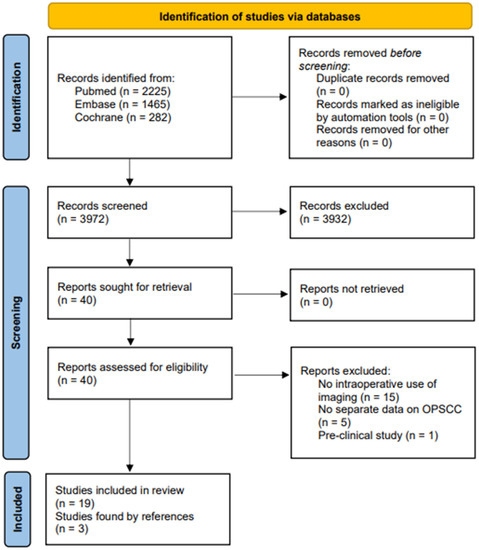
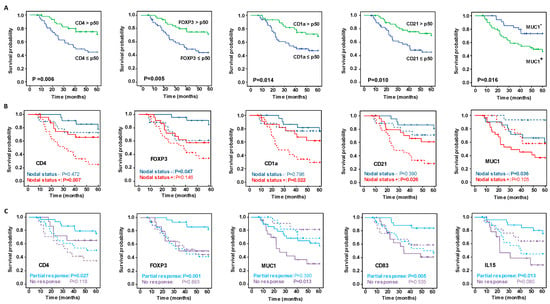
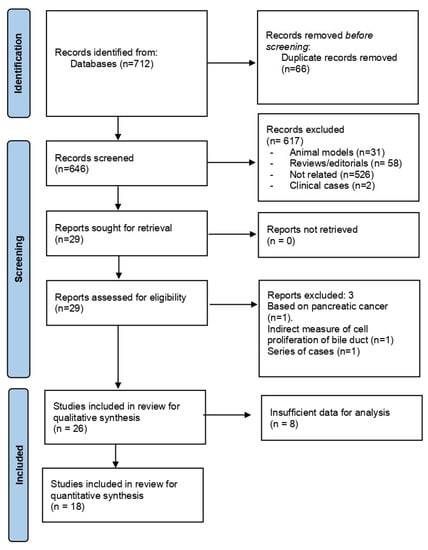
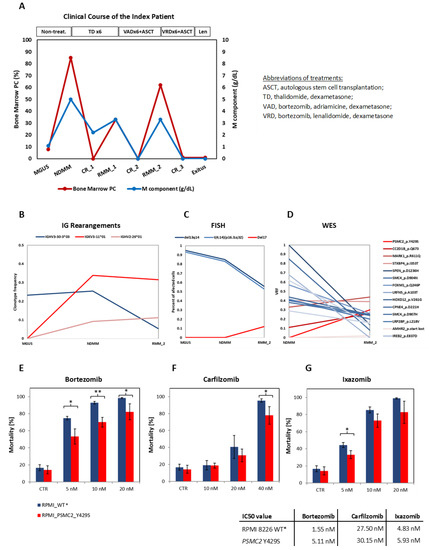
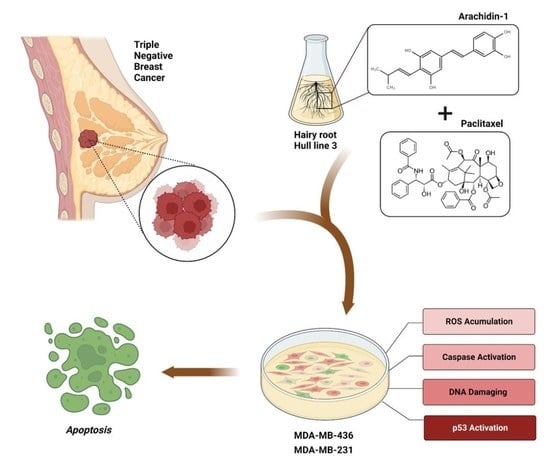
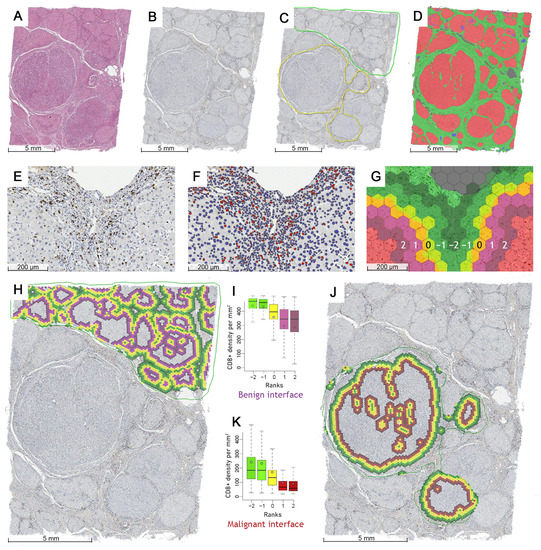
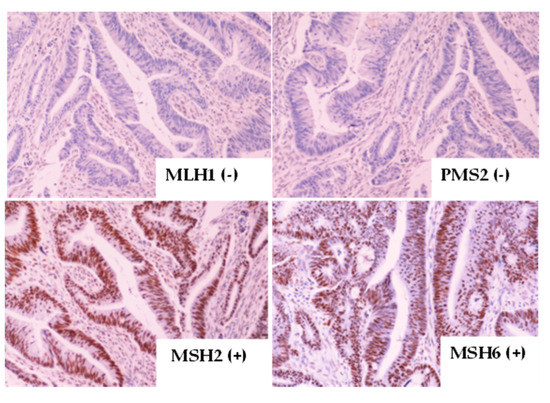
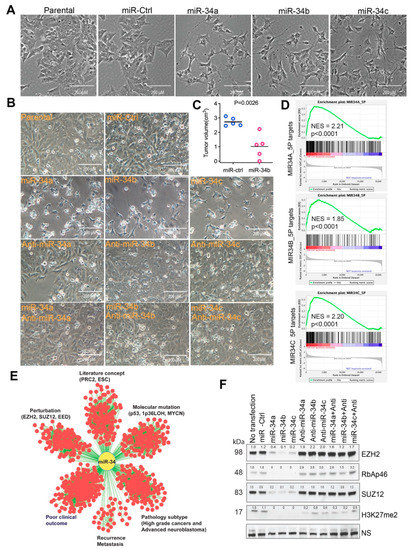
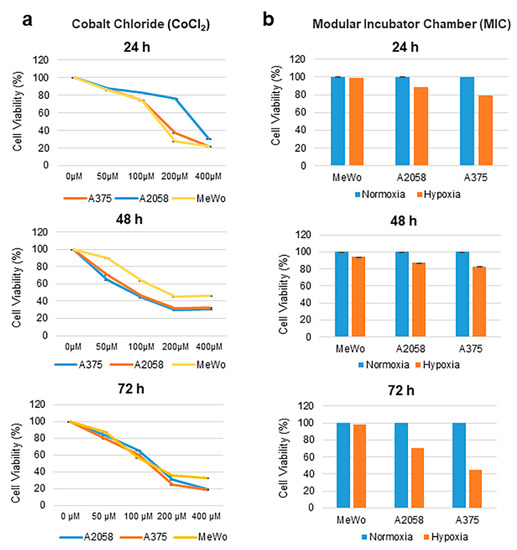
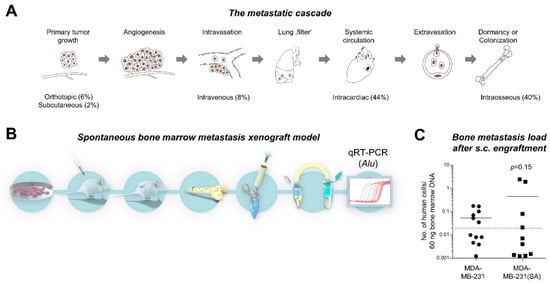
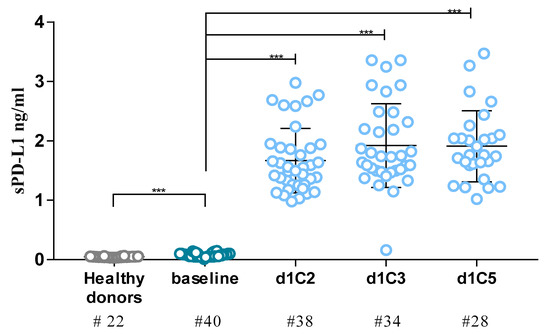
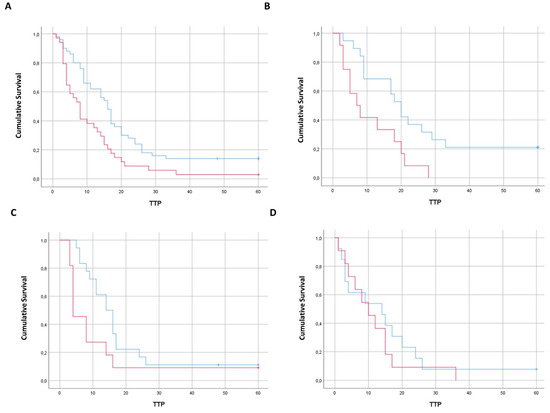
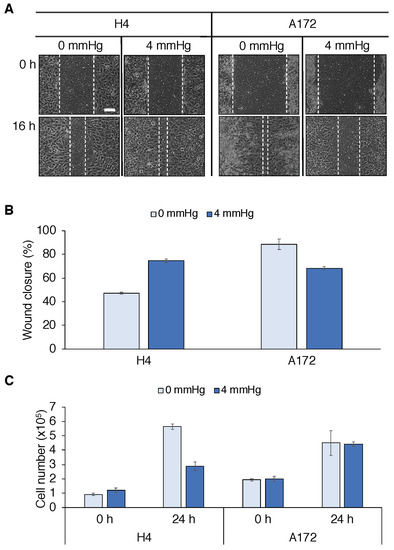
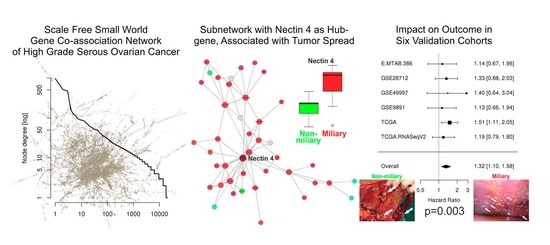
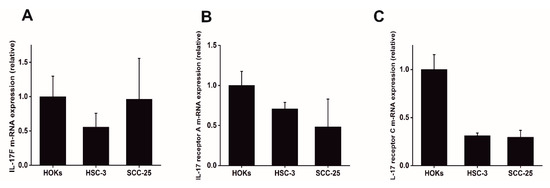
Targeting Solid Tumors
A topical collection in Cancers (ISSN 2072-6694). This collection belongs to the section "Tumor Microenvironment".
Viewed by 378592Editors
Interests: precision medicine; biomarkers; liquid biopsy; brain tumours; Hedgehog-Gli signaling; colorectal cancer; thyroid cancer; melanoma; obesity; diabetes
Special Issues, Collections and Topics in MDPI journals
Interests: sirtuins; hypoxia inflammation; metabolism; autophagy
Special Issues, Collections and Topics in MDPI journals
Topical Collection Information
Dear Colleagues,
