RSV Vaccination Programme for Older Adults: A Scotland-Wide Study on RSVpreF Vaccine Safety
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
- Estimate the OE ratios of pre-specified adverse events of special interest (AESI) following RSV vaccination of older adults, using an OE design.
- Estimate the relative incidence of AESI within pre-defined risk periods following RSV vaccination of older adults compared with pre-vaccination risk periods, using a SCCS design.
2.2. Data Sources
2.3. Exposure of Interest
2.4. Outcomes of Interest
2.5. Statistical Analyses
2.5.1. Observed Versus Expected (OE)
2.5.2. Self-Controlled Case Series (SCCS)
2.5.3. Characterisation of Signals
2.5.4. Attributable Risk
3. Results
3.1. Descriptive Statistics
3.2. Observed Versus Expected (OE Analysis)
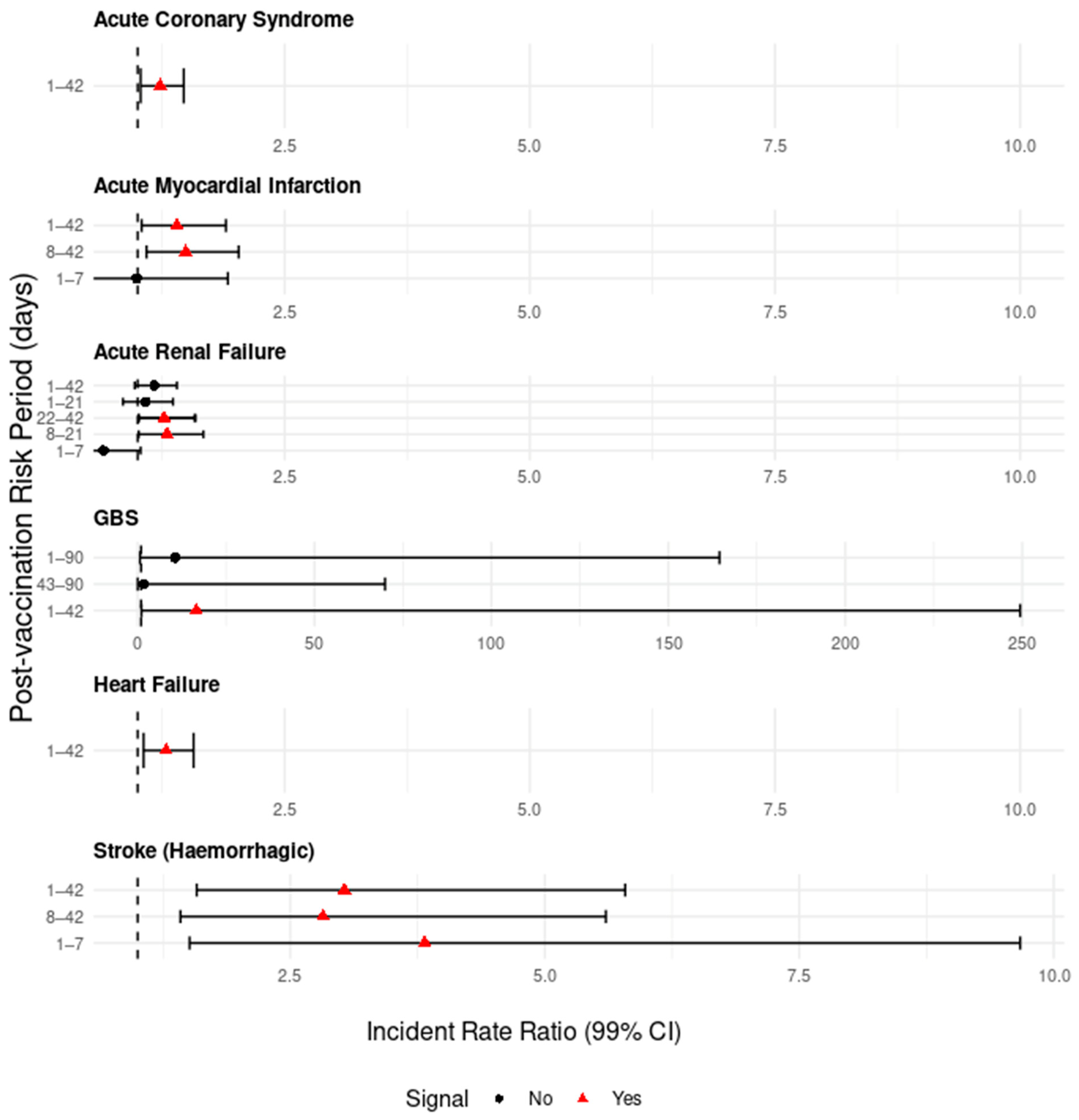
3.3. Self-Controlled Case Series Analysis (SCCS)
3.3.1. Nervous System Signals
3.3.2. Circulatory System Signals
3.3.3. Urinary System Signals
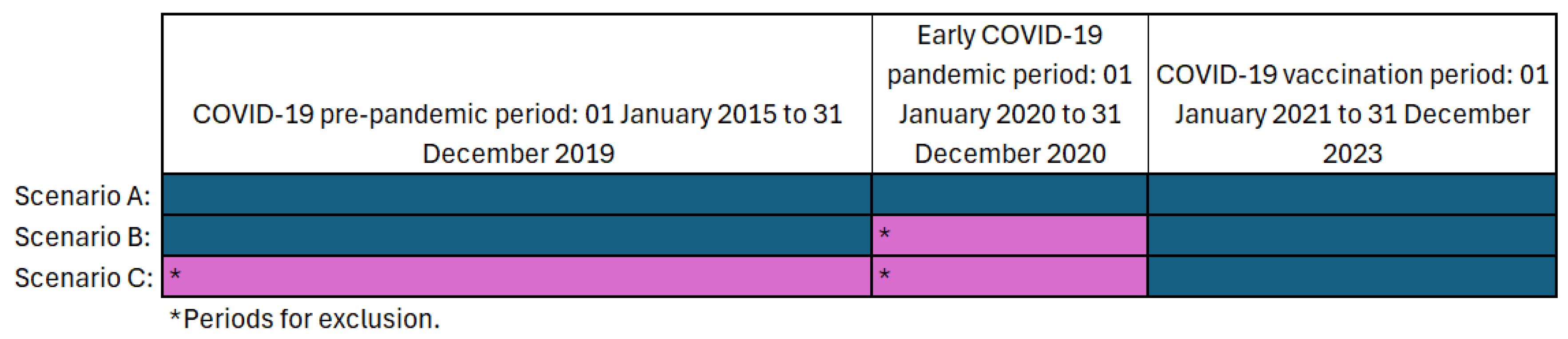
3.3.4. Seasonal Adjustments—Temporal Stratification of Calendar Time
- Temporal stratification of 28 days was used for all AESI except for GBS, which used 56 days. This was due to small numbers.
- The 43–90 day risk period for GBS is not shown due to small numbers producing an infinite upper 99% CI value (Table 4).
