The Role of Cobalt Ions in Angiogenesis—A Review
Abstract
1. Introduction
2. Angiogenesis
2.1. Initiation of Angiogenesis
2.2. Trace Elements Supporting Angiogenesis
2.2.1. Titanium
2.2.2. Copper
2.2.3. Cobalt
3. The Biological Role of Cobalt in the Human Body and Its Toxicity
3.1. Organic Form of Cobalt in Physiology and Its Sources
3.2. Roles of Cbl
3.3. Toxicity of Cobalt
4. Angiogenic Properties of Cobalt
4.1. Role of Cobalt in Angiogenesis
| Cobalt Compound/Biomaterial | Experimental Model | Main Angiogenic Mechanism (s) | Biological Effect (s) | Sources |
|---|---|---|---|---|
| Cobalt chloride (CoCl2) | Endothelial cells, animal models | HIF-1α stabilization, ↑VEGF, ↑FGF, ↑SDF-1; mimics hypoxia | ↑Migration, proliferation, tube formation, angiogenesis | [27,79,84,85,86,87,88,89] |
| Cobalt-doped bioactive glass | hBMSC, HUVEC, animal models | HIF-1α/VEGF, upregulation of bFGF, RUNX2, BMP-2 | ↑VEGF expression, angiogenesis, osteogenesis | [5,6,7,8,9,10,11,75] |
| Cobalt-doped mesoporous silica nanoparticles | rBMSC, rat | HIF-1α/VEGF, CD31 activation | ↑VEGF secretion, neovascularization | [4,7] |
| Cobalt-containing borate glass fibers | HUVEC, wound model | HIF-1α/VEGF, upregulation of angiogenic proteins | ↑Proliferation, migration, accelerated wound healing | [10,14] |
| Cobalt-doped calcium phosphate coatings | Goat (in vivo) | Upregulation of angiogenesis markers | ↑Number and size of blood vessels in tissue | [15] |
| Cobalt protoporphyrin (CoPPIX) | HMEC-1 | HO-1 (HIF-1-independent), ↑VEGF, ↑IL-8 | ↑VEGF and IL-8 expression | [88] |
| Cobalt nanowires (CoNWs) | HUVEC | HIF-1α stabilization | ↑Endothelial cell proliferation | [12] |
| Free cobalt ions | Macrophages, endothelial cells | Integrin-β1-rich exosomes, ↑VEGF, ↑eNOS | ↑Migration, ↑tube formation | [27] |
| Cobalt-substituted β-TCP ceramics | HUVEC, animal models | HIF-1α stabilization, VEGF upregulation | ↑Angiogenesis, ↑VEGF expression | [13] |
| Cobalt-doped borosilicate bioactive glass | BMSC, animal models | HIF-1α/VEGF, SDF-1, BMP-2, RUNX2 upregulation | ↑Angiogenesis, ↑osteogenesis | [90] |
4.2. The Effect of Free Cobalt Ions and Cobalt Chloride on Angiogenesis
4.3. The Effect of Cobalt-Containing Biomaterials on Angiogenesis
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
- The following abbreviations are used in this manuscript:
| Abbreviation | Full Term |
| ACGIH | The American Conference of Governmental Industrial Hygienists |
| Ade | Adenosine |
| aFGF/bFGF | Acidic/Basic Fibroblast Growth Factor |
| ANG-1 | Angiopoietin-1 |
| BAX | Bcl-2-Associated X Protein |
| BH4 | Tetrahydrobiopterin 4 |
| BMSCs | Bone Marrow Stromal Cells |
| CaP | Calcium Phosphate |
| Cbl | Cobalamin (Vitamin B12) |
| CD133+ | Umbilical Cord Blood-Derived Cells |
| Co | Cobalt |
| Co2+ | Cobalt Ion |
| CoCl2 | Cobalt Chloride |
| Co-MMSNs | Cobalt-Doped Mesoporous Silica-Coated Magnetic Nanoparticles |
| CoPPIX | Cobalt Protoporphyrin IX |
| Cu | Copper |
| DMT1 | Divalent Metal Transporter 1 |
| DOAJ | Directory of Open-Access Journals |
| ECs | Endothelial Cells |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| eNOS | Endothelial Nitric Oxide Synthase |
| EPO | Erythropoietin |
| ERK1/2 | Extracellular Signal-Regulated Kinases 1 and 2 |
| FAD | Flavin Adenine Dinucleotide |
| FGF | Fibroblast Growth Factor |
| FMN | Flavin Mononucleotide |
| GLUT1/3 | Glucose Transporter 1/3 |
| GSCbl | Glutathionylcobalamin |
| GSH | Glutathione |
| H2O2 | Hydrogen Peroxide |
| hBMSCs | Human Bone-Marrow-Derived Stem Cells |
| Hcy | Homocysteine |
| HDAC6 | Histone Deacetylase 6 |
| HIF | Hypoxia-Inducible Factor |
| HIF-1α | Hypoxia-Inducible Factor 1-Alpha |
| HIF-1β | Hypoxia-Inducible Factor 1-Beta |
| HMEC-1 | Human Microvascular Endothelial Cells |
| HO-1 | Heme Oxygenase-1 |
| HUVECs | Human Umbilical Vein Endothelial Cells |
| IL-8 | Interleukin-8 |
| iNOS | Inducible Nitric Oxide Synthase |
| LD | Linear Dichroism |
| MBG | Mesoporous Bioactive Glass |
| MCM | Methylmalonyl-CoA Mutase |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MS | Methionine Synthase |
| NADPH | Nicotinamide Adenine Dinucleotide Phosphate |
