Biomarkers Characterizing the Onset of Dietary-Induced Hepatocellular Injury and Visceral Obesity in a Rat Experimental Model: Possible Anti-Inflammatory Effects of Steviol Glycosides
Abstract
1. Introduction
2. Materials and Methods
3. Results
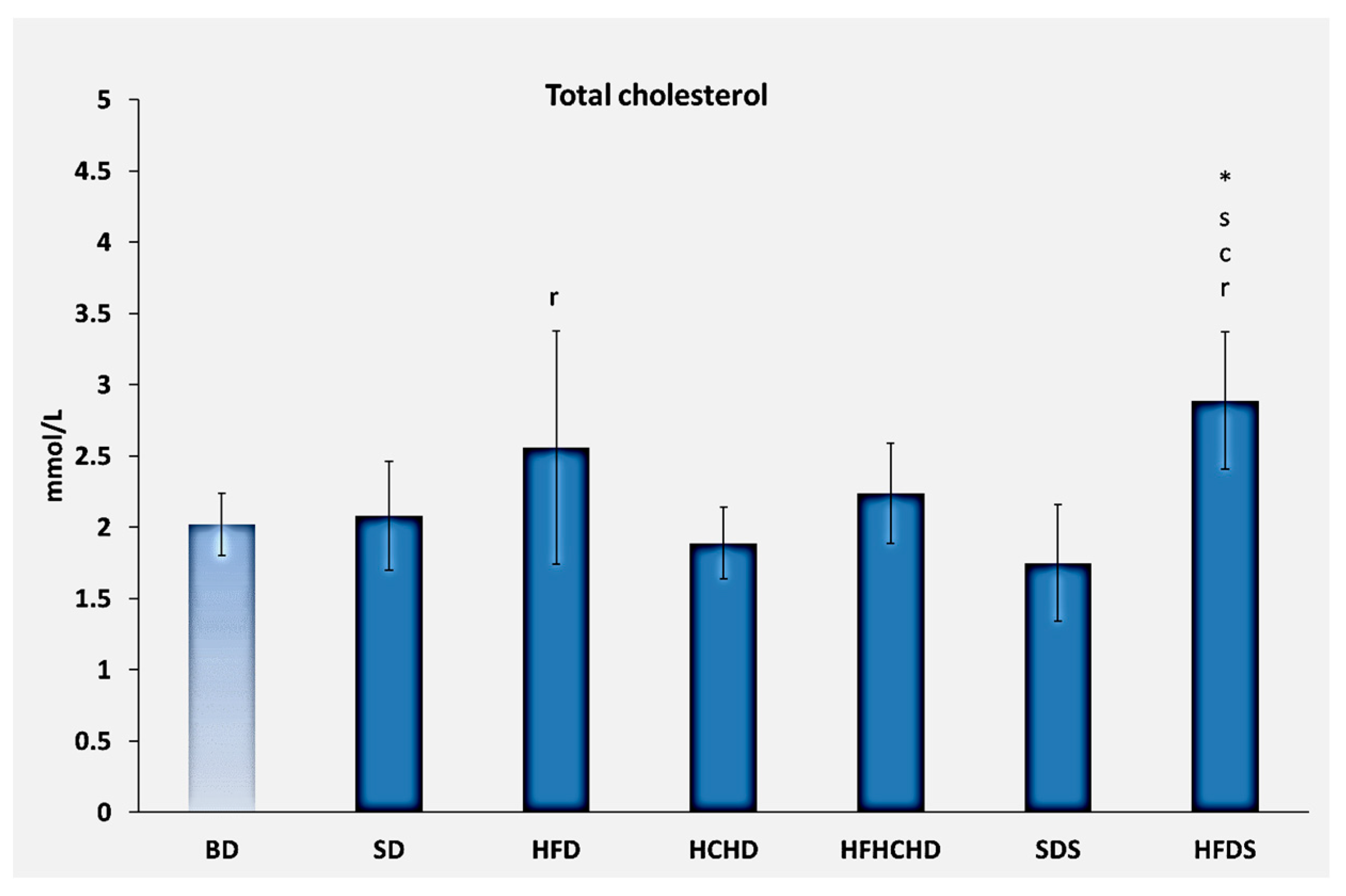
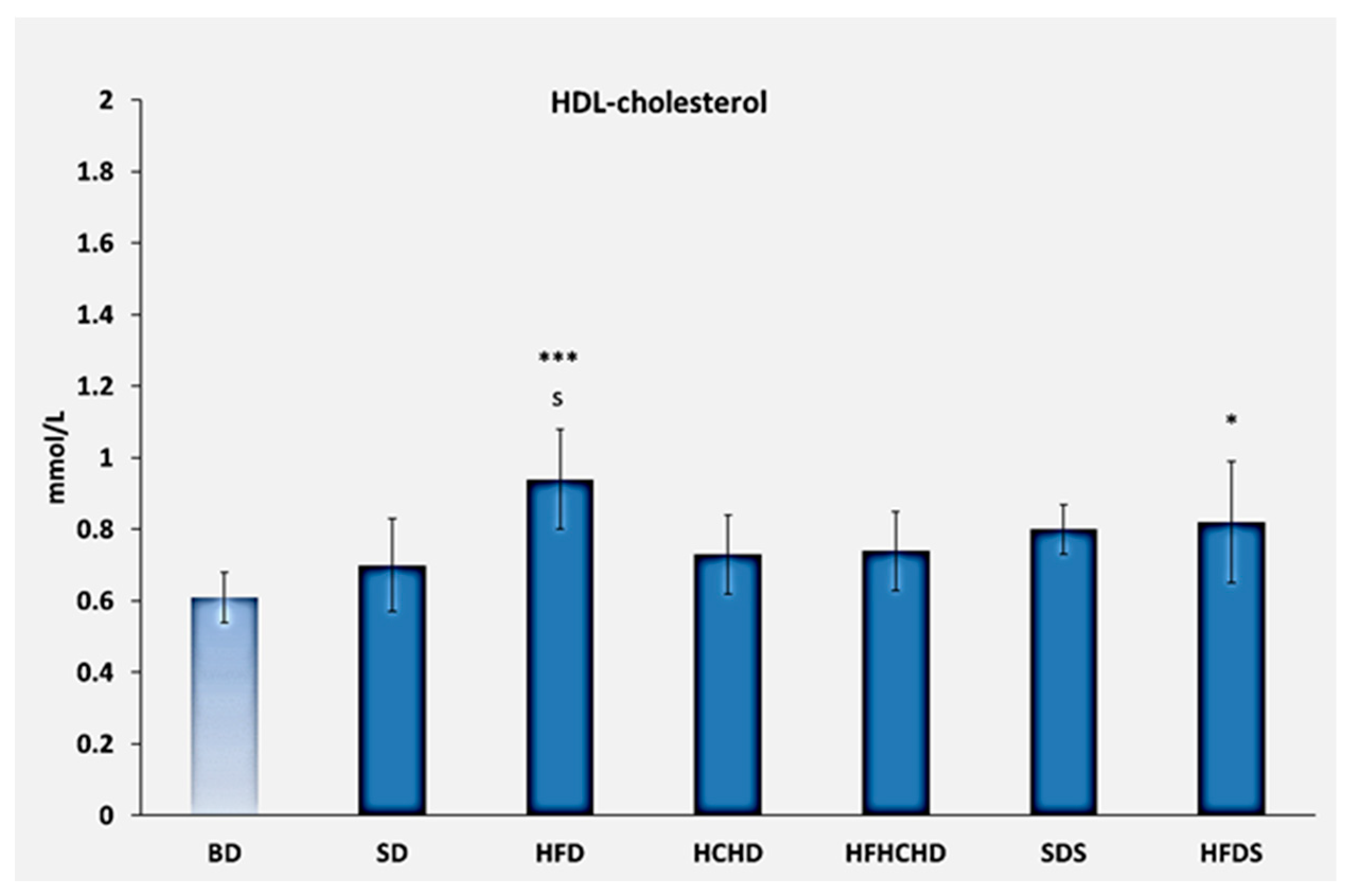
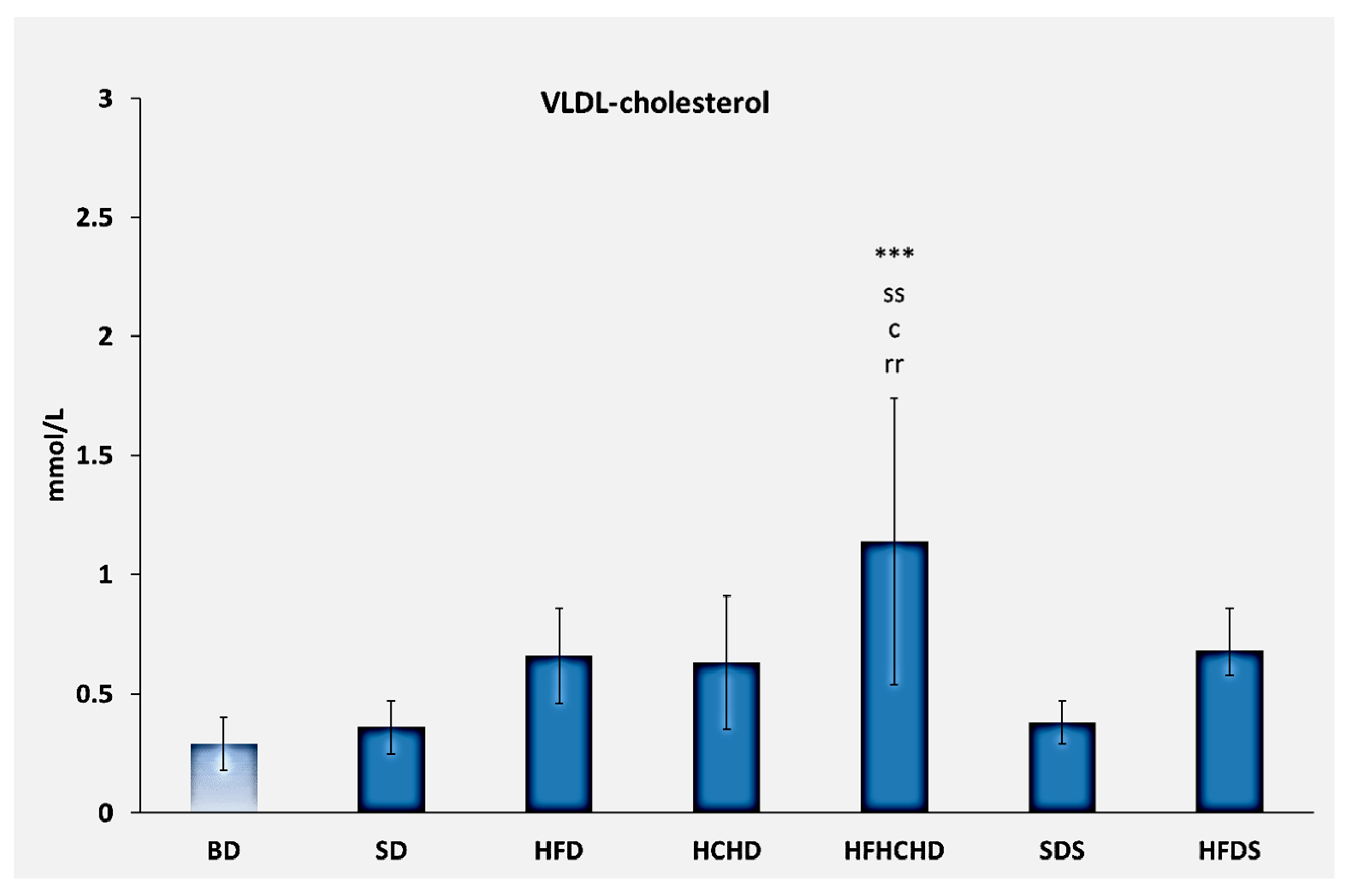
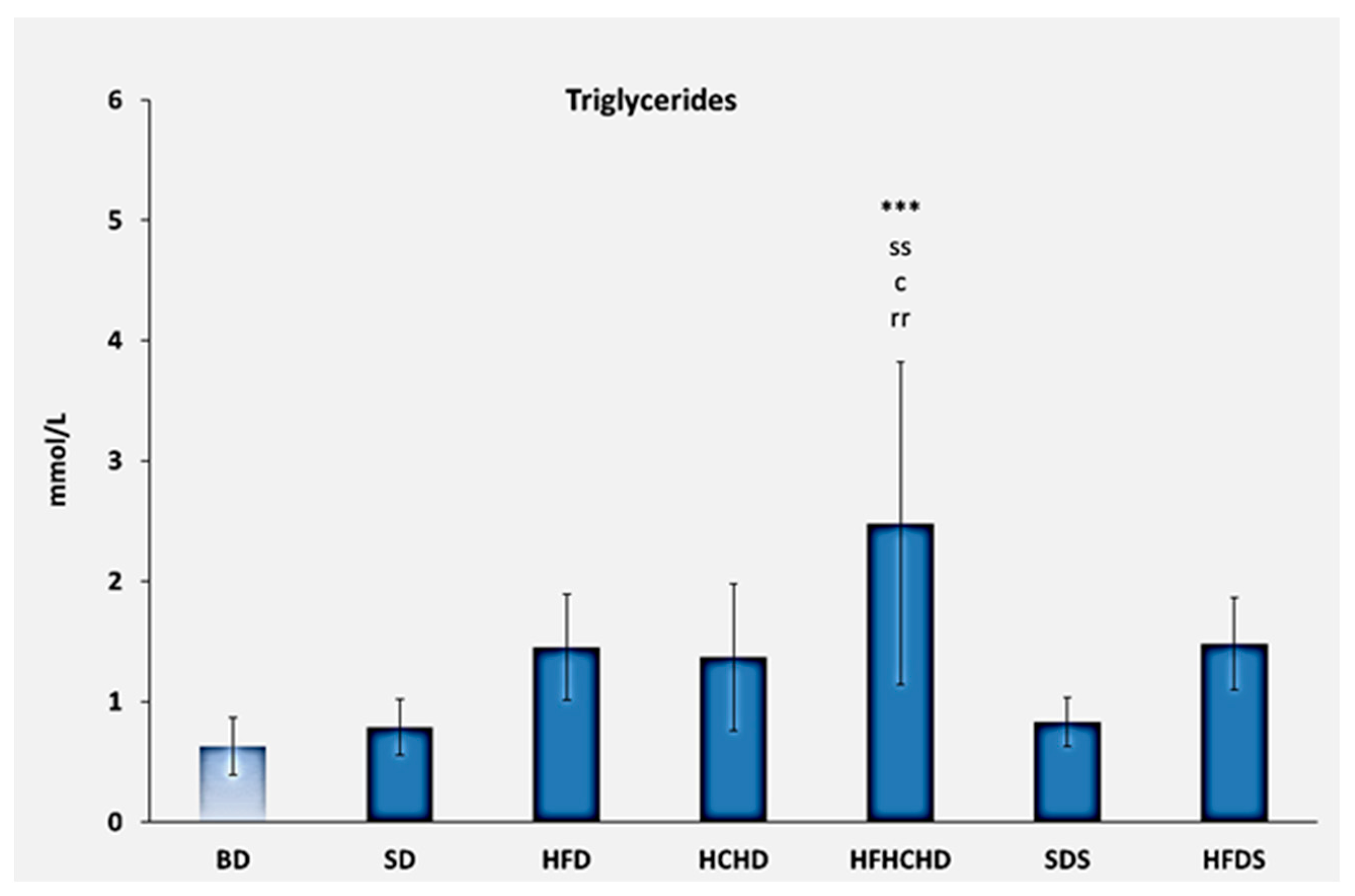
3.1. Lipid Profile Parameters
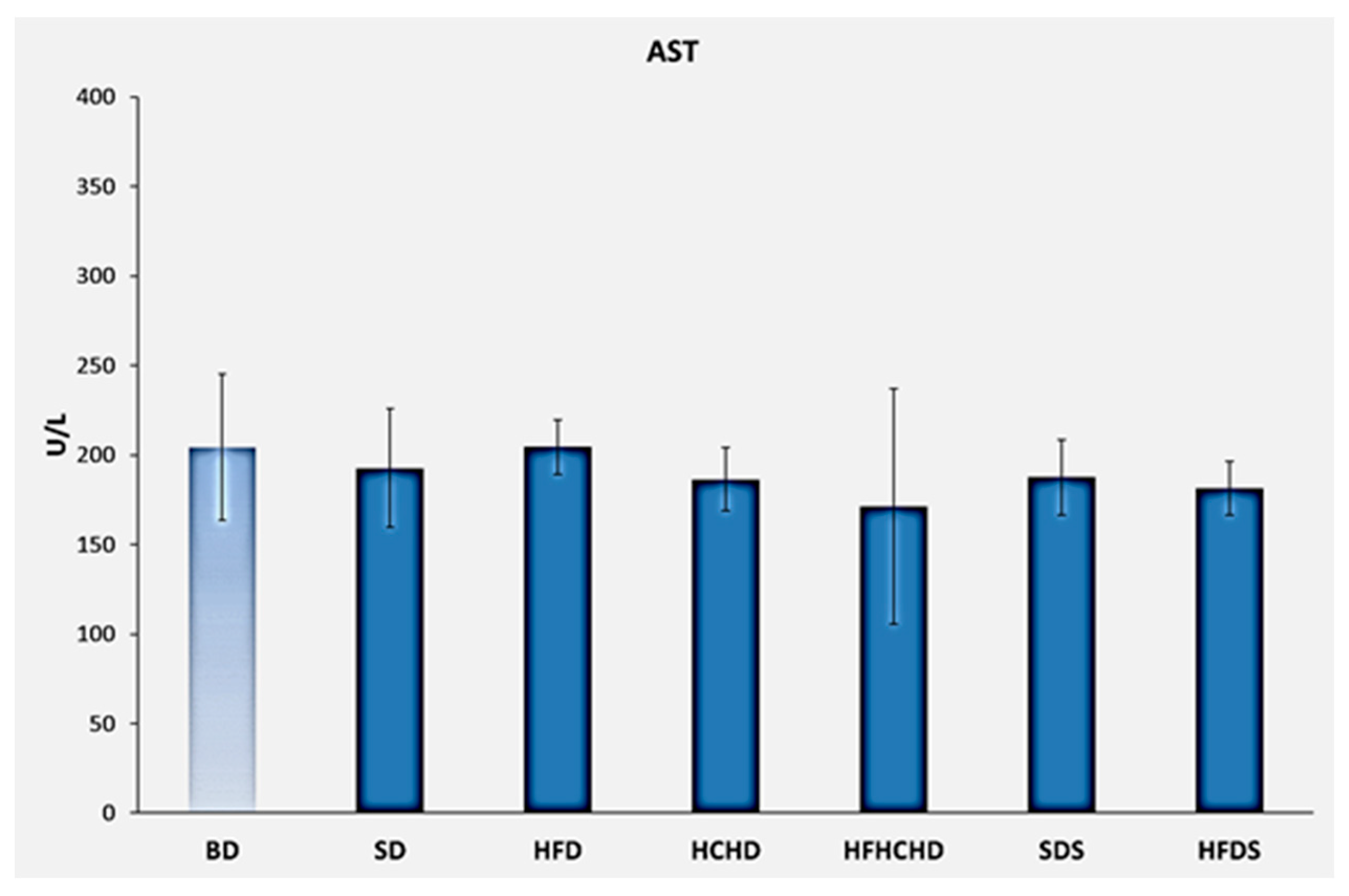
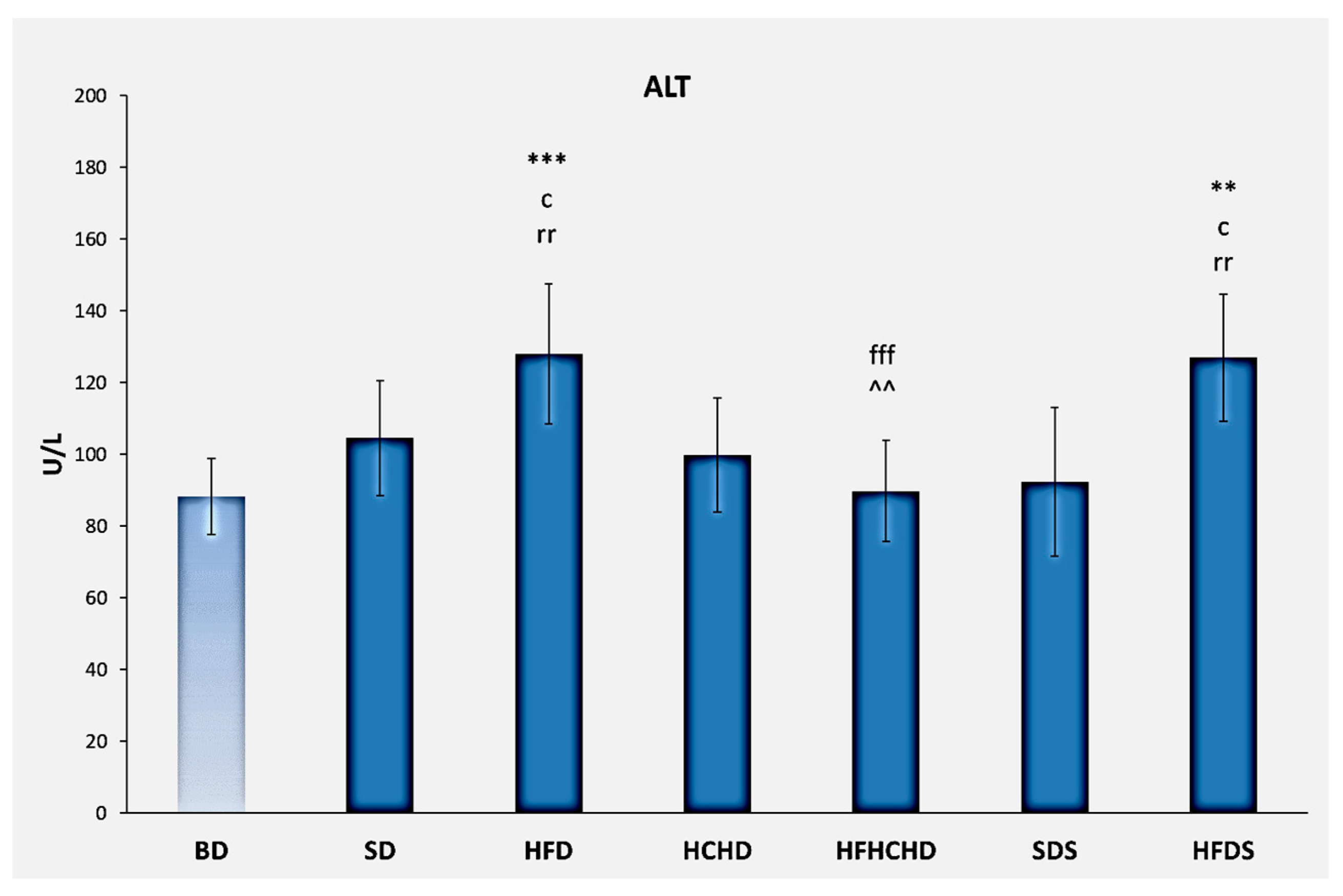
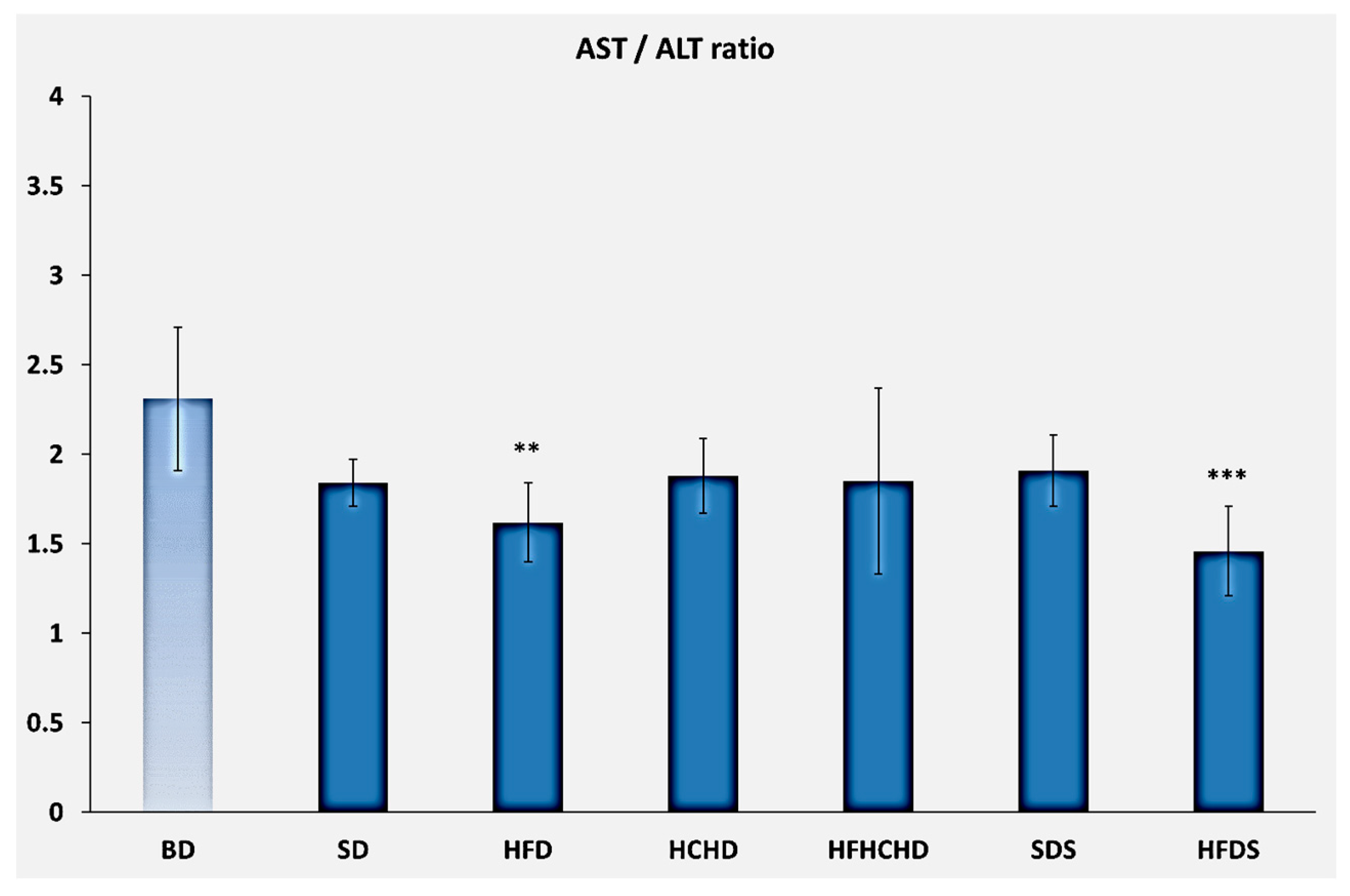
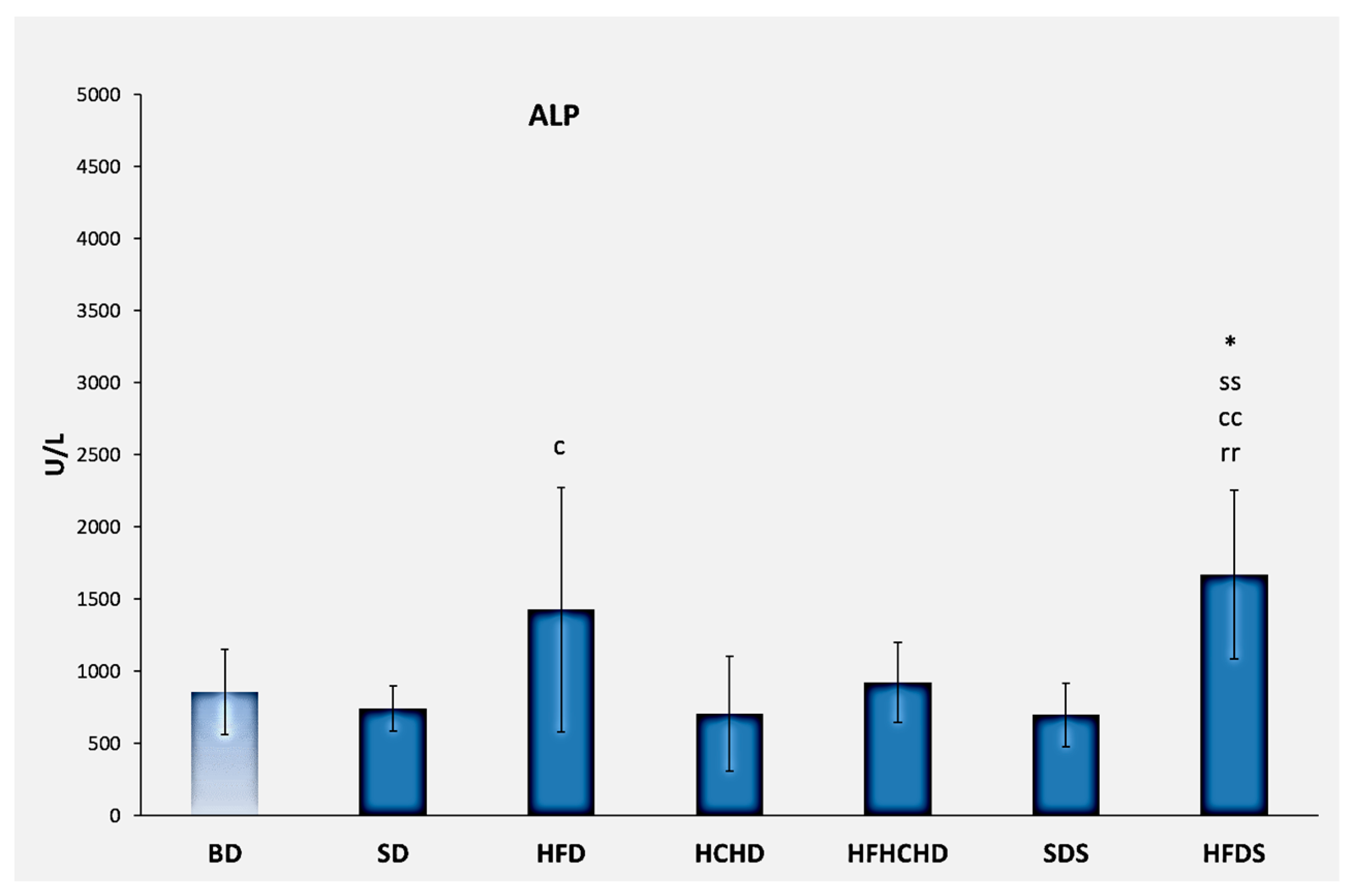
3.2. Liver Enzymes
3.3. Total Bilirubin
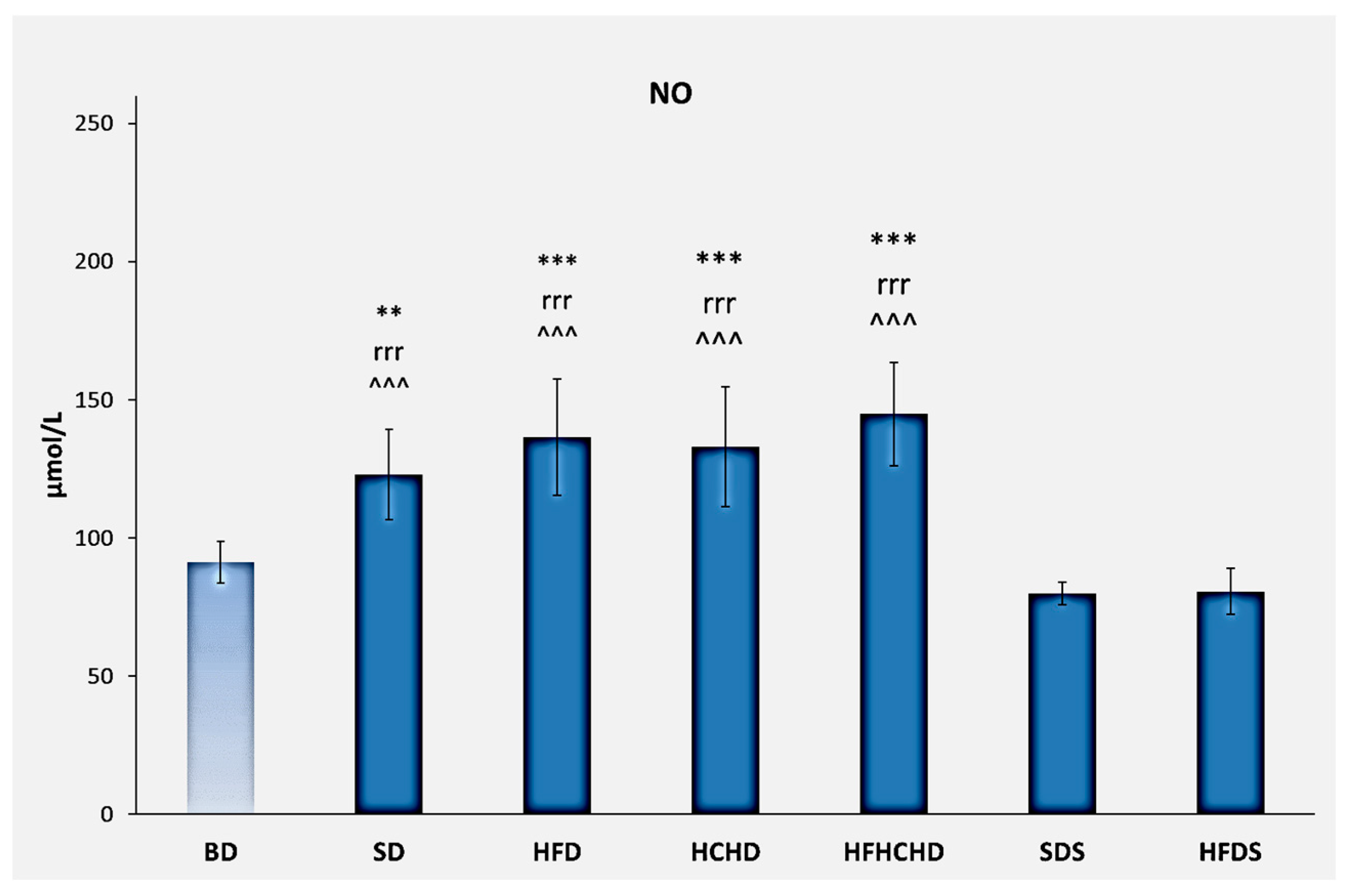
3.4. Nitric Oxide
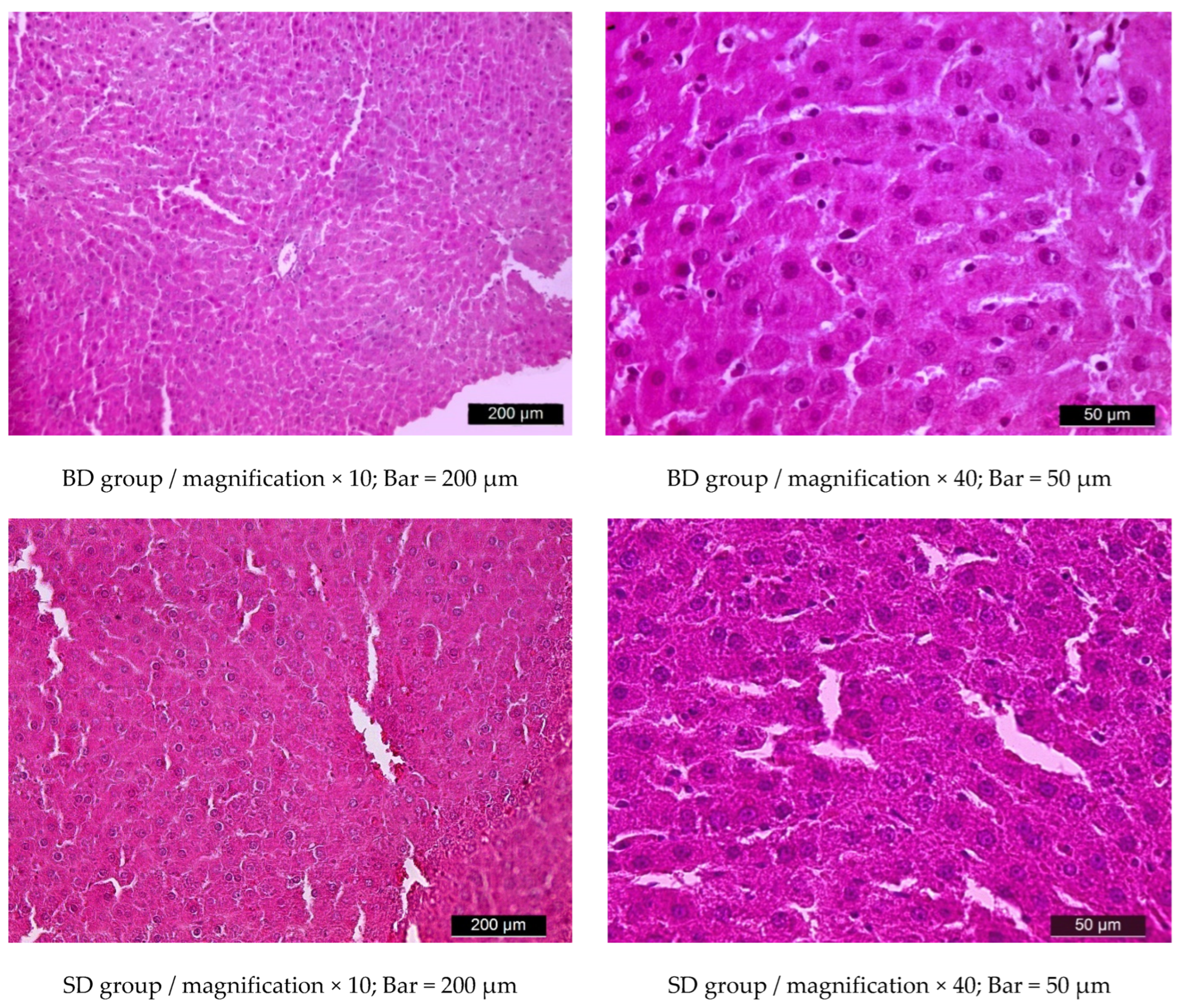
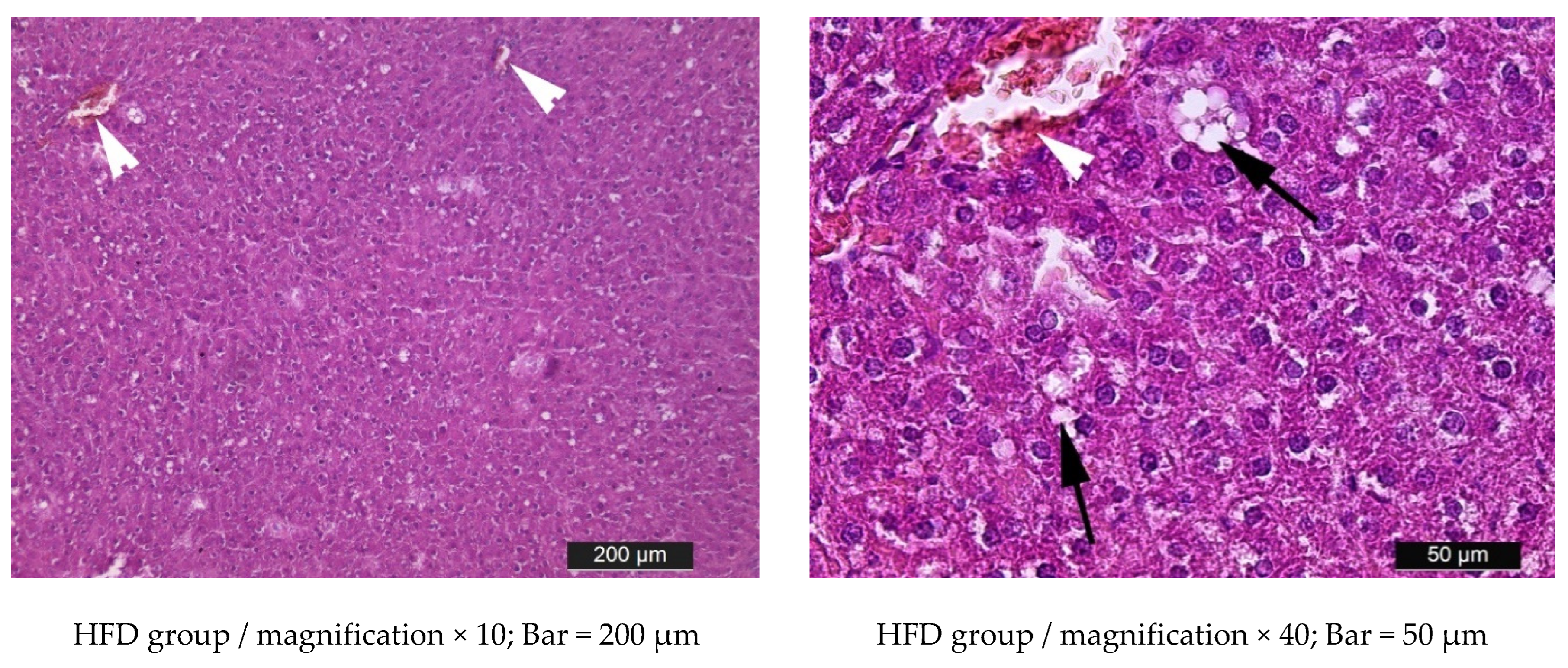
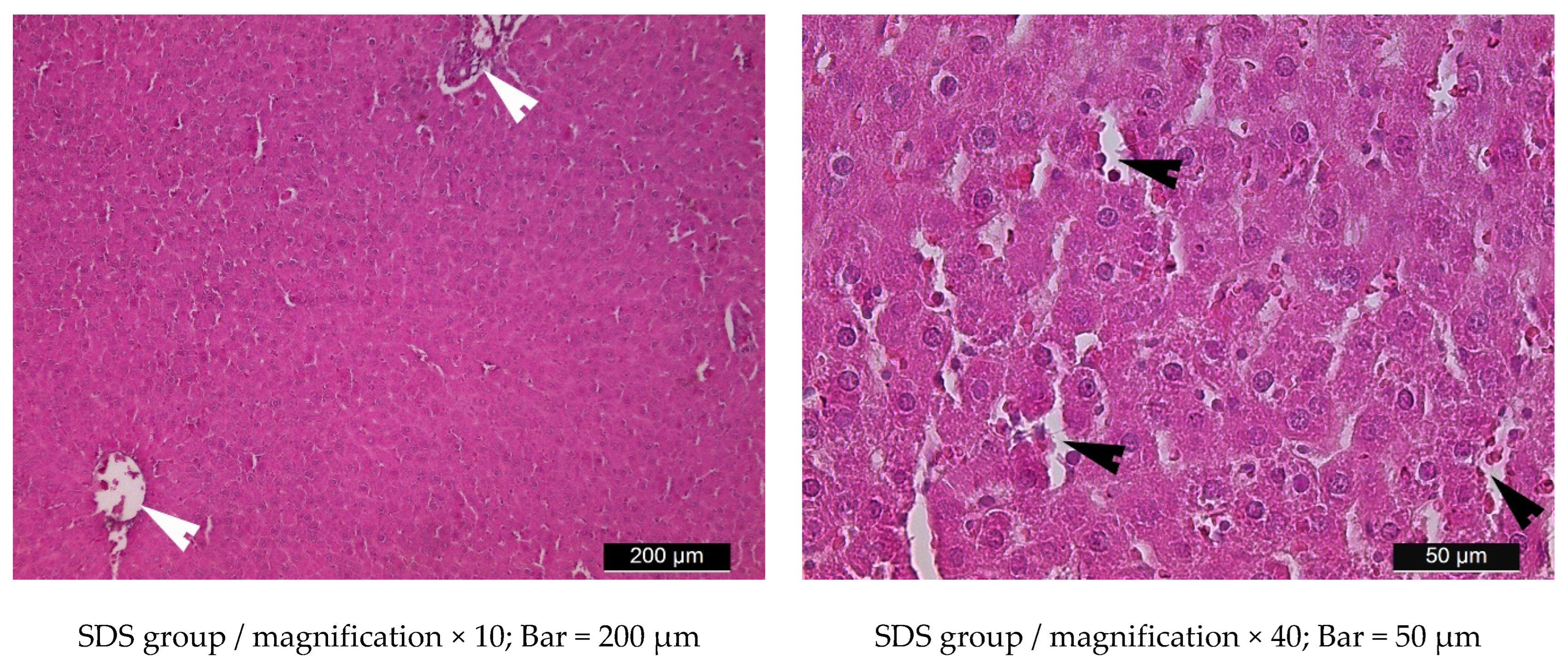
3.5. Liver Histology
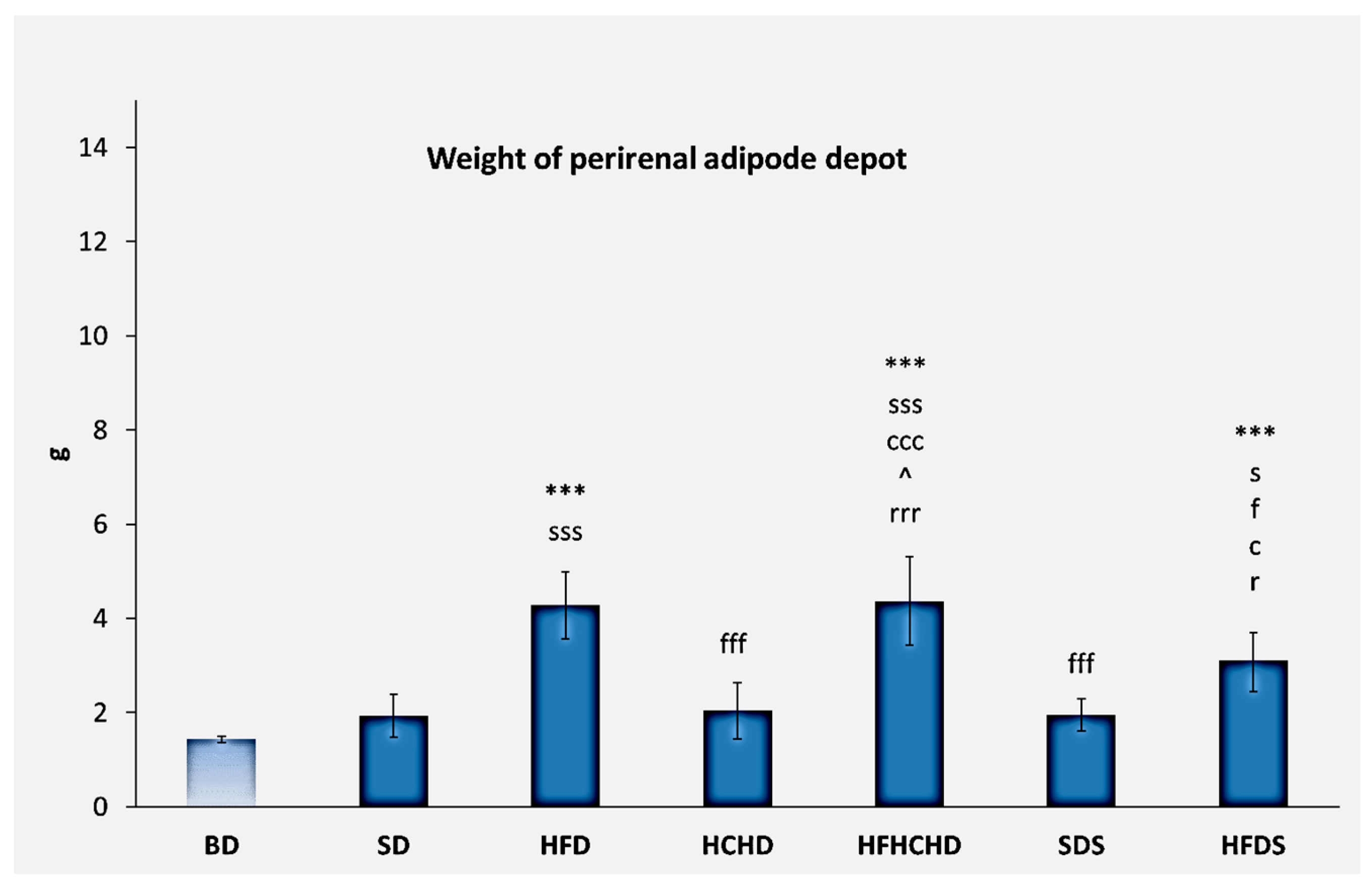
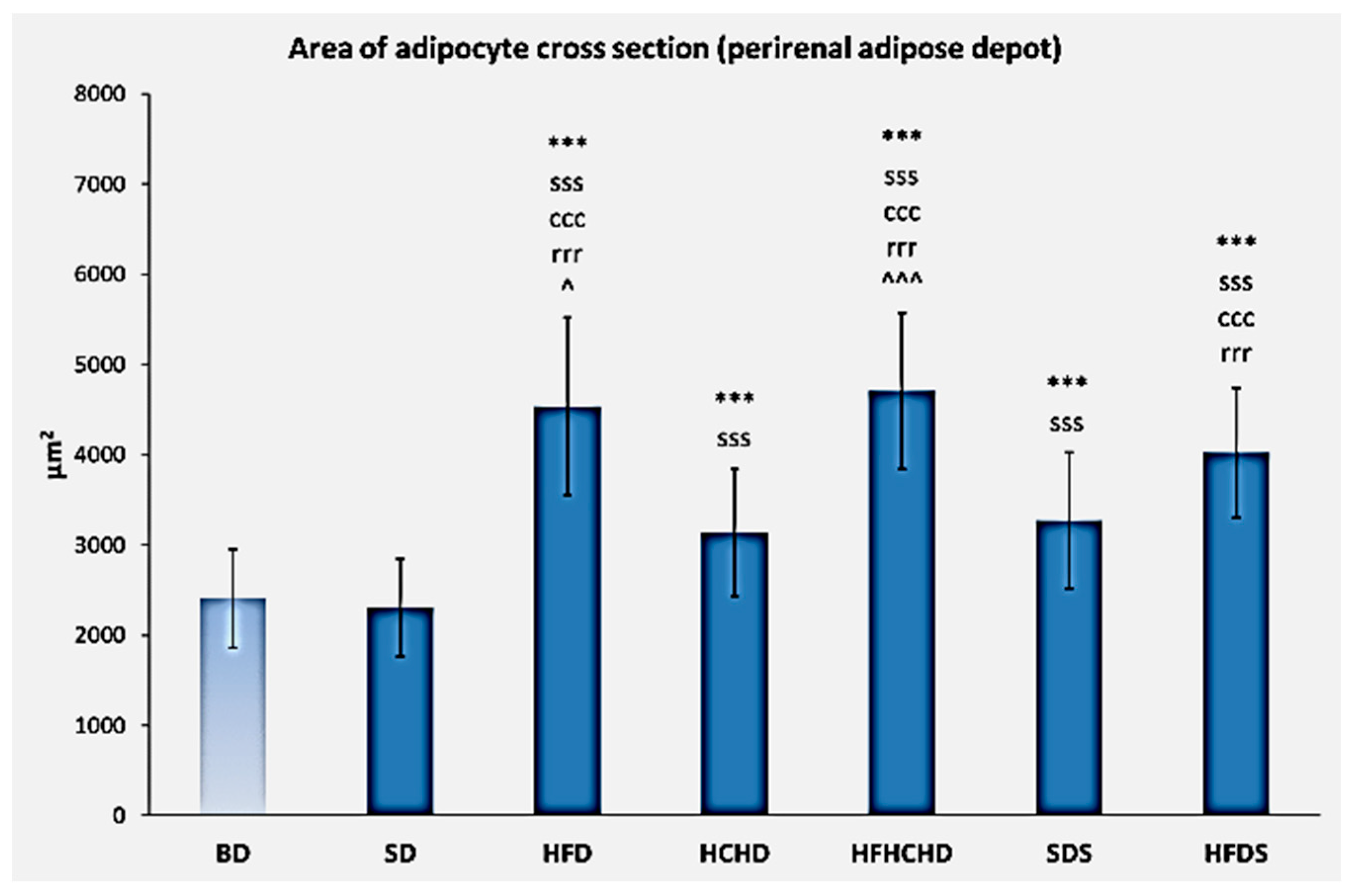
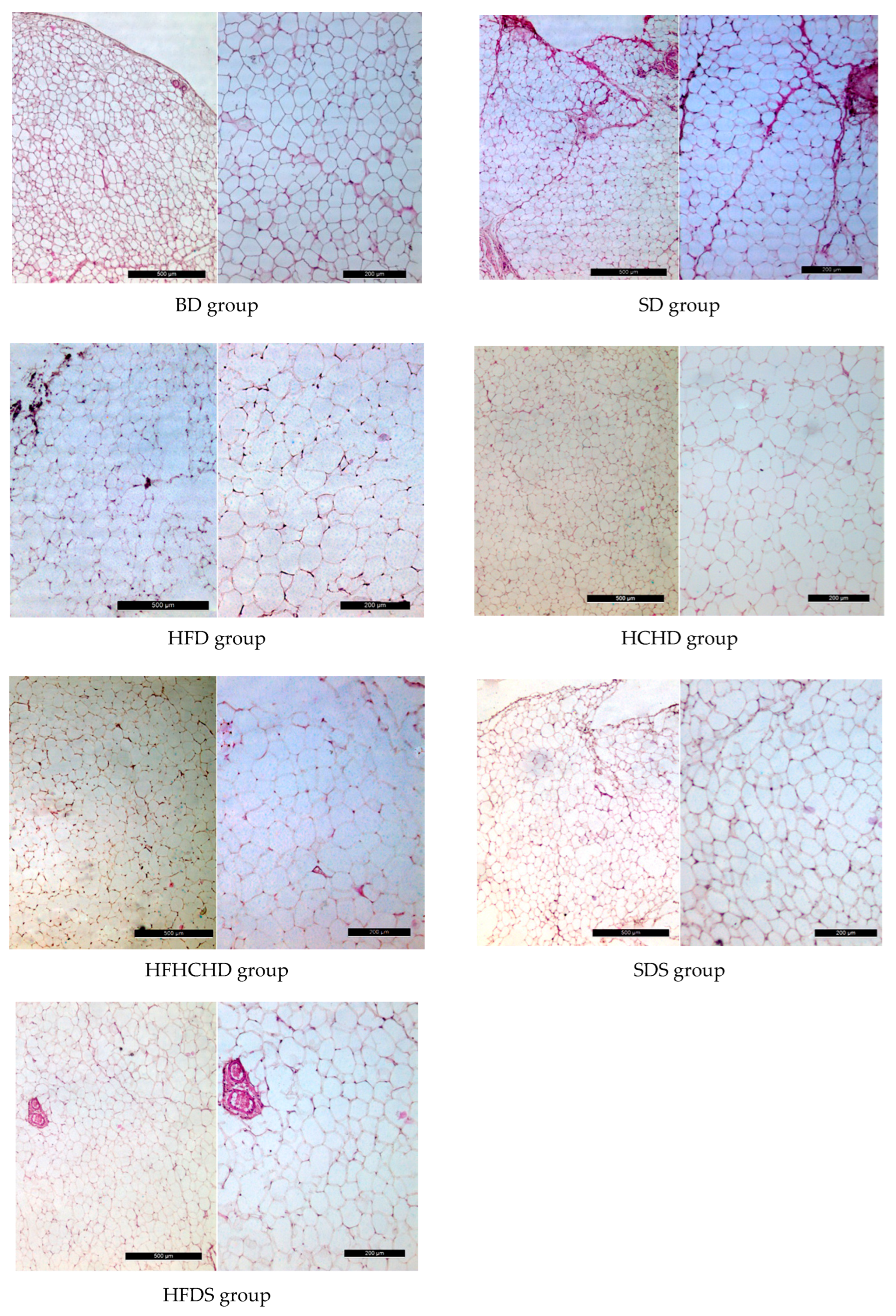
3.6. Perirenal Adipose Depot Parameters and Histology
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ezhilarasan, D. Molecular mechanisms in thioacetamide-induced acute and chronic liver injury models. Environ. Toxicol. Pharmacol. 2023, 99, 104093. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Parikh, N.D. Diagnosis and management of cirrhosis and its complications: A review. JAMA 2023, 329, 1589–1602. [Google Scholar] [CrossRef]
