-
 Mechanotransduction and Skeletal Muscle Atrophy: The Interplay Between Focal Adhesions and Oxidative Stress
Mechanotransduction and Skeletal Muscle Atrophy: The Interplay Between Focal Adhesions and Oxidative Stress -
 Epigenetic Echoes: Bridging Nature, Nurture, and Healing Across Generations
Epigenetic Echoes: Bridging Nature, Nurture, and Healing Across Generations -
 TL1A as a Target in Inflammatory Bowel Disease: Exploring Mechanisms and Therapeutic Potential
TL1A as a Target in Inflammatory Bowel Disease: Exploring Mechanisms and Therapeutic Potential -
 Nanozymes: Innovative Therapeutics in the Battle Against Neurodegenerative Diseases
Nanozymes: Innovative Therapeutics in the Battle Against Neurodegenerative Diseases -
 Breaking the Barrier: The Role of Proinflammatory Cytokines in BBB Dysfunction
Breaking the Barrier: The Role of Proinflammatory Cytokines in BBB Dysfunction
Journal Description
International Journal of Molecular Sciences
International Journal of Molecular Sciences
is an international, peer-reviewed, open access journal providing an advanced forum for biochemistry, molecular and cell biology, molecular biophysics, molecular medicine, and all aspects of molecular research in chemistry, and is published semimonthly online by MDPI. The Australian Society of Plant Scientists (ASPS), Epigenetics Society, European Chitin Society (EUCHIS), Spanish Society for Cell Biology (SEBC) and others are affiliated with IJMS and their members receive a discount on the article processing charges.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, MEDLINE, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q1 (Biochemistry and Molecular Biology) / CiteScore - Q1 (Organic Chemistry)
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 20.5 days after submission; acceptance to publication is undertaken in 2.6 days (median values for papers published in this journal in the first half of 2025).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
- Testimonials: See what our editors and authors say about IJMS.
- Companion journals for IJMS include: Biophysica, Stresses, Lymphatics and SynBio.
Impact Factor:
4.9 (2024);
5-Year Impact Factor:
5.7 (2024)
Latest Articles
Proteomic Insights into Bacterial Responses to Antibiotics: A Narrative Review
Int. J. Mol. Sci. 2025, 26(15), 7255; https://doi.org/10.3390/ijms26157255 (registering DOI) - 27 Jul 2025
Abstract
Antimicrobial resistance is an escalating global threat that undermines the efficacy of modern antibiotics and places a substantial economic burden on healthcare systems—costing Europe alone over EUR 11.7 billion each year due to rising medical expenses and productivity losses. While genomics and transcriptomics
[...] Read more.
Antimicrobial resistance is an escalating global threat that undermines the efficacy of modern antibiotics and places a substantial economic burden on healthcare systems—costing Europe alone over EUR 11.7 billion each year due to rising medical expenses and productivity losses. While genomics and transcriptomics have significantly advanced our understanding of the genetic foundations of resistance, they often fail to capture the dynamic, real-time adaptations that enable bacterial survival. Proteomics, particularly mass spectrometry-based strategies, bridges this gap by uncovering the functional protein-level changes that drive resistance, persistence, and tolerance under antibiotic pressure. In this review, we examine how proteomic approaches provide new insights into resistance mechanisms across various antibiotic classes, with a particular focus on β-lactams, aminoglycosides, and fluoroquinolones, highlighting clinically relevant pathogens, especially members of the ESKAPE group. Finally, we examine future directions, including the integration of proteomics with other omic technologies and the growing role of artificial intelligence in resistance prediction, paving the way for more predictive, personalized, and effective solutions to combat antimicrobial resistance.
Full article
(This article belongs to the Special Issue Proteomics: An Invaluable Tool to Gain Information on Biological Systems and Their Disease Alterations)
Open AccessArticle
Probing the pH Effect on Boehmite Particles in Water Using Vacuum Ultraviolet Single-Photon Ionization Mass Spectrometry
by
Xiao Sui, Bo Xu and Xiao-Ying Yu
Int. J. Mol. Sci. 2025, 26(15), 7254; https://doi.org/10.3390/ijms26157254 (registering DOI) - 27 Jul 2025
Abstract
Boehmite has been widely used in theoretical research and industry, especially for hazardous material processing. For the liquid-phase treating process, the interfacial properties of boehmite are believed to be affected by pH conditions, which change its physicochemical behavior. However, molecular-level detection on cluster
[...] Read more.
Boehmite has been widely used in theoretical research and industry, especially for hazardous material processing. For the liquid-phase treating process, the interfacial properties of boehmite are believed to be affected by pH conditions, which change its physicochemical behavior. However, molecular-level detection on cluster ions is challenging when using bulk approaches. Herein we employ in situ vacuum ultraviolet single-photon ionization mass spectrometry (VUV SPI-MS) coupled with a vacuum-compatible microreactor system for analysis at the liquid–vacuum interface (SALVI) to investigate the solute molecular composition of boehmite under different pH conditions for the first time. The mass spectral results show that more complex clustering of solute molecules exists at the solid–liquid (s–l) interface than conventionally perceived in a “simple” aqueous solution. Besides solute ions, such as boehmite molecules and fragments, the composition and appearance energies of these newly discovered solvated cluster ions are determined by VUV SPI-MS in different pH solutions. We offer new results for the pH-dependent effect of boehmite and provide insights into a more detailed solvation mechanism at the s–l interface. By comparing the key products under different pH conditions, fundamental understanding of boehmite dissolution is revealed to assist the engineering design of waste processing and storage solutions.
Full article
(This article belongs to the Special Issue Ion and Molecule Transport in Membrane Systems, 6th Edition)
►▼
Show Figures

Figure 1
Open AccessReview
Serotonin Modulation of Dorsoventral Hippocampus in Physiology and Schizophrenia
by
Charalampos L. Kandilakis and Costas Papatheodoropoulos
Int. J. Mol. Sci. 2025, 26(15), 7253; https://doi.org/10.3390/ijms26157253 (registering DOI) - 27 Jul 2025
Abstract
The serotonergic system, originating in the raphe nuclei, differentially modulates the dorsal and ventral hippocampus, which are implicated in cognition and emotion, respectively. Emerging evidence from rodent models (e.g., neonatal ventral hippocampal lesion, pharmacological NMDA receptor antagonist exposure) and human postmortem studies indicates
[...] Read more.
The serotonergic system, originating in the raphe nuclei, differentially modulates the dorsal and ventral hippocampus, which are implicated in cognition and emotion, respectively. Emerging evidence from rodent models (e.g., neonatal ventral hippocampal lesion, pharmacological NMDA receptor antagonist exposure) and human postmortem studies indicates dorsoventral serotonergic alterations in schizophrenia. These data include elevated 5-HT1A receptor expression in the dorsal hippocampus, linking serotonergic hypofunction to cognitive deficits, and hyperactive 5-HT2A/3 receptor signaling and denser serotonergic innervation in the ventral hippocampus driving local hyperexcitability associated with psychosis and stress responsivity. These dorsoventral serotonergic alterations are shown to disrupt the excitation–inhibition balance, impair synaptic plasticity, and disturb network oscillations, as established by in vivo electrophysiology and functional imaging. Synthesizing these multi-level findings, we propose a novel “dorsoventral serotonin imbalance” model of schizophrenia, in which ventral hyperactivation predominantly contributes to psychotic symptoms and dorsal hypoactivity underlies cognitive deficits. We further highlight promising preclinical evidence that selective targeting of region- and receptor-specific targeting, using both pharmacological agents and emerging delivery technologies, may offer novel therapeutic opportunities enabling symptom-specific strategies in schizophrenia.
