Factors to Consider for the Correct Use of γH2AX in the Evaluation of DNA Double-Strand Breaks Damage Caused by Ionizing Radiation
Abstract
Simple Summary
Abstract
1. Introduction
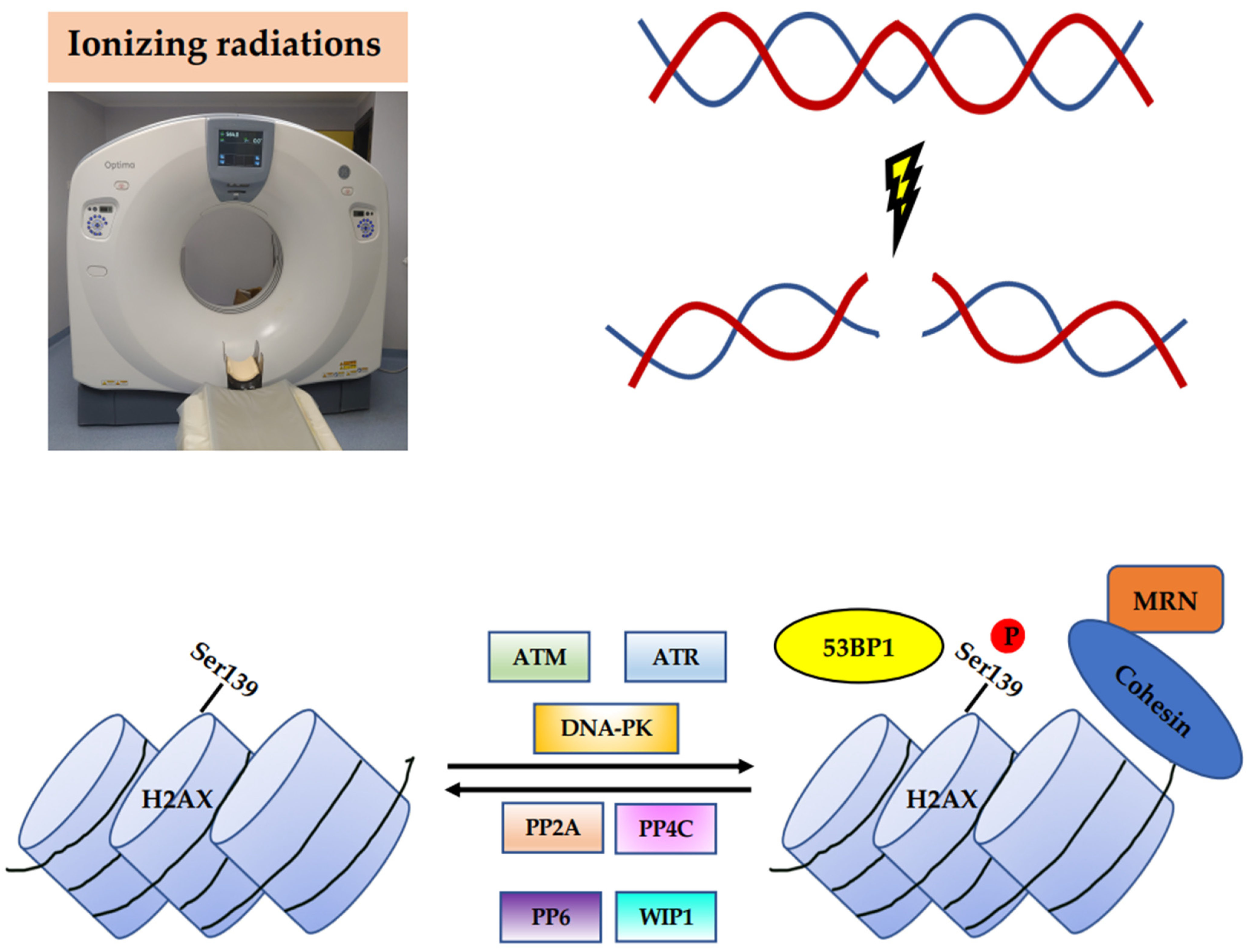
2. Basic Mechanism of H2AX Phosphorylation
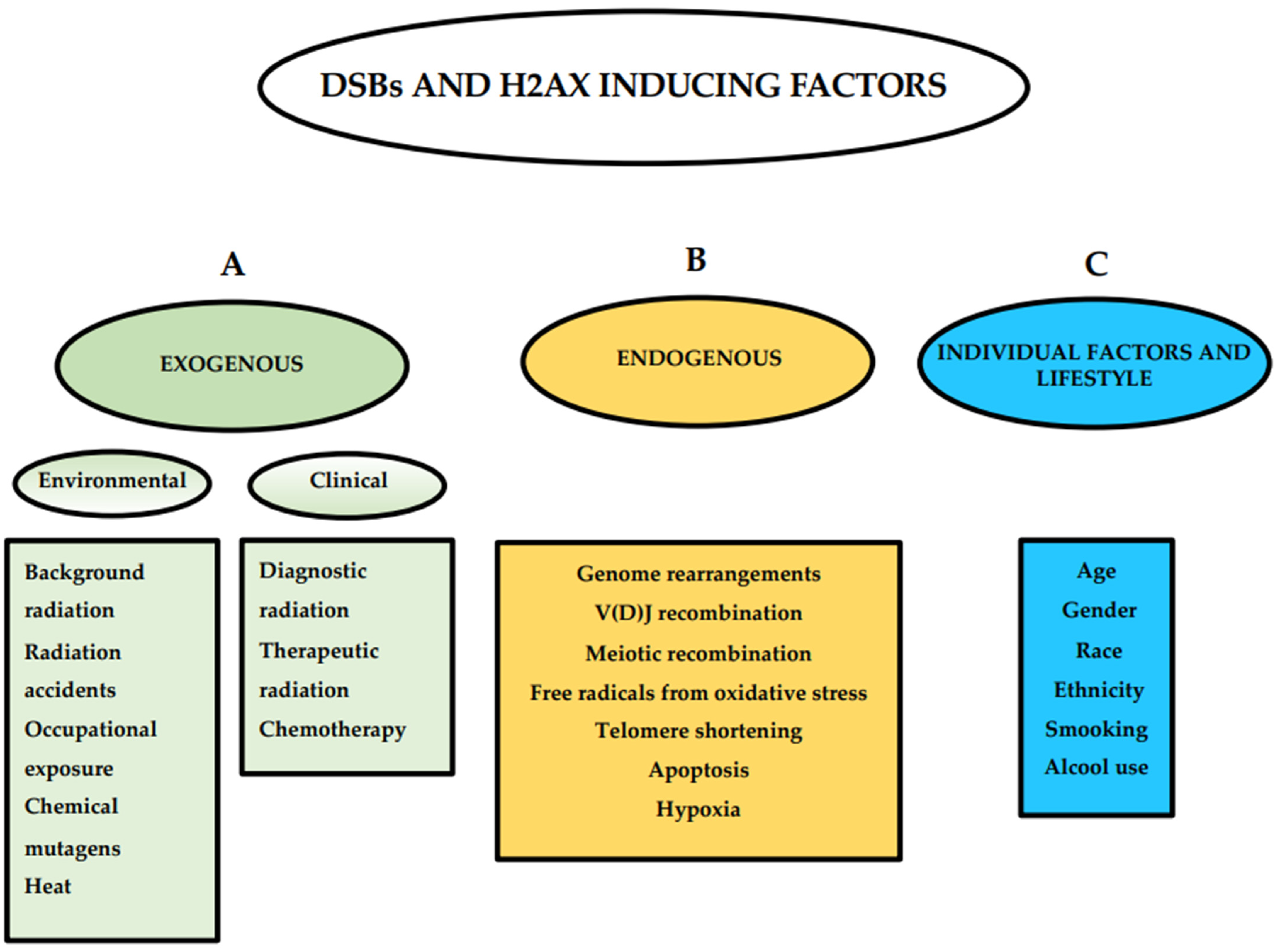
3. Conditions That Affect the Observed Level of H2AX Phosphorylation
3.1. H2AX Phosphorylation and the Cell Cycle
3.2. H2AX Phosphorylation and Oxidative Stress
3.3. H2AX Phosphorylation and Apoptosis
3.4. H2AX Phosphorylation and Hypoxia
3.5. Environmental Factors and Lifestyle Inducing H2AX Phosphorylation
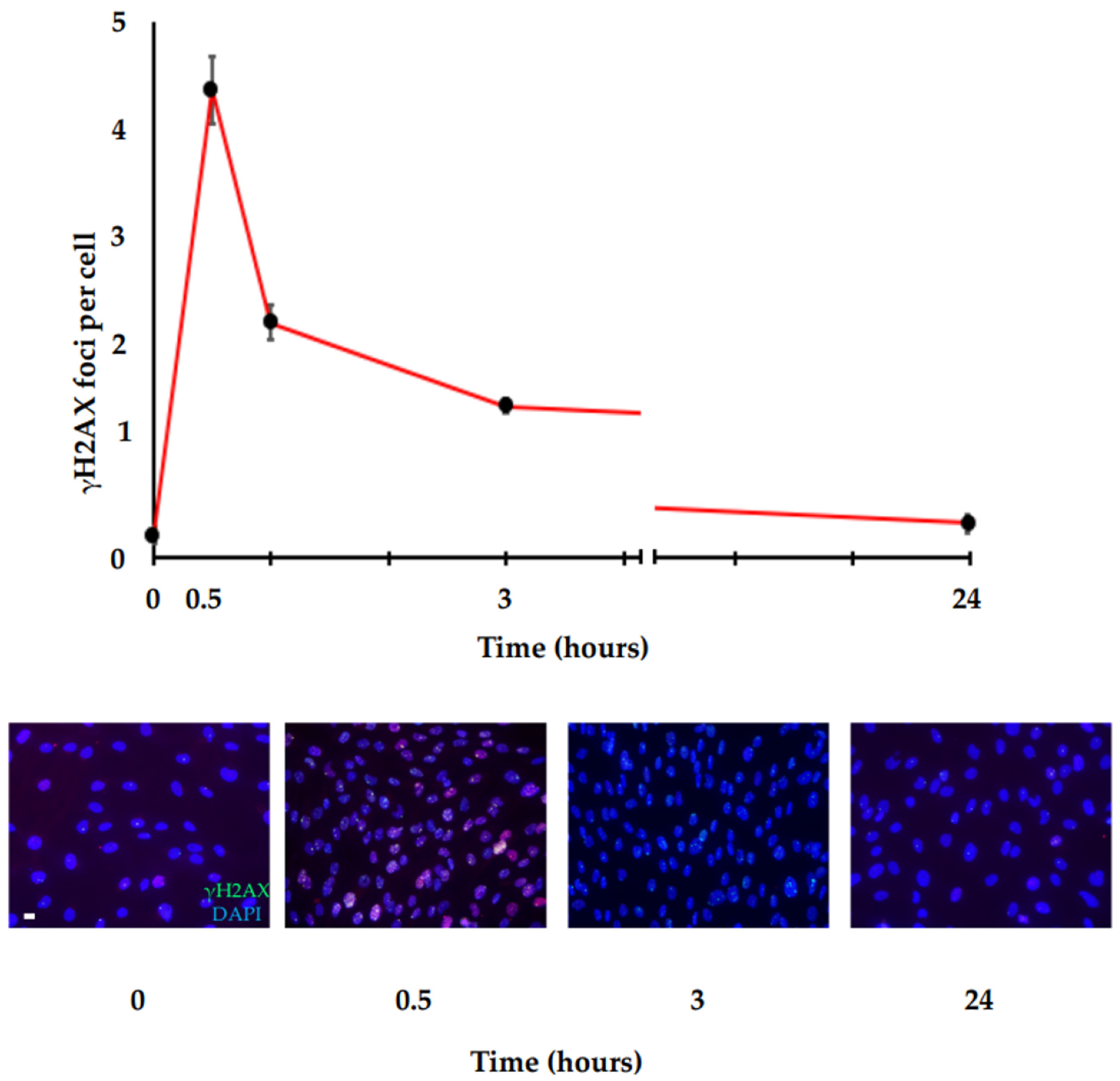
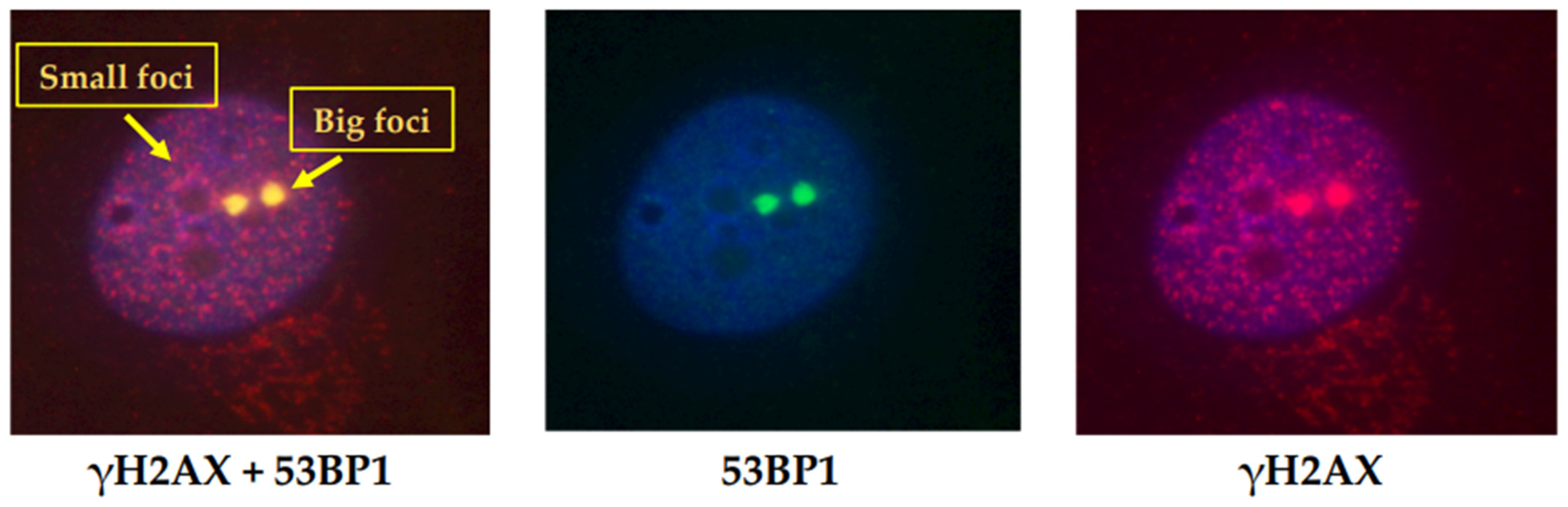
4. Methods for DSB Assessment and Current Problems
5. Applications of γH2AX in Environmental Risk Detection and in Clinical Procedures
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wyman, C.; Kanaar, R. DNA double-strand break repair: All’s well that ends well. Annu. Rev. Genet. 2006, 40, 363–383. [Google Scholar] [PubMed]
- Symington, L.S. DNA repair: Making the cut. Nature 2014, 514, 39–40. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Koliada, A.; Zabuga, O.; Socol, Y. Health Impacts of Low-Dose Ionizing Radiation: Current Scientific Debates and Regulatory Issues. Dose-Response 2018, 16, 1559325818796331. [Google Scholar] [CrossRef] [PubMed]
- Azzam, E.I. What does radiation biology tell us about potential health effects at low dose and low dose rates? J. Radiol. Prot. 2019, 39, S28–S39. [Google Scholar]
- Löbrich, M.; Rief, N.; Kühne, M.; Heckmann, M.; Fleckenstein, J.; Rübe, C.; Uder, M. In vivo formation and repair of DNA double-strand breaks after computed tomography examinations. Proc. Natl. Acad. Sci. USA 2005, 102, 8984–8989. [Google Scholar] [CrossRef]
- Linet, M.S.; Slovis, T.L.; Miller, D.L.; Kleinerman, R.; Lee, C.; Rajaraman, P.; De Gonzalez, A.B. Cancer risks associated with external radiation from diagnostic imaging procedures. CA A Cancer J. Clin. 2012, 62, 75–100. [Google Scholar] [CrossRef]
- Doll, R.; Wakeford, R. Risk of childhood cancer from fetal irradiation. Br. J. Radiol. 1997, 70, 130–139. [Google Scholar] [CrossRef]
- Moores, B.M. A review of the fundamental principles of radiation protection when applied to the patient in diagnostic radiology. Radiat. Prot. Dosim. 2017, 175, 1–9. [Google Scholar] [CrossRef]
- Foucault, A.; Ancelet, S.; Dreuil, S.; Caër-Lorho, S.; Le Pointe, H.D.; Brisse, H.; Chateil, J.-F.; Lee, C.; Leuraud, K.; Bernier, M.-O. Childhood cancer risks estimates following CT scans: An update of the French CT cohort study. Eur. Radiol. 2022, 32, 5491–5498. [Google Scholar] [CrossRef]
- Rühm, W.; Laurier, D.; Wakeford, R. Cancer risk following low doses of ionising radiation—Current epidemiological evidence and implications for radiological protection. Mutat. Res. Toxicol. Environ. Mutagen. 2022, 873, 503436. [Google Scholar] [CrossRef]
- Rehani, M.M.; Hauptmann, M. Estimates of the number of patients with high cumulative doses through recurrent CT exams in 35 OECD countries. Phys. Med. 2020, 76, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; He, N.; Liu, Y.; Wang, Y.; Liu, Q. Research progress on biodosimeters of ionizing radiation damage. Radiat. Med. Prot. 2020, 1, 127–132. [Google Scholar] [CrossRef]
- Raavi, V.; Perumal, V.; Paul, S.F. Potential application of γ-H2AX as a biodosimetry tool for radiation triage. Mutat. Res. Mol. Mech. Mutagen. 2020, 787, 108350. [Google Scholar] [CrossRef] [PubMed]
- Popp, H.D.; Brendel, S.; Hofmann, W.-K.; Fabarius, A. Immunofluorescence Microscopy of γH2AX and 53BP1 for Analyzing the Formation and Repair of DNA Double-strand Breaks. J. Vis. Exp. 2017, 129, e56617. [Google Scholar] [CrossRef]
- Köcher, S.; Volquardsen, J.; Heinsohn, A.P.; Petersen, C.; Roggenbuck, D.; Rothkamm, K.; Mansour, W. Fully automated counting of DNA damage foci in tumor cell culture: A matter of cell separation. DNA Repair 2021, 102, 103100. [Google Scholar] [CrossRef]
