Metabolic Culture Medium Enhances Maturation of Human iPSC-Derived Cardiomyocytes via Cardiac Troponin I Isoform Induction
Abstract
1. Introduction
2. Results
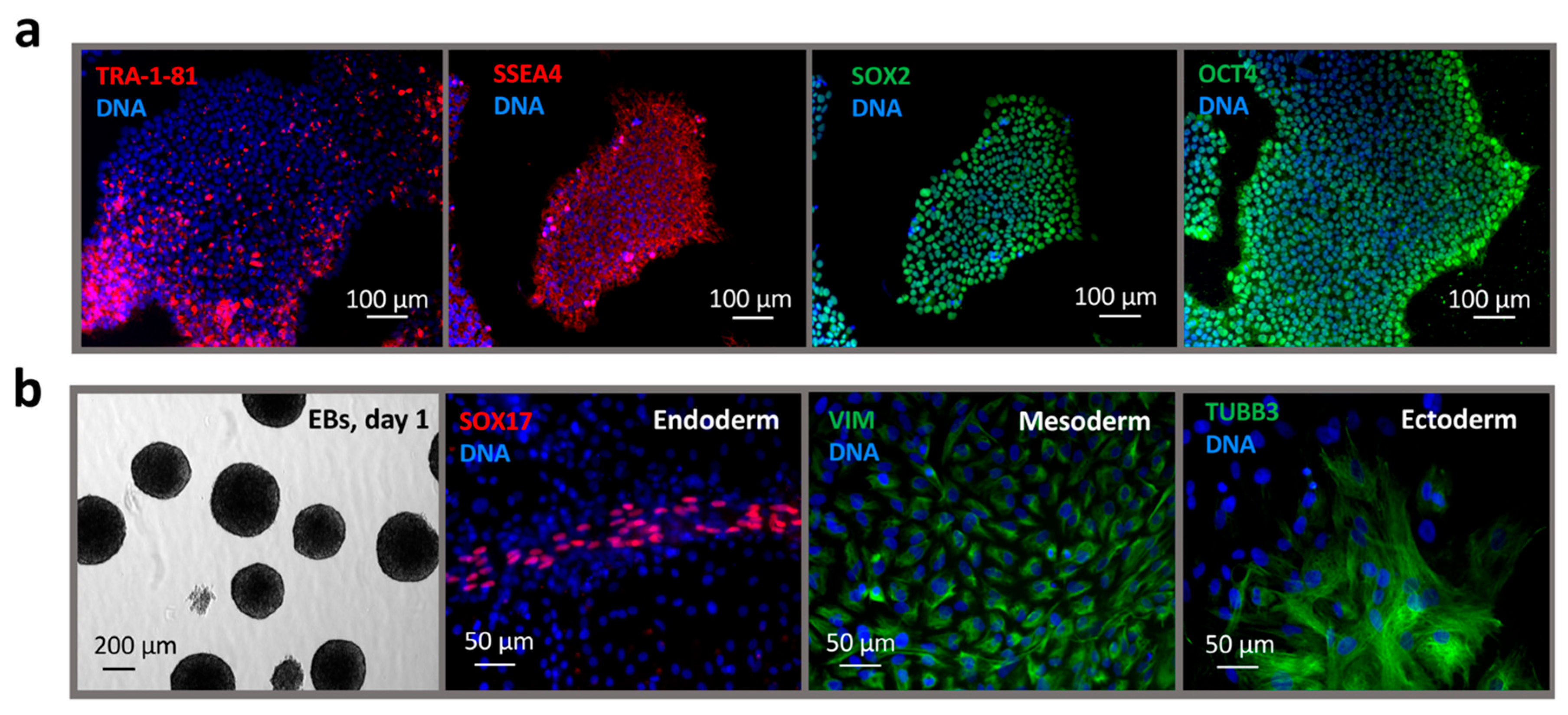
2.1. Reprogramming of Dermal Fibroblasts to a Pluripotent State
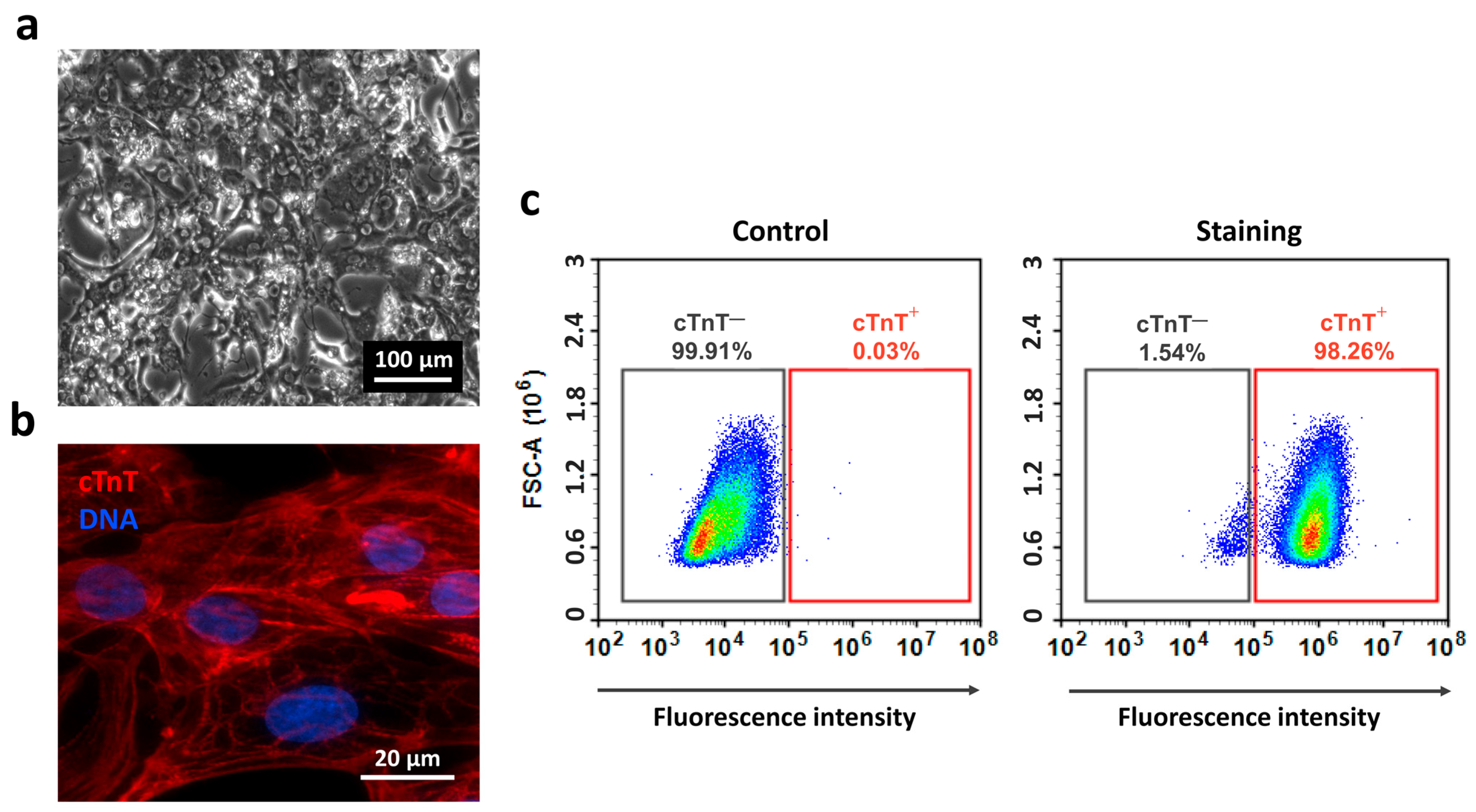
2.2. Differentiation of iPSCs into Cardiomyocytes
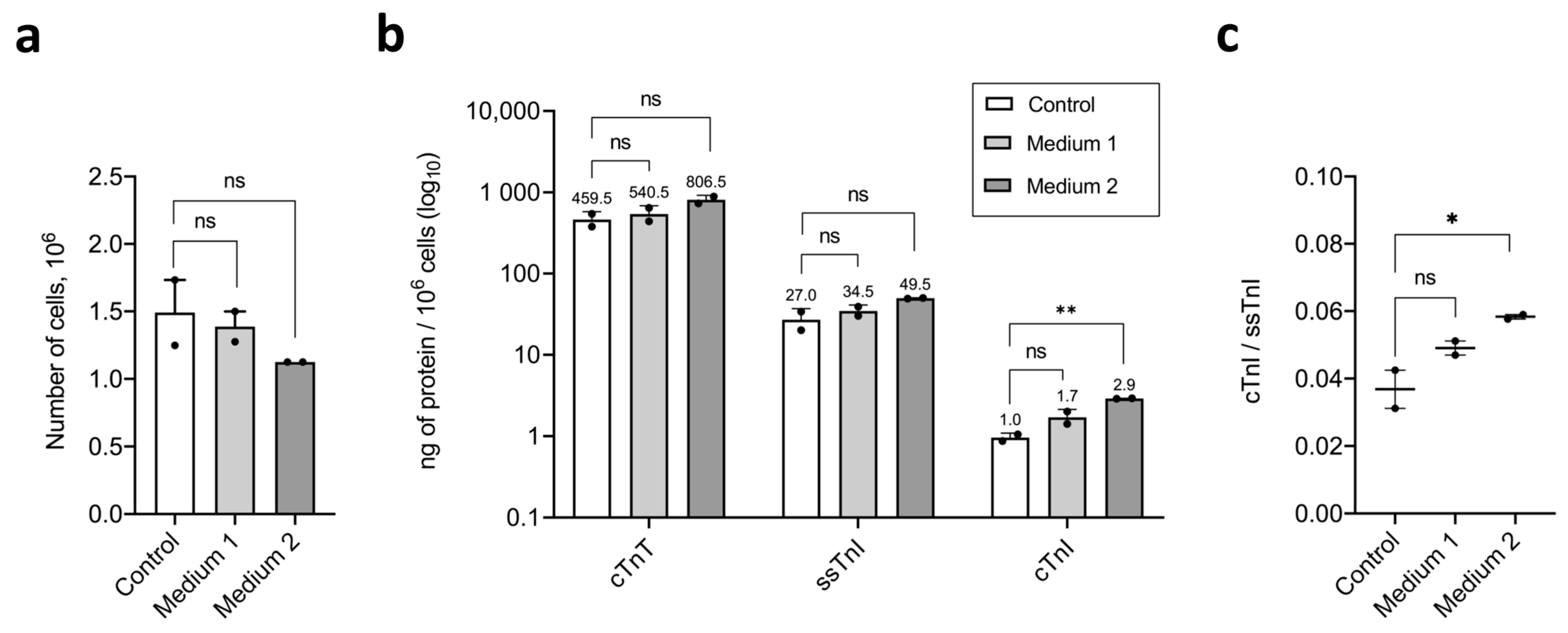
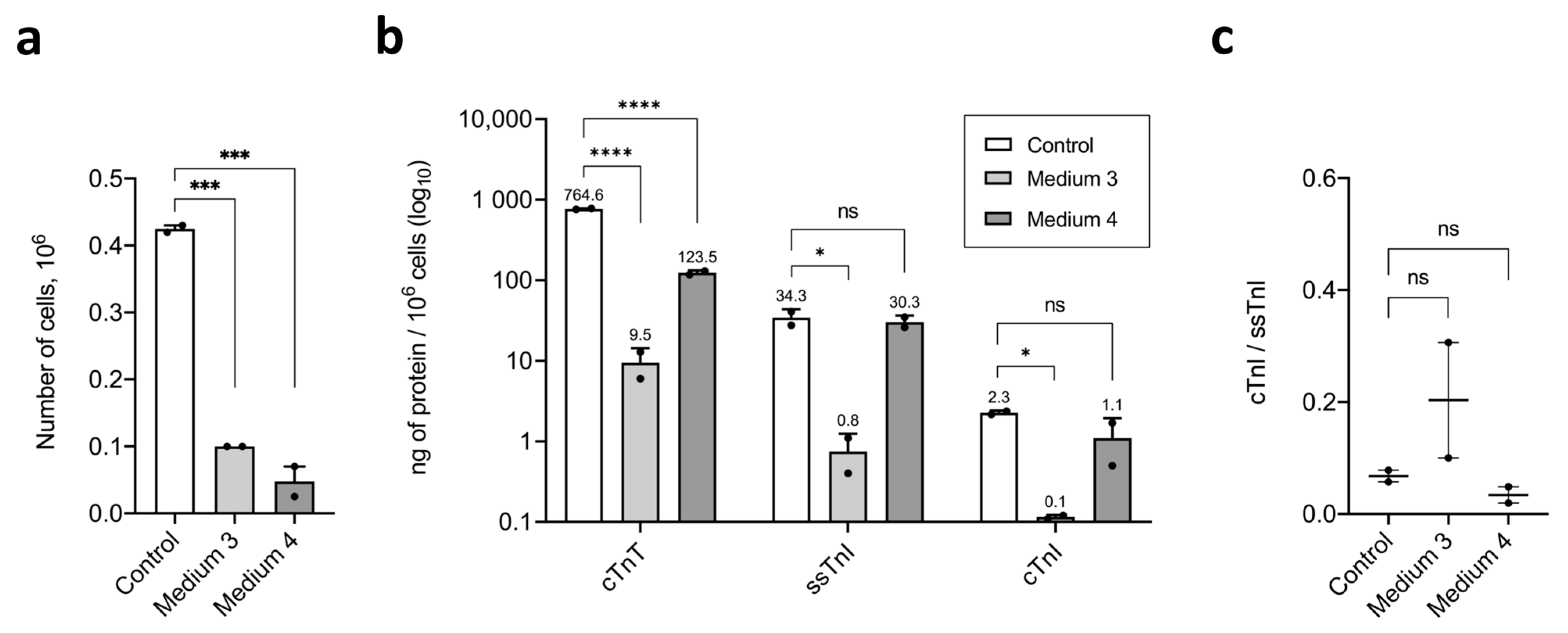