Full article
(This article belongs to the Special Issue Unveiling Mental Disorders: Molecular Mechanisms and Innovative Therapies)
►▼
Show Figures

Figure 1
Open AccessArticle
Low BOK Expression Promotes Epithelial–Mesenchymal Transition and Migration via the Wnt Signaling Pathway in Breast Cancer Cells
by
Ling Liu, Tiantian He, Zhen Zhang, Wenjie Dai, Liyang Ding, Hong Yang, Bo Xu, Yitong Shang, Yu Deng, Xufeng Fu and Xing Du
Int. J. Mol. Sci. 2025, 26(15), 7252; https://doi.org/10.3390/ijms26157252 (registering DOI) - 27 Jul 2025
Abstract
The B-cell lymphoma 2 (Bcl-2)-related ovarian killer (BOK), a member of the Bcl-2 protein family, shares a similar domain structure and amino acid sequence homology with the pro-apoptotic family members BAX and BAK. Although BOK is involved in the development of various types
[...] Read more.
The B-cell lymphoma 2 (Bcl-2)-related ovarian killer (BOK), a member of the Bcl-2 protein family, shares a similar domain structure and amino acid sequence homology with the pro-apoptotic family members BAX and BAK. Although BOK is involved in the development of various types of cancer, its mechanism of action in breast cancer remains unclear. This study found that BOK was involved in the process of MG132, inhibiting the migration and epithelial–mesenchymal transition (EMT) of breast cancer cells induced by transforming growth factor-β. Furthermore, interfering BOK reversed the inhibition of breast cancer cell migration and the EMT process by MG132. Additional studies revealed that BOK silencing promoted the expression of EMT-related markers in breast cancer cells, while BOK overexpression inhibited EMT and migration. Using RNA-seq sequencing and Western blotting, we confirmed that the Wnt signaling pathway is involved in BOK regulating the EMT process in breast cancer cells. Therefore, we conclude that low BOK expression promotes breast cancer EMT and migration by activating the Wnt signaling pathway. This study enhances our understanding of breast cancer pathogenesis and suggests that BOK may serve as a potential prognostic marker and therapeutic target for breast cancer.
Full article
(This article belongs to the Section Molecular Biology)
►▼
Show Figures

Figure 1
Open AccessArticle
The Rapid Activation of MYDGF Is Critical for Cell Survival in the Acute Phase of Retinal Regeneration in Fish
by
Kayo Sugitani, Yuya Omori, Takumi Mokuya, Serika Hosoi, Haruto Kobayashi, Koki Miyata, Yuhei Araiso and Yoshiki Koriyama
Int. J. Mol. Sci. 2025, 26(15), 7251; https://doi.org/10.3390/ijms26157251 (registering DOI) - 27 Jul 2025
Abstract
Myeloid-derived growth factor (MYDGF), named in reference to its secretion from myeloid cells in bone marrow, is a novel protein with anti-apoptotic and tissue-repairing properties. MYDGF is found in various human tissues affected by different diseases. To date, however, MYDGF expression has yet
[...] Read more.
Myeloid-derived growth factor (MYDGF), named in reference to its secretion from myeloid cells in bone marrow, is a novel protein with anti-apoptotic and tissue-repairing properties. MYDGF is found in various human tissues affected by different diseases. To date, however, MYDGF expression has yet to be reported in the nervous system. Herein, we demonstrate for the first time that MYDGF mRNA levels increased in the zebrafish retina 1 h after optic nerve injury (ONI). MYDGF-producing cells were located in the photoreceptors and infiltrating leukocytic cells. We prepared the retina for MYDGF gene knockdown by performing intraocular injections using either MYDGF-specific morpholino or the CRISPR/Cas9 system. Under these MYDGF-knockdown retinal conditions, anti-apoptotic Bcl-2 mRNA was suppressed; in comparison, apoptotic caspase-3 and inflammatory TNFα mRNA were significantly upregulated in the zebrafish retina after ONI compared to the control. Furthermore, heat shock factor 1 (HSF1) was evidently suppressed under these conditions, leading to a significant number of apoptotic neurons. These findings indicate that MYDGF is a key molecule in the stimulation of neuronal regeneration in the central nervous system.
Full article
(This article belongs to the Special Issue Molecular Mechanisms of Neurodegeneration and Neuroprotection for Retinal and Optic Nerve Diseases)
Open AccessArticle
Cepharanthine Promotes Ca2+-Independent Premature Red Blood Cell Death Through Metabolic Insufficiency and p38 MAPK/CK1α/COX/MLKL/PKC/iNOS Signaling
by
Shaymah H. Alruwaili, Jawaher Alsughayyir and Mohammad A. Alfhili
Int. J. Mol. Sci. 2025, 26(15), 7250; https://doi.org/10.3390/ijms26157250 (registering DOI) - 27 Jul 2025
Abstract
Nonspecific toxicity to normal and malignant cells restricts the clinical utility of many anticancer drugs. In particular, anemia in cancer patients develops due to drug-induced toxicity to red blood cells (RBCs). The anticancer alkaloid, cepharanthine (CEP), elicits distinct forms of cell death including
[...] Read more.
Nonspecific toxicity to normal and malignant cells restricts the clinical utility of many anticancer drugs. In particular, anemia in cancer patients develops due to drug-induced toxicity to red blood cells (RBCs). The anticancer alkaloid, cepharanthine (CEP), elicits distinct forms of cell death including apoptosis and autophagy, but its cytotoxicity to RBCs has not been investigated. Colorimetric and fluorometric techniques were used to assess eryptosis and hemolysis in control and CEP-treated RBCs. Cells were labeled with Fluo4/AM and annexin-V-FITC to measure Ca2+ and phosphatidylserine (PS) exposure, respectively. Forward scatter (FSC) was detected to estimate cell size, and extracellular hemoglobin along with lactate dehydrogenase and aspartate transaminase activities were assayed to quantify hemolysis. Physiological manipulation of the extracellular milieu and various signaling inhibitors were tested to dissect the underlying mechanisms of CEP-induced RBC death. CEP increased PS exposure and hemolysis indices and decreased FSC in a concentration-dependent manner with prominent membrane blebbing. Although no Ca2+ elevation was detected, chelation of intracellular Ca2+ by BAPTA-AM reduced hemolysis. Whereas SB203580, D4476, acetylsalicylic acid, necrosulfonamide, and melatonin inhibited both PS exposure and hemolysis, staurosporin, L-NAME, ascorbate, caffeine, adenine, and guanosine only prevented hemolysis. Interestingly, sucrose had a unique dual effect by exacerbating PS exposure and reversing hemolysis. Of note, blocking KCl efflux augmented PS exposure while aggravating hemolysis only under Ca2+-depleted conditions. CEP activates Ca2+-independent pathways to promote eryptosis and hemolysis. The complex cytotoxic profile of CEP can be mitigated by targeting the identified modulatory pathways to potentiate its anticancer efficacy.
