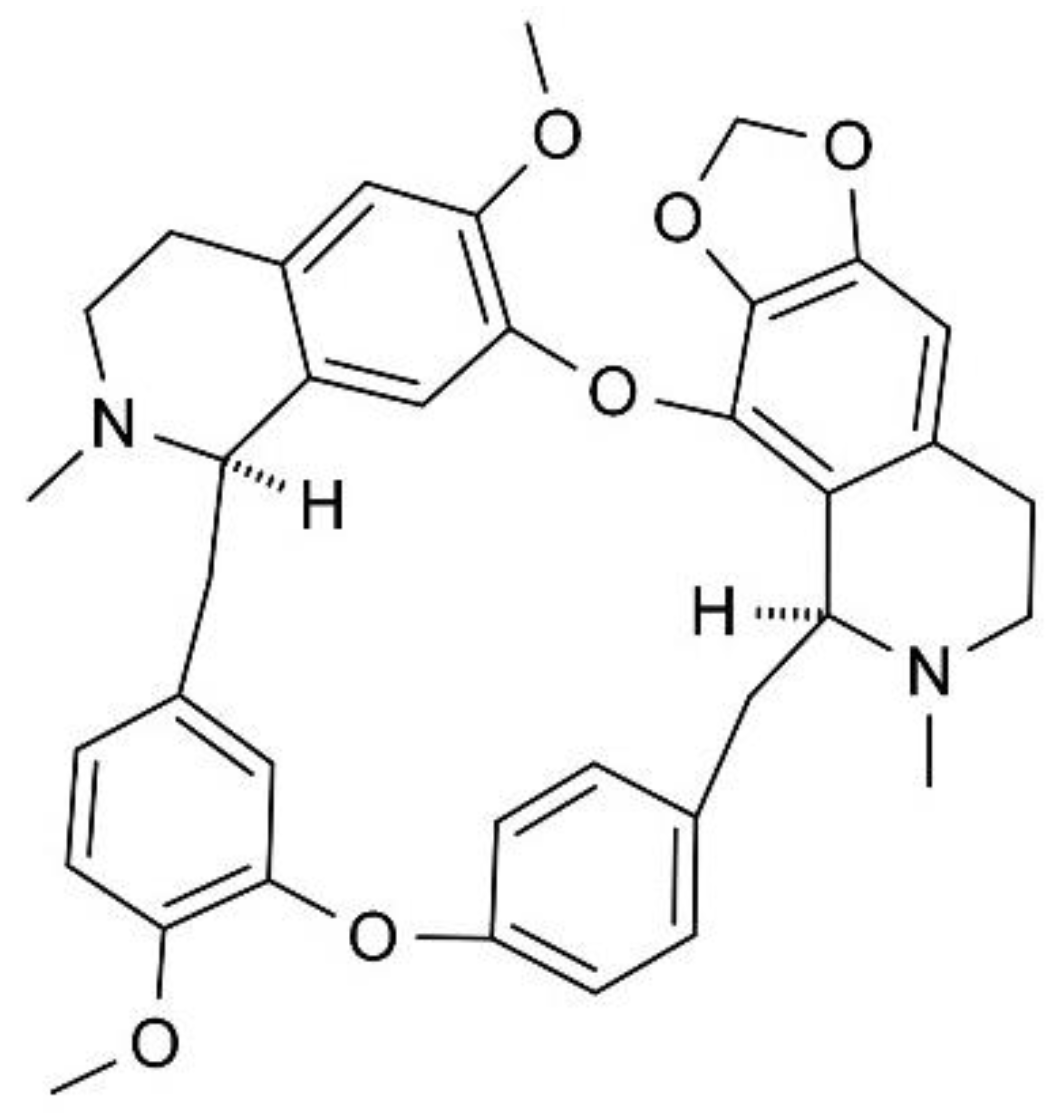
Cepharanthine Promotes Ca2+-Independent Premature Red Blood Cell Death Through Metabolic Insufficiency and p38 MAPK/CK1α/COX/MLKL/PKC/iNOS Signaling
Abstract
1. Introduction
2. Results
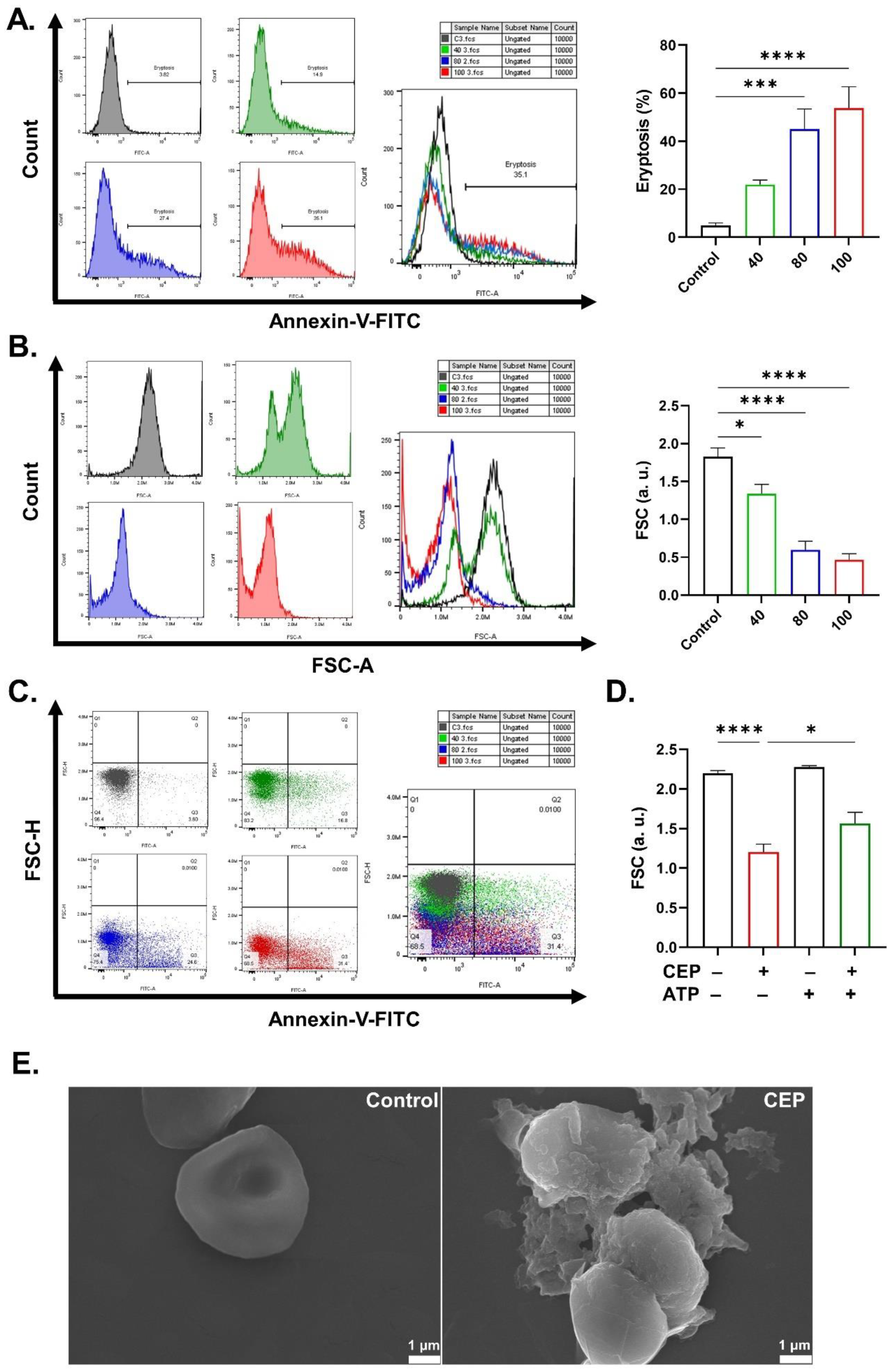
2.1. CEP Promotes Eryptosis
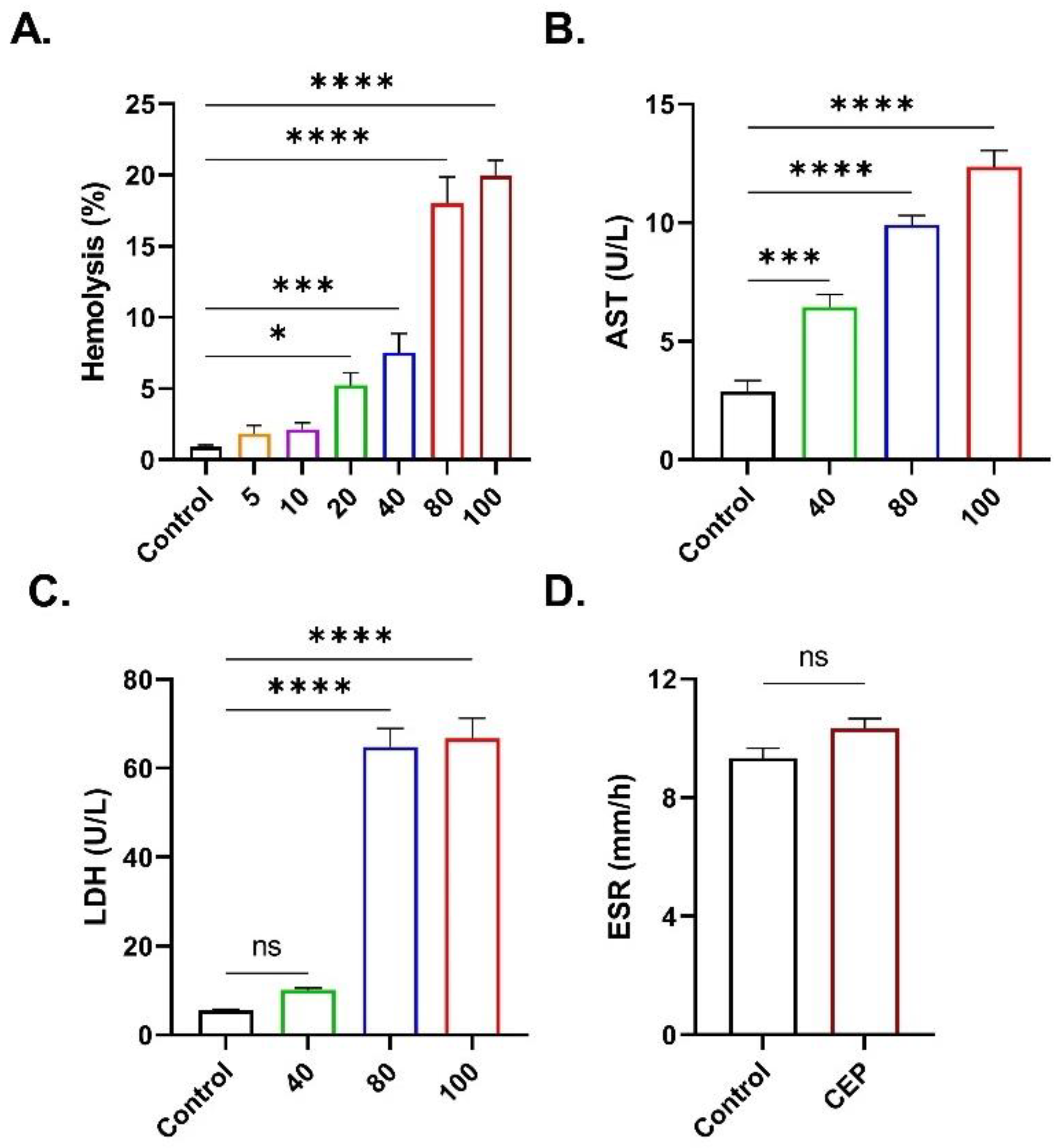
2.2. CEP Promotes Hemolysis
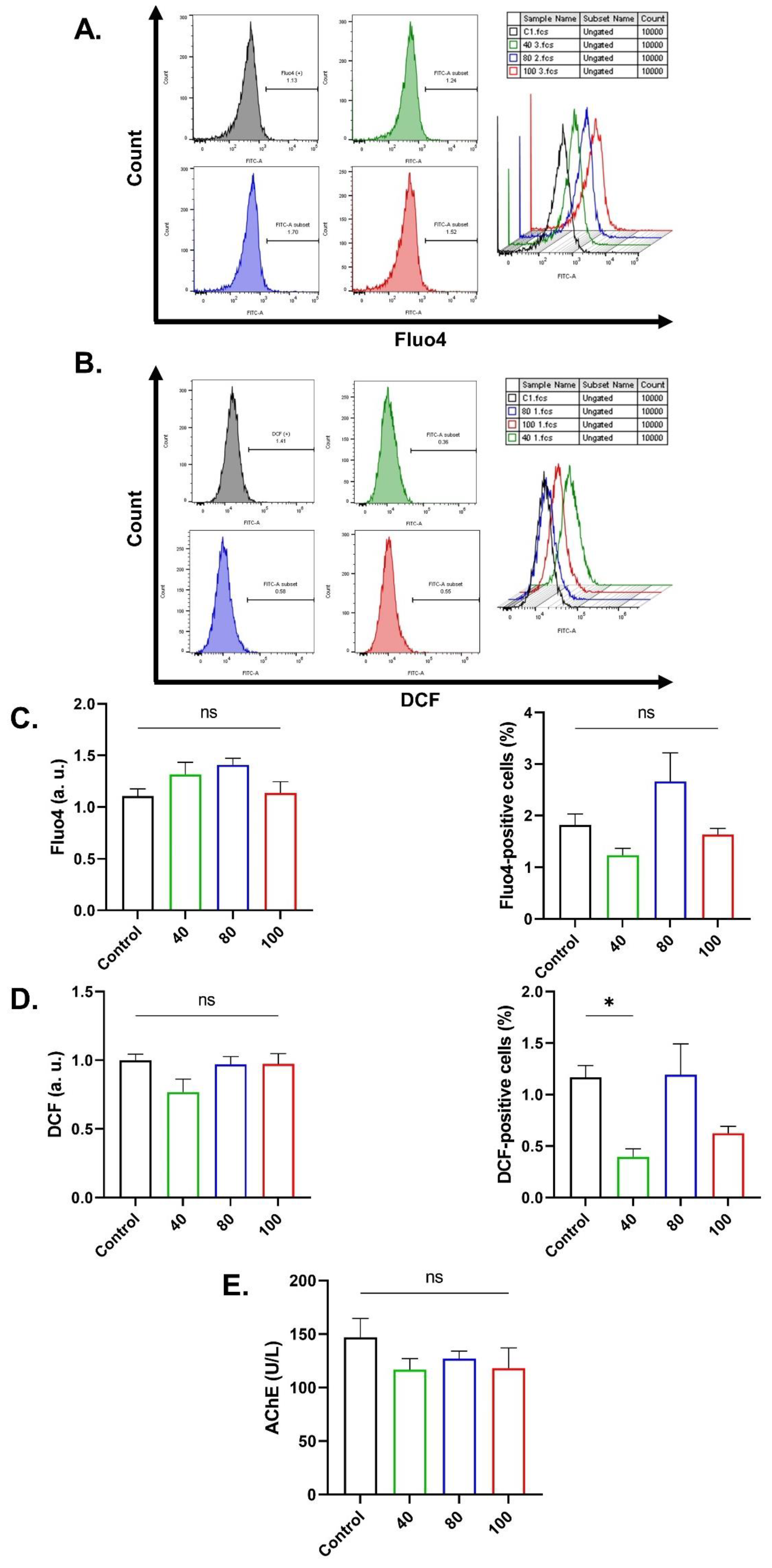
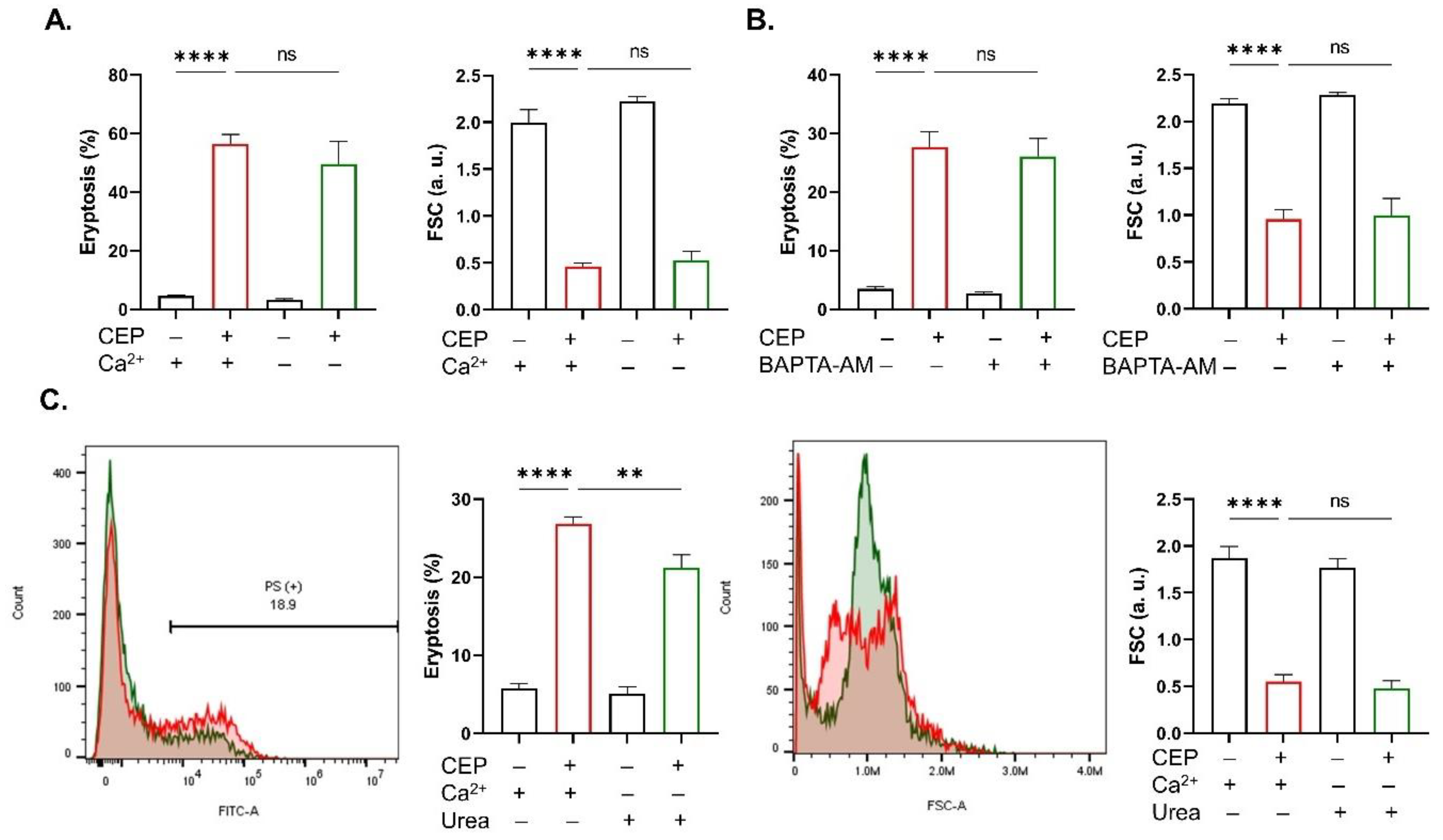
2.3. CEP Cytotoxicity Is Independent of Ca2+ or Oxidative Stress
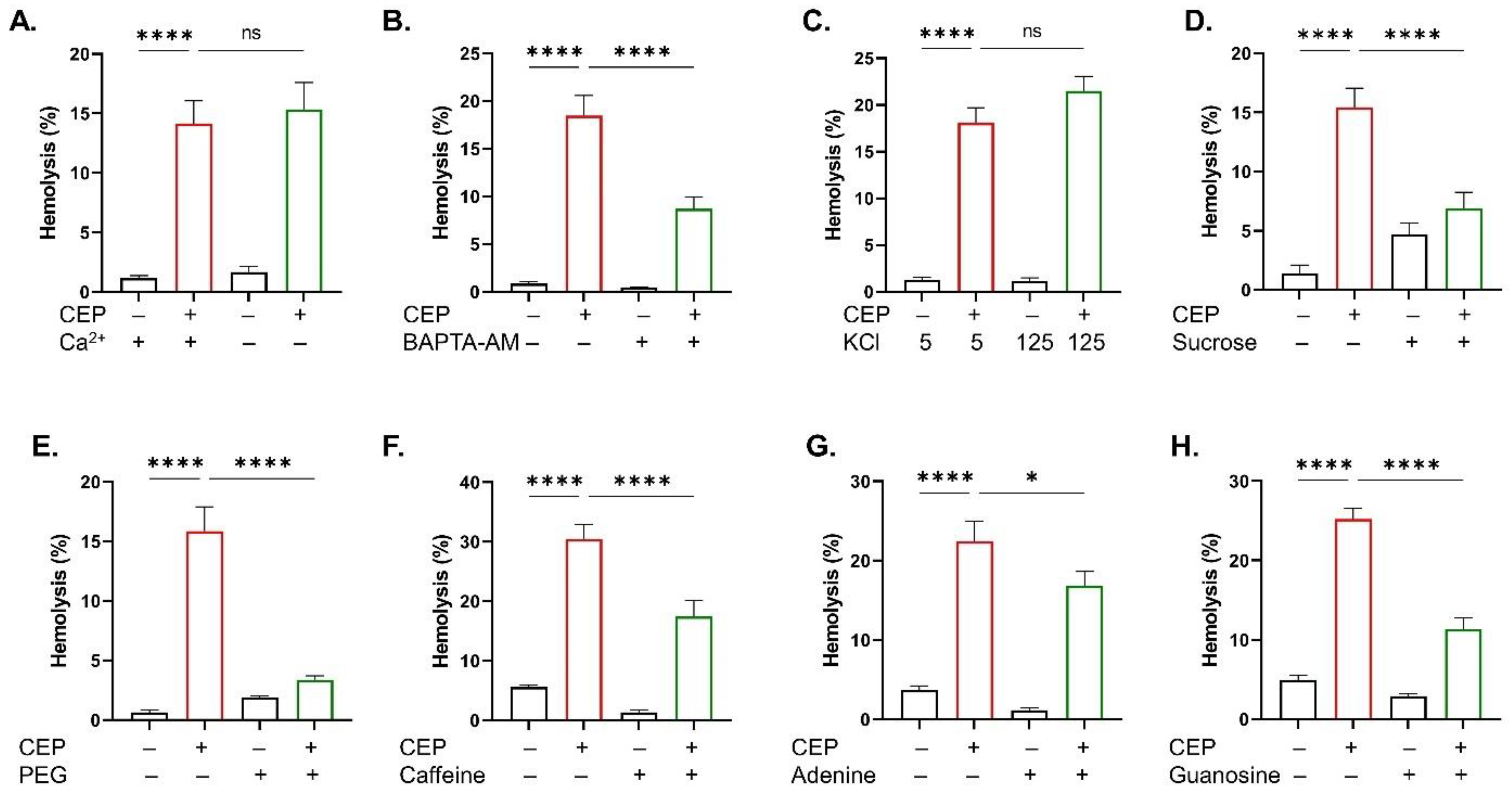
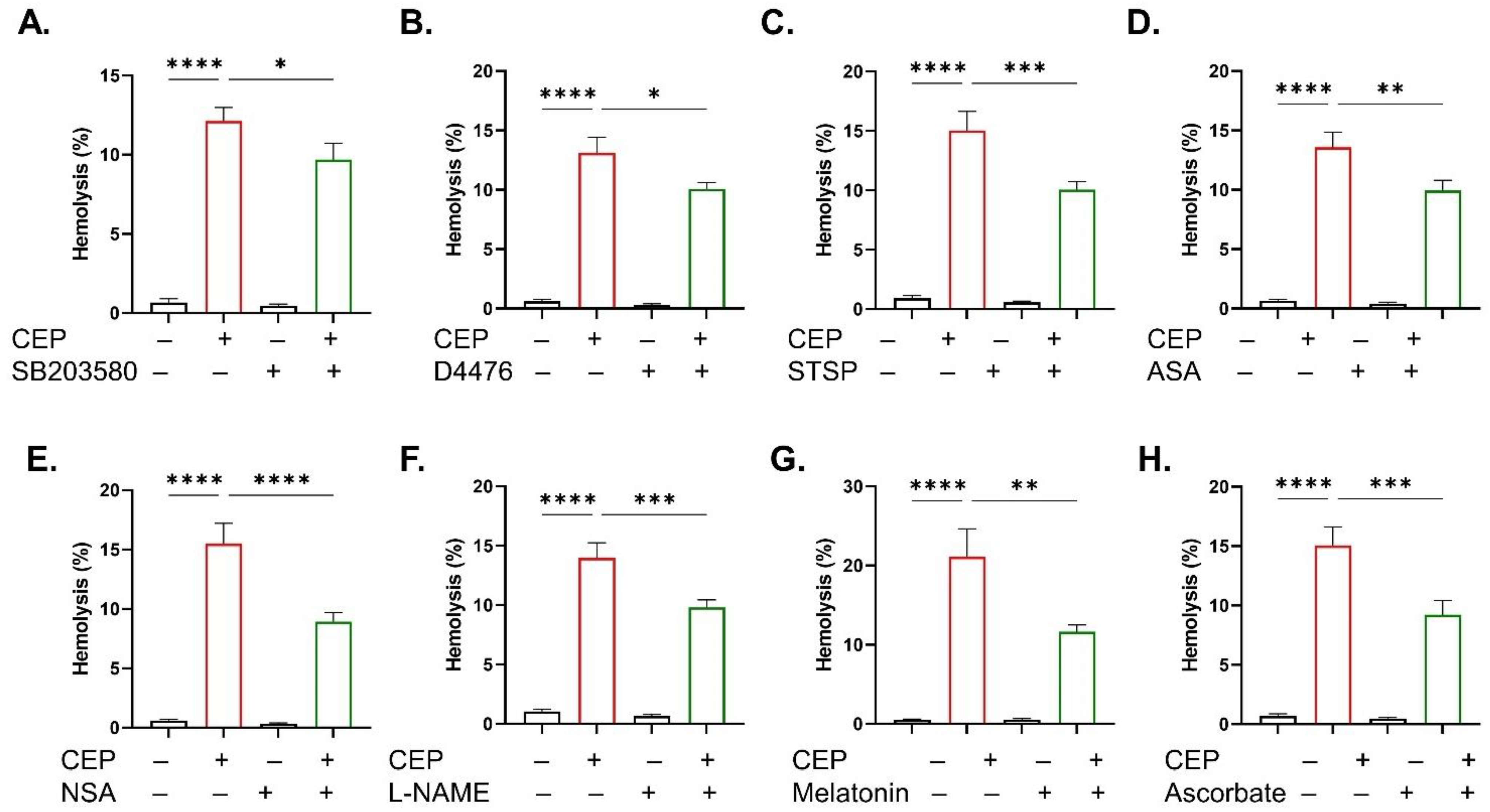
2.4. Inhibitor of CEP-Induced Hemolysis
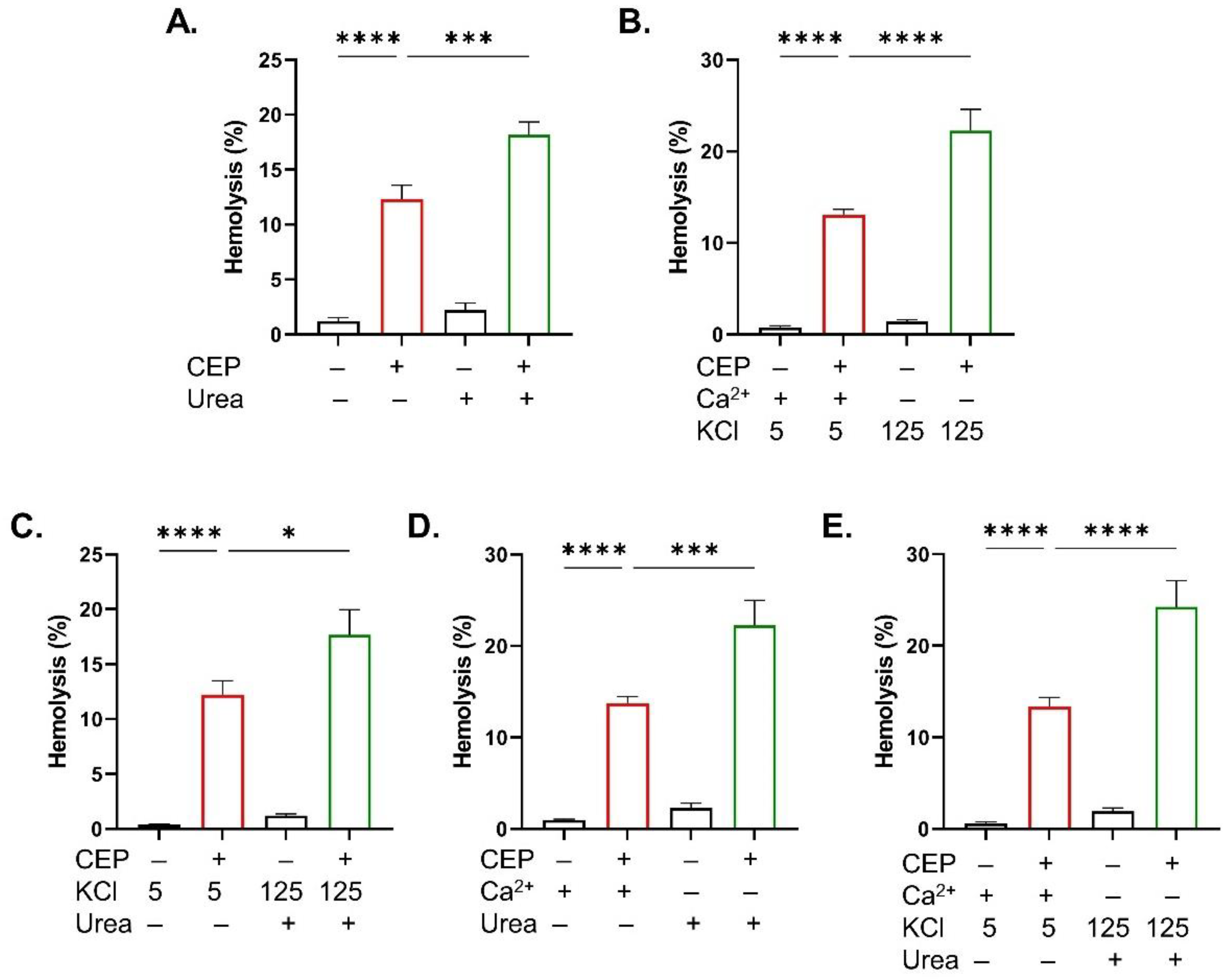
2.5. KCl Enrichment and Sucrose Exacerbate CEP-Induced Eryptosis
2.6. Urea and KCl Enrichment Sensitize RBCs to the Hemolytic Activity of CEP
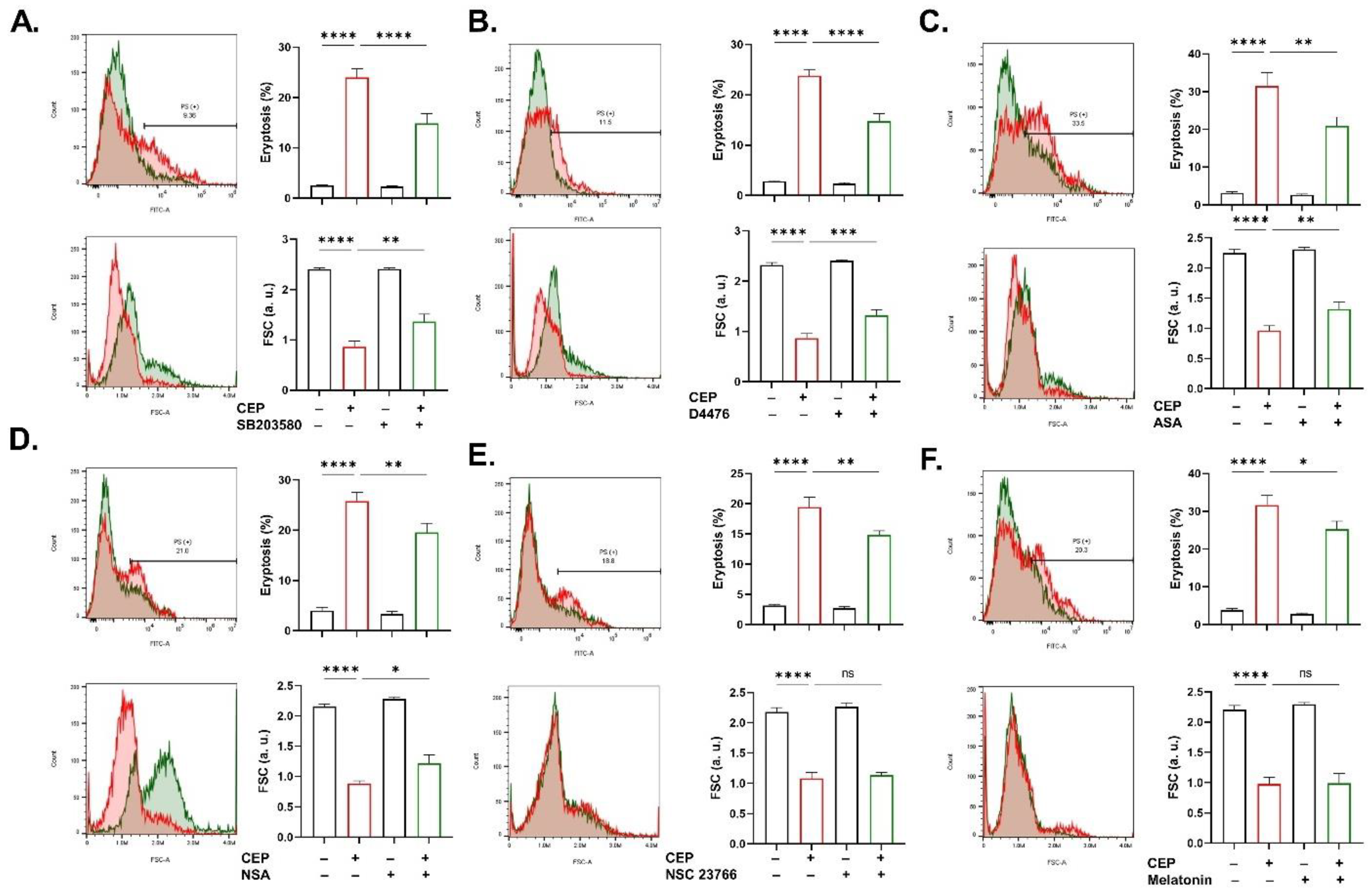
2.7. Signaling Cascades Involved in CEP Cytotoxicity
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Experimental Design
4.3. Hemolysis
4.4. Eryptosis
4.5. Intracellular Ca2+
4.6. Oxidative Stress
4.7. Erythrocyte Sedimentation Rate (ESR)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lang, E.; Bissinger, R.; Qadri, S.M.; Lang, F. Suicidal death of erythrocytes in cancer and its chemotherapy: A potential target in the treatment of tumor-associated anemia. Int. J. Cancer 2017, 141, 1522–1528. [Google Scholar] [CrossRef]
- Jeswani, G.; Alexander, A.; Saraf, S.; Saraf, S.; Qureshi, A. Recent approaches for reducing hemolytic activity of chemotherapeutic agents. J. Control. Release 2015, 211, 10–21. [Google Scholar] [CrossRef]
- Alghareeb, S.A.; Alfhili, M.A.; Fatima, S. Molecular Mechanisms and Pathophysiological Significance of Eryptosis. Int. J. Mol. Sci. 2023, 24, 5079. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, T.; Wang, H.; Liu, W.; Li, X.; Wang, X.; Zhang, Y. A mechanistic updated overview on Cepharanthine as potential anticancer agent. Biomed. Pharmacother. 2023, 165, 115107. [Google Scholar] [CrossRef]
