Anal Cancer in High-Risk Women: The Lost Tribe
Abstract
Simple Summary
Abstract
1. Introduction
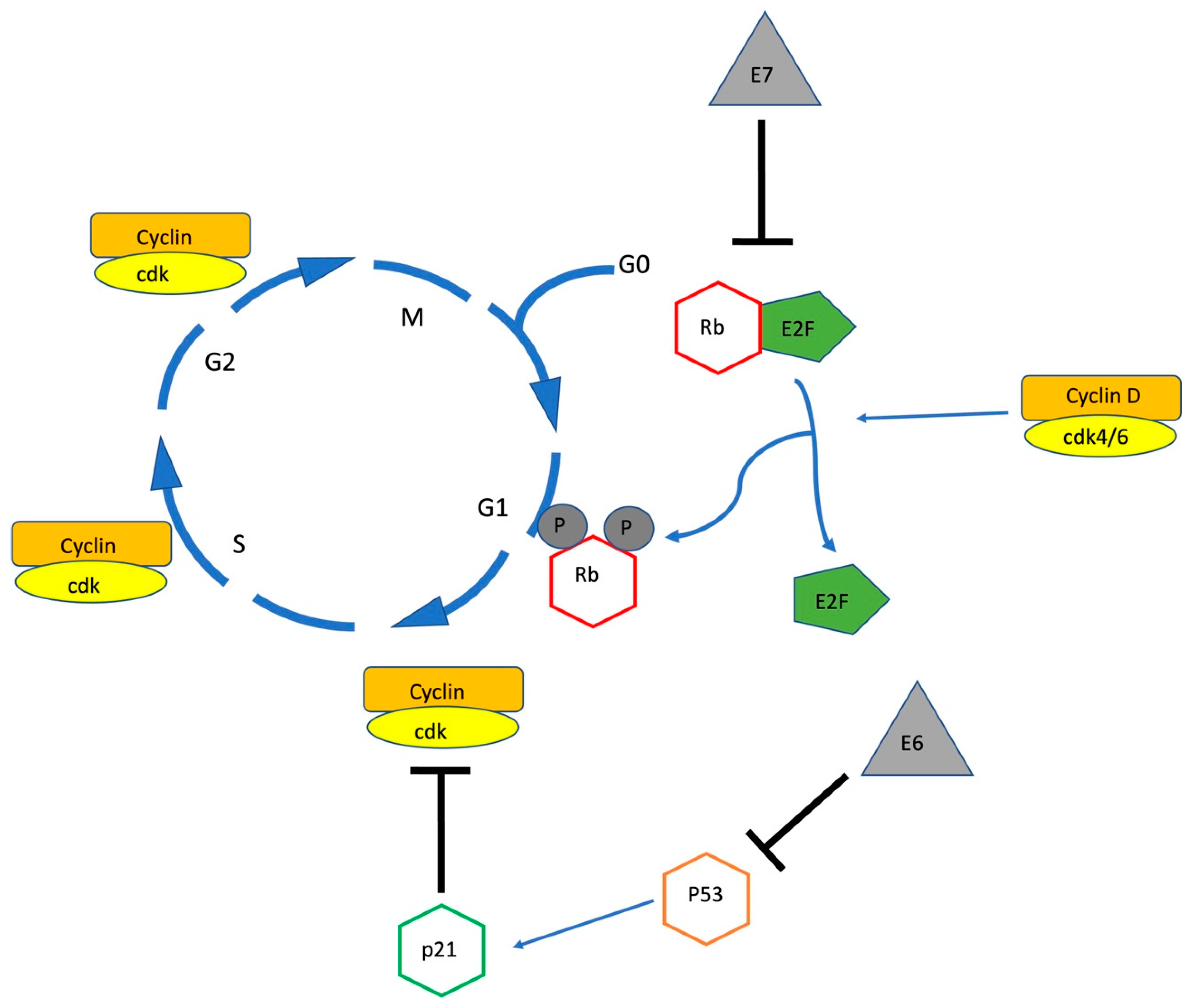
2. High-Risk Human Papillomavirus Pathogenesis
3. Anal Squamous Intraepithelial Lesions and Their Natural History
4. Risk Factors for Anal hrHPV Colonisation in Women
5. Risk Factors for Chronicity of hrHPV Infection in Women
5.1. Smoking
5.2. Immunosuppression
5.3. Human Immunodeficiency Virus
6. The Perineum, a Reservoir for Anogenital hrHPV in Women
7. Understanding Anal Cancer Trends in Women
8. Screening, Surveillance and Treatment of Anal HSIL in High-Risk Women: Is It Necessary?
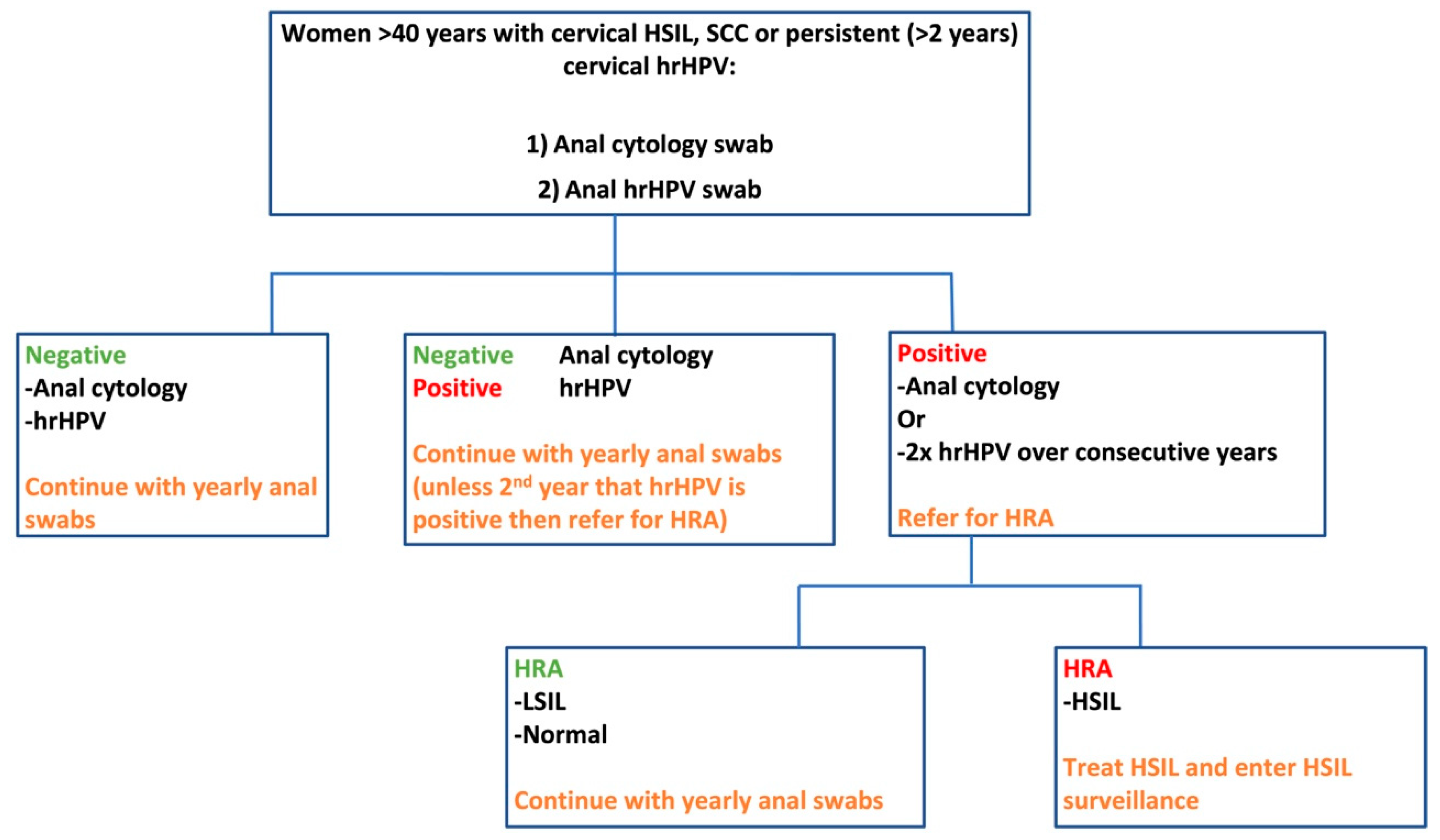
9. A Potential Screening, Treatment and Surveillance Model for High-Risk Women
9.1. Screening
9.1.1. The Digital Anorectal Examination
9.1.2. Anal Cytology and HPV Testing
9.1.3. High Resolution Anoscopy
9.2. Treatment
9.3. Screening and Surveillance Pathways
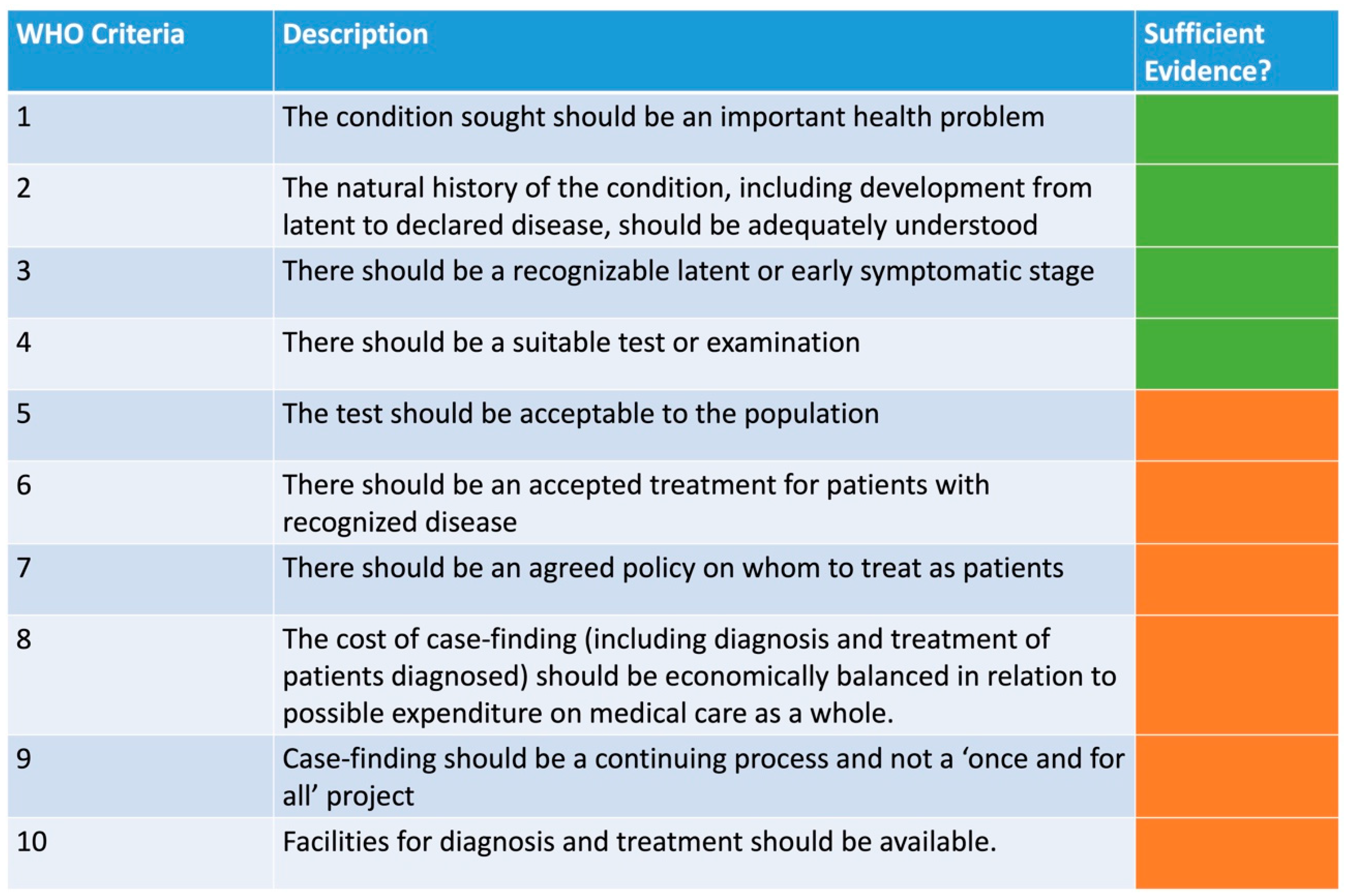
9.4. World Health Organisation (WHO) Screening Criteria for the Secondary Prevention of Anal Cancer
10. The Role of the HPV Vaccination
11. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- National Cancer Institute. Surveillance Epidemiology and End Results Program. Cancer Stat Facts: Anal Cancer . NIH. Available online: https://seer.cancer.gov/statfacts/html/anus.html (accessed on 28 September 2022).
