Probing the pH Effect on Boehmite Particles in Water Using Vacuum Ultraviolet Single-Photon Ionization Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. VUV SPI-MS Spectral and AE Analysis of Boehmite Dissolution
2.2. The pH Effects on Boehmite Dissolution
3. Materials and Methods
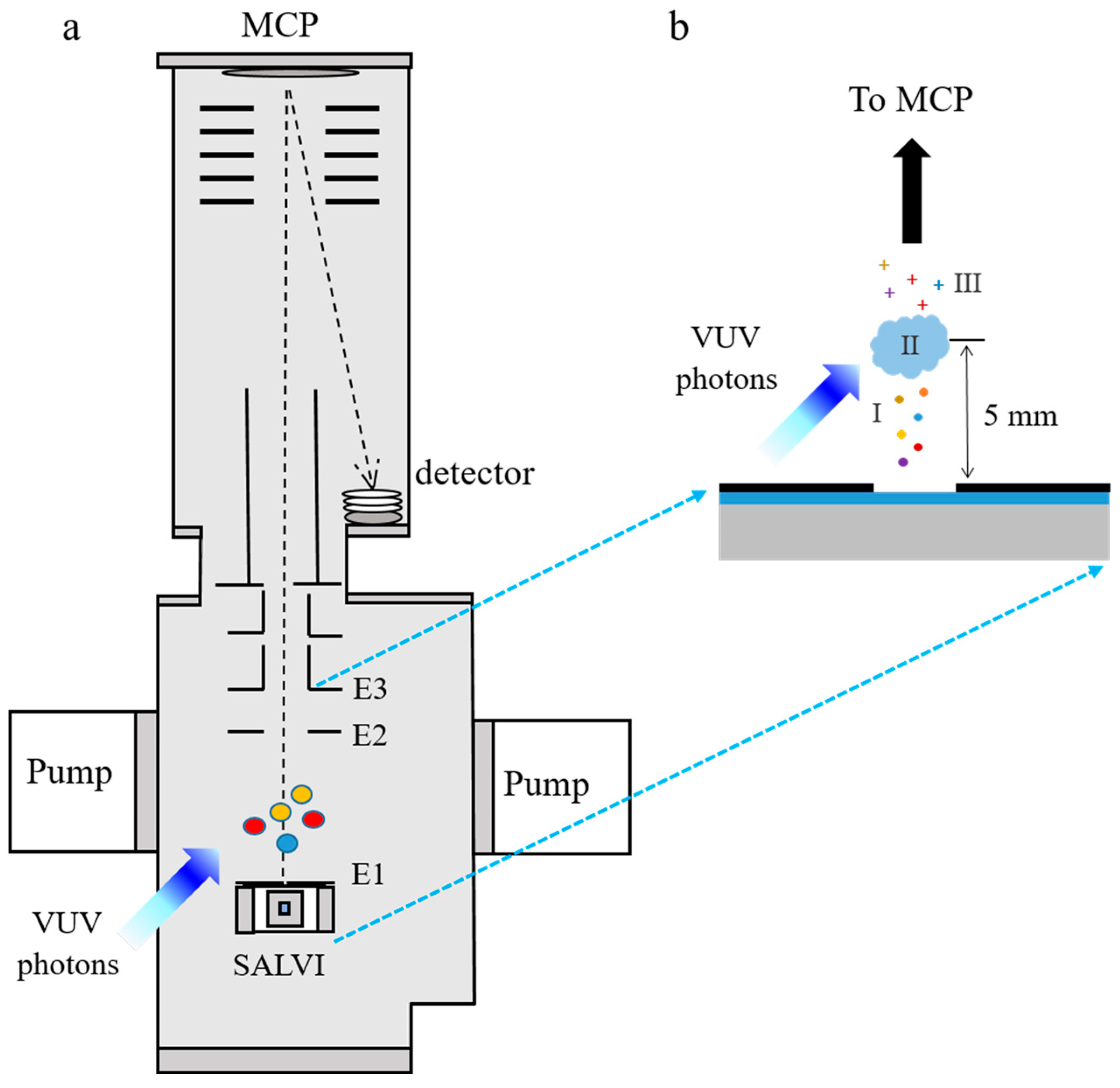
3.1. SALVI Fabrication
3.2. Boehmite Sample Preparation
3.3. VUV SPI-MS Liquid Analysis
3.4. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Truong, T.; Kim, D.-J. Synthesis of high quality boehmite and γ-alumina for phosphorus removal from water works sludge by extraction and hydrothermal treatment. Environ. Res. 2022, 212, 113448. [Google Scholar] [CrossRef]
- Kerisit, S.N.; Shen, Z.; Prange, M.P.; Ilton, E.S. Separation of Radiolytic Species at the Boehmite—Water Interface. J. Phys. Chem. C 2019, 123, 15534–15539. [Google Scholar] [CrossRef]
- Alemi, A.; Hosseinpour, Z.; Dolatyari, M.; Bakhtiari, A. Boehmite (γ-AlOOH) nanoparticles: Hydrothermal synthesis, characterization, pH-controlled morphologies, optical properties, and DFT calculations. Phys. Status. Solidi. B 2012, 249, 1264–1270. [Google Scholar] [CrossRef]
- Nagai, N.; Ihara, K.; Itoi, A.; Kodaira, T.; Takashima, H.; Hakuta, Y.; Bando, K.K.; Itoh, N.; Mizukami, F. Fabrication of boehmite and Al2O3 nonwovens from boehmite nanofibres and their potential as the sorbent. J. Mater. Chem. 2012, 22, 21225. [Google Scholar] [CrossRef]
