Changes in Gastric Pathology after H. pylori Treatment in Community-Driven Research Aimed at Gastric Cancer Prevention
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
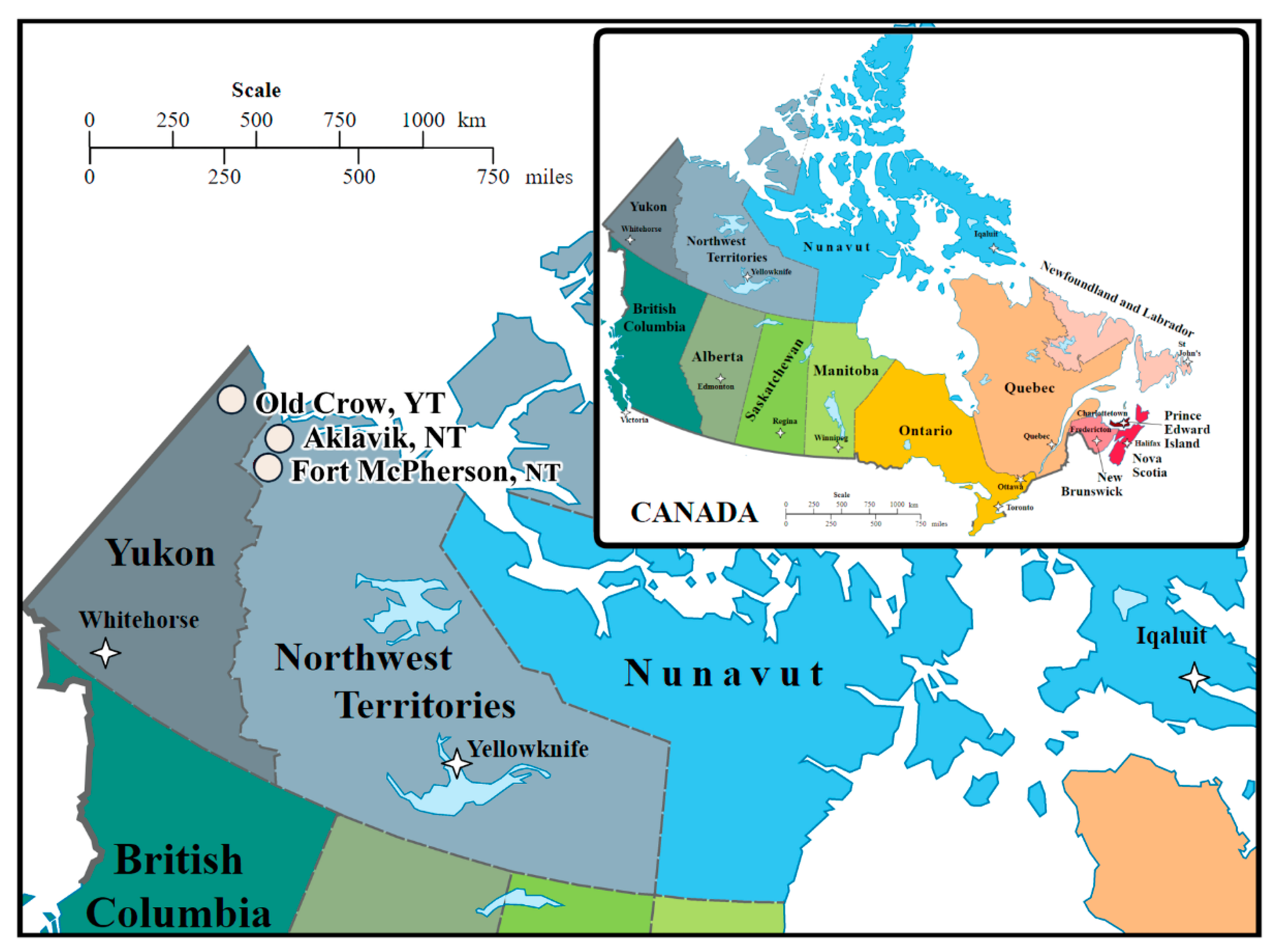
2.1. Participating Communities
2.2. Summary of Research Sought by Communities
2.3. Study Population and Design
2.4. Diagnostic Methods
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Follow-Up Time Period
3.3. Hp Prevalence and Antimicrobial Resistance at Baseline and Follow-Up
3.4. Prevalence of Abnormal Gastric Pathology at Baseline and Follow-Up
3.5. Within-Individual Change in Abnormal Gastric Pathology Severity from Baseline to Follow-Up
3.6. Probability of Changes in Gastric Pathology Severity by Participant Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Baseline | Hp (+) | Hp (−) | ||||
|---|---|---|---|---|---|---|
| Follow-Up | Hp (+) n = 3 | Hp (−) n = 2 | Hp (+) n = 1 | Hp (−) n = 16 | ||
| Chronic gastritis | ||||||
| None at both times | 0 | 0 | 0 | 11 | ||
| Lower | 0 | 2 | 0 | 1 | ||
| Unchanged | 2 | 0 | 0 | 1 | ||
| Higher | 1 | 0 | 1 | 3 | ||
| Active gastritis | ||||||
| None at both times | 0 | 0 | 0 | 16 | ||
| Lower | 0 | 2 | 0 | 0 | ||
| Unchanged | 2 | 0 | 0 | 0 | ||
| Higher | 1 | 0 | 1 | 0 | ||
| Atrophic gastritis | ||||||
| None at both times | 1 | 2 | 0 | 14 | ||
| Lower | 0 | 0 | 0 | 0 | ||
| Unchanged | 0 | 0 | 0 | 0 | ||
| Higher | 2 | 0 | 1 | 2 | ||
| Intestinal metaplasia | ||||||
| None at both times | 2 | 2 | 0 | 14 | ||
| Lower | 0 | 0 | 0 | 0 | ||
| Unchanged | 0 | 0 | 0 | 0 | ||
| Higher | 1 | 0 | 1 | 2 | ||
| Hp Status at Follow-Up | |||||||
|---|---|---|---|---|---|---|---|
| Hp+ | Hp− | ||||||
| % | 95% CI * | % | 95% CI * | ||||
| Chronic gastritis | |||||||
| Negative UBT ≥ 8 weeks after treatment | n = 7 | n = 27 | |||||
| None at both times | 0 | 0, 41 | 0 | 0, 13 | |||
| Lower | 43 | 10, 82 | 100 | 87, 100 | |||
| Unchanged | 57 | 18, 90 | 0 | 0, 13 | |||
| Higher | 0 | 0, 35 | 0 | 0, 13 | |||
| Positive or unknown UBT ≥ 8 weeks after treatment | n = 7 | n = 6 | |||||
| None at both times | 0 | 0, 41 | 0 | 0, 46 | |||
| Lower | 57 | 18, 90 | 100 | 54, 100 | |||
| Unchanged | 28 | 4, 71 | 0 | 0, 46 | |||
| Higher | 14 | 0.4, 58 | 0 | 0, 46 | |||
| Active gastritis | |||||||
| Negative UBT ≥ 8 weeks after treatment | n = 7 | n = 27 | |||||
| None at both times | 0 | 0, 41 | 7 | 0.9, 24 | |||
| Lower | 43 | 10, 82 | 92 | 83, 100 | |||
| Unchanged | 43 | 10, 82 | 0 | 0, 13 | |||
| Higher | 14 | 0.4, 58 | 0 | 0, 13 | |||
| Positive or unknown UBT ≥ 8 weeks after treatment | n = 7 | n = 6 | |||||
| None at both times | 0 | 0, 41 | 0 | 0, 46 | |||
| Lower | 28 | 4, 71 | 100 | 54, 100 | |||
| Unchanged | 57 | 18, 90 | 0 | 0, 46 | |||
| Higher | 14 | 0.4, 58 | 0 | 0, 46 | |||
| Atrophic gastritis | |||||||
| Negative UBT ≥ 8 weeks after treatment | n = 7 | n = 27 | |||||
| None at both times | 28 | 4, 71 | 60 | 41, 78 | |||
| Lower | 43 | 10, 82 | 37 | 19, 55 | |||
| Unchanged | 14 | 0.4, 58 | 0 | 0, 13 | |||
| Higher | 14 | 0.4, 58 | 4 | 0, 19 | |||
| Positive or unknown UBT ≥ 8 weeks after treatment | n = 7 | n = 6 | |||||
