Abstract
Neurodegenerative diseases (NDDs) such as Alzheimer’s, Parkinson’s, ALS, and Huntington’s pose a growing global challenge due to their complex pathobiology and aging demographics. Once considered as cellular debris, small extracellular vesicles (sEVs) are now recognized as active mediators of intercellular signaling in NDD progression. These nanovesicles (~30–150 nm), capable of crossing the blood–brain barrier, carry pathological proteins, RNAs, and lipids, facilitating the spread of toxic species like Aβ, tau, TDP-43, and α-synuclein. sEVs are increasingly recognized as valuable diagnostic tools, outperforming traditional CSF biomarkers in early detection and disease monitoring. On the therapeutic front, engineered sEVs offer a promising platform for CNS-targeted delivery of siRNAs, CRISPR tools, and neuroprotective agents, demonstrating efficacy in preclinical models. However, translational hurdles persist, including standardization, scalability, and regulatory alignment. Promising solutions are emerging, such as CRISPR-based barcoding, which enables high-resolution tracking of vesicle biodistribution; AI-guided analytics to enhance quality control; and coordinated regulatory efforts by the FDA, EMA, and ISEV aimed at unifying identity and purity criteria under forthcoming Minimal Information for Studies of Extracellular Vesicles (MISEV) guidelines. This review critically examines the mechanistic roles, diagnostic potential, and therapeutic applications of sEVs in NDDs, and outlines key strategies for clinical translation.
1. Introduction
Neurodegenerative diseases (NDDs), including Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), Huntington’s disease (HD), and related disorders, affect more than 50 million people worldwide and are poised to triple by 2050 as populations age [1,2,3]. Despite their diverse clinical phenotypes, NDDs share convergent hallmarks: selective neuronal loss, glial activation, and the accumulation of misfolded proteins such as amyloid-β, hyper-phosphorylated tau, α-synuclein, and TDP-43. The classic cell-autonomous paradigm of neurodegeneration is increasingly being replaced by systems-level models that emphasize the trans-synaptic and extracellular propagation of pathogenic proteins through interconnected neuronal and glial networks, as well as crosstalk with peripheral immune pathways [4]. A major driver of this paradigm shift is the discovery that small extracellular vesicles (sEVs) can transport toxic proteins, lipids, and nucleic acids between cells, thereby amplifying pathology while simultaneously providing a molecular “snapshot” of ongoing disease processes [5].
sEVs are 30–150 nm, double-membraned nanovesicles generated by the endosomal pathway and released via multivesicular body fusion or outward budding. Once dismissed as mere debris and labeled “exosomes,” sEVs are now recognized as potent transporters of proteins, lipids, and nucleic acids, distinct from larger microvesicles and apoptotic bodies [6]. First identified in reticulocytes in 1983 and formally termed “exosomes” in 1987 [7,8], their immunological function was firmly established when Raposo and colleagues demonstrated that B-cell-derived sEVs could present antigens to T cells [9]. Over the past decade, interest in sEVs in the context of NDD has expanded dramatically, with PubMed citations linking “exosome AND neurodegenerative” increasing over 30-fold, reflecting their rising significance in basic science, biomarker discovery, and therapeutic development [10].
2. Emerging Roles of sEVs in Neurodegeneration
2.1. Biogenesis and Molecular Cargo
The formation of sEVs is orchestrated through multiple interconnected pathways, including the endosomal sorting complexes required for transport (ESCRT)-dependent mechanism, ESCRT-independent routes involving lipid raft domains, and scaffolding by tetraspanins such as CD9, CD63, and CD81 [11]. Cargo selection is a highly regulated process influenced by post-translational modifications like ubiquitylation and sumoylation, as well as the action of RNA-binding proteins, which facilitate the selective packaging of proteins, lipids, and nucleic acids. This specificity underlies the emergence of disease-relevant signatures, for example, the enrichment of specific microRNAs like miR-9, miR-29, miR-135, and miR-186, which play a crucial role in Aβ generation [12,13] in AD, or phosphorylated Rab GTPases in PD linked to LRRK2 [13,14,15]. Encapsulated within a lipid bilayer, sEVs protect labile molecular contents from enzymatic degradation, allowing them to mediate stable, long-range communication both within the central nervous system (CNS) and between the CNS and peripheral tissues [16].
2.2. Crossing the Blood–Brain Barrier
sEVs possess a unique capacity to cross the blood–brain barrier (BBB) in both directions, distinguishing them from most synthetic nanocarriers. This bidirectional transport occurs through multiple mechanisms, including receptor-mediated transcytosis, adsorptive endocytosis, and trafficking via immune cells such as monocytes and macrophages, a phenomenon often referred to as “cellular hitchhiking” [17,18]. sEVs of CNS origin have been successfully detected in peripheral biofluids such as plasma, saliva, and urine, enabling the development of non-invasive liquid biopsies that reflect ongoing neuropathological processes. These vesicles offer considerable promise for early disease detection, longitudinal monitoring, and personalized therapeutic interventions in neurodegenerative disorders [19] while creating new opportunities for early diagnosis and real-time therapeutic monitoring [20].
2.3. Vectors of Pathology
Compelling evidence now supports the role of sEVs as vectors for the intercellular transmission of misfolded proteins, contributing to the prion-like spread of pathology in various NDDs [21,22] as shown in Scheme 1. Neuron-derived sEVs carrying oligomeric amyloid-β (Aβ) or hyperphosphorylated tau have been shown to enhance the deposition of amyloid plaques and neurofibrillary tangles in AD models [23]. Emerging studies support the existence of a dynamic microbiome-gut-brain axis, wherein microbial-derived metabolites and sEVs can traverse physiological barriers and influence neurodevelopment and neurodegeneration, as demonstrated in a gut-brain axis microfluidic model using human-iPSC-derived neurons, highlighting their potential as modulators of neural growth, synaptic plasticity, and CNS function [23]. There are reports of neuron-derived vesicles laden with oligomeric Aβ or tau seeds accelerating plaque and tangle deposition, while α-synuclein-rich gut sEVs ascend the vagus nerve to initiate PD-like changes in the substantia nigra. In ALS, sEVs secreted by motor neurons have been found to carry pathogenic species such as mutant superoxide dismutase 1 (SOD1) and TAR DNA-binding protein 43 (TDP-43), which elicit neurotoxic responses in astrocytes and microglia, fostering a feed-forward loop of neuroinflammation, thereby amplifying neuroinflammation through a self-reinforcing cycle [23]. These observations align with the emerging concept of sEV-mediated “connectomic spread” in NDDs [4,24].
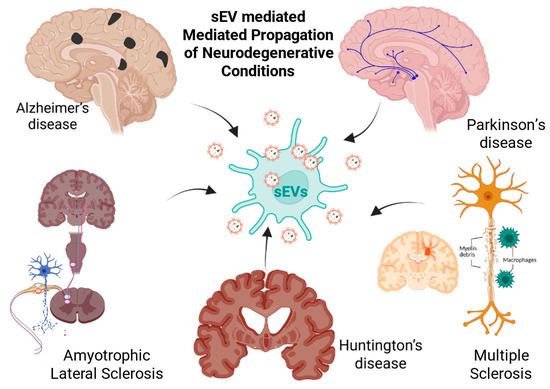
Scheme 1.
Role of sEVs in the spread and progression of NDDs. Pathogenic sEVs released in the CNS can transport misfolded proteins, inflammatory mediators, and nucleic acids that seed or amplify disease-specific lesions. The Scheme highlights five major disorders: Alzheimer’s disease (amyloid-β/tau spread), Parkinson’s disease (α-synuclein propagation), amyotrophic lateral sclerosis (motor neuron degeneration), Huntington’s disease (mutant huntingtin dissemination), and multiple sclerosis (immune-mediated demyelination), illustrating how sEVs contribute to the pathological spread and progression of these neurological conditions. (The scheme was created with BioRender.com. (accessed on 22 July 2025)).
2.4. Liquid Biopsy Potential
Recent technological advancements in immunoaffinity capture techniques targeting CNS-specific markers such as L1CAM and GLAST, along with innovations in microfluidics and single-vesicle omics, have significantly enhanced the ability to isolate and profile brain-derived sEVs from peripheral biofluids [25,26,27]. These brain-enriched sEVs (BEVs) carry disease-relevant molecular cargo, allowing for high-accuracy diagnostic applications. For instance, combined profiling of Aβ42, phosphorylated tau-181 (p-tau181), synaptophysin, and microRNA-21 within BEVs yields over 90% sensitivity and specificity in identifying prodromal AD [28]. Moreover, BEV-associated biomarkers such as neurofilament light chain (NfL) and phosphorylated TDP-43 have demonstrated superior performance over their plasma counterparts in staging and tracking progression in ALS [29]. Ongoing large-scale, longitudinal studies are underway to validate these sEV signatures and establish reference ranges across the aging spectrum [5,25,29].
2.5. Therapeutic Promise
sEVs offer a compelling platform for CNS drug delivery due to their inherent biocompatibility, low immunogenicity, and capacity for surface engineering and cargo customization [11,30]. Advanced bioengineering approaches such as the incorporation of rabies virus glycoprotein (RVG) peptides for neuron-specific targeting and CRISPR-Cas9 or siRNA loading for gene modulation have enabled sEVs to successfully deliver therapeutic payloads across the BBB, mitigating neuropathology in preclinical models of AD and PD [31,32,33]. These vesicles have been used to transport small molecules, nucleic acids, and genome-editing components with high target specificity and minimal off-target effects. Moreover, early-phase clinical trials utilizing mesenchymal stem cell (MSC)-derived sEVs have reported favorable safety profiles and preliminary signs of therapeutic efficacy in neurodegenerative and inflammatory CNS conditions [34,35,36].
2.6. Rationale and Scope of This Review
Amidst the accelerating pace of research into sEVs, there is a critical need for a comprehensive and up-to-date review of current knowledge. In this review, we aim to (i) delineate the molecular mechanisms governing sEV biogenesis and cargo sorting; (ii) critically examine experimental evidence supporting their role in the propagation of neurodegenerative pathology; (iii) assess the diagnostic and prognostic utility of sEV-based biomarkers in biofluids; (iv) explore emerging bioengineering strategies that leverage sEVs for therapeutic delivery across the BBB; and (v) identify key technical, translational, and regulatory hurdles that must be overcome to enable clinical application. Collectively, our objective is to provide a structured framework to harness the potential of sEVs to elucidate and ultimately intervene in the complex pathobiology of neurodegenerative disorders.
3. Determinants of sEV Composition in the CNS: Biogenesis, Cellular Origin, and Disease Modulation
3.1. Biogenesis and Molecular Determinants of Cargo Loading in sEVs
sEV biogenesis is not a random shedding process but a precisely tuned molecular event. Advances in single-vesicle sequencing, CRISPR-based labeling, and high-throughput proteomics have refined our ability to dissect sEV heterogeneity and decode their physiological relevance.
SEVs originate from the endosomal system and are released upon the fusion of multivesicular bodies (MVBs) with the plasma membrane. sEV biogenesis occurs through two major pathways: the ESCRT-dependent pathway and the ceramide-mediated ESCRT-independent pathway [37] as shown in Scheme 2. The ESCRT (endosomal sorting complex required for transport) machinery consists of four protein complexes (ESCRT-0, -I, -II, and -III) and accessory proteins such as ALIX and TSG101, which coordinate cargo recognition, membrane budding, and vesicle scission. Disruption of ESCRT components, such as CHMP2B, has been linked to familial frontotemporal dementia, underscoring the importance of this pathway in neurobiology [38]. In parallel, ESCRT-independent sEV formation is driven by ceramide generation via neutral sphingomyelinase 2 (nSMase2), promoting negative membrane curvature. This mechanism is particularly active in glial cells and can be pharmacologically inhibited using agents like GW4869 [39,40]. Tetraspanins such as CD9, CD63, and CD81 organize into specialized microdomains that scaffold vesicles budding independently of ESCRT components. Super-resolution microscopy has revealed that CD9 modulates the intracellular trafficking of CD63; depletion of CD9 enhances CD63-positive EV secretion, whereas CD9 overexpression suppresses it [41].
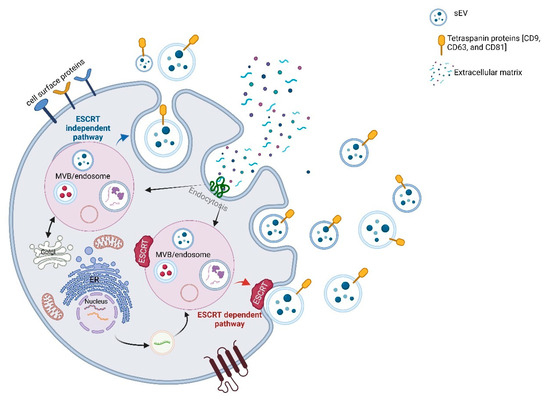
Scheme 2.
Biogenesis and cargo sorting mechanisms of sEVs. sEV formation begins with inward budding of the plasma membrane to generate early endosomes, which mature into multivesicular bodies (MVBs). Within MVBs, intraluminal vesicles (ILVs) are formed via two major pathways: the ESCRT-dependent pathway (red), involving endosomal sorting complexes required for transport, and the ESCRT-independent pathway (blue), driven by tetraspanins and specific lipids. Mature MVBs either fuse with lysosomes for degradation or with the plasma membrane to release ILVs as sEVs into the extracellular space. These vesicles carry proteins, RNAs, and lipids, and can be taken up by recipient cells via endocytosis, mediating intercellular communication. (The scheme was created with BioRender.com. (accessed on 22 July 2025)).
Cargo loading into sEVs is increasingly recognized as a highly regulated process rather than a passive reflection of cytoplasmic contents [42]. Protein inclusion relies on multiple signals, including ubiquitination (which facilitates ESCRT recognition), lipid raft partitioning, and tetraspanin-mediated clustering of membrane proteins such as MHC molecules and L1CAM [37]. Post-translational modifications like phosphorylation and sumoylation further influence cargo sorting under both physiological and pathological conditions, such as misfolded tau or TDP-43, particularly under pathological conditions like ALS or AD [43].
RNA cargo selection, encompassing miRNAs, mRNAs, and non-coding RNAs, is guided by RNA-binding proteins (RBPs) such as hnRNPA2B1, AGO2, and YBX1, which recognize short sequence motifs such as EXOmotifs and mediate vesicular packaging [44]. Disease states such as AD can alter this sorting process, resulting in enrichment of specific miRNAs (e.g., miR-34a, miR-125b, miR-146a) and lipids that may contribute to neuroinflammation and synaptic dysfunction [45,46].