3.3.5. Attributable Risk for GBS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADEM | Acute Disseminated Encephalomyelitis |
| AESI | Adverse Event of Special Interest |
| AMI | Acute Myocardial Infarction |
| CI | Confidence Interval |
| DVT | Deep Vein Thrombosis |
| GBS | Guillain Barré Syndrome |
| ICD | International Classification of Diseases |
| IR | Incidence Rates |
| IRR | Incident Rate Ratio |
| LRTI | Lower Respiratory Tract Infection |
| MHRA | Medicines and Healthcare products Regulatory Agency |
| NCDS | National Clinical Data Store |
| NRS | National Records of Scotland |
| OE | Observed Versus Expected |
| PROMISE | Preparing for RSV Immunisation and Surveillance in Europe |
| RSV | Respiratory Syncytial Virus |
| RSVpreF | Respiratory Syncytial Virus Bivalent Prefusion F Vaccine |
| SCCS | Self-controlled Case Series |
| SMR01 | Scottish Morbidity Record 01 |
| US | United States |
| VMT | Vaccine Management Tool |
References
- UK Health Security Agency. Green Book on Immunisation Chapter 27a Respiratory Syncytial Virus. Available online: https://www.gov.uk/government/publications/respiratory-syncytial-virus-the-green-book-chapter-27a (accessed on 1 July 2025).
- NHS Inform. RSV Vaccine for Adults. Available online: https://www.nhsinform.scot/healthy-living/immunisation/vaccines/rsv-vaccine-for-adults/ (accessed on 7 July 2025).
