Breaking the Barrier: The Role of Proinflammatory Cytokines in BBB Dysfunction
Abstract
1. Introduction
2. Methodology of the Literature Search
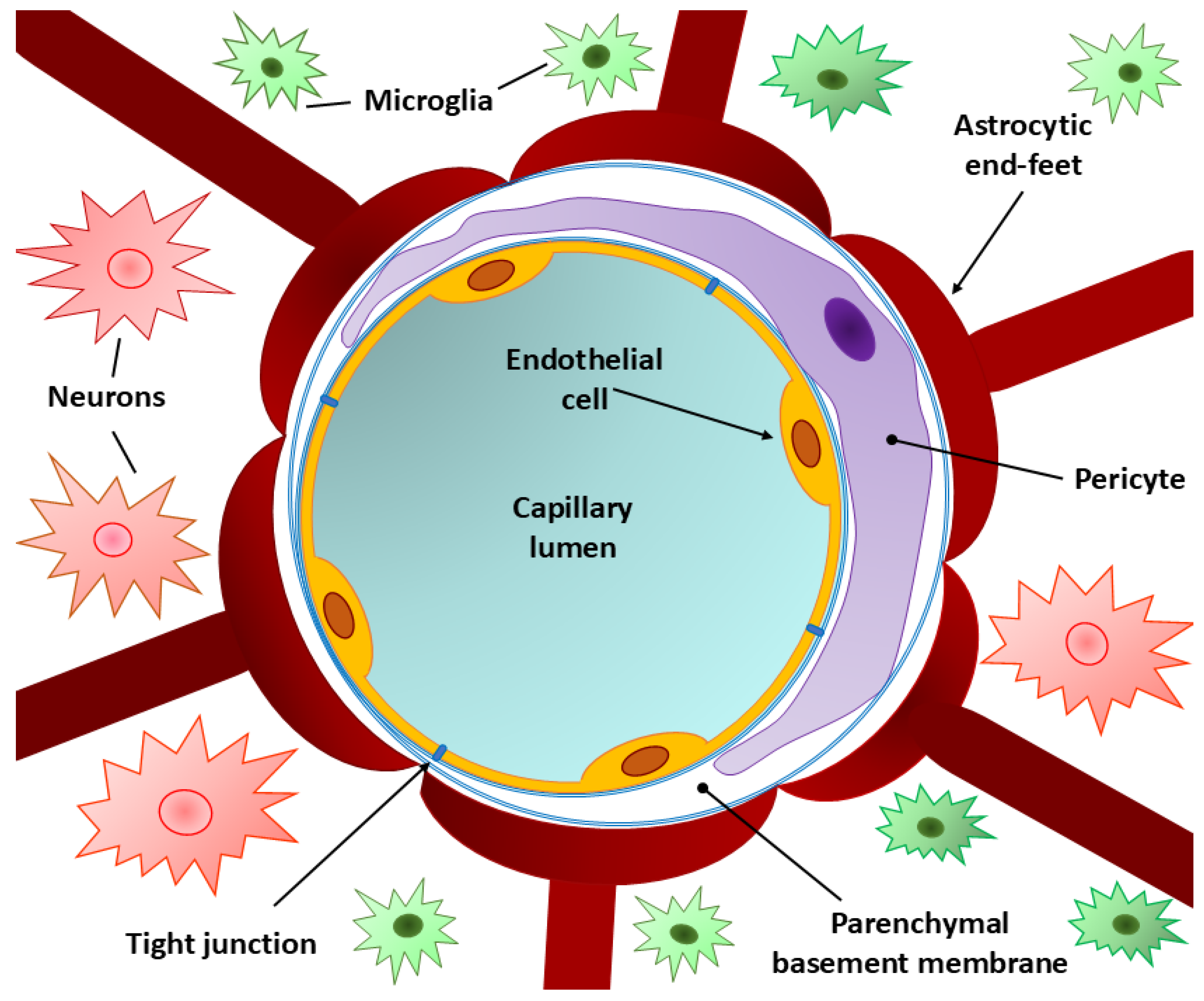
3. Normal BBB Structure
3.1. Endothelial Cells
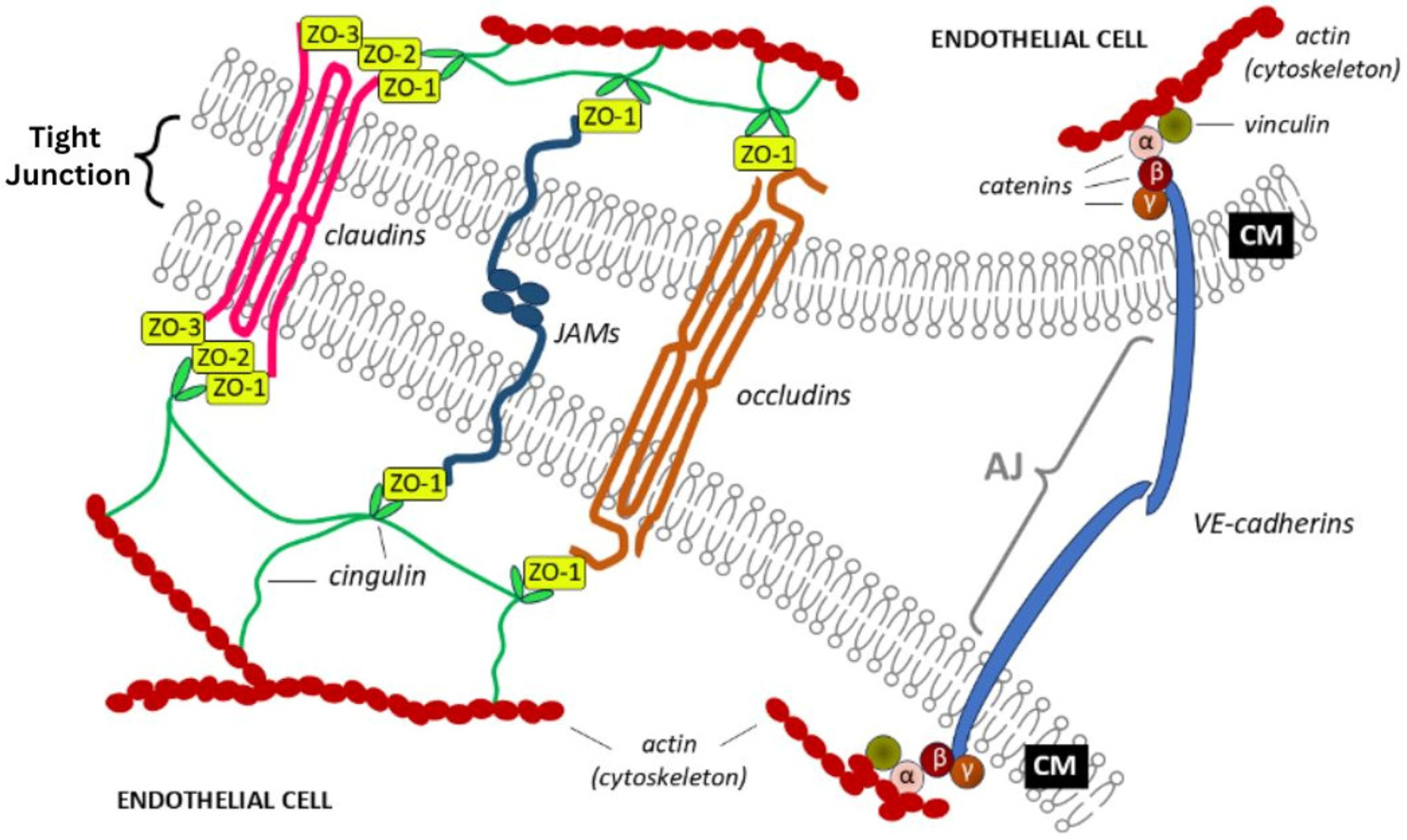
3.2. Tight and Adherence Junctions
3.3. Pericytes
3.4. Basement Membrane
3.5. Perivascular Membrane
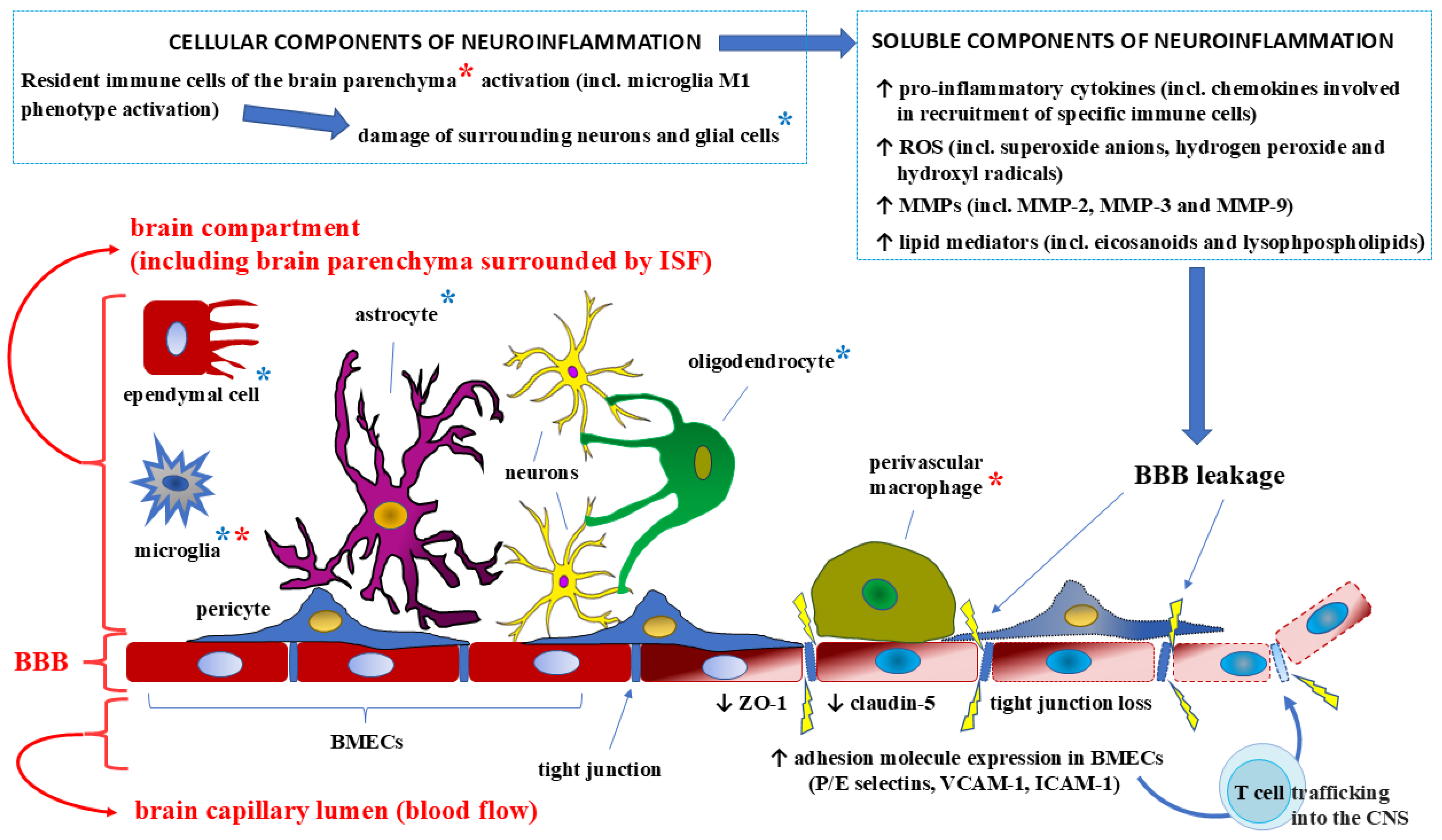
4. Effects of Proinflammatory Cytokines on the BBB Structure and Integrity
5. Proinflammatory Cytokines Within the BBB: Clinical Approaches and Potential Therapies
5.1. Interleukin–6
5.2. Tumor Necrosis Factor-Alpha (TNF-α)
5.3. Interleukin-17A
5.4. Interleukin–4
6. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Dyrna, F.; Hanske, S.; Krueger, M.; Bechmann, I. The blood-brain barrier. J. Neuroimmune Pharmacol. 2013, 8, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Khan, E. An examination of the blood-brain barrier in health and disease. Br. J. Nurs. 2005, 14, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Benz, F.; Liebner, S. Structure and Function of the Blood-Brain Barrier (BBB). Handb. Exp. Pharmacol. 2022, 273, 3–31. [Google Scholar] [CrossRef]
- Kim, J.H.; Byun, H.M.; Chung, E.C.; Chung, H.Y.; Bae, O.N. Loss of Integrity: Impairment of the Blood-brain Barrier in Heavy Metal-associated Ischemic Stroke. Toxicol. Res. 2013, 29, 157–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-Brain Barrier Dysfunction Amplifies the Development of Neuroinflammation: Understanding of Cellular Events in Brain Microvascular Endothelial Cells for Prevention and Treatment of BBB Dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef]
- Zhao, B.; Yin, Q.; Fei, Y.; Zhu, J.; Qiu, Y.; Fang, W.; Li, Y. Research progress of mechanisms for tight junction damage on blood-brain barrier inflammation. Arch. Physiol. Biochem. 2022, 128, 1579–1590. [Google Scholar] [CrossRef]
- Li, X.; Cai, Y.; Zhang, Z.; Zhou, J. Glial and Vascular Cell Regulation of the Blood-Brain Barrier in Diabetes. Diabetes Metab. J. 2022, 46, 222–238. [Google Scholar] [CrossRef]
- Cai, Z.; Qiao, P.F.; Wan, C.Q.; Cai, M.; Zhou, N.K.; Li, Q. Role of Blood-Brain Barrier in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 63, 1223–1234. [Google Scholar] [CrossRef]
- Nikolopoulos, D.; Manolakou, T.; Polissidis, A.; Filia, A.; Bertsias, G.; Koutmani, Y.; Boumpas, D.T. Microglia activation in the presence of intact blood-brain barrier and disruption of hippocampal neurogenesis via IL-6 and IL-18 mediate early diffuse neuropsychiatric lupus. Ann. Rheum. Dis. 2023, 82, 646–657. [Google Scholar] [CrossRef]
