Endothelial Arginine Metabolism in Angiogenesis: Mechanistic Insights from Tissue Repair to Tumor Progression
Abstract
1. Introduction
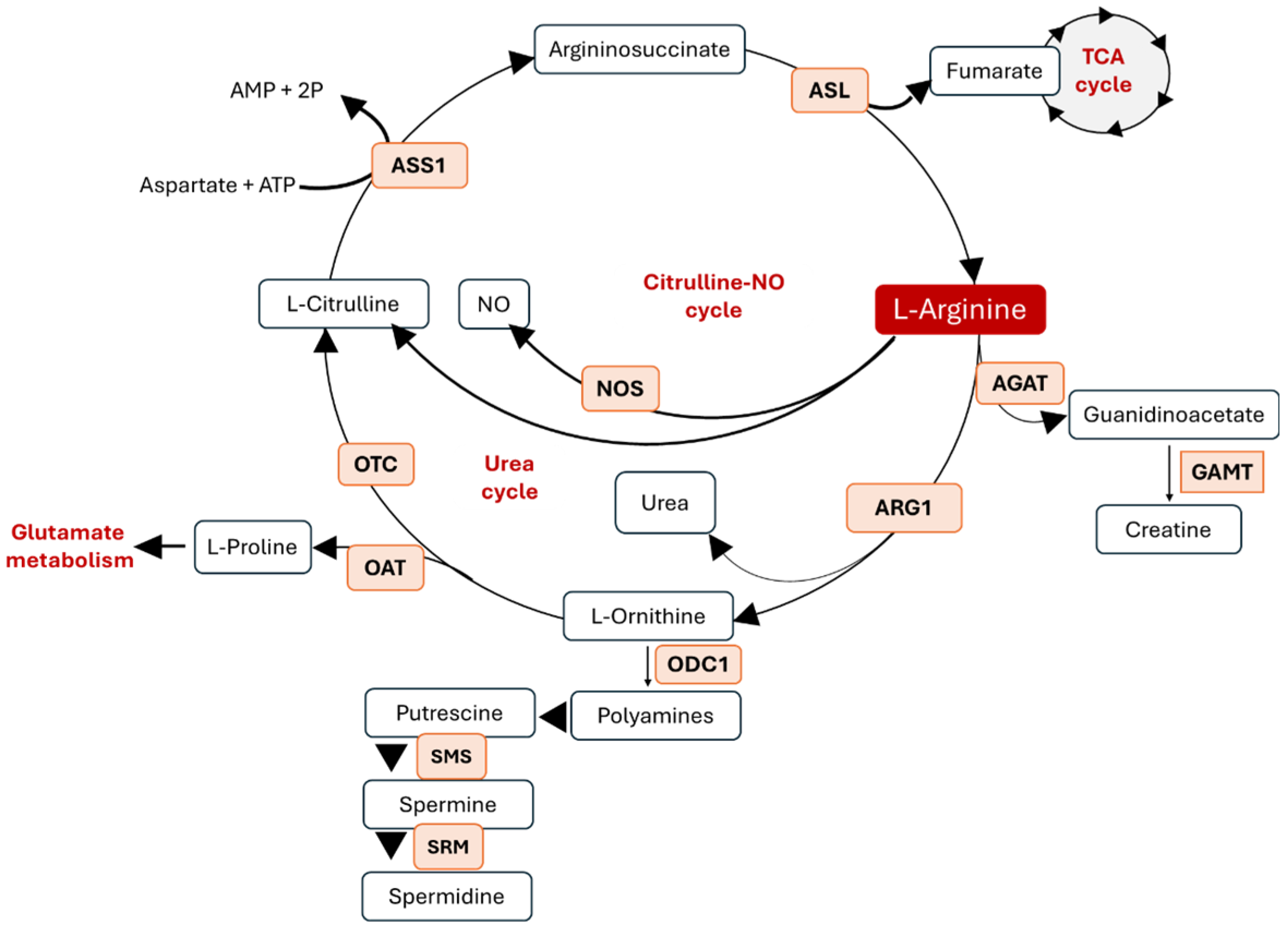
2. Arginine Metabolism and Its Metabolic Crosstalk
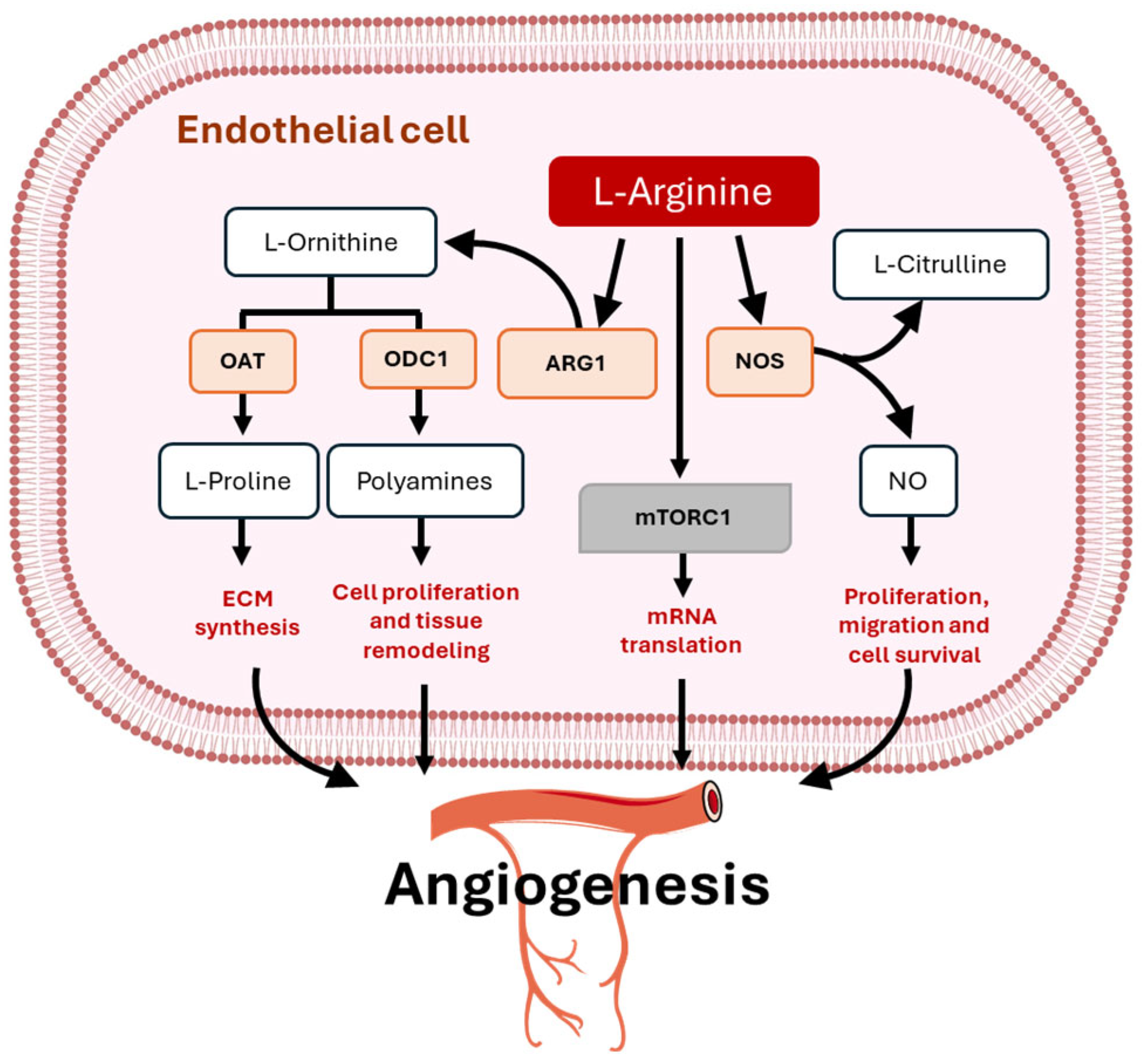
3. Metabolic Functions of Arginine in Physiological Angiogenesis
4. Arginine and Tissue Repair: Unlocking New Therapeutic Horizons
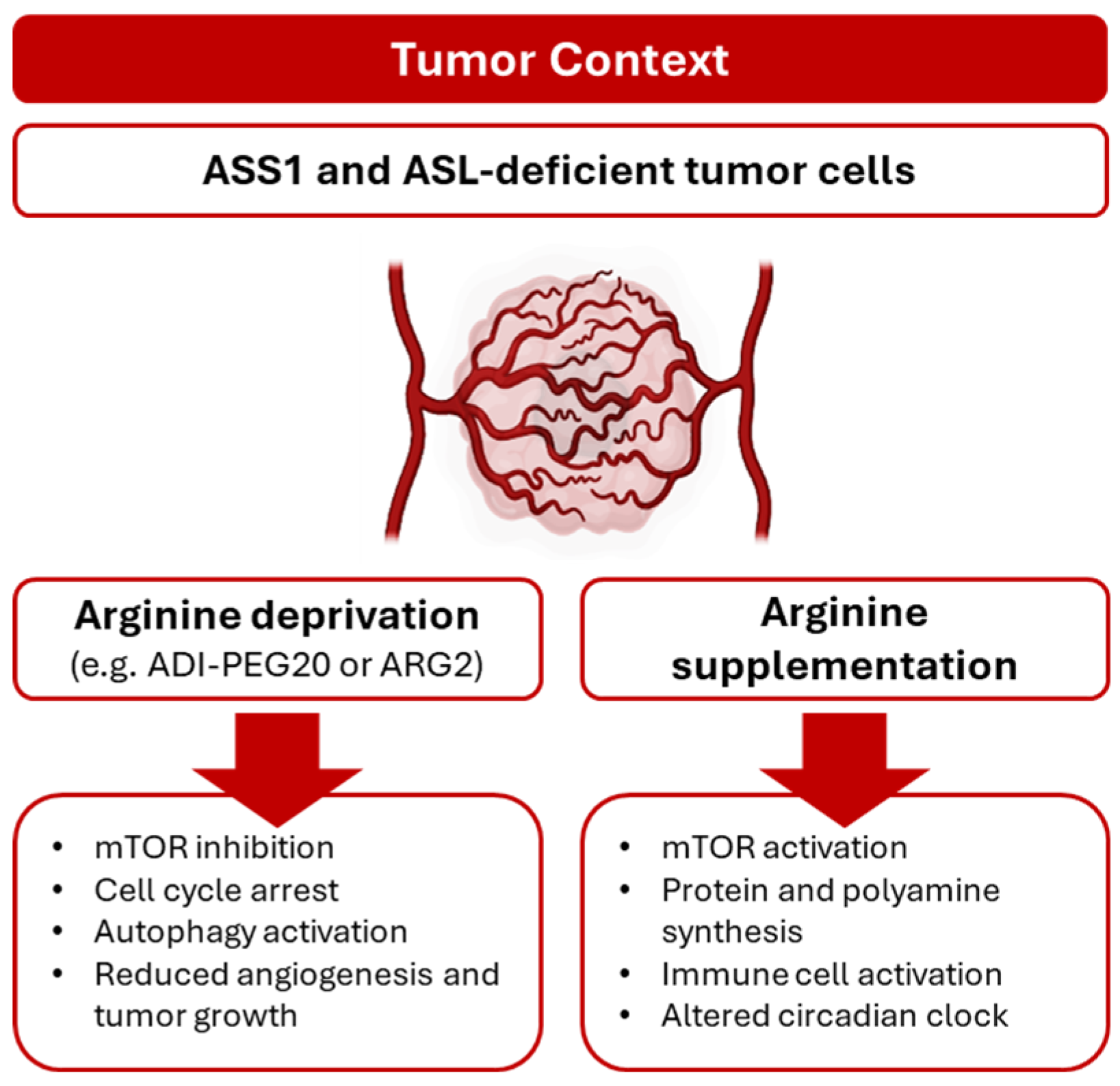
5. Arginine as Tumor Angiogenic Driver
6. Clinical Implications of Arginine in Tumor Angiogenesis
7. Remarkable Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eelen, G.; Treps, L.; Li, X.; Carmeliet, P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020, 127, 310–329. [Google Scholar] [CrossRef]
- Augustin, H.G.; Koh, G.Y. A systems view of the vascular endothelium in health and disease. Cell 2024, 187, 4833–4858. [Google Scholar] [CrossRef]
- Luo, Z.; Yao, J.; Wang, Z.; Xu, J. Mitochondria in endothelial cells angiogenesis and function: Current understanding and future perspectives. J. Transl. Med. 2023, 21, 441. [Google Scholar] [CrossRef] [PubMed]
- Siekmann, A.F. Biology of vascular mural cells. Dev. Camb. Engl. 2023, 150, dev200271. [Google Scholar] [CrossRef]
- Han, C.; Barakat, M.; DiPietro, L.A. Angiogenesis in Wound Repair: Too Much of a Good Thing? Cold Spring Harb. Perspect. Biol. 2022, 14, a041225. [Google Scholar] [CrossRef]
- Chen, H.; Peng, C.; Fang, F.; Li, Y.; Liu, X.; Hu, Y.; Wang, G.; Liu, X.; Shen, Y. Angiogenesis within atherosclerotic plaques: Mechanical regulation, molecular mechanism and clinical diagnosis. Mechanobiol. Med. 2025, 3, 100114. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Albiero, M.; Bonora, B.M.; Avogaro, A. Angiogenic Abnormalities in Diabetes Mellitus: Mechanistic and Clinical Aspects. J. Clin. Endocrinol. Metab. 2019, 104, 5431–5444. [Google Scholar] [CrossRef] [PubMed]
- Corvera, S.; Solivan-Rivera, J.; Yang Loureiro, Z. Angiogenesis in adipose tissue and obesity. Angiogenesis 2022, 25, 439–453. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef]
- Cantelmo, A.R.; Conradi, L.-C.; Brajic, A.; Goveia, J.; Kalucka, J.; Pircher, A.; Chaturvedi, P.; Hol, J.; Thienpont, B.; Teuwen, L.-A.; et al. Inhibition of the Glycolytic Activator PFKFB3 in Endothelium Induces Tumor Vessel Normalization, Impairs Metastasis, and Improves Chemotherapy. Cancer Cell 2016, 30, 968–985. [Google Scholar] [CrossRef]
- De Bock, K.; Georgiadou, M.; Schoors, S.; Kuchnio, A.; Wong, B.W.; Cantelmo, A.R.; Quaegebeur, A.; Ghesquière, B.; Cauwenberghs, S.; Eelen, G.; et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell 2013, 154, 651–663. [Google Scholar] [CrossRef]
- Kalucka, J.; Bierhansl, L.; Conchinha, N.V.; Missiaen, R.; Elia, I.; Brüning, U.; Scheinok, S.; Treps, L.; Cantelmo, A.R.; Dubois, C.; et al. Quiescent Endothelial Cells Upregulate Fatty Acid β-Oxidation for Vasculoprotection via Redox Homeostasis. Cell Metab. 2018, 28, 881–894.e13. [Google Scholar] [CrossRef]
- Bruning, U.; Morales-Rodriguez, F.; Kalucka, J.; Goveia, J.; Taverna, F.; Queiroz, K.C.S.; Dubois, C.; Cantelmo, A.R.; Chen, R.; Loroch, S.; et al. Impairment of Angiogenesis by Fatty Acid Synthase Inhibition Involves mTOR Malonylation. Cell Metab. 2018, 28, 866–880.e15. [Google Scholar] [CrossRef]
- Kim, B.; Li, J.; Jang, C.; Arany, Z. Glutamine fuels proliferation but not migration of endothelial cells. EMBO J. 2017, 36, 2321–2333. [Google Scholar] [CrossRef]
- Huang, H.; Vandekeere, S.; Kalucka, J.; Bierhansl, L.; Zecchin, A.; Brüning, U.; Visnagri, A.; Yuldasheva, N.; Goveia, J.; Cruys, B.; et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017, 36, 2334–2352. [Google Scholar] [CrossRef] [PubMed]
