Melatonin and Inflammatory Cytokines as Modulators of the Interaction Between Gestational Diabetes Mellitus and Pregnancy-Specific Urinary Incontinence
Abstract
1. Introduction
2. Materials and Methods
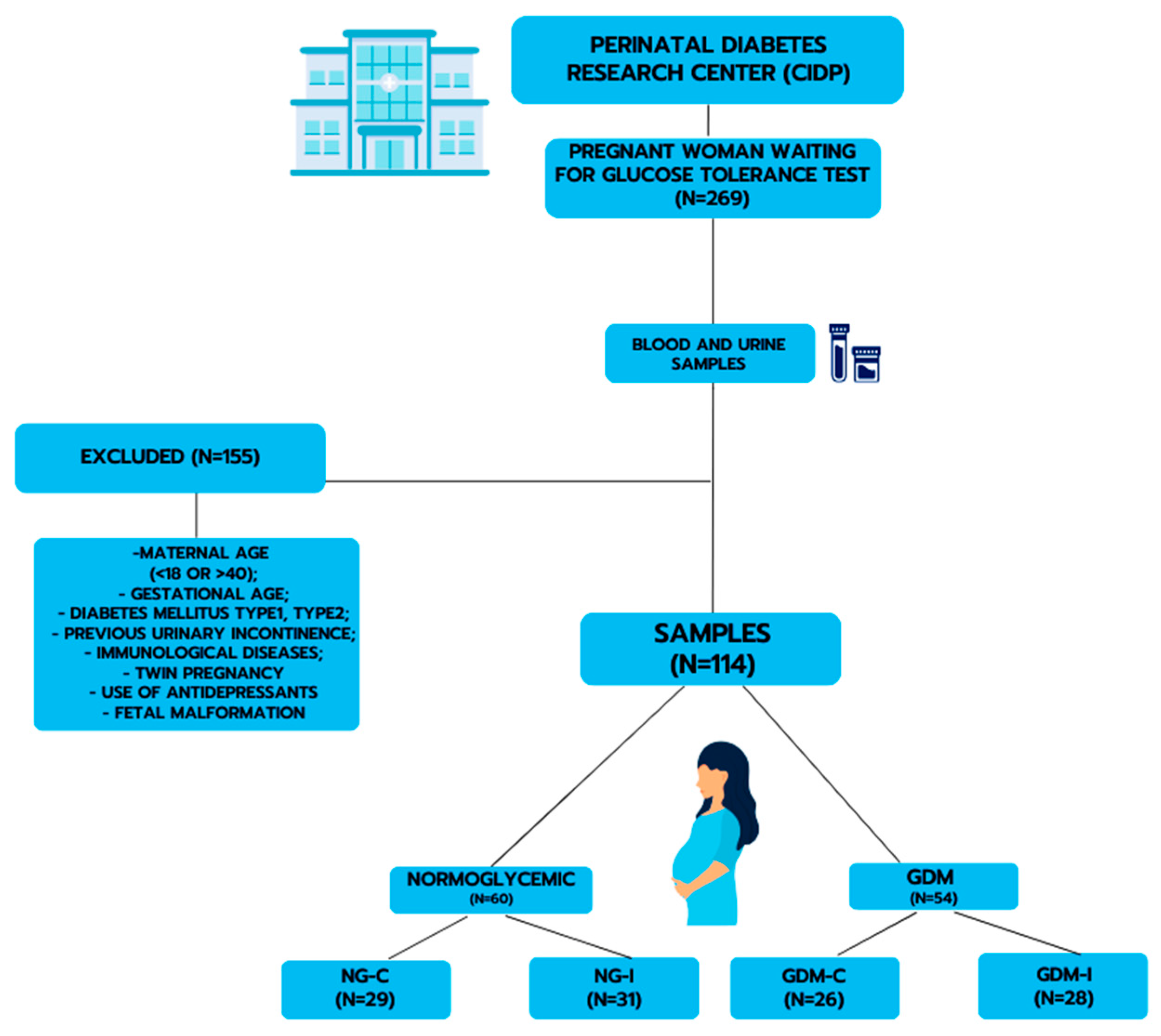
2.1. Study Population
2.2. Blood and Urine Samples
2.3. Melatonin Determination
2.4. Melatonin Receptors Determination
2.5. Urinary Melatonin–Sulfate Measurement
2.6. Cytokines Determination
2.7. Statistics
3. Results
3.1. Study Groups
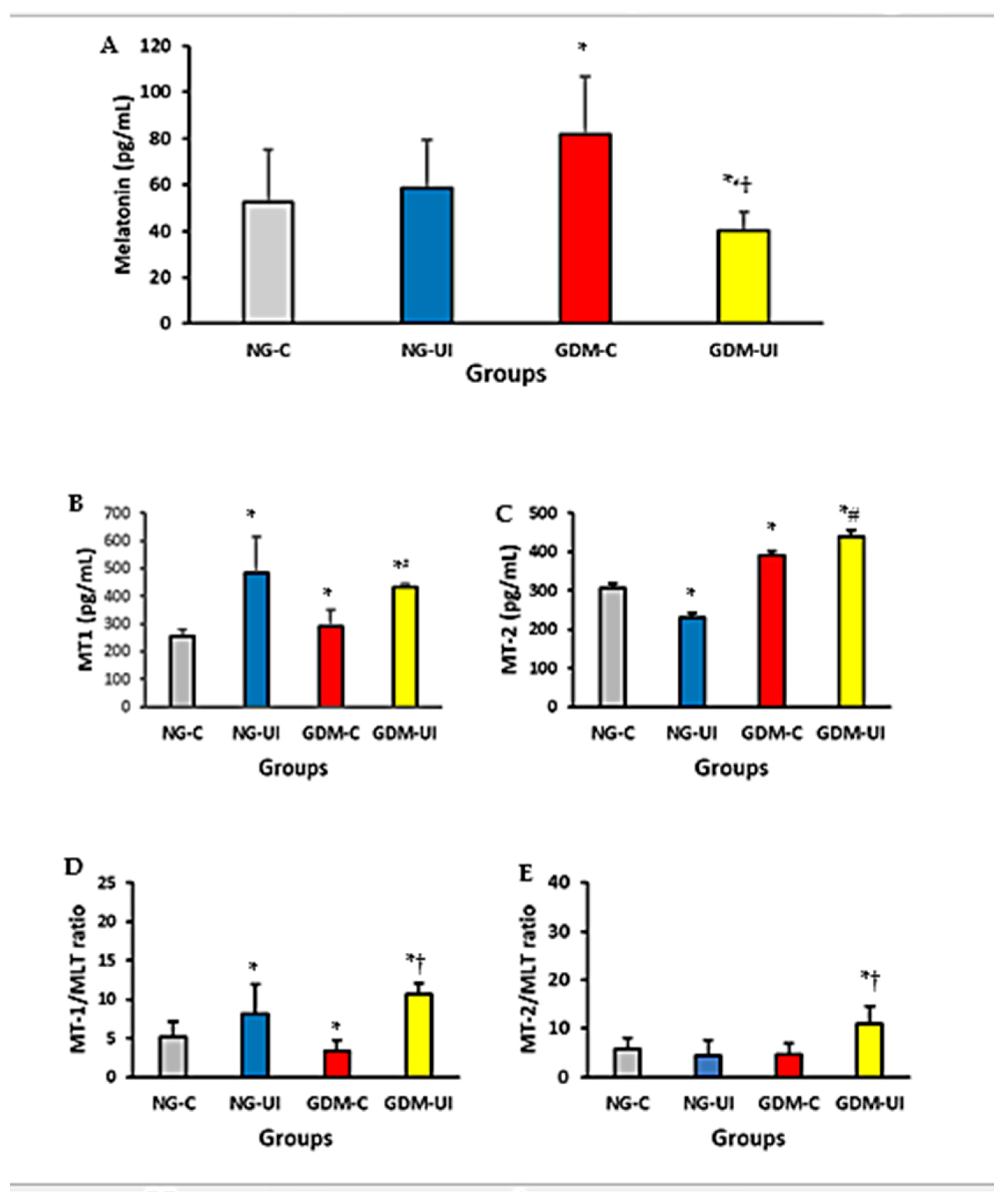
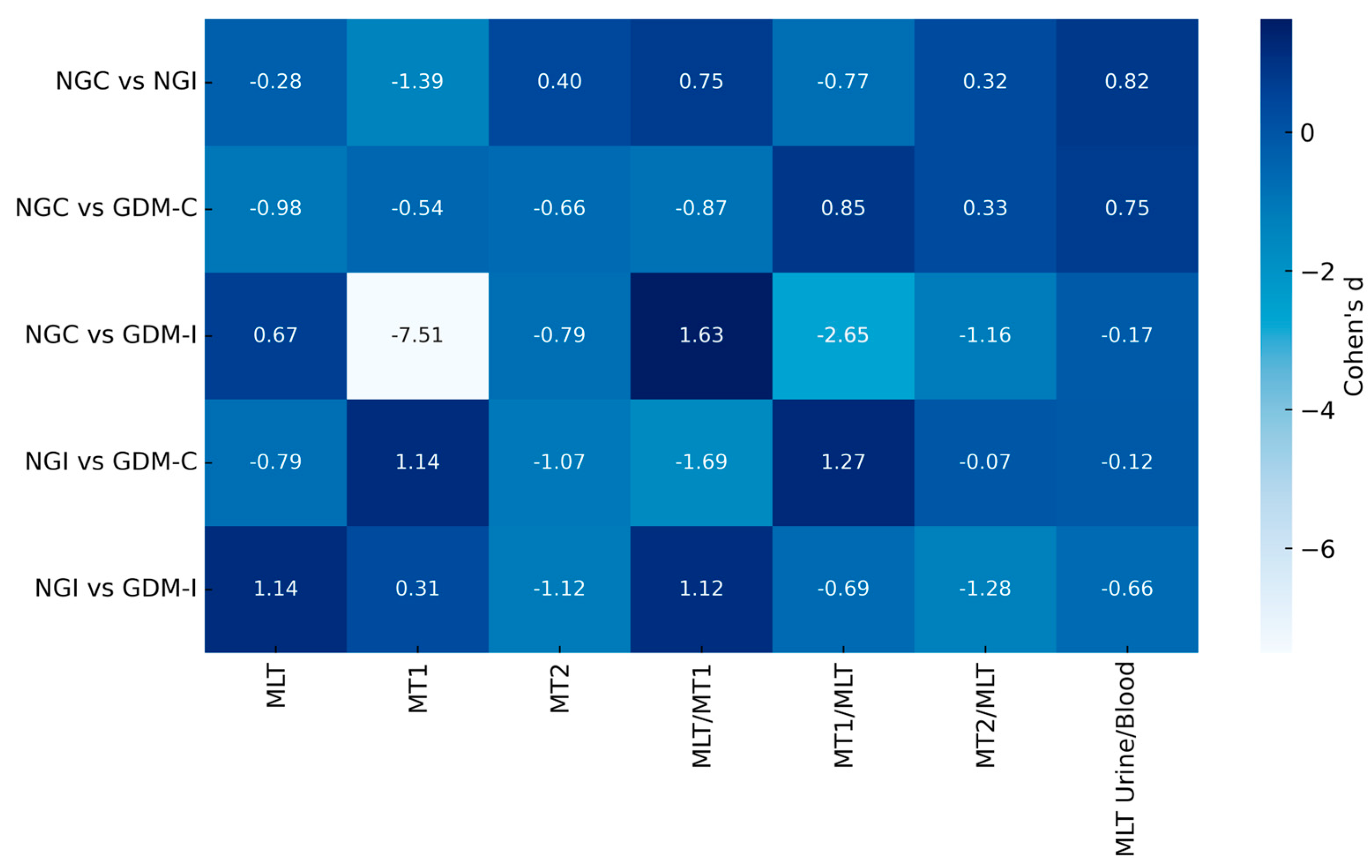
3.2. Blood Melatonin and Melatonin Receptors
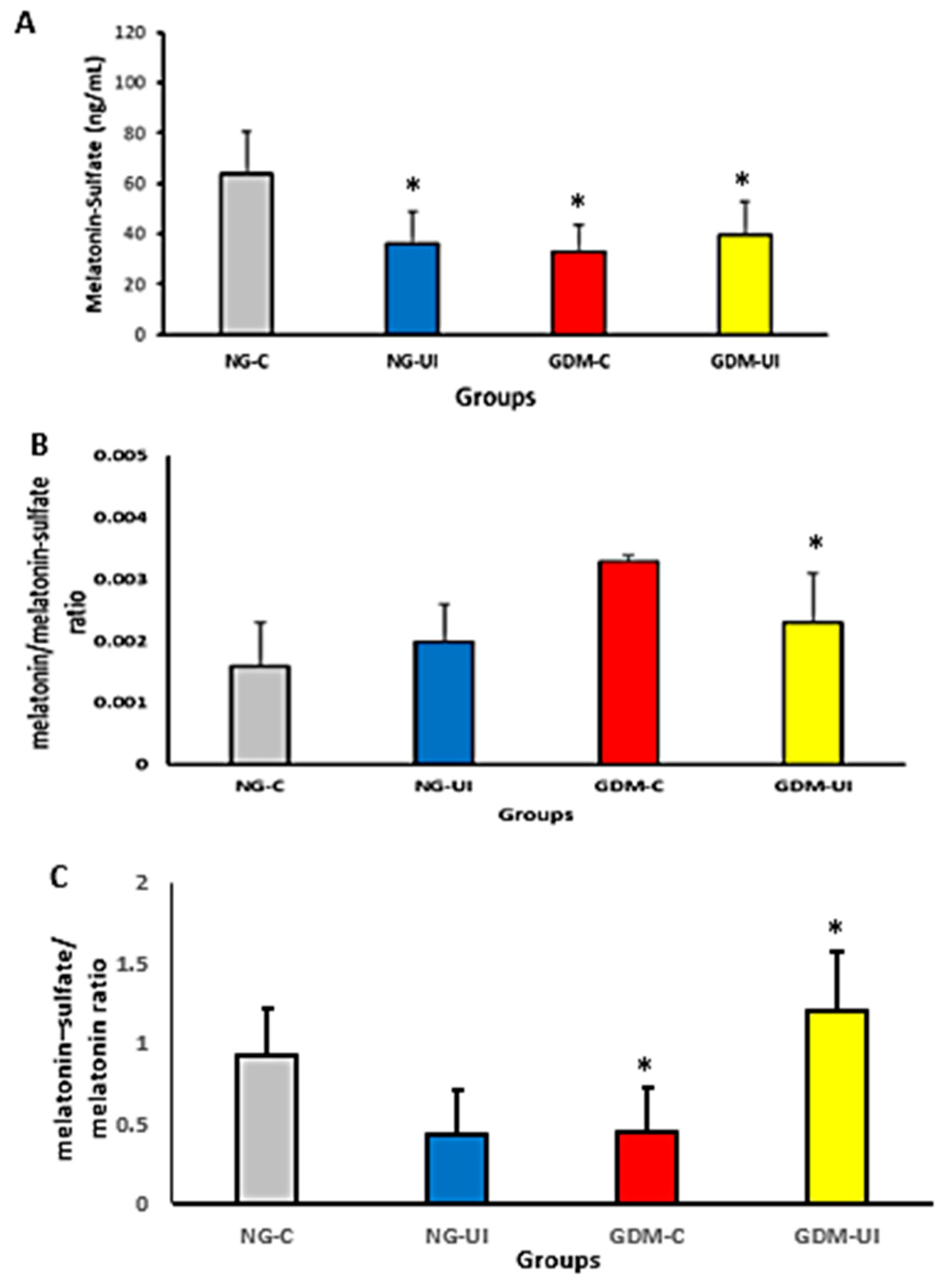
3.3. Urine Melatonin–Sulfate Level
3.4. Serum and Urine Cytokine Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moon, J.H.; Jang, H.C. Gestational Diabetes Mellitus: Diagnostic Approaches and Maternal-Offspring Complications. Diabetes Metab. J. 2022, 46, 3–14. [Google Scholar] [CrossRef]
- McIntyre, H.D.; Catalano, P.; Zhang, C.; Desoye, G.; Mathiesen, E.R.; Damm, P. Gestational diabetes mellitus. Nat. Rev. Dis. Primers 2019, 5, 47. [Google Scholar] [CrossRef]
- Kim, K.S.; Hong, S.; Han, K.; Park, C.Y. The clinical characteristics of gestational diabetes mellitus in Korea: A National Health Information Database Study. Endocrinol. Metab. 2021, 36, 628–636. [Google Scholar] [CrossRef]
- Barbosa, A.M.P.; Enriquez, E.M.A.; Rodrigues, M.R.K.; Prudencio, C.B.; Atallah, A.N.; Reyes, D.R.A.; Hallur, R.L.S.; Nunes, S.K.; Pinheiro, F.A.; Filho, C.I.S.; et al. Effectiveness of the pelvic floor muscle training on muscular dysfunction and pregnancy-specific urinary incontinence in pregnant women with gestational diabetes mellitus: A systematic review protocol. PLoS ONE 2020, 15, e0241962. [Google Scholar] [CrossRef]
- Huang, H.; Han, X.; Liu, Q.; Xue, J.; Yu, Z.; Miao, S. Associations between metabolic syndrome and female stress urinary incontinence: A meta-analysis. Int. Urogynecol. J. 2022, 33, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Nomura, K.; Senga, Y.; Okada, Y.; Kobayashi, K.; Okamoto, S.; Minokoshi, Y.; Imamura, M.; Takeda, S.; Hosooka, T.; et al. Hyperglycemia induces skeletal muscle atrophy via a WWP1/KLF15 axis. JCI Insight 2019, 4, e124952. [Google Scholar] [CrossRef] [PubMed]
