Restricting O-Linked Glycosylation of the Mucin-like Domains Enhances Immunogenicity and Protective Efficacy of a Respiratory Syncytial Virus G Glycoprotein Vaccine Antigen
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Immunogens
2.3. Monoclonal Antibodies (mAbs) and Recombinant CCD-Fc Protein
2.4. Binding-Affinity Analysis
2.5. Mice
2.6. Virus
2.7. Vaccination
2.8. Detection of Anti-RSV Gecto and Anti-CCD Specific IgG by ELISA
2.9. Determining RSV Lung Titers by Plaque Assays
2.10. Detection of Anti-RSV/A2 Antibodies by ELISA
2.11. Bronchoalveolar Lavage (BAL) and Flow Cytometry
2.12. Microneutralization Assay
2.13. Statistical Analysis
3. Results
3.1. Expression and Purification of O-Glycan-Restricted RSV Gecto Glycoproteins
3.2. Antigenicity of O-Glycan-Restricted RSV Gecto Glycoproteins
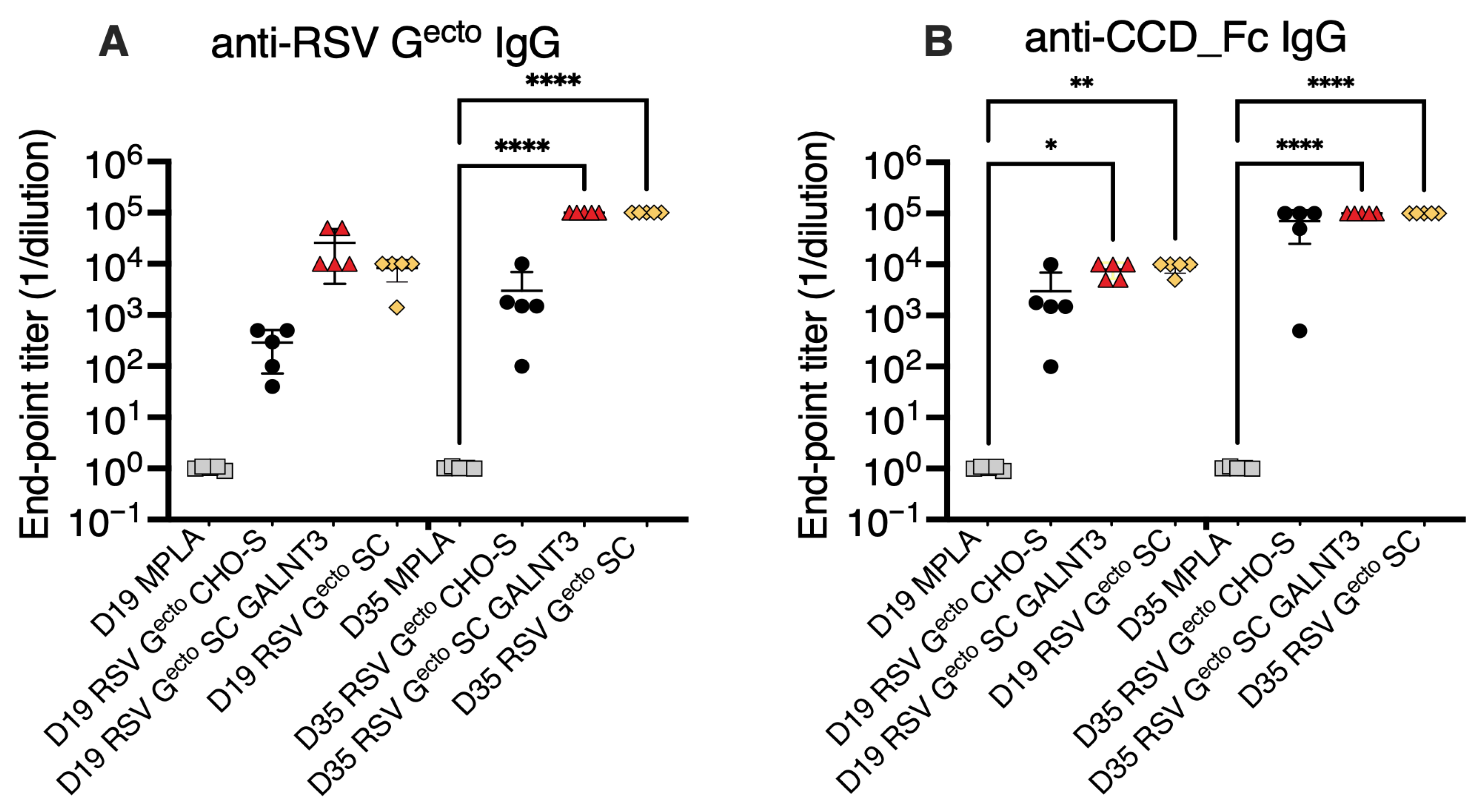
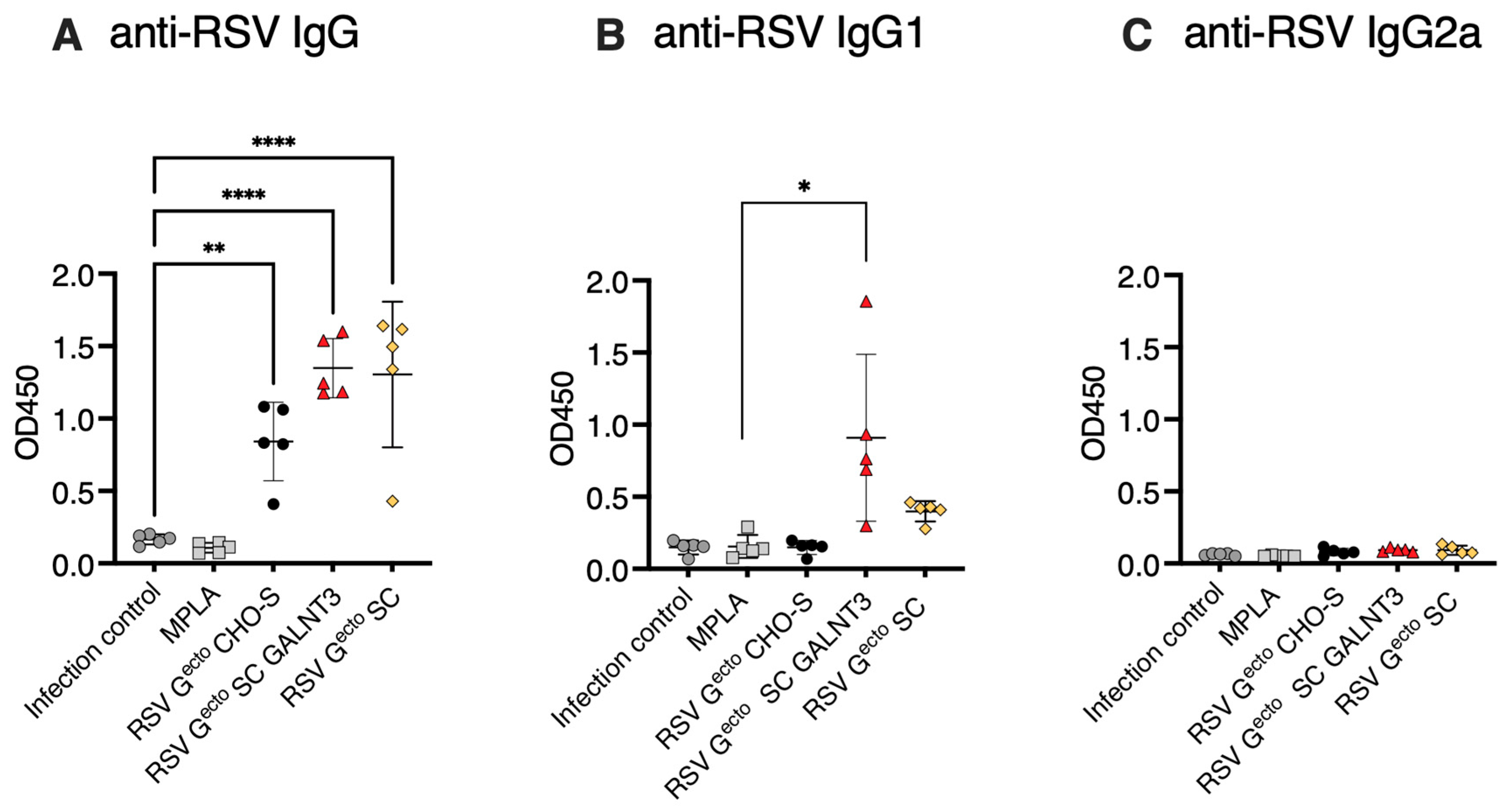
3.3. Immunogenicity of O-Glycan-Restricted RSV Gecto Protein Immunogens
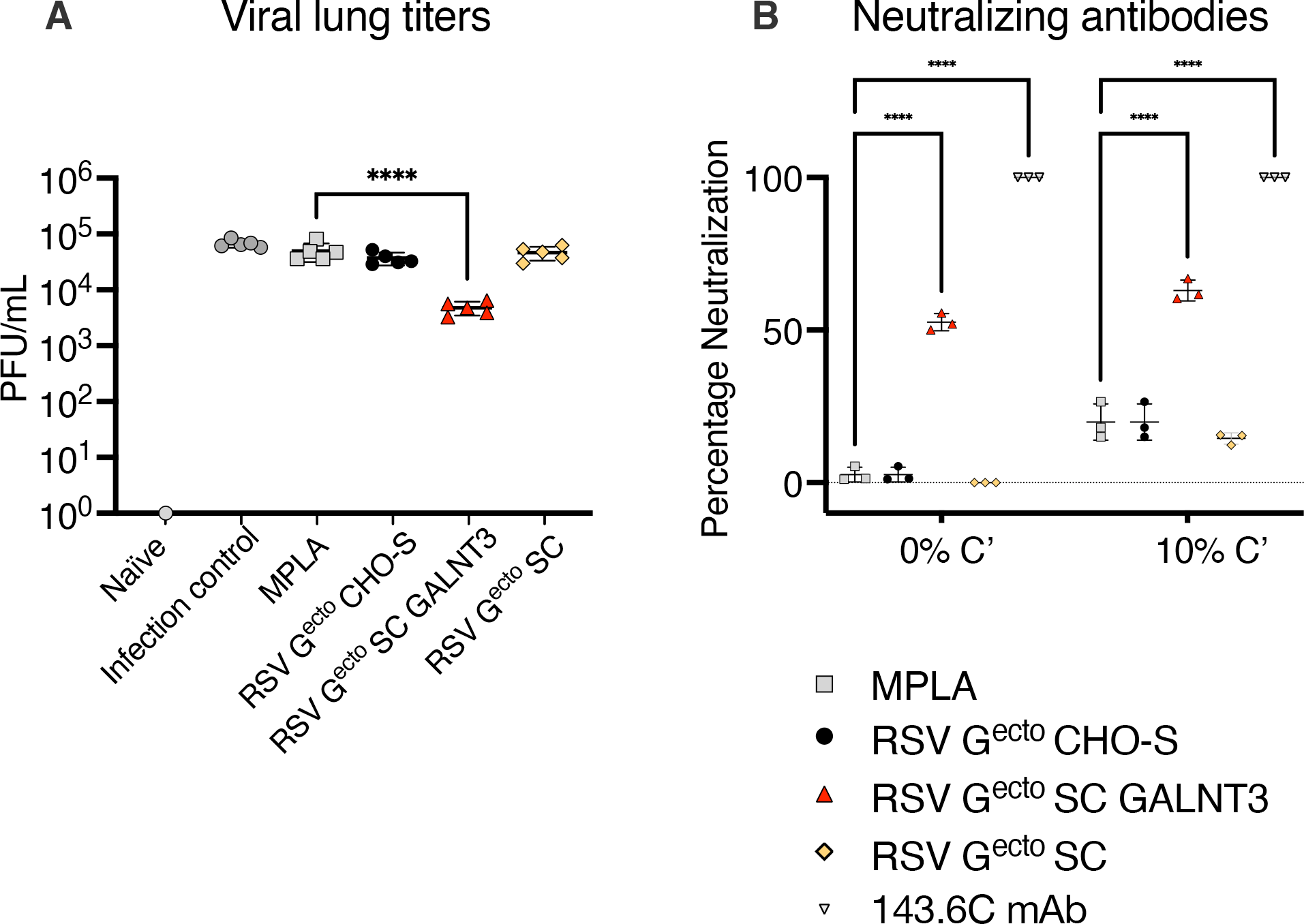
3.4. Evaluation of Post-RSV/A2 Challenge Responses
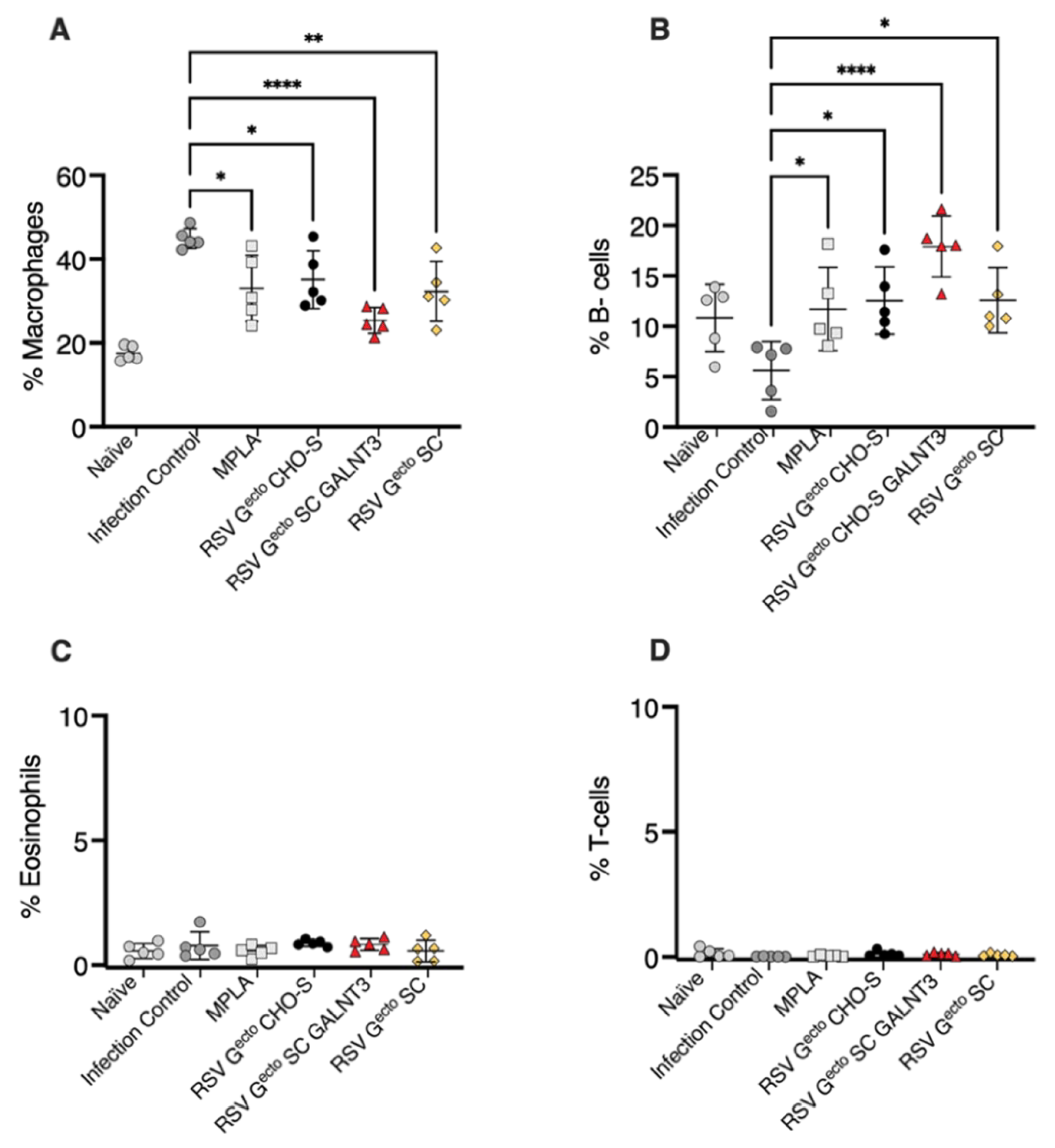
3.5. Evaluation of Lung Biomarkers Associated with Vaccine-Enhanced Disease
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Wang, X.; Blau, D.M.; Caballero, M.T.; Feikin, D.R.; Gill, C.J.; Madhi, S.A.; Omer, S.B.; Simoes, E.A.F.; Campbell, H.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet 2022, 399, 2047–2064. [Google Scholar] [CrossRef]
- McLellan, J.S.; Ray, W.C.; Peeples, M.E. Structure and function of respiratory syncytial virus surface glycoproteins. Curr. Top. Microbiol. Immunol. 2013, 372, 83–104. [Google Scholar] [PubMed]
- Kampmann, B.; Madhi, S.A.; Munjal, I.; Simoes, E.A.F.; Pahud, B.A.; Llapur, C.; Baker, J.; Perez Marc, G.; Radley, D.; Shittu, E.; et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N. Engl. J. Med. 2023, 388, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Ison, M.G.; Langley, J.M.; Lee, D.G.; Leroux-Roels, I.; Martinon-Torres, F.; Schwarz, T.F.; van Zyl-Smit, R.N.; Campora, L.; Dezutter, N.; et al. Respiratory syncytial virus prefusion F protein vaccine in older adults. N. Engl. J. Med. 2023, 388, 595–608. [Google Scholar] [CrossRef]
- Walsh, E.E.; Eiras, D.; Woodside, J.; Jiang, Q.; Patton, M.; Marc, G.P.; Llapur, C.; Ramet, M.; Fukushima, Y.; Hussen, N.; et al. Efficacy, immunogenicity, and safety of the bivalent RSV prefusion F (RSVpreF) vaccine in older adults over 2 RSV seasons. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2025, 388, 1465–1477 . [Google Scholar] [CrossRef]
- Wilson, E.; Goswami, J.; Baqui, A.H.; Doreski, P.A.; Perez-Marc, G.; Zaman, K.; Monroy, J.; Duncan, C.J.A.; Ujiie, M.; Ramet, M.; et al. Efficacy and safety of an mRNA-based RSV preF vaccine in older adults. N. Engl. J. Med. 2023, 389, 2233–2244. [Google Scholar] [CrossRef]
- Anderson, J.; Do, L.A.H.; van Kasteren, P.B.; Licciardi, P.V. The role of respiratory syncytial virus G protein in immune cell infection and pathogenesis. EBioMedicine 2024, 107, 105318. [Google Scholar] [CrossRef]
- Anderson, L.J.; Jadhao, S.J.; Paden, C.R.; Tong, S. Functional features of the respiratory syncytial virus G protein. Viruses 2021, 13, 1214. [Google Scholar] [CrossRef]
- Castilow, E.M.; Olson, M.R.; Varga, S.M. Understanding respiratory syncytial virus (RSV) vaccine-enhanced disease. Immunol. Res. 2007, 39, 225–239. [Google Scholar] [CrossRef]
- Shishir, T.A.; Saha, O.; Rajia, S.; Mondol, S.M.; Masum, M.H.U.; Rahaman, M.M.; Hossen, F.; Bahadur, N.M.; Ahmed, F.; Naser, I.B.; et al. Genome-wide study of globally distributed respiratory syncytial virus (RSV) strains implicates diversification utilizing phylodynamics and mutational analysis. Sci. Rep. 2023, 13, 13531. [Google Scholar] [CrossRef]
