Simple Summary
Brachytherapy for prostate cancer is a method where radiotherapy is directly delivered to the prostate via surgical insertion of the radioactive source. Brachytherapy can increase the amount of radiation delivered to the prostate cancer but sometimes at the cost of increased side effects. Here, we review promising early results from alternative non-invasive techniques that now exist and can deliver similar radiotherapy doses without the need for the surgical procedure required for brachytherapy.
Abstract
Prostate cancer (PC) is the most common malignancy in men. Internal radiotherapy (brachytherapy) has been used to treat PC successfully for over a century. In particular, there is level-one evidence of the benefits of using brachytherapy to escalate the dose of radiotherapy compared with standard external beam radiotherapy approaches. However, the use of PC brachytherapy is declining, despite strong evidence for its improved cancer outcomes. A method using external beam radiotherapy known as virtual high-dose-rate brachytherapy boost (vHDRB) aims to noninvasively mimic a brachytherapy boost radiation dose plan. In this review, we consider the evidence supporting brachytherapy boosts for PC and the continuing evolution of vHDRB approaches, culminating in the current generation of clinical trials, which will help define the role of this emerging modality.
1. Introduction
Radiotherapy is an effective treatment option for men with prostate cancer (PC), with disease control outcomes similar to what can be achieved with surgery [1]. Radiotherapy can be delivered either externally (external beam radiotherapy (EBRT)) or internally (brachytherapy). The advantage of brachytherapy is in the ability to deliver high radiation doses with rapid dose falloff, which improves the potential for tumour control while minimizing the risk of damage to adjacent healthy tissues. Increased radiotherapy doses have been found to be associated with higher tumour control probability (Figure 1). This relationship has been demonstrated in several randomized trials, which have reported better disease control outcomes with higher radiotherapy doses [2].
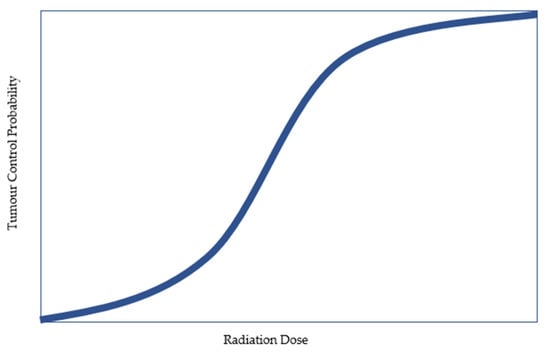
Figure 1.
Idealized tumour control probability curve.
Brachytherapy monotherapy is a proven treatment modality for favourable intermediate risk PC based on NCCN treatment guidelines, with excellent oncological outcomes [3,4]. Higher risk PC necessitates a combined modality approach with brachytherapy boosting and EBRT due to the risk of disease outside the prostatic capsule.
Brachytherapy has been used as a radiation dose-escalation strategy in several randomized trials, consistently demonstrating improved disease control compared with standard doses of EBRT [5,6,7]. Despite this, the use of brachytherapy in the management of PC has markedly declined internationally [8,9]. Simultaneously, technological advances have led to advanced EBRT techniques emerging, which have the ability to deliver higher doses of radiation accurately over a small number of treatment sessions or fractions. This technique is called stereotactic body radiotherapy (SBRT), and it has been widely investigated for the management of PC over the last two decades [10,11,12]. This has naturally led to efforts to apply SBRT techniques in an attempt to mimic the outcomes that can be achieved with brachytherapy. In this review, we focus on the biology and clinical evidence behind dose escalation for PC, aiming at a target audience consisting of all clinicians involved in the management of this disease. Furthermore, we explore the evidence supporting the employment—and, conversely, the declining use—of brachytherapy to achieve this, as well as the investigations into SBRT as an alternative to brachytherapy, which is termed virtual high-dose-rate brachytherapy boost (vHDRB). Our search strategy pursued to achieve this is outlined in Figure 2.
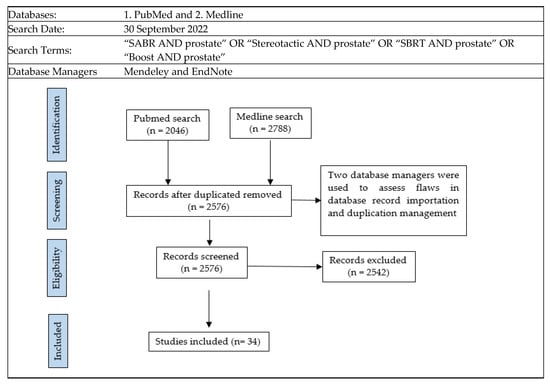
Figure 2.
Search strategy.
Selection Criteria
Titles and abstracts of records were searched to assess inclusion. The radiotherapy studies needed to combine a conventional schedule with a ”boost” schedule, with a high dose per fraction size. These records needed to report a cancer control endpoint and/or late toxicity endpoint. The records also needed to report the radiation dose used. If the abstract and/or title had details suggestive of the inclusion criteria, the full study was obtained and reviewed for further analysis. The criteria excluded records involving stereotactic monotherapy, simultaneous integrated boost (SIB), or radiotherapy delivered in the salvage setting. Only boost studies were selected for review, as this radiotherapy technique has emerged more recently. Both these techniques developed alongside one another; however, we await the results of randomized trials to adequately compare these two [13]. Studies that lacked significant details were also excluded from the analysis. For instance, in a multi-institutional patient registry study [14] that involved 437 patients, only a small proportion (5%) of the patients received a stereotactic boost; therefore, no conclusive findings could be drawn regarding this subgroup.
Thirty-four studies were found to meet the search criteria. Some study populations were described in more than one publication, such as in the work by Kim et al. [15]. For these 34 relevant studies, each publication’s references and citations were further searched and reviewed to assess the strength of the original search strategy. Google Scholar and Scopus were used for the citation search, resulting in a total of 680 citations. No further eligible records were discovered using this citation/reference search.
2. Radiation Dose Escalation—Theory and Evidence
Radiotherapy has been used for PC treatment for over a century. The rationale for dose escalation is that it improves tumour control probability (TCP). Conversely, increased radiation doses to healthy structures lead to higher rates of toxicity, a relationship described by the normal tissue complication probability (NTCP). There is, therefore, always a trade-off between maximizing TCP and ensuring NTCP is not unacceptably high.
Radiotherapy originally evolved from treatment close to normal tissue tolerance. With new technological advancements, higher radiotherapy doses could be given safely and/or NTCP decreased. A significant step forward in the development of EBRT was the advent of three-dimensional conformal radiation therapy and intensity-modulated radiation therapy (IMRT), where the radiotherapy beams are more precisely shaped to the desired target volume. With these developments, dose escalation trials began as early as the 1980s [16]. EBRT dose escalation evolved from a 2 Gy per fraction approach, with improved biochemical control seen across low-, intermediate-, and high-risk PC. The increasing dose has led to better biochemical control, with a plateau yet to be seen [17,18]. An MD Anderson Cancer Centre randomized phase 3 trial from 1993 to 1998 compared a 70 Gy arm to a 78 Gy arm. Using a relatively simple four-field box technique resulted in improved 15 year clinical control (hazard ratio (HR): 0.61, p = 0.042), distant metastasis-free survival rates (HR: 0.33, p = 0.018), and prostate cancer-specific survival (HR: 0.52, p = 0.045) in the 78 Gy arm [19]. The RTOG 0126 randomized trial compared a dose escalation of 70.2 Gy to 79.2 Gy, and 33.8% of the patients were managed with the more contemporary IMRT approach, where the treatment beams are dynamically shaped to better conform to the shape of the desired target. This trial showed, for intermediate-risk PC, improved 8 year biochemical control (HR: 0.54, p < 0.001), as well as a reduced need for salvage therapy (HR 0.63, p < 0.001), in the dose escalation arm [20]. These trials consistently showed increased rates of rectal and/or urinary toxicity, leading to efforts such as rectal spacing and image-guided treatment techniques being introduced to successfully offset such risks [21,22].
