-
 Engineering O-Glycosylation in an RSV G Vaccine Antigen
Engineering O-Glycosylation in an RSV G Vaccine Antigen -
 Microbiome–Immune Interaction and Harnessing for Next-Generation Vaccines Against Highly Pathogenic Avian Influenza in Poultry
Microbiome–Immune Interaction and Harnessing for Next-Generation Vaccines Against Highly Pathogenic Avian Influenza in Poultry -
 Toxoplasma gondii at the Host Interface: Immune Modulation and Translational Strategies for Infection Control
Toxoplasma gondii at the Host Interface: Immune Modulation and Translational Strategies for Infection Control -
 Efficacy and Safety of Anti-Respiratory Syncytial Virus Monoclonal Antibody Nirsevimab in Neonates: A Real-World Monocentric Study
Efficacy and Safety of Anti-Respiratory Syncytial Virus Monoclonal Antibody Nirsevimab in Neonates: A Real-World Monocentric Study
Journal Description
Vaccines
Vaccines
is an international, peer-reviewed, open access journal published monthly online by MDPI.
- Open Access— free for readers, with article processing charges (APC) paid by authors or their institutions.
- High Visibility: indexed within Scopus, SCIE (Web of Science), PubMed, PMC, Embase, CAPlus / SciFinder, and other databases.
- Journal Rank: JCR - Q2 (Medicine, Research and Experimental) / CiteScore - Q1 (Pharmacology (medical))
- Rapid Publication: manuscripts are peer-reviewed and a first decision is provided to authors approximately 19.6 days after submission; acceptance to publication is undertaken in 2.8 days (median values for papers published in this journal in the first half of 2025).
- Recognition of Reviewers: reviewers who provide timely, thorough peer-review reports receive vouchers entitling them to a discount on the APC of their next publication in any MDPI journal, in appreciation of the work done.
Impact Factor:
3.4 (2024);
5-Year Impact Factor:
3.7 (2024)
Latest Articles
Adapting the WHO BeSD COVID-19 Survey to Examine Behavioral and Social Drivers of Vaccine Uptake Among Transgender, Intersex, and Disability Communities in India
Vaccines 2025, 13(11), 1095; https://doi.org/10.3390/vaccines13111095 (registering DOI) - 24 Oct 2025
Abstract
Background: During the COVID-19 pandemic, transgender and gender-diverse (TGD) people and people with disabilities in India faced disproportionate barriers to accessing vaccination services. Building on previous studies, this study explored the experiences of COVID-19 vaccine access in these two marginalized communities, using the
[...] Read more.
Background: During the COVID-19 pandemic, transgender and gender-diverse (TGD) people and people with disabilities in India faced disproportionate barriers to accessing vaccination services. Building on previous studies, this study explored the experiences of COVID-19 vaccine access in these two marginalized communities, using the WHO Behavioral and Social Drivers (BeSD) framework. Methods: Keeping community-based participatory methods (CBPR) at heart, we conducted a survey adapted from the BeSD COVID-19 survey tool. The survey was adapted using insights from a prior study, a literature review, stakeholder consultations, and discussions with a community leadership group (CLG) and an advisory board (AdB). Participants were recruited through transgender, gender-diverse, and disability rights networks. Data were analyzed descriptively, using percent analysis, and psychometrically, using exploratory factor analysis on polychoric correlations. Results: The adapted BeSD survey tool showed a high 0.85 (p < 0.05) internal consistency and criterion validity. Moreover, it showed a high willingness to be vaccinated (for ease of access to other services and community responsibility); however, systemic barriers hindered vaccination access. TGD people and people with disabilities faced multiple barriers in being vaccinated. The TGD community reported documentation mismatches and mistrust in health systems. People with disabilities reported mobility challenges, escort dependence, financial challenges, and variable accessibility at vaccination sites. Both groups faced digital exclusion, received inadequate information that did not address their specific needs, and experienced inconsistent implementation of inclusive policies. Community-led facilitation led to more uptake. Conclusions: Vaccine willingness alone is insufficient to ensure that vaccines reach everyone. Addressing trust deficits, infrastructural barriers, and digital exclusions requires diligent attention and commitment from the government to mitigate broader challenges faced by TGD people and people with disabilities.
Full article
(This article belongs to the Special Issue Inequality in Immunization 2025)
Open AccessArticle
Safety and Immunogenicity of sIPV in Healthy Infants Aged 2 Months Following Sequential Immunization Program Combination with bOPV: A Phase 3, Randomized, Blinded, Parallel Positive-Controlled Clinical Trial
by
Yafei Liu, Xiaodong Liu, Li Zhang, Xianyun Chang, Ping Xiong, Yanxin Guan, Yixin Li, Weiling Zhang, Lili Xuan, Yan Li, Zhifang Ying and Qing Xu
Vaccines 2025, 13(11), 1094; https://doi.org/10.3390/vaccines13111094 (registering DOI) - 24 Oct 2025
Abstract
Objectives: This phase 3 clinical trial aimed to evaluate the safety and immunogenicity of the Sabin inactivated poliovirus vaccine (sIPV) manufactured by Biominhai in healthy infants following a sequential immunization regimen. Methods: A total of 300 healthy infants aged 2 months
[...] Read more.
Objectives: This phase 3 clinical trial aimed to evaluate the safety and immunogenicity of the Sabin inactivated poliovirus vaccine (sIPV) manufactured by Biominhai in healthy infants following a sequential immunization regimen. Methods: A total of 300 healthy infants aged 2 months were randomly divided into the test group (sIPV-sIPV-bOPV) and the control group (wIPV-wIPV-bOPV) according to the ratio of 1:1. Both groups were inoculated under “2IPV + 1bOPV” schedule. Safety was assessed alongside poliovirus antibody levels before and after vaccination. Results: The overall incidence of adverse reactions (AEs) in the test and control groups was 44% and 39%, respectively. AEs in both groups primarily occurred following the first dose, with approximately 30% classified as grade 1 in severity. No significant differences were observed between groups regarding the incidence, severity, and symptoms of AEs. Additionally, no vaccine-related serious adverse events (SAEs) were reported. At 30 days after the last dose, the seroconversion rates of neutralizing antibodies against poliovirus types I and III reached 100% in both groups, while type II rates at 99% for the test group and 95% for the control. Notably, the seroconversion rates for all types in the test group were non-inferior to those in the control group. The geometric mean titers (GMTs) of neutralizing antibodies against poliovirus for type I (8622.64 vs. 2687.65), type II (207.73 vs. 54.06), and type III (2121.74 vs. 1699.12) were significantly higher in the test group (p < 0.0001 for type I and II; p = 0.04 for type III). Conclusions: The study concluded that the trial vaccine sIPV following sequential immunization program demonstrates good safety and immunogenicity, showing non-inferiority to the control vaccine.
Full article
Open AccessArticle
Digital Storytelling to Reduce Hispanic Parents’ COVID-19 Vaccine Hesitancy: A Pilot Randomized Controlled Trial
by
Sunny W. Kim, Fernanda Lozano, Michael Todd, Linda Larkey, Raheleh Bahrami, Kavya Juwadi and Alexis Koskan
Vaccines 2025, 13(11), 1093; https://doi.org/10.3390/vaccines13111093 (registering DOI) - 24 Oct 2025
Abstract
Background/Objectives: Hispanic children in the U.S. experience disproportionately low COVID-19 vaccination rates, largely due to parental vaccine hesitancy. Digital storytelling offers a culturally relevant approach to address concerns through first-hand narratives. This study examined the feasibility and acceptability of a community-driven digital storytelling
[...] Read more.
