Abstract
Natural products demonstrate potent immunomodulatory properties through checkpoint modulation, macrophage polarization, and T cell/natural killer (NK) cell activation. While cancer organoid-immune co-culture platforms enable physiologically relevant modeling of tumor–immune interactions, systematic investigation of natural product immunomodulation in these systems remains entirely unexplored. We conducted a comprehensive literature analysis examining natural products tested in cancer organoids, immunomodulatory mechanisms from traditional models, technical advances in organoid-immune co-cultures, and standardization requirements for clinical translation. Our analysis reveals a critical research gap: no published studies have investigated natural product-mediated immunomodulation using organoid-immune co-culture systems. Even though compounds like curcumin, resveratrol, and medicinal mushroom polysaccharides show extensive immunomodulatory effects in two-dimensional (2D) cultures, and organoid technology achieves high clinical correlation for drug response prediction, all existing organoid studies focus exclusively on direct cytotoxicity. Technical challenges include compound stability, limited matrix penetration requiring substantially higher concentrations than 2D cultures, and maintaining functional immune populations in three-dimensional (3D) systems. The convergence of validated organoid-immune co-culture platforms, Food and Drug Administration (FDA) regulatory support through the Modernization Act 2.0, and extensive natural product knowledge creates unprecedented opportunities. Priority research directions include systematic screening of immunomodulatory natural products in organoid-immune co-cultures, development of 3D-optimized delivery systems, and clinical validation trials. Success requires moving beyond cytotoxicity-focused studies to investigate immunomodulatory mechanisms in physiologically relevant 3D systems, potentially unlocking new precision cancer immunotherapy approaches.
1. Introduction
Cancer immunotherapy has revolutionized oncology treatment, yet its clinical success remains limited, with response rates reaching approximately 20% by 2023 []. This modest efficacy, combined with significant adverse effects and high costs, necessitates innovative approaches to enhance therapeutic outcomes. Natural products, a vital source of anti-cancer drugs, represent a promising yet underexplored avenue for immunomodulation. Compounds such as curcumin, resveratrol, and medicinal mushroom polysaccharides demonstrate potent immunomodulatory properties through multiple mechanisms including checkpoint modulation, macrophage polarization, and immunogenic cell death induction [,,,].
The clinical translation of these immunomodulatory natural products has been severely hampered by inadequate preclinical models. Traditional two-dimensional cell cultures fail to recapitulate tumor architecture and drug responses [] and are insufficient for modeling complex tumor immunobiology, lacking the cellular heterogeneity and three-dimensional structures essential for studying tumor–immune interactions [,]. Although animal models show poor predictive value for human toxicities in oncology trials [], the species-specific immune differences are particularly problematic—mice and humans differ fundamentally in Toll-like receptor (TLR) expression, cytokine responses, and immune cell subset balances [,]. These limitations create a significant translational gap, contributing to the high failure rate of promising compounds in clinical trials.
Cancer organoids—three-dimensional structures derived from patient tumors—offer a transformative solution. Recent breakthroughs have enabled the co-culture of tumor organoids with autologous immune cells, creating physiologically relevant models that preserve tumor heterogeneity and immune interactions. Pioneering work by Dijkstra et al. demonstrated the generation of tumor-reactive T cells through co-culture of peripheral blood lymphocytes (PBL) with patient-derived tumor organoids []. Neal et al. developed an air-liquid interface (ALI) method that maintains diverse immune populations including T cells, B cells, natural killer (NK) cells, and macrophages while preserving native T-cell receptor repertoires []. These platforms achieve remarkable clinical correlation, with Votanopoulos et al. demonstrating strong concordance between organoid responses and patient outcomes in a subset of 15 metastatic cancer patients []. Larger studies in gastrointestinal cancers by Vlachogiannis et al. reported 100% sensitivity and 93% specificity for predicting patient treatment responses across 71 patients [].
Although these technological advances, comprehensive exploration of natural product immunomodulation using organoid-immune co-culture systems remains in its infancy. While studies have demonstrated direct cytotoxic effects of natural compounds like curcumin [] and resveratrol [], the vast majority of existing work either uses simple organoid monocultures or focuses solely on direct anti-cancer effects, with organoid-immune co-culture investigations of natural products remaining absent from the literature. Notable examples include demonstrations of curcumin efficacy in patient-derived colorectal cancer organoids [] and resveratrol effects in breast cancer organoids [], yet thorough examination of immunomodulatory mechanisms—particularly involving immune cell co-cultures—is yet to be pursued.
The intersection of these fields is particularly timely given regulatory changes in 2022. The Food and Drug Administration (FDA) Modernization Act 2.0 now permits the utilization of organoid models as alternatives to animal testing, encouraging sponsors to advance these technologies []. This regulatory shift, combined with diverse opportunities for organoid-based investigations in precision medicine and immunotherapy development, creates significant potential for advancing cancer treatment [].
To our knowledge, no published studies have comprehensively studied natural product-mediated immunomodulation using organoid-immune co-culture systems. Although numerous studies have tested natural compounds in cancer organoids for cytotoxicity and extensive literature exist on immunomodulatory mechanisms in traditional two-dimensional (2D) cultures, the key intersection—evaluating how natural products modulate tumor–immune interactions in three-dimensional (3D) organoid co-cultures— has not been investigated.
This review comprehensively examines the current state and future potential of immunomodulatory natural products in cancer organoid models. We analyze (1) the limited but promising evidence of natural products tested in organoid systems, (2) methodological advances enabling organoid-immune co-culture studies, (3) insights from traditional immunomodulation research to identify promising candidates for organoid investigation, and (4) technical challenges and standardization requirements for clinical translation. By bridging these previously disparate fields, we aim to establish a framework for leveraging organoid technology to unlock the immunotherapeutic potential of natural products in precision oncology (Figure 1).
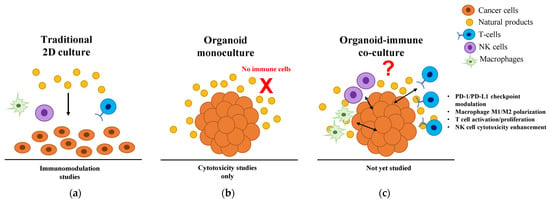
Figure 1.
Current landscape of natural product research across different cancer model systems. (a) Traditional 2D culture systems enable immunomodulation studies but lack physiological relevance due to artificial cell arrangements and uniform drug distribution. (b) Current 3D organoid studies focus exclusively on direct cytotoxicity, with natural products tested only in monocultures lacking immune components (indicated by red X). (c) The proposed organoid-immune co-culture system would enable comprehensive evaluation of immunomodulatory mechanisms, including PD-1/PD-L1 checkpoint modulation, macrophage M1/M2 polarization, T cell activation/proliferation, and NK cell cytotoxicity enhancement. Even with the availability of validated co-culture technologies, no published studies have investigated natural product-mediated immunomodulation in these physiologically relevant 3D systems. 2D, two-dimensional; 3D, three-dimensional; M1, classically activated macrophage; M2, alternatively activated macrophage; NK, natural killer; PD-1, programmed cell death protein 1; PD-L1, programmed death-ligand 1.
2. Background and Current Landscape
2.1. Fundamentals of Cancer Immunomodulation Pathways
The tumor microenvironment contains diverse immune cell populations whose functional states fundamentally determine therapeutic outcomes. Understanding these pathways is essential for developing effective immunomodulatory natural products.
CD8+ cytotoxic T-lymphocytes (CTLs) represent the primary effector cells for tumor elimination, but their function is often impaired through checkpoint molecules. The programmed cell death protein (PD)-1/PD-L1 axis serves as the dominant checkpoint, with PD-L1 expression on tumor cells binding to PD-1 on T cells to induce exhaustion characterized by reduced proliferation, cytokine production, and cytotoxicity [,,]. Cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) provides an earlier checkpoint by competing with CD28 for B7 ligands, preventing T cell activation [,]. Beyond exhaustion, T cells exist in multiple states including naive (CD45RA+CCR7+), central memory (CD45RO+CCR7+), effector memory (CD45RO+CCR7−), and terminally differentiated effector (CD45RA+CCR7−) populations, each with distinct capacities for tumor control [,,].
Tumor-associated macrophages (TAMs) exhibit remarkable plasticity along the classically activated macrophages (M1)-alternatively activated macrophages (M2) polarization spectrum. M1, induced by interferon-gamma (IFN-γ) and lipopolysaccharide (LPS), express high levels of inducible nitric oxide synthase (iNOS), interleukin (IL)-12, and tumor necrosis factor-alpha (TNF-α), promoting anti-tumor immunity through direct cytotoxicity and T cell activation [,,]. Conversely, M2, induced by IL-4/IL-13, express arginase-1, IL-10, and transforming growth factor-beta (TGF-β), supporting tumor growth through immunosuppression, angiogenesis, and tissue remodeling [,]. Yet, this binary classification oversimplifies TAM heterogeneity, as single-cell analyses reveal multiple intermediate states with mixed phenotypes that dynamically respond to microenvironmental cues [,,].
NK cells provide rapid, antigen-independent tumor surveillance through activating receptors (NKG2D, NKp46, NKp30, NKp44) that recognize stress ligands on malignant cells [,]. NK cell cytotoxicity involves perforin/granzyme-mediated apoptosis and death receptor engagement (tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), Fas ligand (FasL)) while activated NK cells produce IFN-γ to enhance adaptive immunity [,,]. Nevertheless, tumors evade NK surveillance through ligand shedding, immunosuppressive cytokines (TGF-β, IL-10), and metabolic reprogramming that impairs NK cell fitness [,,,].
The tumor cytokine milieu orchestrates immune responses through complex feedback loops. Pro-inflammatory cytokines including IL-2, IL-12, and IFN-γ promote anti-tumor immunity by enhancing T cell proliferation, Th1 differentiation, and CTL function [,,]. Conversely, immunosuppressive cytokines such as TGF-β, IL-10, and vascular endothelial growth factor (VEGF) create tolerogenic environments by inducing regulatory T cells (Tregs), myeloid-derived suppressor cells (MDSCs), and M2 macrophages [,,]. IL-6 demonstrates context-dependent effects, promoting both inflammation and tumor progression through signal transducer and activator of transcription 3 (STAT3) signaling [,,,].
These interconnected pathways provide multiple targets for natural product intervention. Successful immunomodulation requires simultaneous engagement of multiple mechanisms—checkpoint inhibition alone may fail without concurrent macrophage reprogramming or cytokine modulation. Organoid-immune co-culture systems uniquely enable investigation of these complex interactions in physiologically relevant contexts, as detailed in subsequent sections.
2.2. Natural Products as Cancer Immunomodulators: Mechanisms Relevant to Organoid Applications
Natural products exhibit immunomodulatory effects through distinct mechanisms that are particularly relevant for organoid-based investigation. Four major categories demonstrate differential activities suitable for 3D culture systems.
Polysaccharides (predominantly β-glucans from medicinal mushrooms) activate pattern recognition receptors on immune cells within the tumor microenvironment. Lentinan, approved in Japan since the 1980s, demonstrates immunomodulatory effects through TLR4/Dectin-1 and spleen tyrosine kinase (Syk)-protein kinase C (PKC)-nuclear factor kappa B (NFκB) signaling pathways []. Polysaccharide-K (PSK), approved in 1977, acts as a novel TLR2 agonist that mediates tumor inhibition via stimulation of CD8+ T cells and NK cells [,]. Notwithstanding their therapeutic potential, these high molecular weight compounds (400–800 kDa for lentinan; 90–100 kDa for PSK) face unique challenges in penetrating dense tumor matrices.
Polyphenols including curcumin, resveratrol, and epigallocatechin-3-gallate (EGCG) modulate multiple immunological pathways simultaneously. These compounds suppress PD-L1 expression through diverse mechanisms—curcumin via CSN5 inhibition leading to PD-L1 destabilization [] and STAT3 pathway blockade [], EGCG through JAK2/STAT1 inhibition [], and resveratrol by disrupting PD-L1 glycosylation and dimerization []. Though traditionally known for anti-inflammatory effects that promote M2 macrophage polarization [,], emerging evidence suggests context-dependent immunomodulation where certain formulations may enhance anti-tumor immunity [,]. This apparent paradox stems from an oversimplified M1/M2 dichotomy, as recent transcriptome-based network analysis of 299 macrophage samples demonstrates that macrophage activation exists along a spectrum with at least nine distinct activation programs rather than binary states []. In this context, polyphenols induce concentration-dependent and context-specific macrophage responses—for example, resveratrol demonstrates biphasic effects where moderate concentrations (20 μM) downregulate M2 markers (MRC1, CCL24, Chi3l3) while higher concentrations favor M2 polarization through phosphatidylinositol 3-kinase (PI3K)/Akt and AMP-activated protein kinase (AMPK) pathways []. This complexity, combined with evidence that M2-like tumor-associated macrophages can be reprogrammed to anti-tumor phenotypes [], highlights how polyphenol immunomodulation cannot be adequately studied in simplified 2D systems. Organoid-immune co-culture platforms offer the unique opportunity to investigate how tumor microenvironmental factors, drug concentration, and exposure timing determine whether polyphenol-induced macrophage modulation ultimately supports or inhibits tumor growth.
Complex extracts demonstrate immunomodulatory effects through their bioactive components. Panax ginseng berry polysaccharides (GBPP) enhance immune responses by activating macrophages and NK cells, achieving significant anti-cancer effects through cytokine production (IL-6, IL-12, and TNF-α) []. Ganoderma lucidum contains both polysaccharides and triterpenoids, with organic solvent extracts showing direct cytotoxicity through IL-6/IL-8 suppression [], commercial extracts inhibiting tumor growth via mechanistic target of rapamycin (mTOR) pathway modulation [], and multiple immunomodulatory mechanisms []. These findings align well with organoid platforms that enable systematic evaluation of immune-tumor interactions [].
The focus on key immunological targets—checkpoint molecules, cytokine networks, macrophage polarization, and T cell/NK cell activation—provides clear endpoints for organoid-based assessment [,]. Importantly, these mechanisms require intact tumor–immune interactions that only 3D systems can adequately model, as demonstrated in patient-derived organoid (PDO) co-culture studies achieving 33–50% success rates for generating tumor-reactive T cells []. The diverse anti-tumor mechanisms of natural products—encompassing direct cytotoxicity, immunomodulation, and tumor microenvironment regulation—underscore their therapeutic potential that demands validation in physiologically relevant 3D systems (Figure 2).
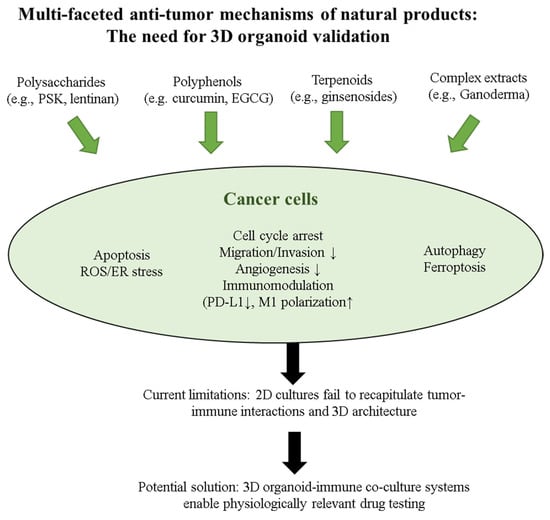
Figure 2.
Multi-faceted anti-tumor mechanisms of natural products requiring validation in 3D organoid-immune co-culture systems. Natural products from four major categories (polysaccharides, polyphenols, terpenoids, and complex extracts) exhibit diverse anti-tumor activities through multiple mechanisms: direct cytotoxicity (apoptosis, cell cycle arrest, autophagy, ferroptosis), oxidative stress induction (ROS/ER stress), immunomodulation (PD-L1 downregulation, M1 macrophage polarization), and tumor microenvironment regulation (migration/invasion and angiogenesis inhibition). While these mechanisms have been extensively characterized in 2D cultures, current models fail to recapitulate tumor–immune interactions and 3D architecture. The implementation of 3D organoid-immune co-culture systems represents a crucial advancement for physiologically relevant drug testing and precision cancer therapy development. 2D, two-dimensional; 3D, three-dimensional; EGCG, epigallocatechin-3-gallate; ER, endoplasmic reticulum; M1, classically activated macrophage; PD-L1, programmed death-ligand 1; PSK, polysaccharide-K; ROS, reactive oxygen species. Symbols: ↓ indicates decreased or downregulated expression, ↑ indicates increased or upregulated expression.
2.3. Limitations of Current Models Driving the Need for Organoid Platforms
In spite of their limitations, two-dimensional cell culture models remain a standard platform for initial natural product screening and mechanistic studies [,]. These models enabled the initial identification of key immunomodulatory compounds, including characterization of curcumin’s NF-κB inhibitory effects [,,], resveratrol’s SIRT1 activation [,,,], and EGCG’s effects on T cell differentiation [,,]. The simplicity, cost-effectiveness, and high-throughput capability of 2D cultures facilitated screening of thousands of natural compounds, establishing fundamental structure-activity relationships that guide current research [,,,]. Moreover, mechanistic insights into checkpoint modulation, cytokine signaling, and immune cell activation pathways by natural products have been extensively studied [,,,].
Nevertheless, two-dimensional cultures fundamentally alter immune-related responses. Comparative studies reveal that 3D spheroids showed relatively lower activities in the AKT-mTOR-S6K signaling pathway compared to cells cultured in two dimensions, with significantly altered drug sensitivities []. The transition from 2D to 3D culture significantly impacts cell proliferation, differentiation, and survival under some circumstances, resulting in cellular behaviors that differ from those in vivo []. For immunomodulatory compounds, this discrepancy is amplified by the absence of physiological cell–cell interactions, gradient formation, and mechanical forces.