- Anderson, L.J.; Hierholzer, J.C.; Tsou, C.; Hendry, R.M.; Fernie, B.F.; Stone, Y.; McIntosh, K. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J. Infect. Dis. 1985, 151, 626–633. [Google Scholar] [CrossRef]
- Martinez, I.; Dopazo, J.; Melero, J.A. Antigenic structure of the human respiratory syncytial virus G glycoprotein and relevance of hypermutation events for the generation of antigenic variants. J. Gen. Virol. 1997, 78 Pt 10, 2419–2429. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, S.; Bally, M.; Trybala, E.; Bergstrom, T. Structure and role of O-linked glycans in viral envelope proteins. Annu. Rev. Virol. 2023, 10, 283–304. [Google Scholar] [CrossRef] [PubMed]
- Wandall, H.H.; Nielsen, M.A.I.; King-Smith, S.; de Haan, N.; Bagdonaite, I. Global functions of O-glycosylation: Promises and challenges in O-glycobiology. FEBS J. 2021, 288, 7183–7212. [Google Scholar] [CrossRef] [PubMed]
- Tripp, R.A.; Jones, L.P.; Haynes, L.M.; Zheng, H.; Murphy, P.M.; Anderson, L.J. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat. Immunol. 2001, 2, 732–738. [Google Scholar] [CrossRef]
- Imai, T.; Hieshima, K.; Haskell, C.; Baba, M.; Nagira, M.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Nomiyama, H.; Schall, T.J.; et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 1997, 91, 521–530. [Google Scholar] [CrossRef]
- Anderson, C.S.; Chirkova, T.; Slaunwhite, C.G.; Qiu, X.; Walsh, E.E.; Anderson, L.J.; Mariani, T.J. CX3CR1 engagement by respiratory syncytial virus leads to induction of nucleolin and dysregulation of cilia-related genes. J. Virol. 2021, 95, A1171. [Google Scholar] [CrossRef]
- Chirkova, T.; Lin, S.; Oomens, A.G.; Gaston, K.A.; Boyoglu-Barnum, S.; Meng, J.; Stobart, C.C.; Cotton, C.U.; Hartert, T.V.; Moore, M.L.; et al. CX3CR1 is an important surface molecule for respiratory syncytial virus infection in human airway epithelial cells. J. Gen. Virol. 2015, 96, 2543–2556. [Google Scholar] [CrossRef]
- Jeong, K.I.; Piepenhagen, P.A.; Kishko, M.; DiNapoli, J.M.; Groppo, R.P.; Zhang, L.; Almond, J.; Kleanthous, H.; Delagrave, S.; Parrington, M. CX3CR1 is expressed in differentiated human ciliated airway cells and co-localizes with respiratory syncytial virus on cilia in a G protein-dependent manner. PLoS ONE 2015, 10, e0130517. [Google Scholar] [CrossRef]
- Johnson, S.M.; McNally, B.A.; Ioannidis, I.; Flano, E.; Teng, M.N.; Oomens, A.G.; Walsh, E.E.; Peeples, M.E. Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. PLoS Pathog. 2015, 11, e1005318. [Google Scholar] [CrossRef]
- Zhivaki, D.; Lemoine, S.; Lim, A.; Morva, A.; Vidalain, P.O.; Schandene, L.; Casartelli, N.; Rameix-Welti, M.A.; Herve, P.L.; Deriaud, E.; et al. Respiratory syncytial virus infects regulatory B cells in human neonates via chemokine receptor CX3CR1 and promotes lung disease severity. Immunity 2017, 46, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Liu, H.; Li, X.; Ming, L. Preliminary functional and phylogeographic analyses of the 72 nucleotide duplication region in the emerging human respiratory syncytial virus ON1 strain attachment glycoprotein gene. Biomed. Pharmacother. 2020, 123, 109800. [Google Scholar] [CrossRef] [PubMed]
- Hotard, A.L.; Laikhter, E.; Brooks, K.; Hartert, T.V.; Moore, M.L. Functional analysis of the 60-nucleotide duplication in the respiratory syncytial virus buenos aires strain attachment glycoprotein. J. Virol. 2015, 89, 8258–8266. [Google Scholar] [CrossRef] [PubMed]