- Islami, F.; Ferlay, J.; Lortet-Tieulent, J.; Bray, F.; Jemal, A. International trends in anal cancer incidence rates. Int. J. Epidemiol. 2017, 46, 924–938. [Google Scholar] [CrossRef]
- Brogden, D.R.; Kontovounisios, C.; Mandalia, S.; Tekkis, P.; Mills, S.C. The Role of Demographics, Social Deprivation and Ethnicity on Anal Squamous Cell Carcinoma Incidence in England. J. Clin. Med. 2021, 10, 3621. [Google Scholar] [CrossRef]
- Clifford, G.M.; Georges, D.; Shiels, M.S.; Engels, E.A.; Albuquerque, A.; Poynten, I.M.; de Pokomandy, A.; Easson, A.M.; Stier, E.A. A meta-analysis of anal cancer incidence by risk group: Toward a unified anal cancer risk scale. Int. J. Cancer 2021, 148, 38–47. [Google Scholar] [CrossRef]
- Reid, E.; Suneja, G.; Ambinder, R.F.; Ard, K.; Baiocchi, R.; Barta, S.K.; Carchman, E.; Cohen, A.; Crysler, O.V.; Gupta, N. AIDS-related Kaposi sarcoma, Version 2.2019. J. Natl. Compr. Cancer Netw. 2019, 17, 171–189. [Google Scholar] [CrossRef]
- European AIDS Clinical Society. European AIDS Clinical Society Clinical Guidelines Version 10.1. Available online: https://www.eacsociety.org/files/guidelines-10.1_5.pdf (accessed on 17 December 2020).