Drug resistance patterns differ markedly between culture dimensions. Triple-negative breast cancer cells demonstrated significantly higher half-maximal inhibitory concentration (IC50) values for epirubicin, cisplatin, and docetaxel in 3D cultures compared to 2D across 13 tested cell lines []. Natural products show similar patterns—marine-derived Caulerpa sertularioides extract exhibited IC50 values of 80.28 μg/mL in 2D versus 530 μg/mL in 3D cultures after 24 h, representing a 6.6-fold increase in resistance [], while crocin treatment showed IC50 values of 3.68 mg/mL in 2D versus 10.12 mg/mL in 3D cultures, representing a 2.77-fold increase that further elevated to 4.34-fold by 72 h []. These findings underscore the inadequacy of monolayer systems for predicting therapeutic responses, failing to achieve clinical approval [].
Animal models have made indispensable contributions to natural product immunology research. Murine models enabled the discovery of immunomodulatory mechanisms for numerous compounds now in clinical development, including demonstration of PSK’s TLR2-mediated immune activation [,,,] and lentinan’s enhancement of anti-tumor immunity through macrophage activation [,,,]. These models provided essential pharmacokinetic and safety data that facilitated the clinical approval of mushroom polysaccharides in Japan [,,]. Furthermore, syngeneic tumor models allowed investigation of complex tumor–immune interactions, revealing how natural products modulate the tumor microenvironment through effects on MDSCs, Tregs, and TAMs [,,]. Mechanistic studies using knockout mice have elucidated specific molecular targets and signaling pathways essential for natural product activity [,,,].
In contrast, animal models present species-specific immune barriers that have led to numerous translational failures in natural product development. Mestas and Hughes cataloged fundamental immunological differences between mice and humans that directly impact natural product efficacy []. These include (1) divergent TLR expression patterns—TLR10 is completely absent (pseudogene) in mice while widely expressed in humans, and TLR9 is expressed on all murine myeloid cells but restricted to B cells and plasmacytoid dendritic cells (DCs) in humans; (2) contrasting cytokine responses—human Th1 cells produce IL-10 while murine Th1 cells do not, and IL-8 (CXCL8), crucial for human neutrophil recruitment, is entirely absent in mice; (3) reversed immune cell subset balances—human blood contains 50–70% neutrophils and 30–50% lymphocytes, while mouse blood shows 10–25% neutrophils and 75–90% lymphocytes, alongside completely different NK cell receptor families (Ly49 in mice vs. killer cell immunoglobulin-like receptor (KIR) in humans) [].
These profound differences have resulted in significant translational failures for natural products. Curcumin demonstrated potent anti-inflammatory effects in murine models but showed minimal bioavailability and efficacy in human trials [,,]. Resveratrol’s robust immunomodulatory activity in mice failed to translate to humans due to species-specific differences in metabolism and SIRT1 pathway activation [,,]. Most strikingly, TLR9 agonists like cytosine-phosphate-guanine (CpG) oligonucleotides that showed promising anti-tumor immunity in mice caused severe adverse events in human trials due to the broader TLR9 expression on human immune cells [,,,,].
PDOs offer a compelling alternative that circumvents these species-specific barriers. PDOs maintain patient-specific genetic backgrounds and tissue architecture while enabling co-culture with autologous human immune cells, thereby preserving human-specific TLR expression patterns, cytokine networks, and immune cell ratios [,,,,]. Unlike murine models, PDO-immune co-cultures accurately recapitulate human IL-8/CXCR1 signaling, human-specific Th1 IL-10 production, and authentic human neutrophil-lymphocyte interactions, providing more predictive platforms for natural product immunomodulator screening [,,,].
Seok et al. demonstrated that genomic responses in mouse models poorly mimic human inflammatory diseases, with key pathways showing opposite directional changes between species []. Meta-analyses show that animal models achieve only 0.65 positive predictive value for human toxicities, likely lower for complex immunomodulatory effects [].
Pharmacokinetic disparities further complicate translation. Natural products face unique challenges: curcumin achieves only nanomolar plasma concentrations (22–41 ng/mL) despite 8 g daily dosing [], while oral polysaccharides show extremely poor oral bioavailability of 0.5–4.9% depending on molecular structure []. These issues cannot be adequately modeled in systems lacking human-specific metabolism and immune components.
2.4. Organoid Platforms: Bridging the Translational Gap
PDOs demonstrate remarkable potential as predictive models for drug response. In a landmark study of metastatic gastrointestinal cancers by Vlachogiannis et al. (n = 71), organoid drug sensitivity testing achieved 100% sensitivity and 93% specificity in predicting treatment responses in 15 patients from their larger cohort (n = 71), establishing a robust foundation for precision medicine applications []. This predictive capability has been further validated in cancer-specific contexts, where organoids maintain essential tumor characteristics essential for accurate modeling. Pancreatic cancer organoids have proven particularly valuable, as they recapitulate the mutational spectrum and transcriptional subtypes of primary tumors through combined genomic, transcriptomic, and therapeutic profiling, enabling comprehensive assessment of treatment vulnerabilities []. Nonetheless, the clinical translation of organoid-based predictions reveals important drug-specific variations in accuracy. Whereas colorectal cancer organoids demonstrate >80% accuracy for irinotecan-based therapies, they notably failed to predict outcome for 5-fluorouracil plus oxaliplatin combinations, highlighting the need for therapy-specific validation of organoid predictive models []. These findings underscore both the promise and limitations of organoid technology, emphasizing that successful clinical implementation requires careful consideration of cancer type, treatment regimen, and individual drug mechanisms when interpreting organoid-based predictions for patient care.
Immune integration breakthroughs have transformed organoid capabilities. The development of ALI cultures by Neal et al. enables maintenance of endogenous tumor-infiltrating lymphocytes, macrophages, and other immune populations for over 10 days with IL-2 supplementation []. Recent innovations include autologous T cell generation achieving 33–50% success rates in colorectal and lung cancers [,], generation of tumor-reactive T cells by co-culture of PBL and tumor organoids, sustained cytokine production matching in vivo patterns, and functional assessment of checkpoint blockade responses [,,].
Technical standardization is rapidly maturing. Korean and Chinese organoid initiatives have published comprehensive guidelines [,,,,,], while automated platforms demonstrate excellent reproducibility with average coefficient of variation (CV) of 3.56% for drug screening []. These advances address previous reproducibility concerns while enabling high-throughput natural product screening.
Clinical correlation validates the platform’s utility. A systematic review of organoid studies demonstrate 81% sensitivity and 74% specificity for predicting patient responses in pooled analyses, with subset analyses of studies containing ≥5 responders and non-responders achieving 84% sensitivity and 81% specificity []. Patient-derived ovarian cancer organoids display inter- and intrapatient drug response heterogeneity, with PDO responses to carboplatin/paclitaxel showing statistically significant correlation (p < 0.01) with clinical outcomes []. Combined with 2–3 week turnaround times, this positions organoid-guided natural product selection as a viable clinical tool.
The combination of preserved architecture, functional immune integration, improving standardization, and clinical validation creates a unique opportunity to systematically evaluate immunomodulatory natural products in human-relevant systems.
3. Natural Products in Cancer Organoid Systems: Current Evidence
The intersection of natural product research and cancer organoid technology remains remarkably limited. Our comprehensive literature analysis reveals a major gap: even as natural compounds have been tested in cancer organoids and their immunomodulatory properties have been extensively studied in conventional systems, the investigation of natural product-mediated immunomodulation using organoid-immune co-culture platforms is yet to be examined. Here we synthesize available evidence from two distinct categories: (1) natural products tested in cancer organoids for cytotoxic effects, and (2) immunomodulatory mechanisms demonstrated in traditional 2D cultures or animal models that require translation to organoid platforms (Table 1).

Table 1.
Comparison of research platforms for natural product evaluation in cancer models.
Important note: Unless otherwise specified, all natural product studies in organoids discussed below evaluated direct cytotoxicity only, without immune cell involvement. This absence of immunomodulatory investigation in 3D co-culture systems represents the fundamental gap this review addresses.
3.1. Direct Evidence from Organoid Studies
3.1.1. Polyphenolic Compounds in PDOs
The landscape of polyphenolic compound testing in PDOs is surprisingly sparse. Even though the extensive literature on natural product anti-cancer activities in traditional models, comprehensive assessment in PDO systems is still at an early stage, with existing studies now expanding beyond cytotoxicity to include diverse endpoints such as signaling pathways, metabolomics, and stemness markers (Table 2).

Table 2.
Natural products tested in patient-derived cancer organoid systems.
Luteolin represents one of the few comprehensively studied flavonoids in PDOs. Hao et al. [] tested luteolin in 11 patient-derived gastric cancer organoids, revealing an IC50 of 27.19 μM for direct cytotoxic effects, which demonstrated superior efficacy compared to carboplatin (IC50: 37.87 μM) but was comparable to norcantharidin (IC50: 23.9 μM). The compound exhibited differential sensitivity patterns across patient samples, highlighting the potential for personalized therapeutic approaches. Other polyphenolic compounds remain largely unexplored in true PDO systems. While numerous studies have investigated compounds such as curcumin, resveratrol, quercetin, and EGCG in cell line-based 3D cultures or spheroids, these models lack the patient-specific heterogeneity and tissue architecture of authentic PDOs. Importantly, EGCG has demonstrated extensive immunomodulatory effects in conventional 2D cancer models, including modulation of cytotoxic lymphocytes, dendritic cells, myeloid-derived suppressor cells, and regulatory T cells [], yet PDO-immune co-culture studies have not been conducted.
The scarcity of polyphenol PDO studies is further highlighted by recent efforts in related natural product research. Studies have explored other natural compounds in organoid systems, such as Chaga mushroom (Inonotus obliquus) extracts in canine bladder cancer organoids [], suggesting the technical feasibility of natural product screening in organoid platforms while underscoring the gap in polyphenolic compound investigation.
The extreme paucity of polyphenolic compound studies in PDOs represents a significant missed opportunity in personalized cancer medicine. PDOs have emerged as powerful platforms for precision oncology, offering multiple advantages for drug testing and therapeutic prediction []. These models maintain patient-specific tumor characteristics while enabling high-throughput screening and mechanistic studies in a controlled environment []. The integration of validated PDO technology with the therapeutic potential of polyphenolic compounds presents an unexplored frontier for developing personalized natural product-based cancer therapies.
3.1.2. Polysaccharides and Complex Extracts
Although extensive evidence of anti-cancer activity in traditional systems, polysaccharide evaluation in true cancer organoid platforms remains remarkably limited, with existing studies focusing exclusively on cytotoxic effects. Natural product screening in PDOs is still an emerging field [], with organoid research only gaining significant momentum after 2015 while most polysaccharide cancer studies predate this technological shift. Modified citrus pectin (MCP) shows promising synergy with conventional therapies in 3D systems, though tested only for direct anti-cancer effects. Pectasol-C MCP (0.025%) combined with paclitaxel in SKOV-3 ovarian cancer spheroids demonstrated significant viability reduction through galectin-3 inhibition and STAT3 pathway disruption []. Still, this represents spheroid rather than true organoid evaluation, and immunomodulatory potential has not been evaluated.
Chaga mushroom extract evaluation in canine bladder cancer organoids represents the most comprehensive 3D study for medicinal mushrooms, assessing cytotoxicity and cell cycle arrest only. The study achieved dose-dependent viability reduction with 25–100 μg/mL inducing G0/G1 arrest after 72 h treatment []. In spite of mushroom polysaccharides’ well-documented immunostimulatory effects in 2D systems, this veterinary model focused solely on direct anti-cancer mechanisms.
Luteolin from plant sources demonstrates exceptional cytotoxic activity in gastric cancer organoids (IC50: 27.2 μM), outperforming carboplatin while maintaining selectivity for malignant cells []. PDO responses correlated with clinical outcomes in 11/12 cases, validating the platform’s predictive value for cytotoxic agents—without any immunomodulatory assessment.
The limited number of organoid studies, particularly for immunomodulatory assessment, likely reflects technical challenges rather than a lack of therapeutic potential. High molecular weight polysaccharides face unique barriers including limited penetration through Matrigel, as Phan et al. demonstrated that “Matrigel does not form representative barriers to ovarian cancer cells in either 2D or 3D culture systems” [], concentration gradients from periphery to center demonstrated through matrix-assisted laser desorption/ionization (MALDI) imaging and microfluidic studies [,], and susceptibility to enzymatic degradation in long-term culture conditions. Although Berlemont comprehensively analyzed fungal polysaccharide-degrading enzymes [], the specific impact on organoid cultures is still unknown. Even with extensive preclinical evidence of immunomodulatory activity in simpler model systems, marine-derived fucoidan (molecular weight (Mw) = 469 kDa) and mushroom β-glucans—with PSK (90–100 kilodalton (kDa)) approved as an oral adjuvant and lentinan (400–800 kDa) as an intravenous formulation in Japan, highlighting how delivery route impacts clinical translation specifically for their immunostimulatory effects [,]—have never been evaluated in organoid-immune co-culture platforms. Dendrobium officinale polysaccharides ranging from 24.89 kDa to 1224.54 kDa have shown anti-cancer effects [], highlighting the diverse molecular weight range of bioactive polysaccharides.
Systematic screening of natural product libraries in organoid platforms represents an unmet need. Even though computational approaches have identified promising candidates from traditional medicine databases, with 5278 anti-cancer compounds predicted from traditional Chinese medicine (TCM) database [], and comprehensive natural product libraries have been established [], their application to organoid-based screening is not yet fully established. The successful application of automated platforms achieving coefficient of variation of 3.56% for size distribution and <9% for cellular composition [] combined with recent standardization guidelines [,,,,,,], now enables high-throughput polysaccharide discovery. This synergy between technical capabilities and regulatory support creates exceptional opportunities for advancing these historically important but understudied compounds.
3.2. Evidence of Immunomodulation from 2D and Animal Models
Although organoid-immune co-culture studies are lacking, extensive studies in 2D cultures and animal models demonstrate significant immunomodulatory potential of natural products. These findings, though not yet validated in organoid platforms, provide a promising rationale and roadmap for future organoid-based investigations. Here we review key immunomodulatory mechanisms that warrant immediate translation to organoid-immune co-culture systems.
3.2.1. Checkpoint Modulation Capabilities
Natural products demonstrate potent checkpoint modulation in traditional systems, yet these mechanisms need validation in organoid-immune co-cultures. Resveratrol inhibits PD-L1 glycosylation in 2D cancer cell cultures, preventing proper membrane localization through endoplasmic reticulum retention []. This mechanism appears particularly relevant for organoid-immune co-cultures where spatial organization and cell–cell interactions are preserved.
Multiple compounds demonstrate checkpoint modulation requiring prompt organoid validation:
- Curcumin: Enhances PD-L1 ubiquitination via CSN5 downregulation in 2D systems []
- Ginsenoside Rh2: Augments anti-PD-L1 immunotherapy by reinvigorating CD8+ T cells through increasing intratumoral CXCL10 signaling in xenograft models []
- Astragalus polysaccharides: Suppress PD-L1 via AKT/mTOR/p70S6K signaling in cancer cell lines []
- EGCG: Inhibits PD-L1/PD-L2 expression through JAK-STAT signaling [] and separately suppresses PD-1 expression via NF-κB pathway modulation []
These mechanisms, demonstrated only in simplified 2D cultures or immunodeficient mouse models, urgently require validation in human-relevant organoid-immune co-culture systems.
Translation to organoid platforms: These checkpoint modulation mechanisms represent priority targets for validation in organoid-immune co-cultures. Future studies should leverage established co-culture protocols [] while addressing the unique challenges of natural product delivery in 3D systems, including optimization of compound concentrations and exposure timing.
3.2.2. Macrophage Polarization in 3D Contexts
Limited organoid-macrophage studies provide proof of concept, though none have evaluated natural product effects. Zhang et al. demonstrated that transplanted intestinal organoids attenuate ischemia/reperfusion injury through endogenous L-malic acid production, which induces M2 macrophage polarization via suppressor of cytokine signaling 2 (SOCS2)-dependent pathways in vivo []. Though this represents an in vivo transplantation model rather than direct natural product testing, it confirms organoids can modulate macrophage phenotypes—a capability yet to be exploited for natural product screening.
Traditional compounds show macrophage modulation in 2D systems that require investigation in organoid systems. Contrary to promoting M1 polarization, both curcumin and EGCG actually suppress M1 and enhance M2 polarization—curcumin reduces inflammatory cytokines while increasing anti-inflammatory markers like IL-10 and arginase-1 (Arg-1) through NF-κB inhibition [,], and EGCG suppresses M1 markers (iNOS, TNF-α) while upregulating M2 markers (Arg-1, Krüppel-like factor 4 (KLF4)) []. Various polysaccharides from medicinal mushrooms promote M1 polarization through TLR4 activation, as demonstrated by Ganoderma lucidum polysaccharides inducing pro-inflammatory cytokines via TLR4-myeloid differentiation primary response 88 (MyD88) signaling [,]. These opposing effects—some compounds promoting anti-tumor M1 polarization while others induce potentially tumor-supportive M2 phenotypes—highlight the vital need for organoid-based evaluation in physiologically relevant tumor microenvironments.
Organoid applications: The successful maintenance of endogenous immune populations in organoid cultures, particularly through ALI platforms [], provides an opportunity to investigate natural product-mediated macrophage polarization in physiologically relevant contexts. Adaptation of existing organoid-immune co-culture methods [] will be essential for translating these immunomodulatory mechanisms.
