Serotonin Modulation of Dorsoventral Hippocampus in Physiology and Schizophrenia
Abstract
1. Introduction
2. Organization of the Hippocampal Serotonergic System
2.1. Structural Features of the Hippocampal Serotonergic System
2.2. Cellular Mechanisms of Serotonin Receptors in the Hippocampus
2.2.1. 5-HT1Rs
2.2.2. 5-HT2Rs
2.2.3. 5-HT3Rs
2.2.4. 5-HT4Rs
2.2.5. 5-HT5Rs
2.2.6. 5-HT6Rs
2.2.7. 5-HT7Rs
3. Serotonergic Regulation of Hippocampal Network Dynamics
3.1. Synaptic Plasticity
3.2. Hippocampal Rhythms
3.2.1. Theta Rhythm
3.2.2. Gamma Rhythm
3.2.3. Sharp Waves and Ripples (SWRs)
3.3. Developmental Aspects of Serotonergic Regulation
4. The Hippocampal Serotonergic System in Schizophrenia
4.1. 5-HTRs in Schizophrenia
4.2. Atypical Antipsychotics and the Hippocampus
4.3. Psychosis of Epilepsy and the Hippocampus
5. The Role of Serotonin in Schizophrenia: A Dorsoventral Hippocampal Perspective
5.1. Positive Symptoms
5.2. Negative Symptoms
5.3. Cognitive Deficits
5.4. Translational Implications
6. Final Remarks
6.1. Controversial Findings
6.2. Limitations, Open Questions, and Future Directions
6.3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5-HT | 5-Hydroxytryptamine |
| 5-HTR | 5-Hydroxytryptamine Receptor |
| AC | Adenyl Cyclase |
| AMPA | α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic Acid |
| BDNF | Brain-Derived Neurotrophic Factor |
| BNST | Bed Nucleus of the Stria Terminalis |
| dRNu | Dorsal Raphe Nucleus |
| CA1/CA3 | Cornu Ammonis Regions 1 and 3 |
| CB1 | Cannabinoid Receptor Type 1 |
| CCK | Cholecystokinin |
| CREB | cAMP Response Element-Binding Protein |
| DAG | Diacylglycerol |
| DG | Dentate Gyrus |
| DH | Dorsal Hippocampus |
| E/I | Excitation/Inhibition |
| EPSP | Excitatory Postsynaptic Potential |
| ERK | Extracellular Signal-Activated Protein Kinase |
| fMRI | Functional Magnetic Resonance Imaging |
| GABA | Gamma-Aminobutyric Acid |
| GIRK | G-Protein-Activated Inwardly Rectifying Potassium Channel |
| GPCR | G-Protein-Coupled Receptor |
| Ih | Hyperpolarization-Activated Current |
| IN | Interneuron |
| LTD | Long-Term Depression |
| LTP | Long-Term Potentiation |
| MAPK | Mitogen-Activated Protein Kinase |
| MDMA | 3,4-Methylenedioxymethamphetamine |
| MK | MK-801 (NMDA Receptor Antagonist) |
| mPFC | Medial Prefrontal Cortex |
| mRNu | Median Raphe Nucleus |
| NMDA | N-Methyl-D-Aspartate |
| NVHL | Neonatal Ventral Hippocampal Lesion |
| PCA | Para-Chloroamphetamine |
| PDE | Phosphodiesterase |
| PIP 3 | Phosphatidylinositol (3,4,5)-triphosphate |
| PKA | Protein Kinase A |
| PLC | Phospholipase C |
| PN | Pyramidal Neuron |
| PPI | Prepulse Inhibition |
| PV | Parvalbumin |
| SERT | Serotonin Transporter |
| SSRIs | Selective Serotonin Reuptake Inhibitors |
| SST | Somatostatin |
| SWRs | Sharp Waves–Ripples |
| TrkB | Tropomyosin Receptor Kinase B Receptor |
| VH | Ventral Hippocampus |
References
- Pytliak, M.; Vargová, V.; Mechírová, V.; Felšöci, M. Serotonin receptors—From molecular biology to clinical applications. Physiol. Res. 2011, 60, 15–25. [Google Scholar] [CrossRef]
- Charnay, Y.; Léger, L. Brain serotonergic circuitries. Dialogues Clin. Neurosci. 2010, 12, 471–487. [Google Scholar] [CrossRef]
- Boess, F.G.; Martin, I.L. Molecular biology of 5-HT receptors. Neuropharmacology 1994, 33, 275–317. [Google Scholar] [CrossRef]
- Mlinar, B.; Corradetti, R. Differential modulation of CA1 impulse flow by endogenous serotonin along the hippocampal longitudinal axis. Hippocampus 2018, 28, 217–225. [Google Scholar] [CrossRef]
- Bombardi, C.; Grandis, A.; Pivac, N.; Sagud, M.; Lucas, G.; Chagraoui, A.; Lemaire-Mayo, V.; De Deurwaerdère, P.; Di Giovanni, G. Serotonin modulation of hippocampal functions: From anatomy to neurotherapeutics. Prog. Brain Res. 2021, 261, 83–158. [Google Scholar] [CrossRef]
- Fink, K.B.; Göthert, M. 5-HT receptor regulation of neurotransmitter release. Pharmacol. Rev. 2007, 59, 360–417. [Google Scholar] [CrossRef]
- Kojima, T.; Matsumoto, M.; Togashi, H.; Tachibana, K.; Kemmotsu, O.; Yoshioka, M. Fluvoxamine suppresses the long-term potentiation in the hippocampal CA1 field of anesthetized rats: An effect mediated via 5-HT1A receptors. Brain Res. 2003, 959, 165–168. [Google Scholar] [CrossRef]
- Gener, T.; Tauste Campo, A.; Alemany-González, M.; Nebot, P.; Delgado-Sallent, C.; Chanovas, J.; Puig, M.V. Serotonin 5-HT1A, 5-HT2A and dopamine D2 receptors strongly influence prefronto-hippocampal neural networks in alert mice: Contribution to the actions of risperidone. Neuropharmacology 2019, 158, 107743. [Google Scholar] [CrossRef]
- Yamazaki, M.; Arai, T.; Yarimizu, J.; Matsumoto, M. 5-HT5A Receptor Antagonist ASP5736 Ameliorates Several Abnormal Behaviors in an Fmr1-Targeted Transgenic Male Rat Model of Fragile X Syndrome. Int. J. Neuropsychopharmacol. 2022, 25, 786–793. [Google Scholar] [CrossRef]
- Wedzony, K.; Maćkowiak, M.; Czyrak, A.; Fijał, K.; Michalska, B. Single doses of MK-801, a non-competitive antagonist of NMDA receptors, increase the number of 5-HT1A serotonin receptors in the rat brain. Brain Res. 1997, 756, 84–91. [Google Scholar] [CrossRef]
- Bai, M.; Zhu, X.Z.; Zhang, Y.; Zhang, S.; Zhang, L.; Xue, L.; Zhong, M.; Zhang, X. Anhedonia was associated with the dysregulation of hippocampal HTR4 and microRNA Let-7a in rats. Physiol. Behav. 2014, 129, 135–141. [Google Scholar] [CrossRef]
- Wegrzyn, D.; Juckel, G.; Faissner, A. Structural and Functional Deviations of the Hippocampus in Schizophrenia and Schizophrenia Animal Models. Int. J. Mol. Sci. 2022, 23, 5482. [Google Scholar] [CrossRef]
- Hjorthøj, C.; Madsen, T.; Starzer, M.; Erlangsen, A.; Nordentoft, M. Mortality in substance-induced psychosis: A register-based national cohort study. Addiction 2021, 116, 3515–3524. [Google Scholar] [CrossRef]
- Harvey, P.D. Assessment of everyday functioning in schizophrenia: Implications for treatments aimed at negative symptoms. Schizophr. Res. 2013, 150, 353–355. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Reis Marques, T.; Howes, O.D. Schizophrenia-An Overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef]
- Białoń, M.; Wąsik, A. Advantages and Limitations of Animal Schizophrenia Models. Int. J. Mol. Sci. 2022, 23, 5968. [Google Scholar] [CrossRef]
- Dean, B.; Hayes, W.; Opeskin, K.; Naylor, L.; Pavey, G.; Hill, C.; Keks, N.; Copolov, D.L. Serotonin2 receptors and the serotonin transporter in the schizophrenic brain. Behav. Brain Res. 1996, 73, 169–175. [Google Scholar] [CrossRef]
- Winblad, B.; Bucht, G.; Gottfries, C.G.; Roos, B.E. Monoamines and monoamine metabolites in brains from demented schizophrenics. Acta Psychiatr. Scand. 1979, 60, 17–28. [Google Scholar] [CrossRef]
- Naylor, L.; Dean, B.; Opeskin, K.; Pavey, G.; Hill, C.; Keks, N.; Copolov, D. Changes in the serotonin transporter in the hippocampus of subjects with schizophrenia identified using [3H]paroxetine. J. Neural Transm. 1996, 103, 749–757. [Google Scholar] [CrossRef]
- Bengtsson, H.J.; Kullberg, A.; Millan, M.J.; Hjorth, S. The role of 5-HT1A autoreceptors and alpha1-adrenoceptors in the modulation of 5-HT release-III. Clozapine and the novel putative antipsychotic S 16924. Neuropharmacology 1998, 37, 349–356. [Google Scholar] [CrossRef]
- Li, Z.; Ichikawa, J.; Dai, J.; Meltzer, H.Y. Aripiprazole, a novel antipsychotic drug, preferentially increases dopamine release in the prefrontal cortex and hippocampus in rat brain. Eur. J. Pharmacol. 2004, 493, 75–83. [Google Scholar] [CrossRef]
- Choi, I.S.; Cho, J.H.; Kim, J.T.; Park, E.J.; Lee, M.G.; Shin, H.I.; Choi, B.J.; Jang, I.S. Serotoninergic modulation of GABAergic synaptic transmission in developing rat CA3 pyramidal neurons. J. Neurochem. 2007, 103, 2342–2353. [Google Scholar] [CrossRef]
- Dayer, A.G.; Jacobshagen, M.; Chaumont-Dubel, S.; Marin, P. 5-HT6 Receptor: A New Player Controlling the Development of Neural Circuits. ACS Chem. Neurosci. 2015, 6, 951–960. [Google Scholar] [CrossRef]
- Chestnykh, D.A.; Amato, D.; Kornhuber, J.; Müller, C.P. Pharmacotherapy of schizophrenia: Mechanisms of antipsychotic accumulation, therapeutic action and failure. Behav. Brain Res. 2021, 403, 113144. [Google Scholar] [CrossRef]
- Meltzer, H.Y. What’s atypical about atypical antipsychotic drugs? Curr. Opin. Pharmacol. 2004, 4, 53–57. [Google Scholar] [CrossRef]
- Blair, H.T.; Fanselow, M.S. Fear and memory: A view of the hippocampus through the lens of the amygdala. In Space, Time and Memory in the Hippocampal Formation; Springer: Berlin/Heidelberg, Germany, 2014; pp. 465–496. [Google Scholar]
- Gulyaeva, N.V.J.B. Stress-associated molecular and cellular hippocampal mechanisms common for epilepsy and comorbid depressive disorders. Biochemistry 2021, 86, 641–656. [Google Scholar] [CrossRef]
- Bannerman, D.M.; Sprengel, R.; Sanderson, D.J.; McHugh, S.B.; Rawlins, J.N.; Monyer, H.; Seeburg, P.H. Hippocampal synaptic plasticity, spatial memory and anxiety. Nat. Rev. Neurosci. 2014, 15, 181–192. [Google Scholar] [CrossRef]
- Okuyama, T.; Kitamura, T.; Roy, D.S.; Itohara, S.; Tonegawa, S. Ventral CA1 neurons store social memory. Science 2016, 353, 1536–1541. [Google Scholar] [CrossRef]
- Shi, H.J.; Wang, S.; Wang, X.P.; Zhang, R.X.; Zhu, L.J. Hippocampus: Molecular, Cellular, and Circuit Features in Anxiety. Neurosci. Bull. 2023, 39, 1009–1026. [Google Scholar] [CrossRef]
- Dedovic, K.; Duchesne, A.; Andrews, J.; Engert, V.; Pruessner, J.C. The brain and the stress axis: The neural correlates of cortisol regulation in response to stress. Neuroimage 2009, 47, 864–871. [Google Scholar] [CrossRef]
- Nelson, M.D.; Saykin, A.J.; Flashman, L.A.; Riordan, H.J. Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: A meta-analytic study. Arch. Gen. Psychiatry 1998, 55, 433–440. [Google Scholar] [CrossRef]
- Brugger, S.P.; Howes, O.D. Heterogeneity and Homogeneity of Regional Brain Structure in Schizophrenia: A Meta-analysis. JAMA Psychiatry 2017, 74, 1104–1111. [Google Scholar] [CrossRef]
- McHugo, M.; Armstrong, K.; Roeske, M.J.; Woodward, N.D.; Blackford, J.U.; Heckers, S. Hippocampal volume in early psychosis: A 2-year longitudinal study. Transl. Psychiatry 2020, 10, 306. [Google Scholar] [CrossRef]
- Dugré, J.R.; Dumais, A.; Tikasz, A.; Mendrek, A.; Potvin, S. Functional connectivity abnormalities of the long-axis hippocampal subregions in schizophrenia during episodic memory. NPJ Schizophr. 2021, 7, 19. [Google Scholar] [CrossRef]
- Donegan, J.J.; Tyson, J.A.; Branch, S.Y.; Beckstead, M.J.; Anderson, S.A.; Lodge, D.J. Stem cell-derived interneuron transplants as a treatment for schizophrenia: Preclinical validation in a rodent model. Mol. Psychiatry 2017, 22, 1492–1501. [Google Scholar] [CrossRef]
- Szeszko, P.R.; Strous, R.D.; Goldman, R.S.; Ashtari, M.; Knuth, K.H.; Lieberman, J.A.; Bilder, R.M. Neuropsychological correlates of hippocampal volumes in patients experiencing a first episode of schizophrenia. Am. J. Psychiatry 2002, 159, 217–226. [Google Scholar] [CrossRef]
- Avery, S.N.; Rogers, B.P.; Heckers, S. Hippocampal Network Modularity Is Associated With Relational Memory Dysfunction in Schizophrenia. eLife 2018, 3, 423–432. [Google Scholar] [CrossRef]
- Amaral, D.G.; Lavenex, P. Hippocampal Neuroanatomy. In The Hippocampus Book; Andersen, P., Morris, R., Amaral, D., Bliss, T., O’Keefe, J., Eds.; Oxford University Press: Oxford, UK, 2007; pp. 37–114. [Google Scholar]
- Strange, B.A.; Witter, M.P.; Lein, E.S.; Moser, E.I. Functional organization of the hippocampal longitudinal axis. Nat. Rev. Neurosci. 2014, 15, 655–669. [Google Scholar] [CrossRef]
- Fanselow, M.S.; Dong, H.W. Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010, 65, 7–19. [Google Scholar] [CrossRef]
- Papatheodoropoulos, C. Electrophysiological evidence for long-axis intrinsic diversification of the hippocampus. Front. Biosci. 2018, 23, 109–145. [Google Scholar] [CrossRef]
- Tannenholz, L.; Jimenez, J.C.; Kheirbek, M.A. Local and regional heterogeneity underlying hippocampal modulation of cognition and mood. Front. Behav. Neurosci. 2014, 8, 147. [Google Scholar] [CrossRef]
- Witter, M.P.; Wouterlood, F.G.; Naber, P.A.; Van Haeften, T. Anatomical organization of the parahippocampal-hippocampal network. Ann. N. Y. Acad. Sci. 2000, 911, 1–24. [Google Scholar] [CrossRef]
- Small, S.A. The longitudinal axis of the hippocampal formation: Its anatomy, circuitry, and role in cognitive function. Rev. Neurosci. 2002, 13, 183–194. [Google Scholar] [CrossRef]
- Roy, D.S.; Kitamura, T.; Okuyama, T.; Ogawa, S.K.; Sun, C.; Obata, Y.; Yoshiki, A.; Tonegawa, S. Distinct Neural Circuits for the Formation and Retrieval of Episodic Memories. Cell 2017, 170, 1000–1012. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Izaki, Y.; Takita, M.; Akema, T. Specific role of the posterior dorsal hippocampus-prefrontal cortex in short-term working memory. Eur. J. Neurosci. 2008, 27, 3029–3034. [Google Scholar] [CrossRef]
- Yoon, S.H.; Song, W.S.; Chung, G.; Kim, S.J.; Kim, M.H. Activity in the dorsal hippocampus-mPFC circuit modulates stress-coping strategies during inescapable stress. Exp. Mol. Med. 2024, 56, 1921–1935. [Google Scholar] [CrossRef]
- Liu, Y.; McAfee, S.S.; Heijden, M.E.; Dhamala, M.; Sillitoe, R.V.; Heck, D.H. Causal Evidence for a Role of Cerebellar Lobulus Simplex in Prefrontal-Hippocampal Interaction in Spatial Working Memory Decision-Making. Cerebellum 2022, 21, 762–775. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Urien, L.; Cohen, S.; Howard, S.; Yakimov, A.; Nordlicht, R.; Bauer, E.P. Aversive Contexts Reduce Activity in the Ventral Subiculum- BNST Pathway. Neuroscience 2022, 496, 129–140. [Google Scholar] [CrossRef]
- Schwarz, K.; Moessnang, C.; Schweiger, J.I.; Harneit, A.; Schneider, M.; Chen, J.; Cao, H.; Schwarz, E.; Witt, S.H.; Rietschel, M.; et al. Ventral Striatal-Hippocampus Coupling During Reward Processing as a Stratification Biomarker for Psychotic Disorders. Biol. Psychiatry 2022, 91, 216–225. [Google Scholar] [CrossRef]
- Hiser, J.; Koenigs, M. The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol. Psychiatry 2018, 83, 638–647. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bakoyiannis, I.; Ducourneau, E.G.; Parkes, S.L.; Ferreira, G. Pathway specific interventions reveal the multiple roles of ventral hippocampus projections in cognitive functions. Rev. Neurosci. 2023, 34, 825–838. [Google Scholar] [CrossRef]
- Bjarkam, C.R.; Sørensen, J.C.; Geneser, F.A. Distribution and morphology of serotonin-immunoreactive axons in the hippocampal region of the New Zealand white rabbit. I. Area dentata and hippocampus. Hippocampus 2003, 13, 21–37. [Google Scholar] [CrossRef]
- Azmitia, E.C.; Segal, M. An autoradiographic analysis of the differential ascending projections of the dorsal and median raphe nuclei in the rat. J. Comp. Neurol. 1978, 179, 641–667. [Google Scholar] [CrossRef]
- Oleskevich, S.; Descarries, L. Quantified distribution of the serotonin innervation in adult rat hippocampus. Neuroscience 1990, 34, 19–33. [Google Scholar] [CrossRef]
- Ott, C.V.; Johnson, C.B.; Macoveanu, J.; Miskowiak, K. Structural changes in the hippocampus as a biomarker for cognitive improvements in neuropsychiatric disorders: A systematic review. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2019, 29, 319–329. [Google Scholar] [CrossRef]
- Solowij, N.; Walterfang, M.; Lubman, D.I.; Whittle, S.; Lorenzetti, V.; Styner, M.; Velakoulis, D.; Pantelis, C.; Yücel, M. Alteration to hippocampal shape in cannabis users with and without schizophrenia. Schizophr. Res. 2013, 143, 179–184. [Google Scholar] [CrossRef]
- Chye, Y.; Lorenzetti, V.; Suo, C.; Batalla, A.; Cousijn, J.; Goudriaan, A.E.; Jenkinson, M.; Martin-Santos, R.; Whittle, S.; Yücel, M.; et al. Alteration to hippocampal volume and shape confined to cannabis dependence: A multi-site study. Addict. Biol. 2019, 24, 822–834. [Google Scholar] [CrossRef]
- Lisman, J.E.; Grace, A.A. The hippocampal-VTA loop: Controlling the entry of information into long-term memory. Neuron 2005, 46, 703–713. [Google Scholar] [CrossRef]
- Guo, G.; Tang, J.; Shi, M.; Yang, C.; Ou, H.; Chen, W. MK212, a 5-hydroxytryptamine 2C receptor agonist, reverses prepulse inhibition deficits in the medial prefrontal cortex and ventral hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 113, 110441. [Google Scholar] [CrossRef]
- Licht, C.L.; Kirkegaard, L.; Zueger, M.; Chourbaji, S.; Gass, P.; Aznar, S.; Knudsen, G.M. Changes in 5-HT4 receptor and 5-HT transporter binding in olfactory bulbectomized and glucocorticoid receptor heterozygous mice. Neurochem. Int. 2010, 56, 603–610. [Google Scholar] [CrossRef]
- Anacker, C.; Hen, R. Adult hippocampal neurogenesis and cognitive flexibility—Linking memory and mood. Nat. Rev. Neurosci. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Liang, S.; Wu, Y.; Hanxiaoran, L.; Greenshaw, A.J.; Li, T. Anhedonia in Depression and Schizophrenia: Brain Reward and Aversion Circuits. Neuropsychiatr. Dis. Treat. 2022, 18, 1385–1396. [Google Scholar] [CrossRef]
