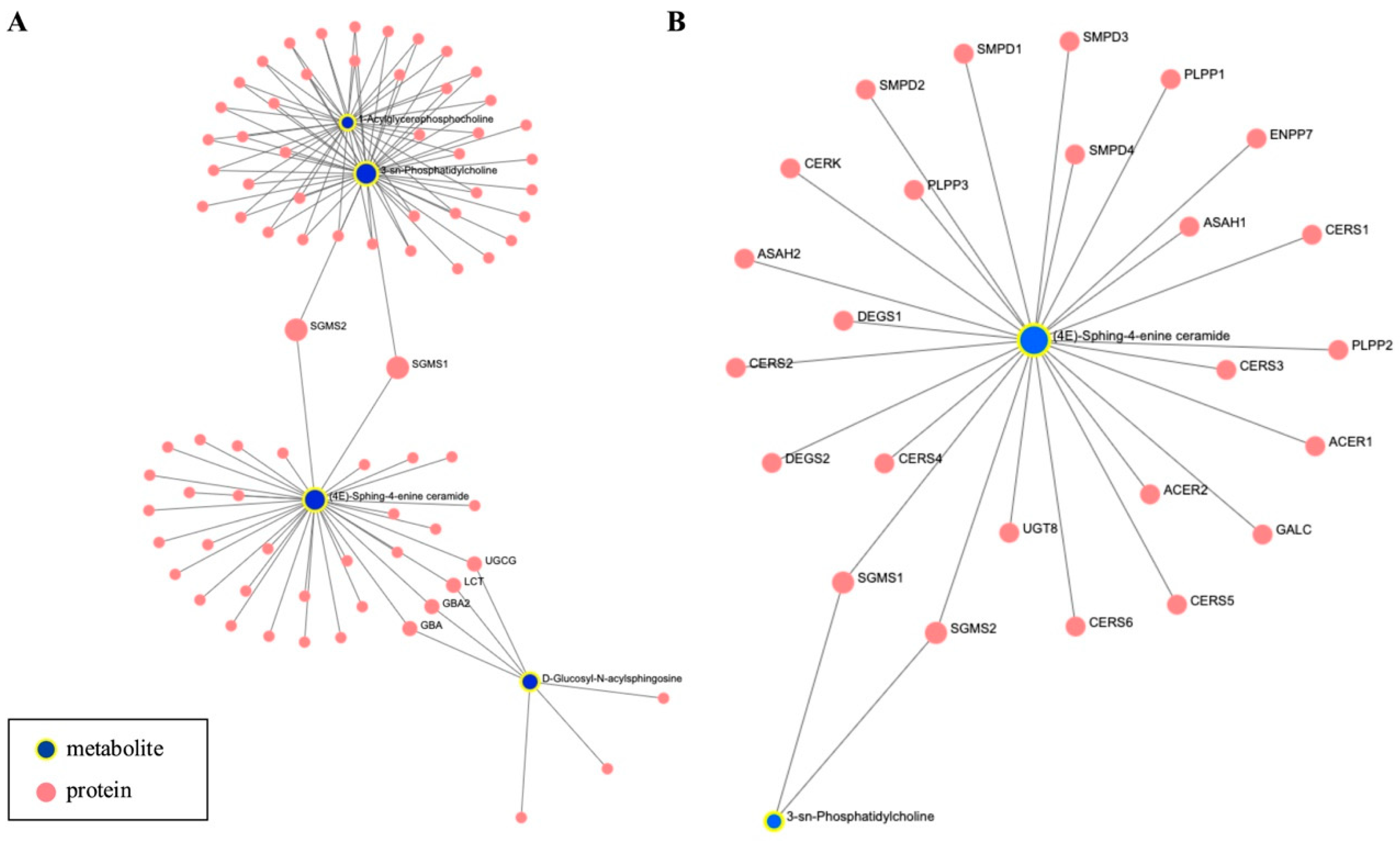
3.3. Network and Pathway Enrichment Analysis
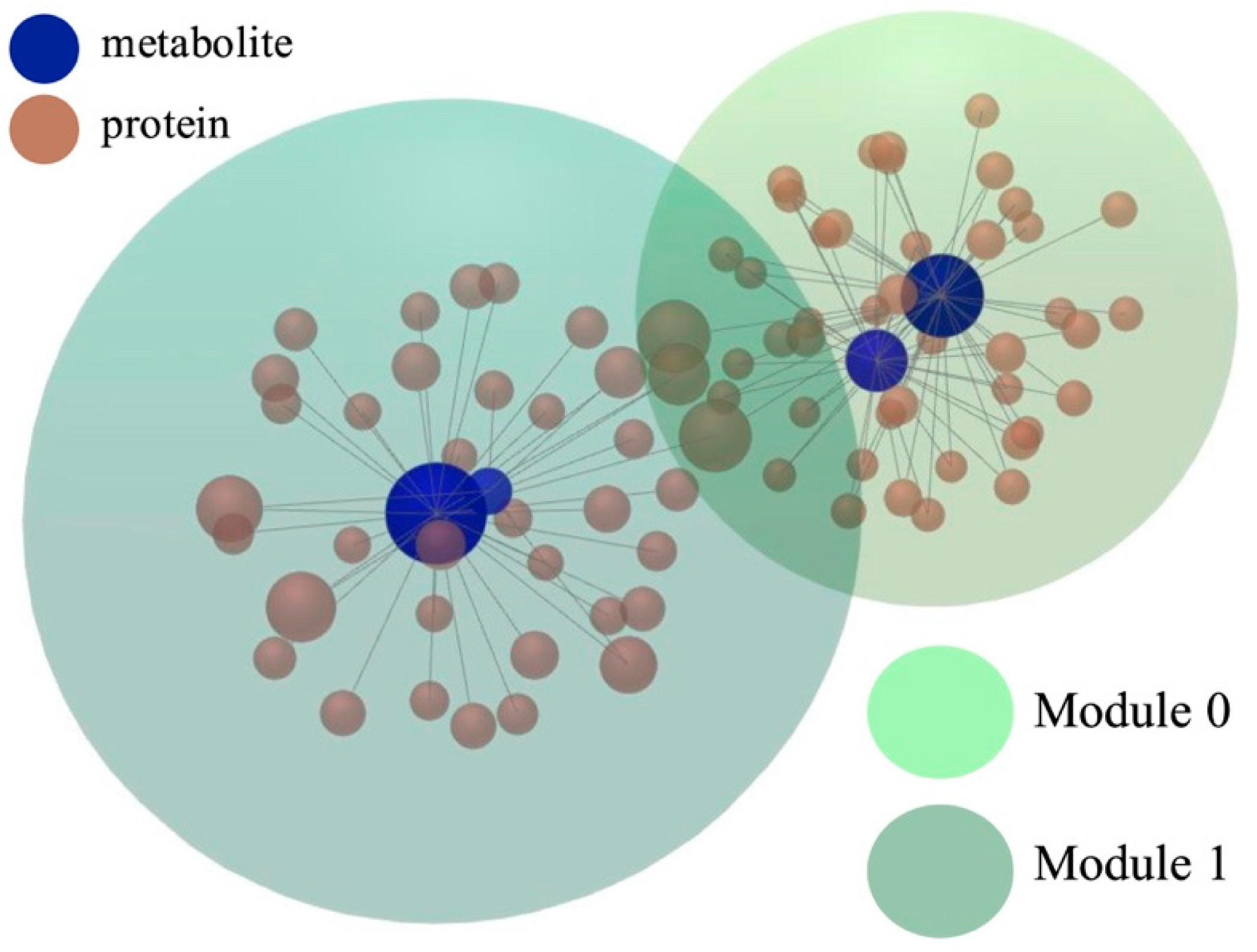
3.4. Functional Module Analysis
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | amino acid |
| ALP | alkaline phosphatase |
| ALT | Alanine Aminotransferase |
| BA | bile acid |
| BSWH | Baylor Scott & White Health |
| BP | biological process |
| CC | cellular component |
| DAG | diacyl-glycerol |
| GO | Gene Ontology |
| HMDB | Human Metabolome Database |
| IQR | interquartile range |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LDL-C | low-density lipoprotein cholesterol |
| LT | Liver Transplantation |
| MELD | Model for End-Stage Liver Disease |
| MF | molecular function |
| mTORC1 | mTOR complex 1 |
| MASLD | Metabolic Dysfunction-Associated Steatotic Liver Disease |
| OLT | Orthotopic Liver Transplantation |
| PEMT | Phosphatidylethanolamine N-methyltransferase |
| PC | phosphatidylcholines |
| PLS-DA | Partial Least Square–Discriminant Analysis |
| SIR | sirolimus |
| S1P | sphingosine-1-phosphate |
| SCFAs | short-chain fatty acids |
| TAC | tacrolimus |
| VIP | Variable Importance in Projection. |
References
- Rana, A.; Ackah, R.L.; Webb, G.J.; Halazun, K.J.; Vierling, J.M.; Liu, H.; Wu, M.-F.; Yoeli, D.; Kueht, M.; Mindikoglu, A.L. No gains in long-term survival after liver transplantation over the past three decades. Ann. Surg. 2019, 269, 20–27. [Google Scholar] [CrossRef]
- Watt, K.D.S.; Pedersen, R.A.; Kremers, W.K.; Heimbach, J.K.; Charlton, M.R. Evolution of Causes and Risk Factors for Mortality Post-Liver Transplant: Results of the NIDDK Long-Term Follow-Up Study. Am. J. Transplant. 2010, 10, 1420–1427. [Google Scholar] [CrossRef]
- Bhat, V.; Tazari, M.; Watt, K.D.; Bhat, M. New-Onset Diabetes and Preexisting Diabetes Are Associated With Comparable Reduction in Long-Term Survival After Liver Transplant: A Machine Learning Approach. Mayo Clin. Proc. 2018, 93, 1794–1802. [Google Scholar] [CrossRef]
- Kotha, S.; Lawendy, B.; Asim, S.; Gomes, C.; Yu, J.; Orchanian-Cheff, A.; Tomlinson, G.; Bhat, M. Impact of immunosuppression on incidence of post-transplant diabetes mellitus in solid organ transplant recipients: Systematic review and meta-analysis. World J. Transpl. 2021, 11, 432–442. [Google Scholar] [CrossRef]
- Azhie, A.; Sheth, P.; Hammad, A.; Woo, M.; Bhat, M. Metabolic Complications in Liver Transplantation Recipients: How We Can Optimize Long-Term Survival. Liver Transpl. 2021, 27, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Bhat, M.; Usmani, S.E.; Azhie, A.; Woo, M. Metabolic Consequences of Solid Organ Transplantation. Endocr. Rev. 2021, 42, 171–197. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.D.; Kim, E.Y.; Yoo, H.; Lee, J.W.; Ryu, D.H.; Noh, D.W.; Park, S.H.; Kim, Y.L.; Hwang, G.S.; Kwon, T.H. Metabonomic analysis of serum metabolites in kidney transplant recipients with cyclosporine A- or tacrolimus-based immunosuppression. Transplantation 2010, 90, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Cortes, M.; Pareja, E.; García-Cañaveras, J.C.; Donato, M.T.; Montero, S.; Mir, J.; Castell, J.V.; Lahoz, A. Metabolomics discloses donor liver biomarkers associated with early allograft dysfunction. J. Hepatol. 2014, 61, 564–574. [Google Scholar] [CrossRef]