- Roy, S.; Maity, A.; Mandal, P.; Chanda, D.K.; Pal, K.; Bardhan, S.; Das, S. Effects of various morphologies on the optical and electrical properties of boehmite nanostructures. CrystEngComm 2018, 20, 6338–6350. [Google Scholar] [CrossRef]
- Kaddissy, J.A.; Esnouf, S.; Durand, D.; Saffre, D.; Foy, E.; Renault, J.-P. Radiolytic Events in Nanostructured Aluminum Hydroxides. J. Phys. Chem. C 2017, 121, 6365–6373. [Google Scholar] [CrossRef]
- Mazloumi, M.; Attarchi, M.; Lak, A.; Mohajerani, M.S.; Kajbafvala, A.; Zanganeh, S.; Sadrnezhaad, S.K. Boehmite nanopetals self assembled to form rosette-like nanostructures. Mater. Lett. 2008, 62, 4184–4186. [Google Scholar] [CrossRef]
- Osmolovskaya, O.M.; Osmolowsky, M.G.; Petrov, M.P.; Voitylov, A.V.; Vojtylov, V.V. Theoretical and experimental approaches to the electro-optical study of boehmite nanoparticles with given morphology. Colloids Surf. A Physicochem. Eng. Asp. 2020, 586, 124095. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.; Xu, Z.; Hu, Z. Preparation of flower-like and rod-like boehmite via a hydrothermal route in a buffer solution. J. Phys. Chem. Solids 2010, 71, 206–209. [Google Scholar] [CrossRef]
- Ghasem Zadeh Khorasani, M.; Silbernagl, D.; Platz, D.; Sturm, H. Insights into Nano-Scale Physical and Mechanical Properties of Epoxy/Boehmite Nanocomposite Using Different AFM Modes. Polymers 2019, 11, 235. [Google Scholar] [CrossRef]
- Kloprogge, J.T.; Wood, B.J. Systematic XPS study of gallium-substituted boehmite. J. Mater. Sci. 2016, 51, 5436–5444. [Google Scholar] [CrossRef]