| NO | Nitric Oxide |
| NOAEL | No-Observed-Adverse-Effect Level |
| NTP | National Toxicology Program |
| PDGF | Platelet-Derived Growth Factor |
| PLA | Poly (Lactic Acid) |
| qPCR/RT-qPCR | Quantitative Real-Time PCR |
| RfD | Reference Dose |
| ROS | Reactive Oxygen Species |
| TCN2 | Transcobalamin II |
| TET | Ten-Eleven Translocation Methylcytosine Dioxygenase |
| TGF-β | Transforming Growth Factor Beta |
| Ti | Titanium |
| TLA | Three-Letter Acronym |
| TLV | Threshold Limit Value |
| VE-cadherin | Vascular Endothelial Cadherin |
| VEGF | Vascular Endothelial Growth Factor |
| VEGFR-1/2 | Vascular Endothelial Growth Factor Receptor 1/2 |
| vWF | von Willebrand Factor |
References
- Bosch-Rué, E.; Díez-Tercero, L.; Rodríguez-González, R.; Bosch-Canals, B.M.; Perez, R.A. Assessing the potential role of copper and cobalt in stimulating angiogenesis for tissue regeneration. PLoS ONE 2021, 16, e0259125. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, L. Hypoxia-mimicking Co doped TiO2 microporous coating on titanium with enhanced angiogenic and osteogenic activities. Acta Biomater. 2016, 43, 358–368. [Google Scholar] [CrossRef]
- Dürig, J.; Calcagni, M.; Buschmann, J. Transition metals in angiogenesis—A narrative review. Mater. Today Bio 2023, 22, 100757. [Google Scholar] [CrossRef]
- Zhao, H.; Jia, Y.; Wang, F.; Chai, Y.; Zhang, C.; Xu, J.; Kang, Q. Cobalt-doped mesoporous silica coated magnetic nanoparticles promoting accelerated bone healing in distraction osteogenesis. Int. J. Nanomed. 2023, 18, 2359–2370. [Google Scholar] [CrossRef]
- Solanki, A.K.; Lali, F.V.; Autefage, H.; Agarwal, S.; Nommeots-Nomm, A.; Metcalfe, A.D.; Stevens, M.M.; Jones, J.R. Bioactive glasses and electrospun composites that release cobalt to stimulate the HIF pathway for wound healing applications. Biomater. Res. 2021, 25, 1. [Google Scholar] [CrossRef]
- Birgani, Z.T.; Gharraee, N.; Malhotra, A.; van Blitterswijk, C.A.; Habibovic, P. Combinatorial incorporation of fluoride and cobalt ions into calcium phosphates to stimulate osteogenesis and angiogenesis. Biomed. Mater. 2016, 11, 015020. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef]
- de Laia, A.G.S.; Valverde, T.M.; Barrioni, B.R.; Cunha, P.S.; de Goes, A.M.; de Miranda, M.C.; Gomes, D.A.; Queiroz-Junior, C.M.; de Sá, M.A.; Pereira, M.M. Cobalt-containing bioactive glass mimics vascular endothelial growth factor A and hypoxia inducible factor 1 function. J. Biomed. Mater. Res. A 2020, 108, 2531–2544. [Google Scholar] [CrossRef]
- Boldbaatar, K.; Dashnyam, K.; Knowles, J.C.; Lee, H.H.; Lee, J.H.; Kim, H.W. Dual-ion delivery for synergistic angiogenesis and bactericidal capacity with silica-based microsphere. Acta Biomater. 2019, 83, 322–333. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, L.; Lv, H.; Cao, Y.; Liu, Y.; Xu, Y.; Ye, G.; Shi, J.; Fan, D.; Wang, L. Lithium and cobalt co-doped mesoporous bioactive glass nanoparticles promote osteogenesis and angiogenesis in bone regeneration. Front. Bioeng. Biotechnol. 2024, 11, 1288393. [Google Scholar] [CrossRef]
- Jiménez-Holguín, J.; Lozano, D.; Saiz-Pardo, M.; de Pablo, D.; Ortega, L.; Enciso, S.; Fernández-Tomé, B.; Díaz-Güemes, I.; Sánchez-Margallo, F.M.; Portolés, M.T.; et al. Osteogenic-angiogenic coupled response of cobalt-containing mesoporous bioactive glasses in vivo. Acta Biomater. 2024, 176, 445–457. [Google Scholar] [CrossRef]
- Zhao, J.; Liu, Y.; Sun, J.; Zhu, H.; Chen, Y.; Dong, T.; Zhang, H.; Wang, C.; Kong, D.; Yi, D. Magnetic targeting cobalt nanowire-based multifunctional therapeutic system for anticancer treatment and angiogenesis. Colloids Surf. B Biointerfaces 2020, 195, 111217. [Google Scholar] [CrossRef]
- Zhang, M.; Lin, K.; Chang, J. Preparation, characterization and in vitro angiogenic capacity of cobalt substituted β-tricalcium phosphate ceramics. J. Mater. Chem. 2012, 22, 21686–21694. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, A.; Ai, F.; Lin, J.; Fu, Q.; Wang, D. Cobalt-containing borate bioactive glass fibers for treatment of diabetic wound. J. Mater. Sci. Mater. Med. 2023, 34, 42. [Google Scholar] [CrossRef]