Background/Objectives: Hispanic children in the U.S. experience disproportionately low COVID-19 vaccination rates, largely due to parental vaccine hesitancy. Digital storytelling offers a culturally relevant approach to address concerns through first-hand narratives. This study examined the feasibility and acceptability of a community-driven digital storytelling intervention to reduce vaccine hesitancy among Hispanic parents. Methods: Ten formerly vaccine-hesitant Hispanic parents developed digital stories about their reasons for vaccinating their child(ren) against COVID-19. We then enrolled 80 Hispanic parents whose children were not up to date with COVID-19 vaccines in a randomized feasibility trial. Intervention group participants (n = 40) viewed four digital stories selected by a community advisory board, while control group participants (n = 40) viewed four length- and format-matched videos about nutrition. Surveys were completed pre-intervention (T1), immediate post-intervention (T2), and at 2-month follow-up (T3). A subsample of intervention participants also joined focus groups at T3. Results: Qualitative data suggested that DST was an acceptable and engaging method of health education. Intervention group parents showed moderately larger increases in intention to vaccinate than did controls at T2 (d = 0.41) and T3 (d = 0.30). At T3, intervention group parents were more likely to have vaccinated their children than were controls (OR = 5.20, 95% CI = 1.63–16.57; RR = 3.10, 95% CI = 1.30–7.37). Conclusions: The community-driven digital storytelling intervention was feasible and acceptable, and findings suggest moderate effects on increasing vaccine intentions and uptake. Future work should evaluate its effectiveness in reducing parental vaccine hesitancy and, in turn, vaccine uptake for other childhood immunizations.
Full article
(This article belongs to the Special Issue Promoting Research, Development and Access to Vaccines to Address Global Inequities)
►▼
Show Figures
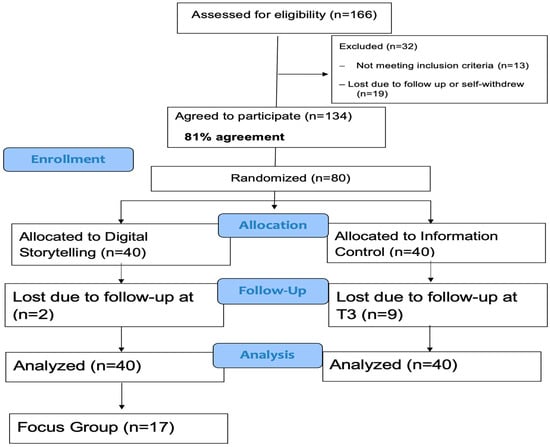
Figure 1
Open AccessArticle
Sporadic Outbreaks of Avian Infectious Bronchitis Viruses Highly Similar to the S95 Live Attenuated Vaccine Strain in Japan: A Comparative Study of Ten Field Isolates and S95
by
Ryohei Nukui, Mari Takahashi, Atsushi Kato, Shiori Oguro, Erika Tanahashi, Takashi Ohmori and Nobuyuki Tsutsumi
Vaccines 2025, 13(11), 1092; https://doi.org/10.3390/vaccines13111092 (registering DOI) - 24 Oct 2025
Abstract
Background: As infectious bronchitis virus (IBV) strains similar to the IBV S95 live attenuated vaccine strain have been occasionally detected in poultry farms in Japan, we investigated the suspicion that outbreaks of the disease were related to the S95 vaccine. Methods: We isolated
[...] Read more.
Background: As infectious bronchitis virus (IBV) strains similar to the IBV S95 live attenuated vaccine strain have been occasionally detected in poultry farms in Japan, we investigated the suspicion that outbreaks of the disease were related to the S95 vaccine. Methods: We isolated ten S95 vaccine-like strains, classified in the JP-I genotype of S1, the VIb (Y-4) genogroup of S2, and the GI-18 lineage, from IBV-affected chickens in Japan between 2020 and 2024. The whole-genome sequence and adaptation to embryonated chicken eggs were investigated. We developed a method for distinguishing the S95 vaccine strain from S95-like wild-type strains using specific primer sets having either the S95 vaccine or S95 parent-specific nucleotide at the 3′ termini of primers on the ORF2 gene. Results: Nine of ten S95 vaccine-like strains lacked identical mutations to the ORF1ab, ORF2, and ORF5a genes that the S95 vaccine strain acquired during attenuation. The remaining S95-like strain, B3389, had identical mutations to the S95 vaccine strain in the ORF1ab and ORF5a genes. The B3389 strain, however, had strain-specific nucleotides that were not found in the S95 vaccine or S95 parent strains, and produced fewer embryonated egg-adapted phenotypes than the S95 vaccine strain. Conclusions: The ten S95-like strains appear not to have emerged from the S95 vaccine strain. Instead, sporadic outbreaks of S95 vaccine-like IBV strains in Japan were indicated. A method for distinguishing and excluding the S95-like wild-type strains as suspected revertants of the S95 vaccine may be utilized for comprehensive IBV surveillance to facilitate development of a vaccination strategy.
Full article
(This article belongs to the Section Veterinary Vaccines)
►▼
Show Figures
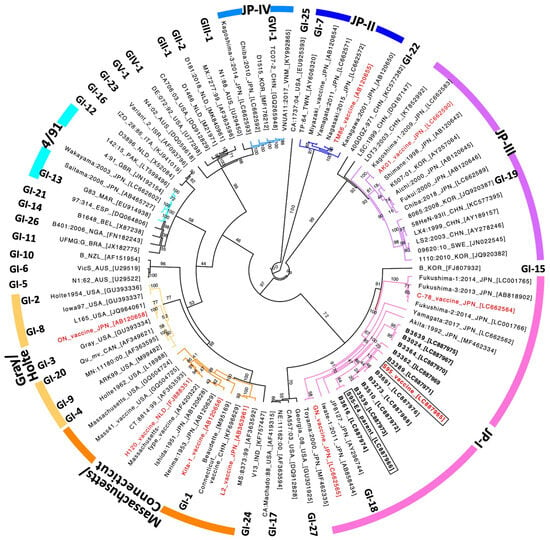
Figure 1
Open AccessArticle
A Multiplex Serological Assay to Evaluate the Antibody Responses to a Set of Plasmodium falciparum Antigens and Their Protective Role Against Malaria in Children Aged 1.5 to 12 Years Living in a Highly Seasonal Malaria Transmission Area of Burkina Faso
by
Sem Ezinmegnon, Issa Nébié, Tegwen Marlais, Daouda Ouattara, Amidou Diarra, Catriona Patterson, Kevin Tetteh, Alphonse Ouédraogo, Chris Drakeley, Alfred B. Tiono and Sodiomon B. Sirima
Vaccines 2025, 13(11), 1091; https://doi.org/10.3390/vaccines13111091 (registering DOI) - 24 Oct 2025
Abstract
Background/Objectives: Understanding the seroepidemiology of P. falciparum antibody responses is essential for assessing the acquisition of natural immunity and may guide interventions that impact the acquisition of immunity against malaria in endemic areas. This study assessed the association between antigen-specific IgG responses and
[...] Read more.