- Mohammadnezhad, G.; Dinari, M.; Nabiyan, A. High Surface Area Nano-Boehmite as Effective Nano-Filler for Preparation of Boehmite-Polyamide-6 Nanocomposites. Adv. Polym. Technol. 2016, 37, 1221–1228. [Google Scholar] [CrossRef]
- Eng, P.J.; Trainor, T.P.; Brown, G.E., Jr.; Waychunas, G.A.; Newville, M.; Sutton, S.R.; Rivers, M.L. Structure of the Hydrated α-Al2O3 (0001) Surface. Science 2000, 288, 1029–1033. [Google Scholar] [CrossRef]
- Lumetta, G.J.; Braley, J.C.; Peterson, J.M.; Bryan, S.A.; Levitskaia, T.G. Separating and Stabilizing Phosphate from High-Level Radioactive Waste: Process Development and Spectroscopic Monitoring. Environ. Sci. Technol. 2012, 46, 6190–6197. [Google Scholar] [CrossRef]
- Bookmeyer, C.; Röhling, U.; Dreisewerd, K.; Soltwisch, J. Single-Photon-Induced Post-Ionization to Boost Ion Yields in MALDI Mass Spectrometry Imaging. Angew. Chem. Int. Ed. 2022, 61, e202202165. [Google Scholar] [CrossRef]
- Kostko, O.; Bandyopadhyay, B.; Ahmed, M. Vacuum Ultraviolet Photoionization of Complex Chemical Systems. Annu. Rev. Phys. Chem. 2016, 67, 19–40. [Google Scholar] [CrossRef]
- Huang, Y.; Xie, C.; Yang, T.; Zhao, G.; Jia, B.; Cheng, P. Boosting the sensitivity of single photon ionization time-of-flight mass spectrometry using a segmented focus quadrupole-ion guide. Talanta 2024, 277, 126327. [Google Scholar] [CrossRef]
- Komorek, R.; Xu, B.; Yao, J.; Kostko, O.; Ahmed, M.; Yu, X.Y. Probing sulphur clusters in a microfluidic electrochemical cell with synchrotron-based photoionization mass spectrometry. Phys. Chem. Chem. Phys. 2020, 22, 14449–14453. [Google Scholar] [CrossRef]
