Hemocyanins: Microscopic Giants with Unique Structural Features for Applications in Biomedicine
Abstract
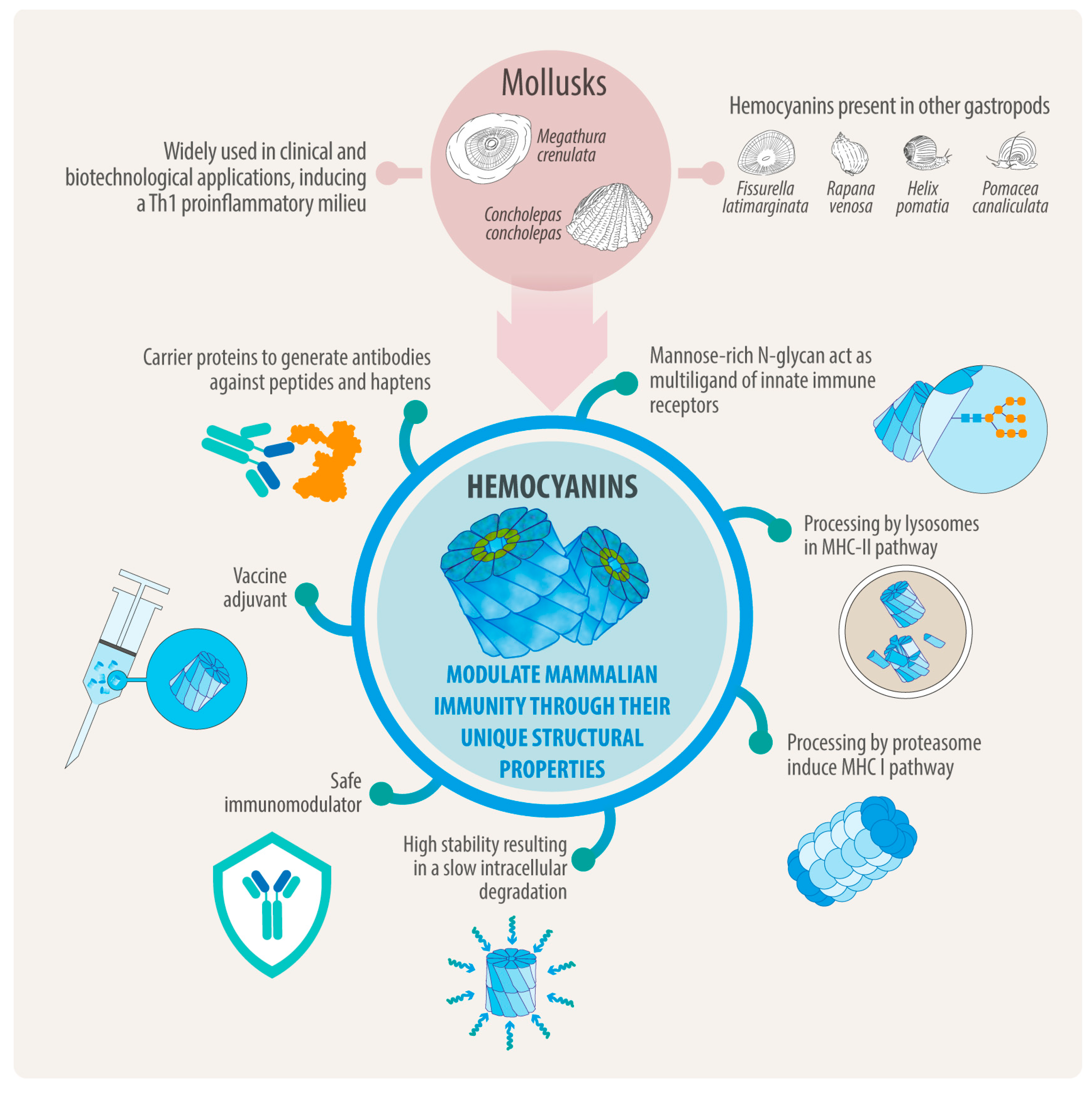
1. Introduction
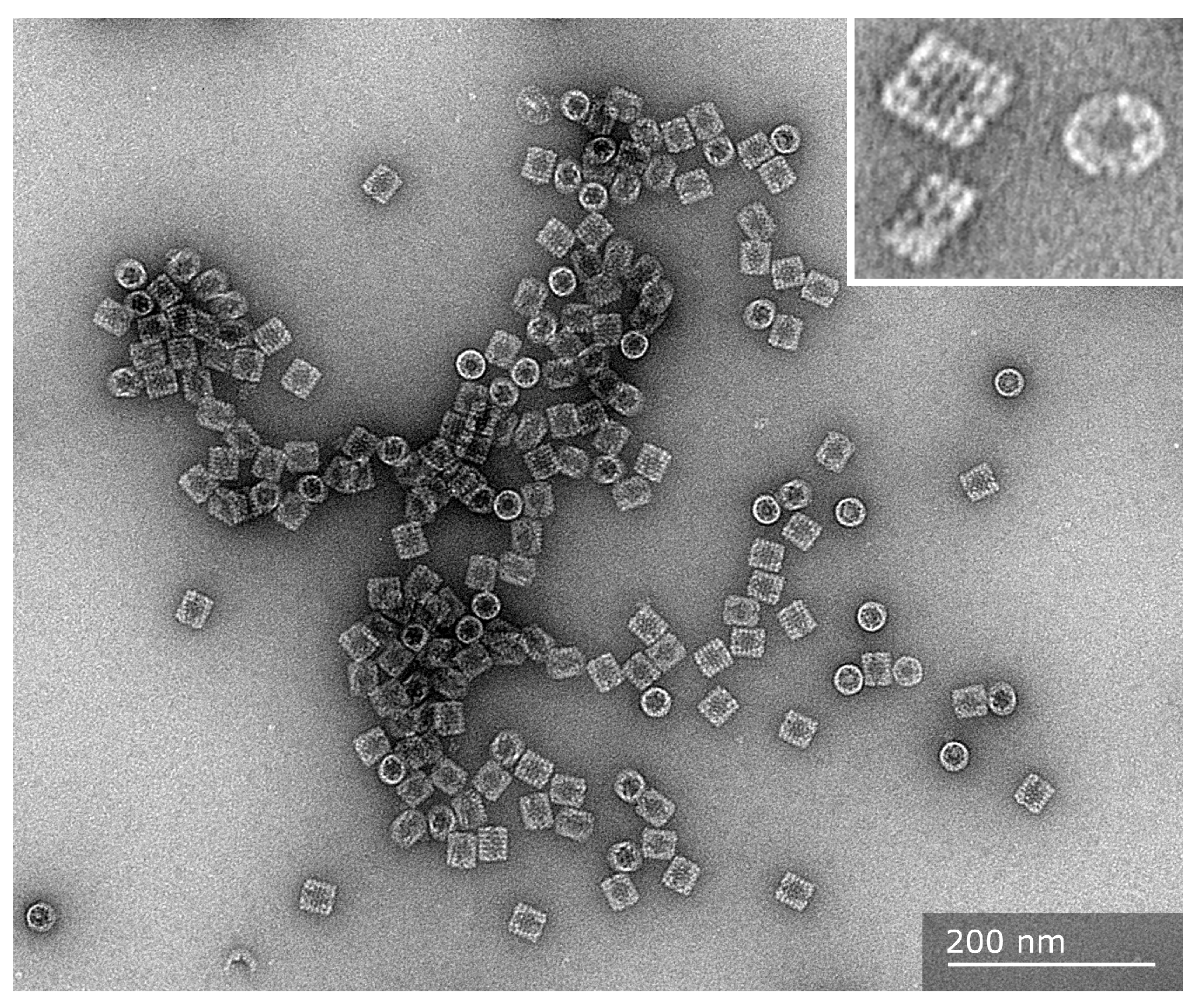
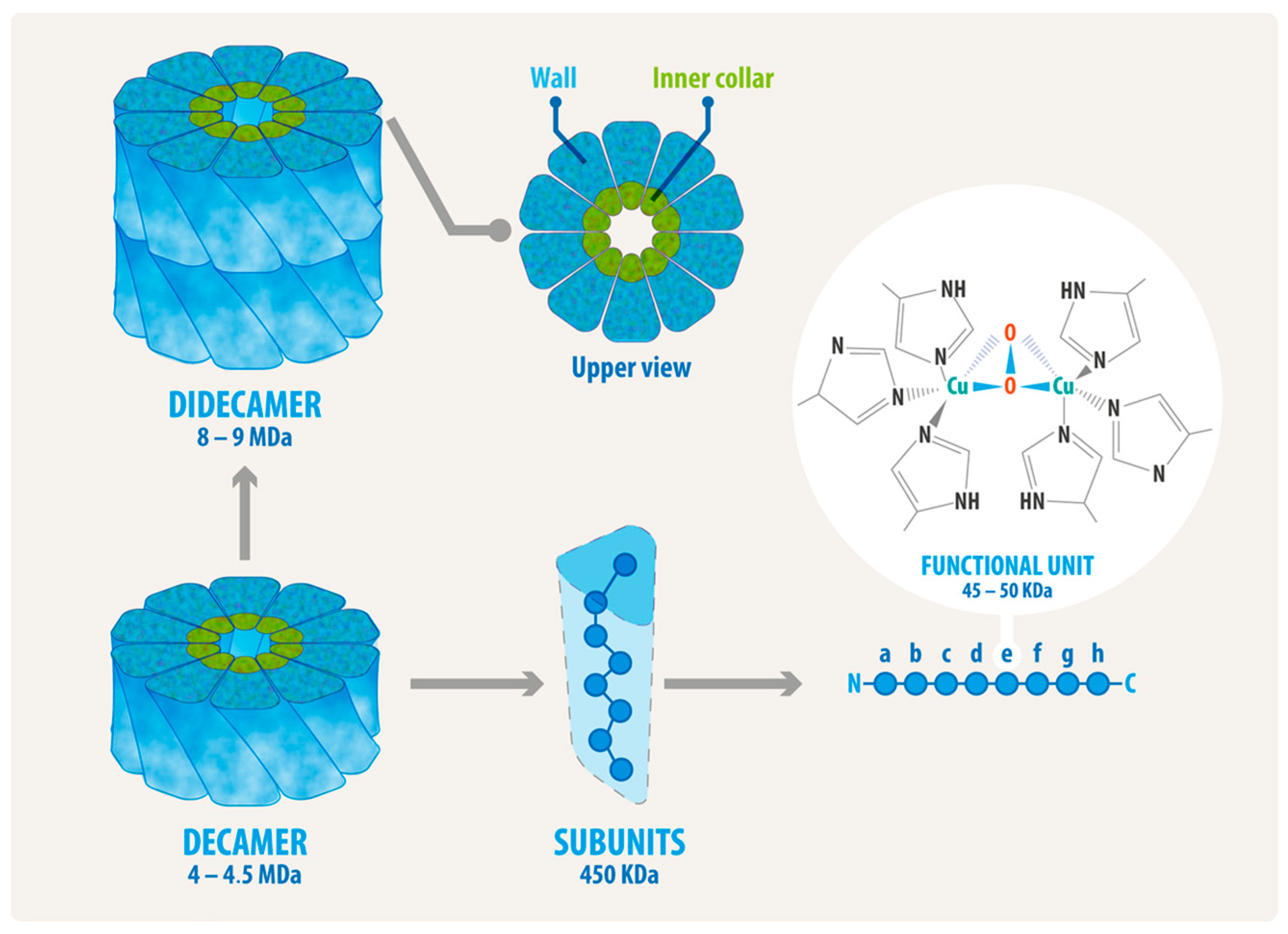
2. Hemocyanin Exhibits Distinctive Structural Characteristics
3. Hemocyanin Size and Structure Disassembly Has No Significant Impact on Immunogenicity, but N-Deglycosylation Does
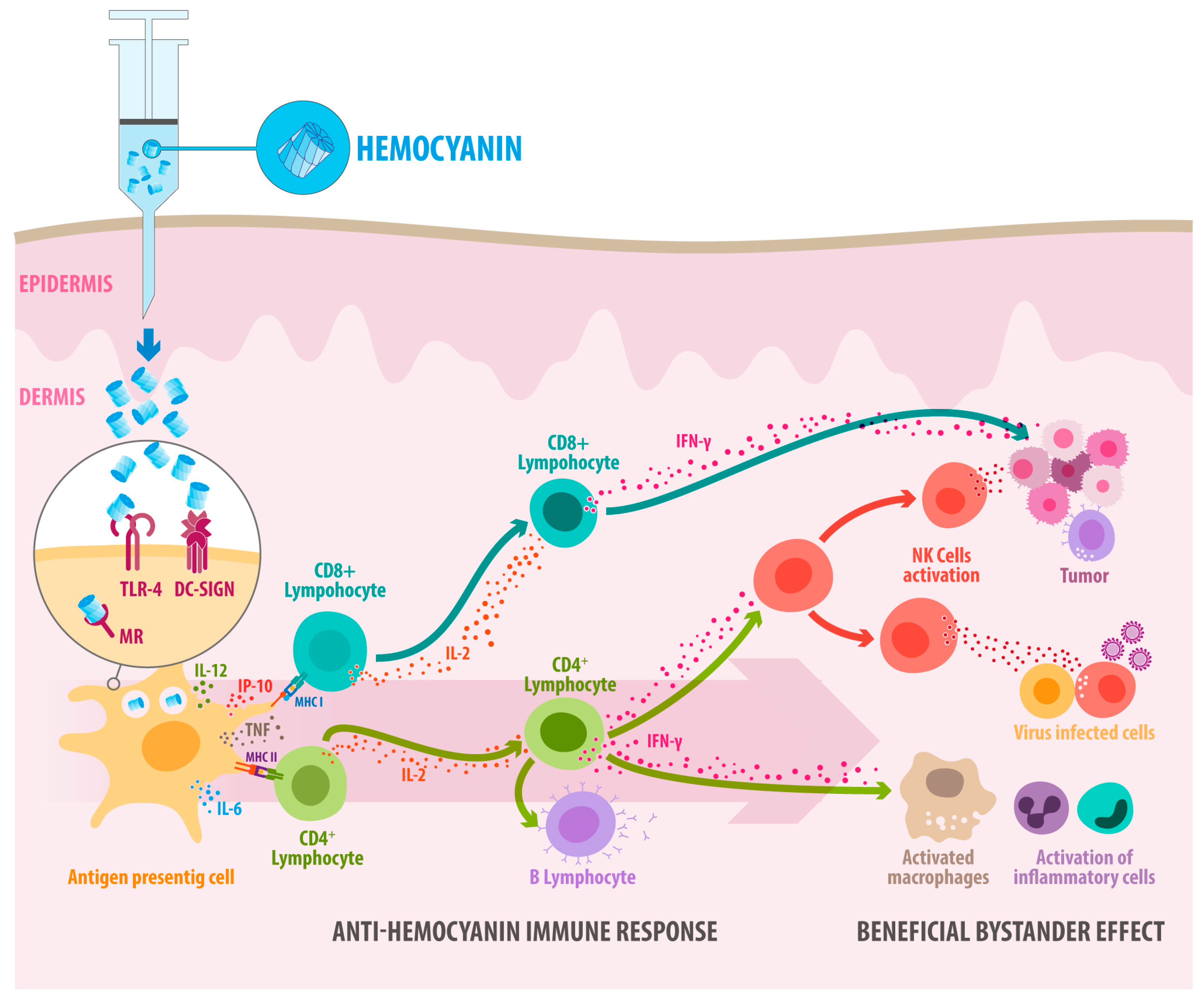
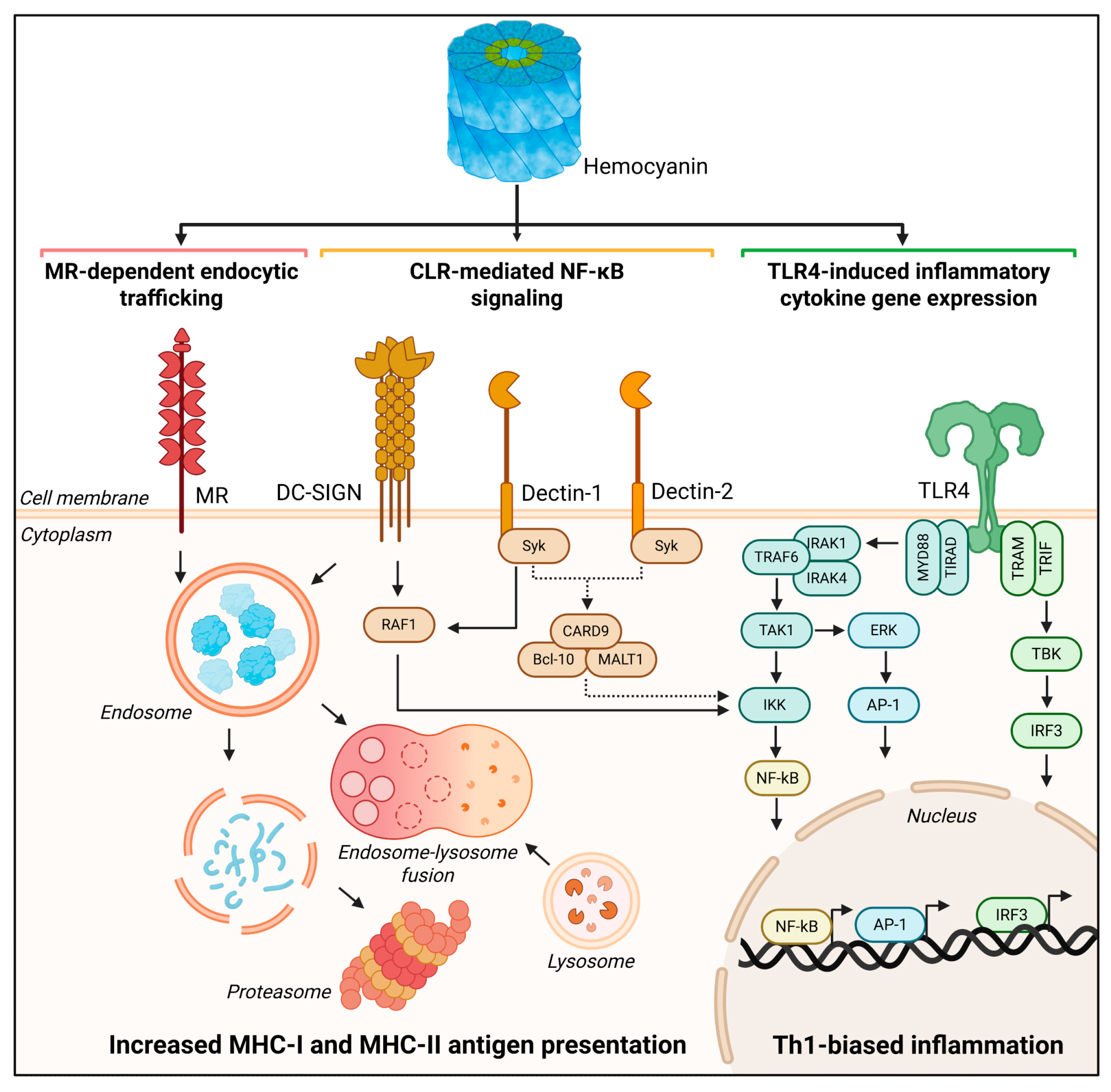
4. Hemocyanins’ Endocytosis Through Binding to Innate Immune Receptors Provides Insights into How They Activate the Immune System
5. As Antigens of Higher Conformational Stability, Hemocyanins Are Slowly Processed into MHC-II and, Surprisingly, MHC-I Pathways to Drive Th1 Immune Responses
6. Hemocyanins’ Interaction with TLR4 Promotes the TLR4 Signaling Pathways Associated with TRIF and MyD88 Adaptor Proteins
7. Hemocyanins in Combination with Adjuvants
8. Conclusions
9. Projections
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting cell |
| AP1 | Activator-protein-1 |
| BMDCs | Bone marrow-derived dendritic cells |
| CCH | Concholepas concholepas hemocyanin |
| CD4+ | Cluster of differentiation 4 (lymphocyte T CD4+) |
| CD8+ | Cluster of differentiation 8 (lymphocyte T CD8+) |
| COX-2 | Cyclooxygenase-2 |
| CD80 | Cluster of differentiation 80 |
| CD86 | Cluster of differentiation 86 |
| CRLs | C-type lectin receptor |
| DC | Dendritic cell |
| DC-SIGN | Dendritic cell-specific ICAM-grabbing non-integrin |
| DTH | Delayed-type hypersensitivity |
| ERK1/2 | Extracellular-signal-regulated kinase 1/2 |
| FLH | Fissurella latimarginata hemocyanin |
| FU | Functional units |
| GD2 | Disialogangloside |
| GM2 | Ganglioside, the M is for monosialic |
| GloboH | Globohexaosil ceramide |
| GMP | Good Manufacturing Practice |
| GTPase | The enzyme that catalyzes the hydrolysis of guanosine triphosphate (GTP) to guanosine diphosphate (GDP) |
| HEK- | Human embryonic kidney cells |
| HpH | Helix pomatia hemocyanin |
| HtH | Haliotis tuberculata hemocyanin |
| Id | Idiotype |
| IFIT1 | Interferon-induced protein with tetratricopeptide repeats 1 |
| IFN-γ | Interferon gamma |
| IL- | Interleukin |
| IP-10 | Interferon gamma-induced protein 10 |
| KLH | Keyhole limpet hemocyanin |
| LAMP-1 | Lysosome-Associated Membrane Protein 1 |
| LPS | Lipopolysaccharide |
| MG-132 | N-Benzyloxycarbonyl-L-leucyl-L-leucyl-L-leucinal |
| MyD88 | Myeloid differentiation primary response 88 |
| MHC-I | Major Histocompatibility Complex I |
| MHC-II | Major Histocompatibility Complex I |
| MGL | Macrophage galactose-type lectin |
| MPLA | Monophosphoryl lipid A |
| MR | Mannose Receptor |
| PBAs | Protein-Based adjuvant |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| OVA | Ovalbumin |
| OTI-I | These mice contain transgenic T-cell receptors that recognize OVA peptide residues 257–264. The CD8 T cells of this mouse primarily recognize OVA257–264 when presented by the MHC I molecule. |
| PcH | Pomacea caniculata hemocyanin |
| Th1 | Type 1 T helper lymphocyte |
| RvH | Rapana venosa hemocyanin |
| Rab5 | Ras-related protein Rab-5; localizes to early endosomes |
| Rab7 | Ras-related protein Rab-7; localizes to late endosomes |
| RtH | Rapana thomasiana hemocyanin |
| SIGNR1 | Mouse homolog of DC-SIGN (CD209b) |
| SIGNR5 | Mouse homolog of DC-SIGN (CD209a) |
| Syk | Spleen tyrosine kinase |
| TAP | Transporter associated with antigen processing |
| TCR | T-cell receptor |
| TH1 | Type of T helper cell, a subset of CD4+ T cells |
| THP-1 | Human monocytic leukemia cell line |
| TIR | Toll-interleukin-1 receptor |
| TLR | Toll-like receptor |
| TNF | Tumor Necrosis Factor |
| TRIF | TIR-domain-containing adapter-inducing interferon-β |
| US EPA | United States Environmental Protection Agency |
References
- Weigle, W.O. Immunochemical Properties of Hemocyanin. Immunochemistry 1964, 1, 295–302. [Google Scholar] [CrossRef]
- Swanson, M.A.; Schwartz, R.S. Immunosuppressive therapy. The relation between clinical response and immunologic competence. N. Engl. J. Med. 1967, 277, 163–170. [Google Scholar] [CrossRef]
- Dixon, F.J.; Jacot-Guillarmod, H.; McConahey, P.J. The antibody responses of rabbits and rats to hemocyanin. J. Immunol. 1966, 97, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.E.; Hersh, E.M.; Harris, J.E.; McBride, C.; Freireich, E.J. The human primary immune response to keyhole limpet haemocyanin: Interrelationships of delayed hypersensitivity, antibody response and in vitro blast transformation. Clin. Exp. Immunol. 1970, 6, 473–491. [Google Scholar] [PubMed]
- Harris, J.R.; Markl, J. Keyhole limpet hemocyanin (KLH): A biomedical review. Micron 1999, 30, 597–623. [Google Scholar] [CrossRef] [PubMed]
- Del Campo, M.; Arancibia, S.; Nova, E.; Salazar, F.; Gonzalez, A.; Moltedo, B.; De Ioannes, P.; Ferreira, J.; Manubens, A.; Becker, M.I. Hemocyanins as immunostimulants. Rev. Med. Chil. 2011, 139, 236–246. [Google Scholar]