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2011, 60, 404–413. [Google Scholar] [CrossRef]
- Mowry, C.J.; Alonso, C.; Iruarrizaga-Lejarreta, M.; Ortiz, P.; Levitsky, J.; Rinella, M. Utility of Metabolomic Biomarkers to Identify Nonalcoholic Fatty Liver Disease in Liver Transplant Recipients. Transpl. Direct 2021, 7, e784. [Google Scholar] [CrossRef] [PubMed]
- Ling, Q.; Han, Y.; Ma, Y.; Wang, X.; Zhu, Z.; Wang, J.; Cao, J.; Lin, X.; Wang, J.; Wang, B. Alterations in the Gut Microbiome in Liver Recipients with Post-Transplant Diabetes Mellitus. Engineering 2023, 31, 98–111. [Google Scholar] [CrossRef]
- Wang, M.; Xu, J.; Yang, N.; Zhang, T.; Zhu, H.; Wang, J. Insight Into the Metabolomic Characteristics of Post-Transplant Diabetes Mellitus by the Integrated LC-MS and GC-MS Approach- Preliminary Study. Front. Endocrinol. 2022, 12, 807318. [Google Scholar] [CrossRef]
- Zhai, L.; Wu, J.; Lam, Y.Y.; Kwan, H.Y.; Bian, Z.X.; Wong, H.L.X. Gut-Microbial Metabolites, Probiotics and Their Roles in Type 2 Diabetes. Int. J. Mol. Sci. 2021, 22, 12846. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, X.; Ling, Q.; Wu, L.; Wu, P.; Tang, R.; Xu, X.; Yang, M.; Zhang, L.; Zhu, W.; et al. Antibiotics-mediated intestinal microbiome perturbation aggravates tacrolimus-induced glucose disorders in mice. Front. Med. 2019, 13, 471–481. [Google Scholar] [CrossRef]
- Lu, J.; Wang, S.; Li, M.; Gao, Z.; Xu, Y.; Zhao, X.; Hu, C.; Zhang, Y.; Liu, R.; Hu, R.; et al. Association of Serum Bile Acids Profile and Pathway Dysregulation With the Risk of Developing Diabetes Among Normoglycemic Chinese Adults: Findings From the 4C Study. Diabetes Care 2021, 44, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Nishida, S.; Ishima, T.; Kimura, N.; Iwami, D.; Nagai, R.; Imai, Y.; Aizawa, K. Metabolomic Profiling of Mice with Tacrolimus-Induced Nephrotoxicity: Carnitine Deficiency in Renal Tissue. Biomedicines 2024, 12, 521. [Google Scholar] [CrossRef] [PubMed]
- Zararsiz, G.E.; Lintelmann, J.; Cecil, A.; Kirwan, J.; Poschet, G.; Gegner, H.M.; Schuchardt, S.; Guan, X.L.; Saigusa, D.; Wishart, D.; et al. Interlaboratory comparison of standardised metabolomics and lipidomics analyses in human and rodent blood using the MxP(®) Quant 500 kit. bioRxiv 2024. [Google Scholar] [CrossRef]
- Pang, Z.; Lu, Y.; Zhou, G.; Hui, F.; Xu, L.; Viau, C.; Spigelman, A.F.; E MacDonald, P.; Wishart, D.S.; Li, S.; et al. MetaboAnalyst 6.0: Towards a unified platform for metabolomics data processing, analysis and interpretation. Nucleic Acids Res. 2024, 52, W398–W406. [Google Scholar] [CrossRef]
