The Activation of the Microglial NLRP3 Inflammasome Is Involved in Tuberous Sclerosis Complex-Related Neuroinflammation
Abstract
1. Introduction
2. Results
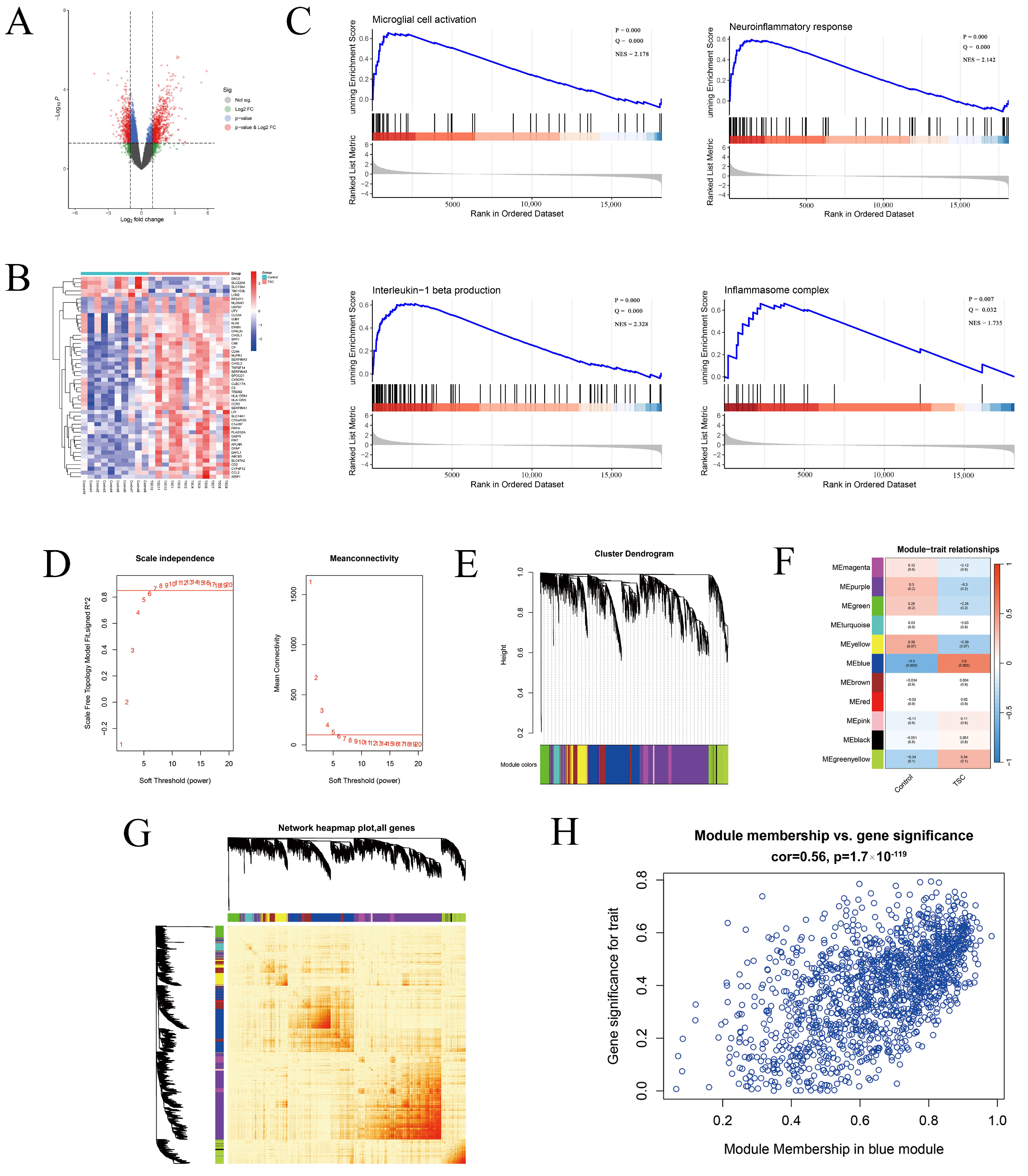
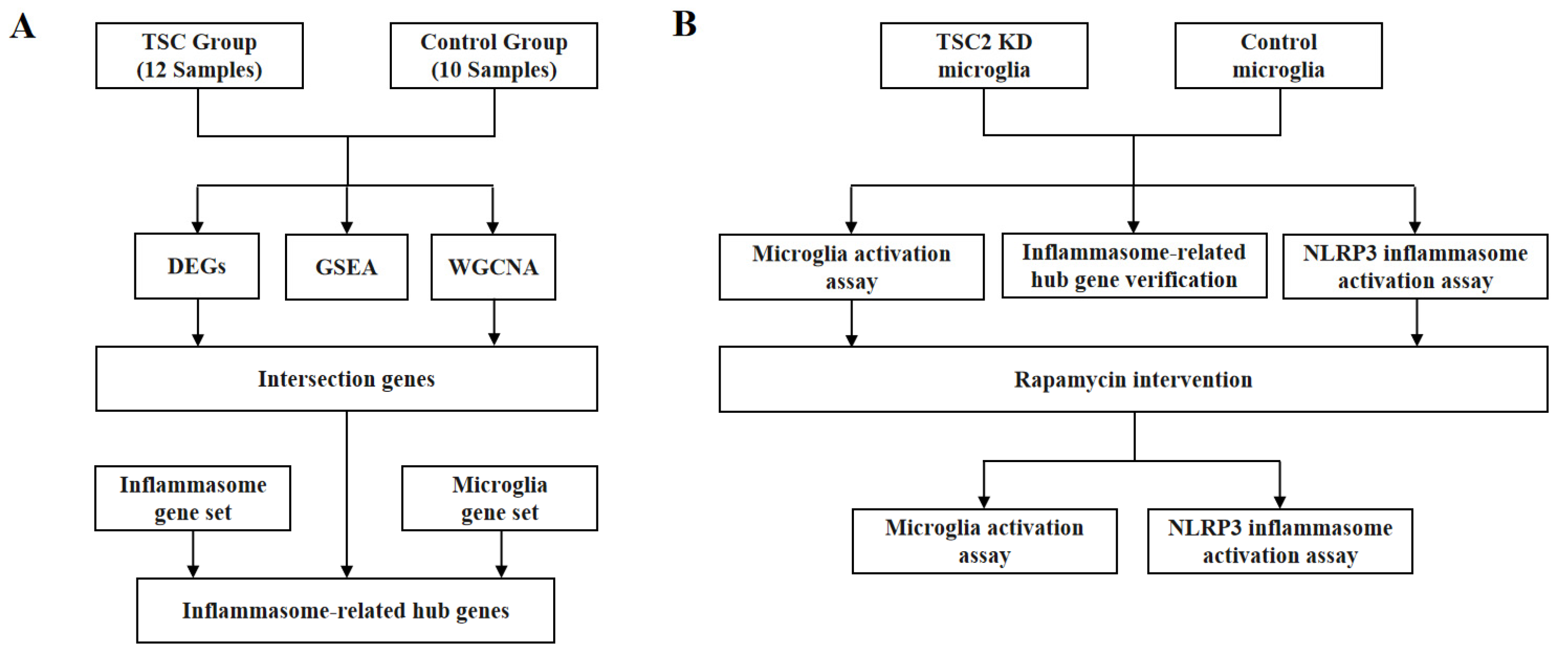
2.1. General Exploration of the Dataset
2.2. Gene Set Enrichment Analysis
2.3. Weighted Gene Co-Expression Network Analysis
2.4. Functional Analysis and Inflammasome-Related Hub Gene Screening
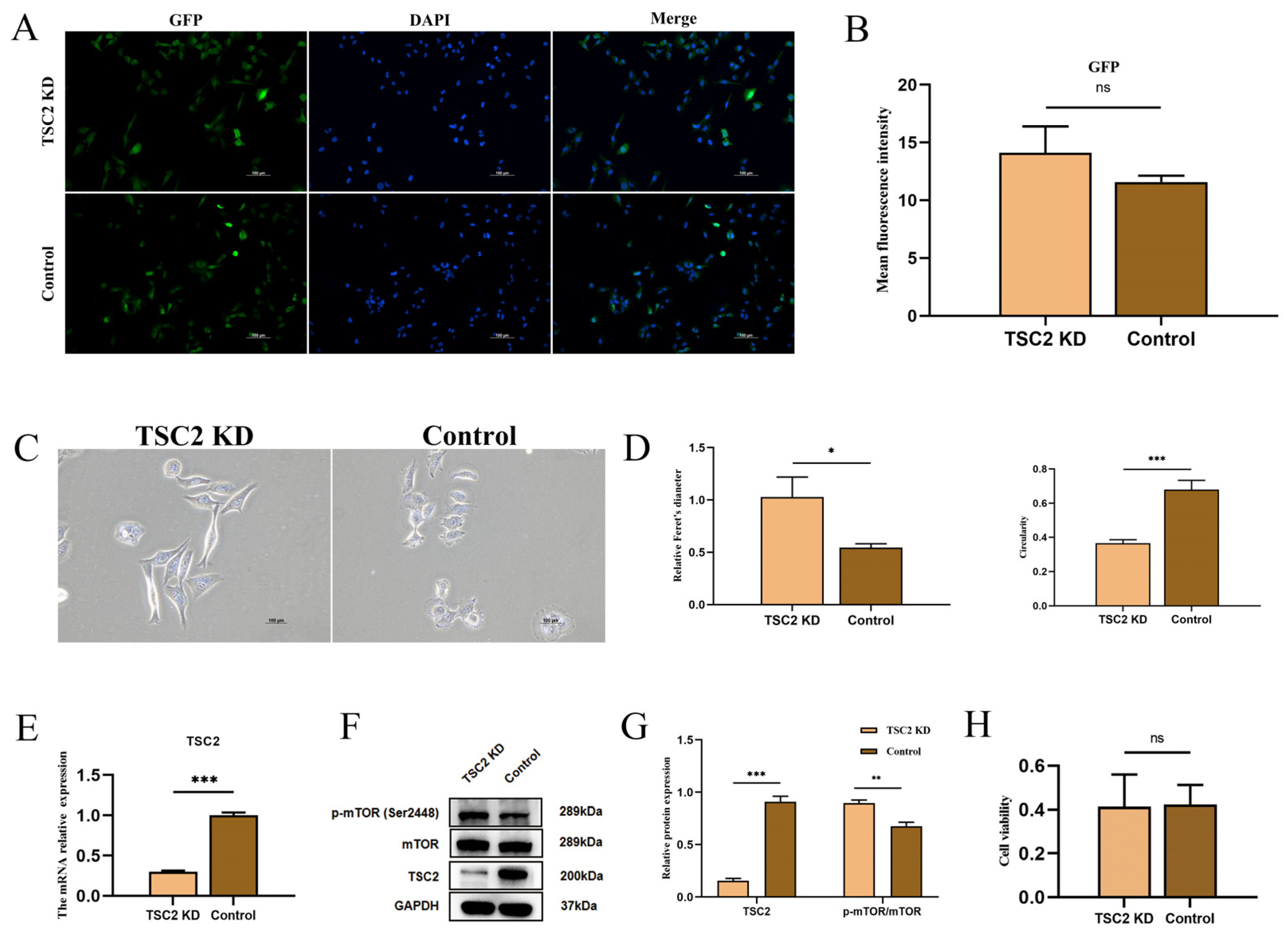
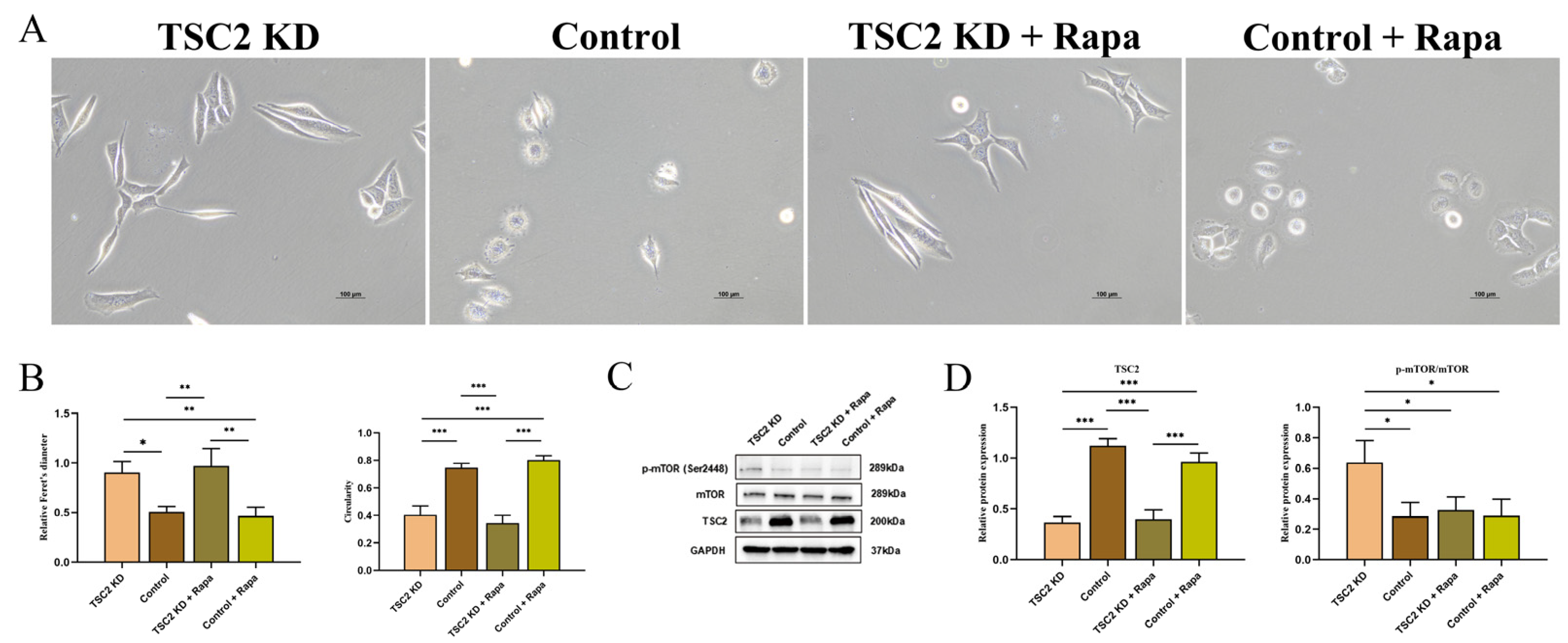
2.5. Establishment of a TSC2 Knockdown Microglial Model
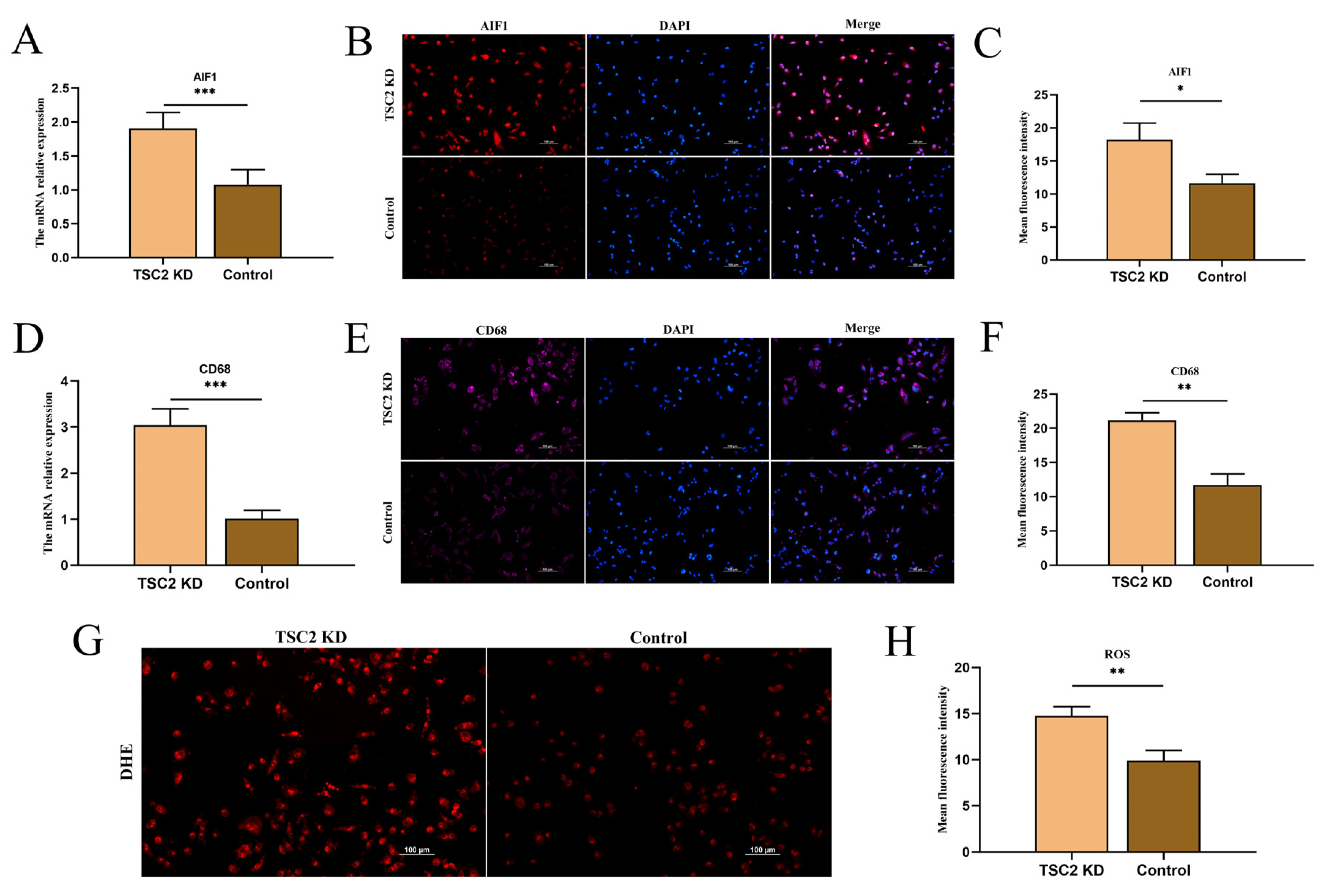
2.6. Microglial Activation Assay
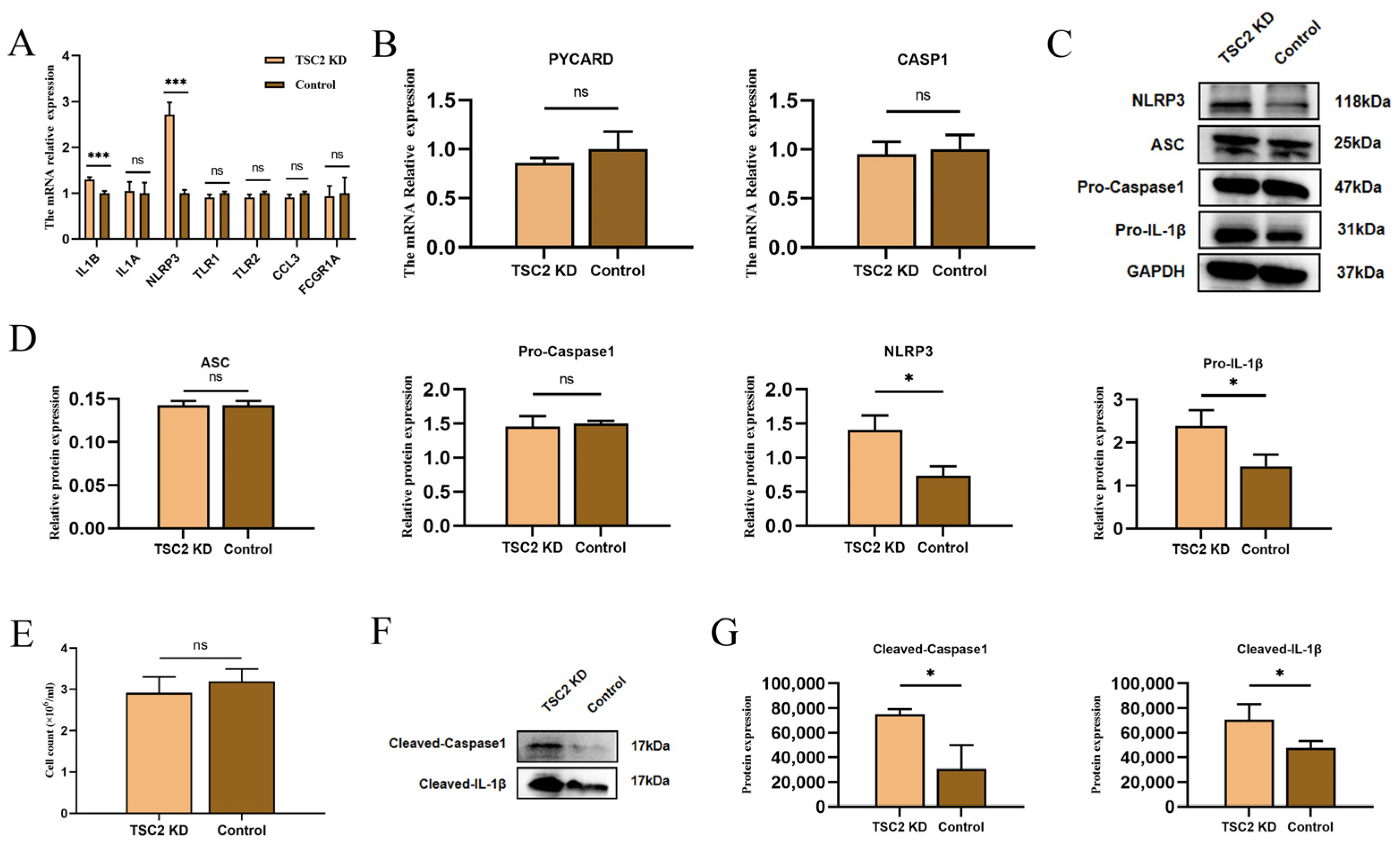
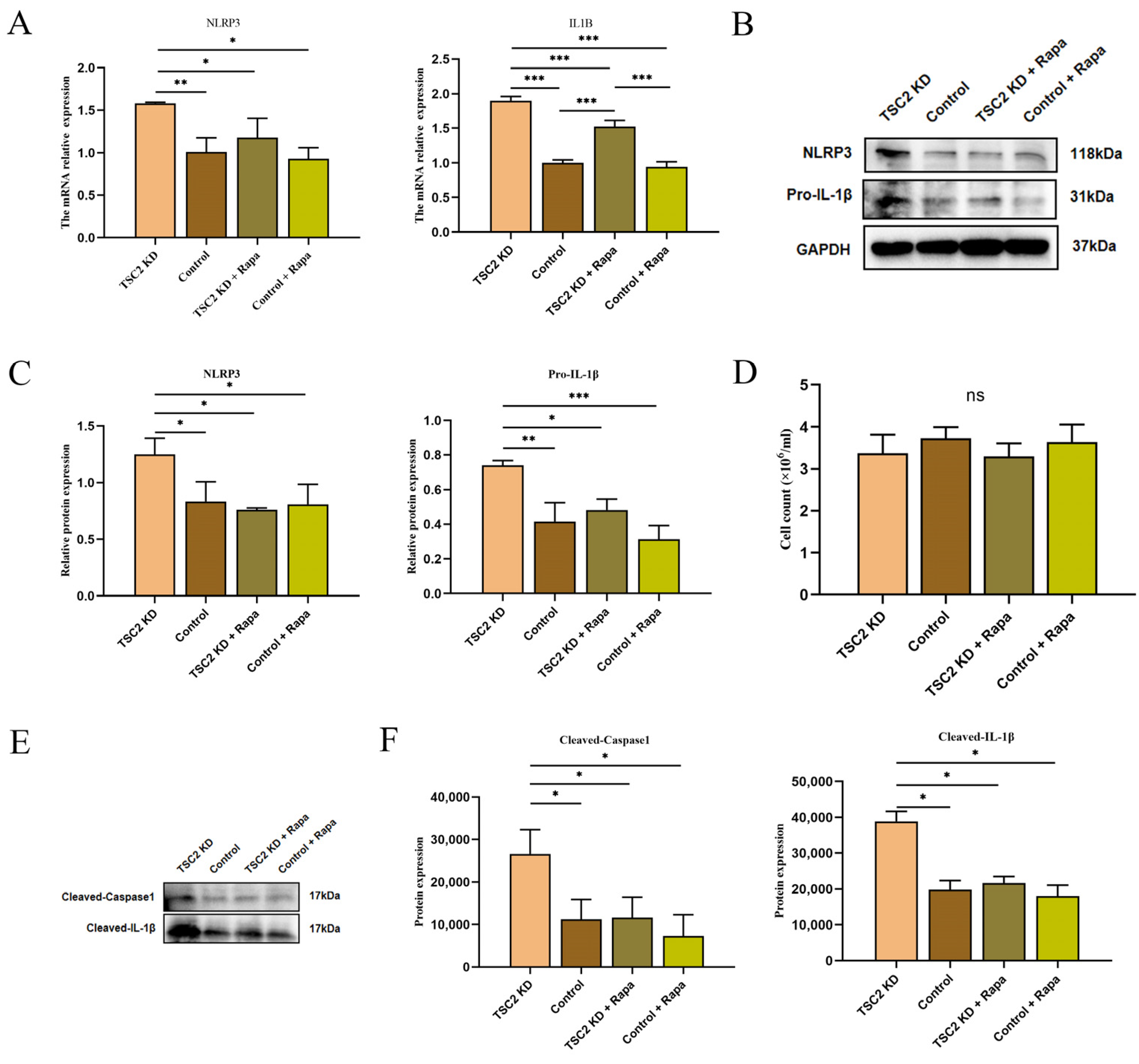
2.7. Validation of Inflammasome-Related Hub Genes and NLRP3 Inflammasome Activation
2.8. Rapamycin Intervention
2.9. Effect of Rapamycin Intervention on Microglial Activation
2.10. Effect of Rapamycin Intervention on the Activation of the NLRP3 Inflammasome in Microglia
3. Discussion
4. Materials and Methods
4.1. Downloading and Processing of the Transcriptome Dataset
4.2. Gene Set Enrichment Analysis
4.3. Weighted Gene Co-Expression Network Analysis
4.4. Intersecting Gene Identification
4.5. Construction of the Protein–Protein Interaction Network and Identification of Inflammasome-Related Hub Genes
4.6. Functional Enrichment Analysis
4.7. Microglial Culture and Construction of the TSC2 Gene Knockdown Model
4.8. Rapamycin Intervention
4.9. Cell Viability Test
4.10. Cell Morphological Analysis
4.11. Real-Time Quantitative Polymerase Chain Reaction
4.12. Western Blot
4.13. Cell Immunofluorescence Staining
4.14. Reactive Oxygen Species Detection
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TSC | Tuberous sclerosis complex |
| IL-1β | Interleukin-1β |
| KD | Knockdown |
| ROS | Reactive oxygen species |
| mTOR | Mammalian target of rapamycin (mTOR) |
| NLRP3 | NOD-like receptor protein 3 |
| ASC | Apoptosis-associated speck-like protein containing a CARD |
| Pro-Caspase 1 | Precursor cysteinyl aspartate-specific proteinase 1 |
| CKO | Conditional knockout |
| Rapa | Rapamycin |
| DEGs | Differentially expressed genes |
| GSEA | Gene set enrichment analysis |
| NES | Normalized enrichment score |
| FC | Fold change |
| WGCNA | Weighted gene co-expression network analysis |
| GAMNIs | Genes associated with microglial NLRP3 inflammasome |
| PPI | Protein–protein interaction |