- Swaminathan, A.; Lucas, R.M.; Dear, K.; McMichael, A.J. Keyhole limpet haemocyanin—A model antigen for human immunotoxicological studies. Br. J. Clin. Pharmacol. 2014, 78, 1135–1142. [Google Scholar] [CrossRef]
- Haque, N.; Parveen, S.; Tang, T.; Wei, J.; Huang, Z. Marine Natural Products in Clinical Use. Mar. Drugs 2022, 20, 528. [Google Scholar] [CrossRef]
- Saghari, M.; Jansen, M.; Grievink, H.W.; Rissmann, R.; Moerland, M. Characterization of KLH-driven immune responses in clinical studies: A systematic review. Front. Drug Discov. 2022, 2, 992087. [Google Scholar] [CrossRef]
- Eveleens Maarse, B.C.; Ronner, M.N.; Jansen, M.A.A.; Niemeyer-van der Kolk, T.; In’t Veld, A.E.; Klaassen, E.S.; Ahmad, S.; Itano, A.; McHale, D.; Moerland, M. Immunomodulating effects of the single bacterial strain therapy EDP1815 on innate and adaptive immune challenge responses—A randomized, placebo-controlled clinical trial. Immunol. Res. 2024, 72, 776–787. [Google Scholar] [CrossRef]
- Olsson, C.A.; Chute, R.; Rao, C.N. Immunologic reduction of bladder cancer recurrence rate. Trans. Am. Assoc. Genitourin. Surg. 1973, 65, 66–72. [Google Scholar] [CrossRef]
- Lammers, R.J.; Witjes, W.P.; Janzing-Pastors, M.H.; Caris, C.T.; Witjes, J.A. Intracutaneous and intravesical immunotherapy with keyhole limpet hemocyanin compared with intravesical mitomycin in patients with non-muscle-invasive bladder cancer: Results from a prospective randomized phase III trial. J. Clin. Oncol. 2012, 30, 2273–2279. [Google Scholar] [CrossRef]
- Arancibia, S.; Salazar, F.; Becker, M. Hemocyanins in the Immunotherapy of Superficial Bladder Cancer. In Bladder Cancer—From Basic Science to Robotic Surgery; InTech: Rijeka, Croatia, 2012. [Google Scholar][Green Version]
- Wimmers, F.; de Haas, N.; Scholzen, A.; Schreibelt, G.; Simonetti, E.; Eleveld, M.J.; Brouwers, H.M.; Beldhuis-Valkis, M.; Joosten, I.; de Jonge, M.I.; et al. Monitoring of dynamic changes in Keyhole Limpet Hemocyanin (KLH)-specific B cells in KLH-vaccinated cancer patients. Sci. Rep. 2017, 7, 43486. [Google Scholar] [CrossRef]
- Drennan, P.G.; Karponis, D.; Richards, D.; Coles, M.; Fullerton, J.N. In vivo human keyhole limpet hemocyanin challenge in early phase drug development: A systematic review. Clin. Transl. Sci. 2023, 16, 357–382. [Google Scholar] [CrossRef]
- Diaz-Dinamarca, D.A.; Salazar, M.L.; Castillo, B.N.; Manubens, A.; Vasquez, A.E.; Salazar, F.; Becker, M.I. Protein-Based Adjuvants for Vaccines as Immunomodulators of the Innate and Adaptive Immune Response: Current Knowledge, Challenges, and Future Opportunities. Pharmaceutics 2022, 14, 1671. [Google Scholar] [CrossRef]
- Holmberg, L.A.; Sandmaier, B.M. Vaccination with Theratope (STn-KLH) as treatment for breast cancer. Expert Rev. Vaccines 2004, 3, 655–663. [Google Scholar] [CrossRef]
- Mettu, R.; Chen, C.Y.; Wu, C.Y. Synthetic carbohydrate-based vaccines: Challenges and opportunities. J. Biomed. Sci. 2020, 27, 9. [Google Scholar] [CrossRef]
- Shivatare, S.S.; Shivatare, V.S.; Wong, C.H. Glycoconjugates: Synthesis, Functional Studies, and Therapeutic Developments. Chem. Rev. 2022, 122, 15603–15671. [Google Scholar] [CrossRef]
- Bagasra, O.; Forman, L.J.; Howeedy, A.; Whittle, P. A potential vaccine for cocaine abuse prophylaxis. Immunopharmacology 1992, 23, 173–179. [Google Scholar] [CrossRef]
- Baruffaldi, F.; Kelcher, A.H.; Laudenbach, M.; Gradinati, V.; Limkar, A.; Roslawski, M.; Birnbaum, A.; Lees, A.; Hassler, C.; Runyon, S.; et al. Preclinical Efficacy and Characterization of Candidate Vaccines for Treatment of Opioid Use Disorders Using Clinically Viable Carrier Proteins. Mol. Pharm. 2018, 15, 4947–4962. [Google Scholar] [CrossRef]
- de Almeida Augusto, P.S.; Pereira, R.L.G.; Caligiorne, S.M.; Sabato, B.; Assis, B.R.D.; do Espirito Santo, L.P.; Dos Reis, K.D.; Castro Goulart, G.A.; de Fatima, A.; de Castro Lourenco das Neves, M.; et al. The GNE-KLH anti-cocaine vaccine protects dams and offspring from cocaine-induced effects during the prenatal and lactating periods. Mol. Psychiatry 2021, 26, 7784–7791. [Google Scholar] [CrossRef]
- Bian, Y.; Ci, Q.; Luo, X.M.; Zhang, C. Precision Adjuvant Strategies in Vaccine Development for Substance Use Disorders: Variability and Mechanistic Insights. Pharmaceutics 2025, 17, 1223. [Google Scholar] [CrossRef]
- Bendandi, M. Idiotype vaccines for lymphoma: Proof-of-principles and clinical trial failures. Nat. Rev. Cancer 2009, 9, 675–681. [Google Scholar] [CrossRef]
- Röllig, C.; Schmidt, C.; Bornhäuser, M.; Ehninger, G.; Schmitz, M.; Auffermann-Gretzinger, S. Induction of cellular immune responses in patients with stage-I multiple myeloma after vaccination with autologous idiotype-pulsed dendritic cells. J. Immunother. 2011, 34, 100–106. [Google Scholar] [CrossRef]