- Li, M.; Chi, X.; Wang, Y.; Setrerrahmane, S.; Xie, W.; Xu, H. Trends in insulin resistance: Insights into mechanisms and therapeutic strategy. Signal Transduct. Target. Ther. 2022, 7, 216. [Google Scholar] [CrossRef]
- Wilcox, G. Insulin and insulin resistance. Clin. Biochem. Rev. 2005, 26, 19. [Google Scholar]
- Mu, W.; Cheng, X.F.; Liu, Y.; Lv, Q.Z.; Liu, G.L.; Zhang, J.G.; Li, X.Y. Potential nexus of non-alcoholic fatty liver disease and type 2 diabetes mellitus: Insulin resistance between hepatic and peripheral tissues. Front. Pharmacol. 2019, 9, 1566. [Google Scholar] [CrossRef]
- Pansuria, M.; Xi, H.; Li, L.; Yang, X.F.; Wang, H. Insulin resistance, metabolic stress, and atherosclerosis. Front. Biosci. (Schol. Ed.). 2012, 4, 916. [Google Scholar]
- Mao, J.; Tan, L.; Tian, C.; Wang, W.; Zhang, H.; Zhu, Z.; Li, Y. Research progress on rodent models and its mechanisms of liver injury. Life Sci. 2024, 337, 122343. [Google Scholar] [CrossRef]