3.3. Cancer Type-Specific Patterns Emerging from Limited Data
Early evidence suggests natural product cytotoxic responses in organoids follow cancer-specific patterns, though immunomodulatory effects have not been studied across all cancer types:
Gastrointestinal cancers demonstrated consistent cytotoxic responses to natural products. Colorectal cancer organoids show varying establishment rates, with Vlachogiannis et al. reporting 70% success rate [] and gastric cancer showing 66.6% success rate in meta-analysis []. Natural products demonstrate consistent cytotoxic responses, with curcumin showing IC50 values of 20–50 μM across colorectal cancer organoids [], and luteolin achieving IC50 of 27.19 μM in gastric cancer organoids []. Regardless of the well-established role of immune dysfunction in GI cancers and extensive evidence of natural product immunomodulation in 2D systems, immune-mediated mechanisms in GI cancer organoid co-cultures have not been investigated.
Breast cancer organoids showed high sensitivity to polyphenol cytotoxicity. Resveratrol induced > 50% cell death in 79.2% (19/24) of treated organoids [], while quercetin demonstrated chemosensitizing effects with IC50 values less than 22 μM, enhancing sensitivity to conventional chemotherapy through PTEN/Akt and ERK1/2 pathway modulation []. Given breast cancer’s known responsiveness to immunotherapy and natural products’ documented effects on tumor-associated macrophages and T cells in 2D models, organoid-immune co-culture studies represent an unexplored opportunity.
Lung cancer models revealed promising cytotoxic responses to marine-derived natural products. Manoalide at 10–15 μM promoted epidermal growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) sensitivity through Kirsten rat sarcoma viral oncogene homolog-extracellular signal-regulated kinase (KRAS-ERK) pathway inhibition and ferroptosis induction []. On the other hand, considering lung cancer’s high mutational burden and potential for immune recognition, the lack of studies examining how natural products might enhance anti-tumor immunity in lung cancer organoids is particularly concerning.
Pancreatic cancer organoids have been successfully established with 75% efficiency from 101 patients [], providing validated platforms for drug testing. Though direct natural product cytotoxicity studies remain limited, the aggressive immunosuppressive microenvironment of pancreatic cancer makes it an ideal candidate for testing immunomodulatory natural products—yet immunomodulatory natural product studies are notably lacking.
The pattern is clear: across all major cancer types, natural product research in organoids has focused exclusively on direct cytotoxicity, overlooking their immunomodulatory potential despite convincing evidence from other model systems.
3.4. Technical Insights from Early Adopters
Pioneering studies reveal important considerations for natural product testing in organoids, though all insights derive from cytotoxicity assessments rather than immunomodulatory investigations.
Concentration dynamics: Organoids consistently require higher concentrations than 2D cultures for cytotoxic effects. Hundsberger et al. demonstrated that quercetin required 31-fold higher concentrations in 3D melanoma spheroids (12.5 μM) compared to 2D cultures (0.4 μM) for caspase 3 activation []. This dramatic difference underscores the importance of concentration optimization when transitioning from traditional screening to organoid platforms [,]. For immunomodulation studies, this poses a serious challenge: the substantially higher concentrations required for tumor effects in 3D may compromise immune cell viability, creating a narrow therapeutic window where anti-tumor effects must be balanced against maintaining functional immune populations.
Temporal patterns: Drug responses in organoids follow time-dependent phases for cytotoxicity. Optical metabolic imaging demonstrates treatment responses within 24 h in pancreatic cancer organoids [], while Chen et al. showed that resveratrol requires 96 h exposure for full anti-cancer effects through ferroptosis induction []. These findings align with optimization studies demonstrating optimal timing for patient response correlation [,].
Formulation requirements: Hydrophobic compounds necessitate specialized delivery systems. Zou et al. demonstrated that liposomal paclitaxel formulations showed superior efficacy compared to albumin-bound forms in gastric cancer organoids, achieving 90% organoid establishment rate []. The additional complexity of maintaining functional immune cells while delivering natural products presents an unexplored technical challenge.
Heterogeneity assessment: Single-organoid analysis reveals cytotoxic response variations. Tebon et al. developed high-speed live cell interferometry for single-organoid drug screening [], while Sharick et al. demonstrated metabolic heterogeneity both between primary sites and within organoid populations []. For immunomodulatory assessment, this heterogeneity would be compounded by immune cell variability [,].
The lack of direct evidence paradoxically highlights the opportunity. With validated organoid-immune co-culture platforms expanding rapidly as reviewed by Papp et al. [] and Wang et al. [], combined with extensive knowledge of natural product immunomodulation from traditional systems used since antiquity [], the field stands poised for rapid advancement through methodical study.
4. Technical Challenges and Standardization
Given that the absence of natural product immunomodulation studies in organoid-immune co-cultures presents a clear opportunity, several technical challenges must be addressed to enable rigorous analysis. These challenges are amplified when considering the unique physicochemical properties of natural products combined with the complexity of maintaining functional immune populations in 3D culture systems.
4.1. Natural Product-Specific Challenges in Organoid Systems
4.1.1. Formulation and Bioavailability Barriers
Natural products face unique physicochemical challenges in 3D organoid matrices that significantly impact their immunomodulatory potential. Hydrophobic compounds exemplify these issues: curcumin’s aqueous solubility (approximately 11 ng/mL at pH 5.0) results in heterogeneous Matrigel distribution, severely impeding its gastrointestinal uptake [,]. VEGF diffusion studies in Matrigel demonstrate the complexity of molecular transport, with coefficients ranging from 0.24 mm2/day (theoretical) to 24 mm2/day (empirical measurements), highlighting the variability in 3D matrix penetration [].
Stability compounds the challenge. Curcumin exhibits pH-dependent stability, retaining only 53–62% at physiological pH (7.0–8.0) after 1 month at 37 °C []. The presence of 10% fetal calf serum (FCS) improves stability (<20% decomposition within 1 h), though 50% degradation still occurs after 8 h even with serum protection []. pH-dependent stability is particularly relevant: curcumin retains > 85% activity at pH < 7 after 1 month at 37 °C, but only 53–62% at physiological pH 7.0–8.0 []. Natural products also demonstrate extensive binding to human serum albumin, which can enhance their stability and bioavailability through protective protein interactions []. This pH sensitivity presents unique challenges for immunomodulation studies. Immune cells require physiological pH for optimal function, yet at this pH curcumin shows significant degradation. This compromise sustained immunomodulatory effects during critical immune activation windows, necessitating either continuous compound replenishment or protective formulations that maintain both compound stability and immune cell viability.
4.1.2. Delivery System Innovations
Recent advances demonstrate quantifiable improvements. At the same time, these delivery systems must consider immune cell compatibility. Different formulation strategies may influence interactions with immune cells in the tumor microenvironment, potentially affecting the intended immunomodulatory outcomes. Polyvinyl alcohol/sodium alginate (PVA/SA) hydrogel systems achieve 60.8 ± 3.7% encapsulation efficiency with zero-order release kinetics (r = 0.993), providing sustained compound delivery essential for immunomodulatory studies []. β-cyclodextrin complexation shows particular promise, with hydroxypropyl-β-cyclodextrin (HPβCD) achieving stability constants of 424 M−1 compared to 401 M−1 for methylated-β-cyclodextrin (MβCD), increasing water solubility of curcumin by 31-fold [,].
Liposomal encapsulation using soya lecithin demonstrates successful delivery of copper nanoparticles in cancer cell systems, achieving cytotoxic effects against MCF-7 breast cancer cells []. Mesoporous silica nanoparticles (MSNs) enable pH/glutathione-responsive release of poorly water-soluble compounds including quercetin, curcumin, and colchicine [,,,]. Amino-functionalized MCM-41 and KCC-1 variants conjugated with folic acid provide targeted delivery with controlled release profiles []. Recent studies demonstrate that surface-modified MSNs (acetylated, succinylated, polyethylene glycol (PEG)ylated) exhibit differential localization in 3D tumor microtissues, with acetylated MSNs showing superior tumor cell targeting in collagen-containing microenvironments, leading to significant reduction in tumor organoid size [].
4.2. Standardization Requirements for Immune-Organoid Studies
4.2.1. Protocol Harmonization
Significant international standardization efforts provide frameworks for organoid research. The Korean Organoid Standards Initiative, launched in September 2023 through partnership between the Ministry of Food and Drug Safety and Sungkyunkwan University [], has published comprehensive guidelines across multiple organs (heart, liver, kidney, lung, brain, skin) with specific protocols for manufacturing, packaging, transport, and storage [,,,,]. Chinese national standards include T/CMBA 017-2022 for gastrointestinal epithelial organoids and T/CSCB 0005-2022/0006-2022 for human intestinal and cancer organoids, which have demonstrably improved gastrointestinal tumor organoid construction success rates from 50% to 90% [].
For natural product-organoid co-culture studies, specific standardization requirements emerge from recent methodological advances. Essential parameters include
- Immune cell ratios: 1:1 to 5:1 T cell:organoid ratios optimized for specific assay types, with 5:1 ratio specifically validated for cytotoxicity assays []
- Culture duration: Short-term exposure (24–48 h) for immediate cytotoxic responses versus extended culture (7–14 days) for T cell expansion and memory formation []
- Medium composition: Balanced mixtures of organoid and T cell media to maintain both populations’ viability []
- Natural product exposure protocols: Pre-treatment strategies demonstrate enhanced immunomodulatory effects in drug studies, including IFN-γ pre-treatment protocols enhancing T cell reactivity [] and small molecule inhibitors improving chimeric antigen receptor T (CAR-T) cell efficacy in organoid models [,], though detailed evaluation of pre-treatment versus co-treatment timing for natural compounds specifically remains, to our knowledge, a significant research gap
- Natural product-specific considerations for immune co-cultures emerge from established protocols and compound characteristics:
- Compound stability varies between culture media, as demonstrated by curcumin’s pH-dependent degradation [,] and the protective effects of serum proteins [].
- Optimization of exposure protocols follows principles established in organoid-T cell co-culture systems [], with timing and sequential exposure requiring empirical determination.
- Matrix penetration challenges, evident from the 6.6–31-fold higher concentrations required in 3D systems [,], are further complicated by immune cell migration and matrix remodeling.
The alignment of these international standards with natural product-specific requirements creates a foundation for reproducible immunomodulatory studies. That said, dedicated protocols addressing the unique physicochemical properties of natural products—particularly regarding compound stability, penetration kinetics, and batch standardization in 3D co-culture systems—require further development to enable reliable multi-center investigations.
4.2.2. Quality Control Metrics
Validated high-throughput platforms demonstrate achievable standards. Van Hemelryk et al. established viability thresholds of 150% (day 10 vs. day 0) with coefficient of variation < 0.22 and Z-factor > 0.4 for prostate cancer organoid drug screening []. Du et al. achieved Z’-factor > 0.5 with signal-to-background ratios of 5–6 in miniaturized 3D organoid platforms [].
Spatial uniformity substantially affects reproducibility. Boehnke et al. demonstrated that proper plate sealing reduces CV from 18.97% (unsealed) to 9.37% (sealed) in high-throughput organoid assays []. Passage consistency requires standardized split ratios (1:3 to 1:4) with quality assessment of marker expression and karyotype analysis every 5–10 passages [,].
4.2.3. Reproducibility Challenges
Inter-laboratory variation remains substantial. Phipson et al. identified experimental batch as the strongest contributor to transcriptional variation in kidney organoids, exceeding biological differences []. Jensen and Little emphasize that organoids are not organs, highlighting sources of variation including matrix effects, passage number, and culture conditions [].
Matrix variability presents particular challenges for natural product studies. Hughes et al. identified >1000 unique proteins in Matrigel with considerable batch-to-batch variability affecting compound diffusion and bioactivity []. Alternative approaches using tissue-derived extracellular matrix hydrogels show promise for reducing variability while maintaining physiological relevance [].
Compound preparation standardization is essential. Verduin et al. note that drug compounds require experimental optimization due to the variable nature of organoids, with natural products presenting additional challenges due to their complex composition and stability issues []. Implementation of standardized protocols could significantly reduce inter-laboratory variation, enabling reliable multi-center studies of immunomodulatory natural products.
These reproducibility challenges are particularly important for immunomodulatory studies, where batch-to-batch variations in both natural products and immune cell preparations could significantly impact outcomes. Long-term co-culture studies face additional challenges including immune cell exhaustion, phenotype drift, and potential loss of cytotoxic function over extended periods. These limitations necessitate careful temporal monitoring of immune cell markers and functional validation throughout experimental timelines using comprehensive workflows designed for organoid-immune co-culture systems (Figure 3).
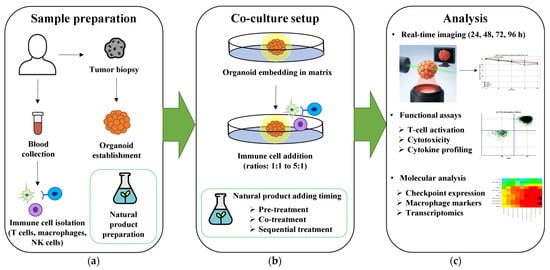
Figure 3.
Proposed workflow for natural product immunomodulation testing in cancer organoid-immune co-culture systems. (a) Sample preparation involves patient tumor biopsy for organoid establishment, peripheral blood collection for immune cell isolation (T cells, macrophages, NK cells), and standardized natural product preparation. (b) Co-culture setup demonstrates organoid embedding in matrix followed by immune cell addition at optimal ratios (1:1 to 5:1), with three alternative natural product treatment strategies: pre-treatment, co-treatment, or sequential treatment protocols. (c) Multi-parametric analysis endpoints include real-time imaging monitoring organoid viability (24–96 h), functional assays measuring T cell activation (CD69/CD25 expression) and cytotoxicity, and molecular profiling using heatmap analysis of cytokine production. Representative/illustrative data shown demonstrate the feasibility of this comprehensive workflow for systematic evaluation of natural product immunomodulation in physiologically relevant 3D systems. 3D, three-dimensional; CD25, interleukin-2 receptor alpha chain; CD69, cluster of differentiation 69; h, hours; NK, natural killer.
4.3. Technological Convergence Enabling Natural Product Discovery
The complete absence of natural product immunomodulation studies in organoid-immune co-cultures, as documented in Section 3, coincides with remarkable technological capabilities that now make such investigations feasible.
4.3.1. Spatial Multi-Omics Integration
Recent advances in spatial transcriptomics offer unparalleled opportunities for understanding natural product mechanisms in organoids. Even as platforms achieving 2-μm resolution were announced for commercial launch in 2024 [], current technologies already demonstrate remarkable capabilities in mapping compound distribution and cellular responses. The foundational spatial transcriptomics platform operates at 100-μm resolution [], while newer methods including Slide-seq (10-μm) [] and MERFISH (single-molecule resolution) [] enable increasingly precise spatial analysis. Wahle et al. successfully combined multiplexed protein mapping (53 antibodies across 21 imaging cycles), single-cell transcriptomics (212,781 cells), and chromatin accessibility analysis (151,684 cells) across retinal organoid development, providing a framework for studying natural product effects at multiple molecular levels []. The application of optogenetics with spatial transcriptomics, as demonstrated by Legnini et al., enables controlled perturbation studies using light-inducible systems (single-cell perturbation transcriptomic screen (SCPTS), photoactivatable Tet-On system (PA-TetON), photoactivatable Cre-Lox system (PA-Cre-Lox)) combined with both 10× Visium and Molecular Cartography platforms that could elucidate natural product mechanisms with exceptional precision [].
Even so, natural products present unique analytical challenges. Their autofluorescence often interferes with imaging modalities, particularly for flavonoids and alkaloids that exhibit intrinsic fluorescence at wavelengths overlapping with common fluorophores [,]. Complex natural product mixtures containing thousands of structurally diverse molecules further complicate molecular tracking, with matrix effects and ion suppression creating additional barriers for accurate spatial quantification [,]. Cell2location and similar Bayesian models show promise for deconvoluting these complex signals, having demonstrated high-sensitivity spatial mapping with superior performance (root mean square error, root mean square error (RMSE) 0.0373) in various tissue types including brain, lymph node, and gut tissues []. Integration costs remain substantial, with commercial platforms requiring $11,000–14,000 per slide [], creating barriers to widespread adoption. Conversely, recent advances in automation demonstrate 35% reduction in sample preparation time [], while comprehensive reviews project continued improvements in efficiency and accessibility through technological maturation [].
4.3.2. Machine Learning for Response Prediction
AI models have achieved remarkable accuracy in predicting drug responses in organoid systems. Kong et al. developed a network-based machine learning approach that predicted anti-cancer drug efficacy with R2 = 0.98 for colorectal cancer and R2 = 0.89 for bladder cancer across 114 colorectal and 77 bladder cancer patients []. Importantly, their model demonstrated clinical relevance with predicted responders showing significantly better survival outcomes (p = 0.014 for colorectal, p = 0.01 for bladder cancer) []. Recent advances include Cellos, which employs deep learning with StarDist-3D using a ResNet backbone for quantitative 3D organoid analysis, enabling high-throughput deconvolution of organoid dynamics at cellular resolution from approximately 100,000 organoids containing 2.35 million cells [].
For natural products, specialized approaches are emerging. The YOLOv8-based Deliod model achieves 87.5% mean average precision at 50% Intersection over Union (IoU) (mAP50) accuracy for intestinal organoid detection, demonstrating the feasibility of automated screening []. OrganoIDNet specifically addresses organoid-immune cell interactions, providing automated analysis of pancreatic ductal adenocarcinoma (PDAC) organoid-peripheral blood mononuclear cell (PBMC) co-cultures from time-resolved imaging data—essential for immunomodulatory natural product assessment []. This platform categorizes organoids by size, health status, and eccentricity while tracking longitudinal responses to chemotherapy and immunotherapy over 100 h periods [].