Background/Objectives: Understanding the seroepidemiology of P. falciparum antibody responses is essential for assessing the acquisition of natural immunity and may guide interventions that impact the acquisition of immunity against malaria in endemic areas. This study assessed the association between antigen-specific IgG responses and protection against P. falciparum infection in children from Burkina Faso. Methods: Children aged 1.5 to 12 years were followed using cross-sectional and longitudinal approaches. IgG responses to 16 P. falciparum antigens were measured using a multiplex assay and analyzed by age group and malaria infection status. Associations between antibody levels and clinical malaria risk were assessed using incidence rate ratios (IRRs), and predictive performance of antibody combinations was evaluated using ROC analysis. Results: IgG responses to AMA1, CSP, and MSP2 CH150 showed weak but significant positive correlation with age. Children aged 5–12 years had higher antibody levels than younger children aged 1.5–5 years. Uninfected children had higher levels of antibodies to EBA181 RIII-V, Rh5.1, and SEA1, while infected children had elevated AMA1 and MSP2 CH150. Anti-GLURP R2 and anti-Rh5.1 antibodies were associated with reduced malaria risk (adjusted IRR = 0.52 and 0.40, respectively). The antibody combination of AMA1, GLURP R2, and Etramp5 Ag1 showed the best predictive performance (AUC = 0.70). Conclusions: This study underlines the value of less-studied antibodies (Etramp5 Ag1, Rh5.1, HSP40 Ag1) for diagnosing and protecting against malaria, opening up prospects for the development of more effective tests and targeted vaccine approaches. The variability of responses according to age and infection status calls for further studies to optimize prevention strategies.
Full article
(This article belongs to the Special Issue Research in Innate and Adaptive Immunity)
►▼
Show Figures
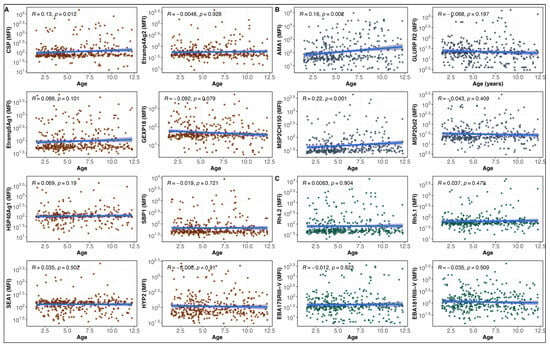
Figure 1
Open AccessArticle
Finding the Sweet Spot: Preferences for Effectiveness, Duration, and Side Effects in a Discrete Choice Experiment Among Uganda’s Key Populations
by
Maiya G. Block Ngaybe, Richard Muhumuza, Mélanie Antunes, Ezra Musingye, Kawoya Kijali Joseph, Betty Nakaggwa, Stephen Mugamba, Bashir Ssuna, Gabriela Valdez, John Ehiri, Maia Ingram, Agnes Kiragga, Grace Mirembe, Betty Mwesigwa, Hannah Kibuuka and Purnima Madhivanan
Vaccines 2025, 13(11), 1090; https://doi.org/10.3390/vaccines13111090 - 24 Oct 2025
Abstract
Background: Human immunodeficiency virus (HIV) affects more than 39 million people worldwide, with Uganda ranked 10th among countries with the highest number of cases. As new preventative HIV injectables emerge, it is vital to think about how best to tailor strategies to promote
[...] Read more.
Background: Human immunodeficiency virus (HIV) affects more than 39 million people worldwide, with Uganda ranked 10th among countries with the highest number of cases. As new preventative HIV injectables emerge, it is vital to think about how best to tailor strategies to promote these injectable drugs, like PrEP and vaccines, when available, to the different populations most in need. Discrete choice experiments (DCEs) are economics-derived methods used to determine factors that influence engagement in a certain behavior. Objective: This study used a DCE to determine the preferences for a preventative HIV injectable drugs/vaccines among people at risk of HIV acquisition in urban and peri-urban areas of Uganda. Methods: In June 2024, we implemented a cross-sectional DCE survey in three urban sites in Uganda in English and Luganda. The survey collected information on demographics, HIV risk, vaccine confidence and responses to the 13 injection product choice tasks presented to determine preferences. We used community-based, respondent-driven sampling methods to recruit participants from three key populations: (1) female sex workers; (2) people who identify as lesbian, gay, bisexual or transgender; and (3) young women (18–24 years). We collected the data on tablets using the Sawtooth Lighthouse Studio software (v. 19.15.6), taking into consideration privacy and confidentiality, given the sensitivity of the information and recent governmental policies in Uganda. Data were analyzed using a split-sample mixed logit regression analysis. The study was approved by local ethical regulatory bodies. Results: From the total of 406 participants screened for this study, 376 participants met the eligibility criteria and were included in the final analysis (85 young women, 159 female sex workers, and 132 who identified as lesbian, gay, bisexual or transgender). The average age was 23.7 (SD: 5.7). The majority of participants had received some secondary school or vocational school (202, 53.7%) The attributes that explained the preferences were primarily severe compared to mild side effects (β: −0.69, 95% CI: −0.78, −0.60), a 30% increase in vaccine/drug effectiveness (β: 0.39, 95% CI: 0.34, 0.44), and a 50,000 UGX (or USD ~13.64) increase in cost (β: −0.22, 95% CI: −0.27, −0.17). There were no significant differences between the preferences for different injectable types. The sensitivity analyses suggested potential differences in preferences by the amount of help participants received from research assistants when completing the survey, although not by income level. Conclusions: Side effects had the greatest impact on participants’ preferences for injectable HIV prevention methods, followed closely by effectiveness and cost. It is therefore essential to develop affordable or free prevention options with minimal side effects. Policymakers should focus on reducing the financial barriers to access and emphasize transparent communication about the effectiveness and safety of these injectables in health promotion campaigns to maximize adoption and improve public health outcomes.
Full article
(This article belongs to the Special Issue Studies of Infectious Disease Epidemiology and Vaccination)
►▼
Show Figures

Figure 1
Open AccessArticle
High-Grade Vaginal Intraepithelial Neoplasia: Clinical Profile, HPV Vaccination Status, and Treatment Outcomes at Two Italian Referral Centers
by
Niccolò Gallio, Mario Preti, Renzo Boldorini, Cristina Cavagnetto, Fulvio Borella, Federica Bevilacqua, Ilaria Barbierato, Raffaella Ribaldone, Enrica Bovio, Chiara Airoldi, Valentino Remorgida, Luca Marozio and Alberto Revelli
Vaccines 2025, 13(11), 1089; https://doi.org/10.3390/vaccines13111089 - 24 Oct 2025
Abstract
Background: There is limited available data about the natural history and clinical characteristics of patients affected by high-grade vaginal intraepithelial neoplasia (or vaginal intraepithelial neoplasia 2/3 or vaginal high-grade squamous intraepithelial lesion, HSIL). The aim of the study was to review clinical
[...] Read more.