- Chirkova, T.; Boyoglu-Barnum, S.; Gaston, K.A.; Malik, F.M.; Trau, S.P.; Oomens, A.G.; Anderson, L.J. Respiratory syncytial virus G protein CX3C motif impairs human airway epithelial and immune cell responses. J. Virol. 2013, 87, 13466–13479. [Google Scholar] [CrossRef]
- Hancock, G.E.; Speelman, D.J.; Heers, K.; Bortell, E.; Smith, J.; Cosco, C. Generation of atypical pulmonary inflammatory responses in BALB/c mice after immunization with the native attachment (G) glycoprotein of respiratory syncytial virus. J. Virol. 1996, 70, 7783–7791. [Google Scholar] [CrossRef]
- Johnson, T.R.; Parker, R.A.; Johnson, J.E.; Graham, B.S. IL-13 is sufficient for respiratory syncytial virus G glycoprotein-induced eosinophilia after respiratory syncytial virus challenge. J. Immunol. 2003, 170, 2037–2045. [Google Scholar] [CrossRef]
- Johnson, T.R.; Varga, S.M.; Braciale, T.J.; Graham, B.S. Vβ14+ T cells mediate the vaccine-enhanced disease induced by immunization with respiratory syncytial virus (RSV) G glycoprotein but not with formalin-inactivated RSV. J. Virol. 2004, 78, 8753–8760. [Google Scholar] [CrossRef]
- Kawahara, E.; Senpuku, K.; Kawaguchi, Y.; Yamamoto, S.; Yasuda, K.; Kuroda, E.; Ouji-Sageshima, N.; Ito, T.; Hirai, T.; Shibata, T.; et al. Recombinant RSV G protein vaccine induces enhanced respiratory disease via IL-13 and mucin overproduction. npj Vaccines 2024, 9, 187. [Google Scholar] [CrossRef]
- Martinez, M.E.; Capella Gonzalez, C.; Huey, D.; Peeples, M.E.; McCarty, D.; Niewiesk, S. Immunogenicity and inflammatory properties of respiratory syncytial virus attachment G protein in cotton rats. PLoS ONE 2021, 16, e0246770. [Google Scholar] [CrossRef]
- Polack, F.P.; Teng, M.N.; Collins, P.L.; Prince, G.A.; Exner, M.; Regele, H.; Lirman, D.D.; Rabold, R.; Hoffman, S.J.; Karp, C.L.; et al. A role for immune complexes in enhanced respiratory syncytial virus disease. J. Exp. Med. 2002, 196, 859–865. [Google Scholar] [CrossRef]
- Stott, E.J.; Taylor, G.; Ball, L.A.; Anderson, K.; Young, K.K.; King, A.M.; Wertz, G.W. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J. Virol. 1987, 61, 3855–3861. [Google Scholar] [CrossRef]
- Bergeron, H.C.; Tripp, R.A. Immunopathology of RSV: An updated review. Viruses 2021, 13, 2478. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Mason, C.S.; Jones, L.P.; Crabtree, J.; Jorquera, P.A.; Tripp, R.A. Antibodies to the central conserved region of respiratory syncytial virus (RSV) G protein block RSV G protein CX3C-CX3CR1 binding and cross-neutralize RSV A and B strains. Viral Immunol. 2012, 25, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Collarini, E.J.; Lee, F.E.; Foord, O.; Park, M.; Sperinde, G.; Wu, H.; Harriman, W.D.; Carroll, S.F.; Ellsworth, S.L.; Anderson, L.J.; et al. Potent high-affinity antibodies for treatment and prophylaxis of respiratory syncytial virus derived from B cells of infected patients. J. Immunol. 2009, 183, 6338–6345. [Google Scholar] [CrossRef] [PubMed]
- Cortjens, B.; Yasuda, E.; Yu, X.; Wagner, K.; Claassen, Y.B.; Bakker, A.Q.; van Woensel, J.B.M.; Beaumont, T. Broadly reactive anti-respiratory syncytial virus G antibodies from exposed individuals effectively inhibit infection of primary airway epithelial cells. J. Virol. 2017, 91, e02357-16. [Google Scholar] [CrossRef]
- Fedechkin, S.O.; George, N.L.; Wolff, J.T.; Kauvar, L.M.; DuBois, R.M. Structures of respiratory syncytial virus G antigen bound to broadly neutralizing antibodies. Sci. Immunol. 2018, 3, eaar3534. [Google Scholar] [CrossRef]