- Wu, C.Y.; Kang, H.Y.; Yang, W.L.; Wu, J.; Jeong, Y.S.; Wang, J.; Chan, C.H.; Lee, S.W.; Zhang, X.; Lamothe, B.; et al. Critical role of monoubiquitination of histone H2AX protein in histone H2AX phosphorylation and DNA damage response. J. Biol. Chem. 2011, 286, 30806–30815. [Google Scholar] [CrossRef]
- Khanna, K.K.; Jackson, S.P. DNA double-strand breaks: Signaling, repair and the cancer connection. Nat. Genet. 2001, 27, 247–254. [Google Scholar] [CrossRef]
- Jaikrishan, G.; Sudheer, K.R.; Andrews, V.J.; Koya, P.K.M.; Madhusoodhanan, M.; Jagadeesan, C.K.; Seshadri, M. Study of stillbirth and major congenital anomaly among newborns in the high-level natural radiation areas of Kerala, India. J. Community Genet. 2013, 4, 21–31. [Google Scholar] [CrossRef]
- Tao, Z.; Cha, Y.; Sun, Q. [Cancer mortality in high background radiation area of Yangjiang, China, 1979–1995]. Zhonghua Yi Xue za zhi 1999, 79, 487–492. [Google Scholar]
- Nakamura, A.J.; Suzuki, M.; Redon, C.E.; Kuwahara, Y.; Yamashiro, H.; Abe, Y.; Takahashi, S.; Fukuda, T.; Isogai, E.; Bonner, W.M.; et al. The Causal Relationship between DNA Damage Induction in Bovine Lymphocytes and the Fukushima Nuclear Power Plant Accident. Radiat. Res. 2017, 187, 630–636. [Google Scholar] [CrossRef]
- Gupta, M.L.; Srivastava, N.N.; Dutta, S.; Shukla, S.K.; Dutta, A.; Verma, S.; Devi, M. Blood biomarkers in metal scrap workers accidentally exposed to ionizing radiation: A case study. Hum. Exp. Toxicol. 2013, 32, 1311–1322. [Google Scholar] [CrossRef] [PubMed]
- Bazyka, D.A.; Muzalevska, K.D.; Maznichenko, O.L.; Belyaev, O.A. Expression of γ-H2AX histone in lymphocytes of the Chornobyl "Shelter" object staff exposed to ionizing radiation in occupational limits. Probl. Radiac. Med. Radiobiol. 2014, 19, 186–191. [Google Scholar]
- Brenner, D.J.; Hall, E.J. Computed Tomography—An Increasing Source of Radiation Exposure. N. Engl. J. Med. 2007, 357, 2277–2284. [Google Scholar] [CrossRef]
- Hodjat, M.; Jourshari, P.B.; Amirinia, F.; Asadi, N. 5-Azacitidine and Trichostatin A induce DNA damage and apoptotic responses in tongue squamous cell carcinoma: An in vitro study. Arch. Oral Biol. 2022, 133, 105296. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, H.; Igarashi, K.; Kataoka, K.; Miura, M. Heat shock induces phosphorylation of histone H2AX in mammalian cells. Biochem. Biophys. Res. Commun. 2005, 328, 1101–1106. [Google Scholar] [CrossRef] [PubMed]
- Smider, V.; Chu, G. The end-joining reaction in V(D)J recombination. Semin. Immunol. 1997, 9, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Keeney, S.; Giroux, C.N.; Kleckner, N. Meiosis-Specific DNA Double-Strand Breaks Are Catalyzed by Spo11, a Member of a Widely Conserved Protein Family. Cell 1997, 88, 375–384. [Google Scholar] [CrossRef]
- Tanaka, T.; Kajstura, M.; Halicka, H.D.; Traganos, F.; Darzynkiewicz, Z. Constitutive histone H2AX phosphorylation and ATM activation are strongly amplified during mitogenic stimulation of lymphocytes. Cell Prolif. 2007, 40, 1–13. [Google Scholar] [CrossRef]
- Li, Z.; Yang, J.; Huang, H. Oxidative stress induces H2AX phosphorylation in human spermatozoa. FEBS Lett. 2006, 580, 6161–6168. [Google Scholar] [CrossRef]
- Rossiello, F.; Jurk, D.; Passos, J.F.; di Fagagna, F.D. Telomere dysfunction in ageing and age-related diseases. Nature 2022, 24, 135–147. [Google Scholar] [CrossRef]
- Raghuram, G.V.; Mishra, P.K. Stress induced premature senescence: A new culprit in ovarian tumorigenesis? Ind. J. Med. Res. 2014, 140 (Suppl. 1), S120–S129. [Google Scholar]