- Upadhyayula, P.S.; Higgins, D.M.; Mela, A.; Banu, M.; Dovas, A.; Zandkarimi, F.; Patel, P.; Mahajan, A.; Humala, N.; Nguyen, T.T.T.; et al. Dietary restriction of cysteine and methionine sensitizes gliomas to ferroptosis and induces alterations in energetic metabolism. Nat. Commun. 2023, 14, 1187. [Google Scholar] [CrossRef]
- Lermant, A.; Murdoch, C.E. Cysteine Glutathionylation Acts as a Redox Switch in Endothelial Cells. Antioxidants 2019, 8, 315. [Google Scholar] [CrossRef] [PubMed]
- Kurhaluk, N.; Tkaczenko, H. L-Arginine and Nitric Oxide in Vascular Regulation—Experimental Findings in the Context of Blood Donation. Nutrients 2025, 17, 665. [Google Scholar] [CrossRef]
- Carlström, M. Nitric oxide signalling in kidney regulation and cardiometabolic health. Nat. Rev. Nephrol. 2021, 17, 575–590. [Google Scholar] [CrossRef]
- Molek, P.; Zmudzki, P.; Wlodarczyk, A.; Nessler, J.; Zalewski, J. The shifted balance of arginine metabolites in acute myocardial infarction patients and its clinical relevance. Sci. Rep. 2021, 11, 83. [Google Scholar] [CrossRef]
- Wu, G.; Meininger, C.J. Arginine Nutrition and Cardiovascular Function. J. Nutr. 2000, 130, 2626–2629. [Google Scholar] [CrossRef] [PubMed]
- Fung, T.S.; Ryu, K.W.; Thompson, C.B. Arginine: At the crossroads of nitrogen metabolism. EMBO J. 2025, 44, 1275–1293. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef] [PubMed]
- Bode-Böger, S.M.; Scalera, F.; Ignarro, L.J. The L-arginine paradox: Importance of the L-arginine/asymmetrical dimethylarginine ratio. Pharmacol. Ther. 2007, 114, 295–306. [Google Scholar] [CrossRef]
- Starikova, E.A.; Rubinstein, A.A.; Mammedova, J.T.; Isakov, D.V.; Kudryavtsev, I.V. Regulated Arginine Metabolism in Immunopathogenesis of a Wide Range of Diseases: Is There a Way to Pass between Scylla and Charybdis? Curr. Issues Mol. Biol. 2023, 45, 3525–3551. [Google Scholar] [CrossRef]
- Kieler, M.; Hofmann, M.; Schabbauer, G. More than just protein building blocks: How amino acids and related metabolic pathways fuel macrophage polarization. FEBS J. 2021, 288, 3694–3714. [Google Scholar] [CrossRef]
- Locke, M.; Ghazaly, E.; Freitas, M.O.; Mitsinga, M.; Lattanzio, L.; Lo Nigro, C.; Nagano, A.; Wang, J.; Chelala, C.; Szlosarek, P.; et al. Inhibition of the Polyamine Synthesis Pathway Is Synthetically Lethal with Loss of Argininosuccinate Synthase 1. Cell Rep. 2016, 16, 1604–1613. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, S.; Cleveland, J.L. Polyamine Homeostasis in Development and Disease. Med. Sci. Basel Switz. 2021, 9, 28. [Google Scholar] [CrossRef]
- Tauer, K.; Theile, C.; Owens, J.W.; Cecil, K.M.; Shillington, A. Arginine, glycine, and creatine supplementation improves symptoms in a female with creatine transporter deficiency. Psychiatr. Genet. 2024, 34, 86–90. [Google Scholar] [CrossRef]
- Diaz, G.A.; Bechter, M.; Cederbaum, S.D. The role and control of arginine levels in arginase 1 deficiency. J. Inherit. Metab. Dis. 2023, 46, 3–14. [Google Scholar] [CrossRef]
- Rabinovich, S.; Adler, L.; Yizhak, K.; Sarver, A.; Silberman, A.; Agron, S.; Stettner, N.; Sun, Q.; Brandis, A.; Helbling, D.; et al. Diversion of aspartate in ASS1-deficient tumours fosters de novo pyrimidine synthesis. Nature 2015, 527, 379–383. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef] [PubMed]
- Takahara, T.; Amemiya, Y.; Sugiyama, R.; Maki, M.; Shibata, H. Amino acid-dependent control of mTORC1 signaling: A variety of regulatory modes. J. Biomed. Sci. 2020, 27, 87. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef]