- Ewald, J.D.; Zhou, G.; Lu, Y.; Kolic, J.; Ellis, C.; Johnson, J.D.; Macdonald, P.E.; Xia, J. Web-based multi-omics integration using the Analyst software suite. Nat. Protoc. 2024, 19, 1467–1497. [Google Scholar] [CrossRef]
- Rosvall, M.; Bergstrom, C.T. Maps of random walks on complex networks reveal community structure. Proc. Natl. Acad. Sci. USA 2008, 105, 1118–1123. [Google Scholar] [CrossRef]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef]
- Issa, D.H.; Alkhouri, N. Long-term management of liver transplant recipients: A review for the internist. Cleve Clin. J. Med. 2015, 82, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Vafadari, R.; Kraaijeveld, R.; Weimar, W.; Baan, C.C. Tacrolimus inhibits NF-κB activation in peripheral human T cells. PLoS ONE 2013, 8, e60784. [Google Scholar] [CrossRef] [PubMed]
- Noble, J.; Terrec, F.; Malvezzi, P.; Rostaing, L. Adverse effects of immunosuppression after liver transplantation. Best. Pract. Res. Clin. Gastroenterol. 2021, 54–55, 101762. [Google Scholar] [CrossRef]
- Vidigal, A.C.; de Lucena, D.D.; Beyerstedt, S.; Rangel, É.B. A comprehensive update of the metabolic and toxicological considerations for immunosuppressive drugs used during pancreas transplantation. Expert. Opin. Drug Metab. Toxicol. 2023, 19, 405–427. [Google Scholar] [CrossRef]
- Ahmed, F.; Zakaria, F.; Enebong Nya, G.; Mouchli, M. Sirolimus vs tacrolimus: Which one is the best therapeutic option for patients undergoing liver transplantation for hepatocellular carcinoma? World J. Gastrointest. Surg. 2022, 14, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Cravedi, P.; Ruggenenti, P.; Remuzzi, G. Sirolimus for calcineurin inhibitors in organ transplantation: Contra. Kidney Int. 2010, 78, 1068–1074. [Google Scholar] [CrossRef]
- Lee, V.W.; Chapman, J.R. Sirolimus: Its role in nephrology. Nephrology 2005, 10, 606–614. [Google Scholar] [CrossRef]
- Sehgal, S.N. Sirolimus: Its discovery, biological properties, and mechanism of action. Transplant. Proc. 2003, 35, S7–S14. [Google Scholar] [CrossRef]
- Staatz, C.E.; Tett, S.E. Pharmacokinetic considerations relating to tacrolimus dosing in the elderly. Drugs Aging 2005, 22, 541–557. [Google Scholar] [CrossRef]
- Kirubakaran, R.; Stocker, S.L.; Hennig, S.; Day, R.O.; Carland, J.E. Population Pharmacokinetic Models of Tacrolimus in Adult Transplant Recipients: A Systematic Review. Clin. Pharmacokinet. 2020, 59, 1357–1392. [Google Scholar] [CrossRef]
- Mao, J.; Cheng, Y.; Liu, D.; Zhang, B.; Li, X. Dosing Regimen Recommendations for Sirolimus in Adult Liver Transplant Recipients: Insights from a Population Pharmacokinetic Model. Drug Des. Devel Ther. 2024, 18, 6379–6388. [Google Scholar] [CrossRef]
- Martínez-Uña, M.; Varela-Rey, M.; Cano, A.; Fernández-Ares, L.; Beraza, N.; Aurrekoetxea, I.; Martínez-Arranz, I.; García-Rodríguez, J.L.; Buqué, X.; Mestre, D.; et al. Excess S-adenosylmethionine reroutes phosphatidylethanolamine towards phosphatidylcholine and triglyceride synthesis. Hepatology 2013, 58, 1296–1305. [Google Scholar] [CrossRef]
