COL7A1 Expression Improves Prognosis Prediction for Patients with Clear Cell Renal Cell Carcinoma Atop of Stage
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Summary of Methods
2.2. Datasets and Processing
2.3. Univariable and Multivariable Cox Model
2.4. Kaplan–Meier Curves
2.5. GSEA
2.6. Statistical Analysis and Data Manipulation
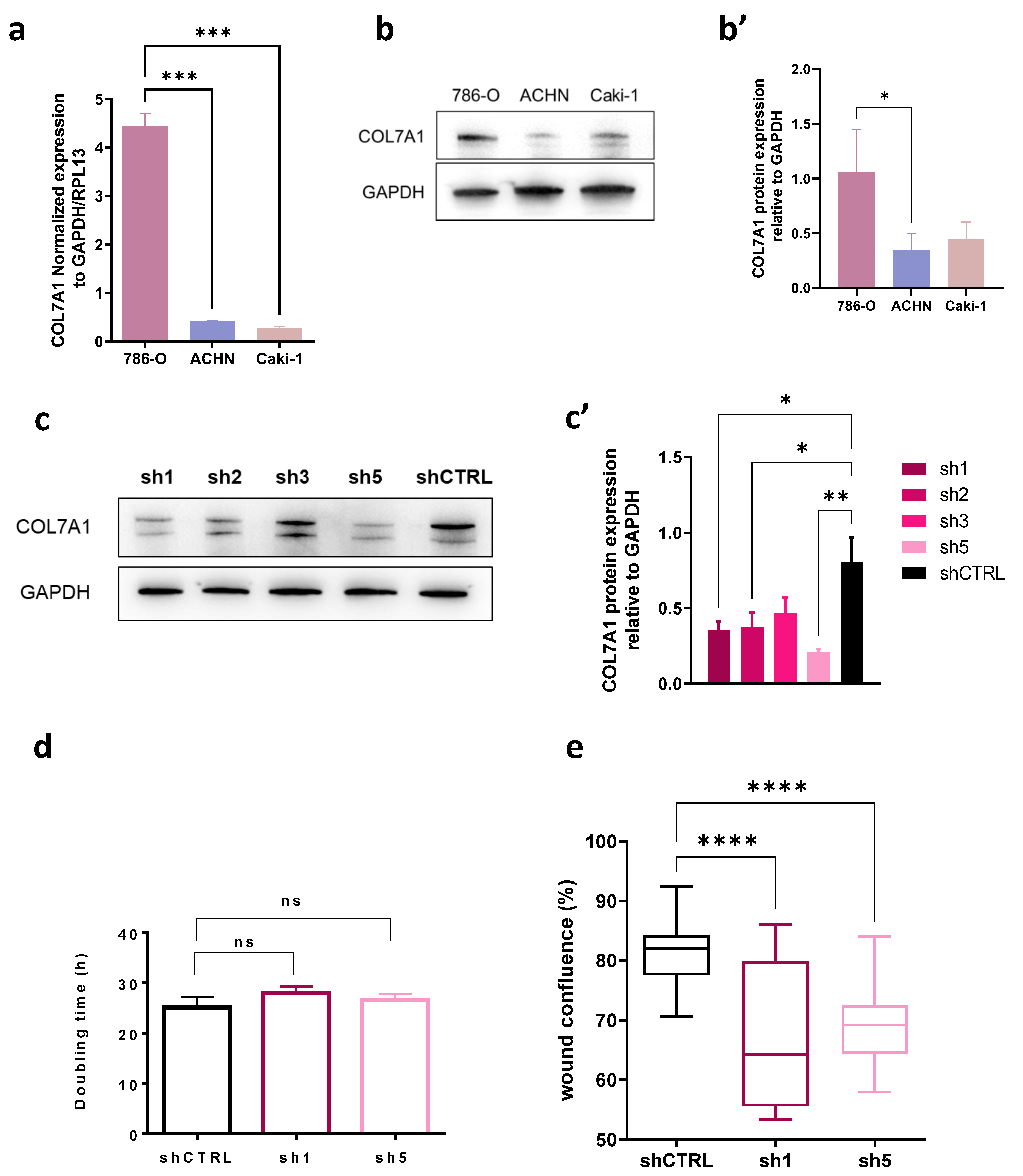
2.7. Cell Culture
2.8. Generation of shCOL7A1 786-O Cells
2.9. Western Blot
2.10. RT-qPCR
2.11. Proliferation and Migration Assay
3. Results
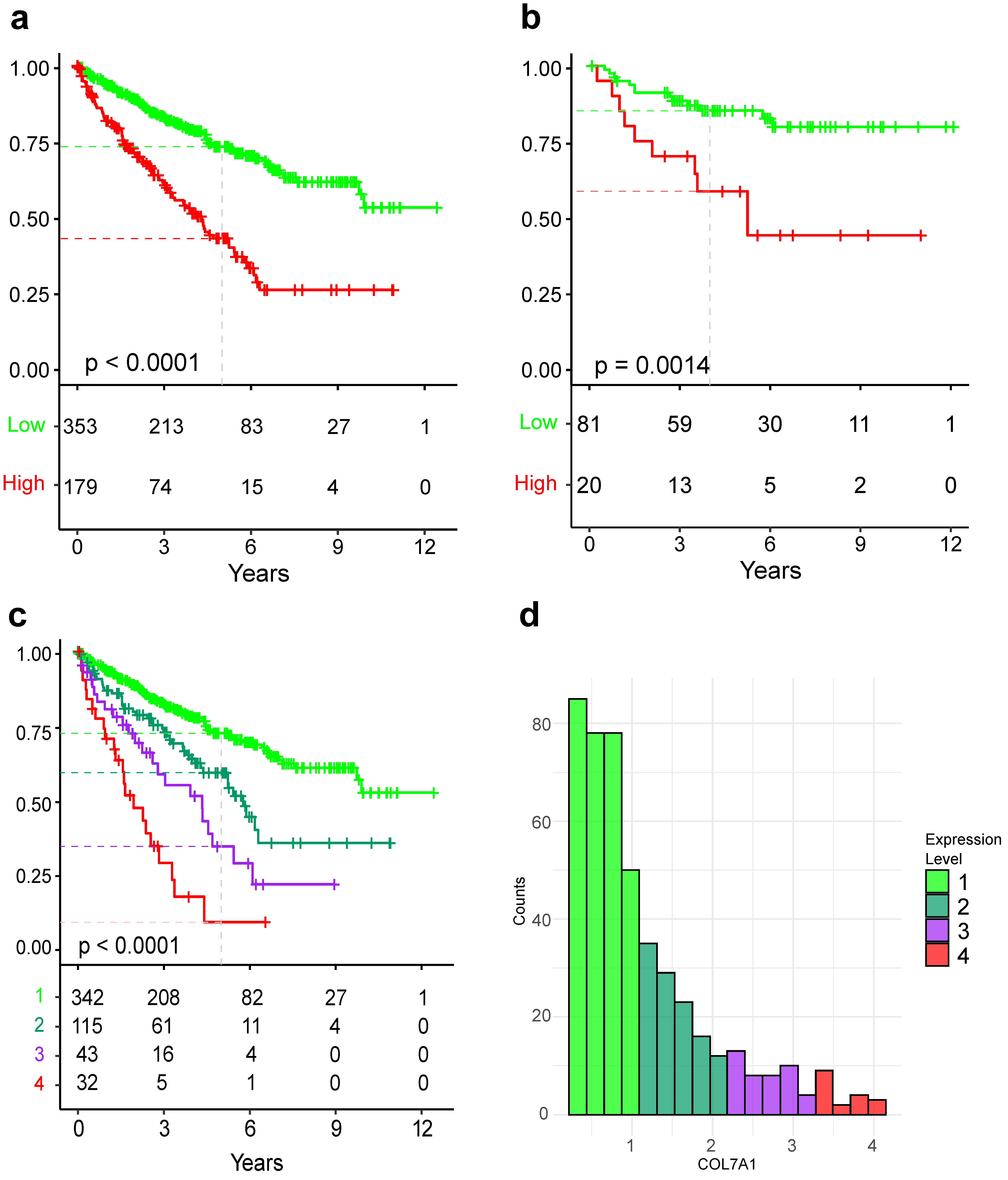
3.1. COL7A1 Expression Is Prognostic of Overall Survival
3.2. COL7A1 Expression Can Predict Patient Survival Atop of Clinical Characteristics
3.3. COL7A1 Expression Can Improve Multivariable Cox Model
3.4. COL7A1 Expression Is Correlated with Genes Involved in Cell Division, Inflammatory Response, and Epithelial to Mesenchymal Transition and Anti-Correlated with Metabolism
3.5. COL7A1 Knocked-Down Cells Show Decreased Migration Rate
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Beltran, A.; Scarpelli, M.; Montironi, R.; Kirkali, Z. 2004 WHO Classification of the Renal Tumors of the Adults. Eur. Urol. 2006, 49, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Turajlic, S.; Xu, H.; Litchfield, K.; Rowan, A.; Horswell, S.; Chambers, T.; O’Brien, T.; Lopez, J.I.; Watkins, T.B.K.; Nicol, D.; et al. Deterministic Evolutionary Trajectories Influence Primary Tumor Growth: TRACERx Renal. Cell 2018, 173, 595–610.e11. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Alva, A.; Baine, M.; Beckermann, K.; Carlo, M.I.