| None at both times | 57 | 18, 90 | 50 | 12, 88 | |||
| Lower | 43 | 10, 82 | 50 | 12, 88 | |||
| Unchanged | 0 | 0, 41 | 0 | 0, 46 | |||
| Higher | 0 | 0, 41 | 0 | 0, 46 | |||
| Intestinal Metaplasia | |||||||
| Negative UBT ≥ 8 weeks after treatment | n = 7 | n = 27 | |||||
| None at both times | 57 | 18, 90 | 93 | 83, 100 | |||
| Lower | 0 | 0, 41 | 4 | 0.1, 19 | |||
| Unchanged | 14 | 0.4, 58 | 0 | 0, 13 | |||
| Higher | 28 | 0.4, 71 | 4 | 0.1, 19 | |||
| Positive or unknown UBT ≥ 8 weeks after treatment | n = 7 | n = 6 | |||||
| None at both times | 43 | 10, 82 | 67 | 23, 96 | |||
| Lower | 14 | 0.4, 58 | 0 | 0, 46 | |||
| Unchanged | 28 | 4, 71 | 17 | 0.4, 64 | |||
| Higher | 14 | 0.4, 58 | 17 | 0.4, 64 | |||
References
- Velazquez, M.; Feirtag, J.M. Helicobacter pylori: Characteristics, pathogenicity, detection methods and mode of transmission implicating foods and water. Int. J. Food Microbiol. 1999, 53, 95–104. [Google Scholar] [CrossRef]
- Brown, L.M. Helicobacter pylori: Epidemiology and routes of transmission. Epidemiol. Rev. 2000, 22, 283–297. [Google Scholar] [CrossRef]
- Goodman, K.J.; Correa, P.; Tengana Aux, H.J.; Ramirez, H.; DeLany, J.P.; Guerrero Pepinosa, O.; Lopez Quiñones, M.; Collazos Parra, T. Helicobacter pylori Infection in the Colombian Andes: A population-based study of transmission pathways. Am. J. Epidemiol. 1996, 144, 290–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plummer, M.; Franceschi, S.; Vignat, J.; Forman, D.; de Martel, C. Global burden of gastric cancer attributable to Helicobacter pylori. Int. J. Cancer 2015, 136, 487–490. [Google Scholar] [CrossRef] [PubMed]
- Correa, P.; Piazuelo, M.B. The gastric precancerous cascade. J. Dig. Dis. 2012, 13, 2–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houghton, J.; Stoicov, C.; Nomura, S.; Rogers, A.B.; Carlson, J.; Li, H.; Cai, X.; Fox, J.G.; Goldenring, J.R.; Wang, T.C. Gastric cancer originating from bone marrow-derived cells. Science 2004, 306, 1568–1571. [Google Scholar] [CrossRef]
- The EUROGAST Study Group. Epidemiology of, and risk factors for, Helicobacter pylori infection among 3194 asymptomatic subjects in 17 populations. The EUROGAST study group. Gut 1993, 34, 1672–1676. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Choi, H.; Leung, K.; Jiang, F.; Graham, D.Y.; Leung, W.K. Global prevalence of Helicobacter pylori infection between 1980 and 2022: A systematic review and meta-analysis. Lancet 2023, 8, 553–564. [Google Scholar] [CrossRef]
- Yuan, C.; Adeloye, D.; Luk, T.T.; Huang, L.; He, Y.; Xu, Y.; Ye, X.; Yi, Q.; Song, P.; Rudan, I.; et al. The global prevalence of and factors associated with Helicobacter pylori infection in children: A systematic review and meta-analysis. Lancet Child Adolesc Health 2022, 6, 185–194. [Google Scholar] [CrossRef]
- Watanabe, M.; Ito, H.; Hosono, S.; Oze, I.; Ashida, C.; Tajima, K.; Katoh, H.; Matsuo, K.; Tanaka, H. Declining trends in prevalence of Helicobacter pylori infection by birth-year in a Japanese population. Cancer Sci. 2015, 106, 1738–1743. [Google Scholar] [CrossRef] [Green Version]