- Wątroba, M.; Grabowska, A.D.; Szukiewicz, D. Effects of Diabetes Mellitus-Related Dysglycemia on the Functions of Blood-Brain Barrier and the Risk of Dementia. Int. J. Mol. Sci. 2023, 24, 10069. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, G.; Wang, Z.; Zhang, X.; Yao, L.; Wang, F.; Liu, S.; Yin, J.; Ling, E.A.; Wang, L.; et al. High glucose-induced expression of inflammatory cytokines and reactive oxygen species in cultured astrocytes. Neuroscience 2012, 202, 58–68. [Google Scholar] [CrossRef]
- Małkiewicz, M.A.; Małecki, A.; Toborek, M.; Szarmach, A.; Winklewski, P.J. Substances of abuse and the blood brain barrier: Interactions with physical exercise. Neurosci. Biobehav. Rev. 2020, 119, 204–216. [Google Scholar] [CrossRef]
- Lesniak, A.; Poznański, P.; Religa, P.; Nawrocka, A.; Bujalska-Zadrozny, M.; Sacharczuk, M. Loss of Brain-Derived Neurotrophic Factor (BDNF) Resulting From Congenital- Or Mild Traumatic Brain Injury-Induced Blood-Brain Barrier Disruption Correlates With Depressive-Like Behaviour. Neuroscience 2021, 458, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bronisz, E.; Cudna, A.; Wierzbicka, A.; Kurkowska-Jastrzębska, I. Blood-Brain Barrier-Associated Proteins Are Elevated in Serum of Epilepsy Patients. Cells 2023, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Łach, A.; Wnuk, A.; Wójtowicz, A.K. Experimental Models to Study the Functions of the Blood-Brain Barrier. Bioengineering 2023, 10, 519. [Google Scholar] [CrossRef]
- Patel, J.P.; Frey, B.N. Disruption in the Blood-Brain Barrier: The Missing Link between Brain and Body Inflammation in Bipolar Disorder? Neural Plast. 2015, 2015, 708306. [Google Scholar] [CrossRef]
- Yarlagadda, A.; Alfson, E.; Clayton, A.H. The blood brain barrier and the role of cytokines in neuropsychiatry. Psychiatry 2009, 6, 18–22. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2801483/ (accessed on 5 February 2025).
- Haley, M.J.; Lawrence, C.B. The blood-brain barrier after stroke: Structural studies and the role of transcytotic vesicles. J. Cereb. Blood Flow Metab. 2017, 37, 456–470. [Google Scholar] [CrossRef]
- Mayer, M.G.; Fischer, T. Microglia at the blood brain barrier in health and disease. Front. Cell. Neurosci. 2024, 18, 1360195. [Google Scholar] [CrossRef]
- Lim, S.H.; Yee, G.T.; Khang, D. Nanoparticle-Based Combinational Strategies for Overcoming the Blood-Brain Barrier and Blood-Tumor Barrier. Int. J. Nanomed. 2024, 19, 2529–2552. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- McCabe, S.M.; Zhao, N. The Potential Roles of Blood-Brain Barrier and Blood-Cerebrospinal Fluid Barrier in Maintaining Brain Manganese Homeostasis. Nutrients 2021, 13, 1833. [Google Scholar] [CrossRef]
- Patabendige, A.; Janigro, D. The role of the blood-brain barrier during neurological disease and infection. Biochem. Soc. Trans. 2023, 51, 613–626. [Google Scholar] [CrossRef]
- Dotiwala, A.K.; McCausland, C.; Samra, N.S. Anatomy, Head and Neck: Blood Brain Barrier. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519556/ (accessed on 5 February 2025).