- Schuster, S.J.; Neelapu, S.S.; Gause, B.L.; Janik, J.E.; Muggia, F.M.; Gockerman, J.P.; Winter, J.N.; Flowers, C.R.; Nikcevich, D.A.; Sotomayor, E.M.; et al. Vaccination with patient-specific tumor-derived antigen in first remission improves disease-free survival in follicular lymphoma. J. Clin. Oncol. 2011, 29, 2787–2794. [Google Scholar] [CrossRef]
- Qazilbash, M.H.; Saini, N.Y.; Cha, S.-C.; Wang, Z.; Stadtmauer, E.A.; Baladandayuthapani, V.; Lin, H.; Tross, B.; Honhar, M.; Rao, S.S.; et al. A randomized phase 2 trial of idiotype vaccination and adoptive autologous T-cell transfer in patients with multiple myeloma. Blood 2022, 139, 1289–1301. [Google Scholar] [CrossRef]
- Tittarelli, A.; González, F.E.; Pereda, C.; Mora, G.; Muñoz, L.; Saffie, C.; García, T.; Díaz, D.; Falcón, C.; Hermoso, M.; et al. Toll-like receptor 4 gene polymorphism influences dendritic cell in vitro function and clinical outcomes in vaccinated melanoma patients. Cancer Immunol. Immunother. 2012, 61, 2067–2077. [Google Scholar] [CrossRef]
- Tittarelli, A.; Pereda, C.; Gleisner, M.A.; López, M.N.; Flores, I.; Tempio, F.; Lladser, A.; Achour, A.; González, F.E.; Durán-Aniotz, C.; et al. Long-Term Survival and Immune Response Dynamics in Melanoma Patients Undergoing TAPCells-Based Vaccination Therapy. Vaccines 2024, 12, 357. [Google Scholar] [CrossRef]
- Dolashka, P.; Dolashki, A.; Van Beeumen, J.; Floetenmeyer, M.; Velkova, L.; Stevanovic, S.; Voelter, W. Antimicrobial Activity of Molluscan Hemocyanins from Helix and Rapana Snails. Curr. Pharm. Biotechnol. 2016, 17, 263–270. [Google Scholar] [CrossRef]
- De Ioannes, P.; Moltedo, B.; Oliva, H.; Pacheco, R.; Faunes, F.; De Ioannes, A.E.; Becker, M.I. Hemocyanin of the molluscan Concholepas concholepas exhibits an unusual heterodecameric array of subunits. J. Biol. Chem. 2004, 279, 26134–26142. [Google Scholar] [CrossRef]
- Matus, S.; Burgos, P.V.; Bravo-Zehnder, M.; Kraft, R.; Porras, O.H.; Farias, P.; Barros, L.F.; Torrealba, F.; Massardo, L.; Jacobelli, S.; et al. Antiribosomal-P autoantibodies from psychiatric lupus target a novel neuronal surface protein causing calcium influx and apoptosis. J. Exp. Med. 2007, 204, 3221–3234. [Google Scholar] [CrossRef]
- Nakamura, S.; Yatabe, R.; Onodera, T.; Toko, K. Sensitive detection of capsaicinoids using a surface plasmon resonance sensor with anti-homovanillic Acid polyclonal antibodies. Biosensors 2013, 3, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Poirier, D.; Theolier, J.; Marega, R.; Delahaut, P.; Gillard, N.; Godefroy, S.B. Evaluation of the discriminatory potential of antibodies created from synthetic peptides derived from wheat, barley, rye and oat gluten. PLoS ONE 2021, 16, e0257466. [Google Scholar] [CrossRef] [PubMed]
- Moltedo, B.; Faunes, F.; Haussmann, D.; De Ioannes, P.; De Ioannes, A.E.; Puente, J.; Becker, M.I. Immunotherapeutic effect of Concholepas hemocyanin in the murine bladder cancer model: Evidence for conserved antitumor properties among hemocyanins. J. Urol. 2006, 176, 2690–2695. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, S.; Del Campo, M.; Nova, E.; Salazar, F.; Becker, M.I. Enhanced structural stability of Concholepas hemocyanin increases its immunogenicity and maintains its non-specific immunostimulatory effects. Eur. J. Immunol. 2012, 42, 688–699. [Google Scholar] [CrossRef]
- Palacios, M.; Tampe, R.; Del Campo, M.; Zhong, T.Y.; Lopez, M.N.; Salazar-Onfray, F.; Becker, M.I. Antitumor activity and carrier properties of novel hemocyanins coupled to a mimotope of GD2 ganglioside. Eur. J. Med. Chem. 2018, 150, 74–86. [Google Scholar] [CrossRef]
- Gleisner, M.A.; Pereda, C.; Tittarelli, A.; Navarrete, M.; Fuentes, C.; Avalos, I.; Tempio, F.; Araya, J.P.; Becker, M.I.; Gonzalez, F.E.; et al. A heat-shocked melanoma cell lysate vaccine enhances tumor infiltration by prototypic effector T cells inhibiting tumor growth. J. Immunother. Cancer 2020, 8, e000999. [Google Scholar] [CrossRef]
- Mora Roman, J.J.; Del Campo, M.; Villar, J.; Paolini, F.; Curzio, G.; Venuti, A.; Jara, L.; Ferreira, J.; Murgas, P.; Lladser, A.; et al. Immunotherapeutic Potential of Mollusk Hemocyanins in Combination with Human Vaccine Adjuvants in Murine Models of Oral Cancer. J. Immunol. Res. 2019, 2019, 7076942. [Google Scholar] [CrossRef]
- Salazar-Onfray, F.; Pereda, C.; Reyes, D.; Lopez, M.N. TAPCells, the Chilean dendritic cell vaccine against melanoma and prostate cancer. Biol. Res. 2013, 46, 431–440. [Google Scholar] [CrossRef][Green Version]
- Reyes, D.; Salazar, L.; Espinoza, E.; Pereda, C.; Castellon, E.; Valdevenito, R.; Huidobro, C.; Ines Becker, M.; Lladser, A.; Lopez, M.N.; et al. Tumour cell lysate-loaded dendritic cell vaccine induces biochemical and memory immune response in castration-resistant prostate cancer patients. Br. J. Cancer 2013, 109, 1488–1497. [Google Scholar] [CrossRef]
- Miller, L.A.; Gionfriddo, J.P.; Fagerstone, K.A.; Rhyan, J.C.; Killian, G.J. The single-shot GnRH immunocontraceptive vaccine (GonaCon) in white-tailed deer: Comparison of several GnRH preparations. Am. J. Reprod. Immunol. 2008, 60, 214–223. [Google Scholar] [CrossRef]