- Reyes, D.R.A.; Barbosa, A.M.P.; Juliana, F.F.; Sofia, Q.B.C.V.; Costa, S.M.B.; Hallur, R.L.S.; Enriquez, E.M.A.; Oliveira, R.G.; Rossignolli, P.S.; Pedroni, C.R.; et al. Viability of ex-vivo myography as a diagnostic tool for rectus abdominis muscle electrical activity collected at Cesarean section within a Diamater cohort study. Biomed. Eng. Online 2022, 21, 76. [Google Scholar] [CrossRef]
- Fagundes, D.L.G.; França, E.L.; Fernandes, R.T.S.; Hara, C.C.P.; Morceli, G.; Honorio-França, A.C.; Calderon, I.M.P. Changes in T cell phenotype and cytokines profile in maternal blood, cord blood and colostrum of diabetic mothers. J. Matern. Fetal Neonatal Med. 2016, 29, 998–1004. [Google Scholar] [CrossRef]
- Kwon, J.; Kim, D.Y.; Cho, K.J.; Hashimoto, M.; Matsuoka, K.; Kamijo, T.; Wang, Z.; Karnup, S.; Robertson, A.M.; Tyagi, P.; et al. Pathophysiology of overactive bladder and pharmacologic treatments including β3-adrenoceptor agonists—Basic research perspectives. Int. Neurourol. J. 2024, 28 (Suppl. 1), S2–S33. [Google Scholar] [CrossRef] [PubMed]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, functions and therapeutic benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- França, D.C.H.; Fujimori, M.; Queiroz, A.A.; Borges, M.D.; Magalhães Neto, A.M.; Camargos, P.J.V.; Ribeiro, E.B.; França, E.L.; Honorio-França, A.C.; Fagundes-Triches, D.L.G. Melatonin and cytokines modulate daily instrumental activities of elderly people with SARS-CoV-2 infection. Int. J. Mol. Sci. 2023, 24, 8647. [Google Scholar] [CrossRef]