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; et al. Kidney Cancer, Version 3.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Canc. Netw. 2022, 20, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Pandolfo, S.D.; Carbonara, U.; Beksac, A.T.; Derweesh, I.; Celia, A.; Schiavina, R.; Elbich, J.; Basile, G.; Hampton, L.J.; Cerrato, C.; et al. Microwave versus Cryoablation and Radiofrequency Ablation for Small Renal Mass: A Multicenter Comparative Analysis. Minerva Urol. Nephrol. 2022, 75, 66–72. [Google Scholar] [CrossRef]
- Uhlig, A.; Hahn, O.; Strauss, A.; Lotz, J.; Trojan, L.; Müller-Wille, R.; Uhlig, J. Treatment for Localized T1a Clear Cell Renal Cell Carcinoma: Survival Benefit for Cryosurgery and Thermal Ablation Compared to Deferred Therapy. Cardiovasc. Intervent. Radiol. 2018, 41, 277–283. [Google Scholar] [CrossRef]
- Makhov, P.; Joshi, S.; Ghatalia, P.; Kutikov, A.; Uzzo, R.G.; Kolenko, V.M. Resistance to Systemic Therapies in Clear Cell Renal Cell Carcinoma: Mechanisms and Management Strategies. Mol. Cancer Ther. 2018, 17, 1355–1364. [Google Scholar] [CrossRef]
- Roelants, C.; Pillet, C.; Franquet, Q.; Sarrazin, C.; Peilleron, N.; Giacosa, S.; Guyon, L.; Fontanell, A.; Fiard, G.; Long, J.-A.; et al. Ex-Vivo Treatment of Tumor Tissue Slices as a Predictive Preclinical Method to Evaluate Targeted Therapies for Patients with Renal Carcinoma. Cancers 2020, 12, 232. [Google Scholar] [CrossRef]
- Nallanthighal, S.; Heiserman, J.P.; Cheon, D.-J. The Role of the Extracellular Matrix in Cancer Stemness. Front. Cell Dev. Biol. 2019, 7, 86. [Google Scholar] [CrossRef]