- Forzano, I.; Avvisato, R.; Varzideh, F.; Jankauskas, S.S.; Cioppa, A.; Mone, P.; Salemme, L.; Kansakar, U.; Tesorio, T.; Trimarco, V.; et al. L-Arginine in diabetes: Clinical and preclinical evidence. Cardiovasc. Diabetol. 2023, 22, 89. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Han, M.; Rezaei, A.; Li, D.; Wu, G.; Ma, X. L-Arginine Modulates Glucose and Lipid Metabolism in Obesity and Diabetes. Curr. Protein Pept. Sci. 2017, 18, 599–608. [Google Scholar] [CrossRef]
- Guo, X.; Xing, Y.; Jin, W. Role of ADMA in the pathogenesis of microvascular complications in type 2 diabetes mellitus. Front. Endocrinol. 2023, 14, 1183586. [Google Scholar] [CrossRef]
- White, P.J.; Wewer Albrechtsen, N.J.; Campbell, J.E. Islet hormones at the intersection of glucose and amino acid metabolism. Nat. Rev. Endocrinol. 2025, 21, 397–412. [Google Scholar] [CrossRef]
- Ali, A.; Liu, X.; Melaku, M.; Lqbal, W.; Yi, B.; Zhong, R.; Chen, L.; Ma, T.; Zhang, H. Effect of arginine supplementation on liver and pectoral muscle: Tissue-specific lipid metabolism in broilers. Poult. Sci. 2025, 104, 105601. [Google Scholar] [CrossRef]
- McKnight, J.R.; Satterfield, M.C.; Jobgen, W.S.; Smith, S.B.; Spencer, T.E.; Meininger, C.J.; McNeal, C.J.; Wu, G. Beneficial effects of L-arginine on reducing obesity: Potential mechanisms and important implications for human health. Amino Acids 2010, 39, 349–357. [Google Scholar] [CrossRef]
- Kalezic, A.; Korac, A.; Korac, B.; Jankovic, A. l-Arginine Induces White Adipose Tissue Browning-A New Pharmaceutical Alternative to Cold. Pharmaceutics 2022, 14, 1368. [Google Scholar] [CrossRef]
- Jobgen, W.S.; Lee, M.-J.; Fried, S.K.; Wu, G. l-Arginine supplementation regulates energy-substrate metabolism in skeletal muscle and adipose tissue of diet-induced obese rats. Exp. Biol. Med. 2023, 248, 209–216. [Google Scholar] [CrossRef]
- Wu, G.; Morris, S.M., Jr. Arginine metabolism: Nitric oxide and beyond. Biochem. J. 1998, 336 Pt 1, 1–17. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Tahmasebinejad, Z.; Azizi, F. Dietary L-arginine intake and the incidence of coronary heart disease: Tehran lipid and glucose study. Nutr. Metab. 2016, 13, 23. [Google Scholar] [CrossRef] [PubMed]
- Mirmiran, P.; Bahadoran, Z.; Gaeini, Z.; Azizi, F. Habitual intake of dietary L-arginine in relation to risk of type 2 diabetes: A prospective study. BMC Endocr. Disord. 2021, 21, 113. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.L.; Oubaha, M.; Cagnone, G.; Boscher, C.; Kim, J.S.; El Bakkouri, Y.; Zhang, Y.; Chidiac, R.; Corriveau, J.; Delisle, C.; et al. eNOS controls angiogenic sprouting and retinal neovascularization through the regulation of endothelial cell polarity. Cell. Mol. Life Sci. 2021, 79, 37. [Google Scholar] [CrossRef]
- Tran, N.; Garcia, T.; Aniqa, M.; Ali, S.; Ally, A.; Nauli, S.M. Endothelial Nitric Oxide Synthase (eNOS) and the Cardiovascular System: In Physiology and in Disease States. Am. J. Biomed. Sci. Res. 2022, 15, 153–177. [Google Scholar] [CrossRef]
- Roy, R.; Wilcox, J.; Webb, A.J.; O’Gallagher, K. Dysfunctional and Dysregulated Nitric Oxide Synthases in Cardiovascular Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 15200. [Google Scholar] [CrossRef]
- Leiper, J.; Nandi, M. The therapeutic potential of targeting endogenous inhibitors of nitric oxide synthesis. Nat. Rev. Drug Discov. 2011, 10, 277–291. [Google Scholar] [CrossRef]
- Ranjbar, K. Improved Cardiac Function Following Ischemia Reperfusion Injury Using Exercise Preconditioning and L-Arginine Supplementation via Oxidative Stress Mitigation and Angiogenesis Amelioration. Cardiovasc. Toxicol. 2022, 22, 736–745. [Google Scholar] [CrossRef]