- Tahmasebi Birgani, Z.; Fennema, E.; Gijbels, M.J.; de Boer, J.; van Blitterswijk, C.A.; Habibovic, P. Stimulatory effect of cobalt ions incorporated into calcium phosphate coatings on neovascularization in an in vivo intramuscular model in goats. Acta Biomater. 2016, 36, 267–276. [Google Scholar] [CrossRef]
- Tonnesen, M.G.; Feng, X.; Clark, R.A.F. Angiogenesis in wound healing. J. Investig. Dermatol. Symp. Proc. 2000, 5, 40–46. [Google Scholar] [CrossRef]
- Ferrara, N.; Davis-Smyth, T. The biology of vascular endothelial growth factor. Endocr. Rev. 1997, 18, 4–25. [Google Scholar] [CrossRef]
- Brown, L.F.; Yeo, K.T.; Berse, B.; Yeo, T.K.; Senger, D.R.; Dvorak, H.F.; Van De Water, L. Expression of vascular permeability factor (vascular endothelial growth factor) by epidermal keratinocytes during wound healing. J. Exp. Med. 1992, 176, 1375–1379. [Google Scholar] [CrossRef]
- Chen, W.; Wu, P.; Yu, F.; Luo, G.; Qing, L.; Tang, J. HIF-1α regulates bone homeostasis and angiogenesis, participating in the occurrence of bone metabolic diseases. Cells 2022, 11, 3552. [Google Scholar] [CrossRef]
- Maxwell, P.; Salnikow, K. HIF-1: An oxygen and metal responsive transcription factor. Cancer Biol. Ther. 2004, 3, 29–35. [Google Scholar] [CrossRef]
- Bir, S.C.; Xiong, Y.; Kevil, C.G.; Luo, J. Emerging role of PKA/eNOS pathway in therapeutic angiogenesis for ischaemic tissue diseases. Cardiovasc. Res. 2012, 95, 7–18. [Google Scholar] [CrossRef]
- Leonard, S.; Gannett, P.M.; Rojanasakul, Y.; Schwegler-Berry, D.; Castranova, V.; Vallyathan, V.; Shi, X. Cobalt-mediated generation of reactive oxygen species and its possible mechanism. J. Inorg. Biochem. 1998, 70, 239–244. [Google Scholar] [CrossRef]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef]
- Raines, A.L.; Berger, M.B.; Schwartz, Z.; Boyan, B.D. Osteoblasts grown on microroughened titanium surfaces regulate angiogenic growth factor production through specific integrin receptors. Acta Biomater. 2019, 97, 578–586. [Google Scholar] [CrossRef]
- Raines, A.L.; Olivares-Navarrete, R.; Wieland, M.; Cochran, D.L.; Schwartz, Z.; Boyan, B.D. Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy. Biomaterials 2010, 31, 4909–4917. [Google Scholar] [CrossRef]
- Zhang, E.; Li, F.; Wang, H.; Liu, J.; Wang, C.; Li, M.; Yang, K. A new antibacterial titanium–copper sintered alloy: Preparation and antibacterial property. Mater. Sci. Eng. C 2013, 33, 4280–4287. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y.; Zhang, Y.; Hang, R.; Yao, X.; Hang, R. Exosomes derived from macrophages upon cobalt ion stimulation promote angiogenesis. Colloids Surf. B Biointerfaces 2021, 203, 111742. [Google Scholar] [CrossRef]
- Wilkinson-Berka, J.L.; Rana, I.; Armani, R.; Agrotis, A. Reactive oxygen species, Nox and angiotensin II in angiogenesis: Implications for retinopathy. Clin. Sci. 2013, 124, 597–615. [Google Scholar] [CrossRef]
- Wheatley, C. The return of the Scarlet Pimpernel: Cobalamin in inflammation II—Cobalamins can both selectively promote all three nitric oxide synthases (NOS), particularly iNOS and eNOS, and, as needed, selectively inhibit iNOS and nNOS. J. Nutr. Environ. Med. 2007, 16, 181–211. [Google Scholar] [CrossRef]
- Fernandes, C.J.d.C.; da Silva, R.A.F.; de Almeida, G.S.; Ferreira, M.R.; de Morais, P.B.; Bezerra, F.; Zambuzzi, W.F. Epigenetic Differences Arise in Endothelial Cells Responding to Cobalt–Chromium. J. Funct. Biomater. 2023, 14, 127. [Google Scholar] [CrossRef]
- Gunshin, H.; MacKenzie, B.; Berger, U.V.; Gunshin, Y.; Romero, M.F.; Boron, W.F.; Nussberger, S.; Gollan, J.L.; Hediger, M.A. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 1997, 388, 482–488. [Google Scholar] [CrossRef]
- Wang, C.Y.; Jenkitkasemwong, S.; Duarte, S.; Sparkman, B.K.; Shawki, A.; Mackenzie, B.; Knutson, M.D. ZIP8 is an iron and zinc transporter whose cell-surface expression is up-regulated by cellular iron loading. J. Biol. Chem. 2012, 287, 34032–34043. [Google Scholar] [CrossRef]
- Quadros, E.V.; Sequeira, J.M. Cellular uptake of cobalamin: Transcobalamin and the TCblR/CD320 receptor. Biochimie 2013, 95, 1008–1018. [Google Scholar] [CrossRef]