Together, the biogenesis and selective cargo loading of sEVs reflect an intricate interplay between endosomal trafficking machinery, membrane dynamics, and molecular recognition systems. These properties not only enable sEVs to serve as intercellular messengers but also position them as potential biomarkers and therapeutic targets in a range of diseases.
3.2. Cell-Type-Specific sEV Profiles in the CNS
In the CNS, sEVs exhibit cell-type-specific molecular compositions that reflect their origin and functional specialization. Major CNS cell types, including neurons, astrocytes, microglia, and oligodendrocytes, release sEVs that contribute to both homeostatic signaling and disease-related processes. Neuronal sEVs are typically characterized by surface markers such as L1CAM and NCAM and are enriched with synaptic proteins including synaptophysin and postsynaptic density protein 95 (PSD-95), as well as neurotransmitter receptors and activity-regulated RNAs. The composition of neuronal sEVs is dynamically regulated by synaptic activity, brain-derived neurotrophic factor (BDNF) signaling, and cellular stressors such as excitotoxicity or oxidative damage [47,48,49,50]. Astrocyte-derived sEVs, often identified by glutamate-aspartate transporter (GLAST) and glial fibrillary acidic protein (GFAP), can transfer neuroprotective molecules including apolipoprotein E (ApoE) and glutamate transporters under physiological conditions. However, in pathological contexts, these vesicles may carry neurotoxic components such as ceramide, pro-inflammatory cytokines, and microRNAs (e.g., miR-21, miR-34a), contributing to neuroinflammation and synaptic dysfunction [51,52,53]. Microglial sEVs play critical roles in neuroimmune communication and synaptic remodeling. These vesicles contain immune-related molecules such as major histocompatibility complex (MHC) class II proteins, interleukin-1β (IL-1β), and pro-inflammatory microRNAs including miR-155, thereby mediating inflammatory responses and microglia–neuron crosstalk [54,55]. Oligodendrocyte-derived sEVs are enriched with myelin-associated proteins such as proteolipid protein (PLP) and myelin oligodendrocyte glycoprotein (MOG), along with glycolytic enzymes that support axonal metabolism [56]. These vesicles have been shown to promote axonal integrity and may exert neuroprotective effects in demyelinating diseases, including experimental models of multiple sclerosis (MS) [56]. Collectively, these distinct cell-specific sEVs profiles underscore the functional heterogeneity of sEVs in CNS intercellular signaling and highlight their potential as biomarkers and therapeutic targets in neurological disorders.
3.3. Influence of Stress and Disease on the Composition of sEVs
The molecular cargo of sEVs is dynamically regulated in response to cellular stress and pathological conditions, reflecting disease-specific changes in their proteomic and transcriptomic content. In AD, sEVs derived from cerebrospinal fluid (CSF) and plasma exhibit increased levels of amyloid-β (Aβ) peptides, phosphorylated tau (p-tau), and regulatory microRNAs such as miR-29a/b and miR-34a, which are implicated in amyloid genesis and synaptic dysfunction [28,57,58]. In PD, dopaminergic neurons subjected to genetic mutations (e.g., LRRK2) or environmental toxins release sEVs enriched in α-synuclein, phosphorylated LRRK2, and Rab10, indicating a role in disease propagation and intracellular trafficking defects [59,60]. In ALS and FTD models, sEVs derived from motor neurons and glial cells have been shown to carry SOD1, TDP-43, and cryptic RNAs associated with C9orf72 repeat expansions, contributing to RNA toxicity and neuroinflammation [51,59,61]. Similarly, in HD, the sEV cargo changes dynamically with motor activity and disease progression, including the presence of mutant huntingtin protein (mHTT), BDNF-modulating microRNAs, and altered synaptic mRNA transcripts, suggesting a role in neuroplasticity and disease modulation [62,63].
4. Physiological Functions of Small EVs in the Healthy CNS
A significant number of studies have convincingly demonstrated that sEVs are not simply a by-product of neural activity; they underpin routine “house-keeping” and long-range communication between cells across the CNS. Below, we elaborate on each major domain in which neuronal and glial nanovesicles shape circuit development and maintain adult neural homeostasis.
4.1. Neuronal sEVs: Tuning Synapse Formation, Maturation, and Plasticity
Neuronal sEVs are emerging as critical regulators of synaptic development, maturation, and plasticity. During early neurodevelopment, neurons secrete sEVs enriched in cell adhesion molecules such as NCAM-1 and L1CAM, Wnt family morphogens, and activity-regulated microRNAs [64]. Studies by Zhang et al. (2021) showed that neuronal sEVs enriched in HDAC2 mediate intercellular signaling that downregulates synaptic gene expression and modulates dendritic spine density during cortical development [65]. These vesicles function in a paracrine manner to guide axonal pathfinding, promote dendritic spine stabilization, and enhance filopodial outgrowth in recipient neurons, thereby facilitating synaptogenesis and circuit assembly. A recent systematic review synthesizing data from over 120 primary studies identified exosome-associated proteins including ephrin-B2, semaphorin-3A, and neuroligin-1 as key contributors to axon guidance and synaptic specificity, functioning in concert with classical chemotropic cues to refine callosal and topographic connectivity [66].
In the mature brain, neuronal sEVs continue to influence synaptic efficacy and plasticity in an activity-dependent manner. High-frequency neuronal firing induces glutamate release and postsynaptic Ca2+ influx, which in turn activates Rab35 and PIKfyve signaling pathways that govern vesicle trafficking and secretion. These activity-evoked sEVs are enriched in microRNAs such as miR-132, miR-134, and miR-212, which are internalized by neighboring postsynaptic compartments. Once delivered, these miRNAs suppress targets such as PTEN and LIMK1, resulting in increased dendritic spine size and elevated thresholds for long-term potentiation (LTP) [67]. This mechanism has been implicated in experience-dependent synaptic remodeling and is thought to underlie aspects of mood regulation and cognitive flexibility [68].
4.2. Microglial sEVs and Complement-Driven Synaptic Pruning
During postnatal development, microglia orchestrate synaptic refinement through complement-mediated pruning, selectively eliminating excess or weak synapses to sculpt neural circuits. Classical complement components C1q and C3 are deposited onto synapses, marking them for engulfment by microglial processes. Emerging evidence reveals that sEVs derived from microglia serve as a conduit for delivering C1q to neuronal targets. A recent study demonstrated that microglia are the predominant source of extracellular C1q in the brain. Once secreted, C1q associates with neuronal ribonucleoprotein granules, promoting their degradation and facilitating synapse elimination by licensing complement receptor interactions on microglia [69]. Beyond contact-dependent mechanisms, microglia also engage in remote, vesicle-mediated synaptic pruning. Pro-inflammatory activation induces the expression of the membrane-associated sialidase Neu3, which is incorporated into fusogenic microglial sEVs. Upon membrane fusion with presynaptic terminals, Neu3 removes terminal sialic acid residues, exposing galactose moieties that enhance complement deposition and subsequent pruning [70]. Functionally, blockade of the complement cascade or genetic ablation of C1q preserves inhibitory synapses following exposure to general anesthetics, indicating that this complement-sEV axis is both physiologically active and pharmacologically modifiable [71].
4.3. Oligodendrocyte-Derived sEVs: Metabolic Lifelines for Axons and Myelin Upkeep
In addition to facilitating saltatory conduction, myelinating oligodendrocytes play a crucial role in maintaining the metabolic integrity of long-projection axons by supplying energetic and antioxidant support. This is achieved through two primary mechanisms: (a) the transfer of metabolic substrates such as lactate and pyruvate via the monocarboxylate transporters MCT1 (on oligodendrocytes) and MCT2 (on axons), and (b) the release of sEVs enriched with glycolytic enzymes, antioxidant proteins, and the mitochondrial deacetylase SIRT2 [72]. Once internalized by neurons, SIRT2 deacetylates adenine nucleotide translocators ANT1 and ANT2, enhancing ADP-ATP exchange across the mitochondrial inner membrane and boosting oxidative phosphorylation efficiency. Impairment of this vesicle-mediated metabolic coupling leads to axonal swellings, impaired mitochondrial function, and conduction failure; conversely, supplementation with purified oligodendrocyte-derived sEVs restores axonal energy homeostasis and conduction fidelity [73].
This support system is dynamically regulated by neuronal activity. Action potential propagation triggers the release of glutamate into the periaxonal space, which in turn activates NMDA and AMPA receptors localized on oligodendrocyte inner processes. This receptor engagement elevates intracellular Ca2+ levels and activates the CaMKK-AMPK signaling cascade, linking neuronal firing to glucose uptake and the biogenesis of metabolically active exosomes [74]. Thus, oligodendrocyte sEV-mediated metabolic support is activity-dependent, preferentially targeting energetically demanding, high-frequency axons and aligning cellular bioenergetics with network dynamics.
4.4. Integrated Glia–Neuron Crosstalk and Myelin Maintenance
Astrocyte-derived sEVs close the metabolic loop by donating glycogen-derived pyruvate to oligodendrocytes and secreting factors (e.g., LIF) that promote myelin sheath stability, evidence that the three glial lineages cooperate through intersecting vesicle channels [75]. Collectively, these data reposition sEVs as pivotal arbiters of CNS physiology, balancing connectivity via microglial pruning, fine-tuning synaptic gain through neuronal miRNAs, and fueling the immense energetic load of myelinated tracts via oligodendrocyte cargo delivery. It is clear that even in the absence of disease, sEVs continually sculpt neural circuits, clear redundant synapses, and safeguard axonal metabolism functions that become maladaptive only when cargo composition or release kinetics go awry.
5. Small EVs in NDD Pathogenesis
sEVs act as vectors of pathogenic proteins and inflammatory signals and as dynamic biomarkers reflecting molecular changes. Recent mechanistic discoveries reveal novel therapeutic and diagnostic strategies across major neurodegenerative disorders.
5.1. Small EVs in Alzheimer’s Disease: Biomarker Signatures, Pathogenic Spread, and Therapeutic Modulation
sEVs play a critical role in AD by carrying pathogenic biomarkers, mediating the spread of tau and amyloid pathology, and offering new therapeutic targets as listed in Table 1. In this section, we highlight their emerging relevance in early detection, disease progression, and intervention strategies, as shown in Scheme 3.
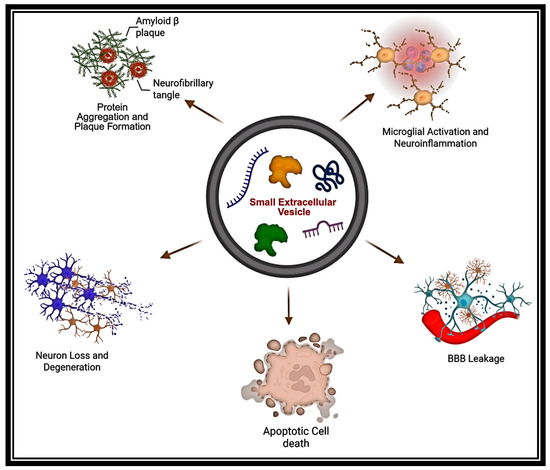
Scheme 3.
Role of sEVs in the propagation of NDDs. This illustration depicts how sEVs contribute to NDD progression by transporting neurotoxic cargo that promotes protein misfolding, neuroinflammation, BBB disruption, and synaptic dysfunction, thereby spreading pathology throughout the CNS. (The Scheme was created with BioRender.com. (accessed on 22 July 2025)).
Cargo signatures that are predictive mRNA and inflammatory protein biomarkers: Plasma-derived BEVs carry mRNAs such as CADM1, GABRB3, and FOXJ3, which, when used in machine learning algorithms, distinguish AD from preclinical stages and accurately predict age at onset (>90% accuracy) [57]. Complementary miRNA profiling confirms increased miR-34a and miR-125b, two regulators of dendritic spine stability and complement activation [46]. Recent studies have shown that EVs facilitate the propagation of inflammasome signaling within both the brain and heart in murine models of AD [76].
Propagating the classical lesions and mediation of tau and amyloid propagation: BEVs from APOE4 carriers carry tau filaments that facilitate trans-neuronal spread of tauopathy, a process enhanced by specific lipidomic cargo [77]. sEVs released from neurons that over-express GSK3β-phosphorylated tau transfer seed-competent tau to neighboring cells, accelerating tangle formation in human-iPSC-derived cortical cultures and wild-type mice [78]. Oligomeric Aβ, in turn, is loaded into astrocyte-derived sEVs, whose CD63-rich surface dramatically enhances neuronal uptake and toxicity; a recent study shows that catalpol + tetramethyl-pyrazine drives astrocytes to secrete CDK5-mRNA-positive sEVs that potentiate STAT3 signaling and axonal sprouting, underscoring how glial vesicles can flip from protective to harmful depending on cargo [79].
Emerging therapeutic angles via sEV modulation. Pharmacological inhibition of neutral sphingomyelinase 2 (nSMase2) has been shown to significantly reduce neuronal exosome secretion and decrease interstitial levels of amyloid-β (Aβ) and phosphorylated tau in 5xFAD AD mouse models. These findings have catalyzed the development of combination therapeutic strategies such as pairing nSMase2 inhibitors with anti-Aβ monoclonal antibodies, which are now advancing through preclinical pipelines as a means to synergistically enhance Aβ clearance and attenuate neurodegeneration [80].
5.2. Parkinson’s Disease (PD)
Similar to as in AD, sEVs have emerged as key mediators in PD, contributing to gut–brain signaling, biomarker discovery, and the development of novel therapeutic strategies.
Gut-brain trafficking of α-synuclein: Enteric-origin α-synuclein loaded into sEVs has been shown to be transmitted via the vagus nerve to the dorsal motor nucleus, thereby providing a physiological vector for Braak stage progression in PD models [81]. Exposure of rodents to rotenone, an environmental mitochondrial toxin, induces α-synuclein release from enteric neurons, and both surgical vagotomy and sEV tetraspanin blockade effectively impede its central propagation [82]. In LRRK2-G2019S mutation carriers, high-resolution phosphoproteomic profiling of urinary and plasma sEVs reveals elevated levels of phosphorylated Rab12 at Ser106; these levels rapidly decline after only one week of treatment with the LRRK2 inhibitor MLi-2, highlighting pSer106-Rab12 as a dynamic pharmacodynamic and biomarker read-out [83].