- Nguyen-Van-Tam, J.S.; O’Leary, M.; Martin, E.T.; Heijnen, E.; Callendret, B.; Fleischhackl, R.; Comeaux, C.; Tran, T.M.P.; Weber, K. Burden of respiratory syncytial virus infection in older and high-risk adults: A systematic review and meta-analysis of the evidence from developed countries. Eur. Respir. Rev. 2022, 31, 220105. [Google Scholar] [CrossRef] [PubMed]
- Pfizer. European Commission Approves Pfizer’s ABRYSVO™ to Help Protect Infants Through Maternal Immunization and Older Adults from RSV. 2023. Available online: https://www.pfizer.com/news/press-release/press-release-detail/european-commission-approves-pfizers-abrysvotm-help-protect (accessed on 4 July 2025).
- Pfizer. U.S. FDA Approves ABRYSVO™, Pfizer’s Vaccine for the Prevention of Respiratory Syncytial Virus (RSV) in Older Adults. 2023. Available online: https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-abrysvotm-pfizers-vaccine-prevention#:~:text=On%20March%2024%2C%202022%2C%20Pfizer,years%20of%20age%20and%20older. (accessed on 2 July 2025).
- UK Health Security Agency. RSV Vaccination of Older Adults: Information for Healthcare Practitioners. Available online: https://www.gov.uk/government/publications/respiratory-syncytial-virus-rsv-programme-information-for-healthcare-professionals/rsv-vaccination-of-older-adults-information-for-healthcare-practioners (accessed on 30 July 2024).
- Ilangovan, K.; Radley, D.; Patton, M.; Shittu, E.; Lino, M.M.; Goulas, C.; Swanson, K.A.; Anderson, A.S.; Gurtman, A.; Munjal, I. Integrated Analysis of the Safety Experience in Adults with the Bivalent Respiratory Syncytial Virus Prefusion F Vaccine. Vaccines 2025, 13, 827. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Marc, G.P.; Zareba, A.M.; Falsey, A.R.; Jiang, Q.; Patton, M.; Polack, F.P.; Llapur, C.; Doreski, P.A.; Ilangovan, K.; et al. Efficacy and Safety of a Bivalent RSV Prefusion F Vaccine in Older Adults. N. Engl. J. Med. 2023, 388, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, P.C.; Shah, P.B.; Zhang, H.T.; Shah, N.; Nair, N.; Wan, Z.; Hu, M.; Clarke, T.C.; Chen, M.; Lin, X.; et al. Evaluation of Guillain-Barré syndrome following Respiratory Syncytial Virus Vaccination among Medicare Beneficiaries 65 Years and Older. medRxiv 2025. [Google Scholar] [CrossRef]
- Fry, S.E.; Terebuh, P.; Kaelber, D.C.; Xu, R.; Davis, P.B. Effectiveness and Safety of Respiratory Syncytial Virus Vaccine for US Adults Aged 60 Years or Older. JAMA Netw. Open 2025, 8, e258322. [Google Scholar] [CrossRef] [PubMed]
- NHS Education for Scotland. Turas Vaccine Management Tool. Available online: https://learn.nes.nhs.scot/42708/turas-vaccination-management-tool/ (accessed on 11 November 2024).
- Public Health Scotland. National Records of Scotland (NRS): Population Estimates. Available online: https://publichealthscotland.scot/resources-and-tools/health-intelligence-and-data-management/national-data-catalogue/national-datasets/search-the-datasets/national-records-of-scotland-nrs-population-estimates/ (accessed on 1 February 2025).
- Public Health Scotland. SMR01-Summary of Rules. Available online: https://publichealthscotland.scot/services/national-data-catalogue/data-dictionary/search-the-data-dictionary/smr01-summary-of-rules/?Search=S&ID=997&Title=SMR01%20-%20Summary%20of%20Rules (accessed on 16 September 2024).
- Public Health Scotland. National Records of Scotland (NRS): Deaths Data. Available online: https://publichealthscotland.scot/resources-and-tools/health-intelligence-and-data-management/national-data-catalogue/national-datasets/search-the-datasets/national-records-of-scotland-nrs-deaths-data/ (accessed on 1 May 2025).
- Cullen, L.; Grange, Z.; Antal, K.; Waugh, L.; Alsina, M.; Gibbons, C.; MacDonald, L.; Robertson, C.; Cameron, J.; Stockton, D.; et al. COVID-19 vaccine safety in Scotland-background rates of adverse events of special interest. Public Heal. 2023, 224, 1–7. [Google Scholar] [CrossRef] [PubMed]
- PROMISE. D2.4 Report on Identification of Adverse Events for Safety Evaluations of RSV Vaccination and Monoclonal Antibodies. 2024. Available online: https://edwebcontent.ed.ac.uk/sites/default/files/atoms/files/promise_d2.4-adverse-events-for-safety-evaluations_v1.0.pdf (accessed on 2 August 2024).