- Lison, D. Cobalt. In Handbook on the Toxicology of Metals, 4th ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 743–763. [Google Scholar]
- Yamada, K. Cobalt: Its role in health and disease. Met. Ions Life Sci. 2013, 13, 295–320. [Google Scholar] [CrossRef]
- Watanabe, F. Vitamin B12 sources and bioavailability. Exp. Biol. Med. 2007, 232, 1266–1274. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes and its Panel on Folate, Other B Vitamins, and Choline. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998. [Google Scholar] [PubMed]
- Geller, M.; Oliveira, L.; Nigri, R.; Mezitis, S.G.E.; Ribeiro, M.G.; de Souza da Fonseca, A.; Guimarães, O.R.; Kaufman, R.; Wajnsztajn, F. B Vitamins for neuropathy and neuropathic pain. Vitam. Miner. 2017, 6, 1000161. [Google Scholar] [CrossRef]
- Mascarenhas, R.; Gouda, H.; Ruetz, M.; Banerjee, R. Human B12-dependent enzymes: Methionine synthase and Methylmalonyl-CoA mutase. Methods Enzym. 2022, 668, 309–326. [Google Scholar] [CrossRef]
- Aslinia, F.; Mazza, J.J.; Yale, S.H. Megaloblastic anemia and other causes of macrocytosis. Clin. Med. Res. 2006, 4, 236–241. [Google Scholar] [CrossRef]
- Hörster, F.; Hoffmann, G.F. Pathophysiology, diagnosis, and treatment of methylmalonic aciduria—Recent advances and new challenges. Pediatr. Nephrol. 2004, 19, 1071–1074. [Google Scholar] [CrossRef]
- Howard, R.; Frieden, I.J.; Crawford, D.; McCalmont, T.; Levy, M.L.; Rosenblatt, D.S.; Sweetman, L.; Goodman, S.I.; Ohnstad, C.; Hart, K.; et al. Methylmalonic acidemia, cobalamin C type, presenting with cutaneous manifestations. Arch. Dermatol. 1997, 133, 1563–1566. [Google Scholar] [CrossRef]
- van der Westhuyzen, J.; Metz, J. The effect of age on serum vitamin B12 and folate levels. Br. J. Nutr. 1983, 50, 325–330. [Google Scholar] [CrossRef]
- Weir, D.G.; Keating, S.; Molloy, A.; McPartlin, J.; Kennedy, S.; Blanchflower, J.; Kennedy, D.G.; Rice, D.; Scott, J.M. Methylation deficiency causes vitamin B12-associated neuropathy in the pig. J. Neurochem. 1988, 51, 1949–1952. [Google Scholar] [CrossRef]
- Yajnik, C.S.; Deshpande, S.S.; Lubree, H.G.; Naik, S.S.; Bhat, D.S.; Uradey, B.S.; Deshpande, J.A.; Rege, S.S.; Refsum, H.; Yudkin, J.S. Vitamin B12 deficiency and hyperhomocysteinemia in rural and urban Indians. J. Assoc. Physicians India 2006, 54, 775–782. [Google Scholar] [PubMed]
- James, S.J.; Cutler, P.; Melnyk, S.; Jernigan, S.; Janak, L.; Gaylor, D.W.; Neubrander, J.A. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr. 2004, 80, 1611–1617. [Google Scholar] [CrossRef]
- de Jager, C.A. Critical levels of brain atrophy associated with homocysteine and cognitive decline. Neurobiol. Aging 2014, 35, S35–S39. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Q.; Chen, Y.; Huang, X.; Yang, I.H.; Cao, L.; Wu, W.K.; Tan, H.M. Homocysteine-impaired angiogenesis is associated with VEGF/VEGFR inhibition. Front. Biosci. 2012, 4, 2525–2535. [Google Scholar] [CrossRef]
- Hutto, B.R. Folate and cobalamin in psychiatric illness. Compr. Psychiatry 1997, 38, 305–314. [Google Scholar] [CrossRef]
- Clarke, R.; Smith, A.D.; Jobst, K.A.; Refsum, H.; Sutton, L.; Ueland, P.M. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch. Neurol. 1998, 55, 1449–1455. [Google Scholar] [CrossRef]
- van Meurs, J.B.; Dhonukshe-Rutten, R.A.; Pluijm, S.M.; van der Klift, M.; de Jonge, R.; Lindemans, J.; de Groot, L.C.; Hofman, A.; Witteman, J.C.; van Leeuwen, J.P.; et al. Homocysteine levels and the risk of osteoporotic fracture. N. Engl. J. Med. 2004, 350, 2033–2041. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Asatourian, A.; Ershadifar, S.; Moghadam, M.M.; Sheibani, N. Vitamins and regulation of angiogenesis: [A, B1, B2, B3, B6, B9, B12, C, D, E, K]. J. Funct. Foods 2017, 38, 180–196. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Provisional Peer-Reviewed Toxicity Values for Cobalt (CASRN 7440-48-4); Superfund Health Risk Technical Support Center, National Center for Environmental Assessment, Office of Research and Development: Cincinnati, OH, USA, 2008. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/cobalt-compounds.pdf (accessed on 15 July 2025).