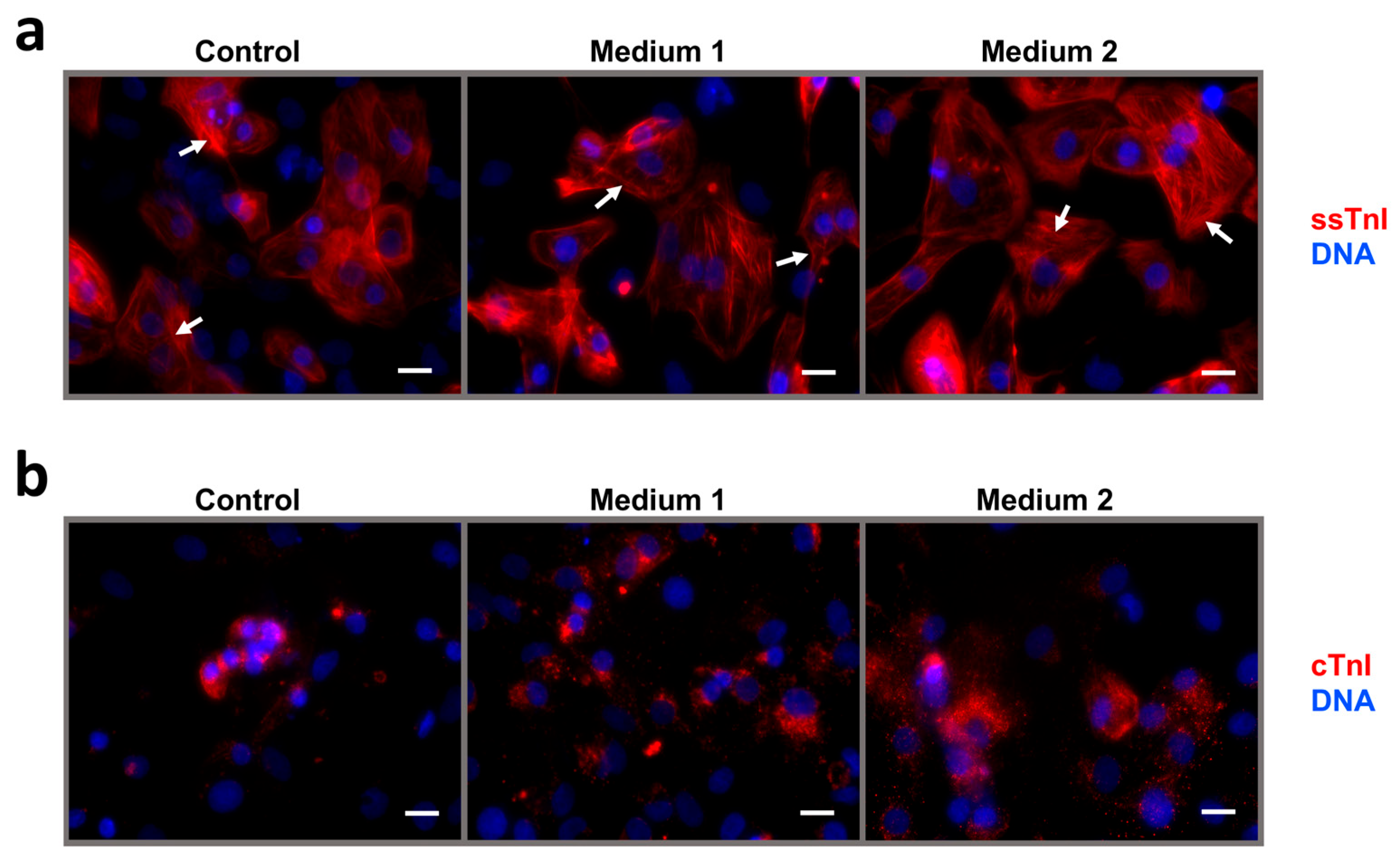
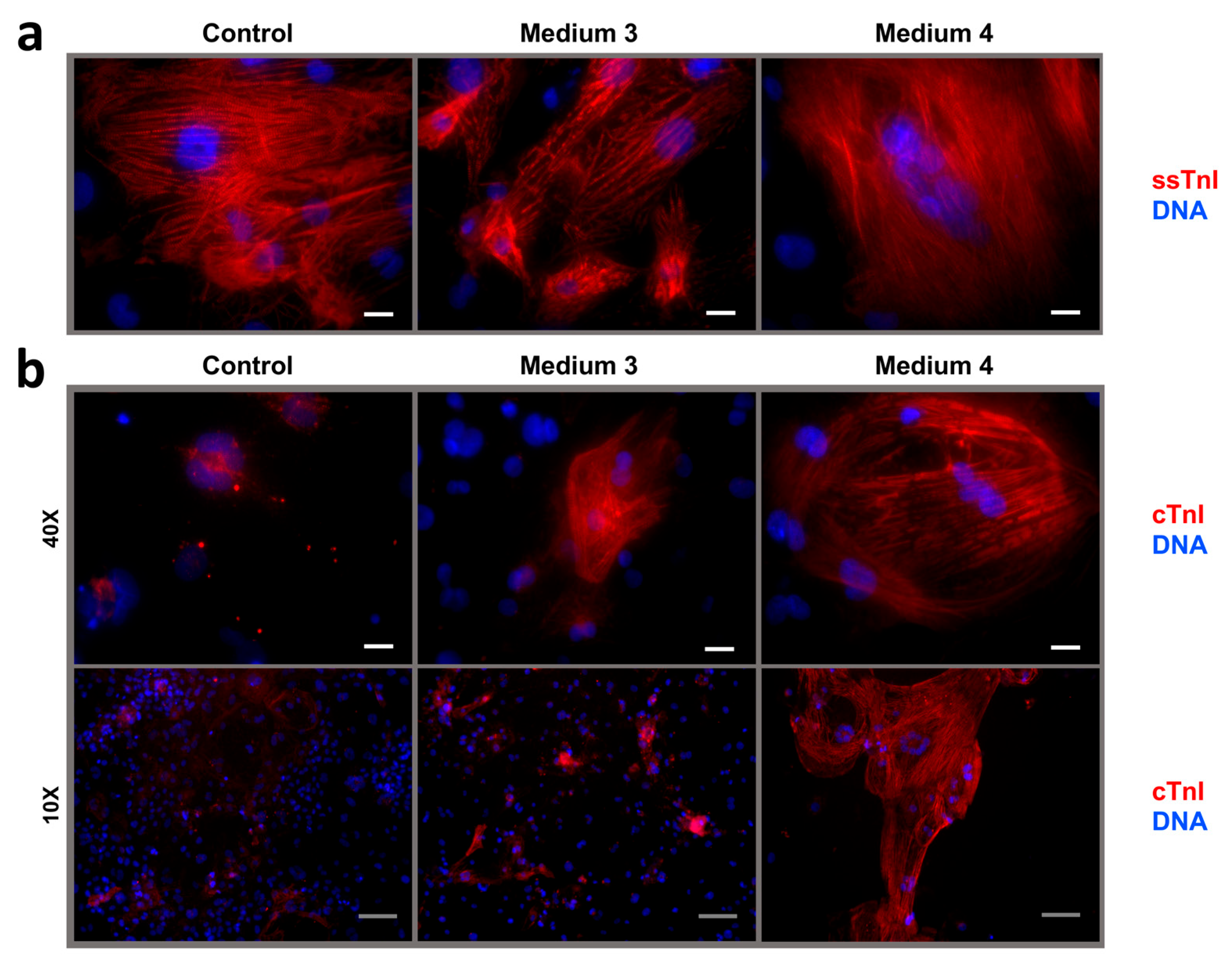
2.3. Maturation and Comparative Analysis of iCMs Maturity
3. Discussion
3.1. Fatty Acids
3.2. T3, IGF-1, and Dexamethasone
3.3. Etoposide
3.4. Metabolic Maturation Medium
3.5. Comparative Effects of Experimental Conditions
4. Materials and Methods
4.1. Cell Material and Troponin-Specific Antibodies
4.2. Isolation and Culture of Fibroblasts
4.3. Generation and Culture of iPSCs
4.4. Validation of iPSC Line
4.5. Differentiation and Culture of iPSC-Derived Cardiomyocytes
4.6. Maturation of iCMs
- Medium 1—STEMdiff Cardiomyocyte Maintenance Medium (Stemcell Technologies, Canada), 100 nM T3 (Sigma-Aldrich, Cat.#T6397, USA), 1 µM Dexamethasone (injectable solution, KRKA, Novo Mesto, Slovenia), 100 ng/mL rhIGF-1 (Stemcell Technologies, Cat.#78022, Canada), 1000× Chemically Defined Lipid Concentrate (Gibco, Cat.#11905-031, USA).