- van der Veen, J.N.; Lingrell, S.; Vance, D.E. The membrane lipid phosphatidylcholine is an unexpected source of triacylglycerol in the liver. J. Biol. Chem. 2012, 287, 23418–23426. [Google Scholar] [CrossRef] [PubMed]
- Quinn, W.J., 3rd; Wan, M.; Shewale, S.V.; Gelfer, R.; Rader, D.J.; Birnbaum, M.J.; Titchenell, P.M. mTORC1 stimulates phosphatidylcholine synthesis to promote triglyceride secretion. J. Clin. Investig. 2017, 127, 4207–4215. [Google Scholar] [CrossRef] [PubMed]
- Maev, I.V.; Samsonov, A.A.; Palgova, L.K.; Pavlov, C.S.; Vovk, E.I.; Shirokova, E.N.; Starostin, K.M. Effectiveness of phosphatidylcholine in alleviating steatosis in patients with non-alcoholic fatty liver disease and cardiometabolic comorbidities (MANPOWER study). BMJ Open Gastroenterol. 2020, 7, e000341. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Chaba, T.; Zhu, L.F.; Jacobs, R.L.; Vance, D.E. Hepatic ratio of phosphatidylcholine to phosphatidylethanolamine predicts survival after partial hepatectomy in mice. Hepatology 2012, 55, 1094–1102. [Google Scholar] [CrossRef]
- Tsai, H.I.; Lo, C.J.; Zheng, C.W.; Lee, C.W.; Lee, W.C.; Lin, J.R.; Shiao, M.S.; Cheng, M.L.; Yu, H.P. A Lipidomics Study Reveals Lipid Signatures Associated with Early Allograft Dysfunction in Living Donor Liver Transplantation. J. Clin. Med. 2018, 8, 30. [Google Scholar] [CrossRef]
- van Schaik, M.; Bredewold, O.W.; Priester, M.; Michels, W.M.; Rabelink, T.J.; Rotmans, J.I.; Teng, Y.K.O. Long-term renal and cardiovascular risks of tacrolimus in patients with lupus nephritis. Nephrol. Dial. Transpl. 2024, 39, 2048–2057. [Google Scholar] [CrossRef]
- Valantine, H. Cardiac allograft vasculopathy after heart transplantation: Risk factors and management. J. Heart Lung Transpl. 2004, 23, S187–S193. [Google Scholar] [CrossRef]
- Napoli, K.L.; Taylor, P.J. From Beach to Bedside: History of the Development of Sirolimus. Ther. Drug Monit. 2001, 23, 559–586. [Google Scholar] [CrossRef]
- Gosis, B.S.; Wada, S.; Thorsheim, C.; Li, K.; Jung, S.; Rhoades, J.H.; Yang, Y.; Brandimarto, J.; Li, L.; Uehara, K.; et al. Inhibition of nonalcoholic fatty liver disease in mice by selective inhibition of mTORC1. Science 2022, 376, eabf8271. [Google Scholar] [CrossRef]
- Sofi, M.H.; Tian, L.; Schutt, S.; Khan, I.; Choi, H.J.; Wu, Y.; Bastian, D.; Ticer, T.; Kassir, M.F.; Atilgan, F.C.; et al. Ceramide synthase 6 impacts T-cell allogeneic response and graft-versus-host disease through regulating N-RAS/ERK pathway. Leukemia 2022, 36, 1907–1915. [Google Scholar] [CrossRef]
- Cruickshanks, N.; Roberts, J.L.; Bareford, M.D.; Tavallai, M.; Poklepovic, A.; Booth, L.; Spiegel, S.; Dent, P. Differential regulation of autophagy and cell viability by ceramide species. Cancer Biol. Ther. 2015, 16, 733–742. [Google Scholar] [CrossRef]