Full article
(This article belongs to the Special Issue Blood Cells in Human Health and Disease)
►▼
Show Figures

Figure 1
Open AccessReview
Spinal Cord Injury Remyelination: Pathways to Therapies
by
Julia K. Kaniuk, Divy Kumar, Joshua Tennyson, Kaitlyn L. Hurka, Alexander Margolis, Andrei Bucaloiu, Ashley Selner and Christopher S. Ahuja
Int. J. Mol. Sci. 2025, 26(15), 7249; https://doi.org/10.3390/ijms26157249 (registering DOI) - 26 Jul 2025
Abstract
Spinal cord injury (SCI) is a debilitating condition that results from a culmination of acute and chronic damage to neural tissue, specifically the myelin sheath, thus impacting neurons’ abilities to synergistically perform their physiological roles. This review explores the molecular underpinnings of myelination,
[...] Read more.
Spinal cord injury (SCI) is a debilitating condition that results from a culmination of acute and chronic damage to neural tissue, specifically the myelin sheath, thus impacting neurons’ abilities to synergistically perform their physiological roles. This review explores the molecular underpinnings of myelination, demyelination, and remyelination, emphasizing the role of oligodendrocyte progenitor cells (OPCs), astrocytes, and microglia in physiological, and pathophysiological, healing. Furthermore, we link these processes with emerging therapeutic strategies currently under investigation in animal and human models, underscoring areas of translational medicine that remain underutilized. The goal of this review is to provide a framework for developing more advanced interventions to restore function and improve outcomes for individuals with SCI.
Full article
(This article belongs to the Special Issue Molecular and Cellular Mechanisms of Spinal Cord Injury and Repair)
Open AccessArticle
Metabolic Culture Medium Enhances Maturation of Human iPSC-Derived Cardiomyocytes via Cardiac Troponin I Isoform Induction
by
Daria V. Goliusova, Agnessa P. Bogomolova, Alina V. Davidenko, Kristina A. Lavrenteva, Margarita Y. Sharikova, Elena A. Zerkalenkova, Ekaterina M. Vassina, Alexandra N. Bogomazova, Maria A. Lagarkova, Ivan A. Katrukha and Olga S. Lebedeva
Int. J. Mol. Sci. 2025, 26(15), 7248; https://doi.org/10.3390/ijms26157248 (registering DOI) - 26 Jul 2025
Abstract
Human induced pluripotent stem cell-derived cardiomyocytes (iCMs) provide a powerful platform for investigating cardiac biology. However, structural, metabolic, and electrophysiological immaturity of iCMs limits their capacity to model adult cardiomyocytes. Currently, no universally accepted criteria or protocols for effective iCMs maturation exist. This
[...] Read more.
Human induced pluripotent stem cell-derived cardiomyocytes (iCMs) provide a powerful platform for investigating cardiac biology. However, structural, metabolic, and electrophysiological immaturity of iCMs limits their capacity to model adult cardiomyocytes. Currently, no universally accepted criteria or protocols for effective iCMs maturation exist. This study aimed to identify practical culture conditions that promote iCMs maturation, thereby generating more physiologically relevant in vitro cardiac models. We evaluated the effects of short- and long-term culture in media supplemented with various stimulatory compounds under 2D conditions, focusing on intracellular content and localization of slow skeletal troponin I (ssTnI) and cardiac troponin I (cTnI) isoforms. Our findings demonstrate that the multicomponent metabolic maturation medium (MM-1) effectively enhances the transition toward a more mature iCM phenotype, as evidenced by increased cTnI expression and formation of cross-striated myofibrils. iCMs cultured in MM-1 more closely resemble adult cardiomyocytes and are compatible with high-resolution single-cell techniques such as electron microscopy and patch-clamp electrophysiology. This work provides a practical and scalable approach for advancing the maturation of iPSC-derived cardiac models, with applications in disease modeling and drug screening.
Full article
(This article belongs to the Special Issue Fundamental and Practical Perspectives in Regenerative Medicine: Proceedings of the VI National Congress of Regenerative Medicine (2024))
►▼
Show Figures

Figure 1
Open AccessReview
Immunomodulatory Natural Products in Cancer Organoid-Immune Co-Cultures: Bridging the Research Gap for Precision Immunotherapy
by
Chang-Eui Hong and Su-Yun Lyu
Int. J. Mol. Sci. 2025, 26(15), 7247; https://doi.org/10.3390/ijms26157247 (registering DOI) - 26 Jul 2025
Abstract
Natural products demonstrate potent immunomodulatory properties through checkpoint modulation, macrophage polarization, and T cell/natural killer (NK) cell activation. While cancer organoid-immune co-culture platforms enable physiologically relevant modeling of tumor–immune interactions, systematic investigation of natural product immunomodulation in these systems remains entirely unexplored. We
[...] Read more.
Natural products demonstrate potent immunomodulatory properties through checkpoint modulation, macrophage polarization, and T cell/natural killer (NK) cell activation. While cancer organoid-immune co-culture platforms enable physiologically relevant modeling of tumor–immune interactions, systematic investigation of natural product immunomodulation in these systems remains entirely unexplored. We conducted a comprehensive literature analysis examining natural products tested in cancer organoids, immunomodulatory mechanisms from traditional models, technical advances in organoid-immune co-cultures, and standardization requirements for clinical translation. Our analysis reveals a critical research gap: no published studies have investigated natural product-mediated immunomodulation using organoid-immune co-culture systems. Even though compounds like curcumin, resveratrol, and medicinal mushroom polysaccharides show extensive immunomodulatory effects in two-dimensional (2D) cultures, and organoid technology achieves high clinical correlation for drug response prediction, all existing organoid studies focus exclusively on direct cytotoxicity. Technical challenges include compound stability, limited matrix penetration requiring substantially higher concentrations than 2D cultures, and maintaining functional immune populations in three-dimensional (3D) systems. The convergence of validated organoid-immune co-culture platforms, Food and Drug Administration (FDA) regulatory support through the Modernization Act 2.0, and extensive natural product knowledge creates unprecedented opportunities. Priority research directions include systematic screening of immunomodulatory natural products in organoid-immune co-cultures, development of 3D-optimized delivery systems, and clinical validation trials. Success requires moving beyond cytotoxicity-focused studies to investigate immunomodulatory mechanisms in physiologically relevant 3D systems, potentially unlocking new precision cancer immunotherapy approaches.