- Shahriyar, S.A.; Woo, S.M.; Seo, S.U.; Min, K.-J.; Kwon, T.K. Cepharanthine Enhances TRAIL-Mediated Apoptosis Through STAMBPL1-Mediated Downregulation of Survivin Expression in Renal Carcinoma Cells. Int. J. Mol. Sci. 2018, 19, 3280. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Ding, X.; Qi, W.; Yang, Q. Cepharanthine Induces Autophagy, Apoptosis and Cell Cycle Arrest in Breast Cancer Cells. Cell Physiol. Biochem. 2017, 41, 1633–1648. [Google Scholar] [CrossRef]
- Lu, Y.-Y.; Zhu, C.-Y.; Ding, Y.-X.; Wang, B.; Zhao, S.-F.; Lv, J.; Chen, S.-M.; Wang, S.-S.; Wang, Y.; Wang, R.; et al. Cepharanthine, a regulator of keap1-Nrf2, inhibits gastric cancer growth through oxidative stress and energy metabolism pathway. Cell Death Discov. 2023, 9, 450. [Google Scholar] [CrossRef]
- Payon, V.; Kongsaden, C.; Ketchart, W.; Mutirangura, A.; Wonganan, P. Mechanism of Cepharanthine Cytotoxicity in Human Ovarian Cancer Cells. Planta Med. 2018, 85, 41–47. [Google Scholar] [CrossRef]
- Rattanawong, A.; Payon, V.; Limpanasittikul, W.; Boonkrai, C.; Mutirangura, A.; Wonganan, P. Cepharanthine exhibits a potent anticancer activity in p53-mutated colorectal cancer cells through upregulation of p21Waf1/Cip1. Oncol. Rep. 2018, 39, 227–238. [Google Scholar] [CrossRef]
- Liu, G.; Wu, D.; Liang, X.; Yue, H.; Cui, Y. Mechanisms and in vitro effects of cepharanthine hydrochloride: Classification analysis of the drug-induced differentially-expressed genes of human nasopharyngeal carcinoma cells. Oncol. Rep. 2015, 34, 2002–2010. [Google Scholar] [CrossRef]