- Sjomina, O.; Pavlova, J.; Niv, Y.; Leja, M. Epidemiology of Helicobacter pylori infection. Helicobacter 2018, 23, e12514. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-J.; Chen, C.-C.; Lin, J.-T.; Lee, Y.-C.; Wu, M.-S.; Liou, J.-M.; Lee, J.-Y.; Yang, T.-H.; Fang, Y.-J.; Yu, J.-J.; et al. Declining trends of prevalence of Helicobacter pylori infection Iand incidence of gastric cancer in Taiwan: An updated cross-sectional survey and meta-analysis. Helicobacter 2022, 27, e12914. [Google Scholar] [CrossRef]
- Agréus, L.; Hellström, P.M.; Talley, N.J.; Wallner, B.; Forsberg, A.; Vieth, M.; Veits, L.; Björkegren, K.; Engstrand, L.; Andreasson, A. Towards a healthy stomach? Helicobacter pylori prevalence has dramatically decreased over 23 years in adults in a Swedish community. UEG J. 2016, 4, 686–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segal, I.; Otley, A.; Issenman, R.; Armstrong, D.; Espinosa, V.; Cawdron, R.; Morshed, M.G.; Jacobson, K. Low prevalence of Helicobacter pylori infection in Canadian children: A cross-sectional analysis. Can. J. Gastroenterol. 2008, 22, 485–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamogawa-Schifter, Y.; Yamaoka, Y.; Uchida, T.; Beer, A.; Tribl, B.; Schöniger-Hekele, M.; Trauner, M.; Dolak, W. Prevalence of Helicobacter pylori and its CagA subtypes in gastric cancer and duodenal ulcer at an Austrian tertiary referral center over 25 years. PLoS ONE 2018, 13, e0197695. [Google Scholar] [CrossRef] [PubMed]
- Shoosanglertwijit, R.; Kamrat, N.; Werawatganon, D.; Chatsuwan, T.; Chaithongrat, S.; Rerknimitr, R. Real-world data of Helicobacter pylori prevalence, eradication regimens, and antibiotic resistance in Thailand, 2013–2018. JGH Open 2020, 4, 49–53. [Google Scholar] [CrossRef] [Green Version]
- Hooi, J.K.Y.; Lai, W.Y.; Ng, W.K.; Suen, M.M.Y.; Underwood, F.E.; Tanyingoh, D.; Malfertheiner, P.; Graham, D.Y.; Wong, V.W.S.; Wu, J.C.Y.; et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017, 153, 420–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everhart, J.E.; Kruszon-Moran, D.; Perez-Perez, G.I.; Tralka, T.S.; McQuillan, G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J. Infect. Dis. 2000, 181, 1359–1363. [Google Scholar] [CrossRef] [Green Version]
- Malcolm, C.A.; MacKay, W.G.; Shepherd, A.; Weaver, L.T. Helicobacter pylori in children is strongly associated with poverty. Strat. Manag. J. 2004, 49, 136–138. [Google Scholar] [CrossRef]
- Wizla-Derambure, N.; Michaud, L.; Ategbo, S.; Vincent, P.; Ganga-Zandzou, S.; Turck, D.; Gottrand, F. Familial and community environmental risk factors for Helicobacter pylori infection in children and adolescents. J. Pediatr. Gastroenterol. Nutr. 2001, 33, 58–63. [Google Scholar] [CrossRef]
- Cromarty, T. Social Inequity, Gender and Helicobacter pylori Infection in Arctic Canada. Master of Science in Epidemiology, University of Alberta, Edmonton. September 2022. Available online: https://era-library-ualberta-ca.login.ezproxy.library.ualberta.ca/items/919332e2-e317-48f8-ab1d-c6b07cb81bc5 (accessed on 28 November 2022).