- Huang, X.; Halicka, H.D.; Traganos, F.; Tanaka, T.; Kurose, A.; Darzynkiewicz, Z. Cytometric assessment of DNA damage in relation to cell cycle phase and apoptosis. Cell Prolif. 2005, 38, 223–243. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Kurose, A.; Tanaka, T.; Traganos, F.; Dai, W.; Darzynkiewicz, Z. Sequential phosphorylation ofSer-10 on histone H3 andser-139 on histone H2AX and ATM activation during premature chromosome condensation: Relationship to cell-cycle phase and apoptosis. Cytom. Part A 2006, 69A, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.M.; Dorie, M.J.; Giaccia, A.J. ATR/ATM Targets Are Phosphorylated by ATR in Response to Hypoxia and ATM in Response to Reoxygenation. J. Biol. Chem. 2003, 278, 12207–12213. [Google Scholar] [CrossRef] [PubMed]
- Bencokova, Z.; Kaufmann, M.R.; Pires, I.; Lecane, P.S.; Giaccia, A.J.; Hammond, E.M. ATM Activation and Signaling under Hypoxic Conditions. Mol. Cell. Biol. 2009, 29, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Bozzella, M.; Seluanov, A.; Gorbunova, V. Comparison of nonhomologous end joining and homologous recombination in human cells. DNA Repair 2008, 7, 1765–1771. [Google Scholar] [CrossRef]
- Scully, R.; Xie, A. Double strand break repair functions of histone H2AX. Mutat. Res. Mol. Mech. Mutagen. 2013, 750, 5–14. [Google Scholar] [CrossRef]
- Marchetti, F.; Coleman, M.A.; Jones, I.M.; Wyrobek, A.J. Candidate protein biodosimeters of human exposure to ionizing radiation. Int. J. Radiat. Biol. 2006, 82, 605–639. [Google Scholar] [CrossRef]
- Olive, P.L.; Banáth, J.P. Phosphorylation of histone H2AX as a measure of radiosensitivity. Int. J. Radiat. Oncol. Biol. Phys. 2004, 58, 331–335. [Google Scholar] [CrossRef]
- Pinto, D.M.; Flaus, A. Structure and function of histone H2AX. Subcell Biochem. 2010, 50, 55–78. [Google Scholar]
- Modesti, M.; Kanaar, R. DNA repair: Spot(light)s on chromatin. Curr. Biol. 2001, 11, R229–R232. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Pilch, D.R.; Orr, A.H.; Ivanova, V.S.; Bonner, W.M. DNA Double-stranded Breaks Induce Histone H2AX Phosphorylation on Serine 139. J. Biol. Chem. 1998, 273, 5858–5868. [Google Scholar] [CrossRef] [PubMed]
- Rogakou, E.P.; Boon, C.; Redon, C.; Bonner, W.M. Megabase Chromatin Domains Involved in DNA Double-Strand Breaks in Vivo. J. Cell Biol. 1999, 146, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Durocher, D.; Jackson, S.P. DNA-PK, ATM and ATR as sensors of DNA damage: Variations on a theme? Curr. Opin. Cell Biol. 2001, 13, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M. Involvement of DNA-PK and ATM in Radiation- and Heat-induced DNA Damage Recognition and Apoptotic Cell Death. J. Radiat. Res. 2010, 51, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, M.; Wang, H.; Böcker, W.; Iliakis, G. Complex H2AX phosphorylation patterns by multiple kinases including ATM and DNA-PK in human cells exposed to ionizing radiation and treated with kinase inhibitors. J. Cell. Physiol. 2005, 202, 492–502. [Google Scholar] [CrossRef]
- Lamarche, B.J.; Orazio, N.I.; Weitzman, M.D. The MRN complex in double-strand break repair and telomere maintenance. FEBS Lett. 2010, 584, 3682–3695. [Google Scholar] [CrossRef]
- Shibata, A.; Jeggo, P.A. Roles for 53BP1 in the repair of radiation-induced DNA double strand breaks. DNA Repair 2020, 93, 102915. [Google Scholar] [CrossRef]
- Ström, L.; Lindroos, H.B.; Shirahige, K.; Sjögren, C. Postreplicative Recruitment of Cohesin to Double-Strand Breaks Is Required for DNA Repair. Mol. Cell 2004, 16, 1003–1015. [Google Scholar] [CrossRef]
- Ünal, E.; Arbel-Eden, A.; Sattler, U.; Shroff, R.; Lichten, M.; Haber, J.E.; Koshland, D. DNA Damage Response Pathway Uses Histone Modification to Assemble a Double-Strand Break-Specific Cohesin Domain. Mol. Cell 2004, 16, 991–1002. [Google Scholar] [CrossRef]
- Foster, E.R.; Downs, J. Histone H2A phosphorylation in DNA double-strand break repair. FEBS J. 2005, 272, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- Bouquet, F.; Muller, C.; Salles, B. The loss of gammaH2AX signal is a marker of DNA double strand breaks repair only at low levels of DNA damage. Cell Cycle 2006, 5, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, D.; Keogh, M.C.; Ishii, H.; Peterson, C.L.; Buratowski, S.; Lieberman, J. gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol. Cell 2005, 20, 801–809. [Google Scholar] [CrossRef]