Toward sEV-guided interventions: A recent review has endorsed BEV panels comprising α-synuclein, DJ-1, and miRNA for early-stage diagnostic use, with superior performance to CSF assays [84]. Moreover, therapeutic strategies leveraging sEV-mediated delivery are advancing: ultrasound-mediated CRISPR interference targeting SNCA within sEVs achieves approximately 70% knockdown of α-synuclein and rescues nigral dopaminergic degeneration in A53T mouse models, while antisense oligonucleotide-loaded, RVG-tagged sEVs similarly mitigate pathology via RNA interference [33,85,86].
5.3. Amyotrophic Lateral Sclerosis (ALS)
Motor-neuron-derived sEVs facilitate intercellular propagation of misfolded TDP-43 and mutant SOD1, which are internalized by astrocytes and microglia, thereby intensifying non-cell-autonomous neuroinflammatory cascades in ALS models [87,88]. Quantitative assays show that plasma sEV-associated TDP-43 discriminates ALS from frontotemporal dementia and healthy controls with an area under the curve (AUC) > 0.9, and these levels correlate longitudinally with motor function decline over 12 months [89]. While serum NfL is an FDA-qualified biomarker, accumulating evidence indicates that BEV NfL increases earlier and more closely tracks corticospinal tract degeneration than serum NfL, suggesting substantial potential to reduce diagnostic latency, a finding emphasized in the 2024 EV-ALS biomarker compendium [90]. Therapeutically, pharmacological blockade of sEV biogenesis using GW4869 in SOD1-G93A mice attenuates weight loss and extends survival, and MSC-derived sEVs engineered to deliver anti-TDP-43 siRNA restore motor neuron electrophysiological function in human cervical spinal cord organoids. Both strategies are now approaching Investigational New Drug-enabling stages, underscoring the translational promise of targeting sEV-mediated mechanisms in ALS [91,92].
5.4. Multiple Sclerosis (MS)
sEVs are increasingly recognized for their dual roles in MS, both as mediators of pathology and as therapeutic vehicles. Pathologically, CNS-derived sEVs traverse the BBB and transfer pro-inflammatory cargo such as myelin antigens and microRNAs that activate peripheral immune cells and exacerbate CNS demyelination and neuroinflammation [93]. Conversely, MSC-derived sEVs exert potent immunomodulatory effects: in vitro, they shift microglia from pro-inflammatory to anti-inflammatory phenotypes, suppress Th17 differentiation, and reprogram T cells toward regulatory subsets, while in vivo, they promote remyelination and reduce clinical disability in experimental autoimmune encephalomyelitis (EAE) models.
Oligodendrocyte-derived sEVs have recently been shown to bridge central and peripheral immunity in MS. These vesicles carry myelin-associated peptides such as MBP, MOG, and PLP that are presented to antigen-presenting cells (APCs) in peripheral lymphoid tissues, thereby priming autoreactive T cells and contributing to CNS-targeted autoimmunity [94]. Clinically, plasma sEVs bearing the integrins CD29, CD31, and adhesion molecule CD44 increase during active demyelinating lesion formation, with elevated levels observable approximately four weeks before corresponding spikes in serum NfL, suggesting a predictive biomarker role for these sEV signatures [95]. These findings have catalyzed the development of sEV-based therapeutic approaches, with MSC-sEVs advancing into early-phase clinical investigation as a cell-free regenerative and immunomodulatory treatment for MS [96].
5.5. Huntington’s Disease (HD)
sEVs, have been increasingly implicated in the intercellular propagation of mHTT, representing a pathogenic mechanism in HD. In vitro and in vivo studies demonstrate that neurons and glial cells secrete mHTT species, both monomers and oligomers within sEVs, which are subsequently internalized by neighboring cells, contributing to non-cell-autonomous neuronal toxicity and neurodegeneration [97]. Notably, LC-MS and imaging analyses have identified mHTT, including full-length (~360 kDa) and ~70 kDa truncated forms, within circulating sEVs from HD patients and transgenic porcine models, indicating systemic release of pathogenic protein via vesicular export [98]. Furthermore, functional studies using human striatal neurons transplanted into mouse brains revealed that sEVs bearing mHTT elicit degeneration of host neurons, a process attenuated by pharmacological inhibition of secretion via sphingosine-1-phosphate receptor antagonists (e.g., FTY720) [99]. Cumulatively, these findings support a model wherein sEV-mediated transfer of mHTT contributes substantively to HD pathogenesis and reinforces the rationale for targeting sEV biogenesis or uptake as novel therapeutic avenues in HD.
Conversely, proteomic analyses of striatal sEVs demonstrate that motor skill training induces a remodeling of vesicle cargo, enriching sEVs with synaptic plasticity proteins such as PSD-95 and Homer1, as well as metabolic enzymes including GAPDH and LDH-B [62]. These training-induced sEVs mitigate mHTT toxicity in neuronal co-culture assays, suggesting that activity-dependent exosome release may contribute to the delayed disease onset observed with environmental enrichment [100]. Concurrent work implicates microglia-derived sEVs enriched in BDNF, specifically splice variant IV, in rescuing corticostriatal synapse loss in HD models, positioning glial sEVs as endogenous modifiers of circuit integrity [101].
5.6. sEVs in Chronic CNS Injury (Chronic SCI and TBI)
sEVs play a dual role in chronic CNS injury such as spinal cord injury (SCI) and traumatic brain injury (TBI), acting both as mediators of secondary neurodegeneration and as vehicles for tissue repair. Pathogenically, sEVs released from injured neural and glial cells carry pro-inflammatory cytokines, damage-associated molecular patterns (DAMPs), and microRNAs that activate microglia and infiltrating immune cells, exacerbating neuroinflammation, excitotoxicity, and neuronal apoptosis in distal brain regions [102].
It has been demonstrated in chronic SCI that sEVs act both as mediators of lasting neurodegenerative signaling and as vehicles for regenerative therapies. Pathogenically, long-term SCI alters tissue- and sex-specific sEV secretion: in chronic SCI models, circulating sEV profiles display sexual dimorphism and correlate with persistent neuroinflammatory markers and cognitive deficits, implicating systemic, sEV-mediated neurodegenerative crosstalk [103]. In parallel, injurious stimuli generate sEVs containing pro-inflammatory cytokines, microRNAs, or DAMPs, which propagate inflammation and neuronal apoptosis beyond the lesion site. Emerging evidence identifies miR-21 as a central modulator in SCI, where it influences key processes such as inflammation, cell death, and repair by regulating molecular targets like PTEN and PDCD4 and shaping post-injury signaling networks [104]. Therapeutically, MSC-derived sEVs have demonstrated potent neuroprotective and reparative capacities: in preclinical SCI models, MSC-sEVs reduce inflammation, inhibit oxidative stress, promote blood–spinal cord barrier restoration, and support axonal regrowth and motor recovery [105,106]. These findings underscore that while aberrant sEV signaling sustains neurodegeneration in chronic SCI, engineered stem-cell-derived sEVs offer a promising cell-free strategy for mitigating pathology and promoting neurological repair.
Similarly, in TBI, circulating sEVs containing neuronal proteins and miRNAs serve as robust biomarkers for injury severity, metabolic dysfunction, and neuroinflammatory states [107]. Studies have reported that sEVs play a central role in propagating inflammasome signaling after CNS injury by carrying inflammasome components such as ASC and caspase-1 [108]. Conversely, sEV-mediated delivery of siRNA can effectively suppress inflammasome activation, offering a promising therapeutic strategy for reducing neuroinflammation and secondary damage following CNS trauma [108]. Recent studies showed that serum sEVs serve as potential biomarkers of TBI. Acute serum exosomal miR-206 and miR-549a-3p predict six-month cognitive outcome; MSC-sEVs curb early neuro-inflammation and white matter loss [109,110].

Table 1.
Roles of sEVs in neurodegenerative and traumatic CNS pathology: functional insights and cargo profiles.
Table 1.
Roles of sEVs in neurodegenerative and traumatic CNS pathology: functional insights and cargo profiles.
| Disease/Condition | sEV Function/Role | Key sEV Cargo | References |
|---|---|---|---|
| Alzheimer’s Disease | Spread of Aβ and tau; modulate microglial inflammation and synaptic dysfunction | Aβ, phosphorylated tau, miR-132-5p, miR-193b, miR-125b, miR-485-5p, miR-23a, miR-125b | [46,111] |
| Parkinson’s Disease | Transmit misfolded α-synuclein; influence dopaminergic neuron survival | α-synuclein, DJ-1, LRRK2, Let-7f-5p and miR-125a-5p, miR-10b-5p and miR-151a-3p, miR-24, miR-195, POU3F3 | [112,113] |
| Amyotrophic Lateral Sclerosis | Transfer TDP-43, SOD1; promote motor neuron degeneration | TDP-43, SOD1, FUS, miR-124, miR-146a-5p; miR-199a-3p; miR-151a-3p; miR-151a-5p; miR-199a-5p, MiR-151a-5p | [114,115] |
| Huntington’s Disease | Disseminate mHTT aggregates; modulate synaptic toxicity and inflammation | mutant huntingtin (mHTT), miR-124 | [116] |
| Multiple Sclerosis | Amplify inflammatory responses; alter T cell and BBB function | miR-155, CD29, CD31, inflammatory cytokines | [95,117,118] |
| Chronic Spinal Cord Injury | Carry pro-inflammatory miRNAs and cytokines; inhibit regeneration, sustain gliosis | miR-21, cytokines (TNF-α, IL-1β), GFAP | [104] |
| Traumatic Brain Injury | Alter neurovascular signaling; deliver injury-responsive miRNAs and DAMPs | miR-873, HSP70, NLRP3, mitochondrial DNA | [119] |
Conversely, therapeutic strategies are leveraging the regenerative capabilities of sEVs. Neural-stem-cell-derived sEVs enhance motor recovery, attenuate inflammatory microglial activation, reduce neuronal apoptosis, and promote autophagy in chronic SCI models [120]. Moreover, MSC-derived sEVs loaded with anti-inflammatory microRNAs or neurotrophic factors mitigate oxidative stress, support BBB repair, and improve functional outcomes in both SCI and TBI paradigms [121]. Intranasal delivery of human MSC-derived sEVs suppress NLRP3 inflammasome activation and downstream p38/MAPK signaling following TBI, thereby mitigating chronic neurological dysfunction [122]. Similarly, sEVs derived from Schwann cells (SCs), the principal myelinating cells of the peripheral nervous system, have demonstrated therapeutic potential in experimental models of both SCI and TBI, highlighting their promise as future candidates for neuroregenerative interventions [123,124,125]. Collectively, these findings highlight that dysregulated, injury-derived sEVs contribute to chronic neurodegeneration, whereas engineered sEVs represent promising cell-free interventions now progressing toward translational studies in repairing chronic CNS damage. sEVs can also provide versatile therapeutic tools to redefine our understanding of disease propagation, enabling minimally invasive biomarker discovery, and paving the way for next-generation, precision-targeted interventions.
6. Small EV Biomarkers: Current Landscape, Technical Hurdles, and Clinical Promise
sEVs represent a transformative advance in NDD diagnostics because they can traverse the BBB intact and carry molecular signatures reflective of the CNS tissue from which they are derived [126]. Immunocapturing these sEVs via cell-type-specific surface markers, such as neuronal L1CAM/CD171, astroglial GLAST, or oligodendroglial MOG, enables a 100-fold enrichment for CNS-derived cargo relative to bulk plasma, allowing ultrasensitive detection via techniques like digital ELISA (Simoa), bead-based flow cytometry, and low-input RNA sequencing [127]. These assays often surpass the performance of matched serum or CSF tests and can detect pathology well ahead of time, before symptom onset. In AD, neuron-derived BEVs routinely show elevated Aβ42 and phosphorylated tau (p-tau181), alongside reduced synaptic proteins such as synaptophysin, with additional increases in miR-21, miR-34a, and miR-125b, capturing neuroinflammatory and synaptic loss signals. In large meta-analyses, a four-analyte BEV panel (Aβ42, p-tau181, synaptophysin, and miR-21) discriminated prodromal AD from controls with 92% accuracy, and p-tau181 levels predicted hippocampal atrophy while synaptophysin tracked cognitive decline [128]. For PD, neuron-derived BEV α-synuclein seed amplification detected misfolded protein up to a decade pre-diagnosis, and sEV phospho-Rab12Ser106 in LRRK2-G2019S carriers reflected response to LRRK2 inhibitor therapy [83,129]. In a recent study of ALS, plasma sEV TDP-43 levels were significantly elevated in ALS compared to healthy controls and other NDD, with AUC values ≥ 0.94 distinguishing ALS from controls. These sEV TDP-43 levels correlated more strongly with disease severity measures, including ALS Functional Rating Scale-Revised (ALS-FRS–R) scores, and cognitive performance than serum NfL alone [89]. Additional applications include determining relapses in MS (miR-155/150 ± CD29/CD31) [130], sEV-associated GFAP as a circulating biomarker of TBI severity correlating with CT-positive lesions post-trauma (EV-GFAP + UCH-L1) [131], and molecular subtyping in frontotemporal dementia via sEV-TDP-43 and tau isoforms [89]. Despite these advances, technical challenges persist, chiefly in ensuring capture specificity, such as avoiding L1CAM expression from peripheral sources via dual epitope strategies [132], controlling pre-analytical variability (centrifugation, freeze–thaw cycles) [133], advancing quantification methods while overcoming critical analytical hurdles with novel emerging platforms such as digital ELISAs for microfluidic proteomics to enable improved sEV quantification [134], and developing standardized reference materials. Looking forward, single-vesicle multi-omics promises to integrate genotype (e.g., APOE, C9orf72), while longitudinal digital phenotyping paired with minimally invasive sEV sampling may enable real-time, adaptive biomarker monitoring [135,136]. In summary, when applied under rigorous MISEV-2023 standards and combined with ultra-sensitive multi-analyte detection, sEV assays stand poised to transition from exploratory research tools to clinically validated diagnostics across NDDs [136].
7. Small EVs as Therapeutic Platforms for CNS Disorders: Engineering, Delivery, and Clinical Translation
sEVs have emerged as promising nanocarriers for CNS therapeutics, primarily due to their innate ability to cross the BBB, low immunogenic profile, and versatile cargo capacity [137] (see Scheme 4). These vesicles leverage endogenous biological pathways, including receptor-mediated transcytosis and immune evasion mechanisms, attributed to host-derived membrane proteins and negligible endotoxin content, allowing for efficient brain-wide delivery of pharmacological payloads at significantly reduced doses [138,139]. Their endogenous origin allows for the encapsulation of small molecules, nucleic acids, proteins, and CRISPR ribonucleoproteins, making them ideal platforms for gene and drug delivery across neural tissues [140].