| GO | Gene Ontology |
| MF | Molecular Function |
| CC | Cellular Component |
| BP | Biological Process |
| HMC3 | Human microglia cell line |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| PBS | Phosphate-buffered saline |
| DHE | Dihydroethidium |
References
- O’Callaghan, F.J.; Shiell, A.W.; Osborne, J.P.; Martyn, C.N. Prevalence of tuberous sclerosis estimated by capture-recapture analysis. Lancet 1998, 351, 1490. [Google Scholar] [CrossRef]
- Meng, Y.; Yu, C.; Chen, M.; Yu, X.; Sun, M.; Yan, H.; Zhao, W.; Yu, S. Mutation landscape of TSC1/TSC2 in Chinese patients with tuberous sclerosis complex. J. Hum. Genet. 2021, 66, 227–236. [Google Scholar] [CrossRef]
- Henske, E.P.; Jóźwiak, S.; Kingswood, J.C.; Sampson, J.R.; Thiele, E.A. Tuberous sclerosis complex. Nat. Rev. Dis. Primer. 2016, 2, 16035. [Google Scholar] [CrossRef]
- Mühlebner, A.; Bongaarts, A.; Sarnat, H.B.; Scholl, T.; Aronica, E. New insights into a spectrum of developmental malformations related to mTOR dysregulations: Challenges and perspectives. J. Anat. 2019, 235, 521–542. [Google Scholar] [CrossRef]
- Guvenc, G.; Kizmazoglu, C.; Sayin, M.; Ture, H.S. Surgically Treated Status Epilepticus due to Large Cortical Tuber and Long-Term Follow-Up Results. Turk. Neurosurg. 2020, 30, 458–461. [Google Scholar] [CrossRef]
- Gruber, V.E.; Luinenburg, M.J.; Colleselli, K.; Endmayr, V.; Anink, J.J.; Zimmer, T.S.; Jansen, F.; Gosselaar, P.; Coras, R.; Scholl, T.; et al. Increased expression of complement components in tuberous sclerosis complex and focal cortical dysplasia type 2B brain lesions. Epilepsia 2022, 63, 364–374. [Google Scholar] [CrossRef]
- Zhang, B.; Zou, J.; Han, L.; Rensing, N.; Wong, M. Microglial activation during epileptogenesis in a mouse model of tuberous sclerosis complex. Epilepsia 2016, 57, 1317–1325. [Google Scholar] [CrossRef]
- van Scheppingen, J.; Mills, J.D.; Zimmer, T.S.; Broekaart, D.W.M.; Iori, V.; Bongaarts, A.; Anink, J.J.; Iyer, A.M.; Korotkov, A.; Jansen, F.E.; et al. miR147b: A novel key regulator of interleukin 1 beta-mediated inflammation in human astrocytes. Glia 2018, 66, 1082–1097. [Google Scholar] [CrossRef]
- Broekaart, D.W.M.; van Scheppingen, J.; Anink, J.J.; Wierts, L.; van Het Hof, B.; Jansen, F.E.; Spliet, W.G.; van Rijen, P.C.; Kamphuis, W.W.; de Vries, H.E.; et al. Increased matrix metalloproteinases expression in tuberous sclerosis complex: Modulation by microRNA 146a and 147b in vitro. Neuropathol. Appl. Neurobiol. 2020, 46, 142–159. [Google Scholar] [CrossRef]