- Esser, S.; Kreuter, A.; Oette, M.; Gingelmaier, A.; Mosthaf, F.; Sautter-Bihl, M.L.; Jongen, J.; Brockmeyer, N.H.; Eldering, G.; Swoboda, J. German-Austrian guidelines on anal dysplasia and anal cancer in HIV-positive individuals: Prevention, diagnosis, and treatment. J. Ger. Soc. Dermatol. 2015, 13, 1302–1319. [Google Scholar] [CrossRef]
- Hirsch, B.E.; McGowan, J.P.; Fine, S.M.; Vail, R.; Merrick, S.T.; Radix, A.; Hoffmann, C.J.; Gonzalez, C.J. Screening for Anal Dysplasia and Cancer in Adults with HIV; Johns Hopkins University: Baltimore, MD, USA, 2022. [Google Scholar]
- Celie, K.-B.; Jackson, C.; Agrawal, S.; Dodhia, C.; Guzman, C.; Kaufman, T.; Hellenthal, N.; Monie, D.; Monzon, J.; Oceguera, L. Socioeconomic and gender disparities in anal cancer diagnosis and treatment. Surg. Oncol. 2017, 26, 212–217. [Google Scholar] [CrossRef]
- Deshmukh, A.A.; Suk, R.; Shiels, M.S.; Damgacioglu, H.; Lin, Y.-Y.; Stier, E.A.; Nyitray, A.G.; Chiao, E.Y.; Nemutlu, G.S.; Chhatwal, J. Incidence trends and burden of human papillomavirus-associated cancers among women in the United States, 2001–2017. J. Natl. Cancer Inst. 2021, 113, 792–796. [Google Scholar] [CrossRef]
- CRUK. Available online: https://www.cancerresearchuk.org/about-cancer/anal-cancer/survival (accessed on 28 September 2022).
- Lee, J.Y.; Lensing, S.Y.; Berry-Lawhorn, J.M.; Jay, N.; Darragh, T.M.; Goldstone, S.E.; Wilkin, T.J.; Stier, E.A.; Einstein, M.; Pugliese, J.C. Design of the ANal Cancer/HSIL Outcomes Research study (ANCHOR study): A randomized study to prevent anal cancer among persons living with HIV. Contemp. Clin. Trials 2022, 113, 106679. [Google Scholar] [CrossRef]
- Palefsky, J.M.; Lee, J.Y.; Jay, N.; Goldstone, S.E.; Darragh, T.M.; Dunlevy, H.A.; Rosa-Cunha, I.; Arons, A.; Pugliese, J.C.; Vena, D. Treatment of anal high-grade squamous intraepithelial lesions to prevent anal cancer. N. Engl. J. Med. 2022, 386, 2273–2282. [Google Scholar] [CrossRef]
- Loopik, D.L.; Ebisch, R.M.; IntHout, J.; Melchers, W.J.; Massuger, L.F.; Bekkers, R.L.; Siebers, A.G. The relative risk of noncervical high-risk human papillomavirus-related (pre) malignancies after recurrent cervical intraepithelial neoplasia grade 3: A population-based study. Int. J. Cancer 2020, 147, 897–900. [Google Scholar] [CrossRef]
- Chia-Ching, J.W.; Sparano, J.; Palefsky, J.M. Human immunodeficiency virus/AIDS, human papillomavirus, and anal cancer. Surg. Oncol. Clin. 2017, 26, 17–31. [Google Scholar]
- Maniar, K.P.; Nayar, R. HPV-related squamous neoplasia of the lower anogenital tract: An update and review of recent guidelines. Adv. Anat. Pathol. 2014, 21, 341–358. [Google Scholar] [CrossRef]
- Leber, K.; van Beurden, M.; Zijlmans, H.; Dewit, L.; Richel, O.; Vrouenraets, S. Screening for intra-anal squamous intra-epithelial lesions in women with a history of human papillomavirus-related vulvar or perianal disease: Results of a screening protocol. Colorectal Dis. 2020, 22, 1991–1998. [Google Scholar] [CrossRef]
- Otter, S.; Whitaker, S.; Chatterjee, J.; Stewart, A. The human papillomavirus as a common pathogen in oropharyngeal, anal and cervical cancers. Clin. Oncol. 2019, 31, 81–90. [Google Scholar] [CrossRef]
- Lin, C.; Franceschi, S.; Clifford, G.M. Human papillomavirus types from infection to cancer in the anus, according to sex and HIV status: A systematic review and meta-analysis. Lancet Infect. Dis. 2018, 18, 198–206. [Google Scholar] [CrossRef]
- Valmary-Degano, S.; Jacquin, E.; Prétet, J.-L.; Monnien, F.; Girardo, B.; Arbez-Gindre, F.; Joly, M.; Bosset, J.-F.; Kantelip, B.; Mougin, C. Signature patterns of human papillomavirus type 16 in invasive anal carcinoma. Hum. Pathol. 2013, 44, 992–1002. [Google Scholar] [CrossRef]
- Giacinti, C.; Giordano, A. RB and cell cycle progression. Oncogene 2006, 25, 5220–5227. [Google Scholar] [CrossRef]
- Zilfou, J.T.; Lowe, S.W. Tumor suppressive functions of p53. Cold Spring Harbor Perspect. Biol. 2009, 1, a001883. [Google Scholar] [CrossRef]
- Araldi, R.P.; Sant’Ana, T.A.; Módolo, D.G.; de Melo, T.C.; Spadacci-Morena, D.D.; de Cassia Stocco, R.; Cerutti, J.M.; de Souza, E.B. The human papillomavirus (HPV)-related cancer biology: An overview. Biomed. Pharmacother. 2018, 106, 1537–1556. [Google Scholar] [CrossRef]
- Scarth, J.A.; Patterson, M.R.; Morgan, E.L.; Macdonald, A. The human papillomavirus oncoproteins: A review of the host pathways targeted on the road to transformation. J. Gen. Virol. 2021, 102, 001540. [Google Scholar] [CrossRef]
- Zur Hausen, H. Papillomavirus infections—A major cause of human cancers. Biochim. Biophys. Acta (BBA)—Rev. Cancer 1996, 1288, F55–F78. [Google Scholar] [CrossRef]
- Saunier, M.; Monnier-Benoit, S.; Mauny, F.; Dalstein, V.; Briolat, J.; Riethmuller, D.; Kantelip, B.; Schwarz, E.; Mougin, C.; Prétet, J.-L. Analysis of human papillomavirus type 16 (HPV16) DNA load and physical state for identification of HPV16-infected women with high-grade lesions or cervical carcinoma. J. Clin. Microbiol. 2008, 46, 3678–3685. [Google Scholar] [CrossRef]
- Cañadas, M.-P.; Darwich, L.; Sirera, G.; Bofill, M.; Piñol, M.; Garcia-Cuyas, F.; Llatjos, M.; Corbasi, P.; Clotet, B.; Videla, S. Human papillomavirus 16 integration and risk factors associated in anal samples of HIV-1 infected men. Sex. Transm. Dis. 2010, 37, 311–315. [Google Scholar] [CrossRef]
- Darragh, T.M.; Colgan, T.J.; Cox, J.T.; Heller, D.S.; Henry, M.R.; Luff, R.D.; McCalmont, T.; Nayar, R.; Palefsky, J.M.; Stoler, M.H. The lower anogenital squamous terminology standardization project for HPV-associated lesions: Background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Arch. Pathol. Lab. Med. 2012, 136, 1266–1297. [Google Scholar] [CrossRef]