- Harcourt, J.L.; Karron, R.A.; Tripp, R.A. Anti-G protein antibody responses to respiratory syncytial virus infection or vaccination are associated with inhibition of G protein CX3C-CX3CR1 binding and leukocyte chemotaxis. J. Infect. Dis. 2004, 190, 1936–1940. [Google Scholar] [CrossRef]
- Boyoglu-Barnum, S.; Gaston, K.A.; Todd, S.O.; Boyoglu, C.; Chirkova, T.; Barnum, T.R.; Jorquera, P.; Haynes, L.M.; Tripp, R.A.; Moore, M.L.; et al. A respiratory syncytial virus (RSV) anti-G protein F(ab′)2 monoclonal antibody suppresses mucous production and breathing effort in RSV rA2-line19F-infected BALB/c mice. J. Virol. 2013, 87, 10955–10967. [Google Scholar] [CrossRef]
- Boyoglu-Barnum, S.; Todd, S.O.; Chirkova, T.; Barnum, T.R.; Gaston, K.A.; Haynes, L.M.; Tripp, R.A.; Moore, M.L.; Anderson, L.J. An anti-G protein monoclonal antibody treats RSV disease more effectively than an anti-F monoclonal antibody in BALB/c mice. Virology 2015, 483, 117–125. [Google Scholar] [CrossRef]
- Caidi, H.; Harcourt, J.L.; Tripp, R.A.; Anderson, L.J.; Haynes, L.M. Combination therapy using monoclonal antibodies against respiratory syncytial virus (RSV) G glycoprotein protects from RSV disease in BALB/c mice. PLoS ONE 2012, 7, e51485. [Google Scholar] [CrossRef]
- Caidi, H.; Miao, C.; Thornburg, N.J.; Tripp, R.A.; Anderson, L.J.; Haynes, L.M. Anti-respiratory syncytial virus (RSV) G monoclonal antibodies reduce lung inflammation and viral lung titers when delivered therapeutically in a BALB/c mouse model. Antivir. Res. 2018, 154, 149–157. [Google Scholar] [CrossRef]
- Haynes, L.M.; Caidi, H.; Radu, G.U.; Miao, C.; Harcourt, J.L.; Tripp, R.A.; Anderson, L.J. Therapeutic monoclonal antibody treatment targeting respiratory syncytial virus (RSV) G protein mediates viral clearance and reduces the pathogenesis of RSV infection in BALB/c mice. J. Infect. Dis. 2009, 200, 439–447. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, J.Y.; Park, M.H.; Kim, J.Y.; Chang, J. Monoclonal antibody against G glycoprotein increases respiratory syncytial virus clearance in vivo and prevents vaccine-enhanced diseases. PLoS ONE 2017, 12, e0169139. [Google Scholar] [CrossRef] [PubMed]
- Miao, C.; Radu, G.U.; Caidi, H.; Tripp, R.A.; Anderson, L.J.; Haynes, L.M. Treatment with respiratory syncytial virus G glycoprotein monoclonal antibody or F(ab′)2 components mediates reduced pulmonary inflammation in mice. J. Gen. Virol. 2009, 90, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Choi, Y.; Haynes, L.M.; Harcourt, J.L.; Anderson, L.J.; Jones, L.P.; Tripp, R.A. Vaccination to induce antibodies blocking the CX3C-CX3CR1 interaction of respiratory syncytial virus G protein reduces pulmonary inflammation and virus replication in mice. J. Virol. 2010, 84, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Boukhvalova, M.S.; Yim, K.C.; Blanco, J. Cotton rat model for testing vaccines and antivirals against respiratory syncytial virus. Antivir. Chem. Chemother. 2018, 26, 2040206618770518. [Google Scholar] [CrossRef]
- Capella, C.; Chaiwatpongsakorn, S.; Gorrell, E.; Risch, Z.A.; Ye, F.; Mertz, S.E.; Johnson, S.M.; Moore-Clingenpeel, M.; Ramilo, O.; Mejias, A.; et al. Prefusion F, postfusion F, G antibodies and disease severity in infants and young children with acute respiratory syncytial virus infection. J. Infect. Dis. 2017, 216, 1398–1406. [Google Scholar] [CrossRef]
- Fuentes, S.; Coyle, E.M.; Beeler, J.; Golding, H.; Khurana, S. Antigenic fingerprinting following primary RSV infection in young children identifies novel antigenic sites and reveals unlinked evolution of human antibody repertoires to fusion and attachment glycoproteins. PLoS Pathog. 2016, 12, e1005554. [Google Scholar] [CrossRef]