- Zhang, B.; Zou, J.; Rensing, N.R.; Yang, M.; Wong, M. Inflammatory mechanisms contribute to the neurological manifestations of tuberous sclerosis complex. Neurobiol. Dis. 2015, 80, 70–79. [Google Scholar] [CrossRef]
- Zhang, B.; Qiao, J.; Li, Q.; Luan, G.; Qin, J. Astrocyte-derived complement C3 is activated in patients with tuberous sclerosis complex and mediates immune injury: An integrated bioinformatics analysis. Transl. Pediatr. 2023, 12, 1098–1109. [Google Scholar] [CrossRef]
- Kagitani-Shimono, K.; Kato, H.; Soeda, F.; Iwatani, Y.; Mukai, M.; Ogawa, K.; Tominaga, K.; Nabatame, S.; Taniike, M. Extension of microglial activation is associated with epilepsy and cognitive dysfunction in Tuberous sclerosis complex: A TSPO-PET study. NeuroImage Clin. 2023, 37, 103288. [Google Scholar] [CrossRef]
- Boer, K.; Jansen, F.; Nellist, M.; Redeker, S.; van den Ouweland, A.M.; Spliet, W.G.; van Nieuwenhuizen, O.; Troost, D.; Crino, P.B.; Aronica, E. Inflammatory processes in cortical tubers and subependymal giant cell tumors of tuberous sclerosis complex. Epilepsy Res. 2008, 78, 7–21. [Google Scholar] [CrossRef]
- Arena, A.; Zimmer, T.S.; van Scheppingen, J.; Korotkov, A.; Anink, J.J.; Mühlebner, A.; Jansen, F.E.; van Hecke, W.; Spliet, W.G.; van Rijen, P.C.; et al. Oxidative stress and inflammation in a spectrum of epileptogenic cortical malformations: Molecular insights into their interdependence. Brain Pathol. 2019, 29, 351–365. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. Shared principles in NF-kappaB signaling. Cell 2008, 132, 344–362. [Google Scholar] [CrossRef]
- Fuso, A.; Iyer, A.M.; van Scheppingen, J.; Maccarrone, M.; Scholl, T.; Hainfellner, J.A.; Feucht, M.; Jansen, F.E.; Spliet, W.G.; Krsek, P.; et al. Promoter-Specific Hypomethylation Correlates with IL-1β Overexpression in Tuberous Sclerosis Complex (TSC). J. Mol. Neurosci. 2016, 59, 464–470. [Google Scholar] [CrossRef]
- Lin, W.; Li, Z.; Liang, G.; Zhou, R.; Zheng, X.; Tao, R.; Huo, Q.; Su, C.; Li, M.; Xu, N.; et al. TNEA therapy promotes the autophagic degradation of NLRP3 inflammasome in a transgenic mouse model of Alzheimer’s disease via TFEB/TFE3 activation. J. Neuroinflamm. 2023, 20, 21. [Google Scholar] [CrossRef]