- França, D.C.H.; Honorio-França, A.C.; Silva, K.M.R.; Alves, F.C.B.; Bueno, G.; Costa, S.M.B.; Cotrim, A.C.d.M.; Barbosa, A.M.P.; França, E.L.; Rudge, M.V.C.; et al. Serotonin and Interleukin-10 Can Influence Blood and Urine Viscosity in Gestational Diabetes Mellitus and Pregnancy-Specific Urinary Incontinence. Int. J. Mol. Sci. 2023, 24, 17125. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.B.; Ali, A.; Bilal, M.; Rashid, S.M.; Wani, A.B.; Bhat, R.R.; Rehman, M.U. Melatonin and health: Insights of melatonin action, biological functions, and associated disorders. Cell. Mol. Neurobiol. 2023, 43, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Jin, Y.; Park, K.; Choi, J.; Kang, H.; Lee, S.-R.; Hong, Y. Elevated serum melatonin under constant darkness enhances neural repair in spinal cord injury through regulation of circadian clock proteins expression. J. Clin. Med. 2019, 8, 135. [Google Scholar] [CrossRef] [PubMed]
- França, D.C.H.; Franca, E.L.; Sobrevia, L.; Barbosa, A.M.P.; Honorio-Franca, A.C.; Rudge, M.V.C.; Diamater Study Group. Integration of nutrigenomics, melatonin, serotonin, and inflammatory cytokines in the pathophysiology of pregnancy-specific urinary incontinence in women with gestational diabetes mellitus. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166737. [Google Scholar] [CrossRef]
- Veen, A.V.; Minović, I.; Faassen, M.V.; Gomes-Neto, A.W.; Berger, S.P.; Bakker, S.J.L.; Kema, I.P. Urinary excretion of 6-sulfatoxymelatonin, the main metabolite of melatonin, and mortality in stable outpatient renal transplant recipients. J. Clin. Med. 2020, 9, 525. [Google Scholar] [CrossRef]
- McMullan, C.J.; Schernhammer, E.S.; Rimm, E.B.; Hu, F.B.; Forman, J.P. Melatonin secretion and the incidence of type 2 diabetes. JAMA 2013, 309, 1388–1396. [Google Scholar] [CrossRef]
- Aylamazyan, E.K.; Evsyukova, I.I.; Yarmolinskaya, M.I. The role of melatonin in the development of gestational diabetes. MOJ Curr. Res. Rev. 2018, 1, 159–162. [Google Scholar] [CrossRef]
- Ramsay, S.; Zagorodnyuk, V. Role of circadian rhythms and melatonin in bladder function in health and diseases. Auton. Neurosci. 2023, 246, 103083. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Scheer, F.A.J.L.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef]