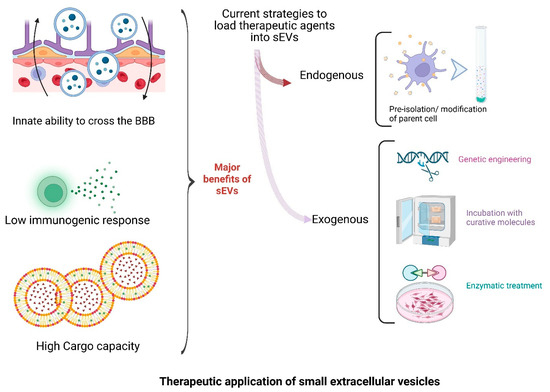
Scheme 4.
Advancing neurodegenerative treatments through sEV-based approaches. sEVs offer significant therapeutic potential due to their innate ability to cross the blood–brain barrier, low immunogenicity, and high cargo capacity. Therapeutic payloads can be introduced via endogenous (parent cell modification) or exogenous (genetic, chemical, or enzymatic) strategies, enabling versatile and targeted delivery platforms for CNS disorders. (The scheme was created with BioRender.com. (accessed on 22 July 2025)).
Various strategies have been developed to load therapeutic agents into sEVs (see Scheme 4). Passive loading through co-incubation or permeabilization permits the incorporation of lipophilic drugs such as rapamycin and curcumin [141,142]. Active methods like sonication or saponin permeabilization further enhance loading efficiency [143]. For example, rapamycin-loaded sEVs restored tight-junction integrity and reduced neuroinflammation in a murine model of BBB dysfunction at one-tenth the conventional dose [144]. Co-loading of polyphenols such as curcumin and methylene blue in MSC-derived sEVs has demonstrated additive effects in reducing Aβ and tau pathology while improving cognitive outcomes in AD mouse models [145]. Advanced microfluidic platforms and electroporation allow for efficient encapsulation of RNA-based cargoes, such as siRNA and CRISPR components, although yield losses remain a limiting factor, spurring innovations like nanoporative chips with up to 80% retention efficiency [146,147].
Surface engineering of sEVs facilitates cell-specific targeting. The fusion of rabies virus glycoprotein (RVG) peptide with Lamp2b, a sEV membrane protein, has been shown to enhance neuronal uptake, enabling delivery of siRNAs targeting BACE1 and α-synuclein, thereby reversing behavioral deficits in NDD models [148,149]. Similarly, engineering donor cells to express anti-CD19 single-chain variable fragments (scFv) resulted in sEVs capable of crossing the BBB and delivering methotrexate to CNS lymphoma sites, achieving therapeutic effects with a 70% lower systemic dose and minimized toxicity [150]. Chemical ligation strategies, such as click-chemistry with RGD or transferrin, are advancing toward GMP-compliant, non-genetically modified vesicle coatings [151]. The therapeutic use of sEVs has extended to nucleic acid delivery and gene editing. For instance, siRNA-loaded vesicles targeting LRRK2 significantly reduced kinase hyperactivity and neuroinflammation in PD models [152]. Similarly, RVG-functionalized sEVs transporting mRNA for BDNF and nerve growth factor (NGF) demonstrated restoration of hippocampal neurogenesis following ischemic injury [153].
Stem-cell-derived sEVs, particularly from MSCs, are naturally enriched with miRNAs such as miR-124-3p and miR-146a-5p, key regulators of synaptic plasticity and NF-κB signaling. Augmenting donor cells to overexpress these miRNAs yields more potent vesicles; for example, miR-124-sEVs restored dendritic spine density and improved cognitive function in 3×Tg-AD mice, while miR-146a-sEVs attenuated astrocyte-mediated cytokine release and improved outcomes in CNS injury models [154,155,156,157]. Infusion of sEVs derived from bone marrow MSCs as a therapeutic approach for ALS reported no serious adverse events [158]. Subsequently, a first human case report demonstrated safety and potential clinical stabilization associated with intravenous infusions of allogeneic SCEVs in a patient with advanced ALS, offering a novel therapeutic avenue targeting this neurological condition [159].
Manufacturing and regulatory challenges remain major bottlenecks in clinical translation. Hollow-fiber bioreactors now enable scalable production of GMP-grade sEVs, yielding >1011 particles/mL while meeting sterility and particle-to-protein ratio requirements [160]. However, no sEV-based therapeutic has received FDA approval as a standalone product marketed for therapeutic use. Regulatory agencies classify sEVs as biologics, requiring IND-level characterization, including identity, purity, potency, and safety data. In conclusion, sEVs represent a clinically promising, biologically compatible platform for targeted CNS therapy. Continued progress in molecular engineering, scalable biomanufacturing, and regulatory harmonization will be essential for realizing their full clinical potential.
8. Comparative Assessment of Diverse Carrier Platforms for CNS Drug Delivery
Delivering therapeutics to the CNS remains a major hurdle because drugs must cross the BBB while retaining bioactivity and safety In addition to cell-derived small extracellular vesicles other drug delivery platforms that are currently under investigation include synthetic liposomes/lipid nanoparticles, biodegradable polymeric nanoparticles, and viral vectors, each offering a unique blend of payload capacity, immunogenicity, manufacturing complexity, and regulatory standing. Table 2 presents these platforms side-by-side, summarizing their principal strengths and limitations to support informed selection or combination for brain-targeted drug-development efforts.

Table 2.
Comparison of CNS-targeted drug delivery platforms.
9. Challenges and Future Directions
The clinical translation of sEVs as diagnostics and therapeutics now hinges more on overcoming methodological limitations than on resolving biological uncertainties. Key barriers lie in several interrelated domains: isolation, precision and reliability, scalable manufacturing, safety and immunogenicity, evolving regulatory landscapes, and analytic innovation. Addressing these bottlenecks through coordinated technical and policy advances will be critical to advancing the field in the coming years.
9.1. Standardization of Isolation and Characterization
The isolation of sEVs remains a major source of variability. Common methods such as differential ultracentrifugation, polymer-based precipitation, size-exclusion chromatography, immune-affinity capture, asymmetric-flow field-flow fractionation, and microfluidic or acoustofluidic platforms enrich for overlapping but distinct vesicle populations. Multicenter studies demonstrate that particle-to-protein ratios can vary by more than two orders of magnitude for a single sample, introducing profound inconsistency in biomarker discovery and therapeutic potency [171,172]. The 2023 update of the MISEV guidelines provides a comprehensive, expert-driven update on current methodologies, best practices, and emerging techniques for sEV production, isolation, characterization, and in vivo study across diverse biological sources [133].
9.2. Scaling Manufacturing Without Compromising Identity
Purifying sEVs hinges on separating intact nanoscale vesicles from cells, protein complexes, and polymers without compromising vesicle integrity or bioactivity. Core workflows fall into six major categories: (i) differential ultracentrifugation, which exploits size- and mass-dependent sedimentation; (ii) density gradient centrifugation, isolating vesicles by buoyant density; (iii) size-exclusion chromatography (SEC), where porous resins partition particles by hydrodynamic radius; (iv) immunoaffinity capture that targets surface antigens for sub-population specificity; (v) polyethylene glycol (PEG) precipitation, which concentrates vesicles via volume exclusion; and (vi) tangential flow filtration (TFF), a scalable cross-flow membrane process that concentrates sEVs and performs buffer exchange under gentle shear. Traditional cell culture flasks yield high-purity but low-volume sEV preparations, typically <1010 particles per liter. Hollow-fiber bioreactors (HFBs) have demonstrated sustained production and increased yields by over 30-fold while maintaining consistent physical and functional properties [173,174]. TFF is replacing ultracentrifugation to prevent shear-induced membrane disruption, and beta-stage perfusion-based systems with in-line diafiltration promise further efficiency and cost reductions [175]. A critical challenge in sEV production is achieving consistent bioactivity and cargo composition, which must be rigorously controlled to meet regulatory standards for safety, potency, and reproducibility. The lack of standardized potency assays remains a major regulatory bottleneck. As no single isolation method optimally balances purity, yield, scalability, and cost, combinatorial or orthogonal strategies are often necessary to align with MISEV guidelines and downstream clinical requirements [176]. Scheme 5 summarizes currently employed sEV purification techniques.
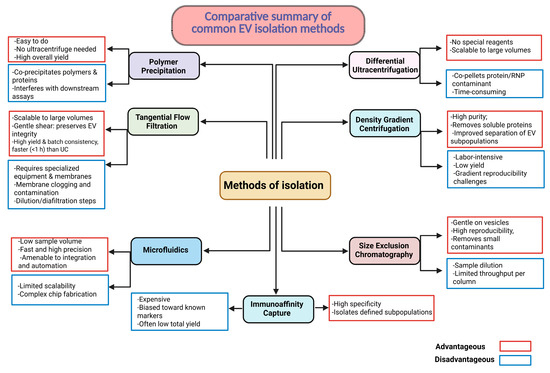
Scheme 5.
sEV isolation methods: advantages vs. limitations. Each method has distinct trade-offs in yield, purity, throughput, cost, and effort; selecting or strategically combining methods is dependent on specific downstream application.
9.3. Immunogenicity and Biodistribution Concerns
Although autologous sEVs are generally well tolerated and exhibit low immunogenicity, engineered or allogeneic vesicles can activate innate immune pathways depending on their cargo and surface composition. For instance, tumor-derived sEVs enriched in miR-21 have been shown to activate Toll-like receptors 7 and 8 (TLR7/8), inducing proinflammatory cytokines such as IL-6 and TNF-α, and promoting cachexia in murine cancer models [10,177]. Similarly, neuronal sEVs containing double-stranded RNA can engage TLR3, amplifying neuroinflammation, particularly in the context of opioid exposure [178]. To counter these effects, preclinical strategies under investigation include CRISPR-mediated depletion of immunostimulatory RNA, partial PEGylation of vesicle membranes to reduce immune detection, and CD47 overexpression to inhibit macrophage-mediated clearance [179,180]. Accurate tracking of sEV biodistribution remains a critical challenge, as conventional lipophilic dyes like DiR and PKH may form micelles, leading to artifactual overestimation of CNS uptake [181,182]. CRISPR-based barcoding methods, such as guiding RNA fusion to the sEV marker CD63, enable high-resolution tracking of individual sEV subtypes through deep sequencing [183]. These tools have revealed distinct in vivo biodistribution patterns between CD9+ and CD63+ sEVs, challenging prior assumptions and highlighting the need for refined, biologically specific tracking approaches in translational sEV research. If these scientific and regulatory challenges can be met, sEVs may soon transition from bench curiosities to front-line nanotherapeutics for complex diseases such as neurodegeneration (See Scheme 6).
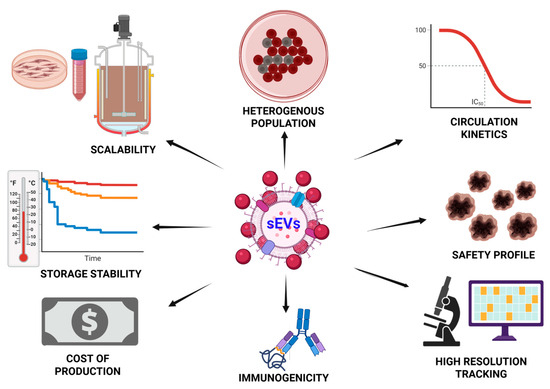
Scheme 6.
Key challenges in the therapeutic application of sEVs. Therapeutic application of sEVs requires comprehensive evaluation of multiple factors including source cell type, production scalability, drug loading efficiency, potency, immune response, biodistribution, stability, cost, and analytical characterization. Optimizing these parameters is essential for successful clinical translation of sEV-based therapeutics. (The scheme was created with BioRender.com. (accessed on 22 July 2025)).
10. Conclusions
In just over a decade, sEVs have emerged as central tools in NDD research, functioning as vectors of pathogenic proteins, diagnostic biomarkers, and therapeutic delivery vehicles. They play key roles in disease propagation, early detection, and targeted treatment. Recent advances include brain-enriched diagnostic panels, engineered therapeutic sEVs, and scalable high-precision manufacturing technologies. While challenges remain such as standardization and in vivo tracking, technological innovations and evolving regulatory frameworks are rapidly addressing them. With ongoing scientific advancements, sEVs are emerging as a pivotal modality in NDD management, ushering in a new era of precision nano-neurotechnology that seamlessly integrates diagnosis and therapy into a single, powerful platform, allowing earlier detection, targeted intervention, and potential disease modification.
Author Contributions
M.G., A.-H.B., and D.D.P. contributed to the conceptualization of the manuscript. M.G. was responsible for the original draft preparation. A.-H.B. and D.D.P. contributed to the review and editing of the manuscript. A.-H.B. and M.G. contributed to the creation and design of the figures and the schemes. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Buoniconti Fund, The Miami Project to Cure Paralysis to M.G., A.-H.B., and D.D.P., and The John M. and Jocelyn H.K. Watkins Distinguished Chair in Cell Therapies to D.D.P.
Data Availability Statement
Not applicable.