- Oh, Y.C.; Jeong, Y.H.; Yang, H.J.; Li, W.; Ma, J.Y. Lumbricus Extract Prevents LPS-Induced Inflammatory Activation of BV2 Microglia and Glutamate-Induced Hippocampal HT22 Cell Death by Suppressing MAPK/NF-κB/NLRP3 Signaling and Oxidative Stress. Curr. Issues Mol. Biol. 2023, 45, 9926–9942. [Google Scholar] [CrossRef]
- Chou, V.; Pearse, R.V., 2nd; Aylward, A.J.; Ashour, N.; Taga, M.; Terzioglu, G.; Fujita, M.; Fancher, S.B.; Sigalov, A.; Benoit, C.R.; et al. INPP5D regulates inflammasome activation in human microglia. Nat. Commun. 2023, 14, 7552. [Google Scholar] [CrossRef]
- MacKeigan, J.P.; Krueger, D.A. Differentiating the mTOR inhibitors everolimus and sirolimus in the treatment of tuberous sclerosis complex. Neuro-Oncol. 2015, 17, 1550–1559. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, D.; Wu, X.; Yue, F.; Yang, H.; Hu, K. Rapamycin ameliorates chronic intermittent hypoxia and sleep deprivation-induced renal damage via the mammalian target of rapamycin (mTOR)/NOD-like receptor protein 3 (NLRP3) signaling pathway. Bioengineered 2022, 13, 5537–5550. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, J.; Xu, Q.; Long, T.; Bi, F.; Chang, C.; Liu, W. Rapamycin improves the neuroprotection effect of inhibition of NLRP3 inflammasome activation after TBI. Brain Res. 2019, 1710, 163–172. [Google Scholar] [CrossRef]
- Aghaie, F.; Moradifar, F.; Hosseini, A. Rapamycin attenuates depression and anxiety-like behaviors through modulation of the NLRP3 pathway in pentylenetetrazole-kindled male Wistar rats. Fundam. Clin. Pharmacol. 2021, 35, 1045–1054. [Google Scholar] [CrossRef]
- Lin, Y.; Cheng, L.; Chen, Y.; Li, W.; Guo, Q.; Miao, Y. TFEB signaling promotes autophagic degradation of NLRP3 to attenuate neuroinflammation in diabetic encephalopathy. Am. J. Physiol. Cell Physiol. 2024, 327, C1481–C1496. [Google Scholar] [CrossRef]
- Gui, J.; Liu, J.; Wang, L.; Yang, X.; Tian, B.; Luo, H.; Huang, D.; Han, Z.; Yang, J.; Ding, R.; et al. Autophagy alleviates hippocampal neuroinflammation by inhibiting the NLRP3 inflammasome in a juvenile rat model exposed particulate matter. Toxicology 2024, 502, 153730. [Google Scholar] [CrossRef]
- Rai, M.A.; Hammonds, J.; Pujato, M.; Mayhew, C.; Roskin, K.; Spearman, P. Comparative analysis of human microglial models for studies of HIV replication and pathogenesis. Retrovirology 2020, 17, 35. [Google Scholar] [CrossRef]
- Etemad, S.; Zamin, R.M.; Ruitenberg, M.J.; Filgueira, L. A novel in vitro human microglia model: Characterization of human monocyte-derived microglia. J. Neurosci. Methods 2012, 209, 79–89. [Google Scholar] [CrossRef]