- Ganong, W.F. Circumventricular organs: Definition and role in the regulation of endocrine and autonomic function. Clin. Exp. Pharmacol. Physiol. 2000, 27, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Verkhratsky, A.; Pivoriūnas, A. Astroglia support, regulate and reinforce brain barriers. Neurobiol. Dis. 2023, 179, 106054. [Google Scholar] [CrossRef]
- Xu, L.; Nirwane, A.; Yao, Y. Basement membrane and blood-brain barrier. Stroke Vasc. Neurol. 2018, 4, 78–82. [Google Scholar] [CrossRef]
- Erickson, M.A.; Banks, W.A. Transcellular routes of blood-brain barrier disruption. Exp. Biol. Med. 2022, 247, 788–796. [Google Scholar] [CrossRef]
- Persidsky, Y.; Ramirez, S.H.; Haorah, J.; Kanmogne, G.D. Blood-brain barrier: Structural components and function under physiologic and pathologic conditions. J. Neuroimmune Pharmacol. 2006, 1, 223–236. [Google Scholar] [CrossRef]
- Weiss, N.; Miller, F.; Cazaubon, S.; Couraud, P.O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim. Biophys. Acta 2009, 1788, 842–857. [Google Scholar] [CrossRef]
- Xie, Y.; He, L.; Lugano, R.; Zhang, Y.; Cao, H.; He, Q.; Chao, M.; Liu, B.; Cao, Q.; Wang, J.; et al. Key molecular alterations in endothelial cells in human glioblastoma uncovered through single-cell RNA sequencing. JCI Insight 2021, 6, e150861. [Google Scholar] [CrossRef] [PubMed]
- Langen, U.H.; Ayloo, S.; Gu, C. Development and Cell Biology of the Blood-Brain Barrier. Annu. Rev. Cell Dev. Biol. 2019, 35, 591–613. [Google Scholar] [CrossRef]
- Ozgür, B.; Helms, H.C.C.; Tornabene, E.; Brodin, B. Hypoxia increases expression of selected blood-brain barrier transporters GLUT-1, P-gp, SLC7A5 and TFRC, while maintaining barrier integrity, in brain capillary endothelial monolayers. Fluids Barriers CNS 2022, 19, 1. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; D’Mello, V.; Caruso, D.; Wallerstein, A.; Abdul-Muneer, P.M. Impairment of pericyte-endothelium crosstalk leads to blood-brain barrier dysfunction following traumatic brain injury. Exp. Neurol. 2019, 317, 260–270. [Google Scholar] [CrossRef]
- Kakava, S.; Schlumpf, E.; Panteloglou, G.; Tellenbach, F.; von Eckardstein, A.; Robert, J. Brain Endothelial Cells in Contrary to the Aortic Do Not Transport but Degrade Low-Density Lipoproteins via Both LDLR and ALK1. Cells 2022, 11, 3044. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Zhong, L.; Luo, Y. Endothelial glycocalyx as an important factor in composition of blood-brain barrier. CNS Neurosci. Ther. 2021, 27, 26–35. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS. 2020, 17, 69. [Google Scholar] [CrossRef]
- Kniesel, U.; Wolburg, H. Tight junctions of the blood-brain barrier. Cell. Mol. Neurobiol. 2000, 20, 57–76. [Google Scholar] [CrossRef]
- Feng, S.; Zou, L.; Wang, H.; He, R.; Liu, K.; Zhu, H. RhoA/ROCK-2 Pathway Inhibition and Tight Junction Protein Upregulation by Catalpol Suppresses Lipopolysaccaride-Induced Disruption of Blood-Brain Barrier Permeability. Molecules 2018, 23, 2371. [Google Scholar] [CrossRef]
- Haseloff, R.F.; Dithmer, S.; Winkler, L.; Wolburg, H.; Blasig, I.E. Transmembrane proteins of the tight junctions at the blood-brain barrier: Structural and functional aspects. Semin. Cell Dev. Biol. 2015, 38, 16–25. [Google Scholar] [CrossRef]
- Koumangoye, R.; Penny, P.; Delpire, E. Loss of NKCC1 function increases epithelial tight junction permeability by upregulating claudin-2 expression. Am. J. Physiol. Cell Physiol. 2022, 323, C1251–C1263. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.M.; Webb, P.G.; Davis, D.M.; Baumgartner, H.K.; Woodruff, E.R.; Guntupalli, S.R.; Neville, M.; Behbakht, K.; Bitler, B.G. Loss of Claudin-4 Reduces DNA Damage Repair and Increases Sensitivity to PARP Inhibitors. Mol. Cancer Ther. 2022, 21, 647–657. [Google Scholar] [CrossRef]
- Kim, N.Y.; Pyo, J.S.; Kang, D.W.; Yoo, S.M. Loss of claudin-1 expression induces epithelial-mesenchymal transition through nuclear factor-κB activation in colorectal cancer. Pathol. Res. Pract. 2019, 215, 580–585. [Google Scholar] [CrossRef]
- Greene, C.; Hanley, N.; Campbell, M. Claudin-5: Gatekeeper of neurological function. Fluids Barriers CNS. 2019, 16, 3. [Google Scholar] [CrossRef]
- Günzel, D.; Fromm, M. Claudins and other tight junction proteins. Compr. Physiol. 2012, 2, 1819–1852. [Google Scholar] [CrossRef] [PubMed]
- Komarova, Y.A.; Kruse, K.; Mehta, D.; Malik, A.B. Protein Interactions at Endothelial Junctions and Signaling Mechanisms Regulating Endothelial Permeability. Circ. Res. 2017, 120, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Y.; Wang, Z.B.; Zhang, L.C.; Wei, X.; Li, L. Tight junction in blood-brain barrier: An overview of structure, regulation, and regulator substances. CNS Neurosci. Ther. 2012, 18, 609–615. [Google Scholar] [CrossRef]
- Bergmann, S.; Lawler, S.E.; Qu, Y.; Fadzen, C.M.; Wolfe, J.M.; Regan, M.S.; Pentelute, B.L.; Agar, N.Y.R.; Cho, C.F. Blood-brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat Protoc. 2018, 13, 2827–2843. [Google Scholar] [CrossRef]
- Hartsock, A.; Nelson, W.J. Adherens and tight junctions: Structure, function and connections to the actin cytoskeleton. Biochim. Biophys. Acta 2008, 1778, 660–669. [Google Scholar] [CrossRef]
- Kuo, W.T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Tietz, S.; Engelhardt, B. Brain barriers: Crosstalk between complex tight junctions and adherens junctions. J. Cell Biol. 2015, 209, 493–506. [Google Scholar] [CrossRef]
- Kushwaha, R.; Li, Y.; Makarava, N.; Pandit, N.P.; Molesworth, K.; Birukov, K.G.; Baskakov, I.V. Reactive astrocytes associated with prion disease impair the blood brain barrier. bioRxiv 2023. bioRxiv:24:2023.03.21.533684. [Google Scholar] [CrossRef]
- Kamouchi, M.; Ago, T.; Kitazono, T. Brain pericytes: Emerging concepts and functional roles in brain homeostasis. Cell. Mol. Neurobiol. 2011, 31, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Sá-Pereira, I.; Brites, D.; Brito, M.A. Neurovascular unit: A focus on pericytes. Mol. Neurobiol. 2012, 45, 327–347. [Google Scholar] [CrossRef] [PubMed]
- Armulik, A.; Genové, G.; Mäe, M.; Nisancioglu, M.H.; Wallgard, E.; Niaudet, C.; He, L.; Norlin, J.; Lindblom, P.; Strittmatter, K.; et al. Pericytes regulate the blood-brain barrier. Nature 2010, 468, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Dudvarski Stankovic, N.; Teodorczyk, M.; Ploen, R.; Zipp, F.; Schmidt, M.H.H. Microglia-blood vessel interactions: A double-edged sword in brain pathologies. Acta Neuropathol. 2016, 131, 347–363. [Google Scholar] [CrossRef]
- Fu, J.; Liang, H.; Yuan, P.; Wei, Z.; Zhong, P. Brain pericyte biology: From physiopathological mechanisms to potential therapeutic applications in ischemic stroke. Front. Cell. Neurosci. 2023, 17, 1267785. [Google Scholar] [CrossRef]
- Su, X.; Huang, L.; Qu, Y.; Xiao, D.; Mu, D. Pericytes in Cerebrovascular Diseases: An Emerging Therapeutic Target. Front. Cell. Neurosci. 2019, 13, 519. [Google Scholar] [CrossRef]
- Brown, L.S.; Foster, C.G.; Courtney, J.M.; King, N.E.; Howells, D.W.; Sutherland, B.A. Pericytes and Neurovascular Function in the Healthy and Diseased Brain. Front. Cell. Neurosci. 2019, 13, 282. [Google Scholar] [CrossRef]
- Alarcon-Martinez, L.; Yemisci, M.; Dalkara, T. Pericyte morphology and function. Histol. Histopathol. 2021, 36, 633–643. [Google Scholar] [CrossRef]
- Armulik, A.; Abramsson, A.; Betsholtz, C. Endothelial/pericyte interactions. Circ. Res. 2005, 97, 512–523. [Google Scholar] [CrossRef]
- Whitehead, B.; Karelina, K.; Weil, Z.M. Pericyte dysfunction is a key mediator of the risk of cerebral ischemia. J. Neurosci. Res. 2023, 101, 1840–1848. [Google Scholar] [CrossRef]