- Miller, L.A.; Fagerstone, K.A.; Eckery, D.C. Twenty years of immunocontraceptive research: Lessons learned. J. Zoo Wildl. Med. 2013, 44, S84–S96. [Google Scholar] [CrossRef]
- Vansandt, L.M.; Kutzler, M.A.; Fischer, A.E.; Morris, K.N.; Swanson, W.F. Safety and effectiveness of a single and repeat intramuscular injection of a GnRH vaccine (GonaCon) in adult female domestic cats. Reprod. Domest. Anim. 2017, 52 (Suppl. 2), 348–353. [Google Scholar] [CrossRef]
- Baker, D.L.; Powers, J.G.; Ransom, J.I.; McCann, B.E.; Oehler, M.W.; Bruemmer, J.E.; Galloway, N.L.; Eckery, D.C.; Nett, T.M. Reimmunization increases contraceptive effectiveness of gonadotropin-releasing hormone vaccine (GonaCon-Equine) in free-ranging horses (Equus caballus): Limitations and side effects. PLoS ONE 2018, 13, e0201570. [Google Scholar] [CrossRef]
- Frey, R.K.; Wehtje, M.E.; Nol, P.; Clarke, P.R.; Rhyan, J.C.; McCollum, M.P.; Miller, L.A.; Eckery, D.C. Effects of the Immunocontraceptive Gonacon on Pregnancy in Brucella-Seropositive American bison (Bison bison). J. Wildl. Dis. 2024, 60, 339–345. [Google Scholar] [CrossRef]
- Benka, V.A.; Levy, J.K. Vaccines for feline contraception: GonaCon GnRH-hemocyanin conjugate immunocontraceptive. J. Feline Med. Surg. 2015, 17, 758–765. [Google Scholar] [CrossRef]
- Arancibia, S.; Espinoza, C.; Salazar, F.; Del Campo, M.; Tampe, R.; Zhong, T.Y.; De Ioannes, P.; Moltedo, B.; Ferreira, J.; Lavelle, E.C.; et al. A novel immunomodulatory hemocyanin from the limpet Fissurella latimarginata promotes potent anti-tumor activity in melanoma. PLoS ONE 2014, 9, e87240. [Google Scholar] [CrossRef]
- Gesheva, V.; Chausheva, S.; Stefanova, N.; Mihaylova, N.; Doumanova, L.; Idakieva, K.; Tchorbanov, A. Helix pomatia hemocyanin—A novel bio-adjuvant for viral and bacterial antigens. Int. Immunopharmacol. 2015, 26, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, E.; Mihaylova, N.; Ralchev, N.; Bradyanova, S.; Manoylov, I.; Raynova, Y.; Idakieva, K.; Tchorbanov, A. Immunotherapeutic Potential of Mollusk Hemocyanins in Murine Model of Melanoma. Mar. Drugs 2024, 22, 220. [Google Scholar] [CrossRef]
- Stoyanova, E.; Mihaylova, N.; Manoylov, I.; Bradyanova, S.; Raynova, Y.; Idakieva, K.; Tchorbanov, A. Intensive therapy with gastropodan hemocyanins increases their antitumor properties in murine model of colon carcinoma. Int. Immunopharmacol. 2020, 84, 106566. [Google Scholar] [CrossRef]
- Tchorbanov, A.; Idakieva, K.; Mihaylova, N.; Doumanova, L. Modulation of the immune response using Rapana thomasiana hemocyanin. Int. Immunopharmacol. 2008, 8, 1033–1038. [Google Scholar] [CrossRef]
- Georgieva, A.; Todorova, K.; Iliev, I.; Dilcheva, V.; Vladov, I.; Petkova, S.; Dolashki, A.; Velkova, L.; Dolashka, P.; Toshkova, R. Assessment of the In Vitro and In Vivo Antitumor Activity of Hemocyanins from Helix aspersa, Helix lucorum, and Rapana venosa in a Graffi Myeloid Tumor Model. Biomedicines 2023, 11, 1545. [Google Scholar] [CrossRef]
- Chiumiento, I.R.; Tricerri, M.A.; Cortez, M.F.; Ituarte, S.; Tau, J.; Marino, K.V.; Smaldini, P.L.; Heras, H.; Dreon, M.S. Pomacea canaliculata hemocyanin as a novel natural immunostimulant in mammals. Front. Immunol. 2024, 15, 1490260. [Google Scholar] [CrossRef]
- Lamm, D.L.; DeHaven, J.I.; Riggs, D.R.; Ebert, R.F. Immunotherapy of murine bladder cancer with keyhole limpet hemocyanin (KLH). J. Urol. 1993, 149, 648–652. [Google Scholar] [CrossRef]
- Harris, J.R.; Markl, J. Keyhole limpet hemocyanin: Molecular structure of a potent marine immunoactivator. A review. Eur. Urol. 2000, 37 (Suppl. 3), 24–33. [Google Scholar] [CrossRef] [PubMed]
- Coates, C.J.; Decker, H. Immunological properties of oxygen-transport proteins: Hemoglobin, hemocyanin and hemerythrin. Cell. Mol. Life Sci. 2017, 74, 293–317. [Google Scholar] [CrossRef] [PubMed]
- van Holde, K.E.; Miller, K.I. Hemocyanins. Adv. Protein Chem. 1995, 47, 1–81. [Google Scholar] [PubMed]
- Markl, J. Evolution of molluscan hemocyanin structures. Biochim. Biophys. Acta 2013, 1834, 1840–1852. [Google Scholar] [CrossRef]
- Kato, S.; Matsui, T.; Tanaka, Y. Molluscan Hemocyanins. Subcell. Biochem. 2020, 94, 195–218. [Google Scholar]
- van Holde, K.E.; Miller, K.I.; Decker, H. Hemocyanins and invertebrate evolution. J. Biol. Chem. 2001, 276, 15563–15566. [Google Scholar] [CrossRef]
- Decker, H.; Hellmann, N.; Jaenicke, E.; Lieb, B.; Meissner, U.; Markl, J. Minireview: Recent progress in hemocyanin research. Integr. Comp. Biol. 2007, 47, 631–644. [Google Scholar] [CrossRef]
- Gatsogiannis, C.; Markl, J. Keyhole limpet hemocyanin: 9-A CryoEM structure and molecular model of the KLH1 didecamer reveal the interfaces and intricate topology of the 160 functional units. J. Mol. Biol. 2009, 385, 963–983. [Google Scholar] [CrossRef]
- Kato, S.; Matsui, T.; Gatsogiannis, C.; Tanaka, Y. Molluscan hemocyanin: Structure, evolution, and physiology. Biophys. Rev. 2018, 10, 191–202. [Google Scholar] [CrossRef]