- Goodman, K.J.; Jacobson, K.; Veldhuyzen van Zanten, S. Helicobacter pylori infection in Canadian and related Arctic Aboriginal populations. Can. J. Gastroenterol. 2008, 22, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Colquhoun, A.; Hannah, H.; Corriveau, A.; Hanley, B.; Yuan, Y.; Goodman, K.J.; The CANHelp Working Group. Gastric Cancer in Northern Canadian Populations: A Focus on Cardia and Non-Cardia Subsites. Cancers 2019, 11, 534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, J.; Goodman, K.J.; Munday, R.; Heavner, K.; Huntington, J.; Morse, J.; van Zanten, S.V.; Fedorak, R.N.; Corriveau, A.; Bailey, R.; et al. Helicobacter pylori infection in Canada’s Arctic: Searching for the solutions. Can. J. Gastroenterol. 2008, 22, 912–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, K.J.; Geary, J.; Walker, E.; Fagan-Garcia, K.; Archie, B.; Lennie, C.; Munday, R.; McAlpine, L.; Colquhoun, A.; Chang, H.J.; et al. Community-driven epidemiologic research: Guiding principles. Glob. Epidemiol. 2019, 1, 100013. [Google Scholar] [CrossRef]
- Cheung, J.; Goodman, K.J.; Girgis, S.; Bailey, R.; Morse, J.; Fedorak, R.N.; Geary, J.; Fagan-Garcia, K.; van Zanten, S.V.; CANHelp Working Group. Disease manifestations of Helicobacter pylori infection in Arctic Canada: Using epidemiology to address community concerns. BMJ Open 2014, 4, e003689. [Google Scholar] [CrossRef] [Green Version]
- Fagan-Garcia, K.; Geary, J.; Chang, H.J.; McAlpine, L.; Walker, E.; Colquhoun, A.; van Zanten, S.; Girgis, S.; Archie, B.; Hanley, B.; et al. Burden of disease from Helicobacter pylori infection in western Canadian Arctic communities. BMC Public Health 2019, 19, 730. [Google Scholar] [CrossRef] [Green Version]
- Walker, E.V.; Girgis, S.; Yuan, Y.; Goodman, K.J. Community-driven research in the Canadian Arctic: Dietary exposure to methylmercury and gastric health outcomes. Int. J. Circumpolar Health 2021, 80, 1889879. [Google Scholar] [CrossRef]
- Infrastructure. Government of Northwest Territories. Available online: https://www.inf.gov.nt.ca/en/transportation (accessed on 30 November 2022).
- Aklavik: About Aklavik. Available online: http://www.aklavik.ca/?p=about_aklavik (accessed on 30 November 2022).
- Northern Research Portal: Transportation in the North. Available online: http://digital.scaa.sk.ca/gallery/northern/content.php?pg=ex10-3&ln= (accessed on 30 November 2022).
- Bowling, E. NNSL Media: Aklavik Ice Road Is Open for Light Traffic. Available online: https://www.nnsl.com/news/aklavik-ice-road-is-open-for-light-traffic/ (accessed on 30 November 2022).
- Old Crow: History. Available online: https://www.oldcrow.ca/history.htm (accessed on 30 November 2022).
- Hamlet of Fort McPherson, Fort McPherson: A Brief History. Available online: http://www.fortmcpherson.ca/AboutUs (accessed on 30 November 2022).
- Gisbert, J.P.; Pajares, J.M. 13C-urea breath test in the diagnosis of Helicobacter pylori infection—A critical review. Aliment. Pharmacol. Ther. 2004, 20, 1001–1017. [Google Scholar] [CrossRef]
- Morse, A.L.; Goodman, K.J.; Munday, R.; Chang, H.J.; Morse, J.W.; Keelan, M.; Geary, J.; Veldhuyzen van Zanten, S.; CANHelp Working Group. A randomized controlled trial comparing sequential with triple therapy for Hp in an Aboriginal community in the Canadian North. Can. J. Gastroenterol. 2013, 27, 701–706. [Google Scholar] [CrossRef] [Green Version]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis. The updated Sydney system. International workshop on the histopathology of gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef]
- Bursac, Z.; Gauss, C.H.; Williams, D.K.; Hosmer, D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008, 3, 17. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, K. The changing prevalence of Helicobacter pylori infection in Canadian children: Should screening be performed in high-risk children? Can. J. Gastroenterol. 2005, 19, 412–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMahon, B.J.; Bruce, M.G.; Hennessy, T.W.; Bruden, D.L.; Sacco, F.; Peters, H.; Hurlburt, D.A.; Morris, J.M.; Reasonover, A.L.; Dailide, G.; et al. Re-infection after successful eradication of Helicobacter pylori: A 2-year prospective study in Alaska Natives. Aliment. Pharmacol. Therapeut. 2009, 23, 1215–1223. [Google Scholar] [CrossRef]