Nonetheless, given these advances, natural products present unique challenges: chemical complexity requiring expanded descriptor sets, limited training data, and batch-to-batch variability affecting model reliability. Realistic near-term applications include structure-activity relationship modeling for compound prioritization and automated quality control for standardization.
4.3.3. Organ-on-Chip Integration
Microfluidic platforms offer solutions to natural product delivery challenges in organoid systems. Schuster et al. developed an automated platform capable of testing 200 organoids simultaneously with real-time analysis, achieving 20 independent fluidic conditions through a gravity-driven flow system []. Recent innovations include perfusable vascular networks that maintain functionality for up to 30 days, as demonstrated by Quintard et al., who achieved long-term perfusion at 1 μL/min in their 10-channel cyclic olefin copolymer (COC) system [].
Technical specifications have been optimized for organoid culture: flow velocities of 100–7500 μm/s enable proper nutrient delivery and waste removal, with physiological shear stress values of 1–10 dynes/cm2 mimicking tumor-vascular interactions []. Although mechanical stimulation has been shown to enhance organoid maturation in various systems, specific parameters for natural product testing in mechanically stimulated organoids require further investigation.
Additionally, natural products pose specific challenges for microfluidic systems. The general review by Dressaire and Sauret highlights clogging as a major challenge in microfluidic systems operating at low Reynolds numbers []. Hydrophobic compounds present absorption challenges in polydimethylsiloxane (PDMS) devices, with small molecules showing >70% loss within 3 h []. Surface modifications using PEG-based coatings reduce PDMS contact angles from 108° ± 5° to 52° ± 3°, significantly improving hydrophilicity and reducing nonspecific absorption [].
Recent advances integrate immune components directly into organ-on-chip systems. Kroll et al. developed millifluidic chips enabling PBMC circulation through kidney organoids for T cell bispecific antibody assessment []. These platforms enable in situ monitoring of immune-tumor interactions through transparent observation windows, allowing real-time confocal microscopy of natural product effects. Emerging organ-on-chip technologies now enable evaluation of natural product immunomodulation in physiologically relevant microenvironments with integrated immune cell populations, bridging the gap between traditional 2D cultures and clinical outcomes.
4.4. Clinical Translation Pathway
The merger of validated organoid-immune co-culture platforms with advanced analytical technologies creates immediate opportunities for translating natural product immunomodulation discoveries into clinical applications.
4.4.1. Regulatory Considerations
The FDA Modernization Act 2.0, signed into law 29 December 2022, authorizes “cell-based assays” and “microphysiological systems” as alternatives to animal testing, which the FDA and scientific community interpret to include organoid technologies [,].
Natural product-organoid combinations face unique regulatory considerations. Quality control requirements are complicated by the lack of defined active pharmaceutical ingredients in natural products. Organoid-based potency assays could establish biological standardization, complementing chemical analysis. The combination of organ-on-chip technology with pharmacokinetic modeling shows promise as a substitute for traditional animal studies, with the FDA aiming to make animal testing “the exception rather than the norm” within 3–5 years [].
Clinical validation pathways are emerging through studies like Narasimhan et al., which demonstrated successful PDO generation in 68% (19/28) of colorectal cancer patients with peritoneal metastases, with one patient showing partial response to PDO-guided gemcitabine-capecitabine therapy []. However, natural product trials require modified endpoints acknowledging longer immunomodulation timelines compared to cytotoxic agents.
4.4.2. Economic Realities
Current cost structures present implementation barriers. Whereas comprehensive precision medicine platforms including genomic and preclinical testing cost $12,743–21,769 per patient (in 2021 Australian dollars) in clinical implementation [], specific organoid-related cost data remains limited. Clinical implementation studies demonstrate 68.8% success rate for completing organoid drug testing within 7 weeks in metastatic colorectal cancer []. This technological advancement, combined with high predictive accuracy (as exemplified by Vlachogiannis et al.’s finding of 100% sensitivity and 93% specificity in predicting treatment responses for 15 patients from their 71-patient cohort) [], demonstrates the potential of organoids to transform drug development by enabling accurate patient-specific treatment selection.
Traditional drug development averages $1.3–2.87 billion per approved drug, with median research and development (R&D) costs of $985 million and mean costs of $1.336 billion (2018 US dollars) [,]. Given that 90% of drugs fail in clinical trials, with lack of efficacy accounting for 40–50% of failures [], organoids’ high predictive accuracy offers significant economic advantages.
For clinical viability, costs must decrease while maintaining quality. Automated organoid platforms achieve coefficient of variation of 3.56% (range 2.2–5.6%), representing up to 5–10-fold improvement in reproducibility compared to other organoid systems []. Cost-reduction strategies such as L-WRN conditioned medium show promise []. Insurance coverage remains a major barrier, with studies showing only 50% or less of personalized medicine tests covered by insurers due to lack of clinical utility evidence [].
4.4.3. Integration with Standard Care
Realistic implementation requires phased approaches. Multiple clinical trials demonstrate organoid utility in treatment selection. The CinClare trial achieved 84.43% accuracy in predicting chemoradiation response in locally advanced rectal cancer patients (n = 80) []. For metastatic gastrointestinal cancers, PDOs demonstrated 100% sensitivity and 93% specificity in predicting treatment responses across patients in phase I/II clinical trials [].
The Drug Efficacy Testing in 3D Cultures (DET3CT) platform represents current best practices for ovarian cancer, achieving 6-day turnaround for drug sensitivity profiling with >90% success rate []. Alternative approaches include micro-organospheres that enable drug testing within 14 days for colorectal and lung cancers []. Molecular tumor boards have been developed to integrate genomic data for clinical decision support, with digital infrastructure platforms like the Molecular Tumor Board Portal enabling automated reporting and virtual collaboration for precision oncology [].
4.5. Priority Research Directions
Based on current evidence and technological capabilities, immediate priorities include:
- Systematic screening: Leverage high-throughput platforms to test natural products specifically in organoid-immune co-cultures, prioritizing compounds with established immunomodulatory effects in 2D systems.
- Mechanistic validation: Apply spatial transcriptomics and live imaging to understand how natural products modulate tumor–immune cell interactions in 3D, not just direct cytotoxicity.
- Formulation optimization: Develop organoid-specific delivery systems utilizing tumor-penetrating peptides and other innovations. Recent advances with internalizing RGD (Arg-Gly-Asp) peptide (iRGD) demonstrate enhanced drug delivery in 3D tumor spheroids [], providing a framework for natural product optimization.
- Clinical correlation: Establish prospective trials comparing organoid predictions with patient responses, following successful frameworks from colorectal and pancreatic cancer studies.
- Standardization: Implement quality control measures achieving coefficient of variation < 15%, as demonstrated in automated screening platforms. Renner et al. [] achieved average CV of 3.56% within batch for organoid size distribution, while Boehnke et al. [] demonstrated CV of 9.37% with proper sealing membranes in high-throughput organoid assays.
4.6. Realistic Outlook
The union of natural products, immunotherapy, and organoid technology offers genuine potential for advancing cancer treatment. Technical barriers are being systematically addressed through platform innovations and standardization efforts. Clinical validation is progressing through multiple prospective trials demonstrating strong organoid-patient correlations, with studies achieving 93–100% sensitivity and specificity for treatment response prediction [,,].
Success depends on avoiding previous pitfalls of natural product research—overpromising based on limited data and focusing solely on cytotoxicity. By systematically investigating immunomodulatory mechanisms in physiologically relevant organoid-immune co-cultures, the field can deliver meaningful clinical advances. Acknowledging that this review highlights promising opportunities, not all natural products will successfully translate to organoid-immune co-culture platforms. Compounds with extreme hydrophobicity, pH sensitivity, or those requiring concentrations toxic to immune cells may prove unsuitable. Future studies should establish clear selection criteria based on physicochemical properties and preliminary toxicity profiles.
5. Conclusions
This review reveals a striking paradox: while natural products demonstrate extensive immunomodulatory potential and organoid technology offers innovative platforms for studying tumor–immune interactions, no published studies have investigated natural product-mediated immunomodulation in organoid-immune co-culture systems. This research gap, despite validated platforms and strong rationale, represents both a significant void and an immediate opportunity.
Key priorities for advancing the field:
- Systematic screening of immunomodulatory natural products in organoid-immune co-cultures—Research teams should prioritize compounds with established 2D immunomodulatory evidence (curcumin, EGCG, resveratrol, medicinal mushroom polysaccharides) using standardized protocols: 1:1 to 5:1 immune:organoid ratios [], 7–14 day culture periods, and multi-parametric readouts including checkpoint expression, cytokine profiles, and functional cytotoxicity.
- Development of natural product-specific delivery systems—Bioengineers must address the unique challenges of hydrophobic compounds (curcumin solubility < 11 ng/mL) [] and high molecular weight polysaccharides (lentinan 400–800 kDa) [] through innovations in nanoparticle formulations, cyclodextrin complexation, and matrix-penetrating peptides optimized for 3D systems.
- Clinical validation through prospective trials—Oncologists should integrate organoid-based natural product screening into clinical workflows, following the successful framework of the TUMOROID [] and CinClare trials [], with endpoints specifically designed for immunomodulatory effects rather than direct cytotoxicity alone.
The path forward is clear: The coming together of validated organoid-immune co-culture platforms (achieving 81–84% clinical correlation in pooled analyses) [], FDA regulatory support through the Modernization Act 2.0 [], and millennia of natural product wisdom creates a transformative opportunity. Success requires moving beyond cytotoxicity-focused studies to systematically investigate immunomodulatory mechanisms in physiologically relevant 3D systems. With organoid establishment now routine (68–90% success rates) [,,] and standardized protocols emerging globally [,,,,,], the field stands ready to unlock the therapeutic potential of natural product immunomodulation for precision cancer therapy.
Author Contributions
Conceptualization: C.-E.H. and S.-Y.L.; Investigation: C.-E.H. and S.-Y.L.; Resources: C.-E.H.; Supervision: S.-Y.L.; Writing—original draft: C.-E.H.; Writing—review and editing: S.-Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are contained within the article.
Acknowledgments
Following results of a study on the “Regional Innovation System & Education” Project, supported by the Ministry of Education.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Haslam, A.; Olivier, T.; Prasad, V. How many people in the US are eligible for and respond to checkpoint inhibitors: An empirical analysis. Int. J. Cancer 2025, 156, 2352–2359. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Pourhanifeh, M.H.; Mirzaei, H.R.; Sahebkar, A.; Asemi, Z.; Mirzaei, H. Targeting regulatory T cells by curcumin: A potential for cancer immunotherapy. Pharmacol. Res. 2019, 147, 104353. [Google Scholar] [CrossRef]
- Mukherjee, S.; Fried, A.; Hussaini, R.; White, R.; Baidoo, J.; Yalamanchi, S.; Banerjee, P. Phytosomal curcumin causes natural killer cell-dependent repolarization of glioblastoma (GBM) tumor-associated microglia/macrophages and elimination of GBM and GBM stem cells. J. Exp. Clin. Cancer Res. 2018, 37, 168. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, J. Resveratrol Activates Natural Killer Cells through Akt- and mTORC2-Mediated c-Myb Upregulation. Int. J. Mol. Sci. 2020, 21, 9575. [Google Scholar] [CrossRef]
- Ying, Y.; Hao, W. Immunomodulatory function and anti-tumor mechanism of natural polysaccharides: A review. Front. Immunol. 2023, 14, 1147641. [Google Scholar] [CrossRef]
- Riedl, A.; Schlederer, M.; Pudelko, K.; Stadler, M.; Walter, S.; Unterleuthner, D.; Unger, C.; Kramer, N.; Hengstschläger, M.; Kenner, L.; et al. Comparison of cancer cells in 2D vs 3D culture reveals differences in AKT-mTOR-S6K signaling and drug responses. J. Cell Sci. 2017, 130, 203–218. [Google Scholar] [CrossRef]
- Papp, D.; Korcsmaros, T.; Hautefort, I. Revolutionizing immune research with organoid-based co-culture and chip systems. Clin. Exp. Immunol. 2024, 218, 40–54. [Google Scholar] [CrossRef]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988.e1916. [Google Scholar] [CrossRef]
- Atkins, J.T.; George, G.C.; Hess, K.; Marcelo-Lewis, K.L.; Yuan, Y.; Borthakur, G.; Khozin, S.; LoRusso, P.; Hong, D.S. Pre-clinical animal models are poor predictors of human toxicities in phase 1 oncology clinical trials. Br. J. Cancer 2020, 123, 1496–1501. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef]