- Ohno, M.; Watanabe, S. Differential effects of 5-HT3 receptor antagonism on working memory failure due to deficiency of hippocampal cholinergic and glutamatergic transmission in rats. Brain Res. 1997, 762, 211–215. [Google Scholar] [CrossRef]
- Buhot, M.C.; Naïli, S. Changes in exploratory activity following stimulation of hippocampal 5-HT1A and 5-HT1B receptors in the rat. Hippocampus 1995, 5, 198–208. [Google Scholar] [CrossRef]
- Dahlström, A.; Fuxe, K. Localization of monoamines in the lower brain stem. Experientia 1964, 20, 398–399. [Google Scholar] [CrossRef]
- Molliver, M.E. Serotonergic neuronal systems: What their anatomic organization tells us about function. J. Clin. Psychopharmacol. 1987, 7, 3s–23s. [Google Scholar] [CrossRef]
- Jacobs, B.L.; Azmitia, E.C. Structure and function of the brain serotonin system. Physiol. Rev. 1992, 72, 165–229. [Google Scholar] [CrossRef]
- McCorvy, J.D.; Roth, B.L. Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 2015, 150, 129–142. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tanaka, K.F.; Samuels, B.A.; Hen, R. Serotonin receptor expression along the dorsal-ventral axis of mouse hippocampus. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2395–2401. [Google Scholar] [CrossRef]
- Kinsey, A.M.; Wainwright, A.; Heavens, R.; Sirinathsinghji, D.J.; Oliver, K.R. Distribution of 5-ht(5A), 5-ht(5B), 5-ht(6) and 5-HT(7) receptor mRNAs in the rat brain. Brain Res. Mol. Brain Res. 2001, 88, 194–198. [Google Scholar] [CrossRef]
- Berumen, L.C.; Rodríguez, A.; Miledi, R.; García-Alcocer, G. Serotonin receptors in hippocampus. Sci. World J. 2012, 2012, 823493. [Google Scholar] [CrossRef]
- Andrade, R.; Nicoll, R.A. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. J. Physiol. 1987, 394, 99–124. [Google Scholar] [CrossRef]
- Penington, N.J.; Kelly, J.S. Serotonin receptor activation reduces calcium current in an acutely dissociated adult central neuron. Neuron 1990, 4, 751–758. [Google Scholar] [CrossRef]
- Yuen, E.Y.; Jiang, Q.; Chen, P.; Gu, Z.; Feng, J.; Yan, Z. Serotonin 5-HT1A receptors regulate NMDA receptor channels through a microtubule-dependent mechanism. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 5488–5501. [Google Scholar] [CrossRef]
- Polter, A.M.; Li, X. 5-HT1A receptor-regulated signal transduction pathways in brain. Cell. Signal. 2010, 22, 1406–1412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zimmer, L.; Rbah, L.; Giacomelli, F.; Le Bars, D.; Renaud, B. A reduced extracellular serotonin level increases the 5-HT1A PET ligand 18F-MPPF binding in the rat hippocampus. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2003, 44, 1495–1501. [Google Scholar]
- Erlander, M.G.; Lovenberg, T.W.; Baron, B.M.; Lecea, L.; Danielson, P.E.; Racke, M.; Slone, A.L.; Siegel, B.W.; Foye, P.E.; Cannon, K. Two members of a distinct subfamily of 5-hydroxytryptamine receptors differentially expressed in rat brain. Proc. Natl. Acad. Sci. USA 1993, 90, 3452–3456. [Google Scholar] [CrossRef]
- Nelson, D.L. 5-HT5 receptors. Curr. Drug Targets CNS Neurol. Disord. 2004, 3, 1. [Google Scholar] [CrossRef]
- Francken, B.J.; Jurzak, M.; Vanhauwe, J.F.; Luyten, W.H.; Leysen, J.E. The human 5-ht5A receptor couples to Gi/Go proteins and inhibits adenylate cyclase in HEK 293 cells. Eur. J. Pharmacol. 1998, 361, 299–309. [Google Scholar] [CrossRef]
- Grailhe, R.; Grabtree, G.W.; Hen, R. Human 5-HT(5) receptors: The 5-HT(5A) receptor is functional but the 5-HT(5B) receptor was lost during mammalian evolution. Eur. J. Pharmacol. 2001, 418, 157–167. [Google Scholar] [CrossRef]
- Noda, M.; Yasuda, S.; Okada, M.; Higashida, H.; Shimada, A.; Iwata, N.; Ozaki, N.; Nishikawa, K.; Shirasawa, S.; Uchida, M.; et al. Recombinant human serotonin 5A receptors stably expressed in C6 glioma cells couple to multiple signal transduction pathways. J. Neurochem. 2003, 84, 222–232. [Google Scholar] [CrossRef]
- Sagi, Y.; Medrihan, L.; George, K.; Barney, M.; McCabe, K.A.; Greengard, P. Emergence of 5-HT5A signaling in parvalbumin neurons mediates delayed antidepressant action. Mol. Psychiatry 2020, 25, 1191–1201. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D’Adamo, M.C.; Catacuzzeno, L.; Di Giovanni, G.; Franciolini, F.; Pessia, M. K(+) channelepsy: Progress in the neurobiology of potassium channels and epilepsy. Front. Cell. Neurosci. 2013, 7, 134. [Google Scholar] [CrossRef]
- Kurrasch-Orbaugh, D.M.; Parrish, J.C.; Watts, V.J.; Nichols, D.E. A complex signaling cascade links the serotonin2A receptor to phospholipase A2 activation: The involvement of MAP kinases. J. Neurochem. 2003, 86, 980–991. [Google Scholar] [CrossRef]
- Kurrasch-Orbaugh, D.M.; Watts, V.J.; Barker, E.L.; Nichols, D.E. Serotonin 5-hydroxytryptamine 2A receptor-coupled phospholipase C and phospholipase A2 signaling pathways have different receptor reserves. J. Pharmacol. Exp. Ther. 2003, 304, 229–237. [Google Scholar] [CrossRef]
- Bécamel, C.; Gavarini, S.; Chanrion, B.; Alonso, G.; Galéotti, N.; Dumuis, A.; Bockaert, J.; Marin, P. The serotonin 5-HT2A and 5-HT2C receptors interact with specific sets of PDZ proteins. J. Biol. Chem. 2004, 279, 20257–20266. [Google Scholar] [CrossRef]
- Vaidya, V.A.; Marek, G.J.; Aghajanian, G.K.; Duman, R.S. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J. Neurosci. 1997, 17, 2785–2795. [Google Scholar] [CrossRef]
- Buckholtz, N.S.; Freedman, D.X.; Middaugh, L.D. Daily LSD administration selectively decreases serotonin2 receptor binding in rat brain. Eur. J. Pharmacol. 1985, 109, 421–425. [Google Scholar] [CrossRef]
- Qu, Y.; Villacreses, N.; Murphy, D.L.; Rapoport, S.I. 5-HT2A/2C receptor signaling via phospholipase A2 and arachidonic acid is attenuated in mice lacking the serotonin reuptake transporter. Psychopharmacology 2005, 180, 12–20. [Google Scholar] [CrossRef]
- Nichols, D.E.; Nichols, C.D. Serotonin receptors. Chem. Rev. 2008, 108, 1614. [Google Scholar] [CrossRef]
- Davies, P.A.; Pistis, M.; Hanna, M.C.; Peters, J.A.; Lambert, J.J.; Hales, T.G.; Kirkness, E.F. The 5-HT3B subunit is a major determinant of serotonin-receptor function. Nature 1999, 397, 359–363. [Google Scholar] [CrossRef]
- Peters, J.A.; Kelley, S.P.; Dunlop, J.I.; Kirkness, E.F.; Hales, T.G.; Lambert, J.J. The 5-hydroxytryptamine type 3 (5-HT3) receptor reveals a novel determinant of single-channel conductance. Biochem. Soc. Trans. 2004, 32, 547–552. [Google Scholar] [CrossRef]
- Reeves, D.C.; Lummis, S.C. The molecular basis of the structure and function of the 5-HT3 receptor: A model ligand-gated ion channel review. Mol. Membr. Biol. 2002, 19, 11–26. [Google Scholar] [CrossRef]
- Barnes, N.M.; Hales, T.G.; Lummis, S.C.; Peters, J.A. The 5-HT3 receptor--the relationship between structure and function. Neuropharmacology 2009, 56, 273–284. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sudweeks, S.N.; Hooft, J.A.; Yakel, J.L. Serotonin 5-HT(3) receptors in rat CA1 hippocampal interneurons: Functional and molecular characterization. J. Physiol. 2002, 544, 715–726. [Google Scholar] [CrossRef]
- Bickmeyer, U.; Heine, M.; Manzke, T.; Richter, D.W. Differential modulation of I(h) by 5-HT receptors in mouse CA1 hippocampal neurons. Eur. J. Neurosci. 2002, 16, 209–218. [Google Scholar] [CrossRef]
- Chapin, E.M.; Haj-Dahmane, S.; Torres, G.; Andrade, R. The 5-HT(4) receptor-induced depolarization in rat hippocampal neurons is mediated by cAMP but is independent of Ih. Neurosci. Lett. 2002, 324, 1–4. [Google Scholar] [CrossRef]
- Torres, G.E.; Chaput, Y.; Andrade, R. Cyclic AMP and protein kinase A mediate 5-hydroxytryptamine type 4 receptor regulation of calcium-activated potassium current in adult hippocampal neurons. Mol. Pharmacol. 1995, 47, 191–197. [Google Scholar] [CrossRef]
- Levallet, G.; Hotte, M.; Boulouard, M.; Dauphin, F. Increased particulate phosphodiesterase 4 in the prefrontal cortex supports 5-HT4 receptor-induced improvement of object recognition memory in the rat. Psychopharmacology 2009, 202, 125–139. [Google Scholar] [CrossRef]
- Pascual-Brazo, J.; Castro, E.; Díaz, A.; Valdizán, E.M.; Pilar-Cuéllar, F.; Vidal, R.; Treceño, B.; Pazos, A. Modulation of neuroplasticity pathways and antidepressant-like behavioural responses following the short-term (3 and 7 days) administration of the 5-HT4 receptor agonist RS67333. Int. J. Neuropsychopharmacol. 2012, 15, 631–643. [Google Scholar] [CrossRef]
- Vidal, R.; Valdizán, E.M.; Mostany, R.; Pazos, A.; Castro, E. Long-term treatment with fluoxetine induces desensitization of 5-HT4 receptor-dependent signalling and functionality in rat brain. J. Neurochem. 2009, 110, 1120–1127. [Google Scholar] [CrossRef]
- Lucas, G.; Compan, V.; Charnay, Y.; Neve, R.L.; Nestler, E.J.; Bockaert, J.; Barrot, M.; Debonnel, G. Frontocortical 5-HT4 receptors exert positive feedback on serotonergic activity: Viral transfections, subacute and chronic treatments with 5-HT4 agonists. Biol. Psychiatry 2005, 57, 918–925. [Google Scholar] [CrossRef]
- Sonnenberg, S.B.; Rauer, J.; Göhr, C.; Gorinski, N.; Schade, S.K.; Galil, D.A.; Naumenko, V.; Zeug, A.; Bischoff, S.C.; Ponimaskin, E.; et al. The 5-HT4 receptor interacts with adhesion molecule L1 to modulate morphogenic signaling in neurons. J. Cell Sci. 2021, 134, jcs249193. [Google Scholar] [CrossRef]
- Ruat, M.; Traiffort, E.; Arrang, J.; Tardivellacombe, J.; Diaz, J.; Leurs, R.; Schwartz, J. A novel rat serotonin (5-HT6) receptor: Molecular cloning, localization and stimulation of cAMP accumulation. Biochem. Biophys. Res. Commun. 1993, 193, 268–276. [Google Scholar] [CrossRef]
- Pereira, M.; Martynhak, B.J.; Andreatini, R.; Svenningsson, P. 5-HT6 receptor agonism facilitates emotional learning. Front. Pharmacol. 2015, 6, 200. [Google Scholar] [CrossRef]
- Marcos, B.; Cabero, M.; Solas, M.; Aisa, B.; Ramirez, M.J. Signalling pathways associated with 5-HT6 receptors: Relevance for cognitive effects. Int. J. Neuropsychopharmacol. 2010, 13, 775–784. [Google Scholar] [CrossRef]
- El Mestikawy, S.; Ruat, M.; Traiffort, E.; Hamon, M.; Martres, M.-P.; Gérard, C.; Lebrand, C.; Adrien, J. Quantitative RT-PCR distribution of serotonin 5-HT6 receptor mRNA in the central nervous system of control or 5,7-dihydroxytryptamine-treated rats. Synapse 1996, 23, 164–173. [Google Scholar]
- Rezaei, R.M.; Shiravi, A.; Seyedinia, S.A.; Kor, N.M.; Vafaei, A.A.; Pour, A.R. Role of Hippocampal 5-HT6 Receptors in Glucocorticoid-Induced Enhancement of Memory Consolidation in Rats. Basic Clin. Neurosci. J. 2020, 11, 507–516. [Google Scholar] [CrossRef]
- Yau, J.L.; Noble, J.; Widdowson, J.; Seckl, J.R. Impact of adrenalectomy on 5-HT6 and 5-HT7 receptor gene expression in the rat hippocampus. Mol. Brain Res. 1997, 45, 182–186. [Google Scholar] [CrossRef]
- Duhr, F.; Déléris, P.; Raynaud, F.; Séveno, M.; Morisset-Lopez, S.; la Cour, C.M.; Millan, M.J.; Bockaert, J.; Marin, P.; Chaumont-Dubel, S. Cdk5 induces constitutive activation of 5-HT6 receptors to promote neurite growth. Nat. Chem. Biol. 2014, 10, 590–597. [Google Scholar] [CrossRef]
- Thomas, D.R.; Middlemiss, D.N.; Taylor, S.G.; Nelson, P.; Brown, A.M. 5-CT stimulation of adenylyl cyclase activity in guinea-pig hippocampus: Evidence for involvement of 5-HT7 and 5-HT1A receptors. Br. J. Pharmacol. 1999, 128, 158–164. [Google Scholar] [CrossRef]
- Kvachnina, E.; Liu, G.; Dityatev, A.; Renner, U.; Dumuis, A.; Richter, D.W.; Dityateva, G.; Schachner, M.; Voyno-Yasenetskaya, T.A.; Ponimaskin, E.G. 5-HT7 receptor is coupled to Gα subunits of heterotrimeric G12-Protein to regulate gene transcription and neuronal morphology. J. Neurosci. 2005, 25, 7821–7830. [Google Scholar] [CrossRef]
- Samarajeewa, A.; Goldemann, L.; Vasefi, M.S.; Ahmed, N.; Gondora, N.; Khanderia, C.; Mielke, J.G.; Beazely, M.A. 5-HT7 receptor activation promotes an increase in TrkB receptor expression and phosphorylation. Front. Behav. Neurosci. 2014, 8, 391. [Google Scholar] [CrossRef]
- Andreetta, F.; Carboni, L.; Grafton, G.; Jeggo, R.; Whyment, A.D.; Top, M.v.D.; Hoyer, D.; Spanswick, D.; Barnes, N.M. Hippocampal 5-HT7 receptors signal phosphorylation of the GluA1 subunit to facilitate AMPA receptor mediated-neurotransmission In Vitro and In Vivo. Br. J. Pharmacol. 2016, 173, 1438–1451. [Google Scholar] [CrossRef]
- Vasefi, M.S.; Kruk, J.S.; Heikkila, J.J.; Beazely, M.A. 5-Hydroxytryptamine type 7 receptor neuroprotection against NMDA-induced excitotoxicity is PDGFβ receptor dependent. J. Neurochem. 2013, 125, 26–36. [Google Scholar] [CrossRef]
- Vasefi, M.S.; Yang, K.; Li, J.; Kruk, J.S.; Heikkila, J.J.; Jackson, M.F.; MacDonald, J.F.; A Beazely, M. Acute 5-HT7 receptor activation increases NMDA-evoked currents and differentially alters NMDA receptor subunit phosphorylation and trafficking in hippocampal neurons. Mol. Brain 2013, 6, 24. [Google Scholar] [CrossRef]
- Tokarski, K.; Zelek-Molik, A.; Duszyńska, B.; Satała, G.; Bobula, B.; Kusek, M.; Chmielarz, P.; Nalepa, I.; Hess, G. Acute and repeated treatment with the 5-HT7 receptor antagonist SB 269970 induces functional desensitization of 5-HT7 receptors in rat hippocampus. Pharmacol. Rep. 2012, 64, 256–265. [Google Scholar] [CrossRef]
- Kvachnina, E.; Dumuis, A.; Wlodarczyk, J.; Renner, U.; Cochet, M.; Richter, D.W.; Ponimaskin, E. Constitutive Gs-mediated, but not G12-mediated, activity of the 5-hydroxytryptamine 5-HT7(a) receptor is modulated by the palmitoylation of its C-terminal domain. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2009, 1793, 1646–1655. [Google Scholar] [CrossRef]
- Renner, U.; Zeug, A.; Woehler, A.; Niebert, M.; Dityatev, A.; Dityateva, G.; Gorinski, N.; Guseva, D.; Abdel-Galil, D.; Fröhlich, M.; et al. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 2012, 125, 2486–2499. [Google Scholar] [CrossRef]
- Kosofsky, B.E.; Molliver, M.E. The serotoninergic innervation of cerebral cortex: Different classes of axon terminals arise from dorsal and median raphe nuclei. Synapse 1987, 1, 153–168. [Google Scholar] [CrossRef]
- Mamounas, L.A.; Molliver, M.E. Evidence for dual serotonergic projections to neocortex: Axons from the dorsal and median raphe nuclei are differentially vulnerable to the neurotoxin p-chloroamphetamine (PCA). Exp. Neurol. 1988, 102, 23–36. [Google Scholar] [CrossRef]
- Molliver, M.E.; Berger, U.V.; Mamounas, L.A.; Molliver, D.C.; O’Hearn, E.; Wilson, M.A. Neurotoxicity of MDMA and Related Compounds: Anatomic Studies. Ann. N. Y. Acad. Sci. 1990, 600, 661–664. [Google Scholar] [CrossRef]
- Descarries, L.; Mechawar, N. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog. Brain Res. 2000, 125, 27–47. [Google Scholar] [CrossRef]
- Varga, V.; Losonczy, A.; Zemelman, B.V.; Borhegyi, Z.; Nyiri, G.; Domonkos, A.; Hangya, B.; Holderith, N.; Magee, J.C.; Freund, T.F. Fast synaptic subcortical control of hippocampal circuits. Science 2009, 326, 449–453. [Google Scholar] [CrossRef]
- De Filippo, R.; Schmitz, D. Transcriptomic mapping of the 5-HT receptor landscape. Patterns 2024, 5, 101048. [Google Scholar] [CrossRef]
- Hannon, J.; Hoyer, D. Molecular biology of 5-HT receptors. Behav. Brain Res. 2008, 195, 198–213. [Google Scholar] [CrossRef]
- Verge, D.; Daval, G.; Marcinkiewicz, M.; Patey, A.; el Mestikawy, S.; Gozlan, H.; Hamon, M. Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J. Neurosci. 1986, 6, 3474–3482. [Google Scholar] [CrossRef]
- Li, Q.; Nakadate, K.; Tanaka-Nakadate, S.; Nakatsuka, D.; Cui, Y.; Watanabe, Y. Unique expression patterns of 5-HT2A and 5-HT2C receptors in the rat brain during postnatal development: Western blot and immunohistochemical analyses. J. Comp. Neurol. 2003, 469, 128–140. [Google Scholar] [CrossRef]
- Mengod, G.; Nguyen, H.; Le, H.; Waeber, C.; Lübbert, H.; Palacios, J. The distribution and cellular localization of the serotonin 1C receptor mRNA in the rodent brain examined by in situ hybridization histochemistry. Comparison with receptor binding distribution. Neuroscience 1990, 35, 577–591. [Google Scholar] [CrossRef]
- Tecott, L.H.; Maricq, A.V.; Julius, D. Nervous system distribution of the serotonin 5-HT3 receptor mRNA. Proc. Natl. Acad. Sci. USA 1993, 90, 1430–1434. [Google Scholar] [CrossRef]
- Kia, H.K.; Miquel, M.C.; Brisorgueil, M.J.; Daval, G.; Riad, M.; El Mestikawy, S.; Hamon, M.; Vergé, D. Immunocytochemical localization of serotonin1A receptors in the rat central nervous system. J. Comp. Neurol. 1996, 365, 289–305. [Google Scholar] [CrossRef]
- Banasr, M.; Hery, M.; Printemps, R.; Daszuta, A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 2004, 29, 450–460. [Google Scholar] [CrossRef]
- Gould, E. Serotonin and hippocampal neurogenesis. Neuropsychopharmacology 1999, 21, 46S–51S. [Google Scholar] [CrossRef]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef]
- Sarnyai, Z.; Sibille, E.L.; Pavlides, C.; Fenster, R.J.; McEwen, B.S.; Tóth, M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin 1A receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 14731–14736. [Google Scholar] [CrossRef]