- Feng, F.; Pan, L.; Wu, J.; Li, L.; Xu, H.; Yang, L.; Xu, K.; Wang, C. Cepharanthine inhibits hepatocellular carcinoma cell growth and proliferation by regulating amino acid metabolism and suppresses tumorigenesis in vivo. Int. J. Biol. Sci. 2021, 17, 4340–4352. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, G.; Zhao, Z.; Xiu, R.; Jia, J.; Chen, P.; Liu, Y.; Wang, Y.; Yi, J. Cepharanthine, a novel selective ANO1 inhibitor with potential for lung adenocarcinoma therapy. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2021, 1868, 119132. [Google Scholar] [CrossRef]
- Tkachenko, A.; Havranek, O. Cell death signaling in human erythron: Erythrocytes lose the complexity of cell death machinery upon maturation. Apoptosis 2025, 30, 652–673. [Google Scholar] [CrossRef]
- Scovino, A.M.; Totino, P.R.R.; Morrot, A. Eryptosis as a New Insight in Malaria Pathogenesis. Front. Immunol. 2022, 13, 855795. [Google Scholar] [CrossRef]
- Bissinger, R.; Bhuyan, A.A.M.; Qadri, S.M.; Lang, F. Oxidative stress, eryptosis and anemia: A pivotal mechanistic nexus in systemic diseases. FEBS J. 2018, 286, 826–854. [Google Scholar] [CrossRef]
- Abed, M.; Towhid, S.T.; Mia, S.; Pakladok, T.; Alesutan, I.; Borst, O.; Gawaz, M.; Gulbins, E.; Lang, F. Sphingomyelinase-induced adhesion of eryptotic erythrocytes to endothelial cells. Am. J. Physiol. Physiol. 2012, 303, C991–C999. [Google Scholar] [CrossRef]
- Proc, J.L.; Kuzyk, M.A.; Hardie, D.B.; Yang, J.; Smith, D.S.; Jackson, A.M.; Parker, C.E.; Borchers, C.H. A Quantitative Study of the Effects of Chaotropic Agents, Surfactants, and Solvents on the Digestion Efficiency of Human Plasma Proteins by Trypsin. J. Proteome Res. 2010, 9, 5422–5437. [Google Scholar] [CrossRef]
- Kameneva, M.V.; Repko, B.M.; Krasik, E.F.; Perricelli, B.C.; Borovetz, H.S. Polyethylene Glycol Additives Reduce Hemolysis in Red Blood Cell Suspensions Exposed to Mechanical Stress. ASAIO J. 2003, 49, 537–542. [Google Scholar] [CrossRef]