- World Health Organisation. International Statistical Classification of Diseases and Related Health Problems 10th Revision: Version 2019. Available online: https://icd.who.int/browse10/2019/en#/ (accessed on 2 August 2024).
- Mahaux, O.; Bauchau, V.; Van Holle, L. Pharmacoepidemiological considerations in observed-to-expected analyses for vaccines. Pharmacoepidemiol. Drug Saf. 2016, 25, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Spencer, F.A.; Goldberg, R.J.; Becker, R.C.; Gore, J.M. Seasonal Distribution of Acute Myocardial Infarction in the Second National Registry of Myocardial Infarction 1. JACC 1998, 31, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Peracha, J.; Pitcher, D.; Casula, A.; Steenkamp, R.; Medcalf, J.F.; Nitsch, D. Seasonal mortality trends for hospitalised patients with acute kidney injury across England. BMC Nephrol. 2023, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Moinuddin, A.; Lewsey, J.; Inglis, S. Effect of seasonal variation on the frequency of incident stroke hospitalizations in Scotland. Saudi J. Heal. Sci. 2015, 4, 23. [Google Scholar] [CrossRef]
- Webb, A.J.S.; A E Brain, S.; Wood, R.; Rinaldi, S.; Turner, M.R. Seasonal variation in Guillain-Barré syndrome: A systematic review, meta-analysis and Oxfordshire cohort study. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Public Health Scotland. Public Health Scotland Vaccination Surveillance Dashboard. Available online: https://scotland.shinyapps.io/phs-vaccination-surveillance/ (accessed on 26 March 2025).
- Hameed, S.S.; Robertson, C.; Morrison, K.; McQueenie, R.; McMenamin, J.; Ghebrehewet, S.; Marsh, K. Early evidence of RSV vaccination impact on hospitalisation rates of older people in Scotland. Lancet Infect. Dis. 2025, 25, 256–258. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Neurological Disorders and Stroke. Guillain-Barré Syndrome. Available online: https://www.ninds.nih.gov/health-information/disorders/guillain-barre-syndrome (accessed on 10 July 2025).
- Galeotti, F.; Massari, M.; D’Alessandro, R.; Beghi, E.; Chiò, A.; Logroscino, G.; Filippini, G.; Benedetti, M.D.; Pugliatti, M.; Santuccio, C.; et al. Risk of Guillain-Barré syndrome after 2010–2011 influenza vaccination. Eur. J. Epidemiol. 2013, 28, 433–444. [Google Scholar] [CrossRef] [PubMed]
- CDC. Guillain-Barré Syndrome and Flu Vaccine. Available online: https://www.cdc.gov/flu/vaccine-safety/guillainbarre.html (accessed on 28 July 2025).
- Medicines and Healthcare Products Regulatory Agency. Abrysvo (Pfizer RSV Vaccine) and Arexvy (GSK RSV Vaccine): Be Alert to a Small Risk of Guillain-Barré Syndrome Following Vaccination in Older Adults. Available online: https://www.gov.uk/drug-safety-update/abrysvov-pfizer-rsv-vaccine-and-arexvyv-gsk-rsv-vaccine-be-alert-to-a-small-risk-of-guillain-barre-syndrome-following-vaccination-in-older-adults (accessed on 8 July 2025).
- UK Health Security Agency. Information for Healthcare Professionals on Guillain-Barré Syndrome (GBS) Following COVID-19 Vaccination. Available online: https://www.gov.uk/government/publications/covid-19-vaccination-guillain-barre-syndrome-information-for-healthcare-professionals/information-for-healthcare-professionals-on-guillain-barre-syndrome-gbs-following-covid-19-vaccination (accessed on 10 July 2025).
- Nakai, M.; Iwanaga, Y.; Sumita, Y.; Miyamoto, Y. Impact of seasonal variation on hospital admission and in-hospital mortality of acute cardiovascular diseases: A contemporary nationwide database study. Ann. Epidemiol. 2023, 85, 100–107.e3. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.-F.; Xiao, Y.-C.; Feng, Y.-F.; Niu, L.-Y.; Tan, X.; Sun, J.-C.; Leng, Y.-Q.; Li, W.-Y.; Wang, W.-Z.; Wang, Y.-K. A systematic review and meta-analysis of cold exposure and cardiovascular disease outcomes. Front. Cardiovasc. Med. 2023, 10, 1084611. [Google Scholar] [CrossRef] [PubMed]
- Public Health Scotland. Viral Respiratory Diseases (Including Influenza and COVID-19) in Scotland Surveillance Report. 2025. Available online: https://publichealthscotland.scot/media/31090/week-2-16-01-25-viral-respiratory-diseases-including-influenza-and-covid-19-in-scotland-surveillance-report.pdf (accessed on 13 August 2025).