- Chowdhury, D.; Xu, X.; Zhong, X.; Ahmed, F.; Zhong, J.; Liao, J.; Dykxhoorn, D.M.; Weinstock, D.M.; Pfeifer, G.P.; Lieberman, J. A PP4-phosphatase complex dephosphorylates gamma-H2AX generated during DNA replication. Mol. Cell 2008, 31, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Nan, A.; Xiao, Y.; Chen, Y.; Lai, Y. PP2A–B56ϵ complex is involved in dephosphorylation of γ-H2AX in the repair process of CPT-induced DNA double-strand breaks. Toxicology 2015, 331, 57–65. [Google Scholar] [CrossRef]
- Xu, Y.; Ayrapetov, M.K.; Xu, C.; Gursoy-Yuzugullu, O.; Hu, Y.; Price, B.D. Histone H2A.Z Controls a Critical Chromatin Remodeling Step Required for DNA Double-Strand Break Repair. Mol. Cell 2012, 48, 723–733. [Google Scholar] [CrossRef]
- Li, L.; Zou, L. Sensing, signaling, and responding to DNA damage: Organization of the checkpoint pathways in mammalian cells. J. Cell. Biochem. 2005, 94, 298–306. [Google Scholar] [CrossRef]
- Mirza-Aghazadeh-Attari, M.; Mohammadzadeh, A.; Yousefi, B.; Mihanfar, A.; Karimian, A.; Majidinia, M. 53BP1: A key player of DNA damage response with critical functions in cancer. DNA Repair 2019, 73, 110–119. [Google Scholar] [CrossRef]
- Lu, C.; Zhu, F.; Cho, Y.-Y.; Tang, F.; Zykova, T.; Ma, W.-Y.; Bode, A.M.; Dong, Z. Cell Apoptosis: Requirement of H2AX in DNA Ladder Formation, but Not for the Activation of Caspase-3. Mol. Cell 2006, 23, 121–132. [Google Scholar] [CrossRef]
- Rogakou, E.P.; Nieves-Neira, W.; Boon, C.; Pommier, Y.; Bonner, W.M. Initiation of DNA Fragmentation during Apoptosis Induces Phosphorylation of H2AX Histone at Serine 139. J. Biol. Chem. 2000, 275, 9390–9395. [Google Scholar] [CrossRef]
- McManus, K.J.; Hendzel, M.J. ATM-dependent DNA Damage-independent Mitotic Phosphorylation of H2AX in Normally Growing Mammalian Cells. Mol. Biol. Cell 2005, 16, 5013–5025. [Google Scholar] [CrossRef] [PubMed]
- Rybak, P.; Hoang, A.; Bujnowicz, L.; Bernas, T.; Berniak, K.; Zarębski, M.; Darzynkiewicz, Z.; Dobrucki, J. Low level phosphorylation of histone H2AX on serine 139 (γH2AX) is not associated with DNA double-strand breaks. Oncotarget 2016, 7, 49574–49587. [Google Scholar] [CrossRef] [PubMed]
- MacPhail, S.H.; Banáth, J.P.; Yu, Y.; Chu, E.; Olive, P.L. Cell Cycle-Dependent Expression of Phosphorylated Histone H2AX: Reduced Expression in Unirradiated but not X-Irradiated G1-Phase Cells. Radiat. Res. 2003, 159, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Darzynkiewicz, Z.; Krassowski, T.; Skopińska, E. Effect of phytomaemagglutinin on synthesis of “rapidly-labelled” ribonucleic acid in human lymphocytes. Nature 1965, 207, 1402–1403. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Traganos, F.; Sharpless, T.; Melamed, M.R. Lymphocyte stimulation: A rapid multiparameter analysis. Proc. Natl. Acad. Sci. USA 1976, 73, 2881–2884. [Google Scholar] [CrossRef]
- Darzynkiewicz, Z.; Staiano-Coico, L.; Melamed, M.R. Increased mitochondrial uptake of rhodamine 123 during lymphocyte stimulation. Proc. Natl. Acad. Sci. USA 1981, 78, 2383–2387. [Google Scholar] [CrossRef]
- Tanaka, T.; Halicka, H.D.; Huang, X.; Traganos, F.; Darzynkiewicz, Z. Constitutive histone H2AX phosphorylation and ATM activation, the reporters of DNA damage by endogenous oxidants. Cell Cycle 2006, 5, 1940–1945. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Kurose, A.; Halicka, H.D.; Traganos, F.; Darzynkiewicz, Z. 2-Deoxy-D-glucose Reduces the Level of Constitutive Activation of ATM and Phosphorylation of Histone H2AX. Cell Cycle 2006, 5, 878–882. [Google Scholar] [CrossRef][Green Version]
- Hernández, L.; Terradas, M.; Martín, M.; Tusell, L.; Genescà, A. Highly Sensitive Automated Method for DNA Damage Assessment: Gamma-H2AX Foci Counting and Cell Cycle Sorting. Int. J. Mol. Sci. 2013, 14, 15810–15826. [Google Scholar] [CrossRef]