- Tkachenko, A.; Havranek, O. Erythronecroptosis: An overview of necroptosis or programmed necrosis in red blood cells. Mol. Cell Biochem. 2024, 479, 3273–3291. [Google Scholar] [CrossRef]
- Floride, E.; FÖLler, M.; Ritter, M.; Lang, F. Caffeine Inhibits Suicidal Erythrocyte Death. Cell Physiol. Biochem. 2008, 22, 253–260. [Google Scholar] [CrossRef]
- Alfhili, M.A.; Alghareeb, S.A.; Alotaibi, G.A.; Alsughayyir, J. Galangin Triggers Eryptosis and Hemolysis Through Ca(2+) Nucleation and Metabolic Collapse Mediated by PKC/CK1alpha/COX/p38/Rac1 Signaling Axis. Int. J. Mol. Sci. 2024, 25, 12267. [Google Scholar] [CrossRef]
- Manica, D.; da Silva, G.B.; de Lima, J.; Cassol, J.; Dallagnol, P.; Narzetti, R.A.; Morena, M.; Bagatini, M.D. Caffeine reduces viability, induces apoptosis, inhibits migration and modulates the CD39/CD73 axis in metastatic cutaneous melanoma cells. Purinergic Signal 2024, 20, 385–397. [Google Scholar] [CrossRef]
- Li, Y.-Z.; Wang, C.; Peng, X.; Wang, B.; Wang, J.-S.; Xie, H.-T.; Zhang, M.-C. Caffeine’s protective role in dry eye disease and meibomian gland dysfunction: Insights from clinical and experimental models. Int. Immunopharmacol. 2024, 146, 113863. [Google Scholar] [CrossRef]
- Barcelos, R.P.; Lima, F.D.; Carvalho, N.R.; Bresciani, G.; Royes, L.F. Caffeine effects on systemic metabolism, oxidative-inflammatory pathways, and exercise performance. Nutr. Res. 2020, 80, 1–17. [Google Scholar] [CrossRef]
- Gatidis, S.; Zelenak, C.; Fajol, A.; Lang, E.; Jilani, K.; Michael, D.; Qadri, S.M.; Lang, F. p38 MAPK Activation and Function following Osmotic Shock of Erythrocytes. Cell Physiol. Biochem. 2011, 28, 1279–1286. [Google Scholar] [CrossRef]
- Zelenak, C.; Eberhard, M.; Jilani, K.; Qadri, S.M.; Macek, B.; Lang, F. Protein kinase CK1alpha regulates erythrocyte survival. Cell Physiol Biochem. 2012, 29, 171–180. [Google Scholar] [CrossRef]