- Lee, G.C.; Kunitake, H.; Milch, H.; Savitt, L.R.; Stafford, C.; Bordeianou, L.G.; Francone, T.D.; Ricciardi, R. What is the risk of anal carcinoma in patients with anal intraepithelial neoplasia III? Dis. Colon Rectum 2018, 61, 1350. [Google Scholar] [CrossRef]
- Fuchs, W.; Wieland, U.; Skaletz-Rorowski, A.; Brockmeyer, N.; Swoboda, J.; Kreuter, A.; Michalik, C.; Potthoff, A.; Competence Network for HIV/AIDS. The male Screen ING Study: Prevalence of HPV-related genital and anal lesions in an urban cohort of HIV-positive men in Germany. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 995–1001. [Google Scholar] [CrossRef]
- Scholefield, J.; Castle, M.; Watson, N. Malignant transformation of high-grade anal intraepithelial neoplasia. J. Br. Surg. 2005, 92, 1133–1136. [Google Scholar] [CrossRef]
- Watson, A.J.; Smith, B.B.; Whitehead, M.R.; Sykes, P.H.; Frizelle, F.A. Malignant progression of anal intra-epithelial neoplasia. ANZ J. Surg. 2006, 76, 715–717. [Google Scholar] [CrossRef]
- Lima, F.D.G.; van der Zee, R.P.; Dick, S.; van Noesel, C.J.; Berkhof, J.; van der Loeff, M.F.S.; Prins, J.M.; Steenbergen, R.D.; de Vries, H.J. DNA Methylation Analysis to predict Regression of high-grade anal Intraepithelial Neoplasia in HIV+ men (MARINE): A cohort study protocol. BMJ Open 2022, 12, e060301. [Google Scholar] [CrossRef]
- Poynten, I.M.; Jin, F.; Roberts, J.M.; Templeton, D.J.; Law, C.; Cornall, A.M.; Molano, M.; Machalek, D.A.; Carr, A.; Farnsworth, A. The natural history of anal high-grade squamous intraepithelial lesions in gay and bisexual men. Clin. Infect. Dis. 2021, 72, 853–861. [Google Scholar] [CrossRef]
- Tong, W.W.; Jin, F.; McHugh, L.C.; Maher, T.; Sinclair, B.; Grulich, A.E.; Hillman, R.J.; Carr, A. Progression to and spontaneous regression of high-grade anal squamous intraepithelial lesions in HIV-infected and uninfected men. Aids 2013, 27, 2233–2243. [Google Scholar] [CrossRef]
- Steenbergen, R.D.; Snijders, P.J.; Heideman, D.A.; Meijer, C.J. Clinical implications of (epi) genetic changes in HPV-induced cervical precancerous lesions. Nat. Rev. Cancer 2014, 14, 395–405. [Google Scholar] [CrossRef]
- Vink, F.; Lissenberg-Witte, B.I.; Meijer, C.; Berkhof, J.; van Kemenade, F.; Siebers, A.; Steenbergen, R.; Bleeker, M.; Heideman, D. FAM19A4/miR124-2 methylation analysis as a triage test for HPV-positive women: Cross-sectional and longitudinal data from a Dutch screening cohort. Clin. Microbiol. Infect. 2021, 27, 125.e121–125.e126. [Google Scholar] [CrossRef]
- Kremer, W.W.; Dick, S.; Heideman, D.A.; Steenbergen, R.D.; Bleeker, M.C.; Verhoeve, H.R.; van Baal, W.M.; van Trommel, N.; Kenter, G.G.; Meijer, C.J. Clinical Regression of High-Grade Cervical Intraepithelial Neoplasia Is Associated with Absence of FAM19A4/miR124-2 DNA Methylation (CONCERVE Study). J. Clin. Oncol. 2022, 40, 3037–3046. [Google Scholar] [CrossRef]
- Van der Zee, R.P.; Richel, O.; van Noesel, C.J.; Ciocănea-Teodorescu, I.; van Splunter, A.P.; ter Braak, T.J.; Nathan, M.; Cuming, T.; Sheaff, M.; Kreuter, A. Cancer risk stratification of anal intraepithelial neoplasia in HIV-positive men by validated methylation markers associated with progression to cancer. Clin. Infect. Dis. 2021, 72, 2154–2163. [Google Scholar] [CrossRef]
- Slama, J.; Sehnal, B.; Dusek, L.; Zima, T.; Cibula, D. Impact of risk factors on prevalence of anal HPV infection in women with simultaneous cervical lesion. Neoplasma 2015, 62, 308–314. [Google Scholar] [CrossRef]
- ElNaggar, A.C.; Santoso, J.T. Risk factors for anal intraepithelial neoplasia in women with genital dysplasia. Obstet. Gynecol. 2013, 122, 218–223. [Google Scholar] [CrossRef]
- Mello, V.; Sundstrom, R. Cervical Intraepithelial Neoplasia; StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Kusters, J.M.; Heijne, J.C.; van Benthem, B.H.; King, A.J.; van der Loeff, M.F.S. Type-specific concurrent anogenital HPV detection among young women and MSM attending Dutch sexual health clinics. Sex. Transm. Infect. 2022, 0, 1–9. [Google Scholar] [CrossRef]
- McCloskey, J.C.; Kast, W.M.; Flexman, J.P.; McCallum, D.; French, M.A.; Phillips, M. Syndemic synergy of HPV and other sexually transmitted pathogens in the development of high-grade anal squamous intraepithelial lesions. Papillomavirus Res. 2017, 4, 90–98. [Google Scholar] [CrossRef]
- Daling, J.R.; Madeleine, M.M.; Johnson, L.G.; Schwartz, S.M.; Shera, K.A.; Wurscher, M.A.; Carter, J.J.; Porter, P.L.; Galloway, D.A.; McDougall, J.K. Human papillomavirus, smoking, and sexual practices in the etiology of anal cancer. Cancer 2004, 101, 270–280. [Google Scholar] [CrossRef]
- Marra, E.; Lin, C.; Clifford, G.M. Type-specific anal human papillomavirus prevalence among men, according to sexual preference and HIV status: A systematic literature review and meta-analysis. J. Infect. Dis. 2019, 219, 590–598. [Google Scholar] [CrossRef]
- Lin, C.; Slama, J.; Gonzalez, P.; Goodman, M.T.; Xia, N.; Kreimer, A.R.; Wu, T.; Hessol, N.A.; Shvetsov, Y.; Ortiz, A.P. Cervical determinants of anal HPV infection and high-grade anal lesions in women: A collaborative pooled analysis. Lancet Infect. Dis. 2019, 19, 880–891. [Google Scholar] [CrossRef]
- Arnold, M.; Liu, L.; Kenter, G.G.; Creutzberg, C.L.; Coebergh, J.W.; Soerjomataram, I. Second primary cancers in survivors of cervical cancer in The Netherlands: Implications for prevention and surveillance. Radiother. Oncol. 2014, 111, 374–381. [Google Scholar] [CrossRef]
- Tseng, H.-F.; Morgenstern, H.; Mack, T.M.; Peters, R.K. Risk factors for anal cancer: Results of a population-based case–control study. Cancer Causes Control 2003, 14, 837–846. [Google Scholar] [CrossRef]