- American Diabetes Association. Standards of Medical Care in Diabetes—2023. Diabetes Care 2023, 46 (Suppl. 1), S1–S194. [Google Scholar] [CrossRef]
- Tamanini, J.T.N.; Dambros, M.; D’Ancona, C.A.L.; Palma, P.C.R.; Netto, R., Jr. Validação para o português do “International Consultation on Incontinence Questionnaire-Short Form” (ICIQ-SF). Rev. Saude Publica 2004, 38, 438–444. [Google Scholar] [CrossRef]
- Pereira, V.S.; Santos, J.Y.C.; Correia, G.N.; Driusso, P. Tradução e validação para a língua portuguesa de um questionário para avaliação da gravidade da incontinência urinária. Rev. Bras. Ginecol. Obstet. 2011, 33, 182–187. [Google Scholar] [CrossRef]
- Muneeb, H.N.; Amjad, M.; Khaliq, H.M.; Shaukat, K.; Shabbir, M.; Shafique, S.; Hamid, M.F. Association between pelvic floor dysfunction and metabolic syndrome: Pelvic floor dysfunction and metabolic syndrome. Pak. J. Med. Sci. 2022, 5, 55–59. [Google Scholar] [CrossRef]
- Hardeland, R. Melatonin and Inflammation—Story of a Double-Edged Blade. J. Pineal Res. 2018, 65, e12525. [Google Scholar] [CrossRef]
- Wang, C.; Jin, L.; Tong, M.; Zhang, J.; Yu, J.; Meng, W.; Jin, L. Prevalence of gestational diabetes mellitus and its determinants among pregnant women in Beijing. J. Matern. Fetal Neonatal Med. 2022, 35, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Garmendia, M.L.; Mondschein, S.; Montiel, B.; Kusanovic, J.P. Trends and predictors of gestational diabetes mellitus in Chile. Int. J. Gynaecol. Obstet. 2020, 148, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Cornejo, M.; Fuentes, G.; Valero, P.; Vega, S.; Grismaldo, A.; Toledo, F.; Pardo, F.; Moore-Carrasco, R.; Subiabre, M.; Casanello, P.; et al. Gestational diabesity and foetoplacental vascular dysfunction. Acta Physiol. 2021, 232, e13671. [Google Scholar] [CrossRef] [PubMed]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The pathophysiology of gestational diabetes mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef]