- Lang, P.A.; Kempe, D.S.; Myssina, S.; Tanneur, V.; Birka, C.; Laufer, S.; Lang, F.; Wieder, T.; Huber, S.M. PGE2 in the regulation of programmed erythrocyte death. Cell Death Differ. 2005, 12, 415–428. [Google Scholar] [CrossRef]
- LaRocca, T.J.; Stivison, E.A.; Hod, E.A.; Spitalnik, S.L.; Cowan, P.J.; Randis, T.M.; Ratner, A.J.; Tweten, R.; Collier, R.J. Human-Specific Bacterial Pore-Forming Toxins Induce Programmed Necrosis in Erythrocytes. mBio 2014, 5, e01251-14. [Google Scholar] [CrossRef]
- Kalfa, T.A.; Pushkaran, S.; Mohandas, N.; Hartwig, J.H.; Fowler, V.M.; Johnson, J.F.; Joiner, C.H.; Williams, D.A.; Zheng, Y. Rac GTPases regulate the morphology and deformability of the erythrocyte cytoskeleton. Blood 2006, 108, 3637–3645. [Google Scholar] [CrossRef]
- Paone, S.; D’aLessandro, S.; Parapini, S.; Celani, F.; Tirelli, V.; Pourshaban, M.; Olivieri, A. Characterization of the erythrocyte GTPase Rac1 in relation to Plasmodium falciparum invasion. Sci. Rep. 2020, 10, 22054. [Google Scholar] [CrossRef]
- Tesoriere, L.; D’ARpa, D.; Conti, S.; Giaccone, V.; Pintaudi, A.M.; Livrea, M.A. Melatonin protects human red blood cells from oxidative hemolysis: New insights into the radical-scavenging activity. J. Pineal Res. 1999, 27, 95–105. [Google Scholar] [CrossRef]
- Tzounakas, V.L.; Anastasiadi, A.T.; Arvaniti, V.-Z.; Lelli, V.; Fanelli, G.; Paronis, E.C.; Apostolidou, A.C.; Balafas, E.G.; Kostomitsopoulos, N.G.; Papageorgiou, E.G.; et al. Supplementation with uric and ascorbic acid protects stored red blood cells through enhancement of non-enzymatic antioxidant activity and metabolic rewiring. Redox Biol. 2022, 57, 102477. [Google Scholar] [CrossRef]
- Savage, R.A.; Zafar, N.; Yohannan, S.; Miller, J.-M.M. Melatonin. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2025. [Google Scholar]