- Seok, J.; Warren, H.S.; Cuenca, A.G.; Mindrinos, M.N.; Baker, H.V.; Xu, W.; Richards, D.R.; McDonald-Smith, G.P.; Gao, H.; Hennessy, L.; et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 2013, 110, 3507–3512. [Google Scholar] [CrossRef]
- Dijkstra, K.K.; Cattaneo, C.M.; Weeber, F.; Chalabi, M.; van de Haar, J.; Fanchi, L.F.; Slagter, M.; van der Velden, D.L.; Kaing, S.; Kelderman, S.; et al. Generation of Tumor-Reactive T Cells by Co-culture of Peripheral Blood Lymphocytes and Tumor Organoids. Cell 2018, 174, 1586–1598.e1512. [Google Scholar] [CrossRef]
- Votanopoulos, K.I.; Forsythe, S.; Sivakumar, H.; Mazzocchi, A.; Aleman, J.; Miller, L.; Levine, E.; Triozzi, P.; Skardal, A. Model of Patient-Specific Immune-Enhanced Organoids for Immunotherapy Screening: Feasibility Study. Ann. Surg. Oncol. 2020, 27, 1956–1967. [Google Scholar] [CrossRef]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef]
- Elbadawy, M.; Hayashi, K.; Ayame, H.; Ishihara, Y.; Abugomaa, A.; Shibutani, M.; Hayashi, S.M.; Hazama, S.; Takenouchi, H.; Nakajima, M.; et al. Anti-cancer activity of amorphous curcumin preparation in patient-derived colorectal cancer organoids. Biomed. Pharmacother. 2021, 142, 112043. [Google Scholar] [CrossRef]
- Ye, H.S.; Gao, H.F.; Li, H.; Nie, J.H.; Li, T.T.; Lu, M.D.; Wu, M.L.; Liu, J.; Wang, K. Higher efficacy of resveratrol against advanced breast cancer organoids: A comparison with that of clinically relevant drugs. Phytother. Res. 2022, 36, 3313–3324. [Google Scholar] [CrossRef]
- Carratt, S.A.; Zuch de Zafra, C.L.; Oziolor, E.; Rana, P.; Vansell, N.R.; Mangipudy, R.; Vaidya, V.S. An industry perspective on the FDA Modernization Act 2.0/3.0: Potential next steps for sponsors to reduce animal use in drug development. Toxicol. Sci. 2025, 203, 28–34. [Google Scholar] [CrossRef]
- Polak, R.; Zhang, E.T.; Kuo, C.J. Cancer organoids 2.0: Modelling the complexity of the tumour immune microenvironment. Nat. Rev. Cancer 2024, 24, 523–539. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Gaber, M.H.; Salama, A.A.; Ali, S.A. Efficacy of copper nanoparticles encapsulated in soya lecithin liposomes in treating breast cancer cells (MCF-7) in vitro. Sci. Rep. 2023, 13, 15576. [Google Scholar] [CrossRef]
- Cao, H.; Wu, T.; Zhou, X.; Xie, S.; Sun, H.; Sun, Y.; Li, Y. Progress of research on PD-1/PD-L1 in leukemia. Front. Immunol. 2023, 14, 1265299. [Google Scholar] [CrossRef]
- Long, Y.; Yu, X.; Chen, R.; Tong, Y.; Gong, L. Noncanonical PD-1/PD-L1 Axis in Relation to the Efficacy of Anti-PD Therapy. Front. Immunol. 2022, 13, 910704. [Google Scholar] [CrossRef]
- Khan, M.; Arooj, S.; Wang, H. Soluble B7-CD28 Family Inhibitory Immune Checkpoint Proteins and Anti-Cancer Immunotherapy. Front. Immunol. 2021, 12, 651634. [Google Scholar] [CrossRef]
- Ma, B.; Kamle, S.; Akosman, B.; Khan, H.; Lee, C.M.; Lee, C.G.; Elias, J.A. CHI3L1 enhances melanoma lung metastasis via regulation of T cell co-stimulators and CTLA-4/B7 axis. Front. Immunol. 2022, 13, 1056397. [Google Scholar] [CrossRef]
- Ko, E.J.; Seo, J.W.; Kim, K.W.; Kim, B.M.; Cho, J.H.; Kim, C.D.; Seok, J.; Yang, C.W.; Lee, S.H.; Chung, B.H. Phenotype and molecular signature of CD8+ T cell subsets in T cell- mediated rejections after kidney transplantation. PLoS ONE 2020, 15, e0234323. [Google Scholar] [CrossRef]
- Fazeli, P.; Talepoor, A.G.; Faghih, Z.; Gholijani, N.; Ataollahi, M.R.; Ali-Hassanzadeh, M.; Moravej, H.; Kalantar, K. The frequency of CD4+ and CD8+ circulating T stem cell memory in type 1 diabetes. Immun. Inflamm. Dis. 2022, 10, e715. [Google Scholar] [CrossRef]
- Fernandes, A.T.G.; Carvalho, M.O.O.; Avvad-Portari, E.; Rocha, N.P.; Russomano, F.; Roma, E.H.; Bonecini-Almeida, M.D.G. A prognostic value of CD45RA(+), CD45RO(+), CCL20(+) and CCR6(+) expressing cells as ‘immunoscore’ to predict cervical cancer induced by HPV. Sci. Rep. 2021, 11, 8782. [Google Scholar] [CrossRef]
- Kashfi, K.; Kannikal, J.; Nath, N. Macrophage Reprogramming and Cancer Therapeutics: Role of iNOS-Derived NO. Cells 2021, 10, 3194. [Google Scholar] [CrossRef]
- Liu, L.; Guo, H.; Song, A.; Huang, J.; Zhang, Y.; Jin, S.; Li, S.; Zhang, L.; Yang, C.; Yang, P. Progranulin inhibits LPS-induced macrophage M1 polarization via NF-кB and MAPK pathways. BMC Immunol. 2020, 21, 32. [Google Scholar] [CrossRef]
- Oyarce, C.; Vizcaino-Castro, A.; Chen, S.; Boerma, A.; Daemen, T. Re-polarization of immunosuppressive macrophages to tumor-cytotoxic macrophages by repurposed metabolic drugs. Oncoimmunology 2021, 10, 1898753. [Google Scholar] [CrossRef]
- Mirlekar, B. Tumor promoting roles of IL-10, TGF-β, IL-4, and IL-35: Its implications in cancer immunotherapy. SAGE Open Med. 2022, 10, 20503121211069012. [Google Scholar] [CrossRef]
- Su, B.; Han, H.; Gong, Y.; Li, X.; Ji, C.; Yao, J.; Yang, J.; Hu, W.; Zhao, W.; Li, J.; et al. Let-7d inhibits intratumoral macrophage M2 polarization and subsequent tumor angiogenesis by targeting IL-13 and IL-10. Cancer Immunol. Immunother. 2021, 70, 1619–1634. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, X.H. Tumor-Associated Neutrophils and Macrophages-Heterogenous but Not Chaotic. Front. Immunol. 2020, 11, 553967. [Google Scholar] [CrossRef]
- Yuan, X.; Wang, J.; Huang, Y.; Shangguan, D.; Zhang, P. Single-Cell Profiling to Explore Immunological Heterogeneity of Tumor Microenvironment in Breast Cancer. Front. Immunol. 2021, 12, 643692. [Google Scholar] [CrossRef]
- Pan, D.; Jia, D. Application of Single-Cell Multi-Omics in Dissecting Cancer Cell Plasticity and Tumor Heterogeneity. Front. Mol. Biosci. 2021, 8, 757024. [Google Scholar] [CrossRef]
- Medjouel Khlifi, H.; Guia, S.; Vivier, E.; Narni-Mancinelli, E. Role of the ITAM-Bearing Receptors Expressed by Natural Killer Cells in Cancer. Front. Immunol. 2022, 13, 898745. [Google Scholar] [CrossRef]
- Diab, M.; Schmiedel, D.; Seidel, E.; Bacharach, E.; Mandelboim, O. Human Metapneumovirus Escapes NK Cell Recognition through the Downregulation of Stress-Induced Ligands for NKG2D. Viruses 2020, 12, 781. [Google Scholar] [CrossRef]
- Ramírez-Labrada, A.; Pesini, C.; Santiago, L.; Hidalgo, S.; Calvo-Pérez, A.; Oñate, C.; Andrés-Tovar, A.; Garzón-Tituaña, M.; Uranga-Murillo, I.; Arias, M.A.; et al. All About (NK Cell-Mediated) Death in Two Acts and an Unexpected Encore: Initiation, Execution and Activation of Adaptive Immunity. Front. Immunol. 2022, 13, 896228. [Google Scholar] [CrossRef]
- Tuomela, K.; Ambrose, A.R.; Davis, D.M. Escaping Death: How Cancer Cells and Infected Cells Resist Cell-Mediated Cytotoxicity. Front. Immunol. 2022, 13, 867098. [Google Scholar] [CrossRef]
- Groth, C.; Maric, J.; Garcés Lázaro, I.; Hofman, T.; Zhang, Z.; Ni, Y.; Keller, F.; Seufert, I.; Hofmann, M.; Neumann-Haefelin, C.; et al. Hepatitis D infection induces IFN-β-mediated NK cell activation and TRAIL-dependent cytotoxicity. Front. Immunol. 2023, 14, 1287367. [Google Scholar] [CrossRef]
- Seliger, B.; Koehl, U. Underlying mechanisms of evasion from NK cells as rationale for improvement of NK cell-based immunotherapies. Front. Immunol. 2022, 13, 910595. [Google Scholar] [CrossRef]
- Jia, H.; Yang, H.; Xiong, H.; Luo, K.Q. NK cell exhaustion in the tumor microenvironment. Front. Immunol. 2023, 14, 1303605. [Google Scholar] [CrossRef]
- You, L.; Wu, W.; Wang, X.; Fang, L.; Adam, V.; Nepovimova, E.; Wu, Q.; Kuca, K. The role of hypoxia-inducible factor 1 in tumor immune evasion. Med. Res. Rev. 2021, 41, 1622–1643. [Google Scholar] [CrossRef]
- Domagala, J.; Lachota, M.; Klopotowska, M.; Graczyk-Jarzynka, A.; Domagala, A.; Zhylko, A.; Soroczynska, K.; Winiarska, M. The Tumor Microenvironment-A Metabolic Obstacle to NK Cells’ Activity. Cancers 2020, 12, 3542. [Google Scholar] [CrossRef]
- Habanjar, O.; Bingula, R.; Decombat, C.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment. Int. J. Mol. Sci. 2023, 24, 4002. [Google Scholar] [CrossRef]
- Ramesh, P.; Shivde, R.; Jaishankar, D.; Saleiro, D.; Le Poole, I.C. A Palette of Cytokines to Measure Anti-Tumor Efficacy of T Cell-Based Therapeutics. Cancers 2021, 13, 821. [Google Scholar] [CrossRef]
- Haist, M.; Stege, H.; Grabbe, S.; Bros, M. The Functional Crosstalk between Myeloid-Derived Suppressor Cells and Regulatory T Cells within the Immunosuppressive Tumor Microenvironment. Cancers 2021, 13, 210. [Google Scholar] [CrossRef]
- Li, Y.; Xiang, S.; Pan, W.; Wang, J.; Zhan, H.; Liu, S. Targeting tumor immunosuppressive microenvironment for pancreatic cancer immunotherapy: Current research and future perspective. Front. Oncol. 2023, 13, 1166860. [Google Scholar] [CrossRef]
- Huang, B.; Lang, X.; Li, X. The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 2022, 12, 1023177. [Google Scholar] [CrossRef]
- Lin, Y.; He, Z.; Ye, J.; Liu, Z.; She, X.; Gao, X.; Liang, R. Progress in Understanding the IL-6/STAT3 Pathway in Colorectal Cancer. Onco Targets Ther. 2020, 13, 13023–13032. [Google Scholar] [CrossRef]
- Manore, S.G.; Doheny, D.L.; Wong, G.L.; Lo, H.W. IL-6/JAK/STAT3 Signaling in Breast Cancer Metastasis: Biology and Treatment. Front. Oncol. 2022, 12, 866014. [Google Scholar] [CrossRef]
- Xu, J.; Lin, H.; Wu, G.; Zhu, M.; Li, M. IL-6/STAT3 Is a Promising Therapeutic Target for Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 760971. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Jiang, Y.; Li, X.; He, Y.; Zeng, P.; Guo, Z.; Chang, Y.; Luo, H.; Liu, Y.; et al. Lentinan as an immunotherapeutic for treating lung cancer: A review of 12 years clinical studies in China. J. Cancer Res. Clin. Oncol. 2018, 144, 2177–2186. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Y.; Gad, E.; Wenner, C.A.; Chang, A.; Larson, E.R.; Dang, Y.; Martzen, M.; Standish, L.J.; Disis, M.L. Polysaccharide krestin is a novel TLR2 agonist that mediates inhibition of tumor growth via stimulation of CD8 T cells and NK cells. Clin. Cancer Res. 2011, 17, 67–76. [Google Scholar] [CrossRef]
- Lu, H.; Yang, Y.; Gad, E.; Inatsuka, C.; Wenner, C.A.; Disis, M.L.; Standish, L.J. TLR2 agonist PSK activates human NK cells and enhances the antitumor effect of HER2-targeted monoclonal antibody therapy. Clin. Cancer Res. 2011, 17, 6742–6753. [Google Scholar] [CrossRef]
- Lim, S.O.; Li, C.W.; Xia, W.; Cha, J.H.; Chan, L.C.; Wu, Y.; Chang, S.S.; Lin, W.C.; Hsu, J.M.; Hsu, Y.H.; et al. Deubiquitination and Stabilization of PD-L1 by CSN5. Cancer Cell 2016, 30, 925–939. [Google Scholar] [CrossRef]
- Hayakawa, T.; Yaguchi, T.; Kawakami, Y. Enhanced anti-tumor effects of the PD-1 blockade combined with a highly absorptive form of curcumin targeting STAT3. Cancer Sci. 2020, 111, 4326–4335. [Google Scholar] [CrossRef]
- Rawangkan, A.; Wongsirisin, P.; Namiki, K.; Iida, K.; Kobayashi, Y.; Shimizu, Y.; Fujiki, H.; Suganuma, M. Green Tea Catechin Is an Alternative Immune Checkpoint Inhibitor that Inhibits PD-L1 Expression and Lung Tumor Growth. Molecules 2018, 23, 2071. [Google Scholar] [CrossRef]
- Verdura, S.; Cuyàs, E.; Cortada, E.; Brunet, J.; Lopez-Bonet, E.; Martin-Castillo, B.; Bosch-Barrera, J.; Encinar, J.A.; Menendez, J.A. Resveratrol targets PD-L1 glycosylation and dimerization to enhance antitumor T-cell immunity. Aging 2020, 12, 8–34. [Google Scholar] [CrossRef]
- Gao, S.; Zhou, J.; Liu, N.; Wang, L.; Gao, Q.; Wu, Y.; Zhao, Q.; Liu, P.; Wang, S.; Liu, Y.; et al. Curcumin induces M2 macrophage polarization by secretion IL-4 and/or IL-13. J. Mol. Cell Cardiol. 2015, 85, 131–139. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, T.; Wang, X.; Wei, X.; Chen, Y.; Guo, L.; Zhang, J.; Wang, C. Curcumin Modulates Macrophage Polarization Through the Inhibition of the Toll-Like Receptor 4 Expression and its Signaling Pathways. Cell Physiol. Biochem. 2015, 36, 631–641. [Google Scholar] [CrossRef]
- Mileo, A.M.; Nisticò, P.; Miccadei, S. Polyphenols: Immunomodulatory and Therapeutic Implication in Colorectal Cancer. Front. Immunol. 2019, 10, 729. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, B.; Wang, N.; Gu, J. Tumor immunomodulatory effects of polyphenols. Front. Immunol. 2022, 13, 1041138. [Google Scholar] [CrossRef]
- Xue, J.; Schmidt, S.V.; Sander, J.; Draffehn, A.; Krebs, W.; Quester, I.; De Nardo, D.; Gohel, T.D.; Emde, M.; Schmidleithner, L.; et al. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 2014, 40, 274–288. [Google Scholar] [CrossRef]
- Wang, P.; Li, Z.; Song, Y.; Zhang, B.; Fan, C. Resveratrol-driven macrophage polarization: Unveiling mechanisms and therapeutic potential. Front. Pharmacol. 2024, 15, 1516609. [Google Scholar] [CrossRef]
- Wang, S.; Wang, J.; Chen, Z.; Luo, J.; Guo, W.; Sun, L.; Lin, L. Targeting M2-like tumor-associated macrophages is a potential therapeutic approach to overcome antitumor drug resistance. NPJ Precis. Oncol. 2024, 8, 31. [Google Scholar] [CrossRef]
- Lee, D.Y.; Park, C.W.; Lee, S.J.; Park, H.R.; Kim, S.H.; Son, S.U.; Park, J.; Shin, K.S. Anti-Cancer Effects of Panax ginseng Berry Polysaccharides via Activation of Immune-Related Cells. Front. Pharmacol. 2019, 10, 1411. [Google Scholar] [CrossRef]
- Barbieri, A.; Quagliariello, V.; Del Vecchio, V.; Falco, M.; Luciano, A.; Amruthraj, N.J.; Nasti, G.; Ottaiano, A.; Berretta, M.; Iaffaioli, R.V.; et al. Anticancer and Anti-Inflammatory Properties of Ganoderma lucidum Extract Effects on Melanoma and Triple-Negative Breast Cancer Treatment. Nutrients 2017, 9, 210. [Google Scholar] [CrossRef]
- Suarez-Arroyo, I.J.; Rosario-Acevedo, R.; Aguilar-Perez, A.; Clemente, P.L.; Cubano, L.A.; Serrano, J.; Schneider, R.J.; Martínez-Montemayor, M.M. Anti-tumor effects of Ganoderma lucidum (reishi) in inflammatory breast cancer in in vivo and in vitro models. PLoS ONE 2013, 8, e57431. [Google Scholar] [CrossRef]
- Sohretoglu, D.; Huang, S. Ganoderma lucidum Polysaccharides as An Anti-cancer Agent. Anticancer Agents Med. Chem. 2018, 18, 667–674. [Google Scholar] [CrossRef]
- Gu, Z.; Wu, Q.; Shang, B.; Zhang, K.; Zhang, W. Organoid co-culture models of the tumor microenvironment promote precision medicine. Cancer Innov. 2024, 3, e101. [Google Scholar] [CrossRef]
- Zhong, Z.; Vong, C.T.; Chen, F.; Tan, H.; Zhang, C.; Wang, N.; Cui, L.; Wang, Y.; Feng, Y. Immunomodulatory potential of natural products from herbal medicines as immune checkpoints inhibitors: Helping to fight against cancer via multiple targets. Med. Res. Rev. 2022, 42, 1246–1279. [Google Scholar] [CrossRef]
- Gomez-Cadena, A.; Barreto, A.; Fioretino, S.; Jandus, C. Immune system activation by natural products and complex fractions: A network pharmacology approach in cancer treatment. Cell Stress 2020, 4, 154–166. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef]