- Musana, K.A.; Yale, S.H.; Abdulkarim, A.S. Tests of liver injury. Clin. Med. Res. 2004, 2, 129–131. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Du, Y.; Zhang, W.; Qiu, H.; Xiao, C.; Shi, J.; Reid, L.M.; He, Z. Mouse models of liver parenchyma injuries and regeneration. Front. Cell Dev. Biol. 2022, 10, 903740. [Google Scholar] [CrossRef]
- Nevzorova, Y.A.; Boyer-Diaz, Z.; Cubero, F.J.; Gracia-Sancho, J. Animal models for liver disease–a practical approach for translational research. J. Hepatol. 2020, 73, 423–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Shen, H.; Seo, W.; Hwang, S. Experimental models of fatty liver diseases: Status and appraisal. Hepatol. Commun. 2023, 7, e00200. [Google Scholar] [CrossRef]
- Van Herck, M.A.; Vonghia, L.; Francque, S.M. Animal models of nonalcoholic fatty liver disease—A starter’s guide. Nutrients 2017, 9, 1072. [Google Scholar] [CrossRef]
- Singh, R.; Gholipourmalekabadi, M.; Shafikhani, S.H. Animal models for type 1 and type 2 diabetes: Advantages and limitations. Front. Endocrinol. 2024, 15, 1359685. [Google Scholar] [CrossRef]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and nutritional overview of diet-induced metabolic syndrome models in rats: What is the best choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Sousa, R.M.L.; Ribeiro, N.L.X.; Pinto, B.A.S.; Sanches, J.R.; da