- Schechter, L.E.; Smith, D.L.; Rosenzweig-Lipson, S.; Sukoff, S.J.; Dawson, L.A.; Marquis, K.; Jones, D.; Piesla, M.; Andree, T.; Nawoschik, S.; et al. Lecozotan (SRA-333): A selective serotonin 1A receptor antagonist that enhances the stimulated release of glutamate and acetylcholine in the hippocampus and possesses cognitive-enhancing properties. J. Pharmacol. Exp. Ther. 2005, 314, 1274–1289. [Google Scholar] [CrossRef]
- Ohno, M.; Watanabe, S. Blockade of 5-HT1A receptors compensates loss of hippocampal cholinergic neurotransmission involved in working memory of rats. Brain Res. 1996, 736, 180–188. [Google Scholar] [CrossRef]
- Carli, M.; Silva, S.; Balducci, C.; Samanin, R. WAY 100635, a 5-HT1A receptor antagonist, prevents the impairment of spatial learning caused by blockade of hippocampal NMDA receptors. Neuropharmacology 1999, 38, 1165–1173. [Google Scholar] [CrossRef]
- Schiapparelli, L.; Del Río, J.; Frechilla, D. Serotonin 5-HT receptor blockade enhances Ca2+/calmodulin-dependent protein kinase II function and membrane expression of AMPA receptor subunits in the rat hippocampus: Implications for memory formation. J. Neurochem. 2005, 94, 884–895. [Google Scholar] [CrossRef]
- Rasmuson, S.; Olsson, T.; Henriksson, B.G.; Kelly, P.A.; Holmes, M.C.; Seckl, M., Jr.; Mohammed, A.H. Environmental enrichment selectively increases 5-HT1A receptor mRNA expression and binding in the rat hippocampus. Mol. Brain Res. 1998, 53, 285–290. [Google Scholar] [CrossRef]
- Afshar, S.; Shahidi, S.; Rohani, A.H.; Asl, S.S.; Komaki, A. Protective effects of 5-HT1A receptor antagonist and 5-HT2A receptor agonist on the biochemical and histological features in a rat model of Alzheimer’s disease. J. Chem. Neuroanat. 2019, 96, 140–147. [Google Scholar] [CrossRef]
- Alves, S.H.; Pinheiro, G.; Motta, V.; Landeira-Fernandez, J.; Cruz, A.P.M. Anxiogenic effects in the rat elevated plus-maze of 5-HT2C agonists into ventral but not dorsal hippocampus. Behav. Pharmacol. 2004, 15, 37–43. [Google Scholar] [CrossRef]
- Sant’aNa, A.B.; Vilela-Costa, H.H.; Vicente, M.A.; Hernandes, P.M.; de Andrade, T.G.C.S.; Zangrossi, H. Role of 5-HT2C receptors of the dorsal hippocampus in the modulation of anxiety- and panic-related defensive responses in rats. Neuropharmacology 2019, 148, 311–319. [Google Scholar] [CrossRef]
- Cremers, T.I.F.H.; Rea, K.; Bosker, F.J.; Wikström, H.V.; Hogg, S.; Mørk, A.; Westerink, B.H.C. Augmentation of SSRI effects on serotonin by 5-HT2C antagonists: Mechanistic studies. Neuropsychopharmacology 2007, 32, 1550–1557. [Google Scholar] [CrossRef]
- Harvey, J.A. Role of the Serotonin 5-HT2A Receptor in Learning. Learn. Mem. 2003, 10, 355–362. [Google Scholar] [CrossRef]
- Zhang, G.; Cinalli, D.; Cohen, S.J.; Knapp, K.D.; Rios, L.M.; Martínez-Hernández, J.; Luján, R.; Stackman, R.W. Examination of the hippocampal contribution to serotonin 5-HT2A receptor-mediated facilitation of object memory in C57BL/6J mice. Neuropharmacology 2016, 109, 332–340. [Google Scholar] [CrossRef]
- Naghdi, N.; Rezaei, M.; Fathollahi, Y. Microinjection of ritanserin into the CA1 region of hippocampus improves scopolamine-induced amnesia in adult male rats. Behav. Brain Res. 2006, 168, 215–220. [Google Scholar] [CrossRef]
- Buchborn, T.; Schröder, H.; Höllt, V.; Grecksch, G. Repeated lysergic acid diethylamide in an animal model of depression: Normalisation of learning behaviour and hippocampal serotonin 5-HT2 signalling. J. Psychopharmacol. 2014, 28, 545–552. [Google Scholar] [CrossRef]
- Zhang, G.; Cinalli, D.; Stackman, R.W. Effect of a hallucinogenic serotonin 5-HT2A receptor agonist on visually guided, hippocampal-dependent spatial cognition in C57BL/6J mice. Hippocampus 2017, 27, 558–569. [Google Scholar] [CrossRef]
- Kondo, M. Molecular Mechanisms of Exercise-induced Hippocampal Neurogenesis and Antidepressant Effects. JMA J. 2023, 6, 114–119. [Google Scholar] [CrossRef]
- Carli, M.; Luschi, R.; Samanin, R. Dose-related impairment of spatial learning by intrahippocampal scopolamine: Antagonism by ondansetron, a 5-HT3 receptor antagonist. Behav. Brain Res. 1997, 82, 185–194. [Google Scholar] [CrossRef]
- Harrell, A.V.; Allan, A.M. Improvements in hippocampal-dependent learning and decremental attention in 5-HT3 receptor overexpressing mice. Learn. Mem. 2003, 10, 410–419. [Google Scholar] [CrossRef]
- Kagami-Ishi, Y.; Shibata, S.; Watanabe, S. Neuroprotective effect of 5-HT3 receptor antagonist on ischemia-induced decrease in CA1 field potential in rat hippocampal slices. Eur. J. Pharmacol. 1992, 224, 51–56. [Google Scholar] [CrossRef]
- Rahimian, R.; Fakhfouri, G.; Mehr, S.E.; Ghia, J.; Genazzani, A.A.; Payandemehr, B.; Dehpour, A.R.; Mousavizadeh, K.; Lim, D. Tropisetron attenuates amyloid-beta-induced inflammatory and apoptotic responses in rats. Eur. J. Clin. Investig. 2013, 43, 1039–1051. [Google Scholar] [CrossRef]
- Wu, Z.-M.; Yang, L.-H.; Cui, R.; Ni, G.-L.; Wu, F.-T.; Liang, Y. Contribution of Hippocampal 5-HT3 Receptors in Hippocampal Autophagy and Extinction of Conditioned Fear Responses after a Single Prolonged Stress Exposure in Rats. Cell. Mol. Neurobiol. 2017, 37, 595–606. [Google Scholar] [CrossRef]
- Kondo, M.; Nakamura, Y.; Ishida, Y.; Yamada, T.; Shimada, S. The 5-HT3A receptor is essential for fear extinction. Learn. Mem. 2013, 21, 1–4. [Google Scholar] [CrossRef]
- Brady, C.A.; Dover, T.J.; Massoura, A.N.; Princivalle, A.P.; Hope, A.G.; Barnes, N.M. Identification of 5-HT3A and 5-HT3B receptor subunits in human hippocampus. Neuropharmacology 2007, 52, 1284–1290. [Google Scholar] [CrossRef]
- Cachard-Chastel, M.; Lezoualc’H, F.; Dewachter, I.; Deloménie, C.; Croes, S.; Devijver, H.; Langlois, M.; Van Leuven, F.; Sicsic, S.; Gardier, A.M. 5-HT4 receptor agonists increase sAPPα levels in the cortex and hippocampus of male C57BL/6j mice. Br. J. Pharmacol. 2007, 150, 883–892. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tesseur, I.; Pimenova, A.A.; Lo, A.C.; Ciesielska, M.; Lichtenthaler, S.F.; De Maeyer, J.H.; Schuurkes, J.A.; D’Hooge, R.; De Strooper, B. Chronic 5-HT4 receptor activation decreases Aβ production and deposition in hAPP/PS1 mice. Neurobiol. Aging 2013, 34, 1779–1789. [Google Scholar] [CrossRef]
- Hashemi-Firouzi, N.; Shahidi, S.; Asl, S.S. Chronic stimulation of the serotonergic 5-HT4 receptor modulates amyloid-beta-related impairments in synaptic plasticity and memory deficits in male rats. Brain Res. 2021, 1773, 147701. [Google Scholar] [CrossRef]
- Licht, C.L.; Marcussen, A.B.; Wegener, G.; Overstreet, D.H.; Aznar, S.; Knudsen, G.M. The brain 5-HT4 receptor binding is down-regulated in the Flinders Sensitive Line depression model and in response to paroxetine administration. J. Neurochem. 2009, 109, 1363–1374. [Google Scholar] [CrossRef]
- Bijak, M.; Zahorodna, A.; Tokarski, K. Opposite effects of antidepressants and corticosterone on the sensitivity of hippocampal CA1 neurons to 5-HT1A and 5-HT4 receptor activation. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2001, 363, 491–498. [Google Scholar] [CrossRef]
- Imoto, Y.; Kira, T.; Sukeno, M.; Nishitani, N.; Nagayasu, K.; Nakagawa, T.; Kaneko, S.; Kobayashi, K.; Segi-Nishida, E. Role of the 5-HT4 receptor in chronic fluoxetine treatment-induced neurogenic activity and granule cell dematuration in the dentate gyrus. Mol. Brain 2015, 8, 29. [Google Scholar] [CrossRef]
- Segi-Nishida, E. The Effect of Serotonin-Targeting Antidepressants on Neurogenesis and Neuronal Maturation of the Hippocampus Mediated via 5-HT1A and 5-HT4 Receptors. Front. Cell. Neurosci. 2017, 11, 142. [Google Scholar] [CrossRef]
- Karayol, R.; Medrihan, L.; Warner-Schmidt, J.L.; Fait, B.W.; Rao, M.N.; Holzner, E.B.; Greengard, P.; Heintz, N.; Schmidt, E.F. Serotonin receptor 4 in the hippocampus modulates mood and anxiety. Mol. Psychiatry 2021, 26, 2334–2349. [Google Scholar] [CrossRef]
- Nasehi, M.; Farrahizadeh, M.; Ebrahimi-Ghiri, M.; Zarrindast, M.-R. Modulation of cannabinoid signaling by hippocampal 5-HT4 serotonergic system in fear conditioning. J. Psychopharmacol. 2016, 30, 936–944. [Google Scholar] [CrossRef]
- Nasehi, M.; Rostam-Nezhad, E.; Ebrahimi-Ghiri, M.; Zarrindast, M.-R. Interaction between hippocampal serotonin and cannabinoid systems in reactivity to spatial and object novelty detection. Behav. Brain Res. 2017, 317, 272–278. [Google Scholar] [CrossRef]
- Volk, B.; Nagy, B.J.; Vas, S.; Kostyalik, D.; Simig, G.; Bagdy, G. Medicinal chemistry of 5-HT5A receptor ligands: A receptor subtype with unique therapeutical potential. Curr. Top. Med. Chem. 2010, 10, 554–578. [Google Scholar] [CrossRef]
- Grailhe, R.; Waeber, C.; Dulawa, S.C.; Hornung, J.P.; Zhuang, X.; Brunner, D.; A Geyer, M.; Hen, R. Increased exploratory activity and altered response to LSD in mice lacking the 5-HT5A receptor. Neuron 1999, 22, 581–591. [Google Scholar] [CrossRef]
- Yamazaki, M.; Okabe, M.; Yamamoto, N.; Yarimizu, J.; Harada, K. Novel 5-HT5A receptor antagonists ameliorate scopolamine-induced working memory deficit in mice and reference memory impairment in aged rats. J. Pharmacol. Sci. 2015, 127, 362–369. [Google Scholar] [CrossRef]
- Aparicio-Nava, L.; Márquez-García, L.; Meneses, A. Effects of 5-HT5A receptor blockade on amnesia or forgetting. Behav. Brain Res. 2019, 358, 98–103. [Google Scholar] [CrossRef]
- Kassai, F.; Schlumberger, C.; Kedves, R.; Pietraszek, M.; Jatzke, C.; Lendvai, B.; Gyertyán, I.; Danysz, W. Effect of 5-HT5A antagonists in animal models of schizophrenia, anxiety and depression. Behav. Pharmacol. 2012, 23, 397–406. [Google Scholar] [CrossRef]
- Nikiforuk, A.; Hołuj, M.; Kos, T.; Popik, P. The effects of a 5-HT5A receptor antagonist in a ketamine-based rat model of cognitive dysfunction and the negative symptoms of schizophrenia. Neuropharmacology 2016, 105, 351–360. [Google Scholar] [CrossRef]
- Meneses, A. Memory formation and memory alterations: 5-HT6 and 5-HT7 receptors, novel alternative. Prog. Neurobiol. 2014, 25, 325–356. [Google Scholar] [CrossRef]
- Nikiforuk, A.; Kos, T.; Wesołowska, A. The 5-HT6 receptor agonist EMD 386088 produces antidepressant and anxiolytic effects in rats after intrahippocampal administration. Psychopharmacology 2011, 217, 411–418. [Google Scholar] [CrossRef]
- Jastrzębska-Więsek, M.; Siwek, A.; Partyka, A.; Antkiewicz-Michaluk, L.; Michaluk, J.; Romańska, I.; Kołaczkowski, M.; Wesołowska, A. Study of a mechanism responsible for potential antidepressant activity of EMD 386088, a 5-HT6 partial agonist in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2016, 389, 839–849. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hirano, K.; Piers, T.M.; Searle, K.L.; Miller, N.D.; Rutter, A.R.; Chapman, P.F. Procognitive 5-HT6 antagonists in the rat forced swimming test: Potential therapeutic utility in mood disorders associated with Alzheimer’s disease. Life Sci. 2009, 84, 558–562. [Google Scholar] [CrossRef]
- Wesołowska, A.; Nikiforuk, A.; Stachowicz, K. Anxiolytic-like and antidepressant-like effects produced by the selective 5-HT6 receptor antagonist SB-258585 after intrahippocampal administration to rats. Behav. Pharmacol. 2007, 18, 439–446. [Google Scholar] [CrossRef]
- Lacroix, L.P.; Dawson, L.A.; Hagan, J.J.; Heidbreder, C.A. 5-HT6 receptor antagonist SB-271046 enhances extracellular levels of monoamines in the rat medial prefrontal cortex. Synapse 2004, 51, 158–164. [Google Scholar] [CrossRef]
- Liu, K.-C.; Li, J.-Y.; Tan, H.-H.; Du, C.-X.; Xie, W.; Zhang, Y.-M.; Ma, W.-L.; Zhang, L. Serotonin6 receptors in the dorsal hippocampus regulate depressive-like behaviors in unilateral 6-hydroxydopamine-lesioned Parkinson’s rats. Neuropharmacology 2015, 95, 290–298. [Google Scholar] [CrossRef]
- Zhang, Y.-M.; Zhang, L.; Wang, Y.; Sun, Y.-N.; Guo, Y.; Du, C.-X.; Zhang, J.; Yao, L.; Yu, S.-Q.; Liu, J. Activation and blockade of prelimbic 5-HT6 receptors produce different effects on depressive-like behaviors in unilateral 6-hydroxydopamine-induced Parkinson’s rats. Neuropharmacology 2016, 110, 25–36. [Google Scholar] [CrossRef]
- Liu, C.; Wen, Y.; Huang, H.; Lin, W.; Huang, M.; Lin, R.; Ma, Y. Over-expression of 5-HT6 Receptor and Activated Jab-1/p-c-Jun Play Important Roles in Pilocarpine-Induced Seizures and Learning-Memory Impairment. J. Mol. Neurosci. 2019, 67, 388–399. [Google Scholar] [CrossRef]
- Zhu, C.; Lin, R.; Liu, C.; Huang, M.; Lin, F.; Zhang, G.; Zhang, Y.; Miao, J.; Lin, W.; Huang, H. The Antagonism of 5-HT6 Receptor Attenuates Current-Induced Spikes and Improves Long-Term Potentiation via the Regulation of M-Currents in a Pilocarpine-Induced Epilepsy Model. Front. Pharmacol. 2020, 11, 475. [Google Scholar] [CrossRef]
- Zareifopoulos, N.; Papatheodoropoulos, C. Effects of 5-HT-7 receptor ligands on memory and cognition. Neurobiol. Learn. Mem. 2016, 136, 204–209. [Google Scholar] [CrossRef]
- Perez-García, G.; Meneses, A. Ex vivo study of 5-HT1A and 5-HT7 receptor agonists and antagonists on cAMP accumulation during memory formation and amnesia. Behav. Brain Res. 2008, 195, 139–146. [Google Scholar] [CrossRef]
- Jafari-Sabet, M.; Nemati, S.; Torab, M. Cross state-dependency of learning between 5-HT1A and/or 5-HT7 receptor agonists and muscimol in the mouse dorsal hippocampus. J. Psychopharmacol. 2019, 33, 722–736. [Google Scholar] [CrossRef]
- Eriksson, T.M.; Holst, S.; Stan, T.L.; Hager, T.; Sjögren, B.; Ögren, S.O.; Svenningsson, P.; Stiedl, O. 5-HT1A and 5-HT7 receptor crosstalk in the regulation of emotional memory: Implications for effects of selective serotonin reuptake inhibitors. Neuropharmacology 2012, 63, 1150–1160. [Google Scholar] [CrossRef]
- Eriksson, T.M.; Golkar, A.; Ekström, J.C.; Svenningsson, P.; Ögren, S.O. 5-HT7 receptor stimulation by 8-OH-DPAT counteracts the impairing effect of 5-HT1A receptor stimulation on contextual learning in mice. Eur. J. Pharmacol. 2008, 596, 107–110. [Google Scholar] [CrossRef]
- Bijata, M.; Bączyńska, E.; Müller, F.E.; Bijata, K.; Masternak, J.; Krzystyniak, A.; Szewczyk, B.; Siwiec, M.; Antoniuk, S.; Roszkowska, M.; et al. Activation of the 5-HT7 receptor and MMP-9 signaling module in the hippocampal CA1 region is necessary for the development of depressive-like behavior. Cell Rep. 2022, 38, 110532. [Google Scholar] [CrossRef]
- Nandam, L.S.; Jhaveri, D.; Bartlett, P. 5-HT7, neurogenesis and antidepressants: A promising therapeutic axis for treating depression. Clin. Exp. Pharmacol. Physiol. 2007, 34, 546–551. [Google Scholar] [CrossRef]
- Dale, E.; Zhang, H.; Leiser, S.C.; Xiao, Y.; Lu, D.; Yang, C.R.; Plath, N.; Sanchez, C. Vortioxetine disinhibits pyramidal cell function and enhances synaptic plasticity in the rat hippocampus. J. Psychopharmacol. 2014, 28, 891–902. [Google Scholar] [CrossRef]
- Du, C.X.; Guo, Y.; Zhang, Q.J.; Zhang, J.; Lv, S.X.; Liu, J. Involvement of prelimbic 5-HT7 receptors in the regulation of anxiety-like behaviors in hemiparkinsonian rats. Neurol. Res. 2018, 40, 847–855. [Google Scholar] [CrossRef]
- De Filippis, B.; Chiodi, V.; Adriani, W.; Lacivita, E.; Mallozzi, C.; Leopoldo, M.; Domenici, M.R.; Fuso, A.; Laviola, G. Long-lasting beneficial effects of central serotonin receptor 7 stimulation in female mice modeling Rett syndrome. Front. Behav. Neurosci. 2015, 9, 86. [Google Scholar] [CrossRef]
- Le Corre, S.; Sharp, T.; Young, A.H.; Harrison, P.J. Increase of 5-HT7 (serotonin-7) and 5-HT1A (serotonin-1A) receptor mRNA expression in rat hippocampus after adrenalectomy. Psychopharmacology 1997, 130, 368–374. [Google Scholar] [CrossRef]
- Laplante, P.; Diorio, J.; Meaney, M.J. Serotonin regulates hippocampal glucocorticoid receptor expression via a 5-HT7 receptor. Dev. Brain Res. 2002, 139, 199–203. [Google Scholar] [CrossRef]
- Beck, S.G.; Goldfarb, J. Serotonin produces a reversible concentration dependent decrease of population spikes in rat hippocampal slices. Life Sci. 1985, 36, 557–563. [Google Scholar] [CrossRef]
- Kasamo, K.; Suzuki, T.; Tada, K.; Ueda, N.; Matsuda, E.; Ishikawa, K.; Kojima, T. Endogenous 5-HT tonically inhibits spontaneous firing activity of dorsal hippocampus CA1 pyramidal neurons through stimulation of 5-HT1A receptors in quiet awake rats In Vivo electrophysiological evidence. Neuropsychopharmacology 2001, 24, 141–151. [Google Scholar] [CrossRef]
- Lu, C.W.; Lin, T.Y.; Huang, S.K.; Wang, S.J. 5-HT1B receptor agonist CGS12066 presynaptically inhibits glutamate release in rat hippocampus. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 86, 122–130. [Google Scholar] [CrossRef]
- Katsurabayashi, S.; Kubota, H.; Tokutomi, N.; Akaike, N. A distinct distribution of functional presynaptic 5-HT receptor subtypes on GABAergic nerve terminals projecting to single hippocampal CA1 pyramidal neurons. Neuropharmacology 2003, 44, 1022–1030. [Google Scholar] [CrossRef]
- Segal, M. Serotonin attenuates a slow inhibitory postsynaptic potential in rat hippocampal neurons. Neuroscience 1990, 36, 631–641. [Google Scholar] [CrossRef]