- Fichman, S.; Niv, Y. Histological changes in the gastric mucosa after Helicobacter pylori eradication. Eur. J. Gastroenterol. Hepatol. 2004, 16, 1183–1188. [Google Scholar] [CrossRef] [PubMed]
- Vannella, L.; Lahner, E.; Bordi, C.; Pilozzi, E.; Di Giulio, E.; Corleto, V.D.; Osborn, J.; Delle Fave, G.; Annibale, B. Reversal of atrophic body gastritis after H. pylori eradication at long-term follow-up. Dig. Liver Dis. 2011, 43, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.O.; Peng, J.Z.; Lam, S.K.; Lai, K.C.; Yuen, M.F.; Cheung, H.K.; Kwong, Y.L.; Rashid, A.; Chan, C.K.; Wong, B.C. Eradication of Helicobacter pylori infection reverses E-cadherin promoter hypermethylation. Gut 2006, 55, 463–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, Y.-J.; Kim, N.; Lee, H.S.; Lee, J.B.; Choi, Y.J.; Yoon, H.; Shin, C.M.; Park, Y.S.; Lee, D.H. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication—A prospective study for up to 10 years. Aliment. Pharmacol. Ther. 2018, 47, 380–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, M.; Haruma, K.; Kamada, T.; Mihara, M.; Kim, S.; Kitadai, Y.; Sumii, M.; Tanaka, S.; Yoshihara, M.; Chayama, K. Helicobacter pylori eradication therapy improves atrophic gastritis and intestinal metaplasia: A 5-year prospective study of patients with atrophic gastritis. Aliment. Pharmacol. Ther. 2002, 16, 1449–1456. [Google Scholar] [CrossRef]
- Ohkusa, T.; Fujiki, K.; Takashimizu, I.; Kumagai, J.; Tanizawa, T.; Eishi, Y.; Yokoyama, T.; Watanabe, M. Improvement in atrophic gastritis and intestinal metaplasia in patients in whom Helicobacter pylori was eradicated. Ann. Intern. Med. 2001, 134, 380–386. [Google Scholar] [CrossRef]
- Massarrat, S.; Haj-Sheykholeslami, A.; Mohamadkhani, A.; Zendehdel, N.; Rakhshani, N.; Stolte, M.; Mirzaei, M.; Saliminejhad, M.; Saeidi, S.; Shahidi, M. Precancerous conditions after H. pylori eradication: A randomized double blind study in first degree relatives of gastric cancer patients. Arch. Iran. Med. 2012, 15, 664–669. [Google Scholar]
- Ford, A.C.; Yuan, Y.; Forman, D.; Hunt, R.; Moayyedi, P. Helicobacter pylori eradication for the prevention of gastric neoplasia. Cochrane Database Syst. Rev. 2020, 7, CD005583. [Google Scholar] [CrossRef] [PubMed]
- IARC Hp Working Group. Hp Eradication as a Strategy for Preventing Gastric Cancer; IARC Working Group Reports, No. 8; International Agency for Research on Cancer: Lyon, France, 2014; pp. 88–94. Available online: www.iarc.fr/en/publications/pdfs-online/wrk/wrk8/index.php (accessed on 1 August 2023).
- Piazuelo, M.B.; Bravo, L.E.; Mera, R.M.; Camargo, M.C.; Bravo, J.C.; Delgado, A.G.; Washington, M.K.; Rosero, A.; Garcia, L.S.; Realpe, J.L.; et al. The Colombian chemoprevention trial. Twenty-year follow-up of a cohort of patients with gastric precancerous lesions. Gastroenterology 2021, 160, 1106–1117.e3. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, N. Diagnosis of Helicobacter pylori by invasive test: Histology. Ann. Transl. Med. 2015, 3, 10. [Google Scholar] [PubMed]
- Carraher, S.; Chang, H.; Munday, R.; Goodman, K.J.; CANHelp Working Group. Hp incidence and re-infection in the Aklavik Hp Project. Int. J. Circumpolar Health 2013, 72, 21594. [Google Scholar] [CrossRef]
- Goodman, K.J.; Veldhuyzen van Zanten, S.; Colquhoun, A.; Corriveau, A.; Hanley, B.; Munday, R.; Geary, J.; CANHelp Working Group. Clinical Management of H. pylori Infection for Indigenous and Northern Communities in Canada: Guidelines Developed from CANHelp Research. 2019. Available online: https://era.library.ualberta.ca/items/71ec103d-38a7-44bc-a179-124a8d7fcc4b (accessed on 25 July 2023).