- Albuquerque, A.; Godfrey, M.A.; Cappello, C.; Pesola, F.; Bowring, J.; Cuming, T.; De Masi, A.; Rosenthal, A.N.; Sasieni, P.; Nathan, M. Multizonal anogenital neoplasia in women: A cohort analysis. BMC Cancer 2021, 21, 232. [Google Scholar] [CrossRef]
- Reinholdt, K.; Thomsen, L.T.; Munk, C.; Dehlendorff, C.; Aalborg, G.L.; Carstensen, B.; Jørgensen, M.E.; Kjaer, S.K. Incidence of human papillomavirus-related anogenital precancer and cancer in women with diabetes: A nationwide registry-based cohort study. Int. J. Cancer 2021, 148, 2090–2101. [Google Scholar] [CrossRef]
- Reinholdt, K.; Thomsen, L.T.; Dehlendorff, C.; Larsen, H.K.; Sørensen, S.S.; Hædersdal, M.; Kjær, S.K. Human papillomavirus-related anogenital premalignancies and cancer in renal transplant recipients: A Danish nationwide, registry-based cohort study. Int. J. Cancer 2020, 146, 2413–2422. [Google Scholar] [CrossRef]
- Segal, J.P.; Askari, A.; Clark, S.K.; Hart, A.L.; Faiz, O.D. The Incidence and Prevalence of Human Papilloma Virus-associated Cancers in IBD. Inflamm. Bowel Dis. 2021, 27, 34–39. [Google Scholar] [CrossRef]
- D’Souza, G.; Wiley, D.J.; Li, X.; Chmiel, J.S.; Margolick, J.B.; Cranston, R.D.; Jacobson, L.P. Incidence and epidemiology of anal cancer in the Multicenter AIDS Cohort Study (MACS). J. Acquir. Immune Defic. Syndr. (1999) 2008, 48, 491. [Google Scholar] [CrossRef]
- Kannappan, S.; Lee, J.H.; Lakshmanakumar, M.; Nesakumar, N.; Rayappan, J.B.B. Cervical Cancer. In Biomarkers and Biosensors for Cervical Cancer Diagnosis; Springer: Singapore, 2021; pp. 13–22. [Google Scholar]
- Bodelon, C.; Madeleine, M.M.; Voigt, L.F.; Weiss, N.S. Is the incidence of invasive vulvar cancer increasing in the United States? Cancer Causes Control 2009, 20, 1779–1782. [Google Scholar] [CrossRef]
- Papatla, K.; Halpern, M.T.; Hernandez, E.; Brown, J.; Benrubi, D.; Houck, K.; Chu, C.; Rubin, S. Second primary anal and oropharyngeal cancers in cervical cancer survivors. Am. J. Obstet. Gynecol. 2019, 221, 478.e471–478.e476. [Google Scholar] [CrossRef]
- Kalliala, I.; Athanasiou, A.; Veroniki, A.; Salanti, G.; Efthimiou, O.; Raftis, N.; Bowden, S.; Paraskevaidi, M.; Aro, K.; Arbyn, M. Incidence and mortality from cervical cancer and other malignancies after treatment of cervical intraepithelial neoplasia: A systematic review and meta-analysis of the literature. Ann. Oncol. 2020, 31, 213–227. [Google Scholar] [CrossRef]
- Jiménez, W.; Paszat, L.; Kupets, R.; Wilton, A.; Tinmouth, J. Presumed previous human papillomavirus (HPV) related gynecological cancer in women diagnosed with anal cancer in the province of Ontario. Gynecol. Oncol. 2009, 114, 395–398. [Google Scholar] [CrossRef]
- Domgue, J.F.; Messick, C.; Milbourne, A.; Guo, M.; Salcedo, M.P.; Dahlstrom, K.R.; Chiao, E.Y.; Deshmukh, A.A.; Sturgis, E.M.; Schmeler, K.M. Prevalence of high-grade anal dysplasia among women with high-grade lower genital tract dysplasia or cancer: Results of a pilot study. Gynecol. Oncol. 2019, 153, 266–270. [Google Scholar] [CrossRef]
- Tatti, S.; Suzuki, V.; Fleider, L.; Maldonado, V.; Caruso, R.; de los Angeles Tinnirello, M. Anal intraepithelial lesions in women with human papillomavirus–related disease. J. Lower Genit. Tract Dis. 2012, 16, 454–459. [Google Scholar] [CrossRef]
- Evans, H.; Newnham, A.; Hodgson, S.; Møller, H. Second primary cancers after cervical intraepithelial neoplasia III and invasive cervical cancer in Southeast England. Gynecol. Oncol. 2003, 90, 131–136. [Google Scholar] [CrossRef]
- Brogden, D.R.; Lupi, M.E.; Warren, O.J.; Kontovounisios, C.; Mills, S.C. Comparing and contrasting clinical consensus and guidelines for anal intraepithelial neoplasia in different geographical regions. Updates Surg. 2021, 73, 2047–2058. [Google Scholar] [CrossRef]
- Koppe, D.C.; Bandeira, C.B.; Rosa, M.R.D.; Cambruzzi, E.; Meurer, L.; Fagundes, R.B. Prevalence of anal intraepithelial neoplasia in women with genital neoplasia. Dis. Colon Rectum 2011, 54, 442–445. [Google Scholar] [CrossRef]
- Proctor, L.; Grennan, T.; Albert, A.; Miller, D.; Sadownik, L.; Lee, M. Screening for anal cancer in women with a history of vulvar high-grade squamous intraepithelial lesions. J. Lower Genit. Tract Dis. 2019, 23, 265–271. [Google Scholar] [CrossRef]
- Scholefield, J.; Talbot, I.; Whatrup, C.; Sonnex, C.; Palmer, J.; Mindel, A.; Northover, J. Anal and cervical intraepithelial neoplasia: Possible parallel. Lancet 1989, 334, 765–769. [Google Scholar] [CrossRef]
- Gravitt, P.E.; Rositch, A.F.; Silver, M.I.; Marks, M.A.; Chang, K.; Burke, A.E.; Viscidi, R.P. A cohort effect of the sexual revolution may be masking an increase in human papillomavirus detection at menopause in the United States. J. Infect. Dis. 2013, 207, 272–280. [Google Scholar] [CrossRef]
- Malhotra, S. Impact of the sexual revolution: Consequences of risky sexual behaviors. J. Am. Physicians Surg. 2008, 13, 88. [Google Scholar]
- Islami, F.; Fedewa, S.A.; Jemal, A. Trends in cervical cancer incidence rates by age, race/ethnicity, histological subtype, and stage at diagnosis in the United States. Prev. Med. 2019, 123, 316–323. [Google Scholar] [CrossRef]
- Khadraoui, H.; Thappa, S.; Smith, M.; Davidov, A.; Castellanos, M.R. Age-associated trends of vulvar cancer in the US. Menopause 2021, 28, 119–125. [Google Scholar] [CrossRef]
- Kang, Y.J.; Smith, M.; Barlow, E.; Coffey, K.; Hacker, N.; Canfell, K. Vulvar cancer in high-income countries: Increasing burden of disease. Int. J. Cancer 2017, 141, 2174–2186. [Google Scholar] [CrossRef]