- Liu, Q.; Yang, Y.; Fan, X. Microvascular pericytes in brain-associated vascular disease. Biomed. Pharmacother. 2020, 121, 109633. [Google Scholar] [CrossRef]
- Anwar, M.M.; Özkan, E.; Gürsoy-Özdemir, Y. The role of extracellular matrix alterations in mediating astrocyte damage and pericyte dysfunction in Alzheimer’s disease: A comprehensive review. Eur. J. Neurosci. 2022, 56, 5453–5475. [Google Scholar] [CrossRef]
- Rivera, F.J.; Hinrichsen, B.; Silva, M.E. Pericytes in Multiple Sclerosis. Adv. Exp. Med. Biol. 2019, 1147, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E. What Are the Roles of Pericytes in the Neurovascular Unit and Its Disorders? Neurology 2023, 100, 970–977. [Google Scholar] [CrossRef]
- Bohannon, D.G.; Long, D.; Kim, W.K. Understanding the Heterogeneity of Human Pericyte Subsets in Blood-Brain Barrier Homeostasis and Neurological Diseases. Cells 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Nwadozi, E.; Rudnicki, M.; Haas, T.L. Metabolic Coordination of Pericyte Phenotypes: Therapeutic Implications. Front. Cell. Dev. Biol. 2020, 8, 77. [Google Scholar] [CrossRef] [PubMed]
- Khalilgharibi, N.; Mao, Y. To form and function: On the role of basement membrane mechanics in tissue development, homeostasis and disease. Open Biol. 2021, 11, 200360. [Google Scholar] [CrossRef]
- Halder, S.K.; Sapkota, A.; Milner, R. The importance of laminin at the blood-brain barrier. Neural Regen. Res. 2023, 18, 2557–2563. [Google Scholar] [CrossRef]
- Boudko, S.P.; Danylevych, N.; Hudson, B.G.; Pedchenko, V.K. Basement membrane collagen IV: Isolation of functional domains. Methods Cell Biol. 2018, 143, 171–185. [Google Scholar] [CrossRef]
- Biswas, S.; Bachay, G.; Chu, J.; Hunter, D.D.; Brunken, W.J. Laminin-Dependent Interaction between Astrocytes and Microglia: A Role in Retinal Angiogenesis. Am. J. Pathol. 2017, 187, 2112–2127. [Google Scholar] [CrossRef]
- Gautam, J.; Zhang, X.; Yao, Y. The role of pericytic laminin in blood brain barrier integrity maintenance. Sci. Rep. 2016, 6, 36450. [Google Scholar] [CrossRef]
- Töpfer, U.; Holz, A. Nidogen in development and disease. Front. Cell. Dev. Biol. 2024, 12, 1380542. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.S.; Routhe, L.J.; Moos, T. The vascular basement membrane in the healthy and pathological brain. J. Cereb. Blood Flow Metab. 2017, 37, 3300–3317. [Google Scholar] [CrossRef] [PubMed]
- Keaney, J.; Campbell, M. The dynamic blood-brain barrier. FEBS J. 2015, 282, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Töpfer, U. Basement membrane dynamics and mechanics in tissue morphogenesis. Biol. Open. 2023, 12, bio059980. [Google Scholar] [CrossRef]
- Clarke, L.E.; Barres, B.A. Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 2013, 14, 311–321. [Google Scholar] [CrossRef]
- Fong, H.; Zhou, B.; Feng, H.; Luo, C.; Bai, B.; Zhang, J.; Wang, Y. Recapitulation of Structure-Function-Regulation of Blood-Brain Barrier under (Patho)Physiological Conditions. Cells 2024, 13, 260. [Google Scholar] [CrossRef]
- Manu, D.R.; Slevin, M.; Barcutean, L.; Forro, T.; Boghitoiu, T.; Balasa, R. Astrocyte Involvement in Blood-Brain Barrier Function: A Critical Update Highlighting Novel, Complex, Neurovascular Interactions. Int. J. Mol. Sci. 2023, 24, 17146. [Google Scholar] [CrossRef] [PubMed]
- Schiera, G.; Di Liegro, C.M.; Schirò, G.; Sorbello, G.; Di Liegro, I. Involvement of Astrocytes in the Formation, Maintenance, and Function of the Blood-Brain Barrier. Cells 2024, 13, 150. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Zhou, Q.G.; Han, F. Cerebrovascular inflammation: A critical trigger for neurovascular injury? Neurochem. Int. 2019, 126, 165–177. [Google Scholar] [CrossRef]
- Quan, N. Immune-to-brain signaling: How important are the blood-brain barrier-independent pathways? Mol. Neurobiol. 2008, 37, 142–152. [Google Scholar] [CrossRef]
- Miyata, S. Glial functions in the blood-brain communication at the circumventricular organs. Front. Neurosci. 2022, 16, 991779. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Eisner, D.; Neher, E.; Taschenberger, H.; Smith, G. Physiology of intracellular calcium buffering. Physiol. Rev. 2023, 103, 2767–2845. [Google Scholar] [CrossRef]
- Schillemans, M.; Karampini, E.; Kat, M.; Bierings, R. Exocytosis of Weibel-Palade bodies: How to unpack a vascular emergency kit. J. Thromb. Haemost. 2019, 17, 6–18. [Google Scholar] [CrossRef]
- Yang, J.; Ran, M.; Li, H.; Lin, Y.; Ma, K.; Yang, Y.; Fu, X.; Yang, S. New insight into neurological degeneration: Inflammatory cytokines and blood-brain barrier. Front. Mol. Neurosci. 2022, 15, 1013933. [Google Scholar] [CrossRef]
- Ransohoff, R.M.; Schafer, D.; Vincent, A.; Blachère, N.E.; Bar-Or, A. Neuroinflammation: Ways in Which the Immune System Affects the Brain. Neurotherapeutics 2015, 12, 896–909. [Google Scholar] [CrossRef]
- Ramesh, G.; MacLean, A.G.; Philipp, M.T. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013, 2013, 480739. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.R.; Liu, J.C.; Bao, J.S.; Bai, Q.Q.; Wang, G.Q. Interaction of Microglia and Astrocytes in the Neurovascular Unit. Front. Immunol. 2020, 11, 1024. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Nighot, M.; Al-Sadi, R.; Gupta, Y.; Viszwapriya, D.; Yochum, G.; Koltun, W.; Ma, T.Y. IL1B Increases Intestinal Tight Junction Permeability by Up-regulation of MIR200C-3p, Which Degrades Occludin mRNA. Gastroenterology 2020, 159, 1375–1389. [Google Scholar] [CrossRef]
- Versele, R.; Sevin, E.; Gosselet, F.; Fenart, L.; Candela, P. TNF-α and IL-1β Modulate Blood-Brain Barrier Permeability and Decrease Amyloid-β Peptide Efflux in a Human Blood-Brain Barrier Model. Int. J. Mol. Sci. 2022, 23, 10235. [Google Scholar] [CrossRef]
- Kaneko, N.; Kurata, M.; Yamamoto, T.; Morikawa, S.; Masumoto, J. The role of interleukin-1 in general pathology. Inflamm. Regen. 2019, 39, 12. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, S.; Sonobe, Y.; Cheng, Y.; Horiuchi, H.; Parajuli, B.; Kawanokuchi, J.; Mizuno, T.; Takeuchi, H.; Suzumura, A. Interleukin-1β induces blood-brain barrier disruption by downregulating Sonic hedgehog in astrocytes. PLoS ONE 2014, 9, e110024. [Google Scholar] [CrossRef]
- Gajtkó, A.; Bakk, E.; Hegedűs, K.; Ducza, L.; Holló, K. IL-1β Induced Cytokine Expression by Spinal Astrocytes Can Play a Role in the Maintenance of Chronic Inflammatory Pain. Front. Physiol. 2020, 11, 543331. [Google Scholar] [CrossRef] [PubMed]
- Sjöström, E.O.; Culot, M.; Leickt, L.; Åstrand, M.; Nordling, E.; Gosselet, F.; Kaiser, C. Transport study of interleukin-1 inhibitors using a human in vitro model of the blood-brain barrier. Brain Behav. Immun. Health. 2021, 16, 100307. [Google Scholar] [CrossRef]
- Fahey, E.; Doyle, S.L. IL-1 Family Cytokine Regulation of Vascular Permeability and Angiogenesis. Front. Immunol. 2019, 10, 1426. [Google Scholar] [CrossRef]
- Souza, P.S.; Gonçalves, E.D.; Pedroso, G.S.; Farias, H.R.; Junqueira, S.C.; Marcon, R.; Tuon, T.; Cola, M.; Silveira, P.C.L.; Santos, A.R.; et al. Physical Exercise Attenuates Experimental Autoimmune Encephalomyelitis by Inhibiting Peripheral Immune Response and Blood-Brain Barrier Disruption. Mol. Neurobiol. 2017, 54, 4723–4737. [Google Scholar] [CrossRef]