- Morceli, G.; Honorio-França, A.C.; Fagundes, D.L.G.; Calderon, I.M.P.; França, E.L. Antioxidant Effect of Melatonin on the Functional Activity of Colostral Phagocytes in Diabetic Women. PLoS ONE 2013, 8, e56915. [Google Scholar] [CrossRef]
- Mendes, L.; Queiroz, M.; Sena, C.M. Melatonin and Vascular Function. Antioxidants 2024, 13, 747. [Google Scholar] [CrossRef]
- Pozo, M.J.; Gomez-Pinilla, P.J.; Camello-Almaraz, C.; Martin-Cano, F.E.; Pascua, P.; Rol, M.A.; Acuna-Castroviejo, D.; Camello, P.J. Melatonin, a Potential Therapeutic Agent for Smooth Muscle-Related Pathological Conditions and Aging. Curr. Med. Chem. 2010, 17, 4150–4165. [Google Scholar] [CrossRef]
- Hanna-Mitchel, A.T.; Robinson, D.; Cardozo, L.; Everaert, K.; Petkov, G.V. Do We Need to Know More about the Effects of Hormones on Lower Urinary Tract Dysfunction? ICI-RS 2014. Neurourol. Urodyn. 2016, 35, 299–303. [Google Scholar] [CrossRef]
- Gomez-Pinilla, P.J.; Pozo, M.J.; Camello, P.J. Aging Impairs Neurogenic Contraction in Guinea Pig Urinary Bladder: Role of Oxidative Stress and Melatonin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R793–R803. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Koppisetti, S.; Jenigiri, B.; Terron, M.P.; Tengattini, S.; Tamura, H.; Flores, L.J.; Tan, D.X.; Reiter, R.J. Reactive Oxygen Species and the Hypomotility of the Gall Bladder as Targets for the Treatment of Gallstones with Melatonin: A Review. Dig. Dis. Sci. 2008, 53, 2592–2603. [Google Scholar] [CrossRef]
- Gomez-Pinilla, P.J.; Gomez, M.F.; Sward, K.; Hedlund, P.; Hellstrand, P.; Camello, P.J.; Andersson, K.E.; Pozo, M.J. Melatonin Restores Impaired Contractility in Aged Guinea Pig Urinary Bladder. J. Pineal Res. 2008, 44, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Owino, S.; Sánchez-Bretaño, A.; Tchio, C.; Cecon, E.; Karamitri, A.; Dam, J.; Jockers, R.; Piccione, G.; Noh, H.L.; Kim, T.; et al. Nocturnal Activation of Melatonin Receptor Type 1 Signaling Modulates Diurnal Insulin Sensitivity via Regulation of PI3K Activity. J. Pineal Res. 2018, 64, e12462. [Google Scholar] [CrossRef]