- World Health Organization. WHO Guideline for Screening and Treatment of Cervical Pre-Cancer Lesions for Cervical Cancer Prevention; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Koh, W.-J.; Greer, B.E.; Abu-Rustum, N.R.; Campos, S.M.; Cho, K.R.; Chon, H.S.; Chu, C.; Cohn, D.; Crispens, M.A.; Dizon, D.S. Vulvar cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 92–120. [Google Scholar] [CrossRef]
- Colombo, N.; Carinelli, S.; Colombo, A.; Marini, C.; Rollo, D.; Sessa, C. Cervical cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2012, 23, vii27–vii32. [Google Scholar] [CrossRef]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Haie-Meder, C.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology guidelines for the management of patients with cervical cancer. Virchows Arch. 2018, 472, 919–936. [Google Scholar] [CrossRef]
- Morrison, J.; Baldwin, P.; Buckley, L.; Cogswell, L.; Edey, K.; Faruqi, A.; Ganesan, R.; Hall, M.; Hillaby, K.; Reed, N. British Gynaecological Cancer Society (BGCS) vulval cancer guidelines: Recommendations for practice. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 502–525. [Google Scholar] [CrossRef]
- Preti, M.; Joura, E.; Vieira-Baptista, P.; Van Beurden, M.; Bevilacqua, F.; Bleeker, M.C.; Bornstein, J.; Carcopino, X.; Chargari, C.; Cruickshank, M.E. The European Society of Gynaecological Oncology (ESGO), the International Society for the Study of Vulvovaginal Disease (ISSVD), the European College for the Study of Vulval Disease (ECSVD) and the European Federation for Colposcopy (EFC) consensus statements on pre-invasive vulvar lesions. Int. J. Gynecol. Cancer 2022, 32, 830–845. [Google Scholar]
- Edgren, G.; Sparén, P. Risk of anogenital cancer after diagnosis of cervical intraepithelial neoplasia: A prospective population-based study. Lancet Oncol. 2007, 8, 311–316. [Google Scholar] [CrossRef]
- Goddard, S.; Templeton, D.; Petoumenos, K.; Jin, F.; Hillman, R.; Law, C.; Roberts, J.; Fairley, C.; Garland, S.; Grulich, A. Association of anal symptoms with anal high grade squamous intraepithelial lesions (HSIL) among men who have sex with men: Baseline data from the study of the prevention of anal cancer (SPANC). Cancer Epidemiol. 2019, 58, 12–16. [Google Scholar] [CrossRef]
- Bauer, P.; Fléjou, J.-F.; Etienney, I. Prospective single-center observational study of routine histopathologic evaluation of macroscopically normal hemorrhoidectomy and fissurectomy specimens in search of anal intraepithelial neoplasia. Dis. Colon Rectum 2015, 58, 692–697. [Google Scholar] [CrossRef]
- Marlow, L.A.V.; Waller, J.; Wardle, J. Barriers to cervical cancer screening among ethnic minority women: A qualitative study. J. Fam. Plan. Reprod. Health Care 2015, 41, 248–254. [Google Scholar] [CrossRef]
- Rawl, S.M.; Dickinson, S.; Lee, J.L.; Roberts, J.L.; Teal, E.; Baker, L.B.; Kianersi, S.; Haggstrom, D.A. Racial and Socioeconomic Disparities in Cancer-Related Knowledge, Beliefs, and Behaviors in Indiana. Cancer Epidemiol. Biomarkers Prev. 2019, 28, 462–470. [Google Scholar] [CrossRef]
- Vrinten, C.; Gallagher, A.; Waller, J.; Marlow, L.A.V. Cancer stigma and cancer screening attendance: A population based survey in England. BMC Cancer 2019, 19, 566. [Google Scholar] [CrossRef]
- Clegg, L.X.; Reichman, M.E.; Miller, B.A.; Hankey, B.F.; Singh, G.K.; Lin, Y.D.; Goodman, M.T.; Lynch, C.F.; Schwartz, S.M.; Chen, V.W.; et al. Impact of socioeconomic status on cancer incidence and stage at diagnosis: Selected findings from the surveillance, epidemiology, and end results: National Longitudinal Mortality Study. Cancer Causes Control 2009, 20, 417–435. [Google Scholar] [CrossRef]
- Beavis, A.L.; Gravitt, P.E.; Rositch, A.F. Hysterectomy-corrected cervical cancer mortality rates reveal a larger racial disparity in the United States. Cancer 2017, 123, 1044–1050. [Google Scholar] [CrossRef]
- Akers, A.Y.; Newmann, S.J.; Smith, J.S. Factors underlying disparities in cervical cancer incidence, screening, and treatment in the United States. Curr. Probl. Cancer 2007, 31, 157–181. [Google Scholar] [CrossRef]
- Marlow, L.; McBride, E.; Varnes, L.; Waller, J. Barriers to cervical screening among older women from hard-to-reach groups: A qualitative study in England. BMC Women’s Health 2019, 19, 38. [Google Scholar] [CrossRef]
- Murfin, J.; Irvine, F.; Meechan-Rogers, R.; Swift, A. Education, income and occupation and their influence on the uptake of cervical cancer prevention strategies: A systematic review. J. Clin. Nurs. 2020, 29, 393–415. [Google Scholar] [CrossRef]
- Costas-Muniz, R.; Leng, J.; Aragones, A.; Ramirez, J.; Roberts, N.; Mujawar, M.I.; Gany, F. Association of socioeconomic and practical unmet needs with self-reported nonadherence to cancer treatment appointments in low-income Latino and Black cancer patients. Ethn. Health 2016, 21, 118–128. [Google Scholar] [CrossRef]
- Shack, L.; Jordan, C.; Thomson, C.S.; Mak, V.; Moller, H.; UK Association of Cancer Registries. Variation in incidence of breast, lung and cervical cancer and malignant melanoma of skin by socioeconomic group in England. BMC Cancer 2008, 8, 271. [Google Scholar] [CrossRef]
- Tron, L.; Belot, A.; Fauvernier, M.; Remontet, L.; Bossard, N.; Launay, L.; Bryere, J.; Monnereau, A.; Dejardin, O.; Launoy, G.; et al. Socioeconomic environment and disparities in cancer survival for 19 solid tumor sites: An analysis of the French Network of Cancer Registries (FRANCIM) data. Int. J. Cancer 2019, 144, 1262–1274. [Google Scholar] [CrossRef]
- Bryere, J.; Dejardin, O.; Launay, L.; Colonna, M.; Grosclaude, P.; Launoy, G.; French Network of Cancer Registries (FRANCIM). Socioeconomic status and site-specific cancer incidence, a Bayesian approach in a French Cancer Registries Network study. Eur. J. Cancer Prev. 2018, 27, 391–398. [Google Scholar] [CrossRef]