- Balzano, T.; Dadsetan, S.; Forteza, J.; Cabrera-Pastor, A.; Taoro-Gonzalez, L.; Malaguarnera, M.; Gil-Perotin, S.; Cubas-Nuñez, L.; Casanova, B.; Castro-Quintas, A.; et al. Chronic hyperammonemia induces peripheral inflammation that leads to cognitive impairment in rats: Reversed by anti-TNF-α treatment. J. Hepatol. 2020, 73, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.R.; Kim, R.K.; Pober, J.S.; Kluger, M.S. Tumor necrosis factor disrupts claudin-5 endothelial tight junction barriers in two distinct NF-κB-dependent phases. PLoS ONE. 2015, 10, e0120075. [Google Scholar] [CrossRef] [PubMed]
- Abd-El-Basset, E.M.; Rao, M.S.; Alshawaf, S.M.; Ashkanani, H.K.; Kabli, A.H. Tumor necrosis factor (TNF) induces astrogliosis, microgliosis and promotes survival of cortical neurons. AIMS Neurosci. 2021, 8, 558–584. [Google Scholar] [CrossRef]
- Muhammad, M. Tumor Necrosis Factor Alpha: A Major Cytokine of Brain Neuroinflammation. In Cytokines; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Chen, A.Q.; Fang, Z.; Chen, X.L.; Yang, S.; Zhou, Y.F.; Mao, L.; Xia, Y.P.; Jin, H.J.; Li, Y.N.; You, M.F.; et al. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis. 2019, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Han, E.C.; Choi, S.Y.; Lee, Y.; Park, J.W.; Hong, S.H.; Lee, H.J. Extracellular RNAs in periodontopathogenic outer membrane vesicles promote TNF-α production in human macrophages and cross the blood-brain barrier in mice. FASEB J. 2019, 33, 13412–13422. [Google Scholar] [CrossRef]
- Ng, C.T.; Fong, L.Y.; Abdullah, M.N.H. Interferon-gamma (IFN-γ): Reviewing its mechanisms and signaling pathways on the regulation of endothelial barrier function. Cytokine 2023, 166, 156208. [Google Scholar] [CrossRef]
- Sonar, S.A.; Shaikh, S.; Joshi, N.; Atre, A.N.; Lal, G. IFN-γ promotes transendothelial migration of CD4+ T cells across the blood-brain barrier. Immunol. Cell Biol. 2017, 95, 843–853. [Google Scholar] [CrossRef]
- Youakim, A.; Ahdieh, M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am. J. Physiol. 1999, 276, G1279–G1288. [Google Scholar] [CrossRef]
- Rahman, M.T.; Ghosh, C.; Hossain, M.; Linfield, D.; Rezaee, F.; Janigro, D.; Marchi, N.; van Boxel-Dezaire, A.H.H. IFN-γ, IL-17A, or zonulin rapidly increase the permeability of the blood-brain and small intestinal epithelial barriers: Relevance for neuro-inflammatory diseases. Biochem. Biophys. Res. Commun. 2018, 507, 274–279. [Google Scholar] [CrossRef]
- Huppert, J.; Closhen, D.; Croxford, A.; White, R.; Kulig, P.; Pietrowski, E.; Bechmann, I.; Becher, B.; Luhmann, H.J.; Waisman, A.; et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010, 24, 1023–1034. [Google Scholar] [CrossRef]
- Furutama, D.; Matsuda, S.; Yamawaki, Y.; Hatano, S.; Okanobu, A.; Memida, T.; Oue, H.; Fujita, T.; Ouhara, K.; Kajiya, M.; et al. IL-6 Induced by Periodontal Inflammation Causes Neuroinflammation and Disrupts the Blood-Brain Barrier. Brain Sci. 2020, 10, 679. [Google Scholar] [CrossRef] [PubMed]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef]
- Banks, W.A.; Kastin, A.J.; Broadwell, R.D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation 1995, 2, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Natesh, K.; Bhosale, D.; Desai, A.; Chandrika, G.; Pujari, R.; Jagtap, J.; Chugh, A.; Ranade, D.; Shastry, P. Oncostatin-M differentially regulates mesenchymal and proneural signature genes in gliomas via STAT3 signaling. Neoplasia 2015, 17, 225–237. [Google Scholar] [CrossRef]
- Wylezinski, L.S.; Hawiger, J. Interleukin 2 Activates Brain Microvascular Endothelial Cells Resulting in Destabilization of Adherens Junctions. J. Biol. Chem. 2016, 291, 22913–22923. [Google Scholar] [CrossRef] [PubMed]
- Waguespack, P.J.; Banks, W.A.; Kastin, A.J. Interleukin-2 does not cross the blood-brain barrier by a saturable transport system. Brain Res. Bull. 1994, 34, 103–109. [Google Scholar] [CrossRef]
- Gao, W.; Li, F.; Zhou, Z.; Xu, X.; Wu, Y.; Zhou, S.; Yin, D.; Sun, D.; Xiong, J.; Jiang, R.; et al. IL-2/Anti-IL-2 Complex Attenuates Inflammation and BBB Disruption in Mice Subjected to Traumatic Brain Injury. Front. Neurol. 2017, 8, 281. [Google Scholar] [CrossRef]
- Yshii, L.; Pasciuto, E.; Bielefeld, P.; Mascali, L.; Lemaitre, P.; Marino, M.; Dooley, J.; Kouser, L.; Verschoren, S.; Lagou, V.; et al. Astrocyte-targeted gene delivery of interleukin 2 specifically increases brain-resident regulatory T cell numbers and protects against pathological neuroinflammation. Nat. Immunol. 2022, 23, 878–891. [Google Scholar] [CrossRef]
- Serna-Rodríguez, M.F.; Bernal-Vega, S.; de la Barquera, J.A.O.; Camacho-Morales, A.; Pérez-Maya, A.A. The role of damage associated molecular pattern molecules (DAMPs) and permeability of the blood-brain barrier in depression and neuroinflammation. J. Neuroimmunol. 2022, 371, 577951. [Google Scholar] [CrossRef]
- Zhang, Y.; Tian, Y.; Wei, J.; Xiang, Y. Relationship of Serum IL-12 to Inflammation, Hematoma Volume, and Prognosis in Patients With Intracerebral Hemorrhage. Emerg. Med. Int. 2022, 2022, 8688413. [Google Scholar] [CrossRef]
- Andreadou, M.; Ingelfinger, F.; De Feo, D.; Cramer, T.L.M.; Tuzlak, S.; Friebel, E.; Schreiner, B.; Eede, P.; Schneeberger, S.; Geesdorf, M.; et al. IL-12 sensing in neurons induces neuroprotective CNS tissue adaptation and attenuates neuroinflammation in mice. Nat. Neurosci. 2023, 26, 1701–1712. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Wu, X.; He, Y.; Hsuchou, H.; Huang, E.Y.; Mishra, P.K.; Kastin, A.J. Brain interleukin-15 in neuroinflammation and behavior. Neurosci. Biobehav. Rev. 2013, 37, 184–192. [Google Scholar] [CrossRef]
- Pan, W.; Hsuchou, H.; Yu, C.; Kastin, A.J. Permeation of blood-borne IL15 across the blood-brain barrier and the effect of LPS. J. Neurochem. 2008, 106, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Han, J.; Ren, H.; Ma, C.G.; Shi, F.D.; Liu, Q.; Li, M. Astrocytic Interleukin-15 Reduces Pathology of Neuromyelitis Optica in Mice. Front. Immunol. 2018, 9, 523. [Google Scholar] [CrossRef]
- Burrack, K.S.; Huggins, M.A.; Taras, E.; Dougherty, P.; Henzler, C.M.; Yang, R.; Alter, S.; Jeng, E.K.; Wong, H.C.; Felices, M.; et al. Interleukin-15 Complex Treatment Protects Mice from Cerebral Malaria by Inducing Interleukin-10-Producing Natural Killer Cells. Immunity 2018, 48, 760–772.e4. [Google Scholar] [CrossRef]
- Jung, H.K.; Ryu, H.J.; Kim, M.J.; Kim, W.I.; Choi, H.K.; Choi, H.C.; Song, H.K.; Jo, S.M.; Kang, T.C. Interleukin-18 attenuates disruption of brain-blood barrier induced by status epilepticus within the rat piriform cortex in interferon-γ independent pathway. Brain Res. 2012, 1447, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Michalak, S.; Kalinowska-Lyszczarz, A.; Rybacka-Mossakowska, J.; Zaborowski, M.; Kozubski, W. The associations between serum vascular endothelial growth factor, tumor necrosis factor and interleukin 4 with the markers of blood-brain barrier breakdown in patients with paraneoplastic neurological syndromes. J. Neural Transm. 2019, 126, 149–158. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Xing, Z.; Zhang, H.; Wen, Y.; Qi, F.; Zuo, Z.; Xu, J.; Yao, Z. IL-4 mediates the delayed neurobehavioral impairments induced by neonatal hepatitis B vaccination that involves the down-regulation of the IL-4 receptor in the hippocampus. Cytokine 2018, 110, 137–149. [Google Scholar] [CrossRef]
- Poliani, P.L.; Brok, H.; Furlan, R.; Ruffini, F.; Bergami, A.; Desina, G.; Marconi, P.C.; Rovaris, M.; Uccelli, A.; Glorioso, J.C.; et al. Delivery to the central nervous system of a nonreplicative herpes simplex type 1 vector engineered with the interleukin 4 gene protects rhesus monkeys from hyperacute autoimmune encephalomyelitis. Hum. Gene Ther. 2001, 12, 905–920. [Google Scholar] [CrossRef]