- Elmetwally, M.A.; Li, X.; Johnson, G.A.; Burghardt, R.C.; Herring, C.M.; Kramer, A.C.; Meininger, C.J.; Bazer, F.W.; Wu, G. Dietary supplementation with L-arginine between days 14 and 25 of gestation enhances NO and polyamine syntheses and the expression of angiogenic proteins in porcine placentae. Amino Acids 2022, 54, 193–204. [Google Scholar] [CrossRef]
- Warden, C.; Zubieta, D.; Brantley, M.A. Citrulline Plus Arginine Induces an Angiogenic Response and Increases Permeability in Retinal Endothelial Cells via Nitric Oxide Production. Int. J. Mol. Sci. 2025, 26, 2080. [Google Scholar] [CrossRef]
- Romero, J.C.; Tonapi, S.S.; Parihar, M.; Loranc, E.; Miller, H.E.; Lawrence, L.A.; Bassani, N.; Robledo, D.G.; Cao, L.; Nie, J.; et al. Loss of CD98HC phosphorylation by ATM impairs antiporter trafficking and drives glutamate toxicity in Ataxia telangiectasia. Nat. Commun. 2025, 16, 5109. [Google Scholar] [CrossRef]
- Fiedler, L.R.; Wojciak-Stothard, B. The DDAH/ADMA pathway in the control of endothelial cell migration and angiogenesis. Biochem. Soc. Trans. 2009, 37, 1243–1247. [Google Scholar] [CrossRef]
- Cefalo, C.M.A.; Riccio, A.; Fiorentino, T.V.; Massimino, M.; Mannino, G.C.; Succurro, E.; Perticone, M.; Sciacqua, A.; Andreozzi, F.; Perticone, F.; et al. Asymmetric dimethylarginine (ADMA) is associated with reduced myocardial mechano-energetic efficiency in hypertensive subjects. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1175–1178. [Google Scholar] [CrossRef]
- Wu, G.; Meininger, C.J.; McNeal, C.J.; Bazer, F.W.; Rhoads, J.M. Role of L-Arginine in Nitric Oxide Synthesis and Health in Humans. Adv. Exp. Med. Biol. 2021, 1332, 167–187. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Fang, B.; Shan, S.; Li, Q. Mechanical stiffness promotes skin fibrosis through Piezo1-mediated arginine and proline metabolism. Cell Death Discov. 2023, 9, 354. [Google Scholar] [CrossRef]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-dependent regulation of collagen metabolism. Cell. Mol. Life Sci. 2020, 77, 1911–1918. [Google Scholar] [CrossRef] [PubMed]
- Karakozova, M.; Kozak, M.; Wong, C.C.L.; Bailey, A.O.; Yates, J.R.; Mogilner, A.; Zebroski, H.; Kashina, A. Arginylation of beta-actin regulates actin cytoskeleton and cell motility. Science 2006, 313, 192–196. [Google Scholar] [CrossRef]
- Kurhaluk, N. The Effectiveness of L-arginine in Clinical Conditions Associated with Hypoxia. Int. J. Mol. Sci. 2023, 24, 8205. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, L.; Ren, Y.; Huang, Y.; Liu, W.; Lv, Z.; Qian, L.; Yu, Y.; Xiong, Y. Arginase: Shedding light on the mechanisms and opportunities in cardiovascular diseases. Cell Death Discov. 2022, 8, 413. [Google Scholar] [CrossRef]
- Kazemi, N.; Javad Mahalati, M.; Kaviani, Y.; Al-Musawi, M.H.; Varshosaz, J.; Soleymani Eil Bakhtiari, S.; Tavakoli, M.; Alizadeh, M.; Sharifianjazi, F.; Salehi, S.; et al. Core-shell nanofibers containing L-arginine stimulates angiogenesis and full thickness dermal wound repair. Int. J. Pharm. 2024, 653, 123931. [Google Scholar] [CrossRef]
- Hussein, Y.; El-Fakharany, E.M.; Kamoun, E.A.; Loutfy, S.A.; Amin, R.; Taha, T.H.; Salim, S.A.; Amer, M. Electrospun PVA/hyaluronic acid/L-arginine nanofibers for wound healing applications: Nanofibers optimization and in vitro bioevaluation. Int. J. Biol. Macromol. 2020, 164, 667–676. [Google Scholar] [CrossRef]