- Zimmer, T.S.; Korotkov, A.; Zwakenberg, S.; Jansen, F.E.; Zwartkruis, F.J.T.; Rensing, N.R.; Wong, M.; Mühlebner, A.; van Vliet, E.A.; Aronica, E.; et al. Upregulation of the pathogenic transcription factor SPI1/PU.1 in tuberous sclerosis complex and focal cortical dysplasia by oxidative stress. Brain Pathol. 2021, 31, e12949. [Google Scholar] [CrossRef]
- Wong, M. The role of glia in epilepsy, intellectual disability, and other neurodevelopmental disorders in tuberous sclerosis complex. J. Neurodev. Disord. 2019, 11, 30. [Google Scholar] [CrossRef]
- Zimmer, T.S.; Broekaart, D.W.M.; Gruber, V.E.; van Vliet, E.A.; Mühlebner, A.; Aronica, E. Tuberous Sclerosis Complex as Disease Model for Investigating mTOR-Related Gliopathy During Epileptogenesis. Front. Neurol. 2020, 11, 1028. [Google Scholar] [CrossRef]
- Boer, K.; Troost, D.; Jansen, F.; Nellist, M.; van den Ouweland, A.M.; Geurts, J.J.; Spliet, W.G.; Crino, P.; Aronica, E. Clinicopathological and immunohistochemical findings in an autopsy case of tuberous sclerosis complex. Neuropathology 2008, 28, 577–590. [Google Scholar] [CrossRef]
- Zhang, B.; Zou, J.; Han, L.; Beeler, B.; Friedman, J.L.; Griffin, E.; Piao, Y.S.; Rensing, N.R.; Wong, M. The specificity and role of microglia in epileptogenesis in mouse models of tuberous sclerosis complex. Epilepsia 2018, 59, 1796–1806. [Google Scholar] [CrossRef]
- Zhao, X.; Liao, Y.; Morgan, S.; Mathur, R.; Feustel, P.; Mazurkiewicz, J.; Qian, J.; Chang, J.; Mathern, G.W.; Adamo, M.A.; et al. Noninflammatory Changes of Microglia Are Sufficient to Cause Epilepsy. Cell Rep. 2018, 22, 2080–2093. [Google Scholar] [CrossRef]
- Sun, F.J.; Zhang, C.Q.; Chen, X.; Wei, Y.J.; Li, S.; Liu, S.Y.; Zang, Z.L.; He, J.J.; Guo, W.; Yang, H. Downregulation of CD47 and CD200 in patients with focal cortical dysplasia type IIb and tuberous sclerosis complex. J. Neuroinflamm. 2016, 13, 85. [Google Scholar] [CrossRef]
- Takanezawa, Y.; Tanabe, S.; Kato, D.; Ozeki, R.; Komoda, M.; Suzuki, T.; Baba, H.; Muramatsu, R. Microglial ASD-related genes are involved in oligodendrocyte differentiation. Sci. Rep. 2021, 11, 17825. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, K.; Yang, X.; Shen, K.; Yang, L.; Ruan, R.; Shi, X.; Wang, M.; Zhu, G.; Yang, M.; et al. Downregulated GPR30 expression in the epileptogenic foci of female patients with focal cortical dysplasia type IIb and tuberous sclerosis complex is correlated with (18) F-FDG PET-CT values. Brain Pathol. 