- Medium 2—STEMdiff Cardiomyocyte Maintenance Medium (Stemcell Technologies, Canada), 25 µM Etoposide (Selleckchem, Houston, TX, Cat.#S1225, USA), 1000× Chemically Defined Lipid Concentrate (Gibco, Cat.#11905-031, USA).
- Medium 3—RPMI-1640 with 11 mM Glucose (Gibco, Cat.#21870-076, USA), 100 nM Triiodothyronine (Sigma-Aldrich, Cat.#T6397, USA), 1 µM Dexamethasone (injectable solution, KRKA, Slovenia), 100 ng/mL rgIGF-1 (Stemcell Technologies, Cat.#78022, Canada), and 50× NeuroMax supplement (PanEco, Cat.#FR-0305, Russia)
- Medium 4—Glucose-free DMEM (Gibco, Cat.#A14430-01, USA) supplemented with 3 mM D-Glucose (injectable solution, Armavir Biological Factory, Armavir, Russia), 4 mM L-Glutamine (Corning, Cat.#25-005-CI, USA), 100× Non-essential amino acids for MEM (PanEco, Cat.#F115/100p, Russia), 10 mM Sodium lactate (PanReac, Cat.#143397.1211, Spain), 5 µg/mL Vitamin B12 (injectable solution, Dalhimfarm, Khabarovsk, Russia), 0.82 µM Biotin (PanReac, Cat.#A0969.0001, Spain), 5 mM Creatine monohydrate (dietary supplement, Evalar, Biysk, Russia), 2 mM L-Taurine (intravitreal solution, Dalhimfarm, Russia), 2 mM L-Carnitine (injectable solution, Ellara, Pokrov, Russia), 0.5 mM Ascorbic acid (Sigma-Aldrich, Cat.#A4544-256, USA), 0.5 mg/mL rhAlbumin (eEnzyme, Cat.#HSA-1g, USA), 1000× Chemically defined lipid concentrate (Gibco, Cat.#11905-031, USA), 50× NeuroMax supplement (PanEco, Cat.#FR-0305, Russia), and 1% KnockOut SR (Gibco, Cat.#10828-028, USA).
4.7. Preparation of iCMs Lysates
4.8. Sandwich Fluoroimmunoassay (FIA)
4.9. Calculation of the cTnI/ssTnI Ratio in iCMs
4.10. Immunocytochemical Staining (ICC)
4.11. Flow Cytometry (FC)
4.12. Statistical Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine triphosphate |
| BSA | bovine serum albumin |
| CDM3L | chemically defined Medium 3 without glucose with lactate |
| cITC | ternary human cardiac troponin complex (I-T-C) |
| cTnI | cardiac troponin I |
| cTnT | cardiac troponin T |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DMEM | Dulbecco’s modified Eagle medium |
| DMSO | dimethyl sulfoxide |
| DNA | deoxyribonucleic acid |
| EBs | embryoid bodies |
| EDTA | ethylenediaminetetraacetic acid |
| FBS | fetal bovine serum |
| FC | flow cytometry |
| FIA | fluoroimmunoassay |
| FSC | forward scatter |
| hc-MYC | human cellular myelocytomatosis oncogene |
| hKLF4 | human Krüppel-like factor-4 |
| hOCT3/4 | human octamer-binding transcription tactor-3/4 |
| hPSC | human pluripotent stem cells |
| hSOX2 | human SRY-box transcription factor 2 |
| ICC | immunocytochemical staining |
| iCMs | induced pluripotent stem cell-derived cardiomyocytes |
| IGF-1 | insulin-like growth factor 1 |
| iPSCs | induced pluripotent stem cells |
| MEM | minimum essential medium |
| MM-1 | metabolic maturation medium 1 |
| PBS | phosphate-buffered saline |
| PEI | polyethyleneimine |
| ROCK | rho-associated protein kinase |
| RPMI | Roswell Park Memorial Institute |
| SEM | standard error of the mean |
| skTnI | skeletal troponin I |
| SOX17 | SRY-box transcription factor 17 |
| SR | serum replacement |
| ssIC | binary human slow skeletal troponin complex (I-C) |
| ssTnI | slow skeletal troponin I |
| STR | short tandem repeat |
| T3 | triiodothyronine |
| TNNI3 | troponin I3, cardiac type |
| TRA-1-81 | podocalyxin-like protein 1 |
References
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev. Reports 2020, 16, 3–32. [Google Scholar] [CrossRef]
- Hui, K.K.; Yamanaka, S. IPS Cell Therapy 2.0: Preparing for next-Generation Regenerative Medicine. BioEssays 2024, 1–23. [Google Scholar] [CrossRef]
- Eremeev, A.V.; Pikina, A.S.; Ruchko, E.S.; Sidorov, V.S.; Ragozin, A.O. Fabrication of Cartilage Tissue Substitutes From Cells With Induced Pluripotency. Extrem. Med. 2022, 30–41. [Google Scholar] [CrossRef]
- Lebedeva, O.S.; Sharova, E.I.; Grekhnev, D.A.; Skorodumova, L.O.; Kopylova, I.V.; Vassina, E.M.; Oshkolova, A.; Novikova, I.V.; Krisanova, A.V.; Olekhnovich, E.I.; et al. An Efficient 2D Protocol for Differentiation of IPSCs into Mature Postmitotic Dopaminergic Neurons: Application for Modeling Parkinson’s Disease. Int. J. Mol. Sci. 2023, 24, 7297. [Google Scholar] [CrossRef]
- Bogomiakova, M.E.; Sekretova, E.K.; Anufrieva, K.S.; Khabarova, P.O.; Kazakova, A.N.; Bobrovsky, P.A.; Grigoryeva, T.V.; Eremeev, A.V.; Lebedeva, O.S.; Bogomazova, A.N.; et al. IPSC-Derived Cells Lack Immune Tolerance to Autologous NK-Cells Due to Imbalance in Ligands for Activating and Inhibitory NK-Cell Receptors. Stem Cell Res. Ther. 2023, 14, 1–18. [Google Scholar] [CrossRef]
- Barsby, T.; Otonkoski, T. Maturation of Beta Cells: Lessons from in Vivo and in Vitro Models. Diabetologia 2022, 65, 917–930. [Google Scholar] [CrossRef]
- Hong, Y.; Zhao, Y.; Li, H.; Yang, Y.; Chen, M.; Wang, X.; Luo, M.; Wang, K. Engineering the Maturation of Stem Cell-Derived Cardiomyocytes. Front. Bioeng. Biotechnol. 2023, 11, 1–19. [Google Scholar] [CrossRef]