- Kishko, M.; Catalan, J.; Swanson, K.; DiNapoli, J.; Wei, C.J.; Delagrave, S.; Chivukula, S.; Zhang, L. Evaluation of the respiratory syncytial virus G-directed neutralizing antibody response in the human airway epithelial cell model. Virology 2020, 550, 21–26. [Google Scholar] [CrossRef]
- Nziza, N.; Jung, W.; Mendu, M.; Chen, T.; Julg, B.; Graham, B.; Ramilo, O.; Mejias, A.; Alter, G. Longitudinal humoral analysis in RSV-infected infants identifies pre-existing RSV strain-specific G and evolving cross-reactive F antibodies. Immunity 2024, 57, 1681–1695.e4. [Google Scholar] [CrossRef]
- Shinoff, J.J.; O’Brien, K.L.; Thumar, B.; Shaw, J.B.; Reid, R.; Hua, W.; Santosham, M.; Karron, R.A. Young infants can develop protective levels of neutralizing antibody after infection with respiratory syncytial virus. J. Infect. Dis. 2008, 198, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Falsey, A.R. Humoral and mucosal immunity in protection from natural respiratory syncytial virus infection in adults. J. Infect. Dis. 2004, 190, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Klenow, L.; Coyle, E.M.; Grubbs, G.; Golding, H.; Khurana, S. Monoclonal antibodies targeting sites in respiratory syncytial virus attachment G protein provide protection against RSV-A and RSV-B in mice. Nat. Commun. 2024, 15, 2900. [Google Scholar] [CrossRef] [PubMed]
- Boukhvalova, M.S.; Prince, G.A.; Soroush, L.; Harrigan, D.C.; Vogel, S.N.; Blanco, J.C. The TLR4 agonist, monophosphoryl lipid A, attenuates the cytokine storm associated with respiratory syncytial virus vaccine-enhanced disease. Vaccine 2006, 24, 5027–5035. [Google Scholar] [CrossRef]
- Cheng, X.; Zhao, G.; Dong, A.; He, Z.; Wang, J.; Jiang, B.; Wang, B.; Wang, M.; Huai, X.; Zhang, S.; et al. A first-in-human trial to evaluate the safety and immunogenicity of a G protein-based recombinant respiratory syncytial virus vaccine in healthy adults 18–45 years of age. Vaccines 2023, 11, 999. [Google Scholar] [CrossRef]
- Hancock, G.E.; Heers, K.M.; Pryharski, K.S.; Smith, J.D.; Tiberio, L. Adjuvants recognized by toll-like receptors inhibit the induction of polarized type 2 T cell responses by natural attachment (G) protein of respiratory syncytial virus. Vaccine 2003, 21, 4348–4358. [Google Scholar] [CrossRef]
- Kawahara, E.; Shibata, T.; Hirai, T.; Yoshioka, Y. Non-glycosylated G protein with CpG ODN provides robust protection against respiratory syncytial virus without inducing eosinophilia. Front. Immunol. 2023, 14, 1282016. [Google Scholar] [CrossRef]
- Li, C.; Zhou, X.; Zhong, Y.; Li, C.; Dong, A.; He, Z.; Zhang, S.; Wang, B. A recombinant G protein plus cyclosporine A-based respiratory syncytial virus vaccine elicits humoral and regulatory T cell responses against infection without vaccine-enhanced disease. J. Immunol. 2016, 196, 1721–1731. [Google Scholar] [CrossRef]
- Garcia-Beato, R.; Martinez, I.; Franci, C.; Real, F.X.; Garcia-Barreno, B.; Melero, J.A. Host cell effect upon glycosylation and antigenicity of human respiratory syncytial virus G glycoprotein. Virology 1996, 221, 301–309. [Google Scholar] [CrossRef]
- Kwilas, S.; Liesman, R.M.; Zhang, L.; Walsh, E.; Pickles, R.J.; Peeples, M.E. Respiratory syncytial virus grown in vero cells contains a truncated attachment protein that alters its infectivity and dependence on glycosaminoglycans. J. Virol. 2009, 83, 10710–10718. [Google Scholar] [CrossRef]
- Olofsson, S.; Blixt, O.; Bergstrom, T.; Frank, M.; Wandall, H.H. Viral O-GalNAc peptide epitopes: A novel potential target in viral envelope glycoproteins. Rev. Med. Virol. 2016, 26, 34–48. [Google Scholar] [CrossRef]