Full article
(This article belongs to the Special Issue Advances in the 3D Culture Systems and Organoids for Disease Modeling and Therapeutics)
►▼
Show Figures
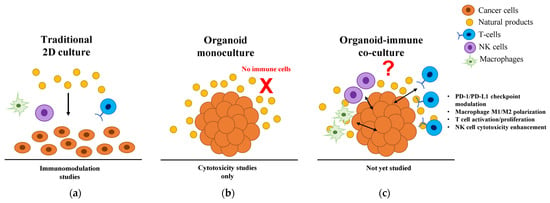
Figure 1
Open AccessReview
Small Extracellular Vesicles in Neurodegenerative Disease: Emerging Roles in Pathogenesis, Biomarker Discovery, and Therapy
by
Mousumi Ghosh, Amir-Hossein Bayat and Damien D. Pearse
Int. J. Mol. Sci. 2025, 26(15), 7246; https://doi.org/10.3390/ijms26157246 (registering DOI) - 26 Jul 2025
Abstract
Neurodegenerative diseases (NDDs) such as Alzheimer’s, Parkinson’s, ALS, and Huntington’s pose a growing global challenge due to their complex pathobiology and aging demographics. Once considered as cellular debris, small extracellular vesicles (sEVs) are now recognized as active mediators of intercellular signaling in NDD
[...] Read more.
Neurodegenerative diseases (NDDs) such as Alzheimer’s, Parkinson’s, ALS, and Huntington’s pose a growing global challenge due to their complex pathobiology and aging demographics. Once considered as cellular debris, small extracellular vesicles (sEVs) are now recognized as active mediators of intercellular signaling in NDD progression. These nanovesicles (~30-150 nm), capable of crossing the blood–brain barrier, carry pathological proteins, RNAs, and lipids, facilitating the spread of toxic species like Aβ, tau, TDP-43, and α-synuclein. sEVs are increasingly recognized as valuable diagnostic tools, outperforming traditional CSF biomarkers in early detection and disease monitoring. On the therapeutic front, engineered sEVs offer a promising platform for CNS-targeted delivery of siRNAs, CRISPR tools, and neuroprotective agents, demonstrating efficacy in preclinical models. However, translational hurdles persist, including standardization, scalability, and regulatory alignment. Promising solutions are emerging, such as CRISPR-based barcoding, which enables high-resolution tracking of vesicle biodistribution; AI-guided analytics to enhance quality control; and coordinated regulatory efforts by the FDA, EMA, and ISEV aimed at unifying identity and purity criteria under forthcoming Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines. This review critically examines the mechanistic roles, diagnostic potential, and therapeutic applications of sEVs in NDDs, and outlines key strategies for clinical translation.
Full article
(This article belongs to the Special Issue Molecular Advances in Neurologic and Neurodegenerative Disorders)
Open AccessArticle
Extracellular Vesicle Mitochondrial DNA Reflects Podocyte Mitochondrial Stress and Is Associated with Relapse in Nephrotic Syndrome
by
Robert L. Myette, Chet E. Holterman, Mayra Trentin-Sonoda, Tyler T. Cooper, Gilles A. Lajoie, George Cairns, Yan Burelle, Nour El Khatib, Joanna Raman-Nair, Dylan Burger and Christopher R. J. Kennedy
Int. J. Mol. Sci. 2025, 26(15), 7245; https://doi.org/10.3390/ijms26157245 (registering DOI) - 26 Jul 2025
Abstract
Idiopathic childhood nephrotic syndrome is a common glomerulopathy comprising proteinuria, hypoalbuminemia, and edema. Podocyte dysfunction is central to this disease process. Extracellular vesicles are released from stressed cells and can represent a molecular snapshot of the parent cell of origin. We previously showed
[...] Read more.
Idiopathic childhood nephrotic syndrome is a common glomerulopathy comprising proteinuria, hypoalbuminemia, and edema. Podocyte dysfunction is central to this disease process. Extracellular vesicles are released from stressed cells and can represent a molecular snapshot of the parent cell of origin. We previously showed that urinary large extracellular vesicles (LEVs) derived from podocytes are increased in patients with nephrotic syndrome relapse. Here, we investigated the role of mitochondrial DNA (mtDNA) within LEVs both in vitro and in vivo, revealing the novel finding that podocytes release LEVs containing mtDNA, driven by mitochondrial stress. A puromycin aminonucleoside nephrosis rat model showed foot process effacement on electron microscopy and urinary LEVs with significantly increased mtDNA. Prednisolone, which drives remission in nephrotic syndrome in children, attenuated mitochondrial stress and reduced the amount of mtDNA content within LEVs in vitro. Lastly, urinary LEVs from children with nephrotic syndrome also contain mtDNA, and it is the podocyte LEV-fraction which is preferentially enriched. Overall, these data support a potential mechanism of podocyte mitochondrial stress in non-genetic, idiopathic pediatric nephrotic syndrome.
Full article
(This article belongs to the Section Molecular Biology)
►▼
Show Figures
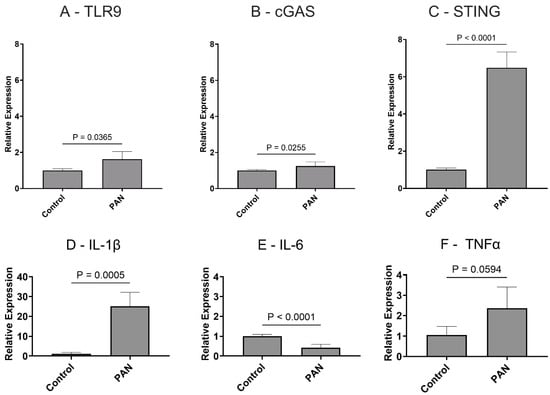
Figure 1
Open AccessArticle
The Activation of the Microglial NLRP3 Inflammasome Is Involved in Tuberous Sclerosis Complex-Related Neuroinflammation
by
Ran Ding, Shengxuan Zhang, Linxue Meng, Lingman Wang, Ziyao Han, Jianxiong Gui, Jiaxin Yang, Li Cheng, Lingling Xie and Li Jiang
Int. J. Mol. Sci. 2025, 26(15), 7244; https://doi.org/10.3390/ijms26157244 (registering DOI) - 26 Jul 2025
Abstract
Tuberous sclerosis complex (TSC) is a systemic disease caused by mutations in either the TSC1 (encoding hamartin) or TSC2 (encoding tuberin) gene, with mutations in the TSC2 gene potentially leading to more severe clinical symptoms. Neurological symptoms are a common clinical manifestation of
[...] Read more.