- Lindstrom, M.; Decleene, N.; Dorsey, H.; Fuster, V.; Johnson, C.O.; Legrand, K.E.; Mensah, G.A.; Razo, C.; Stark, B.; Turco, V.; et al. Summary of Global Burden of Disease Study Methods. J. Am. Coll. Cardiol. 2022, 80, 2372–2425. [Google Scholar] [CrossRef]
- Chong, B.; Jayabaskaran, J.; Jauhari, S.M.; Chan, S.P.; Goh, R.; Kueh, M.T.W.; Li, H.; Chin, Y.H.; Kong, G.; Anand, V.V.; et al. Global Burden of Cardiovascular Diseases: Projections from 2025 to 2050. Eur. J. Prev. Cardiol. 2024, zwae281. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Yan, Y.; Fooladi, S.; Qyang, Y. Advancements in Techniques for Human IPSC-Derived Cardiomyocytes Maturation: Mechanical and Electrical Stimulation Approaches. Biophys. Rev. 2025, 169–183. [Google Scholar] [CrossRef]
- Brodehl, A.; Ebbinghaus, H.; Deutsch, M.A.; Gummert, J.; Gärtner, A.; Ratnavadivel, S.; Milting, H. Human Induced Pluripotent Stem-Cell-Derived Cardiomyocytes as Models for Genetic Cardiomyopathies. Int. J. Mol. Sci. 2019, 20, 4381. [Google Scholar] [CrossRef]
- Peters, N.S.; Severs, N.J.; Rothery, S.M.; Lincoln, C.; Yacoub, M.H.; Green, C.R. Spatiotemporal Relation between Gap Junctions and Fascia Adherens Junctions during Postnatal Development of Human Ventricular Myocardium. Circulation 1994, 90, 713–725. [Google Scholar] [CrossRef]
- Bedada, F.B.; Chan, S.S.K.; Metzger, S.K.; Zhang, L.; Zhang, J.; Garry, D.J.; Kamp, T.J.; Kyba, M.; Metzger, J.M. Acquisition of a Quantitative, Stoichiometrically Conserved Ratiometric Marker of Maturation Status in Stem Cell-Derived Cardiac Myocytes. Stem Cell Reports 2014, 3, 594–605. [Google Scholar] [CrossRef]
- Maroli, G.; Braun, T. The Long and Winding Road of Cardiomyocyte Maturation. Cardiovasc. Res. 2021, 117, 712–726. [Google Scholar] [CrossRef]
- Uscategui Calderon, M.; Gonzalez, B.A.; Yutzey, K.E. Cardiomyocyte-Fibroblast Crosstalk in the Postnatal Heart. Front. Cell Dev. Biol. 2023, 11, 1–12. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Capasso, J.M.; Gerdes, A.M. Rapid Transition of Cardiac Myocytes from Hyperplasia to Hypertrophy during Postnatal Development. J. Mol. Cell. Cardiol. 1996, 28, 1737–1746. [Google Scholar] [CrossRef]
- Mollova, M.; Bersell, K.; Walsh, S.; Savla, J.; Das, L.T.; Park, S.Y.; Silberstein, L.E.; Dos Remedios, C.G.; Graham, D.; Colan, S.; et al. Cardiomyocyte Proliferation Contributes to Heart Growth in Young Humans. Proc. Natl. Acad. Sci. USA 2013, 110, 1446–1451. [Google Scholar] [CrossRef]
- Patterson, M.; Barske, L.; Van Handel, B.; Rau, C.D.; Gan, P.; Sharma, A.; Parikh, S.; Denholtz, M.; Huang, Y.; Yamaguchi, Y.; et al. Frequency of Mononuclear Diploid Cardiomyocytes Underlies Natural Variation in Heart Regeneration. Nat. Genet. 2017, 49, 1346–1353. [Google Scholar] [CrossRef]
- Lopaschuk, G.D.; Jaswal, J.S. Energy Metabolic Phenotype of the Cardiomyocyte during Development, Differentiation, and Postnatal Maturation. J. Cardiovasc. Pharmacol. 2010, 56, 130–140. [Google Scholar] [CrossRef]
- Paredes, A.; Justo-Méndez, R.; Jiménez-Blasco, D.; Núñez, V.; Calero, I.; Villalba-Orero, M.; Alegre-Martí, A.; Fischer, T.; Gradillas, A.; Sant’Anna, V.A.R.; et al. γ-Linolenic Acid in Maternal Milk Drives Cardiac Metabolic Maturation. Nature 2023, 618, 365–373. [Google Scholar] [CrossRef]
- Schwinger, R.H.G.; Böhm, M.; Erdmann, E. Different Negative Inotropic Activity of Ca2+-Antagonists in Human Myocardial Tissue. Klin. Wochenschr. 1990, 68, 797–805. [Google Scholar] [CrossRef]
- Chen, H.S.V.; Kim, C.; Mercola, M. Electrophysiological Challenges of Cell-Based Myocardial Repair. Circulation 2009, 120, 2496–2508. [Google Scholar] [CrossRef]
- Ulmer, B.M.; Eschenhagen, T. Human Pluripotent Stem Cell-Derived Cardiomyocytes for Studying Energy Metabolism. Biochim. Biophys. Acta-Mol. Cell Res. 2020, 1867, 118471. [Google Scholar] [CrossRef]
- Kannan, S.; Miyamoto, M.; Zhu, R.; Lynott, M.; Guo, J.; Chen, E.Z.; Colas, A.R.; Lin, B.L.; Kwon, C. Trajectory Reconstruction Identifies Dysregulation of Perinatal Maturation Programs in Pluripotent Stem Cell-Derived Cardiomyocytes. Cell Rep. 2023, 42, 112330. [Google Scholar] [CrossRef]
- Sugiura, T.; Shahannaz, D.C.; Ferrell, B.E. Global Translational Medicine Advancements in Cardiac Regenerative Therapy: Scalable Human IPSC-Derived Cardiomyocyte Differentiation and Maturation. Glob. Transl. Med. 2025, 4, 1–15. [Google Scholar] [CrossRef]
- Dou, W.; Daoud, A.; Chen, X.; Wang, T.; Malhi, M.; Gong, Z.; Mirshafiei, F.; Zhu, M.; Shan, G.; Huang, X.; et al. Ultrathin and Flexible Bioelectronic Arrays for Functional Measurement of IPSC-Cardiomyocytes under Cardiotropic Drug Administration and Controlled Microenvironments. Nano Lett. 2023, 23, 2321–2331. [Google Scholar] [CrossRef]
- Lopez-Buenafe, G.d.R.; Alonso-Cabrera, J.A.; Marcuello, C.; Ortiz-Perez, M.; Benito-Lopez, F.; Colom, A.; Basabe-Desmonts, L.; Saez, J. Fabrication and Characterization of PEDOT:PSS-Based Microstructured Electrodes for In Vitro Cell Culture. Adv. Mater. Interfaces 2025, 2500097. [Google Scholar] [CrossRef]