- Schmitz, D.; Empson, R.; Heinemann, U. Serotonin reduces inhibition via 5-HT1A receptors in area CA1 of rat hippocampal slices in vitro. J. Neurosci. 1995, 15, 7217–7225. [Google Scholar] [CrossRef]
- Aznar, S.; Qian, Z.; Shah, R.; Rahbek, B.; Knudsen, G.M. The 5-HT1A serotonin receptor is located on calbindin- and parvalbumin-containing neurons in the rat brain. Brain Res. 2003, 959, 58–67. [Google Scholar] [CrossRef]
- Voigt, M.; Laurie, D.; Seeburg, P.; Bach, A. Molecular cloning and characterization of a rat brain cDNA encoding a 5-hydroxytryptamine1B receptor. EMBO J. 1991, 10, 4017–4023. [Google Scholar] [CrossRef]
- Winterer, J.; Stempel, A.V.; Dugladze, T.; Földy, C.; Maziashvili, N.; Zivkovic, A.R.; Priller, J.; Soltesz, I.; Gloveli, T.; Schmitz, D. Cell-type-specific modulation of feedback inhibition by serotonin in the hippocampus. J. Neurosci. 2011, 31, 8464–8475. [Google Scholar] [CrossRef]
- Miles, R.; Tóth, K.; I Gulyás, A.; Hájos, N.; Freund, T.F. Differences between somatic and dendritic inhibition in the hippocampus. Neuron 1996, 16, 815–823. [Google Scholar] [CrossRef]
- Freund, T.F.; Katona, I. Perisomatic inhibition. Neuron 2007, 56, 33–42. [Google Scholar] [CrossRef]
- Lopatina, O.L.; Malinovskaya, N.A.; Komleva, Y.K.; Gorina, Y.V.; Shuvaev, A.N.; Olovyannikova, R.Y.; Belozor, O.S.; Belova, O.A.; Higashida, H.; Salmina, A.B. Excitation/inhibition imbalance and impaired neurogenesis in neurodevelopmental and neurodegenerative disorders. Prog. Neurobiol. 2019, 30, 807–820. [Google Scholar] [CrossRef]
- Ferguson, B.R.; Gao, W.-J. PV Interneurons: Critical Regulators of E/I Balance for Prefrontal Cortex-Dependent Behavior and Psychiatric Disorders. Front. Neural Circuits 2018, 12, 37. [Google Scholar] [CrossRef]
- Kirischuk, S. Keeping Excitation-Inhibition Ratio in Balance. Int. J. Mol. Sci. 2022, 23, 5746. [Google Scholar] [CrossRef]
- Gao, R.; Penzes, P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders. Curr. Mol. Med. 2015, 15, 146–167. [Google Scholar] [CrossRef]
- Bast, T. The hippocampal learning-behavior translation and the functional significance of hippocampal dysfunction in schizophrenia. Curr. Opin. Neurobiol. 2011, 21, 492–501. [Google Scholar] [CrossRef]
- Ballaz, S.J.; Bourin, M. Cholecystokinin-Mediated Neuromodulation of Anxiety and Schizophrenia: A “Dimmer-Switch” Hypothesis. Curr. Neuropharmacol. 2021, 19, 925–938. [Google Scholar] [CrossRef]
- Shen, R.-Y.; Andrade, R. 5-Hydroxytryptamine2 receptor facilitates GABAergic neurotransmission in rat hippocampus. J. Pharmacol. Exp. Ther. 1998, 285, 805–812. [Google Scholar] [CrossRef]
- Wyskiel, D.R.; Andrade, R. Serotonin excites hippocampal CA1 GABAergic interneurons at the stratum radiatum-stratum lacunosum moleculare border. Hippocampus 2016, 26, 1107–1114. [Google Scholar] [CrossRef]
- Luparini, M.R.; Garrone, B.; Pazzagli, M.; Pinza, M.; Pepeu, G. A cortical GABA–5HT interaction in the mechanism of action of the antidepressant trazodone. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 1117–1127. [Google Scholar] [CrossRef]
- Anneken, J.H.; Gudelsky, G.A. MDMA produces a delayed and sustained increase in the extracellular concentration of glutamate in the rat hippocampus. Neuropharmacology 2012, 63, 1022–1027. [Google Scholar] [CrossRef]
- Anneken, J.H.; Cunningham, J.I.; Collins, S.A.; Yamamoto, B.K.; Gudelsky, G.A. MDMA increases glutamate release and reduces parvalbumin-positive GABAergic cells in the dorsal hippocampus of the rat: Role of cyclooxygenase. J. Neuroimmune Pharmacol. 2012, 8, 58–65. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Z.; Sun, J.; Reynolds, G.P. A selective reduction in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia patients. Chin. Med. J. 2002, 115, 819–823. [Google Scholar] [PubMed]
- Kantrowitz, J.T. Targeting Serotonin 5-HT2A Receptors to Better Treat Schizophrenia: Rationale and Current Approaches. CNS Drugs 2020, 34, 947–959. [Google Scholar] [CrossRef]
- Kawa, K. Distribution and functional properties of 5-HT3 receptors in the rat hippocampal dentate gyrus: A patch-clamp study. J. Neurophysiol. 1994, 71, 1935–1947. [Google Scholar] [CrossRef]
- McMahon, L.L.; Kauer, J.A. Hippocampal interneurons express a novel form of synaptic plasticity. Neuron 1997, 18, 295–305. [Google Scholar] [CrossRef]
- Noam, Y.; Wadman, W.J.; Van Hooft, J.A. On the voltage-dependent Ca2+ block of serotonin 5-HT3 receptors: A critical role of intracellular phosphates. J. Physiol. 2008, 586, 3629–3638. [Google Scholar] [CrossRef]
- Koyama, S.; Matsumoto, N.; Murakami, N.; Kubo, C.; Nabekura, J.; Akaike, N. Role of presynaptic 5-HT1A and 5-HT3 receptors in modulation of synaptic GABA transmission in dissociated rat basolateral amygdala neurons. Life Sci. 2002, 72, 375–387. [Google Scholar] [CrossRef]
- Yakel, J.L.; Jackson, M.B. 5-HT3 receptors mediate rapid responses in cultured hippocampus and a clonal cell line. Neuron 1988, 1, 615–621. [Google Scholar] [CrossRef]
- Miquel, M.; Emerit, M.B.; Nosjean, A.; Simon, A.; Rumajogee, P.; Brisorgueil, M.; Doucet, E.; Hamon, M.; Vergé, D. Differential subcellular localization of the 5-HT3-As receptor subunit in the rat central nervous system. Eur. J. Neurosci. 2002, 15, 449–457. [Google Scholar] [CrossRef]
- Ropert, N.; Guy, N. Serotonin facilitates GABAergic transmission in the CA1 region of rat hippocampus in vitro. J. Physiol. 1991, 441, 121–136. [Google Scholar] [CrossRef]
- Morales, M.; Bloom, F.E. The 5-HT3 receptor is present in different subpopulations of GABAergic neurons in the rat telencephalon. J. Neurosci. 1997, 17, 3157–3167. [Google Scholar] [CrossRef]
- Turner, T.; Mokler, D.; Luebke, J. Calcium influx through presynaptic 5-HT3 receptors facilitates GABA release in the hippocampus: In Vitro slice and synaptosome studies. Neuroscience 2004, 129, 703–718. [Google Scholar] [CrossRef]
- McMahon, L.L.; Kauer, J.A. Hippocampal interneurons are excited via serotonin-gated ion channels. J. Neurophysiol. 1997, 78, 2493–2502. [Google Scholar] [CrossRef]
- Morales, M.; Bäckman, C. Coexistence of serotonin 3 (5-HT3) and CB1 cannabinoid receptors in interneurons of hippocampus and dentate gyrus. Hippocampus 2002, 12, 756–764. [Google Scholar] [CrossRef]
- Dorostkar, M.M.; Boehm, S. Opposite effects of presynaptic 5-HT3 receptor activation on spontaneous and action potential-evoked GABA release at hippocampal synapses. J. Neurochem. 2007, 100, 395–405. [Google Scholar] [CrossRef]
- Morales, M.; Hein, K.; Vogel, Z. Hippocampal interneurons co-express transcripts encoding the α7 nicotinic receptor subunit and the cannabinoid receptor 1. Neuroscience 2008, 152, 70–81. [Google Scholar] [CrossRef]
- Freedman, R.; Adams, C.E.; Leonard, S. The α7-nicotinic acetylcholine receptor and the pathology of hippocampal interneurons in schizophrenia. J. Chem. Neuroanat. 2000, 20, 299–306. [Google Scholar] [CrossRef]
- Andrade, R.; Chaput, Y. 5-Hydroxytryptamine4-like receptors mediate the slow excitatory response to serotonin in the rat hippocampus. J. Pharmacol. Exp. Ther. 1991, 257, 930–937. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Haas, H.; Anderson, E. 5-HT1A and 5-HT4 receptor colocalization on hippocampal pyramidal cells. Neuropharmacology 1994, 33, 551–557. [Google Scholar] [CrossRef]
- Egeland, M.; Warner-Schmidt, J.; Greengard, P.; Svenningsson, P. Co-expression of serotonin 5-HT1B and 5-HT4 receptors in p11 containing cells in cerebral cortex, hippocampus, caudate-putamen and cerebellum. Neuropharmacology 2011, 61, 442–450. [Google Scholar] [CrossRef]
- Teixeira, C.M.; Rosen, Z.B.; Suri, D.; Sun, Q.; Hersh, M.; Sargin, D.; Dincheva, I.; Morgan, A.A.; Spivack, S.; Krok, A.C.; et al. Hippocampal 5-HT Input Regulates Memory Formation and Schaffer Collateral Excitation. Neuron 2018, 98, 992–1004.e4. [Google Scholar] [CrossRef]
- Mlinar, B.; Mascalchi, S.; Mannaioni, G.; Morini, R.; Corradetti, R. 5-HT4 receptor activation induces long-lasting EPSP-spike potentiation in CA1 pyramidal neurons. Eur. J. Neurosci. 2006, 24, 719–731. [Google Scholar] [CrossRef]
- Mlinar, B.; Mascalchi, S.; Morini, R.; Giachi, F.; Corradetti, R. MDMA induces EPSP-Spike potentiation in rat ventral hippocampus In Vitro via serotonin and noradrenaline release and coactivation of 5-HT4 and β1 receptors. Neuropsychopharmacology 2008, 33, 1464–1475. [Google Scholar] [CrossRef]
- Tokarski, K.; Zahorodna, A.; Bobula, B.; Hess, G. Comparison of the effects of 5-HT 1A and 5-HT 4 receptor activation on field potentials and epileptiform activity in rat hippocampus. Exp. Brain Res. 2002, 147, 505–510. [Google Scholar] [CrossRef]
- Bianchi, C.; Rodi, D.; Marino, S.; Beani, L.; Siniscalchi, A. Dual effects of 5-HT4 receptor activation on GABA release from guinea pig hippocampal slices. NeuroReport 2002, 13, 2177–2180. [Google Scholar] [CrossRef]
- Hagena, H.; Manahan-Vaughan, D. The serotonergic 5-HT4 receptor: A unique modulator of hippocampal synaptic information processing and cognition. Neurobiol. Learn. Mem. 2017, 138, 145–153. [Google Scholar] [CrossRef]
- Manuel-Apolinar, L.; Rocha, L.; Pascoe, D.; Castillo, E.; Castillo, C.; Meneses, A. Modifications of 5-HT4 receptor expression in rat brain during memory consolidation. Brain Res. 2005, 1042, 73–81. [Google Scholar] [CrossRef]
- Eydipour, Z.; Nasehi, M.; Vaseghi, S.; Jamaldini, S.H.; Zarrindast, M.-R. The role of 5-HT4 serotonin receptors in the CA1 hippocampal region on memory acquisition impairment induced by total (TSD) and REM sleep deprivation (RSD). Physiol. Behav. 2020, 215, 112788. [Google Scholar] [CrossRef]
- Restivo, L.; Roman, F.; Dumuis, A.; Bockaert, J.; Marchetti, E.; Ammassari-Teule, M. The promnesic effect of G-protein-coupled 5-HT4 receptors activation is mediated by a potentiation of learning-induced spine growth in the mouse hippocampus. Neuropsychopharmacology 2008, 33, 2427–2434. [Google Scholar] [CrossRef]
- de Cates, A.N.; Wright, L.C.; Martens, M.A.G.; Gibson, D.; Türkmen, C.; Filippini, N.; Cowen, P.J.; Harmer, C.J.; Murphy, S.E. Déjà-vu? Neural and behavioural effects of the 5-HT4 receptor agonist, prucalopride, in a hippocampal-dependent memory task. Transl. Psychiatry 2021, 11, 497. [Google Scholar] [CrossRef]
- Plassat, J.; Boschert, U.; Amlaiky, N.; Hen, R. The mouse 5HT5 receptor reveals a remarkable heterogeneity within the 5HT1D receptor family. EMBO J. 1992, 11, 4779–4786. [Google Scholar] [CrossRef]
- Matthes, H.; Boschert, U.; Amlaiky, N.; Grailhe, R.; Plassat, J.L.; Muscatelli, F.; Mattei, M.G.; Hen, R. Mouse 5-hydroxytryptamine5A and 5-hydroxytryptamine5B receptors define a new family of serotonin receptors: Cloning, functional expression, and chromosomal localization. Mol. Pharmacol. 1993, 43, 313–319. [Google Scholar] [CrossRef]
- García-Alcocer, G.; Rodríguez, A.; Moreno-Layseca, P.; Berumen, L.C.; Escobar, J.; Miledi, R. Serotonin receptor 5-HT5A in rat hippocampus decrease by leptin treatment. Neurosci. Lett. 2010, 486, 171–173. [Google Scholar] [CrossRef]
- Garza, J.C.; Guo, M.; Zhang, W.; Lu, X.-Y. Leptin increases adult hippocampal neurogenesis In Vivo and In Vitro. J. Biol. Chem. 2008, 283, 18238–18247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yamazaki, M.; Harada, K.; Yamamoto, N.; Yarimizu, J.; Okabe, M.; Shimada, T.; Ni, K.; Matsuoka, N. ASP5736, a novel 5-HT5A receptor antagonist, ameliorates positive symptoms and cognitive impairment in animal models of schizophrenia. Eur. Neuropsychopharmacol. 2014, 24, 1698–1708. [Google Scholar] [CrossRef]
- Helboe, L.; Egebjerg, J.; de Jong, I. Distribution of serotonin receptor 5-HT6 mRNA in rat neuronal subpopulations: A double in situ hybridization study. Neuroscience 2015, 310, 442–454. [Google Scholar] [CrossRef]
- Dupuy, V.; Prieur, M.; Pizzoccaro, A.; Margarido, C.; Valjent, E.; Bockaert, J.; Bouschet, T.; Marin, P.; Chaumont-Dubel, S. Spatiotemporal dynamics of 5-HT6 receptor ciliary localization during mouse brain development. Neurobiol. Dis. 2023, 176, 105949. [Google Scholar] [CrossRef]
- Gérard, C.; Martres, M.-P.; Lefèvre, K.; Miquel, M.-C.; Vergé, D.; Lanfumey, L.; Doucet, E.; Hamon, M.; El Mestikawy, S. Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res. 1997, 746, 207–219. [Google Scholar] [CrossRef]
- Schechter, L.E.; Lin, Q.; Smith, D.L.; Zhang, G.; Shan, Q.; Platt, B.; Brandt, M.R.; Dawson, L.A.; Cole, D.; Bernotas, R.; et al. Neuropharmacological profile of novel and selective 5-HT6 receptor agonists: WAY-181187 and WAY-208466. Neuropsychopharmacology 2008, 33, 1323–1335. [Google Scholar] [CrossRef]
- Wang, H.Y.; Lu, C.W.; Lin, T.Y.; Kuo, J.R.; Wang, S.J. WAY208466 inhibits glutamate release at hippocampal nerve terminals. Eur. J. Pharmacol. 2016, 781, 117–127. [Google Scholar] [CrossRef]
- Lahogue, C.; Billard, J.-M.; Freret, T.; Bouet, V. 5-HT6 Receptors Sex-Dependently Modulate Hippocampal Synaptic Activity through GABA Inhibition. Biomolecules 2023, 13, 751. [Google Scholar] [CrossRef]
- de Bruin, N.; Kruse, C. 5-HT6 Receptor Antagonists: Potential Efficacy for the Treatment of Cognitive Impairment in Schizophrenia. Curr. Pharm. Des. 2015, 21, 3739–3759. [Google Scholar] [CrossRef]
- Gustafson, E.L.; Durkin, M.M.; Bard, J.A.; Zgombick, J.; Branchek, T.A. A receptor autoradiographic and In Situ hybridization analysis of the distribution of the 5-ht7 receptor in rat brain. Br. J. Pharmacol. 1996, 117, 657–666. [Google Scholar] [CrossRef]
- García-Alcocer, G.; Segura, L.C.B.; Peña, M.G.; Martínez-Torres, A.; Miledi, R. Ontogenetic distribution of 5-HT2C, 5-HT5A, and 5-HT7 receptors in the rat hippocampus. Gene Exp. 2006, 13, 53–57. [Google Scholar] [CrossRef]
- Tokarski, K.; Zahorodna, A.; Bobula, B.; Hess, G. 5-HT7 receptors increase the excitability of rat hippocampal CA1 pyramidal neurons. Brain Res. 2003, 993, 230–234. [Google Scholar] [CrossRef]
- Kusek, M.; Sowa, J.; Tokarski, K.; Hess, G. Impaired effect of activation of rat hippocampal 5-HT7 receptors, induced by treatment with the 5-HT7 receptor antagonist SB 269970. J. Physiol. Pharmacol. 2015, 66, 301–308. [Google Scholar]
- Otmakhova, N.A.; Lewey, J.; Asrican, B.; Lisman, J.E. Inhibition of perforant path input to the CA1 region by serotonin and noradrenaline. J. Neurophysiol. 2005, 94, 1413–1422. [Google Scholar] [CrossRef]
- Gill, C.H.; Soffin, E.M.; Hagan, J.J.; Davies, C.H. 5-HT7 receptors modulate synchronized network activity in rat hippocampus. Neuropharmacology 2002, 42, 82–92. [Google Scholar] [CrossRef]
- Ohmura, Y.; Yoshida, T.; Konno, K.; Minami, M.; Watanabe, M.; Yoshioka, M. Serotonin 5-HT7 Receptor in the Ventral Hippocampus Modulates the Retrieval of Fear Memory and Stress-Induced Defecation. Int. J. Neuropsychopharmacol. 2015, 19, pyv131. [Google Scholar] [CrossRef]
- Tokarski, K.; Kusek, M.; Hess, G. 5-HT7 receptors modulate GABAergic transmission in rat hippocampal CA1 area. J. Physiol. Pharmacol. 2011, 62, 535–540. [Google Scholar]
- Sahin, B.; Ozdemir, E.; Gumus, E.; Ergul, M.; Taskiran, A.S. The 5-HT7 receptor antagonist SB-269970 alleviates seizure activity and downregulates hippocampal c-Fos expression in pentylenetetrazole-induced kindled rats. Neurol. Res. 2022, 44, 786–796. [Google Scholar] [CrossRef]
- Núñez-Ochoa, M.A.; Chiprés-Tinajero, G.A.; Medina-Ceja, L. Evaluation of the hippocampal immunoreactivity of the serotonin 5-HT1A, 5-HT2 and 5-HT7 receptors in a pilocarpine temporal lobe epilepsy rat model with fast ripples. NeuroReport 2021, 32, 306–311. [Google Scholar] [CrossRef]
- Okubo, R.; Hasegawa, T.; Fukuyama, K.; Shiroyama, T.; Okada, M. Current Limitations and Candidate Potential of 5-HT7 Receptor Antagonism in Psychiatric Pharmacotherapy. Front. Psychiatry 2021, 12, 623684. [Google Scholar] [CrossRef]
- Alkadhi, K.A. NMDA receptor-independent LTP in mammalian nervous system. Prog. Neurobiol. 2021, 200, 101986. [Google Scholar] [CrossRef]
- Mlinar, B.; Stocca, G.; Corradetti, R. Endogenous serotonin facilitates hippocampal long-term potentiation at CA3/CA1 synapses. J. Neural Transm. 2015, 122, 177–185. [Google Scholar] [CrossRef]
- Klancˇnik, J.M.; Phillips, A.G. Modulation of synaptic plasticity in the dentate gyrus of the rat by electrical stimulation of the median raphe nucleus. Brain Res. 1991, 557, 236–240. [Google Scholar] [CrossRef]
- Bliss, T.V.; Goddard, G.V.; Riives, M. Reduction of long-term potentiation in the dentate gyrus of the rat following selective depletion of monoamines. J. Physiol. 1983, 334, 475–491. [Google Scholar] [CrossRef]
- Corradetti, R.; Ballerini, L.; Pugliese, A.; Pepeu, G. Serotonin blocks the long-term potentiation induced by primed burst stimulation in the CA1 region of rat hippocampal slices. Neuroscience 1992, 46, 511–518. [Google Scholar] [CrossRef]
- Staubli, U.; Otaky, N. Serotonin controls the magnitude of LTP induced by theta bursts via an action on NMDA-receptor-mediated responses. Brain Res. 1994, 643, 10–16. [Google Scholar] [CrossRef]