- O’Leary, N.A.; Wright, M.W.; Brister, J.R.; Ciufo, S.; Haddad, D.; McVeigh, R.; Rajput, B.; Robbertse, B.; Smith-White, B.; Ako-Adjei, D.; et al. Reference Sequence (RefSeq) Database at NCBI: Current Status, Taxonomic Expansion, and Functional Annotation. Nucleic Acids Res. 2016, 44, D733–D745. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Urda, S.; Garcia, J.; Green, C.L.; Chen, L.; Lin, Q.; Veitch, D.P.; Sakai, L.Y.; Lee, H.; Marinkovich, M.P.; Khavari, P.A. Type VII Collagen Is Required for Ras-Driven Human Epidermal Tumorigenesis. Science 2005, 307, 1773–1776. [Google Scholar] [CrossRef] [PubMed]
- Chernov, A.V.; Baranovskaya, S.; Golubkov, V.S.; Wakeman, D.R.; Snyder, E.Y.; Williams, R.; Strongin, A.Y. Microarray-Based Transcriptional and Epigenetic Profiling of Matrix Metalloproteinases, Collagens, and Related Genes in Cancer. J. Biol. Chem. 2010, 285, 19647–19659. [Google Scholar] [CrossRef] [PubMed]
- Oktem, G.; Sercan, O.; Guven, U.; Uslu, R.; Uysal, A.; Goksel, G.; Ayla, S.; Bilir, A. Cancer Stem Cell Differentiation: TGFβ1 and Versican May Trigger Molecules for the Organization of Tumor Spheroids. Oncol. Rep. 2014, 32, 641–649. [Google Scholar] [CrossRef]
- Song, W.; Li, L.; Zhang, J.; Wang, S.; Leng, Z.; Lu, R.; Li, C.; Ma, X.; Wang, T.; Jia, X. Identification of COL7A1 as a Prognostic Biomarker in Lung Squamous Cell Carcinoma. 2022, in review. Available online: https://www.researchsquare.com/article/rs-1767132/v1 (accessed on 9 February 2023).