- Birket, M.J.; Ribeiro, M.C.; Kosmidis, G.; Ward, D.; Leitoguinho, A.R.; van de Pol, V.; Dambrot, C.; Devalla, H.D.; Davis, R.P.; Mastroberardino, P.G.; et al. Contractile Defect Caused by Mutation in MYBPC3 Revealed under Conditions Optimized for Human PSC-Cardiomyocyte Function. Cell Rep. 2015, 13, 733–745. [Google Scholar] [CrossRef]
- Veerman, C.C.; Mengarelli, I.; Lodder, E.M.; Kosmidis, G.; Bellin, M.; Zhang, M.; Dittmann, S.; Guan, K.; Wilde, A.A.M.; Schulze-Bahr, E.; et al. Switch from Fetal to Adult SCN5A Isoform in Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes Unmasks the Cellular Phenotype of a Conduction Disease-Causing Mutation. J. Am. Heart Assoc. 2017, 6. [Google Scholar] [CrossRef]
- Feyen, D.A.M.; McKeithan, W.L.; Bruyneel, A.A.N.; Spiering, S.; Hörmann, L.; Ulmer, B.; Zhang, H.; Briganti, F.; Schweizer, M.; Hegyi, B.; et al. Metabolic Maturation Media Improve Physiological Function of Human IPSC-Derived Cardiomyocytes. Cell Rep. 2020, 32. [Google Scholar] [CrossRef]
- Agarwal, R.; Paulo, J.A.; Toepfer, C.N.; Ewoldt, J.K.; Sundaram, S.; Chopra, A.; Zhang, Q.; Gorham, J.; Depalma, S.R.; Chen, C.S.; et al. Filamin C Cardiomyopathy Variants Cause Protein and Lysosome Accumulation. Circ. Res. 2021, 129, 751–766. [Google Scholar] [CrossRef]
- Peters, M.C.; Maas, R.G.C.; Van Adrichem, I.; Doevendans, P.A.M.; Mercola, M.; Šarić, T.; Buikema, J.W.; Van Mil, A.; Chamuleau, S.A.J.; Sluijter, J.P.G.; et al. Metabolic Maturation Increases Susceptibility to Hypoxia-Induced Damage in Human IPSC-Derived Cardiomyocytes. Stem Cells Transl. Med. 2022, 11, 1040–1051. [Google Scholar] [CrossRef]
- Wang, B.Z.; Nash, T.R.; Zhang, X.; Rao, J.; Abriola, L.; Kim, Y.; Zakharov, S.; Kim, M.; Luo, L.J.; Morsink, M.; et al. Engineered Cardiac Tissue Model of Restrictive Cardiomyopathy for Drug Discovery. Cell Reports Med. 2023, 4, 100976. [Google Scholar] [CrossRef]
- Ohiri, J.C.; Dellefave-Castillo, L.; Tomar, G.; Wilsbacher, L.; Choudhury, L.; Barefield, D.Y.; Fullenkamp, D.; Gacita, A.M.; Monroe, T.O.; Pesce, L.; et al. Reduction of Filamin C Results in Altered Proteostasis, Cardiomyopathy, and Arrhythmias. J. Am. Heart Assoc. 2024, 13, 1–15. [Google Scholar] [CrossRef]
- Bedada, F.B.; Wheelwright, M.; Metzger, J.M. Maturation Status of Sarcomere Structure and Function in Human IPSC-Derived Cardiac Myocytes. Biochim. Biophys. Acta-Mol. Cell Res. 2016, 1863, 1829–1838. [Google Scholar] [CrossRef]
- Li, J.; Feng, X.; Wei, X. Modeling Hypertrophic Cardiomyopathy with Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells. Stem Cell Res. Ther. 2022, 13, 1–20. [Google Scholar] [CrossRef]
- Kermani, F.; Mosqueira, M.; Peters, K.; Lemma, E.D.; Rapti, K.; Grimm, D.; Bastmeyer, M.; Laugsch, M.; Hecker, M.; Ullrich, N.D. Membrane Remodelling Triggers Maturation of Excitation–Contraction Coupling in 3D-Shaped Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Basic Res. Cardiol. 2023, 118, 1–16. [Google Scholar] [CrossRef]
- Deogharia, M.; Venegas-Zamora, L.; Agrawal, A.; Shi, M.; Jain, A.K.; McHugh, K.J.; Altamirano, F.; Marian, A.J.; Gurha, P. Histone Demethylase KDM5 Regulates Cardiomyocyte Maturation by Promoting Fatty Acid Oxidation, Oxidative Phosphorylation, and Myofibrillar Organization. Cardiovasc. Res. 2024, 120, 630–643. [Google Scholar] [CrossRef]
- Kumar, A.; He, S.; Mali, P. Systematic Discovery of Transcription Factors That Improve HPSC-Derived Cardiomyocyte Maturation via Temporal Analysis of Bioengineered Cardiac Tissues. APL Bioeng. 2023, 7, 1–41. [Google Scholar] [CrossRef]
- Kojima, H.; Sadahiro, T.; Muraoka, N.; Yamakawa, H.; Hashimoto, H.; Ishii, R.; Gosho, M.; Abe, Y.; Yamada, Y.; Nakano, K.; et al. MEF2C/P300-Mediated Epigenetic Remodeling Promotes the Maturation of Induced Cardiomyocytes. Stem Cell Reports 2023, 18, 1274–1283. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Schlaeger, T.M.; Daheron, L.; Brickler, T.R.; Entwisle, S.; Chan, K.; Cianci, A.; DeVine, A.; Ettenger, A.; Fitzgerald, K.; Godfrey, M.; et al. A Comparison of Non-Integrating Reprogramming Methods. Nat. Biotechnol. 2015, 33, 58–63. [Google Scholar] [CrossRef]
- Smith, L.; Quelch-Cliffe, R.; Liu, F.; Aguilar, A.H.; Przyborski, S. Evaluating Strategies to Assess the Differentiation Potential of Human Pluripotent Stem Cells: A Review, Analysis and Call for Innovation. Stem Cell Rev. Reports 2024, 107–125. [Google Scholar] [CrossRef]
- Burridge, P.W.; Matsa, E.; Shukla, P.; Lin, Z.C.; Churko, J.M.; Ebert, A.D.; Lan, F.; Diecke, S.; Huber, B.; Mordwinkin, N.M.; et al. Chemically Defined and Small Molecule-Based Generation of Human Cardiomyocytes. Nat Methods 2014, 11, 855–860. [Google Scholar] [CrossRef]
- Bhavsar, P.K.; Dhoot, G.K.; Cumming, D.V.E.; Butler-Browne, G.S.; Yacoub, M.H.; Barton, P.J.R. Developmental Expression of Troponin I Isoforms in Fetal Human Heart. FEBS Lett. 1991, 292, 5–8. [Google Scholar] [CrossRef]