| Baseline | Follow-Up | ||||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Total | 310 | 100 | 69 | 100 | |
| Sex | |||||
| Male | 139 | 45 | 25 | 36 | |
| Female | 171 | 55 | 44 | 64 | |
| Ethnicity | |||||
| Non-Indigenous | 22 | 7 | 3 | 4 | |
| Inuvialuit | 117 | 40 | 26 | 39 | |
| Gwich’in | 139 | 47 | 36 | 54 | |
| Other | 16 | 5 | 2 | 3 | |
| Missing * | 16 | 2 | |||
| Age at endoscopy (years) | |||||
| <30 | 76 | 24 | 3 | 4 | |
| 30–59 | 172 | 56 | 41 | 59 | |
| 60+ | 62 | 20 | 25 | 36 | |
| Baseline (n = 310) | Follow-Up (n = 69) | ||||||
|---|---|---|---|---|---|---|---|
| n | % | 95% CI * | n | % | 95% CI * | ||
| H. pylori infection | 223 | 72 | 66, 77 | 18 | 26 | 16, 36 | |
| Active gastritis | |||||||
| None | 93 | 30 | 25, 35 | 51 | 74 | 64, 84 | |
| Any | 215 | 70 | 65, 75 | 18 | 26 | 16, 36 | |
| Mild | 101 | 33 | 27, 38 | 12 | 17 | 8, 28 | |
| Moderate | 81 | 26 | 21, 31 | 6 | 9 | 3, 18 | |
| Severe | 33 | 11 | 7, 14 | 0 | 0 | 0, 5 | |
| Missing ** | 2 | 0 | |||||
| Chronic gastritis | |||||||
| None | 76 | 25 | 19, 29 | 43 | 62 | 51, 74 | |
| Any | 234 | 75 | 71, 80 | 26 | 38 | 26, 49 | |
| Mild | 28 | 9 | 6, 12 | 12 | 17 | 8, 26 | |
| Moderate | 99 | 32 | 27, 37 | 11 | 16 | 7, 25 | |
| Severe | 107 | 34 | 29, 40 | 3 | 4 | 1, 12 | |
| Atrophic gastritis | |||||||
| None | 214 | 69 | 64, 74 | 59 | 86 | 77, 94 | |
| Any | 96 | 31 | 26, 36 | 10 | 14 | 6, 23 | |
| Mild | 67 | 22 | 17, 26 | 8 | 12 | 5, 22 | |
| Moderate | 24 | 7 | 5, 11 | 2 | 3 | 0, 10 | |
| Severe | 5 | 2 | 0.5, 4 | 0 | 0 | 0, 5 | |
| Intestinal metaplasia | |||||||
| None | 268 | 86 | 83, 90 | 56 | 81 | 72, 90 | |
| Any | 42 | 14 | 10, 17 | 13 | 19 | 10, 28 | |
| Mild | 27 | 9 | 6, 12 | 9 | 13 | 6, 23 | |
| Moderate | 12 | 4 | 2, 6 | 4 | 6 | 2, 14 | |
| Severe | 3 | 1 | 0.2, 3 | 0 | 0 | 0, 5 | |
| Hp− at Follow-Up | Hp+ at Follow-Up † | ||||||
|---|---|---|---|---|---|---|---|
| Hp− at Baseline n = 17 | Hp+ at Baseline ‡ n = 34 | n = 18 | |||||
| % | 95% CI * | % | 95% CI * | % | 95% CI * | ||
| Chronic gastritis | |||||||
| None at both times | 65 | 42, 87 | 0 | 0, 10 | 0 | 0, 19 | |
| Lower | 12 | 1, 36 | 100 | 90, 100 | 39 | 17, 64 | |
| Unchanged | 6 | 0.1, 29 | 0 | 0, 10 | 44 | 22, 69 | |
| Higher | 18 | 4, 43 | 0 | 0, 10 | 17 | 4, 41 | |
| Active gastritis | |||||||
| None at both times | 100 | 80, 100 | 3 | 0.1, 15 | 0 | 0, 19 | |
| Lower | 0 | 0, 20 | 97 | 91, 100 | 28 | 10, 53 | |
| Unchanged | 0 | 0, 20 | 0 | 0, 10 | 50 | 26, 74 | |
| Higher | 0 | 0, 20 | 0 | 0, 10 | 22 | 6, 48 | |
| Atrophic gastritis | |||||||
| None at both times | 88 | 73, 100 | 59 | 42, 75 | 39 | 17, 64 | |
| Lower | 0 | 0, 20 | 38 | 22, 56 | 33 | 13, 59 | |
| Unchanged | 0 | 0, 20 | 0 | 0, 10 | 6 | 0.1, 27 | |
| Higher | 12 | 1, 36 | 3 | 0.1, 15 | 22 | 6, 48 | |
| Intestinal Metaplasia | |||||||
| None at both times | 88 | 73, 100 | 88 | 77, 99 | 50 | 26, 74 | |
| Lower | 0 | 0, 20 | 3 | 0.1, 15 | 6 | 0.1, 27 | |
| Unchanged | 0 | 0, 20 | 3 | 0.1, 15 | 17 | 4, 41 | |
| Higher | 12 | 1, 36 | 6 | 1, 20 | 28 | 10, 53 | |
| n | Risk | 95% CI * | ||
|---|---|---|---|---|
| Chronic gastritis improved among 54 participants with chronic gastritis at baseline | ||||
| Total | 43/54 | 0.80 | 0.66, 0.89 | |
| Sex | ||||
| Male | 16/21 | 0.76 | 0.53, 0.92 | |
| Female | 27/33 | 0.82 | 0.65, 0.93 | |
| Age (years) | ||||
| 27–49 | 14/19 | 0.74 | 0.49, 0.91 | |
| 50–59 | 14/15 | 0.93 | 0.68, 1.0 | |
| 60–78 | 15/20 | 0.75 | 0.51, 0.91 | |
| Ethnicity | ||||
| Gwich’in | 26/30 | 0.87 | 0.69, 0.96 | |
| Inuvialuit | 14/19 | 0.74 | 0.49, 0.91 | |
| Community | ||||
| Aklavik NT | 21/27 | 0.78 | 0.58, 0.91 | |
| Old Crow YT | 8/9 | 0.89 | 0.52, 1.00 | |