Tuberous sclerosis complex (TSC) is a systemic disease caused by mutations in either the TSC1 (encoding hamartin) or TSC2 (encoding tuberin) gene, with mutations in the TSC2 gene potentially leading to more severe clinical symptoms. Neurological symptoms are a common clinical manifestation of TSC, and neuroinflammation is thought to play an important role. Glial cells are a major source of neuroinflammation, but whether microglia are involved in the activation of the NOD-like receptor protein 3 (NLRP3) inflammasome and the expression of interleukin-1β (IL-1β) in TSC patients remains unclear. We used a transcriptome sequencing dataset for bioinformatics analysis to explore the differences in the expression of microglial inflammasome-associated hub genes. TSC2 knockdown (TSC2 KD) microglia (HMC3 cell line) were generated by lentivirus, and the expression of inflammasome-associated hub genes, microglial activation, and NLRP3 inflammasome activation were verified. In addition, experiments were performed to explore the regulatory effects of rapamycin. Bioinformatics analysis identified a total of eight inflammasome-associated hub genes. By detecting GFP fluorescence, TSC2 mRNA, TSC2 protein expression, and the phosphorylation of the mammalian target of rapamycin (p-mTOR)/mTOR, we confirmed that the TSC2 KD microglia model was successfully established. Compared with the control group, the TSC2 KD group presented higher mRNA levels and fluorescence intensities of microglia AIF1 and CD68, as well as greater reactive oxygen species (ROS) production. Eight inflammasome-associated hub gene mRNA assays revealed that the expression of the NLRP3 and IL1B genes was increased. Compared with the control group, the TSC2 KD group presented increased levels of NLRP3 and Pro-IL-1β proteins in cells and Cleaved-Caspase 1 and Cleaved-IL-1β proteins in the supernatant, suggesting NLRP3 inflammasome activation. Rapamycin intervention alleviated these changes, demonstrating that the TSC2 gene regulation of microglial activation and NLRP3 inflammasome activation are correlated with mTOR phosphorylation. In conclusion, microglia are activated in TSC patients and participate in the NLRP3 inflammasome-associated neuroinflammatory response, and rapamycin treatment can alleviate these changes.
Full article
(This article belongs to the Section Molecular Neurobiology)
►▼
Show Figures
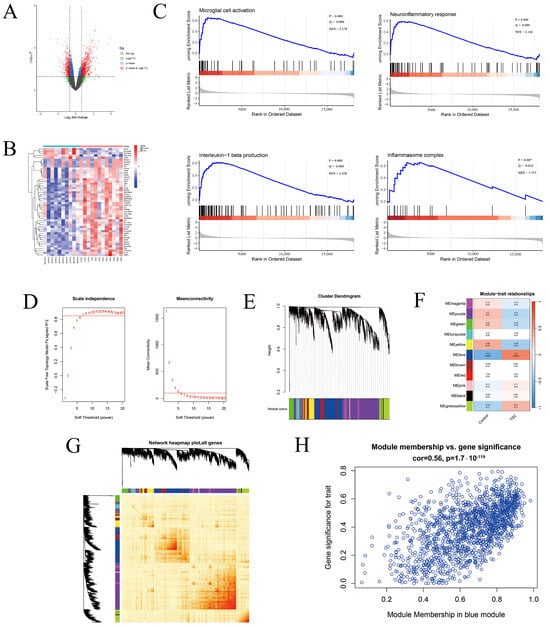
Figure 1
Open AccessArticle
Genome-Wide Identification, Evolution, and Expression Analysis of the DMP Gene Family in Peanut (Arachis hypogaea L.)
by
Pengyu Qu, Lina He, Lulu Xue, Han Liu, Xiaona Li, Huanhuan Zhao, Liuyang Fu, Suoyi Han, Xiaodong Dai, Wenzhao Dong, Lei Shi and Xinyou Zhang
Int. J. Mol. Sci. 2025, 26(15), 7243; https://doi.org/10.3390/ijms26157243 (registering DOI) - 26 Jul 2025
Abstract
Peanut (Arachis hypogaea L.) is a globally important oilseed cash crop, yet its limited genetic diversity and unique reproductive biology present persistent challenges for conventional crossbreeding. Traditional breeding approaches are often time-consuming and inadequate, mitigating the pace of cultivar development. Essential for
[...] Read more.
Peanut (Arachis hypogaea L.) is a globally important oilseed cash crop, yet its limited genetic diversity and unique reproductive biology present persistent challenges for conventional crossbreeding. Traditional breeding approaches are often time-consuming and inadequate, mitigating the pace of cultivar development. Essential for double fertilization and programmed cell death (PCD), DUF679 membrane proteins (DMPs) represent a membrane protein family unique to plants. In the present study, a comprehensive analysis of the DMP gene family in peanuts was conducted, which included the identification of 21 family members. Based on phylogenetic analysis, these genes were segregated into five distinct clades (I–V), with AhDMP8A, AhDMP8B, AhDMP9A, and AhDMP9B in clade IV exhibiting high homology with known haploid induction genes. These four candidates also displayed significantly elevated expression in floral tissues compared to other organs, supporting their candidacy for haploid induction in peanuts. Subcellular localization prediction, confirmed through co-localization assays, demonstrated that AhDMPs primarily localize to the plasma membrane, consistent with their proposed roles in the reproductive signaling process. Furthermore, chromosomal mapping and synteny analyses revealed that the expansion of the AhDMP gene family is largely driven by whole-genome duplication (WGD) and segmental duplication events, reflecting the evolutionary dynamics of the tetraploid peanut genome. Collectively, these findings establish a foundational understanding of the AhDMP gene family and highlight promising targets for future applications in haploid induction-based breeding strategies in peanuts.
Full article
(This article belongs to the Special Issue Advances in Research for Legume Genomics, Genetics, and Breeding, 2nd Edition)
►▼
Show Figures
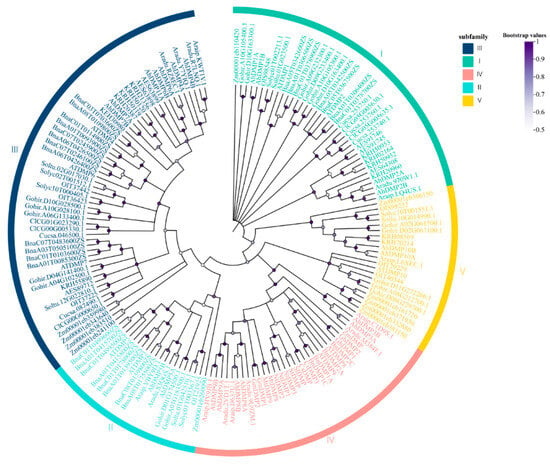
Figure 1
Open AccessReview
Molecular Mechanisms and Clinical Implications of Complex Prehabilitation in Colorectal Cancer Surgery: A Comprehensive Review
by
Jakub Włodarczyk
Int. J. Mol. Sci. 2025, 26(15), 7242; https://doi.org/10.3390/ijms26157242 (registering DOI) - 26 Jul 2025
Abstract
Colorectal cancer (CRC) remains a leading cause of cancer morbidity and mortality worldwide, especially in older adults where frailty complicates treatment outcomes. Multimodal prehabilitation—comprising nutritional support, physical exercise, and psychological interventions—has emerged as a promising strategy to enhance patients’ resilience before CRC surgery.
[...] Read more.