However, in radiotherapy, not every gray (Gy) may be equal, and new radiotherapy methods have been trailed to deliver dose escalation through different approaches. To comprehend this development, it is helpful to have an understanding of the biologically effective dose (BED). In short, a higher dose per fraction size can deliver a higher BED compared to conventional EBRT dose schedules of 2 Gy per fraction. Each tissue and tumour has its own radiation fraction size sensitivity described by the alpha/beta ratio. By using a higher dose per fraction size, theoretically, this should result in a higher biological dose being delivered to the tumour and lead to greater tumour cell death. Table 1 shows examples of BED dosing for different delivered fraction sizes.

Table 1.
BED examples using a prostate cancer alpha/beta ratio of 1.5 [23].
3. Brachytherapy as a Dose-Escalation Strategy
Around a century ago, brachytherapy using radium, which involved permanent low-dose-rate (LDR) implants, emerged as the first successful radiotherapy modality for prostate cancer treatment. Despite the changes in isotopes used, LDR monotherapy has consistently demonstrated excellent oncological outcomes in patients with favourable intermediate-risk PC. In the 1980s, as external beam radiation therapy (EBRT) techniques, such as 3D conformal radiotherapy, evolved, brachytherapy also saw technological advancements, including the introduction of temporary high-dose-rate (HDR) insertions. Following these developments, the combination of these two techniques was pursued to improve oncological outcomes in patients with higher risk PC. The approach involves using brachytherapy to intensify doses in areas with higher disease burden while delivering lower doses via EBRT to microscopic areas. This approach has shown great promise in improving oncological outcomes in patients with higher risk PC. There are three randomized control trials comparing EBRT to EBRT with brachytherapy boost, which have reported improved biochemical control in the brachytherapy boost arms [5,6,7].
The ASCENDE-RT randomized trial compared dose-escalated EBRT (78 Gy in 39 fractions) to EBRT (46 Gy in 23 fractions) + an LDR brachytherapy boost of 115 Gy using Iodine-125 implants. In the brachytherapy boost arm, the 10 year risk for biochemical failure was reduced compared to the dose-escalated EBRT arm (85% vs. 67%, p < 0.001) [24]. The ASCEND-RT trial did report increased toxicity rates in the brachytherapy boost arm compared to the dose-escalated EBRT arm [25]. At 5 years, there was a significant difference in the cumulative incidence of severe grade-three genitourinary (GU) toxicity: 18.4% for the LDR boost arm versus 5.2% in the dose-escalated EBRT arm. These included outcomes such as incontinence and need for catheterization. This trial also summarized toxicities from other brachytherapy boost trials (both HDR and LDR), which showed a wide range in severe late GU toxicity incidence from 1.4% to 31% [25].
4. Diminishing Role of Brachytherapy
Despite the excellent advantages in efficacy from using brachytherapy boost as a dose-escalation strategy, the practice has not been universally adopted and, indeed, is showing a marked decline [8,9]. MD Anderson, in a study from 2010 to 2015, reported that use of prostate brachytherapy was significantly declining both as a monotherapy and as EBRT combination therapy [26]. EBRT use increased during this timeframe. This was not unique and correlated to declining use across Australia [27]. Older age (>70), higher risk subgroups, and treatment at an academic centre correlated with decreased brachytherapy utilization.
The reason for the declining brachytherapy use is likely multifactorial. One factor is the concern amongst radiation oncologists about the high toxicity rates that brachytherapy may cause, resulting in outcomes such as urethral stricture formation. The technical expertise required for optimal outcomes can be a challenge to obtain, with improper needle insertion causing urethral traumatization and catheter migration contributing to higher organ-at-risk (OAR) doses. Further factors may include poor financial remuneration, decreased trainee exposure, and travel distance to expert centres. Further challenges faced by brachytherapy uptake include poor concordance amongst groups in terms of isotope use, prescribed dose, and integration with androgen deprivation therapy (ADT) and a relative lack of level-one evidence comparing EBRT with brachytherapy boost to dose-escalated EBRT. As brachytherapy use has declined, improved EBRT techniques have come under development, such as SBRT, which potentially allows for dose escalation using a less invasive approach.
5. Emergence of SBRT
Early monotherapy SBRT trials looked at keeping an isoequivalent BED using a pure SBRT approach. The HYPO-RT-PC phase 3 randomized controlled trial (RCT) [11] compared 78 Gy in 39 fractions with 42.7 Gy delivered over seven sessions and showed equivalent 5 year biochemical PFS in both approaches at 84% (95% CI: 80–87), with an adjusted HR of 1.002 (95% CI: 0.758–1.325; log-rank p = 0·99). The more common five-fraction approach is also being investigated, with early results available from the PACE-B phase 3 RCT [12], which compared 78 Gy in 39 fractions with 36.25 Gy in 5 fractions. This trial showed that the 2 year RTOG toxicity rates were similar between the two arms, with efficacy data expected in 2023.
Virtual HDR radiotherapy monotherapy trials started as early as 2008, with Fuller [28] undertaking a ten-patient pilot trial utilizing a CyberKnife platform. Shortly after, several small vHDRB trials started between 2010 and 2012, with early investigators including Mirallbel (Spain), Oermann (USA/Washington), Anwar (USA/San Francisco), and Katz and Kang (USA/New York).
This literature review search retrieved 34 relevant records for review. Some researchers used the same study population to publish multiple times. When this was accounted for and these studies were conglomerated, 26 unique patient populations were left. The characteristics and results of these studies are summarized in Table 2.

Table 2.
Studies using vHDRB.
5.1. Biochemical Progression-Free Survival
Despite the large heterogeneity amongst the trials listed in Table 2, excellent bcPFS and other closely associated endpoints can be noted. vHDRB studies included low- to very-high-risk PC patients, with efficacious biochemical control seen across the groups. Paydar [41] showed a 3 year bcPFS of 100% for intermediate-risk PC. Kim’s study [15] without ADT showed an 8 year bcFFS of 100% for intermediate-risk PC and 77.8% for high-risk PC. However, this needs to be taken in the context of the small numbers in this study, which had only 11 high- and 31 intermediate-risk patients. Larger, prospective phase 2 multicentre trials are listed in Table 2, such as the CKNO-PRO and the PROMETHEUS trials. For example, the latter included a mixture of 135 intermediate- and high-risk patients with 2 year bcPFS of 98.6%.
These results are similar to conventional radiotherapy, with high-risk PC patients in the HYPO-PROST trial randomized to a vHDRB arm and conventional dose arm and reports of 5 year bcRFS being 78.2% and 82.9% [57]. This closely follows the ASCENDE-RT brachytherapy boost arm in terms of outcomes.
There are no strong randomized data to compare HDR brachytherapy boost to vHDRB, and future randomized control trials are needed to answer this. The closest data to compare these two approaches come from a propensity score-matched analysis by Chen [59]. This study included 131 patients for the vHDRB and 101 patients for the HDR brachytherapy boost. The median follow-up was 73.4 months for vHDRB and 186 months for the HDR brachytherapy boost. One of the study’s strengths lay in accounting for a large number of known covariates. The majority of PC patients included were high- and very-high-risk PC patients, as defined by NCCN criteria. The five- and ten-year unadjusted bcRFS rates were 88.8% and 85.3% for the vHDRB compared to 91.8% and 74.6% for the HDR boost brachytherapy. Metastasis-free survival was also analysed and, again, no statistical significance was seen in the difference between the two groups. The vHDRB used two dose schedules, 19 Gy and 21 Gy, both in two fractions, with no statistically significant differences between these two.