- U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry. ToxGuide for Cobalt (CAS# 7440-48-4); U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2024. Available online: https://www.atsdr.cdc.gov/toxguides/toxguide-33.pdf (accessed on 15 July 2025).
- Elci, S.G. Determination of cobalt in food by magnetic solid-phase extraction (MSPE) preconcentration by polyaniline (PANI) and polythiophene (PTH) coated magnetic nanoparticles (MNPs) and microsample injection system–flame atomic absorption spectrometry (MIS-FAAS). Instrum. Sci. Technol. 2021, 49, 258–275. [Google Scholar] [CrossRef]
- Paustenbach, D.J.; Galbraith, D.A.; Finley, B.L. Interpreting cobalt blood concentrations in hip implant patients. Clin. Toxicol. 2014, 52, 98–112. [Google Scholar] [CrossRef]
- Sauni, R.; Linna, A.; Oksa, P.; Nordman, H.; Tuppurainen, M.; Uitti, J. Cobalt asthma—A case series from a cobalt plant. Occup. Med. 2010, 60, 301–306. [Google Scholar] [CrossRef]
- Committee for Human Medicinal Products. ICH Guideline Q3D (R1) on Elemental Impurities; European Medicines Agency: Amsterdam, The Netherlands, 2019; EMA/CHMP/ICH/353369/2013. [Google Scholar]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Cobalt, Antimony Compounds, and Weapons-Grade Tungsten Alloy. In IARC Monographs on the Identification of Carcinogenic Hazards to Humans; International Agency for Research on Cancer: Lyon, France, 2023; Volume 131. [Google Scholar]
- NTP (National Toxicology Program). Report on Carcinogens, 15th ed.; U.S. Department of Health and Human Services, Public Health Service: Research Triangle Park, NC, USA, 2021. [Google Scholar] [CrossRef]
- Lison, D. Cobalt. In Handbook on the Toxicology of Metals, 3rd ed.; Nordberg, G.F., Fowler, B.A., Nordberg, M., Friberg, L., Eds.; Academic Press: Cambridge, MA, USA, 2007; pp. 511–528. [Google Scholar]
- Keegan, G.M.; Learmonth, I.D.; Case, C.P. A systematic comparison of the actual, potential, and theoretical health effects of cobalt and chromium exposures from industry and surgical implants. Crit. Rev. Toxicol. 2008, 38, 645–674. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Prevalence of cobalt in the environment and its role in biological processes. Biology 2023, 12, 1335. [Google Scholar] [CrossRef]
- Rolfe, M.W.; Paine, R.; Davenport, R.B.; Strieter, R.M. Hard metal pneumoconiosis and the association of tumor necrosis factor-alpha. Am. Rev. Respir. Dis. 1992, 146, 1600–1602. [Google Scholar] [CrossRef]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Cobalt metabolism and toxicology—A brief update. Sci. Total Environ. 2012, 432, 210–215. [Google Scholar] [CrossRef]