Silva, M.U.; Coêlho, C.F.F.; França, L.M.; de Figueiredo Neto, J.A.; Paes, A.M.D.A. Long-term high-protein diet intake reverts weight gain and attenuates metabolic dysfunction on high-sucrose-fed adult rats. Nutr. Metab. 2018, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Ciapaite, J.; van den Broek, N.M.; Te Brinke, H.; Nicolay, K.; Jeneson, J.A.; Houten, S.M.; Prompers, J.J. Differential effects of short-and long-term high-fat diet feeding on hepatic fatty acid metabolism in rats. Biochim. Biophys. Acta—Mol. Cell Biol. Lipids 2011, 1811, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Panchal, S.K.; Poudyal, H.; Iyer, A.; Nazer, R.; Alam, A.; Diwan, V.; Kauter, K.; Sernia, C.; Campbell, F.; Ward, L.; et al. High-carbohydrate, high-fat diet–induced metabolic syndrome and cardiovascular remodeling in rats. J. Cardiovasc. Pharmacol. 2011, 57, 611–624. [Google Scholar] [CrossRef]
- Raman, B.V.; Krishna, N.V.; Rao, N.B.; Saradhi, P.M.; Rao, B.M.V. Plants with antidiabetic activities and their medicinal values. Int. Res. J. Pharm. 2012, 3, 11–15. [Google Scholar]
- Mejia, E.; Pearlman, M. Natural alternative sweeteners and diabetes management. Curr Diab Rep. 2019, 19, 142. [Google Scholar] [CrossRef]