- Zylinska, L.; Lisek, M.; Guo, F.; Boczek, T. Vitamin C Modes of Action in Calcium-Involved Signaling in the Brain. Antioxidants 2023, 12, 231. [Google Scholar] [CrossRef]
- von Lindern, M.; Egée, S.; Bianchi, P.; Kaestner, L. The Function of Ion Channels and Membrane Potential in Red Blood Cells: Toward a Systematic Analysis of the Erythroid Channelome. Front. Physiol. 2022, 13, 824478. [Google Scholar] [CrossRef]
- Alfhili, M.A.; Alothaimeen, R.F.; Alsughayyir, J. Arctigenin-induced erythrocyte membrane remodelling is mediated through calcium influx, metabolic collapse, and casein kinase 1α. Xenobiotica 2025, 55, 306–316. [Google Scholar] [CrossRef]
- Zelenak, C.; Pasham, V.; Jilani, K.; Tripodi, P.M.; Rosaclerio, L.; Pathare, G.; Lupescu, A.; Faggio, C.; Qadri, S.M.; Lang, F. Tanshinone IIA Stimulates Erythrocyte Phosphatidylserine Exposure. Cell Physiol. Biochem. 2012, 30, 282–294. [Google Scholar] [CrossRef]
- Sprague, R.S.; Ellsworth, M.L.; Stephenson, A.H.; Lonigro, A.J. Participation of cAMP in a signal-transduction pathway relating erythrocyte deformation to ATP release. Am. J. Physiol. Cell Physiol. 2001, 281, C1158–C1164. [Google Scholar] [CrossRef]
- Bennett-Guerrero, E.; Veldman, T.H.; Doctor, A.; Telen, M.J.; Ortel, T.L.; Reid, T.S.; Mulherin, M.A.; Zhu, H.; Buck, R.D.; Califf, R.M.; et al. Evolution of adverse changes in stored RBCs. Proc. Natl. Acad. Sci. USA 2007, 104, 17063–17068. [Google Scholar] [CrossRef]
- Greiner, J.V.; Glonek, T. Intracellular ATP Concentration and Implication for Cellular Evolution. Biology 2021, 10, 1166. [Google Scholar] [CrossRef]
- Evans, B.C.; Nelson, C.E.; Yu, S.S.; Beavers, K.R.; Kim, A.J.; Li, H.; Nelson, H.M.; Giorgio, T.D.; Duvall, C.L. Ex vivo red blood cell hemolysis assay for the evaluation of pH-responsive endosomolytic agents for cytosolic delivery of biomacromolecular drugs. J. Vis. Exp. 2013, 73, 50166. [Google Scholar]