- Caicedo, M.S.; Pennekamp, P.H.; McAllister, K.; Jacobs, J.J.; Hallab, N.J. Soluble ions more than particulate cobalt-alloy implant debris induce monocyte costimulatory molecule expression and release of proinflammatory cytokines critical to metal-induced lymphocyte reactivity. J. Biomed. Mater. Res. A 2010, 93, 1312–1321. [Google Scholar] [CrossRef]
- Barceloux, D.G. Cobalt. Clin. Toxicol. 1999, 37, 201–216. [Google Scholar] [CrossRef]
- Hille, B. Ionic Channels of Excitable Membranes, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 1992; ISBN 978-0878933211. [Google Scholar]
- Akbar, M.; Brewer, J.M.; Grant, M.H. Effect of chromium and cobalt ions on primary human lymphocytes in vitro. J. Immunotoxicol. 2011, 8, 140–149. [Google Scholar] [CrossRef]
- Tang, J.; Li, Y.; Liu, X.; Yu, G.; Zheng, F.; Guo, Z.; Zhang, Y.; Shao, W.; Wu, S.; Li, H. Cobalt induces neurodegenerative damages through impairing autophagic flux by activating hypoxia-inducible factor-1α triggered ROS overproduction. Sci. Total Environ. 2023, 857 Pt 2, 159432. [Google Scholar] [CrossRef]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Passive transport pathways for Ca2+ and Co2+ in human red blood cells. 57Co2+ as a tracer for Ca2+ influx. Blood Cells Mol. Dis. 2011, 47, 214–225. [Google Scholar] [CrossRef]
- Leggett, R.W. The biokinetics of inorganic cobalt in the human body. Sci. Total Environ. 2008, 389, 259–269. [Google Scholar] [CrossRef]
- Schoon, J.; Hesse, B.; Rakow, A.; Ort, M.J.; Lagrange, A.; Jacobi, D.; Winter, A.; Huesker, K.; Reinke, S.; Cotte, M.; et al. Metal-specific biomaterial accumulation in human peri-implant bone and bone marrow. Adv. Sci. 2020, 7, 2000412. [Google Scholar] [CrossRef]
- de Melo Pereira, D.; Schumacher, M.; Habibovic, P. Cobalt-containing calcium phosphate induces resorption of biomineralized collagen by human osteoclasts. Biomater. Res. 2021, 25, 6. [Google Scholar] [CrossRef]
- Baino, F.; Montazerian, M.; Verné, E. Cobalt-doped bioactive glasses for biomedical applications: A review. Materials 2023, 16, 4994. [Google Scholar] [CrossRef]
- Anissian, L.; Stark, A.; Dahlstrand, H.; Granberg, B.; Good, V.; Bucht, E. Cobalt ions influence proliferation and function of human osteoblast-like cells. Acta Orthop. Scand. 2002, 73, 369–374. [Google Scholar] [CrossRef]
- Li, Q.; Ke, Q.; Costa, M. Alterations of histone modifications by cobalt compounds. Carcinogenesis 2009, 30, 1243–1251. [Google Scholar] [CrossRef]
- Chai, Y.C.; Mendes, L.F.; van Gastel, N.; Carmeliet, G.; Luyten, F.P. Fine-tuning pro-angiogenic effects of cobalt for simultaneous enhancement of vascular endothelial growth factor secretion and implant neovascularization. Acta Biomater. 2018, 72, 447–460. [Google Scholar] [CrossRef]