Background: There is limited available data about the natural history and clinical characteristics of patients affected by high-grade vaginal intraepithelial neoplasia (or vaginal intraepithelial neoplasia 2/3 or vaginal high-grade squamous intraepithelial lesion, HSIL). The aim of the study was to review clinical characteristics and treatment outcomes of a large cohort of patients with vaginal HSIL. Methods: A retrospective analysis was performed for patients with histologically confirmed VaIN 2/3 treated at two Italian referral centers from 2003 to 2022. Demographics, referring cytology, associated cervical and vulvar HSIL treatment modalities, follow-up visits, and vaccination status were recorded. The primary outcome was risk of recurrence; the secondary outcome was risk of progression to invasive carcinoma after treatment. Results: 323 women were included in the analysis: 69.7% (225) had VaIN2, and 30.3% (98) had VaIN3. Mean age at diagnosis was 55.43 years (range 24–93 years). 20.4% had a previous hysterectomy, mainly for cervical intraepithelial neoplasia (CIN)/invasive squamous carcinoma (64.6%). In total, 55.2% underwent ablative therapy, and 44.8% underwent excisional treatment. Over a mean follow-up of 62.43 months, 22.0% of patients recurred as vaginal HSIL. At univariate analysis older age, VaIN grade 3, previous hysterectomy, associated cervical lesions, associated vulvar HSIL, and excisional treatment were significantly associated with increased risk of recurrence. At multivariate analysis, only hysterectomy and excisional treatment maintained significance. Five patients progressed to invasive vaginal carcinoma, with a median time to invasion of 87.1 months. Conclusions: The risk of recurrence of vaginal HSIL is higher in patients treated with excisional methods and/or those who have undergone hysterectomy for both benign and preinvasive lesions. Long-term follow-up is essential to monitor clinical outcomes and prevent disease progression.
Full article
(This article belongs to the Special Issue Role of Human Papillomavirus Vaccines in Cervical and Vulvo-Vaginal Diseases)
►▼
Show Figures
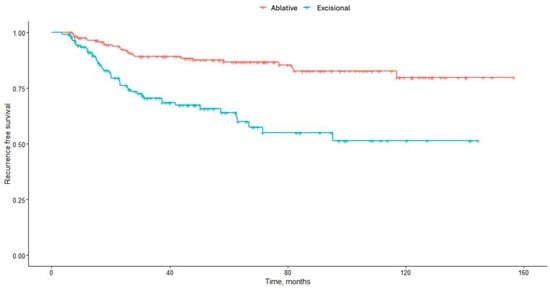
Figure 1
Open AccessArticle
RSV Vaccination Programme for Older Adults: A Scotland-Wide Study on RSVpreF Vaccine Safety
by
Lucy A. Cullen, Cheryl L. Gibbons, Ting Shi, Taimoor Hasan, Christopher Sullivan, Kimberly Marsh, Chris Robertson, Joanne Claire Cameron and Sam Ghebrehewet
Vaccines 2025, 13(11), 1088; https://doi.org/10.3390/vaccines13111088 - 24 Oct 2025
Abstract
►▼
Show Figures
Background/Objectives: Respiratory Syncytial Virus (RSV) is a common respiratory tract infection that accounts for significant morbidity and mortality, particularly among older adults. From 1 August 2024, the bivalent RSVpreF vaccine (Abrysvo®) was introduced in Scotland for eligible older adults. While clinical
[...] Read more.
Background/Objectives: Respiratory Syncytial Virus (RSV) is a common respiratory tract infection that accounts for significant morbidity and mortality, particularly among older adults. From 1 August 2024, the bivalent RSVpreF vaccine (Abrysvo®) was introduced in Scotland for eligible older adults. While clinical trials and post-licensure studies showed a good safety profile of the vaccine, post-marketing observational studies in the United States reported a small increased risk of Guillain–Barré Syndrome (GBS) among older adult populations. Methods: We conducted observed versus expected (OE) and self-controlled case series (SCCS) analyses to retrospectively monitor the incidence of hospital admission for 39 conditions, including GBS. This was undertaken in post-RSV-vaccination periods from 1 August to 31 December 2024, among eligible adults aged 74 to 80 years in Scotland. Results: Observed versus expected analyses identified an increased risk of hospitalisation with GBS in the 1–28-day post-vaccination period. From SCCS analyses, six conditions showed an increased risk in post-vaccination periods (acute coronary syndrome, acute myocardial infarction, acute renal failure, GBS, heart failure and stroke (haemorrhagic)). After temporal adjustment, only GBS remained significant. All 10 hospitalised GBS cases occurred in the 10 to 16 days post-vaccination. Excess risk of GBS was estimated to be 46.1 cases per one million doses. Conclusions: Study results indicated a good safety profile of the RSV vaccine for older eligible adults aged 75 to 79 years, although a small increased risk of GBS was identified in both analyses. Excess risk levels of GBS from vaccination align with other studies.
Full article

Figure 1
Open AccessArticle
Immunogenicity and Safety of Sabin Strain Inactivated Poliovirus Vaccine Booster Dose Administered Separately or Concomitantly with Inactivated Hepatitis A Vaccine or Measles–Mumps–Rubella Combined Attenuated Live Vaccine: An Open-Labelled, Randomized, Controlled, Phase 4 Clinical Trial
by
Jialei Hu, Weixiao Han, Kai Chu, Hengzhen Zhang, Ling Tuo, Xiaoqian Duan, Jun Li, Fang Yuan, Chunfang Luan, Hongxing Pan and Peng Jiao
Vaccines 2025, 13(11), 1087; https://doi.org/10.3390/vaccines13111087 - 23 Oct 2025
Abstract
Objectives: To determine whether a Sabin strain inactivated poliovirus booster (sIPV) given concomitantly (same day, different sites) with MMRV or inactivated hepatitis A vaccine (HepA) is non-inferior in immunogenicity and comparable in safety to separate administration at the 18-month visit. Methods: Open-label, randomized
[...] Read more.
Objectives: To determine whether a Sabin strain inactivated poliovirus booster (sIPV) given concomitantly (same day, different sites) with MMRV or inactivated hepatitis A vaccine (HepA) is non-inferior in immunogenicity and comparable in safety to separate administration at the 18-month visit. Methods: Open-label, randomized Phase IV trial in healthy children (18~22 months), allocated 2:2:2:1:1 to sIPV&MMRV, sIPV&HepA, sIPV-only, MMRV-only, or HepA-only. Primary endpoints were Day-30 seroconversion rates (SCRs) for poliovirus types 1–3 (sIPV arms) and for HepA (HepA arms). Results: Of 892 screened, 889 were randomized; baseline characteristics were balanced. By Day 30, seroprotection was 100% for PV types 1–3 in all sIPV-containing arms. SCRs were high and similar across concomitant vs. separate administration; all pairwise SCR differences met non-inferiority (−10% margin). Adjusted post-vaccination GMTs were comparable across serotypes. For HepA, Day-30 SPoR was 99.3% vs. 100.0%, and adjusted GMC was 412.2 vs. 465.9 (p = 0.2224). For MMRV, Day-30 SPoR was 100% in both groups with similar adjusted GMCs. Within 30 days, overall adverse reactions were 14.5%/17.4%/16.3%/7.3%/10.8% (sIPV&MMRV/sIPV&HepA/sIPV-only/MMRV-only/HepA-only), mostly mild to moderate; no vaccine-related SAEs (NCT06442449). Conclusions: Same-day sIPV co-administration with MMRV or HepA was non-inferior and well tolerated, supporting programmatic adoption.