- Villani, F.; Johnston, D. Serotonin inhibits induction of long-term potentiation at commissural synapses in hippocampus. Brain Res. 1993, 606, 304–308. [Google Scholar] [CrossRef]
- Inoue, S.; Shikanai, H.; Matsumoto, M.; Hiraide, S.; Saito, Y.; Yanagawa, Y.; Yoshioka, M.; Shimamura, K.-I.; Togashi, H. Metaplastic regulation of the median raphe nucleus via serotonin 5-HT1A receptor on hippocampal synaptic plasticity is associated with gender-specific emotional expression in rats. J. Pharmacol. Sci. 2014, 124, 394–407. [Google Scholar] [CrossRef][Green Version]
- Zhang, L.; Wang, X.; Zhao, N.; Wang, Y.; Hu, X.; Ran, Y.; Liu, Y.; Zhang, Y.; Yang, R.; Li, Y. Neurochemical and behavioural effects of hypidone hydrochloride (YL-0919): A novel combined selective 5-HT reuptake inhibitor and partial 5-HT1A agonist. Br. J. Pharmacol. 2017, 174, 769–780. [Google Scholar] [CrossRef]
- Rozas, C.; Loyola, S.; Ugarte, G.; Zeise, M.; Reyes-Parada, M.; Pancetti, F.; Rojas, P.; Morales, B. Acutely applied MDMA enhances long-term potentiation in rat hippocampus involving D1/D5 and 5-HT2 receptors through a polysynaptic mechanism. Eur. Neuropsychopharmacol. 2012, 22, 584–595. [Google Scholar] [CrossRef]
- Ryan, B.K.; Anwyl, R.; Rowan, M.J. 5-HT2 receptor-mediated reversal of the inhibition of hippocampal long-term potentiation by acute inescapable stress. Neuropharmacology 2008, 55, 175–182. [Google Scholar] [CrossRef]
- Yu, Y.; Cao, D.-Q.; Xu, H.-Y.; Sun, M.; Huang, Z.-L.; Yung, W.-H.; Lu, N.; Huang, Y. 5-HT3A receptors are required in long-term depression and AMPA receptor internalization. Neuroscience 2014, 278, 105–112. [Google Scholar] [CrossRef]
- Staubli, U.; Xu, F. Effects of 5-HT3 receptor antagonism on hippocampal theta rhythm, memory, and LTP induction in the freely moving rat. J. Neurosci. 1995, 15, 2445–2452. [Google Scholar] [CrossRef]
- Maeda, T.; Kaneko, S.; Satoh, M. Inhibitory influence via 5-HT3 receptors on the induction of LTP in mossy fiber-CA3 system of guinea-pig hippocampal slices. Neurosci. Res. 1994, 18, 277–282. [Google Scholar] [CrossRef]
- Hao, R.; Qi, Y.; Hou, D.-N.; Ji, Y.-Y.; Zheng, C.-Y.; Li, C.-Y.; Yung, W.-H.; Lu, B.; Huang, Y. BDNF val66met Polymorphism Impairs Hippocampal Long-Term Depression by Down-Regulation of 5-HT3 Receptors. Front. Cell. Neurosci. 2017, 11, 306. [Google Scholar] [CrossRef]
- Kemp, A.; Manahan-Vaughan, D. The 5-hydroxytryptamine4 receptor exhibits frequency-dependent properties in synaptic plasticity and behavioural metaplasticity in the hippocampal CA1 region In Vivo. Cereb. Cortex 2005, 15, 1037–1043. [Google Scholar] [CrossRef]
- Lecouflet, P.; Roux, C.M.; Potier, B.; Leger, M.; Brunet, E.; Billard, J.-M.; Schumann-Bard, P.; Freret, T. Interplay between 5-HT4 Receptors and GABAergic System within CA1 Hippocampal Synaptic Plasticity. Cereb. Cortex 2021, 31, 694–701. [Google Scholar] [CrossRef]
- Twarkowski, H.; Hagena, H.; Manahan-Vaughan, D. The 5-hydroxytryptamine4 receptor enables differentiation of informational content and encoding in the hippocampus. Hippocampus 2016, 26, 875–891. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- West, P.; Marcy, V.; Marino, M.; Schaffhauser, H. Activation of the 5-HT6 receptor attenuates long-term potentiation and facilitates GABAergic neurotransmission in rat hippocampus. Neuroscience 2009, 164, 692–701. [Google Scholar] [CrossRef]
- Costa, L.; Spatuzza, M.; D’ANtoni, S.; Bonaccorso, C.M.; Trovato, C.; Musumeci, S.A.; Leopoldo, M.; Lacivita, E.; Catania, M.V.; Ciranna, L. Activation of 5-HT7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and Fmr1 knockout mice, a model of fragile x syndrome. Biol. Psychiatry 2012, 72, 924–933. [Google Scholar] [CrossRef]
- Hashemi-Firouzi, N.; Komaki, A.; Asl, S.S.; Shahidi, S. The effects of the 5-HT7 receptor on hippocampal long-term potentiation and apoptosis in a rat model of Alzheimer’s disease. Brain Res. Bull. 2017, 135, 85–91. [Google Scholar] [CrossRef]
- Bullmore, E.; Frangou, S.; Murray, R. The dysplastic net hypothesis: An integration of developmental and dysconnectivity theories of schizophrenia. Schizophr. Res. 1997, 28, 143–156. [Google Scholar] [CrossRef]
- Friston, K.J. The disconnection hypothesis. Schizophr. Res. 1998, 30, 115–125. [Google Scholar] [CrossRef]
- Stephan, K.E.; Baldeweg, T.; Friston, K.J. Synaptic plasticity and dysconnection in schizophrenia. Biol. Psychiatry 2006, 59, 929–939. [Google Scholar] [CrossRef]
- Stephan, K.E.; Friston, K.J.; Frith, C.D. Dysconnection in schizophrenia: From abnormal synaptic plasticity to failures of self-monitoring. Schizophr. Bull. 2009, 35, 509–527. [Google Scholar] [CrossRef]
- Bartsch, U.; Simpkin, A.J.; Demanuele, C.; Wamsley, E.; Marston, H.M.; Jones, M.W. Distributed slow-wave dynamics during sleep predict memory consolidation and its impairment in schizophrenia. NPJ Schizophr. 2019, 5, 18. [Google Scholar] [CrossRef]
- Abraham, W.C.; Mason, S.E. Effects of the NMDA receptor/channel antagonists CPP and MK801 on hippocampal field potentials and long-term potentiation in anesthetized rats. Brain Res. 1988, 462, 40–46. [Google Scholar] [CrossRef]
- Wiescholleck, V.; Manahan-Vaughan, D. Persistent deficits in hippocampal synaptic plasticity accompany losses of hippocampus-dependent memory in a rodent model of psychosis. Front. Integr. Neurosci. 2013, 7, 12. [Google Scholar] [CrossRef]
- Wöhrl, R.; Eisenach, S.; Manahan-Vaughan, D.; Heinemann, U.; Von Haebler, D. Acute and long-term effects of MK-801 on direct cortical input evoked homosynaptic and heterosynaptic plasticity in the CA1 region of the female rat. Eur. J. Neurosci. 2007, 26, 2873–2883. [Google Scholar] [CrossRef]
- Buck, N.; Cali, S.; Behr, J. Enhancement of long-term potentiation at CA1-subiculum synapses in MK-801-treated rats. Neurosci. Lett. 2006, 392, 5–9. [Google Scholar] [CrossRef]
- Zorumski, C.F.; Izumi, Y. NMDA receptors and metaplasticity: Mechanisms and possible roles in neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2012, 36, 989–1000. [Google Scholar] [CrossRef]
- Earls, L.R.; Bayazitov, I.T.; Fricke, R.G.; Berry, R.B.; Illingworth, E.; Mittleman, G.; Zakharenko, S.S. Dysregulation of presynaptic calcium and synaptic plasticity in a mouse model of 22q11 deletion syndrome. J. Neurosci. 2010, 30, 15843–15855. [Google Scholar] [CrossRef]
- Fazzari, P.; Snellinx, A.; Sabanov, V.; Ahmed, T.; Serneels, L.; Gartner, A.; Shariati, S.A.M.; Balschun, D.; De Strooper, B. Cell autonomous regulation of hippocampal circuitry via Aph1b-γ-secretase/neuregulin 1 signalling. eLife 2014, 3, e02196. [Google Scholar] [CrossRef]
- Calişkan, G.; Stork, O. Hippocampal network oscillations at the interplay between innate anxiety and learned fear. Psychopharmacology 2019, 236, 321–338. [Google Scholar] [CrossRef]
- Colgin, L.L. Rhythms of the hippocampal network. Nat. Rev. Neurosci. 2016, 17, 239–249. [Google Scholar] [CrossRef]
- Buzsáki, G. Theta oscillations in the hippocampus. Neuron 2002, 33, 325–340. [Google Scholar] [CrossRef]
- Speers, L.J.; Bilkey, D.K. Disorganization of Oscillatory Activity in Animal Models of Schizophrenia. Front. Neural Circuits 2021, 15, 741767. [Google Scholar] [CrossRef]
- Assaf, S.Y.; Miller, J.J. The role of a raphe serotonin system in the control of septal unit activity and hippocampal desynchronization. Neuroscience 1978, 3, 539–550. [Google Scholar] [CrossRef]
- Maru, E.; Takahashi, L.K.; Iwahara, S. Effects of median raphe nucleus lesions on hippocampal EEG in the freely moving rat. Brain Res. 1979, 163, 223–234. [Google Scholar] [CrossRef]
- Kocsis, B.; Varga, V.; Dahan, L.; Sik, A. Serotonergic neuron diversity: Identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc. Natl. Acad. Sci. USA 2006, 103, 1059–1064. [Google Scholar] [CrossRef]
- Gutiérrez-Guzmán, B.E.; Hernández-Pérez, J.J.; González-Burgos, I.; Feria-Velásco, A.; Medina, R.; Guevara, M.Á.; López-Vázquez, M.Á.; Olvera-Cortés, M.E. Hippocampal serotonin depletion facilitates place learning concurrent with an increase in CA1 high frequency theta activity expression in the rat. Eur. J. Pharmacol. 2011, 652, 73–81. [Google Scholar] [CrossRef]
- Shiozaki, H.; Kuga, N.; Kayama, T.; Ikegaya, Y.; Sasaki, T. Selective serotonin reuptake inhibitors suppress sharp wave ripples in the ventral hippocampus. J. Pharmacol. Sci. 2023, 152, 136–143. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, C.; An, L.; Wang, R.; Zhang, T. Effects of Dopamine and Serotonin Systems on Modulating Neural Oscillations in Hippocampus-Prefrontal Cortex Pathway in Rats. Brain Topogr. 2016, 29, 539–551. [Google Scholar] [CrossRef]
- Sörman, E.; Wang, D.; Hajos, M.; Kocsis, B. Control of hippocampal theta rhythm by serotonin: Role of 5-HT2c receptors. Neuropharmacology 2011, 61, 489–494. [Google Scholar] [CrossRef]
- Skovgård, K.; Agerskov, C.; Kohlmeier, K.A.; Herrik, K.F. The 5-HT3 receptor antagonist ondansetron potentiates the effects of the acetylcholinesterase inhibitor donepezil on neuronal network oscillations in the rat dorsal hippocampus. Neuropharmacology 2018, 143, 130–142. [Google Scholar] [CrossRef]
- Johnson, D.E.; Drummond, E.; Grimwood, S.; Sawant-Basak, A.; Miller, E.; Tseng, E.; McDowell, L.L.; Vanase-Frawley, M.A.; Fisher, K.E.; Rubitski, D.M.; et al. The 5-hydroxytryptamine4 receptor agonists prucalopride and PRX-03140 increase acetylcholine and histamine levels in the rat prefrontal cortex and the power of stimulated hippocampal θ oscillations. J. Pharmacol. Exp. Ther. 2012, 341, 681–691. [Google Scholar] [CrossRef]
- Ly, S.; Pishdari, B.; Lok, L.L.; Hajos, M.; Kocsis, B. Activation of 5-HT6 Receptors Modulates Sleep-Wake Activity and Hippocampal Theta Oscillation. ACS Chem. Neurosci. 2013, 4, 191–199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Herrik, K.F.; Mørk, A.; Richard, N.; Bundgaard, C.; Bastlund, J.F.; de Jong, I.E. The 5-HT6 receptor antagonist idalopirdine potentiates the effects of acetylcholinesterase inhibition on neuronal network oscillations and extracellular acetylcholine levels in the rat dorsal hippocampus. Neuropharmacology 2016, 107, 351–363. [Google Scholar] [CrossRef]
- Buzsáki, G.; Wang, X.-J. Mechanisms of gamma oscillations. Annu. Rev. Neurosci. 2012, 35, 203–225. [Google Scholar] [CrossRef]
- Ichim, A.M.; Barzan, H.; Moca, V.V.; Nagy-Dabacan, A.; Ciuparu, A.; Hapca, A.; Vervaeke, K.; Muresan, R.C. The gamma rhythm as a guardian of brain health. eLife 2024, 13, 100238. [Google Scholar] [CrossRef]
- Mably, A.J.; Colgin, L.L. Gamma oscillations in cognitive disorders. Curr. Opin. Neurobiol. 2018, 52, 182–187. [Google Scholar] [CrossRef]
- Wójtowicz, A.M.; Boom, L.v.D.; Chakrabarty, A.; Maggio, N.; Haq, R.U.; Behrens, C.J.; Heinemann, U. Monoamines block kainate- and carbachol-induced γ-oscillations but augment stimulus-induced γ-oscillations in rat hippocampus in vitro. Hippocampus 2009, 19, 273–288. [Google Scholar] [CrossRef]
- Johnston, A.; McBain, C.J.; Fisahn, A. 5-Hydroxytryptamine1A receptor-activation hyperpolarizes pyramidal cells and suppresses hippocampal gamma oscillations via Kir3 channel activation. J. Physiol. 2014, 592, 4187–4199. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Krause, M.; Jia, Y. Serotonergic modulation of carbachol-induced rhythmic activity in hippocampal slices. Neuropharmacology 2005, 48, 381–390. [Google Scholar] [CrossRef]
- Huang, Y.; Yoon, K.; Ko, H.; Jiao, S.; Ito, W.; Wu, J.-Y.; Yung, W.-H.; Lu, B.; Morozov, A. 5-HT3a Receptors Modulate Hippocampal Gamma Oscillations by Regulating Synchrony of Parvalbumin-Positive Interneurons. Cereb. Cortex 2016, 26, 576–585. [Google Scholar] [CrossRef]
- Huang, Y.; Morozov, A.; Hashimoto, K. Hippocampal deletion of BDNF gene attenuates gamma oscillations in area CA1 by up-regulating 5-HT3 receptor. PLoS ONE 2011, 6, e16480. [Google Scholar] [CrossRef]
- Castañé, A.; Cano, M.; Ruiz-Avila, L.; Miquel-Rio, L.; Celada, P.; Artigas, F.; Riga, M.S. Dual 5-HT3 and 5-HT6 Receptor Antagonist FPPQ Normalizes Phencyclidine-Induced Disruption of Brain Oscillatory Activity in Rats. Int. J. Neuropsychopharmacol. 2022, 25, 425–431. [Google Scholar] [CrossRef]
- Buzsáki, G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 2015, 25, 1073–1188. [Google Scholar] [CrossRef]
- Joo, H.R.; Frank, L.M. The hippocampal sharp wave-ripple in memory retrieval for immediate use and consolidation. Nat. Rev. Neurosci. 2018, 19, 744–757. [Google Scholar] [CrossRef]
- Kuga, N.; Nakayama, R.; Morikawa, S.; Yagishita, H.; Konno, D.; Shiozaki, H.; Honjoya, N.; Ikegaya, Y.; Sasaki, T. Hippocampal sharp wave ripples underlie stress susceptibility in male mice. Nat. Commun. 2023, 14, 2105. [Google Scholar] [CrossRef]
- Pfeiffer, B.E. The content of hippocampal “replay”. Hippocampus 2020, 30, 6–18. [Google Scholar] [CrossRef]
- Tomar, A.; Polygalov, D.; Chattarji, S.; McHugh, T.J. Stress enhances hippocampal neuronal synchrony and alters ripple-spike interaction. Neurobiol. Stress 2021, 14, 100327. [Google Scholar] [CrossRef]
- Xie, B.; Zhen, Z.; Guo, O.; Li, H.; Guo, M.; Zhen, J. Progress on the hippocampal circuits and functions based on sharp wave ripples. Brain Res. Bull. 2023, 200, 110695. [Google Scholar] [CrossRef]
- O’CAllaghan, C.; Walpola, I.C.; Shine, J.M. Neuromodulation of the mind-wandering brain state: The interaction between neuromodulatory tone, sharp wave-ripples and spontaneous thought. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20190699. [Google Scholar] [CrossRef]
- Foster, D.J. Replay Comes of Age. Annu. Rev. Neurosci. 2017, 40, 581–602. [Google Scholar] [CrossRef]
- Wilson, M.A.; McNaughton, B.L. Reactivation of hippocampal ensemble memories during sleep. Science 1994, 265, 676–679. [Google Scholar] [CrossRef]
- Kouvaros, S.; Papatheodoropoulos, C. Prominent differences in sharp waves, ripples and complex spike bursts between the dorsal and the ventral rat hippocampus. Neuroscience 2017, 352, 131–143. [Google Scholar] [CrossRef]
- Sosa, M.; Joo, H.R.; Frank, L.M. Dorsal and Ventral Hippocampal Sharp-Wave Ripples Activate Distinct Nucleus Accumbens Networks. Neuron 2019, 105, 725–741. [Google Scholar] [CrossRef]
- Melonakos, E.D.; White, J.A.; Fernandez, F.R. A model of cholinergic suppression of hippocampal ripples through disruption of balanced excitation/inhibition. Hippocampus 2019, 29, 773–786. [Google Scholar] [CrossRef]
- Kenny, A.; Wright, D.; Stanfield, A.C. EEG as a translational biomarker and outcome measure in fragile X syndrome. Transl. Psychiatry 2022, 12, 34. [Google Scholar] [CrossRef]
- Contreras, A.; Djebari, S.; Temprano-Carazo, S.; Múnera, A.; Gruart, A.; Delgado-Garcia, J.M.; Jiménez-Díaz, L.; Navarro-López, J.D. Impairments in hippocampal oscillations accompany the loss of LTP induced by GIRK activity blockade. Neuropharmacology 2023, 238, 109668. [Google Scholar] [CrossRef]
- Hofer, K.T.; Kandrács, Á.; Ulbert, I.; Pál, I.; Szabó, C.; Héja, L.; Wittner, L. The hippocampal CA3 region can generate two distinct types of sharp wave-ripple complexes, in vitro. Hippocampus 2015, 25, 169–186. [Google Scholar] [CrossRef]
- Simeone, T.A.; Simeone, K.A.; Samson, K.K.; Kim, D.Y.; Rho, J.M. Loss of the Kv1.1 potassium channel promotes pathologic sharp waves and high frequency oscillations in in vitro hippocampal slices. Neurobiol. Dis. 2013, 54, 68–81. [Google Scholar] [CrossRef]
- Trompoukis, G.; Rigas, P.; Leontiadis, L.J.; Papatheodoropoulos, C. Ih, GIRK, and KCNQ/Kv7 channels differently modulate sharp wave—Ripples in the dorsal and ventral hippocampus. Mol. Cell. Neurosci. 2020, 107, 103531. [Google Scholar] [CrossRef]
- Wang, D.V.; Yau, H.-J.; Broker, C.J.; Tsou, J.-H.; Bonci, A.; Ikemoto, S. Mesopontine median raphe regulates hippocampal ripple oscillation and memory consolidation. Nat. Neurosci. 2015, 18, 728–735. [Google Scholar] [CrossRef]
- Ponomarenko, A.A.; Knoche, A.; Korotkova, T.M.; Haas, H.L. Aminergic control of high-frequency (approximately 200 Hz) network oscillations in the hippocampus of the behaving rat. Neurosci. Lett. 2003, 348, 101–104. [Google Scholar] [CrossRef]
- Haq, R.U.; Anderson, M.L.; Hollnagel, J.-O.; Worschech, F.; Sherkheli, M.A.; Behrens, C.J.; Heinemann, U. Serotonin dependent masking of hippocampal sharp wave ripples. Neuropharmacology 2016, 101, 188–203. [Google Scholar] [CrossRef]
- Richter-Levin, G.; Segal, M. Effects of serotonin releasers on dentate granule cell excitability in the rat. Exp. Brain Res. 1990, 82, 199–207. [Google Scholar] [CrossRef]
- Ohki, T.; Chao, Z.C.; Takei, Y.; Kato, Y.; Sunaga, M.; Suto, T.; Tagawa, M.; Fukuda, M. Multivariate sharp-wave ripples in schizophrenia during awake state. Psychiatry Clin. Neurosci. 2024, 78, 507–516. [Google Scholar] [CrossRef]
- Munn, R.G.K.; Wolff, A.; Speers, L.J.; Bilkey, D.K. Disrupted hippocampal synchrony following maternal immune activation in a rat model. Hippocampus 2023, 33, 995–1008. [Google Scholar] [CrossRef]
- Gao, M.; Orita, K.; Ikegaya, Y. Maternal Immune Activation in Pregnant Mice Produces Offspring with Altered Hippocampal Ripples. Biol. Pharm. Bull. 2019, 42, 666–670. [Google Scholar] [CrossRef]