- Kita, Y.; Mimori, K.; Tanaka, F.; Matsumoto, T.; Haraguchi, N.; Ishikawa, K.; Matsuzaki, S.; Fukuyoshi, Y.; Inoue, H.; Natsugoe, S.; et al. Clinical Significance of LAMB3 and COL7A1 MRNA in Esophageal Squamous Cell Carcinoma. Eur. J. Surg. Oncol. EJSO 2009, 35, 52–58. [Google Scholar] [CrossRef]
- Oh, S.E.; Oh, M.Y.; An, J.Y.; Lee, J.H.; Sohn, T.S.; Bae, J.M.; Choi, M.-G.; Kim, K.-M. Prognostic Value of Highly Expressed Type VII Collagen (COL7A1) in Patients with Gastric Cancer. Pathol. Oncol. Res. 2021, 125. [Google Scholar] [CrossRef]
- Ding, C.; Yu, Z.; Li, X.; Zhu, J.; Dai, M.; He, Q. Collagen Type VII A1 Chain: A Promising Prognostic and Immune Infiltration Biomarker of Pancreatic Cancer. Oncol. Lett. 2023, 25, 77. [Google Scholar] [CrossRef]
- D’Aniello, C.; Berretta, M.; Cavaliere, C.; Rossetti, S.; Facchini, B.A.; Iovane, G.; Mollo, G.; Capasso, M.; Pepa, C.D.; Pesce, L.; et al. Biomarkers of Prognosis and Efficacy of Anti-Angiogenic Therapy in Metastatic Clear Cell Renal Cancer. Front. Oncol. 2019, 9, 1400. [Google Scholar] [CrossRef]
- Jardillier, R.; Koca, D.; Chatelain, F.; Guyon, L. Prognosis of Lasso-like Penalized Cox Models with Tumor Profiling Improves Prediction over Clinical Data Alone and Benefits from Bi-Dimensional Pre-Screening. BMC Cancer 2022, 22, 1045. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, X.; Yue, C.; Gao, S.; Sun, Z.; Lu, C.; Zuo, L. A Five Collagen-Related Gene Signature to Estimate the Prognosis and Immune Microenvironment in Clear Cell Renal Cell Cancer. Vaccines 2021, 9, 1510. [Google Scholar] [CrossRef]
- Hou, J.; Lv, Z.; Wang, Y.; Wang, X.; Chen, X.; Wang, K. Basement Membrane Genes Can Predict the Prognosis of Patients with Clear Cell Renal Cell Carcinoma (CcRCC) and Are Correlated with Immune Status. Res. Sq. 2022; in review. [Google Scholar] [CrossRef]
- Guo, L.; An, T.; Huang, Z.; Wan, Z.; Chong, T. Comprehensive Analysis of the Collagen Family Members as Prognostic Markers in Clear Cell Renal Cell Carcinoma. Transl. Cancer Res. 2022, 11, 1954–1969. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Fu, S.; Zhao, Z.; Li, Z.; Wu, Y.; Qi, T.; Zheng, J.; Du, Q.; Xu, Z.; Yu, N. Sarcomatoid-Associated Gene Risk Index for Clear Cell Renal Cell Carcinoma. Front. Genet. 2022, 13, 985641B. [Google Scholar] [CrossRef] [PubMed]
- Mounir, M.; Lucchetta, M.; Silva, T.C.; Olsen, C.; Bontempi, G.; Chen, X.; Noushmehr, H.; Colaprico, A.; Papaleo, E. New Functionalities in the TCGAbiolinks Package for the Study and Integration of Cancer Data from GDC and GTEx. PLoS Comput. Biol. 2019, 15, e1006701. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.F.; Parker, J.S.; Reynolds, S.M.; Silva, T.C.; Wang, L.-B.; Zhou, W.; Akbani, R.; Bailey, M.; Balu, S.; Berman, B.P.; et al. Before and after: Comparison of Legacy and Harmonized TCGA Genomic Data Commons’ Data. Cell Syst. 2019, 9, 24–34. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshizato, T.; Shiraishi, Y.; Maekawa, S.; Okuno, Y.; Kamura, T.; Shimamura, T.; Sato-Otsubo, A.; Nagae, G.; Suzuki, H.; et al. Integrated Molecular Analysis of Clear-Cell Renal Cell Carcinoma. Nat. Genet. 2013, 45, 860–867. [Google Scholar] [CrossRef]