Two studies appeared to be divergent from the rest in terms of poor disease control. Koh’s study [38], reported a 50% bcPFS at a median cohort follow-up of 29.3 months. This may have been due to the inclusion of metastatic disease patients and low patient numbers. Khmelevsky [35] reported a 60% 5 year bcRFS, which may have related to the initial proton use and different boost schedules.
Most of these studies staged patients with conventional CT and bone scans. Radiomics has the potential to offset some of the poor sensitivity and specificity issues [62]. PSMA PET staging has been shown to be superior compared to conventional staging [63], and future trials may be expected to have better biochemical control due to improved patient selection.
Despite the heterogeneity in these trials in the magnitude of factors, the oncological results are concordant and excellent. These trials spanned multiple countries and institutions. Certain questions and challenges remain in defining outcomes and measurable endpoints: first, the varied dose schedules and dose distributions, and second, cofounders, such as ADT use/duration and pelvic nodal radiotherapy inclusion. Different endpoints were used in these trials and the definition for biochemical relapse varied, although Phoenix criteria were most commonly used. Many studies were small in patient numbers and had short follow-up times.
5.2. Toxicities
The use of a high dose per fraction size has a long history in radiotherapy, with increased toxicity rates [64]. Table 2 illustrates that, amongst the vHDRB trials, toxicities were generally much lower.
Acute grade 2 (G2) GU toxicities with vHDRB ranged from 17% to 47%, with grade 3 (G3) ranging from 0% to 4% [65]. Acute GI toxicity with vHDRB had a range of 0% to 21% for G2, with no acute G3 being identified [65]. These toxicity rates are similar to that of dose-escalated EBRT.
These studies reported late GU G2 toxicity ranging from 1% to 25% and G3 from 0% to 5%. Most patients in these studies had a late G3 GU toxicity of less than 3%. Late G2 GI toxicities ranged from 0% to 18% and G3 from 0% to 4%.
vHDRB late toxicity data differed from those for conventional fractionation radiotherapy. There appeared to be a flare in subacute toxicity around the 12–18 months range [52]. This was similar to the brachytherapy toxicity data from the ASCENDE-RT study [25]. Even though the cumulative incidence was high in this trial over time, most of the subacute toxicity resolved by the 2 year mark. Interestingly, this 12 month GU flare toxicity seemed to be lower in vHDRB compared to SBRT monotherapy (7.60 ± 0.42 and 9.53 ± 0.47, p = 0.003), as reported by Feng using the American Urological Association (AUA) symptom index [48]. Katz and Kang [31] may also support this, with lower late GU G3 toxicity seen in the vHDRB group compared to the SBRT monotherapy arm (2.3% versus 3.9%) and 2.3% versus 7.8% for late GU G2 toxicity. These data were nonrandomized, and contradictory observations have also been cited from other institutions [37]. Chen [59], using a propensity score-matched analysis to compare vHDRB to HDR brachytherapy boost, found no significant difference in the combined rates of G3+ toxicities between these two groups.
There were five patients in total identified as exhibiting grade-four toxicity. Novikov [61] reported 3 patients out of 51 who developed severe rectal toxicity and required diversion colostomies. One of these three patients also had significant bleeding requiring ICU support. Moreover, one patient in Alayed’s study [49] experienced a rectal fistula, which repeated rectal biopsies were thought to have contributed to. The last patient in Pollack’s study [53] developed sepsis after post-treatment transurethral resection.
Toxicity data from all these studies have multiple contributing factors that are not easily teased out. An example is pelvic nodal irradiation and the effect on GI toxicity. Some studies combine G2+ toxicities, whereas others divide G2 and G3. There were differences in scoring and statistical calculation methods. Some series reported toxicity as the prevalence at a specific time-point while others cited a cumulative toxicity result. Overall, these data support the safety profile for using vHDRB for PC, and this treatment modality may have lower late toxicities compared to brachytherapy. The main caveat is that much of the data came from relatively small, single-institution series and were nonrandomized.
6. SBRT as a Dose-Escalation Strategy—Virtual Boosting
vHDRB can be delivered through a variety of methods. The first key variable is the platform. Although there are various subcategories within each of the following classes, platforms can be broadly categorized as CyberKnife (CK), linear accelerator, or protons/other heavy particles. CK is a unique, compact linear accelerator design with the treatment head mounted on a manoeuvrable robotic arm that allows for radiotherapy delivery to be undertaken at a large number of non-coplanar angles. Each radiotherapy beam delivers a small cylindrical radiotherapy dose profile, with radiation plans being composed of over 100 of these small columnar beams. CK has a unique stereoscopic image guidance system that directs the beam to improve delivery accuracy with motion.
The most common of all radiotherapy machines is the standard linear accelerator. With modern linear accelerators, dose delivery tends to be undertaken with volumetric modulated arc therapy. Radiotherapy is delivered using two to three beam planar arcs, with dose modulation employed from multileaf collimators. Lastly, there are heavy particles, with protons being the most commonly used. Heavy particles theoretically have an improved radiobiology profile compared to the photons described above. Compared to photons, protons have the ability to deliver a large dose at a certain depth. This ability allows for improved sparing of normal tissue; however, the ability to shape the dose tends to be more limited compared to photons.
The second variable is the dose/radiotherapy schedule, and a wide variety of schedules can be seen, as illustrated in Table 2, with the common theme of trying to mimic the physical brachytherapy boost approaches given over two to four sessions.
The last variable is the dose distribution, which varies between centres and trials. Some methods employ a relatively homogeneous whole-gland dose distribution (Figure 3A), whereas others aim to introduce inhomogeneity to replicate a brachytherapy implant with the purposeful aim of creating “hotspots” within defined volumes to receive up to 150% of the prescribed dose. These virtual volumes are intended to direct hotspots towards brachytherapy-type structures, such as spheres or cylinders. The CK platform can better replicate a virtual cylinder dose distribution compared to a standard LINAC due to the large number of non-coplanar angles used during delivery. A “hot shell” distribution aims to create hotspots along the prostate periphery with central urethra cooling (Figure 3B).
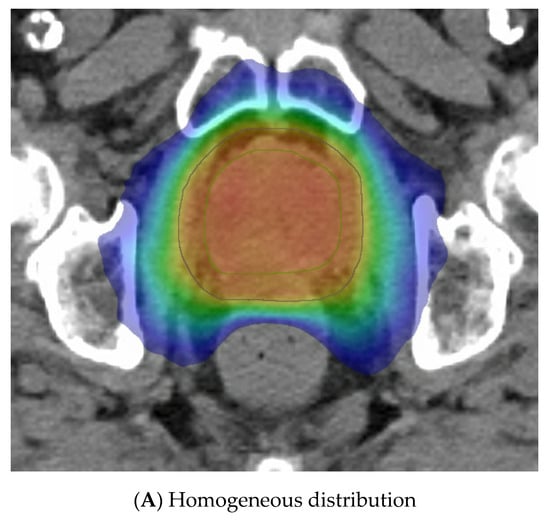
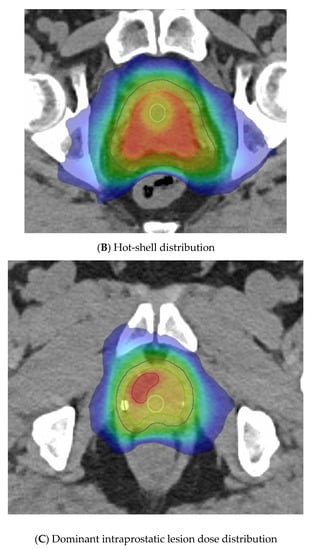
Figure 3.