- Kumar, V.M.; Whynes, D.K. Explaining variation in the uptake of HPV vaccination in England. BMC Public Health 2011, 11, 172. [Google Scholar] [CrossRef]
- Bartlett, J.A.; Peterson, J.A. The uptake of human papillomavirus (HPV) vaccine among adolescent females in the United States: A review of the literature. J. Sch. Nurs. 2011, 27, 434–446. [Google Scholar] [CrossRef]
- Richel, O.; de Vries, H.J.; van Noesel, C.J.; Dijkgraaf, M.G.; Prins, J.M. Comparison of imiquimod, topical fluorouracil, and electrocautery for the treatment of anal intraepithelial neoplasia in HIV-positive men who have sex with men: An open-label, randomised controlled trial. Lancet Oncol. 2013, 14, 346–353. [Google Scholar] [CrossRef]
- Stewart, D.B.; Gaertner, W.B.; Glasgow, S.C.; Herzig, D.O.; Feingold, D.; Steele, S.R. The American Society of Colon and Rectal Surgeons clinical practice guidelines for anal squamous cell cancers (revised 2018). Dis. Colon Rectum 2018, 61, 755–774. [Google Scholar] [CrossRef]
- Steele, S.R.; Varma, M.G.; Melton, G.B.; Ross, H.M.; Rafferty, J.F.; Buie, W.D. Practice parameters for anal squamous neoplasms. Dis. Colon Rectum 2012, 55, 735–749. [Google Scholar] [CrossRef]
- Geh, I.; Gollins, S.; Renehan, A.; Scholefield, J.; Goh, V.; Prezzi, D. Association of Coloproctology of Great Britain & Ireland (ACPGBI): Guidelines for the management of cancer of the colon, rectum and anus (2017)—Anal Cancer. Colorectal Dis. 2017, 19, 82–97. [Google Scholar]
- Scholefield, J.; Harris, D.; Radcliffe, A. Guidelines for management of anal intraepithelial neoplasia. Colorectal Dis. 2011, 13, 3–10. [Google Scholar] [CrossRef]
- Binda, G.A.; Gagliardi, G.; Dal Conte, I.; Verra, M.; Cassoni, P.; Cavazzoni, E.; Stocco, E.; Delmonte, S.; De Nardi, P.; Sticchi, L.; et al. Practice parameters for the diagnosis and treatment of anal intraepithelial neoplasia (AIN) on behalf of the Italian Society of Colorectal Surgery (SICCR). Tech. Coloproctol. 2019, 23, 513–528. [Google Scholar] [CrossRef]
- Giani, I.; Mistrangelo, M.; Fucini, C.; Italian Society of Colo-Rectal Surgery. The treatment of squamous anal carcinoma: Guidelines of the Italian Society of Colo-Rectal Surgery. Tech. Coloproctol. 2013, 17, 171–179. [Google Scholar] [CrossRef]
- Hartschuh, W.; Breitkopf, C.; Lenhard, B.; Wienert, V.; Mlitz, H.; Furtwangler, A. S1 guideline: Anal intraepithelial neoplasia (AIN) and perianal intraepithelial neoplasia (PAIN). J. Dtsch. Dermatol. Ges. 2011, 9, 256–258. [Google Scholar] [CrossRef]
- Chin-Hong, P.V.; Reid, G.E.; AST Infectious Diseases Community of Practice. Human papillomavirus infection in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13590. [Google Scholar] [CrossRef]
- Reid, E.; Suneja, G.; Ambinder, R.F.; Ard, K.; Baiocchi, R.; Barta, S.K.; Carchman, E.; Cohen, A.; Gupta, N.; Johung, K.L.; et al. Cancer in People Living with HIV, Version 1.2018, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2018, 16, 986–1017. [Google Scholar] [CrossRef]
- Gouvas, N.; Gourtsoyianni, S.; Kalogeridi, M.A.; Sougklakos, J.; Vini, L.; Xynos, E. Hellenic society of medical oncology (HESMO) guidelines for the management of anal cancer. Updates Surg. 2020, 73, 7–21. [Google Scholar] [CrossRef]
- Plotzker, R.E.; Barnell, G.M.; Wiley, D.J.; Stier, E.A.; Jay, N. Provider preferences for anal cancer prevention screening: Results of the International Anal Neoplasia Society survey. Tumour Virus Res. 2022, 13, 200235. [Google Scholar] [CrossRef]
- Hillman, R.J.; Berry-Lawhorn, J.M.; Ong, J.J.; Cuming, T.; Nathan, M.; Goldstone, S.; Richel, O.; Barrosso, L.F.; Darragh, T.M.; Law, C. International Anal Neoplasia Society guidelines for the practice of digital anal rectal examination. J. Lower Genit. Tract Dis. 2019, 23, 138–146. [Google Scholar] [CrossRef]
- Albuquerque, A. Cytology in anal cancer screening: Practical review for clinicians. Acta Cytol. 2020, 64, 281–287. [Google Scholar] [CrossRef]
- Clarke, M.A.; Deshmukh, A.A.; Suk, R.; Roberts, J.; Gilson, R.; Jay, N.; Stier, E.A.; Wentzensen, N. A systematic review and meta-analysis of cytology and HPV-related biomarkers for anal cancer screening among different risk groups. Int. J. Cancer 2022, 151, 1889–1901. [Google Scholar] [CrossRef]
- Albuquerque, A.; Sheaff, M.; Stirrup, O.; Cappello, C.; Bowring, J.; Cuming, T.; De Masi, A.; Rosenthal, A.N.; Nathan, M. Performance of anal cytology compared with high-resolution anoscopy and histology in women with lower anogenital tract neoplasia. Clin. Infect. Dis. 2018, 67, 1262–1268. [Google Scholar] [CrossRef]
- Wohlmuth, C.; Ghorab, Z.; Shier, M.; Tinmouth, J.; Salit, I.E.; Covens, A.; Zhang, L.; Vicus, D. Cytology-based screening for anal intraepithelial neoplasia in women with a history of cervical intraepithelial neoplasia or cancer. Cancer Cytopathol. 2020, 129, 140–147. [Google Scholar] [CrossRef]
- Stier, E.A.; Sebring, M.C.; Mendez, A.E.; Ba, F.S.; Trimble, D.D.; Chiao, E.Y. Prevalence of anal human papillomavirus infection and anal HPV-related disorders in women: A systematic review. Am. J. Obstet. Gynecol. 2015, 213, 278–309. [Google Scholar] [CrossRef]
- Palefsky, J.M. Practising high-resolution anoscopy. Sex. Health 2012, 9, 580–586. [Google Scholar] [CrossRef]
- De-Masi, A.; Davis, E.; Cuming, T.; Chindawi, N.; Pesola, F.; Cappello, C.; Chambers, S.; Bowring, J.; Rosenthal, A.N.; Sasieni, P. The acceptability of high resolution anoscopy examination in patients attending a tertiary referral centre. BMC Cancer 2018, 18, 554. [Google Scholar] [CrossRef]