Acknowledgments
Schemes were created using BioRender.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ANT1 | Adenine nucleotide translocators |
| AD | Alzheimer’s disease |
| AMPK | AMP-activated protein kinase |
| ALS | Amyotrophic lateral sclerosis |
| APCs | Antigen-presenting cells |
| ApoE | Apolipoprotein E |
| AGO2 | Argonaute 2 |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| CaMKK | Calcium/calmodulin-dependent protein kinase kinase |
| CADM1 | Cell adhesion molecule 1 |
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| C9orf72 | Chromosome 9 open reading frame 72 |
| CRISPR | Clustered Regularly Interspaced Short Palindromic Repeats |
| CDK5 | Cyclin-dependent kinase 5 |
| DAMPs | Damage-associated molecular patterns |
| ESCRT | Endosomal sorting complexes required for transport |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EMA | European Medicines Agency |
| EAE | Experimental autoimmune encephalomyelitis |
| EV | Extracellular vesicle |
| FDA | Food and Drug Administration |
| FOXJ3 | Forkhead box O3 |
| GABRB3 | Gamma-aminobutyric acid; receptor subunit beta-3 |
| GFAP | Glial fibrillary acidic protein |
| GLAST | Glutamate/aspartate transporter |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GSK3β | Glycogen synthase kinase 3 beta |
| hnRNPA2B1 | Heterogeneous nuclear ribonucleoprotein A2/B1 |
| HDAC2 | Histone deacetylase 2 |
| HD | Huntington’s disease |
| IL-1β | Interleukin-1β |
| ISEV | International Society for Extracellular Vesicles |
| IND | Investigational New Drug |
| L1CAM | L1 cell adhesion molecule |
| L1CAM/CD171 | L1 cell adhesion molecule |
| LRRK2 | Leucine-rich repeat kinase 2 |
| LIMK1 | LIM domain kinase 1 |
| Lamp2b | Lysosomal-associated membrane protein 2 |
| LNP | Lipid nanoparticle |
| MHC | Major histocompatibility complex |
| MSC | Mesenchymal stem cell |
| MSC-EVs | Mesenchymal-stem-cell-derived EVs |
| MISEV-2023 | Minimal Information for Studies of Extracellular Vesicles guidelines 2023 |
| MCT1 | Monocarboxylate transporters |
| MS | Multiple sclerosis |
| MVBs | Multivesicular bodies |
| mHTT | Mutant huntingtin protein |
| MBP | Myelin basic protein |
| MOG | Myelin oligodendrocyte glycoprotein |
| NGF | Nerve growth factor |
| NDDs | Neurodegenerative diseases |
| NFL | Neurofilament light |
| nSMase2 | Neutral sphingomyelinase 2 |
| NMDA | N-methyl-D-aspartate |
| NP | Nanoparticle |
| PD | Parkinson’s disease |
| PTEN | Phosphatase and tensin |
| PEGylation | Polyethylene glycol |
| PSD-95 | Postsynaptic density protein 95 |
| PDCD4 | Programmed cell death protein 4 |
| PLP | Proteolipid protein |
| RVG | Rabies virus glycoprotein |
| RBPs | RNA-binding proteins |
| SCEVs | Schwann cell (SC)-derived exosomal vesicles |
| STAT3 | Signal transducer and activator of transcription 3 |
| sEVs | Small extracellular vesicles |
| SCI | Spinal cord injury |
| SOD1 | Superoxide dismutase 1 |
| TDP-43 | TAR DNA-binding protein 43 |
| TLR7/8 | Toll-like receptors 7 and 8 |
| TBI | Traumatic brain injury |
| TNF-α | Tumor necrosis factor alpha |
| YBX1 | Y-box binding protein 1 |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
References
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chetelat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Pramotton, F.M.; Spitz, S.; Kamm, R.D. Challenges and Future Perspectives in Modeling Neurodegenerative Diseases Using Organ-on-a-Chip Technology. Adv. Sci. 2024, 11, e2403892. [Google Scholar] [CrossRef]
- Ayeni, E.A.; Aldossary, A.M.; Ayejoto, D.A.; Gbadegesin, L.A.; Alshehri, A.A.; Alfassam, H.A.; Afewerky, H.K.; Almughem, F.A.; Bello, S.M.; Tawfik, E.A. Neurodegenerative Diseases: Implications of Environironmental and Climatic Influences on Neurotransmitters and Neuronal Hormones Activities. Int. J. Environ. Res. Public Health 2022, 19, 12495. [Google Scholar] [CrossRef] [PubMed]
- Brettschneider, J.; Del Tredici, K.; Lee, V.M.; Trojanowski, J.Q. Spreading of pathology in neurodegenerative diseases: A focus on human studies. Nat. Rev. Neurosci. 2015, 16, 109–120. [Google Scholar] [CrossRef]
- Satao, K.S.; Doshi, G.M. Intercellular communication via exosomes: A new paradigm in the pathophysiology of neurodegenerative disorders. Life Sci. 2025, 365, 123468. [Google Scholar] [CrossRef] [PubMed]
- Kowal, J.; Tkach, M.; Thery, C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014, 29, 116–125. [Google Scholar] [CrossRef]
- Harding, C.; Heuser, J.; Stahl, P. Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J. Cell Biol. 1983, 97, 329–339. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Nijman, H.W.; Stoorvogel, W.; Liejendekker, R.; Harding, C.V.; Melief, C.J.; Geuze, H.J. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 1996, 183, 1161–1172. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Wang, M.; Qin, L.; Tang, B. MicroRNAs in Alzheimer’s Disease. Front. Genet. 2019, 10, 153. [Google Scholar] [CrossRef]
- Abuelezz, N.Z.; Nasr, F.E.; AbdulKader, M.A.; Bassiouny, A.R.; Zaky, A. MicroRNAs as Potential Orchestrators of Alzheimer’s Disease-Related Pathologies: Insights on Current Status and Future Possibilities. Front. Aging Neurosci. 2021, 13, 743573. [Google Scholar] [CrossRef]
- Meder, D.; Siebner, H.R. Spectral signatures of neurodegenerative diseases: How to decipher them? Brain 2018, 141, 2241–2244. [Google Scholar] [CrossRef]
- Fraser, K.B.; Rawlins, A.B.; Clark, R.G.; Alcalay, R.N.; Standaert, D.G.; Liu, N.; Parkinson’s Disease Biomarker Program, C.; West, A.B. Ser(P)-1292 LRRK2 in urinary exosomes is elevated in idiopathic Parkinson’s disease. Mov. Disord. 2016, 31, 1543–1550. [Google Scholar] [CrossRef] [PubMed]
- Tkach, M.; Thery, C. Communication by Extracellular Vesicles: Where We Are and Where We Need to Go. Cell 2016, 164, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Liu, L.; Ma, F.; Wong, C.W.; Guo, X.E.; Chacko, J.V.; Farhoodi, H.P.; Zhang, S.X.; Zimak, J.; Segaliny, A.; et al. Elucidation of Exosome Migration across the Blood-Brain Barrier Model In Vitro. Cell Mol. Bioeng. 2016, 9, 509–529. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, J.; Stewart, T.; Banks, W.A.; Zhang, J. The Transport Mechanism of Extracellular Vesicles at the Blood-Brain Barrier. Curr. Pharm. Des. 2017, 23, 6206–6214. [Google Scholar] [CrossRef]
- Pulliam, L.; Sun, B.; Mustapic, M.; Chawla, S.; Kapogiannis, D. Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer’s disease. J. Neurovirol. 2019, 25, 702–709. [Google Scholar] [CrossRef]
- Osaid, Z.; Haider, M.; Hamoudi, R.; Harati, R. Exosomes Interactions with the Blood-Brain Barrier: Implications for Cerebral Disorders and Therapeutics. Int. J. Mol. Sci. 2023, 24, 15635. [Google Scholar] [CrossRef]
- Rajendran, L.; Bali, J.; Barr, M.M.; Court, F.A.; Kramer-Albers, E.M.; Picou, F.; Raposo, G.; van der Vos, K.E.; van Niel, G.; Wang, J.; et al. Emerging roles of extracellular vesicles in the nervous system. J. Neurosci. 2014, 34, 15482–15489. [Google Scholar] [CrossRef]
- Guo, J.L.; Lee, V.M. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat. Med. 2014, 20, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Sardar Sinha, M.; Ansell-Schultz, A.; Civitelli, L.; Hildesjo, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Rai, S.; Ray, S.K.; Kanwar, J.R.; Mukherjee, S. Exosome-based therapeutics: Advancing drug delivery for neurodegenerative diseases. Mol. Cell. Neurosci. 2025, 133, 104004. [Google Scholar] [CrossRef]
- Kapogiannis, D.; Mustapic, M.; Shardell, M.D.; Berkowitz, S.T.; Diehl, T.C.; Spangler, R.D.; Tran, J.; Lazaropoulos, M.P.; Chawla, S.; Gulyani, S.; et al. Association of Extracellular Vesicle Biomarkers With Alzheimer Disease in the Baltimore Longitudinal Study of Aging. JAMA Neurol. 2019, 76, 1340–1351. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, F.; Li, C.; Mao, J.; Wang, Y.; Zhou, X.; Xie, H.; Wen, C. Application of Single Extracellular Vesicle Analysis Techniques. Int. J. Nanomed. 2023, 18, 5365–5376. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, M.; Xiao, Q.; Wang, H.; Chi, C.; Lin, T.; Wang, Y.; Yi, X.; Zhu, L. Recent Advances in Microfluidic-Based Extracellular Vesicle Analysis. Micromachines 2024, 15, 630. [Google Scholar] [CrossRef] [PubMed]
- Fiandaca, M.S.; Kapogiannis, D.; Mapstone, M.; Boxer, A.; Eitan, E.; Schwartz, J.B.; Abner, E.L.; Petersen, R.C.; Federoff, H.J.; Miller, B.L.; et al. Identification of preclinical Alzheimer’s disease by a profile of pathogenic proteins in neurally derived blood exosomes: A case-control study. Alzheimers Dement. 2015, 11, 600–607. [Google Scholar] [CrossRef]
- Park, C.; Weerakkody, J.S.; Schneider, R.; Miao, S.; Pitt, D. CNS cell-derived exosome signatures as blood-based biomarkers of neurodegenerative diseases. Front. Neurosci. 2024, 18, 1426700. [Google Scholar] [CrossRef]
- Schwarz, G.; Ren, X.; Xie, W.; Guo, H.; Jiang, Y.; Zhang, J. Engineered exosomes: A promising drug delivery platform with therapeutic potential. Front. Mol. Biosci. 2025, 12, 1583992. [Google Scholar] [CrossRef]
- Lin, E.Y.; Hsu, S.X.; Wu, B.H.; Deng, Y.C.; Wuli, W.; Li, Y.S.; Lee, J.H.; Lin, S.Z.; Harn, H.J.; Chiou, T.W. Engineered Exosomes Containing microRNA-29b-2 and Targeting the Somatostatin Receptor Reduce Presenilin 1 Expression and Decrease the beta-Amyloid Accumulation in the Brains of Mice with Alzheimer’s Disease. Int. J. Nanomed. 2024, 19, 4977–4994. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Feng, T.; Liu, B.; Qiu, F.; Xu, Y.; Zhao, Y.; Zheng, Y. Engineered exosomes: Desirable target-tracking characteristics for cerebrovascular and neurodegenerative disease therapies. Theranostics 2021, 11, 8926–8944. [Google Scholar] [CrossRef]
- Yang, J.; Luo, S.; Zhang, J.; Yu, T.; Fu, Z.; Zheng, Y.; Xu, X.; Liu, C.; Fan, M.; Zhang, Z. Exosome-mediated delivery of antisense oligonucleotides targeting alpha-synuclein ameliorates the pathology in a mouse model of Parkinson’s disease. Neurobiol. Dis. 2021, 148, 105218. [Google Scholar] [CrossRef]
- Kou, M.; Huang, L.; Yang, J.; Chiang, Z.; Chen, S.; Liu, J.; Guo, L.; Zhang, X.; Zhou, X.; Xu, X.; et al. Mesenchymal stem cell-derived extracellular vesicles for immunomodulation and regeneration: A next generation therapeutic tool? Cell Death Dis. 2022, 13, 580. [Google Scholar] [CrossRef]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal stromal/stem cell (MSC)-derived exosomes in clinical trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef]
- Jung, Y.H.; Jo, H.Y.; Kim, D.H.; Oh, Y.J.; Kim, M.; Na, S.; Song, H.Y.; Lee, H.J. Exosome-Mediated Mitochondrial Regulation: A Promising Therapeutic Tool for Alzheimer’s Disease and Parkinson’s Disease. Int. J. Nanomed. 2025, 20, 4903–4917. [Google Scholar] [CrossRef]
- Lee, Y.J.; Shin, K.J.; Chae, Y.C. Regulation of cargo selection in exosome biogenesis and its biomedical applications in cancer. Exp. Mol. Med. 2024, 56, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Skibinski, G.; Parkinson, N.J.; Brown, J.M.; Chakrabarti, L.; Lloyd, S.L.; Hummerich, H.; Nielsen, J.E.; Hodges, J.R.; Spillantini, M.G.; Thusgaard, T.; et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nat. Genet. 2005, 37, 806–808. [Google Scholar] [CrossRef] [PubMed]
- Bianco, F.; Perrotta, C.; Novellino, L.; Francolini, M.; Riganti, L.; Menna, E.; Saglietti, L.; Schuchman, E.H.; Furlan, R.; Clementi, E.; et al. Acid sphingomyelinase activity triggers microparticle release from glial cells. EMBO J. 2009, 28, 1043–1054. [Google Scholar] [CrossRef]
- Liu, X.; Meng, P.; Liu, Z.; Tian, X.; Xi, J.; Du, M.; Yang, H.; Long, Q. New insights on targeting extracellular vesicle release by GW4869 to modulate lipopolysaccharide-induced neuroinflammation in mice model. Nanomedicine 2024, 19, 2619–2632. [Google Scholar] [CrossRef]