- Laskar, R.S.; Li, P.; Ecsedi, S.; Abedi-Ardekani, B.; Durand, G.; Robinot, N.; Hubert, J.-N.; Janout, V.; Zaridze, D.; Mukeria, A.; et al. Sexual Dimorphism in Cancer: Insights from Transcriptional Signatures in Kidney Tissue and Renal Cell Carcinoma. Hum. Mol. Genet. 2021, 30, 343–355. [Google Scholar] [CrossRef]
- Braun, D.A.; Hou, Y.; Bakouny, Z.; Ficial, M.; Sant’ Angelo, M.; Forman, J.; Ross-Macdonald, P.; Berger, A.C.; Jegede, O.A.; Elagina, L.; et al. Interplay of Somatic Alterations and Immune Infiltration Modulates Response to PD-1 Blockade in Advanced Clear Cell Renal Cell Carcinoma. Nat. Med. 2020, 26, 909–918. [Google Scholar] [CrossRef]
- Motzer, R.J.; Robbins, P.B.; Powles, T.; Albiges, L.; Haanen, J.B.; Larkin, J.; Mu, X.J.; Ching, K.A.; Uemura, M.; Pal, S.K.; et al. Avelumab plus Axitinib versus Sunitinib in Advanced Renal Cell Carcinoma: Biomarker Analysis of the Phase 3 JAVELIN Renal 101 Trial. Nat. Med. 2020, 26, 1733–1741. [Google Scholar] [CrossRef]
- Cox, D.R. Regression Models and Life-Tables. J. R. Stat. Soc. Ser. B Methodol. 1972, 34, 187–220. [Google Scholar] [CrossRef]
- Therneau, T.M. R “Survival” Package, version 3.5-5; A Package for Survival Analysis in R. 2022. Available online: https://cran.r-project.org/package=survival (accessed on 9 February 2023).
- Wickham, H. R “ggplot2” Package, v3.4.2; ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; Available online: https://ggplot2.tidyverse.org (accessed on 9 February 2023).
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene Set Enrichment Analysis: A Knowledge-Based Approach for Interpreting Genome-Wide Expression Profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A Universal Enrichment Tool for Interpreting Omics Data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) Hallmark Gene Set Collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.D.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Weaver, C.; Bin Satter, K.; Richardson, K.P.; Tran, L.K.H.; Tran, P.M.H.; Purohit, S. Diagnostic and Prognostic Biomarkers in Renal Clear Cell Carcinoma. Biomedicines 2022, 10, 2953. [Google Scholar] [CrossRef] [PubMed]
- Wan, B.; Liu, B.; Huang, Y.; Yu, G.; Lv, C. Prognostic Value of Immune-Related Genes in Clear Cell Renal Cell Carcinoma. Aging 2019, 11, 11474–11489. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-G.; Su, J.; Liu, H.; Yang, X.-J.; Yang, X.; Wei, Y.; Zhu, X.-Y.; Song, Y.; Zhao, X.-C.; Guo, H.-L. Comprehensive Bioinformatics Analysis of the E2F Family in Human Clear Cell Renal Cell Carcinoma. Oncol. Lett. 2022, 24, 1–10. [Google Scholar] [CrossRef]