- Rana, N.K.; Singh, P.; Koch, B. CoCl2 simulated hypoxia induce cell proliferation and alter the expression pattern of hypoxia associated genes involved in angiogenesis and apoptosis. Biol. Res. 2019, 52, 12. [Google Scholar] [CrossRef]
- Levy, A.P.; Levy, N.S.; Loscalzo, J.; Calderone, A.; Takahashi, N.; Yeo, K.-T.; Koren, G.; Colucci, W.S.; Goldberg, M.A. Regulation of vascular endothelial growth factor in cardiac myocytes. Circ. Res. 1995, 76, 758–766. [Google Scholar] [CrossRef]
- Davis, J.E.; Fields, J.P. Experimental production of polycythemia in humans by administration of cobalt chloride. Proc. Soc. Exp. Biol. Med. 1958, 99, 493–495. [Google Scholar] [CrossRef]
- Fisher, J.W.; Langston, J.W. Effects of testosterone, cobalt and hypoxia on erythropoietin production in the isolated perfused dog kidney. Ann. N. Y. Acad. Sci. 1968, 149, 75–87. [Google Scholar] [CrossRef]
- Lando, D.; Peet, D.J.; Whelan, D.A.; Gorman, J.J.; Whitelaw, M.L. Asparagine hydroxylation of the HIF transactivation domain: A hypoxic switch. Science 2002, 295, 858–861. [Google Scholar] [CrossRef]
- Buttyan, R.; Chichester, P.; Stisser, B.; Matsumoto, S.; Ghafar, M.A.; Levin, R.M. Acute intravesical infusion of a cobalt solution stimulates a hypoxia response, growth and angiogenesis in the rat bladder. J. Urol. 2003, 169, 2402–2406. [Google Scholar] [CrossRef]
- Zan, T.; Guo, L.; Tang, X.; Wang, H. Cobalt chloride enhances angiogenic potential of CD133 cells. Front. Biosci. 2012, 17, 2247–2258. [Google Scholar] [CrossRef]
- Di Mattia, M.; Mauro, A.; Citeroni, M.R.; Drarnia, O.; Peserico, A.; Russo, V.; Cammà, C.; Palazzese, L.; Canciello, A.; Barboni, B. Hypoxia-mimetic CoCl2 agent enhances pro-angiogenic activities in ovine amniotic epithelial cells-derived conditioned medium. Cells 2022, 11, 461. [Google Scholar] [CrossRef]
- Fan, W.; Crawford, R.; Xiao, Y. Enhancing in vivo vascularized bone formation by cobalt chloride-treated bone marrow stromal cells in a tissue engineered periosteum model. Biomaterials 2010, 31, 3580–3589. [Google Scholar] [CrossRef]
- Loboda, A.; Jazwa, A.; Węgiel, B.; Jozkowicz, A.; Dulak, J. Heme oxygenase-1-dependent and -independent regulation of angiogenic genes expression: Effect of cobalt protoporphyrin and cobalt chloride on VEGF and IL-8 synthesis in human microvascular endothelial cells. Cell Mol. Biol. 2005, 51, 347–355. [Google Scholar] [PubMed]
- Tanaka, T.; Kojima, I.; Ohse, T.; Ingelfinger, J.R.; Adler, S.; Fujita, T.; Nangaku, M. Cobalt promotes angiogenesis via hypoxia-inducible factor and protects tubulointerstitium in the remnant kidney model. Lab. Investig. 2005, 85, 1292–1307. [Google Scholar] [CrossRef]