2021, 31, 346–364. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Li, M.; Ju, Y.; Tang, J.; Chen, T.; Lin, X.; Gu, N.; Yang, F. Neuroinflammation catching nanobubbles for microglia-neuron unit modulation against epilepsy. Biomaterials 2023, 302, 122302. [Google Scholar] [CrossRef]
- Hughes, H.K.; Moreno, R.J.; Ashwood, P. Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain Behav. Immun. 2023, 108, 245–254. [Google Scholar] [CrossRef]
- Guttikonda, S.R.; Sikkema, L.; Tchieu, J.; Saurat, N.; Walsh, R.M.; Harschnitz, O.; Ciceri, G.; Sneeboer, M.; Mazutis, L.; Setty, M.; et al. Fully defined human pluripotent stem cell-derived microglia and tri-culture system model C3 production in Alzheimer’s disease. Nat. Neurosci. 2021, 24, 343–354. [Google Scholar] [CrossRef]
- Waltl, I.; Kalinke, U. Beneficial and detrimental functions of microglia during viral encephalitis. Trends Neurosci. 2022, 45, 158–170. [Google Scholar] [CrossRef]
- Shapiro, L.A.; Wang, L.; Ribak, C.E. Rapid astrocyte and microglial activation following pilocarpine-induced seizures in rats. Epilepsia 2008, 49 (Suppl. 2), 33–41. [Google Scholar] [CrossRef]
- Koike-Kumagai, M.; Fujimoto, M.; Wataya-Kaneda, M. Sirolimus relieves seizures and neuropsychiatric symptoms via changes of microglial polarity in tuberous sclerosis complex model mice. Neuropharmacology 2022, 218, 109203. [Google Scholar] [CrossRef]
- Hu, S.; Chen, Y.; Huang, S.; Liu, M.; Liu, Y.; Huang, S. Sodium Danshensu protects against oxygen glucose deprivation/reoxygenation-induced astrocytes injury through regulating NOD-like receptor pyrin domain containing 3 (NLRP3) inflammasome and tuberous sclerosis complex-2 (TSC2)/mammalian target of rapamycin (mTOR) pathways. Ann. Transl. Med. 2022, 10, 1097. [Google Scholar]
- Ghaffari, N.; Mokhtari, T.; Adabi, M.; Ebrahimi, B.; Kamali, M.; Gholaminejhad, M.; Hassanzadeh, G. Neurological recovery and neurogenesis by curcumin sustained-release system cross-linked with an acellular spinal cord scaffold in rat spinal cord injury: Targeting NLRP3 inflammasome pathway. Phytother. Res. PTR 2024, 38, 2669–2686. [Google Scholar] [CrossRef]
- Ge, P.; Duan, H.; Tao, C.; Niu, S.; Hu, Y.; Duan, R.; Shen, A.; Sun, Y.; Sun, W. TMAO Promotes NLRP3 Inflammasome Activation of Microglia Aggravating Neurological Injury in Ischemic Stroke Through FTO/IGF2BP2. J. Inflamm. Res. 2023, 16, 3699–3714. [Google Scholar] [CrossRef]