Colorectal cancer (CRC) remains a leading cause of cancer morbidity and mortality worldwide, especially in older adults where frailty complicates treatment outcomes. Multimodal prehabilitation—comprising nutritional support, physical exercise, and psychological interventions—has emerged as a promising strategy to enhance patients’ resilience before CRC surgery. Clinical studies demonstrate that prehabilitation significantly reduces postoperative complications, shortens hospital stays, and improves functional recovery. Nutritional interventions focus on counteracting malnutrition and sarcopenia through tailored dietary counseling, protein supplementation, and immunonutrients like arginine and glutamine. Physical exercise enhances cardiorespiratory fitness and muscle strength while modulating immune and metabolic pathways critical for surgical recovery. Psychological support reduces anxiety and depression, promoting mental resilience that correlates with better postoperative outcomes. Despite clear clinical benefits, the molecular mechanisms underlying prehabilitation’s effects—such as inflammation modulation, immune activation, and metabolic rewiring—remain poorly understood. This review addresses this knowledge gap by exploring potential biological pathways influenced by prehabilitation, aiming to guide more targeted, personalized approaches in CRC patient management. Advancing molecular insights may optimize prehabilitation protocols and improve survival and quality of life for CRC patients undergoing surgery.
Full article
(This article belongs to the Section Molecular Pathology, Diagnostics, and Therapeutics)
►▼
Show Figures
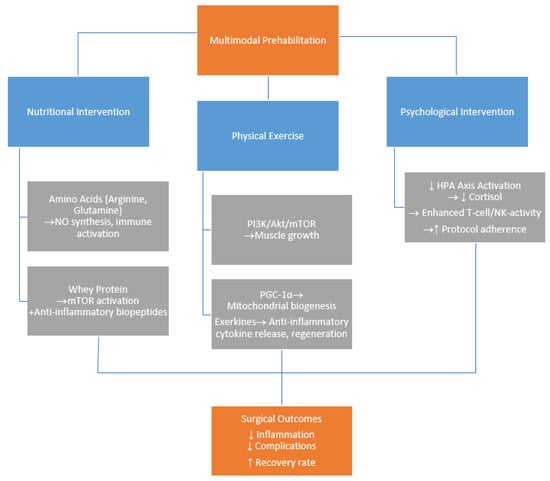
Figure 1
Open AccessReview
Non-Canonical Functions of Adenosine Receptors: Emerging Roles in Metabolism, Immunometabolism, and Epigenetic Regulation
by
Giovanni Pallio and Federica Mannino
Int. J. Mol. Sci. 2025, 26(15), 7241; https://doi.org/10.3390/ijms26157241 (registering DOI) - 26 Jul 2025
Abstract
Adenosine receptors (ARs) are G protein-coupled receptors that are widely expressed across tissues, traditionally associated with cardiovascular, neurological, and immune regulation. Recent studies, however, have highlighted their non-canonical functions, revealing critical roles in metabolism, immunometabolism, and epigenetic regulation. AR subtypes, particularly A2A and
[...] Read more.
Adenosine receptors (ARs) are G protein-coupled receptors that are widely expressed across tissues, traditionally associated with cardiovascular, neurological, and immune regulation. Recent studies, however, have highlighted their non-canonical functions, revealing critical roles in metabolism, immunometabolism, and epigenetic regulation. AR subtypes, particularly A2A and A2B, modulate glucose and lipid metabolism, mitochondrial activity, and energy homeostasis. In immune cells, AR signaling influences metabolic reprogramming and polarization through key regulators such as mTOR, AMPK, and HIF-1α, contributing to immune tolerance or activation depending on the context. Additionally, ARs have been implicated in epigenetic modulation, affecting DNA methylation, histone acetylation, and non-coding RNA expression via metabolite-sensitive mechanisms. Therapeutically, AR-targeting agents are being explored for cancer and chronic inflammatory diseases. While clinical trials with A2A antagonists in oncology show encouraging results, challenges remain due to receptor redundancy, systemic effects, and the need for tissue-specific selectivity. Future strategies involve biased agonism, allosteric modulators, and combination therapies guided by biomarker-based patient stratification. Overall, ARs are emerging as integrative hubs connecting extracellular signals with cellular metabolic and epigenetic machinery. Understanding these non-canonical roles may unlock novel therapeutic opportunities across diverse disease landscapes.
Full article
(This article belongs to the Special Issue Molecular Research on Adenosine Receptors: From Cell Biology to Human Diseases)
Open AccessArticle
Spectral Region Optimization and Machine Learning-Based Nonlinear Spectral Analysis for Raman Detection of Cardiac Fibrosis Following Myocardial Infarction
by
Arno Krause, Marco Andreana, Richard D. Walton, James Marchant, Nestor Pallares-Lupon, Kanchan Kulkarni, Wolfgang Drexler and Angelika Unterhuber
Int. J. Mol. Sci. 2025, 26(15), 7240; https://doi.org/10.3390/ijms26157240 (registering DOI) - 26 Jul 2025
Abstract
Cardiac fibrosis following myocardial infarction plays a critical role in the formation of scar tissue and contributes to ventricular arrhythmias, including ventricular tachycardia and sudden cardiac death. Current clinical diagnostics use electrical and structural markers, but lack precision due to low spatial resolution
[...] Read more.
Cardiac fibrosis following myocardial infarction plays a critical role in the formation of scar tissue and contributes to ventricular arrhythmias, including ventricular tachycardia and sudden cardiac death. Current clinical diagnostics use electrical and structural markers, but lack precision due to low spatial resolution and absence of molecular information. In this paper, we employed line scan Raman microspectroscopy to classify sheep myocardial tissue into muscle, necrotic, granulated, and fibrotic tissue types, using collagen as a molecular biomarker. Three spectral regions were evaluated: region A (
(This article belongs to the Special Issue Raman Spectroscopy and Machine Learning in Human Disease)
Open AccessPerspective
Glucagon-like Peptide-1 Receptor (GLP-1R) Signaling: Making the Case for a Functionally Gs Protein-Selective GPCR
by
Anastasios Lymperopoulos, Victoria L. Altsman and Renee A. Stoicovy
Int. J. Mol. Sci. 2025, 26(15), 7239; https://doi.org/10.3390/ijms26157239 (registering DOI) - 26 Jul 2025
Abstract
Spurred by the enormous therapeutic success of glucagon-like peptide-1 receptor (GLP-1R) agonists (GLP1-RAs) against diabetes and obesity, glucagon family receptor pharmacology has garnered a tremendous amount of interest. Glucagon family receptors, e.g., the glucagon receptor itself (GCGR), the GLP-1R, and the glucose-dependent insulinotropic
[...] Read more.