- Jaenicke, E.; Buchler, K.; Decker, H.; Markl, J.; Schroder, G.F. The refined structure of functional unit h of keyhole limpet hemocyanin (KLH1-h) reveals disulfide bridges. IUBMB Life 2011, 63, 183–187. [Google Scholar] [CrossRef]
- Schutz, J.; Dolashka-Angelova, P.; Abrashev, R.; Nicolov, P.; Voelter, W. Isolation and spectroscopic characterization of the structural subunits of keyhole limpet hemocyanin. Biochim. Biophys. Acta 2001, 1546, 325–336. [Google Scholar] [CrossRef]
- Idakieva, K.; Nikolov, P.; Chakarska, I.; Genov, N.; Shnyrov, V.L. Spectroscopic properties and conformational stability of Concholepas concholepas hemocyanin. J. Fluoresc. 2008, 18, 715–725. [Google Scholar] [CrossRef]
- Salazar, M.L.; Jimenez, J.M.; Villar, J.; Rivera, M.; Baez, M.; Manubens, A.; Becker, M.I. N-Glycosylation of mollusk hemocyanins contributes to their structural stability and immunomodulatory properties in mammals. J. Biol. Chem. 2019, 294, 19546–19564. [Google Scholar] [CrossRef]
- González, A.; Nova, E.; Del Campo, M.; Manubens, A.; De Ioannes, A.; Ferreira, J.; Becker, M.I. The oxygen-binding properties of hemocyanin from the mollusk Concholepas concholepas. Biochim. Biophys. Acta Proteins Proteom. 2017, 1865, 1746–1757. [Google Scholar] [CrossRef]
- Orlova, E.V.; Dube, P.; Harris, J.R.; Beckman, E.; Zemlin, F.; Markl, J.; van Heel, M. Structure of keyhole limpet hemocyanin type 1 (KLH1) at 15 A resolution by electron cryomicroscopy and angular reconstitution. J. Mol. Biol. 1997, 271, 417–437. [Google Scholar] [CrossRef][Green Version]
- Swerdlow, R.D.; Ebert, R.F.; Lee, P.; Bonaventura, C.; Miller, K.I. Keyhole limpet hemocyanin: Structural and functional characterization of two different subunits and multimers. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1996, 113, 537–548. [Google Scholar] [CrossRef]
- Harris, J.R.; Gebauer, W.; Sohngen, S.M.; Nermut, M.V.; Markl, J. Keyhole limpet hemocyanin (KLH), II: Characteristic reassociation properties of purified KLH1 and KLH2. Micron 1997, 28, 43–56. [Google Scholar] [CrossRef]
- Keller, H.; Lieb, B.; Altenhein, B.; Gebauer, D.; Richter, S.; Stricker, S.; Markl, J. Abalone (Haliotis tuberculata) hemocyanin type 1 (HtH1). Organization of the approximately 400 kDa subunit, and amino acid sequence of its functional units f, g and h. Eur. J. Biochem. 1999, 264, 27–38. [Google Scholar] [CrossRef]
- Oliva, H.; Moltedo, B.; De Ioannes, P.; Faunes, F.; De Ioannes, A.E.; Becker, M.I. Monoclonal antibodies to molluskan hemocyanin from Concholepas concholepas demonstrate common and specific epitopes among subunits. Hybrid. Hybridomics 2002, 21, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Dolashka, P.; Daskalova, A.; Dolashki, A.; Voelter, W. De Novo Structural Determination of the Oligosaccharide Structure of Hemocyanins from Molluscs. Biomolecules 2020, 10, 1470. [Google Scholar] [CrossRef]
- Kurokawa, T.; Wuhrer, M.; Lochnit, G.; Geyer, H.; Markl, J.; Geyer, R. Hemocyanin from the keyhole limpet Megathura crenulata (KLH) carries a novel type of N-glycans with Gal(beta1-6)Man-motifs. Eur. J. Biochem. 2002, 269, 5459–5473. [Google Scholar] [CrossRef]
- Staudacher, E.; Stepan, H.; Gutternigg, M. Protein N-Glycosylation of Gastropods. Curr. Top. Biochem. Res. 2009, 11, 29–39. [Google Scholar] [PubMed]
- Wuhrer, M.; Robijn, M.L.; Koeleman, C.A.; Balog, C.I.; Geyer, R.; Deelder, A.M.; Hokke, C.H. A novel Gal(beta1-4)Gal(beta1-4)Fuc(alpha1-6)-core modification attached to the proximal N-acetylglucosamine of keyhole limpet haemocyanin (KLH) N-glycans. Biochem. J. 2004, 378, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Munoz, S.M.; Vallejos-Baccelliere, G.; Manubens, A.; Salazar, M.L.; Nascimento, A.F.Z.; Tapia-Reyes, P.; Meneses, C.; Ambrosio, A.L.B.; Becker, M.I.; Guixe, V.; et al. Structural insights into a functional unit from an immunogenic mollusk hemocyanin. Structure 2024, 32, 812–823.e4. [Google Scholar] [CrossRef]
- Dolashka-Angelova, P.; Lieb, B.; Velkova, L.; Heilen, N.; Sandra, K.; Nikolaeva-Glomb, L.; Dolashki, A.; Galabov, A.S.; Van Beeumen, J.; Stevanovic, S.; et al. Identification of glycosylated sites in Rapana hemocyanin by mass spectrometry and gene sequence, and their antiviral effect. Bioconjug. Chem. 2009, 20, 1315–1322. [Google Scholar] [CrossRef]
- Gai, Z.; Matsuno, A.; Kato, K.; Kato, S.; Khan, M.R.I.; Shimizu, T.; Yoshioka, T.; Kato, Y.; Kishimura, H.; Kanno, G.; et al. Crystal Structure of the 3.8-MDa Respiratory Supermolecule Hemocyanin at 3.0 A Resolution. Structure 2015, 23, 2204–2212. [Google Scholar] [CrossRef]
- Stoeva, S.; Schutz, J.; Gebauer, W.; Hundsdorfer, T.; Manz, C.; Markl, J.; Voelter, W. Primary structure and unusual carbohydrate moiety of functional unit 2-c of keyhole limpet hemocyanin (KLH). Biochim. Biophys. Acta 1999, 1435, 94–109. [Google Scholar] [CrossRef]