- Yang, L.; Yu, X.-Y.; Zhu, Z.; Thevuthasan, T.; Cowin, J.P. Making a hybrid microfluidic platform compatible forin situimaging by vacuum-based techniques. J. Vac. Sci. Technol. A 2011, 29, 061101. [Google Scholar] [CrossRef]
- Lee, M.-T.; Orlando, F.; Artiglia, L.; Chen, S.; Ammann, M. Chemical Composition and Properties of the Liquid–Vapor Interface of Aqueous C1 to C4 Monofunctional Acid and Alcohol Solutions. J. Phys. Chem. A 2016, 120, 9749–9758. [Google Scholar] [CrossRef]
- Yu, X.-Y. In situ, in vivo, and in operando imaging and spectroscopy of liquids using microfluidics in vacuum. J. Vac. Sci. Technol. A 2020, 38. [Google Scholar] [CrossRef]
- Verdier, S.; Metson, J.B.; Dunlop, H.M. Static SIMS studies of the oxides and hydroxides of aluminium. J. Mass. Spectrom. 2007, 42, 11–19. [Google Scholar] [CrossRef]
- Sassi, M.; Wang, Z.; Walter, E.D.; Zhang, X.; Zhang, H.; Li, X.S.; Tuladhar, A.; Bowden, M.; Wang, H.-F.; Clark, S.B.; et al. Surface Hydration and Hydroxyl Configurations of Gibbsite and Boehmite Nanoplates. J. Phys. Chem. C 2020, 124, 5275–5285. [Google Scholar] [CrossRef]
- Strange, L.; Zhang, Y.; Son, J.; Gao, J.; Joshi, V.; Yu, X.-Y. Aluminum hydroxide, bayerite, boehmite, and gibbsite ToF-SIMS spectra in the positive ion mode. II. Surf. Sci. Spectra 2022, 29, 025002. [Google Scholar] [CrossRef]
- Bell, F.; Ruan, Q.N.; Golan, A.; Horn, P.R.; Ahmed, M.; Leone, S.R.; Head-Gordon, M. Dissociative Photoionization of Glycerol and its Dimer Occurs Predominantly via a Ternary Hydrogen-Bridged Ion–Molecule Complex. J. Am. Chem. Soc. 2013, 135, 14229–14239. [Google Scholar] [CrossRef]