Full article
(This article belongs to the Section Vaccine Advancement, Efficacy and Safety)
►▼
Show Figures
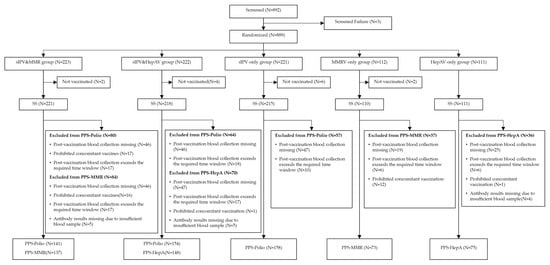
Figure 1
Open AccessReview
Hemocyanins: Microscopic Giants with Unique Structural Features for Applications in Biomedicine
by
Michelle L. Salazar, Diego A. Díaz-Dinamarca, Javier Bustamante, Felipe Vergara, Augusto Manubens, Fabián Salazar and María Inés Becker
Vaccines 2025, 13(11), 1086; https://doi.org/10.3390/vaccines13111086 - 23 Oct 2025
Abstract
Vaccine adjuvants play a crucial role in the field of vaccinology, yet they remain one of the least developed and poorly characterized components of modern biomedical research. The limited availability of clinically approved adjuvants highlights the urgent need for new molecules with well-defined
[...] Read more.
Vaccine adjuvants play a crucial role in the field of vaccinology, yet they remain one of the least developed and poorly characterized components of modern biomedical research. The limited availability of clinically approved adjuvants highlights the urgent need for new molecules with well-defined mechanisms and improved safety profiles. Hemocyanins, large copper-containing metalloglycoproteins found in mollusks, represent a unique class of natural immunomodulators. Hemocyanins serve as carrier proteins that help generate antibodies against peptides and hapten molecules. They also function as non-specific protein-based adjuvants (PBAs) in both experimental human and veterinary vaccines. Their mannose-rich N-glycans allow for multivalent binding to innate immune receptors, including C-type lectin receptors (e.g., MR, DC-SIGN) and Toll-like receptor 4 (TLR4), thereby activating both MyD88- and TRIF-dependent signaling pathways. Hemocyanins consistently favor Th1-skewed immune responses, which is a key characteristic of their adjuvant potential. Remarkably, their conformational stability supports slow intracellular degradation and facilitates dual routing through MHC-II and MHC-I pathways, thereby enhancing both CD4+ and CD8+ T-cell responses. Several hemocyanins are currently being utilized in biomedical research, including Keyhole limpet hemocyanin (KLH) from Megathura crenulata, along with those from other gastropods such as Concholepas concholepas (CCH), Fissurella latimarginata (FLH), Rapana venosa (RvH), and Helix pomatia (HpH), all of which display strong immunomodulatory properties, making them promising candidates as adjuvants for next-generation vaccines against infectious diseases and therapeutic immunotherapies for cancer. However, their structural complexity has posed challenges for their recombinant production, thus limiting their availability from natural sources. This reliance introduces variability, scalability issues, and challenges related to regulatory compliance. Future research should focus on defining the hemocyanin immunopeptidome and isolating minimal peptides that retain their adjuvant activity. Harnessing advances in structural biology, immunology, and machine learning will be critical in transforming hemocyanins into safe, reproducible, and versatile immunomodulators. This review highlights recent progress in understanding how hemocyanins modulate mammalian immunity through their unique structural features and highlights their potential implications as potent PBAs for vaccine development and other biomedical applications. By addressing the urgent need for novel immunostimulatory platforms, hemocyanins could significantly advance vaccine design and immunotherapy approaches.
Full article
(This article belongs to the Section Vaccine Design, Development, and Delivery)
►▼
Show Figures

Figure 1
Open AccessArticle
Parents’ Attitudes and Beliefs Towards Human Papillomavirus Vaccination
by
Ivana Kotromanovic Simic, Darko Kotromanovic, Nika Lovrincevic Pavlovic, Jelena Kovačević, Marija Olujic, Danijela Nujic, Matea Matic Licanin, Ivon Matić, Jelena Sakic Radetic, Ilijan Tomas, Vlatko Kopic, Ivan Miskulin and Maja Miskulin
Vaccines 2025, 13(11), 1085; https://doi.org/10.3390/vaccines13111085 - 22 Oct 2025
Abstract
Background/Objectives: The Human Papillomavirus (HPV) is one of the most common causes of STIs, posing a significant public health problem. Today, the transmission of HPV can be very effectively prevented, making it important to vaccinate the target population at a young age.
[...] Read more.
Background/Objectives: The Human Papillomavirus (HPV) is one of the most common causes of STIs, posing a significant public health problem. Today, the transmission of HPV can be very effectively prevented, making it important to vaccinate the target population at a young age. The aim of this study was to examine the attitudes and beliefs of parents regarding the HPV vaccine and the HPV vaccination of their child. Methods: This cross-sectional study was conducted in Osijek, Croatia, from June 2021 to September 2022 via a self-administered questionnaire and included 215 respondents. Results: The results showed that respondents who would vaccinate their child were significantly more likely to be those who work in the healthcare field, who had heard of the term HPV, who had sought information about the HPV vaccine on their own, and who had received information about vaccination from school doctors. The attitude towards vaccination was more negative among respondents who did not intend to vaccinate their child. In predicting the decision not to vaccinate one’s child against HPV, bivariate logistic regression revealed that the probability of non-vaccination increases with working outside the field of healthcare (OR = 4.61) and a negative attitude towards vaccination (OR = 1.46), while the probability of non-vaccination decreases if information was received from a school doctor (OR = 0.46). Furthermore, multivariate logistic regression showed that there is a significant model in predicting non-vaccination against HPV, consisting of two predictors: working outside the healthcare field (OR = 8.15) and a negative attitude towards vaccination (OR = 1.49). Conclusions: Given that parents are responsible for making the decision about HPV vaccination, it is necessary to invest additional efforts in educating them about the importance of preventing HPV infections and the benefits of HPV vaccination itself.
Full article
(This article belongs to the Special Issue The Role of Vaccination on Public Health and Epidemiology)
Open AccessArticle
FLU-v, a Broad-Spectrum Peptide-Based Influenza Vaccine, Induces NK Cell Activating IgG1 and IgG3 Subclass Antibodies in Humans
by
Lisbeth M. Næss, Hillary Vanderven, Diane Bryant-Bratlie, Ida Laake, Olga Pleguezuelos and Fredrik Oftung
Vaccines 2025, 13(11), 1084; https://doi.org/10.3390/vaccines13111084 - 22 Oct 2025
Abstract
Background/Objectives: FLU-v is a peptide-based broad-spectrum influenza vaccine proven to induce humoral and cellular immune responses in humans. In this study, FLU-v-specific IgG1 and IgG3 subclass antibodies, induced by adjuvanted or non-adjuvanted FLU-v vaccination in healthy adults participating in a phase II clinical
[...] Read more.