Examples of virtual high-dose-rate brachytherapy boost radiotherapy dose distributions, with the colour gradient demonstrating 50% of the dose in blue up to the maximum doses in red. The green outline represents the prostate, the blue outline the planning target volume (PTV), yellow is the urethra, and burgundy the DIL, when outlined. (A) Homogenous distribution with 100% of the dose in red. (B) Hot-shell distribution showing the red 150% dose directed towards the prostate peripheral zone, with lime being the 100% dose. (C) Dominant intraprostatic lesion distribution, with the 150% dose in red directed towards the DIL and 100% dose in lime covering the PTV.
Another method gaining traction due to the FLAME RCT [66] is dominant intraprostatic lesion (DIL) boosting, where the boost dose is concentrated in the image-defined cancer volume (Figure 3C). FLAME used a simultaneous integrated boost and delivered a dose of up to 95 Gy in 35 fractions to the MRI DIL, with the whole prostate gland receiving 77 Gy in 35 fractions. A total of 84% of these patients were high risk and two thirds received ADT for up to 3 years. This trial reported improved 5 year biochemical disease-free survival (bcDFS), increasing from 85% to 92%, in the boost arm (HR: 0.45, 95% CI: 0.28 to 0.71, p < 0.001) without any significant increase in late GI or GU toxicities. The BOOSTER trial [50] integrated DIL boost with vHDRB, with three dose levels given. After the conventional radiotherapy dose of 46 Gy in 23 fractions, the boost used 20 Gy, 22 Gy, or 24 Gy to the prostate and, correspondingly, 25 Gy, 27.5 Gy, and 30 Gy to the DIL, all in two fractions. The highest dose of 30 Gy in two fractions was modelled to reflect the 150% isodose distribution of an HDR plan but preferentially directed towards the DIL.
7. Technical Issues with Virtual Boosting
The ability to implement such high radiotherapy doses per fraction size is linked with the development and application of technological advancements. These can be subdivided into three categories; improved target delineation, enhanced accuracy of treatment delivery, and the use of organ-at-risk (OAR) stabilizers or physical barriers. The stereotactic boost trials shared some similarities with these technological advancements. They often used MRI to better delineate structures, fiducials to help improve accuracy through inter- and intra-fraction motion management, and bladder-filling/rectal-emptying/low-gas-diet protocols.
7.1. Simulation Imaging
Due to its improved ability to accurately define the prostate and other relevant parts of the anatomy [67], MRI has helped substantially in safely delivering stereotactic radiotherapy. The PROMETHEUS trial with 135 patients employed MRI fusion for radiotherapy planning. MRI helps with delineation of all structures, including the DIL (Figure 4). This was a common feature in all of these vHDRB studies. Future studies can also be expected to utilize PSMA staging for both more accurate staging and DIL delineation.
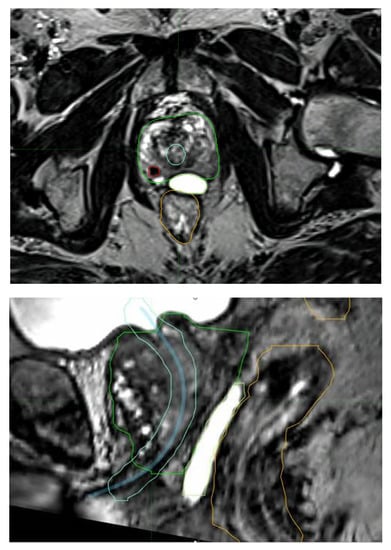
Figure 4.
MRI fusion aiding with the identification of a dominant intraprostatic lesion (red), the urethra with expansion (blue and light blue), rectal sparing hydrogel (lime), the rectum (orange), and the prostrate (green).
7.2. Image Guidance
Intrafraction image guidance is another technological advancement that has helped in the delivery of stereotactic radiotherapy doses whilst minimizing GI and GU toxicity. With fiducial placement and IGRT planning, treatment volumes can be reduced substantially. Most of the trials in Table 2 required fiducial placements and image guidance.
A variety of systems can be employed to help safely reduce margins and improve radiotherapy targeting accuracy. CyberKnife was one of first of such systems utilized. Calypso uses inserted radiofrequency transponders to track and adjust radiation treatment beams. The Varian Truebeam gating is another integrated package commonly used for prostate intrafraction motion management to help enable precision fiducial targeting. Other approaches are also being assessed, such as kilovoltage intrafraction monitoring (KIM), as validated in the TROG 15.01 SPARK trial [10,68]. This technology allows for image verification X-rays to be obtained in real time while prostate radiotherapy is being delivered. One of the advantages of KIM is that it enables strategies such as patient shifting or beam shifting during treatment. KIM has already been shown to have improved treatment accuracy, with the average systematic accuracy measured at 0.46 mm [69], and has the potential for further reductions to the treatment planning margins. As a “proof of principle”, the latter three systems described above were all successfully utilized in the BOOSTER vHDRB trial [50].
The trials mentioned in Table 2 tended to have PTV margins from 5 to 7 mm isometrically, except for the 3 to 5 mm posterior. The exception was the Jabbari/Anwar trial, which utilized a 0 mm PTV margin for part of the cohort and 2 mm isometric margin for the rest. These tight margins are only potentially achievable using real-time image-guided radiotherapy, with recent data suggesting that an MRI-LINAC may permit uniform margin reductions to 2 mm for prostate SBRT, although longer term efficacy data are required to confirm there is no loss of efficacy with this aggressive margin reduction [70].
7.3. OAR Stabilization Devices and Hydrogel Spacers
Endorectal devices were utilized in some of these trials to help minimize rectal toxicity. The endorectal balloon works by inflating a rectal balloon near the prostate, which decreases the radiotherapy dose to the rectum and helps avoid circumferential radiotherapy dose delivery. Miralbell [29] used this approach, stating concerns that the higher late GI toxicities seen may have been caused by the balloon pushing the anterior rectal wall closer to the high-dose prostate radiotherapy region. It was also seen that, in one third of patients, the rectal balloon exhibited systematic errors due to air leakage, suboptimal inflation, or suboptimal rectal emptying.
A rectal displacement device is a rigid device that aims to reduce variations in rectal filling and intrafraction movement. It works by pulling the posterior rectal wall away from the prostate and thereby decreasing the radiation dose to the rectum (Figure 5A) [14,71].
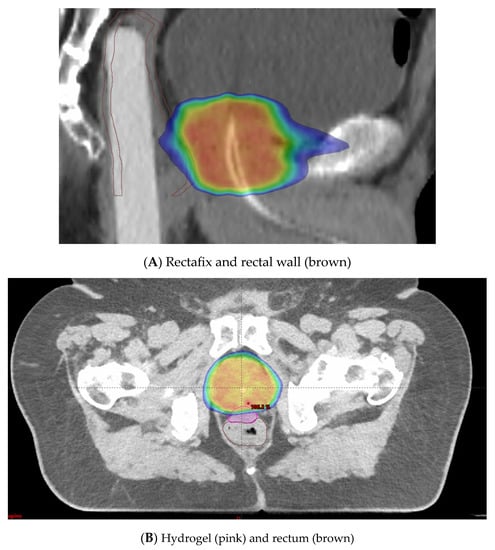
Figure 5.
(A) Rectafix in use to mitigate circumferential rectal radiotherapy doses. (B) Hydrogel in use to prevent high radiotherapy doses to anterior rectal wall. Blue is 50% isodose distribution. Red is 100% isodose distribution.
Hydrogel spacer placement is an alternative method employed to minimize rectal toxicity. The anterior rectal wall lies in close proximity to the prostate, with only 2 to 3 mm typically separating the target volume from this dose-limiting OAR [72]. Injection of a hydrogel spacer to increase this distance can be employed (Figure 5B). This allows the higher radiation doses to fall in the spacer region instead of the anterior rectal wall. A systematic review and meta-analysis on perirectal hydrogel spacer placement with 1011 patients [73] showed that this method was associated with less rectal irradiation, fewer GI toxicities, and higher bowel-related quality of life in the long-term follow-up. This study also showed with 486 patients that the space between the rectum and prostate had a median distance of 11.2 mm. In a multicentre trial, 222 patients were randomized to hydrogel injection and no injection [74]. The trial demonstrated a decrease in rectal dose and a significant reduction in late rectal toxicity (2.0% versus 7.0% in the control group, p = 0.04) with the use of hydrogel insertion.