- Eglen, R.M.; Randle, D.H. Drug Discovery Goes Three-Dimensional: Goodbye to Flat High-Throughput Screening? Assay Drug Dev. Technol. 2015, 13, 262–265. [Google Scholar] [CrossRef]
- Costantino, M.; Corno, C.; Colombo, D.; Perego, P. Curcumin and Related Compounds in Cancer Cells: New Avenues for Old Molecules. Front. Pharmacol. 2022, 13, 889816. [Google Scholar] [CrossRef]
- Keyvani-Ghamsari, S.; Khorsandi, K.; Gul, A. Curcumin effect on cancer cells’ multidrug resistance: An update. Phytother. Res. 2020, 34, 2534–2556. [Google Scholar] [CrossRef]
- Zhu, H.; Wang, X.; Wang, X.; Liu, B.; Yuan, Y.; Zuo, X. Curcumin attenuates inflammation and cell apoptosis through regulating NF-κB and JAK2/STAT3 signaling pathway against acute kidney injury. Cell Cycle 2020, 19, 1941–1951. [Google Scholar] [CrossRef]
- Buhrmann, C.; Shayan, P.; Popper, B.; Goel, A.; Shakibaei, M. Sirt1 Is Required for Resveratrol-Mediated Chemopreventive Effects in Colorectal Cancer Cells. Nutrients 2016, 8, 145. [Google Scholar] [CrossRef]
- Zou, T.; Yang, Y.; Xia, F.; Huang, A.; Gao, X.; Fang, D.; Xiong, S.; Zhang, J. Resveratrol Inhibits CD4+ T cell activation by enhancing the expression and activity of Sirt1. PLoS ONE 2013, 8, e75139. [Google Scholar] [CrossRef]
- Knight, C.M.; Gutierrez-Juarez, R.; Lam, T.K.; Arrieta-Cruz, I.; Huang, L.; Schwartz, G.; Barzilai, N.; Rossetti, L. Mediobasal hypothalamic SIRT1 is essential for resveratrol’s effects on insulin action in rats. Diabetes 2011, 60, 2691–2700. [Google Scholar] [CrossRef]
- Morita, Y.; Wada-Hiraike, O.; Yano, T.; Shirane, A.; Hirano, M.; Hiraike, H.; Koyama, S.; Oishi, H.; Yoshino, O.; Miyamoto, Y.; et al. Resveratrol promotes expression of SIRT1 and StAR in rat ovarian granulosa cells: An implicative role of SIRT1 in the ovary. Reprod. Biol. Endocrinol. 2012, 10, 14. [Google Scholar] [CrossRef]
- Wu, D. Green tea EGCG, T-cell function, and T-cell-mediated autoimmune encephalomyelitis. J. Investig. Med. 2016, 64, 1213–1219. [Google Scholar] [CrossRef]
- Pae, M.; Ren, Z.; Meydani, M.; Shang, F.; Meydani, S.N.; Wu, D. Epigallocatechin-3-gallate directly suppresses T cell proliferation through impaired IL-2 utilization and cell cycle progression. J. Nutr. 2010, 140, 1509–1515. [Google Scholar] [CrossRef]
- Schwager, J.; Seifert, N.; Bompard, A.; Raederstorff, D.; Bendik, I. Resveratrol, EGCG and Vitamins Modulate Activated T Lymphocytes. Molecules 2021, 26, 5600. [Google Scholar] [CrossRef]
- Wilson, B.A.P.; Thornburg, C.C.; Henrich, C.J.; Grkovic, T.; O’Keefe, B.R. Creating and screening natural product libraries. Nat. Prod. Rep. 2020, 37, 893–918. [Google Scholar] [CrossRef]
- Thornburg, C.C.; Britt, J.R.; Evans, J.R.; Akee, R.K.; Whitt, J.A.; Trinh, S.K.; Harris, M.J.; Thompson, J.R.; Ewing, T.L.; Shipley, S.M.; et al. NCI Program for Natural Product Discovery: A Publicly-Accessible Library of Natural Product Fractions for High-Throughput Screening. ACS Chem. Biol. 2018, 13, 2484–2497. [Google Scholar] [CrossRef]
- Henrich, C.J.; Beutler, J.A. Matching the power of high throughput screening to the chemical diversity of natural products. Nat. Prod. Rep. 2013, 30, 1284–1298. [Google Scholar] [CrossRef]
- Ayon, N.J. High-Throughput Screening of Natural Product and Synthetic Molecule Libraries for Antibacterial Drug Discovery. Metabolites 2023, 13, 625. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, F.; Li, G.; Xu, J.; Zhang, J.; Gullen, E.; Yang, J.; Wang, J. From immune checkpoints to therapies: Understanding immune checkpoint regulation and the influence of natural products and traditional medicine on immune checkpoint and immunotherapy in lung cancer. Front. Immunol. 2024, 15, 1340307. [Google Scholar] [CrossRef]
- Yu, W.K.; Xu, Z.Y.; Yuan, L.; Mo, S.; Xu, B.; Cheng, X.D.; Qin, J.J. Targeting β-Catenin Signaling by Natural Products for Cancer Prevention and Therapy. Front. Pharmacol. 2020, 11, 984. [Google Scholar] [CrossRef]
- Zhang, W.; Li, S.; Li, C.; Li, T.; Huang, Y. Remodeling tumor microenvironment with natural products to overcome drug resistance. Front. Immunol. 2022, 13, 1051998. [Google Scholar] [CrossRef]
- Chen, L.; Yu, J. Modulation of Toll-like receptor signaling in innate immunity by natural products. Int. Immunopharmacol. 2016, 37, 65–70. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Muguruma, M.; Teraoka, S.; Miyahara, K.; Ueda, A.; Asaoka, M.; Okazaki, M.; Kawate, T.; Kuroda, M.; Miyagi, Y.; Ishikawa, T. Differences in drug sensitivity between two-dimensional and three-dimensional culture systems in triple-negative breast cancer cell lines. Biochem. Biophys. Res. Commun. 2020, 533, 268–274. [Google Scholar] [CrossRef]
- Agena, R.; Cortés-Sánchez, A.J.; Hernández-Sánchez, H.; Álvarez-Salas, L.M.; Martínez-Rodríguez, O.P.; García, V.H.R.; Jaramillo Flores, M.E. Pro-Apoptotic Activity and Cell Cycle Arrest of Caulerpa sertularioides against SKLU-1 Cancer Cell in 2D and 3D Cultures. Molecules 2023, 28, 4361. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, S.; Liu, H.; Wang, Y.; Jiao, Z.; Nie, Y.; Wang, H.; Liu, T.; Song, K. Evaluation of anti-tumor effects of crocin on a novel 3D tissue-engineered tumor model based on sodium alginate/gelatin microbead. Int. J. Biol. Macromol. 2021, 174, 339–351. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Yang, Y.; Inatsuka, C.; Gad, E.; Disis, M.L.; Standish, L.J.; Pugh, N.; Pasco, D.S.; Lu, H. Protein-bound polysaccharide-K induces IL-1β via TLR2 and NLRP3 inflammasome activation. Innate Immun. 2014, 20, 857–866. [Google Scholar] [CrossRef]
- Inatsuka, C.; Yang, Y.; Gad, E.; Rastetter, L.; Disis, M.L.; Lu, H. Gamma delta T cells are activated by polysaccharide K (PSK) and contribute to the anti-tumor effect of PSK. Cancer Immunol. Immunother. 2013, 62, 1335–1345. [Google Scholar] [CrossRef]
- Ahn, H.; Jeon, E.; Kim, J.C.; Kang, S.G.; Yoon, S.I.; Ko, H.J.; Kim, P.H.; Lee, G.S. Lentinan from shiitake selectively attenuates AIM2 and non-canonical inflammasome activation while inducing pro-inflammatory cytokine production. Sci. Rep. 2017, 7, 1314. [Google Scholar] [CrossRef]
- Ina, K.; Kataoka, T.; Ando, T. The use of lentinan for treating gastric cancer. Anticancer Agents Med. Chem. 2013, 13, 681–688. [Google Scholar] [CrossRef]
- Xu, X.; Pan, C.; Zhang, L.; Ashida, H. Immunomodulatory beta-glucan from Lentinus edodes activates mitogen-activated protein kinases and nuclear factor-kappaB in murine RAW 264.7 macrophages. J. Biol. Chem. 2011, 286, 31194–31198. [Google Scholar] [CrossRef]
- Nishitani, Y.; Zhang, L.; Yoshida, M.; Azuma, T.; Kanazawa, K.; Hashimoto, T.; Mizuno, M. Intestinal anti-inflammatory activity of lentinan: Influence on IL-8 and TNFR1 expression in intestinal epithelial cells. PLoS ONE 2013, 8, e62441. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, Q.; Rong, C.; Wang, S.; Zhao, Z.; Liu, Y.; Xu, J. Immunomodulatory Effects of Edible and Medicinal Mushrooms and Their Bioactive Immunoregulatory Products. J. Fungi 2020, 6, 269. [Google Scholar] [CrossRef]
- Ren, L.; Perera, C.; Hemar, Y. Antitumor activity of mushroom polysaccharides: A review. Food Funct. 2012, 3, 1118–1130. [Google Scholar] [CrossRef]
- Huo, J.L.; Fu, W.J.; Liu, Z.H.; Lu, N.; Jia, X.Q.; Liu, Z.S. Research advance of natural products in tumor immunotherapy. Front. Immunol. 2022, 13, 972345. [Google Scholar] [CrossRef]
- Song, P.; Song, F.; Shao, T.; Wang, P.; Li, R.; Chen, Z.S.; Zhang, Z.; Xue, G. Natural products: Promising therapeutics for targeting regulatory immune cells in the tumor microenvironment. Front. Pharmacol. 2024, 15, 1481850. [Google Scholar] [CrossRef]
- Desamero, M.J.M.; Chung, S.H.; Kakuta, S. Insights on the Functional Role of Beta-Glucans in Fungal Immunity Using Receptor-Deficient Mouse Models. Int. J. Mol. Sci. 2021, 22, 4778. [Google Scholar] [CrossRef]
- Wang, M.X.; Luo, W.; Ye, L.; Jin, L.M.; Yang, B.; Zhang, Q.H.; Qian, J.C.; Wang, Y.; Zhang, Y.; Liang, G. Dectin-1 plays a deleterious role in high fat diet-induced NAFLD of mice through enhancing macrophage activation. Acta Pharmacol. Sin. 2023, 44, 120–132. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Matsushita, T.; Takayama, K.; Matsumoto, T.; Nishida, K.; Kuroda, R.; Kurosaka, M. Disruption of Sirt1 in chondrocytes causes accelerated progression of osteoarthritis under mechanical stress and during ageing in mice. Ann. Rheum. Dis. 2014, 73, 1397–1404. [Google Scholar] [CrossRef]
- Jiménez-Flores, L.M.; López-Briones, S.; Macías-Cervantes, M.H.; Ramírez-Emiliano, J.; Pérez-Vázquez, V. A PPARγ, NF-κB and AMPK-dependent mechanism may be involved in the beneficial effects of curcumin in the diabetic db/db mice liver. Molecules 2014, 19, 8289–8302. [Google Scholar] [CrossRef]
- Khosravi, M.A.; Seifert, R. Clinical trials on curcumin in relation to its bioavailability and effect on malignant diseases: Critical analysis. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 3477–3491. [Google Scholar] [CrossRef]
- Scazzocchio, B.; Minghetti, L.; D’Archivio, M. Interaction between Gut Microbiota and Curcumin: A New Key of Understanding for the Health Effects of Curcumin. Nutrients 2020, 12, 2499. [Google Scholar] [CrossRef]
- Kroon, M.; Berbee, J.K.; Majait, S.; Swart, E.L.; van Tellingen, O.; van Laarhoven, H.W.M.; Kemper, E.M. Non-therapeutic plasma levels in individuals utilizing curcumin supplements in daily life. Front. Nutr. 2023, 10, 1267035. [Google Scholar] [CrossRef]
- Chhabra, G.; Singh, C.K.; Amiri, D.; Akula, N.; Ahmad, N. Recent Advancements on Immunomodulatory Mechanisms of Resveratrol in Tumor Microenvironment. Molecules 2021, 26, 1343. [Google Scholar] [CrossRef]
- Chen, L.; Musa, A.E. Boosting immune system against cancer by resveratrol. Phytother. Res. 2021, 35, 5514–5526. [Google Scholar] [CrossRef]
- Rogina, B.; Tissenbaum, H.A. SIRT1, resveratrol and aging. Front. Genet. 2024, 15, 1393181. [Google Scholar] [CrossRef]
- Wang, Y.; Qiao, S.L.; Wang, J.; Yu, M.Z.; Wang, N.N.; Mamuti, M.; An, H.W.; Lin, Y.X.; Wang, H. Engineered CpG-Loaded Nanorobots Drive Autophagy-Mediated Immunity for TLR9-Positive Cancer Therapy. Adv. Mater. 2024, 36, e2306248. [Google Scholar] [CrossRef]
- Gong, H.; Griffin, J.D.; Groer, C.E.; Wu, S.; Downes, G.M.; Markum, G.; Abdelaziz, M.M.; Alhakamy, N.A.; Forrest, M.L.; Berkland, C.J. Glatiramer Acetate Complexes CpG Oligodeoxynucleotides into Nanoparticles and Boosts Their TLR9-Driven Immunity. Mol. Pharm. 2024, 21, 6323–6338. [Google Scholar] [CrossRef]
- Karapetyan, L.; Luke, J.J.; Davar, D. Toll-Like Receptor 9 Agonists in Cancer. Onco Targets Ther. 2020, 13, 10039–10060. [Google Scholar] [CrossRef]
- Montamat, G.; Leonard, C.; Poli, A.; Klimek, L.; Ollert, M. CpG Adjuvant in Allergen-Specific Immunotherapy: Finding the Sweet Spot for the Induction of Immune Tolerance. Front. Immunol. 2021, 12, 590054. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J.I. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2021, 11, 316–329. [Google Scholar] [CrossRef]
- Jeong, S.R.; Kang, M. Exploring Tumor-Immune Interactions in Co-Culture Models of T Cells and Tumor Organoids Derived from Patients. Int. J. Mol. Sci. 2023, 24, 14609. [Google Scholar] [CrossRef]
- Zhou, G.; Lieshout, R.; van Tienderen, G.S.; de Ruiter, V.; van Royen, M.E.; Boor, P.P.C.; Magré, L.; Desai, J.; Köten, K.; Kan, Y.Y.; et al. Modelling immune cytotoxicity for cholangiocarcinoma with tumour-derived organoids and effector T cells. Br. J. Cancer 2022, 127, 649–660. [Google Scholar] [CrossRef]
- Magré, L.; Verstegen, M.M.A.; Buschow, S.; van der Laan, L.J.W.; Peppelenbosch, M.; Desai, J. Emerging organoid-immune co-culture models for cancer research: From oncoimmunology to personalized immunotherapies. J. Immunother. Cancer 2023, 11, e006290. [Google Scholar] [CrossRef]
- Holokai, L.; Chakrabarti, J.; Lundy, J.; Croagh, D.; Adhikary, P.; Richards, S.S.; Woodson, C.; Steele, N.; Kuester, R.; Scott, A.; et al. Murine- and Human-Derived Autologous Organoid/Immune Cell Co-Cultures as Pre-Clinical Models of Pancreatic Ductal Adenocarcinoma. Cancers 2020, 12, 3816. [Google Scholar] [CrossRef]
- Yuki, K.; Cheng, N.; Nakano, M.; Kuo, C.J. Organoid Models of Tumor Immunology. Trends Immunol. 2020, 41, 652–664. [Google Scholar] [CrossRef]
- Zhou, Z.; Cong, L.; Cong, X. Patient-Derived Organoids in Precision Medicine: Drug Screening, Organoid-on-a-Chip and Living Organoid Biobank. Front. Oncol. 2021, 11, 762184. [Google Scholar] [CrossRef]
- Monnier, M.; Paolini, L.; Vinatier, E.; Mantovani, A.; Delneste, Y.; Jeannin, P. Antitumor strategies targeting macrophages: The importance of considering the differences in differentiation/polarization processes between human and mouse macrophages. J. Immunother. Cancer 2022, 10, e005560. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Rice, P.J.; Adams, E.L.; Ozment-Skelton, T.; Gonzalez, A.J.; Goldman, M.P.; Lockhart, B.E.; Barker, L.A.; Breuel, K.F.; Deponti, W.K.; Kalbfleisch, J.H.; et al. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J. Pharmacol. Exp. Ther. 2005, 314, 1079–1086. [Google Scholar] [CrossRef]
- Tiriac, H.; Belleau, P.; Engle, D.D.; Plenker, D.; Deschênes, A.; Somerville, T.D.D.; Froeling, F.E.M.; Burkhart, R.A.; Denroche, R.E.; Jang, G.H.; et al. Organoid Profiling Identifies Common Responders to Chemotherapy in Pancreatic Cancer. Cancer Discov. 2018, 8, 1112–1129. [Google Scholar] [CrossRef]
- Ooft, S.N.; Weeber, F.; Dijkstra, K.K.; McLean, C.M.; Kaing, S.; van Werkhoven, E.; Schipper, L.; Hoes, L.; Vis, D.J.; van de Haar, J.; et al. Patient-derived organoids can predict response to chemotherapy in metastatic colorectal cancer patients. Sci. Transl. Med. 2019, 11, eaay2574. [Google Scholar] [CrossRef]
- Cattaneo, C.M.; Dijkstra, K.K.; Fanchi, L.F.; Kelderman, S.; Kaing, S.; van Rooij, N.; van den Brink, S.; Schumacher, T.N.; Voest, E.E. Tumor organoid-T-cell coculture systems. Nat. Protoc. 2020, 15, 15–39. [Google Scholar] [CrossRef]
- Zeng, G.; Yu, Y.; Wang, M.; Liu, J.; He, G.; Yu, S.; Yan, H.; Yang, L.; Li, H.; Peng, X. Advancing cancer research through organoid technology. J. Transl. Med. 2024, 22, 1007. [Google Scholar] [CrossRef]
- Recaldin, T.; Steinacher, L.; Gjeta, B.; Harter, M.F.; Adam, L.; Kromer, K.; Mendes, M.P.; Bellavista, M.; Nikolaev, M.; Lazzaroni, G.; et al. Human organoids with an autologous tissue-resident immune compartment. Nature 2024, 633, 165–173. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Aref, A.R.; Lizotte, P.H.; Ivanova, E.; Stinson, S.; Zhou, C.W.; Bowden, M.; Deng, J.; Liu, H.; Miao, D.; et al. Ex Vivo Profiling of PD-1 Blockade Using Organotypic Tumor Spheroids. Cancer Discov. 2018, 8, 196–215. [Google Scholar] [CrossRef]