- Suh, J.; Foster, D.J.; Davoudi, H.; Wilson, M.A.; Tonegawa, S. Impaired hippocampal ripple-associated replay in a mouse model of schizophrenia. Neuron 2013, 80, 484–493. [Google Scholar] [CrossRef]
- Altimus, C.; Harrold, J.; Jaaro-Peled, H.; Sawa, A.; Foster, D.J. Disordered ripples are a common feature of genetically distinct mouse models relevant to schizophrenia. Complex Psychiatry 2015, 1, 52–59. [Google Scholar] [CrossRef]
- Hunt, M.J.; Falinska, M.; Łęski, S.; Wójcik, D.K.; Kasicki, S. Differential effects produced by ketamine on oscillatory activity recorded in the rat hippocampus, dorsal striatum and nucleus accumbens. J. Psychopharmacol. 2011, 25, 808–821. [Google Scholar] [CrossRef]
- Lidov, H.G.; Molliver, M.E. An immunohistochemical study of serotonin neuron development in the rat: Ascending pathways and terminal fields. Brain Res. Bull. 1982, 8, 389–430. [Google Scholar] [CrossRef]
- Rind, H.; Russo, A.; Whittemore, S. Developmental regulation of tryptophan hydroxylase messenger RNA expression and enzyme activity in the raphe and its target fields. Neuroscience 2000, 101, 665–677. [Google Scholar] [CrossRef]
- Mitchell, J.B.; Iny, L.J.; Meaney, M.J. The role of serotonin in the development and environmental regulation of type II corticosteroid receptor binding in rat hippocampus. Dev. Brain Res. 1990, 55, 231–235. [Google Scholar] [CrossRef]
- Vitalis, T.; Fouquet, C.; Alvarez, C.; Seif, I.; Price, D.; Gaspar, P.; Cases, O. Developmental expression of monoamine oxidases A and B in the central and peripheral nervous systems of the mouse. J. Comp. Neurol. 2002, 442, 331–347. [Google Scholar] [CrossRef]
- Lebrand, C.; Cases, O.; Wehrlé, R.; Blakely, R.D.; Edwards, R.H.; Gaspar, P. Transient developmental expression of monoamine transporters in the rodent forebrain. J. Comp. Neurol. 1998, 401, 506–524. [Google Scholar] [PubMed]
- Kozono, N.; Ohtani, A.; Shiga, T. Roles of the serotonin 5-HT4 receptor in dendrite formation of the rat hippocampal neurons in vitro. Brain Res. 2017, 1655, 114–121. [Google Scholar] [CrossRef]
- Volpicelli, F.; Speranza, L.; Pulcrano, S.; De Gregorio, R.; Crispino, M.; De Sanctis, C.; Leopoldo, M.; Lacivita, E.; di Porzio, U.; Bellenchi, G.C.; et al. The microRNA-29a Modulates Serotonin 5-HT7 Receptor Expression and Its Effects on Hippocampal Neuronal Morphology. Mol. Neurobiol. 2019, 56, 8617–8627. [Google Scholar] [CrossRef]
- Matsumoto, M.; Higuchi, K.; Togashi, H.; Koseki, H.; Yamaguchi, T.; Kanno, M.; Yoshioka, M. Early postnatal stress alters the 5-HTergic modulation to emotional stress at postadolescent periods of rats. Hippocampus 2005, 15, 775–781. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Izumi, T.; Horinouchi, T.; Boku, S.; Inoue, T.; Yamaguchi, T.; Yoshida, T.; Matsumoto, M.; Togashi, H.; Miwa, S.; et al. Juvenile stress attenuates the dorsal hippocampal postsynaptic 5-HT1A receptor function in adult rats. Psychopharmacology 2011, 214, 329–337. [Google Scholar] [CrossRef]
- Vázquez, D.M.; López, J.F.; Van Hoers, H.; Watson, S.J.; Levine, S. Maternal deprivation regulates serotonin 1A and 2A receptors in the infant rat. Brain Res. 2000, 855, 76–82. [Google Scholar] [CrossRef]
- Gruber, D.; Gilling, K.; Albrecht, A.; Bartsch, J.; Çalışkan, G.; Richter-Levin, G.; Stork, O.; Heinemann, U.; Behr, J. 5-HT receptor-mediated modulation of granule cell inhibition after juvenile stress recovers after a second exposure to adult stress. Neuroscience 2015, 293, 67–79. [Google Scholar] [CrossRef]
- Browne, C.A.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Differential stress-induced alterations in tryptophan hydroxylase activity and serotonin turnover in two inbred mouse strains. Neuropharmacology 2011, 60, 683–691. [Google Scholar] [CrossRef]
- Jaaro-Peled, H.; Sawa, A. Neurodevelopmental Factors in Schizophrenia. Psychiatr. Clin. North Am. 2020, 43, 263–274. [Google Scholar] [CrossRef]
- Schobel, S.A.; Chaudhury, N.H.; Khan, U.A.; Paniagua, B.; Styner, M.A.; Asllani, I.; Inbar, B.P.; Corcoran, C.M.; Lieberman, J.A.; Moore, H. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 2013, 78, 81–93. [Google Scholar] [CrossRef]
- Eggers, A.E. An explanation of why schizophrenia begins with excitotoxic damage to the hippocampus. Med. Hypotheses 2013, 81, 1056–1058. [Google Scholar] [CrossRef]
- Müller, N.; Schwarz, M.J. The immune-mediated alteration of serotonin and glutamate: Towards an integrated view of depression. Mol. Psychiatry 2007, 12, 988–1000. [Google Scholar] [CrossRef]
- Traktirov, D.S.; Nazarov, I.R.; Artemova, V.S.; Gainetdinov, R.R.; Pestereva, N.S.; Karpenko, M.N. Alterations in Serotonin Neurotransmission in Hyperdopaminergic Rats Lacking the Dopamine Transporter. Biomedicines 2023, 11, 2881. [Google Scholar] [CrossRef]
- Fournet, V.; Jany, M.; Fabre, V.; Chali, F.; Orsal, D.; Schweitzer, A.; Andrieux, A.; Messanvi, F.; Giros, B.; Hamon, M.; et al. The deletion of the microtubule-associated STOP protein affects the serotonergic mouse brain network. J. Neurochem. 2010, 115, 1579–1594. [Google Scholar] [CrossRef]
- Marsden, C.A.; King, M.V.; Fone, K.C. Influence of social isolation in the rat on serotonergic function and memory—Relevance to models of schizophrenia and the role of 5-HT6 receptors. Neuropharmacology 2011, 61, 400–407. [Google Scholar] [CrossRef]
- Bielas, H.; Arck, P.; Bruenahl, C.; Walitza, S.; Grünblatt, E. Prenatal stress increases the striatal and hippocampal expression of correlating c-FOS and serotonin transporters in murine offspring. Int. J. Dev. Neurosci. 2014, 38, 30–35. [Google Scholar] [CrossRef]
- Dias, C.T.; Curi, H.T.; Payolla, T.B.; Lemes, S.F.; Pavan, I.C.B.; Torsoni, M.A.; Simabuco, F.M.; Lambertucci, R.H.; da Silva, C.M. Maternal high-fat diet stimulates proinflammatory pathway and increases the expression of Tryptophan Hydroxylase 2 (TPH2) and brain-derived neurotrophic factor (BDNF) in adolescent mice hippocampus. Neurochem. Int. 2020, 139, 104781. [Google Scholar] [CrossRef]
- García-Osta, A.; Del Río, J.; Frechilla, D. Increased CRE-binding activity and tryptophan hydroxylase mRNA expression induced by 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) in the rat frontal cortex but not in the hippocampus. Mol. Brain Res. 2004, 126, 181–187. [Google Scholar] [CrossRef]
- Kobayashi, T.; Washiyama, K.; Ikeda, K. Inhibition of G-protein-activated inwardly rectifying K+ channels by the selective norepinephrine reuptake inhibitors atomoxetine and reboxetine. Neuropsychopharmacology 2010, 35, 1560–1569. [Google Scholar] [CrossRef]
- Adams, W.; Kusljic, S.; Buuse, M.v.D. Serotonin depletion in the dorsal and ventral hippocampus: Effects on locomotor hyperactivity, prepulse inhibition and learning and memory. Neuropharmacology 2008, 55, 1048–1055. [Google Scholar] [CrossRef]
- Freedman, R.; Hall, M.; Adler, L.E.; Leonard, S. Evidence in postmortem brain tissue for decreased numbers of hippocampal nicotinic receptors in schizophrenia. Biol. Psychiatry 1995, 38, 22–33. [Google Scholar] [CrossRef]
- Stevens, K.E.; Freedman, R.; Collins, A.C.; Hall, M.; Leonard, S.; Marks, M.J.; Rose, G.M. Genetic correlation of inhibitory gating of hippocampal auditory evoked response and α-bungarotoxin-binding nicotinic cholinergic receptors in inbred mouse strains. Neuropsychopharmacology 1996, 15, 152–162. [Google Scholar] [CrossRef]
- Hajos, M.; Rogers, B.N. Targeting α7 nicotinic acetylcholine receptors in the treatment of schizophrenia. Curr. Pharm. Des. 2010, 16, 538–554. [Google Scholar] [CrossRef]
- Paul, S.M.; Yohn, S.E.; Popiolek, M.; Miller, A.C.; Felder, C.C. Muscarinic Acetylcholine Receptor Agonists as Novel Treatments for Schizophrenia. Am. J. Psychiatry 2022, 179, 611–627. [Google Scholar] [CrossRef]
- Scarr, E.; Pavey, G.; Copolov, D.; Dean, B. Hippocampal 5-hydroxytryptamine receptors: Abnormalities in postmortem brain from schizophrenic subjects. Schizophr. Res. 2004, 71, 383–392. [Google Scholar] [CrossRef]
- Tsotsokou, G.; Kouri, V.; Papatheodoropoulos, C. α7 nicotinic acetylcholine receptors induce long-term synaptic enhancement in the dorsal but not ventral hippocampus. Synapse 2024, 78, e22285. [Google Scholar] [CrossRef]
- Stoiljkovic, M.; Kelley, C.; Nagy, D.; Hurst, R.; Hajós, M. Activation of alpha 7 nicotinic acetylcholine receptors facilitates long-term potentiation at the hippocampal-prefrontal cortex synapses in vivo. Eur. Neuropsychopharmacol. 2016, 26, 2018–2023. [Google Scholar] [CrossRef]
- Horan, W.P.; Sauder, C.; Harvey, P.D.; Ramsay, I.S.; Yohn, S.E.; Keefe, R.S.E.; Davis, V.G.; Paul, S.M.; Brannan, S.K. The Impact of Xanomeline and Trospium Chloride on Cognitive Impairment in Acute Schizophrenia: Replication in Pooled Data From Two Phase 3 Trials. Am. J. Psychiatry 2024, 182, 297–306. [Google Scholar] [CrossRef]
- Joyce, J.N.; Shane, A.; Lexow, N.; Winokur, A.; Casanova, M.F.; Kleinman, J.E. Serotonin uptake sites and serotonin receptors are altered in the limbic system of schizophrenics. Neuropsychopharmacology 1993, 8, 315–336. [Google Scholar] [CrossRef]
- Wedzony, K.; Wdzony, K.; Maćkowiak, M.; Zajczkowski, W.; Fijal, K.; Chocyk, A.; Czyrak, A. WAY 100135, an antagonist of 5-HT1A serotonin receptors, attenuates psychotomimetic effects of MK-801. Neuropsychopharmacology 2000, 23, 547–559. [Google Scholar] [CrossRef][Green Version]
- Nikolaus, S.; Wittsack, H.-J.; Beu, M.; Hautzel, H.; Antke, C.; Mamlins, E.; Cardinale, J.; Decheva, C.; Huston, J.P.; Antoch, G.; et al. The 5-HT1A receptor antagonist WAY-100635 decreases motor/exploratory behaviors and nigrostriatal and mesolimbocortical dopamine D2/3 receptor binding in adult rats. Pharmacol. Biochem. Behav. 2022, 215, 173363. [Google Scholar] [CrossRef]
- Belcheva, I.; Belcheva, S.; Hadjiivanova, C.; Petkov, V.D. Behavorial responses to the 5-HT1A receptor antagonist NAN190 injected into rat CA1 hippocampal area. Gen. Pharmacol. Vasc. Syst. 1997, 28, 435–441. [Google Scholar] [CrossRef]
- Gogos, A.; Kusljic, S.; Buuse, M. 8-OH-DPAT-induced effects on prepulse inhibition: Pre- vs. post-synaptic 5-HT1A receptor activation. Pharmacol. Biochem. Behav. 2005, 81, 664–672. [Google Scholar] [CrossRef]
- López-Figueroa, A.L.; Norton, C.S.; O López-Figueroa, M.; Armellini-Dodel, D.; Burke, S.; Akil, H.; López, J.F.; Watson, S.J. Serotonin 5-HT1A, 5-HT1B, and 5-HT2A receptor mRNA expression in subjects with major depression, bipolar disorder, and schizophrenia. Biol. Psychiatry 2004, 55, 225–233. [Google Scholar] [CrossRef]
- Gozzi, A.; Crestan, V.; Turrini, G.; Clemens, M.; Bifone, A. Antagonism at serotonin 5-HT2A receptors modulates functional activity of frontohippocampal circuit. Psychopharmacology 2010, 209, 37–50. [Google Scholar] [CrossRef]
- Shi, M.; Tang, J.; Yang, C.; Guo, G.; Ou, H.; Chen, W. Pimavanserin, a 5-hydroxytryptamine 2A receptor inverse agonist, reverses prepulse inhibition deficits in the nucleus accumbens and ventral hippocampus. Neuropharmacology 2021, 201, 108838. [Google Scholar] [CrossRef]
- Ou, H.; Tang, J.; Guo, G.; Shi, M.; Yang, C.; Chen, W. TCB-2, a 5-hydroxytryptamine2A receptor agonist, disrupts prepulse inhibition in the ventral pallidum and nucleus accumbens. Behav. Brain Res. 2023, 437, 114127. [Google Scholar] [CrossRef]
- Nikolaus, S.; Wittsack, H.-J.; Antke, C.; Beu, M.; Hautzel, H.; Decheva, C.; Mamlins, E.; Mori, Y.; Huston, J.P.; Antoch, G.; et al. Serotonergic Modulation of Nigrostriatal and Mesolimbic Dopamine and Motor/Exploratory Behaviors in the Rat. Front. Neurosci. 2021, 15, 682398. [Google Scholar] [CrossRef]
- Maćkowiak, M. Psychedelics action and schizophrenia. Pharmacol. Rep. 2023, 75, 1350–1361. [Google Scholar] [CrossRef]
- Wildeboer, K.M.; Zheng, L.; Choo, K.S.; Stevens, K.E. Ondansetron results in improved auditory gating in DBA/2 mice through a cholinergic mechanism. Brain Res. 2009, 1300, 41–50. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mohamed, R.A.; Galal, O.; Mohammed, A.R.; El-Abhar, H.S. Tropisetron modulates peripheral and central serotonin/insulin levels via insulin and nuclear factor kappa B/receptor for advanced glycation end products signalling to regulate type-2 diabetes in rats. RSC Adv. 2018, 8, 11908–11920. [Google Scholar] [CrossRef]
- Fakhfouri, G.; Rahimian, R.; Dyhrfjeld-Johnsen, J.; Zirak, M.R.; Beaulieu, J.-M. 5-HT3 Receptor Antagonists in Neurologic and Neuropsychiatric Disorders: The Iceberg Still Lies beneath the Surface. Pharmacol. Rev. 2019, 71, 383–412. [Google Scholar] [CrossRef]
- Dubertret, C.; Hanoun, N.; Adès, J.; Hamon, M.; Gorwood, P. Family-based association studies between 5-HT5A receptor gene and schizophrenia. J. Psychiatr. Res. 2004, 38, 371–376. [Google Scholar] [CrossRef]
- East, S.; Burnet, P.; Kerwin, R.; Harrison, P. An RT-PCR study of 5-HT6 and 5-HT7 receptor mRNAs in the hippocampal formation and prefrontal cortex in schizophrenia. Schizophr. Res. 2002, 57, 15–26. [Google Scholar] [CrossRef]
- Dawson, L. The 5-HT6 receptor antagonist SB-271046 selectively enhances excitatory neurotransmission in the rat frontal cortex and hippocampus. Neuropsychopharmacology 2001, 25, 662–668. [Google Scholar] [CrossRef]
- Shortall, S.E.; Brown, A.M.; Newton-Mann, E.; Dawe-Lane, E.; Evans, C.; Fowler, M.; King, M.V. Calbindin Deficits May Underlie Dissociable Effects of 5-HT6 and mGlu7 Antagonists on Glutamate and Cognition in a Dual-Hit Neurodevelopmental Model for Schizophrenia. Mol. Neurobiol. 2020, 57, 3439–3457. [Google Scholar] [CrossRef]
- Tamminga, C.A.; Stan, A.D.; Wagner, A.D. The hippocampal formation in schizophrenia. Am. J. Psychiatry 2010, 167, 1178–1193. [Google Scholar] [CrossRef]
- Healy, D.J.; Meador-Woodruff, J.H. Ionotropic glutamate receptor modulation of 5-HT6 and 5-HT7 mRNA expression in rat brain. Neuropsychopharmacology 1999, 21, 341–351. [Google Scholar] [CrossRef]
- Shortall, S.E.; Negm, O.H.; Fowler, M.; Fairclough, L.C.; Tighe, P.J.; Wigmore, P.M.; King, M.V. Characterization of Behavioral, Signaling and Cytokine Alterations in a Rat Neurodevelopmental Model for Schizophrenia, and Their Reversal by the 5-HT6 Receptor Antagonist SB-399885. Mol. Neurobiol. 2018, 55, 7413–7430. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lieben, C.K.; Blokland, A.; Şık, A.; Sung, E.; van Nieuwenhuizen, P.; Schreiber, R. The selective 5-HT6 receptor antagonist Ro4368554 restores memory performance in cholinergic and serotonergic models of memory deficiency in the rat. Neuropsychopharmacology 2005, 30, 2169–2179. [Google Scholar] [CrossRef]
- Leng, A.; Ouagazzal, A.; Feldon, J.; A Higgins, G. Effect of the 5-HT6 receptor antagonists Ro04-6790 and Ro65-7199 on latent inhibition and prepulse inhibition in the rat: Comparison to clozapine. Pharmacol. Biochem. Behav. 2003, 75, 281–288. [Google Scholar] [CrossRef]
- Tajiri, M.; Hayata-Takano, A.; Seiriki, K.; Ogata, K.; Hazama, K.; Shintani, N.; Baba, A.; Hashimoto, H. serotonin 5-HT7 receptor blockade reverses behavioral abnormalities in PACAP-deficient mice and receptor activation promotes neurite extension in primary embryonic hippocampal neurons. J. Mol. Neurosci. 2012, 48, 473–481. [Google Scholar] [CrossRef]
- Notter, T. Astrocytes in schizophrenia. Brain Neurosci. Adv. 2021, 5, 23982128211009148. [Google Scholar] [CrossRef]
- E Terrillion, C.; Abazyan, B.; Yang, Z.; Crawford, J.; Shevelkin, A.V.; Jouroukhin, Y.; Yoo, K.H.; Cho, C.H.; Roychaudhuri, R.; Snyder, S.H.; et al. DISC1 in Astrocytes Influences Adult Neurogenesis and Hippocampus-Dependent Behaviors in Mice. Neuropsychopharmacology 2017, 42, 2242–2251. [Google Scholar] [CrossRef]
- Shevelkin, A.V.; E Terrillion, C.; Hasegawa, Y.; A Mychko, O.; Jouroukhin, Y.; Sawa, A.; Kamiya, A.; Pletnikov, M.V. Astrocyte DISC1 contributes to cognitive function in a brain region-dependent manner. Hum. Mol. Genet. 2020, 29, 2936–2950. [Google Scholar] [CrossRef]
- Azmitia, E.; Gannon, P.; Kheck, N.; Whitakerazinitia, P. Cellular localization of the 5-HT receptor in primate brain neurons and glial cells. Neuropsychopharmacology 1996, 14, 35–46. [Google Scholar] [CrossRef]
- Xu, T.; Pandey, S.C. Cellular localization of serotonin2A (5HT2A) receptors in the rat brain. Brain Res. Bull. 2000, 51, 499–505. [Google Scholar] [CrossRef]
- Carson, M.J.; Thomas, E.A.; Danielson, P.E.; Sutcliffe, J.G. The 5-HT5A serotonin receptor is expressed predominantly by astrocytes in which it inhibits cAMP accumulation: A mechanism for neuronal suppression of reactive astrocytes. Glia 1996, 17, 317–326. [Google Scholar] [CrossRef]
- Madhavan, L.; Freed, W.J.; Anantharam, V.; Kanthasamy, A.G. 5-hydroxytryptamine1A receptor activation protects against N-methyl-D-aspartate-induced apoptotic cell death in striatal and mesencephalic cultures. J. Pharmacol. Exp. Ther. 2003, 304, 913–923. [Google Scholar] [CrossRef]
- Ansari, Z.; Pawar, S.; Seetharaman, R. Neuroinflammation and oxidative stress in schizophrenia: Are these opportunities for repurposing? Postgrad. Med. 2022, 134, 187–199. [Google Scholar] [CrossRef]