- Lehmann, H.C.; Hartung, H.-P.; Kieseier, B.C.; Hughes, R.A. Guillain-Barré syndrome after exposure to influenza virus. Lancet Infect. Dis. 2010, 10, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Tam, C.C.; O’Brien, S.J.; Petersen, I.; Islam, A.; Hayward, A.; Rodrigues, L.C. Guillain-Barré syndrome and preceding infection with campylobacter, influenza and Epstein-Barr virus in the general practice research database. PLoS ONE 2007, 2, e344. [Google Scholar] [CrossRef] [PubMed]
- Estabragh, Z.R.; Mamas, M.A. The cardiovascular manifestations of influenza: A systematic review. Int. J. Cardiol. 2013, 167, 2397–2403. [Google Scholar] [CrossRef] [PubMed]

| Statistical Method | Vaccinated Population (n) to 31 December 2024 | Male Sex (n, %) | Mean Age in Years (Min–Max) |
|---|---|---|---|
| Observed vs. Expected | 168,665 1 | 80,949 (48.0%) | 76.8 (75–79) |
| Self-controlled Case Series | 204,140 1 | 98,123 (48.1%) | 76.4 (74–80) |
| Adverse Event of Special Interest | Observed Events in Risk Period 1 | Expected Events in Risk Period 1 | OE Ratio (95% CI) |
|---|---|---|---|
| Acute and Subacute Hepatic Failure | 1 | 0.99 | 1.01 (0.03–5.65) |
| Acute Coronary Syndrome b | 155 | 188.19 | 0.82 (0.70–0.96) |
| Acute Myocardial Infarction b | 78 | 74.91 | 1.04 (0.82–1.30) |
| Acute Renal Failure b | 176 | 238.73 | 0.74 (0.63–0.85) |
| Anaphylactic Shock a | 0 | 0.03 | 0.00 (0.00–121.58) |
| Angina Pectoris b | 80 | 112.46 | 0.71 (0.56–0.89) |
| Atrial Fibrillation a | 251 | 330.42 | 0.76 (0.67–0.86) |
| Autoimmune Thyroiditis a | 0 | 0.51 | 0.00 (0.00–7.26) |
| Bulbar Palsy a | 4 | 3.39 | 1.18 (0.32–3.02) |
| Chronic Fatigue Syndrome a | 0 | 0.54 | 0.00 (0.00–6.89) |
| Demyelination a | 0 | 0.10 | 0.00 (0.00–37.64) |
| Disseminated Intravascular Coagulation a | 0 | 0.40 | 0.00 (0.00–9.18) |
| Deep Vein Thrombosis and Pulmonary Embolism b | 25 | 48.35 | 0.52 (0.33–0.76) |
| Encephalitis including ADEM a | 0 | 0.74 | 0.00 (0.00–4.98) |
| Facial Nerve Disorders inc. Bell’s Palsy b | 2 | 3.66 | 0.55 (0.07–1.98) |
| Fibromyalgia b | 6 | 8.86 | 0.68 (0.25–1.47) |
| GBS 2 a | 9 | 0.74 | 12.1 (5.53–22.96) |
| Heart Failure c | 114 | 146.91 | 0.18 (0.64–0.93) |
| Intracranial Venous Thrombosis a | 0 | 0.35 | 0.00 (0.00–10.52) |
| Lymphadenopathy a | 8 | 13.17 | 0.61 (0.26–1.2) |
| Multiple Sclerosis a | 8 | 6.09 | 1.31 (0.57–2.59) |
| Myasthenia Gravis a | 2 | 2.27 | 0.88 (0.11–3.18) |
| Myocarditis and Pericarditis b | 0 | 2.51 | 0.00 (0.00–1.47) |
| Narcolepsy a | 0 | 0.03 | 0.00 (0.00–112.75) |
| Neuromyelitis Optica a | 0 | 0.00 | 0.00 (0.00–4158.15) |
| Optic Neuritis a | 2 | 0.18 | 11.38 (1.38–41.10) |