- Ray, J.; Kumar, S.; Laor, D.; Shereen, N.; Nwamaghinna, F.; Thomson, A.; Perez, J.; Soni, L.; McFarlane, S.I. Effects of Stevia rebaudiana on glucose homeostasis, blood pressure and inflammation: A critical review of past and current research evidence. Int. J. Clin. Res. Trials. 2020, 5, 142. [Google Scholar] [CrossRef]
- Miranda, K.M.; Espey, M.G.; Wink, D.A. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 2001, 5, 62–71. [Google Scholar] [CrossRef]
- Jia, Y.J.; Liu, J.; Guo, Y.L.; Xu, R.X.; Sun, J.; Li, J.J. Dyslipidemia in rat fed with high-fat diet is not associated with PCSK9-LDL-receptor pathway but ageing. J. Geriatr. Cardiol. 2013, 10, 361. [Google Scholar]
- Sour, S.; Belarbi, M.; Sari, N.; Benammar, C.H.; Baghdad, C.H.; Visioli, F. Argan oil reduces, in rats, the high fat diet-induced metabolic effects of obesity. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Rašković, A.; Martić, N.; Tomas, A.; Andrejić-Višnjić, B.; Bosanac, M.; Atanasković, M.; Nemet, M.; Popović, R.; Krstić, M.; Vukmirović, S.; et al. Carob extract (Ceratonia siliqua L.): Effects on dyslipidemia and obesity in a high-fat diet-fed rat model. Pharmaceutics 2023, 15, 2611. [Google Scholar] [CrossRef]
- Bailey, A.; Mohiuddin, S.S. Biochemistry, High Density Lipoprotein; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK549802/ (accessed on 15 July 2025).