- Williams, J.L.; Holman, D.W.; Klein, R.S. Chemokines in the balance: Maintenance of homeostasis and protection at CNS barriers. Front. Cell. Neurosci. 2014, 8, 154. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, L.; Bian, C.; Liang, Y.; Xing, R.; Yishakea, M.; Dong, J. Role of CX3CL1 in Diseases. Arch. Immunol. Ther. Exp. 2016, 64, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Bachstetter, A.D.; Morganti, J.M.; Jernberg, J.; Schlunk, A.; Mitchell, S.H.; Brewster, K.W.; Hudson, C.E.; Cole, M.J.; Harrison, J.K.; Bickford, P.C.; et al. Fractalkine and CX 3 CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol. Aging 2011, 32, 2030–2044. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.T.; Morganti, J.M.; Bachstetter, A.D.; Hudson, C.E.; Peters, M.M.; Grimmig, B.A.; Weeber, E.J.; Bickford, P.C.; Gemma, C. CX3CR1 deficiency leads to impairment of hippocampal cognitive function and synaptic plasticity. J. Neurosci. 2011, 31, 16241–16250. [Google Scholar] [CrossRef]
- Ancuta, P.; Moses, A.; Gabuzda, D. Transendothelial migration of CD16+ monocytes in response to fractalkine under constitutive and inflammatory conditions. Immunobiology 2004, 209, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bertin, J.; Jalaguier, P.; Barat, C.; Roy, M.A.; Tremblay, M.J. Exposure of human astrocytes to leukotriene C4 promotes a CX3CL1/fractalkine-mediated transmigration of HIV-1-infected CD4+ T cells across an in vitro blood-brain barrier model. Virology 2014, 454–455, 128–138. [Google Scholar] [CrossRef]
- Bañuelos-Cabrera, I.; Valle-Dorado, M.G.; Aldana, B.I.; Orozco-Suárez, S.A.; Rocha, L. Role of histaminergic system in blood-brain barrier dysfunction associated with neurological disorders. Arch. Med. Res. 2014, 45, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Tan, Y.; Huan, R.; Guo, J.; Yang, S.; Deng, M.; Xiong, Y.; Han, G.; Liu, L.; Liu, J.; et al. Mast cell activation mediates blood-brain barrier impairment and cognitive dysfunction in septic mice in a histamine-dependent pathway. Front. Immunol. 2023, 14, 1090288. [Google Scholar] [CrossRef] [PubMed]
- Alstadhaug, K.B. Histamine in migraine and brain. Headache 2014, 54, 246–259. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Rochfort, K.D.; Cummins, P.M. The blood-brain barrier endothelium: A target for pro-inflammatory cytokines. Biochem. Soc. Trans. 2015, 43, 702–706. [Google Scholar] [CrossRef]
- Lee, J.I.; Choi, J.H.; Kwon, T.W.; Jo, H.S.; Kim, D.G.; Ko, S.G.; Song, G.J.; Cho, I.H. Neuroprotective effects of bornyl acetate on experimental autoimmune encephalomyelitis via anti-inflammatory effects and maintaining blood-brain-barrier integrity. Phytomedicine 2023, 112, 154569. [Google Scholar] [CrossRef] [PubMed]
- Pawluk, H.; Woźniak, A.; Grześk, G.; Kołodziejska, R.; Kozakiewicz, M.; Kopkowska, E.; Grzechowiak, E.; Kozera, G. The Role of Selected Pro-Inflammatory Cytokines in Pathogenesis of Ischemic Stroke. Clin. Interv. Aging. 2020, 15, 469–484. [Google Scholar] [CrossRef]
- Soltani Khaboushan, A.; Yazdanpanah, N.; Rezaei, N. Neuroinflammation and Proinflammatory Cytokines in Epileptogenesis. Mol. Neurobiol. 2022, 59, 1724–1743. [Google Scholar] [CrossRef] [PubMed]
- Granata, T.; Fusco, L.; Matricardi, S.; Tozzo, A.; Janigro, D.; Nabbout, R. Inflammation in pediatric epilepsies: Update on clinical features and treatment options. Epilepsy Behav. 2022, 131, 107959. [Google Scholar] [CrossRef]
- Savotchenko, A. Blood-brain barrier disfunction and development of epileptic seizures: According to the materials of scientific report at the meeting of the Presidium of the NAS of Ukraine, December 23, 2020. Visn. Nac. Akad. Nauk Ukr. 2021, 1, 53–61. [Google Scholar] [CrossRef]
- Kamali, A.N.; Zian, Z.; Bautista, J.M.; Hamedifar, H.; Hossein-Khannazer, N.; Hosseinzadeh, R.; Yazdani, R.; Azizi, G. The Potential Role of Pro-Inflammatory and Anti-Inflammatory Cytokines in Epilepsy Pathogenesis. Endocr. Metab. Immune Disord Drug Targets. 2021, 21, 1760–1774. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.P.; Leeman-Markowski, B.A. Proposed mechanisms of tau: Relationships to traumatic brain injury, Alzheimer’s disease, and epilepsy. Front. Neurol. 2024, 14, 1287545. [Google Scholar] [CrossRef]
- Langerscheidt, F.; Wied, T.; Al Kabbani, M.A.; van Eimeren, T.; Wunderlich, G.; Zempel, H. Genetic forms of tauopathies: Inherited causes and implications of Alzheimer’s disease-like TAU pathology in primary and secondary tauopathies. J. Neurol. 2024, 271, 2992–3018. [Google Scholar] [CrossRef]
- Michalicova, A.; Majerova, P.; Kovac, A. Tau Protein and Its Role in Blood-Brain Barrier Dysfunction. Front. Mol. Neurosci. 2020, 13, 570045. [Google Scholar] [CrossRef]
- Diener, H.C.; Gaul, C.; Holle-Lee, D.; Jürgens, T.P.; Kraya, T.; Kurth, T.; Nägel, S.; Neeb, L.; Straube, A. Kopfschmerzen—Update 2018 [Headache—An Update 2018]. Laryngorhinootologie 2019, 98, 192–217. (In German) [Google Scholar] [CrossRef]
- Huang, J.; Ding, J.; Wang, X.; Gu, C.; He, Y.; Li, Y.; Fan, H.; Xie, Q.; Qi, X.; Wang, Z.; et al. Transfer of neuron-derived α-synuclein to astrocytes induces neuroinflammation and blood-brain barrier damage after methamphetamine exposure: Involving the regulation of nuclear receptor-associated protein 1. Brain Behav. Immun. 2022, 106, 247–261. [Google Scholar] [CrossRef]
- Jayanthi, S.; Daiwile, A.P.; Cadet, J.L. Neurotoxicity of methamphetamine: Main effects and mechanisms. Exp. Neurol. 2021, 344, 113795. [Google Scholar] [CrossRef] [PubMed]
- Zareifopoulos, N.; Skaltsa, M.; Dimitriou, A.; Karveli, M.; Efthimiou, P.; Lagadinou, M.; Tsigkou, A.; Velissaris, D. Converging dopaminergic neurotoxicity mechanisms of antipsychotics, methamphetamine and levodopa. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4514–4519. [Google Scholar] [CrossRef] [PubMed]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef]
- van der Vorst, E.P.; Döring, Y.; Weber, C. Chemokines. Arterioscler. Thromb Vasc. Biol. 2015, 35, e52–e56. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Mayo, K.H. Chemokines from a Structural Perspective. Int. J. Mol. Sci. 2017, 18, 2088. [Google Scholar] [CrossRef]
- Vérité, J.; Janet, T.; Chassaing, D.; Fauconneau, B.; Rabeony, H.; Page, G. Longitudinal chemokine profile expression in a blood-brain barrier model from Alzheimer transgenic versus wild-type mice. J. Neuroinflamm. 2018, 15, 182. [Google Scholar] [CrossRef] [PubMed]
- Legler, D.F.; Thelen, M. Chemokines: Chemistry, Biochemistry and Biological Function. Chimia 2016, 70, 856–859. [Google Scholar] [CrossRef]
- Curtaz, C.J.; Schmitt, C.; Herbert, S.L.; Feldheim, J.; Schlegel, N.; Gosselet, F.; Hagemann, C.; Roewer, N.; Meybohm, P.; Wöckel, A.; et al. Serum-derived factors of breast cancer patients with brain metastases alter permeability of a human blood-brain barrier model. Fluids Barriers CNS. 2020, 17, 31. [Google Scholar] [CrossRef]
- Abbott, N.J. Inflammatory mediators and modulation of blood-brain barrier permeability. Cell. Mol. Neurobiol. 2000, 20, 131–147. [Google Scholar] [CrossRef]
- Sharma, H.S.; Vannemreddy, P.; Patnaik, R.; Patnaik, S.; Mohanty, S. Histamine receptors influence blood-spinal cord barrier permeability, edema formation, and spinal cord blood flow following trauma to the rat spinal cord. Acta Neurochir. Suppl. 2006, 96, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cai, X.J.; Qin, J.; Xie, F.J.; Han, N.; Lu, H.Y. The role of histamine in opening blood-tumor barrier. Oncotarget 2016, 7, 31299–31310. [Google Scholar] [CrossRef][Green Version]