- Palomo, C.; Cane, P.A.; Melero, J.A. Evaluation of the antibody specificities of human convalescent-phase sera against the attachment (G) protein of human respiratory syncytial virus: Influence of strain variation and carbohydrate side chains. J. Med. Virol. 2000, 60, 468–474. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, G.; Cheng, X.; Wang, S.; Wang, J.; Huai, X.; Xia, Y.; Xiao, Y.; Ren, S.; Zhang, S.; et al. A phase ii random, double-blind, placebo-controlled study of the safety and immunogenicity of a recombinant G protein-based respiratory syncytial virus vaccine in healthy older adults. Vaccines 2025, 13, 885. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Lee, J.H.; Doores, K.J.; Murin, C.D.; Julien, J.P.; McBride, R.; Liu, Y.; Marozsan, A.; Cupo, A.; Klasse, P.J.; et al. Supersite of immune vulnerability on the glycosylated face of HIV-1 envelope glycoprotein gp120. Nat. Struct. Mol. Biol. 2013, 20, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Byrne, G.; O’Rourke, S.M.; Alexander, D.L.; Yu, B.; Doran, R.C.; Wright, M.; Chen, Q.; Azadi, P.; Berman, P.W. CRISPR/Cas9 gene editing for the creation of an MGAT1-deficient CHO cell line to control HIV-1 vaccine glycosylation. PLoS Biol. 2018, 16, e2005817. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.M.; Huber, M.; Doores, K.J.; Falkowska, E.; Pejchal, R.; Julien, J.P.; Wang, S.K.; Ramos, A.; Chan-Hui, P.Y.; Moyle, M.; et al. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 2011, 477, 466–470. [Google Scholar] [CrossRef]
- Clo, E.; Kracun, S.K.; Nudelman, A.S.; Jensen, K.J.; Liljeqvist, J.A.; Olofsson, S.; Bergstrom, T.; Blixt, O. Characterization of the viral O-glycopeptidome: A novel tool of relevance for vaccine design and serodiagnosis. J. Virol. 2012, 86, 6268–6278. [Google Scholar] [CrossRef]
- D’Arrigo, I.; Clo, E.; Bergstrom, T.; Olofsson, S.; Blixt, O. Diverse IgG serum response to novel glycopeptide epitopes detected within immunodominant stretches of Epstein-Barr virus glycoprotein 350/220: Diagnostic potential of O-glycopeptide microarrays. Glycoconj. J. 2013, 30, 633–640. [Google Scholar] [CrossRef]
- Steentoft, C.; Vakhrushev, S.Y.; Joshi, H.J.; Kong, Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Lavrsen, K.; Dabelsteen, S.; Pedersen, N.B.; Marcos-Silva, L.; et al. Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology. EMBO J. 2013, 32, 1478–1488. [Google Scholar] [CrossRef]
- Steentoft, C.; Vakhrushev, S.Y.; Vester-Christensen, M.B.; Schjoldager, K.T.; Kong, Y.; Bennett, E.P.; Mandel, U.; Wandall, H.; Levery, S.B.; Clausen, H. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat. Methods 2011, 8, 977–982. [Google Scholar] [CrossRef]
- Yang, Z.; Halim, A.; Narimatsu, Y.; Jitendra Joshi, H.; Steentoft, C.; Schjoldager, K.T.; Alder Schulz, M.; Sealover, N.R.; Kayser, K.J.; Paul Bennett, E.; et al. The GalNAc-type O-glycoproteome of CHO cells characterized by the SimpleCell strategy. Mol. Cell. Proteom. MCP 2014, 13, 3224–3235. [Google Scholar] [CrossRef]
- Clausen, H.; Wandall, H.H.; DeLisa, M.P.; Stanley, P.; Schnaar, R.L. Glycosylation engineering. In Essentials of Glycobiology, 4th ed.; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Mohnen, D., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2022. [Google Scholar]
- Kato, K.; Jeanneau, C.; Tarp, M.A.; Benet-Pages, A.; Lorenz-Depiereux, B.; Bennett, E.P.; Mandel, U.; Strom, T.M.; Clausen, H. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis. Secretion of fibroblast growth factor 23 requires O-glycosylation. J. Biol. Chem. 2006, 281, 18370–18377. [Google Scholar] [CrossRef]