Despite the fact that scientific achievements in the studies and therapies for cancer have reached levels that were unthinkable a few years ago, we are very far from an effective cure for solid tumors. The reason for this may be ascribed to many different aspects of such tumors, which are unraveling as we speak.
In particular, tumor microenvironment, metabolic and epigenetic changes, and immune evasion and adaptation are some of the aspects that render solid tumor eradication a real challenge.
In that respect, the current Topical Collection invites contributions aimed at exploring the different characteristics of solid tumors, focused on, but not limited to, the microenvironment, such as hypoxia, alteration of tumor vasculature; extracellular matrix; pH; epigenetic changes, such as methylation, acetylation, and so on; metabolic changes, such as glucose uptake, glutamine metabolism, and so on; modification of microRNA signature; and interaction with the immune system. At the same time, contributions should provide the state-of-the-art of the therapies associated to or proposed for each solid tumor aspect discussed.
Prof. Dr. Elisabetta Ferretti
Dr. Marco Tafani
Collection Editors
Manuscript Submission Information
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. All submissions that pass pre-check are peer-reviewed. Accepted papers will be published continuously in the journal (as soon as accepted) and will be listed together on the collection website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 250 words) can be sent to the Editorial Office for assessment.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are thoroughly refereed through a single-blind peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Cancers is an international peer-reviewed open access semimonthly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. The Article Processing Charge (APC) for publication in this open access journal is 2900 CHF (Swiss Francs). Submitted papers should be well formatted and use good English. Authors may use MDPI's English editing service prior to publication or during author revisions.
Keywords
- solid tumors
- targeted therapies
- tumor microenvironment (TME)
- immune evasion
- epigenetic changes
- microRNA signature
- metabolic features
- precision therapy
- drug resistance