- Ahn, S.J.; Lee, S.; Kwon, D.; Oh, S.; Park, C.; Jeon, S.; Lee, J.H.; Kim, T.S.; Oh, I.U. Essential Guidelines for Manufacturing and Application of Organoids. Int. J. Stem Cells 2024, 17, 102–112. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, H.; Zhao, L.; Hong, F.; Hao, J.; Zhang, Z.; Sheng, W.; Song, L.; Deng, C.X.; Zhao, B.; et al. Standard: Human intestinal organoids. Cell Regen. 2023, 12, 23. [Google Scholar] [CrossRef]
- Lin, H.; Wang, Y.; Cheng, C.; Qian, Y.; Hao, J.; Zhang, Z.; Sheng, W.; Song, L.; Deng, C.X.; Zhao, B.; et al. Standard: Human intestinal cancer organoids. Cell Regen. 2023, 12, 24. [Google Scholar] [CrossRef]
- Yang, R.; Qi, Y.; Zhang, X.; Gao, H.; Yu, Y. Living biobank: Standardization of organoid construction and challenges. Chin. Med. J. 2024, 137, 3050–3060. [Google Scholar] [CrossRef]
- Ahn, S.J. Standards for Organoids. Int. J. Stem Cells 2024, 17, 99–101. [Google Scholar] [CrossRef]
- Lee, H.A.; Woo, D.H.; Lim, D.S.; Oh, J.; Kim, C.Y.; Bae, O.N.; Ahn, S.J. Guidelines for Manufacturing and Application of Organoids: Heart. Int. J. Stem Cells 2024, 17, 130–140. [Google Scholar] [CrossRef]
- Moon, H.R.; Mun, S.J.; Kim, T.H.; Kim, H.; Kang, D.; Kim, S.; Shin, J.H.; Choi, D.; Ahn, S.J.; Son, M.J. Guidelines for Manufacturing and Application of Organoids: Liver. Int. J. Stem Cells 2024, 17, 120–129. [Google Scholar] [CrossRef]
- Renner, H.; Grabos, M.; Becker, K.J.; Kagermeier, T.E.; Wu, J.; Otto, M.; Peischard, S.; Zeuschner, D.; TsyTsyura, Y.; Disse, P.; et al. A fully automated high-throughput workflow for 3D-based chemical screening in human midbrain organoids. elife 2020, 9, e52904. [Google Scholar] [CrossRef]
- Wensink, G.E.; Elias, S.G.; Mullenders, J.; Koopman, M.; Boj, S.F.; Kranenburg, O.W.; Roodhart, J.M.L. Patient-derived organoids as a predictive biomarker for treatment response in cancer patients. NPJ Precis. Oncol. 2021, 5, 30. [Google Scholar] [CrossRef]
- de Witte, C.J.; Espejo Valle-Inclan, J.; Hami, N.; Lõhmussaar, K.; Kopper, O.; Vreuls, C.P.H.; Jonges, G.N.; van Diest, P.; Nguyen, L.; Clevers, H.; et al. Patient-Derived Ovarian Cancer Organoids Mimic Clinical Response and Exhibit Heterogeneous Inter- and Intrapatient Drug Responses. Cell Rep. 2020, 31, 107762. [Google Scholar] [CrossRef]
- Jiang, K.L.; Wang, X.X.; Liu, X.J.; Guo, L.K.; Chen, Y.Q.; Jia, Q.L.; Yang, K.M.; Ling, J.H. Success rate of current human-derived gastric cancer organoids establishment and influencing factors: A systematic review and meta-analysis. World J. Gastrointest. Oncol. 2024, 16, 1626–1646. [Google Scholar] [CrossRef]
- Kroll, K.T.; Mata, M.M.; Homan, K.A.; Micallef, V.; Carpy, A.; Hiratsuka, K.; Morizane, R.; Moisan, A.; Gubler, M.; Walz, A.C.; et al. Immune-infiltrated kidney organoid-on-chip model for assessing T cell bispecific antibodies. Proc. Natl. Acad. Sci. USA 2023, 120, e2305322120. [Google Scholar] [CrossRef]
- Hao, X.; Zu, M.; Ning, J.; Zhou, X.; Gong, Y.; Han, X.; Meng, Q.; Li, D.; Ding, S. Antitumor effect of luteolin proven by patient-derived organoids of gastric cancer. Phytother. Res. 2023, 37, 5315–5327. [Google Scholar] [CrossRef]
- Chen, L.; Dai, Z.; Ge, C.; Huang, D.; Zhou, X.; Pan, K.; Xu, W.; Fu, J.; Du, J.L. Specific metabolic response of patient-derived organoids to curcumin of colorectal cancer. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2022, 1203, 123260. [Google Scholar] [CrossRef]
- Chen, X.; Lu, J.L.; Li, H.; Liu, G.Y.; Li, T.T.; Xiao, K.H.; Ye, H.S.; Li, S.; Chen, X.; Liu, J. Resveratrol Induces Oxidative Stress and Downregulates GPX4 and xCT to Activate the Ferroptosis Pathway for Anti-Bladder Cancer Organoids. J. Cancer 2025, 16, 2613–2625. [Google Scholar] [CrossRef]
- Meng, S.; Cao, Y.; Lu, L.; Li, X.; Sun, S.; Jiang, F.; Lu, J.; Fan, D.; Han, X.; Yao, T. Quercetin Promote the Chemosensitivity in Organoids Derived from Patients with Breast Cancer. Breast Cancer Targets Ther. 2024, 16, 993–1004. [Google Scholar] [CrossRef]
- Ni, Y.; Liu, J.; Zeng, L.; Yang, Y.; Liu, L.; Yao, M.; Chai, L.; Zhang, L.; Li, Y.; Zhang, L.; et al. Natural product manoalide promotes EGFR-TKI sensitivity of lung cancer cells by KRAS-ERK pathway and mitochondrial Ca(2+) overload-induced ferroptosis. Front. Pharmacol. 2022, 13, 1109822. [Google Scholar] [CrossRef]
- Abugomaa, A.; Elbadawy, M.; Ishihara, Y.; Yamamoto, H.; Kaneda, M.; Yamawaki, H.; Shinohara, Y.; Usui, T.; Sasaki, K. Anti-cancer activity of Chaga mushroom (Inonotus obliquus) against dog bladder cancer organoids. Front. Pharmacol. 2023, 14, 1159516. [Google Scholar] [CrossRef]
- Li, D.; Cao, D.; Sun, Y.; Cui, Y.; Zhang, Y.; Jiang, J.; Cao, X. The roles of epigallocatechin gallate in the tumor microenvironment, metabolic reprogramming, and immunotherapy. Front. Immunol. 2024, 15, 1331641. [Google Scholar] [CrossRef]
- Tong, L.; Cui, W.; Zhang, B.; Fonseca, P.; Zhao, Q.; Zhang, P.; Xu, B.; Zhang, Q.; Li, Z.; Seashore-Ludlow, B.; et al. Patient-derived organoids in precision cancer medicine. Med 2024, 5, 1351–1377. [Google Scholar] [CrossRef]
- Yan, H.H.N.; Chan, A.S.; Lai, F.P.; Leung, S.Y. Organoid cultures for cancer modeling. Cell Stem Cell 2023, 30, 917–937. [Google Scholar] [CrossRef]
- Cong, R.; Lu, C.; Li, X.; Xu, Z.; Wang, Y.; Sun, S. Tumor organoids in cancer medicine: From model systems to natural compound screening. Pharm. Biol. 2025, 63, 89–109. [Google Scholar] [CrossRef]
- Hossein, G.; Halvaei, S.; Heidarian, Y.; Dehghani-Ghobadi, Z.; Hassani, M.; Hosseini, H.; Naderi, N.; Sheikh Hassani, S. Pectasol-C Modified Citrus Pectin targets Galectin-3-induced STAT3 activation and synergize paclitaxel cytotoxic effect on ovarian cancer spheroids. Cancer Med. 2019, 8, 4315–4329. [Google Scholar] [CrossRef]
- Phan, N.; Hong, J.J.; Tofig, B.; Mapua, M.; Elashoff, D.; Moatamed, N.A.; Huang, J.; Memarzadeh, S.; Damoiseaux, R.; Soragni, A. A simple high-throughput approach identifies actionable drug sensitivities in patient-derived tumor organoids. Commun. Biol 2019, 2, 78. [Google Scholar] [CrossRef]
- Mulholland, T.; McAllister, M.; Patek, S.; Flint, D.; Underwood, M.; Sim, A.; Edwards, J.; Zagnoni, M. Drug screening of biopsy-derived spheroids using a self-generated microfluidic concentration gradient. Sci. Rep. 2018, 8, 14672. [Google Scholar] [CrossRef]
- Liu, X.; Weaver, E.M.; Hummon, A.B. Evaluation of therapeutics in three-dimensional cell culture systems by MALDI imaging mass spectrometry. Anal. Chem. 2013, 85, 6295–6302. [Google Scholar] [CrossRef]
- Berlemont, R. Distribution and diversity of enzymes for polysaccharide degradation in fungi. Sci. Rep. 2017, 7, 222. [Google Scholar] [CrossRef]
- Maehara, Y.; Tsujitani, S.; Saeki, H.; Oki, E.; Yoshinaga, K.; Emi, Y.; Morita, M.; Kohnoe, S.; Kakeji, Y.; Yano, T.; et al. Biological mechanism and clinical effect of protein-bound polysaccharide K (KRESTIN(®)): Review of development and future perspectives. Surg. Today 2012, 42, 8–28. [Google Scholar] [CrossRef]
- Tao, S.; Ren, Z.; Yang, Z.; Duan, S.; Wan, Z.; Huang, J.; Liu, C.; Wei, G. Effects of Different Molecular Weight Polysaccharides From Dendrobium officinale Kimura & Migo on Human Colorectal Cancer and Transcriptome Analysis of Differentially Expressed Genes. Front. Pharmacol. 2021, 12, 704486. [Google Scholar] [CrossRef]
- Dai, S.X.; Li, W.X.; Han, F.F.; Guo, Y.C.; Zheng, J.J.; Liu, J.Q.; Wang, Q.; Gao, Y.D.; Li, G.H.; Huang, J.F. In silico identification of anti-cancer compounds and plants from traditional Chinese medicine database. Sci. Rep. 2016, 6, 25462. [Google Scholar] [CrossRef]
- Huang, M.Y.; Chen, Y.C.; Lyu, W.Y.; He, X.Y.; Ye, Z.H.; Huang, C.Y.; He, X.L.; Chen, X.; Chen, X.; Zhang, B.; et al. Ginsenoside Rh2 augmented anti-PD-L1 immunotherapy by reinvigorating CD8(+) T cells via increasing intratumoral CXCL10. Pharmacol. Res. 2023, 198, 106988. [Google Scholar] [CrossRef]
- Chang, H.L.; Kuo, Y.H.; Wu, L.H.; Chang, C.M.; Cheng, K.J.; Tyan, Y.C.; Lee, C.H. The extracts of Astragalus membranaceus overcome tumor immune tolerance by inhibition of tumor programmed cell death protein ligand-1 expression. Int. J. Med. Sci. 2020, 17, 939–945. [Google Scholar] [CrossRef]
- Ravindran Menon, D.; Li, Y.; Yamauchi, T.; Osborne, D.G.; Vaddi, P.K.; Wempe, M.F.; Zhai, Z.; Fujita, M. EGCG Inhibits Tumor Growth in Melanoma by Targeting JAK-STAT Signaling and Its Downstream PD-L1/PD-L2-PD1 Axis in Tumors and Enhancing Cytotoxic T-Cell Responses. Pharmaceuticals 2021, 14, 1081. [Google Scholar] [CrossRef]
- Li, Z.D.; Liu, F.; Zeng, Y.; Liu, Y.; Luo, W.; Yuan, F.; Li, S.; Li, Q.; Chen, J.; Fujita, M.; et al. EGCG suppresses PD-1 expression of T cells via inhibiting NF-κB phosphorylation and nuclear translocation. Int. Immunopharmacol. 2024, 133, 112069. [Google Scholar] [CrossRef]
- Zhang, F.L.; Hu, Z.; Wang, Y.F.; Zhang, W.J.; Zhou, B.W.; Sun, Q.S.; Lin, Z.B.; Liu, K.X. Organoids transplantation attenuates intestinal ischemia/reperfusion injury in mice through L-Malic acid-mediated M2 macrophage polarization. Nat. Commun. 2023, 14, 6779. [Google Scholar] [CrossRef]
- Abdollahi, E.; Johnston, T.P.; Ghaneifar, Z.; Vahedi, P.; Goleij, P.; Azhdari, S.; Moghaddam, A.S. Immunomodulatory Therapeutic Effects of Curcumin on M1/M2 Macrophage Polarization in Inflammatory Diseases. Curr. Mol. Pharmacol. 2023, 16, 2–14. [Google Scholar] [CrossRef]
- Mohammadi, A.; Blesso, C.N.; Barreto, G.E.; Banach, M.; Majeed, M.; Sahebkar, A. Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent. J. Nutr. Biochem. 2019, 66, 1–16. [Google Scholar] [CrossRef]
- Almatroodi, S.A.; Almatroudi, A.; Alsahli, M.A.; Aljasir, M.A.; Syed, M.A.; Rahmani, A.H. Epigallocatechin-3-Gallate (EGCG), an Active Compound of Green Tea Attenuates Acute Lung Injury Regulating Macrophage Polarization and Krüpple-Like-Factor 4 (KLF4) Expression. Molecules 2020, 25, 2853. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Hua, K.F.; Lin, C.C.; Lin, C.H.; Hsu, J.; Wong, C.H. Extract of Reishi polysaccharides induces cytokine expression via TLR4-modulated protein kinase signaling pathways. J. Immunol. 2004, 173, 5989–5999. [Google Scholar] [CrossRef]
- Wang, S.Y.; Hsu, M.L.; Hsu, H.C.; Tzeng, C.H.; Lee, S.S.; Shiao, M.S.; Ho, C.K. The anti-tumor effect of Ganoderma lucidum is mediated by cytokines released from activated macrophages and T lymphocytes. Int. J. Cancer 1997, 70, 699–705. [Google Scholar] [CrossRef]
- Hundsberger, H.; Stierschneider, A.; Sarne, V.; Ripper, D.; Schimon, J.; Weitzenböck, H.P.; Schild, D.; Jacobi, N.; Eger, A.; Atzler, J.; et al. Concentration-Dependent Pro- and Antitumor Activities of Quercetin in Human Melanoma Spheroids: Comparative Analysis of 2D and 3D Cell Culture Models. Molecules 2021, 26, 0717. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Fong, E.L.S.; Balachander, G.M.; Chen, Z.; Soragni, A.; et al. Organoids. Nat. Rev. Methods Primers 2022, 2, 94. [Google Scholar] [CrossRef]
- Gassl, V.; Aberle, M.R.; Boonen, B.; Vaes, R.D.W.; Olde Damink, S.W.M.; Rensen, S.S. Chemosensitivity of 3D Pancreatic Cancer Organoids Is Not Affected by Transformation to 2D Culture or Switch to Physiological Culture Medium. Cancers 2022, 14, 5617. [Google Scholar] [CrossRef]
- Sharick, J.T.; Walsh, C.M.; Sprackling, C.M.; Pasch, C.A.; Pham, D.L.; Esbona, K.; Choudhary, A.; Garcia-Valera, R.; Burkard, M.E.; McGregor, S.M.; et al. Metabolic Heterogeneity in Patient Tumor-Derived Organoids by Primary Site and Drug Treatment. Front. Oncol. 2020, 10, 553. [Google Scholar] [CrossRef]
- Smabers, L.P.; Wensink, E.; Verissimo, C.S.; Koedoot, E.; Pitsa, K.C.; Huismans, M.A.; Higuera Barón, C.; Doorn, M.; Valkenburg-van Iersel, L.B.; Cirkel, G.A.; et al. Organoids as a biomarker for personalized treatment in metastatic colorectal cancer: Drug screen optimization and correlation with patient response. J. Exp. Clin. Cancer Res. 2024, 43, 61. [Google Scholar] [CrossRef]
- Zou, J.; Wang, S.; Chai, N.; Yue, H.; Ye, P.; Guo, P.; Li, F.; Wei, B.; Ma, G.; Wei, W.; et al. Construction of gastric cancer patient-derived organoids and their utilization in a comparative study of clinically used paclitaxel nanoformulations. J. Nanobiotechnol. 2022, 20, 233. [Google Scholar] [CrossRef]
- Tebon, P.J.; Wang, B.; Markowitz, A.L.; Davarifar, A.; Tsai, B.L.; Krawczuk, P.; Gonzalez, A.E.; Sartini, S.; Murray, G.F.; Nguyen, H.T.L.; et al. Drug screening at single-organoid resolution via bioprinting and interferometry. Nat. Commun. 2023, 14, 3168. [Google Scholar] [CrossRef]
- Qu, S.; Xu, R.; Yi, G.; Li, Z.; Zhang, H.; Qi, S.; Huang, G. Patient-derived organoids in human cancer: A platform for fundamental research and precision medicine. Mol. Biomed. 2024, 5, 6. [Google Scholar] [CrossRef]
- Wang, J.; Tao, X.; Zhu, J.; Dai, Z.; Du, Y.; Xie, Y.; Chu, X.; Fu, G.; Lei, Z. Tumor organoid-immune co-culture models: Exploring a new perspective of tumor immunity. Cell Death Discov. 2025, 11, 195. [Google Scholar] [CrossRef]
- Tewari, D.; Rawat, P.; Singh, P.K. Adverse drug reactions of anticancer drugs derived from natural sources. Food Chem. Toxicol. 2019, 123, 522–535. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Zamanian, M.Y.; Al-Ani, A.M.; Jabbar, T.L.; Kareem, A.K.; Aghaei, Z.H.; Tahernia, H.; Hjazi, A.; Jissir, S.A.; Hakimizadeh, E. Targeting STAT3 signaling pathway by curcumin and its analogues for breast cancer: A narrative review. Animal Model. Exp. Med. 2024, 7, 853–867. [Google Scholar] [CrossRef]
- Stasiłowicz, A.; Tykarska, E.; Lewandowska, K.; Kozak, M.; Miklaszewski, A.; Kobus-Cisowska, J.; Szymanowska, D.; Plech, T.; Jenczyk, J.; Cielecka-Piontek, J. Hydroxypropyl-β-cyclodextrin as an effective carrier of curcumin-piperine nutraceutical system with improved enzyme inhibition properties. J. Enzyme Inhib. Med. Chem. 2020, 35, 1811–1821. [Google Scholar] [CrossRef]
- Miura, T.; Tanaka, R. In vitro Vasculogenesis Models Revisited-Measurement of VEGF Diffusion in Matrigel. Math. Model. Nat. Phenom. 2009, 4, 118–130. [Google Scholar] [CrossRef]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef]
- Bahadoran, M.; Shamloo, A.; Nokoorani, Y.D. Development of a polyvinyl alcohol/sodium alginate hydrogel-based scaffold incorporating bFGF-encapsulated microspheres for accelerated wound healing. Sci. Rep. 2020, 10, 7342. [Google Scholar] [CrossRef]