- Zhang, X.; Cui, W.; Page, K.L.; Pearce, C.I.; Bowden, M.E.; Graham, T.R.; Shen, Z.; Li, P.; Wang, Z.; Kerisit, S.; et al. Size and Morphology Controlled Synthesis of Boehmite Nanoplates and Crystal Growth Mechanisms. Cryst. Growth. Des. 2018, 18, 3596–3606. [Google Scholar] [CrossRef]
- Komorek, R.; Xu, B.; Yao, J.; Ablikim, U.; Troy, T.P.; Kostko, O.; Ahmed, M.; Yu, X.Y. Enabling liquid vapor analysis using synchrotron VUV single photon ionization mass spectrometry with a microfluidic interface. Rev. Sci. Instrum. 2018, 89, 115105. [Google Scholar] [CrossRef]
- Sui, X.; Xu, B.; Yao, J.; Kostko, O.; Ahmed, M.; Yu, X.Y. New Insights into Secondary Organic Aerosol Formation at the Air-Liquid Interface. J. Phys. Chem. Lett. 2021, 12, 324–329. [Google Scholar] [CrossRef]
- Liu, L.; Chun, J.; Zhang, X.; Sassi, M.; Stack, A.G.; Pearce, C.I.; Clark, S.B.; Rosso, K.M.; Yoreo, J.J.D.; Kimmel, G.A. Radiolysis and Radiation-Driven Dynamics of Boehmite Dissolution Observed by In Situ Liquid-Phase TEM. Environ. Sci. Technol. 2022, 56, 5029–5036. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Yao, J.; Arey, B.; Zhu, Z.; Chun, J. In Situ Correlative Imaging and Spectroscopy of Boehmite Particles in Liquid. Microsc. Microanal. 2018, 24, 368–369. [Google Scholar] [CrossRef]
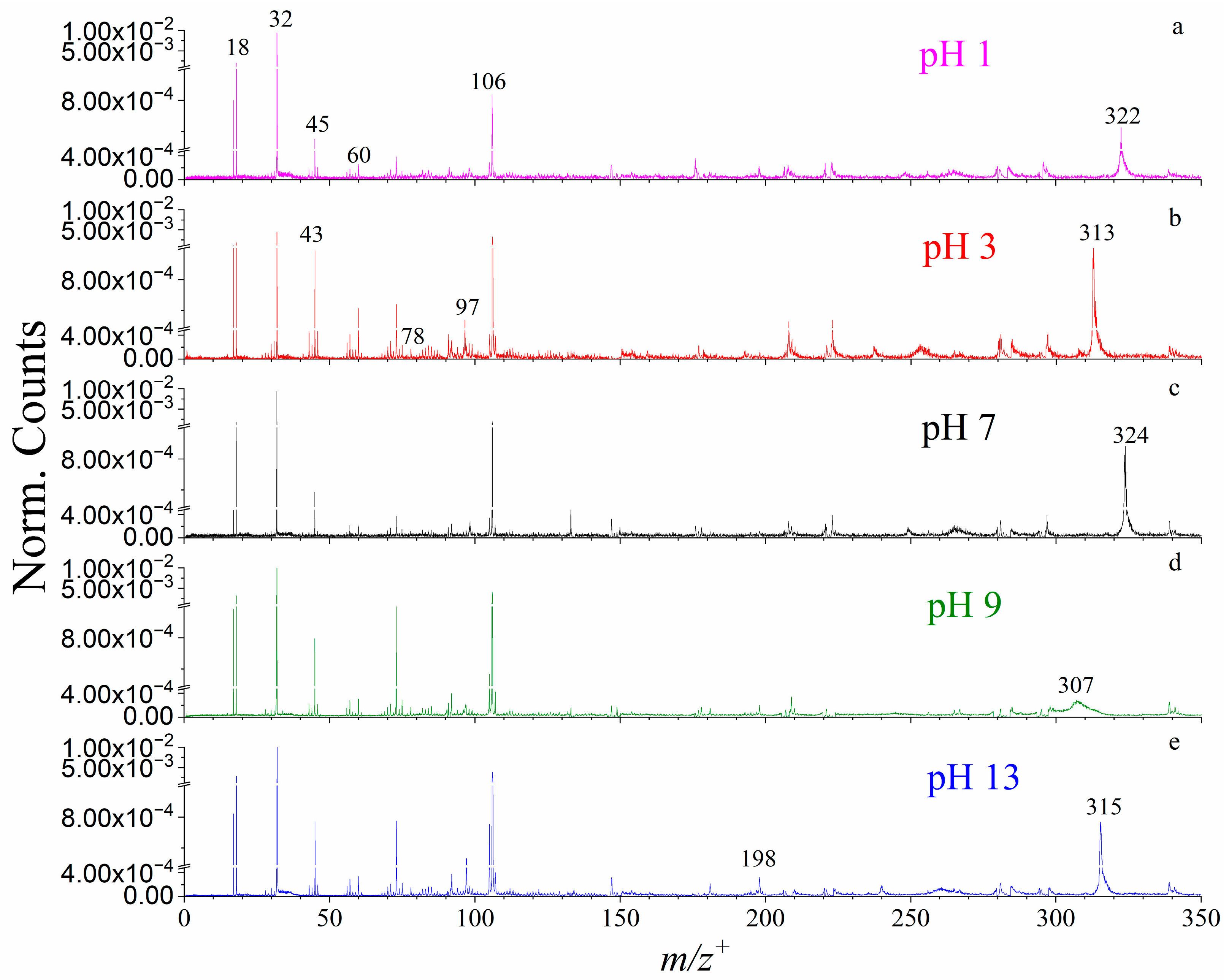

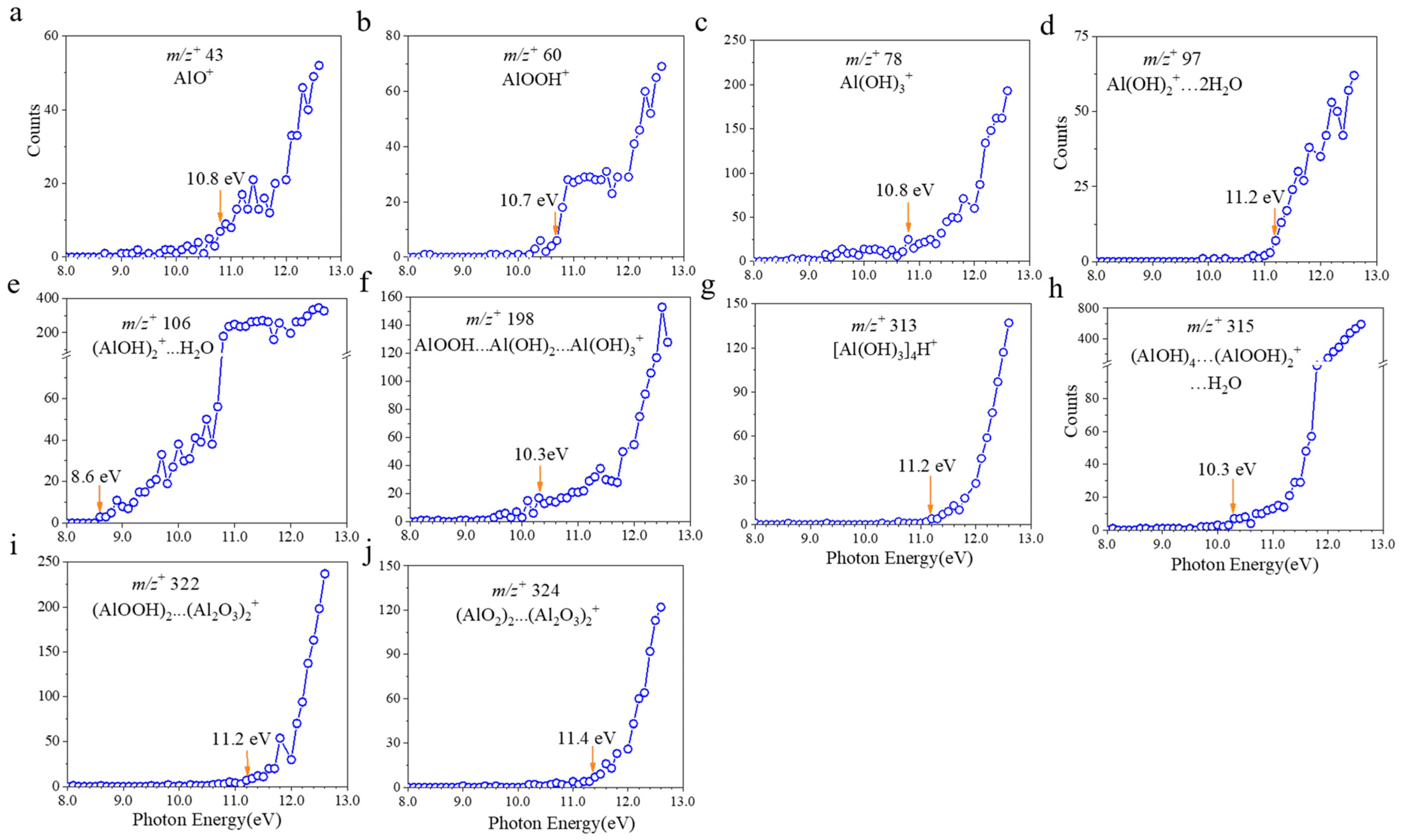