- Duke, L.C.; Cone, A.S.; Sun, L.; Dittmer, D.P.; Meckes, D.G., Jr.; Tomko, R.J., Jr. Tetraspanin CD9 alters cellular trafficking and endocytosis of tetraspanin CD63, affecting CD63 packaging into small extracellular vesicles. J. Biol. Chem. 2025, 301, 108255. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhao, Y.; Yin, Y.; Jia, X.; Mao, L. Mechanism of cargo sorting into small extracellular vesicles. Bioengineered 2021, 12, 8186–8201. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Yang, Z.; Wang, B.; Gong, H.; Zhang, K.; Lin, Y.; Sun, M. Extracellular vesicles: Biological mechanisms and emerging therapeutic opportunities in neurodegenerative diseases. Transl. Neurodegener. 2024, 13, 60. [Google Scholar] [CrossRef]
- Fabbiano, F.; Corsi, J.; Gurrieri, E.; Trevisan, C.; Notarangelo, M.; D’Agostino, V.G. RNA packaging into extracellular vesicles: An orchestra of RNA-binding proteins? J. Extracell. Vesicles 2020, 10, e12043. [Google Scholar] [CrossRef]
- Long, Y.; Liu, J.; Wang, Y.; Guo, H.; Cui, G. The complex effects of miR-146a in the pathogenesis of Alzheimer’s disease. Neural Regen. Res. 2025, 20, 1309–1323. [Google Scholar] [CrossRef]
- Alhenaky, A.; Alhazmi, S.; Alamri, S.H.; Alkhatabi, H.A.; Alharthi, A.; Alsaleem, M.A.; Abdelnour, S.A.; Hassan, S.M. Exosomal MicroRNAs in Alzheimer’s Disease: Unveiling Their Role and Pioneering Tools for Diagnosis and Treatment. J. Clin. Med. 2024, 13, 6960. [Google Scholar] [CrossRef]
- Faure, J.; Lachenal, G.; Court, M.; Hirrlinger, J.; Chatellard-Causse, C.; Blot, B.; Grange, J.; Schoehn, G.; Goldberg, Y.; Boyer, V.; et al. Exosomes are released by cultured cortical neurones. Mol. Cell Neurosci. 2006, 31, 642–648. [Google Scholar] [CrossRef]
- Lachenal, G.; Pernet-Gallay, K.; Chivet, M.; Hemming, F.J.; Belly, A.; Bodon, G.; Blot, B.; Haase, G.; Goldberg, Y.; Sadoul, R. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol. Cell Neurosci. 2011, 46, 409–418. [Google Scholar] [CrossRef]
- Antoniou, A.; Auderset, L.; Kaurani, L.; Sebastian, E.; Zeng, Y.; Allahham, M.; Cases-Cunillera, S.; Schoch, S.; Grundemann, J.; Fischer, A.; et al. Neuronal extracellular vesicles and associated microRNAs induce circuit connectivity downstream BDNF. Cell Rep. 2023, 42, 112063. [Google Scholar] [CrossRef] [PubMed]
- Nogueras-Ortiz, C.J.; Eren, E.; Yao, P.; Calzada, E.; Dunn, C.; Volpert, O.; Delgado-Peraza, F.; Mustapic, M.; Lyashkov, A.; Rubio, F.J.; et al. Single-extracellular vesicle (EV) analyses validate the use of L1 Cell Adhesion Molecule (L1CAM) as a reliable biomarker of neuron-derived EVs. J. Extracell. Vesicles 2024, 13, e12459. [Google Scholar] [CrossRef] [PubMed]
- Basso, M.; Pozzi, S.; Tortarolo, M.; Fiordaliso, F.; Bisighini, C.; Pasetto, L.; Spaltro, G.; Lidonnici, D.; Gensano, F.; Battaglia, E.; et al. Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes: Implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J. Biol. Chem. 2013, 288, 15699–15711. [Google Scholar] [CrossRef]
- Zhao, S.; Sheng, S.; Wang, Y.; Ding, L.; Xu, X.; Xia, X.; Zheng, J.C. Astrocyte-derived extracellular vesicles: A double-edged sword in central nervous system disorders. Neurosci. Biobehav. Rev. 2021, 125, 148–159. [Google Scholar] [CrossRef]
- Wang, G.; Dinkins, M.; He, Q.; Zhu, G.; Poirier, C.; Campbell, A.; Mayer-Proschel, M.; Bieberich, E. Astrocytes secrete exosomes enriched with proapoptotic ceramide and prostate apoptosis response 4 (PAR-4): Potential mechanism of apoptosis induction in Alzheimer disease (AD). J. Biol. Chem. 2012, 287, 21384–21395. [Google Scholar] [CrossRef]
- Aires, I.D.; Santiago, A.R. Microglial exosomes in retinal neuroinflammation: Focus in glaucoma. Neural Regen. Res. 2021, 16, 1801–1802. [Google Scholar] [CrossRef]
- Ghosh, M.; Pearse, D.D. The Yin and Yang of Microglia-Derived Extracellular Vesicles in CNS Injury and Diseases. Cells 2024, 13, 1834. [Google Scholar] [CrossRef]
- Fruhbeis, C.; Kuo-Elsner, W.P.; Muller, C.; Barth, K.; Peris, L.; Tenzer, S.; Mobius, W.; Werner, H.B.; Nave, K.A.; Frohlich, D.; et al. Oligodendrocytes support axonal transport and maintenance via exosome secretion. PLoS Biol. 2020, 18, e3000621. [Google Scholar] [CrossRef]
- Bolivar, D.A.; Mosquera-Heredia, M.I.; Vidal, O.M.; Barcelo, E.; Allegri, R.; Morales, L.C.; Silvera-Redondo, C.; Arcos-Burgos, M.; Garavito-Galofre, P.; Velez, J.I. Exosomal mRNA Signatures as Predictive Biomarkers for Risk and Age of Onset in Alzheimer’s Disease. Int. J. Mol. Sci. 2024, 25, 12293. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Geng, D. Key developments and hotspots of exosomes in Alzheimer’s disease: A bibliometric study spanning 2003 to 2023. Front. Aging Neurosci. 2024, 16, 1377672. [Google Scholar] [CrossRef] [PubMed]
- Almasi, F.; Abbasloo, F.; Soltani, N.; Dehbozorgi, M.; Moghadam Fard, A.; Kiani, A.; Ghasemzadeh, N.; Mesgari, H.; Zadeh Hosseingholi, E.; Payandeh, Z.; et al. Biology, Pathology, and Targeted Therapy of Exosomal Cargoes in Parkinson’s Disease: Advances and Challenges. Mol. Neurobiol. 2025, 62, 8381–8399. [Google Scholar] [CrossRef] [PubMed]
- Akbari-Gharalari, N.; Ghahremani-Nasab, M.; Naderi, R.; Chodari, L.; Nezhadshahmohammad, F. The potential of exosomal biomarkers: Revolutionizing Parkinson’s disease: How do they influence pathogenesis, diagnosis, and therapeutic strategies? AIMS Neurosci. 2024, 11, 374–397. [Google Scholar] [CrossRef]
- Bao, F.; Fleming, J.C.; Golshani, R.; Pearse, D.D.; Kasabov, L.; Brown, A.; Weaver, L.C. A selective phosphodiesterase-4 inhibitor reduces leukocyte infiltration, oxidative processes, and tissue damage after spinal cord injury. J. Neurotrauma 2011, 28, 1035–1049. [Google Scholar] [CrossRef]
- Solana-Balaguer, J.; Garcia-Segura, P.; Campoy-Campos, G.; Chicote-Gonzalez, A.; Fernandez-Irigoyen, J.; Santamaria, E.; Perez-Navarro, E.; Masana, M.; Alberch, J.; Malagelada, C. Motor skill learning modulates striatal extracellular vesicles’ content in a mouse model of Huntington’s disease. Cell Commun. Signal 2024, 22, 321. [Google Scholar] [CrossRef]
- Ananbeh, H.; Vodicka, P.; Kupcova Skalnikova, H. Emerging Roles of Exosomes in Huntington’s Disease. Int. J. Mol. Sci. 2021, 22, 4085. [Google Scholar] [CrossRef]
- Huo, L.; Du, X.; Li, X.; Liu, S.; Xu, Y. The Emerging Role of Neural Cell-Derived Exosomes in Intercellular Communication in Health and Neurodegenerative Diseases. Front. Neurosci. 2021, 15, 738442. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lin, T.V.; Yuan, Q.; Sadoul, R.; Lam, T.T.; Bordey, A. Small Extracellular Vesicles Control Dendritic Spine Development through Regulation of HDAC2 Signaling. J. Neurosci. 2021, 41, 3799–3807. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Teng, T. Exosomes: New targets for understanding axon guidance in the developing central nervous system. Front. Cell Dev. Biol. 2024, 12, 1510862. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Chen, J.; Huang, D.; Feng, S.; Yang, T.; Li, Z.; Wang, X.; Zhao, M.; Wu, J.; Zhong, T. Current perspectives on clinical use of exosomes as novel biomarkers for cancer diagnosis. Front. Oncol. 2022, 12, 966981. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, Y.F.; Lei, L.; Zhang, Y. MicroRNA-specific targets for neuronal plasticity, neurotransmitters, neurotrophic factors, and gut microbes in the pathogenesis and therapeutics of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2025, 136, 111186. [Google Scholar] [CrossRef]
- Scott-Hewitt, N.; Mahoney, M.; Huang, Y.; Korte, N.; Yvanka de Soysa, T.; Wilton, D.K.; Knorr, E.; Mastro, K.; Chang, A.; Zhang, A.; et al. Microglial-derived C1q integrates into neuronal ribonucleoprotein complexes and impacts protein homeostasis in the aging brain. Cell 2024, 187, 4193–4212 e4124. [Google Scholar] [CrossRef]
- Delaveris, C.S.; Wang, C.L.; Riley, N.M.; Li, S.; Kulkarni, R.U.; Bertozzi, C.R. Microglia mediate contact-independent neuronal pruning via secreted Neuraminidase-3 associated with extracellular vesicles. bioRxiv 2023. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.Y.; Meng, X.W.; Chen, Y.; Zhao, W.M.; Li, W.T.; Xu, H.B.; Peng, K.; Ji, F.H. Complement C1q-mediated microglial synaptic elimination by enhancing desialylation underlies sevoflurane-induced developmental neurotoxicity. Cell Biosci. 2024, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, X.H.; Xu, S.X.; Wang, C.; Sun, S.; Song, X.; Li, R.; Li, N.; Feng, Y.; Duan, H.; et al. Oligodendrocyte-derived exosomes-containing SIRT2 ameliorates depressive-like behaviors and restores hippocampal neurogenesis and synaptic plasticity via the AKT/GSK-3beta pathway in depressed mice. CNS Neurosci. Ther. 2024, 30, e14661. [Google Scholar] [CrossRef]
- Chamberlain, K.A.; Huang, N.; Xie, Y.; LiCausi, F.; Li, S.; Li, Y.; Sheng, Z.H. Oligodendrocytes enhance axonal energy metabolism by deacetylation of mitochondrial proteins through transcellular delivery of SIRT2. Neuron 2021, 109, 3456–3472 e3458. [Google Scholar] [CrossRef]
- Fruhbeis, C.; Frohlich, D.; Kuo, W.P.; Kramer-Albers, E.M. Extracellular vesicles as mediators of neuron-glia communication. Front. Cell. Neurosci. 2013, 7, 182. [Google Scholar] [CrossRef]
- Li, S.; Sheng, Z.H. Oligodendrocyte-derived transcellular signaling regulates axonal energy metabolism. Curr. Opin. Neurobiol. 2023, 80, 102722. [Google Scholar] [CrossRef]
- Cyr, B.; Cabrera Ranaldi, E.; Hadad, R.; Dietrich, W.D.; Keane, R.W.; de Rivero Vaccari, J.P. Extracellular vesicles mediate inflammasome signaling in the brain and heart of Alzheimer’s disease mice. Front. Mol. Neurosci. 2024, 17, 1369781. [Google Scholar] [CrossRef]
- Ikezu, T.; Zhang, Z.; Yu, K.; Bao, H.; Ikezu, T.; Ravula, A.R.; Melvin, B.; Scholes, C.; You, Y.; Ellison, J.; et al. Unique lipid cargoes in APOE4 human brain-derived extracellular vesicles recruit cell adhesion molecules and promote tauopathy in Alzheimer’s disease. Res. Sq. 2025, rs.3, rs-6508552. [Google Scholar]
- Song, L.; Oseid, D.E.; Wells, E.A.; Robinson, A.S. The Interplay between GSK3beta and Tau Ser262 Phosphorylation during the Progression of Tau Pathology. Int. J. Mol. Sci. 2022, 23, 11610. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Deng, C.; Meng, Z.; Zhu, M.; Yang, R.; Yuan, J.; Meng, S. Combined Catalpol and Tetramethylpyrazine Promote Axonal Plasticity in Alzheimer’s Disease by Inducing Astrocytes to Secrete Exosomes Carrying CDK5 mRNA and Regulating STAT3 Phosphorylation. Mol. Neurobiol. 2024, 61, 10770–10791. [Google Scholar] [CrossRef]
- Dinkins, M.B.; Enasko, J.; Hernandez, C.; Wang, G.; Kong, J.; Helwa, I.; Liu, Y.; Terry, A.V., Jr.; Bieberich, E. Neutral Sphingomyelinase-2 Deficiency Ameliorates Alzheimer’s Disease Pathology and Improves Cognition in the 5XFAD Mouse. J. Neurosci. 2016, 36, 8653–8667. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, K.W.; Lee, E.J. Gut-brain axis and Environironmental factors in Parkinson’s disease: Bidirectional link between disease onset and progression. Neural Regen. Res. 2025, 20, 3416–3429. [Google Scholar] [CrossRef] [PubMed]
- Pan-Montojo, F.; Schwarz, M.; Winkler, C.; Arnhold, M.; O’Sullivan, G.A.; Pal, A.; Said, J.; Marsico, G.; Verbavatz, J.M.; Rodrigo-Angulo, M.; et al. Environironmental toxins trigger PD-like progression via increased alpha-synuclein release from enteric neurons in mice. Sci. Rep. 2012, 2, 898. [Google Scholar] [CrossRef]
- Cortes, A.; Phung, T.K.; de Mena, L.; Garrido, A.; Infante, J.; Ruiz-Martinez, J.; Galmes-Ordinas, M.A.; Glendinning, S.; Perez, J.; Roig, A.; et al. In-depth mass-spectrometry reveals phospho-RAB12 as a blood biomarker of G2019S LRRK2-driven Parkinson’s disease. Brain 2025, 148, 2075–2092. [Google Scholar] [CrossRef]
- Zhang, S.; Li, J.; Hu, X.; Liu, H.; Yu, Q.; Kuang, G.; Liu, L.; Yu, D.; Lin, Z.; Xiong, N. Brain-derived extracellular vesicles: A promising avenue for Parkinson’s disease pathogenesis, diagnosis, and treatment. Neural Regen. Res. 2025, 21, 1447–1467. [Google Scholar] [CrossRef]
- Kong, W.; Li, X.; Guo, X.; Sun, Y.; Chai, W.; Chang, Y.; Huang, Q.; Wang, P.; Wang, X. Ultrasound-Assisted CRISPRi-Exosome for Epigenetic Modification of alpha-Synuclein Gene in a Mouse Model of Parkinson’s Disease. ACS Nano 2024, 18, 7837–7851. [Google Scholar] [CrossRef]