| Other Arterial Thromboembolism b | 4 | 9.19 | 0.44 (0.12–1.11) |
| Other Venous Thromboembolism a | 4 | 6.42 | 0.62 (0.17–1.59) |
| Polyneuropathies and Peripheral Neuropathies b | 14 | 8.89 | 1.57 (0.86–2.64) |
| Respiratory Failure b | 23 | 31.51 | 0.73 (0.46–1.10) |
| Rheumatoid Arthritis and Polyarthritis b | 17 | 29.22 | 0.58 (0.34–0.93) |
| Seizures b | 9 | 15.69 | 0.57 (0.26–1.09) |
| Stroke (Haemorrhagic) a | 25 | 23.94 | 1.04 (0.68–1.54) |
| Stroke (Ischaemic) b | 52 | 95.19 | 0.55 (0.41–0.72) |
| Subsequent Myocardial Infarction a | 0 | 1.15 | 0.00 (0.00–3.20) |
| Thrombocytopenia a | 8 | 11.99 | 0.67 (0.29–1.31) |
| Transient Ischaemic Attack b | 22 | 21.44 | 1.03 (0.64–1.55) |
| Transverse Myelitis a | 0 | 0.05 | 0.00 (0.00–76.21) |
| Vasculitis a | 2 | 1.04 | 1.93 (0.23–6.96) |
| Interval in Days | Events/Person Days | Incidence Rate Ratio (95% CI) | p |
|---|---|---|---|
| Acute Coronary Syndrome | |||
| Baseline (−75 to −15) | 474/100,711 | Ref | - |
| Clearance (−14 to −1) | 77/23,114 | 0.71 (0.51–0.97) | 0.0048 |
| 0 | 1/1651 | 0.13 (0.01–1.69) | 0.0402 |
| 1–42 | 398/68,684 | 1.23 (1.03–1.47) | 0.0023 |
| Acute Myocardial Infarction | |||
| Baseline (−75 to −15) | 149/35,990 | Ref | - |
| Clearance (−14 to −1) | 10/8260 | 0.29 (0.13–0.68) | 0.0002 |
| 0 | 1/590 | 0.41 (0.03–5.43) | 0.3732 |
| 1–7 | 17/4124 | 0.99 (0.51–1.92) | 0.9820 |
| 8–42 | 125/20,406 | 1.49 (1.09–2.03) | 0.0011 |
| Baseline (−75 to −15) | 149/35,990 | Ref | - |
| Clearance (−14 to −1) | 10/8260 | 0.29 (0.13–0.68) | 0.0002 |
| 0 | 1/590 | 0.41 (0.03–5.43) | 0.3732 |
| 1–42 | 142/24,530 | 1.40 (1.04–1.90) | 0.0039 |
| Acute Renal Failure | |||
| Baseline (−75 to −15) | 457/107,909 | Ref | - |
| Clearance (−14 to −1) | 55/24,766 | 0.52 (0.36–0.76) | 0.0000 |
| 0 | 2/1769 | 0.27 (0.04–1.66) | 0.0623 |
| 1–7 | 34/12,350 | 0.65 (0.41–1.03) | 0.0157 |
| 8–21 | 133/24,461 | 1.30 (1.01–1.67) | 0.0085 |
| 22–42 | 189/36,117 | 1.27 (1.01–1.59) | 0.0065 |
| beyond risk periods | 826/131,701 | 1.59 (1.36–1.86) | 0.0000 |
| Baseline (−75 to −15) | 457/107,909 | Ref | - |
| Clearance (−14 to −1) | 55/24,766 | 0.52 (0.36–0.76) | 0.0000 |
| 0 | 2/1769 | 0.27 (0.04–1.66) | 0.0623 |
| 1–21 | 167/36,811 | 1.08 (0.85–1.36) | 0.4050 |
| 22–42 | 189/36,117 | 1.27 (1.01–1.58) | 0.0066 |
| beyond risk periods | 826/131,701 | 1.59 (1.36–1.86) | 0.0000 |
| Baseline (−75 to −15) | 457/107,909 | Ref | - |
| Clearance (−14 to −1) | 55/24,766 | 0.52 (0.36–0.76) | 0.0000 |
| 0 | 2/1769 | 0.27 (0.04–1.66) | 0.0623 |
| 1–42 | 356/72,928 | 1.17 (0.97–1.40) | 0.0269 |
| beyond risk periods | 826/131,701 | 1.59 (1.36–1.85) | 0.0000 |
| Guillain–Barré Syndrome | |||
| Baseline (−75 to −15) | 1/976 | Ref | - |