- Mullen, T.D.; Hannun, Y.A.; Obeid, L.M. Ceramide synthases at the centre of sphingolipid metabolism and biology. Biochem. J. 2012, 441, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, J.I.; Inamori, K.I.; Kabayama, K.; Nagafuku, M.; Uemura, S.; Go, S.; Suzuki, A.; Ohno, I.; Kanoh, H.; Shishido, F. Biology of GM3 Ganglioside. Prog. Mol. Biol. Transl. Sci. 2018, 156, 151–195. [Google Scholar] [CrossRef] [PubMed]
- Mücke, V.T.; Gerharz, J.; Jakobi, K.; Thomas, D.; Ferreirós Bouzas, N.; Mücke, M.M.; Trötschler, S.; Weiler, N.; Welker, M.W.; Zeuzem, S.; et al. Low Serum Levels of (Dihydro-)Ceramides Reflect Liver Graft Dysfunction in a Real-World Cohort of Patients Post Liver Transplantation. Int. J. Mol. Sci. 2018, 19, 991. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Yi, Y.; Kang, Y.; Kim, S.J.; Yoon, Y.I.; Tran, P.H.; Kang, T.; Kim, M.K.; Han, J.; Tak, E.; et al. Reduced Ceramides Are Associated with Acute Rejection in Liver Transplant Patients and Skin Graft and Hepatocyte Transplant Mice, Reducing Tolerogenic Dendritic Cells. Mol. Cells 2023, 46, 688–699. [Google Scholar] [CrossRef]
- Burghelea, D.; Moisoiu, T.; Ivan, C.; Elec, A.; Munteanu, A.; Iancu, Ș.D.; Truta, A.; Kacso, T.P.; Antal, O.; Socaciu, C.; et al. The Use of Machine Learning Algorithms and the Mass Spectrometry Lipidomic Profile of Serum for the Evaluation of Tacrolimus Exposure and Toxicity in Kidney Transplant Recipients. Biomedicines 2022, 10, 1157. [Google Scholar] [CrossRef]
- Thomas, R.L., Jr.; Matsko, C.M.; Lotze, M.T.; Amoscato, A.A. Mass spectrometric identification of increased C16 ceramide levels during apoptosis. J. Biol. Chem. 1999, 274, 30580–30588. [Google Scholar] [CrossRef]
- Osawa, Y.; Uchinami, H.; Bielawski, J.; Schwabe, R.F.; Hannun, Y.A.; Brenner, D.A. Roles for C16-ceramide and sphingosine 1-phosphate in regulating hepatocyte apoptosis in response to tumor necrosis factor-alpha. J. Biol. Chem. 2005, 280, 27879–27887. [Google Scholar] [CrossRef] [PubMed]
- Mesicek, J.; Lee, H.; Feldman, T.; Jiang, X.; Skobeleva, A.; Berdyshev, E.V.; Haimovitz-Friedman, A.; Fuks, Z.; Kolesnick, R. Ceramide synthases 2, 5, and 6 confer distinct roles in radiation-induced apoptosis in HeLa cells. Cell. Signal. 2010, 22, 1300–1307. [Google Scholar]
- Karahatay, S.; Thomas, K.; Koybasi, S.; Senkal, C.E.; Elojeimy, S.; Liu, X.; Bielawski, J.; Day, T.A.; Gillespie, M.B.; Sinha, D.; et al. Clinical relevance of ceramide metabolism in the pathogenesis of human head and neck squamous cell carcinoma (HNSCC): Attenuation of C(18)-ceramide in HNSCC tumors correlates with lymphovascular invasion and nodal metastasis. Cancer Lett. 2007, 256, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Dany, M.; Ogretmen, B. Ceramide induced mitophagy and tumor suppression. Biochim. Biophys. Acta 2015, 1853, 2834–2845. [Google Scholar] [CrossRef]
- Tamura, A.; Li, X.K.; Funeshima, N.; Enosawa, S.; Amemiya, H.; Kitajima, M.; Suzuki, S. Immunosuppressive therapy using FTY720 combined with tacrolimus in rat liver transplantation. Surgery 2000, 127, 47–54. [Google Scholar] [CrossRef]