- Lu, C.; Diehl, S.A.; Noubade, R.; Ledoux, J.; Nelson, M.T.; Spach, K.; Zachary, J.F.; Blankenhorn, E.P.; Teuscher, C. Endothelial histamine H1 receptor signaling reduces blood-brain barrier permeability and susceptibility to autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2010, 107, 18967–18972. [Google Scholar] [CrossRef]
- Scammell, T.E.; Jackson, A.C.; Franks, N.P.; Wisden, W.; Dauvilliers, Y. Histamine: Neural circuits and new medications. Sleep 2019, 42, zsy183. [Google Scholar] [CrossRef]
- Brown, R.E.; Stevens, D.R.; Haas, H.L. The physiology of brain histamine. Prog. Neurobiol. 2001, 63, 637–672. [Google Scholar] [CrossRef] [PubMed]
- Saxton, R.A.; Glassman, C.R.; Garcia, K.C. Emerging principles of cytokine pharmacology and therapeutics. Nat. Rev. Drug Discov. 2023, 22, 21–37. [Google Scholar] [CrossRef]
- Holder, P.G.; Lim, S.A.; Huang, C.S.; Sharma, P.; Dagdas, Y.S.; Bulutoglu, B.; Sockolosky, J.T. Engineering interferons and interleukins for cancer immunotherapy. Adv. Drug Deliv. Rev. 2022, 182, 114112. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, G.; Cataldi, M.; Taglialatela, M. Neurological risks and benefits of cytokine-based treatments in coronavirus disease 2019: From preclinical to clinical evidence. Br. J. Pharmacol. 2022, 179, 2149–2174. [Google Scholar] [CrossRef]
- Ge, Y.; Wu, J.; Zhang, L.; Huang, N.; Luo, Y. A New Strategy for the Regulation of Neuroinflammation: Exosomes Derived from Mesenchymal Stem Cells. Cell. Mol. Neurobiol. 2024, 44, 24. [Google Scholar] [CrossRef]
- Klein, R.S.; Hunter, C.A. Protective and Pathological Immunity during Central Nervous System Infections. Immunity 2017, 46, 891–909. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Shahrizaila, N.; Lehmann, H.C.; Kuwabara, S. Guillain-Barré syndrome. Lancet 2021, 397, 1214–1228. [Google Scholar] [CrossRef]
- Gilhus, N.E.; Verschuuren, J.J. Myasthenia gravis: Subgroup classification and therapeutic strategies. Lancet Neurol. 2015, 14, 1023–1036. [Google Scholar] [CrossRef]
- Younger, D.S. The Blood-Brain Barrier: Implications for Vasculitis. Neurol. Clin. 2019, 37, 235–248. [Google Scholar] [CrossRef]
- Ortiz, G.G.; Pacheco-Moisés, F.P.; Macías-Islas, M.Á.; Flores-Alvarado, L.J.; Mireles-Ramírez, M.A.; González-Renovato, E.D.; Hernández-Navarro, V.E.; Sánchez-López, A.L.; Alatorre-Jiménez, M.A. Role of the blood-brain barrier in multiple sclerosis. Arch. Med. Res. 2014, 45, 687–697. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.W.; Farooq, M.; Hwang, M.J.; Haseeb, M.; Choi, S. Autoimmune Neuroinflammatory Diseases: Role of Interleukins. Int. J. Mol. Sci. 2023, 24, 7960. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Kamath, U.; Pai, A.R.; Rao, P. Serum Inflammatory Markers in Patients with Guillain Barre Syndrome. Neurol. India. 2022, 70, 2082–2085. [Google Scholar] [CrossRef]
- Uzawa, A.; Kanai, T.; Kawaguchi, N.; Oda, F.; Himuro, K.; Kuwabara, S. Changes in inflammatory cytokine networks in myasthenia gravis. Sci. Rep. 2016, 6, 25886. [Google Scholar] [CrossRef]
- Amoriello, R.; Memo, C.; Ballerini, L.; Ballerini, C. The brain cytokine orchestra in multiple sclerosis: From neuroinflammation to synaptopathology. Mol. Brain. 2024, 17, 4. [Google Scholar] [CrossRef]
- Schreiner, T.G.; Romanescu, C.; Popescu, B.O. The Blood-Brain Barrier—A Key Player in Multiple Sclerosis Disease Mechanisms. Biomolecules 2022, 12, 538. [Google Scholar] [CrossRef]
- Takeshita, Y.; Fujikawa, S.; Serizawa, K.; Fujisawa, M.; Matsuo, K.; Nemoto, J.; Shimizu, F.; Sano, Y.; Tomizawa-Shinohara, H.; Miyake, S.; et al. New BBB Model Reveals That IL-6 Blockade Suppressed the BBB Disorder, Preventing Onset of NMOSD. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e1076. [Google Scholar] [CrossRef] [PubMed]
- Corti, A.; Calimeri, T.; Curnis, F.; Ferreri, A.J.M. Targeting the Blood-Brain Tumor Barrier with Tumor Necrosis Factor-α. Pharmaceutics 2022, 14, 1414. [Google Scholar] [CrossRef]
- Hurtado-Alvarado, G.; Becerril-Villanueva, E.; Contis-Montes de Oca, A.; Domínguez-Salazar, E.; Salinas-Jazmín, N.; Pérez-Tapia, S.M.; Pavon, L.; Velázquez-Moctezuma, J.; Gómez-González, B. The yin/yang of inflammatory status: Blood-brain barrier regulation during sleep. Brain Behav. Immun. 2018, 69, 154–166. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, L.; Bao, L.; Liu, J.; Zhu, H.; Lv, Q.; Liu, R.; Chen, W.; Tong, W.; Wei, Q.; et al. SARS-CoV-2 crosses the blood-brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct. Target Ther. 2021, 6, 337. [Google Scholar] [CrossRef] [PubMed]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Parra, H.; Reyes-Hernández, O.D.; Figueroa-González, G.; González-Del Carmen, M.; González-Torres, M.; Peña-Corona, S.I.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Alteration of the blood-brain barrier by COVID-19 and its implication in the permeation of drugs into the brain. Front. Cell. Neurosci. 2023, 17, 1125109. [Google Scholar] [CrossRef] [PubMed]
- Loiola, R.A.; Nguyen, C.; Dib, S.; Saint-Pol, J.; Dehouck, L.; Sevin, E.; Naudot, M.; Landry, C.; Pahnke, J.; Pot, C.; et al. 25-Hydroxycholesterol attenuates tumor necrosis factor alpha-induced blood-brain barrier breakdown in vitro. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167479. [Google Scholar] [CrossRef]
- Sun, Z.W.; Wang, X.; Zhao, Y.; Sun, Z.X.; Wu, Y.H.; Hu, H.; Zhang, L.; Wang, S.D.; Li, F.; Wei, A.J.; et al. Blood-brain barrier dysfunction mediated by the EZH2-Claudin-5 axis drives stress-induced TNF-α infiltration and depression-like behaviors. Brain Behav. Immun. 2024, 115, 143–156. [Google Scholar] [CrossRef]
- Zhao, Y.; Gan, L.; Ren, L.; Lin, Y.; Ma, C.; Lin, X. Factors influencing the blood-brain barrier permeability. Brain Res. 2022, 1788, 147937. [Google Scholar] [CrossRef]
- Trindade, P.; Loiola, E.C.; Gasparotto, J.; Ribeiro, C.T.; Cardozo, P.L.; Devalle, S.; Salerno, J.A.; Ornelas, I.M.; Ledur, P.F.; Ribeiro, F.M.; et al. Short and long TNF-alpha exposure recapitulates canonical astrogliosis events in human-induced pluripotent stem cells-derived astrocytes. Glia 2020, 68, 1396–1409. [Google Scholar] [CrossRef]
- Huang, X.; Wei, P.; Fang, C.; Yu, M.; Yang, S.; Qiu, L.; Wang, Y.; Xu, A.; Hoo, R.L.C.; Chang, J. Compromised endothelial Wnt/β-catenin signaling mediates the blood-brain barrier disruption and leads to neuroinflammation in endotoxemia. J. Neuroinflammation. 2024, 21, 265. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Wang, Y.; Yang, Y. Neuroprotective effect of interleukin-6 in a rat model of cerebral ischemia. Exp. Ther. Med. 2015, 9, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sadowska, G.B.; Chen, X.; Park, S.Y.; Kim, J.E.; Bodge, C.A. Anti-IL-6 neutralizing antibody modulates blood-brain barrier function in the ovine fetus. FASEB J. 2015, 29, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, K.; Bennett, J.L.; de Seze, J.; Haramura, M.; Kleiter, I.; Weinshenker, B.G. Interleukin-6 in neuromyelitis optica spectrum disorder pathophysiology. Neurol. Neuroimmunol. Neuroinflammation 2020, 7, e841. [Google Scholar] [CrossRef]
- Hirohata, S.; Arinuma, Y.; Takeno, M.; Hasegawa, M. Association of blood-brain barrier breakdown with cerebrospinal fluid cytokine levels and disease activity in neuropsychiatric lupus erythematosus. J. Neuroimmunol. 2020, 346, 577282. [Google Scholar] [CrossRef]
- Silva, N.M.; Gonçalves, R.A.; Pascoal, T.A.; Lima-Filho, R.A.S.; Resende, E.P.F.; Vieira, E.L.M. Pro-inflammatory interleukin-6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer’s disease. Transl. Psychiatry 2021, 11, 251. [Google Scholar] [CrossRef]