7.4. Radiotherapy Platform
The trials identified in Table 2 used mixed radiotherapy delivery systems. Some studies used CyberKnife and others used conventional LINAC. Radiotherapy boost was utilized in the proton and carbon ion settings. The results showed no obvious indications as to which platform performed better with regard to the oncological outcomes. For this to be seen, one would assume that much larger patient numbers would be needed. The PACE-B trial [12] mentioned some differences in toxicities seen between CyberKnife and standard linear accelerator approaches; however, the low numbers precluded statistical analysis. The NINJA RCT included both CK and LINAC patients, so it should be informative as to whether any clinically relevant differences exist between these two particular approaches [13].
8. Future Directions
High-quality prospective randomized data are needed before vHDRB can be considered the standard of care. Currently active trials were identified using the ClinicalTrials.gov database with the disease term “Prostate cancer” and the other term “boost”. This resulted in 162 studies for screening. The same eligibility criteria as listed above were applied. Fourteen relevant trials were identified from the ClinicalTrials.gov database that involved delivery of a vHDRB for treatment of PC. These results are shown in Table 3.

Table 3.
Future stereotactic boost trials identified from the ClinicalTrials.gov database.
Four randomized trials stood out as helping to evaluate the true impact of a vHDRB on PC. The first was NCT03380806 from Hamilton, Canada. This trial of 100 high-risk patients looked into delivering conventional dosing of 45 Gy in 25 fractions, then randomized patients to either further conventional radiotherapy of 32–33 Gy in 15–16 fractions or a vHDRB of 19.5–21 Gy in 3 fractions. ADT will be given for a total of 3 years and outcome measures will be quality of life, safety, and efficacy.
The second study (NCT01839994), from Gliwice, Poland, aimed to recruit 350 intermediate- and high-risk patients to a phase 3 randomized trial. The trial aimed to randomize patients to a cohort with 76–78 Gy in 38–39 fractions and a cohort with 50 Gy in 25 fractions + boost. The boost radiotherapy decision will be made using a nonrandomized approach between either HDR brachytherapy (20 Gy in two fractions) or vHDRB (20 Gy in two fractions). ADT will also be given for 3 years for high-risk patients. The primary endpoint will be freedom from biochemical failure at 3 years. The trial allows for PSMA PET staging. This study will hopefully shed more light on the difference between SBRT vs. brachytherapy boost. Another future trial that will help discern the differences between brachytherapy and SBRT is the ASCENDE-SBRT trial [75]. This trial aims to randomize 710 unfavourable intermediate-risk and high-risk PC patients to either a schedule with whole pelvic radiotherapy + brachytherapy boost with 46 Gy in 23 fractions or an ultra-hypofractionated schedule of 25 Gy in 5 fractions administered to the whole pelvis + a vHDRB boost of 40 Gy in 5 fractions.
The final study (NINJA/TROG 18.01) is an international, multicentre trial looking to randomize 472 unfavourable intermediate-risk and high-risk patients between a monotherapy SBRT arm with 40 Gy in 5 fractions and an arm with 36 Gy in 12 fractions that includes a 20 Gy vHDRB in 2 fractions. For high-risk patients, PSMA PET staging is included. Another new technological advancement in this study is the use of only MRI planning. Radiotherapy plans are created with a synthetic CT derived from the MRI [76,77]. ADT is given for 6 months. The primary endpoint will be biochemical control at 5 years. This study will aim to tease out whether there are any efficacy and/or toxicity differences between SBRT monotherapy and vHDRB.
9. Conclusions
This review summarizes results from the published literature regarding the use of vHDRB in the treatment of PC. Although most of the series are relatively small, single-centre trials, emerging multicentre data from larger series with longer follow-ups suggest that vHDRB has high efficacy and a favourable toxicity profile. Current randomized trials will help determine whether vHDRB has a wider role as an option in the future standard management of prostate cancer.
Author Contributions
E.W.: Conceptualization; Formal analysis; Methodology; Supervision; Validation; Visualization; Writing—original draft; Writing—review and editing. J.S.: Visualization; Writing—review and editing. M.S.: Validation; Writing—review and editing. Y.T.: Validation; Writing—review and editing. S.S.: Validation; Writing—review and editing. S.D.: Validation; Writing—review and editing. N.M.: Validation; Writing—review and editing. J.M.M.: Conceptualization; Formal analysis; Methodology; Supervision; Validation; Visualization; Writing—original draft; Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no potential conflict of interest with respect to this article’s research, authorship, and/or publication.
References
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef] [PubMed]
- Trada, Y.; Plank, A.; Martin, J. Defining a dose-response relationship for prostate external beam radiotherapy. J. Med. Imaging Radiat. Oncol. 2013, 57, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Matzkin, H.; Chen, J.; Agai, R.; Ziv-Baran, T.; Mabjeesh, N.J. Long-term biochemical progression-free survival following brachytherapy for prostate cancer: Further insight into the role of short-term androgen deprivation and intermediate risk group subclassification. PLoS ONE 2019, 14, e0215582. [Google Scholar] [CrossRef]
- Lazarev, S.; Thompson, M.R.; Stone, N.N.; Stock, R.G. Low-dose-rate brachytherapy for prostate cancer: Outcomes at >10 years of follow-up. BJU Int. 2018, 121, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Dayes, I.S.; Parpia, S.; Gilbert, J.; Julian, J.A.; Davis, I.R.; Levine, M.N.; Sathya, J. Long-Term Results of a Randomized Trial Comparing Iridium Implant Plus External Beam Radiation Therapy with External Beam Radiation Therapy Alone in Node-Negative Locally Advanced Cancer of the Prostate. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 90–93. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, P.J.; Rojas, A.M.; Ostler, P.J.; Bryant, L.; Lowe, G.J. Randomised trial of external-beam radiotherapy alone or with high-dose-rate brachytherapy for prostate cancer: Mature 12-year results. Radiother. Oncol. 2021, 154, 214–219. [Google Scholar] [CrossRef]
- Morris, W.J.; Tyldesley, S.; Rodda, S.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; Murray, N. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 275–285. [Google Scholar] [CrossRef]
- Roberts, M.J.; Papa, N.; Perera, M.; Scott, S.; Teloken, P.E.; Joshi, A.; Vela, I.; Pryor, D.; Martin, J.; Woo, H. A contemporary, nationwide analysis of surgery and radiotherapy treatment for prostate cancer. BJU Int. 2019, 124 (Suppl. S1), 31–36. [Google Scholar] [CrossRef]