- Shroff, E.H.; Eberlin, L.S.; Dang, V.M.; Gouw, A.M.; Gabay, M.; Adam, S.J.; Bellovin, D.I.; Tran, P.T.; Philbrick, W.M.; Garcia-Ocana, A.; et al. MYC Oncogene Overexpression Drives Renal Cell Carcinoma in a Mouse Model through Glutamine Metabolism. Proc. Natl. Acad. Sci. USA 2015, 112, 6539–6544. [Google Scholar] [CrossRef]
- Motzer, R.J.; Banchereau, R.; Hamidi, H.; Powles, T.; McDermott, D.; Atkins, M.B.; Escudier, B.; Liu, L.-F.; Leng, N.; Abbas, A.R.; et al. Molecular Subsets in Renal Cancer Determine Outcome to Checkpoint and Angiogenesis Blockade. Cancer Cell 2020, 38, 803–817.e4. [Google Scholar] [CrossRef]
- Hell, M.P.; Duda, M.; Weber, T.C.; Moch, H.; Krek, W. Tumor Suppressor VHL Functions in the Control of Mitotic Fidelity. Cancer Res. 2014, 74, 2422–2431. [Google Scholar] [CrossRef]
- Tun, H.W.; Marlow, L.A.; von Roemeling, C.A.; Cooper, S.J.; Kreinest, P.; Wu, K.; Luxon, B.A.; Sinha, M.; Anastasiadis, P.Z.; Copland, J.A. Pathway Signature and Cellular Differentiation in Clear Cell Renal Cell Carcinoma. PLoS ONE 2010, 5, e10696. [Google Scholar] [CrossRef]
- Shi, R.; Zhang, Z.; Zhu, A.; Xiong, X.; Zhang, J.; Xu, J.; Sy, M.; Li, C. Targeting Type I Collagen for Cancer Treatment. Int. J. Cancer 2022, 151, 665–683. [Google Scholar] [CrossRef] [PubMed]
- Lucarelli, G.; Loizzo, D.; Franzin, R.; Battaglia, S.; Ferro, M.; Cantiello, F.; Castellano, G.; Bettocchi, C.; Ditonno, P.; Battaglia, M. Metabolomic Insights into Pathophysiological Mechanisms and Biomarker Discovery in Clear Cell Renal Cell Carcinoma. Expert Rev. Mol. Diagn. 2019, 19, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, H.; Lindgren, D.; Mandahl Forsberg, A.; Mulder, H.; Axelson, H.; Johansson, M.E. Primary Clear Cell Renal Carcinoma Cells Display Minimal Mitochondrial Respiratory Capacity Resulting in Pronounced Sensitivity to Glycolytic Inhibition by 3-Bromopyruvate. Cell Death Dis. 2015, 6, e1585. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Medina Bengtsson, L.; Wang, X.; Huang, T.; Liu, G.; Murphy, S.; Wang, C.; Koren, J.; Schafer, Z.; Lu, X. UQCRH Downregulation Promotes Warburg Effect in Renal Cell Carcinoma Cells. Sci. Rep. 2020, 10, 15021. [Google Scholar] [CrossRef]


| TCGA:KIRC | E-MTAB-1980 | GSE167093 | Braun et al. | JAVELIN Renal 101 | |
|---|---|---|---|---|---|
| Total samples | 532 | 101 | 604 | 225 | 726 |
| Median age at diagnosis years (range) | 61 (26–90) | 64 (35–91) | 62 (23–85) | 62 (30–88) | 61(27–88) |
| Sex, N (%) | |||||
| Female | 187 (35.15) | 24 (23.8) | 247 (41) | 61 (27.1) | 178 (24.5) |
| Male | 345 (64.85) | 77 (76.2) | 357 (59) | 164 (72.9) | 548(75.5) |
| Tumor grade a, N (%) | - | ||||
| I | 14 (2.7) | 13 (13.1) | 100 (18.8) | - | - |
| II | 228 (43.5) | 59 (59.6) | 304 (57) | - | - |
| III | 206 (39.3) | 22 (22.2) | 105 (19.7) | - | - |
| IV | 76 (14.5) | 5 (5.1) | 24 (4.5) | - | - |
| Missing | 8 | 2 | 71 | 225 | 726 |
| Tumor stage a, N (%) | |||||
| I | 266 (50.3) | 66 (65.3) | 306 (50.7) | 0 | - |