- Rousset, X.; Vaisman, B.; Amar, M.; Sethi, A.A.; Remaley, A.T. Lecithin: Cholesterol acyltransferase: From biochemistry to role in cardiovascular disease. Curr. Opin. Endocrinol. Diabetes Obes. 2009, 16, 163. [Google Scholar] [CrossRef] [PubMed]
- Rosenson, R.S.; Brewer Jr, H.B.; Ansell, B.J.; Barter, P.; Chapman, M.J.; Heinecke, J.W.; Kontush, A.; Tall, A.R.; Webb, N.R. Dysfunctional HDL and atherosclerotic cardiovascular disease. Nat. Rev. Cardiol. 2016, 13, 48–60. [Google Scholar] [CrossRef] [PubMed]
- Morgantini, C.; Trifirò, S.; Tricò, D.; Meriwether, D.; Baldi, S.; Mengozzi, A.; Reddy, S.T.; Natali, A. A short-term increase in dietary cholesterol and fat intake affects high-density lipoprotein composition in healthy subjects. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 575–581. [Google Scholar] [CrossRef]
- Ahmad, U.; Ahmad, R.S.; Arshad, M.S.; Mushtaq, Z.; Hussain, S.M.; Hameed, A. Antihyperlipidemic efficacy of aqueous extract of Stevia rebaudiana Bertoni in albino rats. Lipids Health Dis. 2018, 17, 175. [Google Scholar] [CrossRef]