- Owino, S.; Buonfiglio, D.D.C.; Tchio, C.; Tosini, G. Melatonin Signaling: A Key Regulator of Glucose Homeostasis and Energy Metabolism. Front. Endocrinol. 2019, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Carlomagno, G.; Minini, M.; Tilotta, M.; Unfer, V. From Implantation to Birth: Insight into Molecular Melatonin Functions. Int. J. Mol. Sci. 2018, 19, 2802. [Google Scholar] [CrossRef]
- Man, G.C.W.; Zhang, T.; Chen, X.; Wang, J.; Wu, F.; Liu, Y.; Wang, C.C.; Cheong, Y.; Li, T.C. The Regulations and Role of Circadian Clock and Melatonin in Uterine Receptivity and Pregnancy—An Immunological Perspective. Am. J. Reprod. Immunol. 2017, 78, e12715. [Google Scholar] [CrossRef]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology Meets Neuropsychopharmacology: Translational Implications of the Impact of Inflammation on Behavior. Neuropsychopharmacology 2012, 37, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Chess-Williams, R.; McDermott, C.; Sellers, D.J.; West, E.G.; Mills, K.A. Chronic Psychological Stress and Lower Urinary Tract Symptoms. Low. Urin. Tract Symptoms 2021, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Jhang, J.F.; Hsu, Y.H.; Ho, H.C.; Wu, Y.H.; Kuo, H.C. Urine Cytokines as Biomarkers for Diagnosing Interstitial Cystitis/Bladder Pain Syndrome and Mapping Its Clinical Characteristics. Am. J. Physiol. Renal Physiol. 2020, 318, F1391–F1399. [Google Scholar] [CrossRef] [PubMed]
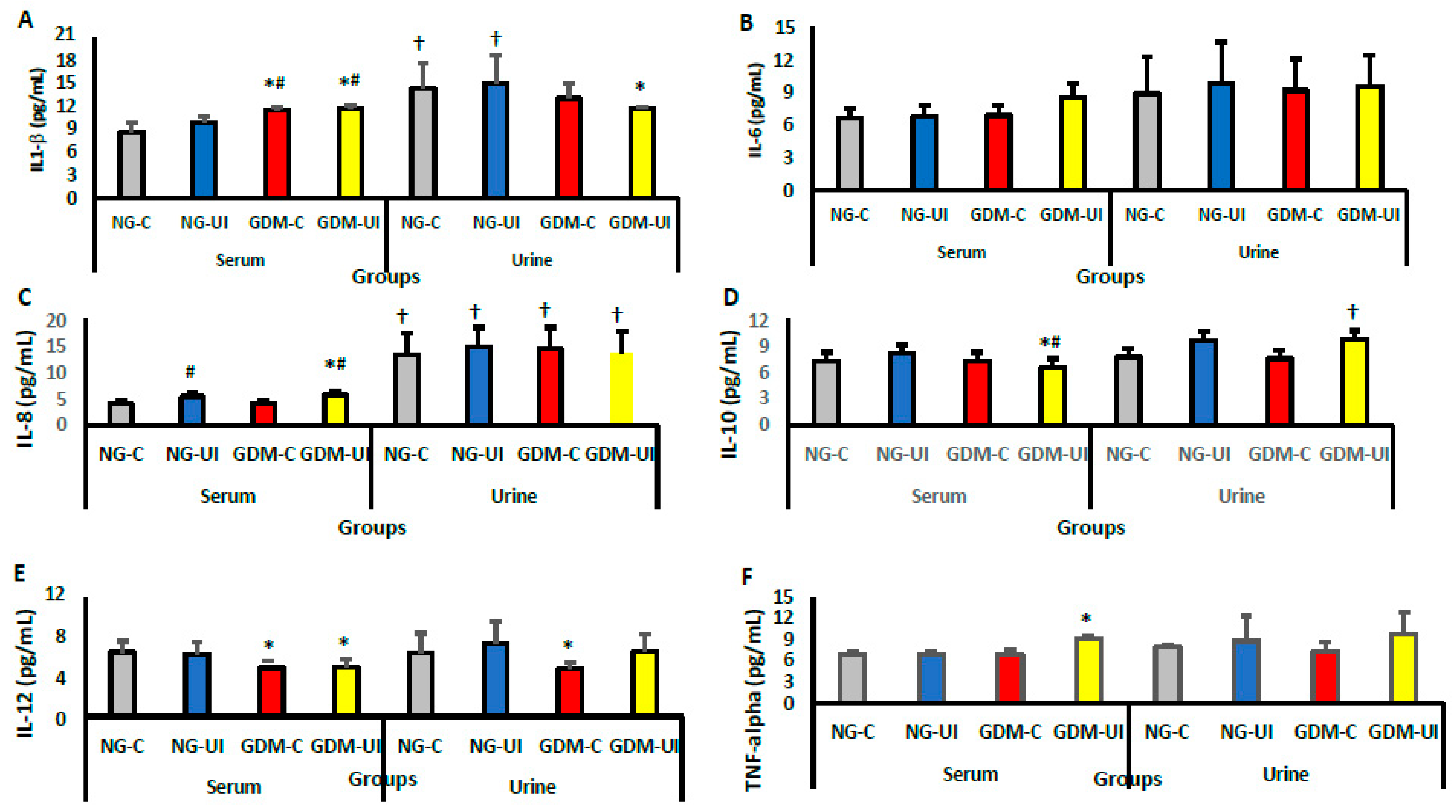
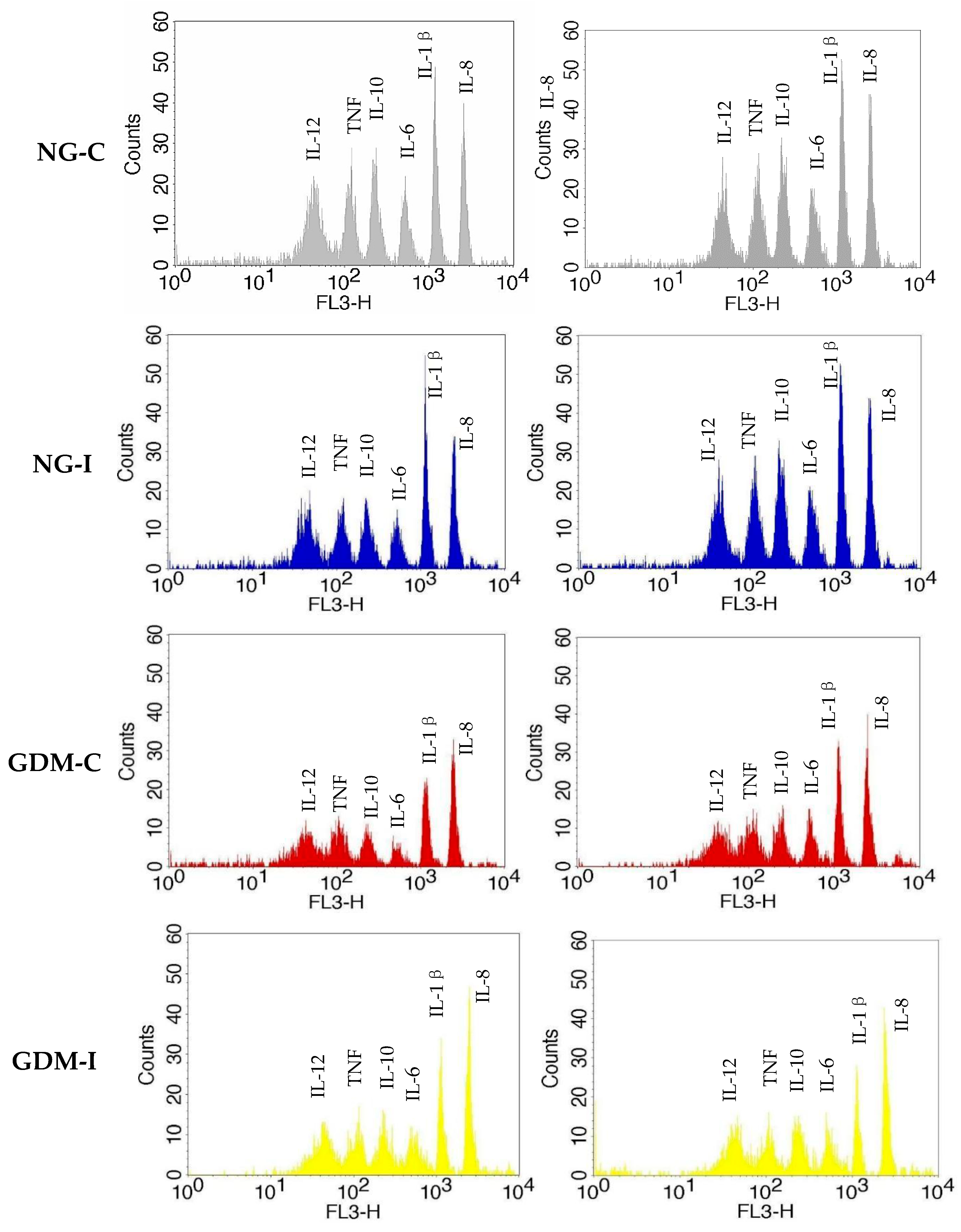
| NG-C (n = 29) | NG-UI (n = 31) | GDM-C (n = 26) | GDM-UI (n = 28) | |
|---|---|---|---|---|
| Age | 25.0 ± 4.6 | 27.1 ± 7.2 | 30.5 ± 6.1 * | 30.9 ± 6.3 *† |
| BMI | 29.0 ± 4.2 | 30.0 ± 5.5 | 34.7 ± 8.2 * | 36.0 ± 7.1 *† |
| Glucose (mg/dL) | 76.2 ± 6.9 | 76.2 ± 7.9 | 93.2 ± 10.6 * | 92.0 ± 10.3 *† |
| HbA1c (%) | 4.9 ± 0.3 | 5.0 ± 0.3 | 5.0 ± 0.4 | 5.2 ± 0.4 *† |
| Urinary density | 1.0 ± 0.1 | 1.01 ± 0.1 | 1.0 ± 0.1 | 1.2 ± 0.1 |