- Becker, M.I.; Fuentes, A.; Del Campo, M.; Manubens, A.; Nova, E.; Oliva, H.; Faunes, F.; Valenzuela, M.A.; Campos-Vallette, M.; Aliaga, A.; et al. Immunodominant role of CCHA subunit of Concholepas hemocyanin is associated with unique biochemical properties. Int. Immunopharmacol. 2009, 9, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Wirguin, I.; Suturkova-Milosevic, L.; Briani, C.; Latov, N. Keyhole limpet hemocyanin contains Gal(beta 1-3)-GalNAc determinants that are cross-reactive with the T antigen. Cancer Immunol. Immunother. 1995, 40, 307–310. [Google Scholar] [PubMed]
- Geyer, H.; Wuhrer, M.; Resemann, A.; Geyer, R. Identification and characterization of keyhole limpet hemocyanin N-glycans mediating cross-reactivity with Schistosoma mansoni. J. Biol. Chem. 2005, 280, 40731–40748. [Google Scholar] [CrossRef]
- Pizarro-Bauerle, J.; Maldonado, I.; Sosoniuk-Roche, E.; Vallejos, G.; Lopez, M.N.; Salazar-Onfray, F.; Aguilar-Guzman, L.; Valck, C.; Ferreira, A.; Becker, M.I. Molluskan Hemocyanins Activate the Classical Pathway of the Human Complement System through Natural Antibodies. Front. Immunol. 2017, 8, 188. [Google Scholar] [CrossRef]
- Lieb, B.; Altenhein, B.; Markl, J. The sequence of a gastropod hemocyanin (HtH1 from Haliotis tuberculata). J. Biol. Chem. 2000, 275, 5675–5681. [Google Scholar] [CrossRef]
- Boisguérin, V. Recombinant Expression of Molluscan Hemocyanin (KLH) Substructures in a Prokaryotic System: E. coli. Ph.D. Thesis, Johannes Gutenberg-Universität, Mainz, Germany, 2006. [Google Scholar]
- Villar, J.; Salazar, M.L.; Jimenez, J.M.; Campo, M.D.; Manubens, A.; Gleisner, M.A.; Avalos, I.; Salazar-Onfray, F.; Salazar, F.; Mitchell, D.A.; et al. C-type lectin receptors MR and DC-SIGN are involved in recognition of hemocyanins, shaping their immunostimulatory effects on human dendritic cells. Eur. J. Immunol. 2021, 51, 1715–1731. [Google Scholar] [CrossRef]
- Shimizu, K.; Thomas, E.K.; Giedlin, M.; Mule, J.J. Enhancement of tumor lysate- and peptide-pulsed dendritic cell-based vaccines by the addition of foreign helper protein. Cancer Res. 2001, 61, 2618–2624. [Google Scholar] [PubMed]
- Becker, M.I.; Arancibia, S.; Salazar, F.; Del Campo, M.; De Ioannes, A. Mollusk Hemocyanins as Natural Immunostimulants in Biomedical Applications. In Immune Response Activation; InTechOpen: Rijeka, Croatia, 2014. [Google Scholar]
- Zhong, T.Y.; Arancibia, S.; Born, R.; Tampe, R.; Villar, J.; Del Campo, M.; Manubens, A.; Becker, M.I. Hemocyanins Stimulate Innate Immunity by Inducing Different Temporal Patterns of Proinflammatory Cytokine Expression in Macrophages. J. Immunol. 2016, 196, 4650–4662. [Google Scholar] [CrossRef]
- Sarker, M.M.; Zhong, M. Keyhole limpet hemocyanin augmented the killing activity, cytokine production and proliferation of NK cells, and inhibited the proliferation of Meth A sarcoma cells in vitro. Indian J. Pharmacol. 2014, 46, 40–45. [Google Scholar] [CrossRef]
- Jurincic-Winkler, C.; Metz, K.A.; Beuth, J.; Engelmann, U.; Klippel, K.F. Immunohistological findings in patients with superficial bladder carcinoma after intravesical instillation of keyhole limpet haemocyanin. Br. J. Urol. 1995, 76, 702–707. [Google Scholar] [CrossRef]
- Meier, S.L.; Satpathy, A.T.; Wells, D.K. Bystander T cells in cancer immunology and therapy. Nat. Cancer 2022, 3, 143–155. [Google Scholar] [CrossRef]
- Hoving, J.C.; Wilson, G.J.; Brown, G.D. Signalling C-type lectin receptors, microbial recognition and immunity. Cell. Microbiol. 2014, 16, 185–194. [Google Scholar] [CrossRef]
- Presicce, P.; Taddeo, A.; Conti, A.; Villa, M.L.; Della Bella, S. Keyhole limpet hemocyanin induces the activation and maturation of human dendritic cells through the involvement of mannose receptor. Mol. Immunol. 2008, 45, 1136–1145. [Google Scholar] [CrossRef]
- van Kooyk, Y. C-type lectins on dendritic cells: Key modulators for the induction of immune responses. Biochem. Soc. Trans. 2008, 36, 1478–1481. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; Gringhuis, S.I. Signalling through C-type lectin receptors: Shaping immune responses. Nat. Rev. Immunol. 2009, 9, 465–479. [Google Scholar] [CrossRef]
- Pishesha, N.; Harmand, T.J.; Ploegh, H.L. A guide to antigen processing and presentation. Nat. Rev. Immunol. 2022, 22, 751–764. [Google Scholar] [CrossRef]
- Martinez-Pomares, L. The mannose receptor. J. Leukoc. Biol. 2012, 92, 1177–1186. [Google Scholar] [CrossRef] [PubMed]
- van Kooyk, Y.; Unger, W.W.; Fehres, C.M.; Kalay, H.; Garcia-Vallejo, J.J. Glycan-based DC-SIGN targeting vaccines to enhance antigen cross-presentation. Mol. Immunol. 2013, 55, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, J.M.; Salazar, M.L.; Arancibia, S.; Villar, J.; Salazar, F.; Brown, G.D.; Lavelle, E.C.; Martinez-Pomares, L.; Ortiz-Quintero, J.; Lavandero, S.; et al. TLR4, but Neither Dectin-1 nor Dectin-2, Participates in the Mollusk Hemocyanin-Induced Proinflammatory Effects in Antigen-Presenting Cells From Mammals. Front. Immunol. 2019, 10, 1136. [Google Scholar] [CrossRef] [PubMed]