- Klempin, F.; Beis, D.; Mosienko, V.; Kempermann, G.; Bader, M.; Alenina, N. Serotonin is required for exercise-induced adult hippocampal neurogenesis. J. Neurosci. 2013, 33, 8270–8275. [Google Scholar] [CrossRef]
- Yuan, T.-F.; Paes, F.; Arias-Carrión, O.; Rocha, N.F.; Filho, A.d.S.; Machado, S. Neural Mechanisms of Exercise: Anti-Depression, Neurogenesis, and Serotonin Signaling. CNS Neurol. Disord. Drug Targets 2015, 14, 1307–1311. [Google Scholar] [CrossRef]
- Firth, J.; Stubbs, B.; Rosenbaum, S.; Vancampfort, D.; Malchow, B.; Schuch, F.; Elliott, R.; Nuechterlein, K.H.; Yung, A.R. Aerobic Exercise Improves Cognitive Functioning in People With Schizophrenia: A Systematic Review and Meta-Analysis. Schizophr. Bull. 2017, 43, 546–556. [Google Scholar] [CrossRef]
- Abbas, M.S.; Nassar, S.T.; Tasha, T.; Desai, A.; Bajgain, A.; Ali, A.; Dutta, C.; Pasha, K.; Paul, S.; Venugopal, S. Exercise as an Adjuvant Treatment of Schizophrenia: A Review. Cureus 2023, 15, e42084. [Google Scholar] [CrossRef]
- Creese, I.; Burt, D.R.; Snyder, S.H.; Burt, D.R. Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 1976, 192, 481–483. [Google Scholar] [CrossRef]
- Bacqué-Cazenave, J.; Bharatiya, R.; Barrière, G.; Delbecque, J.-P.; Bouguiyoud, N.; Di Giovanni, G.; Cattaert, D.; De Deurwaerdère, P. Serotonin in Animal Cognition and Behavior. Int. J. Mol. Sci. 2020, 21, 1649. [Google Scholar] [CrossRef]
- Huang, M.; He, W.; Kiss, B.; Farkas, B.; Adham, N.; Meltzer, H.Y. The Role of Dopamine D3 Receptor Partial Agonism in Cariprazine-Induced Neurotransmitter Efflux in Rat Hippocampus and Nucleus Accumbens. J. Pharmacol. Exp. Ther. 2019, 371, 517–525. [Google Scholar] [CrossRef]
- Herrmann, A.P.; Lunardi, P.; Pilz, L.K.; Tramontina, A.C.; Linck, V.M.; Okunji, C.O.; Gonçalves, C.A.; Elisabetsky, E. Effects of the putative antipsychotic alstonine on glutamate uptake in acute hippocampal slices. Neurochem. Int. 2012, 61, 1144–1150. [Google Scholar] [CrossRef]
- Huang, M.; Horiguchi, M.; Felix, A.R.; Meltzer, H.Y. 5-HT1A and 5-HT7 receptors contribute to lurasidone-induced dopamine efflux. NeuroReport 2012, 23, 436–440. [Google Scholar] [CrossRef]
- Shirazi-Southall, S.; Rodriguez, D.E.; Nomikos, G.G. Effects of typical and atypical antipsychotics and receptor selective compounds on acetylcholine efflux in the hippocampus of the rat. Neuropsychopharmacology 2002, 26, 583–594. [Google Scholar] [CrossRef]
- Newman-Tancredi, A.; Rivet, J.-M.; Cussac, D.; Touzard, M.; Chaput, C.; Marini, L.; Millan, M.J. Comparison of hippocampal G protein activation by 5-HT 1A receptor agonists and the atypical antipsychotics clozapine and S16924. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2003, 368, 188–199. [Google Scholar] [CrossRef]
- Han, M.; Huang, X.; du Bois, T.; Deng, C. The effects of antipsychotic drugs administration on 5-HT1A receptor expression in the limbic system of the rat brain. Neuroscience 2009, 164, 1754–1763. [Google Scholar] [CrossRef]
- Choi, Y.K.; Gardner, M.P.; Tarazi, F.I. Developmental effects of antipsychotic drugs on serotonin receptor subtypes. Synapse 2017, 71, e21988. [Google Scholar] [CrossRef]
- Hill, X.L.; Richeri, A.; Scorza, M.C. Clozapine blockade of MK-801-induced learning/memory impairment in the mEPM: Role of 5-HT1A receptors and hippocampal BDNF levels. Physiol. Behav. 2017, 179, 346–352. [Google Scholar] [CrossRef]
- Heiser, P.; Schulte, E.; Hausmann, C.; Becker, R.; Remschmidt, H.; Krieg, J.-C.; Vedder, H. Effects of clozapine and its metabolites on the 5-HT2 receptor system in cortical and hippocampal cells in vitro. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2004, 28, 297–302. [Google Scholar] [CrossRef]
- Tarazi, F.I.; Zhang, K.; Baldessarini, R.J. Long-term effects of olanzapine, risperidone, and quetiapine on serotonin 1A, 2A and 2C receptors in rat forebrain regions. Psychopharmacology 2002, 161, 263–270. [Google Scholar] [CrossRef]
- Castellano, O.; Arji, M.; Sancho, C.; Carro, J.; Riolobos, A.; Molina, V.; Gmez-Nieto, R.; Horta, J.d.A.d.C.e.; Herrero-Turrin, M.; Lpez, D. Chronic administration of risperidone in a rat model of schizophrenia: A behavioural, morphological and molecular study. Behav. Brain Res. 2013, 242, 178–190. [Google Scholar] [CrossRef]
- Piontkewitz, Y.; Bernstein, H.-G.; Dobrowolny, H.; Bogerts, B.; Weiner, I.; Keilhoff, G. Effects of risperidone treatment in adolescence on hippocampal neurogenesis, parvalbumin expression, and vascularization following prenatal immune activation in rats. Brain, Behav. Immun. 2012, 26, 353–363. [Google Scholar] [CrossRef]
- Schotte, A.; Janssen, P.F.M.; Gommeren, W.; Luyten, W.H.M.L.; Van Gompel, P.; Lesage, A.S.; De Loore, K.; Leysen, J.E. Risperidone compared with new and reference antipsychotic drugs: In Vitro and in vivo receptor binding. Psychopharmacology 1996, 124, 57–73. [Google Scholar] [CrossRef]
- Frederick, J.A.; Meador-Woodruff, J.H. Effects of clozapine and haloperidol on 5-HT6 receptor mRNA levels in rat brain. Schizophr. Res. 1999, 38, 7–12. [Google Scholar] [CrossRef]
- Li, Z.; Huang, M.; Prus, A.J.; Dai, J.; Meltzer, H.Y. 5-HT6 receptor antagonist SB-399885 potentiates haloperidol and risperidone-induced dopamine efflux in the medial prefrontal cortex or hippocampus. Brain Res. 2007, 1134, 70–78. [Google Scholar] [CrossRef]
- Chlan-Fourney, J.; Ashe, P.; Nylen, K.; Juorio, A.V.; Li, X.-M. Differential regulation of hippocampal BDNF mRNA by typical and atypical antipsychotic administration. Brain Res. 2002, 954, 11–20. [Google Scholar] [CrossRef]
- Duinkerke, S.J.; Botter, P.A.; Jansen, A.A.I.; Van Dongen, P.A.M.; Van Haaften, A.J.; Boom, A.J.; Van Laarhoven, J.H.M.; Busard, H.L.S.M. Ritanserin, a Selective 5-HT2/1C Antagonist, and Negative Symptoms in Schizophrenia. Br. J. Psychiatry 1993, 163, 451–455. [Google Scholar] [CrossRef]
- Fumagalli, F.; Calabrese, F.; Luoni, A.; Bolis, F.; Racagni, G.; Riva, M.A. Modulation of BDNF expression by repeated treatment with the novel antipsychotic lurasidone under basal condition and in response to acute stress. Int. J. Neuropsychopharmacol. 2012, 15, 235–246. [Google Scholar] [CrossRef]
- Bai, O.; Chlan-Fourney, J.; Bowen, R.; Keegan, D.; Li, X. Expression of brain-derived neurotrophic factor mRNA in rat hippocampus after treatment with antipsychotic drugs. J. Neurosci. Res. 2003, 71, 127–131. [Google Scholar] [CrossRef]
- Fumagalli, F.; Molteni, R.; Roceri, M.; Bedogni, F.; Santero, R.; Fossati, C.; Gennarelli, M.; Racagni, G.; Riva, M.A. Effect of antipsychotic drugs on brain-derived neurotrophic factor expression under reduced N-methyl-D-aspartate receptor activity. J. Neurosci. Res. 2003, 72, 622–628. [Google Scholar] [CrossRef]
- Czubak, A.; Nowakowska, E.; Kus, K.; Burda, K.; Metelska, J.; Baer-Dubowska, W.; Cichocki, M. Influences of chronic venlafaxine, olanzapine and nicotine on the hippocampal and cortical concentrations of brain-derived neurotrophic factor (BDNF). Pharmacol. Rep. 2009, 61, 1017–1023. [Google Scholar] [CrossRef]
- Xu, H.; Chen, Z.; He, J.; Haimanot, S.; Li, X.; Dyck, L.; Li, X. Synergetic effects of quetiapine and venlafaxine in preventing the chronic restraint stress-induced decrease in cell proliferation and BDNF expression in rat hippocampus. Hippocampus 2006, 16, 551–559. [Google Scholar] [CrossRef]
- Xu, H.; Qing, H.; Lu, W.; Keegan, D.; Richardson, J.; Chlan-Fourney, J.; Li, X.-M. Quetiapine attenuates the immobilization stress-induced decrease of brain-derived neurotrophic factor expression in rat hippocampus. Neurosci. Lett. 2002, 321, 65–68. [Google Scholar] [CrossRef]
- Fumagalli, F.; Molteni, R.; Bedogni, F.; Gennarelli, M.; Perez, J.; Racagni, G.; Riva, M.A. Quetiapine regulates FGF-2 and BDNF expression in the hippocampus of animals treated with MK-801. NeuroReport 2004, 15, 2109–2112. [Google Scholar] [CrossRef]
- Lipska, B.K.; Khaing, Z.Z.; Weickert, C.S.; Weinberger, D.R. BDNF mRNA expression in rat hippocampus and prefrontal cortex: Effects of neonatal ventral hippocampal damage and antipsychotic drugs. Eur. J. Neurosci. 2001, 14, 135–144. [Google Scholar] [CrossRef]
- Delgado-Sallent, C.; Nebot, P.; Gener, T.; Fath, A.B.; Timplalexi, M.; Atypical, P.M.V.; Typical, N. Antipsychotic Drugs Reduce Hypersynchronized Prefrontal-Hippocampal Circuits during Psychosis-Like States in Mice: Contribution of 5-HT2A and 5-HT1A Receptors. Cereb. Cortex 2022, 32, 3472–3487. [Google Scholar] [CrossRef]
- Schulz, S.B.; E Heidmann, K.; Mike, A.; Klaft, Z.; Heinemann, U.; Gerevich, Z. First and second generation antipsychotics influence hippocampal gamma oscillations by interactions with 5-HT3 and D3 receptors. Br. J. Pharmacol. 2012, 167, 1480–1491. [Google Scholar] [CrossRef]
- Mulert, C.; Kirsch, V.; Pascual-Marqui, R.; McCarley, R.W.; Spencer, K.M. Long-range synchrony of gamma oscillations and auditory hallucination symptoms in schizophrenia. Int. J. Psychophysiol. 2011, 79, 55–63. [Google Scholar] [CrossRef]
- Lee, S.-H.; Kim, D.-W.; Kim, E.-Y.; Kim, S.; Im, C.-H. Dysfunctional gamma-band activity during face structural processing in schizophrenia patients. Schizophr. Res. 2010, 119, 191–197. [Google Scholar] [CrossRef]
- Clancy, M.J.; Clarke, M.C.; Connor, D.J.; Cannon, M.; Cotter, D.R. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry 2014, 14, 75. [Google Scholar] [CrossRef]
- Borah, A.; Kalita, A.; Dutta, S. Clozapine-induced seizure. Indian J. Pharmacol. 2019, 51, 410–412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Whitson, J.; Agrawal, N. Epilepsy and psychosis. In The Comorbidities of Epilepsy; Academic Press: Cambridge, MA, USA, 2019; pp. 315–342. [Google Scholar]
- de Toffol, B. Epilepsy and psychosis. Rev. Neurol. 2024, 180, 298–307. [Google Scholar] [CrossRef]
- Landolt, H. Serial EEG investigations during psychotic episodes in epileptic patients and during schizophrenic attacks. In Lectures on Epilepsy; Haas Am, L., Ed.; Elsevier: Amsterdam, The Netherlands, 1958. [Google Scholar]
- Chen, Z.; Lusicic, A.; O’Brien, T.J.; Velakoulis, D.; Adams, S.J.; Kwan, P. Psychotic disorders induced by antiepileptic drugs in people with epilepsy. Brain 2016, 139, 2668–2678. [Google Scholar] [CrossRef]
- Akanuma, N.; Adachi, N.; Fenwick, P.; Ito, M.; Okazaki, M.; Hara, K.; Ishii, R.; Sekimoto, M.; Kato, M.; Onuma, T. Individual vulnerabilities to psychosis after antiepileptic drug administration. BMJ Neurol. Open 2020, 2, e000036. [Google Scholar] [CrossRef]
- Scharfman, H.E.; Kanner, A.M.; Friedman, A.; Blümcke, I.; Crocker, C.E.; Cendes, F.; Diaz-Arrastia, R.; Förstl, H.; Fenton, A.A.; Grace, A.A.; et al. Epilepsy as a Network Disorder (2): What can we learn from other network disorders such as dementia and schizophrenia, and what are the implications for translational research? Epilepsy Behav. 2018, 78, 302–312. [Google Scholar] [CrossRef]
- Kandratavicius, L.; Lopes-Aguiar, C.; Bueno-Júnior, L.S.; Romcy-Pereira, R.N.; Hallak, J.E.C.; Leite, J.P. Psychiatric comorbidities in temporal lobe epilepsy: Possible relationships between psychotic disorders and involvement of limbic circuits. Rev. Bras. Psiquiatr. 2012, 34, 454–466. [Google Scholar] [CrossRef]
- Cascella, N.G.; Schretlen, D.J.; Sawa, A. Schizophrenia and epilepsy: Is there a shared susceptibility? Neurosci. Res. 2009, 63, 227–235. [Google Scholar] [CrossRef]
- Bauer, J.P.; Rader, S.L.; Joffe, M.E.; Kwon, W.; Quay, J.; Seanez, L.; Zhou, C.; Conn, P.J.; Lewis, A.S. Modeling intrahippocampal effects of anterior hippocampal hyperactivity relevant to schizophrenia using chemogenetic excitation of long axis–projecting mossy cells in the mouse dentate gyrus. Biol. Psychiatry Glob. Open Sci. 2021, 1, 101–111. [Google Scholar] [CrossRef]
- Sharp, F.R.; Hendren, R.L. Psychosis: Atypical limbic epilepsy versus limbic hyperexcitability with onset at puberty? Epilepsy Behav. 2007, 10, 515–520. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chatzikonstantinou, A. Epilepsy and the hippocampus. Front. Neurol. Neurosci. 2014, 34, 121–142. [Google Scholar] [CrossRef]
- Schwartzkroin, P.A. Role of the hippocampus in epilepsy. Hippocampus 1994, 4, 239–242. [Google Scholar] [CrossRef]
- Green, J.D.; Shimamoto, T. Hippocampal seizures and their propagation. Arch. Neurol. Psychiatry 1953, 70, 687–702. [Google Scholar] [CrossRef]
- Oyebode, F. The neurology of psychosis. Med. Princ. Prac. 2008, 17, 263–269. [Google Scholar] [CrossRef]
- Scheibel, A.B. Are complex partial seizures a sequela of temporal lobe dysgenesis? Neurology 1991, 55, 59–77. [Google Scholar]
- Suckling, J.; Roberts, H.; Walker, M.; Highley, F., Jr.; Fenwick, P.; Oxbury, J.; Esiri, M.M. Temporal lobe epilepsy with and without psychosis: Exploration of hippocampal pathology including that in subpopulations of neurons defined by their content of immunoreactive calcium-binding proteins. Acta Neuropathol. 2000, 99, 547–554. [Google Scholar] [CrossRef]
- Maier, M.; Mellers, J.; Toone, B.; Trimble, M.; Ron, M.A. Schizophrenia, temporal lobe epilepsy and psychosis: An in vivo magnetic resonance spectroscopy and imaging study of the hippocampus/amygdala complex. Psychol. Med. 2000, 30, 571–581. [Google Scholar] [CrossRef]
- Shaw, P.; Mellers, J.; Henderson, M.; Polkey, C.; David, A.S.; Toone, B.K. Schizophrenia-like psychosis arising de novo following a temporal lobectomy: Timing and risk factors. J. Neurol. Neurosurg. Psychiatry 2004, 75, 1003–1008. [Google Scholar] [CrossRef]
- Calle-López, Y.; Ladino, L.D.; Benjumea-Cuartas, V.; Castrillón-Velilla, D.M.; Téllez-Zenteno, J.F.; Wolf, P. Forced normalization: A systematic review. Epilepsia 2019, 60, 1610–1618. [Google Scholar] [CrossRef]
- Walker, M.C. Hippocampal Sclerosis: Causes and Prevention. Semin. Neurol. 2015, 35, 193–200. [Google Scholar] [CrossRef]
- Zhong, Q.; Ren, B.-X.; Tang, F.-R. Neurogenesis in the Hippocampus of Patients with Temporal Lobe Epilepsy. Curr. Neurol. Neurosci. Rep. 2016, 16, 20. [Google Scholar] [CrossRef]
- Parent, J.M.; Timothy, W.Y.; Leibowitz, R.T.; Geschwind, D.H.; Sloviter, R.S.; Lowenstein, D.H. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997, 17, 3727–3738. [Google Scholar] [CrossRef]
- da Fonseca, N.C.; Joaquim, H.P.; Talib, L.L.; de Vincentiis, S.; Gattaz, W.F.; Valente, K.D. Hippocampal serotonin depletion is related to the presence of generalized tonic-clonic seizures, but not to psychiatric disorders in patients with temporal lobe epilepsy. Epilepsy Res. 2015, 111, 18–25. [Google Scholar] [CrossRef]
- Zis, A.P.; Nomikos, G.G.; E Brown, E.; Damsma, G.; Fibiger, H.C. Neurochemical effects of electrically and chemically induced seizures: An in vivo microdialysis study in the rat hippocampus. Neuropsychopharmacology 1992, 7, 189–195. [Google Scholar]
- Guiard, B.P.; Di Giovanni, G. Central serotonin-2A (5-HT2A) receptor dysfunction in depression and epilepsy: The missing link? Front. Pharmacol. 2015, 6, 46. [Google Scholar] [CrossRef]
- Li, B.; Wang, L.; Sun, Z.; Zhou, Y.; Shao, D.; Zhao, J.; Song, Y.; Lv, J.; Dong, X.; Liu, C.; et al. The anticonvulsant effects of SR 57227 on pentylenetetrazole-induced seizure in mice. PLoS ONE 2014, 9, e93158. [Google Scholar] [CrossRef]
- Yan, Q.S.; Mishra, P.K.; Burger, R.L.; Bettendorf, A.F.; Jobe, P.C.; Dailey, J.W. Evidence that carbamazepine and antiepilepsirine may produce a component of their anticonvulsant effects by activating serotonergic neurons in genetically epilepsy-prone rats. J. Pharmacol. Exp. Ther. 1992, 261, 652–659. [Google Scholar] [CrossRef]
- Dailey, J.W.; Seo, D.O.; Yan, Q.-S.; Ko, K.H.; Jo, M.; Jobe, P.C. The anticonvulsant effect of the broad spectrum anticonvulsant loreclezole may be mediated in part by serotonin in rats: A microdialysis study. Neurosci. Lett. 1994, 178, 179–183. [Google Scholar] [CrossRef]
- Ben Menachem, E.; Persson, L.I.; Schechter, P.J.; Haegele, K.D.; Huebert, N.; Hardenberg, J.; Dahlgren, L.; Mumford, J.P. Effects of single doses of vigabatrin on CSF concentrations of GABA, homocarnosine, homovanillic acid and 5-hydroxyindoleacetic acid in patients with complex partial epilepsy. Epilepsy Res. 1988, 2, 96–101. [Google Scholar] [CrossRef]
- Nyakas, C.; Oosterink, B.; Keijser, J.; Felszeghy, K.; de Jong, G.; Korf, J.; Luiten, P. Selective decline of 5-HT1A receptor binding sites in rat cortex, hippocampus and cholinergic basal forebrain nuclei during aging. J. Chem. Neuroanat. 1997, 13, 53–61. [Google Scholar] [CrossRef]
- Meltzer, C.C.; Smith, G.; Price, J.C.; Reynolds, C.F.; Mathis, C.A.; Greer, P.; Lopresti, B.; Mintun, M.A.; Pollock, B.G.; Ben-Eliezer, D.; et al. Reduced binding of [18F]altanserin to serotonin type 2A receptors in aging: Persistence of effect after partial volume correction. Brain Res. 1998, 813, 167–171. [Google Scholar] [CrossRef]
- Buckmaster, P.S.; Reyes, B.; Kahn, T.; Wyeth, M. Ventral Hippocampal Formation Is the Primary Epileptogenic Zone in a Rat Model of Temporal Lobe Epilepsy. J. Neurosci. 2022, 42, 7482–7495. [Google Scholar] [CrossRef]