| 1 m/z+obs | 2 m/z+the | Possible Identification | 3 AEs (eV) |
|---|---|---|---|
| 43 | 42.98 | AlO+ | 10.8 |
| 60 | 59.99 | AlOOH+ | 10.7 |
| 78 | 78.00 | Al(OH)3+ | 10.8 |
| 97 | 96.94 | Al(OH)2+…2H2O | 11.2 |
| 106 | 105.99 | (AlOH)2+…H2O | 8.6 |
| 198 | 197.98 | AlOOH…Al(OH)2…Al(OH)3+ | 10.3 |
| 313 | 313.02 | [Al(OH)3]4H+ | 11.2 |
| 315 | 314.93 | (AlOH)4…(AlOOH)2…H3O+ | 10.3 |
| 322 | 321.88 | (AlO2)2…(Al2O3)2+ | 11.4 |
| 324 | 323.90 | (AlOOH)2…(Al2O3)2+ | 11.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, X.; Xu, B.; Yu, X.-Y. Probing the pH Effect on Boehmite Particles in Water Using Vacuum Ultraviolet Single-Photon Ionization Mass Spectrometry. Int. J. Mol. Sci. 2025, 26, 7254. https://doi.org/10.3390/ijms26157254
Sui X, Xu B, Yu X-Y. Probing the pH Effect on Boehmite Particles in Water Using Vacuum Ultraviolet Single-Photon Ionization Mass Spectrometry. International Journal of Molecular Sciences. 2025; 26(15):7254. https://doi.org/10.3390/ijms26157254
Chicago/Turabian StyleSui, Xiao, Bo Xu, and Xiao-Ying Yu. 2025. "Probing the pH Effect on Boehmite Particles in Water Using Vacuum Ultraviolet Single-Photon Ionization Mass Spectrometry" International Journal of Molecular Sciences 26, no. 15: 7254. https://doi.org/10.3390/ijms26157254
APA StyleSui, X., Xu, B., & Yu, X.-Y. (2025). Probing the pH Effect on Boehmite Particles in Water Using Vacuum Ultraviolet Single-Photon Ionization Mass Spectrometry. International Journal of Molecular Sciences, 26(15), 7254. https://doi.org/10.3390/ijms26157254