- Arribas-López, E.; Zand, N.; Ojo, O.; Snowden, M.J.; Kochhar, T. The Effect of Amino Acids on Wound Healing: A Systematic Review and Meta-Analysis on Arginine and Glutamine. Nutrients 2021, 13, 2498. [Google Scholar] [CrossRef]
- Goli, P.; Yazdi, M.; Heidari-Beni, M.; Kelishadi, R. Growth Hormone Response to L-Arginine Alone and Combined with Different Doses of Growth Hormone-Releasing Hormone: A Systematic Review and Meta-Analysis. Int. J. Endocrinol. 2022, 2022, 8739289. [Google Scholar] [CrossRef]
- Leigh, B.; Desneves, K.; Rafferty, J.; Pearce, L.; King, S.; Woodward, M.C.; Brown, D.; Martin, R.; Crowe, T.C. The effect of different doses of an arginine-containing supplement on the healing of pressure ulcers. J. Wound Care 2012, 21, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Cheshmeh, S.; Hojati, N.; Mohammadi, A.; Rahmani, N.; Moradi, S.; Pasdar, Y.; Elahi, N. The use of oral and enteral tube-fed arginine supplementation in pressure injury care: A systematic review and meta-analysis. Nurs. Open 2022, 9, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Sonenberg, N.; Tahmasebi, S. mRNA translational control of regeneration. Curr. Opin. Genet. Dev. 2025, 93, 102367. [Google Scholar] [CrossRef]
- Blanc, R.S.; Richard, S. Arginine Methylation: The Coming of Age. Mol. Cell 2017, 65, 8–24. [Google Scholar] [CrossRef]
- Zaccarelli-Magalhães, J.; Citadin, C.T.; Langman, J.; Smith, D.J.; Matuguma, L.H.; Lin, H.W.; Udo, M.S.B. Protein arginine methyltransferases as regulators of cellular stress. Exp. Neurol. 2025, 384, 115060. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.; Maetzel, D.; Maddocks, O.D.; Otten, G.; Ratcliff, M.; Smith, G.R.; Dunlop, E.A.; Passos, J.F.; Davies, O.R.; Jaenisch, R.; et al. Control of TSC2-Rheb signaling axis by arginine regulates mTORC1 activity. eLife 2016, 5, e11058. [Google Scholar] [CrossRef]
- Wang, Z.; Pan, Z.; Adhikari, S.; Harada, B.T.; Shen, L.; Yuan, W.; Abeywardana, T.; Al-Hadid, Q.; Stark, J.M.; He, C.; et al. m6A Deposition is Regulated by PRMT1-Mediated Arginine Methylation of METTL14 in Its Disordered C-Terminal Region. EMBO J. 2021, 40, e106309. Available online: https://www.embopress.org/doi/full/10.15252/embj.2020106309 (accessed on 15 September 2025). [CrossRef]
- Sarandy, M.M.; Pelinsari, S.M.; de Souza, L.M.; Novaes, R.D.; Zanuncio, V.V.; Gonçalves, R.V. l-arginine and l-citrulline supplementation accelerates second intention wound healing in iNOS knockout mice. J. Funct. Foods 2023, 100, 105395. [Google Scholar] [CrossRef]
- Torsy, T.; Tency, I.; Beeckman, D.; Isoherranen, K.; Litchford, M.; De Vylder, F. The Role of Glutamine and Arginine in Wound Healing of Pressure Ulcers: A Systematic Review. Wound Repair Regen. 2025, 33, e70077. [Google Scholar] [CrossRef]
- Ding, Q.; Li, R.; Wang, Q.; Yu, L.; Zi, F. A pan-cancer analysis of the role of argininosuccinate synthase 1 in human tumors. Front. Oncol. 2023, 13, 1049147. [Google Scholar] [CrossRef]
- Chan, P.Y.; Phillips, M.M.; Ellis, S.; Johnston, A.; Feng, X.; Arora, A.; Hay, G.; Cohen, V.M.L.; Sagoo, M.S.; Bomalaski, J.S.; et al. A Phase 1 study of ADI-PEG20 (pegargiminase) combined with cisplatin and pemetrexed in ASS1-negative metastatic uveal melanoma. Pigment Cell Melanoma Res. 2022, 35, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Przystal, J.M.; Hajji, N.; Khozoie, C.; Renziehausen, A.; Zeng, Q.; Abaitua, F.; Hajitou, A.; Suwan, K.; Want, E.; Bomalaski, J.; et al. Efficacy of arginine depletion by ADI-PEG20 in an intracranial model of GBM. Cell Death Dis. 2018, 9, 1192. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-J.; Hsiao, H.-H.; Hsu, Y.-T.; Liu, Y.-C.; Kao, H.-W.; Liu, T.-C.; Cho, S.-F.; Feng, X.; Johnston, A.; Bomalaski, J.S.; et al. Phase I study of ADI-PEG20 plus low-dose cytarabine for the treatment of acute myeloid leukemia. Cancer Med. 2021, 10, 2946–2955. [Google Scholar] [CrossRef] [PubMed]