- Dröge, W. Free Radicals in the Physiological Control of Cell Function. Physiol. Rev. 2002, 82, 47–95. [Google Scholar] [CrossRef]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and Prevention of Chronic Disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Genestra, M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell Signal. 2007, 19, 1807–1819. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Biochemistry of oxidative stress. Biochem. Soc.Trans. 2007, 35(Pt. 5), 1147–1150. [Google Scholar] [CrossRef] [PubMed]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, A.; Yamamoto, K. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Nohl, H. Generation of superoxide radicals as byproduct of cellular respiration. Ann. Biol. Clin. 1994, 52, 199–204. [Google Scholar]
- Møller, P.; Loft, S. Interventions with antioxidants and nutrients in relation to oxidative DNA damage and repair. Mutat. Res. Mol. Mech. Mutagen. 2004, 551, 79–89. [Google Scholar] [CrossRef]
- Beckman, K.B.; Ames, B.N. Oxidative decay of DNA. J. Biol. Chem. 1997, 272, 19633–19636. [Google Scholar] [CrossRef]
- Vilenchik, M.M.; Knudson, A.G. Endogenous DNA double-strand breaks: Production, fidelity of repair, and induction of cancer. Proc. Natl. Acad. Sci. USA 2003, 100, 12871–12876. [Google Scholar] [CrossRef]
- Huang, X.; Tanaka, T.; Kurose, A.; Traganos, F.; Darzynkiewicz, Z. Constitutive histone H2AX phosphorylation on Ser-139 in cells untreated by genotoxic agents is cell-cycle phase specific and attenuated by scavenging reactive oxygen species. Int. J. Oncol. 2006, 29, 495–501. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ichijima, Y.; Sakasai, R.; Okita, N.; Asahina, K.; Mizutani, S.; Teraoka, H. Phosphorylation of histone H2AX at M phase in human cells without DNA damage response. Biochem. Biophys. Res. Commun. 2005, 336, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Solier, S.; Pommier, Y. The apoptotic ring: A novel entity with phosphorylated histones H2AX and H2B, and activated DNA damage response kinases. Cell Cycle 2009, 8, 1853–1859. [Google Scholar] [CrossRef]
- Solier, S.; Sordet, O.; Kohn, K.W.; Pommier, Y. Death Receptor-Induced Activation of the Chk2- and Histone H2AX-Associated DNA Damage Response Pathways. Mol. Cell Biol. 2009, 29, 68–82. [Google Scholar] [CrossRef]
- Solier, S.; Pommier, Y. The nuclear γ-H2AX apoptotic ring: Implications for cancers and autoimmune diseases. Cell Mol. Life Sci. 2014, 71, 2289–2297. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, R.; Solary, E.; O’Connor, P.; Kohn, K.W.; Pommier, Y. Induction of a Common Pathway of Apoptosis by Staurosporine. Exp. Cell Res. 1994, 211, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, M.D.; Burne, J.F.; Raff, M.C. Programmed cell death and Bcl-2 protection in the absence of a nucleus. EMBO J. 1994, 13, 1899–1910. [Google Scholar] [CrossRef]
- Bonner, W.M.; Redon, C.E.; Dickey, J.S.; Nakamura, A.J.; Sedelnikova, O.A.; Solier, S.; Pommier, Y. GammaH2AX and cancer. Nat. Rev. Cancer 2008, 8, 957–967. [Google Scholar] [CrossRef]
- Solier, S.; Kohn, K.W.; Scroggins, B.; Xu, W.; Trepel, J.; Neckers, L.; Pommier, Y. Heat shock protein 90α (HSP90α), a substrate and chaperone of DNA-PK necessary for the apoptotic response. Proc. Natl. Acad. Sci. USA 2012, 109, 12866–12872. [Google Scholar] [CrossRef]
- Solier, S.; Pommier, Y. MDC1 Cleavage by Caspase-3: A Novel Mechanism for Inactivating the DNA Damage Response during Apoptosis. Cancer Res. 2011, 71, 906–913. [Google Scholar] [CrossRef]
- Matsumoto, S.; Yasui, H.; Mitchell, J.B.; Krishna, M.C. Imaging Cycling Tumor Hypoxia. Cancer Res 2010, 70, 10019–10023. [Google Scholar] [CrossRef] [PubMed]
- Yasui, H.; Matsumoto, S.; Devasahayam, N.; Munasinghe, J.P.; Choudhuri, R.; Saito, K.; Subramanian, S.; Mitchell, J.B.; Krishna, M.C. Low-Field Magnetic Resonance Imaging to Visualize Chronic and Cycling Hypoxia in Tumor-Bearing Mice. Cancer Res. 2010, 70, 6427–6436. [Google Scholar] [CrossRef] [PubMed]