Background/Objectives: FLU-v is a peptide-based broad-spectrum influenza vaccine proven to induce humoral and cellular immune responses in humans. In this study, FLU-v-specific IgG1 and IgG3 subclass antibodies, induced by adjuvanted or non-adjuvanted FLU-v vaccination in healthy adults participating in a phase II clinical study, were quantitated. The ability of these antibodies to induce NK cell activation was investigated. Methods: An ELISA was developed to quantify FLU-v-specific IgG1 and IgG3 antibodies in serum. A flowcytometric assay based on an NK cell line was used to evaluate NK cell activation by expression of degranulation marker CD107a. Results: In the adjuvanted FLU-v group, IgG1 and IgG3 seroconversion on day 42 was 88.5% and 86.5% compared to 53.4% and 29.3% in the non-adjuvanted FLU-v group, which was significantly different from the respective placebo groups (0–6.3%). Adjuvanted FLU-v vaccination induced a raise in median IgG1 and IgG3 levels from 435 and 167 ng/mL pre vaccination to 4422 and 2020 ng/mL 42 days post vaccination, representing a fold increase of 16.3 for IgG1 and 11.6 for IgG3, which was sustained on day 180 post vaccination (10.4-fold and 5.0-fold, respectively). Non-adjuvanted vaccination induced a more modest increase in IgG1 and IgG3 from 655 and 206 ng/mL pre vaccination to 1808 and 264 ng/mL 42 days post vaccination. A correlation between levels of FLU-v-specific IgG, IgG1, or IgG3 and their ability to induce NK cell activation was demonstrated. Conclusions: A single dose of adjuvanted FLU-v induced high levels of long-lasting antigen-specific IgG1 and IgG3 antibodies with NK cell-mediated effector functions relevant to protection against influenza disease.
Full article
(This article belongs to the Section Influenza Virus Vaccines)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Assessing Vaccine Confidence Using the Vaccine Hesitancy Scale Among Adolescent Girls and Young Women at Risk of HIV Acquisition Living in Uganda, Zambia, and South Africa
by
Nasimu Kyakuwa, Ali Ssetaala, Matt A. Price, Annet Nanvubya, Joel M. Abyesiza, Geofrey Basalirwa, Brenda Okech, Juliet Mpendo, Mubiana Inambao, Kawela Mumba-Mwangelwa, Chishiba Kabengele, Suzanna C. Francis, Pholo Maenetje, Ken Ondeng’e, Vinodh Edward, William Kilembe and Monica O. Kuteesa
Vaccines 2025, 13(11), 1083; https://doi.org/10.3390/vaccines13111083 (registering DOI) - 22 Oct 2025
Abstract
Background: Vaccine hesitancy (VH) remains a major threat to global health that can reverse the progress in tackling vaccine-preventable diseases. Vaccine uptake among adolescent Girls and young women (AGYW) is often low. We assessed VH using a validated scale among AGYW in Uganda,
[...] Read more.
Background: Vaccine hesitancy (VH) remains a major threat to global health that can reverse the progress in tackling vaccine-preventable diseases. Vaccine uptake among adolescent Girls and young women (AGYW) is often low. We assessed VH using a validated scale among AGYW in Uganda, Zambia, and South Africa. Methods: From June 2023 to February 2024, we recruited AGYW from fishing communities in Uganda, urban and peri-urban locations in Lusaka and Ndola, Zambia, and mining communities in Rustenburg, South Africa. Eligible participants were aged 15–24 years, sexually active, and HIV-negative but at risk for HIV acquisition. We collected demographic, HIV-related behavioral data, and vaccine hesitancy data using a structured questionnaire. Vaccine confidence was assessed using the 10-question Vaccine Hesitancy Scale that describes two factors, i.e., “vaccine confidence” and “risk tolerance”. Exploratory and Confirmatory Factor Analyses were performed to assess scale validity and internal consistency. Logistic regression was used to determine associations between demographics and vaccine confidence. Results: A total of 1213 AGYW participated in the study, with a mean age of 19.4 (SD ± 2.6) years. More than half (54%) were aged between 15 and 19 years. The majority of AGYW (94%) strongly believed that vaccines were important for their health and the community and that vaccination is a good way to protect them from diseases. About two-thirds of the AGYW (66%) indicated that they were concerned about the adverse effects of vaccines, while 30% responded that they did not need vaccines for diseases that were not common. We observed that 951 (78%) of the AGYW reported high vaccine confidence, while 494 (41%) reported low concerns over risks. Vaccine confidence varied across countries, with Zambia and Uganda showing lower vaccine confidence (adjusted odds ratios of 0.28 and 0.45, respectively, p < 0.005) in comparison to South Africa. Conclusions: A high level of vaccine confidence was observed among AGYW. Vaccine confidence among AGYW was driven more by the trust in vaccine safety and the need to protect communities against diseases. These findings suggest the potential for acceptance of vaccines, including future HIV vaccines, among AGYW. Despite high levels of vaccine confidence, concerns over vaccine risks remain substantial and must be addressed.
Full article
(This article belongs to the Special Issue Acceptance and Hesitancy in Vaccine Uptake: 2nd Edition)
►▼
Show Figures

Figure 1
Open AccessReview
Intracellular Parasitic Infections Caused by Plasmodium falciparum, Leishmania spp., Toxoplasma gondii, Echinococcus multilocularis, Among Key Pathogens: Global Burden, Transmission Dynamics, and Vaccine Advances—A Narrative Review with Contextual Insights from Armenia
by
Tatevik Sargsyan, Lala Stepanyan, Avetis Tsaturyan, Rosanna Palumbo, Caterina Vicidomini and Giovanni N. Roviello
Vaccines 2025, 13(11), 1082; https://doi.org/10.3390/vaccines13111082 - 22 Oct 2025
Abstract
Intracellular parasitic infections continue to pose significant public health and veterinary challenges globally, driven by their ability to evade immune responses, persist within host cells, and spread through complex transmission pathways. Caused by a diverse array of protozoan, helminthic, and arthropod-borne parasites, these
[...] Read more.
Intracellular parasitic infections continue to pose significant public health and veterinary challenges globally, driven by their ability to evade immune responses, persist within host cells, and spread through complex transmission pathways. Caused by a diverse array of protozoan, helminthic, and arthropod-borne parasites, these infections, such as toxoplasmosis, leishmaniasis, and tick-borne diseases, remain prevalent across many regions, often exacerbated by environmental, socio-economic, and ecological factors. This review explores the current knowledge on intracellular parasitic diseases, outlining parasite classification, immune evasion mechanisms, diagnostic difficulties, and control strategies. Special attention is given to recent advancements in vaccine development, with a focus on experimental and licensed vaccines targeting intracellular pathogens. Additionally, the review highlights the importance of a ‘One Health’ approach, integrating human, animal, and environmental health efforts to address the multifaceted nature of parasitic transmission and control. Within this global context, Armenia serves as a case study, offering insight into how local ecological conditions, vector distribution, public health capacity, and social determinants shape the national burden of these infections. Challenges in Armenia, such as limited access to advanced diagnostics, underreporting, and the need for robust surveillance systems, underscore broader regional needs for investment in research, infrastructure, and cross-sectoral collaboration.
Full article
(This article belongs to the Special Issue Intracellular Parasites: Immunology, Resistance, and Therapeutics)
►▼
Show Figures

Figure 1
Open AccessCase Report
Observations of Wart Clearance Following COVID-19 Vaccination: Coincidence or Missed Immunologic Signals?
by
Qiwei Wilton Sun, Caroline A. Nelson and Howard P. Forman
Vaccines 2025, 13(11), 1081; https://doi.org/10.3390/vaccines13111081 - 22 Oct 2025
Abstract
Background/Objectives: The COVID-19 vaccines have been extensively studied for their potential adverse side effects. However, reports of unexpected but potentially beneficial immune responses have received comparatively less attention. Methods: In this case series, a PubMed search was conducted using the terms “warts”, “verruca”,
[...] Read more.