Spurred by the enormous therapeutic success of glucagon-like peptide-1 receptor (GLP-1R) agonists (GLP1-RAs) against diabetes and obesity, glucagon family receptor pharmacology has garnered a tremendous amount of interest. Glucagon family receptors, e.g., the glucagon receptor itself (GCGR), the GLP-1R, and the glucose-dependent insulinotropic peptide receptor (GIPR), belong to the incretin receptor superfamily, i.e., receptors that increase blood glucose-dependent insulin secretion. All incretin receptors are class B1 G protein-coupled receptors (GPCRs), coupling to the Gs type of heterotrimeric G proteins which activates adenylyl cyclase (AC) to produce cyclic adenosine monophosphate (cAMP). Most GPCRs undergo desensitization, i.e., uncouple from G proteins and internalize, thanks to interactions with the βarrestins (arrestin-2 and -3). Since the βarrestins can also mediate their own G protein-independent signaling, any given GPCR can theoretically signal (predominantly) either via G proteins or βarrestins, i.e., be a G protein- or βarrestin-“biased” receptor, depending on the bound ligand. A plethora of experimental evidence suggests that the GLP-1R does not undergo desensitization in physiologically relevant tissues in vivo, but rather, it produces robust and prolonged cAMP signals. A particular property of constant cycling between the cell membrane and caveolae/lipid rafts of the GLP-1R may underlie its lack of desensitization. In contrast, GIPR signaling is extensively mediated by βarrestins and the GIPR undergoes significant desensitization, internalization, and downregulation, which may explain why both agonists and antagonists of the GIPR exert the same physiological effects. Here, we discuss this evidence and make a case for the GLP-1R being a phenotypically or functionally Gs-selective receptor. We also discuss the implications of this for the development of GLP-1R poly-ligands, which are increasingly pursued for the treatment of obesity and other diseases.
Full article
(This article belongs to the Collection Feature Papers in Molecular Pharmacology)
►▼
Show Figures

Figure 1
Open AccessArticle
RNA Sequencing Reveals Inflammatory and Metabolic Changes in the Lung and Brain After Carbon Black and Naphthalene Whole Body Inhalation Exposure in a Rodent Model of Military Burn Pit Exposures
by
Allison M Haaning, Brian J Sandri, Henry L Wyneken, William T. Goldsmith, Joshua P Nixon, Timothy R Nurkiewicz, Chris H Wendt, Paul Barach, Janeen H Trembley and Tammy A Butterick
Int. J. Mol. Sci. 2025, 26(15), 7238; https://doi.org/10.3390/ijms26157238 (registering DOI) - 26 Jul 2025
Abstract
Military personnel deployed to Iraq and Afghanistan were exposed to emissions from open-air burn pits, where plastics, metals, and medical waste were incinerated. These exposures have been linked to deployment-related respiratory diseases (DRRD) and may also impact neurological health via the lung–brain axis.
[...] Read more.
Military personnel deployed to Iraq and Afghanistan were exposed to emissions from open-air burn pits, where plastics, metals, and medical waste were incinerated. These exposures have been linked to deployment-related respiratory diseases (DRRD) and may also impact neurological health via the lung–brain axis. To investigate molecular mechanisms, adult male rats were exposed to filtered air, naphthalene (a representative volatile organic compound), or a combination of naphthalene and carbon black (surrogate for particulate matter; CBN) via whole-body inhalation (six hours/day, three consecutive days). Lung, brain, and plasma samples were collected 24 h after the final exposure. Pro-inflammatory biomarkers were assessed using multiplex electrochemiluminescence and western blot. Differentially expressed genes (DEGs) were identified by RNA sequencing, and elastic net modeling was used to define exposure-predictive gene signatures. CBN exposure altered inflammatory biomarkers across tissues, with activation of nuclear factor kappa B (NF-κB) signaling. In the lung, gene set enrichment revealed activated pathways related to proliferation and inflammation, while epithelial–mesenchymal transition (EMT) and oxidative phosphorylation were suppressed. In the brain, EMT, inflammation, and senescence pathways were activated, while ribosomal function and oxidative metabolism were downregulated. Elastic net modeling identified a lung gene signature predictive of CBN exposure, including Kcnq3, Tgfbr1, and Tm4sf19. These findings demonstrate that inhalation of a surrogate burn pit mixture induces inflammatory and metabolic gene expression changes in both lung and brain tissues, supporting the utility of this animal model for understanding systemic effects of airborne military toxicants and for identifying potential biomarkers relevant to DRRD and Veteran health.
Full article
(This article belongs to the Section Molecular Pathology, Diagnostics, and Therapeutics)
►▼
Show Figures
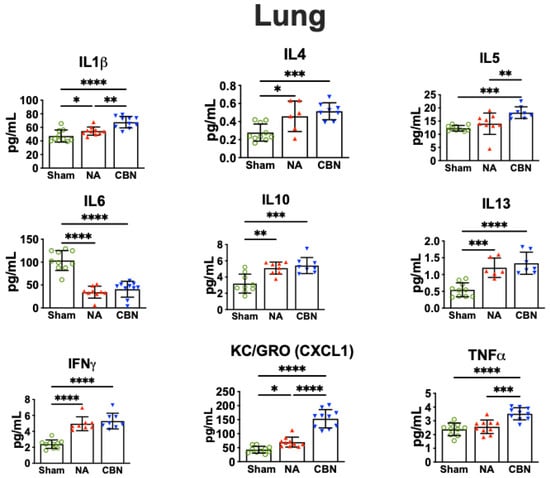
Figure 1
Open AccessReview
Recent Advances in Experimental Functional Characterization of GWAS Candidate Genes in Osteoporosis
by
Petra Malavašič, Jasna Lojk, Marija Nika Lovšin and Janja Marc
Int. J. Mol. Sci. 2025, 26(15), 7237; https://doi.org/10.3390/ijms26157237 (registering DOI) - 26 Jul 2025
Abstract
Osteoporosis is a multifactorial, polygenic disease characterized by reduced bone mineral density (BMD) and increased fracture risk. Genome-wide association studies (GWASs) have identified numerous loci associated with BMD and/or bone fractures, but functional characterization of these target genes is essential to understand the
[...] Read more.
Osteoporosis is a multifactorial, polygenic disease characterized by reduced bone mineral density (BMD) and increased fracture risk. Genome-wide association studies (GWASs) have identified numerous loci associated with BMD and/or bone fractures, but functional characterization of these target genes is essential to understand the biological mechanisms underlying osteoporosis. This review focuses on current methodologies and key examples of successful functional studies aimed at evaluating gene function in osteoporosis research. Functional evaluation typically follows a multi-step approach. In silico analyses using omics datasets expression quantitative trait loci (eQTLs), protein quantitative trait loci (pQTLs), and DNA methylation quantitative trait loci (mQTLs) help prioritize candidate genes and predict relevant biological pathways. In vitro models, including immortalized bone-derived cell lines and primary mesenchymal stem cells (MSCs), are used to explore gene function in osteogenesis. Advanced three-dimensional culture systems provide additional physiological relevance for studying bone-related cellular processes. In situ analyses of patient-derived bone and muscle tissues offer validation in a disease-relevant context, while in vivo studies using mouse and zebrafish models enable comprehensive assessment of gene function in skeletal development and maintenance. Integration of these complementary methodologies helps translate GWAS findings into biological insights and supports the identification of novel therapeutic targets for osteoporosis.