- Gill, D.J.; Clausen, H.; Bard, F. Location, location, location: New insights into O-GalNAc protein glycosylation. Trends Cell Biol. 2011, 21, 149–158. [Google Scholar] [CrossRef]
- Peluso, G.; Tian, E.; Abusleme, L.; Munemasa, T.; Mukaibo, T.; Ten Hagen, K.G. Loss of the disease-associated glycosyltransferase Galnt3 alters Muc10 glycosylation and the composition of the oral microbiome. J. Biol. Chem. 2020, 295, 1411–1425. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, I.; Garg, R.; van Drunen Littel-van den Hurk, S. Selection of adjuvants for vaccines targeting specific pathogens. Expert Rev. Vaccines 2019, 18, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Mountford, A.P.; Fisher, A.; Wilson, R.A. The profile of IgG1 and IgG2a antibody responses in mice exposed to Schistosoma mansoni. Parasite Immunol. 1994, 16, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Schjoldager, K.T.; Joshi, H.J.; Kong, Y.; Goth, C.K.; King, S.L.; Wandall, H.H.; Bennett, E.P.; Vakhrushev, S.Y.; Clausen, H. Deconstruction of O-glycosylation—GalNAc-T isoforms direct distinct subsets of the O-glycoproteome. EMBO Rep. 2015, 16, 1713–1722. [Google Scholar] [CrossRef]
- Boukhvalova, M.S.; Mbaye, A.; Kovtun, S.; Yim, K.C.; Konstantinova, T.; Getachew, T.; Khurana, S.; Falsey, A.R.; Blanco, J.C.G. Improving ability of RSV microneutralization assay to detect G-specific and cross-reactive neutralizing antibodies through immortalized cell line selection. Vaccine 2018, 36, 4657–4662. [Google Scholar] [CrossRef]
- Anderson, L.J.; Bingham, P.; Hierholzer, J.C. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J. Virol. 1988, 62, 4232–4238. [Google Scholar] [CrossRef]
- Juarez, M.G.; O’Rourke, S.M.; Dzimianski, J.V.; Gagnon, D.; Penunuri, G.; Serrao, V.H.B.; Corbett-Detig, R.B.; Kauvar, L.M.; DuBois, R.M. Structures of respiratory syncytial virus G bound to broadly reactive antibodies provide insights into vaccine design. Sci. Rep. 2025, 15, 8666. [Google Scholar] [CrossRef]
- Nuñez Castrejon, A.M.; O’Rourke, S.M.; Kauvar, L.M.; DuBois, R.M. Structure-based design and antigenic validation of respiratory syncytial virus G immunogens. J. Virol. 2022, 96, e0220121. [Google Scholar] [CrossRef] [PubMed]
- Burg, J.S.; Ingram, J.R.; Venkatakrishnan, A.J.; Jude, K.M.; Dukkipati, A.; Feinberg, E.N.; Angelini, A.; Waghray, D.; Dror, R.O.; Ploegh, H.L.; et al. Structural biology. Structural basis for chemokine recognition and activation of a viral G protein-coupled receptor. Science 2015, 347, 1113–1117. [Google Scholar] [CrossRef]
- Tripp, R.A.; Moore, D.; Jones, L.; Sullender, W.; Winter, J.; Anderson, L.J. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J. Virol. 1999, 73, 7099–7107. [Google Scholar] [CrossRef]
- Shambaugh, C.; Azshirvani, S.; Yu, L.; Pache, J.; Lambert, S.L.; Zuo, F.; Esser, M.T. Development of a high-throughput respiratory syncytial virus fluorescent focus-based microneutralization assay. Clin. Vaccine Immunol. CVI 2017, 24, e00225-17. [Google Scholar] [CrossRef]
- Altamirano-Lagos, M.J.; Diaz, F.E.; Mansilla, M.A.; Rivera-Perez, D.; Soto, D.; McGill, J.L.; Vasquez, A.E.; Kalergis, A.M. Current animal models for understanding the pathology caused by the respiratory syncytial virus. Front. Microbiol. 2019, 10, 873. [Google Scholar] [CrossRef]
- Graham, B.S.; Perkins, M.D.; Wright, P.F.; Karzon, D.T. Primary respiratory syncytial virus infection in mice. J. Med. Virol. 1988, 26, 153–162. [Google Scholar] [CrossRef]
- Openshaw, P.J.; Tregoning, J.S. Immune responses and disease enhancement during respiratory syncytial virus infection. Clin. Microbiol. Rev. 2005, 18, 541–555. [Google Scholar] [CrossRef]