- Gomez-Iturriaga, A.; Keyes, M.; Martin, J.; Spratt, D.E. Should brachytherapy be added to external beam radiotherapy for prostate cancer? Lancet Oncol. 2022, 23, 23–25. [Google Scholar] [CrossRef]
- Keall, P.; Nguyen, D.T.; O’Brien, R.; Hewson, E.; Ball, H.; Poulsen, P.; Booth, J.; Greer, P.; Hunter, P.; Wilton, L.; et al. Real-Time Image Guided Ablative Prostate Cancer Radiation Therapy: Results from the TROG 15.01 SPARK Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 530–538. [Google Scholar] [CrossRef]
- Widmark, A.; Gunnlaugsson, A.; Beckman, L.; Thellenberg-Karlsson, C.; Hoyer, M.; Lagerlund, M.; Kindblom, J.; Ginman, C.; Johansson, B.; Bjornlinger, K.; et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019, 394, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Tree, A.C.; Ostler, P.; van der Voet, H.; Chu, W.; Loblaw, A.; Ford, D.; Tolan, S.; Jain, S.; Martin, A.; Staffurth, J.; et al. Intensity-modulated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): 2-year toxicity results from an open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2022, 23, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Keall, P.; Siva, S.; Greer, P.; Christie, D.; Moore, K.; Dowling, J.; Pryor, D.; Chong, P.; McLeod, N.; et al. TROG 18.01 phase III randomised clinical trial of the Novel Integration of New prostate radiation schedules with adJuvant Androgen deprivation: NINJA study protocol. BMJ Open 2019, 9, e030731. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.; Sharma, S.; Shumway, R.; Perry, D.; Bydder, S.; Simpson, C.K.; D’Ambrosio, D. Stereotactic Body Radiotherapy for Clinically Localized Prostate Cancer: Toxicity and Biochemical Disease-Free Outcomes from a Multi-Institutional Patient Registry. Cureus 2015, 7, e395. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.S.; Kim, W.C. Long-term outcome of whole pelvis radiotherapy and stereotactic body radiotherapy boost for intermediate and high risk prostate cancer. Int. J. Radiat. Res. 2022, 20, 37–41. [Google Scholar] [CrossRef]
- Kupelian, P.A.; Ciezki, J.; Reddy, C.A.; Klein, E.A.; Mahadevan, A. Effect of increasing radiation doses on local and distant failures in patients with localized prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 16–22. [Google Scholar] [CrossRef]
- Viani, G.A.; Stefano, E.J.; Afonso, S.L. Higher-than-conventional radiation doses in localized prostate cancer treatment: A meta-analysis of randomized, controlled trials. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1405–1418. [Google Scholar] [CrossRef]
- Diez, P.; Vogelius, I.S.; Bentzen, S.M. A new method for synthesizing radiation dose-response data from multiple trials applied to prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1066–1071. [Google Scholar] [CrossRef]
- Pasalic, D.; Kuban, D.A.; Allen, P.K.; Tang, C.; Mesko, S.M.; Grant, S.R.; Augustyn, A.A.; Frank, S.J.; Choi, S.; Hoffman, K.E.; et al. Dose Escalation for Prostate Adenocarcinoma: A Long-Term Update on the Outcomes of a Phase 3, Single Institution Randomized Clinical Trial. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 790–797. [Google Scholar] [CrossRef]
- Michalski, J.M.; Moughan, J.; Purdy, J.; Bosch, W.; Bruner, D.W.; Bahary, J.P.; Lau, H.; Duclos, M.; Parliament, M.; Morton, G.; et al. Effect of Standard vs Dose-Escalated Radiation Therapy for Patients with Intermediate-Risk Prostate Cancer: The NRG Oncology RTOG 0126 Randomized Clinical Trial. JAMA Oncol. 2018, 4, e180039. [Google Scholar] [CrossRef]
- Hamstra, D.A.; Mariados, N.; Sylvester, J.; Shah, D.; Karsh, L.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, R.A.; et al. Continued Benefit to Rectal Separation for Prostate Radiation Therapy: Final Results of a Phase III Trial. Int. J. Radiat. Oncol. Biol. Phys. 2017, 97, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, G.; Martin, J.; Plank, A.; Wong, W. Incremental changes verses a technological quantum leap: The additional value of intensity-modulated radiotherapy beyond image-guided radiotherapy for prostate irradiation. J. Med. Imaging Radiat. Oncol. 2014, 58, 503–510. [Google Scholar] [CrossRef]
- Fowler, J.F. The radiobiology of prostate cancer including new aspects of fractionated radiotherapy. Acta Oncol. 2005, 44, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Tyldesley, S.; Pai, H.; McKenzie, M.; Halperin, R.; Duncan, G.; Morton, G.; Keyes, M.; Hamm, J.; Morris, W.J. An Updated Analysis of the Survival Endpoints of ASCENDE-RT. Int. J. Radiat. Oncol. Biol. Phys. 2022, 115, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Rodda, S.; Tyldesley, S.; Morris, W.J.; Keyes, M.; Halperin, R.; Pai, H.; McKenzie, M.; Duncan, G.; Morton, G.; Hamm, J.; et al. ASCENDE-RT: An Analysis of Treatment-Related Morbidity for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost with a Dose-Escalated External Beam Boost for High- and Intermediate-Risk Prostate Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2017, 98, 286–295. [Google Scholar] [CrossRef]
- Andring, L.; Yoder, A.; Pezzi, T.; Tang, C.; Kumar, R.; Mahmood, U.; Walker, G.V. PSA: Declining utilization of prostate brachytherapy. Brachytherapy 2022, 21, 6–11. [Google Scholar] [CrossRef]
- Ong, W.L.; Evans, S.M.; Millar, J.L. Under-utilisation of high-dose-rate brachytherapy boost in men with intermediate-high risk prostate cancer treated with external beam radiotherapy. J. Med. Imaging Radiat. Oncol. 2018, 62, 256–261. [Google Scholar] [CrossRef]
- Fuller, D.B.; Naitoh, J.; Lee, C.; Hardy, S.; Jin, H. Virtual HDR CyberKnife treatment for localized prostatic carcinoma: Dosimetry comparison with HDR brachytherapy and preliminary clinical observations. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 1588–1597. [Google Scholar] [CrossRef]
- Miralbell, R.; Moll, M.; Rouzaud, M.; Hidalgo, A.; Toscas, J.I.; Lozano, J.; Sanz, S.; Ares, C.; Jorcano, S.; Linero, D.; et al. Hypofractionated boost to the dominant tumor region with intensity modulated stereotactic radiotherapy for prostate cancer: A sequential dose escalation pilot study. Int. J. Radiat. Oncol. Biol. Phys. 2010, 78, 50–57. [Google Scholar] [CrossRef]
- Oermann, E.K.; Slack, R.S.; Hanscom, H.N.; Lei, S.; Suy, S.; Park, H.U.; Kim, J.S.; Sherer, B.A.; Collins, B.T.; Satinsky, A.N.; et al. A Pilot Study of Intensity Modulated Radiation Therapy with Hypofractionated Stereotactic Body Radiation Therapy (SBRT) Boost in the Treatment of Intermediate- to High-Risk Prostate Cancer. Technol. Cancer Res. Treat. 2010, 9, 453–462. [Google Scholar] [CrossRef]