- Park, I.-S.; Kang, S.-W.; Shin, Y.-J.; Chae, K.-Y.; Park, M.-O.; Kim, M.-Y.; Wheatley, D.N.; Min, B.-H. Arginine deiminase: A potential inhibitor of angiogenesis and tumour growth. Br. J. Cancer 2003, 89, 907–914. [Google Scholar] [CrossRef]
- Ben-Sahra, I.; Manning, B.D. mTORC1 signaling and the metabolic control of cell growth. Curr. Opin. Cell Biol. 2017, 45, 72–82. [Google Scholar] [CrossRef]
- Duysak, T.; Kim, K.; Yun, M.; Jeong, J.-H.; Choy, H.E. Enhanced anti-cancer efficacy of arginine deaminase expressed by tumor-seeking Salmonella Gallinarum. Oncogene 2024, 43, 3378–3387. [Google Scholar] [CrossRef] [PubMed]
- Prudner, B.C.; Rathore, R.; Robinson, A.M.; Godec, A.; Chang, S.F.; Hawkins, W.G.; Hirbe, A.C.; Van Tine, B.A. Arginine Starvation and Docetaxel Induce c-Myc–Driven hENT1 Surface Expression to Overcome Gemcitabine Resistance in ASS1-Negative Tumors. Clin. Cancer Res. 2019, 25, 5122–5134. [Google Scholar] [CrossRef]
- Rogers, L.C.; Kremer, J.C.; Brashears, C.B.; Lin, Z.; Hu, Z.; Bastos, A.C.S.; Baker, A.; Fettig, N.; Zhou, D.; Shoghi, K.I.; et al. Discovery and Targeting of a Noncanonical Mechanism of Sarcoma Resistance to ADI-PEG20 Mediated by the Microenvironment. Clin. Cancer Res. 2023, 29, 3189–3202. [Google Scholar] [CrossRef]
- Giatromanolaki, A.; Harris, A.L.; Koukourakis, M.I. The prognostic and therapeutic implications of distinct patterns of argininosuccinate synthase 1 (ASS1) and arginase-2 (ARG2) expression by cancer cells and tumor stroma in non-small-cell lung cancer. Cancer Metab. 2021, 9, 28. [Google Scholar] [CrossRef]
- Jungnickel, K.E.J.; Parker, J.L.; Newstead, S. Structural basis for amino acid transport by the CAT family of SLC7 transporters. Nat. Commun. 2018, 9, 550. [Google Scholar] [CrossRef]
- Visigalli, R.; Barilli, A.; Parolari, A.; Sala, R.; Rotoli, B.M.; Bussolati, O.; Gazzola, G.C.; Dall’Asta, V. Regulation of arginine transport and metabolism by protein kinase Calpha in endothelial cells: Stimulation of CAT2 transporters and arginase activity. J. Mol. Cell. Cardiol. 2010, 49, 260–270. [Google Scholar] [CrossRef]
- Zani, B.G.; Bohlen, H.G. Transport of extracellular l-arginine via cationic amino acid transporter is required during in vivo endothelial nitric oxide production. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1381–H1390. [Google Scholar] [CrossRef] [PubMed]
- Banjarnahor, S.; Rodionov, R.N.; König, J.; Maas, R. Transport of L-Arginine Related Cardiovascular Risk Markers. J. Clin. Med. 2020, 9, 3975. [Google Scholar] [CrossRef]
- Ikeda, A.; Nagayama, S.; Sumazaki, M.; Konishi, M.; Fujii, R.; Saichi, N.; Muraoka, S.; Saigusa, D.; Shimada, H.; Sakai, Y.; et al. Colorectal Cancer–Derived CAT1-Positive Extracellular Vesicles Alter Nitric Oxide Metabolism in Endothelial Cells and Promote Angiogenesis. Mol. Cancer Res. 2021, 19, 834–846. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Visigalli, R.; Barilli, A.; Bussolati, O.; Sala, R.; Gazzola, G.C.; Parolari, A.; Tremoli, E.; Simon, A.; Closs, E.I.; Dall’Asta, V. Rapamycin stimulates arginine influx through CAT2 transporters in human endothelial cells. Biochim. Biophys. Acta 2007, 1768, 1479–1487. [Google Scholar] [CrossRef][Green Version]
- Ong, Y.T.; Andrade, J.; Armbruster, M.; Shi, C.; Castro, M.; Costa, A.S.H.; Sugino, T.; Eelen, G.; Zimmermann, B.; Wilhelm, K.; et al. A YAP/TAZ-TEAD signalling module links endothelial nutrient acquisition to angiogenic growth. Nat. Metab. 2022, 4, 672–682. [Google Scholar] [CrossRef]