| Fort McPherson NT | 14/18 | 0.78 | 0.52, 0.94 | |
| Baseline Hp density | ||||
| No bacteria observed | 2/3 | 0.67 | 0.09, 0.99 | |
| Mild | 12/17 | 0.71 | 0.44, 0.90 | |
| Moderate | 18/21 | 0.85 | 0.64, 0.97 | |
| Marked | 11/13 | 0.85 | 0.55, 0.98 | |
| Baseline Hp resistance | ||||
| To metronidazole | 13/17 | 0.76 | 0.49, 0.92 | |
| To clarithrymycin | 7/7 | 1 | 0.53, 1 | |
| To 1+ antibiotics | 15/19 | 0.79 | 0.53, 0.92 | |
| To 2+ antibiotics | 5/6 | 0.83 | 0.23, 0.99 | |
| Baseline chronic gastritis severity | ||||
| Mild | 4/8 | 0.50 | 0.16, 0.84 | |
| Moderate | 18/23 | 0.78 | 0.56, 0.93 | |
| Severe | 21/23 | 0.91 | 0.72, 0.99 | |
| Treated to eliminate Hp before follow-up | ||||
| No | 3/6 | 0.50 | 0.12, 0.88 | |
| Yes | 40/48 | 0.83 | 0.70, 0.93 | |
| Hp-negative breath test before follow-up | ||||
| No | 12/18 | 0.67 | 0.41, 0.87 | |
| Yes | 31/36 | 0.86 | 0.71, 0.95 | |
| Hp density improved at follow-up | ||||
| No | 4/13 | 0.20 | 0.03, 0.56 | |
| Yes | 39/41 | 0.95 | 0.84, 0.99 | |
| Hp density at follow-up | ||||
| No bacteria observed | 36/37 | 0.97 | 0.86, 1.0 | |
| Mild | 2/5 | 0.40 | 0.05, 0.85 | |
| Moderate | 4/9 | 0.44 | 0.14, 0.79 | |
| Marked | 0/2 | 0 | 0, 0.85 † | |
| Active gastritis improved among 50 participants with active gastritis at baseline | ||||
| Total | 36/50 | 0.72 | 0.56, 0.84 | |
| Sex | ||||
| Male | 14/20 | 0.70 | 0.46, 0.88 | |
| Female | 22/30 | 0.73 | 0.54, 0.88 | |
| Age (years) | ||||
| 27–49 | 10/19 | 0.53 | 0.29, 0.76 | |
| 50–59 | 13/14 | 0.93 | 0.66, 1.00 | |
| 60–78 | 13/17 | 0.76 | 0.50, 0.93 | |
| Ethnicity | ||||
| Gwichin | 21/27 | 0.78 | 0.58, 0.91 | |
| Inuvialuit | 14/18 | 0.78 | 0.52, 0.94 | |
| Community | ||||
| Aklavik NT | 18/26 | 0.69 | 0.48, 0.86 | |
| Old Crow YT | 6/9 | 0.67 | 0.30, 0.93 | |
| Fort McPherson NT | 12/15 | 0.80 | 0.52, 0.96 | |
| Baseline Hp density | ||||
| Mild | 12/16 | 0.75 | 0.48, 0.93 | |
| Moderate | 16/21 | 0.76 | 0.53, 0.92 | |
| Marked | 8/13 | 0.62 | 0.32, 0.86 | |
| Baseline Hp resistance | ||||
| To metronidazole | 11/17 | 0.65 | 0.38, 0.84 | |
| To clarithrymycin | 7/7 | 1 | 0.53, 1 | |
| To 1+ antibiotics | 13/19 | 0.68 | 0.43, 0.86 | |
| To 2+ antibiotics | 6/6 | 1 | 0.48, 1 | |
| Baseline active gastritis severity | ||||
| Mild | 20/27 | 0.74 | 0.54, 0.89 | |
| Moderate | 12/18 | 0.67 | 0.41, 0.87 | |
| Marked | 4/5 | 0.80 | 0.28, 0.99 | |
| Treated to eliminate Hp before follow-up | ||||
| No | 3/4 | 0.75 | 0.19, 0.99 | |
| Yes | 33/46 | 0.72 | 0.57, 0.84 | |
| Hp-negative breath test before follow-up | ||||
| No | 10/18 | 0.56 | 0.31, 0.78 | |
| Yes | 26/32 | 0.81 | 0.64, 0.93 | |
| Hp density improved at follow-up | ||||
| No | 3/10 | 0.30 | 0.07, 0.65 | |
| Yes | 33/40 | 0.83 | 0.67, 0.93 | |
| Hp density at follow-up | ||||
| No bacteria observed | 33/33 | 1 | 0.89, 1 † | |
| Mild | 0/5 | 0 | 0, 0.52 † | |
| Moderate | 7/9 | 0.22 | 0.03, 0.60 | |
| Marked | 2/4 | 0.50 | 0.01, 0,99 | |
| Atrophic gastritis improved among 20 participants with atrophic gastritis at baseline | ||||
| Total | 19/20 | 0.95 | 0.75, 1 † | |
| Treated to eliminate Hp before follow-up | ||||
| No | 0/20 | -- | -- | |
| Yes | 20/20 | 0.95 | 0.75, 1 † | |
| Hp-negative breath test before follow-up | ||||
| No | 6/6 | 1 | 0.54, 1 † | |
| Yes | 13/14 | 0.93 | 0.66, 1.00 | |
| Hp density improved at follow-up | ||||
| No | 3/3 | 1 | 0.29, 1 † | |
| Yes | 16/17 | 0.94 | 0.71, 1.00 | |
| Atrophic gastritis and/or intestinal metaplasia progressed (including new onsets and six co-occurring cases) among 69 participants assessed at baseline | ||||
| Total | 10/69 | 0.14 | 0.07, 0.25 | |
| Sex | ||||
| Male | 3/25 | 0.12 | 0.03, 0.31 | |