- Yin, Y.; Yu, Z.; Xia, M.; Luo, X.; Lu, X.; Ling, W. Vitamin D attenuates high fat diet–induced hepatic steatosis in rats by modulating lipid metabolism. Eur. J. Clin. Investig. 2012, 42, 1189–1196. [Google Scholar] [CrossRef]
- Munshi, R.P.; Joshi, S.G.; Rane, B.N. Development of an experimental diet model in rats to study hyperlipidemia and insulin resistance, markers for coronary heart disease. Indian J. Pharmacol. 2014, 46, 270–276. [Google Scholar] [CrossRef]
- Gancheva, S.; Zhelyazkova-Savova, M.; Galunska, B.; Chervenkov, T. Experimental models of metabolic syndrome in rats. Scr. Sci. Med. 2015, 47, 14–21. [Google Scholar] [CrossRef]
- Fernandez, M.L.; West, K.L. Mechanisms by which dietary fatty acids modulate plasma lipids. J. Nutr. 2005, 135, 2075–2078. [Google Scholar] [CrossRef] [PubMed]
- Ble-Castillo, J.L.; Aparicio-Trapala, M.A.; Juárez-Rojop, I.E.; Torres-Lopez, J.E.; Mendez, J.D.; Aguilar-Mariscal, H.; Olvera-Hernández, V.; Palma-Cordova, L.C.; Diaz-Zagoya, J.C. Differential effects of high-carbohydrate and high-fat diet composition on metabolic control and insulin resistance in normal rats. Int. J. Environ. Res. Public Health 2012, 9, 1663–1676. [Google Scholar] [PubMed]
- Lala, V.; Zubair, M.; Minter, D.A. Liver Function Tests; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482489/ (accessed on 17 July 2025).