- Oberkersch, R.E.; Santoro, M.M. YAP/TAZ–TEAD link angiogenesis to nutrients. Nat. Metab. 2022, 4, 645–646. [Google Scholar] [CrossRef]
- Lidonnici, J.; Oberkersch, R.E. Reciprocal Dynamics of Metabolism and mRNA Translation in Tumor Angiogenesis. Int. J. Mol. Sci. 2024, 25, 11284. [Google Scholar] [CrossRef]
- Chantranupong, L.; Scaria, S.M.; Saxton, R.A.; Gygi, M.P.; Shen, K.; Wyant, G.A.; Wang, T.; Harper, J.W.; Gygi, S.P.; Sabatini, D.M. The CASTOR Proteins Are Arginine Sensors for the mTORC1 Pathway. Cell 2016, 165, 153–164. [Google Scholar] [CrossRef]
- Iadevaia, V.; Liu, R.; Proud, C.G. mTORC1 signaling controls multiple steps in ribosome biogenesis. Semin. Cell Dev. Biol. 2014, 36, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Darnell, A.M.; Subramaniam, A.R.; O’Shea, E.K. Translational Control through Differential Ribosome Pausing during Amino Acid Limitation in Mammalian Cells. Mol. Cell 2018, 71, 229–243.e11. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.J.; Gao, J.; Yamaguchi, N.; Pinzaru, A.; Wu, Q.; Mandayam, N.; Liberti, M.; Heissel, S.; Alwaseem, H.; Tavazoie, S.; et al. Arginine limitation drives a directed codon-dependent DNA sequence evolution response in colorectal cancer cells. Sci. Adv. 2023, 9, eade9120. Available online: https://www.science.org/doi/10.1126/sciadv.ade9120 (accessed on 12 September 2025). [CrossRef] [PubMed]
- Hsu, D.J.; Tavazoie, S.F. Cysteine substitutants emerge in lung cancer proteomes during arginine restriction. Mol. Cell 2024, 84, 1819–1821. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Pataskar, A.; Feng, X.; Montenegro Navarro, J.; Paniagua, I.; Jacobs, J.J.L.; Zaal, E.A.; Berkers, C.R.; Bleijerveld, O.B.; Agami, R. Arginine deprivation enriches lung cancer proteomes with cysteine by inducing arginine-to-cysteine substitutants. Mol. Cell 2024, 84, 1904–1916.e7. [Google Scholar] [CrossRef]
- Oberkersch, R.E.; Pontarin, G.; Astone, M.; Spizzotin, M.; Arslanbaeva, L.; Tosi, G.; Panieri, E.; Ricciardi, S.; Allega, M.F.; Brossa, A.; et al. Aspartate metabolism in endothelial cells activates the mTORC1 pathway to initiate translation during angiogenesis. Dev. Cell 2022, 57, 1241–1256.e8. [Google Scholar] [CrossRef]
- Wu, R.; Zhong, J.; Song, L.; Zhang, M.; Chen, L.; Zhang, L.; Qiu, Z. Untargeted metabolomic analysis of ischemic injury in human umbilical vein endothelial cells reveals the involvement of arginine metabolism. Nutr. Metab. 2023, 20, 17. [Google Scholar] [CrossRef]
- Schibalski, R.S.; Shulha, A.S.; Tsao, B.P.; Palygin, O.; Ilatovskaya, D.V. The role of polyamine metabolism in cellular function and physiology. Am. J. Physiol.-Cell Physiol. 2024, 327, C341–C356. [Google Scholar] [CrossRef]
- Cohen, E.B.; Geck, R.C.; Toker, A. Metabolic pathway alterations in microvascular endothelial cells in response to hypoxia. PLoS ONE 2020, 15, e0232072. [Google Scholar] [CrossRef] [PubMed]
- Astone, M.; Oberkersch, R.E.; Tosi, G.; Biscontin, A.; Santoro, M.M. The circadian protein BMAL1 supports endothelial cell cycle during angiogenesis. Cardiovasc. Res. 2023, 119, 1952–1968. [Google Scholar] [CrossRef] [PubMed]
- Fortin, B.M.; Mahieu, A.L.; Fellows, R.C.; Kang, Y.; Lewis, A.N.; Ead, A.S.; Lamia, K.A.; Cao, Y.; Pannunzio, N.R.; Masri, S. The diverse roles of the circadian clock in cancer. Nat. Cancer 2025, 6, 753–767. [Google Scholar] [CrossRef]