- Mathe, D.; Adam, R.; Malmendier, C.; Gigou, M.; Lontie, J.F.; Dubois, D.; Martin, C.; Bismuth, H.; Jacotot, B. Prevalence of dyslipidemia in liver transplant recipients. Transplantation 1992, 54, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Hüsing, A.; Kabar, I.; Schmidt, H.H. Lipids in liver transplant recipients. World J. Gastroenterol. 2016, 22, 3315–3324. [Google Scholar] [CrossRef]
- Morrisett, J.D.; Abdel-Fattah, G.; Hoogeveen, R.; Mitchell, E.; Ballantyne, C.M.; Pownall, H.J.; Opekun, A.R.; Jaffe, J.S.; Oppermann, S.; Kahan, B.D. Effects of sirolimus on plasma lipids, lipoprotein levels, and fatty acid metabolism in renal transplant patients. J. Lipid Res. 2002, 43, 1170–1180. [Google Scholar] [CrossRef]
- Savino, A.; Loglio, A.; Neri, F.; Camagni, S.; Pasulo, L.; Lucà, M.G.; Trevisan, R.; Fagiuoli, S.; Viganò, M. Metabolic-Dysfunction-Associated Steatotic Liver Disease (MASLD) after Liver Transplantation: A Narrative Review of an Emerging Issue. J. Clin. Med. 2024, 13, 3871. [Google Scholar] [CrossRef]
- Ling, Q.; Huang, H.; Han, Y.; Zhang, C.; Zhang, X.; Chen, K.; Wu, L.; Tang, R.; Zheng, Z.; Zheng, S.; et al. The tacrolimus-induced glucose homeostasis imbalance in terms of the liver: From bench to bedside. Am. J. Transpl. 2020, 20, 701–713. [Google Scholar] [CrossRef]
- Tory, R.; Sachs-Barrable, K.; Goshko, C.B.; Hill, J.S.; Wasan, K.M. Tacrolimus-induced elevation in plasma triglyceride concentrations after administration to renal transplant patients is partially due to a decrease in lipoprotein lipase activity and plasma concentrations. Transplantation 2009, 88, 62–68. [Google Scholar] [CrossRef]
- Trusch, F.; Loebach, L.; Wawra, S.; Durward, E.; Wuensch, A.; Iberahim, N.A.; de Bruijn, I.; MacKenzie, K.; Willems, A.; Toloczko, A.; et al. Cell entry of a host-targeting protein of oomycetes requires gp96. Nat. Commun. 2018, 9, 2347. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Peiffer, C.; Craig, D.L.; Biden, T.J. Ceramide generation is sufficient to account for the inhibition of the insulin-stimulated PKB pathway in C2C12 skeletal muscle cells pretreated with palmitate. J. Biol. Chem. 1999, 274, 24202–24210. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Echtermeyer, F.; Alozie, A.; Brands, K.; Buddecke, E. Plasmin- and thrombin-accelerated shedding of syndecan-4 ectodomain generates cleavage sites at Lys(114)-Arg(115) and Lys(129)-Val(130) bonds. J. Biol. Chem. 2005, 280, 34441–34446. [Google Scholar] [CrossRef]
- Hannun, Y.A.; Obeid, L.M. Principles of bioactive lipid signalling: Lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 2008, 9, 139–150. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, M.; Xiong, X.; Du, Y.; Li, D.; Wang, Z.; Ge, W.; Zhu, Y. Plasma metabolomic profiling reveals factors associated with dose-adjusted trough concentration of tacrolimus in liver transplant recipients. Front. Pharmacol. 2022, 13, 1045843. [Google Scholar] [CrossRef]