| Female | 7/44 | 0.16 | 0.07, 0.30 | |
| Age (years) | ||||
| 27–49 | 5/21 | 0.24 | 0.08, 0.47 | |
| 50–59 | 1/23 | 0.04 | 0.00, 0.22 | |
| 60–78 | 4/25 | 0.16 | 0.05, 0.36 | |
| Community | ||||
| Aklavik NT | 9/39 | 0.23 | 0.11, 0.39 | |
| Old Crow YT | 0/9 | 0 | 0, 0.34 † | |
| Fort McPherson NT | 1/21 | 0.05 | 0.00, 0.24 | |
| Baseline Hp density | ||||
| No bacteria observed | 3/18 | 0.17 | 0.04, 0.41 | |
| Mild | 2/17 | 0.12 | 0.01, 0.36 | |
| Moderate | 4/21 | 0.19 | 0.05, 0.42 | |
| Marked | 1/13 | 0.08 | 0.00, 0.36 † | |
| Treated to eliminate Hp before follow-up | ||||
| No | 5/21 | 0.24 | 0.08, 0.47 | |
| Yes | 5/48 | 0.10 | 0.03, 0.23 | |
| Hp-negative breath test before follow-up | ||||
| No | 4/18 | 0.22 | 0.06, 0.48 | |
| Yes | 6/51 | 0.12 | 0.04, 0.24 | |
| Hp density at follow-up | ||||
| No bacteria observed | 4/51 | 0.08 | 0.02, 0.19 | |
| Mild | 0/6 | 0 | 0, 0.46 † | |
| Moderate | 5/10 | 0.50 | 0.19, 0.81 | |
| Marked | 1/2 | 0.50 | 0.01, 0.99 | |
| * Adjusted Risk Difference | 95% CI | ||||
|---|---|---|---|---|---|
| Probability of improved chronic gastritis among 54 participants with chronic gastritis at baseline | |||||
| Treated to eliminate Hp at baseline | |||||
| No | Referent | ||||
| Yes | 0.29 | 0.04, 0.53 | |||
| Hp density improved at follow-up | |||||
| No | Referent | ||||
| Yes | 0.74 | 0.50, 0.99 | |||
| Probability of improved active gastritis among 50 participants with active gastritis at baseline | |||||
| Baseline Hp density | |||||
| Mild | Referent | ||||
| Moderate | −0.15 | −0.31, −0.01 | |||
| Marked | −0.35 | −0.58, −0.13 | |||
| Hp density improved at follow-up | |||||
| No | Referent | ||||
| Yes | 0.63 | 0.39, 0.87 | |||
| Progression in atrophic gastritis and/or intestinal metaplasia (including new onsets and six co-occurring cases) among 69 participants assessed at baseline | |||||
| Hp observed on histology at baseline | |||||
| No | Referent | ||||
| Yes | 0.19 | 0.12, 0.27 | |||
| Treated to eliminate Hp at baseline | |||||
| No | Referent | ||||
| Yes | −0.29 | −0.45, −0.13 | |||
| Hp observed on histology at follow-up | |||||
| No | Referent | ||||
| Yes | 0.23 | 0.00, 0.45 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Girgis, S.; Chang, H.-J.; Assi, A.; Fagan-Garcia, K.; Cromarty, T.; Munday, R.; Goodman, K.J.; Veldhuyzen van Zanten, S.; the CANHelp Working Group. Changes in Gastric Pathology after H. pylori Treatment in Community-Driven Research Aimed at Gastric Cancer Prevention. Cancers 2023, 15, 3950. https://doi.org/10.3390/cancers15153950
Wang T, Girgis S, Chang H-J, Assi A, Fagan-Garcia K, Cromarty T, Munday R, Goodman KJ, Veldhuyzen van Zanten S, the CANHelp Working Group. Changes in Gastric Pathology after H. pylori Treatment in Community-Driven Research Aimed at Gastric Cancer Prevention. Cancers. 2023; 15(15):3950. https://doi.org/10.3390/cancers15153950
Chicago/Turabian StyleWang, Ting, Safwat Girgis, Hsiu-Ju Chang, Ali Assi, Katharine Fagan-Garcia, Taylor Cromarty, Rachel Munday, Karen J. Goodman, Sander Veldhuyzen van Zanten, and the CANHelp Working Group. 2023. "Changes in Gastric Pathology after H. pylori Treatment in Community-Driven Research Aimed at Gastric Cancer Prevention" Cancers 15, no. 15: 3950. https://doi.org/10.3390/cancers15153950
APA StyleWang, T., Girgis, S., Chang, H.-J., Assi, A., Fagan-Garcia, K., Cromarty, T., Munday, R., Goodman, K. J., Veldhuyzen van Zanten, S., & the CANHelp Working Group. (2023). Changes in Gastric Pathology after H. pylori Treatment in Community-Driven Research Aimed at Gastric Cancer Prevention. Cancers, 15(15), 3950. https://doi.org/10.3390/cancers15153950