- Katz, A.; Kang, J. Stereotactic body radiotherapy with or without external beam radiation as treatment for organ confined high-risk prostate carcinoma: A six year study. Radiat. Oncol. 2014, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.J.; Santoro, M.; Ashley, R.; Diblasio, F.; Witten, M. Stereotactic Body Radiotherapy as Boost for Organ-confined Prostate Cancer. Technol. Cancer Res. Treat. 2010, 9, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, S.; Weinberg, V.K.; Kaprealian, T.; Hsu, I.C.; Ma, L.; Chuang, C.; Descovich, M.; Shiao, S.; Shinohara, K.; Roach, M.; et al. Stereotactic body radiotherapy as monotherapy or post-external beam radiotherapy boost for prostate cancer: Technique, early toxicity, and PSA response. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Anwar, M.; Weinberg, V.; Seymour, Z.; Hsu, I.J.; Roach, M.; Gottschalk, A.R. Outcomes of hypofractionated stereotactic body radiotherapy boost for intermediate and high-risk prostate cancer. Radiat. Oncol. 2016, 11, 8. [Google Scholar] [CrossRef]
- Khmelevsky, E.V.; Kancheli, I.N.; Khoroshkov, V.S.; Kaprin, A.D. Morbidity dynamics in proton–photon or photon radiation therapy for locally advanced prostate cancer. Rep. Pract. Oncol. Radiother. 2018, 23, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Khmelevskiĭ, E.V.; Pan’shin, G.A.; Kancheli, I.N.; Khoroshkov, V.S. Options of hypofractionation of proton boost in locally advanced prostate cancer. Vopr. Onkol. 2012, 58, 787–794. [Google Scholar]
- Lin, Y.W.; Lin, L.C.; Lin, K.L. The early result of whole pelvic radiotherapy and stereotactic body radiotherapy boost for high-risk localized prostate cancer. Front. Oncol. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Koh, D.-H.; Kim, J.-B.; Kim, H.-W.; Chang, Y.-S.; Kim, H.J. Clinical Outcomes of CyberKnife Radiotherapy in Prostate Cancer Patients: Short-term, Single-Center Experience. Korean J. Urol. 2014, 55, 172–177. [Google Scholar] [CrossRef]
- Freeman, D.; Dickerson, G.; Perman, M. Multi-Institutional Registry for Prostate Cancer Radiosurgery: A Prospective Observational Clinical Trial. Front. Oncol. 2015, 4, 369. [Google Scholar] [CrossRef]
- Mercado, C.; Kress, M.A.; Cyr, R.A.; Chen, L.N.; Yung, T.M.; Bullock, E.G.; Lei, S.; Collins, B.T.; Satinsky, A.N.; Harter, K.W.; et al. Intensity-modulated radiation therapy with stereotactic body radiation therapy boost for unfavorable prostate cancer: The Georgetown University experience. Front. Oncol. 2016, 6, 114. [Google Scholar] [CrossRef]
- Paydar, I.; Pepin, A.; Cyr, R.A.; King, J.; Yung, T.M.; Hartsell, W.F.; Collins, S.P. Intensity-Modulated Radiation Therapy with Stereotactic Body Radiation Therapy Boost for Unfavorable Prostate Cancer: A Report on 3-Year Toxicity. Front. Oncol. 2017, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Pontoriero, A.; Iatì, G.; Mondello, S.; Midili, F.; Siragusa, C.; Brogna, A.; Ielo, I.; Anastasi, G.; Magno, C.; Pergolizzi, S.; et al. High-Dose Robotic Stereotactic Body Radiotherapy in the Treatment of Patients With Prostate Cancer: Preliminary Results in 26 Patients. Technol. Cancer Res. Treat. 2016, 15, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Phak, J.H.; Kim, W.C. Prostate-specific antigen kinetics following hypofractionated stereotactic body radiotherapy boost and whole pelvic radiotherapy for intermediate- and high-risk prostate cancer. Asia-Pac. J. Clin. Oncol. 2017, 13, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Phak, J.H.; Kim, W.C. Clinical outcomes of whole pelvis radiotherapy and stereotactic body radiotherapy boost for intermediate- and high-risk prostate cancer. Asia-Pac. J. Clin. Oncol. 2017, 13, e342–e347. [Google Scholar] [CrossRef]
- Phak, J.H.; Kim, H.J.; Kim, W.C. Prostate-specific antigen kinetics following hypofractionated stereotactic body radiotherapy boost as post-external beam radiotherapy versus conventionally fractionated external beam radiotherapy for localized prostate cancer. Prostate Int. 2016, 4, 25–29. [Google Scholar] [CrossRef]
- Pasquier, D.; Nickers, P.; Peiffert, D.; Maingon, P.; Pommier, P.; Lacornerie, T.; Martinage, G.; Tresch, E.; Lartigau, E. Hypofractionated stereotactic boost in intermediate risk prostate carcinoma: Preliminary results of a multicenter phase II trial (CKNO-PRO). PLoS ONE 2017, 12, e0187794. [Google Scholar] [CrossRef]
- Pasquier, D.; Peiffert, D.; Nickers, P.; Maingon, P.; Pommier, P.; Lacornerie, T.; Martinage, G.; Tresch, E.; Lartigau, E. A Multicenter Phase 2 study of Hypofractionated Stereostatic Boost in Intermediate Risk Prostate Carcinoma: A 5-Year Analysis of the CKNO-PRO Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 116–123. [Google Scholar] [CrossRef]
- Feng, L.R.; Suy, S.; Collins, S.P.; Lischalk, J.W.; Yuan, B.; Saligan, L.N. Comparison of Late Urinary Symptoms Following SBRT and SBRT with IMRT Supplementation for Prostate Cancer. Curr. Urol. 2018, 11, 218–224. [Google Scholar] [CrossRef]
- Alayed, Y.; Loblaw, A.; Chu, W.; Al-Hanaqta, M.; Chiang, A.; Jain, S.; Chung, H.; Vesprini, D.; Morton, G.; Ravi, A.; et al. Stereotactic Body Radiation Therapy Boost for Intermediate-Risk Prostate Cancer: A Phase 1 Dose-Escalation Study. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 1066–1073. [Google Scholar] [CrossRef]
- Eade, T.; Hruby, G.; Booth, J.; Bromley, R.; Guo, L.; O’Toole, A.; Le, A.; Wu, K.; Whitaker, M.; Rasiah, K.; et al. Results of a Prospective Dose Escalation Study of Linear Accelerator-Based Virtual Brachytherapy (BOOSTER) for Prostate Cancer; Virtual HDR Brachytherapy for Prostate Cancer. Adv. Radiat. Oncol. 2019, 4, 623–630. [Google Scholar] [CrossRef]
- Johansson, S.; Isacsson, U.; Sandin, F.; Turesson, I. High efficacy of hypofractionated proton therapy with 4 fractions of 5 Gy as a boost to 50 Gy photon therapy for localized prostate cancer. Radiother. Oncol. Journal. Eur. Soc. Ther. Radiol. Oncol. 2019, 141, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Pryor, D.; Sidhom, M.; Arumugam, S.; Bucci, J.; Gallagher, S.; Smart, J.; Grand, M.; Greer, P.; Keats, S.; Wilton, L.; et al. Phase 2 Multicenter Study of Gantry-Based Stereotactic Radiotherapy Boost for Intermediate and High Risk Prostate Cancer (PROMETHEUS). Front. Oncol. 2019, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Pollack, A.; Chinea, F.M.; Bossart, E.; Kwon, D.; Abramowitz, M.C.; Lynne, C.; Jorda, M.; Marples, B.; Patel, V.N.; Wu, X.; et al. Phase I Trial of MRI-Guided Prostate Cancer Lattice Extreme Ablative Dose (LEAD) Boost Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2020, 107, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-C.; Ting, W.-C.; Chang, Y.-C.; Yang, C.-C.; Lin, L.-C.; Ho, H.-W.; Chu, S.-S.; Lin, Y.-W. Whole Pelvic Radiotherapy with Stereotactic Body Radiotherapy Boost vs. Conventionally Fractionated Radiotherapy for Patients with High or Very High-Risk Prostate Cancer. Front. Oncol. 2020, 10, 814. [Google Scholar] [CrossRef]
- Narang, K.; Kadian, M.; Venkatesan, K.; Mishra, S.; Bisht, S.; Gupta, D.; Banerjee, S.; Kataria, T. Phase I/II Study of Extreme Hypofractionated Stereotactic Body Radiation Therapy Boost to Prostate for Locally Advanced, Node-Positive and Oligometastatic Cancer. Cureus 2020, 12, e11751. [Google Scholar] [CrossRef]