| Clearance (−14 to −1) | 0/224 | 0.00 (0.00–Inf) | 0.9984 |
| 0 | 0/16 | 0.00 (0.00–Inf) | 0.9996 |
| 1–42 | 10/634 | 16.60 (1.10–249.41) | 0.0076 |
| 43–90 | 1/672 | 1.76 (0.04–69.94) | 0.6942 |
| Baseline (−75 to −15) | 1/976 | Ref | - |
| Clearance (−14 to −1) | 0/224 | 0.00 (0.00–Inf) | 0.9985 |
| 0 | 0/16 | 0.00 (0.00–Inf) | 0.9996 |
| 1–90 | 11/1306 | 10.62 (0.69–164.46) | 0.0263 |
| Heart Failure | |||
| Baseline (−75 to −15) | 367/76,128 | Ref | - |
| Clearance (−14 to −1) | 58/17,472 | 0.69 (0.48–0.99) | 0.0082 |
| 0 | 0/1248 | 0.00 (0.00–Inf) | 0.9835 |
| 1–42 | 318/51,590 | 1.29 (1.06–1.57) | 0.0010 |
| Stroke (Haemorrhagic) | |||
| Baseline (−75 to −15) | 26/9150 | Ref | - |
| Clearance (−14 to −1) | 3/2100 | 0.50 (0.10–2.42) | 0.2594 |
| 0 | 0/150 | 0.00 (0.00–Inf) | 0.9953 |
| 1–7 | 11/1042 | 3.82 (1.51–9.67) | 0.0002 |
| 8–42 | 35/4981 | 2.82 (1.42–5.60) | 0.0001 |
| Baseline (−75 to −15) | 26/9150 | Ref | - |
| Clearance (−14 to −1) | 3/2100 | 0.50 (0.10–2.42) | 0.2594 |
| 0 | 0/150 | 0.00 (0.00–Inf) | 0.9952 |
| 1–42 | 46/6023 | 3.03 (1.58–5.79) | 0.0000 |
| Interval in Days | Events/Person Days | Incidence Rate Ratio (95% CI) | p |
|---|---|---|---|
| Acute Coronary Syndrome | |||
| Baseline (−75 to −15) | 474/100,711 | Ref | - |
| Clearance (−14 to −1) | 77/23,114 | 0.68 (0.46–1.01) | 0.0122 |
| 0 | 1/1651 | 0.12 (0.01–1.61) | 0.0355 |
| 1–42 | 398/68,684 | 1.16 (0.75–1.81) | 0.3748 |
| Acute Myocardial Infarction | |||
| Baseline (−75 to −15) | 149/35,990 | Ref | - |
| Clearance (−14 to −1) | 10/8260 | 0.29 (0.11–0.75) | 0.0008 |
| 0 | 1/590 | 0.39 (0.03–5.55) | 0.3650 |
| 1–7 | 17/4124 | 0.95 (0.39–2.33) | 0.8893 |
| 8–42 | 125/20,406 | 1.58 (0.68–3.65) | 0.1603 |
| Baseline (−75 to −15) | 149/35,990 | Ref | - |
| Clearance (−14 to −1) | 10/8260 | 0.28 (0.11–0.71) | 0.0005 |
| 0 | 1/590 | 0.36 (0.03–5.11) | 0.3246 |
| 1–42 | 142/24,530 | 1.24 (0.57–2.70) | 0.4721 |
| Acute Renal Failure | |||
| Baseline (−75 to −15) | 457/107,909 | Ref | - |
| Clearance (−14 to −1) | 55/24,766 | 0.49 (0.32–0.75) | 0.0000 |
| 0 | 2/1769 | 0.25 (0.04–1.60) | 0.0551 |
| 1–7 | 34/12,350 | 0.63 (0.36–1.10) | 0.0317 |
| 8–21 | 133/24,461 | 1.36 (0.88–2.12) | 0.0717 |
| 22–42 | 189/36,117 | 1.54 (0.91–2.60) | 0.0338 |
| beyond risk periods | 826/131,701 | 2.16 (1.17–3.97) | 0.0012 |
| Baseline (−75 to −15) | 457/107,909 | Ref | - |
| Clearance (−14 to −1) | 55/24,766 | 0.48 (0.31–0.74) | 0.0000 |
| 0 | 2/1769 | 0.24 (0.04–1.54) | 0.0486 |
| 1–21 | 167/36,811 | 1.03 (0.68–1.55) | 0.8682 |
| 22–42 | 189/36,117 | 1.35 (0.80–2.27) | 0.1394 |
| beyond risk periods | 826/131,701 | 1.85 (1.01–3.39) | 0.0088 |