| Cytokines | Blood/Urine Ratio | Statistical | |||
|---|---|---|---|---|---|
| NG-C | NG-UI | GDM-C | GDM-UI | ||
| IL-1 β | 0.63 ± 0.14 | 0.88 ± 0.31 * | 0.89 ± 0.10 * | 1.06 ± 0.04 * | F = 17.6607; |
| p = 0.0001 | |||||
| IL-6 | 0.86 ± 0.32 | 0.92 ± 0. 50 | 0.80 ± 0.30 | 0.86 ± 0.28 | F = 0.1333; |
| p = 0.9387 | |||||
| IL-8 | 0.39 ± 0.12 | 0.38 ± 0.11 | 0.37 ± 0.09 | 0.46 ± 0.22 | F = 0.3809; |
| p = 0.7704 | |||||
| IL-10 | 0.97 ± 0.27 | 0.90 ± 0.22 | 0.96 ± 0.15 | 0.87 ± 0.22 | F = 0.4176; |
| p = 0.7450 | |||||
| IL-12 | 1.01 ± 0.41 | 0.99 ± 0.34 | 0.94 ± 0.22 | 1.20 ± 0.15 * | F = 2.3077 |
| p = 0.0482 | |||||
| TNF-α | 0.86 ± 0.05 | 0.85 ± 0.18 | 0.98 ± 0.15 | 0.90 ± 0.33 | F = 1.2564; |
| p = 0.3088 | |||||
| Melatonin | NG-C | NG-UI | GDM-C | GDM-UI |
|---|---|---|---|---|
| IL-1 β | r = −0.4102 p = 0.3607 | r = −0.6301 p = 0.1292 | r = −0.6414 p = 0.1204 | r = 0.2530 p = 0.5840 |
| IL-6 | r = 0.0710 p = 0.8797 | r = 0.0690 p = 0.8831 | r = −0.2908 p = 0.5269 | r = 0.2798 p = 0.5433 |
| IL-8 | r = 0.4632 p = 0.2951 | r = 0.2298 p = 0.6200 | r = 0.2911 p = 0.5265 | r = 0.0346 p = 0.9413 |
| IL-10 | r = 0.2824 p = 0.5394 | r = −0.2685 p = 0.5605 | r = 0.5486 p = 0.2021 | r = 0.8980 * p = 0.0470 |
| IL-12 | r = −0.4874 p = 0.2672 | r = −0.0890 p = 0.8496 | r = 0.4474 p = 0.3141 | r = −0.0698 p = 0.8818 |
| TNF-α | r = 0.2669 p = 0.5628 | r = 0.4940 p = 0.2598 | r = 0.4033 p = 0.3696 | r = −0.9310 * p = 0.0427 |
| Melatonin–sulfate | NG-C | NG-UI | GDM-C | GDM-UI |
|---|---|---|---|---|
| IL-1 β | r = −0.1597 p = 0.7056 | r = −0.1267 p = 0.7649 | r = −0.2110 p = 0.6159 | r = −0.4096 p = 0.3135 |
| IL-6 | r = −0.1461 p = 0.7298 | r = 0.2588 p = 0.5360 | r = 0.0018 p = 0.9966 | r = 0.8984 * p = 0.0024 |
| IL-8 | r = 0.4240 p = 0.2941 | r = 0.4627 p = 0.2482 | r = −0.0991 p = 0.9829 | r = 0.0278 p = 0.9480 |
| IL-10 | r = 0.3738 p = 0.3617 | r = 0.1833 p = 0.6639 | r = −0.6275 p = 0.0958 | r = 0.7669 * p = 0.0263 |
| IL-12 | r = −0.0266 p = 0.9501 | r = 0.1666 p = 0.8496 | r = −0.2638 p = 0.5279 | r = −0.4411 p = 0.2739 |