- Hou, B.; Yin, J.; Liu, S.; Guo, J.; Zhang, B.; Zhang, Z.; Yang, L.; Tan, X.; Long, Y.; Feng, S.; et al. Inhibiting the NLRP3 Inflammasome with MCC950 Alleviates Neurological Impairment in the Brain of EAE Mice. Mol. Neurobiol. 2024, 61, 1318–1330. [Google Scholar] [CrossRef]
- Mahawar, S.; Rakshit, D.; Patel, I.; Gore, S.K.; Sen, S.; Ranjan, O.P.; Mishra, A. Fisetin-loaded chitosan nanoparticles ameliorate pilocarpine-induced temporal lobe epilepsy and associated neurobehavioral alterations in mice: Role of ROS/TNF-α-NLRP3 inflammasomes pathway. Nanomed 2024, 59, 102752. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, X.; Cui, X.; Su, S.; Li, L.; Chen, Y.; Wang, N.; Sun, L.; Zhao, J.; Zhang, J.; et al. Inhibiting the IRAK4/NF-κB/NLRP3 signaling pathway can reduce pyroptosis in hippocampal neurons and seizure episodes in epilepsy. Exp. Neurol. 2024, 377, 114794. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Y.; Liu, N.; Hai, D.; Wei, W.; Liu, Y.; Lan, X.; Jin, X.; Yu, J.; Ma, L. Mechanism of NLRP3 Inflammasome in Epilepsy and Related Therapeutic Agents. Neuroscience 2024, 546, 157–177. [Google Scholar] [CrossRef]
- Su, L.; Guo, P.; Guo, X.; He, Z.; Zhao, Y.; Zong, Y.; Li, J.; Chen, W.; Du, R. Paeoniflorin alleviates depression by inhibiting the activation of NLRP3 inflammasome via promoting mitochondrial autophagy. Chin. J. Nat. Med. 2024, 22, 515–529. [Google Scholar] [CrossRef]
- Mills, J.D.; Iyer, A.M.; van Scheppingen, J.; Bongaarts, A.; Anink, J.J.; Janssen, B.; Zimmer, T.S.; Spliet, W.G.; van Rijen, P.C.; Jansen, F.E.; et al. Coding and small non-coding transcriptional landscape of tuberous sclerosis complex cortical tubers: Implications for pathophysiology and treatment. Sci. Rep. 2017, 7, 8089, Erratum in Sci. Rep. 2022, 12, 15457. [Google Scholar] [CrossRef]


| NO. | 5′ | STEM | LOOP | STEM | 3′ |
|---|---|---|---|---|---|
| TSC2-RNAi (16136-1)-a | Ccgg | ACGAGTCAAACAAGCCAAT | CTCGAG | ATTGGCTTGTTTGACTCGT | TTTTTg |
| TSC2-RNAi (16136-1)-b | aattcaaaaa | ACGAGTCAAACAAGCCAAT | CTCGAG | ATTGGCTTGTTTGACTCGT | |
| GV112-NC-1 | CCGG | TTCTCCGAACGTGTCACGT | TTCAAGAGA | ACGTGACACGTTCGGAGAA | TTTTTG |
| GV112-NC-2 | AATTCAAAAA | TTCTCCGAACGTGTCACGT | TCTCTTGAA | ACGTGACACGTTCGGAGAA |
| Primer Name | Sequence (5′ → 3′) | |
|---|---|---|
| IL1B | Forward | GACCTGGACCTCTGCCCTCTG |