- Akaike, K.; Tanaka, S.; Tojo, H.; Fukumoto, S.-I.; Imamura, S.-I.; Takigawa, M. Kainic acid-induced dorsal and ventral hippocampal seizures in rats. Brain Res. 2001, 900, 65–71. [Google Scholar] [CrossRef]
- Häussler, U.; Bielefeld, L.; Froriep, U.P.; Wolfart, J.; Haas, C.A. Septotemporal position in the hippocampal formation determines epileptic and neurogenic activity in temporal lobe epilepsy. Cereb. Cortex 2012, 22, 26–36. [Google Scholar] [CrossRef]
- Racine, R.; Rose, P.A.; Burnham, W.M. Afterdischarge thresholds and kindling rates in dorsal and ventral hippocampus and dentate gyrus. Can. J. Neurol. Sci. 1977, 4, 273–278. [Google Scholar] [CrossRef]
- Lee, P.H.; Xie, C.W.; Lewis, D.V.; A Wilson, W.; Mitchell, C.L.; Hong, J.S. Opioid-induced epileptiform bursting in hippocampal slices: Higher susceptibility in ventral than dorsal hippocampus. J. Pharmacol. Exp. Ther. 1990, 253, 545–551. [Google Scholar] [CrossRef]
- Papatheodoropoulos, C. Higher intrinsic network excitability in ventral compared with the dorsal hippocampus is controlled less effectively by GABAB receptors. BMC Neurosci. 2015, 16, 75. [Google Scholar] [CrossRef]
- Tseng, K.Y.; Chambers, R.A.; Lipska, B.K. The neonatal ventral hippocampal lesion as a heuristic neurodevelopmental model of schizophrenia. Behav. Brain Res. 2009, 204, 295–305. [Google Scholar] [CrossRef]
- Grace, A.A. Dopamine system dysregulation by the hippocampus: Implications for the pathophysiology and treatment of schizophrenia. Neuropharmacology 2012, 62, 1342–1348. [Google Scholar] [CrossRef]
- Perez, M.M.; Trimble, M.R. Epileptic psychosis-diagnostic comparison with process schizophrenia. Br. J. Psychiatry 1980, 137, 245–249. [Google Scholar] [CrossRef]
- Ganzola, R.; Maziade, M.; Duchesne, S. Hippocampus and amygdala volumes in children and young adults at high-risk of schizophrenia: Research synthesis. Schizophr. Res. 2014, 156, 76–86. [Google Scholar] [CrossRef]
- Li, W.; Yang, Y.; Lin, J.; Wang, S.; Zhao, J.; Yang, G.; Wang, X.; Ding, M.; Zhang, H.; Lv, L. Association of serotonin transporter gene (SLC6A4) polymorphisms with schizophrenia susceptibility and symptoms in a Chinese-Han population. Prog. Neuro-Psychopharmacology Biol. Psychiatry 2013, 44, 290–295. [Google Scholar] [CrossRef]
- Kiemes, A.; Gomes, F.V.; Cash, D.; Uliana, D.L.; Simmons, C.; Singh, N.; Vernon, A.C.; Turkheimer, F.; Davies, C.; Stone, J.M.; et al. GABA(A) and NMDA receptor density alterations and their behavioral correlates in the gestational methylazoxymethanol acetate model for schizophrenia. Neuropsychopharmacology 2022, 47, 687–695. [Google Scholar] [CrossRef]
- Li, J.; Cao, D.; Dimakopoulos, V.; Shi, W.; Yu, S.; Fan, L.; Stieglitz, L.; Imbach, L.; Sarnthein, J.; Jiang, T. Anterior-Posterior Hippocampal Dynamics Support Working Memory Processing. J. Neurosci. 2021, 42, 443–453. [Google Scholar] [CrossRef]
- Lodge, D.J.; Grace, A.A. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J. Neurosci. 2007, 27, 11424–11430. [Google Scholar] [CrossRef]
- Kätzel, D.; Wolff, A.R.; Bygrave, A.M.; Bannerman, D.M. Hippocampal Hyperactivity as a Druggable Circuit-Level Origin of Aberrant Salience in Schizophrenia. Front. Pharmacol. 2020, 11, 486811. [Google Scholar] [CrossRef]
- Wolff, A.R.; Bygrave, A.M.; Sanderson, D.J.; Boyden, E.S.; Bannerman, D.M.; Kullmann, D.M.; Kätzel, D. Optogenetic induction of the schizophrenia-related endophenotype of ventral hippocampal hyperactivity causes rodent correlates of positive and cognitive symptoms. Sci. Rep. 2018, 8, 12871. [Google Scholar] [CrossRef]
- Pourhamzeh, M.; Moravej, F.G.; Arabi, M.; Shahriari, E.; Mehrabi, S.; Ward, R.; Ahadi, R.; Joghataei, M.T. The Roles of Serotonin in Neuropsychiatric Disorders. Cell. Mol. Neurobiol. 2022, 42, 1671–1692. [Google Scholar] [CrossRef]
- Kranz, G.; Kasper, S.; Lanzenberger, R. Reward and the serotonergic system. Neuroscience 2010, 166, 1023–1035. [Google Scholar] [CrossRef]
- Anacker, C.; Luna, V.M.; Stevens, G.S.; Millette, A.; Shores, R.; Jimenez, J.C.; Chen, B.; Hen, R. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 2018, 559, 98–102. [Google Scholar] [CrossRef]
- Muir, J.; Tse, Y.C.; Iyer, E.S.; Biris, J.; Cvetkovska, V.; Lopez, J.; Bagot, R.C. Ventral Hippocampal Afferents to Nucleus Accumbens Encode Both Latent Vulnerability and Stress-Induced Susceptibility. Biol. Psychiatry 2020, 88, 843–854. [Google Scholar] [CrossRef]
- Knight, S.; McCutcheon, R.; Dwir, D.; Grace, A.A.; O’Daly, O.; McGuire, P.; Modinos, G. Hippocampal circuit dysfunction in psychosis. Transl. Psychiatry 2022, 12, 344. [Google Scholar] [CrossRef]
- Van den Hove, D.L.; Lauder, J.M.; Scheepens, A.; Prickaerts, J.; Blanco, C.E.; Steinbusch, H.W. Prenatal stress in the rat alters 5-HT1A receptor binding in the ventral hippocampus. Brain Res. 2006, 1090, 29–34. [Google Scholar] [CrossRef]
- Rinaldi, R.; Lefebvre, L. Goal-directed behaviors in patients with schizophrenia: Concept relevance and updated model. Psychiatry Clin. Neurosci. 2016, 70, 394–404. [Google Scholar] [CrossRef]
- Yoshida, K.; Drew, M.R.; Mimura, M.; Tanaka, K.F. Serotonin-mediated inhibition of ventral hippocampus is required for sustained goal-directed behavior. Nat. Neurosci. 2019, 22, 770–777. [Google Scholar] [CrossRef]
- Zoratto, F.; Tringle, A.L.; Bellenchi, G.; Speranza, L.; Travaglini, D.; di Porzio, U.; Perrone-Capano, C.; Laviola, G.; Dreyer, J.-L.; Adriani, W. Impulsivity and home-cage activity are decreased by lentivirus-mediated silencing of serotonin transporter in the rat hippocampus. Neurosci. Lett. 2013, 548, 38–43. [Google Scholar] [CrossRef]
- Risold, P.Y.; Swanson, L.W. Structural evidence for functional domains in the rat hippocampus. Science 1996, 272, 1484–1486. [Google Scholar] [CrossRef]
- Poppenk, J.; Evensmoen, H.R.; Moscovitch, M.; Nadel, L. Long-axis specialization of the human hippocampus. Trends Cogn. Sci. 2013, 17, 230–240. [Google Scholar] [CrossRef]
- Cembrowski, M.S.; Spruston, N. Heterogeneity within classical cell types is the rule: Lessons from hippocampal pyramidal neurons. Nat. Rev. Neurosci. 2019, 20, 193–204. [Google Scholar] [CrossRef]
- van Strien, N.M.; Cappaert, N.L.M.; Witter, M.P. The anatomy of memory: An interactive overview of the parahippocampal–hippocampal network. Nat. Rev. Neurosci. 2009, 10, 272–282. [Google Scholar] [CrossRef]
- Pitkänen, A.; Pikkarainen, M.; Nurminen, N.; Ylinen, A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat: A review. Ann. New York Acad. Sci. 2000, 911, 369–391. [Google Scholar] [CrossRef]
- Jin, J.; Maren, S. Prefrontal-Hippocampal Interactions in Memory and Emotion. Front. Syst. Neurosci. 2015, 9, 170. [Google Scholar] [CrossRef]
- Edwards, K.A.; Zup, S.L. Serotonin Pretreatment Abolishes Sex-specific NMDA-induced Seizure Behavior in Developing Rats. Neuroscience 2021, 463, 184–196. [Google Scholar] [CrossRef]
- Wu, Y.C.; Hill, R.A.; Klug, M.; Buuse, M.v.D. Sex-specific and region-specific changes in BDNF–TrkB signalling in the hippocampus of 5-HT1A receptor and BDNF single and double mutant mice. Brain Res. 2012, 1452, 10–17. [Google Scholar] [CrossRef]
- Boulle, F.; Pawluski, J.L.; Homberg, J.R.; Machiels, B.; Kroeze, Y.; Kumar, N.; Steinbusch, H.W.M.; Kenis, G.; Hove, D.L.A.V.D. Prenatal stress and early-life exposure to fluoxetine have enduring effects on anxiety and hippocampal BDNF gene expression in adult male offspring. Dev. Psychobiol. 2016, 58, 427–438. [Google Scholar] [CrossRef]
- Maher, R.; Moreno-Borrallo, A.; Jindal, D.; Mai, B.T.; Ruiz-Hernandez, E.; Harkin, A. Intranasal Polymeric and Lipid-Based Nanocarriers for CNS Drug Delivery. Pharmaceutics 2023, 15, 746. [Google Scholar] [CrossRef]
- Ohmura, Y.; Tanaka, K.F.; Tsunematsu, T.; Yamanaka, A.; Yoshioka, M. Optogenetic activation of serotonergic neurons enhances anxiety-like behaviour in mice. Int. J. Neuropsychopharmacol. 2014, 17, 1777–1783. [Google Scholar] [CrossRef]
- Ozawa, A.; Arakawa, H. Chemogenetics drives paradigm change in the investigation of behavioral circuits and neural mechanisms underlying drug action. Behav. Brain Res. 2021, 406, 113234. [Google Scholar] [CrossRef]
- Patrono, E.; Hrůzova, K.; Svoboda, J.; Stuchlík, A. The role of optogenetic stimulations of parvalbumin-positive interneurons in the prefrontal cortex and the ventral hippocampus on an acute MK-801 model of schizophrenia-like cognitive inflexibility. Schizophr. Res. 2023, 252, 198–205. [Google Scholar] [CrossRef]
- Bifone, A.; Gozzi, A. Neuromapping techniques in drug discovery: Pharmacological MRI for the assessment of novel antipsychotics. Expert Opin. Drug Discov. 2012, 7, 1071–1082. [Google Scholar] [CrossRef]
- Minzenberg, M.J. Pharmacological MRI approaches to understanding mechanisms of drug action. Curr. Top. Behav. Neurosci. 2012, 11, 365–388. [Google Scholar] [CrossRef]
- Seo, Y.; Chang, K.W.; Lee, J.; Kong, C.; Shin, J.; Chang, J.W.; Na, Y.C.; Chang, W.S. Optimal timing for drug delivery into the hippocampus by focused ultrasound: A comparison of hydrophilic and lipophilic compounds. Heliyon 2024, 10, e29480. [Google Scholar] [CrossRef]
- Cohen, H.; Kaplan, Z.; Kozlovsky, N.; Gidron, Y.; Matar, M.A.; Zohar, J. Hippocampal microinfusion of oxytocin attenuates the behavioural response to stress by means of dynamic interplay with the glucocorticoid-catecholamine responses. J. Neuroendocr. 2010, 22, 889–904. [Google Scholar] [CrossRef]
- Rezai, A.R.; Ranjan, M.; D’hAese, P.-F.; Haut, M.W.; Carpenter, J.; Najib, U.; Mehta, R.I.; Chazen, J.L.; Zibly, Z.; Yates, J.R.; et al. Noninvasive hippocampal blood−brain barrier opening in Alzheimer’s disease with focused ultrasound. Proc. Natl. Acad. Sci. USA 2020, 117, 9180–9182. [Google Scholar] [CrossRef]
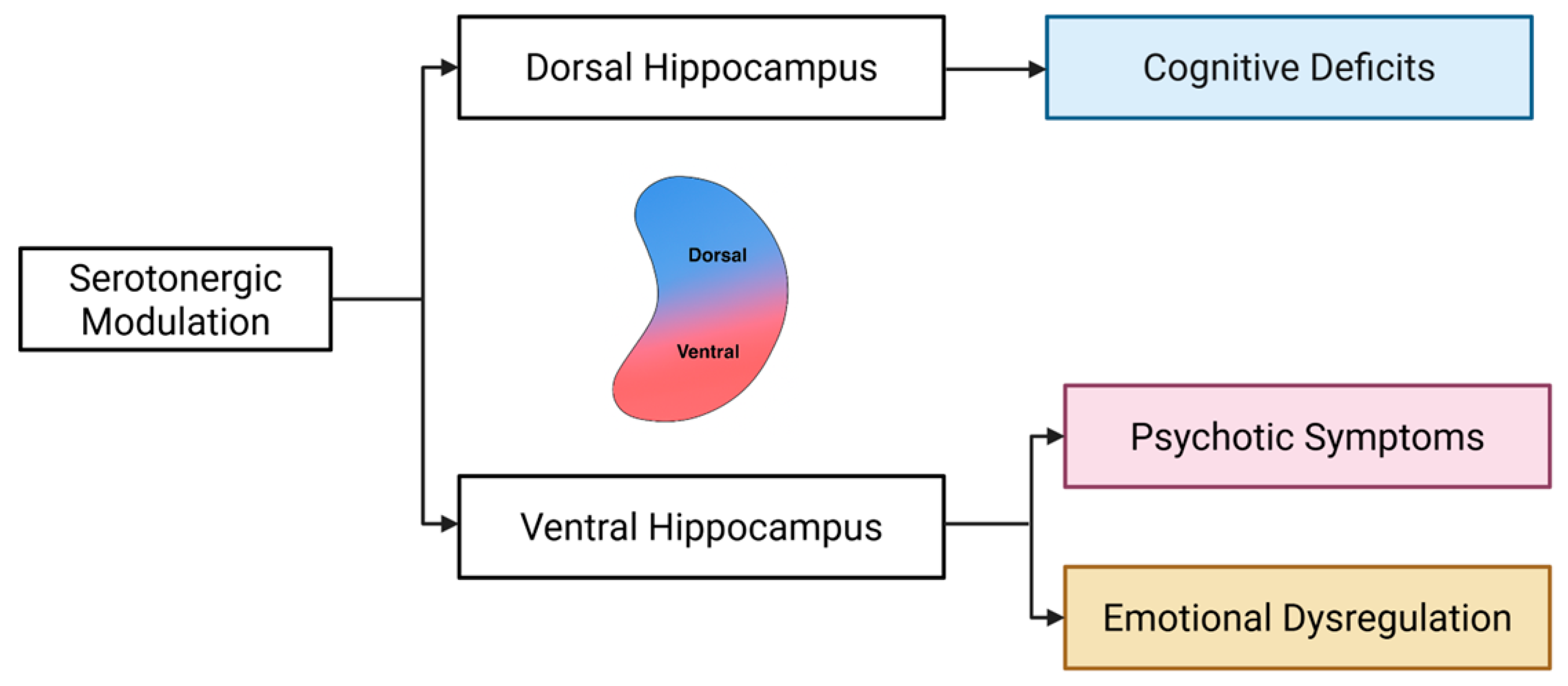
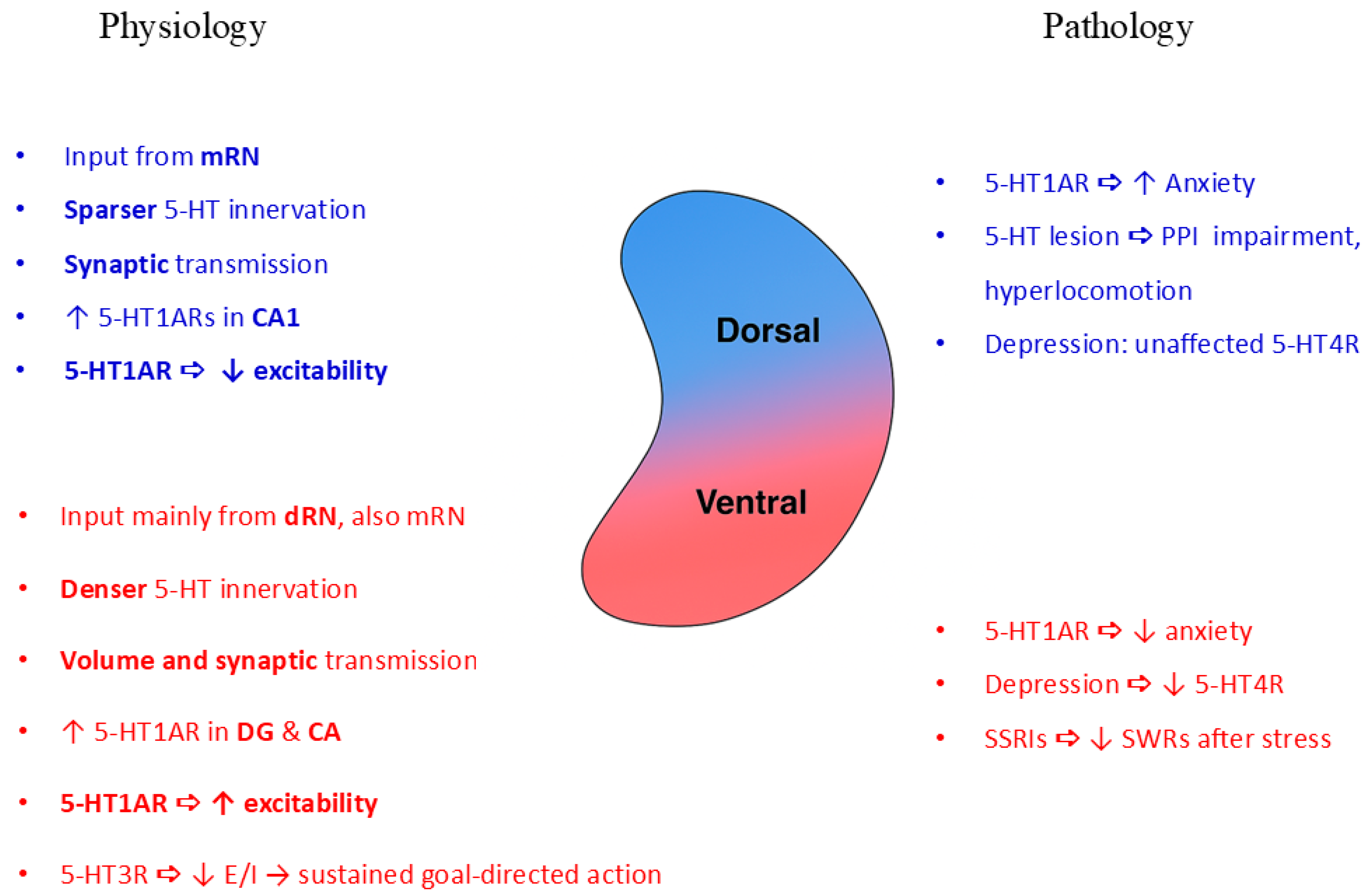

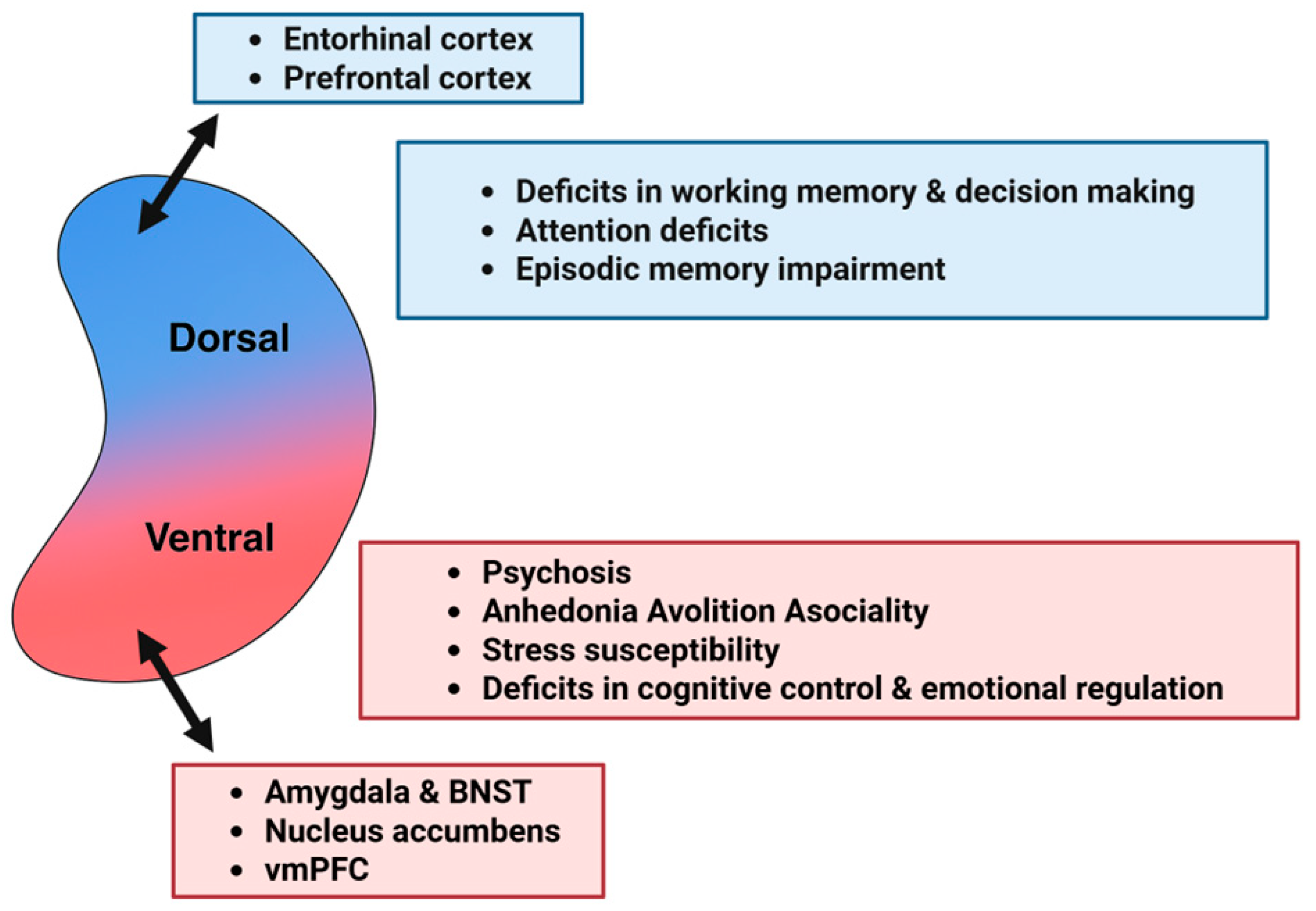
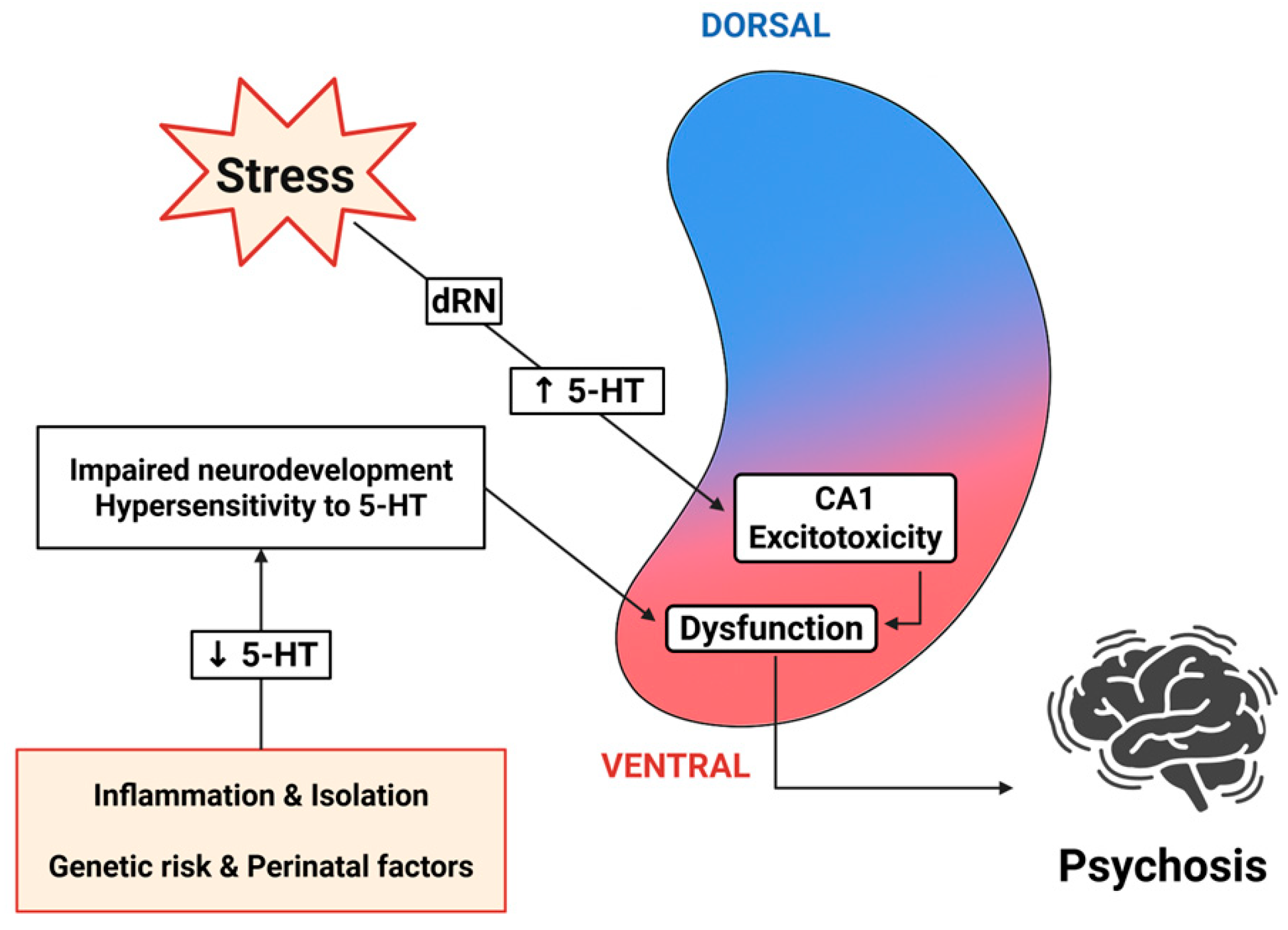
| Serotonin | Dopamine | Acetylcholine | Noradrenaline | |
|---|---|---|---|---|
| 5-HT1AR | ↓ (receptors in raphe nuclei) | ↑ | ↓ | ↑ |
| 5-HT1BR | ↓ | Unknown | ↓ | Unknown |
| 5-HT2R | ↓ | ↓ | ↓, ↑ | ↓ |
| 5-HT3R | ↑ | Unknown | ↑, ↓ | ↑, ↓ |
| 5-HT4R | ↑ | Unknown | ↑ | Unknown |
| 5-HT5R | Unknown | Unknown | Unknown | Unknown |
| 5-HT6R | Unknown | ↑, ↓ | ↓ | ↑ |
| 5-HT7R | ↑ | ↑ | Unknown | ↑ |
| Receptor | Regional Localization | Cellular Expression | Molecular Targets | Physiological Roles | References |
|---|---|---|---|---|---|
| 5-HT1A/B | DG, CA3, CA1 | PN and IN | Gi/o, GIRK, Ca2+ channels, NMDA, AMPA, MAPK-ERK | Neuroprotection, neurogenesis, emotion, cognition | [74,75,76,77,78,134,135,136,137,138,139,140,141,142] |
| 5-HT2A/C | DG, CA3, CA1 | PN and IN | Gq/11, PLC- PIP3-DAG, K+ channels, PLA2-AA, ERK, PDZ, BDNF | Neuroprotection, mood, anxiety, learning, memory | [85,86,87,88,89,90,91,143,144,145,146,147,148,149,150,151] |
| 5-HT3 | DG, CA3, CA1 | IN | Cation channel (Na+, K+, Ca2+), IGF-1 | Neuroprotection, neurogenesis, fear extinction, working memory, learning | [65,92,93,94,95,96,97,152,153,154,155,156,157,158,159] |
| 5-HT4 | DG, CA3 | PN | Gs, PKA-AC-cAMP, K+ channels, PDE, β-catenin, CREB, BDNF, AKT | Neuroprotection, emotion, cognition | [62,98,99,100,101,102,103,104,105,160,161,162,163,164,165,166,167,168,169] |
| 5-HT5 | CA1 | PN | Gi/o, GIRK | (No studies in the hippocampus) | [9,106,107,108,109,110,111,112,170,171,172,173,174,175] |
| 5-HT6 | DG, CA3, CA1 | PN and IN | Gs, PKA-AC-cAMP, ERK1/2, BDNF | Mood, anxiety, memory consolidation | [113,114,115,116,117,118,119,120,121,176,177,178,179,180,181,182,183,184,185] |
| 5-HT7 | DG, CA3, CA1 | PN | Gs, PKA-AC-cAMP, TrkB, AMPA, NMDA, CREB, 5-HT1A | Neuroprotection, emotional learning, stress regulation, cognition | [79,80,81,82,83,84,111,176,186,187,188,189,190,191,192,193,194,195,196,197] |
| Receptor | Dorsal Hippocampus | Ventral Hippocampus | Main Expression | Principal Circuit Actions | Symptoms Linked to Schizophrenia | Therapeutics (Potential/Existing) |
|---|---|---|---|---|---|---|
| 5-HT1A | High (CA1, DG); extrasynaptic | Moderate (CA3, DG) | PNs, INs | Inhibitory role in dorsal hippocampus; excitatory role in ventral hippocampus | Cognitive deficits (dorsal hippocampus); positive symptoms (ventral hippocampus) | Antagonists (cognitive rescue); partial agonists |
| 5-HT2A | Moderate | Moderate–High (CA3, CA1) | PNs, INs | ↑ Glutamate ↑ GABA | Psychosis, emotional dysregulation | Antagonists (atypical antipsychotics), inverse agonists |
| 5-HT2C | High (Str. oriens/radiatum CA1) | High (CA3) | PNs, INs | Similar to 5-HT2A | Mood/anxiety symptoms, sensorimotor gating | Agonists/antagonists (experimental) |
| 5-HT3 | Moderate (INs) | Moderate | INs; PNs (in humans) | Fast excitation of INs | Working memory, negative symptoms | Antagonists (ondansetron, tropisetron) |
| 5-HT4 | Moderate (CA3, DG) | Moderate | PNs | Excitatory ↑ LTP, ↑ ACh | Cognitive, emotional, neurogenic roles | Agonists/antagonists (early clinical stage) |
| 5-HT5A/B | Low–Moderate (DG, CA1, CA3) | Low–Moderate | PNs, INs | Unknown/potentially inhibitory | Cognition, social behavior | Blockers (preclinical, memory/social rescue) |
| 5-HT6 | Moderate (DG, CA1, INs) | Moderate | PNs, CCK+ INs | Inhibitory; modulate LTP | Memory, mood, negative symptoms | Antagonists (SB-742457, clinical trials) |
| 5-HT7 | CA3 > CA1 > DG | Moderate | PNs | Excitatory | Cognitive flexibility, stress | Agonists/antagonists (experimental) |
| LTP/LTD | Theta Rhythm | Gamma Rhythm | SWRs | E/I | |
|---|---|---|---|---|---|
| 5-HT1R | ↓ CA1 [7] ↑ DG [280] | ↓ [8] | ↓ [8,314,324] | ↑ sharp waves [348] ↓ ripples [347] | ↓ dorsal hippocampus ↑ ventral hippocampus |
| 5-HT2R | ↑ CA1 [281,282] | ↑ 5-HT2A, [8] ↓ 5-HT2C [315] | ↑ [325] ↑ 5-HT2A [8] | ↓ sharp waves [348] | ↑ |
| 5-HT3R | ↓ CA1, [283,284] ↓ CA3 [285,286] | ↓ [283,284,316] | ↓ [316,326,327] ↑ 5-HT3 + 5-HT6 [328] | ↓ ripples [347] no effect on sharp waves [348] | ↓ |
| 5-HT4R | ↑ DG, ↓ CA3 [244] | ↑ [324] | unknown | no effect on sharp waves [348] | ↑ |
| 5-HT5R | unknown | unknown | unknown | unknown | |
| 5-HT6R | ↓ CA1 [290] ↑ DG [289] | ↓ [318,319] | ↓ [319] ↑ 5-HT3 + 5-HT6 [328] | unknown | ↓ |
| 5-HT7R | ↑ CA1 [291] ↑ DG [292] | unknown | unknown | unknown | ↑ |
| Finding | Condition | Relevance to Schizophrenia | References | |
|---|---|---|---|---|
| 5-HT levels | Reduced | Neuroinflammation, social isolation, STOP mutation, hyperdopaminergic state | Risk factors | [371,372,373,374] |
| TPH2 | Increased | Maternal inflammation | Risk factors | [376] |
| Decreased | STOP mutation | [373] | ||
| 5-HT1A | Increased | Isolation rearing, prenatal infection, MK-801 administration, postmortem human studies | Translational importance | [10,374,388,393] |
| 5-HT1B | Increased | Postmortem human studies | Human findings | [388] |
| 5-HT2A | Increased | Postmortem human studies | Human findings | [388] |
| Decreased | [384,393] | |||
| 5-HT4 | Reduced | Animal model for anhedonia | Negative symptoms | [11] |
| 5-HT6 | Reduced | Postmortem human studies | Human findings | [403] |
| Receptor Manipulation | Therapeutic Effect | Relevance to Schizophrenia | References |
|---|---|---|---|
| 5-HT1A blockage | Restores PPI, hyperlocomotion, working memory performance | Positive and cognitive symptoms | [137,138,389] |
| 5-HT2A blockage/partial agonism | Restoration of frontoseptohippocampal circuit activity, reverses PPI deficits | Positive and cognitive symptoms | [394,395] |
| 5-HT3 blockage | Antipsychotic actions, improvement of learning and working memory | Positive and cognitive symptoms | [65,153,399,401] |
| 5-HT5 modulators | Procognitive effects, emotional regulation | Cognitive and negative symptoms | [253] |
| 5-HT6 blockage | Procognitive, anxiolytic, antidepressant, and antiepileptic effects | Cognitive and negative symptoms | [113,176,177,178,184,185,408,409] |
| 5-HT7 blockage | Procognitive effects | Cognitive symptoms | [411] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kandilakis, C.L.; Papatheodoropoulos, C. Serotonin Modulation of Dorsoventral Hippocampus in Physiology and Schizophrenia. Int. J. Mol. Sci. 2025, 26, 7253. https://doi.org/10.3390/ijms26157253
Kandilakis CL, Papatheodoropoulos C. Serotonin Modulation of Dorsoventral Hippocampus in Physiology and Schizophrenia. International Journal of Molecular Sciences. 2025; 26(15):7253. https://doi.org/10.3390/ijms26157253
Chicago/Turabian StyleKandilakis, Charalampos L., and Costas Papatheodoropoulos. 2025. "Serotonin Modulation of Dorsoventral Hippocampus in Physiology and Schizophrenia" International Journal of Molecular Sciences 26, no. 15: 7253. https://doi.org/10.3390/ijms26157253
APA StyleKandilakis, C. L., & Papatheodoropoulos, C. (2025). Serotonin Modulation of Dorsoventral Hippocampus in Physiology and Schizophrenia. International Journal of Molecular Sciences, 26(15), 7253. https://doi.org/10.3390/ijms26157253