| TNF-α | r = −0.2657 p = 0.5247 | r = 0.0696 p = 0.6933 | r = 0.2398 p = 0.5672 | r = −0.5681 p = 0.1417 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
França, D.C.H.; França, E.L.; Honório-França, A.C.; Silva, K.M.R.; Queiroz, A.A.d.; Morais, T.C.; França, E.C.H.; de Carvalho, C.N.F.; Fagundes-Triches, D.L.G.; Barbosa, A.M.P.; et al. Melatonin and Inflammatory Cytokines as Modulators of the Interaction Between Gestational Diabetes Mellitus and Pregnancy-Specific Urinary Incontinence. Metabolites 2025, 15, 699. https://doi.org/10.3390/metabo15110699
França DCH, França EL, Honório-França AC, Silva KMR, Queiroz AAd, Morais TC, França ECH, de Carvalho CNF, Fagundes-Triches DLG, Barbosa AMP, et al. Melatonin and Inflammatory Cytokines as Modulators of the Interaction Between Gestational Diabetes Mellitus and Pregnancy-Specific Urinary Incontinence. Metabolites. 2025; 15(11):699. https://doi.org/10.3390/metabo15110699
Chicago/Turabian StyleFrança, Danielle Cristina Honório, Eduardo Luzia França, Adenilda Cristina Honório-França, Kênia Maria Rezende Silva, Adriele Ataídes de Queiroz, Tassiane Cristina Morais, Emanuelle Carolina Honorio França, Carolina Neiva Frota de Carvalho, Danny Laura Gomes Fagundes-Triches, Angélica Mércia Pascon Barbosa, and et al. 2025. "Melatonin and Inflammatory Cytokines as Modulators of the Interaction Between Gestational Diabetes Mellitus and Pregnancy-Specific Urinary Incontinence" Metabolites 15, no. 11: 699. https://doi.org/10.3390/metabo15110699
APA StyleFrança, D. C. H., França, E. L., Honório-França, A. C., Silva, K. M. R., Queiroz, A. A. d., Morais, T. C., França, E. C. H., de Carvalho, C. N. F., Fagundes-Triches, D. L. G., Barbosa, A. M. P., Calderon, I. d. M. P., Sobrevia, L., & Rudge, M. V. C. (2025). Melatonin and Inflammatory Cytokines as Modulators of the Interaction Between Gestational Diabetes Mellitus and Pregnancy-Specific Urinary Incontinence. Metabolites, 15(11), 699. https://doi.org/10.3390/metabo15110699