| II | 57 (10.8) | 20 (19.8) | 98 (16.2) | 0 | - |
| III | 123 (23.2) | 3 (3) | 138 (22.8) | 0 | - |
| IV | 83 (15.7) | 12 (11.9) | 62 (10.3) | 225 (100) | 726 (100) |
| Missing | 3 | 0 | 0 | 0 | 0 |
| EVENTS | 175 (32.9) | 23 (22.78) | - | 173 (76.9) | 358 (49.3) b |
| 5 year survival probability % (95% CI) | 62.9 (58.2–68.1) | 79.6 (71.7–88.3) | - | 20.4 (15.5–26.9) | - |
| Gene Symbol | p-Value | Coefficient | HR | Rank |
|---|---|---|---|---|
| COL7A1 | 5.1 × 10−19 | 0.44 | 1.56 | 1 |
| CDCA3 | 6.2 × 10−19 | 0.74 | 2.09 | 2 |
| CRB3 | 2.1 × 10−18 | −0.50 | 0.60 | 3 |
| SOWAHB | 2.5 × 10−18 | −0.52 | 0.60 | 4 |
| SLC16A12 | 4.3 × 10−17 | −0.28 | 0.75 | 5 |
| SORBS2 | 1.9 × 10−16 | −0.49 | 0.61 | 6 |
| CYFIP2 | 2.3 × 10−16 | −0.59 | 0.55 | 7 |
| METTL7A | 2.7 × 10−16 | −0.55 | 0.57 | 8 |
| TROAP | 5.2 × 10−16 | 0.61 | 1.83 | 9 |
| RGS17 | 1.1 × 10−15 | 0.76 | 2.14 | 10 |
| Univariable Cox Model | Multivariable Cox Model b | Anova | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Covariate | Coefficient (β) | HR [exp(β)] | p-Value | Score Test | Coefficient (β) | HR [exp(β)] | p-Value | Chisq | p-Value |
| STAGE | - | - | 1.1 × 10−26 | 123.8 | - | - | - | 95.22 | 1.7 × 10−20 |
| II | 0.22 | 1.245 | 0.486 | - | 0.18 | 1.20 | 0.56 | ||
| III | 0.91 | 2.48 | 1.2 × 10−05 | - | 0.55 | 1.73 | 0.013 | ||
| IV | 1.84 | 6.32 | 1.4 × 10−21 | - | 1.44 | 4.23 | 1.8 × 10−10 | ||
| GRADEa | - | - | 5.6 × 10−18 | 79.46 | - | - | - | 14.2 | 8.3 × 10−4 |
| III | 0.64 | 1.90 | 6.9 × 10−4 | - | 0.36 | 1.43 | 0.067 | ||
| IV | 1.63 | 5.10 | 6.7 × 10−16 | - | 0.60 | 1.83 | 0.011 | ||
| AGE | 0.035 | 1.03 | 2.5 × 10−06 | 22.51 | 0.036 | 1.036 | 1.4 × 10−6 | 22.67 | 1.9 × 10−6 |
| COL7A1 | 0.44 | 1.56 | 5.8 × 10−20 | 83.67 | 0.32 | 1.37 | 6.9 × 10−9 | 28.45 | 9.6 × 10−8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koca, D.; Séraudie, I.; Jardillier, R.; Cochet, C.; Filhol, O.; Guyon, L. COL7A1 Expression Improves Prognosis Prediction for Patients with Clear Cell Renal Cell Carcinoma Atop of Stage. Cancers 2023, 15, 2701. https://doi.org/10.3390/cancers15102701
Koca D, Séraudie I, Jardillier R, Cochet C, Filhol O, Guyon L. COL7A1 Expression Improves Prognosis Prediction for Patients with Clear Cell Renal Cell Carcinoma Atop of Stage. Cancers. 2023; 15(10):2701. https://doi.org/10.3390/cancers15102701
Chicago/Turabian StyleKoca, Dzenis, Irinka Séraudie, Rémy Jardillier, Claude Cochet, Odile Filhol, and Laurent Guyon. 2023. "COL7A1 Expression Improves Prognosis Prediction for Patients with Clear Cell Renal Cell Carcinoma Atop of Stage" Cancers 15, no. 10: 2701. https://doi.org/10.3390/cancers15102701
APA StyleKoca, D., Séraudie, I., Jardillier, R., Cochet, C., Filhol, O., & Guyon, L. (2023). COL7A1 Expression Improves Prognosis Prediction for Patients with Clear Cell Renal Cell Carcinoma Atop of Stage. Cancers, 15(10), 2701. https://doi.org/10.3390/cancers15102701