- Liu, A.; Jacobs-McFarlane, C.; Sebastiani, P.; Glassberg, J.; McCuskee, S.; Curtis, S. Plasma free hemoglobin is associated with LDH, AST, total bilirubin, reticulocyte count, and the hemolysis score in patients with sickle cell anemia. Ann. Hematol. 2025, 104, 2221–2228. [Google Scholar] [CrossRef]
- Singh, M.; Karthikeyan, C.; Waiker, D.K.; Tiwari, A.; Shrivastava, S.K.; Sousa, S.F.; Kiriwan, D.; Martins, F.G.; Moorthy, N.S.H.N. Design, synthesis, and pharmacological evaluation of heteroaryl thiol-linked kojic acid derivatives as a novel class of acetylcholinesterase inhibitors for Alzheimer’s disease therapy. 3 Biotech 2025, 15, 134. [Google Scholar] [CrossRef]
- Kot, Y.; Prokopiuk, V.; Klochkov, V.; Tryfonyuk, L.; Maksimchuk, P.; Aslanov, A.; Kot, K.; Avrunin, O.; Demchenko, L.; Kurmangaliyeva, S.; et al. Mn3O4 Nanocrystal-Induced Eryptosis Features Ca2+ Overload, ROS and RNS Accumulation, Calpain Activation, Recruitment of Caspases, and Changes in the Lipid Order of Cell Membranes. Int. J. Mol. Sci. 2025, 26, 3284. [Google Scholar] [CrossRef]
- İpek, Ö.Y.; Abbas, F.; Sajidy, H.; Canepari, M. Fast Neuronal Calcium Signals in Brain Slices Loaded With Fluo-4 AM Ester. Eur. J. Neurosci. 2025, 61, e16657. [Google Scholar] [CrossRef]
- Lee, T.L.; Shen, W.C.; Chen, Y.C.; Lai, T.C.; Lin, S.R.; Lin, S.W.; Yu, I.S.; Yeh, Y.H.; Li, T.K.; Lee, I.T.; et al. Mir221- and Mir222-enriched adsc-exosomes mitigate PM exposure-exacerbated cardiac ischemia-reperfusion injury through the modulation of the BNIP3-MAP1LC3B-BBC3/PUMA pathway. Autophagy 2025, 21, 374–393. [Google Scholar] [CrossRef]
- Lorubbio, M.; Diamanti, D.; Pieroni, C.; Gialli, E.; Pettinari, M.; Bassi, S.; Gorini, G.; Carniani, S.; Saracini, A.; Meloni, P.; et al. Erythrocyte Sedimentation Rate Reference Intervals Determined via VES-MATIC 5 and CUBE 30 Touch with Respect to the Westergren Method. Diagnostics 2025, 15, 1101. [Google Scholar] [CrossRef]