- Yadav, V.R.; Suresh, S.; Devi, K.; Yadav, S. Effect of cyclodextrin complexation of curcumin on its solubility and antiangiogenic and anti-inflammatory activity in rat colitis model. AAPS PharmSciTech 2009, 10, 752–762. [Google Scholar] [CrossRef]
- Mangolim, C.S.; Moriwaki, C.; Nogueira, A.C.; Sato, F.; Baesso, M.L.; Neto, A.M.; Matioli, G. Curcumin-β-cyclodextrin inclusion complex: Stability, solubility, characterisation by FT-IR, FT-Raman, X-ray diffraction and photoacoustic spectroscopy, and food application. Food Chem. 2014, 153, 361–370. [Google Scholar] [CrossRef]
- Saputra, O.A.; Lestari, W.A.; Kurniansyah, V.; Lestari, W.W.; Sugiura, T.; Mukti, R.R.; Martien, R.; Wibowo, F.R. Organically surface engineered mesoporous silica nanoparticles control the release of quercetin by pH stimuli. Sci. Rep. 2022, 12, 20661. [Google Scholar] [CrossRef]
- Hasan, A.A.; Tatarskiy, V.; Kalinina, E. Synthetic Pathways and the Therapeutic Potential of Quercetin and Curcumin. Int. J. Mol. Sci. 2022, 23, 14413. [Google Scholar] [CrossRef]
- Xu, Y.; Xiao, L.; Chang, Y.; Cao, Y.; Chen, C.; Wang, D. pH and Redox Dual-Responsive MSN-S-S-CS as a Drug Delivery System in Cancer Therapy. Materials 2020, 13, 1279. [Google Scholar] [CrossRef]
- Gracheva, I.A.; Shchegravina, E.S.; Schmalz, H.G.; Beletskaya, I.P.; Fedorov, A.Y. Colchicine Alkaloids and Synthetic Analogues: Current Progress and Perspectives. J. Med. Chem. 2020, 63, 10618–10651. [Google Scholar] [CrossRef]
- AbouAitah, K.; Lojkowski, W. Delivery of Natural Agents by Means of Mesoporous Silica Nanospheres as a Promising Anticancer Strategy. Pharmaceutics 2021, 13, 143. [Google Scholar] [CrossRef]
- Prabhakar, N.; Långbacka, E.; Özliseli, E.; Mattsson, J.; Mahran, A.; Suleymanova, I.; Sahlgren, C.; Rosenholm, J.M.; Åkerfelt, M.; Nees, M. Surface Modification of Mesoporous Silica Nanoparticles as a Means to Introduce Inherent Cancer-Targeting Ability in a 3D Tumor Microenvironment. Small Sci. 2024, 4, 2400084. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, D.; Lee, H.B.; Jeon, S.; Park, C.; Kim, T.S.; Lee, J.H.; Oh, I.U.; Ahn, S.J. Guidelines for Packaging, Transport, and Storage of Source Cells for Organoids. Int. J. Stem Cells 2024, 17, 113–119. [Google Scholar] [CrossRef]
- Schnalzger, T.E.; de Groot, M.H.; Zhang, C.; Mosa, M.H.; Michels, B.E.; Röder, J.; Darvishi, T.; Wels, W.S.; Farin, H.F. 3D model for CAR-mediated cytotoxicity using patient-derived colorectal cancer organoids. EMBO J. 2019, 38, e100928. [Google Scholar] [CrossRef]
- Michie, J.; Beavis, P.A.; Freeman, A.J.; Vervoort, S.J.; Ramsbottom, K.M.; Narasimhan, V.; Lelliott, E.J.; Lalaoui, N.; Ramsay, R.G.; Johnstone, R.W.; et al. Antagonism of IAPs Enhances CAR T-cell Efficacy. Cancer Immunol. Res. 2019, 7, 183–192. [Google Scholar] [CrossRef]
- Van Hemelryk, A.; Erkens-Schulze, S.; Lim, L.; de Ridder, C.M.A.; Stuurman, D.C.; Jenster, G.W.; van Royen, M.E.; van Weerden, W.M. Viability Analysis and High-Content Live-Cell Imaging for Drug Testing in Prostate Cancer Xenograft-Derived Organoids. Cells 2023, 12, 1377. [Google Scholar] [CrossRef]
- Du, Y.; Li, X.; Niu, Q.; Mo, X.; Qui, M.; Ma, T.; Kuo, C.J.; Fu, H. Development of a miniaturized 3D organoid culture platform for ultra-high-throughput screening. J. Mol. Cell Biol. 2020, 12, 630–643. [Google Scholar] [CrossRef]
- Boehnke, K.; Iversen, P.W.; Schumacher, D.; Lallena, M.J.; Haro, R.; Amat, J.; Haybaeck, J.; Liebs, S.; Lange, M.; Schäfer, R.; et al. Assay Establishment and Validation of a High-Throughput Screening Platform for Three-Dimensional Patient-Derived Colon Cancer Organoid Cultures. J. Biomol. Screen. 2016, 21, 931–941. [Google Scholar] [CrossRef]
- Yao, Q.; Cheng, S.; Pan, Q.; Yu, J.; Cao, G.; Li, L.; Cao, H. Organoids: Development and applications in disease models, drug discovery, precision medicine, and regenerative medicine. MedComm (2020) 2024, 5, e735. [Google Scholar] [CrossRef]
- Zhang, J.H.; Chung, T.D.; Oldenburg, K.R. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. J. Biomol. Screen. 1999, 4, 67–73. [Google Scholar] [CrossRef]
- Phipson, B.; Er, P.X.; Combes, A.N.; Forbes, T.A.; Howden, S.E.; Zappia, L.; Yen, H.J.; Lawlor, K.T.; Hale, L.J.; Sun, J.; et al. Evaluation of variability in human kidney organoids. Nat. Methods 2019, 16, 79–87. [Google Scholar] [CrossRef]
- Jensen, K.B.; Little, M.H. Organoids are not organs: Sources of variation and misinformation in organoid biology. Stem Cell Rep. 2023, 18, 1255–1270. [Google Scholar] [CrossRef]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Kim, S.; Min, S.; Choi, Y.S.; Jo, S.H.; Jung, J.H.; Han, K.; Kim, J.; An, S.; Ji, Y.W.; Kim, Y.G.; et al. Tissue extracellular matrix hydrogels as alternatives to Matrigel for culturing gastrointestinal organoids. Nat. Commun. 2022, 13, 1692. [Google Scholar] [CrossRef]
- Verduin, M.; Hoeben, A.; De Ruysscher, D.; Vooijs, M. Patient-Derived Cancer Organoids as Predictors of Treatment Response. Front. Oncol. 2021, 11, 641980. [Google Scholar] [CrossRef]
- Williams, C.G.; Lee, H.J.; Asatsuma, T.; Vento-Tormo, R.; Haque, A. An introduction to spatial transcriptomics for biomedical research. Genome Med. 2022, 14, 68. [Google Scholar] [CrossRef]
- Ståhl, P.L.; Salmén, F.; Vickovic, S.; Lundmark, A.; Navarro, J.F.; Magnusson, J.; Giacomello, S.; Asp, M.; Westholm, J.O.; Huss, M.; et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 2016, 353, 78–82. [Google Scholar] [CrossRef]
- Rodriques, S.G.; Stickels, R.R.; Goeva, A.; Martin, C.A.; Murray, E.; Vanderburg, C.R.; Welch, J.; Chen, L.M.; Chen, F.; Macosko, E.Z. Slide-seq: A scalable technology for measuring genome-wide expression at high spatial resolution. Science 2019, 363, 1463–1467. [Google Scholar] [CrossRef]
- Chen, K.H.; Boettiger, A.N.; Moffitt, J.R.; Wang, S.; Zhuang, X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 2015, 348, aaa6090. [Google Scholar] [CrossRef]
- Wahle, P.; Brancati, G.; Harmel, C.; He, Z.; Gut, G.; Del Castillo, J.S.; Xavier da Silveira Dos Santos, A.; Yu, Q.; Noser, P.; Fleck, J.S.; et al. Multimodal spatiotemporal phenotyping of human retinal organoid development. Nat. Biotechnol. 2023, 41, 1765–1775. [Google Scholar] [CrossRef]
- Legnini, I.; Emmenegger, L.; Zappulo, A.; Rybak-Wolf, A.; Wurmus, R.; Martinez, A.O.; Jara, C.C.; Boltengagen, A.; Hessler, T.; Mastrobuoni, G.; et al. Spatiotemporal, optogenetic control of gene expression in organoids. Nat. Methods 2023, 20, 1544–1552. [Google Scholar] [CrossRef]
- Hwang, W.; McPartland, T.; Jeong, S.; Evans, C.L. A robust method for autofluorescence-free immunofluorescence using high-speed fluorescence lifetime imaging microscopy. Sci. Rep. 2025, 15, 5503. [Google Scholar] [CrossRef]
- Razgonova, M.P.; Zinchenko, Y.N.; Kozak, D.K.; Kuznetsova, V.A.; Zakharenko, A.M.; Ercisli, S.; Golokhvast, K.S. Autofluorescence-Based Investigation of Spatial Distribution of Phenolic Compounds in Soybeans Using Confocal Laser Microscopy and a High-Resolution Mass Spectrometric Approach. Molecules 2022, 27, 8228. [Google Scholar] [CrossRef]
- Spraker, J.E.; Luu, G.T.; Sanchez, L.M. Imaging mass spectrometry for natural products discovery: A review of ionization methods. Nat. Prod. Rep. 2020, 37, 150–162. [Google Scholar] [CrossRef]
- Lopes, N.P.; Roberto da Silva, R. From structural determination of natural products in complex mixtures to single cell resolution: Perspectives on advances and challenges for mass spectrometry. Front. Nat. Prod. 2023, 2, 1109557. [Google Scholar] [CrossRef]
- Kleshchevnikov, V.; Shmatko, A.; Dann, E.; Aivazidis, A.; King, H.W.; Li, T.; Elmentaite, R.; Lomakin, A.; Kedlian, V.; Gayoso, A.; et al. Cell2location maps fine-grained cell types in spatial transcriptomics. Nat. Biotechnol. 2022, 40, 661–671. [Google Scholar] [CrossRef]
- Juwayria; Shrivastava, P.; Yadav, K.; Das, S.; Mittal, S.; Kumar, S.; Jain, D.; Malik, P.S.; Gupta, I. Microarray integrated spatial transcriptomics (MIST) for affordable and robust digital pathology. NPJ Syst. Biol. Appl. 2024, 10, 142. [Google Scholar] [CrossRef]
- Berglund, E.; Saarenpää, S.; Jemt, A.; Gruselius, J.; Larsson, L.; Bergenstråhle, L.; Lundeberg, J.; Giacomello, S. Automation of Spatial Transcriptomics library preparation to enable rapid and robust insights into spatial organization of tissues. BMC Genom. 2020, 21, 298. [Google Scholar] [CrossRef]
- Lewis, S.M.; Asselin-Labat, M.L.; Nguyen, Q.; Berthelet, J.; Tan, X.; Wimmer, V.C.; Merino, D.; Rogers, K.L.; Naik, S.H. Spatial omics and multiplexed imaging to explore cancer biology. Nat. Methods 2021, 18, 997–1012. [Google Scholar] [CrossRef]
- Kong, J.; Lee, H.; Kim, D.; Han, S.K.; Ha, D.; Shin, K.; Kim, S. Network-based machine learning in colorectal and bladder organoid models predicts anti-cancer drug efficacy in patients. Nat. Commun. 2020, 11, 5485. [Google Scholar] [CrossRef]
- Mukashyaka, P.; Kumar, P.; Mellert, D.J.; Nicholas, S.; Noorbakhsh, J.; Brugiolo, M.; Courtois, E.T.; Anczukow, O.; Liu, E.T.; Chuang, J.H. High-throughput deconvolution of 3D organoid dynamics at cellular resolution for cancer pharmacology with Cellos. Nat. Commun. 2023, 14, 8406. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Huang, F.; Gao, Q.; Li, P.; Li, D.; Luo, G. Deliod a lightweight detection model for intestinal organoids based on deep learning. Sci. Rep. 2025, 15, 5040. [Google Scholar] [CrossRef]
- Ferreira, N.; Kulkarni, A.; Agorku, D.; Midelashvili, T.; Hardt, O.; Legler, T.J.; Ströbel, P.; Conradi, L.C.; Alves, F.; Ramos-Gomes, F.; et al. OrganoIDNet: A deep learning tool for identification of therapeutic effects in PDAC organoid-PBMC co-cultures from time-resolved imaging data. Cell Oncol. 2025, 48, 101–122. [Google Scholar] [CrossRef]
- Schuster, B.; Junkin, M.; Kashaf, S.S.; Romero-Calvo, I.; Kirby, K.; Matthews, J.; Weber, C.R.; Rzhetsky, A.; White, K.P.; Tay, S. Automated microfluidic platform for dynamic and combinatorial drug screening of tumor organoids. Nat. Commun. 2020, 11, 5271. [Google Scholar] [CrossRef]
- Quintard, C.; Tubbs, E.; Jonsson, G.; Jiao, J.; Wang, J.; Werschler, N.; Laporte, C.; Pitaval, A.; Bah, T.S.; Pomeranz, G.; et al. A microfluidic platform integrating functional vascularized organoids-on-chip. Nat. Commun. 2024, 15, 1452. [Google Scholar] [CrossRef]
- Bang, S.; Lee, S.R.; Ko, J.; Son, K.; Tahk, D.; Ahn, J.; Im, C.; Jeon, N.L. A Low Permeability Microfluidic Blood-Brain Barrier Platform with Direct Contact between Perfusable Vascular Network and Astrocytes. Sci. Rep. 2017, 7, 8083. [Google Scholar] [CrossRef]
- Dressaire, E.; Sauret, A. Clogging of microfluidic systems. Soft Matter 2016, 13, 37–48. [Google Scholar] [CrossRef]
- Toepke, M.W.; Beebe, D.J. PDMS absorption of small molecules and consequences in microfluidic applications. Lab. Chip 2006, 6, 1484–1486. [Google Scholar] [CrossRef]
- Kovach, K.M.; Capadona, J.R.; Gupta, A.S.; Potkay, J.A. The effects of PEG-based surface modification of PDMS microchannels on long-term hemocompatibility. J. Biomed. Mater. Res. A 2014, 102, 4195–4205. [Google Scholar] [CrossRef]
- Han, J.J. FDA Modernization Act 2.0 allows for alternatives to animal testing. Artif. Organs 2023, 47, 449–450. [Google Scholar] [CrossRef]
- Zushin, P.H.; Mukherjee, S.; Wu, J.C. FDA Modernization Act 2.0: Transitioning beyond animal models with human cells, organoids, and AI/ML-based approaches. J. Clin. Invest. 2023, 133, e175824. [Google Scholar] [CrossRef]
- FDA pushes to replace animal testing. Nat. Biotechnol. 2025, 43, 655. [CrossRef]
- Narasimhan, V.; Wright, J.A.; Churchill, M.; Wang, T.; Rosati, R.; Lannagan, T.R.M.; Vrbanac, L.; Richardson, A.B.; Kobayashi, H.; Price, T.; et al. Medium-throughput Drug Screening of Patient-derived Organoids from Colorectal Peritoneal Metastases to Direct Personalized Therapy. Clin. Cancer Res. 2020, 26, 3662–3670. [Google Scholar] [CrossRef]
- Owens, C.E.L.; Tan, O.; Kuroiwa-Trzmielina, J.; Shrestha, R.N.; O’Brien, T.; Tyrrell, V.; Schofield, D.J. The economic costs of precision medicine for clinical translational research among children with high-risk cancer. NPJ Precis. Oncol. 2024, 8, 224. [Google Scholar] [CrossRef]
- Tan, T.; Mouradov, D.; Lee, M.; Gard, G.; Hirokawa, Y.; Li, S.; Lin, C.; Li, F.; Luo, H.; Wu, K.; et al. Unified framework for patient-derived, tumor-organoid-based predictive testing of standard-of-care therapies in metastatic colorectal cancer. Cell Rep. Med. 2023, 4, 101335. [Google Scholar] [CrossRef]
- Wouters, O.J.; McKee, M.; Luyten, J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA 2020, 323, 844–853. [Google Scholar] [CrossRef]
- DiMasi, J.A.; Grabowski, H.G.; Hansen, R.W. Innovation in the pharmaceutical industry: New estimates of R&D costs. J. Health Econ. 2016, 47, 20–33. [Google Scholar] [CrossRef]
- Takahashi, Y.; Inoue, Y.; Sato, S.; Okabe, T.; Kojima, H.; Kiyono, H.; Shimizu, M.; Yamauchi, Y.; Sato, R. Drug cytotoxicity screening using human intestinal organoids propagated with extensive cost-reduction strategies. Sci. Rep. 2023, 13, 5407. [Google Scholar] [CrossRef]
- Hresko, A.; Haga, S.B. Insurance coverage policies for personalized medicine. J. Pers. Med. 2012, 2, 201–216. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.; Yang, L.; Zhu, J.; Wan, J.; Shen, L.; Xia, F.; Fu, G.; Deng, Y.; Pan, M.; et al. Patient-Derived Organoids Predict Chemoradiation Responses of Locally Advanced Rectal Cancer. Cell Stem Cell 2020, 26, 17–26.e16. [Google Scholar] [CrossRef]
- Åkerlund, E.; Gudoityte, G.; Moussaud-Lamodière, E.; Lind, O.; Bwanika, H.C.; Lehti, K.; Salehi, S.; Carlson, J.; Wallin, E.; Fernebro, J.; et al. The drug efficacy testing in 3D cultures platform identifies effective drugs for ovarian cancer patients. NPJ Precis. Oncol. 2023, 7, 111. [Google Scholar] [CrossRef]
- Ding, S.; Hsu, C.; Wang, Z.; Natesh, N.R.; Millen, R.; Negrete, M.; Giroux, N.; Rivera, G.O.; Dohlman, A.; Bose, S.; et al. Patient-derived micro-organospheres enable clinical precision oncology. Cell Stem Cell 2022, 29, 905–917.e906. [Google Scholar] [CrossRef]
- Tamborero, D.; Dienstmann, R.; Rachid, M.H.; Boekel, J.; Lopez-Fernandez, A.; Jonsson, M.; Razzak, A.; Braña, I.; De Petris, L.; Yachnin, J.; et al. The Molecular Tumor Board Portal supports clinical decisions and automated reporting for precision oncology. Nat. Cancer 2022, 3, 251–261. [Google Scholar] [CrossRef]
- Peng, Z.H.; Kopeček, J. Enhancing Accumulation and Penetration of HPMA Copolymer-Doxorubicin Conjugates in 2D and 3D Prostate Cancer Cells via iRGD Conjugation with an MMP-2 Cleavable Spacer. J. Am. Chem. Soc. 2015, 137, 6726–6729. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).