- Schulte, S.; Sukhova, G.K.; Libby, P. Genetically programmed biases in Th1 and Th2 immune responses modulate atherogenesis. Am. J. Pathol. 2008, 172, 1500–1508. [Google Scholar] [CrossRef]
- Fuentes, S.; Coyle, E.M.; Golding, H.; Khurana, S. Nonglycosylated G-protein vaccine protects against homologous and heterologous respiratory syncytial virus (RSV) challenge, while glycosylated G enhances RSV lung pathology and cytokine levels. J. Virol. 2015, 89, 8193–8205. [Google Scholar] [CrossRef] [PubMed]
- Aegerter, H.; Lambrecht, B.N.; Jakubzick, C.V. Biology of lung macrophages in health and disease. Immunity 2022, 55, 1564–1580. [Google Scholar] [CrossRef] [PubMed]
- Norden, R.; Nilsson, J.; Samuelsson, E.; Risinger, C.; Sihlbom, C.; Blixt, O.; Larson, G.; Olofsson, S.; Bergstrom, T. Recombinant glycoprotein e of varicella zoster virus contains glycan-peptide motifs that modulate B cell epitopes into discrete immunological signatures. Int. J. Mol. Sci. 2019, 20, 954. [Google Scholar] [CrossRef]
- Fuentes, S.; Klenow, L.; Golding, H.; Khurana, S. Preclinical evaluation of bacterially produced RSV-G protein vaccine: Strong protection against RSV challenge in cotton rat model. Sci. Rep. 2017, 7, 42428. [Google Scholar] [CrossRef]
- Lee, J.; Klenow, L.; Coyle, E.M.; Golding, H.; Khurana, S. Protective antigenic sites in respiratory syncytial virus G attachment protein outside the central conserved and cysteine noose domains. PLoS Pathog. 2018, 14, e1007262. [Google Scholar] [CrossRef]
- Spiess, M. The asialoglycoprotein receptor: A model for endocytic transport receptors. Biochemistry 1990, 29, 10009–10018. [Google Scholar] [CrossRef]
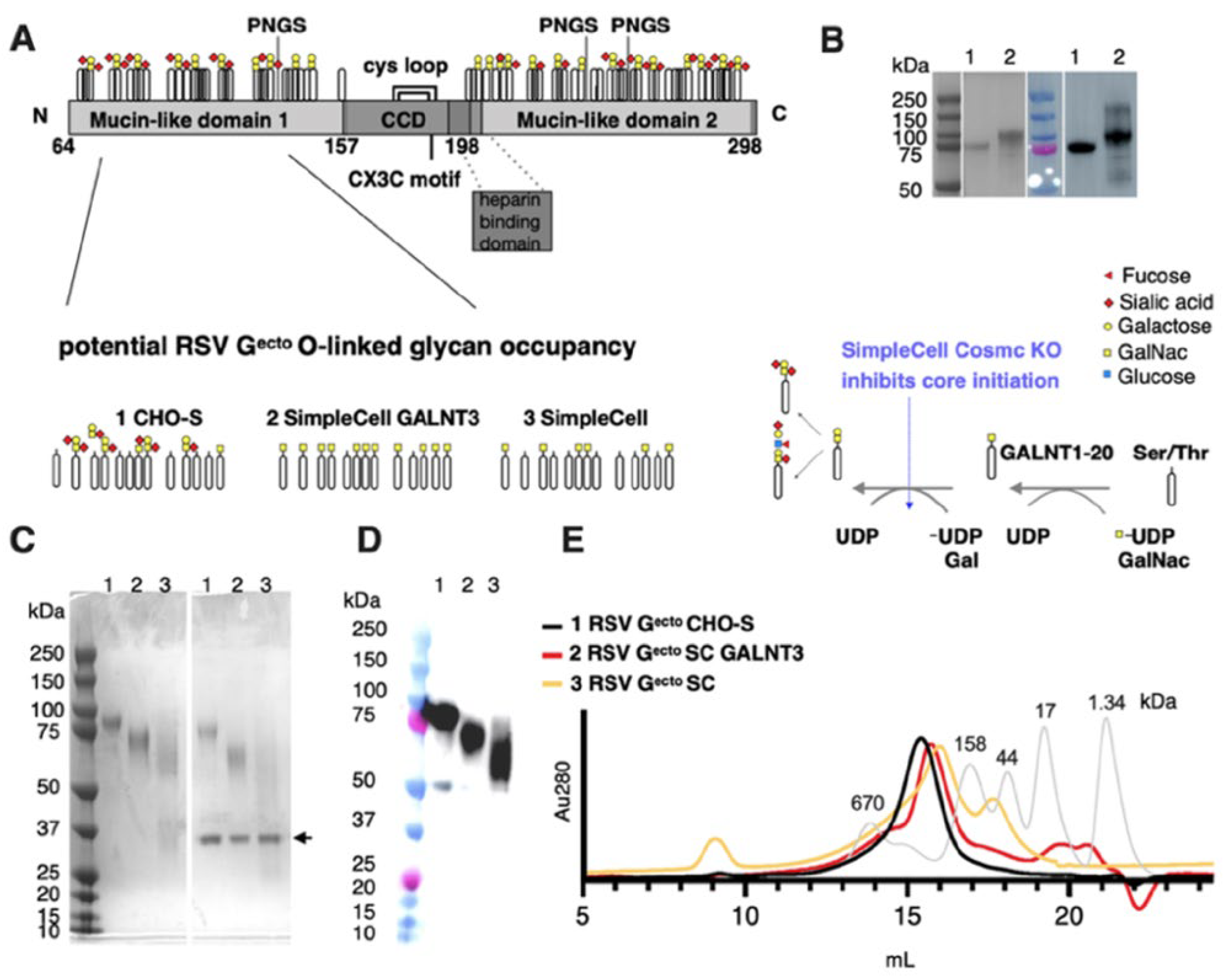
| mAb | KD (pM) * | KD SD (pM) | ka (105 M−1s−1) | kd (10−7s−1) | R2 | x2 | |
|---|---|---|---|---|---|---|---|
| CHO-S Gecto | 3D3 | <1.0 | 3.0 | 1.00 | <1 | 0.9927 | 0.0562 |
| CHO-S Gecto | 2D10 | <1.0 | 1.7 | 5.00 | <1 | 0.9947 | 0.1022 |
| SC GALNT3 Gecto | 3D3 | 1.0 | <1.0 | 6.00 | <1 | 0.9906 | 0.0667 |
| SC GALNT3 Gecto | 2D10 | <1.0 | <1.0 | 2.00 | <1 | 0.9943 | 0.0468 |
| SC Gecto | 3D3 | <1.0 | <1.0 | 8.00 | <1 | 0.9738 | 0.1689 |
| SC Gecto | 2D10 | 2.0 | 1.6 | 5.00 | <1 | 0.9888 | 0.0300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Rourke, S.M.; Murray, J.; Juarez, M.G.; Tripp, R.A.; DuBois, R.M. Restricting O-Linked Glycosylation of the Mucin-like Domains Enhances Immunogenicity and Protective Efficacy of a Respiratory Syncytial Virus G Glycoprotein Vaccine Antigen. Vaccines 2025, 13, 1004. https://doi.org/10.3390/vaccines13101004
O’Rourke SM, Murray J, Juarez MG, Tripp RA, DuBois RM. Restricting O-Linked Glycosylation of the Mucin-like Domains Enhances Immunogenicity and Protective Efficacy of a Respiratory Syncytial Virus G Glycoprotein Vaccine Antigen. Vaccines. 2025; 13(10):1004. https://doi.org/10.3390/vaccines13101004
Chicago/Turabian StyleO’Rourke, Sara M., Jackelyn Murray, Maria G. Juarez, Ralph A. Tripp, and Rebecca M. DuBois. 2025. "Restricting O-Linked Glycosylation of the Mucin-like Domains Enhances Immunogenicity and Protective Efficacy of a Respiratory Syncytial Virus G Glycoprotein Vaccine Antigen" Vaccines 13, no. 10: 1004. https://doi.org/10.3390/vaccines13101004
APA StyleO’Rourke, S. M., Murray, J., Juarez, M. G., Tripp, R. A., & DuBois, R. M. (2025). Restricting O-Linked Glycosylation of the Mucin-like Domains Enhances Immunogenicity and Protective Efficacy of a Respiratory Syncytial Virus G Glycoprotein Vaccine Antigen. Vaccines, 13(10), 1004. https://doi.org/10.3390/vaccines13101004