| Reverse | GCCTGCCTGAAGCCCTTGC | |
| IL1A | Forward | GACCAACCAGTGCTGCTGAAGG |
| Reverse | GCCGTGAGTTTCCCAGAAGAAGAG | |
| NLRP3 | Forward | CCTGGTCTGCTGGATCGTGTG |
| Reverse | CGGTGGTGGTCTTGGATGTCTG | |
| AIF1 | Forward | AGGATGATGCTGGGCAAGAGATC |
| Reverse | TCAGGGCAACTCAGAGATAGCTTTC | |
| TLR1 | Forward | CAATGCTGCTGTTCAGCTCTTCT |
| Reverse | GGTGCCCAATATGCCTTTGTTATCC | |
| TLR2 | Forward | CTACCAGATGCCTCCCTCTTACCC |
| Reverse | TGCCACCAGCTTCCAAAGTCTTC | |
| CCL3 | Forward | TCTGCAACCAGTTCTCTGCATC |
| Reverse | TGCTCGTCTCAAAGTAGTCAGC | |
| FCGR1A | Forward | CTCCTTCTACATGGGCAGCAAGAC |
| Reverse | GCAGCCTCGCACCAGTATAACC | |
| TSC2 | Forward | CAGACAATGGGAGACACATCACCTAC |
| Reverse | CCAAGTTCACCAGCACCAGAAGG | |
| CASP1 | Forward | CACACCGCCCAGAGCACAAG |
| Reverse | TCCCACAAATGCCTTCCCGAATAC | |
| PYCARD | Forward | GCCCACCAACCCAAGCAAGATG |
| Reverse | CTCCGCTCCAGGTCCTCCAC | |
| GAPDH | Forward | GCACCGTCAAGGCTGAGAAC |
| Reverse | TGGTGAAGACGCCAGTGGA | |
| CD68 | Forward | CTTCTCTCATTCCCCTATGGACA |
| Reverse | GAAGGACACATTGTACTCCACC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, R.; Zhang, S.; Meng, L.; Wang, L.; Han, Z.; Gui, J.; Yang, J.; Cheng, L.; Xie, L.; Jiang, L. The Activation of the Microglial NLRP3 Inflammasome Is Involved in Tuberous Sclerosis Complex-Related Neuroinflammation. Int. J. Mol. Sci. 2025, 26, 7244. https://doi.org/10.3390/ijms26157244
Ding R, Zhang S, Meng L, Wang L, Han Z, Gui J, Yang J, Cheng L, Xie L, Jiang L. The Activation of the Microglial NLRP3 Inflammasome Is Involved in Tuberous Sclerosis Complex-Related Neuroinflammation. International Journal of Molecular Sciences. 2025; 26(15):7244. https://doi.org/10.3390/ijms26157244
Chicago/Turabian StyleDing, Ran, Shengxuan Zhang, Linxue Meng, Lingman Wang, Ziyao Han, Jianxiong Gui, Jiaxin Yang, Li Cheng, Lingling Xie, and Li Jiang. 2025. "The Activation of the Microglial NLRP3 Inflammasome Is Involved in Tuberous Sclerosis Complex-Related Neuroinflammation" International Journal of Molecular Sciences 26, no. 15: 7244. https://doi.org/10.3390/ijms26157244
APA StyleDing, R., Zhang, S., Meng, L., Wang, L., Han, Z., Gui, J., Yang, J., Cheng, L., Xie, L., & Jiang, L. (2025). The Activation of the Microglial NLRP3 Inflammasome Is Involved in Tuberous Sclerosis Complex-Related Neuroinflammation. International Journal of Molecular Sciences, 26(15), 7244. https://doi.org/10.3390/ijms26157244