- Wrann, S.; Kaufmann, M.R.; Wirthner, R.; Stiehl, D.P.; Wenger, R.H. HIF mediated and DNA damage independent histone H2AX phosphorylation in chronic hypoxia. Biol. Chem. 2013, 394, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Pospelova, T.V.; Demidenko, Z.N.; Bukreeva, E.I.; Pospelov, V.A.; Gudkov, A.; Blagosklonny, M.V. Pseudo-DNA damage response in senescent cells. Cell Cycle 2009, 8, 4112–4118. [Google Scholar] [CrossRef]
- Sedelnikova, O.A.; Horikawa, I.; Redon, C.; Nakamura, A.; Zimonjic, D.B.; Popescu, N.C.; Bonner, W.M. Delayed kinetics of DNA double-strand break processing in normal and pathological aging. Aging Cell 2008, 7, 89–100. [Google Scholar] [CrossRef]
- Schurman, S.H.; Dunn, C.A.; Greaves, R.; Yu, B.; Ferrucci, L.; Croteau, D.L.; Seidman, M.M.; Bohr, V.A. Age-Related Disease Association of Endogenous γ-H2AX Foci in Mononuclear Cells Derived from Leukapheresis. PLoS ONE 2012, 7, e45728. [Google Scholar] [CrossRef]
- Mah, L.J.; El-Osta, A.; Karagiannis, T.C. GammaH2AX as a molecular marker of aging and disease. Epigenetics 2010, 5, 129–136. [Google Scholar] [CrossRef]
- Sharma, P.M.; Ponnaiya, B.; Taveras, M.; Shuryak, I.; Turner, H.; Brenner, D.J. High throughput measurement of γH2AX DSB repair kinetics in a healthy human population. PLoS ONE 2015, 10, e0121083. [Google Scholar] [CrossRef]
- Albino, A.; Huang, X.; Jorgensen, E.; Yang, J.; Gietl, D.; Traganos, F.; Darzynkiewicz, Z. Induction of H2AX Phosphorylation in Pulmonary Cells by Tobacco Smoke: A New Assay for Carcinogens. Cell Cycle 2004, 3, 1062–1068. [Google Scholar] [CrossRef]
- Albino, A.P.; Jorgensen, E.D.; Rainey, P.; Gillman, G.; Clark, T.J.; Gietl, D.; Zhao, H.; Traganos, F.; Darzynkiewicz, Z. gammaH2AX: A potential DNA damage response biomarker for assessing toxicological risk of tobacco products. Mutat. Res. 2009, 678, 43–52. [Google Scholar] [CrossRef]
- Jorgensen, E.D.; Zhao, H.; Traganos, F.; Albino, A.P.; Darzynkiewicz, Z. DNA damage response induced by exposure of human lung adenocarcinoma cells to smoke from tobacco- and nicotine-free cigarettes. Cell Cycle 2010, 9, 2170–2176. [Google Scholar] [CrossRef]
- Ishida, M.; Ishida, T.; Tashiro, S.; Uchida, H.; Sakai, C.; Hironobe, N.; Miura, K.; Hashimoto, Y.; Arihiro, K.; Chayama, K.; et al. Smoking Cessation Reverses DNA Double-Strand Breaks in Human Mononuclear Cells. PLoS ONE 2014, 9, e103993. [Google Scholar] [CrossRef] [PubMed]
- Ismail, I.H.; Wadhra, T.I.; Hammarsten, O. An optimized method for detecting gamma-H2AX in blood cells reveals a significant interindividual variation in the gamma-H2AX response among humans. Nucleic Acids Res. 2007, 35, e36. [Google Scholar] [CrossRef] [PubMed]
- Paull, T.T.; Rogakou, E.P.; Yamazaki, V.; Kirchgessner, C.U.; Gellert, M.; Bonner, W.M. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 2000, 10, 886–895. [Google Scholar] [CrossRef]
- Hahn, H.; Neitzel, C.; Kopečná, O.; Heermann, D.W.; Falk, M.; Hausmann, M. Topological Analysis of γH2AX and MRE11 Clusters Detected by Localization Microscopy during X-ray-Induced DNA Double-Strand Break Repair. Cancers 2021, 13, 5561. [Google Scholar] [CrossRef] [PubMed]
- Barroso, S.I.; Aguilera, A. Detection of DNA Double-Strand Breaks by γ-H2AX Immunodetection. Methods Mol. Biol. 2020, 2153, 1–8. [Google Scholar] [CrossRef]
- Böcker, W.; Iliakis, G. Computational Methods for analysis of foci: Validation for radiation-induced gamma-H2AX foci in human cells. Radiat. Res. 2006, 165, 113–124. [Google Scholar] [CrossRef]
- Runge, R.; Hiemann, R.; Wendisch, M.; Kasten-Pisula, U.; Storch, K.; Zoephel, K.; Fritz, C.; Roggenbuck, D.; Wunderlich, G.; Conrad, K.; et al. Fully automated interpretation of ionizing radiation-induced γH2AX foci by the novel pattern recognition system AKLIDES®. Int. J. Radiat. Biol. 2012, 88, 439–447. [Google Scholar] [CrossRef]