- Diebold, S.S. Activation of dendritic cells by toll-like receptors and C-type lectins. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 3–30. [Google Scholar]
- Nakaira-Takahagi, E.; Golim, M.A.; Bannwart, C.F.; Puccia, R.; Peracoli, M.T. Interactions between TLR2, TLR4, and mannose receptors with gp43 from Paracoccidioides brasiliensis induce cytokine production by human monocytes. Med. Mycol. 2011, 49, 694–703. [Google Scholar]
- Dias-Melicio, L.A.; Fernandes, R.K.; Rodrigues, D.R.; Golim, M.A.; Soares, A.M. Interleukin-18 increases TLR4 and mannose receptor expression and modulates cytokine production in human monocytes. Mediat. Inflamm. 2015, 2015, 236839. [Google Scholar] [CrossRef] [PubMed]
- Burgdorf, S.; Kurts, C. Endocytosis mechanisms and the cell biology of antigen presentation. Curr. Opin. Immunol. 2008, 20, 89–95. [Google Scholar] [CrossRef] [PubMed]
- van der Zande, H.J.P.; Nitsche, D.; Schlautmann, L.; Guigas, B.; Burgdorf, S. The Mannose Receptor: From Endocytic Receptor and Biomarker to Regulator of (Meta)Inflammation. Front. Immunol. 2021, 12, 765034. [Google Scholar] [CrossRef]
- Zehner, M.; Burgdorf, S. Regulation of antigen transport into the cytosol for cross-presentation by ubiquitination of the mannose receptor. Mol. Immunol. 2013, 55, 146–148. [Google Scholar] [CrossRef]
- Tacken, P.J.; Ginter, W.; Berod, L.; Cruz, L.J.; Joosten, B.; Sparwasser, T.; Figdor, C.G.; Cambi, A. Targeting DC-SIGN via its neck region leads to prolonged antigen residence in early endosomes, delayed lysosomal degradation, and cross-presentation. Blood 2011, 118, 4111–4119. [Google Scholar] [CrossRef]
- Kotsias, F.; Cebrian, I.; Alloatti, A. Antigen processing and presentation. Int. Rev. Cell Mol. Biol. 2019, 348, 69–121. [Google Scholar] [PubMed]
- Bet, A.; Sterrett, S.; Sato, A.; Bansal, A.; Goepfert, P.A. Characterization of T-cell responses to cryptic epitopes in recipients of a noncodon-optimized HIV-1 vaccine. J. Acquir. Immune Defic. Syndr. 2014, 65, 142–150. [Google Scholar] [CrossRef][Green Version]
- Ascough, S.; Ingram, R.J.; Chu, K.K.; Musson, J.A.; Moore, S.J.; Gallagher, T.; Baillie, L.; Williamson, E.D.; Robinson, J.H.; Maillere, B.; et al. CD4+ T Cells Targeting Dominant and Cryptic Epitopes from Bacillus anthracis Lethal Factor. Front. Microbiol. 2015, 6, 1506. [Google Scholar] [CrossRef]
- Wolpert, E.Z.; Grufman, P.; Sandberg, J.K.; Tegnesjo, A.; Karre, K. Immunodominance in the CTL response against minor histocompatibility antigens: Interference between responding T cells, rather than with presentation of epitopes. J. Immunol. 1998, 161, 4499–4505. [Google Scholar] [CrossRef]
- Oyama, K.; Ueda, T. Relationship between protein conformational stability and its immunogenicity when administering antigens to mice using adjuvants-Analysis employed the CH2 domain in human antibodies. PLoS ONE 2024, 19, e0307320. [Google Scholar] [CrossRef] [PubMed]
- Mouritsen, S.; Meldal, M.; Christiansen-Brams, I.; Elsner, H.; Werdelin, O. Attachment of oligosaccharides to peptide antigen profoundly affects binding to major histocompatibility complex class II molecules and peptide immunogenicity. Eur. J. Immunol. 1994, 24, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Werdelin, O.; Meldal, M.; Jensen, T. Processing of glycans on glycoprotein and glycopeptide antigens in antigen-presenting cells. Proc. Natl. Acad. Sci. USA 2002, 99, 9611–9613. [Google Scholar] [CrossRef]
- Sun, L.; Middleton, D.R.; Wantuch, P.L.; Ozdilek, A.; Avci, F.Y. Carbohydrates as T-cell antigens with implications in health and disease. Glycobiology 2016, 26, 1029–1040. [Google Scholar] [CrossRef]
- Salazar, M.L.; Bustamante, J.; d’Alencon, C.; Díaz-Dinamarca, D.; Manubens, A.; Salazar, F.; Becker, M.I. The intracellular processing of mollusk hemocyanins from Concholepas concholepas (CCH) and Megathura crenulata (KLH) modulates their proinflammatory effects in murine dendritic cells. In Proceedings of the XLVII Annual Meeting Chilean Society for Biochemistry and Molecular Biology, La Serena, Chile, 30 September–4 October 2024; p. 195. [Google Scholar]
- Delamarre, L.; Pack, M.; Chang, H.; Mellman, I.; Trombetta, E.S. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 2005, 307, 1630–1634. [Google Scholar] [CrossRef]
- Scheiblhofer, S.; Laimer, J.; Machado, Y.; Weiss, R.; Thalhamer, J. Influence of protein fold stability on immunogenicity and its implications for vaccine design. Expert. Rev. Vaccines 2017, 16, 479–489. [Google Scholar] [CrossRef]
- Saylor, K.; Gillam, F.; Lohneis, T.; Zhang, C. Designs of Antigen Structure and Composition for Improved Protein-Based Vaccine Efficacy. Front. Immunol. 2020, 11, 283. [Google Scholar] [CrossRef]
- Colbert, J.D.; Cruz, F.M.; Rock, K.L. Cross-presentation of exogenous antigens on MHC I molecules. Curr. Opin. Immunol. 2020, 64, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Suresh, M. Vaccine adjuvants to engage the cross-presentation pathway. Front. Immunol. 2022, 13, 940047. [Google Scholar] [CrossRef]