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic steatohepatitis: A review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef]
- Kurek, J.M.; Król, E.; Krejpcio, Z. Steviol glycosides supplementation affects lipid metabolism in high-fat fed STZ-induced diabetic rats. Nutrients 2021, 13, 112. [Google Scholar] [CrossRef]
- Odiegwu, C.N.C.; Chianella, I.; Azubike, N.C.; Odiegwu, U.O.; Ogbuowelu, O.S. Liver Function Tests Values in Albino Wistar Rats Administered with Isolated Nigeria Achatina achatina Snail Lectin. GSC Biol. Pharm. Sci. 2021, 15, 092–102. [Google Scholar] [CrossRef]
- McAtee, B.B.; Lidbury, J.A. Liver Enzyme Interpretation and Liver Function Tests. Today’s Vet. Pract. 2017, 7, 29–38. [Google Scholar]
- Aksoy, N.H.; Karaşahin, T.; Dursun, Ş.; Akbulut, N.K.; Haydardedeoğlu, A.E.; İlgün, R.; Büyükleblebici, O. Comparative investigation of some liver enzyme functions considering age and gender distinctions in healthy Akkaraman sheep. J. Exp. Clin. Med. 2018, 35, 71–75. [Google Scholar]
- Lowe, D.; Sanvictores, T.; Zubair, M.; John, S. Alkaline Phosphatase; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459201/ (accessed on 1 August 2025).
- Jones, W.; Rockey, D.C. An observational study of the causes of an isolated elevated alkaline phosphatase level of unclear etiology. Am. J. Med. Sci. 2025, 369, 354–358. [Google Scholar] [CrossRef]
- Lasker, S.; Rahman, M.; Parvez, F.; Zamila, M.; Miah, P.; Nahar, K.; Kabir, F.; Sharmin, S.; Subhan, N.; Ahsan, G.; et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci. Rep. 2019, 9, 20026. [Google Scholar] [CrossRef]
- Guerra Ruiz, A.R.; Crespo, J.; López Martínez, R.M.; Iruzubieta, P.; Casals Mercadal, G.; Lalana Garcés, M.; Lavin, B.; Morales Ruiz, M. Measurement and clinical usefulness of bilirubin in liver disease. Adv. Lab. Med. 2021, 2, 352–361. [Google Scholar] [CrossRef]
- Gordon, D.M.; Neifer, K.L.; Hamoud, A.R.A.; Hawk, C.F.; Nestor-Kalinoski, A.L.; Miruzzi, S.A.; Morran, M.P.; Adeosun, S.O.; Sarver, J.G.; Erhardt, P.W.; et al. Bilirubin remodels murine white adipose tissue by reshaping mitochondrial activity and the coregulator profile of peroxisome proliferator–activated receptor α. J. Biol. Chem. 2020, 295, 9804–9822. [Google Scholar] [CrossRef]
- Xu, J.; Stanislaus, S.; Chinookoswong, N.; Lau, Y.Y.; Hager, T.; Patel, J.; Ge, H.; Weiszmann, J.; Lu, S.C.; Graham, M.; et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models—Association with liver and adipose tissue effects. Am. J. Physiol. Endocrinol. Metab. 2009, 297, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, T.W.; Saleh, M.; Higginson, D.S.; Paul, B.D.; Juluri, K.R.; Snyder, S.H. Bilirubin and glutathione have complementary antioxidant and cytoprotective roles. Proc. Natl. Acad. Sci. USA 2009, 106, 5171–5176. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.T.; DelCimmuto, N.R.; Flack, K.D.; Stec, D.E.; Hinds Jr, T.D. Reactive oxygen species (ROS) and antioxidants as immunomodulators in exercise: Implications for heme oxygenase and bilirubin. Antioxidants 2022, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Stec, D.E.; John, K.; Trabbic, C.J.; Luniwal, A.; Hankins, M.W.; Baum, J.; Hinds Jr, T.D. Bilirubin binding to PPARα inhibits lipid accumulation. PLoS ONE 2016, 11, e0153427. [Google Scholar] [CrossRef]
- Khanna, D.; Khanna, S.; Khanna, P.; Kahar, P.; Patel, B.M. Obesity: A chronic low-grade inflammation and its markers. Cureus 2022, 14, 22711. [Google Scholar] [CrossRef]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- da Silva-Santi, L.G.; Antunes, M.M.; Caparroz-Assef, S.M.; Carbonera, F.; Masi, L.N.; Curi, R.; Visentainer, J.V.; Bazotte, R.B. Liver fatty acid composition and inflammation in mice fed with high-carbohydrate diet or high-fat diet. Nutrients 2016, 8, 682. [Google Scholar] [CrossRef]
- Hoffman, W.P.; Ness, D.K.; Van Lier, R.B. Analysis of rodent growth data in toxicology studies. Toxicol. Sci. 2002, 66, 313–319. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jeddi, S.; Kashfi, K. The laboratory rat: Age and body weight matter. EXCLI J. 2021, 20, 1431. [Google Scholar]
- Papaefthimiou, M.; Kontou, P.I.; Bagos, P.G.; Braliou, G.G. Antioxidant activity of leaf extracts from Stevia rebaudiana Bertoni exerts attenuating effect on diseased experimental rats: A systematic review and meta-analysis. Nutrients 2023, 15, 3325. [Google Scholar] [CrossRef]
- Orellana-Paucar, A.M. Steviol Glycosides from Stevia rebaudiana: An Updated Overview of Their Sweetening Activity, Pharmacological Properties, and Safety Aspects. Molecules 2023, 28, 1258. [Google Scholar] [CrossRef]
- Carlucci, V.; Milella, L.; Tzvetkov, N.T.; Bhattacharya, S.; Ponticelli, M.; Russo, D. Not just sweetness, the anti-inflammatory potential of Stevia rebaudiana Bertoni and its major glycosides: A systematic review of in vivo investigations. Food Nutr. 2025, 1, 100035. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal models of metabolic syndrome: A review. Nutr. Metab. 2016, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, A.; Kalita, H.; Boruah, D.C.; Kalita, M.C.; Devi, R. Pathophysiology of metabolic syndrome: The onset of natural recovery on withdrawal of a high-carbohydrate, high-fat diet. Nutrition 2016, 32, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Cota, S.D.J.; Aguilar-Medina, E.M.; Ramos-Payán, R.; Ruiz-Quiñónez, A.K.; Romero-Quintana, J.G.; Montes-Avila, J.; Rendón-Maldonado, J.G.; Sánchez-López, A.; Centurión, D.; Osuna-Martínez, U. Histopathological and biochemical changes in the development of nonalcoholic fatty liver disease induced by high-sucrose diet at different times. Can. J. Physiol. Pharmacol. 2019, 97, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Gluchowski, N.L.; Becuwe, M.; Walther, T.C.; Farese, R.V. Lipid droplets and liver disease: From basic biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 343–355. [Google Scholar] [CrossRef]
- Jensen, V.S.; Hvid, H.; Damgaard, J.; Nygaard, H.; Ingvorsen, C.; Wulff, E.M.; Lykkesfeldt, J.; Fledelius, C. Dietary fat stimulates development of NAFLD more potently than dietary fructose in Sprague–Dawley rats. Diabetol. Metab. Syndr. 2018, 10, 4. [Google Scholar] [CrossRef]
- Cole, C.L.; Beck, C.A.; Robinson, D.; Ye, J.; Mills, B.; Gerber, S.A.; Schwarz, E.M.; Linehan, D. Dual Energy X-ray Absorptiometry (DEXA) as a longitudinal outcome measure of cancer-related muscle wasting in mice. PLoS ONE 2020, 15, e0230695. [Google Scholar] [CrossRef]
- Bagchi, D.P.; MacDougald, O.A. Identification and dissection of diverse mouse adipose depots. J. Vis. Exp. 2019, 149, e59499. [Google Scholar]
- Wronska, A.; Sledzinski, T.; Goyke, E.; Lawniczak, A.; Wierzbicki, P.; Kmiec, Z. Short-term calorie restriction and refeeding differently affect lipogenic enzymes in major white adipose tissue depots of young and old rats. J. Physiol. Pharmacol. 2014, 65, 117–126. [Google Scholar] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trifonova, K.; Yonkova, P.; Dzhelebov, P. Biomarkers Characterizing the Onset of Dietary-Induced Hepatocellular Injury and Visceral Obesity in a Rat Experimental Model: Possible Anti-Inflammatory Effects of Steviol Glycosides. Metabolites 2025, 15, 656. https://doi.org/10.3390/metabo15100656
Trifonova K, Yonkova P, Dzhelebov P. Biomarkers Characterizing the Onset of Dietary-Induced Hepatocellular Injury and Visceral Obesity in a Rat Experimental Model: Possible Anti-Inflammatory Effects of Steviol Glycosides. Metabolites. 2025; 15(10):656. https://doi.org/10.3390/metabo15100656
Chicago/Turabian StyleTrifonova, Krastina, Penka Yonkova, and Petko Dzhelebov. 2025. "Biomarkers Characterizing the Onset of Dietary-Induced Hepatocellular Injury and Visceral Obesity in a Rat Experimental Model: Possible Anti-Inflammatory Effects of Steviol Glycosides" Metabolites 15, no. 10: 656. https://doi.org/10.3390/metabo15100656
APA StyleTrifonova, K., Yonkova, P., & Dzhelebov, P. (2025). Biomarkers Characterizing the Onset of Dietary-Induced Hepatocellular Injury and Visceral Obesity in a Rat Experimental Model: Possible Anti-Inflammatory Effects of Steviol Glycosides. Metabolites, 15(10), 656. https://doi.org/10.3390/metabo15100656