Background/Objectives: The COVID-19 vaccines have been extensively studied for their potential adverse side effects. However, reports of unexpected but potentially beneficial immune responses have received comparatively less attention. Methods: In this case series, a PubMed search was conducted using the terms “warts”, “verruca”, “HPV”, “COVID-19”, “SARS-CoV-2”, “immunization”, and “vaccination.” All reported cases of wart clearance temporally linked to COVID-19 vaccination were identified and summarized, including patient demographics, vaccine type, number of doses, timing of clearance, and follow-up duration. Results: Five cases were identified. Patients varied in age, sex, comorbidity, and immunologic status. Warts were long-standing and treatment-resistant in all cases. Clearance occurred within approximately 2–4 weeks following the second or third vaccine dose (either mRNA-based [BNT162b2, mRNA-1273] or adenoviral vector [ChAdOx1-S]) and was sustained for 2–8 months of follow-up with no recurrences reported. Conclusions: While causality cannot be determined, the convergence of reports across diverse patients, consistent timing of clearance, and plausible immunologic pathways suggest that COVID-19 vaccination may, in rare instances, trigger beneficial immune activation against HPV-infected keratinocytes. Recognition of such unexpected outcomes underscores the need for broader vaccine safety and efficacy surveillance that includes both adverse and beneficial immune effects.
Full article
(This article belongs to the Section COVID-19 Vaccines and Vaccination)
Open AccessArticle
Norovirus in Pediatric Gastroenteritis: A Study in Argentine Hospitals Before and After the Introduction of Universal Rotavirus Vaccination
by
Karina A. Gomes, Karina A. Rivero, Christian Barrios Mathieur, Juan I. Degiuseppe, Paulo R. Cortes, Patricia A. Gonzalez, Abel Zurschmitten, María P. Castro, Viviana Parreño, Marina V. Mozgovoj and Juan A. Stupka
Vaccines 2025, 13(11), 1080; https://doi.org/10.3390/vaccines13111080 - 22 Oct 2025
Abstract
Norovirus (NoV) is a leading cause of acute gastroenteritis (AGE) in young children worldwide. Following the introduction of universal rotavirus (RVA) vaccination in Argentina in 2015, the role of NoV in pediatric AGE warrants evaluation. This study aimed to assess the prevalence, clinical
[...] Read more.
Norovirus (NoV) is a leading cause of acute gastroenteritis (AGE) in young children worldwide. Following the introduction of universal rotavirus (RVA) vaccination in Argentina in 2015, the role of NoV in pediatric AGE warrants evaluation. This study aimed to assess the prevalence, clinical characteristics, and molecular diversity of NoV in children under five years of age, comparing the periods before and after RVA vaccine implementation. Methods: A descriptive observational study was conducted in two pediatric hospitals in Argentina. Stool samples were obtained from both outpatient and hospitalized children presenting with acute gastroenteritis (AGE) during two distinct one-year periods: 285 samples from the pre-vaccination period (2011–2012) and 212 samples from the post-vaccination period (2019–2020). NoV, RVA and other viral enteropathogens were detected by RT-qPCR or immunoassay. Positive NoV samples were genotyped by Sanger sequencing of the ORF1/ORF2 junction. Results: NoV was detected in 30.1% (86/285) and 23.5% (50/212) of cases in the pre- and post-vaccination periods, respectively. Children under two years of age and inpatients had significantly higher NoV detection in both periods. NoV mono-infections were more frequent in post-vaccination period (72% vs. 50%). NoV GII predominated in both periods, with increased genotype diversity observed post-vaccination, including GII.3[P12], GII.4 Sydney[P16], GII.6[P7], and GII.2[P16]. Conclusions: NoV remains a major cause of pediatric AGE in Argentina, particularly in children under two years old. Although NoV prevalence did not increase after RVA vaccine introduction, its clinical relevance persists. Continued molecular surveillance is essential to monitor genotype dynamics and implement prevention strategies.
Full article
(This article belongs to the Special Issue Epidemiology, Immunology, and Therapeutic Vaccines of Enteric Pathogens)
►▼
Show Figures

Graphical abstract
Open AccessArticle
Invasive Meningococcal Disease in the Post–COVID-19 Era in South America
by
Marco A. P. Sáfadi, Juan Francisco Falconi, Maria Gabriela Abalos, Lidia Serra, Angela Gentile, Alejandro Diaz, Claudia P. Cortes and Rodolfo Villena
Vaccines 2025, 13(11), 1079; https://doi.org/10.3390/vaccines13111079 - 22 Oct 2025
Abstract
Background: During the COVID-19 pandemic, reductions in cases of bacterial diseases transmitted via the respiratory route were reported by the Invasive Respiratory Infection Surveillance Consortium. Here, we evaluate the epidemiology of invasive meningococcal disease (IMD) in Argentina, Brazil, Chile, and Colombia during and
[...] Read more.
Background: During the COVID-19 pandemic, reductions in cases of bacterial diseases transmitted via the respiratory route were reported by the Invasive Respiratory Infection Surveillance Consortium. Here, we evaluate the epidemiology of invasive meningococcal disease (IMD) in Argentina, Brazil, Chile, and Colombia during and after the COVID-19 pandemic. Methods: The epidemiology of meningococcal disease was reviewed in selected South American countries through 2023 from publicly available national surveillance system databases. Results: The incidence of IMD decreased substantially in 2020 in Argentina, Brazil, Chile, and Colombia and was followed by a trend of increased disease. Similarly to observations in several European countries, the post-pandemic rebounds in cases of IMD in the four South American countries included in this analysis were mainly caused by serogroup B, that became one of the predominant serogroups causing IMD in all four countries. Conclusions: Enhanced surveillance of IMD, including genomic characterization of strains, is needed to inform public health policymakers and guide future vaccination strategies in the region.
Full article
(This article belongs to the Section Epidemiology and Vaccination)
►▼
Show Figures
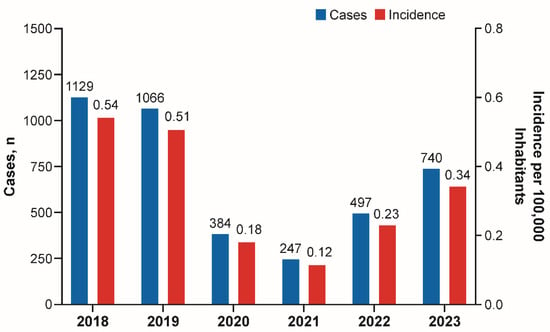
Figure 1
Open AccessPerspective
Balancing Innovation and Equity: A Successful Dynamic Between Private and Public Sectors Is Essential to Ensure True Pandemic Influenza Preparedness
by
Lyn Morgan Marsden and Marie Mazur
Vaccines 2025, 13(11), 1078; https://doi.org/10.3390/vaccines13111078 - 22 Oct 2025
Abstract
The COVID-19 pandemic demonstrated both the transformative capacity of vaccine innovation and the persistent inequities that accompany emergency access, underscoring the critical need for stronger collaboration between global health governance and the vaccine industry. Influenza pandemics remain inevitable threats. The continued emergence of
[...] Read more.