Full article
(This article belongs to the Special Issue Highlights in Pathophysiology of the Musculoskeletal System, 2nd Edition)
►▼
Show Figures
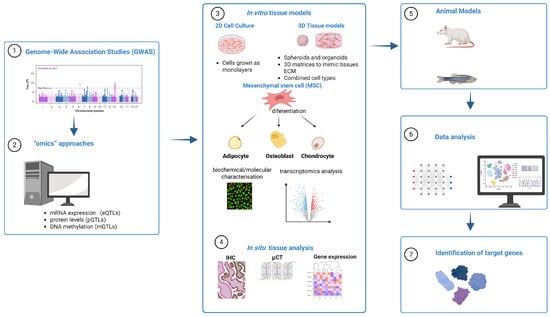
Figure 1
Open AccessReview
The Role of Cobalt Ions in Angiogenesis—A Review
by
Wiktor Gregorowicz and Lukasz Pajchel
Int. J. Mol. Sci. 2025, 26(15), 7236; https://doi.org/10.3390/ijms26157236 (registering DOI) - 26 Jul 2025
Abstract
Cobalt is an essential trace element involved in key biological processes. It serves most notably as a component of vitamin B12 (cobalamin) and a regulator of erythropoiesis. While cobalt deficiency can lead to disorders such as megaloblastic anemia, excess cobalt poses toxicological
[...] Read more.
Cobalt is an essential trace element involved in key biological processes. It serves most notably as a component of vitamin B12 (cobalamin) and a regulator of erythropoiesis. While cobalt deficiency can lead to disorders such as megaloblastic anemia, excess cobalt poses toxicological risks to the thyroid, cardiovascular, and hematopoietic systems. In recent years, cobalt ions (Co2+) have gained attention for their ability to mimic hypoxia and promote angiogenesis. This represents a crucial mechanism for tissue regeneration. Cobalt mediates this effect mainly by stabilizing hypoxia-inducible factor 1α (HIF-1α) under normoxic conditions, thereby upregulating angiogenic genes, including VEGF, FGF, and EPO. Experimental studies—from cell culture to animal models—have demonstrated cobalt-induced enhancement of endothelial proliferation, migration, and microvascular formation. Emerging evidence also indicates that Co2+-stimulated macrophages secrete integrin-β1-rich exosomes. These exosomes enhance endothelial motility and tubulogenesis independently of VEGF. Furthermore, cobalt-modified biomaterials have been developed to deliver cobalt ions in a controlled manner. Examples include cobalt-doped β-tricalcium phosphate or bioactive glasses. These materials support both angiogenesis and osteogenesis.This review summarizes current findings on cobalt’s role in angiogenesis. The emphasis is on its potential in cobalt-based biomaterials for tissue engineering and regenerative medicine.
Full article
(This article belongs to the Special Issue Translational Research in Angiogenesis—a Key Tool for Developing Therapeutic Strategies Towards Tissue Regeneration)
►▼
Show Figures
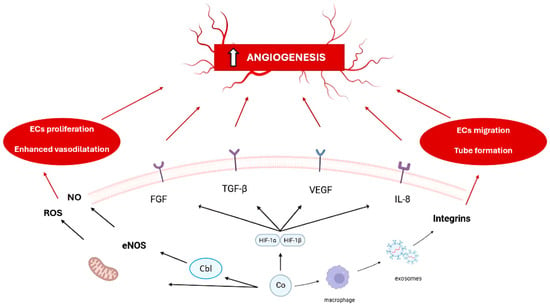
Figure 1

Journal Menu
► ▼ Journal Menu-
- IJMS Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Society Collaborations
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal Browser-
arrow_forward_ios
Forthcoming issue
arrow_forward_ios Current issue - Vol. 26 (2025)
- Vol. 25 (2024)
- Vol. 24 (2023)
- Vol. 23 (2022)
- Vol. 22 (2021)
- Vol. 21 (2020)
- Vol. 20 (2019)
- Vol. 19 (2018)
- Vol. 18 (2017)
- Vol. 17 (2016)
- Vol. 16 (2015)
- Vol. 15 (2014)
- Vol. 14 (2013)
- Vol. 13 (2012)
- Vol. 12 (2011)
- Vol. 11 (2010)
- Vol. 10 (2009)
- Vol. 9 (2008)
- Vol. 8 (2007)
- Vol. 7 (2006)
- Vol. 6 (2005)
- Vol. 5 (2004)
- Vol. 4 (2003)
- Vol. 3 (2002)
- Vol. 2 (2001)
- Vol. 1 (2000)
Highly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Electrochem, IJMS, Molecules, Polymers, Separations
Advances in Chemistry and Chemical Engineering, 2nd Edition
Topic Editors: Cristina Orbeci, Cristian Pirvu, Ileana Rau, Stefania Stoleriu, Maria-Cristina Todasca, Elena Iuliana BîruDeadline: 31 July 2025
Topic in
Energies, IJMS, Membranes, Separations, Water
Membrane Separation Technology Research
Topic Editors: Chenxiao Jiang, Zhe Yang, Ying MeiDeadline: 15 September 2025
Topic in
Biomolecules, IJMS, Molecules, Sci. Pharm., Marine Drugs, Plants
Antioxidant Activity of Natural Products—2nd Edition
Topic Editors: José Virgílio Santulhão Pinela, Maria Inês Moreira Figueiredo Dias, Carla Susana Correia Pereira, Alexandra PlácidoDeadline: 30 September 2025
Topic in
Biomedicines, JCM, Molecules, Pharmaceutics, Sci. Pharm., IJMS
Cannabis, Cannabinoids and Its Derivatives
Topic Editors: Melanie Kelly, Christian LehmannDeadline: 31 October 2025

Conferences
Special Issues
Special Issue in
IJMS
Skin Diseases: Molecular Prediction, Diagnosis, Pathogenesis and Treatment
Guest Editor: Youwen ZhouDeadline: 28 July 2025
Special Issue in
IJMS
New Advances in DNA Nanotechnology
Guest Editor: Zhaoqi YangDeadline: 29 July 2025
Special Issue in
IJMS
Hyperelastic Structures
Guest Editors: Hossein Khaniki, Mergen GhayeshDeadline: 30 July 2025
Special Issue in
IJMS
The Role of Inflammation in the Etiology of Precancerous Lesions, Carcinogenesis and Cancer Treatment
Guest Editors: Boguslaw Nedoszytko, Leszek Kalinowski, Luigi MaranoDeadline: 30 July 2025
Topical Collections
Topical Collection in
IJMS
State-of-the-Art Molecular Endocrinology and Metabolism in Japan
Collection Editor: Koichi Fujisawa
Topical Collection in
IJMS
Immunopathology and Immunosenescence
Collection Editors: Calogero Caruso, Giuseppina Candore, Anna Aiello, Giulia Accardi
Topical Collection in
IJMS
Novel Insights into the Sleeping, Waking, and Dreaming Brain
Collection Editor: Michael Lazarus
Topical Collection in
IJMS
Latest Review Papers in Molecular Nanoscience
Collection Editors: Andre Lee, Maciej Monedeiro-Milanowski