- Barabási, B.; Barna, L.; Santa-Maria, A.R.; Harazin, A.; Molnár, R.; Kincses, A. Role of interleukin-6 and interleukin-10 in morphological and functional changes of the blood-brain barrier in hypertriglyceridemia. Fluids Barriers CNS 2023, 20, 15. [Google Scholar] [CrossRef]
- Galea, I. The blood-brain barrier in systemic infection and inflammation. Cell. Mol. Immunol. 2021, 18, 2489–2501. [Google Scholar] [CrossRef]
- Vezzani, A.; Viviani, B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 2015, 96, 70–82. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, M.; Qiu, W.; Ma, H.; Zhang, X.; Zhu, Z. Safety and efficacy of tocilizumab versus azathioprine in highly relapsing neuromyelitis optica spectrum disorder (TANGO): An open-label, multicenter, randomized, phase 2 trial. Lancet Neurol. 2020, 19, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Traboulsee, A.; Greenberg, B.M.; Bennett, J.L.; Sato, D.K.; Yamamura, T.; Brassat, D. Safety and efficacy of satralizumab in NMOSD: Results from two phase 3 trials. Lancet Neurol. 2020, 19, 391–401. [Google Scholar] [CrossRef]
- Choy, E.H.; De Benedetti, F.; Takeuchi, T.; Hashizume, M.; John, M.R.; Kishimoto, T. Translating IL-6 biology into effective treatments. Nat. Rev. Rheumatol. 2020, 16, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Leng, K.; Park, J.; Sorets, A.G.; Kim, S.; Shostak, A.; Embalabala, R.J.; Mlouk, K.; Katdare, K.A.; Rose, I.V.L.; et al. Reactive astrocytes transduce inflammation in a blood-brain barrier model through a TNF-STAT3 signaling axis and secretion of alpha 1-antichymotrypsin. Nat. Commun. 2022, 13, 6581. [Google Scholar] [CrossRef]
- Chen, H.; Tang, X.; Li, J.; Hu, B.; Yang, W.; Zhan, M.; Ma, T.; Xu, S. IL-17 crosses the blood-brain barrier to trigger neuroinflammation: A novel mechanism in nitroglycerin-induced chronic migraine. J. Headache Pain. 2022, 23, 1. [Google Scholar] [CrossRef]
| Molecule | Category of Action | Impact on BBB (Structures) | Clinical Relevance (Pathology, Therapy) | References |
|---|---|---|---|---|
| IL-1 | Proinflammatory | Disrupts tight junctions of capillary endothelial cells; activates other proinflammatory cytokines; abolishes the protective effect of astrocytes on BBB integrity by suppressing astrocytic sonic hedgehog (SHH) production; stimulates astrocytes to produce potential neurotoxic substances; stimulates vascular permeability and angiogenesis | Involvement in neuroinflammation; potential therapeutic target | [94,95,96,97,98,99,100] |
| IL-1β | Proinflammatory | Disrupts tight junctions of capillary endothelial cells; damages astrocytes by downregulating SHH; increases the secretion of other proinflammatory cytokines | Involvement in neuroinflammation; potential therapeutic target | [97,98,101,102] |
| TNF-α | Proinflammatory | Damages tight junctions; induces astrocyte dysfunction; alters BBB morphology | Pathogenesis of MS, NMOSD, depressive states; therapeutic target | [103,104,105,106,107] |
| IFN-γ | Proinflammatory | Disrupts tight junctions of endothelial cells; induces transendothelial migration of CD4+ T cells to the basement membrane and promotes the transcellular route of this migration; induces a change in the C6 of ZO-1 and decreases mRNA and protein levels of ZO-1 in epithelial cells | Autoimmune CNS diseases; potential therapeutic target | [108,109,110,111] |
| IL-17A | Proinflammatory | Disrupts the tight junctions of endothelial cells by downregulating the expression of occludin; enhances neuroinflammatory signaling pathways | Migraine, MS, neuropsychiatric lupus; promising therapeutic target | [112] |
| IL-6 | Mixed | Disrupts tight junctions of capillary endothelial cells; reduces tight junction proteins β-catenin; may act anti-inflammatory by reducing the secretion of other proinflammatory cytokines | NMOSD, MS, Alzheimer’s; potential therapeutic target | [11,90,113,114,115,116] |
| IL-2 | Mixed | Activates endothelium, may increase BBB permeability; supports BBB repair in certain conditions; astrocyte-targeted IL-2 gene delivery may protect against neuroinflammation and BBB disruption by an increase in brain resident regulatory T cell number | MS, Treg-based therapies; potential therapeutic target | [117,118,119,120] |
| IL-12 | Mixed | Promotes neuroinflammation and BBB disruption; in autoimmune neuroinflammation in mice, plays a neuroprotective role that is mediated by neuroectoderm-derived cells, specifically neurons, and not immune cells | MS, anti-IL-12/23 therapies | [121,122,123] |
| IL-15 | Mixed | Has a low level of permeability through the BBB; reduces astrocyte damage and death, increasing resistance to cytotoxicity; IL-15 complex treatment during experimental cerebral malaria in mice reduces BBB permeability and prevents BBB breakdown (the effect related to the induction of IL-10-producing NK cells) | HIV, MS, potential therapeutic application | [124,125,126,127] |
| IL-18 | Mixed | Mediated up-regulation of dystrophin expression may play either a direct or indirect role in the maintenance of BBB function following status epilepticus (prevents increased BBB permeability leading to vasogenic edema) | Inflammatory states in epilepsy, MS; potential therapeutic target | [128] |
| IL-4 | Anti-inflammatory | May influence BBB permeability through modulation of the immune response and interaction with microglial cells | Elevated IL-4 levels in serum are associated with markers of BBB damage in paraneoplastic syndromes; neutralization of IL-4 may protect dopaminergic neurons and reduce BBB damage in Parkinson’s disease models | [129,130,131] |
| CCL2 (MCP-1—Monocyte Chemoattractant Protein-1) | Proinflammatory | Attracts monocytes to the BBB, facilitates transmigration | MS, neuroinfections, stroke; therapeutic target | [97] |
| CXCL12 (SDF-1—Stromal Cell-Derived Factor 1) | Proinflammatory | Mobilization of progenitor cells, angiogenesis, regulation of migration of stem cells and lymphocytes | Cancer metastasis, immune responses | [132] |
| CCL20 (MIP-3α—Macrophage Inflammatory Protein-3 alpha) | Proinflammatory | Facilitates Th17 cell migration across the BBB | MS, therapeutic target | [132] |
| CX3CL1 (Fractalkine) | Proinflammatory | Attracts neutrophils, damages endothelial cells | Meningitis, stroke; potential therapeutic target | [133,134,135,136,137] |
| Histamine | Proinflammatory | Vasodilation, increased vascular permeability, recruitment of inflammatory cells | Allergic reactions, inflammation, regulation of immune response | [138,139,140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryka-Marton, M.; Grabowska, A.D.; Szukiewicz, D. Breaking the Barrier: The Role of Proinflammatory Cytokines in BBB Dysfunction. Int. J. Mol. Sci. 2025, 26, 3532. https://doi.org/10.3390/ijms26083532
Gryka-Marton M, Grabowska AD, Szukiewicz D. Breaking the Barrier: The Role of Proinflammatory Cytokines in BBB Dysfunction. International Journal of Molecular Sciences. 2025; 26(8):3532. https://doi.org/10.3390/ijms26083532
Chicago/Turabian StyleGryka-Marton, Małgorzata, Anna D. Grabowska, and Dariusz Szukiewicz. 2025. "Breaking the Barrier: The Role of Proinflammatory Cytokines in BBB Dysfunction" International Journal of Molecular Sciences 26, no. 8: 3532. https://doi.org/10.3390/ijms26083532
APA StyleGryka-Marton, M., Grabowska, A. D., & Szukiewicz, D. (2025). Breaking the Barrier: The Role of Proinflammatory Cytokines in BBB Dysfunction. International Journal of Molecular Sciences, 26(8), 3532. https://doi.org/10.3390/ijms26083532