- Bahadorani, M.; Nasiri, M.; Dellinger, K.; Aravamudhan, S.; Zadegan, R. Engineering Exosomes for Therapeutic Applications: Decoding Biogenesis, Content Modification, and Cargo Loading Strategies. Int. J. Nanomed. 2024, 19, 7137–7164. [Google Scholar] [CrossRef]
- Darabi, S.; Ariaei, A.; Rustamzadeh, A.; Afshari, D.; Charkhat Gorgich, E.A.; Darabi, L. Cerebrospinal fluid and blood exosomes as biomarkers for amyotrophic lateral sclerosis; a systematic review. Diagn. Pathol. 2024, 19, 47. [Google Scholar] [CrossRef]
- Carata, E.; Muci, M.; Di Giulio, S.; Di Giulio, T.; Mariano, S.; Panzarini, E. The Neuromuscular Disorder Mediated by Extracellular Vesicles in Amyotrophic Lateral Sclerosis. Curr. Issues Mol. Biol. 2024, 46, 5999–6017. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ozdemir, S.; Fritz, C.; Mobius, W.; Kleineidam, L.; Mandelkow, E.; Biernat, J.; Dogdu, C.; Peters, O.; Cosma, N.C.; et al. Plasma extracellular vesicle tau and TDP-43 as diagnostic biomarkers in FTD and ALS. Nat. Med. 2024, 30, 1771–1783. [Google Scholar] [CrossRef] [PubMed]
- Menke, R.A.; Gray, E.; Lu, C.H.; Kuhle, J.; Talbot, K.; Malaspina, A.; Turner, M.R. CSF neurofilament light chain reflects corticospinal tract degeneration in ALS. Ann. Clin. Transl. Neurol. 2015, 2, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wang, H.; Li, Q.; Chen, Z. Extracellular Vesicles: A Review of Their Therapeutic Potentials, Sources, Biodistribution, and Administration Routes. Int. J. Nanobiotechnol. 2025, 20, 3175–3199. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, F.; Jia, B.; Wu, Z.; Huang, Z.; He, M.; Weng, H.; So, K.F.; Qu, W.; Fu, Q.L.; et al. Intranasal delivery of small extracellular vesicles reduces the progress of amyotrophic lateral sclerosis and the overactivation of complement-coagulation cascade and NF-kB signaling in SOD1(G93A) mice. J. Nanobiotechnol. 2024, 22, 503. [Google Scholar] [CrossRef]
- Manna, I.; De Benedittis, S.; Porro, D. Extracellular Vesicles in Multiple Sclerosis: Their Significance in the Development and Possible Applications as Therapeutic Agents and Biomarkers. Genes 2024, 15, 772. [Google Scholar] [CrossRef]
- Casella, G.; Rasouli, J.; Boehm, A.; Zhang, W.; Xiao, D.; Ishikawa, L.L.W.; Thome, R.; Li, X.; Hwang, D.; Porazzi, P.; et al. Oligodendrocyte-derived extracellular vesicles as antigen-specific therapy for autoimmune neuroinflammation in mice. Sci. Transl. Med. 2020, 12, eaba0599. [Google Scholar] [CrossRef] [PubMed]
- Lim Falk, V.; Mueller-Wirth, N.; Karathanasis, D.; Evangelopoulos, M.E.; Maleska Maceski, A.; Zadic, A.; Kuhle, J.; Schlup, C.; Marti, S.; Guse, K.; et al. Extracellular Vesicle Marker Changes Associated With Disease Activity in Relapsing-Remitting Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflamm. 2025, 12, e200404. [Google Scholar] [CrossRef]
- Krakenes, T.; Sandvik, C.E.; Ytterdal, M.; Gavasso, S.; Evjenth, E.C.; Bo, L.; Kvistad, C.E. The Therapeutic Potential of Exosomes from Mesenchymal Stem Cells in Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 10292. [Google Scholar] [CrossRef]
- Tang, B.L. Unconventional Secretion and Intercellular Transfer of Mutant Huntingtin. Cells 2018, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Ananbeh, H.; Novak, J.; Juhas, S.; Juhasova, J.; Klempir, J.; Doleckova, K.; Rysankova, I.; Turnovcova, K.; Hanus, J.; Hansikova, H.; et al. Huntingtin Co-Isolates with Small Extracellular Vesicles from Blood Plasma of TgHD and KI-HD Pig Models of Huntington’s Disease and Human Blood Plasma. Int. J. Mol. Sci. 2022, 23, 5598. [Google Scholar] [CrossRef]
- Miguez, A.; Gomis, C.; Vila, C.; Monguio-Tortajada, M.; Fernandez-Garcia, S.; Bombau, G.; Galofre, M.; Garcia-Bravo, M.; Sanders, P.; Fernandez-Medina, H.; et al. Soluble mutant huntingtin drives early human pathogenesis in Huntington’s disease. Cell Mol. Life Sci. 2023, 80, 238. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhong, S.; Zhang, R.; Kang, K.; Zhang, X.; Xu, Y.; Zhao, C.; Zhao, M. Functional analysis of brain derived neurotrophic factor (BDNF) in Huntington’s disease. Aging 2021, 13, 6103–6114. [Google Scholar] [CrossRef]
- Wilton, D.K.; Mastro, K.; Heller, M.D.; Gergits, F.W.; Willing, C.R.; Fahey, J.B.; Frouin, A.; Daggett, A.; Gu, X.; Kim, Y.A.; et al. Microglia and complement mediate early corticostriatal synapse loss and cognitive dysfunction in Huntington’s disease. Nat. Med. 2023, 29, 2866–2884. [Google Scholar] [CrossRef]
- Guo, M.; Wang, L.; Yin, Z.; Chen, F.; Lei, P. Small extracellular vesicles as potential theranostic tools in central nervous system disorders. Biomed. Pharmacother. 2023, 167, 115407. [Google Scholar] [CrossRef]
- Li, Y.; Khan, N.; Ritzel, R.M.; Lei, Z.; Allen, S.; Faden, A.I.; Wu, J. Sexually dimorphic extracellular vesicle responses after chronic spinal cord injury are associated with neuroinflammation and neurodegeneration in the aged brain. J. Neuroinflamm. 2023, 20, 197. [Google Scholar] [CrossRef]
- Hasan, A.; Ardizzone, A.; Giosa, D.; Scuderi, S.A.; Calcaterra, E.; Esposito, E.; Capra, A.P. The Therapeutic Potential of MicroRNA-21 in the Treatment of Spinal Cord Injury. Curr. Issues Mol. Biol. 2025, 47, 70. [Google Scholar] [CrossRef]
- Lin, M.; Alimerzaloo, F.; Wang, X.; Alhalabi, O.; Krieg, S.M.; Skutella, T.; Younsi, A. Harnessing stem cell-derived exosomes: A promising cell-free approach for spinal cord injury. Stem Cell Res. Ther. 2025, 16, 182. [Google Scholar] [CrossRef] [PubMed]
- Nakazaki, M.; Yokoyama, T.; Lankford, K.L.; Hirota, R.; Kocsis, J.D.; Honmou, O. Mesenchymal Stem Cells and Their Extracellular Vesicles: Therapeutic Mechanisms for Blood-Spinal Cord Barrier Repair Following Spinal Cord Injury. Int. J. Mol. Sci. 2024, 25, 13460. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.A.; Asim, M.; El-Menyar, A.; Biswas, K.H.; Rizoli, S.; Al-Thani, H. The evolving role of extracellular vesicles (exosomes) as biomarkers in traumatic brain injury: Clinical perspectives and therapeutic implications. Front. Aging Neurosci. 2022, 14, 933434. [Google Scholar] [CrossRef]
- de Rivero Vaccari, J.P.; Brand, F., 3rd; Adamczak, S.; Lee, S.W.; Perez-Barcena, J.; Wang, M.Y.; Bullock, M.R.; Dietrich, W.D.; Keane, R.W. Exosome-mediated inflammasome signaling after central nervous system injury. J. Neurochem. 2016, 136 (Suppl. S1), 39–48. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Li, P.; Bai, F.; Liu, C.; Huang, X. Serum exosomes miR-206 and miR-549a-3p as potential biomarkers of traumatic brain injury. Sci. Rep. 2024, 14, 10082. [Google Scholar]
- Liu, M.W.; Li, H.; Xiong, G.F.; Zhang, B.R.; Zhang, Q.J.; Gao, S.J.; Zhu, Y.L.; Zhang, L.M. Mesenchymal stem cell exosomes therapy for the treatment of traumatic brain injury: Mechanism, progress, challenges and prospects. J. Transl. Med. 2025, 23, 427. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Gu, Y.; Zhang, Q.; Liu, H.; Liu, Y. The Potential Roles of Exosomes Carrying APP and Tau Cleavage Products in Alzheimer’s Disease. J. Clin. Med. 2023, 12, 1883. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Chen, Z.T.; Zhou, R.L.; Zhang, X.; Ye, Q.Y.; Wang, Y.Z. Increased DJ-1 and alpha-Synuclein in Plasma Neural-Derived Exosomes as Potential Markers for Parkinson’s Disease. Front. Aging Neurosci. 2018, 10, 438. [Google Scholar]
- Zhang, P.; Rasheed, M.; Liang, J.; Wang, C.; Feng, L.; Chen, Z. Emerging Potential of Exosomal Non-coding RNA in Parkinson’s Disease: A Review. Front. Aging Neurosci. 2022, 14, 819836. [Google Scholar] [CrossRef]
- Gagliardi, D.; Bresolin, N.; Comi, G.P.; Corti, S. Extracellular vesicles and amyotrophic lateral sclerosis: From misfolded protein vehicles to promising clinical biomarkers. Cell Mol. Life Sci. 2021, 78, 561–572. [Google Scholar] [CrossRef]
- Banack, S.A.; Dunlop, R.A.; Cox, P.A. An miRNA fingerprint using neural-enriched extracellular vesicles from blood plasma: Towards a biomarker for amyotrophic lateral sclerosis/motor neuron disease. Open Biol. 2020, 10, 200116. [Google Scholar] [CrossRef]
- Lee, S.T.; Im, W.; Ban, J.J.; Lee, M.; Jung, K.H.; Lee, S.K.; Chu, K.; Kim, M. Exosome-Based Delivery of miR-124 in a Huntington’s Disease Model. J. Mov. Disord. 2017, 10, 45–52. [Google Scholar] [CrossRef]
- Mazzucco, M.; Mannheim, W.; Shetty, S.V.; Linden, J.R. CNS endothelial derived extracellular vesicles are biomarkers of active disease in multiple sclerosis. Fluids Barriers CNS 2022, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Maciak, K.; Dziedzic, A.; Miller, E.; Saluk-Bijak, J. miR-155 as an Important Regulator of Multiple Sclerosis Pathogenesis. A Review. Int. J. Mol. Sci. 2021, 22, 4332. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ye, L.; Sun, S.; Wang, Y.; Chen, Y.; Chang, J.; Yin, R.; Liu, X.; Zuo, W.; Xu, H.; et al. Molecular intersections of traumatic brain injury and Alzheimer’s disease: The role of ADMSC-derived exosomes and hub genes in microglial polarization. Metab. Brain Dis. 2024, 40, 77. [Google Scholar] [CrossRef]
- Rong, Y.; Liu, W.; Wang, J.; Fan, J.; Luo, Y.; Li, L.; Kong, F.; Chen, J.; Tang, P.; Cai, W. Neural stem cell-derived small extracellular vesicles attenuate apoptosis and neuroinflammation after traumatic spinal cord injury by activating autophagy. Cell Death Dis. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, Z.; Bao, Q.; Chen, S.; Xu, W.; Jiang, J. Extracellular Vesicles as Emerging Therapeutic Strategies in Spinal Cord Injury: Ready to Go. Biomedicines 2025, 13, 1262. [Google Scholar] [CrossRef] [PubMed]
- Kodali, M.; Madhu, L.N.; Reger, R.L.; Milutinovic, B.; Upadhya, R.; Gonzalez, J.J.; Attaluri, S.; Shuai, B.; Gitai, D.L.G.; Rao, S.; et al. Intranasally administered human MSC-derived extracellular vesicles inhibit NLRP3-p38/MAPK signaling after TBI and prevent chronic brain dysfunction. Brain Behav. Immun. 2023, 108, 118–134. [Google Scholar] [CrossRef]
- Blaya, M.O.; Pressman, Y.; Andreu, M.; Moreno, W.J.; Sanchez-Molano, J.; Kerr, N.A.; Umland, O.; Khan, A.; Bramlett, H.M.; Dietrich, W.D. Human Schwann cell exosome treatment attenuates secondary injury mechanisms, histopathological consequences, and behavioral deficits after traumatic brain injury. Neurotherapeutics 2025, 22, e00555. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, K.; Sanchez-Molano, J.; Kerr, N.; Pressman, Y.; Silvera, R.; Khan, A.; Gajavelli, S.; Bramlett, H.M.; Dietrich, W.D. Beneficial Effects of Human Schwann Cell-Derived Exosomes in Mitigating Secondary Damage After Penetrating Ballistic-Like Brain Injury. J. Neurotrauma 2024, 41, 2395–2412. [Google Scholar] [CrossRef]
- Ghosh, M.; Pearse, D.D. Schwann Cell-Derived Exosomal Vesicles: A Promising Therapy for the Injured Spinal Cord. Int. J. Mol. Sci. 2023, 24, 17317. [Google Scholar] [CrossRef]
- Ramos-Zaldivar, H.M.; Polakovicova, I.; Salas-Huenuleo, E.; Corvalan, A.H.; Kogan, M.J.; Yefi, C.P.; Andia, M.E. Extracellular vesicles through the blood-brain barrier: A review. Fluids Barriers CNS 2022, 19, 60. [Google Scholar] [CrossRef]
- Bravo-Miana, R.D.C.; Arizaga-Echebarria, J.K.; Otaegui, D. Central nervous system-derived extracellular vesicles: The next generation of neural circulating biomarkers? Transl. Neurodegener. 2024, 13, 32. [Google Scholar] [CrossRef]
- Utz, J.; Berner, J.; Munoz, L.E.; Oberstein, T.J.; Kornhuber, J.; Herrmann, M.; Maler, J.M.; Spitzer, P. Cerebrospinal Fluid of Patients With Alzheimer’s Disease Contains Increased Percentages of Synaptophysin-Bearing Microvesicles. Front. Aging Neurosci. 2021, 13, 682115. [Google Scholar] [CrossRef]
- Kluge, A.; Schaeffer, E.; Bunk, J.; Sommerauer, M.; Rottgen, S.; Schulte, C.; Roeben, B.; von Thaler, A.K.; Welzel, J.; Lucius, R.; et al. Detecting Misfolded alpha-Synuclein in Blood Years before the Diagnosis of Parkinson’s Disease. Mov. Disord. 2024, 39, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- Scaroni, F.; Visconte, C.; Serpente, M.; Golia, M.T.; Gabrielli, M.; Huiskamp, M.; Hulst, H.E.; Carandini, T.; De Riz, M.; Pietroboni, A.; et al. miR-150-5p and let-7b-5p in Blood Myeloid Extracellular Vesicles Track Cognitive Symptoms in Patients with Multiple Sclerosis. Cells 2022, 11, 1551. [Google Scholar] [CrossRef]