| Baseline (−75 to −15) | 457/107,909 | Ref | - |
| Clearance (−14 to −1) | 55/24,766 | 0.47 (0.30–0.72) | 0.0000 |
| 0 | 2/1769 | 0.23 (0.04–1.48) | 0.0420 |
| 1–42 | 356/72,928 | 1.04 (0.69–1.57) | 0.8146 |
| beyond risk periods | 826/131,701 | 1.48 (0.86–2.54) | 0.0622 |
| Guillain–Barré Syndrome | |||
| Baseline (−75 to −15) | 1/976 | Ref | - |
| Clearance (−14 to −1) | 0/224 | 0.00 (0.00–Inf) | 0.9991 |
| 0 | 0/16 | 0.00 (0.00–Inf) | 0.9999 |
| 1–42 | 10/634 | 21.21 (1.15–389.63) | 0.0069 |
| 43–90 | 1/672 | 0.00 (0.00–Inf) | 0.9992 |
| Baseline (−75 to −15) | 1/976 | Ref | - |
| Clearance (−14 to −1) | 0/224 | 0.00 (0.00–Inf) | 0.9987 |
| 0 | 0/16 | 0.00 (0.00–Inf) | 0.9999 |
| 1–90 | 11/1306 | 23.95 (1.28–447.52) | 0.0052 |
| Heart Failure | |||
| Baseline (−75 to −15) | 367/76,128 | Ref | - |
| Clearance | 58/17,472 | 0.72 (0.45–1.13) | 0.0593 |
| 0 | 0/1248 | 0.00 (0.00–Inf) | 0.9894 |
| 1–42 | 318/51,590 | 1.18 (0.70–1.98) | 0.4052 |
| Stroke (Haemorrhagic) | |||
| Baseline (−75 to −15) | 26/9150 | Ref | - |
| Clearance (−14 to −1) | 3/2100 | 0.61 (0.10–3.82) | 0.4914 |
| 0 | 0/150 | 0.00 (0.00–Inf) | 0.9954 |
| 1–7 | 11/1042 | 5.55 (1.00–30.85) | 0.0100 |
| 8–42 | 35/4981 | 3.79 (0.57–25.39) | 0.0713 |
| Baseline (−75 to −15) | 26/9150 | Ref | - |
| Clearance (−14 to −1) | 3/2100 | 0.66 (0.11–4.09) | 0.5577 |
| 0 | 0/150 | 0.00 (0.00–Inf) | 0.9954 |
| 1–42 | 46/6023 | 5.05 (0.89–28.71) | 0.0164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cullen, L.A.; Gibbons, C.L.; Shi, T.; Hasan, T.; Sullivan, C.; Marsh, K.; Robertson, C.; Cameron, J.C.; Ghebrehewet, S. RSV Vaccination Programme for Older Adults: A Scotland-Wide Study on RSVpreF Vaccine Safety. Vaccines 2025, 13, 1088. https://doi.org/10.3390/vaccines13111088
Cullen LA, Gibbons CL, Shi T, Hasan T, Sullivan C, Marsh K, Robertson C, Cameron JC, Ghebrehewet S. RSV Vaccination Programme for Older Adults: A Scotland-Wide Study on RSVpreF Vaccine Safety. Vaccines. 2025; 13(11):1088. https://doi.org/10.3390/vaccines13111088
Chicago/Turabian StyleCullen, Lucy A., Cheryl L. Gibbons, Ting Shi, Taimoor Hasan, Christopher Sullivan, Kimberly Marsh, Chris Robertson, Joanne Claire Cameron, and Sam Ghebrehewet. 2025. "RSV Vaccination Programme for Older Adults: A Scotland-Wide Study on RSVpreF Vaccine Safety" Vaccines 13, no. 11: 1088. https://doi.org/10.3390/vaccines13111088
APA StyleCullen, L. A., Gibbons, C. L., Shi, T., Hasan, T., Sullivan, C., Marsh, K., Robertson, C., Cameron, J. C., & Ghebrehewet, S. (2025). RSV Vaccination Programme for Older Adults: A Scotland-Wide Study on RSVpreF Vaccine Safety. Vaccines, 13(11), 1088. https://doi.org/10.3390/vaccines13111088