- Hunkeler, N.M.; Kullman, J.; Murphy, A.M. Troponin I Isoform Expression in Human Heart. Circ. Res. 1991, 69, 1409–1415. [Google Scholar] [CrossRef]
- Sasse, S.; Brand, N.J.; Kyprianou, P.; Dhoot, G.K.; Wade, R.; Arai, M.; Periasamy, M.; Yacoub, M.H.; Barton, P.J.R. Troponin I Gene Expression during Human Cardiac Development and in End-Stage Heart Failure. Circ. Res. 1993, 72, 932–938. [Google Scholar] [CrossRef]
- Siedner, S.; Krüger, M.; Schroeter, M.; Metzler, D.; Roell, W.; Fleischmann, B.K.; Hescheler, J.; Pfitzer, G.; Stehle, R. Developmental Changes in Contractility and Sarcomeric Proteins from the Early Embryonic to the Adult Stage in the Mouse Heart. J. Physiol. 2003, 548, 493–505. [Google Scholar] [CrossRef]
- Katrukha, I.A. Human Cardiac Troponin Complex. Structure and Functions. Biochem. 2013, 78, 1447–1465. [Google Scholar] [CrossRef]
- Wei, B.; Jin, J.P. TNNT1, TNNT2, and TNNT3: Isoform Genes, Regulation, and Structure-Function Relationships. Gene 2016, 582, 1–13. [Google Scholar] [CrossRef]
- Anderson, P.A.W.; Malouf, N.N.; Oakeley, A.E.; Pagani, E.D.; Allen, P.D. Troponin T Isoform Expression in Humans. A Comparison among Normal and Failing Adult Heart, Fetal Heart, and Adult and Fetal Skeletal Muscle. Circ. Res. 1991, 69, 1226–1233. [Google Scholar] [CrossRef]
- Jin, J.P.; Lin, J.J. Rapid Purification of Mammalian Cardiac Troponin T and Its Isoform Switching in Rat Hearts during Development. J. Biol. Chem. 1988, 263, 7309–7315. [Google Scholar] [CrossRef]
- Jin, J.P. Alternative RNA Splicing-Generated Cardiac Troponin T Isoform Switching: A Non-Heart-Restricted Genetic Programming Synchronized in Developing Cardiac and Skeletal Muscles. Biochem. Biophys. Res. Commun. 1996, 225, 883–889. [Google Scholar] [CrossRef]
- Bogomolova, A.P.; Katrukha, I.A.; Emelin, A.M.; Zabolotsky, A.I.; Bereznikova, A.V.; Lebedeva, O.S.; Deev, R.V.; Katrukha, A.G. Development of Immunochemical Systems for Detection of Human Skeletal Troponin I Isoforms. Biochem. 2025, 90, 349–363. [Google Scholar] [CrossRef]
- Paterson, N.; Biggart, E.M.; Chapman, R.S.; Beastall, G.H. Evaluation of a Time-Resolved Immunofluorometric Assay for Serum Thyroid Stimulating Hormone. Ann. Clin. Biochem. 1985, 22, 606–611. [Google Scholar] [CrossRef]
- Vylegzhanina, A.V.; Kogan, A.E.; Katrukha, I.A.; Koshkina, E.V.; Bereznikova, A.V.; Filatov, V.L.; Bloshchitsyna, M.N.; Bogomolova, A.P.; Katrukha, A.G. Full-Size and Partially Truncated Cardiac Troponin Complexes in the Blood of Patients with Acute Myocardial Infarction. Clin. Chem. 2019, 65, 882–892. [Google Scholar] [CrossRef]
- Brewer, G.J.; Cotman, C.W. Survival and Growth of Hippocampal Neurons in Defined Medium at Low Density: Advantages of a Sandwich Culture Technique or Low Oxygen. Brain Res. 1989, 494, 65–74. [Google Scholar] [CrossRef]
- Makinde, A.O.; Kantor, P.F.; Lopaschuk, G.D. Maturation of Fatty Acid and Carbohydrate Metabolism in the Newborn Heart. Mol. Cell. Biochem. 1998, 188, 49–56. [Google Scholar] [CrossRef]
- Knight, W.E.; Cao, Y.; Dillon, P.; Song, K. A Simple Protocol to Produce Mature Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. STAR Protoc. 2021, 2, 100912. [Google Scholar] [CrossRef]
- Yang, X.; Rodriguez, M.; Pabon, L.; Fischer, K.A.; Reinecke, H.; Regnier, M.; Sniadecki, N.J.; Ruohola-Baker, H.; Murry, C.E. Tri-Iodo-l-Thyronine Promotes the Maturation of Human Cardiomyocytes-Derived from Induced Pluripotent Stem Cells. J. Mol. Cell. Cardiol. 2014, 72, 296–304. [Google Scholar] [CrossRef]
- Parikh, S.S.; Blackwell, D.J.; Gomez-Hurtado, N.; Frisk, M.; Wang, L.; Kim, K.; Dahl, C.P.; Fiane, A.; Tønnessen, T.; Kryshtal, D.O.; et al. Thyroid and Glucocorticoid Hormones Promote Functional T-Tubule Development in Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Circ. Res. 2017, 121, 1323–1330. [Google Scholar] [CrossRef]
- Horikoshi, Y.; Yan, Y.; Terashvili, M.; Wells, C.; Horikoshi, H.; Fujita, S.; Bosnjak, Z.J.; Bai, X. Fatty Acid-Treated Induced Pluripotent Stem Cell-Derived Human Cardiomyocytes Exhibit Adult Cardiomyocyte-Like Energy Metabolism Phenotypes. Cells 2019, 8, 1095. [Google Scholar] [CrossRef]
- Forhead, A.J.; Fowden, A.L. Thyroid Hormones in Fetal Growth and Prepartum Maturation. J. Endocrinol. 2014, 221. [Google Scholar] [CrossRef]
- Gomez-Garcia, M.J.; Quesnel, E.; Al-attar, R.; Laskary, A.R.; Laflamme, M.A. Maturation of Human Pluripotent Stem Cell Derived Cardiomyocytes in Vitro and in Vivo. Semin. Cell Dev. Biol. 2021, 118, 163–171. [Google Scholar] [CrossRef]
- Ivy, J.R.; Carter, R.N.; Zhao, J.F.; Buckley, C.; Urquijo, H.; Rog-Zielinska, E.A.; Panting, E.; Hrabalkova, L.; Nicholson, C.; Agnew, E.J.; et al. Glucocorticoids Regulate Mitochondrial Fatty Acid Oxidation in Fetal Cardiomyocytes. J. Physiol. 2021, 599, 4901–4924. [Google Scholar] [CrossRef]
- McMullen, J.R.; Shioi, T.; Huang, W.Y.; Zhang, L.; Tarnavski, O.; Bisping, E.; Schinke, M.; Kong, S.; Sherwood, M.C.; Brown, J.; et al. The Insulin-like Growth Factor 1 Receptor Induces Physiological Heart Growth via the Phosphoinositide 3-Kinase(P110α) Pathway. J. Biol. Chem. 2004, 279, 4782–4793. [Google Scholar] [CrossRef]