| Inhibitor | PS Externalization | Cell Shrinkage | Hemolysis |
|---|---|---|---|
| SB203580 | |||
| D4476 | |||
| Acetylsalicylic acid | |||
| Necrosulfonamide | |||
| Melatonin | |||
| NSC23766 | |||
| ATP | |||
| BAPTA-AM | |||
| Staurosporin | |||
| L-NAME | |||
| Ascorbic acid | |||
| Caffeine | |||
| Adenine | |||
| Guanosine | |||
| Polyethylene glycol 8000 | |||
| Sucrose | |||
| KCl | |||
| Urea | |||
| Ca2+ elimination | |||
| Glucose |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alruwaili, S.H.; Alsughayyir, J.; Alfhili, M.A. Cepharanthine Promotes Ca2+-Independent Premature Red Blood Cell Death Through Metabolic Insufficiency and p38 MAPK/CK1α/COX/MLKL/PKC/iNOS Signaling. Int. J. Mol. Sci. 2025, 26, 7250. https://doi.org/10.3390/ijms26157250
Alruwaili SH, Alsughayyir J, Alfhili MA. Cepharanthine Promotes Ca2+-Independent Premature Red Blood Cell Death Through Metabolic Insufficiency and p38 MAPK/CK1α/COX/MLKL/PKC/iNOS Signaling. International Journal of Molecular Sciences. 2025; 26(15):7250. https://doi.org/10.3390/ijms26157250
Chicago/Turabian StyleAlruwaili, Shaymah H., Jawaher Alsughayyir, and Mohammad A. Alfhili. 2025. "Cepharanthine Promotes Ca2+-Independent Premature Red Blood Cell Death Through Metabolic Insufficiency and p38 MAPK/CK1α/COX/MLKL/PKC/iNOS Signaling" International Journal of Molecular Sciences 26, no. 15: 7250. https://doi.org/10.3390/ijms26157250
APA StyleAlruwaili, S. H., Alsughayyir, J., & Alfhili, M. A. (2025). Cepharanthine Promotes Ca2+-Independent Premature Red Blood Cell Death Through Metabolic Insufficiency and p38 MAPK/CK1α/COX/MLKL/PKC/iNOS Signaling. International Journal of Molecular Sciences, 26(15), 7250. https://doi.org/10.3390/ijms26157250