- Richter, N.; Raddatz, G.; Graeter, T.; Schäfers, H.J.; Schlitt, H.J. Allogeneic lymphocyte chimerism after clinical lung transplantation. Transpl. Immunol. 1995, 3, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Schlitt, H.J.; Kanehiro, H.; Raddatz, G.; Steinhoff, G.; Richter, N.; Nashan, B.; Ringe, B.; Wonigeit, K.; Pichlmayr, R. Persistence of donor lymphocytes in liver allograft recipients. Transplantation 1993, 56, 1001–1007. [Google Scholar] [CrossRef]
| Characteristic | N | SIR, N = 44 1 | TAC, N = 84 1 | p-Value 2 |
|---|---|---|---|---|
| RECIPIENT | ||||
| Age at LT (years) | 128 | 57.50 (53.00, 62.00) | 52.00 (47.00, 59.25) | 0.004 |
| Sex | 128 | 0.97 | ||
| Female | 14 (32%) | 27 (32%) | ||
| Laboratory readings | ||||
| ALT (U/L) | 128 | 60.00 (30.50, 198.00) | 94.00 (39.00, 457.75) | 0.24 |
| AST (U/L) | 128 | 81.50 (53.00, 381.00) | 136.50 (63.50, 646.75) | 0.23 |
| ALP (U/L) | 128 | 115.50 (93.00, 163.50) | 128.50 (88.50, 181.75) | 0.54 |
| Creatinine (mg/dL) | 126 | 1.40 (1.00, 1.83) | 1.23 (0.80, 1.70) | 0.24 |
| MELD at transplant | 125 | 21.50 (13.00, 29.25) | 23.00 (17.00, 28.00) | 0.44 |
| MELD Na | 125 | 24.10 (14.80, 29.95) | 24.40 (18.50, 30.70) | 0.49 |
| Primary diagnosis | 125 | 0.12 | ||
| Acute Hepatic Necrosis | 0 (0%) | 6 (7.3%) | ||
| Alcohol-related | 13 (30%) | 25 (30%) | ||
| Autoimmune | 2 (4.7%) | 2 (2.4%) | ||
| Biliary | 1 (2.3%) | 10 (12%) | ||
| Cryptogenic | 5 (12%) | 11 (13%) | ||
| Other | 3 (7.0%) | 7 (8.5%) | ||
| Viral Hepatitis | 19 (44%) | 21 (26%) | ||
| Past Medical History | ||||
| Hypertension | 128 | 16 (36%) | 27 (32%) | 0.63 |
| BMI | 121 | 27.49 (25.41, 31.30) | 28.64 (25.32, 32.93) | 0.70 |
| Obesity | 121 | 0.50 | ||
| BMI <= 30 | 29 (66%) | 46 (60%) | ||
| BMI > 30 | 15 (34%) | 31 (40%) | ||
| DONOR | ||||
| Age (years) | 128 | 49.00 (31.25, 60.00) | 43.00 (29.75, 56.00) | 0.27 |
| Sex | 128 | 0.95 | ||
| Female | 17 (39%) | 32 (38%) | ||
| Male | 27 (61%) | 52 (62%) | ||
| Past Medical History | ||||
| Diabetes mellitus–insulin dependent | 128 | 2 (4.5%) | 3 (3.6%) | >0.99 |
| Diabetes mellitus— Non-insulin dependent | 128 | 4 (9.1%) | 8 (9.5%) | >0.99 |
| Hypertension | 128 | 15 (34%) | 36 (43%) | 0.34 |
| Pathway | Total | Hits | FDR |
|---|---|---|---|
| Glycerophospholipid metabolism | 97 | 46 | 1.12 × 10−70 |
| Ether lipid metabolism | 47 | 34 | 8.65 × 10−60 |
| Sphingolipid metabolism | 47 | 30 | 1.08 × 10−49 |
| alpha-Linolenic acid metabolism | 25 | 21 | 2.08 × 10−38 |
| Linoleic acid metabolism | 29 | 21 | 5.51 × 10−36 |
| EGFR tyrosine kinase inhibitor resistance | 1490 | 65 | 6.78 × 10−35 |
| Arachidonic acid metabolism | 63 | 21 | 2.02 × 10−26 |
| Alzheimer disease | 41 | 14 | 1.91 × 10−17 |
| Axon guidance | 132 | 19 | 2.78 × 10−16 |
| p53 signaling pathway | 119 | 18 | 8.22 × 10−16 |
| Module | FA | Pathway | Hits (Range)/Total | FDR (Range) |
|---|---|---|---|---|
| 0 | GO:BP | cellular biogenic amine metabolic process | 28/167 | 8.29 × 10−43 |