- Diaz-Dinamarca, D.A.; Salazar, M.L.; Escobar, D.F.; Castillo, B.N.; Valdebenito, B.; Diaz, P.; Manubens, A.; Salazar, F.; Troncoso, M.F.; Lavandero, S.; et al. Surface immunogenic protein from Streptococcus agalactiae and Fissurella latimarginata hemocyanin are TLR4 ligands and activate MyD88- and TRIF dependent signaling pathways. Front. Immunol. 2023, 14, 1186188. [Google Scholar] [CrossRef]
- Svajger, U.; Anderluh, M.; Jeras, M.; Obermajer, N. C-type lectin DC-SIGN: An adhesion, signalling and antigen-uptake molecule that guides dendritic cells in immunity. Cell. Signal. 2010, 22, 1397–1405. [Google Scholar] [CrossRef] [PubMed]
- Villar, J. Participación de Receptores Lectina Tipo C en el Reconocimiento de Hemocianinas de Moluscos por Células Presentadoras de Antígeno y Consecuencias en la Presentación Antigénica. Master’s Thesis, Universidad de Chile, Santiago, Chile, May 2016. [Google Scholar]
- Becker, M.I.; Salazar, M.L.; Díaz-Dinamarca, D.; Vásquez, A.; Villar, J.; Alvarado, A.; Castillo, B.; Navarro, D.; Salazar, F.; Manubens, A. Mechanisms underlying the mollusk hemocyanin processing and presentation through MHC-dependent pathways in antigen presenting cells of mammals. J. Immunol. 2022, 208 (Suppl. 1), 102.26. [Google Scholar]
- van Montfoort, N.; Camps, M.G.; Khan, S.; Filippov, D.V.; Weterings, J.J.; Griffith, J.M.; Geuze, H.J.; van Hall, T.; Verbeek, J.S.; Melief, C.J.; et al. Antigen storage compartments in mature dendritic cells facilitate prolonged cytotoxic T lymphocyte cross-priming capacity. Proc. Natl. Acad. Sci. USA 2009, 106, 6730–6735. [Google Scholar] [CrossRef]
- Embgenbroich, M.; Burgdorf, S. Current Concepts of Antigen Cross-Presentation. Front. Immunol. 2018, 9, 1643. [Google Scholar] [CrossRef]
- Yasuda, K.; Ushio, H. Keyhole limpet hemocyanin induces innate immunity via Syk and Erk phosphorylation. EXCLI J. 2016, 15, 474–481. [Google Scholar]
- Mata-Haro, V.; Cekic, C.; Martin, M.; Chilton, P.M.; Casella, C.R.; Mitchell, T.C. The vaccine adjuvant monophosphoryl lipid A as a TRIF-biased agonist of TLR4. Science 2007, 316, 1628–1632. [Google Scholar] [CrossRef]
- Wang, G.; Wang, Y.; Ma, F. Exploiting bacterial-origin immunostimulants for improved vaccination and immunotherapy: Current insights and future directions. Cell Biosci. 2024, 14, 24. [Google Scholar] [CrossRef]
- Kuzmich, N.N.; Sivak, K.V.; Chubarev, V.N.; Porozov, Y.B.; Savateeva-Lyubimova, T.N.; Peri, F. TLR4 Signaling Pathway Modulators as Potential Therapeutics in Inflammation and Sepsis. Vaccines 2017, 5, 34. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.T.; Duthie, M.S.; Windish, H.P.; Lucas, E.A.; Guderian, J.A.; Hudson, T.E.; Shaverdian, N.; O’Donnell, J.; Desbien, A.L.; Reed, S.G.; et al. MyD88 and TRIF synergistic interaction is required for TH1-cell polarization with a synthetic TLR4 agonist adjuvant. Eur. J. Immunol. 2013, 43, 2398–2408. [Google Scholar] [CrossRef]
- Le Moigne, V. Absence of amplification role of the protein KLH on antibody response generated by a MAP Staphyloccocus aureus enterotoxin A (SEA) peptide comparing with the corresponding monomeric peptide. J. Immunol. Methods 2011, 365, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Curtsinger, J.; Berthold, M.; Malvey, K.; Bliss, R.L.; Le, C.T.; Fautsch, S.K.; Dudek, A.Z.; Blazar, B.R.; Panoskaltsis-Mortari, A. Diminished neo-antigen response to keyhole limpet hemocyanin (KLH) vaccines in patients after treatment with chemotherapy or hematopoietic cell transplantation. Clin. Immunol. 2005, 117, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- Ochando, J.; Mulder, W.J.M.; Madsen, J.C.; Netea, M.G.; Duivenvoorden, R. Trained immunity–Basic concepts and contributions to immunopathology. Nat. Rev. Nephrol. 2023, 19, 23–37. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salazar, M.L.; Díaz-Dinamarca, D.A.; Bustamante, J.; Vergara, F.; Manubens, A.; Salazar, F.; Becker, M.I. Hemocyanins: Microscopic Giants with Unique Structural Features for Applications in Biomedicine. Vaccines 2025, 13, 1086. https://doi.org/10.3390/vaccines13111086
Salazar ML, Díaz-Dinamarca DA, Bustamante J, Vergara F, Manubens A, Salazar F, Becker MI. Hemocyanins: Microscopic Giants with Unique Structural Features for Applications in Biomedicine. Vaccines. 2025; 13(11):1086. https://doi.org/10.3390/vaccines13111086
Chicago/Turabian StyleSalazar, Michelle L., Diego A. Díaz-Dinamarca, Javier Bustamante, Felipe Vergara, Augusto Manubens, Fabián Salazar, and María Inés Becker. 2025. "Hemocyanins: Microscopic Giants with Unique Structural Features for Applications in Biomedicine" Vaccines 13, no. 11: 1086. https://doi.org/10.3390/vaccines13111086
APA StyleSalazar, M. L., Díaz-Dinamarca, D. A., Bustamante, J., Vergara, F., Manubens, A., Salazar, F., & Becker, M. I. (2025). Hemocyanins: Microscopic Giants with Unique Structural Features for Applications in Biomedicine. Vaccines, 13(11), 1086. https://doi.org/10.3390/vaccines13111086