- Pineda, C.E.; Berry, J.M.; Jay, N.; Palefsky, J.M.; Welton, M.L. High-resolution anoscopy targeted surgical destruction of anal high-grade squamous intraepithelial lesions: A ten-year experience. Dis. Colon Rectum 2008, 51, 829–837. [Google Scholar] [CrossRef]
- Heráclio, S.A.; Schettini, J.; Oliveira, M.L.; Souza, A.S.R.; Souza, P.R.E.; Amorim, M.M.R. High-resolution anoscopy in women with cervical neoplasia. Int. J. Gynecol. Obstet. 2015, 128, 216–219. [Google Scholar] [CrossRef]
- Hillman, R.J.; Cuming, T.; Darragh, T.; Nathan, M.; Berry-Lawthorn, M.; Goldstone, S.; Law, C.; Palefsky, J.; Barroso, L.F.; Stier, E.A. 2016 IANS international guidelines for practice standards in the detection of anal cancer precursors. J. Lower Genit. Tract Dis. 2016, 20, 283–291. [Google Scholar] [CrossRef]
- Brogden, D.R.; Walsh, U.; Pellino, G.; Kontovounisios, C.; Tekkis, P.; Mills, S.C. Evaluating the efficacy of treatment options for anal intraepithelial neoplasia: A systematic review. Int. J. Colorectal Dis. 2021, 36, 213–226. [Google Scholar] [CrossRef]
- Brown, S.; Skinner, P.; Tidy, J.; Smith, J.; Sharp, F.; Hosie, K. Outcome after surgical resection for high-grade anal intraepithelial neoplasia (Bowen’s disease). Br. J. Surg. 1999, 86, 1063–1066. [Google Scholar] [CrossRef]
- Cromwell, I.; Gaudet, M.; Peacock, S.; Aquino-Parsons, C. Cost-effectiveness analysis of anal cancer screening in women with cervical neoplasia in British Columbia, Canada. BMC Health Serv. Res. 2016, 16, 206. [Google Scholar] [CrossRef]
- Ehrenpreis, E.D.; Smith, D.G. Patients with newly diagnosed cervical cancer should be screened for anal human papilloma virus and anal dysplasia: Results of a pilot study using a STELLA computer simulation and economic model. Papillomavirus Res. 2018, 5, 38–45. [Google Scholar] [CrossRef]
- Nitkowski, J.; Giuliano, A.; Ridolfi, T.; Chiao, E.; Fernandez, M.; Schick, V.; Swartz, M.D.; Smith, J.S.; Schneider, E.A.; Brzezinski, B. Effect of the Environment on Home-Based Self-Sampling Kits for Anal Cancer Screening. J. Virol. Methods 2022, 310, 114616. [Google Scholar] [CrossRef]
- Sagan, A.; McDaid, D.; Rajan, S.; Farrington, J.; McKee, M. European Observatory Policy Briefs. In Screening: When Is It Appropriate and How Can We Get It Right? European Observatory on Health Systems and Policies: Copenhagen, Denmark, 2020. [Google Scholar]
- Kaufman, E.; De Castro, C.; Williamson, T.; Lessard, B.; Munoz, M.; Mayrand, M.; Burchell, A.; Klein, M.; Charest, L.; Auger, M. Acceptability of anal cancer screening tests for women living with HIV in the EVVA study. Curr. Oncol. 2020, 27, 19–26. [Google Scholar] [CrossRef]
- Lam, J.; Barnell, G.; Merchant, M.; Ellis, C.; Silverberg, M. Acceptability of high-resolution anoscopy for anal cancer screening in HIV-infected patients. HIV Med. 2018, 19, 716–723. [Google Scholar] [CrossRef]
- Falcaro, M.; Castañon, A.; Ndlela, B.; Checchi, M.; Soldan, K.; Lopez-Bernal, J.; Elliss-Brookes, L.; Sasieni, P. The effects of the national HPV vaccination programme in England, UK, on cervical cancer and grade 3 cervical intraepithelial neoplasia incidence: A register-based observational study. Lancet 2021, 398, 2084–2092. [Google Scholar] [CrossRef]
- Joura, E.A.; Garland, S.M.; Paavonen, J.; Ferris, D.G.; Perez, G.; Ault, K.A.; Huh, W.K.; Sings, H.L.; James, M.K.; Haupt, R.M. Effect of the human papillomavirus (HPV) quadrivalent vaccine in a subgroup of women with cervical and vulvar disease: Retrospective pooled analysis of trial data. BMJ 2012, 344, e1401. [Google Scholar] [CrossRef]
- Swedish, K.A.; Factor, S.H.; Goldstone, S.E. Prevention of recurrent high-grade anal neoplasia with quadrivalent human papillomavirus vaccination of men who have sex with men: A nonconcurrent cohort study. Clin. Infect. Dis. 2012, 54, 891–898. [Google Scholar] [CrossRef]
- Kechagias, K.S.; Kalliala, I.; Bowden, S.J.; Athanasiou, A.; Paraskevaidi, M.; Paraskevaidis, E.; Dillner, J.; Nieminen, P.; Strander, B.; Sasieni, P. Role of human papillomavirus (HPV) vaccination on HPV infection and recurrence of HPV related disease after local surgical treatment: Systematic review and meta-analysis. BMJ 2022, 378, e070135. [Google Scholar] [CrossRef]
- Wilkin, T.J.; Chen, H.; Cespedes, M.S.; Leon-Cruz, J.T.; Godfrey, C.; Chiao, E.Y.; Bastow, B.; Webster-Cyriaque, J.; Feng, Q.; Dragavon, J. A randomized, placebo-controlled trial of the quadrivalent human papillomavirus vaccine in human immunodeficiency virus-infected adults aged 27 years or older: AIDS Clinical Trials Group Protocol A5298. Clin. Infect. Dis. 2018, 67, 1339–1346. [Google Scholar] [CrossRef]
- Karita, H.C.S.; Hauge, K.; Magaret, A.; Mao, C.; Schouten, J.; Grieco, V.; Xi, L.F.; Galloway, D.A.; Madeleine, M.M.; Wald, A. Effect of human papillomavirus vaccine to interrupt recurrence of vulvar and anal neoplasia (VIVA): A trial protocol. JAMA Netw. Open 2019, 2, e190819. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupi, M.; Brogden, D.; Howell, A.-M.; Tekkis, P.; Mills, S.; Kontovounisios, C. Anal Cancer in High-Risk Women: The Lost Tribe. Cancers 2023, 15, 60. https://doi.org/10.3390/cancers15010060
Lupi M, Brogden D, Howell A-M, Tekkis P, Mills S, Kontovounisios C. Anal Cancer in High-Risk Women: The Lost Tribe. Cancers. 2023; 15(1):60. https://doi.org/10.3390/cancers15010060
Chicago/Turabian StyleLupi, Micol, Danielle Brogden, Ann-Marie Howell, Paris Tekkis, Sarah Mills, and Christos Kontovounisios. 2023. "Anal Cancer in High-Risk Women: The Lost Tribe" Cancers 15, no. 1: 60. https://doi.org/10.3390/cancers15010060
APA StyleLupi, M., Brogden, D., Howell, A.-M., Tekkis, P., Mills, S., & Kontovounisios, C. (2023). Anal Cancer in High-Risk Women: The Lost Tribe. Cancers, 15(1), 60. https://doi.org/10.3390/cancers15010060