- Jucha, A.; Wegierek-Ciuk, A.; Koza, Z.; Lisowska, H.; Wojcik, A.; Wojewodzka, M.; Lankoff, A. FociCounter: A freely available PC programme for quantitative and qualitative analysis of gamma-H2AX foci. Mutat. Res. Toxicol. Environ. Mutagen. 2010, 696, 16–20. [Google Scholar] [CrossRef]
- González, J.E.; Lee, M.; Barquinero, J.F.; Valente, M.; Roch-Lefèvre, S.; García, O. Quantitative image analysis of gamma-H2AX foci induced by ionizing radiation applying open source programs. Anal. Quant. Cytol. Histol. 2012, 34, 66–71. [Google Scholar]
- Garty, G.; Chen, Y.; Salerno, A.; Turner, H.; Zhang, J.; Lyulko, O.; Bertucci, A.; Xu, Y.; Wang, H.; Simaan, N.; et al. The RABIT: A rapid automated biodosimetry tool for radiological triage. Health Phys. 2010, 98, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Garty, G.; Chen, Y.; Turner, H.C.; Zhang, J.; Lyulko, O.V.; Bertucci, A.; Xu, Y.; Wang, H.; Simaan, N.; Randers-Pehrson, G.; et al. The RABiT: A rapid automated biodosimetry tool for radiological triage. II. Technological developments. Int. J. Radiat. Biol. 2011, 87, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Wang, H.; Garty, G.; Xu, Y.; Lyulko, O.V.; Turner, H.C.; Randers-Pehrson, G.; Simaan, N.; Yao, Y.L.; et al. Design and Preliminary Validation of a Rapid Automated Biodosimetry Tool for High Througput Radiological Triage. Proc. ASME Des. Eng. Tech. Conf. 2009, 3, 61–67. [Google Scholar] [PubMed]
- Ivashkevich, A.; Redon, C.E.; Nakamura, A.J.; Martin, R.F.; Martin, O.A. Use of the γ-H2AX assay to monitor DNA damage and repair in translational cancer research. Cancer Lett. 2012, 327, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Avondoglio, D.; Scott, T.; Kil, W.J.; Sproull, M.; Tofilon, P.J.; Camphausen, K. High throughput evaluation of gamma-H2AX. Radiat. Oncol. 2009, 4, 31. [Google Scholar] [CrossRef] [PubMed]
- Tommasino, F.; Friedrich, T.; Jakob, B.; Meyer, B.; Durante, M.; Scholz, M. Induction and Processing of the Radiation-Induced Gamma-H2AX Signal and its Link to the Underlying Pattern of DSB: A Combined Experimental and Modelling Study. PLoS ONE 2015, 10, e0129416. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barbieri, S.; Babini, G.; Morini, J.; Friedland, W.; Buonanno, M.; Grilj, V.; Brenner, D.J.; Ottolenghi, A.; Baiocco, G. Predicting DNA damage foci and their experimental readout with 2D microscopy: A unified approach applied to photon and neutron exposures. Sci. Rep. 2019, 9, 14019. [Google Scholar] [CrossRef]
- Ainsbury, E.A.; Moquet, J.; Sun, M.; Barnard, S.; Ellender, M.; Lloyd, D. The future of biological dosimetry in mass casualty radiation emergency response, personalized radiation risk estimation and space radiation protection. Int. J. Radiat. Biol. 2022, 98, 421–427. [Google Scholar] [CrossRef]
- Blakely, W.F.; Salter, C.A.; Prasanna, P.G. Early-response biological dosimetry???Recommended countermeasure enhancements for mass-casualty radiological incidents and terrorism. Health Phys. 2005, 89, 494–504. [Google Scholar] [CrossRef]
- Lassmann, M.; Hänscheid, H.; Gassen, D.; Biko, J.; Meineke, V.; Reiners, C.; Scherthan, H. In vivo formation of gamma-H2AX and 53BP1 DNA repair foci in blood cells after radioiodine therapy of differentiated thyroid cancer. J. Nucl. Med. 2010, 51, 1318–1325. [Google Scholar] [CrossRef]
- Kawanishi, M.; Watanabe, T.; Hagio, S.; Ogo, S.; Shimohara, C.; Jouchi, R.; Takayama, S.; Hasei, T.; Hirayama, T.; Oda, Y.; et al. Genotoxicity of 3,6-dinitrobenzo[e]pyrene, a novel mutagen in ambient air and surface soil, in mammalian cells in vitro and in vivo. Mutagenesis 2009, 24, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Toyooka, T.; Ibuki, Y. Co-exposure to benzo[a]pyrene and UVA induces phosphorylation of histone H2AX. FEBS Lett. 2005, 579, 6338–6342. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, A.; Lundstedt, S.; Stenius, U. Exposure of HepG2 cells to low levels of PAH-containing extracts from contaminated soils results in unpredictable genotoxic stress responses. Environ. Mol. Mutagen. 