The COVID-19 pandemic demonstrated both the transformative capacity of vaccine innovation and the persistent inequities that accompany emergency access, underscoring the critical need for stronger collaboration between global health governance and the vaccine industry. Influenza pandemics remain inevitable threats. The continued emergence of avian influenza strains such as H5N1 reinforces the necessity of robust preparedness. This perspective examines the underutilization of private sector vaccine manufacturers in current pandemic influenza frameworks and identifies three central areas where industry participation is indispensable: predictable vaccine demand through robust seasonal influenza programs, economic incentives that de-risk investments in research and development, and diversification of vaccine platforms to expand response capacity. In addition, regionalizing manufacturing, advancing collaborative regulatory models, and negotiating export waivers are presented as potential mechanisms to strengthen equity and supply security. The review highlights demand-based tiered pricing and Advance Purchase Agreements as practical tools to align commercial incentives with public health priorities. Furthermore, it makes the case for embedding private sector representation and knowledge into top-level decision-making and preparedness planning, ensuring investment in innovation is aligned with global health objectives. Ultimately, true pandemic influenza readiness depends on building a sustained seasonal influenza market, embedding private sector engagement into governance structures, and fostering mutual trust to ensure timely access and equitable protection for populations worldwide.
Full article
(This article belongs to the Special Issue Pandemic Influenza Vaccination)
►▼
Show Figures
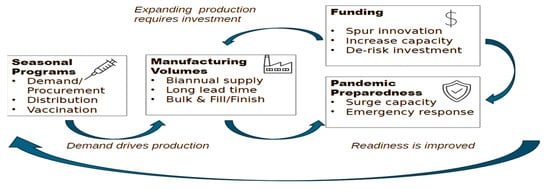
Figure 1
Open AccessReview
Construction and Research Progress of Animal Models and Mouse Adapted Strains of Seasonal Influenza Virus
by
Haijun Zhu, Siyu Pu, Peiqing He, Junhao Luo and Rongbao Gao
Vaccines 2025, 13(10), 1077; https://doi.org/10.3390/vaccines13101077 - 21 Oct 2025
Abstract
Influenza viruses, featured by high variability, pose a persistent public health threat because of an annual seasonal epidemic in the world and irregular global pandemic, requiring animal models to elucidate their pathogenic mechanisms and advance preventive strategies. Mice have been selected as the
[...] Read more.
Influenza viruses, featured by high variability, pose a persistent public health threat because of an annual seasonal epidemic in the world and irregular global pandemic, requiring animal models to elucidate their pathogenic mechanisms and advance preventive strategies. Mice have been selected as the primary animal model, although several experimental animals have been used in studies of the influenza virus. However, the limited susceptibility of wild-type influenza viruses to mice poses significant challenges for studying pathogenesis and intervention strategies. Here, to help understand the construction of mouse-adapted influenza viruses, we reviewed the recent research progress in constructing mouse-adapted influenza virus strains to overcome species-specific barriers.
Full article
(This article belongs to the Special Issue Immunity to Influenza Viruses and Vaccines)
►▼
Show Figures
Figure 1
Open AccessArticle
Efficacy of a Novel PCV2d and Mycoplasma hyopneumoniae Combined Vaccine in Piglets with High and Low Levels of PCV2 Maternally Derived Antibodies at Vaccination
by
Mònica Sagrera, Laura Garza-Moreno, Àlex Cobos, Anna Maria Llorens, Eva Huerta, Mónica Pérez, Diego Pérez, David Espigares, Joaquim Segalés and Marina Sibila
Vaccines 2025, 13(10), 1076; https://doi.org/10.3390/vaccines13101076 - 21 Oct 2025
Abstract
Background/Objectives: Maternally derived antibody (MDA) levels of porcine circovirus 2 (PCV2) may eventually interfere with humoral response and vaccination efficacy. This study aimed to evaluate the efficacy of a ready-to-use PCV2d and Mycoplasma hyopneumoniae combined vaccine in piglets with different PCV2 MDA levels
[...] Read more.
Background/Objectives: Maternally derived antibody (MDA) levels of porcine circovirus 2 (PCV2) may eventually interfere with humoral response and vaccination efficacy. This study aimed to evaluate the efficacy of a ready-to-use PCV2d and Mycoplasma hyopneumoniae combined vaccine in piglets with different PCV2 MDA levels at vaccination in an experimental inoculation with a heterologous viral genotype. Methods: Forty-eight piglets were allocated into vaccinated (V) and non-vaccinated (NV) groups with high (H) and low (L) PCV2 MDA subgroups (H-V, H-NV, L-V, L-NV). At 3 weeks of age, the piglets received either one dose of vaccine or placebo. Five weeks later, all animals were intranasally challenged with a PCV2b inoculum. Body weight was registered at different time points. Blood samples, peripheral blood mononuclear cells and tracheobronchial lymph nodes (TBLN) were collected and used to assess viraemia, viral load, humoral and cellular responses and histological lesions. Results: The V group showed higher PCV2 antibody levels from challenge onwards, along with a lower percentage of viraemic pigs and reduced viral load in serum at 2 and 3 weeks post-challenge (wpc) and in TBLN tissues compared to the NV group. The H-V group had the highest antibody levels post-challenge, showed no detectable viraemia and had a lower overall amount of virus in tissues. The NV group (especially H-NV) exhibited increased levels of IFN-γ, IFN-α and TNF-α post-challenge. Conclusions: The tested vaccine elicited humoral and cellular immune responses and reduced viral presence in serum and tissues, demonstrating efficacy in a PCV2 subclinical infection model despite high MDA levels at the time of vaccination. Understanding both humoral and cellular immune responses according to different MDA levels can help design more effective vaccination strategies against PCV2.
Full article
(This article belongs to the Section Veterinary Vaccines)
►▼
Show Figures
Figure 1

Journal Menu
► ▼ Journal Menu-
- Vaccines Home
- Aims & Scope
- Editorial Board
- Reviewer Board
- Topical Advisory Panel
- Instructions for Authors
- Special Issues
- Topics
- Sections & Collections
- Article Processing Charge
- Indexing & Archiving
- Editor’s Choice Articles
- Most Cited & Viewed
- Journal Statistics
- Journal History
- Journal Awards
- Conferences
- Editorial Office
Journal Browser
► ▼ Journal BrowserHighly Accessed Articles
Latest Books
E-Mail Alert
News
Topics
Topic in
Animals, Arthropoda, Insects, Vaccines, Veterinary Sciences, Pathogens
Ticks and Tick-Borne Pathogens: 2nd Edition
Topic Editors: Alina Rodriguez-Mallon, Alejandro Cabezas-CruzDeadline: 31 March 2026

Conferences
Special Issues
Special Issue in
Vaccines
Viral Vector-Based Vaccines and Therapeutics
Guest Editor: Sujit PujhariDeadline: 31 October 2025
Special Issue in
Vaccines
Immunization Strategies for Animal Health
Guest Editors: Eva Calvo-Pinilla, Alejandro Marin-LopezDeadline: 31 October 2025
Special Issue in
Vaccines
Research in Vaccine Adjuvants: Innovations and Challenges
Guest Editors: Haibo Li, Hongwu SunDeadline: 31 October 2025
Special Issue in
Vaccines
Pandemic Influenza Vaccination
Guest Editors: Francisco Nogareda, Shoshanna GoldinDeadline: 31 October 2025
Topical Collections
Topical Collection in
Vaccines
Research on Monoclonal Antibodies and Antibody Engineering
Collection Editor: Tatsuya Yamazaki
Topical Collection in
Vaccines
COVID-19 Vaccine Development and Vaccination
Collection Editors: Ralph Tripp, Scott Anthony
Topical Collection in
Vaccines
Topic Advisory Panel Members’ Collection Series: Immunization and Vaccines for Infectious Diseases
Collection Editors: Shumaila Hanif, Ravinder Kumar