- Lin, R.; Mo, Y.; Zha, H.; Qu, Z.; Xie, P.; Zhu, Z.-J.; Xu, Y.; Xiong, Y.; Guan, K.-L. CLOCK Acetylates ASS1 to Drive Circadian Rhythm of Ureagenesis. Mol. Cell 2017, 68, 198–209.e6. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, Y.; Cortes, I.M.; Coleman, D.N.; Dai, H.; Liang, Y.; Parys, C.; Fernandez, C.; Wang, M.; Loor, J.J. Supply of methionine and arginine alters phosphorylation of mechanistic target of rapamycin (mTOR), circadian clock proteins, and α-s1-casein abundance in bovine mammary epithelial cells. Food Funct. 2020, 11, 883–894. [Google Scholar] [CrossRef]
- Long, Y.; Tsai, W.-B.; Chang, J.T.; Estecio, M.; Wangpaichitr, M.; Savaraj, N.; Feun, L.G.; Chen, H.H.W.; Kuo, M.T. Cisplatin-induced synthetic lethality to arginine-starvation therapy by transcriptional suppression of ASS1 is regulated by DEC1, HIF-1α, and c-Myc transcription network and is independent of ASS1 promoter DNA methylation. Oncotarget 2016, 7, 82658–82670. [Google Scholar] [CrossRef]
- Mun, G.I.; Kim, I.-S.; Lee, B.-H.; Boo, Y.C. Endothelial argininosuccinate synthetase 1 regulates nitric oxide production and monocyte adhesion under static and laminar shear stress conditions. J. Biol. Chem. 2011, 286, 2536–2542. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, Z.; Du, X.; Lin, X.; Liang, Z.-M.; Chen, S.; Sun, Y.; Wang, Y.; Na, Z.; Wu, Z.; et al. Cancer cell-derived arginine fuels polyamine biosynthesis in tumor-associated macrophages to promote immune evasion. Cancer Cell 2025, 43, 1045–1060.e7. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.-Y.; Chiang, N.J.; Wu, S.Y.; Yen, C.J.; Chen, S.H.; Yeh, Y.M.; Li, C.F.; Feng, X.X.; Wu, K.; Johnston, A.; et al. Phase 1b study of pegylated arginine deiminase (ADI-PEG 20) plus Pembrolizumab in advanced solid cancers. Oncoimmunology 2021, 10, 1943253. [Google Scholar] [CrossRef]
- Zhang, Y.; Chung, S.-F.; Tam, S.-Y.; Leung, Y.-C.; Guan, X. Arginine deprivation as a strategy for cancer therapy: An insight into drug design and drug combination. Cancer Lett. 2021, 502, 58–70. [Google Scholar] [CrossRef]
- Suo, X.; Lu, X.; Hu, T.; Ma, G.; Su, Z. A solid-phase adsorption method for PEGylation of human serum albumin and staphylokinase: Preparation, purification and biochemical characterization. Biotechnol. Lett. 2009, 31, 1191–1196. [Google Scholar] [CrossRef]
- Ni, Y.; Schwaneberg, U.; Sun, Z.-H. Arginine deiminase, a potential anti-tumor drug. Cancer Lett. 2008, 261, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hulin, J.-A.; Gubareva, E.A.; Jarzebska, N.; Rodionov, R.N.; Mangoni, A.A.; Tommasi, S. Inhibition of Dimethylarginine Dimethylaminohydrolase (DDAH) Enzymes as an Emerging Therapeutic Strategy to Target Angiogenesis and Vasculogenic Mimicry in Cancer. Front Oncol. 2019, 9, 1455. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arce-Recatala, C.; Oberkersch, R.E. Endothelial Arginine Metabolism in Angiogenesis: Mechanistic Insights from Tissue Repair to Tumor Progression. Metabolites 2025, 15, 694. https://doi.org/10.3390/metabo15110694
Arce-Recatala C, Oberkersch RE. Endothelial Arginine Metabolism in Angiogenesis: Mechanistic Insights from Tissue Repair to Tumor Progression. Metabolites. 2025; 15(11):694. https://doi.org/10.3390/metabo15110694
Chicago/Turabian StyleArce-Recatala, Cristina, and Roxana Elena Oberkersch. 2025. "Endothelial Arginine Metabolism in Angiogenesis: Mechanistic Insights from Tissue Repair to Tumor Progression" Metabolites 15, no. 11: 694. https://doi.org/10.3390/metabo15110694
APA StyleArce-Recatala, C., & Oberkersch, R. E. (2025). Endothelial Arginine Metabolism in Angiogenesis: Mechanistic Insights from Tissue Repair to Tumor Progression. Metabolites, 15(11), 694. https://doi.org/10.3390/metabo15110694