2009, 50, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Yang, J.; Zeng, Q.-L.; Zhu, X.-M.; Qian, Y.-L.; Huang, H.-F. 50-Hertz Electromagnetic Fields Induce gammaH2AX Foci Formation in Mouse Preimplantation Embryos In Vitro1. Biol. Reprod. 2006, 75, 673–680. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Markovà, E.; Hillert, L.; Malmgren, L.; Persson, B.R.; Belyaev, I.Y. Microwaves from GSM mobile telephones affect 53BP1 and gamma-H2AX foci in human lymphocytes from hypersensitive and healthy persons. Environ. Health Perspect. 2005, 113, 1172–1177. [Google Scholar] [CrossRef]
- Hunt, C.R.; Pandita, R.K.; Laszlo, A.; Higashikubo, R.; Agarwal, M.; Kitamura, T.; Gupta, A.; Rief, N.; Horikoshi, N.; Baskaran, R.; et al. Hyperthermia Activates a Subset of Ataxia-Telangiectasia Mutated Effectors Independent of DNA Strand Breaks and Heat Shock Protein 70 Status. Cancer Res. 2007, 67, 3010–3017. [Google Scholar] [CrossRef]
- Banáth, J.P.; Olive, P.L. Expression of phosphorylated histone H2AX as a surrogate of cell killing by drugs that create DNA double-strand breaks. Cancer Res. 2003, 63, 4347–4435. [Google Scholar]
- Wasco, M.J.; Pu, R.T. Utility of antiphosphorylated H2AX antibody (gamma-H2AX) in diagnosing metastatic renal cell carcinoma. Appl. Immunohistochem. Mol. Morphol. 2008, 16, 349–356. [Google Scholar] [CrossRef]
- Halm, B.M.; Franke, A.; Lai, J.F.; Li, X.; Custer, L.J.; Pagano, I.; Cooney, R.V.; Turner, H.C.; Brenner, D.J. Pilot study for the establishment of biomarkers for radiation damage after computed tomography in children. Hawaii J. Med. Public Health A J. Asia Pac. Med. Public Health 2015, 74, 112–119. [Google Scholar]
- Beels, L.; Bacher, K.; De Wolf, D.; Werbrouck, J.; Thierens, H. gamma-H2AX foci as a biomarker for patient X-ray exposure in pediatric cardiac catheterization: Are we underestimating radiation risks? Circulation 2009, 120, 1903–1909. [Google Scholar] [CrossRef]
- Kuefner, M.A.; Grudzenski, S.; Schwab, S.A.; Wiederseiner, M.; Heckmann, M.; Bautz, W.; Lobrich, M.; Uder, M. DNA Double-Strand Breaks and Their Repair in Blood Lymphocytes of Patients Undergoing Angiographic Procedures. Investig. Radiol. 2009, 44, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.S.; Mason, J.; Lobachevsky, P.N.; Munforte, L.; Selbie, L.; Ball, D.L.; Martin, R.F.; Leong, T.; Siva, S.; Martin, O.A. Radiation Therapy Modulates DNA Repair Efficiency in Peripheral Blood Mononuclear Cells of Patients With Non-Small Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 521–531. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valente, D.; Gentileschi, M.P.; Guerrisi, A.; Bruzzaniti, V.; Morrone, A.; Soddu, S.; Verdina, A. Factors to Consider for the Correct Use of γH2AX in the Evaluation of DNA Double-Strand Breaks Damage Caused by Ionizing Radiation. Cancers 2022, 14, 6204. https://doi.org/10.3390/cancers14246204
Valente D, Gentileschi MP, Guerrisi A, Bruzzaniti V, Morrone A, Soddu S, Verdina A. Factors to Consider for the Correct Use of γH2AX in the Evaluation of DNA Double-Strand Breaks Damage Caused by Ionizing Radiation. Cancers. 2022; 14(24):6204. https://doi.org/10.3390/cancers14246204
Chicago/Turabian StyleValente, Davide, Maria Pia Gentileschi, Antonino Guerrisi, Vicente Bruzzaniti, Aldo Morrone, Silvia Soddu, and Alessandra Verdina. 2022. "Factors to Consider for the Correct Use of γH2AX in the Evaluation of DNA Double-Strand Breaks Damage Caused by Ionizing Radiation" Cancers 14, no. 24: 6204. https://doi.org/10.3390/cancers14246204
APA StyleValente, D., Gentileschi, M. P., Guerrisi, A., Bruzzaniti, V., Morrone, A., Soddu, S., & Verdina, A. (2022). Factors to Consider for the Correct Use of γH2AX in the Evaluation of DNA Double-Strand Breaks Damage Caused by Ionizing Radiation. Cancers, 14(24), 6204. https://doi.org/10.3390/cancers14246204