- Deng, Z.; Lin, B.; Jiang, Z.; Huang, W.; Li, J.; Zeng, X.; Wang, H.; Wang, D.; Zhang, Y. Hypoxia-mimicking cobalt-doped borosilicate bioactive glass scaffolds with enhanced angiogenic and osteogenic capacity for bone regeneration. Int. J. Biol. Sci. 2019, 15, 1113–1124. [Google Scholar] [CrossRef]
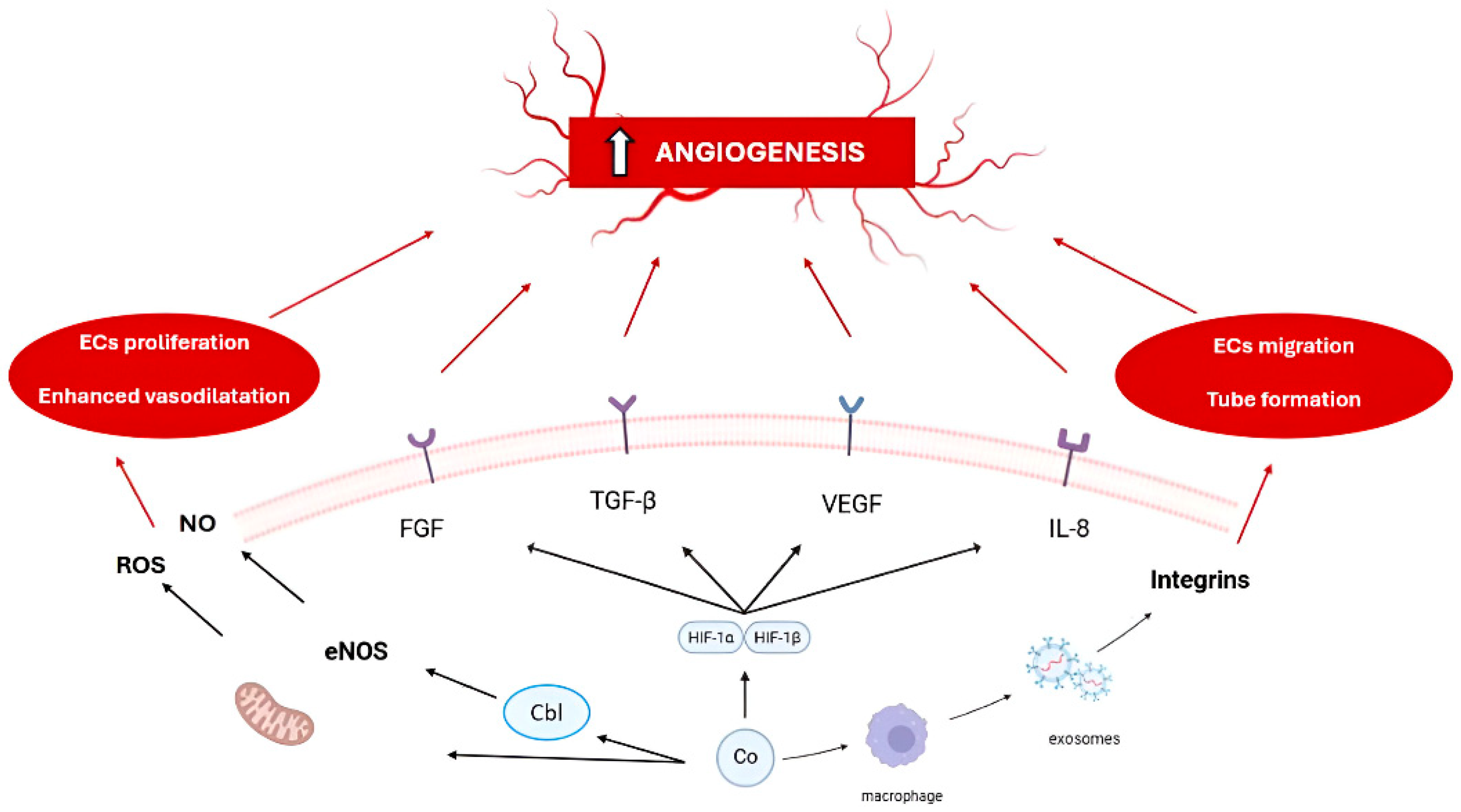
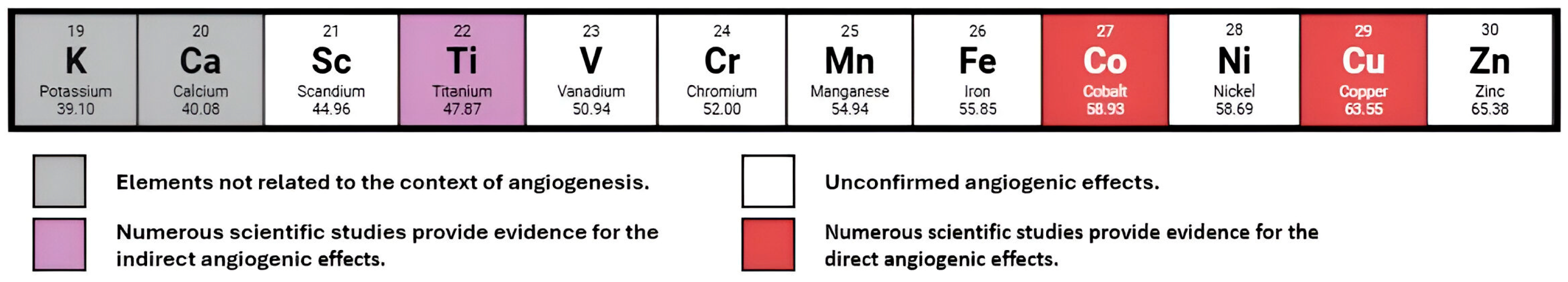

| Element | Primary Mechanism of Action in Angiogenesis | Key Molecular Targets/Pathways | Notes on Toxicity |
|---|---|---|---|
| Co | Mimics hypoxia and stabilizes HIF-1α, leading to VEGF upregulation and macrophage-mediated secretion of pro-angiogenic factors | ↑ HIF-1α→↑ VEGF-A; macrophage activation; angiogenic cytokine secretion | Dose-dependent cytotoxicity; embedding in biomaterials reduces adverse effects |
| Cu | Upregulates VEGF expression via ROS signaling (H2O2), supports EC migration and proliferation, and catalyzes pro-angiogenic reactions | ↑ VEGF, FGF-1, angiogenin, HIF-1α, eNOS; enhanced VEGF in keratinocytes with ROS interaction | Excess causes oxidative stress; essential in small amounts but toxic at high concentrations |
| Ti | Surface topography influences osteoblast and EC response; activates integrin-dependent signaling pathways | ↑ VEGF-A, FGF-2, ANG-1, VEGFR-1/2, eNOS, iNOS; enhanced NO production and EC activation | Low inherent toxicity: roughened surfaces increase bioactivity without raising toxicity |
| Mechanism | Description | Source |
|---|---|---|
| Stabilization of HIF-1α | Co2+, as an inducer of the hypoxic response, triggers a coordinated activation of a wide array of HIF-dependent genes, mostly VEGF | [1,4,5,6,7,8,9,10,11,12,13,14,20,27,82,83,84,85,86,87,88,89,90] |
| Induction of heme oxygenase-1 (HO-1) expression | Co2+ significantly upregulated VEGF and IL-8 via HO-1 activation, independently of the HIF-pathway | [88] |
| Influence on ECs causing high integrin-β1 expression | Integrin-β1 plays a crucial role in cell–cell and cell–matrix interactions, highly influencing angiogenesis | [9,27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregorowicz, W.; Pajchel, L. The Role of Cobalt Ions in Angiogenesis—A Review. Int. J. Mol. Sci. 2025, 26, 7236. https://doi.org/10.3390/ijms26157236
Gregorowicz W, Pajchel L. The Role of Cobalt Ions in Angiogenesis—A Review. International Journal of Molecular Sciences. 2025; 26(15):7236. https://doi.org/10.3390/ijms26157236
Chicago/Turabian StyleGregorowicz, Wiktor, and Lukasz Pajchel. 2025. "The Role of Cobalt Ions in Angiogenesis—A Review" International Journal of Molecular Sciences 26, no. 15: 7236. https://doi.org/10.3390/ijms26157236
APA StyleGregorowicz, W., & Pajchel, L. (2025). The Role of Cobalt Ions in Angiogenesis—A Review. International Journal of Molecular Sciences, 26(15), 7236. https://doi.org/10.3390/ijms26157236