- Kim, Y.J.; Ahn, H.; Kim, C.-S.; Kim, Y.S. Phase I/IIa trial of androgen deprivation therapy, external beam radiotherapy, and stereotactic body radiotherapy boost for high-risk prostate cancer (ADEBAR). Radiat. Oncol. 2020, 15, 234. [Google Scholar] [CrossRef] [PubMed]
- Milecki, P.; Antczak, A.; Milecki, T.; Gluszak, P.; Piotrowski, T.G.; Rucinska, A.; Malicki, J. Ultra-hypofractionated versus Conventionally Fractionated Radiation Therapy Boost for Patients with High-Risk, Localized Prostate Cancer: A 5-Year Results from Randomized HYPO-PROST Trial. Int. J. Radiat. Oncol. Biol. Phys. 2020 108, S62–S63. [CrossRef]
- Turna, M.; Akboru, H.; Ermis, E.; Oskeroglu, S.; Dincer, S.; Altin, S. Stereotactic body radiotherapy as a boost after external beam radiotherapy for high-risk prostate cancer patients. Indian J. Cancer 2021, 58, 518–524. [Google Scholar] [CrossRef]
- Chen, W.C.; Li, Y.; Lazar, A.; Altun, A.; Descovich, M.; Nano, T.; Ziemer, B.; Sudhyadhom, A.; Cunha, A.; Thomas, H.; et al. Stereotactic Body Radiation Therapy and High-Dose-Rate Brachytherapy Boost in Combination with Intensity Modulated Radiation Therapy for Localized Prostate Cancer: A Single-Institution Propensity Score Matched Analysis. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 429–437. [Google Scholar] [CrossRef]
- Phuong, C.; Chan, J.W.; Ni, L.; Wall, P.; Mohamad, O.; Wong, A.C.; Hsu, I.C.; Chang, A.J. Safety of accelerated hypofractionated whole pelvis radiation therapy prior to high dose rate brachytherapy or stereotactic body radiation therapy prostate boost. Radiat. Oncol. 2022, 17, 12. [Google Scholar] [CrossRef]
- Novikov, S.N.; Novikov, R.V.; Merezhko, Y.O.; Gotovchikova, M.Y.; Ilin, N.D.; Melnik, Y.S.; Kanaev, S.V. A comparison between high dose rate brachytherapy and stereotactic body radiotherapy boost after elective pelvic irradiation for high and very high-risk prostate cancer. Radiat. Oncol. J. 2022, 40, 200–207. [Google Scholar] [CrossRef]
- Ferro, M.; de Cobelli, O.; Musi, G.; Del Giudice, F.; Carrieri, G.; Busetto, G.M.; Falagario, U.G.; Sciarra, A.; Maggi, M.; Crocetto, F.; et al. Radiomics in prostate cancer: An up-to-date review. Ther. Adv. Urol. 2022, 14, 17562872221109020. [Google Scholar] [CrossRef]
- Hofman, M.S.; Lawrentschuk, N.; Francis, R.J.; Tang, C.; Vela, I.; Thomas, P.; Rutherford, N.; Martin, J.M.; Frydenberg, M.; Shakher, R.; et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomised, multicentre study. Lancet 2020, 395, 1208–1216. [Google Scholar] [CrossRef]
- Koontz, B.F.; Bossi, A.; Cozzarini, C.; Wiegel, T.; D’Amico, A. A systematic review of hypofractionation for primary management of prostate cancer. Eur. Urol. 2015, 68, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Mesci, A.; Isfahanian, N.; Dayes, I.; Lukka, H.; Tsakiridis, T. The Journey of Radiotherapy Dose Escalation in High Risk Prostate Cancer; Conventional Dose Escalation to Stereotactic Body Radiotherapy (SBRT) Boost Treatments. Clin. Genitourin. Cancer 2022, 20, e25–e38. [Google Scholar] [CrossRef] [PubMed]
- Kerkmeijer, L.G.W.; Groen, V.H.; Pos, F.J.; Haustermans, K.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; de Boer, J.C.J.; van der Voort van Zijp, J.; van Vulpen, M.; et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients with Localized Prostate Cancer: Results from the FLAME Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 787–796. [Google Scholar] [CrossRef]
- Khoo, E.L.; Schick, K.; Plank, A.W.; Poulsen, M.; Wong, W.W.; Middleton, M.; Martin, J.M. Prostate contouring variation: Can it be fixed? Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Keall, P.; Nguyen, D.T.; O’Brien, R.; Booth, J.; Greer, P.; Poulsen, P.; Gebski, V.; Kneebone, A.; Martin, J. Stereotactic prostate adaptive radiotherapy utilising kilovoltage intrafraction monitoring: The TROG 15.01 SPARK trial. BMC Cancer 2017, 17, 180. [Google Scholar] [CrossRef]
- Ng, J.A.; Booth, J.T.; Poulsen, P.R.; Fledelius, W.; Worm, E.S.; Eade, T.; Hegi, F.; Kneebone, A.; Kuncic, Z.; Keall, P.J. Kilovoltage intrafraction monitoring for prostate intensity modulated arc therapy: First clinical results. Int. J. Radiat. Oncol. Biol. Phys. 2012, 84, e655–e661. [Google Scholar] [CrossRef]
- Kishan, A.U.; Ma, T.M.; Lamb, J.M.; Casado, M.; Wilhalme, H.; Low, D.A.; Sheng, K.; Sharma, S.; Nickols, N.G.; Pham, J.; et al. Magnetic Resonance Imaging-Guided vs Computed Tomography-Guided Stereotactic Body Radiotherapy for Prostate Cancer: The MIRAGE Randomized Clinical Trial. JAMA Oncol. 2023, 9, 365–373. [Google Scholar] [CrossRef]
- de Leon, J.; Jameson, M.G.; Rivest-Henault, D.; Keats, S.; Rai, R.; Arumugam, S.; Wilton, L.; Ngo, D.; Liney, G.; Moses, D.; et al. Reduced motion and improved rectal dosimetry through endorectal immobilization for prostate stereotactic body radiotherapy. Br. J. Radiol. 2019, 92, 20190056. [Google Scholar] [CrossRef] [PubMed]
- Wilton, L.; Richardson, M.; Keats, S.; Legge, K.; Hanlon, M.C.; Arumugam, S.; Hunter, P.; Evans, T.J.; Sidhom, M.; Martin, J. Rectal protection in prostate stereotactic radiotherapy: A retrospective exploratory analysis of two rectal displacement devices. J. Med. Radiat. Sci. 2017, 64, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.E.; Efstathiou, J.A.; Bhattacharyya, S.K.; Payne, H.A.; Woodward, E.; Pinkawa, M. Association of the Placement of a Perirectal Hydrogel Spacer with the Clinical Outcomes of Men Receiving Radiotherapy for Prostate Cancer: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e208221. [Google Scholar] [CrossRef] [PubMed]
- Mariados, N.; Sylvester, J.; Shah, D.; Karsh, L.; Hudes, R.; Beyer, D.; Kurtzman, S.; Bogart, J.; Hsi, R.A.; Kos, M.; et al. Hydrogel Spacer Prospective Multicenter Randomized Controlled Pivotal Trial: Dosimetric and Clinical Effects of Perirectal Spacer Application in Men Undergoing Prostate Image Guided Intensity Modulated Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 971–977. [Google Scholar] [CrossRef]
- Correa, R.J.M.; Loblaw, A. Stereotactic Body Radiotherapy: Hitting Harder, Faster, and Smarter in High-Risk Prostate Cancer. Front. Oncol. 2022, 12, 889132. [Google Scholar] [CrossRef]
- Dowling, J.A.; Sun, J.; Pichler, P.; Rivest-Henault, D.; Ghose, S.; Richardson, H.; Wratten, C.; Martin, J.; Arm, J.; Best, L.; et al. Automatic Substitute Computed Tomography Generation and Contouring for Magnetic Resonance Imaging (MRI)-Alone External Beam Radiation Therapy from Standard MRI Sequences. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 1144–1153. [Google Scholar] [CrossRef]
- Greer, P.; Martin, J.; Sidhom, M.; Hunter, P.; Pichler, P.; Choi, J.H.; Best, L.; Smart, J.; Young, T.; Jameson, M.; et al. A Multi-center Prospective Study for Implementation of an MRI-Only Prostate Treatment Planning Workflow. Front. Oncol. 2019, 9, 826. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).