- Babaee, A.; Wichmann, T.O.; Rasmussen, M.M.; Brink, O.; Olsen, D.A.; Borris, L.C.; Lesbo, M.; Rasmussen, R.W.; Salomon, C.; Handberg, A.; et al. Extracellular Vesicle Glial Fibrillary Acidic Protein as a Circulating Biomarker of Traumatic Brain Injury Severity. J. Mol. Neurosci. 2025, 75, 69. [Google Scholar] [CrossRef]
- Ljungstrom, M.; Oltra, E. Methods for Extracellular Vesicle Isolation: Relevance for Encapsulated miRNAs in Disease Diagnosis and Treatment. Genes 2025, 16, 330. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrugger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Ramirez, M.I.; Amorim, M.G.; Gadelha, C.; Milic, I.; Welsh, J.A.; Freitas, V.M.; Nawaz, M.; Akbar, N.; Couch, Y.; Makin, L.; et al. Technical challenges of working with extracellular vesicles. Nanoscale 2018, 10, 881–906. [Google Scholar] [CrossRef]
- Saftics, A.; Purnell, B.; Beres, B.; Thompson, S.; Jiang, N.; Ghaeli, I.; Lima, C.; Armstrong, B.; Van Keuren-Jensen, K.; Jovanovic-Talisman, T. Single Extracellular VEsicle Nanoscopy-Universal Protocol (SEVEN-UP): Accessible Imaging Platform for Quantitative Characterization of Single Extracellular Vesicles. Anal. Chem. 2025, 97, 1654–1664. [Google Scholar] [CrossRef]
- Cohn, W.; Melnik, M.; Huang, C.; Teter, B.; Chandra, S.; Zhu, C.; McIntire, L.B.; John, V.; Gylys, K.H.; Bilousova, T. Multi-Omics Analysis of Microglial Extracellular Vesicles From Human Alzheimer’s Disease Brain Tissue Reveals Disease-Associated Signatures. Front. Pharmacol. 2021, 12, 766082. [Google Scholar] [CrossRef]
- Heidarzadeh, M.; Gursoy-Ozdemir, Y.; Kaya, M.; Eslami Abriz, A.; Zarebkohan, A.; Rahbarghazi, R.; Sokullu, E. Exosomal delivery of therapeutic modulators through the blood-brain barrier; promise and pitfalls. Cell Biosci. 2021, 11, 142. [Google Scholar] [CrossRef]
- Yanez-Mo, M.; Siljander, P.R.; Andreu, Z.; Zavec, A.B.; Borras, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Balaraman, A.K.; Babu, M.A.; Moglad, E.; Mandaliya, V.; Rekha, M.M.; Gupta, S.; Prasad, G.V.S.; Kumari, M.; Chauhan, A.S.; Ali, H.; et al. Exosome-mediated delivery of CRISPR-Cas9: A revolutionary approach to cancer gene editing. Pathol. Res. Pract. 2025, 266, 155785. [Google Scholar] [CrossRef]
- Song, L.L.; Tang, Y.P.; Qu, Y.Q.; Yun, Y.X.; Zhang, R.L.; Wang, C.R.; Wong, V.K.W.; Wang, H.M.; Liu, M.H.; Qu, L.Q.; et al. Exosomal delivery of rapamycin modulates blood-brain barrier penetration and VEGF axis in glioblastoma. J. Control. Release 2025, 381, 113605. [Google Scholar] [CrossRef]
- Bashirrohelleh, M.A.; Bavarsad, K.; Khodadadi, A.; Shohan, M.; Asadirad, A. Curcumin-enhanced stem cell exosomes: A novel approach to modulating neuroinflammation and improving cognitive function in a rat model of Alzheimer’s disease. Eur. J. Pharmacol. 2025, 999, 177695. [Google Scholar] [CrossRef]
- Han, Y.; Jones, T.W.; Dutta, S.; Zhu, Y.; Wang, X.; Narayanan, S.P.; Fagan, S.C.; Zhang, D. Overview and Update on Methods for Cargo Loading into Extracellular Vesicles. Process 2021, 9, 356. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Z.; Wu, L.; Zhu, L.; Lu, M.; Zhang, Z.; Lv, Y.; Zhu, X.; Yao, H. Rapamycin-induced small extracellular vesicles under GelMA scaffolds facilitate diabetic wound repair through accelerating angiogenesis and alleviating macrophage-mediated inflammation via PI3K/Akt signaling pathway. Mater. Today Bio 2025, 32, 101846. [Google Scholar] [CrossRef]
- Yang, D.; Deng, Z.; Zhou, H.; Zhang, Q.; Zhang, X.; Gong, J. Exosome-mediated dual drug delivery of curcumin and methylene blue for enhanced cognitive function and mechanistic elucidation in Alzheimer’s disease therapy. Front. Cell Dev. Biol. 2025, 13, 1562565. [Google Scholar] [CrossRef]
- Fajrial, A.K.; He, Q.Q.; Wirusanti, N.I.; Slansky, J.E.; Ding, X. A review of emerging physical transfection methods for CRISPR/Cas9-mediated gene editing. Theranostics 2020, 10, 5532–5549. [Google Scholar] [CrossRef]
- Hur, J.; Chung, A.J. Microfluidic and Nanofluidic Intracellular Delivery. Adv. Sci. 2021, 8, e2004595. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, S.; Qin, F.; Fu, A.; Fu, C. Application progress of RVG peptides to facilitate the delivery of therapeutic agents into the central nervous system. RSC Adv. 2021, 11, 8505–8515. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Q.; Chai, Y.; Rong, R.; He, L.; Zhang, Y.; Cui, H.; Xu, H.; Zhang, X.; Wang, Z.; et al. An anti-CD19-exosome delivery system navigates the blood-brain barrier for targeting of central nervous system lymphoma. J. Nanobiotechnol. 2025, 23, 173. [Google Scholar] [CrossRef]
- N’Diaye, E.R.; Orefice, N.S.; Ghezzi, C.; Boumendjel, A. Chemically Modified Extracellular Vesicles and Applications in Radiolabeling and Drug Delivery. Pharmaceutics 2022, 14, 653. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, P.; Chen, T.; Lin, L.; Ji, X.; Liu, J.; Huang, H.; Saravanan, T.; Fuehrer, H.; Ross, C.A.; et al. In vivo self-assembled siRNAs within small extracellular vesicles attenuate LRRK2-induced neurodegeneration in Parkinson’s disease models. J. Control. Release 2025, 378, 1139–1153. [Google Scholar] [CrossRef]
- Yang, J.; Wu, S.; Hou, L.; Zhu, D.; Yin, S.; Yang, G.; Wang, Y. Therapeutic Effects of Simultaneous Delivery of Nerve Growth Factor mRNA and Protein via Exosomes on Cerebral Ischemia. Mol. Ther. Nucleic Acids 2020, 21, 512–522. [Google Scholar] [CrossRef]
- Evora, A.; Garcia, G.; Rubi, A.; De Vitis, E.; Matos, A.T.; Vaz, A.R.; Gervaso, F.; Gigli, G.; Polini, A.; Brites, D. Exosomes enriched with miR-124-3p show therapeutic potential in a new microfluidic triculture model that recapitulates neuron-glia crosstalk in Alzheimer’s disease. Front. Pharmacol. 2025, 16, 1474012. [Google Scholar] [CrossRef]
- Han, D.; Dong, X.; Zheng, D.; Nao, J. MiR-124 and the Underlying Therapeutic Promise of Neurodegenerative Disorders. Front. Pharmacol. 2019, 10, 1555. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, Y.; Wang, L.; Liu, Y.; Wang, X.; Li, Y.; Chi, G. miR-124: A Promising Therapeutic Target for Central Nervous System Injuries and Diseases. Cell Mol. Neurobiol. 2022, 42, 2031–2053. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, W.; Ke, H.; Lan, C. Optical imaging detection of extracellular vesicles of miR-146 modified bone marrow mesenchymal stem cells promoting spinal cord injury repair. SLAS Technol. 2024, 29, 100172. [Google Scholar] [CrossRef]
- Crose, J.J.; Crose, A.; Ransom, J.T.; Lightner, A.L. Bone marrow mesenchymal stem cell-derived extracellular vesicle infusion for amyotrophic lateral sclerosis. Neurodegener. Dis. Manag. 2024, 14, 111–117. [Google Scholar] [CrossRef]
- Goldschmidt-Clermont, P.J.; Khan, A.; Jimsheleishvili, G.; Graham, P.; Brooks, A.; Silvera, R.; Goldschmidt, A.J.P.; Pearse, D.D.; Dietrich, W.D.; Levi, A.D.; et al. Treating amyotrophic lateral sclerosis with allogeneic Schwann cell-derived exosomal vesicles: A case report. Neural Regen. Res. 2025, 20, 1207–1216. [Google Scholar] [CrossRef]
- Grangier, A.; Branchu, J.; Volatron, J.; Piffoux, M.; Gazeau, F.; Wilhelm, C.; Silva, A.K.A. Technological advances towards extracellular vesicles mass production. Adv. Drug Deliv. Rev. 2021, 176, 113843. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Ichihara, M.; Wang, X.; Yamamoto, K.; Kimura, J.; Majima, E.; Kiwada, H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J. Control. Release 2006, 112, 15–25. [Google Scholar] [CrossRef]
- Beloqui, A.; Solinis, M.A.; Rodriguez-Gascon, A.; Almeida, A.J.; Preat, V. Nanostructured lipid carriers: Promising drug delivery systems for future clinics. Nanomedicine 2016, 12, 143–161. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Preat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Begines, B.; Ortiz, T.; Perez-Aranda, M.; Martinez, G.; Merinero, M.; Arguelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomater 2020, 10, 1403. [Google Scholar] [CrossRef]
- Mingozzi, F.; High, K.A. Immune responses to AAV vectors: Overcoming barriers to successful gene therapy. Blood 2013, 122, 23–36. [Google Scholar] [CrossRef]
- Naso, M.F.; Tomkowicz, B.; Perry, W.L., 3rd; Strohl, W.R. Adeno-Associated Virus (AAV) as a Vector for Gene Therapy. BioDrugs 2017, 31, 317–334. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef]
- Torres Crigna, A.; Fricke, F.; Nitschke, K.; Worst, T.; Erb, U.; Karremann, M.; Buschmann, D.; Elvers-Hornung, S.; Tucher, C.; Schiller, M.; et al. Inter-Laboratory Comparison of Extracellular Vesicle Isolation Based on Ultracentrifugation. Transfus. Med. Hemother 2021, 48, 48–59. [Google Scholar] [CrossRef]
- Jia, Y.; Yu, L.; Ma, T.; Xu, W.; Qian, H.; Sun, Y.; Shi, H. Small extracellular vesicles isolation and separation: Current techniques, pending questions and clinical applications. Theranostics 2022, 12, 6548–6575. [Google Scholar] [CrossRef]
- Garcia, S.G.; Sanroque-Munoz, M.; Clos-Sansalvador, M.; Font-Moron, M.; Monguio-Tortajada, M.; Borras, F.E.; Franquesa, M. Hollow fiber bioreactor allows sustained production of immortalized mesenchymal stromal cell-derived extracellular vesicles. Extracell. Vesicles Circ. Nucl. Acids 2024, 5, 201–220. [Google Scholar] [CrossRef]
- Yan, L.; Wu, X. Exosomes produced from 3D cultures of umbilical cord mesenchymal stem cells in a hollow-fiber bioreactor show improved osteochondral regeneration activity. Cell Biol. Toxicol. 2020, 36, 165–178. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Stoppato, M.; Sere, Y.Y.; Coles, A.; Didiot, M.C.; Wollacott, R.; Sapp, E.; Dubuke, M.L.; Li, X.; et al. Exosomes Produced from 3D Cultures of MSCs by Tangential Flow Filtration Show Higher Yield and Improved Activity. Mol. Ther. 2018, 26, 2838–2847. [Google Scholar] [CrossRef]
- Batrakova, E.V.; Kim, M.S. Development and regulation of exosome-based therapy products. Wiley Interdiscip. Rev. Nanomed. Nanobiotechn. 2016, 8, 744–757. [Google Scholar] [CrossRef]
- Marzan, A.L.; Chitti, S.V. Unravelling the Role of Cancer Cell-Derived Extracellular Vesicles in Muscle Atrophy, Lipolysis, and Cancer-Associated Cachexia. Cells 2023, 12, 2598. [Google Scholar] [CrossRef]
- Wang, B.; Le, D.S.; Liu, L.; Zhang, X.X.; Yang, F.; Lai, G.R.; Zhang, C.; Zhao, M.L.; Shen, Y.P.; Liao, P.S.; et al. Targeting exosomal double-stranded RNA-TLR3 signaling pathway attenuates morphine tolerance and hyperalgesia. Cell Rep. Med. 2024, 5, 101782. [Google Scholar] [CrossRef]
- Muskan, M.; Abeysinghe, P.; Cecchin, R.; Branscome, H.; Morris, K.V.; Kashanchi, F. Therapeutic potential of RNA-enriched extracellular vesicles: The next generation in RNA delivery via biogenic nanoparticles. Mol. Ther. 2024, 32, 2939–2949. [Google Scholar] [CrossRef]
- Zhang, E.; Phan, P.; Zhao, Z. Cellular nanovesicles for therapeutic immunomodulation: A perspective on engineering strategies and new advances. Acta Pharm. Sin. B 2023, 13, 1789–1827. [Google Scholar] [CrossRef]
- Wan, Z.; Liu, T.; Xu, N.; Zhu, W.; Zhang, X.; Liu, Q.; Wang, H.; Wang, H. PKH Dyes Should Be Avoided in the EVs Biodistribution Study of the Brain: A Call for Caution. Int. J. Nanomed. 2024, 19, 10885–10898. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Wiklander, O.P.B.; Wood, M.J.A.; El-Andaloussi, S. Biodistribution of therapeutic extracellular vesicles. Extracell. Vesicles Circ. Nucl. Acids 2023, 4, 170–190. [Google Scholar] [CrossRef] [PubMed]
- Kunitake, K.; Mizuno, T.; Hattori, K.; Oneyama, C.; Kamiya, M.; Ota, S.; Urano, Y.; Kojima, R. Barcoding of small extracellular vesicles with CRISPR-gRNA enables comprehensive, subpopulation-specific analysis of their biogenesis and release regulators. Nat. Commun. 2024, 15, 9777. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).