- Laustsen, P.G.; Russell, S.J.; Cui, L.; Entingh-Pearsall, A.; Holzenberger, M.; Liao, R.; Kahn, C.R. Essential Role of Insulin and Insulin-Like Growth Factor 1 Receptor Signaling in Cardiac Development and Function. Mol. Cell. Biol. 2007, 27, 1649–1664. [Google Scholar] [CrossRef]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA Topoisomerases and Their Poisoning by Anticancer and Antibacterial Drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef]
- Choudhari, A.S.; Mandave, P.C.; Deshpande, M.; Ranjekar, P.; Prakash, O. Phytochemicals in Cancer Treatment: From Preclinical Studies to Clinical Practice. Front. Pharmacol. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Nemade, H.; Chaudhari, U.; Acharya, A.; Hescheler, J.; Hengstler, J.G.; Papadopoulos, S.; Sachinidis, A. Cell Death Mechanisms of the Anti-Cancer Drug Etoposide on Human Cardiomyocytes Isolated from Pluripotent Stem Cells. Arch. Toxicol. 2018, 92, 1507–1524. [Google Scholar] [CrossRef]
- Fetterman, K.A.; Blancard, M.; Lyra-Leite, D.M.; Vanoye, C.G.; Fonoudi, H.; Jouni, M.; DeKeyser, J.M.L.; Lenny, B.; Sapkota, Y.; George, A.L.; et al. Independent Compartmentalization of Functional, Metabolic, and Transcriptional Maturation of HiPSC-Derived Cardiomyocytes. Cell Rep. 2024, 43, 114160. [Google Scholar] [CrossRef]
- Goliusova, D.V.; Lebedeva, O.S.; Sharikova, M.Y.; Kopylova, I.V.; Teryakova, M.V. Derivation of RCPCMi011-A Induced Pluripotent Stem Cell Line from Fibroblasts of a Patient with Restrictive Cardiomyopathy Caused by c. 7416 _ 7418delGAA Mutation in the FLNC Gene. Russ. J. Dev. Biol. 2024, 55, 347–355. [Google Scholar] [CrossRef]


| Name | Basal Medium | Maturation Factors | Maturation Protocol |
|---|---|---|---|
| Medium 1 | STEMdiff Cardiomyocyte Maintenance Medium | Triiodothyronine (T3), dexamethasone, insulin-like growth factor 1 (IGF-1), chemically defined lipid concentrate | Short (4-day) |
| Medium 2 | STEMdiff Cardiomyocyte Maintenance Medium | Etoposide, chemically defined lipid concentrate | Short (4-day) |
| Medium 3 | RPMI-1640 with Glucose | T3, dexamethasone, IGF-1, NeuroMax supplement (NeuroMax) | Long (22-day) |
| Medium 4 (MM-1) | DMEM without Glucose | D-glucose, L-glutamine, non-essential amino acids, sodium lactate, vitamin B12, biotin, creatine monohydrate, L-taurine, L-carnitine, ascorbic acid, albumin, chemically defined lipid concentrate, NeuroMax, KnockOut serum replacement (KnockOut SR) | Long (22-day) |
| Target Protein | Antibody | Manufacturer, Cat.#, or Reference | Dilution |
|---|---|---|---|
| FIA | |||
| ssTnI | skTnI58 | [55] | - |
| skTnI27 | [55] | - | |
| cTnI | Y306 | HyTest #RC4T21 | - |
| Y603 | HyTest #RC4T21 | - | |
| cTnT | 329 | HyTest #4T19cc | - |
| 406 | HyTest #4T19cc | - | |
| ICC | |||
| ssTnI | skTnI30 | [55] | 1:1000 |
| cTnI | MF4 | HyTest #4T21 | 1:1000 |
| Goat anti-Mouse IgG (H + L) | Alexa Fluor 555-conjugated secondary antibody | Invitrogen #A-21422 | 1:1000 |
| FC | |||
| cTnT | 406 | HyTest #4T19CC | 1:1000 |
| Goat anti-Mouse IgG (H + L) | Alexa Fluor 488-conjugated secondary antibody | Invitrogen #A-11001 | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goliusova, D.V.; Bogomolova, A.P.; Davidenko, A.V.; Lavrenteva, K.A.; Sharikova, M.Y.; Zerkalenkova, E.A.; Vassina, E.M.; Bogomazova, A.N.; Lagarkova, M.A.; Katrukha, I.A.; et al. Metabolic Culture Medium Enhances Maturation of Human iPSC-Derived Cardiomyocytes via Cardiac Troponin I Isoform Induction. Int. J. Mol. Sci. 2025, 26, 7248. https://doi.org/10.3390/ijms26157248
Goliusova DV, Bogomolova AP, Davidenko AV, Lavrenteva KA, Sharikova MY, Zerkalenkova EA, Vassina EM, Bogomazova AN, Lagarkova MA, Katrukha IA, et al. Metabolic Culture Medium Enhances Maturation of Human iPSC-Derived Cardiomyocytes via Cardiac Troponin I Isoform Induction. International Journal of Molecular Sciences. 2025; 26(15):7248. https://doi.org/10.3390/ijms26157248
Chicago/Turabian StyleGoliusova, Daria V., Agnessa P. Bogomolova, Alina V. Davidenko, Kristina A. Lavrenteva, Margarita Y. Sharikova, Elena A. Zerkalenkova, Ekaterina M. Vassina, Alexandra N. Bogomazova, Maria A. Lagarkova, Ivan A. Katrukha, and et al. 2025. "Metabolic Culture Medium Enhances Maturation of Human iPSC-Derived Cardiomyocytes via Cardiac Troponin I Isoform Induction" International Journal of Molecular Sciences 26, no. 15: 7248. https://doi.org/10.3390/ijms26157248
APA StyleGoliusova, D. V., Bogomolova, A. P., Davidenko, A. V., Lavrenteva, K. A., Sharikova, M. Y., Zerkalenkova, E. A., Vassina, E. M., Bogomazova, A. N., Lagarkova, M. A., Katrukha, I. A., & Lebedeva, O. S. (2025). Metabolic Culture Medium Enhances Maturation of Human iPSC-Derived Cardiomyocytes via Cardiac Troponin I Isoform Induction. International Journal of Molecular Sciences, 26(15), 7248. https://doi.org/10.3390/ijms26157248