| glycerophospholipid metabolic process | 32/327 | 8.29 × 10−43 | ||
| phospholipid metabolic process | 34/463 | 3.36 × 10−42 | ||
| glycerophospholipid biosynthetic process | 30/255 | 3.36 × 10−42 | ||
| phospholipid biosynthetic process | 30/285 | 8.98 × 10−41 | ||
| 1 | GO:BP | sphingolipid metabolic process | 25/173 | 2.19 × 10−41 |
| membrane lipid metabolic process | 25/233 | 2.98 × 10−38 | ||
| ceramide metabolic process | 20/77 | 2.28 × 10−37 | ||
| phospholipid metabolic process | 27/463 | 2.2 × 10−35 | ||
| sphingolipid biosynthetic process | 19/80 | 1.39 × 10−34 | ||
| 0 | GO:MF | phospholipase activity | 29/112 | 2.61 × 10−52 |
| lipase activity | 29/136 | 7.65 × 10−50 | ||
| phospholipase A2 activity | 21/32 | 2.24 × 10−47 | ||
| hydrolase activity, acting on ester bonds | 29/1010 | 5.71 × 10−24 | ||
| calcium ion binding | 16/673 | 4.28 × 10−10 | ||
| 1 | GO:MF | phosphoric ester hydrolase activity | 13/496 | 2.09 × 10−9 |
| hydrolase activity, acting on ester bonds | 13/1010 | 5.21 × 10−6 | ||
| N-acyltransferase activity | 6/97 | 5.21 × 10−6 | ||
| phosphatase activity | 8/373 | 6.21 × 10−5 | ||
| transferase activity, transferring acyl groups other than amino-acyl groups | 6/193 | 1.83 × 10−3 | ||
| 0, 1 | GO:CC | endoplasmic reticulum membrane | (13–14)/872 | (1.6–7.1) × 10−7 |
| nuclear outer membrane–endoplasmic reticulum membrane network | (13–14)/894 | (1.6–7.1) × 10−7 | ||
| endoplasmic reticulum part | (13–14)/1060 | 7.93 × 10−7–2.89 × 10−6 | ||
| endomembrane system | 17/2160 | 9.65 × 10−7–1 × 10−4 | ||
| endoplasmic reticulum | (1417)/1660 | (1.2–2.9) × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baciu, C.; Hasjim, B.J.; Maleki, S.; Pasini, E.; Patel, M.K.; Shojaee, M.; Azhie, A.; Saracino, G.; Asrani, S.K.; Bhat, M. Metabolomics Analysis Uncovers Distinct Profiles of Liver Post-Transplant Patients by Immunosuppression Regimen. Metabolites 2025, 15, 700. https://doi.org/10.3390/metabo15110700
Baciu C, Hasjim BJ, Maleki S, Pasini E, Patel MK, Shojaee M, Azhie A, Saracino G, Asrani SK, Bhat M. Metabolomics Analysis Uncovers Distinct Profiles of Liver Post-Transplant Patients by Immunosuppression Regimen. Metabolites. 2025; 15(11):700. https://doi.org/10.3390/metabo15110700
Chicago/Turabian StyleBaciu, Cristina, Bima J. Hasjim, Saba Maleki, Elisa Pasini, Meera Kennedybhai Patel, Maryam Shojaee, Amirhossein Azhie, Giovanna Saracino, Sumeet K. Asrani, and Mamatha Bhat. 2025. "Metabolomics Analysis Uncovers Distinct Profiles of Liver Post-Transplant Patients by Immunosuppression Regimen" Metabolites 15, no. 11: 700. https://doi.org/10.3390/metabo15110700
APA StyleBaciu, C., Hasjim, B. J., Maleki, S., Pasini, E., Patel, M. K., Shojaee, M., Azhie, A., Saracino, G., Asrani, S. K., & Bhat, M. (2025). Metabolomics Analysis Uncovers Distinct Profiles of Liver Post-Transplant Patients by Immunosuppression Regimen. Metabolites, 15(11), 700. https://doi.org/10.3390/metabo15110700







